- Department of Urology, Zhongshan Hospital, Fudan University, Shanghai, China
Introduction: Vimentin, a classical marker of epithelial–mesenchymal transition, reflects the invasiveness of cancer cells. Its role in the genesis and progression of tumor has been reported in various cancers, including renal cell carcinoma. However, the impact of vimentin on tumor microenvironment, particularly its implication with tumor-infiltrating immune cells, is unknown.
Methods: We conducted this study in 231 patients with metastatic renal cell carcinoma (mRCC) to determine the potential relationship between vimentin and immune status. Using immunohistochemical staining, expression of vimentin, CD8, FOXP3, programmed cell death protein 1 (PD-1), and programmed cell death ligand 1 (PD-L1) were evaluated in resected tumor tissue. Kaplan–Meier analysis and Cox regression models were used for survival analysis. Chi-square test, Fisher exact test, and Mann–Whitney U-test were used for comparison between vimentin high and low groups.
Results: High expression of vimentin, stroma PD-L1, and PD-1 indicated poor overall survival, whereas low regulatory T cell or high CD8+ T cell infiltration indicated long overall survival. Stroma PD-L1 (P = 0.030), vimentin (P = 0.026) expression, and CD8+ T cell infiltration (P < 0.001) were independent prognostic factors in mRCC. High vimentin expression was accompanied by high PD-1, PD-L1 expression, and increased regulatory T cell infiltration (all P < 0.001), indicating immunosuppression in the tumor microenvironment.
Conclusions: We revealed that vimentin expression was associated with immunosuppression in mRCC, and the immune-suppressive status might be possibly posed by PD-1/PD-L1. Patients with high vimentin expression may acquire potential benefit from the recently approved PD-1/PD-L1 inhibitors. However, further clinical trials are needed to validate our findings.
Introduction
Renal cell carcinoma (RCC) is one of the most common urological cancers, which accounts for 2–3% of all malignant tumors in adults (1). Radical surgery is effective in localized RCC, but once metastasized, the prognosis is poor. The median survival of patients with recurrence and metastasis is only about 26 months treated with sunitinib (2). Currently, tyrosine kinase inhibitor (TKI) is one of the first-line agents for metastatic RCC (mRCC), with a response rate of <30% (3). With the development of a variety of targeted drugs and sequential TKI therapy regimen, survival of mRCC patients is prolonging. Most of these patients have received multi-line TKI therapy and lack new available drugs (4). Studies have shown abundant immune cell infiltration in the RCC microenvironment. Thus, interferon-alpha and interleukin-2 were used to treat RCC (5). Immunotherapy with immune checkpoint inhibitors (monoclonal antibodies) of programmed cell death protein 1 (PD-1)/programmed cell death ligand 1 (PD-L1) has also shown a good effect in recent trials, particularly in high-risk mRCC (6).
Epithelial–mesenchymal transition (EMT) contributes to the proliferation, invasion, and metastasis of carcinoma (7). EMT makes epithelial cells lose their polarity, decrease cell-to-cell and cell-to-extracellular matrix adhesion, and increase the invasiveness of tumor cells (8). Vimentin, a component of mesenchymal cell skeleton, increased expression during the EMT process. Previous studies have suggested that vimentin indicates EMT, which affects tumor progression by increasing the invasive ability of the tumor (9). Also, it is closely related to the ability of tumor invasion and metastasis. Currently, studies in malignant tumors have shown that vimentin plays an important role in cell cycle regulation, migration, adhesion, and EMT of carcinoma (10). The latest report shows that vimentin or EMT can affect the infiltration of immune cells in tumor microenvironment (11).
Whether EMT is associated with anti-tumor immunity in RCC is yet unknown. In this study, expression of vimentin, immune checkpoint molecules (PD-1 and PD-L1), tumor-infiltrating CD8+ T cells, and regulatory T cells (Tregs) were assessed by immunohistochemical staining to reflect immune status in RCC. Also, we aimed to evaluate the prognostic value of vimentin expression and its association with the tumor immune environment in an mRCC cohort.
Materials and Methods
Clinical Data and Specimen Collection
Patients with mRCCs from Zhongshan Hospital, Fudan University were enrolled in the study from June 2007 to June 2017. All patients received first-line sunitinib or sorafenib therapy, and characteristics are summarized in Table 1. Clinical data and follow-up state were collected. Progression-free survival (PFS) was evaluated from the introduction of system therapy to the date of progression, and overall survival (OS) was from the introduction of system therapy to the date of death or last follow-up. The follow-up period ranged from 3 to 111 months (median 26 months). No patients received preoperative radiotherapy or chemotherapy. The ethics committee of Zhongshan Hospital, Fudan University, approved the study, and all patients signed informed consent.
Tissue Samples and Immunohistochemical Staining
Archival anonymized, formalin-fixed, paraffin-embedded (FFPE) tumor tissue samples obtained from nephrectomy or tumor biopsy specimens of patients were enrolled in this study. Immunohistochemical staining was performed as previously described (12). A total of five immunohistochemistry (IHC) assays were developed with serial sections: Primary antibodies used were mouse anti-human CD8 (ab17147, Abcam, concentration 1:400), mouse anti-human FOXP3 (ab20034, Abcam, concentration 1:100), rabbit anti-human vimentin (ab92547, Abcam, concentration 1:200), mouse anti-human PD-1 (ab52587, Abcam, concentration 1:100), and rabbit anti-human PD-L1 (ab205921, Abcam, concentration 1:400). In total, 231 individual patient formalin-fixed, paraffin-embedded blocks were selected for IHC staining of PD-L1, PD-1, vimentin, CD8, and FOXP3. IHC-stained slides were reviewed and evaluated by two independent investigators; the mean of two evaluations was recorded as the final score for each specimen. Staining intensity was classified as negative for 0 points, weakly positive for 1 point, positive for 2 points, and strongly positive for 3 points. The PD-L1 expression on both stromal and tumor cells were calculated. Staining intensity score multiplied the percentage of positive cells (1 point for 1 percentage) to get the final PD-L1 H-score. Vimentin expression was assessed using a similar H-score semiquantitative method as PD-L1. Final immunohistochemical scores were staining intensity multiplied to positive cell percentage. The percentage of vimentin-positive cells was scored 1 point for positive cell percentage ≤ 10%, 2 points for percentage 11 to 40%, 3 points for percentage 41 to 75%, and 4 points for percentage ≥75%. Scores of 0 to 7 were considered low expression and scores of 8 to 12 considered high expression for vimentin (cut-off by median) (13). Five independent areas of each slide were examined under a microscope to calculate the counts of FOXP3+, CD8+, or PD-1+ tumor-infiltrating cells. Median of Treg density (16/mm2), CD8+ cell density (143/mm2), and PD-1+ cell density (82/mm2) were set as the cutoff point for high and low infiltration.
Statistical Analysis
Chi-squared test, Fisher exact test, and Mann–Whitney U-test were performed to compare qualitative and quantitative parameters. Kaplan–Meier curves were compared using the log-rank test. Univariate and multivariable Cox proportional hazards models were used to explore the potential predictive effect of the biomarker (vimentin, CD8+ T cell density [high/low], or PD-L1, PD-1 status [+/–]), and other characteristics. Variables with P < 0.1 in the univariate analysis entered the multivariate model. A two-sided p < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS 21.0 software (SPSS Inc., Chicago, IL, USA).
Results
Association of Vimentin and Clinical Characteristics of Metastatic Renal Cell Carcinoma Patients
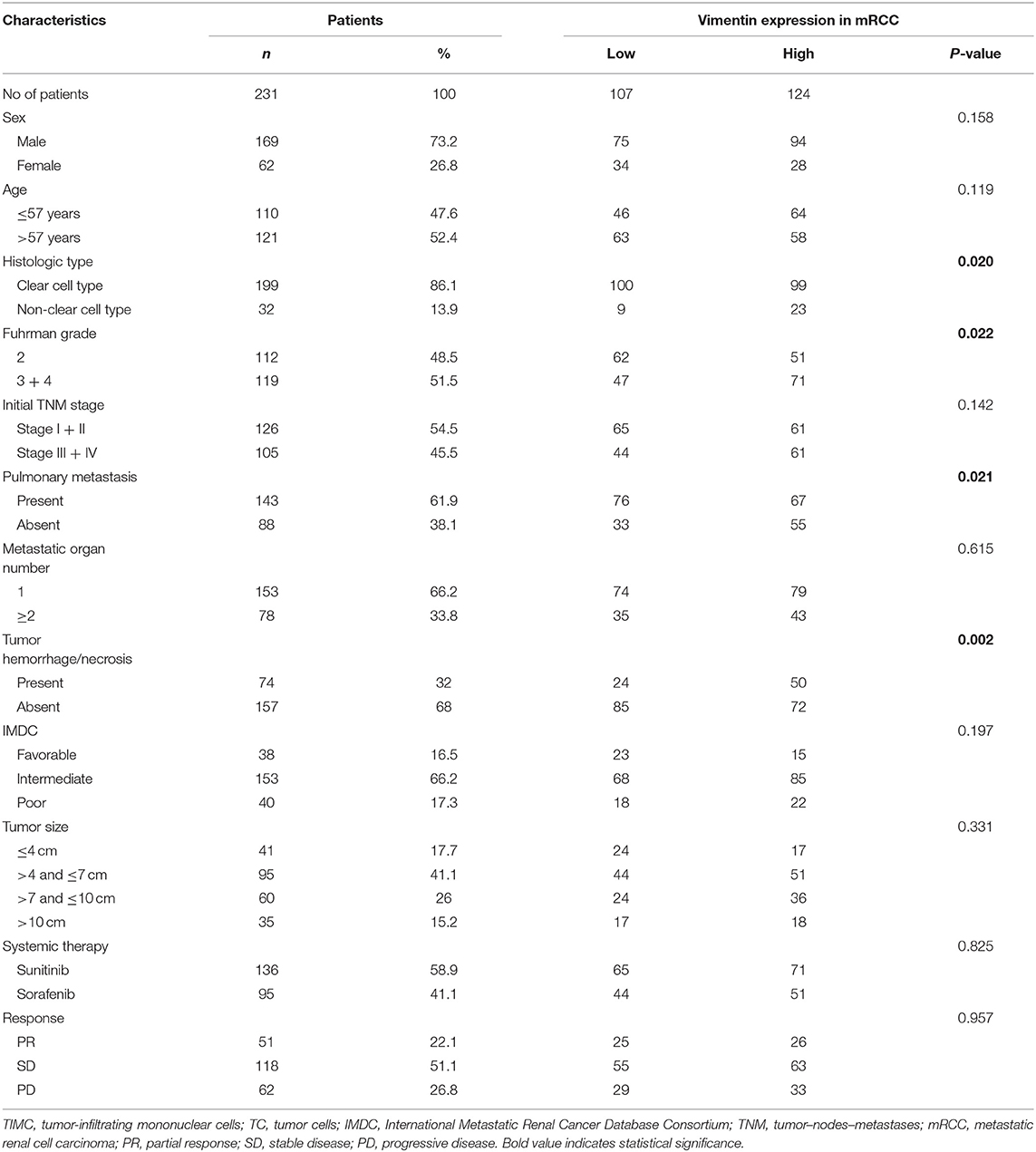
A total of 231 cases were enrolled, 169 males and 62 females, aged from 14 to 87 years old; mean age was 57.2 years old. Typical vimentin immunostaining is shown in Figures 1A,B. Overall, 46.32% (n = 107) of mRCC cases were considered to have low vimentin expression, whereas 53.68% (n = 124) of mRCC cases were considered to have high vimentin expression. Characteristics of low or high vimentin expression group are summarized and compared in Table 1. The expression of vimentin in 231 cases of mRCC had no significant relation with sex, age, and initial tumor–nodes–metastases stage. With the Fuhrman grade increasing, the expression of vimentin also increased. High vimentin expression was associated with high Fuhrman grade (P = 0.022), non-clear-cell histologic type (P = 0.020), absent of pulmonary metastasis (P = 0.021), and tumor hemorrhage/necrosis (P = 0.002).
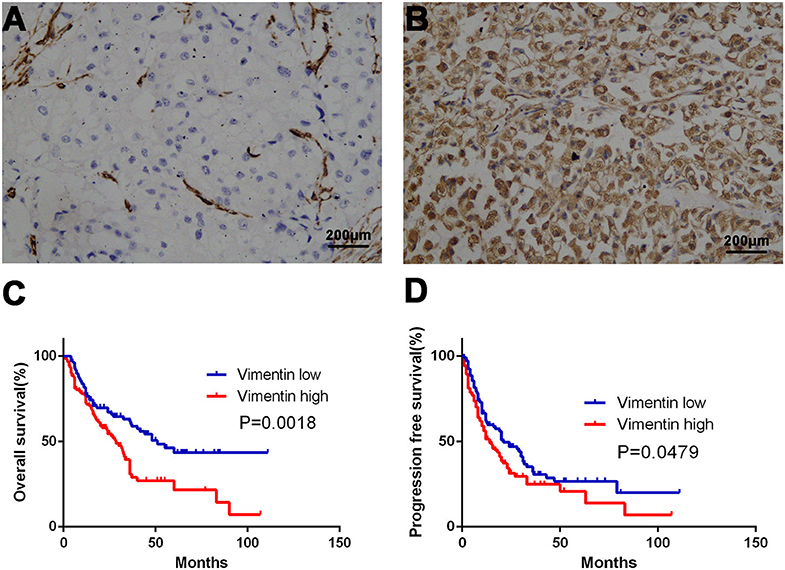
Figure 1. Association of vimentin expression and prognosis. Typical images of low vimentin expression (A) (negative expression in tumor cells and positive expression in endothelial cells) and high expression in tissue microarray. (B) Kaplan–Meier curve of overall survival (OS) (C) and progression-free survival (PFS) (D) according to vimentin expression. Original magnification: ×100. P-value calculated by log-rank test.
Association of Vimentin and Immune Status in Metastatic Renal Cell Carcinoma
Typical images of tumor PD-L1 (tPD-L1), stroma PD-L1 (sPD-L1), and PD-1 staining are shown in Figures 2E,G,I. PD-1 and PD-L1 (both sPD-L1 and tPD-L1) overexpression were associated with high vimentin expression (Figures 2F,H,J; all P < 0.001). Typical images of tumor-infiltrating CD8+ T cells and Tregs are also shown in Figures 2A,C. The association between vimentin expression and CD8+ T cell infiltration number was statistically insignificant (Figure 2B; P = 0.145). However, high vimentin expression was significantly associated with increased Treg infiltration (Figure 2D; P < 0.001).
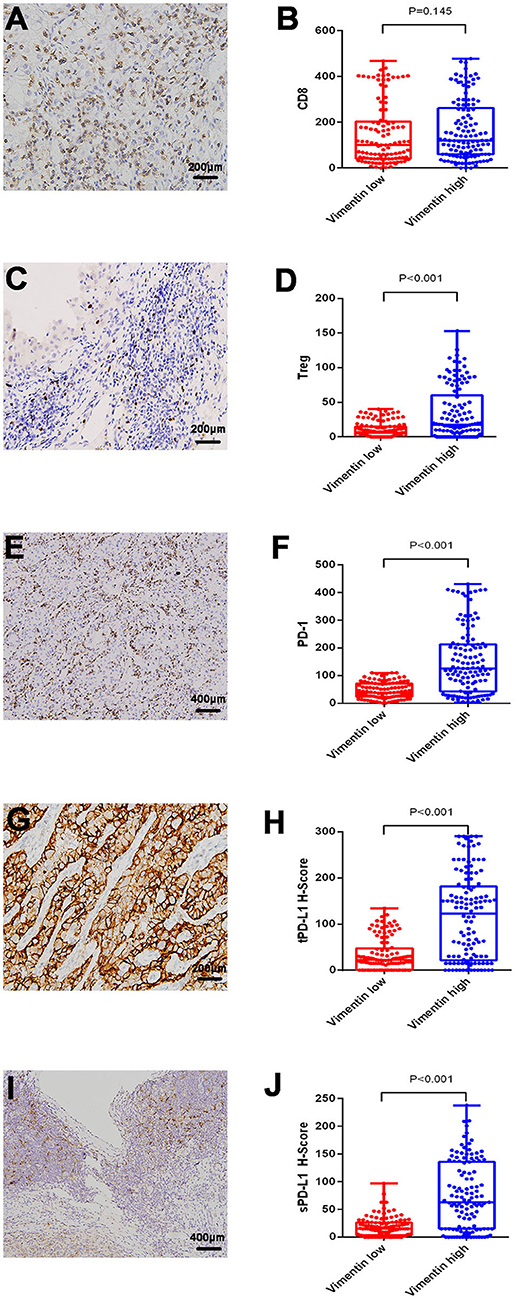
Figure 2. Association of vimentin expression and immune status. Typical images of infiltrating CD8+ T cell (A) and regulatory T cell (Treg) (C). Typical images of programmed cell death protein 1 (PD-1) expression (E), tumor PD-L1 (tPD-L1) (G), stroma PD-L1 (sPD-L1) (I). CD8+ T cell (B), and Treg (D) infiltrating levels in vimentin low and high groups. PD-1 (F), tPD-L1 (H), and sPD-L1 (J) expression levels in vimentin low and high groups.
Impact of Vimentin and Immune Status on Prognosis of Metastatic Renal Cell Carcinoma
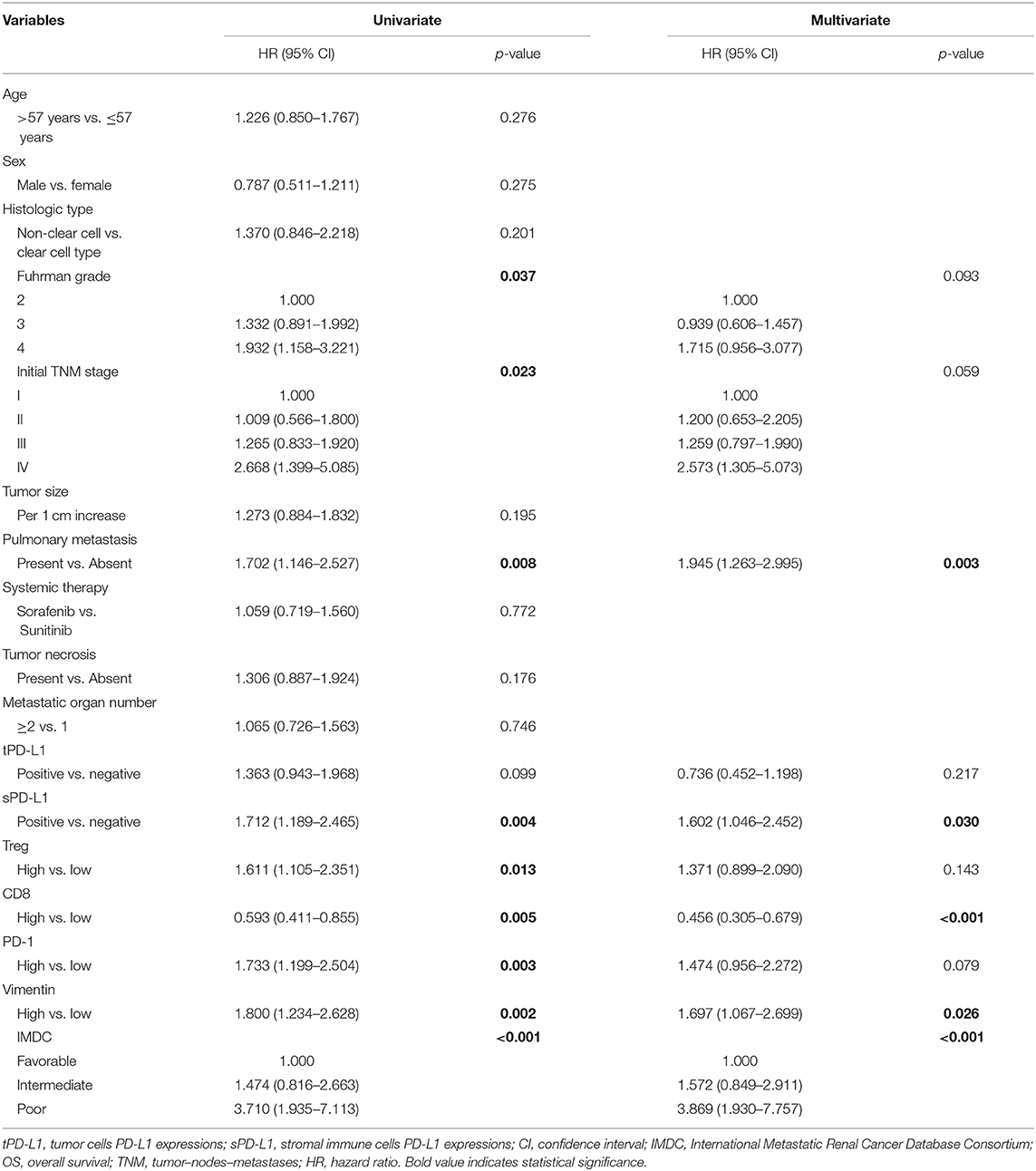
Univariate analysis demonstrated that vimentin, sPD-L1, PD-1 expression, and Treg, CD8+ T cell infiltration were possible prognostic factors for OS. Clinical factors of initial tumor–nodes–metastases stage, pulmonary metastasis, and International Metastatic Renal Cancer Database Consortium risk group were possible prognostic factors for OS as well. Multivariate analysis indicated that vimentin expression [hazard ratio (HR) = 1.697, 95% confidence interval (CI): 1.067–2.699, P = 0.026] was an independent prognostic factor for OS of the patients of mRCC, along with sPD-L1 expression (HR = 1.602, 95% CI: 1.046–2.452, P = 0.030) and CD8+ T cell infiltration (HR = 0.456, 95% CI: 0.305–0.679, P < 0.001, Table 2). Clinical factors of pulmonary metastasis and International Metastatic Renal Cancer Database Consortium risk group remained to be independent prognostic factors of OS (P = 0.003 and P < 0.001, respectively). Kaplan–Meier survival analysis also showed that patients with high vimentin, sPD-L1, and PD-1 expression had significantly poorer OS (P = 0.0018, P = 0.0031, and P = 0.0028, respectively, Figures 1C, 3C,E). Patients with low Treg infiltration or high CD8+ T cell infiltration showed longer OS than those with high Treg infiltration or low CD8+ T cell infiltration (P = 0.0115, P = 0.0043, respectively, Figures 3A,B). No association were observed between OS and tPD-L1 expression (P = 0.0943; Figure 3D). Although high vimentin expression indicated poor PFS in Kaplan–Meier survival analysis (cut-off by median, P = 0.0479, Figure 1D), it was not an independent prognostic factor for PFS (Table S1).
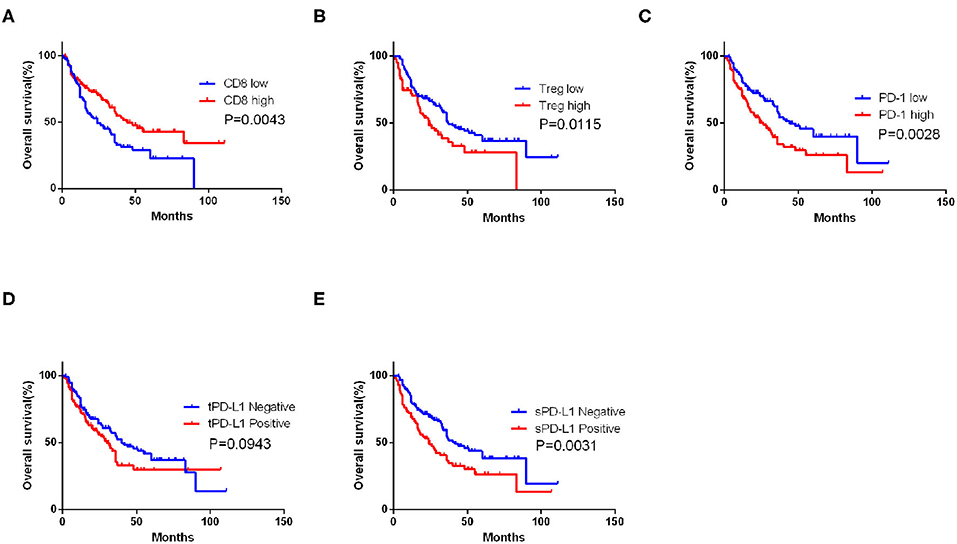
Figure 3. Impact of immune features on overall survival (OS) of metastatic renal cell carcinoma (mRCC) patients. Kaplan–Meier curve of OS according to the CD8+ T cell (A), Treg infiltrating number (B), programmed cell death protein 1 (PD-1) (C), tumor PD-L1 (tPD-L1) (D), and stroma PD-L1 (sPD-L1) (E) expression in mRCC patients. P-value calculated by log-rank test.
Discussion
Vimentin is a canonical marker of EMT. It is an important regulator of cellular motility, ubiquitously expressed in normal mesenchymal cells to maintain cellular integrity (14). Increased vimentin expression has been reported in various cancers, such as prostate cancer, gastric cancer, and esophageal squamous cell carcinoma, and correlates with tumor growth, invasion, migration, and poor prognosis (15–17). Williams et al. (18) also found that expression of vimentin was closely related to low differentiation, strong invasiveness, and easy metastasis in chromophobe RCC. We confirmed that high expression of vimentin indicated poor OS in this mRCC cohort mainly composed of patients with clear-cell RCC.
CD8+ T cell is the main effector of anti-tumor immunity. PD-1/PD-L1 acts as inhibitory molecules reflecting the immunosuppressive state in tumor microenvironment (19). PD-1- or PD-L1-positive Tregs inhibit the expansion of immune response and reduce anti-tumor immunity in microenvironment (20). Exhaustion of CD8+ T cell, characterized by increased expression of inhibitory checkpoint receptors, is a gradual loss of the effector function and the proliferation potential (21). Overexpression of PD-L1 on tumor cells can inhibit cytotoxicity of CD8+ T cells (22). PD-1/PD-L1 pathway also affects the suppressive properties of the Tregs (23). The blockade of the PD-1/PD-L1 pathway prevents the conversion of naive Th cells to a Treg phenotype and rejuvenates CD8+ T cells (24). Therefore, PD-1/PD-L1-targeted immune checkpoint inhibitors have been approved for the treatment of advanced melanoma, kidney cancer, head and neck cancer, and non-small cell lung cancer (25). Consistent with the previously mentioned theories, CD8+ T cell was associated with favorable survival, whereas PD-1, sPD-L1, and Tregs were associated with unfavorable survival in our cohort.
Whether vimentin is related to immune status in the microenvironment of RCC has not been reported yet. Asgarova et al. (26) reported that EMT can induce PD-L1 promoter demethylation and upregulate the expression of PD-L1, which may be one of the possible mechanisms of vimentin-induced immunosuppression. It has also been reported that vimentin is involved in the migration of immune cells, such as lymphocytes (27). Our study indicated that high expression of vimentin in mRCC was associated with immunosuppressive status. Patients with high vimentin expression were accompanied by high expression of sPD-L1, tPD-L1, and PD-1. These patients also had more plentiful Treg infiltration (P < 0.001), but CD8+ T cell infiltration was comparative (P = 0.145). Because Tregs and PD-1/PD-L1 play immunosuppressive roles in the microenvironment, high levels of these factors indicate an immunosuppressive status. Although the number of infiltrating CD8+ T cells was similar in patients with vimentin high and low expression, the function of CD8+ T cells was probably inhibited.
Tumor-derived PD-L1 is capable of immunosuppression, but we suppose that stromal-derived PD-L1 plays a more important role in CD8+ T cell inhibition because sPD-L1 has direct contact with CD8+ T cells. In accordance with our speculation, sPD-L1 was an independent prognostic factor for OS, whereas tPD-L1 was not. sPD-L1 mainly expresses on the surface of Tregs and myeloid-derived suppressor cells (28). We did not evaluate infiltration of myeloid-derived suppressor cells in this cohort because the marker of this cell population for immunohistochemical staining is not available at present. For Tregs (marked by FOXP3), high infiltration suggested poor survival in our cohort. As the receptor of PD-L1, high PD-1 expression also indicated poor survival. Results of univariate COX regression analysis were concordant with survival analysis. After adjusting for various clinical and immunological factors in multivariate COX regression model, sPD-L1, vimentin, and CD8+ T cells remained to be independent prognostic factors.
A previous study has found a deleterious impact of vimentin expression on clinical outcome of localized RCC (29). Our study extended this conclusion to an mRCC cohort. Also, our results further suggested the association between vimentin expression and immune-suppressive status. Recruitment of Tregs, but not CD8+ T cells, may be affected by tumor vimentin expression. High vimentin expression may also induce increased PD-L1 expression both in tumor and stromal cells. However, the mechanisms behind the phenomenon were still vague. In addition to its well-known role in EMT, vimentin was also suggested to have another possible tumor-promoting mechanism in our study: it may induce immunosuppression via the PD-1/PD-L1 axis and inhibit CD8+ T cell function. With the fast development and application of PD-1/PD-L1-targeted immunotherapy in oncology, patients with high vimentin expression mRCC may be appropriate candidates due to accompanied high PD-1/PD-L1 expression.
There are several limitations to this study. First, this retrospective study was conducted in a single center with limited sample size; external validation with a prospective design is needed to confirm our findings. Second, all mRCC patients included in our study received sunitinib/sorafenib as first-line treatment. The results of our study should be generalized with caution for patients receiving other therapy, particularly for those receiving immune checkpoint inhibitors. Third, relationships between other EMT markers, such as E-cadherin, N-cadherin, fibronectin, etc., and immunosuppression status were not investigated in this study, and the association of vimentin and PD-1/PD-L1 axis was only demonstrated in human tissue level. The underlying mechanism should further be confirmed in an intervention experiment. Fourth, intra-tumor heterogeneity may bias our evaluation of vimentin expression. For example, the center of the tumor and the invasive border may have a different level of vimentin expression.
In summary, the present study confirmed in mRCC that high vimentin expression indicated poor OS. Vimentin expression positively associated with PD-1, PD-L1 expression, and Treg infiltration. In addition to EMT, vimentin may induce immunosuppression via PD-1/PD-L1. Patients with high vimentin expression may have a potential benefit from the recently approved PD-1/PD-L1 inhibitors.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.
Ethics Statement
The study was performed according to the principles of the Declaration of Helsinki. The study was approved by the ethics committee of Zhongshan Hospital, Fudan University and all patients signed informed consent.
Author Contributions
Acquisition of data, analysis, interpretation of data, statistical analysis, and drafting of the manuscript were carried out by JY, XC, and YZ. YZ and HW provided technical and material support. XH and JG were responsible for the study concept and design, analysis and interpretation of data, drafting of the manuscript, obtained funding, and study supervision. All authors read and approved the final manuscript.
Funding
This study was funded by grants from the National Natural Science Foundation of China (81772696 and 81472376), Zhongshan Hospital, Fudan University (2020ZSQN-ZPJJ-086), and Higher education innovation development fund of Gansu Province (2020B-200).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.01181/full#supplementary-material
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. (2019) 69:7–34. doi: 10.3322/caac.21551
2. Motzer RJ, Tannir NM, McDermott DF, Aren Frontera O, Melichar B, Choueiri TK, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. (2018) 378:1277–90. doi: 10.1056/NEJMoa1712126
3. Barata PC, De Liano AG, Mendiratta P, Crolley V, Szabados B, Morrison L, et al. The efficacy of VEGFR TKI therapy after progression on immune combination therapy in metastatic renal cell carcinoma. Br J Cancer. (2018) 119:160–3. doi: 10.1038/s41416-018-0104-z
4. Ralla B, Erber B, Goranova I, von der Aue L, Floercken A, Hinz S, et al. Efficacy of fourth-line targeted therapy in patients with metastatic renal cell carcinoma: a retrospective analysis. World J Urol. (2016) 34:1147–54. doi: 10.1007/s00345-015-1740-z
5. Passalacqua R, Caminiti C, Buti S, Porta C, Camisa R, Braglia L, et al. Adjuvant low-dose interleukin-2 (IL-2) plus interferon-alpha (IFN-alpha) in operable renal cell carcinoma (RCC): a phase III, randomized, multicentre trial of the Italian oncology group for clinical research (GOIRC). J Immunother. (2014) 37:440–7. doi: 10.1097/CJI.0000000000000055
6. Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. (2019) 380:1116–27. doi: 10.1056/NEJMoa1816714
7. Fantozzi A, Gruber DC, Pisarsky L, Heck C, Kunita A, Yilmaz M, et al. VEGF-mediated angiogenesis links EMT-induced cancer stemness to tumor initiation. Cancer Res. (2014) 74:1566–75. doi: 10.1158/0008-5472.CAN-13-1641
8. Cho ES, Kang HE, Kim NH, Yook JI. Therapeutic implications of cancer epithelial-mesenchymal transition (EMT). Arch Pharm Res. (2019) 16:14–24. doi: 10.1007/s12272-018-01108-7
9. Monteiro-Reis S, Lobo J, Henrique R, Jeronimo C. Epigenetic mechanisms influencing epithelial to mesenchymal transition in bladder cancer. Int J Mol Sci. (2019) 20:297. doi: 10.3390/ijms20020297
10. Felipe Lima J, Nofech-Mozes S, Bayani J, Bartlett JM. EMT in breast carcinoma-a review. J Clin Med. (2016) 5:65. doi: 10.3390/jcm5070065
11. Dutsch-Wicherek M, Lazar A, Tomaszewska R. The potential role of MT and vimentin immunoreactivity in the remodeling of the microenvironment of parotid adenocarcinoma. Cancer Microenviron. (2010) 4:105–13. doi: 10.1007/s12307-010-0058-z
12. Yao J, Xi W, Zhu Y, Wang H, Hu X, Guo J. Checkpoint molecule PD-1-assisted CD8(+) T lymphocyte count in tumor microenvironment predicts overall survival of patients with metastatic renal cell carcinoma treated with tyrosine kinase inhibitors. Cancer Manag Res. (2018) 10:3419–31. doi: 10.2147/CMAR.S172039
13. Barrett MT, Lenkiewicz E, Malasi S, Basu A, Yearley JH, Annamalai L, et al. The association of genomic lesions and PD-1/PD-L1 expression in resected triple-negative breast cancers. Breast Cancer Res. (2018) 20:71. doi: 10.1186/s13058-018-1004-0
14. Przybyla L, Muncie JM, Weaver VM. Mechanical control of epithelial-to-mesenchymal transitions in development and cancer. Annu Rev Cell Dev Biol. (2016) 32:527–54. doi: 10.1146/annurev-cellbio-111315-125150
15. Vyas AR, Singh SV. Functional relevance of D,L-sulforaphane-mediated induction of vimentin and plasminogen activator inhibitor-1 in human prostate cancer cells. Eur J Nutr. (2014) 53:843–52. doi: 10.1007/s00394-013-0588-5
16. Zhou LH, Yang YC, Zhang RY, Wang P, Pang MH, Liang LQ. CircRNA_0023642 promotes migration and invasion of gastric cancer cells by regulating EMT. Eur Rev Med Pharmacol Sci. (2018) 22:2297–303. doi: 10.26355/eurrev_201804_14818
17. Tanaka M, Kijima H, Shimada H, Makuuchi H, Ozawa S, Inokuchi S. Expression of podoplanin and vimentin is correlated with prognosis in esophageal squamous cell carcinoma. Mol Med Rep. (2015) 12:4029–36. doi: 10.3892/mmr.2015.3966
18. Williams AA, Higgins JP, Zhao H, Ljunberg B, Brooks JD. CD 9 and vimentin distinguish clear cell from chromophobe renal cell carcinoma. BMC Clin Pathol. (2009) 9:1472–6890. doi: 10.1186/1472-6890-9-9
19. Gibbons Johnson RM, Dong H. Functional expression of programmed death-ligand 1 (B7-H1) by immune cells and tumor cells. Front Immunol. (2017) 8:961. doi: 10.3389/fimmu.2017.00961
20. Boussiotis VA, Chatterjee P, Li L. Biochemical signaling of PD-1 on T cells and its functional implications. Cancer J. (2014) 20:265–71. doi: 10.1097/PPO.0000000000000059
21. Doering TA, Crawford A, Angelosanto JM, Paley MA, Ziegler CG, Wherry EJ. Network analysis reveals centrally connected genes and pathways involved in CD8+ T cell exhaustion versus memory. Immunity. (2012) 37:1130–44. doi: 10.1016/j.immuni.2012.08.021
22. Juneja VR, McGuire KA, Manguso RT, LaFleur MW, Collins N, Haining WN, et al. PD-L1 on tumor cells is sufficient for immune evasion in immunogenic tumors and inhibits CD8 T cell cytotoxicity. J Exp Med. (2017) 214:895–904. doi: 10.1084/jem.20160801
23. Habicht A, Dada S, Jurewicz M, Fife BT, Yagita H, Azuma M, et al. A link between PDL1 and T regulatory cells in fetomaternal tolerance. J Immunol. (2007) 179:5211–9. doi: 10.4049/jimmunol.179.8.5211
24. Tripathi S, Guleria I. Role of PD1/PDL1 pathway, and TH17 and treg cells in maternal tolerance to the fetus. Biomed J. (2015) 38:25–31. doi: 10.4103/2319-4170.143511
25. Yu Y. Molecular classification and precision therapy of cancer: immune checkpoint inhibitors. Front Med. (2018) 12:229–35. doi: 10.1007/s11684-017-0581-0
26. Asgarova A, Asgarov K, Godet Y, Peixoto P, Nadaradjane A, Boyer-Guittaut M, et al. PD-L1 expression is regulated by both DNA methylation and NF-kB during EMT signaling in non-small cell lung carcinoma. Oncoimmunology. (2018) 7:1423170. doi: 10.1080/2162402X.2017.1423170
27. Ivaska J, Pallari HM, Nevo J, Eriksson JE. Novel functions of vimentin in cell adhesion, migration, and signaling. Exp Cell Res. (2007) 313:2050–62. doi: 10.1016/j.yexcr.2007.03.040
28. Weber R, Fleming V, Hu X, Nagibin V, Groth C, Altevogt P, et al. Myeloid-derived suppressor cells hinder the anti-cancer activity of immune checkpoint inhibitors. Front Immunol. (2018) 9:1310. doi: 10.3389/fimmu.2018.01310
29. Ingels A, Hew M, Algaba F, de Boer OJ, van Moorselaar RJ, Horenblas S, et al. Vimentin over-expression and carbonic anhydrase IX under-expression are independent predictors of recurrence, specific and overall survival in non-metastatic clear-cell renal carcinoma: a validation study. World J Urol. (2017) 35:81–7. doi: 10.1007/s00345-016-1854-y
Keywords: metastatic renal cell carcinoma, prognosis, immunohistochemistry, vimentin, immunosuppression
Citation: Yao J, Chen X, Zhu Y, Wang H, Hu X and Guo J (2020) Prognostic Value of Vimentin Is Associated With Immunosuppression in Metastatic Renal Cell Carcinoma. Front. Oncol. 10:1181. doi: 10.3389/fonc.2020.01181
Received: 26 April 2019; Accepted: 10 June 2020;
Published: 04 August 2020.
Edited by:
Ronald M. Bukowski, Cleveland Clinic, United StatesReviewed by:
Kouji Izumi, Kanazawa University, JapanHaris Zahoor, University of Southern California, United States
Sanja Štifter, University of Rijeka, Croatia
Copyright © 2020 Yao, Chen, Zhu, Wang, Hu and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiao yi Hu, aHUueGlhb3lpJiN4MDAwNDA7enMtaG9zcGl0YWwuc2guY24=; Jian ming Guo, Z3VvLmppYW5taW5nJiN4MDAwNDA7enMtaG9zcGl0YWwuc2guY24=
†These authors have contributed equally to this work
 Jia xi Yao
Jia xi Yao Xiang Chen
Xiang Chen Yan jun Zhu
Yan jun Zhu Hang Wang
Hang Wang Jian ming Guo
Jian ming Guo