
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Oncol., 04 August 2020
Sec. Hematologic Malignancies
Volume 10 - 2020 | https://doi.org/10.3389/fonc.2020.01294
This article is part of the Research TopicAcute Promyelocytic Leukemia - Towards a Chemotherapy-Free Approach to Cure in All PatientsView all 11 articles
Various forms of arsenic were used in China and elsewhere for over 5,000 years. Following the initial success of intravenous arsenic trioxide (i.v. As2O3), we revived an oral formulation of pure As2O3 in 1998 for the treatment of acute promyelocytic leukemia (APL). We were the first to produce a 1 mg/ml oral-As2O3 solution and showed that it had comparable bioavailability to i.v. As2O3. Moreover, we also reported that intracellular arsenic concentrations were considerably higher than the corresponding plasma values. Our oral-As2O3 was patented internationally and registered in Hong Kong for the treatment of APL. Safety, tolerability and clinical efficacy was confirmed in long-term follow-up studies. We have extended the use of oral-As2O3 to frontline induction of newly diagnosed APL. With these findings, we are moving toward an era of completely oral and chemotherapy-free management of APL.
Over many centuries and even millennia, numerous accounts have attested that imbibing arsenicals was a powerful means of poisoning as well as a purported remedy for treating many diseases (1–4). Arsenic first appeared in Western Medicine in the eighteenth century. It was first patented in 1771 by Thomas Wilson for the treatment of malaria and agues. Thomas Fowler from Edinburgh subsequently produced a 1% solution of potassium arsenite, known as “Fowler's solution” (1) From the 1830s to the 1930s, oral arsenic was predominantly used for the management of syphilis, parasitic infestations, chronic skin conditions, and asthma (4). In Hematology, oral arsenic was first reported in the treatment of chronic myeloid leukemia from the 1860s to 1920s in Germany and Boston (1, 4). This practice was phased out following World War II with the development of alkylating chemotherapy and radiotherapy. Oral Fowler's solution, known as “liquor arsenicalis” was produced in Queen Mary Hospital, Hong Kong until the mid-1950s when its use as an anti-leukemic agent was replaced by chemotherapy and radiotherapy (1).
Pure intravenous pure As2O3 solution was first used in Harbin, China in 1973. Data on the mechanism, pharmacokinetics, and clinical efficacy were extensively published in 1996. Similar treatment results were confirmed around the world (5–7). Moreover, as the Chinese had described using intravenous (i.v.) treatment, the Food and Drug Administration (FDA) in the US agreed to license an American company to produce an i.v. formulation of As2O3 (Trisenox®) for treating APL. Treatment with Trisenox® was inconvenient, cumbersome and prohibitively expensive. Depending on the source, current monthly costs of i.v.-As2O3-based regimens typically used during induction or re-induction of APL may amount to ~10,000–11,000 U.S. dollars, though more affordable generic formulations are increasingly available (8, 9). Moreover, besides the burdensome quality of life impairments and medication costs of such recurrent i.v. treatment, patients inevitably incurred additional expenses. The latter would be to cover the costs of hospital admissions or day-care attendances, medical and nursing staff, i.v. infusion equipment and fluids, as well as to deal with infusion site-related complications. In addition, the patients would incur necessary travel expenses and loss of earnings due to absence from work.
With memories of Fowler's solution, we revived oral-As2O3 or the “modern” liquor arsenicalis in 1998 as a means of treating APL patients. This stemmed from two sets of key historical observations. Both of them could be regarded as bedside experiences and inferences that led to laboratory testing and bench-side work, the fruits of which were eventually passed on to patients at the bedside:
1. Meticulously chronicled medical records of Hong Kong CML patients cared for in the 1950s, consistently detailed objective benefits after treatment with Fowler's solution. Accordingly, researchers set out to reinvestigate a possible role for oral-As2O3 as part of the modern management of APL patients. Treating patients with an oral As2O3 formulation manufactured in accordance with Good Manufacturing Practice (GMP) could therefore have the potential to confer important benefits with a degree of confidence and safety that was never attained by Fowler's solution.
2. Meanwhile, important bedside observations and correlations arose from advances in molecular genetics, in-vitro studies describing As2O3 induced apoptosis and differentiation of APL cells, and an understanding of chromosomal mutations that accompany aging and disease (10, 11). Notably, APL is almost always associated with the specific chromosomal translocations, t(15;17)(q24;q21), and there was mounting evidence that their presence identified patients who respond much more favorably to treatments based on arsenic and all-trans-retinoic acid (ATRA) than to conventional treatment (12, 13).
In contrast to using the i.v. route, treatment with a safe and reliable oral-As2O3 formulation whose production conformed to GMP standards, obviously had the potential to vastly improve quality of life and treatment affordability for APL patients. At the same time, it could also harness the newly realized benefits of avoiding conventional chemotherapy. Another important advantageous distinguishing feature of oral as opposed to i.v. dosing was that it appeared to be less cardiotoxic. Potentially and actually fatal cardiac arrhythmias associated with excessive electrocardiographic QTc interval prolongation were a recognized feature of parenteral treatment (14–16).
The primary objective of this initiative was to determine whether the systemic bioavailability of an in-house locally developed oral As2O3 formulation would be comparable to that following commercially available i.v. dosing, when administered to patients with relapsed/refractory APL or acute myeloid leukemia. A secondary objective was to ascertain the extent of arsenic accumulation in the non-cellular and cellular components of blood. However, redeveloping such an oral formulation and determining its systemic bioavailability whilst ensuring acceptability for clinical use in very sick patients posed a number of significant challenges. These challenges and how they were addressed are listed:
1. In the absence of any readily available commercially produced pharmaceutical grade As2O3 powder, a good quality substitute was eventually sourced from Sigma (U.S.A.).
2. Being a sparingly soluble powder, a suspension was prepared in sterile water and subjected to manipulation of its pH to produce a clear colorless solution with a pH 7.2 that contained 1 mg/ml of As2O3. Contrary to recommendations in available pharmacopeias, fungicide was not added as the entire preparation process was conducted in a pharmaceutical isolator. Samples subsequently submitted to microbiology and chemical testing yielded no fungi and the solute concentration remained unchanged; its shelf-life exceeded 6 months and very likely extended to more than 1 year.
3. As a means of investigating As2O3 bioavailability in fairly sick hospitalized patients, the study protocol was necessarily unconventional and did not entail tolerability testing, randomization, a crossover design, or any form of blinding. In all, 9 patients (aged 17–67 years; mean weight of 58 Kg; 6 men and 3 women) were recruited using predefined inclusion/exclusion criteria and asked to refrain from seafood (an arsenic source) in the preceding week. None of them had antecedent renal or liver function test abnormalities. Each patient received a 10 mg i.v. infusion of As2O3 over 1 h on day 1, followed by a 10 mg oral dose 24 h later. Venous blood samples were drawn from each patient, just before initiating i.v. dosing and at predefined times over the next 48 h. To maximize retrieval of potentially useful data from each sample, aliquots of whole blood and freshly separated plasma were stored and subsequently analyzed in batches using well-established methods.
4. Institutional Ethics Committee approval of the proposed unconventional bioavailability study protocol was achieved after drawing attention to several compelling issues. First, all the patients would have relapsed or refractory disease that was an indication for i.v. As2O3 treatment and second, they would have to give written informed consent. The committee was also informed that recruiting healthy volunteers to take oral and i.v. arsenic to conduct a formal bioavailability study would prove daunting and nor could it reveal how the oral-As2O3 would be tolerated by diseased patients.
5. Not surprisingly, publication of the study findings based on such an unconventional protocol was treated with skepticism by many journals. One journal editor however, appreciated the special circumstances constraining administration of arsenic to very sick patients and saw fit to allow publication of the study findings, despite advice to the contrary from some reviewers.
For each patient, systemic bioavailability following i.v. and oral treatment involved comparison of corresponding area under the curve (AUC) for arsenic concentration vs. time plots attributable to each form of dosing (17). The relevant AUCs were derived using standard computer software incorporating the trapezoidal rule.
Figure 1 shows plasma arsenic concentrations (in excess of basal levels) prevailing between 0 and 48 h in one representative patient (17). It illustrates how i.v. and oral bioavailability (AUC over the first 24 h following each dose) was inferred, assuming first order terminal elimination of arsenic. The net attributable 0–24 h AUC after oral dosing was taken to be the difference between the gross 24–48 h AUC and the extrapolated 24–48 h AUC attributed to i.v. dosing. In the same way, plasma and whole blood arsenic concentration AUCs were computed for all 9 patients, and the ensuing mean ± standard error of mean (SEM) results and 95% confidence intervals (C.I.) were calculated.
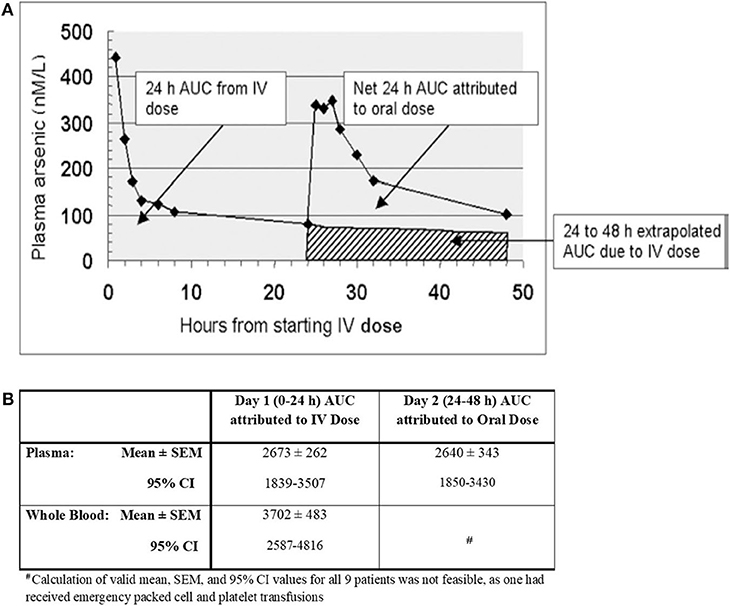
Figure 1. (A) Area under the curve (AUC) of arsenic levels attributed to intravenous (i.v.) and oral dosing with arsenic trioxide in a single patient; (B) Area Under the Curve (AUC) of Arsenic Concentrations (nanomolar-hours) [adapted from Kumana et al. (17) with permission].
These pharmacokinetic studies indicated that, first, the oral formulation and i.v. dosing achieved comparable systemic bioavailability, and secondly, arsenic concentrations in the cellular component of blood were considerably higher than in plasma. Since then, the main finding of this study, namely that orally administered (though differently formulated) As2O3 attains virtually the same systemic bioavailability as i.v. dosing was confirmed by others (18, 19). The latter researchers also pointed out that compared to i.v. dosing, oral dosing was well-tolerated, more convenient, and equally safe. The observation that arsenic attains higher concentrations in the cellular components of blood than in plasma has also been reported (20). Furthermore, due to arsenic existing as III and V forms, its speciation, pharmacokinetics, and metabolic profiling is complex and confusing (10, 19–22). Yet, based on the latter available pharmacokinetic findings, there are reasonable grounds for accepting that the terminal phase of arsenic elimination from plasma approximates to a first order process, and supports extrapolation of 24–48 h AUCs attributable to i.v. dosing. As indicated by the CIs detailed in Figure 1B, there was substantial inter-patient variation in both plasma and whole blood AUCs. Though not tabulated for individual patients, up to ~5 and 10-fold variations, respectively, were encountered between individuals, whilst inter-day variation was much less marked. Interestingly, since considerable inter-patient plasma level/AUC variations have also been encountered after i.v. dosing (21, 22), differences in arsenic absorption capacity from the gut is unlikely to be the main explanation. Thus, conceivable reasons for such variations include saturable tissue binding of As2O3, dietary indiscretions by individual patients, and inherent disease state/physiological differences. It is nevertheless evident that repeated courses of oral As2O3 with these types of therapeutic doses are safe (18, 19, 23). The optimal dose of oral-As2O3 we suggest is 10 mg (0.15-0.2 mg/kg) per day in adult patients (≥50 kg) with normal renal function. In adults weighing less than 50 kg and in pediatric patients, oral-As2O3 solutions at 0.15 mg/kg are advised. With plasma and intracellular arsenic level monitoring, we were also able to administer oral-As2O3 safely at a lower doses for patients with end-stage renal failure or patients dialysis (24, 25).
Interestingly, standard i.v.-As2O3 dosing regimens have been repeatedly incriminated as a cause of cardiac arrhythmias and sudden death, possibly associated with electrocardiographic QTc interval prolongation induced by arsenic (14–16). The latter phenomena have been linked to excessive levels of plasma arsenic. Oral dosing seems to mitigate this cardiac risk, possibly because arsenic's entry into the circulation from the gut is much more gradual and the peak concentrations attained are consequently much lower (26). Based on having demonstrated this particular likely pharmacokinetic advantage of the aforementioned oral-As2O3 formulation, in 2009 its inventors were granted a U.S. patent for treating APL patients. Thereafter, patents for this formulation were also granted by the European Union, China, and Japan. Nevertheless, it should be noted that there has been a single case report of such cardiac arrhythmias occurring transiently after oral therapy of a patient with known chemotherapy-induced dilated cardiomylopathy (27).
A plethora of well-conducted phase 2 studies followed. Our group demonstrated excellent long-term outcomes in APL patients treated with oral-As2O3-based regimens (28–31). In a 15-year prospective follow-up study in 73 patients with relapsed APL, idarubicin (6 mg/m2/day for 5 days) plus oral-As2O3 (10 mg/day), all-trans-retinoic acid (45 mg/m2/day) and ascorbic acid (1 g/day) (AAA) for 42 days resulted in a 100% molecular remission rate (28). Ascorbic acid was used in the AAA regimen due to its synergism with As2O3 which has been shown in-vitro and clinically (30, 32, 33). Following second complete remission (CR2), 2 monthly cycles of idarubicin (6 mg/m2/day for 3 days) plus AAA for 7 days followed by 12 cycles of AAA maintenance (given for 2 weeks every 2 months for 2 years) resulted in 5-year and 10-year overall survival (OS) of 79.5 and 67.3%, respectively (28). Importantly, this was achieved without hematopoietic stem cell transplantation (HSCT) in CR2. This shows that prolonged AAA maintenance is an effective post-remission strategy following CR2, obviating the need for HSCT, a procedure still considered a standard for managing such patients in many places around the world. We then moved AAA forward as post-remission maintenance following CR1, which resulted in a 5-year leukemia-free survival (LFS) and OS of 90% and 97%, respectively (29–31). Most recently, we incorporated AAA (given for 42 days) into frontline induction for newly diagnosed APL with daunorubicin (50 mg/m2/day for 3 days) followed by 2 cycles of consolidation with daunorubicin (50 mg/m2/day for 2 days) and cytarabine (100 mg/m2/day for 5 days) and 2 years of AAA maintenance. Both LFS and OS were 100% at 5 years (29). In patients aged 70 or above or those with medical co-morbidities, chemotherapy was omitted and patients were treated with an entirely oral regimen comprising 42 days of AAA and no relapses have been observed so far (29). With AAA-based regimens, outcome for both newly diagnosed and relapsed APL were independent of the conventional risk scores. With LFS plateauing 2 years after completion of maintenance both in CR1 or CR2, long-term molecular monitoring is not necessary 2 years beyond completion of AAA maintenance following CR1 or CR2 (28, 29). Even in conventional high-risk patients, our strategy of incorporating oral-As2O3 to frontline induction achieved excellent long-term outcomes similar to those achieved in low-risk patients. It remains to be seen whether maintenance is necessary in low-risk patients treated with frontline oral-As2O3-based induction. We are currently testing frontline induction with AAA in APL (ClinicalTrials.gov Identifier: NCT03624270) in a risk-adapted manner incorporating a chemotherapy-free approach.
In our oral-As2O3 studies, both short-term and long-term cardiac safety was confirmed. QTc prolongation, ventricular arrhythmias and cardiac failure were not observed. QTc prolongation occurred in 16% of patients given i.v. As2O3 which was significantly higher than that observed with our regimen (12, 34, 35). We have demonstrated lower peak plasma arsenic levels with oral-As2O3 dosing that probably accounted for the cardiac safety. Drug-induced transaminitis (Grade 1–2: 31%; Grade 3–4: 26%) were all reversible with transient dose reductions or interupptions (29). Upon normalization of liver enzymes, all patients were able to tolerate oral-As2O3 at 10 mg/day without recurrence of transaminitis. Our regimen of AAA showed similar or lower rates of hepatoxicity than with i.v. As2O3-ATRA treatment, the latter being associated with transaminitis that occurred in 44–71% of patients (12, 34, 35). Acyclovir prophylaxis is used universally in all patients on oral-As2O3 due to the risk of herpes zoster that was demonstrated in our earlier studies (36). Differentiation syndrome (DS) occurred in 26 and 12% in patients with newly diagnosed APL and APL in first relapse (R1), respectively, whilst no induction deaths were observed. With early recognition, cytoreduction and the use of dexemathasone, interruption of oral-As2O3 or ATRA was not necessary in our studies. Other common side-effects of the AAA regimen included headache (Grade 1–2: 32%) and upper gastrointestinal upset (Grade 1–2: 11%) most of which were either self-limiting or controlled with simple analgesics and antacids.
In our studies involving relapsed APL patients receiving oral-As2O3, as with i.v.-As2O3 treatment – there was a high risk of central nervous system disease in those with severe relapses (28, 37). We also showed that after an oral administration, meaningful cerebrospinal fluid (CSF) levels of arsenic were achieved implying its benefit in the prophylaxis or treatment of central nervous system (CNS) disease (38, 39). CSF and plasma arsenic levels were linearly correlated with CSF arsenic levels at about 18% of the levels in plasma levels (38). Frontline use in newly diagnosed APL may ameliorate the risk. Since 2013, none of our patients treated with frontline oral-As2O3 had evidence of CNS relapse (29). There is limited data on CSF arsenic levels in patients treated with i.v. As2O3. The concurrent use of intravenous mannitol may significantly increase CSF arsenic levels comparable to those in blood, thus providing the prospect of managing CNS APL effectively (40).
Realgar-Indigo naturalis formula (RIF) was developed in the 1980s entirely based on traditional Chinese Medicine (TCM) concepts and comprises realgar, Indigo naturalis, Salvia miltiiorrhiza and Radix psudostellariae (40) and was launched in Mainland China in 2009. Tetraarsenic tetrasulphide (As4S4), indirubin and tanshinone IIA are the active ingredients of RIF, which show in-vitro synergism (40, 41). RIF showed comparable efficacy to i.v. As2O3 (42, 43), together with improved quality of life and reduced costs. Hepatotoxicity is frequently reported and occurs in about 58–65% (Grade 1–2: 49–55%; Grade 3–4: 9–10%) of patients treated with RIF plus ATRA (42, 43). Another common toxicity of RIF is diarrhoea which occurs in about 15% of patients. Prolonged QTc intervals are uncommon with RIF at dosages of 60 mg/kg/day but occurs in about 24% of patients given a high dose of 7.5 g/day (44). Clinically significant arrhythmias are nevertheless rare. Groups in Australia (ANZCTR registration number: ACTRN12616001022459 and the United States (ClinicalTrials.gov Identifier: NCT03048344) have developed novel formulations of oral As2O3. For instance, ORH-2014 was recently shown to be safe in patients with leukaemia and had comparable bioavailability to i.v. As2O3 (19).
The history of APL and development of arsenic trioxide has formed a paradigm for targeted therapy in cancer (Figure 2). There is every reason to believe that the form of oral treatment described above can offer therapeutic benefits equivalent to dosing with i.v. As2O3, with the added advantages of far superior convenience (enabling injection free home treatment with outpatient supervision), greater affordability, and a lower risk of cardiac incidents. Moreover, though reverting to oral treatment can be considered as only a small incremental step in the overall context of treating haematogical malignancies such as APL, for individual patients it can nevertheless be regarded as an important and life-changing advance. A commercially available oral-As2O3 in the not-too-distant future will pave the way for this inexpensive and convenient form of As2O3 to be available worldwide.

Figure 2. Timeline of advances in acute promyelocytic leukaemia (APL) treatment and the development of oral arsenic trioxide (ATO) in Hong Kong. ATRA, all-trans-retinoic acid; chemo., chemotherapy; HK, Hong Kong; i.v., intravenous; CR1, first completeremission; CR2, second complete remission; HSCT, hematopoietic stem cell transplantation.
CK, Y-LK, and HG: conception, manuscript writing, and final approval of manuscript. RM: manuscript writing and final approval of manuscript. All authors: contributed to the article and approved the submitted version.
The University of Hong Kong currently holds two United States (US) patents (7,521,071 B2 and 8,906,422 B2), one Japanese patent (4786341) and one European patent (EP 1562616 B1) for the use of oral-As2O3 in the treatment of leukemias and lymphomas. HG, CK, and Y-LK are employed by or associated with the University of Hong Kong.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Kwong YL, Todd D. Delicious poison: arsenic trioxide for the treatment of leukemia. Blood. (1997) 89:3487–8. doi: 10.1182/blood.V89.9.3487
2. Hoonjan M, Jadhav V, Bhatt P. Arsenic trioxide: insights into its evolution to an anticancer agent. J Biol Inorg Chem. (2018) 23:313–29. doi: 10.1007/s00775-018-1537-9
3. Karamanou M. Arsenic powder in the treatment of cancer: the invention of French physician Pierre Alliot (1610–1685). J BUON. (2019) 24:2583–6.
4. Au WY. A biography of arsenic and medicine in Hong Kong and China. Hong Kong Med J. (2011) 17:507–13.
5. Soignet SL, Maslak P, Wang ZG, Jhanwar S, Calleja E, Dardashti LJ, et al. Complete remission after treatment of acute promyelocytic leukemia with arsenic trioxide. N Engl J Med. (1998) 339:1341–8. doi: 10.1056/NEJM199811053391901
6. Wang ZY. Ham-Wasserman lecture: treatment of acute leukemia by inducing differentiation and apoptosis. Hematol Am Soc Hematol Educ Program. (2003) 1–13. doi: 10.1182/asheducation-2003.1.1
7. Douer D, Tallman MS. Arsenic trioxide: new clinical experience with an old medication in hematologic malignancies. J Clin Oncol. (2005) 23:2396–410. doi: 10.1200/JCO.2005.10.217
8. Bankar A, Korula A, Kulkarni UP, Devasia AJ, Na F, Lionel S, et al. Resource utilization and cost effectiveness of treating acute promyelocytic leukaemia using generic arsenic trioxide. Br J Haematol. (2020) 189:269–78. doi: 10.1111/bjh.16343
9. Chen X, Hong Y, Zheng P, You X, Feng J, Huang Z, et al. The economic research of arsenic trioxide for the treatment of newly diagnosed acute promyelocytic leukemia in China. Cancer. (2020) 126:311–21. doi: 10.1002/cncr.32519
10. Lengfelder E, Hofmann WK, Nowak D. Impact of arsenic trioxide in the treatment of acute promyelocytic leukemia. Leukemia. (2012) 26:433–42. doi: 10.1038/leu.2011.245
11. Papaemmanuil E, Gerstung M, Bullinger L, Gaidzik VI, Paschka P, Roberts ND, et al. Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med. (2016) 374:2209–21. doi: 10.1056/NEJMoa1516192
12. Burnett AK, Russell NH, Hills RK, Bowen D, Kell J, Knapper S, et al. Arsenic trioxide and all-trans retinoic acid treatment for acute promyelocytic leukaemia in all risk groups (AML17): results of a randomised, controlled, phase 3 trial. Lancet Oncol. (2015) 16:1295–305. doi: 10.1016/S1470-2045(15)00193-X
13. Ma Y, Liu L, Jin J, Lou Y. All-trans retinoic acid plus arsenic trioxide versus all-trans retinoic acid plus chemotherapy for newly diagnosed acute promyelocytic leukemia: a meta-analysis. PLoS ONE. (2016) 11:e0158760. doi: 10.1371/journal.pone.0158760
14. Ohnishi K, Yoshida H, Shigeno K, Nakamura S, Fujisawa S, Naito K, et al. Prolongation of the QT interval and ventricular tachycardia in patients treated with arsenic trioxide for acute promyelocytic leukemia. Ann Intern Med. (2000) 133:881–5. doi: 10.7326/0003-4819-133-11-200012050-00012
15. Unnikrishnan D, Dutcher JP, Varshneya N, Lucariello R, Api M, Garl S, et al. Torsades de pointes in 3 patients with leukemia treated with arsenic trioxide. Blood. (2001) 97:1514–6. doi: 10.1182/blood.V97.5.1514
16. Westervelt P, Brown RA, Adkins DR, Khoury H, Curtin P, Hurd D, et al. Sudden death among patients with acute promyelocytic leukemia treated with arsenic trioxide. Blood. (2001) 98:266–71. doi: 10.1182/blood.V98.2.266
17. Kumana CR, Au WY, Lee NS, Kou M, Mak RW, Lam CW, et al. Systemic availability of arsenic from oral arsenic-trioxide used to treat patients with hematological malignancies. Eur J Clin Pharmacol. (2002) 58:521–6. doi: 10.1007/s00228-002-0514-x
18. Firkin F. Oral administration of arsenic trioxide in the treatment of acute promyelocytic leukaemia and accelerated phase chronic myeloid leukaemia: an Australian single-centre study. Intern Med J. (2012) 42:948–52. doi: 10.1111/j.1445-5994.2012.02852.x
19. Ravandi F, Koumenis I, Johri A, Tallman M, Roboz GJ, Strickland S, et al. Oral arsenic trioxide ORH-2014 pharmacokinetic and safety profile in patients with advanced hematologic disorders. Haematologica. (2019) 105:1567–47. doi: 10.3324/haematol.2019.229583
20. Iriyama N, Yoshino Y, Yuan B, Horikoshi A, Hirabayashi Y, Hatta Y, et al. Speciation of arsenic trioxide metabolites in peripheral blood and bone marrow from an acute promyelocytic leukemia patient. J Hematol Oncol. (2012) 5:1. doi: 10.1186/1756-8722-5-1
21. Shen Y, Shen ZX, Yan H, Chen J, Zeng XY, Li JM, et al. Studies on the clinical efficacy and pharmacokinetics of low-dose arsenic trioxide in the treatment of relapsed acute promyelocytic leukemia: a comparison with conventional dosage. Leukemia. (2001) 15:735–41. doi: 10.1038/sj.leu.2402106
22. Fujisawa S, Ohno R, Shigeno K, Sahara N, Nakamura S, Naito K, et al. Pharmacokinetics of arsenic species in Japanese patients with relapsed or refractory acute promyelocytic leukemia treated with arsenic trioxide. Cancer Chemother Pharmacol. (2007) 59:485–93. doi: 10.1007/s00280-006-0288-4
23. Au WY, Kwong YL. Arsenic trioxide: safety issues and their management. Acta Pharmacol Sin. (2008) 29:296–304. doi: 10.1111/j.1745-7254.2008.00771.x
24. Au WY, Cheung GT, Yuen TW, Kumana CR, Kwong YL. Successful treatment of relapsed acute promyelocytic leukemia in a patient receiving continuous ambulatory peritoneal dialysis with oral arsenic trioxide. Arch Intern Med. (2005) 165:1067–8. doi: 10.1001/archinte.165.9.1067
25. Au WY, Fong BM, Tam S, Kwong YL. Feasibility of oral arsenic trioxide treatment for acute promyelocytic leukemia during hemodialysis. Ann Hematol. (2013) 92:417–8. doi: 10.1007/s00277-012-1576-1
26. Siu CW, Au WY, Yung C, Kumana CR, Lau CP, Kwong YL, et al. Effects of oral arsenic trioxide therapy on QT intervals in patients with acute promyelocytic leukemia: implications for long-term cardiac safety. Blood. (2006) 108:103–6. doi: 10.1182/blood-2006-01-0054
27. Hai JJ, Gill H, Tse HF, Kumana CR, Kwong YL, Siu CW. Torsade de pointes during oral arsenic trioxide therapy for acute promyelocytic leukemia in a patient with heart failure. Ann Hematol. (2015) 94:501–3. doi: 10.1007/s00277-014-2174-1
28. Gill H, Yim R, Lee HKK, Mak V, Lin SY, Kho B, et al. Long-term outcome of relapsed acute promyelocytic leukemia treated with oral arsenic trioxide-based reinduction and maintenance regimens: a 15-year prospective study. Cancer. (2018) 124:2316–26. doi: 10.1002/cncr.31327
29. Gill H, Kumana CR, Yim R, Hwang YY, Chan TSY, Yip SF, et al. Oral arsenic trioxide incorporation into frontline treatment with all-trans retinoic acid and chemotherapy in newly diagnosed acute promyelocytic leukemia: aS 5-year prospective study. Cancer. (2019) 125:3001–12. doi: 10.1002/cncr.32180
30. Au WY, Kumana CR, Lee HK, Lin SY, Liu H, Yeung DY, et al. Oral arsenic trioxide-based maintenance regimens for first complete remission of acute promyelocytic leukemia: a 10-year follow-up study. Blood. (2011) 118:6535–43. doi: 10.1182/blood-2011-05-354530
31. Gill HS, Yim R, Kumana CR, Tse E, Kwong YL. Oral arsenic trioxide, all-trans retinoic acid, and ascorbic acid maintenance after first complete remission in acute promyelocytic leukemia: long-term results and unique prognostic indicators. Cancer. (2020) 126:3244–54. doi: 10.1002/cncr.32937
32. Dai J, Weinberg RS, Waxman S, Jing Y. Malignant cells can be sensitized to undergo growth inhibition and apoptosis by arsenic trioxide through modulation of the glutathione redox system. Blood. (1999) 93:268–77. doi: 10.1182/blood.V93.1.268.401a21_268_277
33. Grad JM, Bahlis NJ, Reis I, Oshiro MM, Dalton WS, Boise LH. Ascorbic acid enhances arsenic trioxide-induced cytotoxicity in multiple myeloma cells. Blood. (2001) 98:805–13. doi: 10.1182/blood.V98.3.805
34. Abaza Y, Kantarjian H, Garcia-Manero G, Estey E, Borthakur G, Jabbour E, et al. Long-term outcome of acute promyelocytic leukemia treated with all-trans-retinoic acid, arsenic trioxide, and gemtuzumab. Blood. (2017) 129:1275–83. doi: 10.1182/blood-2016-09-736686
35. Lo-Coco F, Avvisati G, Vignetti M, Thiede C, Orlando SM, Iacobelli S, et al. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N Engl J Med. (2013) 369:111–21. doi: 10.1056/NEJMoa1300874
36. Au WY, Kwong YL. Frequent varicella zoster reactivation associated with therapeutic use of arsenic trioxide: portents of an old scourge. J Am Acad Dermatol. (2005) 53:890–2. doi: 10.1016/j.jaad.2005.07.030
37. Gill H, Ip HW, Pang AW, Sum J, Leung AY, Kwong YL. FLT3 internal tandem duplication in acute promyelocytic leukemia: central nervous system relapse. Ann Hematol. (2015) 94:1049–51. doi: 10.1007/s00277-014-2281-z
38. Au WY, Tam S, Fong BM, Kwong YL. Determinants of cerebrospinal fluid arsenic concentration in patients with acute promyelocytic leukemia on oral arsenic trioxide therapy. Blood. (2008) 112:3587–90. doi: 10.1182/blood-2008-06-161000
39. Au WY, Tam S, Kwong YL. Entry of elemental arsenic into the central nervous system in patients with acute promyelocytic leukemia during arsenic trioxide treatment. Leuk Res. (2008) 32:357–8. doi: 10.1016/j.leukres.2007.06.005
40. Wang L, Zhou GB, Liu P, Song JH, Liang Y, Yan XJ, et al. Dissection of mechanisms of Chinese medicinal formula Realgar-Indigo naturalis as an effective treatment for promyelocytic leukemia. Proc Natl Acad Sci USA. (2008) 105:4826–31. doi: 10.1073/pnas.0712365105
41. Wu J, Shao Y, Liu J, Chen G, Ho PC. The medicinal use of realgar (As(4)S(4)) and its recent development as an anticancer agent. J Ethnopharmacol. (2011) 135:595–602. doi: 10.1016/j.jep.2011.03.071
42. Zhu HH, Wu DP, Jin J, Li JY, Ma J, Wang JX, et al. Oral tetra-arsenic tetra-sulfide formula versus intravenous arsenic trioxide as first-line treatment of acute promyelocytic leukemia: a multicenter randomized controlled trial. J Clin Oncol. (2013) 31:4215–21. doi: 10.1200/JCO.2013.48.8312
43. Zhu HH, Wu DP, Du X, Zhang X, Liu L, Ma J, et al. Oral arsenic plus retinoic acid versus intravenous arsenic plus retinoic acid for non-high-risk acute promyelocytic leukaemia: a non-inferiority, randomised phase 3 trial. Lancet Oncol. (2018) 19:871–9. doi: 10.1016/S1470-2045(18)30295-X
Keywords: oral arsenic trioxide, acute promyelocitic leukaemia, history, pharmacokinetics, clinical applications
Citation: Kumana CR, Mak R, Kwong Y-L and Gill H (2020) Resurrection of Oral Arsenic Trioxide for Treating Acute Promyelocytic Leukaemia: A Historical Account From Bedside to Bench to Bedside. Front. Oncol. 10:1294. doi: 10.3389/fonc.2020.01294
Received: 09 May 2020; Accepted: 22 June 2020;
Published: 04 August 2020.
Edited by:
Massimo Breccia, Sapienza University of Rome, ItalyReviewed by:
Hong-Hu Zhu, Zhejiang University, ChinaCopyright © 2020 Kumana, Mak, Kwong and Gill. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Harinder Gill, Z2lsbGhzaEBoa3UuaGs=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.