- Department of Obstetrics and Gynecology, Shengjing Hospital of China Medical University, Shenyang, China
Background: The comparison of survival outcomes between minimally invasive surgery and open surgery for cervical cancer patients remains controversial. We evaluated the survival outcomes of cervical cancer patients who underwent different surgical approaches.
Methods: A literature search was performed in PubMed, Embase, and Cochrane databases up to February 2020, using the MESH terms “minimally invasive surgical procedures” and “Uterine Cervical Neoplasms.” Included were all original comparative studies and trials both published and unpublished in English that were related to minimally invasive surgery and open surgery for cervical cancer patients with International Federation of Gynecology and Obstetrics (FIGO) 2009 stage < IIB. Begg's and Egger's regressions were used to evaluate publication bias.
Results: This meta-analysis included 28 studies enrolling 18,961 patients with cervical cancer. The overall analyses indicated that cervical cancer patients with FIGO 2009 stage < IIB who underwent minimally invasive surgery had a lower rate of OS (HR = 1.43, 95% CI = 1.06–1.92, P = 0.019) and DFS (HR = 1.50, 95% CI = 1.21–1.85, P < 0.001) than those who underwent open surgery. Moreover, minimally invasive surgery could lower OS (HR = 2.30, 95% CI = 1.50–3.52, P < 0.001) and DFS (HR = 1.94, 95% CI = 1.36–2.76, P < 0.001) of cervical cancer patients with FIGO 2009 stage ≤ IB1 compared to open surgery. However, there were no significant differences in OS (HR = 1.07, 95% CI = 0.65–1.76, P = 0.801) and DFS (HR = 1.20, 95% CI = 0.65–2.19, P = 0.559) in patients with tumors < 2 cm between the two groups.
Conclusions: Minimally invasive radical hysterectomy was associated with poor survival outcomes compared to open surgery. Patients with FIGO 2009 stage ≤ IB1 cervical cancer who underwent minimally invasive surgery have lower OS and DFS rates than those who underwent open surgery. Therefore, open surgery should be performed for cervical cancer patients. However, patients with tumors < 2 cm might take the most advantage of minimally invasive surgery without increasing poor prognosis. There are some limitations in the meta-analysis, which needs further high-quality multicenter studies to confirm and update our findings.
Introduction
Cervical cancer is the fourth most common cancer and the fourth leading cause of cancer death in women worldwide (1). In 2020, it was estimated that there will be 13,800 new cases and 4,290 deaths in the United States, and in women aged 20–39 years, cervical cancer is the second leading cause of cancer death (2). Radical hysterectomy with pelvic lymphadenectomy is the standard recommended surgical treatment for early-stage cervical cancer patients. Traditionally, laparotomy has been deemed as the gold standard treatment for early cervical cancer (3). With the development of laparoscopic surgery, minimally invasive radical hysterectomy has ever been the standard surgical approach in patients with early-stage cervical cancer (4). Since 2018, the guidelines from the National Comprehensive Cancer Network (NCCN) advise that patients should be carefully informed about the risks and benefits of the different surgical approaches due to the findings of poorer survival outcomes with laparoscopy compared to laparotomy in the Laparoscopic Approach to Cervical Cancer (LACC) Trial (5). However, the latest guidelines from the NCCN advise that abdominal radical hysterectomy is the standard surgical treatment for early-stage cervical cancer patients (6).
Several meta-analyses have compared minimally invasive surgery (laparoscopic or robot-assisted radical hysterectomy) with open surgery (abdominal radical hysterectomy) in cervical cancer patients, showing that minimally invasive surgery is safe and has fewer perioperative complications and faster recovery than open surgery (7–9). Only a few studies included in previous meta-analyses looked at the rate of overall survival (OS) or disease-free survival (DFS), but neither laparoscopic nor robot-assisted radical hysterectomy has been associated with lower rates of OS or DFS (10–15). Instead, the evidence in support of minimally invasive surgery has been based mainly on observational studies.
A phase 3, multicenter, randomized trial of minimally invasive surgery vs. open surgery in patients with early-stage cervical cancer was published (5). The LACC trial showed that minimally invasive surgery could lower the rate of OS and DFS relative to open surgery in cervical cancer patients with International Federation of Gynecology and Obstetrics (FIGO) 2009 stage IA1 with lymphovascular space invasion (LVSI) to IB1. However, there were some limitations in the trial. The LACC trial didn't reach its preconcerted enrollment. And final results from LACC could not be generalized to patients with “low-risk” cervical cancer such as tumor size < 2 cm.
The oncologic outcomes of minimally invasive surgery compared to open surgery remain controversial. Therefore, we conducted a meta-analysis to observe OS and DFS in cervical cancer patients with FIGO 2009 stage < IIB between open and minimally invasive surgery, which might provide the evidence to choose the better surgical approach.
Methods
Search Strategy
This study was conducted in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, which was listed in Supplementary Table 1. We searched PubMed, Embase, and Cochrane databases for both published and unpublished trials up to February, 2020. The following MeSH and main keywords were used: “minimally invasive surgical procedures,” “minimally invasive surgery,” “procedure, minimal surgical,” “laparoscopy,” “robotic surgical procedures,” “robotic surgery,” and associated terms; and “uterine cervical neoplasms,” “cervical cancer,” “cancer of cervix,” “cervical neoplasm,” and associated terms. The language was restricted to English. For multiple-arm comparative studies, we extracted data only from the arms that matched our eligibility criteria. We also performed manual searches of the reference lists in the selected studies to retrieve all relevant data.
Inclusion and Exclusion Criteria
Studies were selected according to PICOS (population, intervention, comparison, outcomes, and study design) guidelines if they met the following inclusion criteria: (1) population: cervical cancer patients with clinical FIGO 2009 stage < IIB; (2) intervention: radical hysterectomy was the primary treatment; (3) comparison: minimally invasive surgery vs. open surgery (both groups with or without adjuvant therapy); (4) outcomes: survival outcomes (OS and DFS) compared between two groups; (5) study design: studies were comparative (randomized control trials [RCTs] and observational studies).
Exclusion criteria were as follows: (1) population: patients with advanced cervical cancer who could not undergo surgery; (2) intervention: radiation or chemoradiation therapy was used as the primary treatment; (3) comparison: laparoscopic radical hysterectomy vs. robot-assisted radical hysterectomy or minimally invasive surgery vs. patients without open surgery; (4) outcomes: studies with insufficiently detailed data or lacking the outcomes of interest; (5) study design: single-arm study or review.
Data Extraction and Quality Assessment of Included Studies
Two independent authors assessed the identified studies and the abstracts were reviewed to select full papers. All the authors evaluated the included studies for inclusion. The Jadad scale (16) and the Newcastle-Ottawa Scale (NOS) (17) were used to evaluate the quality of RCTs and observation studies, respectively. Discussion was performed among all the authors to resolve any disagreements.
Statistical Analysis
The primary endpoints (time-to-event outcomes) of this meta-analysis were assessed using hazard ratios (HRs). If the HRs were not provided directly, we used Kaplan–Meier curves to get an estimated HR (18). Stata software, version 12.0 (2011; Stata Corp., College Station, TX, USA) was used to perform the meta-analysis. HRs are presented with 95% confidence intervals (CIs), and the two-tailed P-values of <0.05 were considered significant. We used Cochran's Q-test and the I2 statistic to evaluate the heterogeneity among the studies, and a P < 0.1 was considered as statistically significant (19, 20). The robustness of the results was assessed using sensitivity analyses (21). Finally, Begg's and Egger's regressions were used to evaluate publication bias (22, 23).
Results
Study Selection
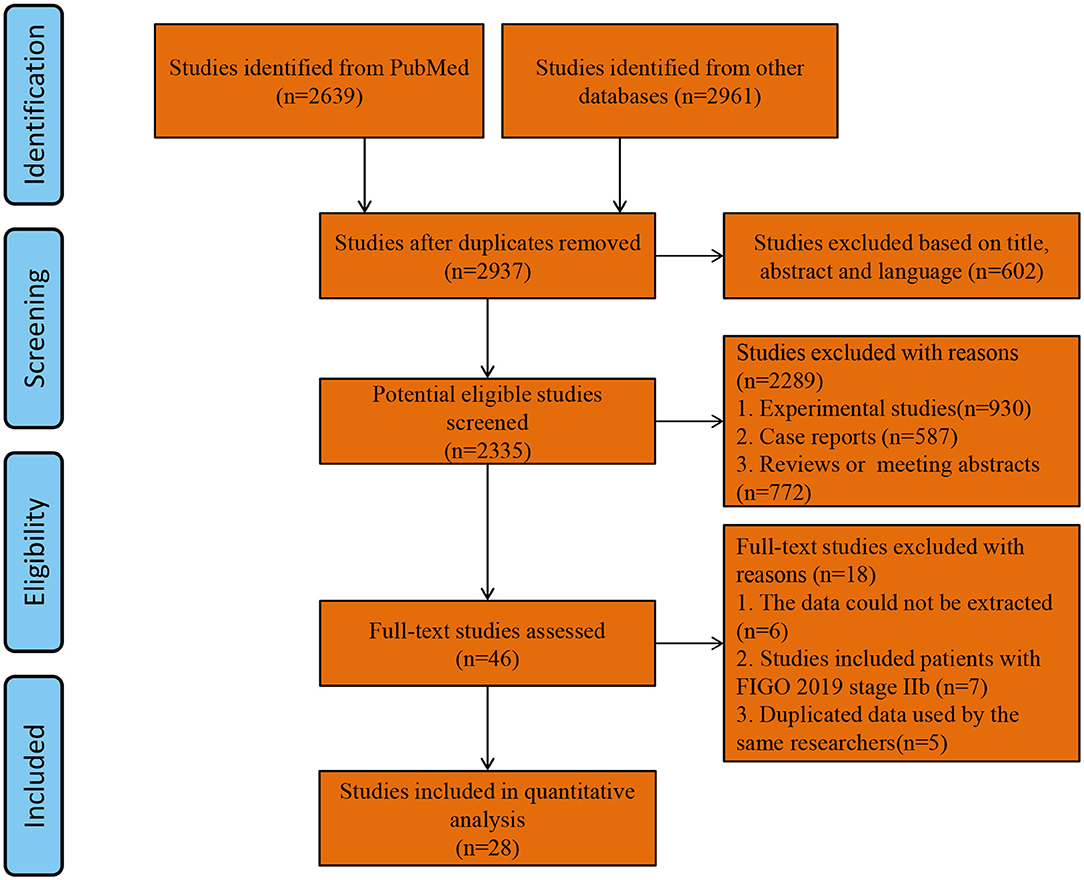
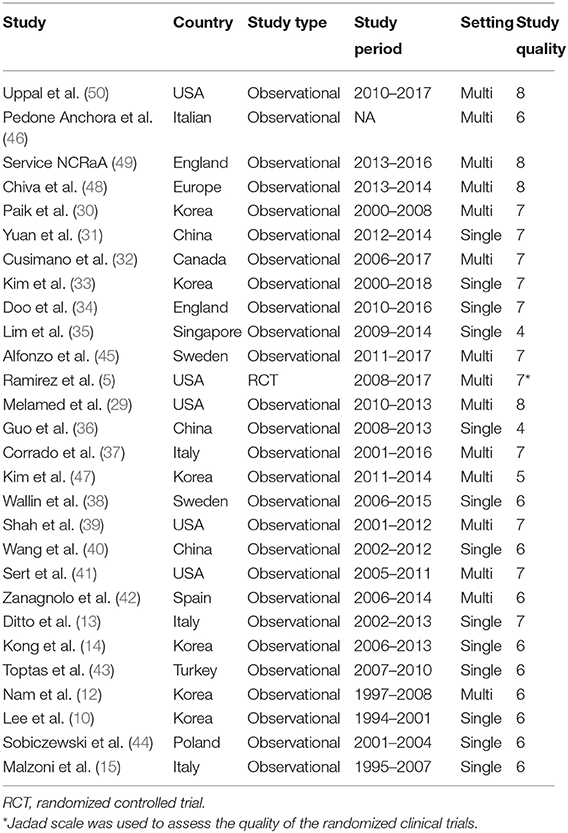
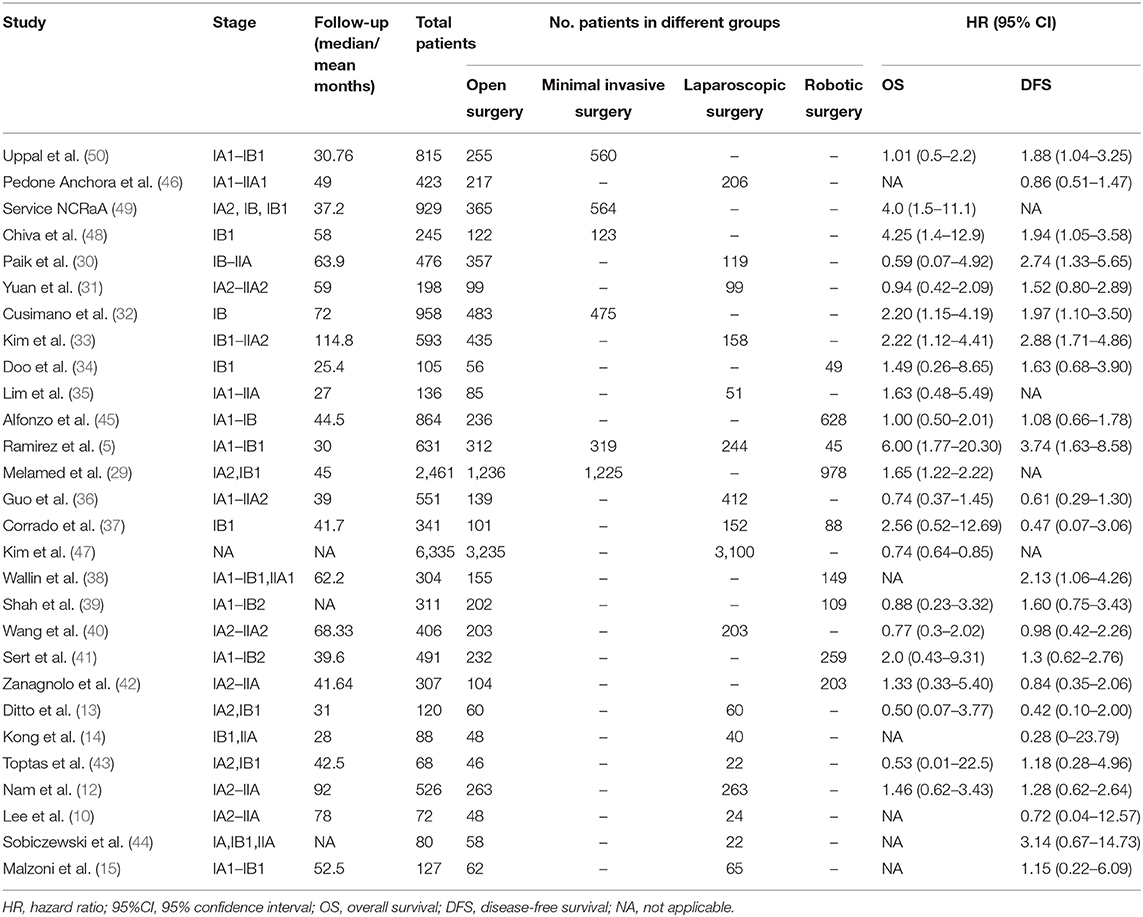
Two thousand and nine hundred and thirty-seven studies were retrieved using our search strategy. After screening of the abstracts or titles, the full texts of 33 studies were further reviewed. Amongst these, five publications were excluded for the duplicated data used by the same researchers (24–28). Finally, 28 comparative studies which met the study inclusion criteria were selected for analysis (minimally invasive surgery group = 9,747, open surgery group = 9,214; total = 18,961 patients) (5, 10, 12–15, 29–50). A flow diagram of the meta-analysis process is illustrated in Figure 1. For one observational study in which the HR and 95% CIs were reported separately for laparoscopic surgery vs. open surgery and robot-assisted surgery vs. open surgery (37), we handled each surgical approach as a separate study in our meta-analysis. Tables 1, 2 show the main characteristics and quality scores of studies.
Minimally Invasive Surgery vs. Open Surgery for Cervical Cancer
The OS data was provided in 23 studies, and the HR was derived based on OS. Based on our pooled analysis, patients who underwent minimally invasive surgery had a lower rate of OS than those who underwent open surgery for cervical cancer (HR = 1.43, 95% CI = 1.06–1.92, P = 0.019; Figure 2A). In addition, 25 studies provided DFS data, and our pooled analysis indicated an inferior DFS in patients who underwent minimally invasive surgery than those who underwent open surgery (HR = 1.50, 95% CI = 1.21–1.85, P < 0.001; Figure 2B).
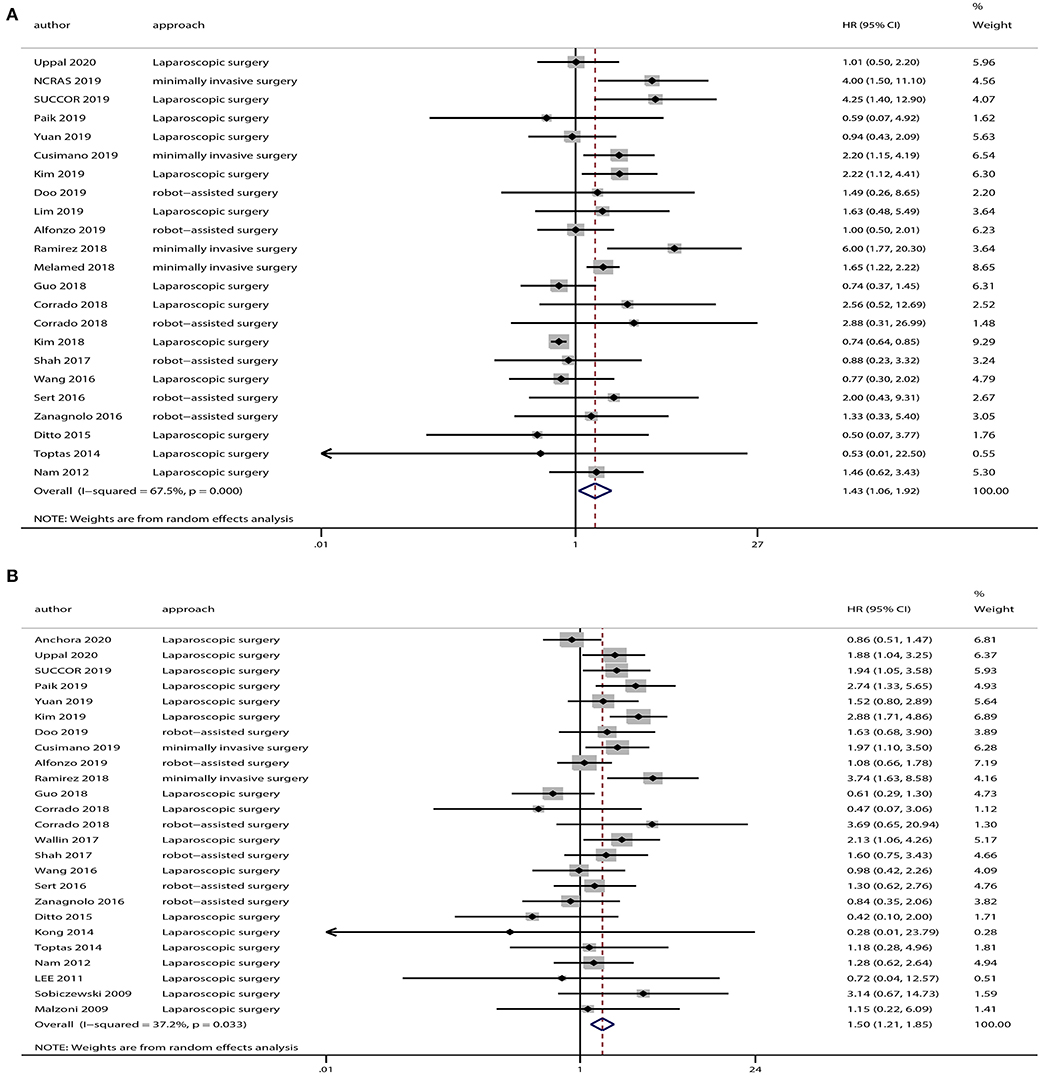
Figure 2. Overall analyses of minimally invasive surgery vs. open surgery for cervical cancer patients. (A) Overall survival; (B) disease-free survival.
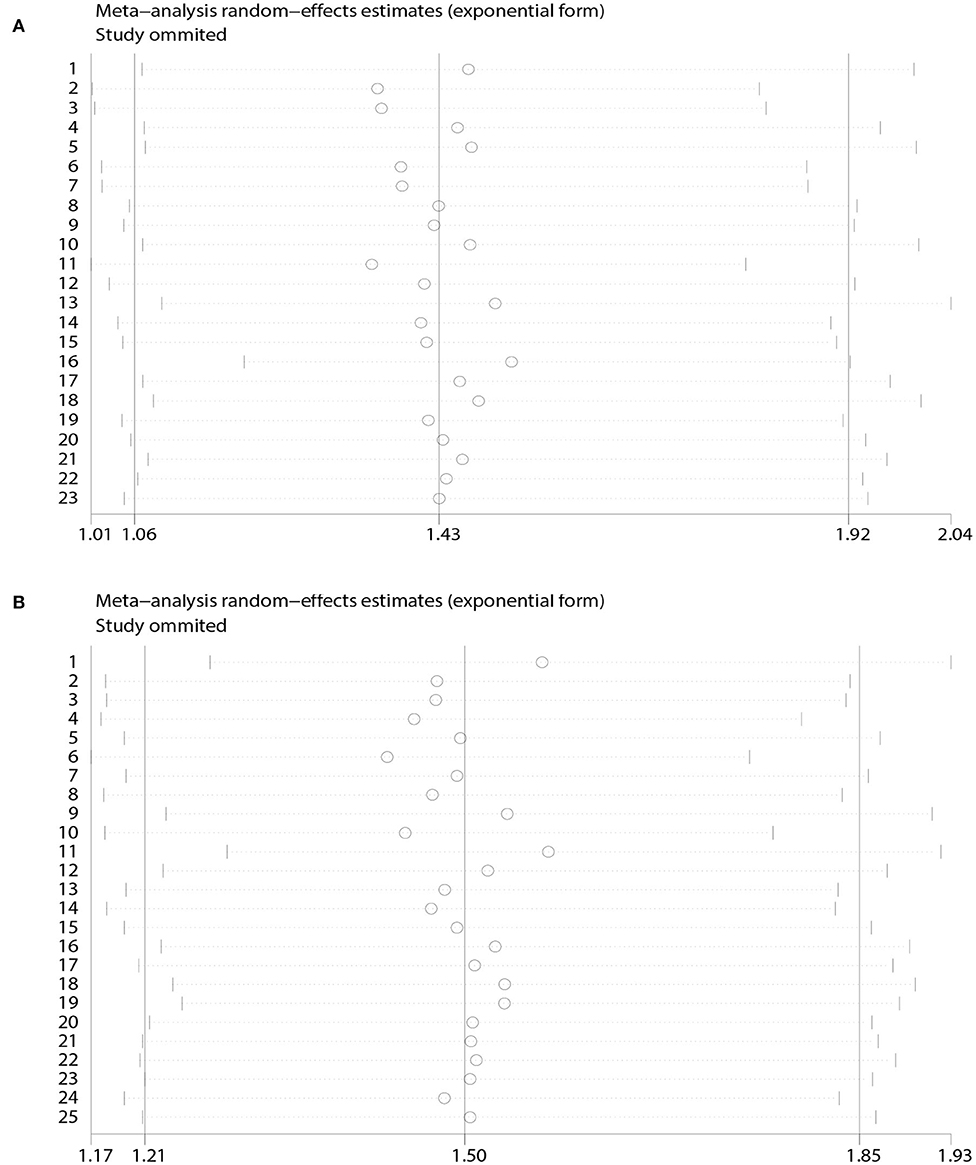
A heterogeneity was seen amongst the studies in terms of OS (χ2 = 67.64, P < 0.01, I2 = 67.5%) and DFS (χ2 = 38.24, P = 0.03, I2 = 37.2%). Hence, we conducted sensitivity analysis which showed that omitting any single study did not alter the corresponding pooled HRs of OS or DFS significantly (Figure 3).
The funnel plot showed potential publication bias in terms of OS [Begg's test: P = 0.67, (Figure 4A); Egger's test: P = 0.01, (Figure 4B)] but not of DFS [Begg's test: P = 0.41, (Figure 4C); Egger's test: P = 0.37, (Figure 4D)].
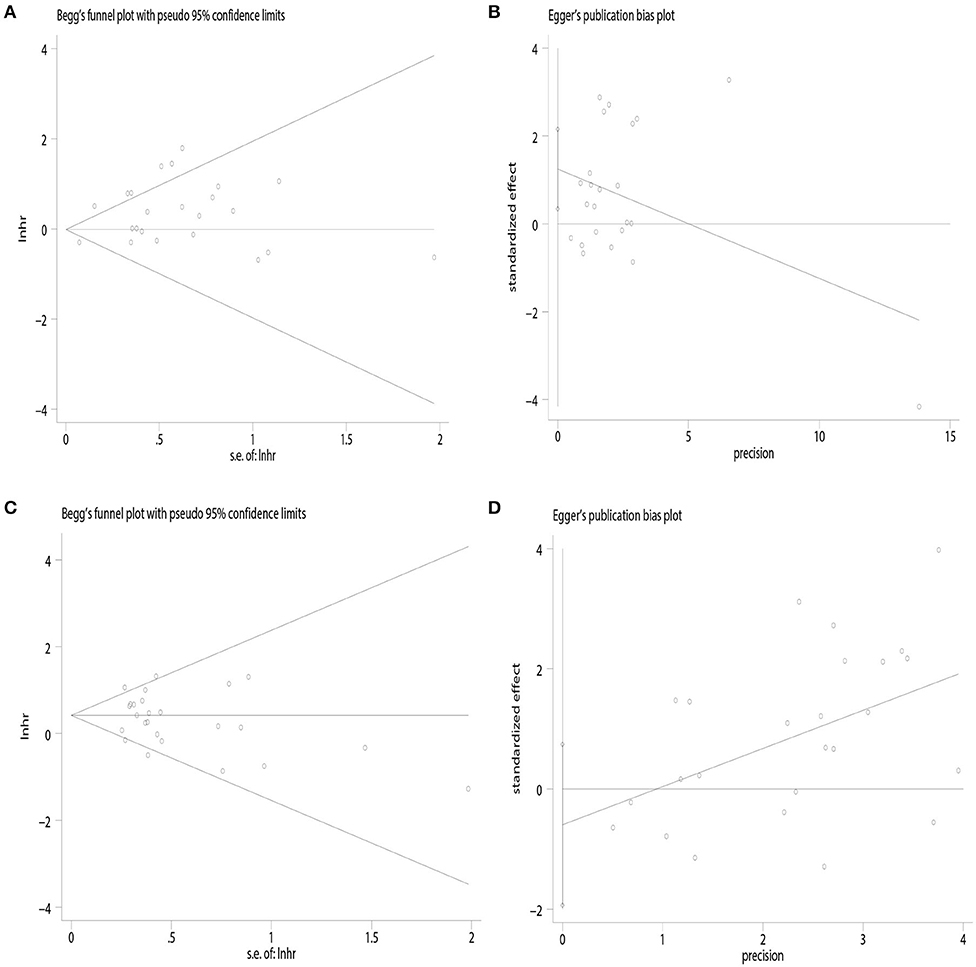
Figure 4. Publication bias. (A) Begg's test of overall survival; (B) Egger's test of overall survival; (C) Begg's test of disease-free survival; (D) Egger's test of disease-free survival.
Survival Outcomes for Patients With Stage ≤ IB1 Cervical Cancer
We extracted OS and DFS data from the studies including patients with stage ≤ IB1 cervical cancer. And there were eight studies provided OS and DFS data of FIGO 2009 stage ≤ IB1. Our results demonstrated that patients in the minimally invasive surgery group had a lower rate of OS (HR = 2.30, 95% CI = 1.50–3.52, P < 0.001) and DFS (HR = 1.94, 95% CI = 1.36–2.76, P < 0.001) compared with those in the open surgery group, as shown in Figure 5.
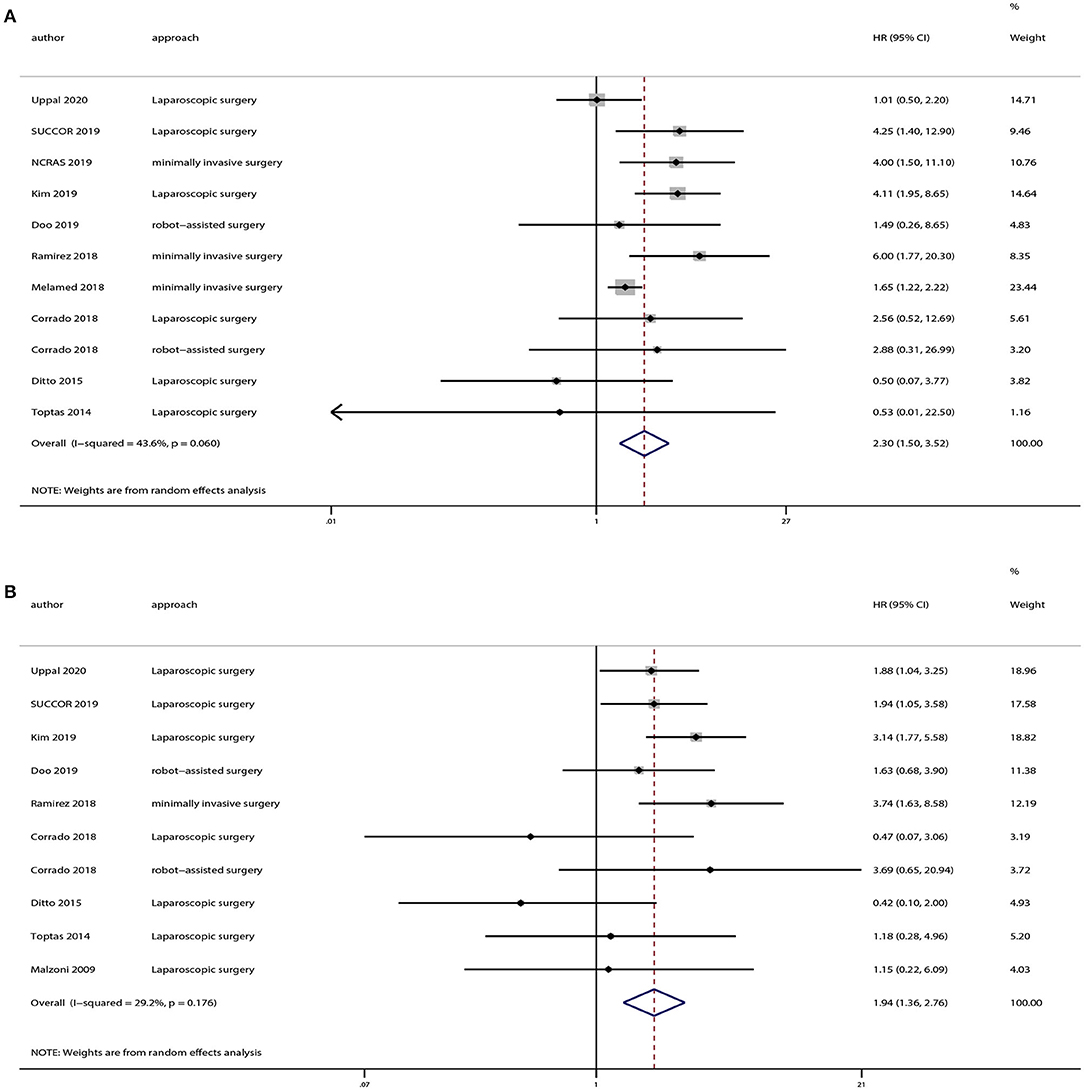
Figure 5. Subgroup analyses of patients with International Federation of Gynecology and Obstetrics (FIGO) 2009 stage ≤ IB1 cervical cancer between minimally invasive surgery group and open surgery group. (A) Overall survival; (B) disease-free survival.
Subgroup Analyses Based on Tumor Dimension
There were 12 strudies provided the data of tumors <2 or >2 cm. And we also extracted OS and DFS data from these studies. Eight studies provided OS and 10 studies provided DFS of tumors <2 cm, and the pooled results indicated no statistically significant difference in OS (HR = 1.07, 95% CI = 0.65–1.76, P = 0.801) and DFS (HR = 1.20, 95% CI = 0.65–2.19, P = 0.559) between the minimally invasive surgery group and open surgery group (Figure 6). With regard to patients with tumors >2 cm, seven studies provided OS and eight studies provided DFS. And the pooled results demonstrated that minimally invasive surgery could lower OS (HR = 1.52, 95% CI = 1.15–2.02, P = 0.003) and DFS (HR = 1.63, 95% CI = 1.12–2.38, P = 0.011) compared to the open surgery group (Figure 7).
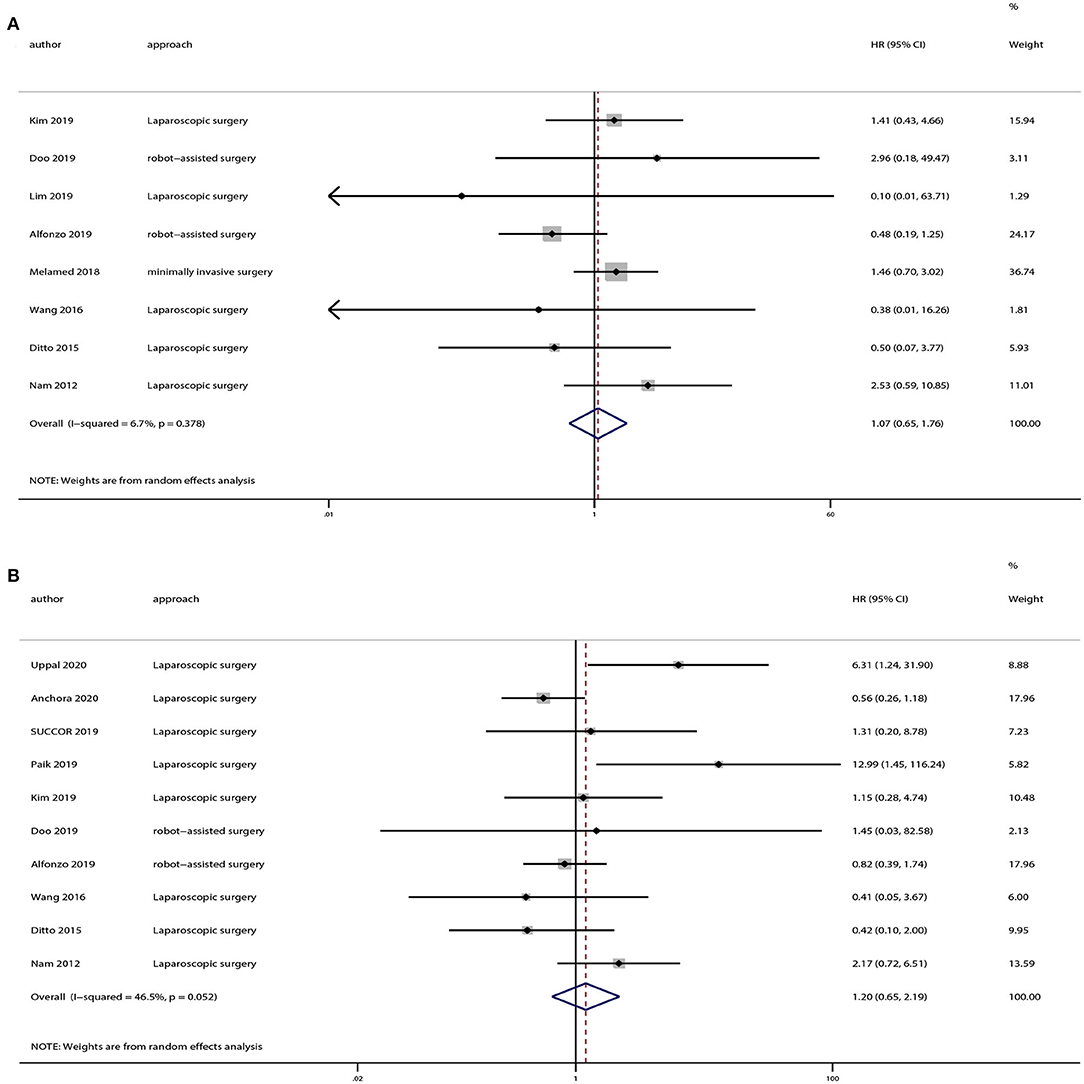
Figure 6. Subgroup analyses of patients with tumor size <2 cm between minimally invasive surgery group and open surgery group. (A) Overall survival; (B) disease-free survival.
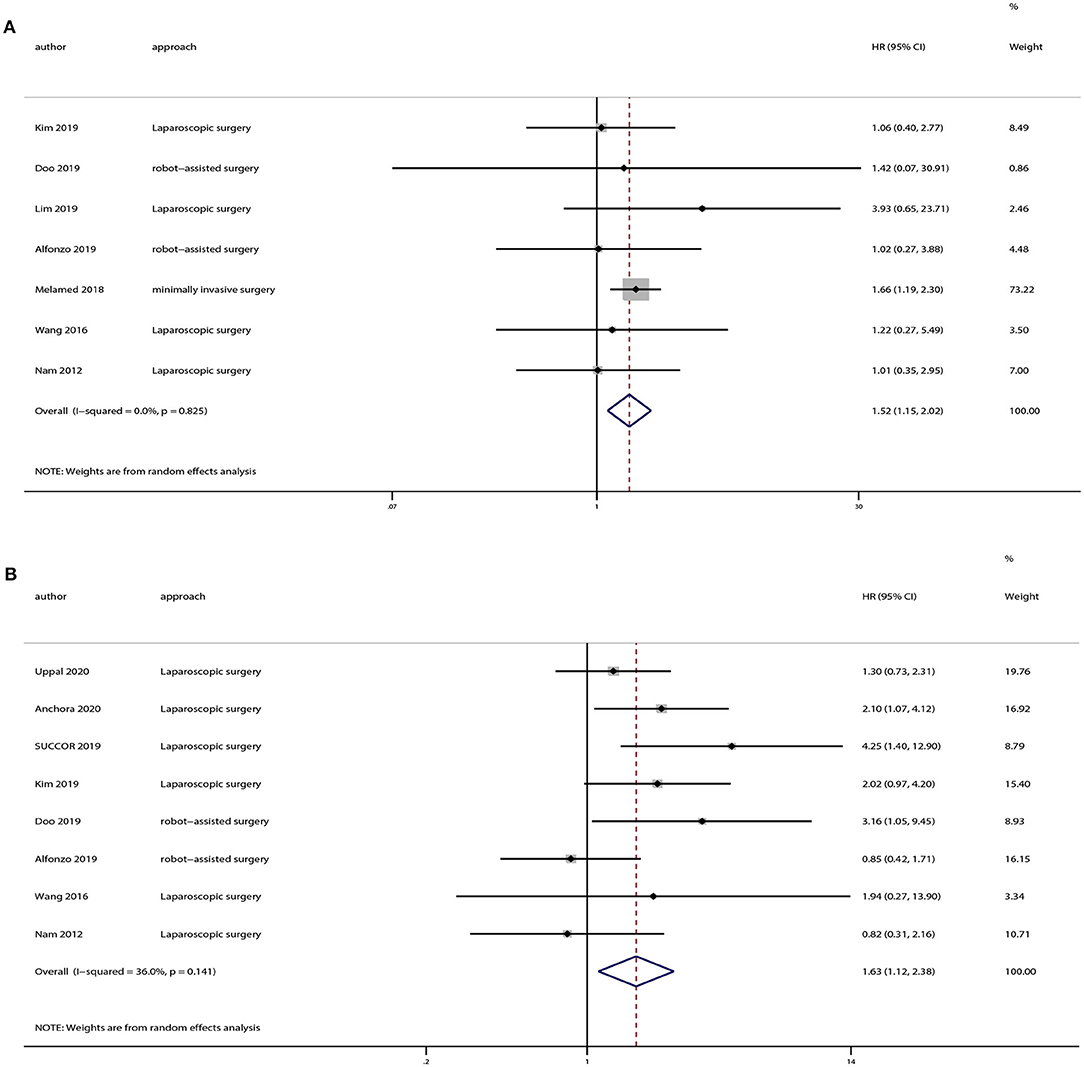
Figure 7. Subgroup analyses of patients with tumor size > 2 cm between minimally invasive surgery group and open surgery group. (A) Overall survival; (B) disease-free survival.
Discussion
Since 1975, survival rates have increased significantly in all of the most common cancers except for cervical and uterine cancer (51). Radical hysterectomy with pelvic lymphadenectomy remains the primary surgical treatment for cervical cancer (52). Since the first case of laparoscopic radical hysterectomy with pelvic lymphadenectomy was reported (53), minimally invasive surgery has developed gradually. Numerous studies (54–57) have stated its advantages of fewer perioperative complications and improved quality of life as compared with open surgery, but they did not report the oncologic outcomes.
Over the past decade, some studies have compared the minimally invasive and open approach, and found no differences in oncologic outcomes. In 2015, Wang et al. (8) and Cao et al. (9) performed separate meta-analyses evaluating the perioperative outcomes, efficiency, and prognostic results of traditional and minimally invasive techniques. However, these studies analyzed fewer than ten studies with survival outcomes, and all were based on retrospective cohorts. With the publication of the first RCT results of oncologic outcomes for different surgical approaches, the previous findings might be questioned. Thus, the time is right to evaluate systematically the survival outcomes associated with the minimally invasive approach.
Our meta-analysis included 28 studies enrolling 18,961 patients with cervical cancer. Based on our overall meta-analysis results, minimally invasive radical hysterectomy lowered the OS or DFS rate as compared with the open approach for patients with cervical cancer. Twenty-five studies reported DFS and 23 studies reported OS, including only one RCT. Ramirez et al. (5) reported that minimally invasive radical hysterectomy could lower the rate of OS and DFS as compared with the open approach. The RCT included women with stage ≤ IB1 cervical cancer and primarily evaluated survival outcomes. Hence, we analyzed the studies enrolling patients with stage ≤ IB1 cervical cancer, and found that the minimally invasive surgery group had a lower rate of OS and DFS in comparison with the open surgery group.
When compared to other prognostic stratification, the use of the tumor dimension appears to be the most reliable (46). And we also conducted subgroup based on tumor dimension. The results indicated an improved prognosis in patients with tumors >2 cm who underwent open surgery compared to those underwent minimally invasive surgery. However, there were no significant differences in OS or DFS in patients with tumors <2 cm between the two groups.
Before the LACC trial, a majority of the previous retrospective studies reached conclusions contrary to the RCT, we should consider the reasons why they may have done so. Open radical hysterectomy and pelvic lymphadenectomy treatment for cervical cancer has had a long history since the 1930s (58), and the minimally invasive approach was only reported much later, in the 1990s (53). During 2006–2010 (59), only 15.0% of all patients with cervical cancer who underwent radical hysterectomy underwent the minimally invasive approach, a proportion that increased to 45% during 2012–2015 (60). Most of the retrospective studies involved in our meta-analysis did not match the two groups in a same time frame, and open surgery was performed much more during an earlier time, when the criteria of adjuvant therapy was not defined clearly or carried out routinely (5). In addition, while small tumors would mostly likely be resected by the minimally invasive approach, more patients with large tumors may undergo open surgery (47). Differences in the tumor characters of the two surgery groups may have led to selection bias, resulting in a seemingly poorer survival outcome in the open surgery group. Meanwhile, we observed in many retrospective studies that patients who underwent minimally invasive surgery had a significantly shorter follow-up time than patients who underwent open surgery (11, 13, 14, 28, 37, 38, 41, 61). All of the above might create bias in calculating oncological outcomes.
On the other hand, when convinced by the result of the LACC trial by Ramirez et al. (5) or the recently high-quality observational studies (29), the latest NCCN guidelines have been updated to state that open abdominal surgery was the standard approach for radical hysterectomy. In terms of the poorer survival outcomes in the minimally invasive surgery group, we can offer some explanation. Some investigators have postulated that dissemination of malignant cells or increased lymph-vascular space invasion might occur with the use of the uterine manipulator (5, 62–64). And ESGO 2019 SUCCOR study showed significative difference in patients using or not a uterine manipulator (48). Meanwhile, experimental animal studies observed that CO2 pneumoperitoneum might promote intraperitoneal tumor dissemination or implantation (65–67). Finally, in the study by Sobiczewski et al. (44), we included, two patients in the laparoscopic surgery group were found to have intraperitoneal spread. However, with regard to the patients with tumor diameter smaller than 2 cm, we can't give a reasonable explanation for the non-significant difference between the two groups. And some authors explained that in case of larger tumors, the use of a uterine manipulator may squeeze them, which may result cancer spread (34, 46).
There are some limitations to our meta-analysis. First, only one RCT was included in the analysis. The majority of the studies involved were single center and retrospective observational studies with high risk for patients' selection bias, heterogeneity in the choice of postoperative therapy, and differences in surgeons' skills. Also, the criteria for candidate selection for radical hysterectomy may differ between centers and surgeons. The heterogeneity between-studies could have great influence in analyzing the median overall survival. Second, the reported tumor characteristics varied between studies, preventing independent comparisons of tumor size, histology, FIGO stage, and adjuvant treatment between the two groups. For example, some studies didn't state whether FIGO stage IA1 without LVSI is included (35, 36, 38, 39, 45). Most studies were not intended to analyze the impact of different type of radical hysterectomy on overall survival. And only a few studies stated that the patients were comparable in terms of histologic subtypes, rate of LVSI, tumor size, and grade and rate of use of adjuvant therapy (5, 13, 29, 34, 40, 45, 46, 50). Therefore, the results could not be combined because of such differences in the included studies. Third, when we analyzed the survival outcomes of patients with stage ≤ IB1 cervical cancer and tumor size by surgical approach, the number of studies included was relatively small. Fourth, although there was no significance in Begg's test based on the overall survival, Egger's test was statistically significant, which indicated a potential publication bias. Finally, data collected in our meta-analysis covered a particularly long timeframe during which minimally invasive surgery techniques have evolved considerably, which might not reflect changing survival outcomes over time.
Conclusion
Minimally invasive radical hysterectomy was associated with inferior survival to open radical hysterectomy in patients with cervical cancer. At the same time, minimally invasive surgery may lower the rate of OS and DFS in comparison with open surgery for cervical cancer patients with FIGO 2009 stage ≤ IB1. However, patients with tumors <2 cm who underwent minimally invasive surgery didn't suffer inferior prognosis compared to those underwent open surgery.
Data Availability Statement
All datasets analyzed for this study are included in the article/Supplementary Material.
Author Contributions
YW and KL designed the research. YW and BL conducted the research and extracted the data. FR, ZS, LO, and KL evaluated the included studies and provided specific support in quantitative data analysis. YW drafted the first version of the manuscript. All authors read and approved the final version of the manuscript.
Funding
This study was funded by National Natural Science Foundation of China (Grant number: 81501235), Liaoning province Department of Education fund (Grant number: JC2019012), and Science and Technology Planned Project of Shenyang (Grant number: 19-112-4-020).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.01236/full#supplementary-material
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, (2020). CA Cancer J Clin. (2020) 70:7–30. doi: 10.3322/caac.21590
3. Abu-Rustum NR, Hoskins WJ. Radical abdominal hysterectomy. Surg Clin North Am. (2001) 81:815–28. doi: 10.1016/S0039-6109(05)70167-5
4. Cibula D, Potter R, Planchamp F, Avall-Lundqvist E, Fischerova D, Haie Meder C, et al. The European Society of Gynaecological Oncology/European Society for Radiotherapy and Oncology/European Society of Pathology guidelines for the management of patients with cervical cancer. Int J Gynecol Cancer. (2018) 28:641–55. doi: 10.1097/IGC.0000000000001216
5. Ramirez PT, Frumovitz M, Pareja R, Lopez A, Vieira M, Ribeiro R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. (2018) 379:1895–904. doi: 10.1056/NEJMoa1806395
7. Shazly SA, Murad MH, Dowdy SC, Gostout BS, Famuyide AO. Robotic radical hysterectomy in early stage cervical cancer: a systematic review and meta-analysis. Gynecol Oncol. (2015) 138:457–71. doi: 10.1016/j.ygyno.2015.06.009
8. Wang YZ, Deng L, Xu HC, Zhang Y, Liang ZQ. Laparoscopy versus laparotomy for the management of early stage cervical cancer. BMC Cancer. (2015) 15:928. doi: 10.1186/s12885-015-1818-4
9. Cao T, Feng Y, Huang Q, Wan T, Liu J. Prognostic and safety roles in laparoscopic versus abdominal radical hysterectomy in cervical cancer: a meta-analysis. J Laparoendosc Adv Surg Tech A. (2015) 25:990–98. doi: 10.1089/lap.2015.0390
10. Lee EJ, Kang H, Kim DH. A comparative study of laparoscopic radical hysterectomy with radical abdominal hysterectomy for early-stage cervical cancer: a long-term follow-up study. Eur J Obstet Gynecol Reprod Biol. (2011) 156:83–6. doi: 10.1016/j.ejogrb.2010.12.016
11. Bogani G, Cromi A, Uccella S, Serati M, Casarin J, Pinelli C, et al. Laparoscopic versus open abdominal management of cervical cancer: long-term results from a propensity-matched analysis. J Minim Invasive Gynecol. (2014) 21:857–62. doi: 10.1016/j.jmig.2014.03.018
12. Nam JH, Park JY, Kim DY, Kim JH, Kim YM, Kim YT. Laparoscopic versus open radical hysterectomy in early-stage cervical cancer: long-term survival outcomes in a matched cohort study. Ann Oncol. (2012) 23:903–11. doi: 10.1093/annonc/mdr360
13. Ditto A, Martinelli F, Bogani G, Gasparri ML, Di Donato V, Zanaboni F, et al. Implementation of laparoscopic approach for type B radical hysterectomy: a comparison with open surgical operations. Eur J Surg Oncol. (2015) 41:34–9. doi: 10.1016/j.ejso.2014.10.058
14. Kong TW, Chang SJ, Lee J, Paek J, Ryu HS. Comparison of laparoscopic versus abdominal radical hysterectomy for FIGO stage IB and IIA cervical cancer with tumor diameter of 3 cm or greater. Int J Gynecol Cancer. (2014) 24:280–8. doi: 10.1097/IGC.0000000000000052
15. Malzoni M, Tinelli R, Cosentino F, Fusco A, Malzoni C. Total laparoscopic radical hysterectomy versus abdominal radical hysterectomy with lymphadenectomy in patients with early cervical cancer: our experience. Ann Surg Oncol. (2009) 16:1316–23. doi: 10.1245/s10434-009-0342-7
16. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. (1996) 17:1–2. doi: 10.1016/0197-2456(95)00134-4
17. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
18. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. (2007) 8:16. doi: 10.1186/1745-6215-8-16
19. Laird NM, Mosteller F. Some statistical methods for combining experimental results. Int J Technol Assess Health Care. (1990) 6:5–30. doi: 10.1017/S0266462300008916
20. Dickersin K, Berlin JA. Meta-analysis: state-of-the-science. Epidemiol Rev. (1992) 14:154–76. doi: 10.1093/oxfordjournals.epirev.a036084
21. Copas J, Shi JQ. Meta-analysis, funnel plots and sensitivity analysis. Biostatistics. (2000) 1:247–62. doi: 10.1093/biostatistics/1.3.247
22. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
23. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. (1994) 50:1088–101. doi: 10.2307/2533446
24. Park JY, Kim DY, Kim JH, Kim YM, Kim YT, Nam JH. Laparoscopic compared with open radical hysterectomy in obese women with early-stage cervical cancer. Obstet Gynecol. (2012) 119:1201–9. doi: 10.1097/AOG.0b013e318256ccc5
25. Park JY, Kim DY, Kim JH, Kim YM, Kim YT, Nam JH. Laparoscopic versus open radical hysterectomy for elderly patients with early-stage cervical cancer. Am J Obstet Gynecol. (2012) 207:195.e191–8. doi: 10.1016/j.ajog.2012.06.081
26. Park JY, Kim DY, Kim JH, Kim YM, Kim YT, Nam JH. Laparoscopic versus open radical hysterectomy in patients with stage IB2 and IIA2 cervical cancer. J Surg Oncol. (2013) 108:63–9. doi: 10.1002/jso.23347
27. Kim SI, Lee M, Lee S, Suh DH, Kim HS, Kim K, et al. Impact of laparoscopic radical hysterectomy on survival outcome in patients with FIGO stage IB cervical cancer: a matching study of two institutional hospitals in Korea. Gynecol Oncol. (2019) 155:75–82. doi: 10.1016/j.ygyno.2019.07.019
28. Park JY, Kim D, Suh DS, Kim JH, Kim YM, Kim YT, et al. The role of laparoscopic radical hysterectomy in early-stage adenocarcinoma of the uterine cervix. Ann Surg Oncol. (2016) 23(Suppl 5):825–33. doi: 10.1245/s10434-016-5489-4
29. Melamed A, Margul DJ, Chen L, Keating NL, Del Carmen MG, Yang J, et al. Survival after minimally invasive radical hysterectomy for early-stage cervical cancer. N Engl J Med. (2018) 379:1905–14. doi: 10.1056/NEJMoa1804923
30. Paik ES, Lim MC, Kim M-H, Kim YH, Song ES, Seong SJ, et al. Comparison of laparoscopic and abdominal radical hysterectomy in early stage cervical cancer patients without adjuvant treatment: ancillary analysis of a Korean Gynecologic Oncology Group Study (KGOG 1028). Gynecol Oncol. (2019) 154:547–53. doi: 10.1016/j.ygyno.2019.06.023
31. Yuan Z, Cao D, Yang J, Yu M, Shen K, Yang J, et al. Laparoscopic vs. open abdominal radical hysterectomy for cervical cancer: a single-institution, propensity score matching study in China. Front Oncol. (2019) 9:1107. doi: 10.3389/fonc.2019.01107
32. Cusimano MC, Baxter NN, Gien LT, Moineddin R, Liu N, Dossa F, et al. Impact of surgical approach on oncologic outcomes in women undergoing radical hysterectomy for cervical cancer. Am J Obstet Gynecol. (2019) 221:619.e1–9.e24. doi: 10.1016/j.ajog.2019.07.009
33. Kim SI, Cho JH, Seol A, Kim YI, Lee M, Kim HS, et al. Comparison of survival outcomes between minimally invasive surgery and conventional open surgery for radical hysterectomy as primary treatment in patients with stage IB1-IIA2 cervical cancer. Gynecol Oncol. (2019) 153:3–12. doi: 10.1016/j.ygyno.2019.01.008
34. Doo DW, Kirkland CT, Griswold LH, McGwin G, Huh WK, Leath CA, et al. Comparative outcomes between robotic and abdominal radical hysterectomy for IB1 cervical cancer: results from a single high volume institution. Gynecol Oncol. (2019) 153:242–7. doi: 10.1016/j.ygyno.2019.03.001
35. Lim TYK, Lin KKM, Wong WL, Aggarwal IM, Yam PKL. Surgical and oncological outcome of total laparoscopic radical hysterectomy versus radical abdominal hysterectomy in early cervical cancer in Singapore. Gynecol Minim Invasive Ther. (2019) 8:53–8. doi: 10.4103/GMIT.GMIT_43_18
36. Guo J, Yang L, Cai J, Xu L, Min J, Shen Y, et al. Laparoscopic procedure compared with open radical hysterectomy with pelvic lymphadenectomy in early cervical cancer: a retrospective study. OncoTargets Ther. (2018) 11:5903–8. doi: 10.2147/OTT.S156064
37. Corrado G, Vizza E, Legge F, Pedone Anchora L, Sperduti I, Fagotti A, et al. Comparison of different surgical approaches for stage IB1 cervical cancer patients: a multi-institution study and a review of the literature. Int J Gynecol Cancer. (2018) 28:1020–8. doi: 10.1097/IGC.0000000000001254
38. Wallin E, Floter Radestad A, Falconer H. Introduction of robot-assisted radical hysterectomy for early stage cervical cancer: impact on complications, costs and oncologic outcome. Acta Obstet Gynecol Scand. (2017) 96:536–42. doi: 10.1111/aogs.13112
39. Shah CA, Beck T, Liao JB, Giannakopoulos NV, Veljovich D, Paley P. Surgical and oncologic outcomes after robotic radical hysterectomy as compared to open radical hysterectomy in the treatment of early cervical cancer. J Gynecol Oncol. (2017) 28:e82. doi: 10.3802/jgo.2017.28.e82
40. Wang W, Chu HJ, Shang CL, Gong X, Liu TY, Zhao YH, et al. Long-term oncological outcomes after laparoscopic versus abdominal radical hysterectomy in stage IA2 to IIA2 cervical cancer: a matched cohort study. Int J Gynecol Cancer. (2016) 26:1264–73. doi: 10.1097/IGC.0000000000000749
41. Sert BM, Boggess JF, Ahmad S, Jackson AL, Stavitzski NM, Dahl AA, et al. Robot-assisted versus open radical hysterectomy: a multi-institutional experience for early-stage cervical cancer. Eur J Surg Oncol. (2016) 42:513–22. doi: 10.1016/j.ejso.2015.12.014
42. Zanagnolo V, Minig L, Rollo D, Tomaselli T, Aletti G, Bocciolone L, et al. Clinical and oncologic outcomes of robotic versus abdominal radical hysterectomy for women with cervical cancer: experience at a referral cancer center. Int J Gynecol Cancer. (2016) 26:568–74. doi: 10.1097/IGC.0000000000000645
43. Toptas T, Simsek T. Total laparoscopic versus open radical hysterectomy in stage IA2-IB1 cervical cancer: disease recurrence and survival comparison. J Laparoendosc Adv Surg Tech A. (2014) 24:373–8. doi: 10.1089/lap.2013.0514
44. Sobiczewski P, Bidzinski M, Derlatka P, Panek G, Danska-Bidzinska A, Gmyrek L, et al. Early cervical cancer managed by laparoscopy and conventional surgery: comparison of treatment results. Int J Gynecol Cancer. (2009) 19:1390–5. doi: 10.1111/IGC.0b013e3181ba5e88
45. Alfonzo E, Wallin E, Ekdahl L, Staf C, Rådestad AF, Reynisson P, et al. No survival difference between robotic and open radical hysterectomy for women with early-stage cervical cancer: results from a nationwide population-based cohort study. Eur J Cancer. (2019) 116:169–77. doi: 10.1016/j.ejca.2019.05.016
46. Pedone Anchora L, Turco LC, Bizzarri N, Capozzi VA, Lombisani A, Chiantera V, et al. How to select early-stage cervical cancer patients still suitable for laparoscopic radical hysterectomy: a propensity-matched study. Ann Surg Oncol. (2020) 27:1947–55. doi: 10.1245/s10434-019-08162-5
47. Kim JH, Kim K, Park SJ, Lee JY, Kim K, Lim MC, et al. Comparative effectiveness of abdominal versus laparoscopic radical hysterectomy for cervical cancer in the postdissemination era. Cancer Res Treat. (2019) 51:788–96. doi: 10.4143/crt.2018.120
48. Chiva L, Zanagnolo V, Kucukmetin A, Chakalova G, Raspagliesi F, Narducci F, et al. SUCCOR Study. An International European Cohort Observational Study comparing minimally invasive surgery versus open abdominal Radical Hysterectomy in patients with stage IB1 cervical cancer operated in 2013–2014. (2019) 29(Suppl 4):A1–2. doi: 10.1136/ijgc-2019-ESGO.1
49. Service NCRaA. Comparisons of Overall Survival in Women Diagnosed with Early Stage Cervical Cancer during 2013–2016 treated by Radical Hysterectomy Using Minimal Access or Open Approach. (2019). Available online at: wwwbgcsorguk/wp-content/uploads/2019/07/NCRAS-cervical-cancer-surgery-analysis-May-2019-finalpdf
50. Uppal S, Gehrig PA, Peng K, Bixel KL, Matsuo K, Vetter MH, et al. Recurrence rates in patients with cervical cancer treated with abdominal versus minimally invasive radical hysterectomy: a multi-institutional retrospective review study. J Clin Oncol. (2020) 38:1030–40. doi: 10.1200/JCO.19.03012
51. Jemal A, Ward EM, Johnson CJ, Cronin KA, Ma J, Ryerson B, et al. Annual Report to the Nation on the Status of Cancer, 1975–2014, featuring survival. J Natl Cancer Inst. (2017) 109:djx030. doi: 10.1093/jnci/djx030
52. Rizou N, Moris D, Pikoulis E, Dimitrokallis N, Mpaili E, Felekouras E, et al. Minimally invasive lymphadenectomy in uterine cervical cancer: a systematic review. Anticancer Res. (2017) 37:335–42. doi: 10.21873/anticanres.11326
53. Nezhat CR, Burrell MO, Nezhat FR, Benigno BB, Welander CE. Laparoscopic radical hysterectomy with paraaortic and pelvic node dissection. Am J Obstet Gynecol. (1992) 166:864–5. doi: 10.1016/0002-9378(92)91351-A
54. Lim YK, Chia YN, Yam KL. Total laparoscopic Wertheim's radical hysterectomy versus Wertheim's radical abdominal hysterectomy in the management of stage I cervical cancer in Singapore: a pilot study. Singapore Med J. (2013) 54:683–8. doi: 10.11622/smedj.2013242
55. Frumovitz M, dos Reis R, Sun CC, Milam MR, Bevers MW, Brown J, et al. Comparison of total laparoscopic and abdominal radical hysterectomy for patients with early-stage cervical cancer. Obstet Gynecol. (2007) 110:96–102. doi: 10.1097/01.AOG.0000268798.75353.04
56. Zakashansky K, Chuang L, Gretz H, Nagarsheth NP, Rahaman J, Nezhat FR. A case-controlled study of total laparoscopic radical hysterectomy with pelvic lymphadenectomy versus radical abdominal hysterectomy in a fellowship training program. Int J Gynecol Cancer. (2007) 17:1075–82. doi: 10.1111/j.1525-1438.2007.00921.x
57. Campos LS, Limberger LF, Stein AT, Kalil AN. Postoperative pain and perioperative outcomes after laparoscopic radical hysterectomy and abdominal radical hysterectomy in patients with early cervical cancer: a randomised controlled trial. Trials. (2013) 14:293. doi: 10.1186/1745-6215-14-293
58. Meigs JV. Radical hysterectomy with bilateral pelvic lymph node dissections; a report of 100 patients operated on five or more years ago. Am J Obstet Gynecol. (1951) 62:854–70. doi: 10.1016/0002-9378(51)90175-5
59. Wright JD, Herzog TJ, Neugut AI, Burke WM, Lu YS, Lewin SN, et al. Comparative effectiveness of minimally invasive and abdominal radical hysterectomy for cervical cancer. Gynecol Oncol. (2012) 127:11–7. doi: 10.1016/j.ygyno.2012.06.031
60. Uppal S, Rebecca Liu J, Kevin Reynolds R, Rice LW, Spencer RJ. Trends and comparative effectiveness of inpatient radical hysterectomy for cervical cancer in the United States (2012-2015). Gynecol Oncol. (2019) 152:133–8. doi: 10.1016/j.ygyno.2018.09.027
61. Xiao M, Zhang Z. Total laparoscopic versus laparotomic radical hysterectomy and lymphadenectomy in cervical cancer: an observational study of 13-year experience. Medicine. (2015) 94:e1264. doi: 10.1097/MD.0000000000001264
62. Logani S, Herdman AV, Little JV, Moller KA. Vascular “pseudo invasion” in laparoscopic hysterectomy specimens: a diagnostic pitfall. Am J Surg Pathol. (2008) 32:560–5. doi: 10.1097/PAS.0b013e31816098f0
63. Krizova A, Clarke BA, Bernardini MQ, James S, Kalloger SE, Boerner SL, et al. Histologic artifacts in abdominal, vaginal, laparoscopic, and robotic hysterectomy specimens: a blinded, retrospective review. Am J Surg Pathol. (2011) 35:115–26. doi: 10.1097/PAS.0b013e31820273dc
64. Dewdney SB, Jiao Z, Roma AA, Gao F, Rimel BJ, Thaker PH, et al. The prognostic significance of lymphovascular space invasion in laparoscopic versus abdominal hysterectomy for endometrioid endometrial cancer. Eur J Gynaecol Oncol. (2014) 35:7–10.
65. Canis M, Botchorishvili R, Wattiez A, Mage G, Pouly JL, Bruhat MA. Tumor growth and dissemination after laparotomy and CO2 pneumoperitoneum: a rat ovarian cancer model. Obstet Gynecol. (1998) 92:104–8. doi: 10.1016/S0029-7844(98)00145-8
66. Volz J, Koster S, Spacek Z, Paweletz N. The influence of pneumoperitoneum used in laparoscopic surgery on an intraabdominal tumor growth. Cancer. (1999) 86:770–4. doi: 10.1002/(SICI)1097-0142(19990901)86:5<770::AID-CNCR11>3.0.CO;2-3
Keywords: cervical cancer, minimally invasive surgery, open surgery, radical hysterectomy, meta-analysis
Citation: Wang Y, Li B, Ren F, Song Z, Ouyang L and Liu K (2020) Survival After Minimally Invasive vs. Open Radical Hysterectomy for Cervical Cancer: A Meta-Analysis. Front. Oncol. 10:1236. doi: 10.3389/fonc.2020.01236
Received: 19 March 2020; Accepted: 16 June 2020;
Published: 24 July 2020.
Edited by:
Stefano Cianci, University of Campania Luigi Vanvitelli, ItalyReviewed by:
Giuseppe Vizzielli, Catholic University of the Sacred Heart, ItalyLuigi Pedone Anchora, Catholic University of the Sacred Heart, Italy
Copyright © 2020 Wang, Li, Ren, Song, Ouyang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kuiran Liu, liukr@sj-hospital.org
 Yizi Wang
Yizi Wang Bo Li
Bo Li Fang Ren
Fang Ren Zixuan Song
Zixuan Song Ling Ouyang
Ling Ouyang Kuiran Liu
Kuiran Liu