
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol., 11 August 2020
Sec. Head and Neck Cancer
Volume 10 - 2020 | https://doi.org/10.3389/fonc.2020.01394
This article is part of the Research TopicAdvances in the Involvement of Human Papilloma Virus in Head and Neck Squamous Cell CarcinomaView all 15 articles
 Anna Beltz1
Anna Beltz1 Stefanie Zimmer2
Stefanie Zimmer2 Ioannis Michaelides3
Ioannis Michaelides3 Katja Evert4
Katja Evert4 Georgios Psychogios5
Georgios Psychogios5 Christopher Bohr3
Christopher Bohr3 Julian Künzel3*
Julian Künzel3*Squamous cell carcinomas of the head and neck are the subject of numerous current studies, especially in view of the increasing incidence of tumors induced by human papillomavirus (HPV) and the latest changes to the TNM classification of oropharyngeal squamous cell carcinoma (OPSCC). In addition to HPV status, the presence of extranodal extension of lymph node metastases represents an important risk and prognostic factor, which has now been integrated into the staging algorithm of the eighth edition of TNM classification for HPV-negative OPSCC. In the past numerous studies had shown a lack of prognostic significance of extranodal extension in HPV-associated tumors. However, extranodal extension–as a possible risk factor even in HPV-positive OPSCC–remains an important subject of current studies, which are now particularly characterized by high numbers of cases. In this paper, diagnostic methods and the prognostic significance of extranodal extension in surgically treated HPV-positive OPSCC are presented and discussed based on relevant literature, and the results of current publications are summarized. Further development of diagnostic criteria and procedures as well as international standardization of clinical diagnostics of extranodal extension should be encouraged. Several studies demonstrate that extranodal extension results in worse survival outcomes even in HPV-positive tumors, in contrast to results of previous studies. Consequently, whether the prognostic significance of extranodal extension is not actually relevant to outcome and the staging algorithm of HPV-positive OPSCC should be questioned and further analyzed.
The role of HPV in OPSCC has gained a great deal of attention in recent years. In addition to its causative role, HPV infection also proved to have a clear prognostic value (1). With the introduction of the eighth edition of the TNM classification (2017) a distinction is being made for the first time between HPV-positive and HPV-negative squamous cell carcinomas by the use of p16 immunohistochemistry (p16 IHC) as part of the staging of oropharyngeal squamous cell carcinoma (OPSCC). Furthermore, the prognostic influence of extranodal extension (ENE) of lymph node metastases for HPV-negative OPSCC was integrated into the staging algorithm. The prognostic influence of ENE has been analyzed in several studies and it was recognized as an essential prognostic factor, which should facilitate an even more accurate estimate of the risk of regional disease recurrences or distant metastases (1, 2). In the clinical staging of lymph node metastasis, defined criteria must be fulfilled for the diagnosis of clinical ENE. According to the new edition of the TNM classification, ENE in p16-positive OPSCC compared to HPV-negative tumors does not result in prognostic upstaging with regard to the N category or UICC stage. Several current studies focus on evaluating extensively the prognostic influence of ENE in HPV-positive tumors. The diagnostic methods and significance of ENE—with particular attention to surgically treated HPV-positive OPSCC—are presented and discussed below.
Extranodal extension was first described in 1930 by Rupert A. Willis in the context of autopsies on patients with advanced head and neck squamous cell carcinoma (HNSCC) (1, 3). It is generally defined as the spread of tumor tissue or neoplastic cells outside the lymph node capsule with infiltration of perinodal soft tissue (4). By means of histopathologic examination (lymph node metastasis without ENE; Figure 1A), ENE can additionally be subdivided into the categories “microscopic” (≤2 mm beyond the lymph node capsule; Figure 1B) and “macroscopic” (>2 mm beyond the lymph node capsule; Figure 1C) (4). For instance, Bauer et al. in their 2019 study illustrated the importance of the extent of ENE in patients with OPSCC (5). Patients were classified into the categories ENE-negative, microscopic ENE, and macroscopic ENE, and patients with microscopic ENE showed significantly reduced survival compared to patients with negative ENE status (hazard ratio = HR = 1.52; 95% CI = 1.00–2.31; p = 0.048) (5). Patients with macroscopic ENE had the worst outcome (HR = 2.50; 95% CI = 1.39–4.51; p = 0.002) (5). In addition to that, recent data even differentiate between the categories “no ENE,” “minimal ENE” (≤1 mm beyond the lymph node capsule”), and “>1 mm beyond the lymph node capsule” (6).
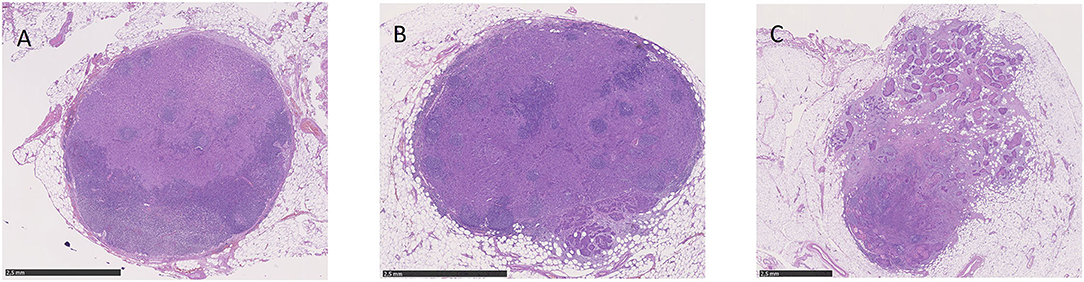
Figure 1. Microscopic view of lymph node metastases without ENE (A), with microscopic ENE (B) and with macroscopic ENE (C).
Although these subcategories have not yet been applied for the purpose of pN classification, they are recommended by the AJCC for data collection and analyses and find application in recent studies (4). In addition to patients with diagnosed lymph node metastases and ENE of metastases, it is reported that a proportion of 10.5–25% of patients exhibit microscopic ENE despite having a clinically unremarkable lymph node status (1, 7). Thus, microscopic ENE, micrometastases, or soft tissue deposits can cause an underestimate of the incidence of ENE—especially with regard to patients with primary radiotherapy that are classified within the cTNM-classification system (1, 7).
In addition to the widely used diagnostic methods involved in postoperative histopathologic examination, various imaging techniques can be applied, such as ultrasound, magnetic resonance imaging (MRI), and computed tomography (CT). Clinical diagnosis of ENE of lymph node metastases presupposes that clear, defined criteria are met (8). Clinical or radiologic signs of tumor invasion alone (including the skin and surrounding soft tissue) as well as clinical symptoms of neural involvement (e.g., paresis of cranial nerves) are defined as clinical ENE in the new TNM classification (9). The following criteria are used for radiologic diagnosis of ENE for both CT and MRI: presence of irregular nodal capsular enhancement, loss of distinct nodal margins and infiltration into adjacent structures (fatty tissue, muscle, blood vessels) (1, 10, 11). Generally speaking, however, the limited sensitivity and specificity of the methods used in relation to the clinical diagnosis of ENE need to be discussed (8). For example, Steinkamp et al. showed in several publications a sensitivity of ~80.9% with specificity of 72.2% for CT investigations and sensitivity of ~74.4% and specificity of 72.2% for MRI imaging (1, 12, 13). Clinical diagnosis of ENE by ultrasound, with a sensitivity of 78.6% and specificity of 81.8%, achieved slightly better results than CT or MRI (1, 14). With regard to diagnosing ENE by contrast-enhanced CT imaging, the values quoted in the literature according to Faraji et al. and others range from 75 to 86% for the accuracy of predicting pathologic ENE, from 65 to 90% for sensitivity and 73–91% for the specificity of the imaging method (12, 15–21). As clinical diagnosis of ENE, for example, does not differentiate between microscopic and macroscopic ENE so far, the data of patient collectives with primary surgery and collectives with primary radiotherapy are not readily comparable.
For a long time there have been strong demands for standardization and further development of investigation methods and internationally recognized diagnostic criteria for the imaging modalities of ultrasound, CT and MRI (1). Only recently Kann et al. published their study on the diagnosis of lymph node metastases and ENE in HNSCC by means of pretreatment CT images and three-dimensional deep learning neural networks (22). In this study they trained the neural network using a data set of 2,875 CT-segmented lymph node specimens and achieved diagnostic results which exceeded those of human clinicians (22). The area under the receiver operating characteristics curve for diagnosing ENE and lymph node metastases was 0.91 (95% CI = 0.85–0.97)—ENE of lymph node metastases could be predicted with a sensitivity of 88% and specificity of 85% (22). The diagnosis of ENE by means of CT was additionally the subject of the recently published work by Faraji et al. (15). Seventy-three patients with HPV-positive OPSCC treated by primary surgery and neck dissection were reviewed for the presence of seven defined criteria of CT imaging (15). The pretreatment CT scans were evaluated by two radiologists who were blinded to the pathologic ENE results (15). In the evaluations, the presence of irregular nodal margins (highest specificity of 94% for examiner A and 95% for examiner B) and the absence of perinodal fatty tissue (highest sensitivity of 87% for examiner A and 96% for examiner B) showed a significant association with ENE (15).
In 2018 Hararah et al. published initial attempts at pretreatment prediction of ENE and positive surgical margins for OPSCC (23). In the course of analyzing prognostic parameters of 5,056 patients (3,336 HPV-positive), Hararah et al. developed nomograms for the parameters ENE and/or positive resection status for HPV-negative as well as HPV-positive OPSCC (23). Regarding the prediction of postoperative ENE, for HPV-positive tumors clinical ENE, cN staging, cT staging, age, and tumor grading were integrated into the nomogram as predictive parameters (AUC ROC = 0.66; p < 0.01; 95% CI = 0.64–0.68) (23). Hararah et al. are thus presenting additional approaches to diagnosing ENE which, as a whole, could potentially facilitate clinical decision-making regarding primary treatment. However, they particularly stress the current aspiration to further develop clinical (and pathologic) diagnostic methods.
Further studies and more prolonged use of the new TNM classification are required to show how far the new clinical N classification, or particularly the clinical diagnosis of ENE defined therein, can succeed in everyday clinical practice and result in reliable identification of ENE status or whether new diagnostic methods will prevail in future. Understaging or upstaging of patients not surgically treated should be avoided where the prognostic influence of ENE is proven. Improving the modalities for clinical diagnosis of ENE and standardizing ENE diagnosis in general will thus continue to be the objective over the next few years.
The aim of this paper is to provide a structured overview of current study results on the topic “Prognostic influence of ENE in surgically treated HPV-positive OPSCC.” In order to investigate a possible prognostic influence of ENE in HPV-positive OPSCC, a literature research was conducted with PubMed. Using the PubMed Search Builder the following search term was created: ((((((((((extracapsular spread[Title/Abstract]) OR perinodal spread[Title/Abstract]) OR transcapsular spread[Title/Abstract]) OR extranodal spread[Title/Abstract]) OR extracapsular extension[Title/Abstract]) OR extranodal extension[Title/Abstract]) OR perinodal extension[Title/Abstract]) OR transcapsular extension[Title/Abstract])) AND (((hpv[Title/Abstract]) OR human papilloma virus[Title/Abstract]) OR p16[Title/Abstract])) AND (((((head and neck squamous cell carcinoma)) OR hnscc) OR oropharyngeal squamous cell carcinoma) OR opscc). The process of literature research is illustrated in Figure 2. 109 of 110 results were available in English. The included studies were published before July 2020.
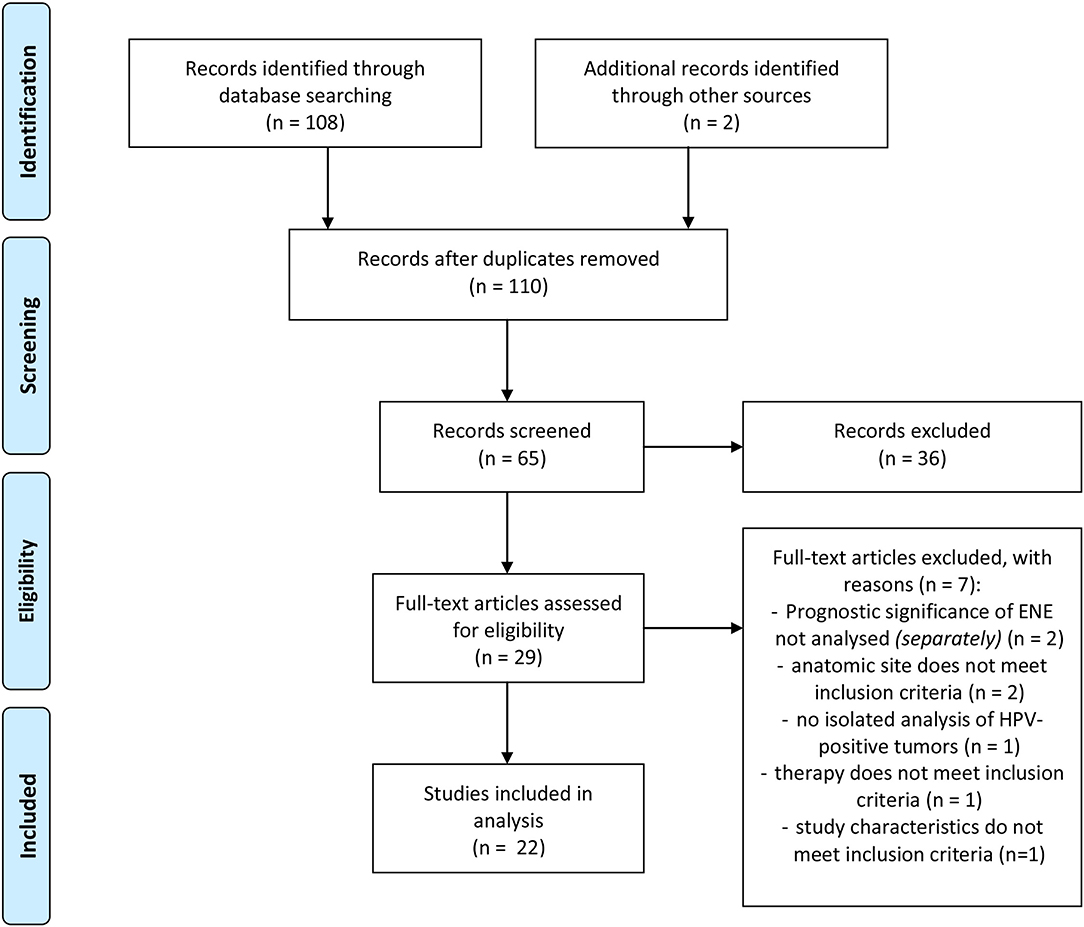
Figure 2. Flow-chart of literature research adapted from PRISMA (24).
Before screening the records, the following inclusion criteria were defined: (a) OPSCC, (b) patient collective contains HPV-positive tumors, (c) surgically treated collective/Neck Dissection (with or without adjuvant therapy), (d) ENE-Status available, (e) statement on the prognostic influence of ENE, (f) original research paper. Full-text articles assessed for eligibility were screened for further relevant publications. The publications included in analysis were screened for their study results referring to the prognostic impact of ENE in HPV-positive OPSCC. The relevant results as well as characteristics of the studies and number of cases are shown in Tables 1, 2 in the following.
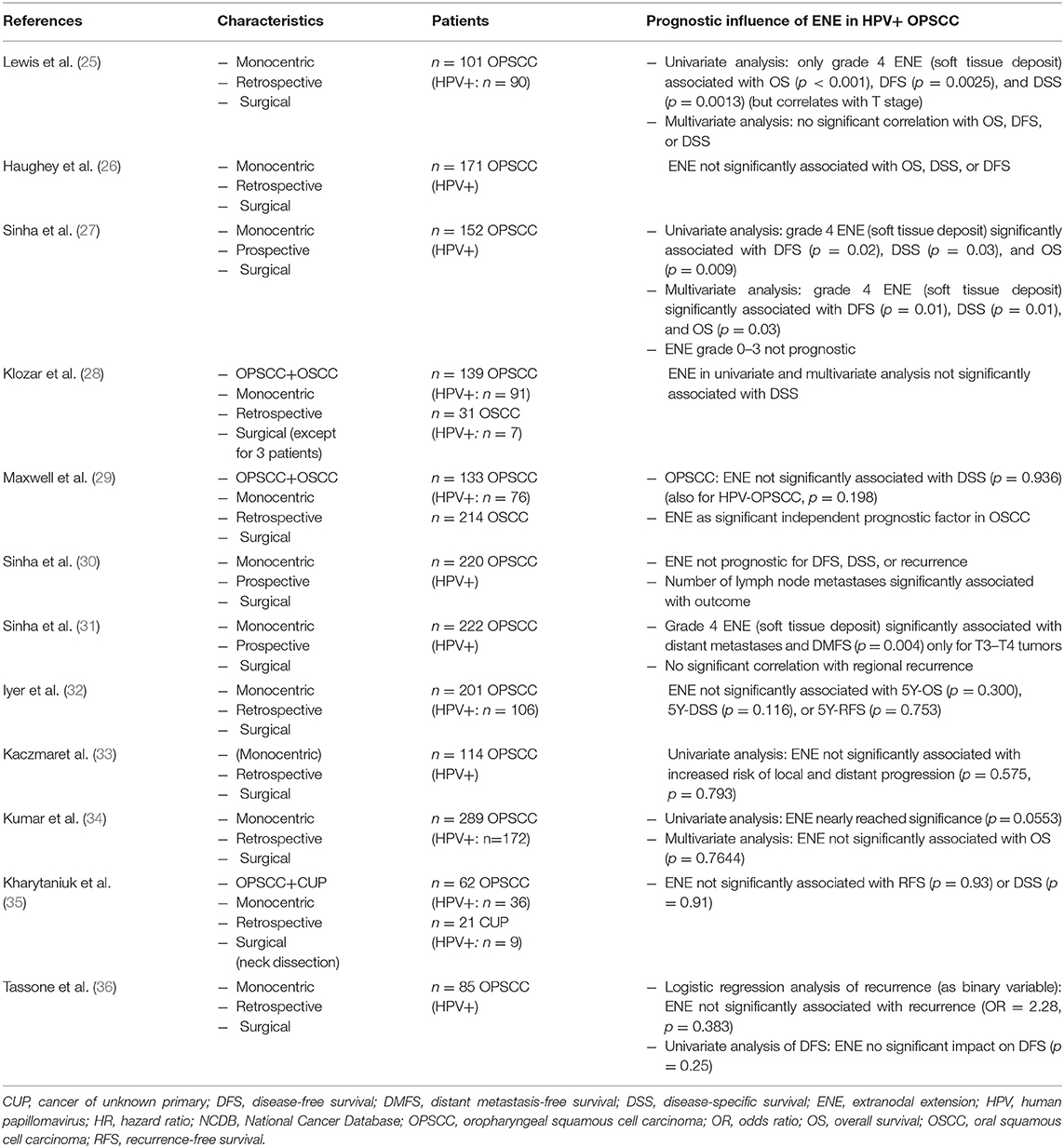
Table 1. Summary of the described studies on the prognostic influence of ENE in HPV-positive OPSCC—part 1.
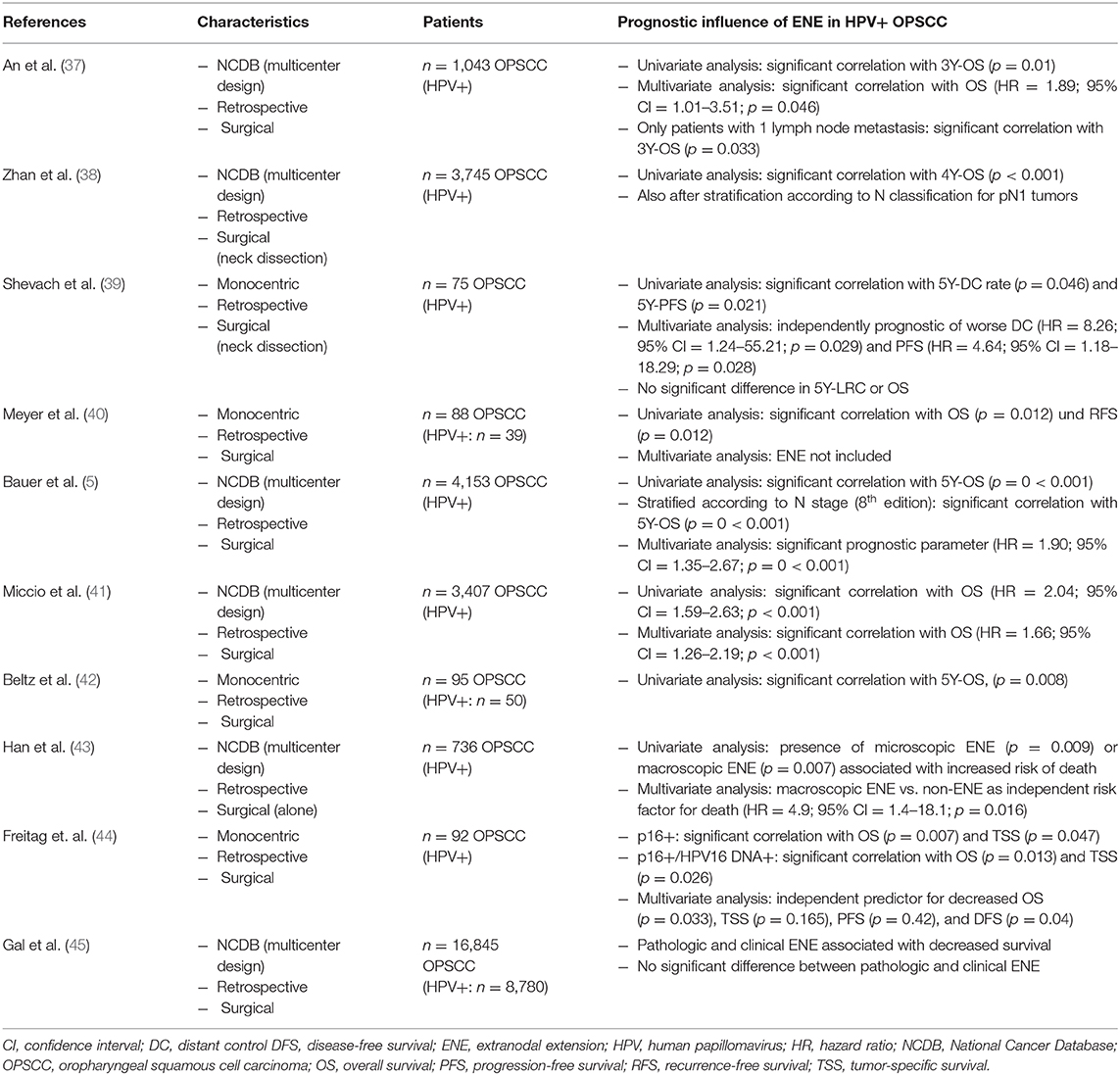
Table 2. Summary of the described studies on the prognostic influence of ENE in HPV-positive OPSCC—part 2.
Numerous studies in the past have shown that the presence of ENE additionally worsens the prognosis of patients with HNSCC (1, 8, 46–50). In a 2006 meta-analysis by Dunne et al. involving 1,620 patients with diagnosed HNSCC and lymph node metastasization, 5-year overall survival deteriorated to 30.7% in the presence of ENE compared to 58.1% in the absence of ENE (1, 50).
Furthermore, the correlation between ENE and locoregional recurrence and distant metastases has been studied in recent years. For example, Myers et al. showed in their publication of 2001 on 266 patients with squamous cell carcinoma of the tongue that ENE was the most significant prognostic parameter for the risk of regional recurrence and distant metastases in their population (1, 2). The meta-analysis by Mermod et al. from 2016 showed an odds ratio of 2.18 (95% CI = 1.23–3.87) for the correlation between ENE and distant metastases (1).
In 2014 Künzel et al. analyzed, among other things, the influence of ENE on the disease-specific survival of patients with OPSCC not stratified to HPV status. The study analyzed 384 patients first diagnosed between 1980 and 2010 (48). One of the findings was that ENE is associated with significantly worse disease-specific survival of 50% compared to 81% in the absence of ENE (p < 0.001) (48).
A few studies, however, demonstrated a lack of significant worsening of outcome due to ENE in HPV-positive OPSCC (1, 25, 27, 29, 30). In particular, the research group of Sinha et al. investigated the influence of ENE in HPV-induced OPSCC in detail (1, 25–27, 30, 31). In 2012, with regard to a group of 171 patients with p16-positive surgically treated OPSCC (adjuvant RT: n = 73, adjuvant CRT: n = 69), they published a lack of significant impact of ENE on overall and disease-specific survival (1, 26). In their multivariate analysis from 2012 (27), ENE (except for soft tissue deposits) did not prove prognostic in HPV-positive OPSCC in a prospective transoral laser surgery database (n = 152 patients—adjuvant RT: n = 66, adjuvant CRT: n = 67) (1, 27). In 2015 in their multivariate analysis of p16-positive OPSCC treated by surgery and neck dissection (n = 220 patients—RT: n = 97, CRT: n = 75), one of their findings was that the number of lymph node metastases (≥5)—but not ENE—was an independent prognostic factor (1, 30).
Their analyses of 2011, 2012, and 2015 additionally investigated the significance of the extent of ENE and its prognostic influence: Thus Lewis et al. also described a lack of significant influence of ENE on overall survival, disease-free survival (DFS), and disease-specific survival (DSS) in surgically treated HPV-positive OPSCC (n = 101 patients–postoperative radiation therapy: n = 100, postoperative chemotherapy: n = 44) (25). In fact, a significant correlation was found between the presence of soft tissue deposits (defined as grade 4 ENE) and overall survival, DSS and DFS. Given a correlation with the T stage, it was not confirmed in the multivariate analysis (25). In their 2012 publication, Sinha et al. then confirmed a significant influence of soft tissue deposits on overall survival, DFS and DSS (27). In 2015 they again published results which showed a significant correlation between soft tissue deposits and distant metastasis-free survival for T3–T4 tumors only (n = 222 patients, adjuvant RT: n = 97, adjuvant CRT: n = 78) (31).
In this connection Maxwell et al. reached the following conclusion in their 2013 publication (29): In their analysis of 133 patients with OPSCC and 214 patients with carcinoma of the oral cavity (surgically treated in the years 1983–2009), they found no significant association between ENE status and DSS for HPV-positive and HPV-negative patients (OPSCC: adjuvant radiation: n = 111, adjuvant chemotherapy: n = 40).
The investigation of potential prognostic parameters of HPV-positive and HPV-negative OPSCC and oral carcinomas of 170 patients (OPSCC: n = 139, 65.5% HPV-positive) was also the purpose of the study by Klozar et al. published in 2013 (28). For HPV-negative tumors, univariate analysis showed UICC stage, Pt, and pN classification, number of positive lymph nodes and ENE to be significant prognostic parameters. Except for ENE, these were confirmed in the multivariate analysis. For HPV-positive tumors, by contrast, none of these parameters showed a significant correlation with the DSS of patients (28).
In 2015 Iyer et al. published the following results: While ENE, resection status, lymph vessel invasion, and pN category were independent predictors of survival in the case of HPV-negative OPSCC, they were not prognostic for HPV-positive tumors [in respect of recurrence-free survival (RFS), DSS, and OS] (n = 201 patients, adjuvant RT: n = 138) (32). In addition to that, Kumar et al. (34) showed that ENE (p = 0.0021) and advanced T-classification represent significant predictors of survival in HPV-negative surgically treated OPSCC (most patients treated with adjuvant RT/RCT based on NCCN guidelines). While ENE nearly reached significance in the univariate analysis of HPV-positive OPSCC (p = 0.0553), multivariate analysis revealed that ENE was not significantly associated with survival (p = 0.7644) (34). Kharytaniuk et al. in their 2016 analysis of 83 patients (n = 62 with OPSCC, n = 21 with cancer of unknown primary = CUP) with neck dissection as part of primary definitive treatment—also confirmed that ENE is not a negative prognostic factor for HPV-positive OPSCC (in respect of RFS and DSS) (35) (surgery only: n = 8, RT: n = 50, CRT: n = 25). Kaczmar et al. showed that ENE does not correlate with increased risk of local as well as distant progression in HPV-positive OPSCC (114 surgical patients, 89 with adjuvant radiation, 54 with adjuvant chemotherapy) in univariate analysis (33). In addition to that, Tassone et al. confirmed that ENE is not significantly associated with recurrence and DFS in a retrospective analysis of 85 surgically treated HPV-positive OPSCC (adjuvant RT: n = 81, adjuvant systemic therapy: n = 52) (36). Table 1 summarizes the studies described in this chapter showing no or weak prognostic influence of ENE in primarily surgically treated HPV-positive OPSCC.
According to the 8th edition of the TNM classification, the presence of ENE leads to distinct upstaging solely in HPV-negative OPSCC. For HPV-positive tumors, only the number of positive lymph nodes is decisive in terms of pTNM staging. This is why the prognostic influence of ENE in HPV-induced tumors is currently the focus of a number of studies. In a 2019 study by the present authors, the application and prognostic impact of the new TNM classification as well as the factors HPV status and ENE were examined in a group of 255 patients with OPSCC first diagnosed in the years 2008–2015 (42). This included analyzing the overall survival of HPV-positive patients with negative vs. positive ENE status treated with surgery alone or surgery combined with adjuvant radiation/chemoradiation. In this cohort adjuvant therapy was standard in case of pathologically proven ENE. This study addressed, among other things, the question of whether ENE can actually be ignored in HPV-mediated OPSCC (42). Ninety five patients met the inclusion criteria for ENE analysis. The Kaplan-Meier curves presented (Figure 3) and the log rank test revealed a statistically significant deterioration of overall survival in the presence of ENE for HPV-positive patients in the univariate analysis (ENE-negative: OS = 92.9%, ENE-positive: 68.0%, p = 0.008) (42).
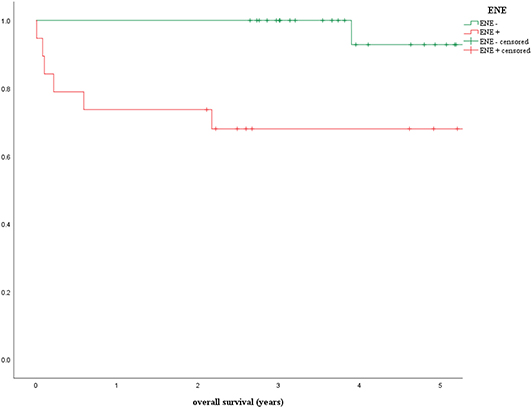
Figure 3. Prognostic influence of extranodal extension in patients with p16-positive oropharyngeal carcinoma (ENE, extranodal extension) (42).
The univariate analysis of the study by Meyer et al. (40), which examined the prognostic influence of the lymph node ratio, also showed a significant influence of ENE on overall survival of HPV-positive patients with surgically treated OPSCC (p = 0.012; ENE not included in the multivariate analysis) (surgery: n = 21, surgery + R(C)T: n = 67).
In 2017 Zhan et al. published results of their validation of the new staging system based on 3,745 cases of HPV-positive OPSCC treated by surgery and neck dissection from the National Cancer Database (NCDB) for the years 2010–2014 (surgery only: n = 642, surgery + RT: n = 1,005, surgery + CRT: n = 1,773) (38). As well as a general evaluation of the new staging algorithm, the study focused on analyzing the prognostic influence of ENE in HPV-positive OPSCC. During the course of their analyses, Zhan et al. demonstrated a statistically significant influence of ENE on 4-year overall survival in HPV-positive OPSCC (ENE-negative: 92% vs. ENE-positive: 85%, p < 0.001) (38). Upon stratification according to pN classification, ENE proved to be a significant prognostic parameter for the 4-year overall survival of HPV-positive patients with pN1 stage [pN1: ENE-negative 92%, ENE-positive 87% (p = 0.004); pN2: ENE-negative 88%, ENE-positive 77% (p = 0.061)] (38).
The described results are consistent with the results of analyses by An et al. published in 2017 (37): In their study the prognostic value of ENE was examined in a group of 1,043 patients with HPV-positive OPSCC (pT1–T4, pN1–N3, M0, R0) who underwent primary surgical treatment (adjuvant RT: n = 306, adjuvant CRT: n = 498). Patients who met the defined inclusion criteria were identified via the NCDB for the years 2010–2012 (37). In the course of their analyses An et al. demonstrated that a positive ENE status is associated with a significant deterioration of overall survival of HPV-positive patients (3-year overall survival: 89.3 vs. 93.6%, p = 0.01) (37). No significant difference in overall survival was found between cases with microscopic vs. macroscopic ENE (37). Furthermore, An et al. also demonstrated in the multivariate analysis that rather than the presence of ≥5 lymph node metastases—as integrated into the TNM classification for HPV-positive OPSCC—it is the presence of ENE that is significantly associated with a deterioration of overall survival (HR = 1.89; 95% CI = 1.01–3.51; p = 0.046) (37). Hence the results to some extent contradict the current system of N classification of p16-positive OPSCC (42). For HPV-positive OPSCC patients who have undergone primary surgical treatment, it is only the absolute number of lymph nodes involved (cut-off between pN1 and pN2: ≥5 involved lymph nodes) which has the decisive prognostic influence on determining the pN category according to the 8th edition (42). Given comparable hazard ratios (≥5 lymph node metastases: HR = 1.81, p = 0.086 vs. ENE: HR = 1.89, p = 0.046), An et al. do not question the cut-off of ≥5 lymph node metastases as a prognostic parameter for HPV-positive OPSCC. However, they do advocate evaluation of both parameters as potential prognostic factors—especially since a higher number of lymph node metastases is associated with the presence of ENE with a greater probability (37).
In order to eliminate the possible confounding variable of “total number of lymph node metastases” from the analysis and to investigate the relationship between ENE and overall survival in isolation, overall survival was analyzed only in patients with one lymph node metastasis: once again a deterioration of 3-year overall survival to 90.8% compared to 96.0% (p = 0.033) was observed (37).
In addition to that, Shevach et al. (39) published results showing that ENE-positive status is significantly associated with a deterioration of distant control and progression-free survival in univariate and multivariate analysis (39). They evaluated a collective of 75 patients with HPV-mediated OPSCC treated with surgery, respectively, neck dissection followed by adjuvant radiotherapy/chemoradiotherapy. However, there was no significant difference in OS and locoregional control between ENE-negative and -positive patients (39).
Bauer et al. recently showed that ENE represents a significant prognostic parameter in respect of overall survival in HPV-mediated OPSCC (5). In their paper published in 2019, they analyzed the prognostic significance of ENE in a group of 4,153 patients with HPV-positive OPSCC from the NCDB who were treated surgically and by neck dissection (N0 = 531, ENE-positive: 1,429, ENE-negative: 2,193—surgery only: n = 923, surgery/radiation: n = 1,403, surgery/radiation/chemo: n = 1,827) (5). The univariate analysis revealed a statistically significant correlation between ENE and overall survival in HPV-positive patients (p < 0.001) with 5-year overall survival of 92.6% (95% CI = 90.5–94.7%) for negative ENE status compared to 84.0% (95% CI = 80.7–87.4%) for ENE-positive tumors (5). Furthermore, when stratified according to N stage (8th edition), tumors classified as N1/ENE-negative showed the highest 5-year overall survival rate of 93.4% (95% CI = 91.3–95.5%), whereas N2/ENE-negative and N1/ENE-positive tumors had similar 5-year overall survival of 87.8 and 87.3%, respectively, (5). The multivariate analysis (with age, gender, population group, morbidity, T stage, treatment, and resection status as possible confounding variables) revealed in respect of mortality risk a hazard ratio of 1.90 (95% CI = 1.35–2.67) in the presence of ENE vs. ENE-negative OPSCC (p < 0.001). The pathologic N stage—or hence the number of positive lymph nodes—was significantly associated with patients' outcome (pN2 vs. pN1: HR = 1.53) (5). Furthermore, Bauer et al. demonstrated that—when combining pN category and ENE status—tumors classified as N2/ENE-positive had the lowest 5-year overall survival rate (HR = 2.93; 95% CI = 1.94–4.43; p < 0.001) in comparison with N1/ENE-negative OPSCC (HR = 1.00) (5). OPSCC classified as N1/ENE-positive also showed nearly twice as high mortality risk (HR = 1.88; 95% CI = 1.26–2.80; p = 0.002) as ENE-negative pN1 tumors. Bauer et al. thus concluded in the course of their evaluation that ENE is prognostic irrespective of the number of positive lymph nodes and that the combination of ENE status and number of positive lymph nodes (pN category) particularly leads to an improved picture of mortality risk (5). All in all, the work by Bauer et al. including 4,153 patients represents a large study in this field for HPV-positive OPSCC and—as a result of the length of follow-up—also allows the prognostic influence of ENE on 5-year overall survival to be evaluated. As Bauer et al. also stress, this is particularly significant in view of the relatively good prognosis of HPV-positive tumors. Furthermore, a large number of possible confounding variables were integrated into the multivariate analysis. In summary, the large number of cases included and the length of follow-up made it possible for the results to reach statistical significance (5).
Furthermore, Miccio et al. recently published their study on the influence of contralateral lymph node metastasization and ENE on survival in HPV-mediated OPSCC (41). Three thousand four hundred seven patients from the NCDB (2010–2015) with surgically treated, HPV-positive OPSCC and a minimum of 10 lymph nodes removed made up the study population (adjuvant RT: n = 1,262, adjuvant CRT: n = 1,501, unknown: n = 78) (41). In their evaluation, the research group of Miccio et al. concluded that, in both the univariate analysis (HR = 2.04; 95% CI = 1.59–2.63; p < 0.001) and the multivariate analysis (HR = 1.66; 95% CI = 1.26–02.19; p < 0.001), the presence of ENE is associated statistically highly significantly with a deterioration of overall survival in HPV-positive tumors and it should be included in future staging algorithms (41).
In 2019, Han et al. published a retrospective analysis of 736 patients with only surgically treated HPV-positive OPSCC from the NCDB (2010–2014) (43). Among other things, they showed that microscopic or macroscopic ENE results in a significantly worse OS when compared to positive lymph nodes without ENE (5J-OS: 91% vs. 78%; p < 0.0001) (43). In addition, Freitag et al. recently published their analysis of a cohort of 92 patients with surgically treated HPV-mediated OPSCC (IC+OP+RT: n = 8, OP: n = 21, OP+RT: n = 23, OP+RCT: n = 39, OP+RT+Cetuximab: n = 1) (44). Their multivariate analysis showed that ENE represents an independent predictor for decreased OS (p = 0.033), tumor-specific survival (p = 0.165), progression-free survival (p = 0.42), and DFS (p = 0.04) (44). The results of their investigation as a whole led them to the conclusion that ENE (as well as HPV16 DNA status) should be integrated in the prognostic staging algorithm of HPV-mediated OPSCC (44). Furthermore, Gal et al. recently showed a decreased survival in the presence of clinical and pathological ENE compared to the absence of ENE. Their retrospective SEER database study analyzed 16,845 primarily surgical treated patients with tonsillar and base of the tongue primaries (45).
As a whole the results described above contrast with the conclusions of the 2016 review by Mermod et al. which included an analysis of the prognostic significance of histopathologically proven ENE in HPV-positive OPSCC (1). Individual results of the studies included have already been presented: overall, the analyses had shown a lack of negative influence of ENE in HPV-positive OPSCC (1). Compared with the monocentric design of the studies analyzed by Mermod et al. (1) and the maximum number of 222 patients included Sinha et al. the great strengths of the studies by Zhan et al. (38), An et al. (37), Bauer et al. (5), Miccio et al. (41), and Gal et al. (45) are the case numbers of 3,745, 1,043, 4,153, 3,407, and 16,845 patients, respectively, and hence their power as well as their multicenter design.
Table 2 summarizes the described studies showing significant influence on prognosis of ENE in HPV-positive OPSCC.
The possible influence of tobacco consumption of patients was not analyzed because of the lack of recording in the NCDB. The influence of nicotine consumption on the risk and prognostic profile of HPV-positive OPSCC, however, is a relevant parameter according to the results of Ang et al. (51) and should be considered in future prospective analyses. Kompelli et al. recently published an analysis of patients with HPV-related OPSCC (52): Aim of this study was to investigate the impact of pathologic prognostic factors in the context of chronic tobacco use. The results show that, among other things, presence of ENE did not significantly affect survival in HPV-positive heavy smokers (≥20 pack years) (52). However, HPV-positive ENE-positive heavy smokers had a significant decrease in survival (similar to HPV-negative patients) compared to HPV-positive non-smokers with positive ENE-status (52). In the future, the prognostic impact of ENE should also be evaluated in the context of tobacco consumption and the prognostic influence of tobacco abuse in HPV-positive OPSCC should be examined in detail further on.
All in all, the current study results from various publications presented here emphasize that ENE is a risk and prognostic factor, including HPV-positive OPSCC, which to date has not been integrated into the staging algorithm of the TNM classification. Possible reasons for the different results of the studies mentioned are discussed by Bauer et al. (5), An et al. (37), and Zhan et al. (38). For example, the excellent prognosis of HPV-positive OPSCC could lead to the fact that only studies with a higher number of cases can reveal statistically significant differences between ENE-positive and—negative tumors (5). An et al. stress the greater power achieved by larger patient collectives as well (37). In addition, Zhan et al. support this argumentation with their data–with a moderate effect size on OS (5–11% on 4Y-OS), significant results are only likely in high numbers of cases (38), such as those made possible by the NCDB. Nonetheless, the prognostic influence of ENE in surgically treated HPV-positive OPSCC remains a topic that should be analyzed in further (prospective) multicenter studies.
Limitations of this work are that only studies that explicitly examined ENE in HPV-positive OPSCC (e.g., ENE terms in title/abstract) were included—therefore other possibly relevant research results, which were not identified through literature research or references, could have been missed. The aim of this publication was to provide the reader with an overview of the current status of research in this field in a structured form. Due to a limited number of studies that explicitly focus on this issue, as well as partly limited comparability, we did not choose a systematic review, but a structured review according to the PRISMA guidelines. We performed our literature research as structured and traceable as possible. Furthermore, no quality assessment of the included studies was carried out. Despite the focus on surgically treated collectives, it can be assumed that the therapy algorithms (especially regarding adjuvant therapy) vary to a certain extent from institute to institute, which influences the comparability of the studies. As histopathologically proven ENE is an accepted risk factor in HNSCC in general and de-escalation strategies in HPV-positive tumors have not been integrated into clinical practice, the majority of patients of all discussed studies should have been treated by adjuvant radiotherapy. As a topic that is currently gaining more and more interest, a future meta-analysis—including possible additional publications of the next months/years—should be considered.
Whether the prognostic influence of ENE of lymph node metastases can actually be ignored for HPV-positive tumors in the TNM classification system should be reevaluated in detail in the context of prospective multicenter studies. According to the study results presented here, it also seems necessary to record ENE in the tumor documentation for HPV-positive tumors. Furthermore, it is also the extent of ENE (macroscopic vs. microscopic—ENE ≤/>1 mm, respectively, ENE ≤/>2 mm) that should be examined and documented as it represents an additional prognostic factor. The methods applied (including ultrasound, CT, MRI) in the clinical diagnosing of ENE have limited sensitivity and specificity. In this regard initial attempts at computer-aided analysis of image data should be pursued. Furthermore, clinical diagnostic criteria should be standardized overall at the international level.
JK came up with the idea and planned the manuscript. AB and JK collected the data. AB drafted the manuscript and performed the systematic review and its analysis. AB provided the original data presented. SZ and KE provided expert opinion on histopathology and high resolution histopathologic pictures. IM helped with editing the manuscript. GP, CB, and JK reviewed the manuscript. All authors finally checked the manuscript and provided critical review of its content.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This paper contains essential parts of the doctoral thesis of AB.
1. Mermod M, Tolstonog G, Simon C, Monnier Y. Extracapsular spread in head and neck squamous cell carcinoma: a systematic review and meta-analysis. Oral Oncol. (2016) 62:60–71. doi: 10.1016/j.oraloncology.2016.10.003
2. Myers JN, Greenberg JS, Mo V, Roberts D. Extracapsular spread. A significant predictor of treatment failure in patients with squamous cell carcinoma of the tongue. Cancer. (2001) 92:3030–6. doi: 10.1002/1097-0142(20011215)92:12<3030::AID-CNCR10148>3.0.CO;2-P
3. Willis RA. Epidermoid carcinoma of the head and neck, with special reference to metastasis. J. Pathol. (1930) 33:501–26. doi: 10.1002/path.1700330302
4. Bullock MJ, Beitler JJ, Carlson DL, Fonseca I, Hunt JL, Katabi N, et al. Data set for the reporting of nodal excisions and neck dissection specimens for head and neck tumors: explanations and recommendations of the guidelines from the international collaboration on cancer reporting. Arch Pathol Lab Med. (2019) 143:452–62. doi: 10.5858/arpa.2018-0421-SA
5. Bauer E, Mazul A, Chernock R, Rich J, Jackson RS, Paniello R, et al. Extranodal extension is a strong prognosticator in HPV-positive oropharyngeal squamous cell carcinoma. Laryngoscope. (2019) 130:939–45. doi: 10.1002/lary.28059
6. Ferris RL, Flamand Y, Weinstein GS, Li S, Quon H, Mehra R, et al. Transoral robotic surgical resection followed by randomization to low- or standard-dose IMRT in resectable p16+ locally advanced oropharynx cancer: a trial of the ECOG-ACRIN cancer research group (E3311). JCO. (2020) 38(15 suppl):6500. doi: 10.1200/JCO.2020.38.15_suppl.6500
7. Coatesworth AP, MacLennan K. Squamous cell carcinoma of the upper aerodigestive tract: the prevalence of microscopic extracapsular spread and soft tissue deposits in the clinically N0 neck. Head Neck. (2002) 24:258–61. doi: 10.1002/hed.10020
8. Beltz A, Gösswein D, Zimmer S, Stauber RH, Hagemann J, Strieth S, et al. Staging von oropharynxkarzinomen: neue TNM-klassifikation als herausforderung für kopf-hals-tumorzentren. HNO. (2018) 66:375–82. doi: 10.1007/s00106-018-0499-0
9. Lydiatt WM, Patel SG, O'Sullivan B, Brandwein MS, Ridge JA, Migliacci JC, et al. Head and neck cancers-major changes in the American joint committee on cancer eighth edition cancer staging manual. CA Cancer J Clin. (2017) 67:122–37. doi: 10.3322/caac.21389
10. Som PM. Lymph nodes of the neck. Radiology. (1987) 165:593–600. doi: 10.1148/radiology.165.3.3317494
11. King AD, Tse GM, Yuen EH, To EW, Vlantis AC, Zee B, et al. Comparison of CT and MR imaging for the detection of extranodal neoplastic spread in metastatic neck nodes. Eur J Radiol. (2004) 52:264–70. doi: 10.1016/j.ejrad.2004.03.004
12. Steinkamp HJ, van der Hoeck E, Böck JC, Felix R. Kapseldurchbrüche zervikaler lymphknotenmetastasen: diagnostischer stellenwert der computertomographie. Rofo. (1999) 170:457–62. doi: 10.1055/s-2007-1011073
13. Steinkamp HJ, Beck A, Werk M, Felix R. Kapseldurchbrüche zervikaler lymphknotenmetastasen: diagnostischer stellenwert der magnetresonanztomographie. Rofo. (2002) 174:50–5. doi: 10.1055/s-2002-19533
14. Steinkamp HJ, Beck A, Werk M, Rademaker J, Felix R. Kapseldurchbrüche zervikaler lymphknotenmetastasen: diagnostischer stellenwert der sonographie. Ultraschall Med. (2003) 24:323–30. doi: 10.1055/s-2003-42914
15. Faraji F, Aygun N, Coquia SF, Gourin CG, Tan M, Rooper LM, et al. Computed tomography performance in predicting extranodal extension in HPV-positive oropharynx cancer. Laryngoscope. (2019) 130:1479–86. doi: 10.1002/lary.28237
16. Yousem DM, Som PM, Hackney DB, Schwaibold F, Hendrix RA. Central nodal necrosis and extracapsular neoplastic spread in cervical lymph nodes: MR imaging versus CT. Radiology. (1992) 182:753–9. doi: 10.1148/radiology.182.3.1535890
17. Souter MA, Allison RS, Clarkson JH, Cowan IA, Coates MH, Wells JE. Sensitivity and specificity of computed tomography for detection of extranodal spread from metastatic head and neck squamous cell carcinoma. J Laryngol Otol. (2009) 123:778–82. doi: 10.1017/S0022215109004332
18. Url C, Schartinger VH, Riechelmann H, Glückert R, Maier H, Trumpp M, et al. Radiological detection of extracapsular spread in head and neck squamous cell carcinoma (HNSCC) cervical metastases. Eur J Radiol. (2013) 82:1783–7. doi: 10.1016/j.ejrad.2013.04.024
19. Prabhu RS, Magliocca KR, Hanasoge S, Aiken AH, Hudgins PA, Hall WA, et al. Accuracy of computed tomography for predicting pathologic nodal extracapsular extension in patients with head-and-neck cancer undergoing initial surgical resection. Int J Radiat Oncol Biol Phys. (2014) 88:122–9. doi: 10.1016/j.ijrobp.2013.10.002
20. Chai RL, Rath TJ, Johnson JT, Ferris RL, Kubicek GJ, Duvvuri U, et al. Accuracy of computed tomography in the prediction of extracapsular spread of lymph node metastases in squamous cell carcinoma of the head and neck. JAMA Otolaryngol Head Neck Surg. (2013) 139:1187–94. doi: 10.1001/jamaoto.2013.4491
21. Carlton JA, Maxwell AW, Bauer LB, McElroy SM, Layfield LJ, Ahsan H, et al. Computed tomography detection of extracapsular spread of squamous cell carcinoma of the head and neck in metastatic cervical lymph nodes. Neuroradiol J. (2017) 30:222–9. doi: 10.1177/1971400917694048
22. Kann BH, Aneja S, Loganadane GV, Kelly JR, Smith SM, Decker RH, et al. Pretreatment identification of head and neck cancer nodal metastasis and extranodal extension using deep learning neural networks. Sci Rep. (2018) 8:14036. doi: 10.1038/s41598-018-32441-y
23. Hararah MK, Stokes WA, Jones BL, Oweida A, Ding D, McDermott J, et al. Nomogram for preoperative prediction of nodal extracapsular extension or positive surgical margins in oropharyngeal squamous cell carcinoma. Oral Oncol. (2018) 83:73–80. doi: 10.1016/j.oraloncology.2018.06.005
24. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
25. Lewis JS, Carpenter DH, Thorstad WL, Zhang Q, Haughey BH. Extracapsular extension is a poor predictor of disease recurrence in surgically treated oropharyngeal squamous cell carcinoma. Mod Pathol. (2011) 24:1413–20. doi: 10.1038/modpathol.2011.105
26. Haughey BH, Sinha P. Prognostic factors and survival unique to surgically treated p16+ oropharyngeal cancer. Laryngoscope. (2012) 122(Suppl. 2):13–33. doi: 10.1002/lary.23493
27. Sinha P, Lewis JS, Piccirillo JF, Kallogjeri D, Haughey BH. Extracapsular spread and adjuvant therapy in human papillomavirus-related, p16-positive oropharyngeal carcinoma. Cancer. (2012) 118:3519–30. doi: 10.1002/cncr.26671
28. Klozar J, Koslabova E, Kratochvil V, Salakova M, Tachezy R. Nodal status is not a prognostic factor in patients with HPV-positive oral/oropharyngeal tumors. J Surg Oncol. (2013) 107:625–33. doi: 10.1002/jso.23292
29. Maxwell JH, Ferris RL, Gooding W, Cunningham D, Mehta V, Kim S, et al. Extracapsular spread in head and neck carcinoma: impact of site and human papillomavirus status. Cancer. (2013) 119:3302–8. doi: 10.1002/cncr.28169
30. Sinha P, Kallogjeri D, Gay H, Thorstad WL, Lewis JS, Chernock R, et al. High metastatic node number, not extracapsular spread or N-classification is a node-related prognosticator in transorally-resected, neck-dissected p16-positive oropharynx cancer. Oral Oncol. (2015) 51:514–20. doi: 10.1016/j.oraloncology.2015.02.098
31. Sinha P, Lewis JS, Kallogjeri D, Nussenbaum B, Haughey BH. Soft tissue metastasis in p16-positive oropharynx carcinoma: prevalence and association with distant metastasis. Oral Oncol. (2015) 51:778–86. doi: 10.1016/j.oraloncology.2015.05.004
32. Iyer NG, Dogan S, Palmer F, Rahmati R, Nixon IJ, Lee N, et al. Detailed analysis of clinicopathologic factors demonstrate distinct difference in outcome and prognostic factors between surgically treated HPV-positive and negative oropharyngeal cancer. Ann Surg Oncol. (2015) 22:4411–21. doi: 10.1245/s10434-015-4525-0
33. Kaczmar JM, Tan KS, Heitjan DF, Lin A, Ahn PH, Newman JG, et al. HPV-related oropharyngeal cancer: risk factors for treatment failure in patients managed with primary transoral robotic surgery. Head Neck. (2016) 38:59–65. doi: 10.1002/hed.23850
34. Kumar B, Cipolla MJ, Old MO, Brown NV, Kang SY, Dziegielewski PT, et al. Surgical management of oropharyngeal squamous cell carcinoma: survival and functional outcomes. Head Neck. (2016) 38(Suppl. 1):E1794–802. doi: 10.1002/hed.24319
35. Kharytaniuk N, Molony P, Boyle S, O'Leary G, Werner R, Heffron C, et al. Association of extracapsular spread with survival according to human papillomavirus status in oropharynx squamous cell carcinoma and carcinoma of unknown primary site. JAMA Otolaryngol Head Neck Surg. (2016) 142:683–90. doi: 10.1001/jamaoto.2016.0882
36. Tassone P, Crawley M, Bovenzi C, Zhan T, Keane W, Cognetti D, et al. Pathologic markers in surgically treated HPV-associated oropharyngeal cancer: retrospective study, systematic review, and meta-analysis. Ann Otol Rhinol Laryngol. (2017) 126:365–74. doi: 10.1177/0003489417693014
37. An Y, Park HS, Kelly JR, Stahl JM, Yarbrough WG, Burtness BA, et al. The prognostic value of extranodal extension in human papillomavirus-associated oropharyngeal squamous cell carcinoma. Cancer. (2017) 123:2762–72. doi: 10.1002/cncr.30598
38. Zhan KY, Eskander A, Kang SY, Old MO, Ozer E, Agrawal AA, et al. Appraisal of the AJCC 8th edition pathologic staging modifications for HPV-positive oropharyngeal cancer, a study of the national cancer data base. Oral Oncol. (2017) 73:152–9. doi: 10.1016/j.oraloncology.2017.08.020
39. Shevach J, Bossert A, Bakst RL, Liu J, Misiukiewicz K, Beyda J, et al. Extracapsular extension is associated with worse distant control and progression-free survival in patients with lymph node-positive human papillomavirus-related oropharyngeal carcinoma. Oral Oncol. (2017) 74:56–61. doi: 10.1016/j.oraloncology.2017.09.014
40. Meyer MF, Meinrath J, Seehawer J, Lechner A, Odenthal M, Quaas A, et al. The relevance of the lymph node ratio as predictor of prognosis is higher in HPV-negative than in HPV-positive oropharyngeal squamous cell carcinoma. Clin Otolaryngol. (2018) 43:192–8. doi: 10.1111/coa.12938
41. Miccio JA, Verma V, Kelly J, Kann BH, An Y, Park HS, et al. Impact of contralateral lymph nodal involvement and extranodal extension on survival of surgically managed HPV-positive oropharyngeal cancer staged with the AJCC eighth edition. Oral Oncol. (2019) 99:104447. doi: 10.1016/j.oraloncology.2019.104447
42. Beltz A, Gösswein D, Zimmer S, Limburg I, Wünsch D, Gribko A, et al. Staging of oropharyngeal squamous cell carcinoma of the head and neck: prognostic features and power of the 8th edition of the UICC staging manual. Eur J Surg Oncol. (2019) 45:1046–53. doi: 10.1016/j.ejso.2019.02.032
43. Han M, Stanford-Moore GB, Larson AR, Schoppy DW, Cognetti DM, Joshi AS, et al. Predictors of mortality in HPV-associated oropharynx carcinoma treated with surgery alone. Laryngoscope. (2019) 130:E423–35. doi: 10.1002/lary.28344
44. Freitag J, Wald T, Kuhnt T, Gradistanac T, Kolb M, Dietz A, et al. Extracapsular extension of neck nodes and absence of human papillomavirus 16-DNA are predictors of impaired survival in p16-positive oropharyngeal squamous cell carcinoma. Cancer. (2020) 126:1856–72. doi: 10.1002/cncr.32667
45. Gal TJ, O'Brien KJ, Chen Q, Huang B. Clinical vs microscopic extranodal extension and survival in oropharyngeal carcinoma in the human papillomavirus era. Otolaryngol Head Neck Surg. (2020) 162:693–701. doi: 10.1177/0194599820910431
46. Alvi A, Johnson JT. Extracapsular spread in the clinically negative neck (N0): implications and outcome. Otolaryngol Head Neck Surg. (1996) 114:65–70. doi: 10.1016/S0194-5998(96)70285-1
47. Johnson JT, Myers EN, Bedetti CD, Barnes EL, Schramm VL, Thearle PB. Cervical lymph node metastases. Incidence and implications of extracapsular carcinoma. Arch Otolaryngol. (1985) 111:534–7. doi: 10.1001/archotol.1985.00800100082012
48. Künzel J, Psychogios G, Mantsopoulos K, Grundtner P, Waldfahrer F, Iro H. Lymph node ratio as a predictor of outcome in patients with oropharyngeal cancer. Eur Arch Otorhinolaryngol. (2014) 271:1171–80. doi: 10.1007/s00405-013-2513-1
49. Künzel J, Mantsopoulos K, Psychogios G, Grundtner P, Koch M, Iro H. Lymph node ratio as a valuable additional predictor of outcome in selected patients with oral cavity cancer. Oral Surg Oral Med Oral Pathol Oral Radiol. (2014) 117:677–84. doi: 10.1016/j.oooo.2014.02.032
50. Dünne AA, Müller HH, Eisele DW, Kessel K, Moll R, Werner JA. Meta-analysis of the prognostic significance of perinodal spread in head and neck squamous cell carcinomas (HNSCC) patients. Eur J Cancer. (2006) 42:1863–8. doi: 10.1016/j.ejca.2006.01.062
51. Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tân PF, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. (2010) 363:24–35. doi: 10.1056/NEJMoa0912217
Keywords: extranodal extension, TNM classification, human papilloma virus, oropharyngeal carcinoma, HPV, OPSCC
Citation: Beltz A, Zimmer S, Michaelides I, Evert K, Psychogios G, Bohr C and Künzel J (2020) Significance of Extranodal Extension in Surgically Treated HPV-Positive Oropharyngeal Carcinomas. Front. Oncol. 10:1394. doi: 10.3389/fonc.2020.01394
Received: 14 January 2020; Accepted: 02 July 2020;
Published: 11 August 2020.
Edited by:
Francois Mouawad, Centre Hospitalier Regional et Universitaire de Lille, FranceReviewed by:
Panagiotis Balermpas, University Hospital Zürich, SwitzerlandCopyright © 2020 Beltz, Zimmer, Michaelides, Evert, Psychogios, Bohr and Künzel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Julian Künzel, anVsaWFuLmt1ZW56ZWxAdWtyLmRl
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.