
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 25 August 2020
Sec. Gastrointestinal Cancers
Volume 10 - 2020 | https://doi.org/10.3389/fonc.2020.01406
This article is part of the Research TopicMolecular Biomarkers for Gastric CancerView all 36 articles
 Yixian Guo1†
Yixian Guo1† Xu Liu1†
Xu Liu1† Danhua Xu1
Danhua Xu1 Chen Huang1
Chen Huang1 Zeyu Wang1
Zeyu Wang1 Xiang Xia1
Xiang Xia1 Chunchao Zhu1
Chunchao Zhu1 Jia Xu1
Jia Xu1 Zizhen Zhang1
Zizhen Zhang1 Yanying Shen2
Yanying Shen2 Wenyi Zhao1*
Wenyi Zhao1* Gang Zhao1*
Gang Zhao1*Background: Gastric cancer (GC) remains a refractory cancer particularly in Eastern Asia. Large tumor suppressor kinases 1/2 (LATS1/2) are core members of the Hippo pathway. The role of LATS1/2 in the prognosis of different subtypes of advanced gastric cancer and its relationship with the tumor immune microenvironment in GC remain unknown. Exploring the role of LATS1/2 in GC might provide potential immunotherapeutic approaches for treating GC.
Methods: Four hundred and ninety surgically resected primary GC samples were assessed for LATS1/2, CD8, FOXP3, and CD163. Correlations between LATS1/2 expression and immune-related markers were investigated and the prognoses of patients with different GC subtypes were analyzed.
Results: CD8 and CD163 appeared to be favorable and adverse prognostic factors, respectively. LATS1/2 and FOXP3 did not predict patients' overall survival. However, in microsatellite-stable GC patients, high LATS1/2 and FOXP3 expression and low CD8 expression predicted poor prognoses. Furthermore, high LATS1/2 expression was significantly correlated with decreased CD8 and increased FOXP3. Combined analysis of LATS1/2, CD8, and FOXP3 had better prognostic accuracy than did each marker individually.
Conclusions: Different biological molecules can predict the prognoses of different types of GC patients. LATS1/2, core kinases in the Hippo pathway, are closely related to CD8 and FOXP3. Further understanding the mechanisms of LATS1/2 in CD8+ T cells and FOXP3+ Treg cells provides further theoretical basis and potential targets for GC immunotherapy.
Gastric cancer (GC) is a serious malignant tumor with the fifth highest global incidence rate and the third highest mortality rate (1). Asian countries have high incidences of GC. The 5–year survival rate of GC patients in China is only 35.9% (2). GC patients are often diagnosed at advanced stages because of the lack of early characteristic symptoms and frequent recurrence and distant metastasis that occurs after surgical resection (3, 4). According to the Cancer Genome Atlas database, Bass et al. established a new molecular classification of GC (5). Cristescu et al. found that the prognoses of microsatellite-stable (MSS) and microsatellite-instable (MSI) GC patients differed in which patients with MSS GC had worse prognoses (6); however, the reason for this remains unclear. Therefore, using biological markers to analyze the prognoses of patients with different GC subtypes may provide clues for exploring the pathogenesis and clinical treatment of GC.
The Hippo pathway is a tumor-suppressive pathway, and its inactivation is associated with the progression and metastasis of various cancers (7, 8). Large tumor suppressor kinases 1/2 (LATS1/2) are core members of the Hippo pathway, and their activation is the major functional output of this pathway. LATS1 and LATS2 have the same function and are both expressed in GC (9, 10). Although LATS1/2 are traditionally believed to inhibit tumor growth (11, 12), Pan et al. found that LATS1/2 deletion inhibits the growth of murine MC38 colon cancer cells (13). Moreover, LATS1/2 inhibit antitumor immunity by suppressing CD8 cytotoxicity. Mechanistically, LATS1/2-null tumor cells secrete nucleic acid-rich extracellular vesicles, which induce a type I interferon response via the Toll-like receptor-MYD88/TRIF pathway (14). Therefore, the role of LATS1/2 in a tumor microenvironment remains controversial. To explore the relationship between LATS1/2 and a tumor microenvironment in advanced GC, we used tumor immune-related markers including CD8, FOXP3, and CD163, representing CD8+ T cells, FOXP3+ Treg cells, and CD163+ M2 macrophages, respectively, and all played important roles in a tumor immune microenvironment (15–20). We sought to identify novel strategies to obtain more accurate prognoses in advanced GC patients by analyzing different biological marker combinations.
We found that different biological markers predicted the prognoses of patients with different types of advanced GC. LATS1/2, important kinases in the Hippo pathway, were closely related to CD8 and FOXP3. Furthermore, we identified novel strategies for obtaining more accurate prognoses in GC patients by analyzing LATS1/2 in combination with immune-related markers including CD8 and FOXP3.
This study was conducted on a cohort of 490 patients with advanced GC [American Joint Committee on Cancer (AJCC) stages T2–T4]. All samples were retrieved from patients who underwent primary tumor resection between June 2006 and December 2016 at the Department of Gastrointestinal Surgery, Renji Hospital, School of Medicine, Shanghai Jiao Tong University. All samples were definitively diagnosed as advanced GC by the Department of Pathology. The clinical criteria for patient recruitment were as follows: (i) patients had complete clinical information, postoperative pathological diagnoses, and follow-up data; (ii) patients had not received preoperative radiotherapy, chemotherapy, hormonal therapy, or any other anticancer therapy before surgery; (iii) patients had undergone non-neoplastic resection such as laparotomy or palliative gastrointestinal bypass surgery; (iv) patients had non-adenocarcinoma (gastrointestinal stromal tumors); and (v) the primary tumor involved only one regional site at the site of occurrence (21, 22).
Overall survival time was defined as the interval between gastrectomy and either patient death or the last follow-up. The final follow–up date was February 25, 2020. All patients received standard treatments such as D2 radical resection and adjuvant chemotherapy or palliative tumor resection for patients with stage IV GC according to the National Comprehensive Cancer Network (NCCN) guidelines. Patients' tumors were staged in accordance with the AJCC 8th edition staging system. Two senior pathologists confirmed the diagnosis in each case from the hematoxylin and eosin-stained slides.
Formalin-fixed, paraffin-embedded (FFPE) tissue samples were sliced in consecutive 3.0-μm-thick sections, which were dewaxed in xylene and rehydrated in graded ethanol. Immunohistochemical staining was then performed as per the Dako REAL EnVision Detection System (K5007, Dako) manual. The following primary antibodies were used:
Anti-LATS1/2 (1:100; ab111344, Abcam), Anti-CD163 (1:100, ab87099, Abcam), Anti-CD8 (1:100, ab4055, Abcam), Anti-FOXP3 (1:100, ab20034, Abcam), Anti-MLH1 (1:50, clone ES05, DAKO), Anti-PMS2 (1:40, clone EP51, DAKO), Anti-MSH2 (1:50, clone FE11, DAKO), and Anti-MSH6 (1:50, clone EP49, DAKO).
Paraffin-embedded sections (3.0 μm) were prepared for immunohistochemical analyses. After deparaffinization, all antigens except nestin were retrieved at 120°C for 15 min in a sodium citrate buffer solution (pH 6.0). Tissues were incubated with 0.3% hydrogen peroxide for 30 min and then blocked with 1% bovine serum albumin (Sangon, Shanghai, China) overnight at 4°C. The peroxidase reaction was developed using a 3,3-diaminobenzidine (DAB) chromogen solution in a DAB buffer substrate and then counterstained with hematoxylin.
Five random fields per section were viewed under a light microscope (Axioskop 40; Zeiss GmbH, Jena, Germany) at 400× magnification. Three investigators, who were blinded to the clinical features and outcomes, independently examined and scored the sections. After counting the cells, the cell density was calculated as mm2 for further analysis.
In a two-category immunoscore analysis, patients were dichotomized into the high- and low-density groups according to the median number of stained cells. The cutoffs were as follows: 17/mm2 for CD8, 25/mm2 for FOXP3, and 20/mm2 for CD163 (23, 24). LATS1/2 expressions in the samples were considered high when they were expressed in at least 10% of the samples (25).
All statistical analyses were performed using SPSS 23.0 and GraphPad Prism 8.0. The Spearman's correlation coefficient was calculated to examine associations between continuous variables. Chi-square tests were performed to analyze relationships between categorical variables. Kaplan–Meier univariate and multivariate prognostic analyses of the Cox proportional hazards regression model were performed to assess the influence of each variable on survival. Hazard ratios (HRs) with 95% confidence intervals (CIs) were calculated as correlation estimates. A two-tailed P < 0.05 was considered statistically significant.
Four hundred ninety surgically resected FFPE primary advanced GC samples were assessed for LATS1/2, CD8, FOXP3, and CD163 via tissue microarrays. The patients included 337 men (68.78%) and 153 women (31.22%). The median age at diagnosis was 62 years old (range: 22–88 years). One hundred seventy-two patients (35.10%) were <60 years old, and 318 (64.90%) were >60 years old. The median overall survival time was 43 months (range: 0–123 months). One hundred seventeen GC lesions (23.88%) occurred in the upper stomach, 157 (32.04%) occurred in the middle stomach, and 216 (44.08%) occurred in the lower stomach. The tumors were classified based on the 8th AJCC gastric cancer staging manual: 150 were stage II (30.61%), 266 were stage III (54.29%), and 74 were stage IV (15.10%) (26). According to the 8th AJCC staging, lymph node metastasis occurred in 366 cases (74.69%), while distant metastasis occurred in 74 cases (15.10%). The tissue samples comprised 200 cases (40.82%) of intestinal type carcinoma and 290 cases (59.18%) of diffused gastric carcinoma. According to the microsatellite stability classification, 364 cases were MSI (74.29%) and 126 were MSS (25.71%; Table 1).
We classified biomarkers according to the expression level via microscopic observation. Figures S1, S2 show the LATS1/2, CD8, FOXP3, and CD163 expression profiles. LATS1/2 were highly expressed in 226 cases (46.12%) and lowly expressed in 264 cases (53.88%). CD8 was highly expressed in 245 cases (50%) and lowly expressed in 245 cases (50%). CD163 was lowly expressed in 257 cases (52.44%) and highly expressed in 233 cases (47.55%). FOXP3 was highly expressed in 213 cases (43.46%) and lowly expressed in 277 cases (56.54%; Table S1).
Table S1 shows the association between LATS1/2, CD8, FOXP3, CD163, and the pathological features. LATS1/2 were significantly positively correlated with AJCC stage (P = 0.049) and microsatellite stability (P = 0.041). CD8 expression was significantly negatively associated with the AJCC stage (P = 0.039), the advanced tumor stage (P = 0.035), distant metastasis (P = 0.023), the Lauren classification (P = 0.043), and microsatellite stability (P = 0.032). FOXP3 was significantly correlated with microsatellite stability (P = 0.042). CD163 was not correlated with any of the pathological features.
We analyzed the relationship between prognosis and clinical features in patients with GC via Cox regression analysis. The tumor node metastasis (TNM) stage (HR = 1.353, 95% CI: 1.116–1.640, P = 0.002), lymph node metastasis (HR = 1.827, 95% CI: 1.363–2.451, P = 0.000), distance metastasis (HR = 3.377, 95% CI: 2.546–4.480, P = 0.000), Lauren classification (HR = 1.530, 95% CI: 1.324–1.885, P = 0.000), and microsatellite classification (HR = 1.336, 95% CI: 1.083–1.729, P = 0.009) were significantly associated with the overall survival (Table S2). Cox regression analysis was performed to evaluate the prognostic roles of LATS1/2, CD8, FOXP3, and CD163. Univariate analysis showed that CD8 (HR = 0.706, 95% CI: 0.559–0.893, P = 0.004) and CD163 (HR = 1.222, 95% CI: 1.089–1.417, P = 0.033) predicted patients' prognoses. However, LATS1/2 (HR = 1.157, 95% CI: 0.917–1.461, P = 0.219) and FOXP3 (HR = 1.110, 95% CI: 0.878–1.402, P = 0.384) expressions did not significantly affect the overall survival.
Variables with P < 0.05 in the univariate analysis were included in multivariate analyses. Because TNM staging included both lymph node metastasis and distant metastasis, they were excluded. The TNM stage (HR = 1.316, 95% CI: 1.087–1.599, P = 0.046) and CD8 (HR = 0.705, 95% CI: 0.556–0.893, P = 0.004) were independent factors for predicting the overall survival in the multivariate analysis. LATS1/2 and FOXP3 did not predict patient prognoses (Figure 1A, Table S2).
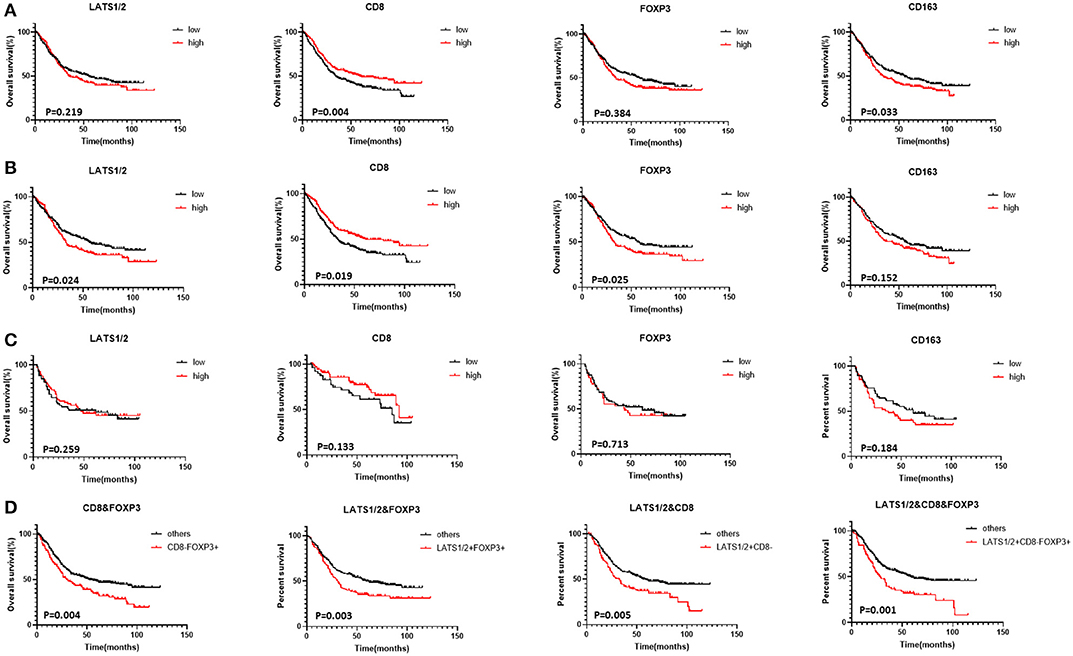
Figure 1. Correlation between LATS1/2, CD8, FOXP3, and CD163 and GC patients' overall survival (Kaplan–Meier survival curves): (A) patients with advanced GC, (B) patients with advanced MSS GC, (C) patients with advanced MSI GC, and (D) combination of LATS1/2, CD8, and FOXP3 in all patients with advanced GC.
Patients were then divided into MSS and MSI subgroups according to MLH1, MSH2, MSH6, and PMS2 expressions (27, 28). FOXP3 and CD163 expressions were significantly higher in MSS GC patients than in MSI GC patients while CD8 expression was significantly lower in MSS GC patients (Table S1). Although LATS1/2 and FOXP3 expressions did not predict advanced GC, high LATS1/2 (HR = 1.304, 95% CI: 1.035–1.643, P = 0.024) and FOXP3 (HR = 1.320, 95% CI: 1.047–1.665, P = 0.019) expressions predicted shorter overall survival in patients with MSS GC. In patients with MSI GC, LATS1/2, CD8, FOX3, and CD163 expressions did not significantly affect the overall survival (Figures 1B,C, Table S3).
To evaluate LATS1/2 expression in cells in the immune microenvironment and the relationship between LATS1/2 and immune cells, we first analyzed the relationship between LATS1/2, FOXP3, CD163, and CD8 (Table S4). High LATS1/2 expression was significantly correlated with low CD8 expression (P = 0.008) and high FOXP3 expression (P = 0.012), but LATS1/2 and CD163 were not correlated. Thus, we combined LATS1/2 with CD8 and FOXP3 for prognostic analysis and divided them into four subtypes: subtype 1 with LATS1/2 and CD8; subtype 2 with LATS1/2 and FOXP3; subtype 3 with CD8 and FOXP3; and subtype 4 with LATS1/2, CD8, and FOXP3. The survival curve revealed that the LATS1/2highCD8low, LATS1/2highFOXP3high, CD8lowFOXP3high, and LATS1/2highCD8lowFOXP3high subgroups in each subtype have the worst overall survival (Figures S3A–D). We then compared them with other subgroups (Figure 1D, Table S5). Combined analysis of the three indicators, LATS1/2, CD8, and FOXP3, had better prognostic accuracy than did the combination of any two indicators (HR = 2.207, 95% CI: 1.653–2.959, P = 0.001). Thus, the combined analysis of LATS1/2, CD8, and FOXP3 may be a good prognostic factor for patients with advanced GC.
LATS1/2 are key kinases in the Hippo signaling pathway. LATS1/2 activation can inhibit tumor growth (29, 30); however, Toshiro et al. recently reported that suppressing LATS1/2 exhibited antitumor immunity (14); therefore, the roles of LATS1/2 in the tumor microenvironment remain controversial. Here, we selected tumor immunity-related biological markers, including CD8, FOXP3, and CD163, which, respectively, represented CD8+ T cells, FOXP3+ Treg cells, and CD163+ M2 macrophages to analyze different advanced GC types. We focused on the relationship between LATS1/2 and these tumor immunity-related biological markers in a tumor immune microenvironment by analyzing 490 immunohistochemically stained samples from advanced GC patients.
First, we analyzed the correlation between LATS1/2, CD8, FOXP3, CD163, and clinicopathological features and prognosis of patients with advanced GC. CD8 and CD163 represented favorable and adverse prognostic factors, respectively. High LATS1/2 expression in GC has been reported to yield better prognoses (9). However, LATS1/2 and FOXP3 expressions did not predict the overall survival in patients with advanced GC in this study. The results revealed that high LATS1/2 expression was related to TNM stage progression. Different GC types may have different prognosis-related biological indicators (24). Therefore, LATS1/2 may differently predict the prognosis in different GC subtypes. Because LATS1/2 is significantly related to GC microsatellite stability, we then analyzed LATS1/2 expressions in MSS and MSI patients.
Molecular classification is essential for subtyping GC (5, 6). The prognoses differ among patients with different molecular subtypes, but the reason remains unclear (6). Therefore, we defined patients with MSS GC and MSI GC according to the immunohistochemical expressions of MLH1, MSH2, MSH6, and PMS2 (28, 29). Patients with MSS GC had poor prognoses, which is consistent with previous reports (29). In addition, LATS1/2 and FOXP3 expressions were increased while CD8 expression was decreased in patients with MSS GC. CD163 expression did not significantly differ between MSS GC and MSI GC patients. Next, we separately analyzed the prognoses of MSS and MSI GC patients according to LATS1/2, CD8, FOXP3, and CD163 expressions. High CD163 expression indicated a poor prognosis for GC patients. Subgroup analysis revealed that CD163 did not predict the prognoses of MSS and MSI patients, but the results revealed that its survival prognosis trend was consistent with that of the overall analysis and is likely because the decreased sample sizes resulted in no statistical differences after grouping. Therefore, in future studies, we should further analyze the role of CD163 in different GC subgroups by increasing the sample size and continuing to follow the patients. Furthermore, in MSS GC patients, high LATS1/2 and FOXP3 expressions and low CD8 expression predicted adverse patient prognoses, whereas in MSI GC, only CD8 predicted patient prognoses. In patients with MSS GC, LATS1/2 signaling pathway activation was also correlated with an adverse prognosis, indicating that LATS1/2 activation might suppress the antitumor effect of CD8+ T cells and activate immunosuppressive effects in FOXP3+ Treg cells. However, its deep mechanism remains unclear. Ma et al. reported that PD-L1 was always expressed in MSI and EBV(+) GC. Kim et al. found that anti-PD1 treatment was more effective against MSI and EBV(+) GC (31, 32), but less attention was paid to MSS GC. Of note, we found that LATS1/2 were more highly expressed in MSS GC than in MSI GC and could be regarded as adverse prognostic factors in MSS GC, suggesting that LATS1/2 might be a new target for MSS GC treatment.
Recent evidence revealed that combined analysis of multiple biological markers has a superior prognostic value compared with that of analyzing individual biological markers (23). We found that LATS1/2 were negatively correlated with CD8 and positively correlated with FOXP3. We combined LATS1/2, CD8, and FOXP3 to analyze the prognoses of advanced GC patients and found that combined analysis of LATS1/2, CD8, and FOXP3 predicted patient prognoses, and the HR of the combined analysis of the three indicators was better than the combination of any two indicators, suggesting that LATS1/2 might play an important role in CD8+ T cells and FOXP3+ Treg cells in a tumor immune microenvironment. CD8+ T cells and FOXP3+ Treg cells are reported to be closely related and play important roles in tumor development and immune escape in breast, ovarian, and gastric cancers (33–35). In a tumor immune microenvironment, LATS1/2 knockout in tumor cells weakened the CD8+T cell functions, leading to tumor immune escape (13). In GC, a previous report indicated that LATS2 was positively correlated with FOXP3 (36), but the function of LATS1/2 in FOXP3+ Treg cells is unreported and may represent a future research direction. Thus, combined analysis of LATS1/2, CD8, and FOXP3 might be a good strategy for obtaining an accurate prognosis in advanced GC patients.
This study has several limitations. First, the data in our analysis was from a single center without an external validation cohort and needs to be jointly verified by multiple centers. Second, this was a retrospective study and thus was inherently subject to selection bias.
In summary, LATS1/2, CD8, and FOXP3 expressions may be used as prognostic markers in advanced GC patients. Our study identified the novel individual marker— LATS1/2, for obtaining a prognosis in MSS GC, and combining LATS1/2, CD8, and FOXP3 may serve as a prognostic marker in advanced GC. These findings contribute to better understand advanced GC, and further investigation is needed to elucidate the underlying mechanisms of these markers.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Ethical Committee of the Shanghai Jiao Tong University School of Medicine, Renji Hospital. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
XL and DX collected the data and wrote the manuscript. YG, CH, and ZW analyzed the data and contributed in writing the manuscript. GZ and WZ performed the design and oversaw the project. YS provided the immunohistochemical analysis. XX, CZ, ZZ, JX, and GZ substantially contributed to the study design, performed the surgery, and revised the manuscript. All authors contributed to the article and approved the submitted version.
This study was funded by the Academic Clinician Team of the Shanghai Jiaotong University School of Medicine (20191905), the National Natural Science Foundation (31872740), the project of Shanghai Jiao Tong University (YG2016QN48), and the project of Shanghai Municipal Health Commission (201740049).
The opinions, conclusions, or assertions contained herein are the private views of the authors and are not to be construed as official nor reflect the views of the Department of Gastrointestinal Surgery, Renji Hospital, School of Medicine, Shanghai Jiao Tong University.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors thank all the patients who participated in this study.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.01406/full#supplementary-material
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
3. Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, et al. CONCORD Working Group, Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. (2018) 391:1023–75. doi: 10.1016/S0140-6736(17)33326-3
4. Kerkar SP, Kemp CD, Duffy A, Kammula US, Schrump DS, Kwong KF, et al. The GYMSSA trial: a prospective randomized trial comparing gastrectomy, metastasectomy plus systemic therapy versus systemic therapy alone. Trials. (2009) 10:121. doi: 10.1186/1745-6215-10-121
5. Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. (2014) 513:202–9. doi: 10.1038/nature13480
6. Cristescu R, Lee J, Nebozhyn M, Kim KM, Ting JC, Wong SS, et al. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat Med. (2015) 21:449–56. doi: 10.1038/nm.3850
7. Yu FX, Guan KL. The Hippo pathway: regulators and regulations. Genes Dev. (2013) 27:355–71. doi: 10.1101/gad.210773.112
8. Harvey KF, Zhang X, Thomas DM. The Hippo pathway and human cancer. Nat Rev Cancer Correlation. (2013) 13:246–57. doi: 10.1038/nrc3458
9. Son MW, Song GJ, Jang SH, Hong SA, Oh MH, Lee JH, et al. Clinicopathological significance of large tumor suppressor (LATS) expression in gastric cancer. J Gastric Cancer. (2017) 17:363–73. doi: 10.5230/jgc.2017.17.e41
10. Ma S, Meng Z, Chen R, Guan KL. The Hippo pathway: biology and pathophysiology. Annu Rev Biochem. (2019) 88:577–604. doi: 10.1146/annurev-biochem-013118-111829
11. Li J, Chen X, Ding X, Cheng Y, Zhao B, Lai ZC, et al. LATS2 suppresses oncogenic Wnt signaling by disrupting β-catenin/BCL9 interaction. Cell Rep. (2013) 5:1650–63. doi: 10.1016/j.celrep.2013.11.037
12. Xu B, Sun D, Wang Z, Weng H, Wu D, Zhang X, et al. Expression of LATS family proteins in ovarian tumors and its significance. Hum Pathol. (2015) 46:858–67. doi: 10.1016/j.humpath.2015.02.012
13. Pan WW, Moroishi T, Koo JH, Guan KL. Cell type-dependent function of LATS1/2 in cancer cell growth. Oncogene. (2019) 38:2595–610. doi: 10.1038/s41388-018-0610-8
14. Moroishi T, Hayashi T, Pan WW, Fujita Y, Holt MV, Qin J, et al. The Hippo pathway kinases LATS1/2 suppress cancer immunity. Cell. (2016) 167:1525–39. doi: 10.1016/j.cell.2016.11.005
15. Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. (2013) 14:1014–22. doi: 10.1038/ni.2703
16. Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. (2008) 133:775–87. doi: 10.1016/j.cell.2008.05.009
17. Li Z, Li D, Tsun A, Li B. FOXP3+regulatory T cells and their functional regulation. Cell Mol Immunol. (2015) 12:558–65. doi: 10.1038/cmi.2015.10
18. Ngambenjawong C, Gustafson HH, Pun SH. Progress in tumor-associated macrophage (TAM)-targeted therapeutics. Adv Drug Deliv Rev. (2017) 114:206–21. doi: 10.1016/j.addr.2017.04.010
19. Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. (2006) 66:605–12. doi: 10.1158/0008-5472.CAN-05-4005
20. Alvarado-Vazquez PA, Bernal L, Paige CA, Grosick RL, Moracho Vilrriales C, Ferreira DW, et al. Macrophage-specifc nanotechnology-driven CD163 overexpression in human macrophages results in an M2 phenotype under inflammatory conditions. Immunobiology. (2017) 222:900–12. doi: 10.1016/j.imbio.2017.05.011
21. McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM. Reporting recommendations for tumour marker prognostic studies (REMARK). Br J Cancer. (2005) 93:387–91. doi: 10.1038/sj.bjc.6602678
22. Wang Y, Zhu C, Song W, Li J, Zhao G, Cao H. PD-L1 expression and CD8+T cell infiltration predict a favorable prognosis in advanced gastric cancer. J Immunol Res. (2018) 2018:4180517. doi: 10.1155/2018/4180517
23. Takada K, Kashiwagi S, Goto W, Asano Y, Takahashi K, Takashima T, et al. Use of the tumor-infiltrating CD8 to FOXP3 lymphocyte ratio in predicting treatment responses to combination therapy with pertuzumab, trastuzumab, and docetaxel for advanced HER2-positive breast cancer. J Transl Med. (2018) 16:86. doi: 10.1186/s12967-018-1460-4
24. Liu X, Xu D, Huang C, Guo Y, Wang S, Zhu C, et al. Regulatory T cells and M2 macrophages present diverse prognostic value in gastric cancer patients with different clinicopathologic characteristics and chemotherapy strategies. J Transl Med. (2019) 17:192. doi: 10.1186/s12967-019-1929-9
25. Ercolani C, Di Benedetto A, Terrenato I, Pizzuti L, Di Lauro L, Sergi D, et al. Expression of phosphorylated Hippo pathway kinases (MST1/2 and LATS1/2) in HER2-positive and triple-negative breast cancer patients treated with neoadjuvant therapy. Cancer Biol Ther. (2017) 18:339–46. doi: 10.1080/15384047.2017.1312230
26. In H, Solsky I, Palis B, Langdon-Embry M, Ajani J, Sano T. Validation of the 8th edition of the AJCC TNM staging system for gastric cancer using the national cancer database. Ann Surg Oncol. (2017) 24:3683–91. doi: 10.1245/s10434-017-6078-x
27. Halling KC, Harper J, Moskaluk CA, Thibodeau SN, Petroni GR, Yustein AS, et al. Origin of microsatellite instability in gastric cancer. Am J Pathol. (1999) 155:205–11. doi: 10.1016/S0002-9440(10)65114-0
28. Wu CW, Chen GD, Jiang KC, Li AF, Chi CW, Lo SS, et al. A genome-wide study of microsatellite instability in advanced gastric carcinoma. Cancer. (2001) 92:92–101. doi: 10.1002/1097-0142(20010701)92:1<92::AID-CNCR1296>3.0.CO;2-W
29. Hao Y, Chun A, Cheung K, Rashidi B, Yang X. Tumor suppressor LATS1 is a negative regulator of oncogene YAP. J Biol Chem. (2008) 283:5496–509. doi: 10.1074/jbc.M709037200
30. Hay BA, Guo M. Coupling cell growth, proliferation, and death. Hippo weighs in. Dev Cell. (2003) 5:361–3. doi: 10.1016/S1534-5807(03)00270-3
31. Ma C, Patel K, Singhi AD, Ren B, Zhu B, Shaikh F, et al. Programmed death-ligand 1 expression is common in gastric cancer associated with Epstein-Barr virus or microsatellite instability. Am J Surg Pathol. (2016) 40:1496–506. doi: 10.1097/PAS.0000000000000698
32. Kim ST, Cristescu R, Bass AJ, Kim KM, Odegaard JI, Kim K, et al. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat Med. (2018) 24:1449–58. doi: 10.1038/s41591-018-0101-z
33. Ali HR, Provenzano E, Dawson SJ, Blows FM, Liu B, Shah M, et al. Association between CD8+ T-cell infiltration and breast cancer survival in 12,439 patients. Ann Oncol. (2014) 25:1536–43. doi: 10.1093/annonc/mdu191
34. Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Version 2. Proc Natl Acad Sci USA. (2005) 102:18538–43. doi: 10.1073/pnas.0509182102
35. Shen Z, Zhou S, Wang Y, Li RL, Zhong C, Liang C, et al. Higher intratumoral infiltrated FOXP3+ Treg numbers and FOXP3+/CD8+ ratio are associated with adverse prognosis in resectable gastric cancer. J Cancer Res Clin Oncol. (2010) 136:1585–95. doi: 10.1007/s00432-010-0816-9
Keywords: gastric cancer, prognosis, LATS1/2, CD8, FOXP3, CD163, microsatellite stability
Citation: Guo Y, Liu X, Xu D, Huang C, Wang Z, Xia X, Zhu C, Xu J, Zhang Z, Shen Y, Zhao W and Zhao G (2020) Role of LATS1/2 in Prognosis of Advanced Gastric Cancer and Its Relationship With the Tumor Immune Microenvironment. Front. Oncol. 10:1406. doi: 10.3389/fonc.2020.01406
Received: 19 May 2020; Accepted: 03 July 2020;
Published: 25 August 2020.
Edited by:
Fenglin Liu, Fudan University, ChinaCopyright © 2020 Guo, Liu, Xu, Huang, Wang, Xia, Zhu, Xu, Zhang, Shen, Zhao and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenyi Zhao, emhhb3d5MndpbkAxNjMuY29t; Gang Zhao, emhhb2dhbmc3NDMxM0BhbGl5dW4uY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.