
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol., 29 October 2020
Sec. Surgical Oncology
Volume 10 - 2020 | https://doi.org/10.3389/fonc.2020.01639
This article is part of the Research TopicRadiofrequency Ablation in Liver Cancers : Investigations of Efficacy as Monotherapy and PolytherapyView all 7 articles
 Yang-Xun Pan1,2,3
Yang-Xun Pan1,2,3 Yi-Zhen Fu1,2
Yi-Zhen Fu1,2 Dan-Dan Hu1,2
Dan-Dan Hu1,2 Qian Long1,4
Qian Long1,4 Jun-Cheng Wang1,2
Jun-Cheng Wang1,2 Mian Xi1,5
Mian Xi1,5 Shi-Liang Liu1,4
Shi-Liang Liu1,4 Li Xu1,2
Li Xu1,2 Meng-Zhong Liu1,4
Meng-Zhong Liu1,4 Min-Shan Chen1,2
Min-Shan Chen1,2 Yao-Jun Zhang1,2*
Yao-Jun Zhang1,2*Background: Both stereotactic body radiotherapy (SBRT) and radiofrequency ablation (RFA) are effective local treatments for hepatocellular carcinoma (HCC), but whether RFA is superior to SBRT is still controversial. Therefore, we performed a meta-analysis to compare the treatment outcomes of SBRT with RFA as curable or bridge intention.
Methods: We searched online databases for studies that compared treatment outcomes for SBRT and RFA. Eligibility criteria included evaluation of local control, overall survival (OS), transplant rate, and post-transplant pathological necrosis.
Results: As no randomized clinical trials met the criteria, 10 retrospective studies with a total of 2,732 patients were included. Two studies were in favor of SBRT in local control, two studies preferred RFA in OS, and others reported comparable outcomes for both. SBRT demonstrated significantly higher 1- and 3-year local control than RFA [odds ratio (OR) 0.42, 95% CI 0.24–0.74, P = 0.003; and OR 0.54, 95% CI 0.37–0.80, P = 0.002, respectively]. However, SBRT reported significantly shorter 1- and 2-year OS (OR 1.52, 95% CI 1.21–1.90, P = 0.0003; and OR 1.66, 95% CI 1.38–2.01, P < 0.00001, respectively). As bridge treatment, no significant difference was shown in transplant rate and post-transplant pathological necrosis rate (OR 0.57, 95% CI 0.32–1.03, P = 0.060; and OR 0.49, 95% CI 0.13–1.82, P = 0.290, respectively).
Conclusions: This study demonstrates SBRT is able to complete a better local control for HCC than RFA, though the OS is inferior to RFA because of tumor burden or liver profiles of the enrolled studies. Well-designed, randomized, multicenter trials will be required to further investigate the conclusion.
Liver transplantation is the most beneficial therapy for early hepatocellular carcinoma (HCC) patients, as it removes both the tumor and the cirrhotic liver (1). However, given organ availability limitation, a number of patients who may benefit from this treatment have to stay on the waiting list for a long time. Therefore, stereotactic body radiotherapy (SBRT) and radiofrequency ablation (RFA) are offered as potential alternative local control modalities for patients in the waiting list (2, 3).
RFA induces coagulative necrosis of tumor through thermal effect and is the first-line treatment for small HCC (≤3 cm), providing comparable long-term outcome with resection (4). However, RFA has several contraindications, including large tumor size and lesions adjacent to major vessels or close to the liver hilum. The above circumstances may result in incomplete ablation, which potentially leads to worse prognosis (5–9).
SBRT is an advanced technology that delivers ablative radiation doses to tumors in a few fractions while minimizing the dose to normal liver tissue. Early results with SBRT have shown considerable local control even for large tumors or HCC ineligible for surgery (10). Moreover, SBRT has been frequently used as an alternative to RFA for small HCC patients with tumors near critical anatomical structures or major vessels due to the heat sink effect that can occur with RFA (11).
Recent publications have reported either comparable outcomes between the two treatment modalities or favorable outcomes for one to the other (3, 10, 12–19). Most of the published studies retrospectively reviewed clinical data in one single center; the results of these observational studies could have been strongly affected by several biases, and hence, the efficacy of these two treatments regarding disease control, long-term survival, and treatment related complications is ununified. With the absence of randomized data, meta-analysis might be able to draw a relative objective and reliable conclusion by integrating data from different clinical centers. The aim of this meta-analysis is mainly to compare the benefits of SBRT and RFA in the local progression (LP) control and overall survival (OS) in the treatment for HCC and to further help in clinical decision making.
The inclusion criteria of this meta-analysis were as follows: (1) diagnosed primary liver cancer definitively and patients diagnosed with HCC based on pathological evidence from fine-needle aspiration (FNA) or in the absence of biopsy evidence, based on imaging techniques including contrast-enhanced ultrasonography (CEUS), computed tomography (CT), and magnetic resonance imaging (MRI) companied with alpha-fetoprotein elevation; (2) no evidence of invasion into the major portal/hepatic vein branches or extrahepatic metastasis based on radiologic imaging; (3) patients without previous treatment of transcatheter arterial chemoembolization (TACE), surgery, chemotherapy, or other antitumor treatment; (4) documented indications for SBRT and RFA clearly; (5) either randomized controlled trials (RCTs) or retrospective studies were candidates; (6) patients of two groups with comparable basic clinical characters; and (7) studies with outcome information regarding LP rates, OS rates, and/or transplant rates.
Studies with following characteristics were excluded: (1) studies that did not report original data, including abstracts, case reports, expert opinions, editorials, reviews, or letters; (2) either group in the studies or combined other therapies; and (3) studies based on the same cohort.
A systematic online databases search of PubMed Central, Embase, Cochrane Library, and Google Scholar was separately conducted by two reviewers to identify all relevant availability of studies until August 26, 2019.
This meta-analysis was performed consistent with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement checklist. The subject headings (MeSH) search included “radiofrequency ablation,” “stereotactic radiation therapy,” and “hepatocellular carcinoma”; and keywords search was used, including “radiofrequency ablation,” “stereotactic body radiation therapy,” and “hepatocellular carcinoma.” These terms were used in different combinations. Only studies on humans and English-language studies were included. A manual research was performed by browsing all references of all identified studies. This progressed research was repeated to ensure to include the whole relevant studies. The research was completed by two reviewers before the data analysis independently (Y-XP and MX). If a study was controversial, the corresponding author was asked to judge (Y-JZ).
Data were extracted from the included studies, including number of patients in the SBRT and RFA groups; age; gender; primary tumor size; number of tumors; median dose of SBRT; Child–Pugh class; median follow-up time; 1-, 2-, and 3-year LP rates; 1-, 2-, 3-, and 5-year OS rates; post-transplant necrosis rates; and time to liver transplant.
LP was defined as the recurrence of lesion in the treatment area by imaging studies. And LP time was the period from the initial treatment to the discovery of LP or last follow-up. The OS was defined the period from the date of initial treatment of the HCC to the date of death related to any cause or last follow-up. Transplant rate was the proportion of patients who received liver transplantation after SBRT or RFA therapies. Post-transplant pathological necrosis was evaluated by post-transplant pathology.
All analyses were performed with the help of statistical software, named Review Manager, version 5.3 (Nordic Cochrane Center; Oxford, England). For data evaluation, patients were assigned into two groups: the SBRT-treated group and the RFA-treated group. The odds ratio (OR) and/or hazard ratios (HRs) accompanying 95% confidence interval (95% CI) were calculated for dichotomous and univariable analysis outcomes in terms of LP, OS, and prognostic factor on treatment allocation. Meanwhile, we assessed the heterogeneity among trials according to the chi-squared (χ2) test including the inconsistency factor (I2). The heterogeneity was defined as a P < 0.05 or an I2 >40% (20). Given the small number of included studies, though the heterogeneity was not high, the random effects model was applied throughout to enhance the reliability of results. A potential publication bias was assessed by visually inspecting the Begg funnel plots in which the standard error (SE) of log OR or log HR was plotted against the OR or HR, respectively.
A total of 440 studies were identified for the first time from PubMed by the search strategy previously established, and 269 studies were identified via other sources or review. Subsequently, 11 studies were deleted for duplication with the help of Mendeley (Elsevier Inc., Atlanta, GA, USA). The titles and abstracts of 270 studies were then screened for inclusion. The full texts of 36 studies were read; and, finally, we included 10 non-RCT studies that met the present meta-analysis criteria (3, 10, 13–16, 18, 19, 21, 22). The details of the PRISMA flow diagram of the literature for meta-analysis is shown in Supplementary Figure 1 (23).
In the present analysis, three studies were based on already existing database (13, 15, 19), and the remaining seven studies were based on retrospective studies (3, 10, 16–18, 21, 22). Two studies conducted SBRT or RFA for transplant intent. Five studies were performed in the USA (10, 13, 15, 18, 19), two in Japan (12, 16), one in Canada (3), one in South Korea (17), and one in China (22). Out of 2,732 patients from the 10 included studies, 859 patients were classified into the SBRT group, and the rest of the 1,873 patients were classified into the RFA group. The Newcastle Ottawa Scale (NOS) (24) were used to assess the quality of non-randomized studies. Although the qualities of selections and outcomes were relatively appropriate in terms of each study, over 50% of included studies were medium-score studies because of inconsistent comparability. Therefore, we believed that the present meta-analysis possesses a medium class of quality (Supplementary Table 1).
Among included studies, two studies were in favor of SBRT on local control (10, 21), two studies preferred RFA on OS (15, 19), and others reported comparable outcomes between groups (3, 13, 16–18, 22). Notably, according to baseline characteristics, several studies enrolled patients in SBRT group were prone to suffer from larger tumor diameter (3, 10, 16, 19) and higher proportion of Child–Pugh C patients [(18); Table 1]. These groups were evaluated for therapeutic efficacy in treating HCC patients. The details of the studies included in the present meta-analysis are listed in Table 1.
Six out of the 10 studies illustrated 1-, 2-, and 3-year LP rates (10, 16–18, 21, 22). Our pooled results showed that the SBRT group had significantly better 1- and 3-year local control rates than the RFA group (OR 0.47, 95% CI 0.26–0.83, P = 0.010; and OR 0.55, 95% CI 0.37–0.81, P = 0.003, respectively). However, the 2-year LP rate showed marginal benefit of SBRT group than the RFA group (OR 0.67, 95% CI 0.43–1.05, P = 0.080). No heterogeneity was shown among the studies of 1-, 2-, and 3-year LP rates (χ2 = 2.93, I2 = 0%; χ2 = 0.33, I2 = 0%; and χ2 = 2.66, I2 = 0%, respectively) (Figure 1).
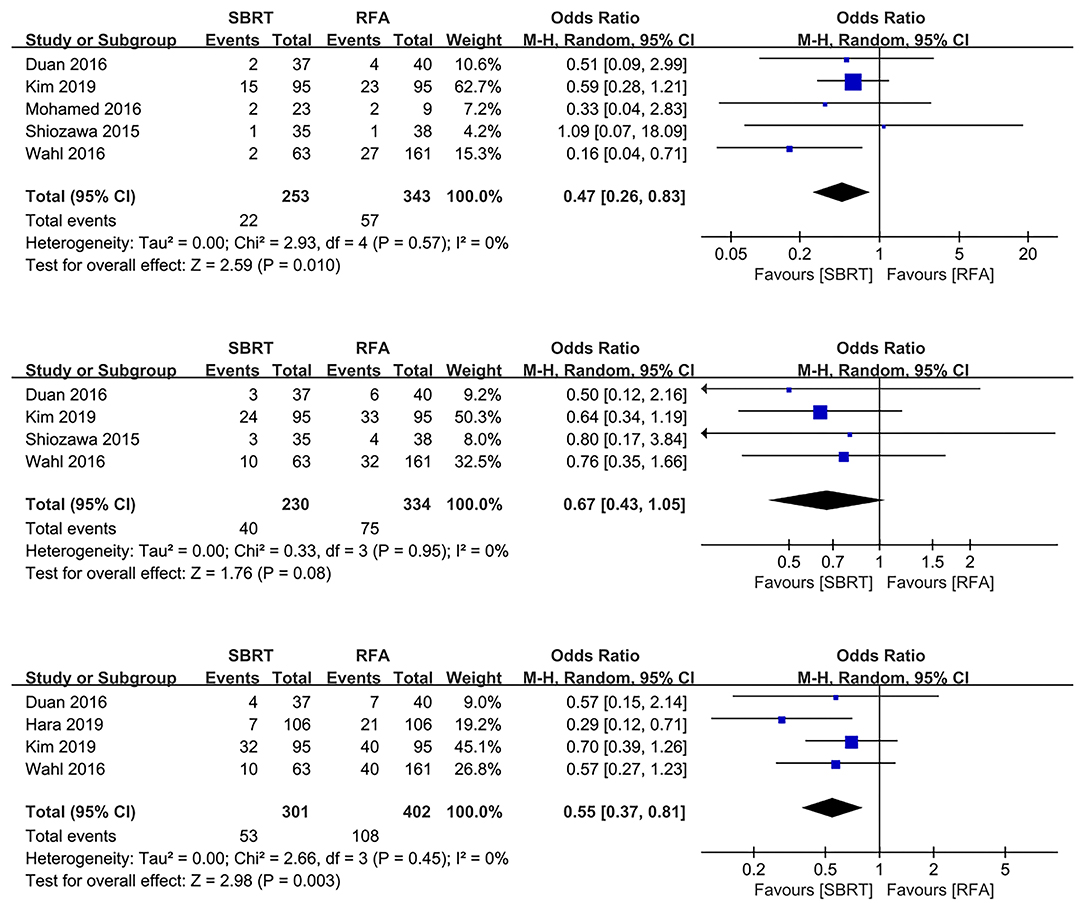
Figure 1. Forest plots demonstrating 1-, 2-, and 3-year LP in SBRT and RFA for HCC. LP, local progression; SBRT, stereotactic body radiotherapy; RFA, radiofrequency ablation; HCC, hepatocellular carcinoma.
Nine studies with 2,700 patients compared OS rates of SBRT group with RFA group (3, 10, 13, 15–17, 19, 21, 22), one study with 280 patients were excluded as both SBRT and RFA were applied as bridge therapies before transplantation, and the actual OS rates of SBRT or RFA might be affected by subsequent transplantation (3). The pool results showed that 2-year OS rates of RFA group were better than those of the SBRT group (OR 1.57, 95% CI 1.23–2.00, P < 0.0003), whereas there were no differences for 1-, 3-, and 5-year OS rates in both groups (OR 1.38, 95% CI 1.00–1.93, P = 0.050; OR 1.44, 95% CI 0.90–2.33, P = 0.130; and OR 1.35, 95% CI 0.81–2.26, P = 0.250, respectively; Figure 2).
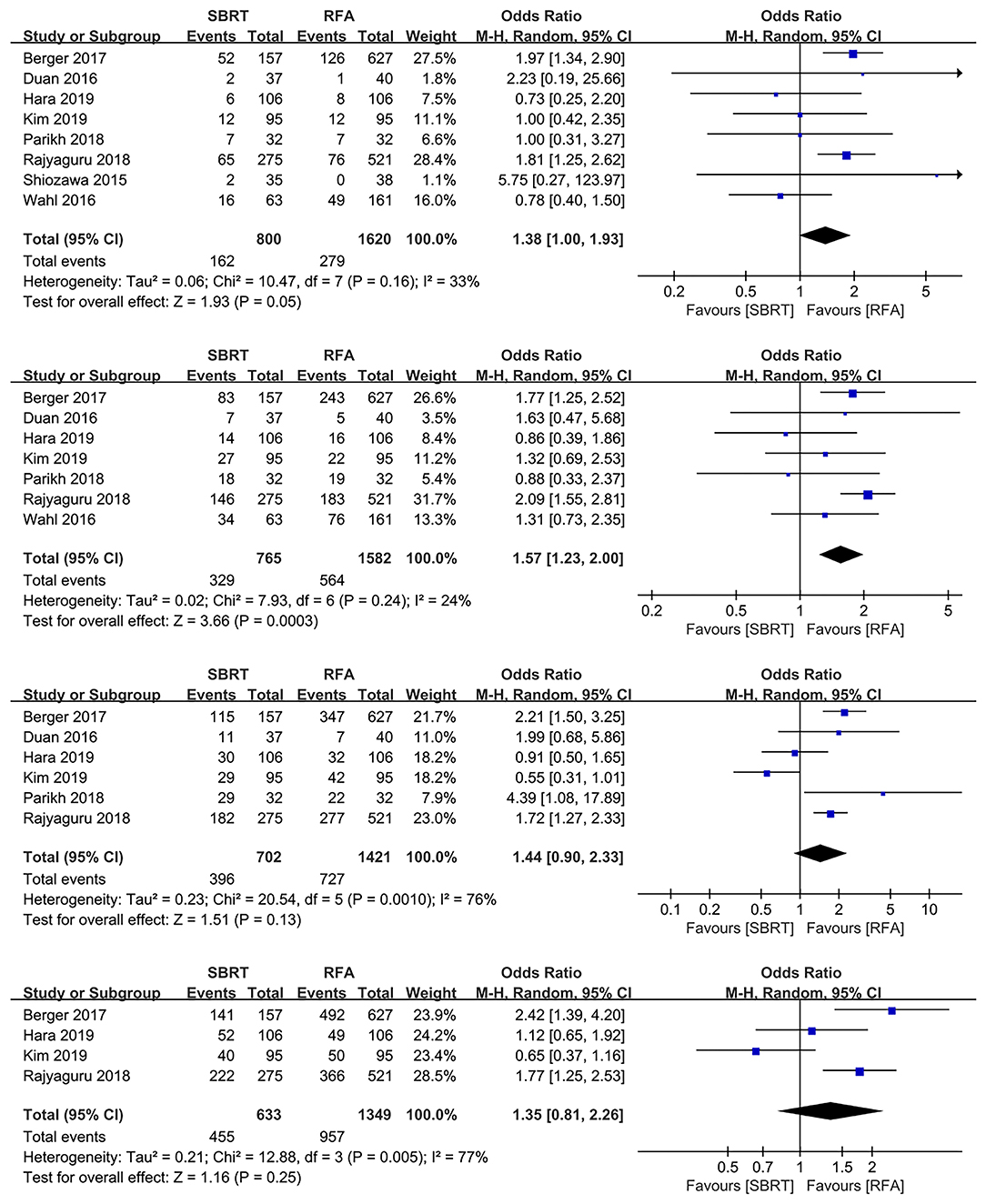
Figure 2. Forest plots demonstrating 1-, 2-, 3-, and 5-year LP in SBRT and RFA for HCC. LP, local progression; SBRT, stereotactic body radiotherapy; RFA, radiofrequency ablation; HCC, hepatocellular carcinoma.
Additionally, a secondary analysis was performed to control the potential report bias, and we enrolled the studies that reported outcomes of both LP and OS. As a result, the 1-, 2-, 3-, and 5-year OS rates indicated no significant difference between both groups (OR 0.96, 95% CI 0.59–1.57, P = 0.870; OR 1.35, 95% CI 0.89–2.03, P = 0.160; OR 0.97, 95% CI 0.28–3.36, P = 0.960; and OR 0.65, 95% CI 0.37–1.16, P = 0.150, respectively; Supplementary Figure 2).
Three and five studies evaluated the results of treatment allocation as a prognostic factor for LP (10, 16, 17) and OS (13, 15, 17, 19, 21), respectively. The treatment allocation was not a prognostic factor for LP (HR 0.72, 95% IC 0.42–1.25, P = 0.240). However, RFA group was more favorable than SBRT group for OS benefits (HR 1.43, 95% IC 1.24–1.64, P < 0.00001; Figure 3).
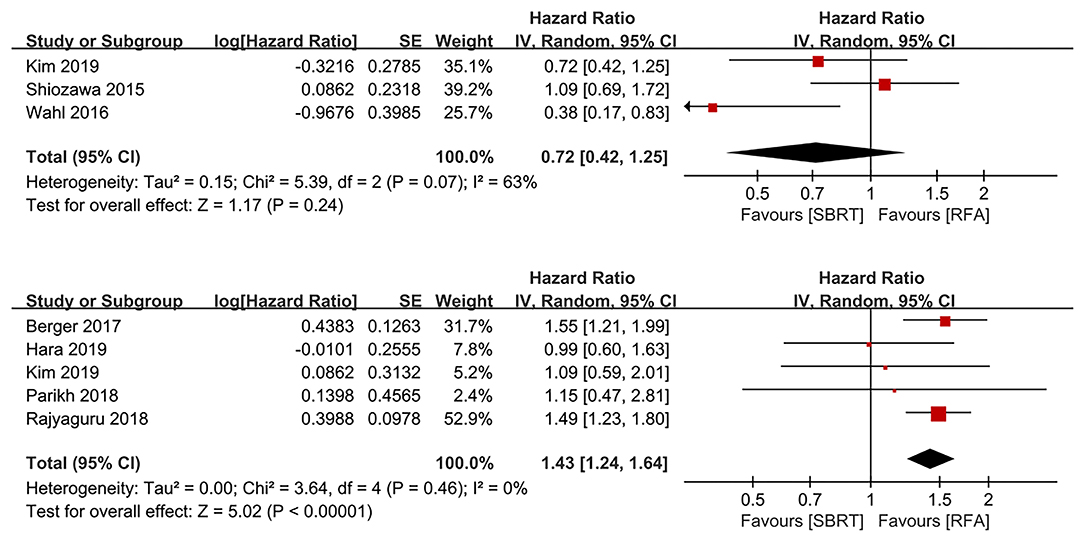
Figure 3. Forest plots demonstrating prognostic factor for LP and OS regarding to treatment allocation. LP, local progression; OS, overall survival.
Three and two studies reported the transplant rate and post-transplant pathological necrosis rate, respectively (3, 10, 18). There were no significant differences in transplant rate and post-transplant pathological necrosis rate for both SBRT and RFA (OR 0.65, 95% CI 0.24–1.79, P = 0.040; and OR 0.46, 95% CI 0.13–1.63, P = 0.230, respectively; Figure 4).
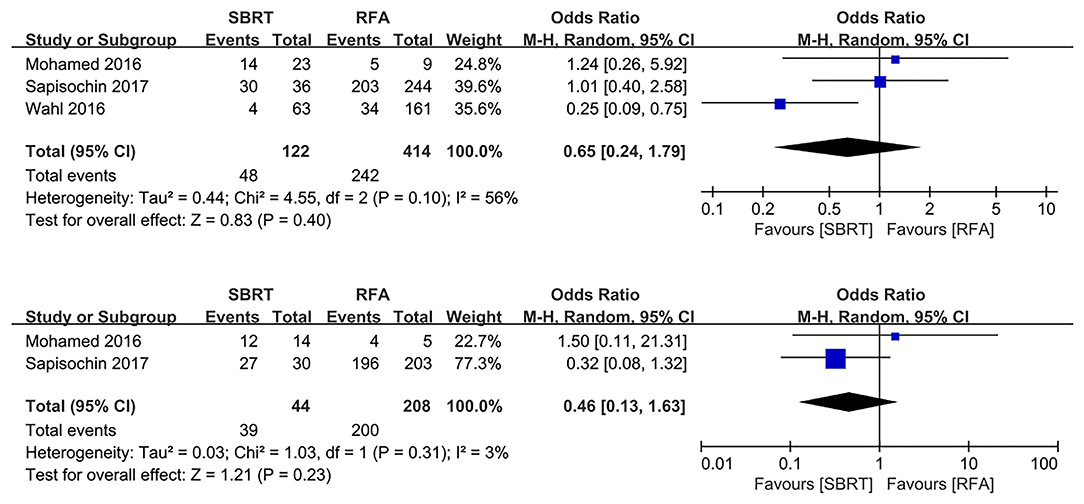
Figure 4. Forest plot demonstrating transplant rate and post-transplant pathologic necrosis in SBRT and RFA for HCC. SBRT, stereotactic body radiotherapy; RFA, radiofrequency ablation; HCC, hepatocellular carcinoma.
The Begg funnel plot was used to evaluate the reliability of publication bias in our meta-analysis (25). The shape of 11 funnel plots was basically inverted and bilateral symmetry. Therefore, these results indicated that there was little publication bias among all comparisons in this meta-analysis (Supplementary Figure 3).
The main finding of this meta-analysis is that SBRT showed a better local control than RFA for patients with HCC, though the 2-year OS rates of SBRT were inferior because of the tumor burden and liver profiles. Recently, RFA, as a traditional curable treatment, was challenged by SBRT (15, 21, 22). With the development of imaging technique, such as four-dimensional CT (4DCT), SBRT is able to provide a more precise picture of HCC for treatment design (26). This improvement of SBRT effectively fixed the deficiencies of the high incomplete ablation rate of RFA under several specific conditions (7).
In 2006, Romero et al. (27) firstly applied the radiational technique of SBRT as a salvage treatment to control the primary and metastatic liver tumors. Although only 25 patients with 45 lesions that were unfit for other local control treatment were included in this study, the results preliminarily indicated that SBRT was feasible with acceptable toxicity and local control efficacy. They additionally pointed out that patients with Child B level were accompanied by high toxicity risks, and the optimal dose-fractionation schemes had to be found. Inspired by Romero et al., other investigators mainly focused on the application of SBRT on unresectable HCC and its effect in combination with other therapies (28–30). All these studies proved that SBRT was safe and provided satisfying local control for HCC, and this encouraged the temptation of expanding the indications of SBRT from a salvage or bridge treatment to curable intention.
RFA has long been applied as the first-line treatment for small HCC according to several clinical practice guidelines, including the European Society for Medical Oncology (ESMO) and American Association for the Study of Liver Disease (AASLD) (31, 32). However, RFA still suffers from high incidence of local incomplete ablation because of technical limitations [varies from 2 to 60% (5–9)], and it required additional or combinational therapies (33). As an advanced technique that shows reliable local control and safety on HCC, SBRT has been considered as a potential alternative therapy to RFA.
Many observational or retrospective studies in recent years have indicated that SBRT showed a non-inferior local control rate as compared with RFA (10, 16–18, 22). Shiozawa et al. (16) first compared the clinical outcomes between SBRT and RFA for HCC patients in a pilot study in 2015 and reported that SBRT was likely to become an important option for local treatment of early HCC. The subsequent studies indicated that SBRT appeared to be a reasonable alternative treatment of inoperable HCC in 2016 (10, 22). Subsequently, several large-volume validation studies based on online database had also proved comparable outcomes between SBRT and RFA regarding local control (13, 19). Moreover, in 2019, Kim et al. (17) retrospectively reviewed the institutional database for RFA and SBRT with curative intent, and they revealed that SBRT appears to be an effective alternative treatment for HCC when RFA is not feasible due to tumor location or size. However, another database validation from the American National Cancer Database revealed better OS of RFA than SBRT (15). A meta-analysis is needed to attain definitive proof to solve these debates. Thus, we performed this meta-analysis to help identify the advantages and disadvantages of SBRT and RFA in HCC.
In the present meta-analysis, with respect to local control, SBRT showed significantly lower 1- and 3-year LP rates, which indicated that SBRT achieved superior local control to RFA in treating HCC. In detail, there were five and four studies that enrolled the 1- and 3-year LP rate analyses, respectively. Among these studies, studies from Wahl et al. (10) and Duan et al. (22) were based on patients with inoperable HCC, studies of Shiozawa et al. (16) and Hara et al. (21) were based on patients with early-stage HCC, and studies from Kim et al. (17) and Mohamed et al. (18) did not specify patients characteristics. Most of the enrolled studies tended to draw the conclusions with respect to local control that supported SBRT. Moreover, Wahl et al. (10) and Hara et al. (21) reached significantly favorable results for SBRT in treating inoperable and early-stage HCC, respectively. However, only Kim et al. (17) clarified the information of HCC location; others did not refer to this critical factor, which might influence the local control of RFA in our previous study (34). Additionally, treatment allocation was not a significance prognostic factor on the basis of prognostic analysis. Therefore, further studies are needed to guarantee the appropriate individual treatment allocation.
Although SBRT enjoyed higher local control rates than RFA in the present study, the 2-year OS rates of SBRT were significantly lower than RFA. Notably, Berger et al. (19) and Rajyaguru et al. (15), who reported favorable OS rates of RFA with large sample volume, did not illustrate the LP rates correspondingly. There were two main reasons to the contradictory results between OS rates and LP rates. Firstly, there might be report bias when analyzing LP rates for both groups, which might result in inconsistent outcomes between LP rates and OS rates because of unreported LP. Therefore, we conducted the secondary analysis on OS rates that included the studies that reported both LP rates and OS rates, and we found that the 1-, 2-, 3-, and 5-year OS rates were comparable between SBRT and RFA (Supplementary Figure 2). Secondly, as the first-line treatment, RFA is more likely to be assigned to patients with better conditions. Patients who underwent SBRT were prone to suffer from larger tumor size and worse liver function, which indicated worse prognosis and decreased OS. Interestingly, the 3- and 5-year OS rates showed no significance in both groups, indicating that patients who did not die of tumor burden or liver functions in a short time might finally benefit from both treatments similarly. Meanwhile, RFA showed significant survival benefit for prognostic analysis on treatment allocation, which also could be explained by the reasons above. Therefore, we believed that the real effects of SBRT and RFA on long-term survival need to be further validated by high-level evidence, including RCTs.
As for bridge therapies to transplant, both SBRT and RFA provided a similar effect on patients waiting for transplantation. And the post-transplant pathological necrosis rate was comparable between both groups. These outcomes indicated that SBRT can be safely utilized as a bridge treatment to patients in the waiting list for transplantation with HCC, as an alternative to conventional bridging therapies. However, only three studies and 536 patients in total compared these parameters, and more solid studies were needed to be enrolled in the future.
This meta-analysis suffered from several limitations. First of all, only retrospective studies were available, resulting in relatively low quality of the evidence for the whole pooled results. Secondly, the results from this study should be interpreted carefully, because the sample sizes of four studies were relatively small, which is supposed to affect the reliability. And a further sensitivity analysis on the factors affecting outcomes could not be applied. Additionally, some studies were shorter follow-up in the SBRT group, which could result in obscuring late effects. Therefore, some well-designed, large, prospective, and multicenter studies are desperately needed to obtain more solid evidence.
In sum, our meta-analysis shows that SBRT provided better local control than RFA, and it could be used as a potential alternative local control treatment for HCC.
Y-XP and D-DH designed the experiments and drafted the manuscript. Y-ZF, QL, J-CW, and MX were responsible for data collection and statistical analysis. S-LL, LX, and M-ZL helped revise the manuscript. M-SC and Y-JZ approved the final version. All authors contributed to the article and approved the submitted version.
This work was supported by the National Science and Technology Major Project of China (2018ZX10723204 and 2018ZX10302205).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors acknowledge and express their deepest gratitude to the participants of this study. This manuscript has been revised by a native English speaker for effective communication to a professional medical audience.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.01639/full#supplementary-material
1. Sapisochin G, Bruix J. Liver transplantation for hepatocellular carcinoma: outcomes and novel surgical approaches. Nat Rev Gastroenterol Hepatol. (2017) 14:203–17. doi: 10.1038/nrgastro.2016.193
2. Panel NHC. Hepatobiliary cancers, V.1.2010. Nccn Guidelines. (2019) 1:1–142. doi: 10.6004/jnccn.2019.0019
3. Sapisochin G, Barry A, Doherty M, Fischer S, Goldaracena N, Rosales R, et al. Stereotactic body radiotherapy vs. TACE or RFA as a bridge to transplant in patients with hepatocellular carcinoma. An intention-to-treat analysis. J Hepatol. (2017) 67:92–99. doi: 10.1016/j.jhep.2017.02.022
4. Chen M-S, Li J-Q, Zheng Y, Guo R-P, Liang H-H, Zhang Y-Q, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. (2006) 243:321–8. doi: 10.1097/01.sla.0000201480.65519.b8
5. N'Kontchou G, Mahamoudi A, Aout M, Ganne-Carrie N, Grando V, Coderc E, et al. Radiofrequency ablation of hepatocellular carcinoma: long-term results and prognostic factors in 235 Western patients with cirrhosis. Hepatology. (2009) 50:1475–83. doi: 10.1002/hep.23181
6. Tateishi R, Shiina S, Teratani T, Obi S, Sato S, Koike Y, et al. Percutaneous radiofrequency ablation for hepatocellular carcinoma. An analysis of 1000 cases. Cancer. (2005) 103:1201–9. doi: 10.1002/cncr.20892
7. Waki K, Aikata H, Katamura Y, Kawaoka T, Takaki S, Hiramatsu A, et al. Percutaneous radiofrequency ablation as first-line treatment for small hepatocellular carcinoma: results and prognostic factors on long-term follow up. J Gastroenterol Hepatol. (2010) 25:597–604. doi: 10.1111/j.1440-1746.2009.06125.x
8. Granata V, Petrillo M, Fusco R, Setola SV, de Lutio di Castelguidone E, Catalano O, et al. Surveillance of HCC patients after liver RFA: role of MRI with hepatospecific contrast versus three-phase CT scan-experience of high volume oncologic institute. Gastroenterol Res Pract. (2013) 2013:469097. doi: 10.1155/2013/469097
9. Doyle A, Gorgen A, Muaddi H, Aravinthan AD, Issachar A, Mironov O, et al. Outcomes of radiofrequency ablation as first-line therapy for hepatocellular carcinoma less than 3 cm in potentially transplantable patients. J Hepatol. (2019) 70:866–73. doi: 10.1016/j.jhep.2018.12.027
10. Wahl DR, Stenmark MH, Tao Y, Pollom EL, Caoili EM, Lawrence TS, et al. Outcomes after stereotactic body radiotherapy or radiofrequency ablation for hepatocellular carcinoma. J Clin Oncol. (2016) 34:452–9. doi: 10.1200/JCO.2015.61.4925
11. Feng M, Suresh K, Schipper MJ, Bazzi L, Ben-Josef E, Matuszak MM, et al. Individualized adaptive stereotactic body radiotherapy for liver tumors in patients at high risk for liver damage: a phase 2 clinical trial. JAMA Oncol. (2018) 4:40–47. doi: 10.1001/jamaoncol.2017.2303
12. Hara K, Takeda A, Tsurugai Y, Sanuki N, Saigusa Y, Maeda S, et al. Clinical outcomes after treatment for hepatocellular carcinoma, stereotactic body radiotherapy vs radiofrequency ablation: a propensity score-matched analysis. Hepatology. (2018) 68:848A. doi: 10.1002/hep.30257
13. Parikh ND, Marshall VD, Green M, Lawrence TS, Razumilava N, Owen D, et al. Effectiveness and cost of radiofrequency ablation and stereotactic body radiotherapy for treatment of early-stage hepatocellular carcinoma: an analysis of SEER-medicare. J Med Imaging Radiat Oncol. (2018) 62:673–81. doi: 10.1111/1754-9485.12754
14. Wahl D, Stenmark M, Pollom E, Tao Y, Lee O, Schipper M, et al. SBRT provides equivalent local control compared to RFA for the treatment of hepatocellular carcinoma with minimal toxicity. Int J Radiat Oncol Biol Phys. (2014) 90:S378–9. doi: 10.1016/j.ijrobp.2014.05.1218
15. Rajyaguru DJ, Borgert AJ, Smith AL, Thomes RM, Conway PD, Halfdanarson TR, et al. Radiofrequency ablation versus stereotactic body radiotherapy for localized hepatocellular carcinoma in nonsurgically managed patients: analysis of the national cancer database. J Clin Oncol. (2018) 36:600–8. doi: 10.1200/JCO.2017.75.3228
16. Shiozawa K, Watanabe M, Ikehara T, Matsukiyo Y, Kogame M, Kishimoto Y, et al. Comparison of percutaneous radiofrequency ablation and CyberKnife® for initial solitary hepatocellular carcinoma: a pilot study. World J Gastroenterol. (2015) 21:13490–9. doi: 10.3748/wjg.v21.i48.13490
17. Kim N, Kim HJ, Won JY, Kim DY, Han K-H, Jung I, et al. Retrospective analysis of stereotactic body radiation therapy efficacy over radiofrequency ablation for hepatocellular carcinoma. Radiother Oncol. (2019) 131:81–87. doi: 10.1016/j.radonc.2018.12.013
18. Mohamed M, Katz AW, Tejani MA, Sharma AK, Kashyap R, Noel MS, et al. Comparison of outcomes between SBRT, yttrium-90 radioembolization, transarterial chemoembolization, and radiofrequency ablation as bridge to transplant for hepatocellular carcinoma. Adv Radiat Oncol. (2016) 1:35–42. doi: 10.1016/j.adro.2015.12.003
19. Berger NG, Tanious MN, Hammad AY, Miura JT, Mogal H, Clarke CN, et al. External radiation or ablation for solitary hepatocellular carcinoma: a survival analysis of the SEER database. J Surg Oncol. (2017) 116:307–12. doi: 10.1002/jso.24661
20. Melsen WG, Bootsma MCJ, Rovers MM, Bonten MJM. The effects of clinical and statistical heterogeneity on the predictive values of results from meta-analyses. Clin Microbiol Infect. (2014) 20:123–9. doi: 10.1111/1469-0691.12494
21. Hara K, Takeda A, Tsurugai Y, Saigusa Y, Sanuki N, Eriguchi T, et al. Radiotherapy for hepatocellular carcinoma results in comparable survival to radiofrequency ablation: a propensity score analysis. Hepatology. (2019) 69:2533–45. doi: 10.1002/hep.30591
22. Duon X, Zhang T, Xie H, Sun J, He W, Xu H. Stereotactic body radiotherapy vs. radiofrequency ablation in the Treatment of small hepatocellular Carcinoma. Hepatology. (2016) 64:653–4A. doi: 10.1002/hep.28799
23. Tian J, Zhang J, Ge L, Yang K, Song F. The methodological and reporting quality of systematic reviews from China and the USA are similar. J Clin Epidemiol. (2017) 85:50–58. doi: 10.1016/j.jclinepi.2016.12.004
24. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
25. Higgins JPT, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
26. Couri T, Pillai A. Goals and targets for personalized therapy for HCC. Hepatol Int. (2019) 13:125–37. doi: 10.1007/s12072-018-9919-1
27. Romero AM, Wunderink W, Hussain SM, De Pooter JA, Heijmen BJ, Nowak PC, et al. Stereotactic body radiation therapy for primary and metastatic liver tumors: a single institution phase i-ii study. Acta Oncol. (2006) 45:831–7. doi: 10.1080/02841860600897934
28. Goyal K, Einstein D, Yao M, Kunos C, Barton F, Singh D, et al. Cyberknife stereotactic body radiation therapy for nonresectable tumors of the liver: preliminary results. HPB Surgery. (2010) 2010:309780. doi: 10.1155/2010/309780
29. Bae SH, Kwon JH, Jang JW, Song MJ, Choi JY, Yoon SK. Stereotactic body radiation therapy using Cyberknife for primary hepatocellular carcinoma ineligible for local ablation therapy or surgical resection. Hepatology. (2009) 50:1100A. doi: 10.1002/hep.23307
30. Choi BO, Choi IB, Jang HS, Kang YN, Jang JS, Bae SH, et al. Stereotactic body radiation therapy with or without transarterial chemoembolization for patients with primary hepatocellular carcinoma: Preliminary analysis. BMC Cancer. (2008) 8:351. doi: 10.1186/1471-2407-8-351
31. Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. (2018) 67:358–80. doi: 10.1002/hep.29086
32. Vogel A, Cervantes A, Chau I, Daniele B, Llovet J, Meyer T, et al. Hepatocellular carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up†. Ann Oncol. (2018) 29:iv238–55. doi: 10.1093/annonc/mdy308
33. Peng Z-W, Zhang Y-J, Chen M-S, Xu L, Liang H-H, Lin X-J, et al. Radiofrequency ablation with or without transcatheter arterial chemoembolization in the treatment of hepatocellular carcinoma: a prospective randomized trial. J Clin Oncol. (2013) 31:426–32. doi: 10.1200/JCO.2012.42.9936
Keywords: minimally invasive treatment, meta-analysis, hepatocellular carcinoma, stereotactic body radiotherapy, radiofrequency ablation
Citation: Pan Y-X, Fu Y-Z, Hu D-D, Long Q, Wang J-C, Xi M, Liu S-L, Xu L, Liu M-Z, Chen M-S and Zhang Y-J (2020) Stereotactic Body Radiotherapy vs. Radiofrequency Ablation in the Treatment of Hepatocellular Carcinoma: A Meta-Analysis. Front. Oncol. 10:1639. doi: 10.3389/fonc.2020.01639
Received: 27 December 2019; Accepted: 27 July 2020;
Published: 29 October 2020.
Edited by:
Aali Jan Sheen, Manchester Royal Infirmary, United KingdomReviewed by:
Alex Nicolas Gordon-Weeks, University of Oxford, United KingdomCopyright © 2020 Pan, Fu, Hu, Long, Wang, Xi, Liu, Xu, Liu, Chen and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yao-Jun Zhang, emhhbmd5dWpAc3lzdWNjLm9yZy5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.