- 1Department of Oncology, Beijing Mentougou District Hospital, Beijing, China
- 2Department of Immunotherapy, Affiliated Cancer Hospital of Zhengzhou University and Henan Cancer Hospital, Zhengzhou, China
- 3Department of Radiotherapy, Affiliated Cancer Hospital of Zhengzhou University and Henan Cancer Hospital, Zhengzhou, China
Background: Previous studies have suggested that an elevated pre-treatment neutrophil-to-lymphocyte ratio (NLR) is associated with worse outcomes in patients with a variety of cancers. The purpose of this retrospective analysis is to investigate the prognostic value of the NLR in a Chinese melanoma population.
Methods: Melanoma patients were divided into two groups based on pre-treatment NLR values (≥3 vs. <3). Cox proportional hazard regression analysis and the Kaplan-Meier method were employed to study the prognostic role of the NLR for overall survival (OS) and progression-free survival (PFS).
Results: A total of 159 melanoma patients were included in this study, including 40 patients treated with PD-1 inhibitor and 119 patients treated with chemotherapy. In the PD-1 inhibitor group, the median OS was 18.0 months in the low NLR subgroup and 5.6 months in the high NLR subgroup; the median PFS was 7.0 and 2.2 months, respectively. In chemotherapy group, the median OS was 23.0 months in the low NLR group and 8.0 months in the high NLR group, and the median PFS was 9.0 and 4.0 months, respectively. Multivariate analysis showed that the NLR was significantly associated with OS and PFS in melanoma patients treated with either PD-1 inhibitor immunotherapy or chemotherapy.
Conclusion: In the Chinese population, an elevated NLR was closely related to worse survival in patients with melanoma treated with either PD-1 inhibitor monotherapy or chemotherapy.
Introduction
Melanoma is a highly aggressive malignant tumor which occurs most frequently in skin, digestive tract, genitals, nasal cavity and eye. Although the incidence of melanoma is lower in China compared to that in American and European countries, it is growing at an annual rate of 3–5%, and ~20,000 new cases are reported each year (1). In China, the mortality of melanoma also has been increasing rapidly, and most melanoma patients were diagnosed with stage III/IV disease with a really poor prognosis (2). The prognosis of patients with advanced melanoma is poor and the 5-year survival is <5% (3). Furthermore, traditional treatments, such as chemotherapy and targeted therapy, are often ineffective and susceptible to drug resistance. In addition to providing early diagnosis, determining readily, available and reliable biomarkers that can predict patient's prognosis is of great significance for choosing appropriate treatment and improving prognosis.
The clinical application of programmed cell death protein 1 (PD-1)/programmed death-ligand 1 (PD-L1) inhibitors has revolutionarily transformed cancer treatment. Recent studies have demonstrated that a variety of tumors have responses to PD-1/PD-L1 inhibitors. Moreover, if these inhibitors are effective for patients, durable responses can be observed and long-term survival is possible in these populations (4). However, a tremendous limitation of PD-1/PD-L1 inhibitors is the very low response rate (<20%), which means that most patients cannot benefit from the treatment (5, 6). Moreover, these agents have a relatively long median response time, which may cause some patients to miss the optimal treatment window. In addition, although anti-PD-1/PD-L1 antibodies have a low incidence of immune-related adverse events (irAEs), some are serious or even life-threatening (7). Given the low response rate, high cost, and risk of developing rare, yet serious irAEs of novel anti-PD-1/PD-L1 agents, it is important to investigate reliable markers to identify the most suitable patients for such therapy while sparing non-responders from toxicity.
Several predictive markers for anti-PD-1 antibody therapy are currently used in clinical practice, including PD-L1 expression, tumor mutational burden (TMB), and microsatellite instability (MSI). Among them, PD-L1 expression is the most extensively studied. Studies have shown that high PD-L1 expression (tumor proportion score [TPS] ≥ 50%) may be associated with a high response rate and long progression-free survival (PFS) and overall survival (OS) (8, 9). Other studies have reached different conclusions, showing that some patients with negative (TPS <1%)/weak (TPS = 1–49%) PD-L1 expression also have response to PD-1 blockers, whereas some patients with positive PD-L1 expression do not respond to PD-1 blockers (10). Meanwhile, PD-L1 testing requires a biopsy, which is not feasible in some patients. Moreover, PD-L1 expression is biologically heterogeneous and may be modified by previous treatments and different test methods often have different standards (11–14). This marker also has other limitations, such as high costs, and complex procedures. Therefore, alternative markers that are simpler and easier to use, economical and reliable need to be identified.
Accumulating evidence suggests that chronic inflammation plays an important role in cancer progression. As a parameter in routine blood tests, neutrophil is an important indicator of inflammation and has been extensively described in the pathophysiology of autoimmunity and infection. In the cancer setting, the traditional function of neutrophils is being challenged by recent studies, and a novel comprehension of neutrophils has arisen. Emerging evidence indicates that various cancer types often have increased neutrophil infiltration (15). Elevated neutrophils are involved in almost every stage of tumor pathologies, including but not limited to tumor growth, proliferation, invasion, metastasis and angiogenesis (16–18). Lymphocytes, as the body's most important immune cells, play a pivotal role during the antitumour immune response.
The neutrophil-to-lymphocyte ratio (NLR) is closely related to adverse prognosis in patients with various tumors, including lung cancer, colorectal cancer and renal cell carcinoma (19). Although there are also a few studies have been conducted to investigate the prognostic value of NLR in melanoma patients treated with anti-PD-1 antibodies, these studies evaluated the caucasian population, and there are no data from Chinese melanoma patients. There is a big difference between melanoma in China and Caucasians in Europe and America. The differences in genetic etiological mechanisms, pathological morphology, biological behavior, treatment methods and prognosis are quite different (20, 21). Therefore, in order to investigate the relationship between NLR and the response to anti-PD-1 antibody in Chinese melanoma patients, and to investigate the prognostic value of NLR for melanoma patients treated with chemotherapy, we conducted this retrospective study.
Patients and Methods
We retrospectively analyzed the clinical data of patients with advanced melanoma treated at our hospital between January 2010 and June 2018 and included patients treated with anti-PD-1 antibodies or chemotherapy in this study. The inclusion criteria were as follows: (1) pathologically confirmed melanoma; (2) at least 2 cycles of chemotherapy or anti-PD-1 antibody therapy; (3) at least 1 evaluable lesion; (4) available pretreatment baseline computed tomography (CT) or magnetic resonance imaging (MRI) data; (5) no uncontrolled infections or hematological diseases; and (6) initiation of PD-1 inhibitor is at least more than 4 weeks from the last treatment.
Clinicopathologic Variables
Clinicopathologic variables for the patients included age, sex, smoking history, drinking history, body mass index (BMI), pathological classification (mucosal vs. non-mucosal), Eastern Cooperative Oncology Group (ECOG) performance status (PS) score, treatment plan (chemotherapy or PD-1 inhibitors), and NLR. NLR was calculated by dividing the absolute number of neutrophils by the absolute number of lymphocytes obtained from a peripheral complete blood cell count. Complete blood cell counts were obtained from all patients within 3 weeks of therapy administration. According to Law of the People's Republic of China on the Protection of the Rights and Interests of the Elderly, we defined patients aged 60 years and older are the elderly. In this study, we used 3.0 as the cut-off value for NLR based on the median and mean of NLR and results reported in previous studies (22, 23).
Efficacy Evaluation
Efficacy was evaluated based on the Response Evaluation Criteria in Solid Tumors 1.1 (RECIST 1.1) (24). CT/MR examination was performed within 3 weeks before the treatment to assess the patients' baseline condition, and then the assessment was performed every 2 cycles of treatment or whenever patients showed apparent symptoms. OS was defined as the time from the initiation of treatment until death. PFS was defined as the time from the start of treatment to disease progression. Surviving patients and progression-free patients as of the last follow-up were defined as censored.
Statistical Analysis
The Pearson Chi-square or Fisher' exact test was used to assess the association of baseline NLR with clinical characteristics. OS and PFS were conducted and compared using the Kaplan-Meier method and the log-rank test. The prognostic value of each variable was evaluated with univariate Cox proportional hazards model and the significant univariate factors (P < 0.1) were included into multivariate. Statistical analysis was performed with SPSS version 21.0 (IBM Corp, USA). All statistical tests were two-sided, and a P ≤ 0.05 was considered as a statistically significant difference.
Results
Characteristics of the Patients
The characteristics of the patients with melanoma are shown in Table 1. A total of 159 patients were included in this retrospective analysis, including 76 males and 83 females. Most patients (76.1%) were diagnosed as non-mucosal melanoma, and 23.9% were diagnosed as mucosal melanoma. A large majority (81.1%) of patients presented with stage IV disease. Most patients received chemotherapy, and only a small minority (25.2%) of patients received PD-1 inhibitor therapy. We divided the patients into the high-NLR group (NLR ≥ 3; n = 47) and the low-NLR group (NLR <3; n = 112). No significant differences were observed in baseline data, such as sex, age, smoking history, drinking history, BMI, ECOG PS, stage, anatomic location or treatment, between the high-NLR group and the low-NLR group (Table 1).
An Elevated NLR Is Associated With Worse OS and PFS
The median OS was 22.0 months (95% confidence interval (CI): 18.2–25.9 months) in the-low NLR group and only 7.0 months (95% CI: 4.1–9.9 months) in the-high NLR group (P < 0.001) (Figure 1A), and the median PFS was 8.0 months (95% CI: 5.7–10.3 months) and 4.0 months (95% CI: 3.4–4.6 months), respectively (P < 0.001) (Figure 1B). Univariate survival analysis showed that smoking history, BMI, stage, anatomic location and NLR were related to poorer OS, whereas BMI, stage, anatomic location, treatment, and NLR were related to shorter PFS (Tables 2, 3). In multivariate analysis, NLR was still an independent factor for OS and PFS. The risk of mortality was nearly 2.5 times (hazard ratio (HR): 2.484, 95% CI: 1.706–3.615, P < 0.001) higher and the risk of disease progression was 1.9 times (HR: 1.885, 95% CI: 1.300–2.732, P = 0.001) higher for patients with elevated NLR (Tables 2, 3). In addition, multivariate analysis showed that smoking history, BMI and stage were also factors affecting OS, and stage was another factor affecting PFS (Tables 2, 3).
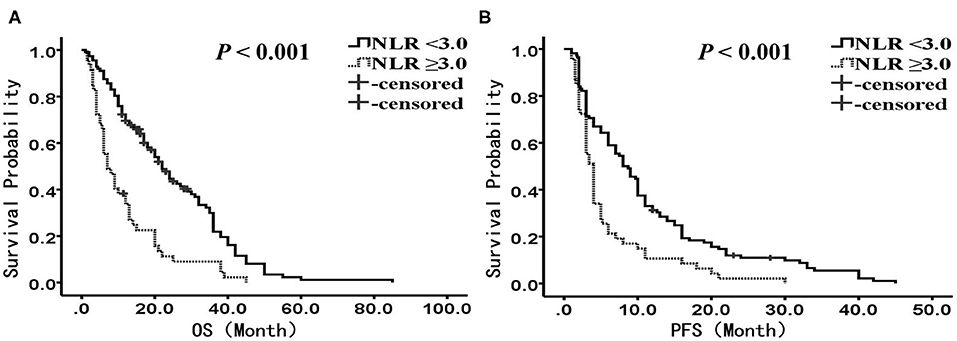
Figure 1. Kaplan-Meier analysis of OS and PFS for the entire cohort. (A) OS curve (P < 0.001). (B) PFS curve (P < 0.001). There are 112 patients presented with NLR <3, and 47 presented with NLR ≥ 3. OS, overall survival; PFS, progression-free survival; NLR, neutrophil to lymphocyte ratio.
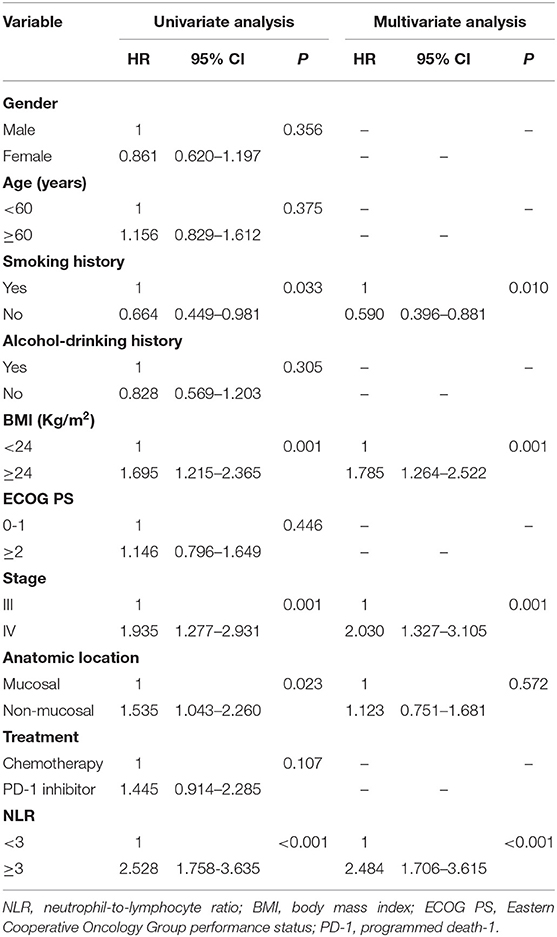
Table 2. Univariate and multivariate analysis of factors associated with overall survival for all patients.
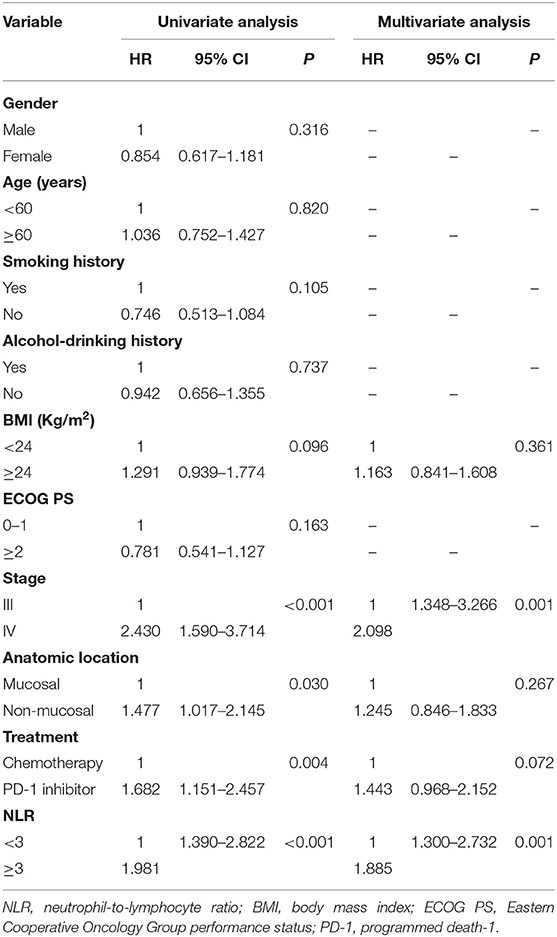
Table 3. Univariate and multivariate analysis of factors associated with progression free survival for all patients.
We performed subgroup analysis to further investigate the prognostic value of NLR in patients receiving different treatments. In the PD-1 inhibitor group (n = 40), there were 27 patients with NLR <3.0, and 13 patients with NLR ≥3.0, respectively. The median OS was 18.0 months (95% CI: 12.4–23.6 months) in the low NLR subgroup and 5.6 months (95% CI: 2.1–9.1 months) in the high NLR subgroup (P < 0.001) (Figure 2A); the median PFS was 7.0 months (95% CI: 1.8–12.2 months) and 2.2 months (95% CI: 1.0–3.4 months), respectively, with a significant difference (P < 0.001) (Figure 2B). Multivariate analysis showed that NLR was the only factor closely related to poor OS and PFS (Tables 4, 5).
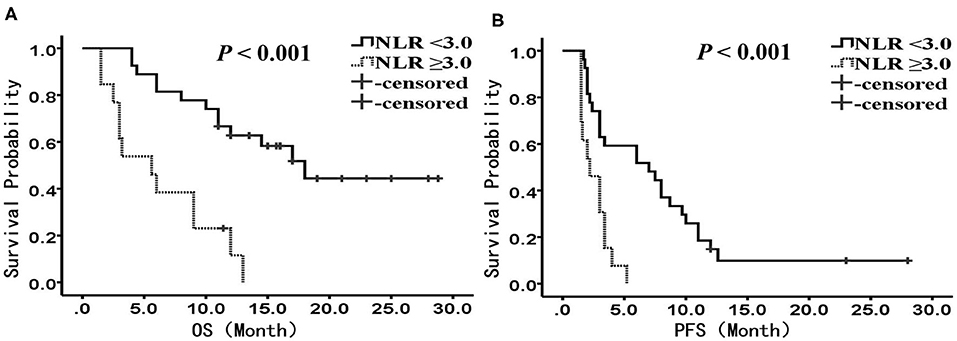
Figure 2. Kaplan-Meier analysis of OS and PFS for patients treated with PD-1 inhibitor. (A) OS curve (P < 0.001). (B) PFS curve (P < 0.001). There are 27 patients presented with NLR <3, and 13 presented with NLR ≥ 3. OS, overall survival; PFS, progression-free survival; NLR, neutrophil to lymphocyte ratio.
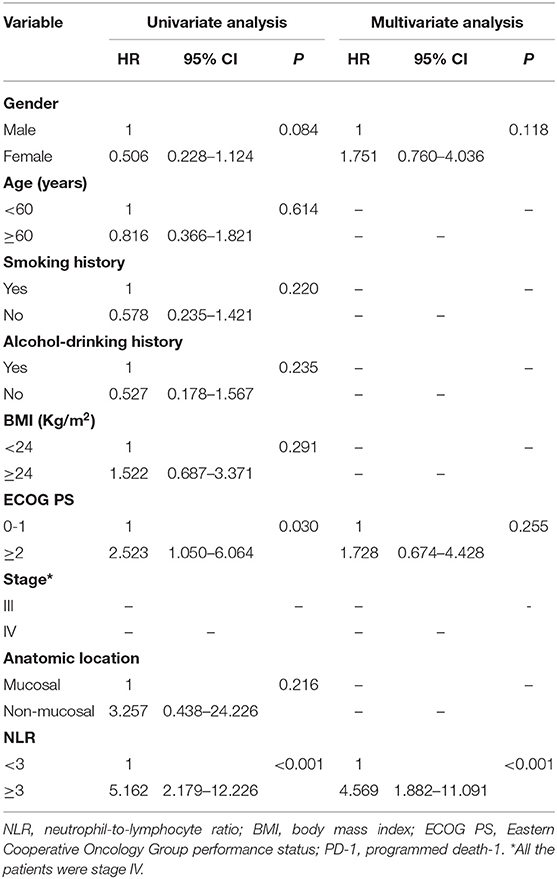
Table 4. Univariate and multivariate analysis of factors associated with overall survival for patients treated with PD-1 inhibitor.
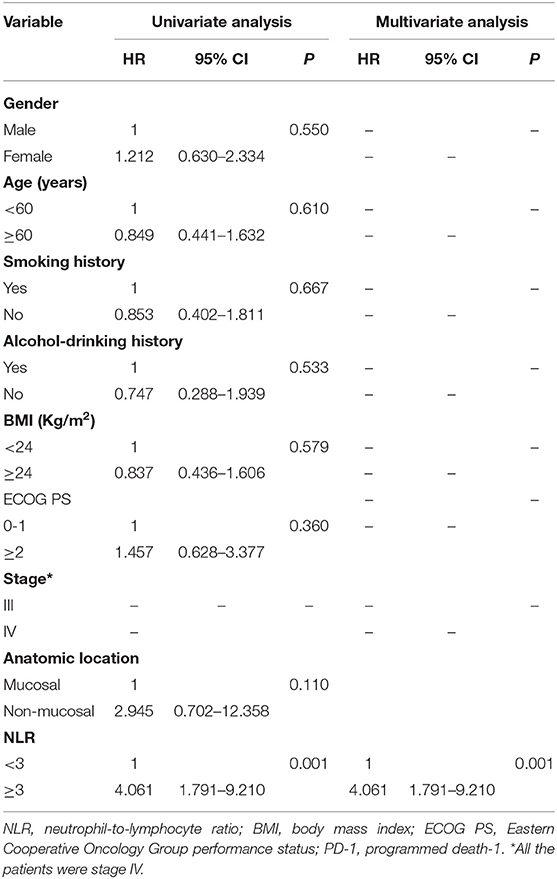
Table 5. Univariate and multivariate analysis of factors associated with progression free survival for patients treated with PD-1 inhibitor.
In the chemotherapy group (n = 119), there were 85 patients with NLR <3.0, and 34 patients with NLR ≥3.0, respectively. The median OS was 23.0 months (95% CI: 18.5–27.5 months) in the low NLR group and 8.0 months (95% CI: 4.2–11.8 months) in the high NLR group, with a significant difference (P < 0.001) (Figure 3A), and the median PFS was 9.0 months (95% CI: 7.1–11.0 months) and 4.0 months (95% CI: 3.2–4.8 months), respectively (P = 0.003) (Figure 3B). Multivariate analysis showed that NLR, smoking history, BMI, and stage were independent prognostic factors for OS, while NLR, smoking history, and stage were independent prognostic factors for PFS (Tables 6, 7).
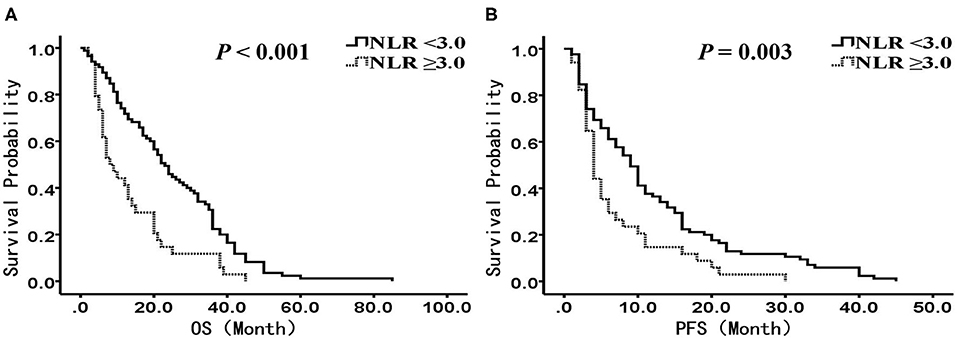
Figure 3. Kaplan-Meier analysis of OS and PFS for patients treated with chemotherapy. (A) OS curve (P < 0.001). (B) PFS curve (P = 0.003). There are 85 patients presented with NLR <3, and 34 presented with NLR ≥ 3. OS, overall survival; PFS, progression-free survival; NLR, neutrophil to lymphocyte ratio.
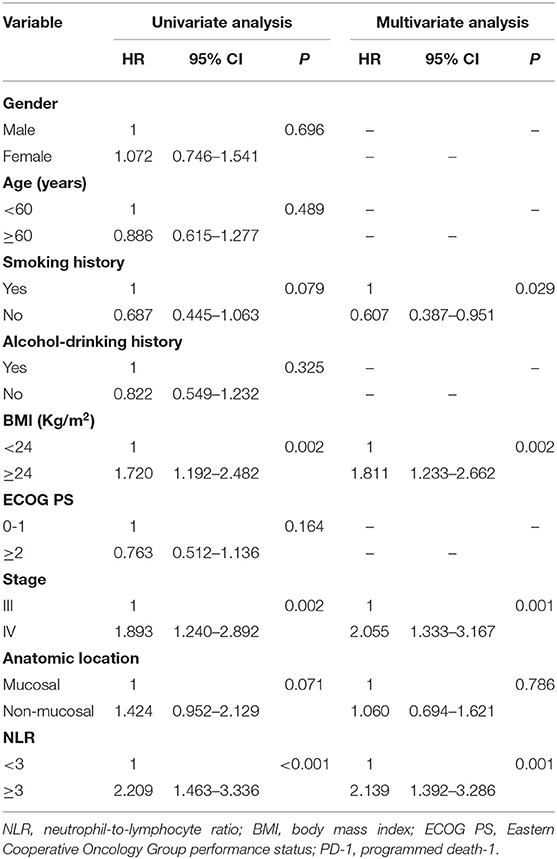
Table 6. Univariate and multivariate analysis of factors associated with overall survival for patients treated with chemotherapy.
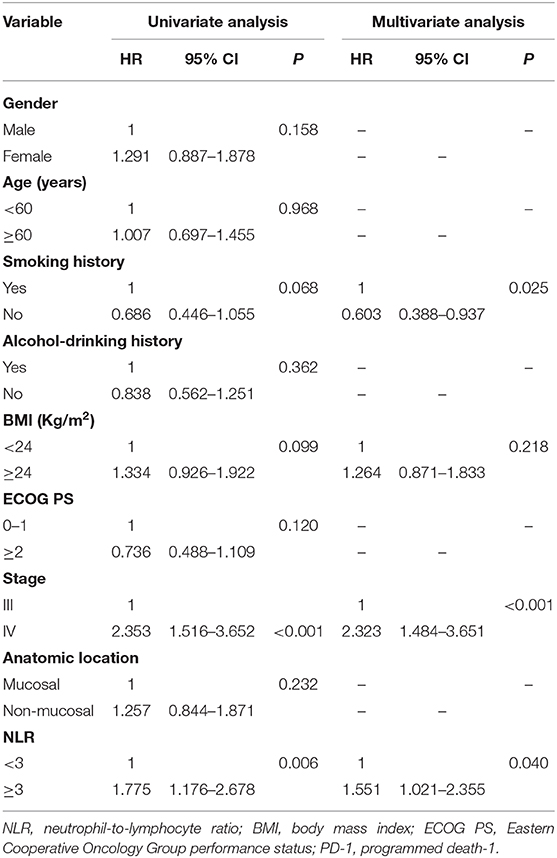
Table 7. Univariate and multivariate analysis of factors associated with progression free survival for patients treated with chemotherapy.
Discussion
In recent years, immunotherapy, especially represented by PD-1 inhibitors, has achieved significant advancement, and in certain cancers, immunotherapy has outperformed traditional chemotherapy and targeted therapy (8, 25, 26). In the development of PD-1 inhibitors, melanoma has become one of the first tumor species to undergo clinical trials. These agents have been approved as first-line and second-line treatments for metastatic melanoma based on their significant clinical efficacy. In December 2017, nivolumab was approved as a postoperative adjuvant therapy for lymph node or metastatic melanoma. These clinical data indicate that PD-1 inhibitors are highly effective. However, a significant portion of patients do not benefit from this treatment. Given that PD-1 inhibitor immunotherapy can be associated with a low response rate, significant toxicity and high treatment costs, it is important to investigate predictive biomarkers of the therapeutic response to identify patients who would maximally benefit from such therapy.
Inflammation plays an important role in many pathological processes of tumourigenesis, including but not limited to tumor initiation, proliferation, invasion, metastasis, and angiogenesis (27–29). Neutrophils are the first responders to inflammation, infection, and injury. As one of the most important and abundant types of leukocytes in the immune system, neutrophils play a vital role in these processes (30–32). As a new inflammatory marker, NLR has not been widely used as a prognostic marker, but many studies have shown that an elevated NLR is closely related to inferior outcomes for many tumors, including lung cancer (33), renal cell carcinoma (34), esophageal cancer (35), and breast cancer (36). A meta-analysis of NLR was performed including 40,559 patients with solid tumors, such asgastric cancer, liver cancer, non-small cell lung cancer, pancreatic cancer, breast cancer, soft tissue sarcoma, ovarian cancer, and cervical cancernasopharyngeal carcinoma (37). The result revealed that an elevated NLR was associated with a hazard ratio for OS of 1.81 (95% CI: 1.67–1.97, P < 0.001), an effect observed for all disease subgroups, sites, and stages. Hazard ratios for NLR greater than the cut-off for PFS and disease-free survival were 1.63 and 2.27, respectively (all P < 0.001). Currently, most studies focus on the relationship between NLR and prognosis after conventional treatments such as surgery and chemotherapy. With the widespread clinical application of PD-1 inhibitors, current data have indicated that NLR has the potential to become a promising prognostic marker.
A retrospective analysis of 97 patients with stage IV melanoma who received nivolumab immunotherapy showed a significant difference in OS (2.9 vs. 16.0 months, P < 0.0001) and PFS (2.0 vs. 9.0 months, P < 0.0001) between the high NLR group (NLR ≥ 5) and the low NLR group (NLR <5) (38). The response of patients with an NLR <5 was also better than those with an NLR ≥ 5. Multivariate analysis showed that NLR was closely related to OS, PFS and clinical response and that an elevated NLR was a predictor of poor outcomes. In another cohort containing 101 patients with unresectable stage III or IV melanoma treated with anti-PD-1 antibodies, the results showed that along with known prognostic factors, such as lactate dehydrogenase (LDH), PS score, and symptomatic brain metastasis, NLR was an independent prognostic factor for patient outcomes and was closely related to worse survival (39). Similarly, in a recent publication of 224 patients with stage IV melanoma treated with anti-PD-1 immunotherapy (nivolumab or pembrolizumab) as the initial treatment, the baseline NLR was closely related to ECOG PS and the number of metastatic sites, and elevated NLR was associated with lower ECOG PS and more metastatic sites (40). Survival analysis showed that an elevated baseline NLR was independently and significantly associated with an increased risk of death and disease progression in patients with either mucosal melanoma or non-mucosal melanoma, regardless of the mutation status of v-raf murine sarcoma viral oncogene homolog B1 (BRAF) gene. Furthermore, NLR dynamic monitoring showed that an increase in NLR by 30% from baseline after 2 cycles of treatment was also associated with treatment failure and death. Other studies showed that neutrophils were also closely related to tumor ulceration and tumor thickness, both of which were independent negative prognostic factors previously confirmed in melanoma patients (41–43).
To investigate the prognostic value of NLR in Chinese melanoma patients, we conducted this retrospective analysis. In this study, the basic clinical data of 159 patients with advanced melanoma were analyzed. The results indicated that elevated NLR was closely related to worse OS and PFS. The risk of death was nearly 2.5 times higher and the risk of disease progression was 1.9 times higher in the high-NLR group (NLR ≥ 3) compared with the low-NLR group (NLR <3). We further performed subgroup analysis to investigate the relationship between NLR and clinical outcomes of treatment with anti-PD-1 antibodies. Forty patients with stage IV melanoma received anti-PD-1 antibodies after failure of multiple lines of chemotherapy. We analyzed the effect of sex, age, smoking history, drinking history, BMI, ECOG PS, stage, anatomic site, and the NLR on prognosis. Multivariate analysis showed that NLR was the only independent prognostic factor for OS and PFS. The risk of mortality was 4.6 times higher and the risk of disease progression was 4.1 times higher in the high NLR group than in the low NLR group. Surprisingly, we found that an elevated NLR was also an independent prognostic factor in patients receiving chemotherapy and that the risk of mortality was 2.0 times higher and the risk of disease progression was 1.6 time higher in the high-NLR group than in the low-NLR group. To the best of our knowledge, this is the first study to confirm that an elevated NLR is associated with worse clinical outcomes in melanoma patients treated with either anti-PD-1 antibody therapy or chemotherapy in a Chinese population. These results provide strong evidence for the clinical use of NLR as a prognostic marker to optimize patient benefit.
However, the actual mechanism underlying the relationship between an elevated NLR and poor outcomes remains unclear. Several possible explanations have been reported. Melanoma is highly invasive and is prone to metastases. Most patients die due to metastases via the lymphatic and circulatory systems. During these processes, neutrophils play an important role. As an important chemokine, C-X-C motif chemokine ligand 5 (CXCL5) is a critical factor in the distant metastasis of various tumors including melanoma, and the role of CXCL5 in tumor metastasis depends on neutrophils (41, 44). CXCL5 expression is significantly increased in patients with advanced melanoma. Compared with melanoma cells with no or low CXCL5 expression, melanoma cells with high CXCL5 expression have significantly greater neutrophil infiltration, suggesting that CXCL5 recruits a large number of neutrophils to melanomas and that the interaction between neutrophils and melanoma cells further promotes tumor cell migration to lymphatic vessels, ultimately leading to lymph node metastasis.
Neutrophils promote not only lymphatic metastasis but also blood metastasis of melanoma. Activated neutrophils have potent adhesion and migration capacities, where β2 integrins play an important role. Intercellular adhesion molecule-1 (ICAM-1) is expressed on the surface of melanoma cells. As a ligand of β2 integrin, ICAM-1 binds to β2 integrin, thereby forming a complex between melanoma cells and neutrophils, which enhances melanoma adhesion to and migration through vascular endothelial cells (45, 46). Moreover, many studies have reported that melanoma can continuously secrete chemokines such as interleukin-8 (IL-8), which upregulates the activity of β2 integrin on the surface of neutrophils, further promoting the binding between ICAM-1 on the surface of melanoma cells and β2 integrin on the surface of neutrophils (47–49). Through this mechanism, neutrophils enhance melanoma adhesion to the vascular endothelial cells, thereby promoting distant metastasis of melanoma via blood circulation.
Another important mechanism involves neutrophil extracellular traps (NETs), fibrillar structures, secreted by neutrophils under the effect of various stimulating factors and involved in the innate immune response (50, 51). Many recent studies have shown that in addition to the innate immune response, NETs initiate and promote tumor progression and metastasis via several mechanisms (52–54). NETs are composed of depolymerized chromatin and different protein particles, including a loose DNA skeleton, matrix metalloproteinase-9 (MMP-9), neutrophil elastase (NE), cathepsin G (CG), and myeloperoxidase (50). The extracellular matrix (ECM) acts as a barrier during tumor cell invasion and blocks tumor cells from entering the circulatory system. Therefore, degradation of the ECM will facilitate tumor metastasis. As a primary component of NETs, MMP-9 is mainly secreted by neutrophils and plays an important role in ECM remodeling and membrane protein cleavage, promoting cancer cell invasion, migration, metastasis, angiogenesis, inflammation, and proliferation (55–59).
NE is another important protease released by NETs. As a protease implicated in matrix remodeling in various stages of tumor progression, its expression and actively is up regulated in a variety of human tumors, and it correlates with not only disease state but also disease progression (60). NE can activate the extracellular transactivation of membrane receptors such as epidermal growth factor receptor (EGFR) and Toll-like receptor 4 (TLR4), inducing mitogen activated protein kinase (MAPK) signaling and downstream effects, thereby directly promoting cancer cell proliferation. In a mouse model of lung adenocarcinoma [Lox-Stop-Stop (LsL)-K-ras], NE can enter tumor cells to directly induce tumor cell proliferation (61). In addition, NE can indirectly promote tumor cell growth and proliferation by inactivating tumor suppressors, such as Elastin Microfibril Interface Located Protein 1 (EMILIN1) and thrombospondin-1 (62, 63).
NETs can also promote the adhesion of circulating tumor cells to vascular endothelial cells, thereby destroying the integrity of vascular endothelial cells, increasing vascular permeability, and promoting blood metastasis (64–66). The mesh structure of NETs has the capacity to trap tumor cells to form a physical barrier between immune cells and tumor cells, thereby allowing tumor cells to escape surveillance and attack by the immune system (67). These studies indicate that the function of neutrophils is not limited to the processes of inflammation; neutrophils also play a very important role in the stages of tumor origination, growth, proliferation, invasion, metastasis, and immune escape through various complex mechanisms.
The main limitations of this study are related to its retrospective nature and it is a mono-center study. Therefore, there is a risk of bias and confounding factors in patient selection. To control for this, we developed strict inclusion and exclusion criteria, and our results were consistent with those of previous studies. In addition, the sample size of this study was not very large, especially the number of patients receiving PD-1 inhibitor treatment. Third, for objective reasons, we lost some clinical and pathological data, such as LDH, BRAF mutation status, and PD-L1 expression. Last, at present, there is no unified standard for the calculation of the NLR cut-off value. We used a value of 3.0 as the cut-off in this analysis based on our data analysis and previous studies. However, further research is needed to validate this cut-off value. Taking into account these factors, we hope that in the future more prospective, randomized and large-scale clinical trials will be conducted and provide more reliable data.
This study suggests that an elevated NLR is closely and independently related to poor clinical outcomes in advanced melanoma patients receiving either PD-1 inhibitor immunotherapy or chemotherapy. Notably, NLR testing is economic, readily available, non-invasive, biopsy-independent, repeatable, and dynamic. Therefore, NLR may be an ideal prognostic indicator for patients with melanomas. In the future, prospective, multi-center clinical trials are needed to confirm this conclusion, and basic studies are also needed to further clarify the mechanism by which neutrophils promote tumor progression to enable NLR application in clinical practice as soon as possible, helping clinicians to develop more suitable and effective treatment regimens for patients.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by The Ethics Committee of Henan Cancer Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
YQ and YZ performed the literature search and designed the study. YQ, DL, and DM contributed to the acquisition, analysis, and interpretation of the data. DM and DL contributed to data collection. YQ wrote the original draft of the manuscript. YZ, DL, and YL edited the manuscript. All authors read and approved the final version.
Funding
This study was supported by Henan Provincial Scientific and Technological Project (grant no. 162300410095), and Henan Medical Science and Technique Foundation (grant no. 201701030).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Guo J, Qin S, Liang J, Lin T, Si L, Chen X, et al. Chinese guidelines on the diagnosis and treatment of melanoma (2015 edition). Chin Clin Oncol. (2016) 5:57. doi: 10.21037/cco.2015.12.02
2. Yu J, Luo X, Huang H, Zhai Z, Shen Z, Lin H. Clinical characteristics of malignant melanoma in Southwest China: a single-center series of 82 consecutive cases and a meta-analysis of 958 reported cases. PLoS ONE. (2016) 11:e165591. doi: 10.1371/journal.pone.0165591
3. Leichsenring J, Stögbauer F, Volckmar AL, Buchhalter I, Oliveira C, Kirchner M, et al. Genetic profiling of melanoma in routine diagnostics: assay performance and molecular characteristics in a consecutive series of 274 cases. Pathology. (2018) 50:703–10. doi: 10.1016/j.pathol.2018.08.004
4. Betof WA, Palmer JS, Shoushtari AN, Goldman DA, Panageas KS, Hayes SA, et al. Long-term outcomes and responses to retreatment in patients with melanoma treated with PD-1 blockade. J Clin Oncol. (2020) 38:O1901464. doi: 10.1200/JCO.19.01464
5. Gettinger S, Horn L, Jackman D, Spigel D, Antonia S, Hellmann M, et al. Five-year follow-up of nivolumab in previously treated advanced non-small-cell lung cancer: results from the CA209-003 study. J Clin Oncol. (2018) 36:1675–84. doi: 10.1200/JCO.2017.77.0412
6. Vokes EE, Ready N, Felip E, Horn L, Burgio MA, Antonia SJ, et al. Nivolumab versus docetaxel in previously treated advanced non-small-cell lung cancer (CheckMate 017 and CheckMate 057): 3-year update and outcomes in patients with liver metastases. Ann Oncol. (2018) 29:959–65. doi: 10.1093/annonc/mdy041
7. Sacks D, Baxter B, Campbell B, Carpenter JS, Cognard C, Dippel D, et al. Multisociety consensus quality improvement revised consensus statement for endovascular therapy of acute ischemic stroke. Int J Stroke. (2018) 13:612–32. doi: 10.1177/1747493018778713
8. Antonia SJ, Borghaei H, Ramalingam SS, Horn L, De Castro CJ, Pluzanski A, et al. Four-year survival with nivolumab in patients with previously treated advanced non-small-cell lung cancer: a pooled analysis. Lancet Oncol. (2019) 20:1395–408. doi: 10.1016/S1470-2045(19)30407-3
9. Tray N, Weber JS, Adams S. Predictive biomarkers for checkpoint immunotherapy: current status and challenges for clinical application. Cancer Immunol Res. (2018) 6:1122–8. doi: 10.1158/2326-6066.CIR-18-0214
10. Evans M, O'Sullivan B, Hughes F, Mullis T, Smith M, Trim N, et al. The clinicopathological and molecular associations of PD-L1 Expression in non-small cell lung cancer: analysis of a series of 10,005 cases tested with the 22c3 assay. Pathol Oncol Res. (2020) 26:79–89. doi: 10.1007/s12253-018-0469-6
11. Rebelatto MC, Midha A, Mistry A, Sabalos C, Schechter N, Li X, et al. Development of a programmed cell death ligand-1 immunohistochemical assay validated for analysis of non-small cell lung cancer and head and neck squamous cell carcinoma. Diagn Pathol. (2016) 11:95. doi: 10.1186/s13000-016-0545-8
12. Ilie M, Hofman V, Dietel M, Soria JC, Hofman P. Assessment of the PD-L1 status by immunohistochemistry: challenges and perspectives for therapeutic strategies in lung cancer patients. Virchows Arch. (2016) 468:511–25. doi: 10.1007/s00428-016-1910-4
13. Tsunoda A, Morikawa K, Inoue T, Miyazawa T, Hoshikawa M, Takagi M, et al. A prospective observational study to assess PD-L1 expression in small biopsy samples for non-small-cell lung cancer. Bmc Cancer. (2019) 19:546. doi: 10.1186/s12885-019-5773-3
14. Kerr KM, Tsao MS, Nicholson AG, Yatabe Y, Wistuba II, Hirsch FR. Programmed death-ligand 1 immunohistochemistry in lung cancer: in what state is this art? J Thorac Oncol. (2015) 10:985–9. doi: 10.1097/JTO.0000000000000526
15. Lerman I, Garcia-Hernandez ML, Rangel-Moreno J, Chiriboga L, Pan C, Nastiuk KL, et al. Infiltrating myeloid cells exert protumorigenic actions via neutrophil elastase. Mol Cancer Res. (2017) 15:1138–52. doi: 10.1158/1541-7786.MCR-17-0003
16. Coffelt SB, Wellenstein MD, de Visser KE. Neutrophils in cancer: neutral no more. Nat Rev Cancer. (2016) 16:431–46. doi: 10.1038/nrc.2016.52
17. Moses K, Brandau S. Human neutrophils: their role in cancer and relation to myeloid-derived suppressor cells. Semin Immunol. (2016) 28:187–96. doi: 10.1016/j.smim.2016.03.018
18. Dumitru CA, Lang S, Brandau S. Modulation of neutrophil granulocytes in the tumor microenvironment: mechanisms and consequences for tumor progression. Semin Cancer Biol. (2013) 23:141–8. doi: 10.1016/j.semcancer.2013.02.005
19. Moschetta M, Uccello M, Kasenda B, Mak G, McClelland A, Boussios S, et al. Dynamics of neutrophils-to-lymphocyte ratio predict outcomes of PD-1/PD-L1 blockade. Biomed Res Int. (2017) 2017:1506824. doi: 10.1155/2017/1506824
20. Luo Y, Zhang Z, Liu J, Li L, Xu X, Yao X, et al. Characterizations of gene alterations in melanoma patients from Chinese population. Biomed Res Int. (2020) 2020:6096814. doi: 10.1155/2020/6096814
21. Cormier JN, Xing Y, Ding M, Lee JE, Mansfield PF, Gershenwald JE, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. (2006) 166:1907–14. doi: 10.1001/archinte.166.17.1907
22. Zhan H, Ma JY, Jian QC. Prognostic significance of pretreatment neutrophil-to-lymphocyte ratio in melanoma patients: a meta-analysis. Clin Chim Acta. (2018) 484:136–40. doi: 10.1016/j.cca.2018.05.055
23. Ding Y, Zhang S, Qiao J. Prognostic value of neutrophil-to-lymphocyte ratio in melanoma: evidence from a PRISMA-compliant meta-analysis. Medicine. (2018) 97:e11446. doi: 10.1097/MD.0000000000011446
24. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. (2009) 45:228–47. doi: 10.1016/j.ejca.2008.10.026
25. Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. (2015) 372:2521–32. doi: 10.1056/NEJMoa1503093
26. Motzer RJ, Tannir NM, McDermott DF, Frontera OA, Melichar B, Choueiri TK, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. (2018) 378:1277–90. doi: 10.1056/NEJMoa1712126
27. Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. (2014) 15:e493–503. doi: 10.1016/S1470-2045(14)70263-3
28. Crusz SM, Balkwill FR. Inflammation and cancer: advances and new agents. Nat Rev Clin Oncol. (2015) 12:584–96. doi: 10.1038/nrclinonc.2015.105
29. Murata M. Inflammation and cancer. Environ Health Prev Med. (2018) 23:50. doi: 10.1186/s12199-018-0740-1
30. Swierczak A, Mouchemore KA, Hamilton JA, Anderson RL. Neutrophils: important contributors to tumor progression and metastasis. Cancer Metastasis Rev. (2015) 34:735–51. doi: 10.1007/s10555-015-9594-9
31. Zhang X, Zhang W, Yuan X, Fu M, Qian H, Xu W. Neutrophils in cancer development and progression: roles, mechanisms, and implications (Review). Int J Oncol. (2016) 49:857–67. doi: 10.3892/ijo.2016.3616
32. Wu L, Saxena S, Singh RK. Neutrophils in the tumor microenvironment. Adv Exp Med Biol. (2020) 1224:1–20. doi: 10.1007/978-3-030-35723-8_1
33. Russo A, Russano M, Franchina T, Migliorino MR, Aprile G, Mansueto G, et al. Neutrophil-to-Lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and outcomes with nivolumab in pretreated non-small cell lung cancer (NSCLC): a large retrospective multicenter study. Adv Ther. (2020) 37:1145–55. doi: 10.1007/s12325-020-01229-w
34. Lalani AA, Xie W, Martini DJ, Steinharter JA, Norton CK, Krajewski KM, et al. Change in neutrophil-to-lymphocyte ratio (NLR) in response to immune checkpoint blockade for metastatic renal cell carcinoma. J Immunother Cancer. (2018) 6:5. doi: 10.1186/s40425-018-0315-0
35. Li KJ, Xia XF, Su M, Zhang H, Chen WH, Zou CL. Predictive value of lymphocyte-to-monocyte ratio (LMR) and neutrophil-to-lymphocyte ratio (NLR) in patients with oesophageal cancer undergoing concurrent chemoradiotherapy. Bmc Cancer. (2019) 19:1004. doi: 10.1186/s12885-019-6157-4
36. Moon G, Noh H, Cho IJ, Lee JI, Han A. Prediction of late recurrence in patients with breast cancer: elevated neutrophil to lymphocyte ratio (NLR) at 5 years after diagnosis and late recurrence. Breast Cancer-Tokyo. (2020) 27:54–61. doi: 10.1007/s12282-019-00994-z
37. Templeton AJ, McNamara MG, Šeruga B, Vera-Badillo FE, Aneja P, Ocaña A, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. (2014) 106:u124. doi: 10.1093/jnci/dju124
38. Capone M, Giannarelli D, Mallardo D, Madonna G, Festino L, Grimaldi AM, et al. Baseline neutrophil-to-lymphocyte ratio (NLR) and derived NLR could predict overall survival in patients with advanced melanoma treated with nivolumab. J Immunother Cancer. (2018) 6:74. doi: 10.1186/s40425-018-0383-1
39. Garnier M, Zaragoza J, Bénéton N, Bens G, Meurisse V, Samimi M, et al. High neutrophil-to-lymphocyte ratio before starting anti-programmed cell death 1 immunotherapy predicts poor outcome in patients with metastatic melanoma. J Am Acad Dermatol. (2018) 79:165–7. doi: 10.1016/j.jaad.2018.03.006
40. Bartlett EK, Flynn JR, Panageas KS, Ferraro RA, Sta CJ, Postow MA, et al. High neutrophil-to-lymphocyte ratio (NLR) is associated with treatment failure and death in patients who have melanoma treated with PD-1 inhibitor monotherapy. Cancer-Am Cancer Soc. (2020) 126:76–85. doi: 10.1002/cncr.32506
41. Soler-Cardona A, Forsthuber A, Lipp K, Ebersberger S, Heinz M, Schossleitner K, et al. CXCL5 facilitates melanoma cell-neutrophil interaction and lymph node metastasis. J Invest Dermatol. (2018) 138:1627–35. doi: 10.1016/j.jid.2018.01.035
42. Bønnelykke-Behrndtz ML, Steiniche T. Ulcerated Melanoma: Aspects and Prognostic Impact, In Ward WH, Farma JM editors. Cutaneous Melanoma: Etiology and Therapy (Brisbane, AU: Codon Publications). (2017) 5:67–75. doi: 10.15586/codon.cutaneousmelanoma.2017.ch5
43. Mandalà M, Massi D. Tissue prognostic biomarkers in primary cutaneous melanoma. Virchows Arch. (2014) 464:265–81. doi: 10.1007/s00428-013-1526-x
44. Forsthuber A, Lipp K, Andersen L, Ebersberger S, Graña-Castro Ellmeier W, et al. CXCL5 as regulator of neutrophil function in cutaneous melanoma. J Invest Dermatol. (2019) 139:186–94. doi: 10.1016/j.jid.2018.07.006
45. Liang S, Slattery MJ, Dong C. Shear stress and shear rate differentially affect the multi-step process of leukocyte-facilitated melanoma adhesion. Exp Cell Res. (2005) 310:282–92. doi: 10.1016/j.yexcr.2005.07.028
46. Slattery MJ, Liang S, Dong C. Distinct role of hydrodynamic shear in leukocyte-facilitated tumor cell extravasation. Am J Physiol Cell Physiol. (2005) 288:C831–9. doi: 10.1152/ajpcell.00439.2004
47. Tobin RP, Jordan KR, Kapoor P, Spongberg E, Davis D, Vorwald VM, et al. IL-6 and IL-8 are linked with myeloid-derived suppressor cell accumulation and correlate with poor clinical outcomes in melanoma patients. Front Oncol. (2019) 9:1223. doi: 10.3389/fonc.2019.01223
48. Peng HH, Liang S, Henderson AJ, Dong C. Regulation of interleukin-8 expression in melanoma-stimulated neutrophil inflammatory response. Exp Cell Res. (2007) 313:551–9. doi: 10.1016/j.yexcr.2006.10.030
49. Seo SM, McIntire LV, Smith CW. Effects of IL-8, Gro-alpha, and LTB(4) on the adhesive kinetics of LFA-1 and Mac-1 on human neutrophils. Am J Physiol Cell Physiol. (2001) 281:C1568–78. doi: 10.1152/ajpcell.2001.281.5.C1568
50. Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science. (2004) 303:1532–5. doi: 10.1126/science.1092385
51. Burgener SS, Schroder K. Neutrophil extracellular traps in host defense. Cold Spring Harb Perspect Biol. (2019) doi: 10.1101/cshperspect.a037028
52. Demers M, Wong SL, Martinod K, Gallant M, Cabral JE, Wang Y, et al. Priming of neutrophils toward NETosis promotes tumor growth. Oncoimmunology. (2016) 5:e1134073. doi: 10.1080/2162402X.2015.1134073
53. Takesue S, Ohuchida K, Shinkawa T, Otsubo Y, Matsumoto S, Sagara A, et al. Neutrophil extracellular traps promote liver micrometastasis in pancreatic ductal adenocarcinoma via the activation of cancer-associated fibroblasts. Int J Oncol. (2020) 56:596–605. doi: 10.3892/ijo.2019.4951
54. Erpenbeck L, Schön MP. Neutrophil extracellular traps: protagonists of cancer progression? Oncogene. (2017) 36:2483–90. doi: 10.1038/onc.2016.406
55. Vandooren J, Van den Steen PE, Opdenakker G. Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9): the next decade. Crit Rev Biochem Mol Biol. (2013) 48:222–72. doi: 10.3109/10409238.2013.770819
56. Farina AR, Mackay AR. Gelatinase B/MMP-9 in tumour pathogenesis and progression. Cancers. (2014) 6:240–96. doi: 10.3390/cancers6010240
57. Huang H. Matrix metalloproteinase-9 (MMP-9) as a cancer biomarker and MMP-9 biosensors: recent advances. Sensors. (2018) 18. doi: 10.3390/s18103249
58. Hsu CC, Huang SF, Wang JS, Chu WK, Nien JE, Chen WS, et al. Interplay of N-Cadherin and matrix metalloproteinase 9 enhances human nasopharyngeal carcinoma cell invasion. BMC Cancer. (2016) 16:800. doi: 10.1186/s12885-016-2846-4
59. Qorri B, Kalaydina RV, Velickovic A, Kaplya Y, Decarlo A, Szewczuk MR. Agonist-biased signaling via matrix metalloproteinase-9 promotes extracellular matrix remodeling. Cells-Basel. (2018) 7:117. doi: 10.3390/cells7090117
60. Lerman I, Hammes SR. Neutrophil elastase in the tumor microenvironment. Steroids. (2018) 133:96–101. doi: 10.1016/j.steroids.2017.11.006
61. Houghton AM, Rzymkiewicz DM, Ji H, Gregory AD, Egea EE, Metz HE, et al. Neutrophil elastase-mediated degradation of IRS-1 accelerates lung tumor growth. Nat Med. (2010) 16:219–23. doi: 10.1038/nm.2084
62. Maiorani O, Pivetta E, Capuano A, Modica TM, Wassermann B, Bucciotti F, et al. Neutrophil elastase cleavage of the gC1q domain impairs the EMILIN1-α4β1 integrin interaction, cell adhesion and anti-proliferative activity. Sci Rep. (2017) 7:39974. doi: 10.1038/srep39974
63. El RT, Catena R, Lee S, Stawowczyk M, Joshi N, Fischbach C, et al. Lung inflammation promotes metastasis through neutrophil protease-mediated degradation of Tsp-1. Proc Natl Acad Sci USA. (2015) 112:16000–5. doi: 10.1073/pnas.1507294112
64. Kolaczkowska E, Jenne CN, Surewaard BG, Thanabalasuriar A, Lee WY, Sanz MJ, et al. Molecular mechanisms of NET formation and degradation revealed by intravital imaging in the liver vasculature. Nat Commun. (2015) 6:6673. doi: 10.1038/ncomms7673
65. Park J, Wysocki RW, Amoozgar Z, Maiorino L, Fein MR, Jorns J, et al. Cancer cells induce metastasis-supporting neutrophil extracellular DNA traps. Sci Transl Med. (2016) 8:138r−361. doi: 10.1126/scitranslmed.aag1711
66. Najmeh S, Cools-Lartigue J, Rayes RF, Gowing S, Vourtzoumis P, Bourdeau F, et al. Neutrophil extracellular traps sequester circulating tumor cells via β1-integrin mediated interactions. Int J Cancer. (2017) 140:2321–30. doi: 10.1002/ijc.30635
Keywords: neutrophil-to-lymphocyte ratio, PD-1 inhibitor, melanoma, biomarker, prognosis
Citation: Qi Y, Liao D, Mei D, Zhang Y and Liu Y (2020) Elevated Neutrophil-to-Lymphocyte Ratio Is Associated With Poor Outcomes for Melanoma Patients Treated With PD-1 Inhibitor or Chemotherapy in a Chinese Population. Front. Oncol. 10:1752. doi: 10.3389/fonc.2020.01752
Received: 19 May 2020; Accepted: 05 August 2020;
Published: 11 September 2020.
Edited by:
Todd Schell, Pennsylvania State University, United StatesReviewed by:
Camelia Quek, Melanoma Institute Australia, AustraliaGagan Chhabra, University of Wisconsin-Madison, United States
Copyright © 2020 Qi, Liao, Mei, Zhang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong Zhang, emx5eXp5MjgzMkB6enUuZWR1LmNu; Yang Liu, c3Vubnk1Nzk5QDEyNi5jb20=
†These authors have contributed equally to this work
 Yalong Qi
Yalong Qi Daixiang Liao
Daixiang Liao Dinglian Mei
Dinglian Mei Yong Zhang
Yong Zhang Yang Liu3*
Yang Liu3*