- 1Department of Radiation Oncology, Sunnybrook Health Sciences Centre, Toronto, ON, Canada
- 2Faculty of Medicine, University of Toronto, Toronto, ON, Canada
Radiotherapy (RT) and chemotherapy continue to be widely utilized in small cell lung cancer (SCLC) management. In most limited stage (LS)-SCLC cases, the standard initial therapy remains concurrent chemoradiotherapy (CRT), typically with an etoposide and platinum-based regimen. Hyperfractionated twice daily (BID) RT remains the standard of care, though conventional daily (QD) RT is now a viable alternative supported by randomized evidence. In LS-SCLC patients who experienced good response to CRT, prophylactic cranial irradiation (PCI) remains the standard of care. Brain imaging, ideally with MRI, should be performed prior to PCI to screen for clinically apparent brain metastases that may require a higher dose of cranial irradiation. Platinum doublet chemotherapy alone is the historic standard initial therapy in extensive stage (ES)-SCLC. Addition of immunotherapy such as atezolizumab and durvalumab to chemotherapy is now recommended after their benefits were demonstrated in recent trials. In patients with response to chemotherapy, consolidation thoracic RT and PCI could be considered, though with caveats. Emergence of hippocampal avoidance cranial irradiation and SRS in SCLC patients may supplant whole cranial irradiation as future standards of care. Incorporation of novel systemic therapies such as immunotherapies has changed the treatment paradigm and overall outlook of patients with SCLC. This narrative review summarizes the current state, ongoing trials, and future directions of radiotherapy in management of SCLC.
Introduction
Small cell lung cancer (SCLC) is an aggressive form of lung cancer that accounts for ~15% of all lung cancer diagnoses, with over 30,000 new cases per year in the United States (1–4). Histologically, it is a high-grade neuroendocrine tumor, appearing under the microscope as small round blue malignant cells that stain positive for chromogranin A, synaptophysin and a high Ki-67 index (5–7). It clinically differentiates itself from the more prevalent non-small cell lung cancer (NSCLC) by having a rapid doubling time and high growth rate, with over 70% of patients being diagnosed with metastatic disease at the time of diagnosis (1, 2, 8). Though SCLC is typically responsive to initial therapy, recurrences are common and the prognosis of SCLC patients remains poor with 5-year overall survival rates of under 8% (1, 2, 8).
SCLC is usually categorized as either limited stage (LS) or extensive stage (ES), according to the Veterans' Affairs Lung Study Group (VALSG) classification (9). LS-SCLC is defined as disease that is confined to the ipsilateral hemithorax and regional lymph nodes that can be safely encompassed by a single radiation field, and ES-SCLC consists of the remainder cases that could not be safely treated with radiotherapy initially (10). More recently, the International Association for the Study of Lung Cancer (IASLC) TNM staging system has been shown to further prognosticate SCLC outcomes beyond LS and ES designations, and its use has been recommended for current clinical decision making and clinical trials (11, 12). The use of radiation therapy for SCLC is continuing to evolve due to advances in imaging and radiation delivery techniques. Controversy still exists over the optimal fractionation schedule for concurrent chemoradiotherapy (CRT) in the treatment of LS-SCLC (3, 7, 13). Stereotactic ablative radiotherapy (SABR) is increasingly considered as an alternative to surgery in node-negative LS-SCLC (7, 14). Additionally, prospective data has resulted in enthusiasm to re-evaluate the role of brain MRI surveillance instead of PCI (7, 15). The wider availability of stereotactic radiosurgery (SRS) for brain metastases also raises questions regarding the most appropriate brain-directed radiation strategy (16). This review aims to summarize the current state of radiotherapy in management of LS and ES-SCLC, as well as ongoing clinical trials and future directions.
Management of Limited Stage-Small Cell Lung Cancer (LS-SCLC)
Early Stage LS-SCLC
In patients with cT1-2N0 SCLC, surgical resection with lobectomy and mediastinal nodal sampling is recommended as the preferred radical therapy as per National Comprehensive Cancer Network (NCCN) (17, 18). In a National Cancer Data Base (NCDB) propensity-matched review of 2301 cT1-2N0 SCLC patients, surgery and adjuvant chemotherapy were associated with superior overall survival (OS) than concurrent thoracic chemoradiation (CRT) (5-year OS 47.6 vs. 29.8%, p < 0.01) (19). Adjuvant chemotherapy without thoracic radiotherapy (TRT) can be given to pN0 and select pN1 patients (20, 21), while patients with pN2 disease should receive thoracic CRT similar to patients with more advanced LS-SCLC at clinical staging (18). For medically inoperable cT1-2 N0 patients, concurrent CRT has been the historical standard. Considering the encouraging results in early stage NSCLC, stereotactic ablative radiotherapy (SABR) is increasingly being utilized for well-staged medically inoperable SCLC patients. In another NCDB study, 2107 histologically confirmed cT1-2N0 SCLC patients did not demonstrate any difference in survival when comparing SABR followed by chemotherapy in those who were eligible, compared to CRT (22). A multi-institutional series of SABR for 74 cT1-2N0 SCLC patients yielded 3-year OS, disease-free survival (DFS), and local control (LC) rates of 34.0, 53.2, and 96.1%, respectively (23). The high rates of LC and relatively high DFS in this series demonstrated the SABR as a standard option for medically inoperable early stage SCLC (23).
Sequence and Timing of TRT and Chemotherapy
In more advanced LS-SCLC (clinical Stage II-III), concurrent CRT is the current standard of care (18). Concurrent CRT where RT starts with an early cycle (1st or 2nd) of chemotherapy is more effective compared to delayed-start RT or sequential CRT. A non-significant trend toward better survival with concurrent CRT (45 Gy BID at cycle 1 chemotherapy) was shown compared to sequential chemotherapy followed by TRT in a Japan Clinical Oncology Group trial (24). Early TRT yielded better survival compared to delayed TRT (e.g., at cycle 4 of chemotherapy) in 2 meta-analyses (25, 26).
In the meta-analysis by Fried et al., there was a 2-year OS benefit with early TRT, with relative risk (RR): 1.17 (p = 0.03), and non-significant trend toward better 3-year OS with RR: 1.13 (p = 0.20) when including all seven identified trials. Subset analysis showed the survival benefit was demonstrated in the five trials using platinum-based chemotherapy: 2-year OS RR: 1.30 (p = 0.002) and 3-year OS RR: 1.35 (p = 0.01), but not in the remaining trials that employed non-platinum chemotherapy (25). In a second meta-analysis by De Ruysscher et al., early TRT did not show OS benefit when including all seven identified trials, but showed significant 5-year OS benefit with odds ratio (OR): 0.64 (p = 0.02), and non-significant trend toward better 2-year OS with OR: 0.73 (p = 0.07) when excluding 1 trial using non-platinum chemotherapy (26). Shorter period (<30 days) between the start of any treatment until the end of radiotherapy (SER) was shown to predict better 5-year OS, with decrease of 1.83% was shown for each week of SER extension beyond 30 days (27). An updated meta-analysis of individualized patient data from 9 trials further supported early (i.e., within 9 weeks of chemotherapy initiation) and short TRT, but at the cost of increased acute esophagitis (28).
Optimal Dose and Fractionation
The current standard of care of thoracic CRT dose fractionation was established in the landmark Intergroup 0096 prospective randomized controlled trial (RCT) by Turrisi et al. (29) in 1999, which demonstrated superiority of concurrent hyperfractionated twice daily (BID) TRT (45 Gy/30 fractions BID in 3 weeks) compared to daily (QD) TRT (45 Gy/25 fractions QD in 5 weeks). BID fractionation was associated with an OS benefit (26 vs. 16% at 5 years, p = 0.04), but was associated with increased grade 3 acute esophagitis (27 vs. 11%, p < 0.001) (29). Following this trial, BID fractionation was not adapted universally. Reasons include the inconvenience of BID treatments and increased toxicity (30, 31). In addition, a common criticism of this trial was that the QD TRT arm employed a lower biologically equivalent dose (BED compared to the BID fractionation (32).
Following this era, two RCTs have compared the Turrisi BID fractionation with higher BED QD regimens (Table 1) (33, 34). The CONVERT trial was reported by Faivre-Finn et al. (34) in 2017, the trial randomized 547 LS-SCLC patients with good performance status to Turrisi BID regimen or QD TRT (66 Gy/33 fractions in 6.5 weeks). The trial was designed with a superiority endpoint for the QD regimen (34). There was no difference in OS between treatment arms, with BID regimen showing a trend toward improved OS (median OS 30 vs. 25 months, p = 0.14). Toxicity was similar in both arms (34). The study concluded that BID fractionation should remain the standard of care (32, 34). However, considering the lack of significant survival and toxicity difference between treatment arms, some have argued that the CONVERT QD regimen is a reasonable alternative (35). Considering the lower BED of BID regimen, it is interesting that the CONVERT QD regimen did not improve outcomes. Indeed, as SCLC is both highly proliferative and radiosensitive, malignant repopulation occurs rapidly after each radiation fraction, and therefore may favor shorter time between fractions and a shorter overall RT regimen (32). A CALGB 30610/RTOG 0538 RCT comparing Turrisi regimen against an even higher BED QD regimen (70 Gy/35 fractions in 7 weeks) is ongoing (NCT00632853).
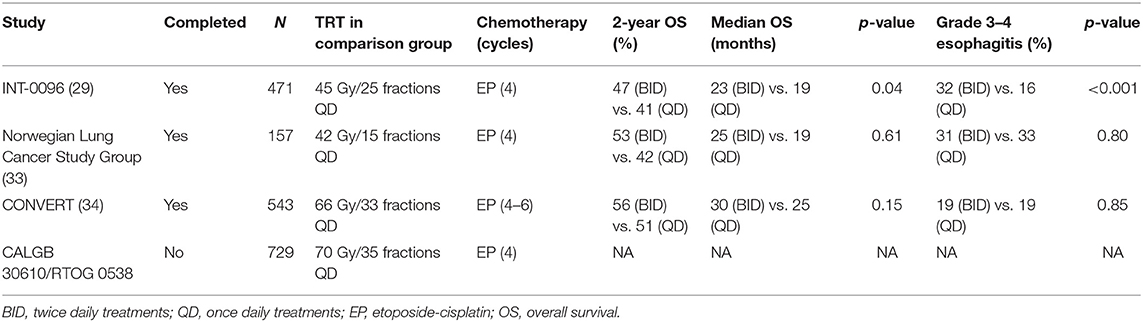
Table 1. Selected trials of chemoradiation for LS-SCLC comparing 45 Gy/30 fractions BID regimen with QD TRT regimens.
Accelerated hypofractionation is a historical option (36) and is still considered a CRT standard option in some parts of the world (37). Recently, a Phase II Scandinavian RCT published by Grønberg et al. in 2015 randomized 157 LS-SCLC patients to either BID (45 Gy/30 fractions BID) or an accelerated hypofractionated QD regimen (42 Gy/15 fractions) (33). The BID fractionation had a numerically higher, though non-significant median OS (25.1 vs. 18.8 months; p = 0.61). Furthermore, BID fractionation was associated with higher rates of complete response (CR) compared to QD (33 vs. 13%; p = 0.003). There were no differences in severe toxicities. The study's conclusion was that the Turrisi BID fractionation should remain the standard of care (33). Nonetheless proponents of the hypofractionated QD schedule, argue that it is a reasonable option if the BID regimen is logistically difficult, and may be preferred in scenarios wherein a shorter overall treatment course is more viable for the patient.
Prophylactic Cranial Irradiation in LS-SCLC
Brain metastases (BM) are the most common mode of distant spread in SCLC, with a reported 2-year incidence of approximately 50% among patients not receiving PCI (38, 39). In the Auperin meta-analysis of 7 trials comparing PCI or no PCI among 987 SCLC patients (86% LS-SCLC) with complete response (CR) following initial therapy (57% CRT, 18% chemotherapy alone, 25% chemotherapy ±TRT), the absolute OS benefit of PCI was estimated to be 5.4% at 3 years. The reduction of BM risk was nearly 2-fold [33.3 vs. 58.6%, relative risk (RR): 0.46] (40). The PCI regimens used in the trials were heterogeneous, ranging from 8 Gy/1 fraction to 40 Gy/20 fractions (40). Although this meta-analysis demonstrated the efficacy of PCI in reducing BM and improving OS (41, 42), reasons to withhold PCI include its negative impact on neurocognition and quality of life (QoL) (43, 44). In an RCT of 720 LS-SCLC with CR following CRT, standard-dose PCI (25 Gy/10 fractions) was compared to high-dose PCI (36 Gy/18 fractions), with the standard dose demonstrating improved 2-year OS (42 vs. 37%, p = 0.05). The higher PCI dose strategy did not reduce the incidence of BM (23 vs. 29% with standard dose at 2 years, p = 0.18) and was also associated with increased neurocognitive toxicity (45).
Given the potential negative effects of PCI on neurocognition, QoL, as well as acute effects such as: nausea, hair loss, and fatigue; there is interest to revisit the role of PCI in LS-SCLC (41). In two series of surgically resected Stage I-III SCLC patients, PCI improved OS for p-Stage II-III, but not p-Stage I patients (39, 46). The lower BM incidence in resected Stage I patients (range 0–15.4%) may explain the purported lack of PCI benefit in this setting (47). These retrospective data suggested that PCI may be omitted in surgically resected p-Stage I SCLC patients, provided that there is brain-directed imaging surveillance (35, 39, 46–48).
The trials in the Auperin meta-analysis were conducted in era prior to the routine use of brain magnetic resonance imaging (MRI) in staging, with CT or clinical neurologic symptoms used to screen for BM prior to PCI (40, 41). Seute et al.'s study demonstrated improved sensitivity of BM detection with MRI (24%, of which 11% were asymptomatic) compared to 10% (all symptomatic) with CT (49). Had patients in the Auperin meta-analysis undergone brain MRI during staging or post-CRT, a proportion may have had BM detected. These patients, therefore, would have received whole brain radiation therapy (WBRT) for undetected, subclinical BM instead of PCI. The use of brain MR surveillance with or without PCI in SCLC patients is the subject of a new Southwest Oncology Group Phase III RCT, MAVERICK (NCT04155034). Based on current data, surgically resected p-Stage I SCLC patients aside, PCI should be offered for all LS-SCLC patients treated with reasonable performance status and no contraindications (e.g., severe cognitive impairment) (47, 48).
Management of Extensive Stage-Small Cell Lung Cancer (ES-SCLC)
Consolidative Thoracic Radiation Therapy
The role of TRT is well-established in the management of LS-SCLC, where the early initiation of TRT concurrently with etoposide-carboplatin (EC) or etoposide-cisplatin (EP) has demonstrated improved local tumor control and survival (18, 50, 51). Historically, RT for ES-SCLC was reserved for palliation in the setting of symptomatic locoregional and/or distant disease. The observation that a large proportion of ES patients had recurrent, persistent and/or progressive intrathoracic disease following initial chemotherapy led to a single-institution phase III RCT investigating consolidative TRT in this population (52). In their pivotal RCT, Jeremic et al. randomized 109 patients [with a CR distantly and at least a partial response (PR) in the thorax following 3 cycles of EP] to either further EP alone or consolidative TRT and EP (52). It should be noted that this patient population was carefully selected, with 90% of patients having only 1–2 sites of extrathoracic metastatic disease prior to initial chemotherapy (52, 53). Consolidative TRT (CTRT) was delivered in combination with EC (using an accelerated twice-daily regimen of 54 Gy in 36 fractions) and all patients received prophylactic cranial irradiation (PCI) to a dose of 25 Gy in 10 fractions (52). The investigators found significant improvements in median OS (17 vs. 11 months, p = 0.041) and a trend toward improved 5-year local relapse free survival (20 vs. 8.1%; p = 0.06) with consolidative TRT (52). Although nearly 1 in four patients (27%) experienced acute grade 3 esophagitis with consolidative TRT, no treatment interruptions were reported, and CTRT was generally well tolerated (52).
Following this RCT, consolidative TRT was not routinely administered following initial chemotherapy, though a few other retrospective and non-randomized prospective studies recapitulated similar findings of a potential benefit as in the Jeremic study (54–57). More recently, the CREST RCT by Slotman et al. randomized 495 ES-SCLC patients with any response to 4–6 cycles of EP to either consolidative TRT (with 30 Gy in 10 fractions) and PCI or PCI alone (58). Although the primary endpoint of 1-year OS was not found to be significantly different between the groups, on secondary analysis, 2-year OS was significantly improved in consolidative TRT patients (13 vs. 3%; p = 0.004) (58). Patients receiving consolidative TRT had a near 50% reduction in intrathoracic progression (43.7 vs. 79.8%; p < 0.0001) with no significant toxic effects reported (58). In fact, only 4 of 247 patients receiving consolidative TRT experienced grade 3 or greater esophagitis, and the only grade 4 toxicity reported was fatigue in a patient enrolled in the control arm (59), Despite the CREST study not meeting its primary endpoint, the authors concluded that consolidative TRT may improve long-term survival and should be considered for ES-SCLC patients who have had any response to initial chemotherapy (58). This “all-or-none” conclusion drew several criticisms, particularly given the trial's negative primary endpoint, unplanned secondary analysis of 2-year OS, and relatively short median follow-up of 24 months (60). Subgroup analyses of the CREST trial suggest that patients with residual intrathoracic disease (a stratification factor at the time of randomization) benefited the most from consolidative TRT, with a statistically significant difference in OS (HR = 0.81, 95% CI 0.66–0.98, p = 0.03) when compared to patients with an intrathoracic CR following chemotherapy (61, 62). In a separate secondary analysis of a subset of CREST patients (89% of whom had intrathoracic residual disease), patients with 2 or fewer metastases had improved OS and progression-free survival (PFS), and the presence of liver and/or bone metastases was a negative prognostic factor for OS (63). These updated analyses suggest that the presence of intrathoracic residual disease, in addition to overall metastatic disease burden, are important factors to consider when identifying ES patients that are most likely to benefit from consolidative TRT (61–63).
Although ES-SCLC generally has a limited prognosis of 8–10 months with chemotherapy alone, Jeremic et al. demonstrated that patients with limited extrathoracic metastatic disease may achieve survival nearing LS-SCLC if PCI and consolidative TRT is delivered (52). This observation, coupled with the finding that disease relapse in patients undergoing multimodality therapy occurs mostly outside of the irradiated brain and thorax, led to the hypothesis that extrathoracic consolidative RT may control limited distant metastases and improve survival (58, 64). To that end, RTOG 0937 was a phase II trial that randomized oligometastatic ES-SCLC patients to either PCI (25 Gy in 10 fractions) or PCI and consolidative RT to the thorax and metastatic sites (30–45 Gy in 10–15 fractions), following a response to initial chemotherapy (64). Unfortunately, the study crossed the futility boundary for the primary endpoint of 1-year OS, and closed after accruing 86 out of a planned 154 patients (64). Recognizing several caveats of a trial that did not complete accrual, as well as imbalances in treatment arms with respect to age, performance status, and disease burden; RTOG 0937 did demonstrate that consolidative RT to residual sites of disease reduced the risk of intrathoracic progression from 83 to 26% (64). Considering that intrathoracic progression in the CREST study was 44% (with 30 Gy in 10 fractions), one interpretation is that higher radiation doses (such as the preferred dose of 45 Gy in 15 fractions used in RTOG 0937) may achieve better local control rates, which may have an effect on survival outcomes. In fact, retrospective series have demonstrated that consolidative TRT doses with a BED with α/β= 10 (BED10)> 50 Gy10 are associated with improved intrathoracic control and OS (65, 66).
To summarize, future prospective studies should aim to further identify patients routinely benefitting from consolidative TRT, as well as the optimal radiation dose, fractionation, and timing for the safest and most effective treatment. Evidence-based guidelines recommend an individualized approach to clinical decision-making, where consolidative TRT is best suited for patients who respond to initial chemotherapy, present with residual intrathoracic disease, and have minimal extrathoracic disease burden (18, 50). In general, 30 Gy in 10 fractions is considered an acceptable and well-tolerated CTRT dose; however, higher doses may be considered in select patients (18).
Prophylactic Cranial Irradiation in ES-SCLC
Although more than 50% of patients with SCLC will eventually develop intracranial metastases, the role of PCI in ES-SCLC is often debated, especially in the present era of MRI imaging (53). Although only a minority of the patients in the previously discussed landmark Auperin meta-analysis had ES disease (140 vs. 847 LS patients), subgroup analysis demonstrated a persistent benefit of PCI regardless of the initial extent of disease in patients with a CR to initial chemotherapy with or without TRT (40).
To assess the role of PCI in ES-SCLC, the EORTC conducted a phase III RCT that randomized 286 patients with any response to initial chemotherapy to either PCI (20 Gy in 5–8; 24 Gy in 12; 25 Gy in 10; or 30 Gy in 10–12 fractions) or no additional therapy (67). Pre-treatment brain imaging was not required and was only performed if symptoms of brain metastases were apparent. PCI was found to significantly reduce the incidence of symptomatic brain metastases (15 vs. 40%) and doubled OS (27 vs. 13%) at 1-year (67). A major critique of the EORTC study, however, was that the absence of pre-treatment imaging may have resulted in the treatment of subclinical intracranial metastases with PCI, leading to the modest improvement in median OS observed (6.7 vs. 5.4 months, p = 0.003). An additional criticism is the use of several different PCI dose/fractionation regimens, which limits the ability to make conclusions regarding optimal radiation delivery. In terms of tolerability, there was no statistically significant difference between global health status between each arm (p = 0.10). Nevertheless, PCI was associated with significantly more fatigue and hair loss, with exploratory analyses demonstrating higher rates of decreased appetite, nausea/vomiting, and leg weakness in those who underwent PCI (p < 0.001) (67). Additionally, as many QoL assessments were of low frequency and/or missing due to the overall deterioration of the patients, the EORTC authors commented that the limited number of QoL assessments may have underpowered the ability to detect any potential significant difference in global health status between arms (67).
A subsequent study performed by Japanese investigators addressed many of the concerns raised following the EORTC study (68). In this, phase III RCT, 224 ES-SCLC patients with any response to initial platinum-doublet chemotherapy (and without evidence of brain metastases on MRI) were randomized to PCI (25 Gy in 10 fractions) or MRI surveillance (every 3 months in year 1, and then every 6 months until 24 months) (68). The study was terminated early following an interim analysis of the first 163 patients that revealed futility of the PCI intervention for the primary endpoint of OS. While PCI was found to decrease the incidence of brain metastases (69–48%; p < 0.001), there was no difference in median OS (11.6 months with PCI and 13.7 months with observation, p = 0.094) (68).
Given the limitations of the EORTC study and the following results of the Japanese trial, a more reserved stance on routine PCI use in ES-SCLC has generally been adopted., Modern surveys of practice patterns indicate that ~50% of radiation oncologists would still offer PCI to ES patients responding to initial chemotherapy (37, 69, 70). Evidence-based guidelines recommend an individualized patient approach, whereby a discussion regarding the potential benefits (e.g., reduced risk for the development of brain metastases) and detriments of PCI (e.g., increased risk of neurocognitive toxicity) should be central to shared clinical decision making (18). In most clinical practices, 25 Gy in 10 fractions appears to be a preferred PCI regimen, with treatment delivered after recovery from initial chemotherapy (18, 50, 51). Higher PCI doses, concurrent chemotherapy, and the treatment of elderly patients and/or those with poor performance status should be avoided given the potential for increased toxicity (18, 37, 45, 70). Hippocampal avoidance and the use of memantine (an NMDA antagonist) have shown promise in reducing neurotoxicity associated with whole-brain RT; though further evidence is required before these techniques become routinely adopted (59, 71, 72). For ES patients undergoing CNS surveillance rather than PCI, it is recommended to perform MRI (preferred) or CT imaging with contrast according to the protocol outlined by Takahashi et al. (68).
Future Directions of Radiotherapy in SCLC
Immunotherapy and Radiotherapy in Small Cell Lung Cancer
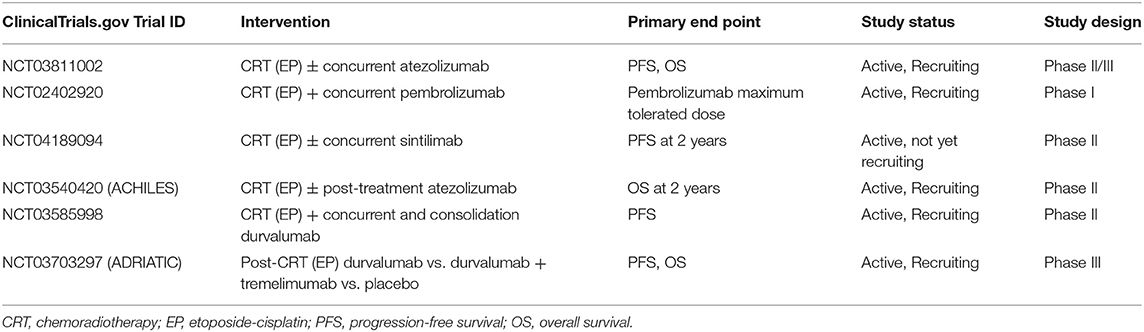
The advances in cancer immunotherapies have resulted in significant outcome improvements in multiple cancers (73–76). Immunotherapy, predominantly immune-check point inhibitors (ICIs) enhance immune-mediated anticancer activity by blocking immune-attenuating interactions of CTLA-4/B7 or PD-1/PD-L1 receptors between T-lymphocytes and cancer cells (77). Several ongoing studies are being conducted to evaluate the addition of immunotherapy, both concurrently and after CRT for LS-SCLC (Table 2). NRG-LU005 is an active phase II/III trial (NCT03811002) examining the use of atezolizumab with CRT and its effects on PFS and OS. Finally, the ADRIATIC trial—an active phase III, randomized, double-blind, placebo controlled multi-center study (NCT03703297)—that investigates durvalumab and tremelimumab in patients without progression following CRT, with PFS and OS as primary outcomes.
For ES-SCLC, various attempts of combining novel therapies with standard chemotherapy including rilotumumab, ganitumab, and ipilimumab failed to show an OS benefit (78–80), IMpower133 was the first major development of combining ICI and chemotherapy in ES-SCLC, demonstrating significant OS and PFS benefits with the addition of atezolizumab (81). There has been increasing interest to investigate immunotherapy and chemotherapy combinations, such as nivolumab in relapsed ES-SCLC (Checkmate 331), nivolumab ± ipilimumab after chemotherapy (Checkmate 451), ipilimumab alone with chemotherapy (NCT01450761), and pembrolizumab in various regimens (Keynote 028, 159, 604, NCT02359019). Similar to IMpower133, the CASPIAN trial was a three-arm RCT, evaluating the addition of durvalumab with or without tremelimumab to EP chemotherapy. Presently, the arm adding durvalumab has been reported, and demonstrated an improved median OS from 10.3 to 13 months and improved 18-month OS from 25 to 34% (p= 0.0047), with no increase in grade 3–4 toxicities (82). Finally, a recently published phase I trial examining pembrolizumab concurrent with the CRT regimen in ES-SCLC demonstrated its safety as a combined regimen, with no grade 4–5 toxicities, and only 6% (n = 2) grade 3 adverse effects (83).
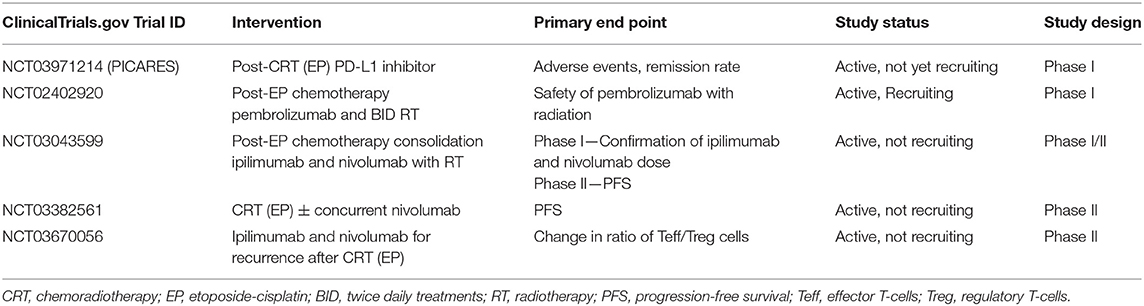
A secondary analysis from IMpower133 also demonstrated safety of palliative thoracic radiotherapy among ES-SCLC patients following chemotherapy and immunotherapy. Several ongoing trials are examining the addition of immunotherapy to CRT in ES-SCLC (Table 3). The PICARES study (NCT03971214) is a prospective pilot trial examining consolidation therapy with PD-L1 inhibitors after CRT. Similarly, another phase I trial (NCT02402920) is currently examining the role of concurrent pembrolizumab with RT. Other ongoing studies include examining CRT with nivolumab (NCT03382561) and nivolumab with ipilimumab (NCT03043599), as well as nivolumab and ipilimumab in recurrent ES-SCLC after CRT (NCT03670056). The results of these studies will provide significant insight into the emerging field of combination immuno-chemoradiotherapy, and will help further delineate benefits, dose, timing, toxicities, and indications/contraindications for its use in ES-SCLC.
Hippocampal Avoidance Cranial RT
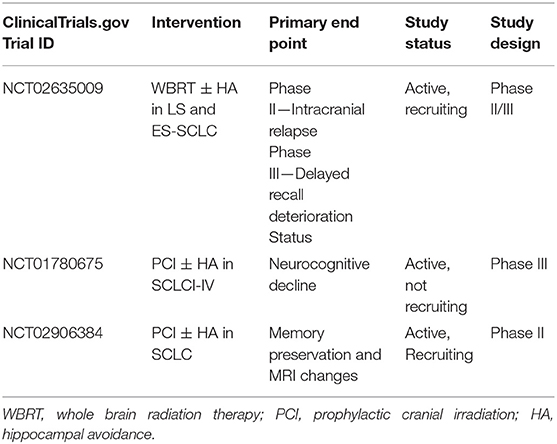
There has been growing interest in hippocampal-sparing technique during cranial RT to reduce its associated acute side effects, neurocognitive toxicity and QoL detriments (Table 4) (84–89). A phase II trial (90) and subsequent study from Redmond et al. demonstrated that conformal avoidance of the hippocampus during WBRT/PCI was associated with improved memory and QoL, with only 10% of patients developing new BM in the underdosed area, which were amenable to stereotactic radiosurgery (SRS) (59, 90). The recently completed phase III PREMER-TRIAL also demonstrated that compared to conventional PCI, hippocampal avoidance PCI improved free delayed recall at 3 months (21.7 vs. 5.1%), 6 months (32.6 vs. 7.3%), and 12 months (18.5 vs. 3.8%) (91). These encouraging results have led to the development of NRG-CC003, an active, randomized phase II/III trial of WBRT with or without hippocampal avoidance in patients with both LS-SCLC and ES-SCLC (NCT02635009). The trial's primary endpoints are 12-month intracranial relapse and 6-month deterioration of the Hopkins Verbal Learning Test-Revised (HVLT-R) delayed recall.
Stereotactic Radiosurgery in SCLC
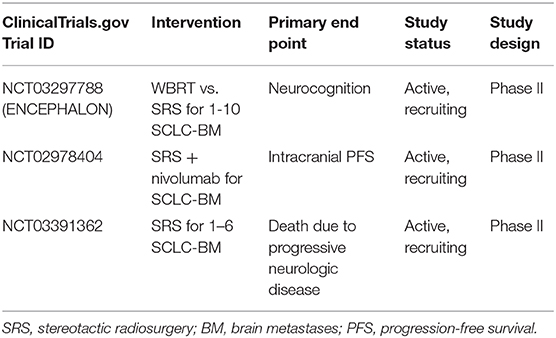
The increasing use of SRS in brain metastases has also provided opportunities to examine its efficacy in SCLC (Table 5). Initial studies demonstrated its efficacy in patients who had previously received PCI/WBRT (41, 92–98), with Rava et al. demonstrating that SRS yields excellent LC (81 and 69% at 6 and 12 months, respectively) for small lesions <2 cm (99). Some studies supported the viability of omitting of PCI/WBRT in favor of active MRI surveillance and SRS as first-line therapy for emerging brain metastases (41, 97, 98, 100, 101). Ozawa et al. demonstrated that MRI surveillance and SRS for BM had an equivalent OS to initial PCI for LS-SCLC (102), while Chang et al. demonstrated that SRS alone without PCI/WBRT is associated with better neurocognition, learning and memory function (84). Nonetheless, as of 2020, the NCCN guidelines do not suggest the use of SRS alone given the high rate of brain metastases in SCLC (18). Rather, SRS is preferred (if feasible) in patients who develop BM after PCI, particularly if there is a prolonged time between PCI and BM occurrence and if extracranial disease is controlled (18). The results of ongoing trials will further inform the role of SRS in SCLC patients. In particular, ENCEPHALON (NCT03297788) is an ongoing phase II trial examining WBRT vs. SRS for SCLC with 1–10 BM. Similarly, NCT03391362 is single arm, phase II trial examining SRS in SCLC pts with 1–6 BM. Investigations of SRS with other therapies are also ongoing, such as the use of SRS and nivolumab (NCT02978404) and SRS with the medical device NovoTTF-200A (NCT03488472). NovoTTF-200A is a battery-operated, portable device that produces changing electrical fields (known as Tumor Treatment Fields) through ceramic disks placed on the head to stop the growth of brain tumor cells, and potentially sensitize tumor cells to immunotherapies. An active ongoing trial is currently examining its use, feasibility, and compliance in ES-SCLC for prevention of BM (NCT03607682).
SCLC continues to be associated with poor prognosis. However, there continues to be promising progress in its multidisciplinary management involving radiotherapy, systemic therapies, medical imaging, and surgery. While randomized data supports the addition of immunotherapy to standard chemotherapy in extensive stage disease, its role in limited stage disease has not yet been established. In addition, novel applications of radiation technologies such as SABR and hippocampal avoidance cranial irradiation hold promise for the radical, palliative, and preventative management of this disease.
Author Contributions
MT: designing and planning of the review, conducting literature review, synthesis of literature findings, writing, and editing manuscript. AL: supervising the project, designing and planning of the review, conducting literature review, synthesis of literature findings, writing, and editing manuscript. HC, GL, JS, and DM: conducting literature review, synthesis of literature findings, writing, and editing manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
AL has received honoraria from RefleXion, Varian Medical Systems Inc. and AstraZeneca.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Govindan R, Page N, Morgensztern D, Read W, Tierney R, Vlahiotis A, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol. (2006) 24:4539–44. doi: 10.1200/JCO.2005.04.4859
2. Yin X, Yan D, Qiu M, Huang L, Yan SX. Prophylactic cranial irradiation in small cell lung cancer: a systematic review and meta-analysis. BMC Cancer. (2019) 19:95. doi: 10.1186/s12885-018-5251-3
3. Zhao H, Ren D, Liu H, Chen J. Comparison and discussion of the treatment guidelines for small cell lung cancer. Thorac Cancer. (2018) 9:769–74. doi: 10.1111/1759-7714.12765
4. Oronsky B, Reid TR, Oronsky A, Carter CA. What's new in SCLC? A review. Neoplasia. (2017) 19:842–7. doi: 10.1016/j.neo.2017.07.007
5. Pelosi G, Rindi G, Travis WD, Papotti M. Ki-67 antigen in lung neuroendocrine tumors: unraveling a role in clinical practice. J Thorac Oncol. (2014) 9:273–84. doi: 10.1097/JTO.0000000000000092
6. Taneja TK, Sharma SK. Markers of small cell lung cancer. World J Surg Oncol. (2004) 2:1–5. doi: 10.1186/1477-7819-2-1
7. Kalemkerian GP, Loo BW, Akerley W, Attia A, Bassetti M, Boumber Y, et al. NCCN guidelines® insights: small cell lung cancer, version 2.2018 featured updates to the NCCN guidelines. JNCCN J Natl Compr Cancer Netw. (2018) 16:1171–82. doi: 10.6004/jnccn.2018.0079
8. National Cancer Institute. SEER* Explorer: An Interactive Website for SEER Cancer Statistics. Surveill Res Progr. (2020).
9. Kalemkerian GP. Staging and imaging of small cell lung cancer. Cancer Imaging. (2011) 11:253–8. doi: 10.1102/1470-7330.2011.0036
10. Nosaki K, Seto T. The role of radiotherapy in the treatment of small-cell lung cancer. Curr Treat Options Oncol. (2015) 16:e87–94. doi: 10.1007/s11864-015-0372-2
11. Shepherd FA, Crowley J, Van Houtte P, Postmus PE, Carney D, Chansky K, et al. The international association for the study of lung cancer lung cancer staging project: proposals regarding the clinical staging of small cell lung cancer in the forthcoming (seventh) edition of the tumor, node, metastasis classification for lung cancer. J Thorac Oncol. (2007) 2:1067–77. doi: 10.1097/JTO.0b013e31815bdc0d
12. Vallières E, Shepherd FA, Crowley J, Van Houtte P, Postmus PE, Carney D, et al. The IASLC lung cancer staging project: proposals regarding the relevance of TNM in the pathologic staging of small cell lung cancer in the forthcoming (seventh) edition of the TNM classification for lung cancer. J Thorac Oncol. (2009) 4:1049–59. doi: 10.1097/JTO.0b013e3181b27799
13. Zhao S, Zhou T, Ma S, Zhao Y, Zhan J, Fang W, et al. Effects of thoracic radiotherapy timing and duration on progression-free survival in limited-stage small cell lung cancer. Cancer Med. (2018) 7:4208–16. doi: 10.1002/cam4.1616
14. Rathod S, Koul R, Bashir B, Chowdhury A, Dubey A. Role of stereotactic body radiation therapy in early stage small cell lung cancer in the era of lung cancer screening. Am J Clin Oncol Cancer Clin Trials. (2019) 42:123–30. doi: 10.1097/COC.0000000000000489
15. Rusthoven CG. Small cell lung cancer: pci uncertainty and emerging radiosurgery interest. Int J Radiat Oncol Biol Phys. (2019) 103:1034–5. doi: 10.1016/j.ijrobp.2018.12.036
16. Jiang W, Haque W, Verma V, Butler B, Teh BS. Stereotactic radiosurgery for brain metastases from newly diagnosed small cell lung cancer: practice patterns and outcomes. Acta Oncol. (2019) 58:491–8. doi: 10.1080/0284186X.2018.1562207
17. Combs SE, Hancock JG, Boffa DJ, Decker RH, Detterbeck FC, Kim AW. Bolstering the case for lobectomy in stages I, II, and IIIA small-cell lung cancer using the national cancer data base. J Thorac Oncol. (2015) 10:316–23. doi: 10.1097/JTO.0000000000000402
18. NCCN.org. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Small Cell Lung Cancer Version 2.2020 (2020).
19. Yang CFJ, Chan DY, Shah SA, Yerokun BA, Wang XF, D'Amico TA, et al. Long-term survival after surgery compared with concurrent chemoradiation for node-negative small cell lung cancer. Ann Surg. (2018) 268:1105–12. doi: 10.1097/SLA.0000000000002287
20. Yang CFJ, Chan DY, Speicher PJ, Gulack BC, Wang X, Hartwig MG, et al. Role of adjuvant therapy in a population-based cohort of patients with early-stage small-cell lung cancer. J Clin Oncol. (2016) 34:1057–64. doi: 10.1200/JCO.2015.63.8171
21. Marr AS, Ganti AK. Resected small cell lung cancer-what do we do next? Ann Transl Med. (2016) 4:288. doi: 10.21037/atm.2016.05.41
22. Verma V, Hasan S, Wegner RE, Abel S, Colonias A. Stereotactic ablative radiation therapy versus conventionally fractionated radiation therapy for stage I small cell lung cancer. Radiother Oncol. (2019) 131:145–9. doi: 10.1016/j.radonc.2018.12.006
23. Verma V, Simone CB, Allen PK, Gajjar SR, Shah C, Zhen W, et al. Multi-institutional experience of stereotactic ablative radiation therapy for stage i small cell lung cancer. Int J Radiat Oncol. (2017) 97:362–71. doi: 10.1016/j.ijrobp.2016.10.041
24. Takada M, Fukuoka M, Kawahara M, Sugiura T, Yokoyama A, Yokota S, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with cisplatin and etoposide for limited-stage small-cell lung cancer: results of the Japan Clinical Oncology Group Study 9104. J Clin Oncol. (2002) 20:3054–60. doi: 10.1200/JCO.2002.12.071
25. Fried DB, Morris DE, Poole C, Rosenman JG, Halle JS, Detterbeck FC, et al. Systematic review evaluating the timing of thoracic radiation therapy in combined modality therapy for limited-stage small-cell lung cancer. J Clin Oncol. (2004) 22:4785–93. doi: 10.1200/JCO.2004.01.178
26. De Ruysscher D, Pijls-Johannesma M, Vansteenkiste J, Kester A, Rutten I, Lambin P. Systematic review and meta-analysis of randomised, controlled trials of the timing of chest radiotherapy in patients with limited-stage, small-cell lung cancer. Ann Oncol. (2006) 17:543–52. doi: 10.1093/annonc/mdj094
27. De Ruysscher D, Pijls-Johannesma M, Bentzen SM, Minken A, Wanders R, Lutgens L, et al. Time between the first day of chemotherapy and the last day of chest radiation is the most important predictor of survival in limited-disease small-cell lung cancer. J Clin Oncol. (2006) 24:1057–63. doi: 10.1200/JCO.2005.02.9793
28. De Ruysscher D, Lueza B, Le Péchoux C, Johnson DH, O'Brien M, Murray N, et al. Impact of thoracic radiotherapy timing in limited-stage small-cell lung cancer: usefulness of the individual patient data meta-analysis. Ann Oncol. (2016) 27:1818–28. doi: 10.1093/annonc/mdw263
29. Turrisi AT, Kim K, Blum R, Sause WT, Livingston RB, Komaki R, et al. Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med. (1999) 340:265–71. doi: 10.1056/NEJM199901283400403
30. Glatzer M, Schmid S, Radovic M, Früh M, Putora PM. The role of radiation therapy in the management of small cell lung cancer. Breathe. (2017) 13:e87–e94. doi: 10.1183/20734735.009617
31. Komaki R, Khalid N, Langer C, Kong F, Owen J, Crozier C, et al. Penetration of recommended procedures for lung cancer staging and management in the United States over 10 years: a quality research in radiation oncology survey. Int J Radiat Oncol Biol Phys. (2013) 85:1082–9. doi: 10.1016/j.ijrobp.2012.10.016
32. Slotman B. What is the optimal radiotherapy schedule for limited stage small cell lung cancer? Lung Cancer. (2017) 105:52–3. doi: 10.1016/j.lungcan.2017.01.002
33. Grønberg BH, Halvorsen TO, Fløtten Ø, Brustugun OT, Brunsvig PF, Aasebø U, et al. Randomized phase II trial comparing twice daily hyperfractionated with once daily hypofractionated thoracic radiotherapy in limited disease small cell lung cancer. Acta Oncol. (2016) 55:591–7. doi: 10.3109/0284186X.2015.1092584
34. Faivre-Finn C, Snee M, Ashcroft L, Appel W, Barlesi F, Bhatnagar A, et al. Concurrent once-daily versus twice-daily chemoradiotherapy in patients with limited-stage small-cell lung cancer (CONVERT): an open-label, phase 3, randomised, superiority trial. Lancet Oncol. (2017) 18:1116–25. doi: 10.1016/S1470-2045(17)30318-2
35. Simone CB, Bogart JA, Cabrera AR, Megan E, Denunzio NJ, Detterbeck F, et al. Radiation therapy for small cell lung cancer : An ASTRO clinical practice guideline. Pract Radiat Oncol. (2020) 10:158–73. doi: 10.1016/j.prro.2020.02.009
36. Murray N, Coy P, Pater JL, Hodson I, Arnold A, Zee BC, et al. Importance of timing for thoracic irradiation in the combined modality treatment of limited-stage small-cell lung cancer. The National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. (1993) 11:336–44. doi: 10.1200/JCO.1993.11.2.336
37. Shahi J, Wright JR, Gabos Z, Swaminath A. Management of small-cell lung cancer with radiotherapy-a pan-Canadian survey of radiation oncologists. Curr Oncol. (2016) 23:184–95. doi: 10.3747/co.23.3023
38. Komaki R, Cox JD, Whitson W. Risk of brain metastasis from small cell carcinoma of the lung related to length of survival and prophylactic irradiation. Cancer Treat Rep. (1981) 65:811–4.
39. Zhu H, Guo H, Shi F, Zhu K, Luo J, Liu X, et al. Prophylactic cranial irradiation improved the overall survival of patients with surgically resected small cell lung cancer, but not for stage I disease. Lung Cancer. (2014) 86:334–8. doi: 10.1016/j.lungcan.2014.09.019
40. Aupérin A, Arriagada R, Pignon J, Le Péchoux. C, Gregor A, Stephens R, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. N Engl J Med. (1999) 341:476–84. doi: 10.1056/NEJM199908123410703
41. Rusthoven CG, Kavanagh BD. Prophylactic cranial irradiation (PCI) versus active MRI surveillance for small cell lung cancer: the case for equipoise. J Thorac Oncol. (2017) 12:1746–54. doi: 10.1016/j.jtho.2017.08.016
42. Rodriguez de Dios N, Calvo P, Rico M, Martín M, Couñago F, Sotoca A, et al. Recent developments in radiotherapy for small-cell lung cancer: a review by the Oncologic Group for the Study of Lung Cancer (Spanish Radiation Oncology Society). Clin Transl Oncol. (2017) 19:1183–92. doi: 10.1007/s12094-017-1667-5
43. Le Péchoux C, Laplanche A, Faivre-Finn C, Ciuleanu T, Wanders R, Lerouge D, et al. Clinical neurological outcome and quality of life among patients with limited small-cell cancer treated with two different doses of prophylactic cranial irradiation in the intergroup phase III trial (PCI99-01, EORTC 22003-08004, RTOG 0212 and IFCT 99-01). Ann Oncol. (2011) 22:1154–63. doi: 10.1093/annonc/mdq576
44. Lok BH, Ma J, Foster A, Perez CA, Shi W, Zhang Z, et al. Factors influencing the utilization of prophylactic cranial irradiation in patients with limited-stage small cell lung cancer. Adv Radiat Oncol. (2017) 2:548–54. doi: 10.1016/j.adro.2017.08.001
45. Le Péchoux C, Dunant A, Senan S, Wolfson A, Quoix E, Faivre-Finn C, et al. Standard-dose versus higher-dose prophylactic cranial irradiation (PCI) in patients with limited-stage small-cell lung cancer in complete remission after chemotherapy and thoracic radiotherapy (PCI 99-01, EORTC 22003-08004, RTOG 0212, and IFCT 99-01): a randomised clinical trial. Lancet Oncol. (2009) 10:467–74. doi: 10.1016/S1470-2045(09)70101-9
46. Xu J, Yang H, Fu X, Jin B, Lou Y, Zhang Y, et al. Prophylactic cranial irradiation for patients with surgically resected small cell lung cancer. J Thorac Oncol. (2017) 12:347–53. doi: 10.1016/j.jtho.2016.09.133
47. Eze C, Roengvoraphoj O, Manapov F. Prophylactic cranial irradiation in resected early-stage small cell lung cancer. Int J Radiat Oncol Biol Phys. (2017) 98:612–4. doi: 10.1016/j.ijrobp.2017.03.002
48. Le Péchoux C, Sun A, Slotman BJ, De Ruysscher D, Belderbos J, Gore EM. Prophylactic cranial irradiation for patients with lung cancer. Lancet Oncol. (2016) 17:e277–93. doi: 10.1016/S1470-2045(16)30065-1
49. Seute T, Leffers P, Ten Velde GPM, Twijnstra A. Detection of brain metastases from small cell lung cancer: consequences of changing imaging techniques (CT versus MRI). Cancer. (2008) 112:1827–34. doi: 10.1002/cncr.23361
50. Jett JR, Schild SE, Kesler KA, Kalemkerian GP. Treatment of small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. (2013) 143:e400S−19S. doi: 10.1378/chest.12-2363
51. Früh M, De Ruysscher D, Popat S, Crinò L, Peters S, Felip E. Small-cell lung cancer (SCLC): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2013) 24:vi99–105. doi: 10.1093/annonc/mdt178
52. Jeremic B, Shibamoto Y, Nikolic N, Milicic B, Milisavljevic S, Dagovic A, et al. Role of radiation therapy in the combined-modality treatment of patients with extensive disease small-cell lung cancer: a randomized study. J Clin Oncol. (1999) 17:2092–9. doi: 10.1200/JCO.1999.17.7.2092
53. Jeremic B, Gomez-Caamano A, Dubinsky P, Cihoric N, Casas F, Filipovic N. Radiation therapy in extensive stage small cell lung cancer. Front Oncol. (2017) 7:169. doi: 10.3389/fonc.2017.00169
54. Ou S-HI, Ziogas A, Zell JA. Prognostic factors for survival in extensive stage small cell lung cancer (ED-SCLC): the importance of smoking history, socioeconomic and marital statuses, and ethnicity. J Thorac Oncol. (2009) 4:37–43. doi: 10.1097/JTO.0b013e31819140fb
55. Giuliani ME, Atallah S, Sun A, Bezjak A, Le LW, Brade A, et al. Clinical outcomes of extensive stage small cell lung carcinoma patients treated with consolidative thoracic radiotherapy. Clin Lung Cancer. (2011) 12:375–9. doi: 10.1016/j.cllc.2011.03.028
56. Zhu H, Zhou Z, Wang Y, Bi N, Feng Q, Li J, et al. Thoracic radiation therapy improves the overall survival of patients with extensive-stage small cell lung cancer with distant metastasis. Cancer. (2011) 117:5423–431. doi: 10.1002/cncr.26206
57. Yee D, Butts C, Reiman A, Joy A, Smylie M, Fenton D, et al. Clinical trial of post-chemotherapy consolidation thoracic radiotherapy for extensive-stage small cell lung cancer. Radiother Oncol. (2012) 102:234–8. doi: 10.1016/j.radonc.2011.08.042
58. Slotman BJ, Van Tinteren H, Praag JO, Knegjens JL, El Sharouni SY, Hatton M, et al. Use of thoracic radiotherapy for extensive stage small-cell lung cancer: a phase 3 randomised controlled trial. Lancet. (2015) 385:36–42. doi: 10.1016/S0140-6736(14)61085-0
59. Redmond KJ, Hales RK, Anderson-Keightly H, Zhou XC, Kummerlowe M, Sair HI, et al. Prospective study of hippocampal-sparing prophylactic cranial irradiation in limited-stage small cell lung cancer. Int J Radiat Oncol. (2017) 98:603–11. doi: 10.1016/j.ijrobp.2017.03.009
60. Corkum MT, Rodrigues GB. Patient selection for thoracic radiotherapy in extensive-stage small-cell lung cancer. Lung Cancer Manag. (2017) 6:47–53. doi: 10.2217/lmt-2017-0006
61. Slotman BJ, van Tinteren H. Which patients with extensive stage small-cell lung cancer should and should not receive thoracic radiotherapy? Transl lung cancer Res. (2015) 4:292–4. doi: 10.3978/j.issn.2218-6751.2015.04.07
62. Slotman BJ, van Tinteren H, Praag JO, Knegjens JL, Sharouni SY El, Hatton M, et al. Radiotherapy for extensive stage small-cell lung cancer – Authors' reply. Lancet. (2015) 385:1292–3. doi: 10.1016/S0140-6736(15)60679-1
63. Slotman BJ, Faivre-Finn C, van Tinteren H, Keijser A, Praag J, Knegjens J, et al. Which patients with ES-SCLC are most likely to benefit from more aggressive radiotherapy: a secondary analysis of the Phase III CREST trial. Lung Cancer. (2017) 108:150–3. doi: 10.1016/j.lungcan.2017.03.007
64. Gore EM, Hu C, Sun AY, Grimm DF, Ramalingam SS, Dunlap NE, et al. Randomized phase II study comparing prophylactic cranial irradiation alone to prophylactic cranial irradiation and consolidative extracranial irradiation for extensive-disease small cell lung cancer (ED SCLC): NRG oncology RTOG 0937. J Thorac Oncol. (2017) 12:1561–70. doi: 10.1016/j.jtho.2017.06.015
65. Yoon HG, Noh JM, Ahn YC, Oh D, Pyo H, Kim H. Higher thoracic radiation dose is beneficial in patients with extensive small cell lung cancer. Radiat Oncol J. (2019) 37:185–92. doi: 10.3857/roj.2019.00192
66. Li-Ming X, Zhao L-J, Simone CB, Cheng C, Kang M, Wang X, et al. Receipt of thoracic radiation therapy and radiotherapy dose are correlated with outcomes in a retrospective study of three hundred and six patients with extensive stage small-cell lung cancer. Radiother Oncol. (2017) 125:331–7. doi: 10.1016/j.radonc.2017.10.005
67. Slotman B, Faivre-Finn C, Kramer G, Rankin E, Snee M, Hatton M, et al. Prophylactic cranial irradiation in extensive small-cell lung cancer. N Engl J Med. (2007) 357:664–72. doi: 10.1056/NEJMoa071780
68. Takahashi T, Yamanaka T, Seto T, Harada H, Nokihara H, Saka H, et al. Prophylactic cranial irradiation versus observation in patients with extensive-disease small-cell lung cancer: a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. (2017) 18:663–71. doi: 10.1016/S1470-2045(17)30230-9
69. Gjyshi O, Ludmir EB, Pezzi TA, Boyce-Fappiano D, Dursteler AE, Mitin T, et al. Evolving practice patterns in the use of prophylactic cranial irradiation for extensive-stage small cell lung cancer. JAMA Netw open. (2019) 2:e199135. doi: 10.1001/jamanetworkopen.2019.9135
70. Wolfson AH, Bae K, Komaki R, Meyers C, Movsas B, Le Pechoux C, et al. Primary analysis of a phase II randomized trial Radiation Therapy Oncology Group (RTOG) 0212: impact of different total doses and schedules of prophylactic cranial irradiation on chronic neurotoxicity and quality of life for patients with limited-disease small-cell lung cancer. Int J Radiat Oncol Biol Phys. (2011) 81:77–84. doi: 10.1016/j.ijrobp.2010.05.013
71. Brown PD, Pugh S, Laack NN, Wefel JS, Khuntia D, Meyers C, et al. Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: a randomized, double-blind, placebo-controlled trial. Neuro Oncol. (2013) 15:1429–37. doi: 10.1093/neuonc/not114
72. Kundapur V, Ellchuk T, Ahmed S, Gondi V. Risk of hippocampal metastases in small cell lung cancer patients at presentation and after cranial irradiation: a safety profile study for hippocampal sparing during prophylactic or therapeutic cranial irradiation. Int J Radiat Oncol. (2015) 91:781–6. doi: 10.1016/j.ijrobp.2014.12.026
73. Motzer RJ, Penkov K, Haanen J, Rini B, Albiges L, Campbell MT, et al. Avelumab plus Axitinib versus Sunitinib for advanced renal-cell carcinoma. N Engl J Med. (2019) 380:1103–15. doi: 10.1056/NEJMoa1816047
74. Burtness B, Harrington KJ, Greil R, Soulières D, Tahara M, de Castro G, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. (2019) 394:1915–28. doi: 10.1016/S0140-6736(19)32591-7
75. Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Lao CD, et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. (2019) 381:1535–46. doi: 10.1056/NEJMoa1910836
76. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab after chemoradiotherapy in stage III non–small-cell lung cancer. N Engl J Med. (2017) 377:1919–29. doi: 10.1056/NEJMoa1709937
77. Buchbinder EI, Desai A. CTLA-4 and PD-1 pathways similarities, differences, and implications of their inhibition. Am J Clin Oncol Cancer Clin Trials. (2016) 39:98–106. doi: 10.1097/COC.0000000000000239
78. Glisson B, Besse B, Dols MC, Dubey S, Schupp M, Jain R, et al. A randomized, placebo-controlled, phase 1b/2 study of rilotumumab or ganitumab in combination with platinum-based chemotherapy as first-line treatment for extensive-stage small-cell lung cancer. Clinical Lung Cancer. (2017) 18:615–25.e8. doi: 10.1016/j.cllc.2017.05.007
79. Arriola E, Wheater M, Galea I, Cross N, Maishman T, Hamid D, et al. Outcome and biomarker analysis from a multicenter phase 2 study of ipilimumab in combination with carboplatin and etoposide as first-line therapy for extensive-stage SCLC. J Thorac Oncol. (2016) 11:1511–21. doi: 10.1016/j.jtho.2016.05.028
80. Reck M, Luft A, Szczesna A, Havel L, Kim SW, Akerley W, et al. Phase III randomized trial of ipilimumab plus etoposide and platinum versus placebo plus etoposide and platinum in extensive-stage small-cell lung cancer. J Clin Oncol. (2016) 34:3740–8. doi: 10.1200/JCO.2016.67.6601
81. Horn L, Mansfield AS, Szczȩsna A, Havel L, Krzakowski M, Hochmair MJ, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. (2018) 379:2220–9. doi: 10.1056/NEJMoa1809064
82. Paz-Ares L, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, et al. Durvalumab plus platinum–etoposide versus platinum–etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet. (2019) 394:1929–39. doi: 10.1016/S0140-6736(19)32222-6
83. Welsh JW, Heymach JV, Chen D, Verma V, Cushman TR, Hess KR, et al. Phase I trial of pembrolizumab and radiation therapy after induction chemotherapy for extensive-stage small cell lung cancer. J Thorac Oncol. (2020) 15:266–73. doi: 10.1016/j.jtho.2019.10.001
84. Chang EL, Wefel JS, Hess KR, Allen PK, Lang FF, Kornguth DG, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. (2009) 10:1037–44. doi: 10.1016/S1470-2045(09)70263-3
85. DeAngelis LM, Delattre JY, Posner JB. Radiation-induced dementia in patients cured of brain metastases. Neurology. (1989) 39:789–96. doi: 10.1212/WNL.39.6.789
86. Welzel G, Fleckenstein K, Schaefer J, Hermann B, Kraus-Tiefenbacher U, Mai SK, et al. Memory function before and after whole brain radiotherapy in patients with and without brain metastases. Int J Radiat Oncol Biol Phys. (2008) 72:1311–8. doi: 10.1016/j.ijrobp.2008.03.009
87. Fisher BJ, Sieferheld W, Schultz C, DeAngelis L, Nelson D, Schold SC, et al. Secondary analysis of RTOG 9310: an intergroup phase II combined modality treatment of primary central nervous system lymphoma with chemotherapy and hyperfractionated radiotherapy. Int J Radiat Oncol. (2001) 51:166. doi: 10.1016/S0360-3016(01)02125-3
88. Gavrilovic IT, Hormigo A, Yahalom J, DeAngelis LM, Abrey LE. Long-term follow-up of high-dose methotrexate-based therapy with and without whole brain irradiation for newly diagnosed primary CNS lymphoma. J Clin Oncol. (2006) 24:4570–4. doi: 10.1200/JCO.2006.06.6910
89. Li J, Bentzen SM, Li J, Renschler M, Mehta MP. Relationship between neurocognitive function and quality of life after whole-brain radiotherapy in patients with brain metastasis. Int J Radiat Oncol Biol Phys. (2008) 71:64–70. doi: 10.1016/j.ijrobp.2007.09.059
90. Gondi V, Pugh SL, Tome WA, Caine C, Corn B, Kanner A, et al. Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): a phase II multi-institutional trial. J Clin Oncol. (2014) 32:3810–6. doi: 10.1200/JCO.2014.57.2909
91. De Dios NR, Murcia M, Counago F, Lopez J, Oses MR, Ots PMS, et al. Phase III trial of prophylactic cranial irradiation with or without hippocampal avoidance for small-cell lung cancer. Int J Radiat Oncol. (2019) 105:S35–6. doi: 10.1016/j.ijrobp.2019.06.451
92. Bernhardt D, Bozorgmehr F, Adeberg S, Opfermann N, von Eiff D, Rieber J, et al. Outcome in patients with small cell lung cancer re-irradiated for brain metastases after prior prophylactic cranial irradiation. Lung Cancer. (2016) 101:76–81. doi: 10.1016/j.lungcan.2016.09.010
93. Harris S, Chan MD, Lovato JF, Ellis TL, Tatter SB, Bourland JD, et al. Gamma knife stereotactic radiosurgery as salvage therapy after failure of whole-brain radiotherapy in patients with small-cell lung cancer. Int J Radiat Oncol Biol Phys. (2012) 83:e53–9. doi: 10.1016/j.ijrobp.2011.11.059
94. Nakazaki K, Higuchi Y, Nagano O, Serizawa T. Efficacy and limitations of salvage gamma knife radiosurgery for brain metastases of small-cell lung cancer after whole-brain radiotherapy. Acta Neurochir. (2013) 155:107–13. doi: 10.1007/s00701-012-1520-0
95. Olson AC, Wegner RE, Rwigema JCM, Heron DE, Burton SA, Mintz AH. Clinical outcomes of reirradiation of brain metastases from small cell lung cancer with Cyberknife stereotactic radiosurgery. J Cancer Res Ther. (2012) 8:411–6. doi: 10.4103/0973-1482.103522
96. Sheehan J, Kondziolka D, Flickinger J, Lunsford LD. Radiosurgery for patients with recurrent small cell lung carcinoma metastatic to the brain: outcomes and prognostic factors. J Neurosurg. (2005) 102:247–54. doi: 10.3171/sup.2005.102.s_supplement.0247
97. Wegner RE, Olson AC, Kondziolka D, Niranjan A, Lundsford LD, Flickinger JC. Stereotactic radiosurgery for patients with brain metastases from small cell lung cancer. Int J Radiat Oncol Biol Phys. (2011) 81:e21–7. doi: 10.1016/j.ijrobp.2011.01.001
98. Yomo S, Hayashi M. Is stereotactic radiosurgery a rational treatment option for brain metastases from small cell lung cancer? A retrospective analysis of 70 consecutive patients. BMC Cancer. (2015) 15:95. doi: 10.1186/s12885-015-1103-6
99. Rava P, Sioshansi S, DiPetrillo T, Cosgrove R, Melhus C, Wu J, et al. Local recurrence and survival following stereotactic radiosurgery for brain metastases from small cell lung cancer. Pract Radiat Oncol. (2015) 5:e37–44. doi: 10.1016/j.prro.2014.03.006
100. Jo KW, Kong DS, Lim DH, Ahn YC, Nam DH, Lee J-Il. The role of radiosurgery in patients with brain metastasis from small cell lung carcinoma. J Korean Neurosurg Soc. (2011) 50:99–102. doi: 10.3340/jkns.2011.50.2.99
101. Yomo S, Hayashi M. Upfront stereotactic radiosurgery in patients with brain metastases from small cell lung cancer: retrospective analysis of 41 patients. Radiat Oncol. (2014) 9:152. doi: 10.1186/1748-717X-9-152
102. Ozawa Y, Omae M, Fujii M, Matsui T, Kato M, Sagisaka S, et al. Management of brain metastasis with magnetic resonance imaging and stereotactic irradiation attenuated benefits of prophylactic cranial irradiation in patients with limited-stage small cell lung cancer. BMC Cancer. (2015) 15:589. doi: 10.1186/s12885-015-1593-2
Keywords: small cell lung cancer (SCLC), Radiotherapy—Chemotherapy, review (article), immunotherapy, stereotactic ablative body radiation, radiotherapy—adverse effects
Citation: Tjong MC, Mak DY, Shahi J, Li GJ, Chen H and Louie AV (2020) Current Management and Progress in Radiotherapy for Small Cell Lung Cancer. Front. Oncol. 10:1146. doi: 10.3389/fonc.2020.01146
Received: 26 March 2020; Accepted: 08 June 2020;
Published: 14 July 2020.
Edited by:
Lizza E. L. Hendriks, Maastricht University Medical Centre, NetherlandsReviewed by:
David Ball, Peter MacCallum Cancer Centre, AustraliaMatteo Giaj Levra, Centre Hospitalier Universitaire de Grenoble, France
Copyright © 2020 Tjong, Mak, Shahi, Li, Chen and Louie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alexander V. Louie, YWxleGFuZGVyLmxvdWllQHN1bm55YnJvb2suY2E=
 Michael C. Tjong
Michael C. Tjong David Y. Mak
David Y. Mak Jeevin Shahi
Jeevin Shahi George J. Li2
George J. Li2 Alexander V. Louie
Alexander V. Louie