- 1Department of General Surgery, Shanghai Jiao Tong University Affiliated Sixth People's Hospital, Shanghai, China
- 2Guangdong Provincial Key Laboratory of Colorectal and Pelvic Floor Diseases, Department of Colorectal Surgery, Guangdong Institute of Gastroenterology, The Sixth Affiliated Hospital, Sun Yat-sen University, Guangzhou, China
- 3Department of General Surgery, The Second Affiliated Hospital of Harbin Medical University, Harbin, China
Background: Carcinoembryonic antigen (CEA) is the most common serum tumor marker in colorectal cancer (CRC). Nevertheless, few previous studies demonstrated the impacts of postoperative CEA and post-preoperative CEA increment on prognosis of CRC.
Methods: Patients with stage II and III CRC were included from January 2009 to December 2015. All clinical and follow-up data were collected. Patients were divided into four different groups according to the levels of postoperative serum CEA and post-preoperative CEA trends. Chi-square test was used to analyze the relationship between clinical variables and categorized postoperative CEA and CEA increment. Cox proportional hazard regression was used for univariate and multivariable analyses. The log-rank test was performed to compare PFS and OS among groups.
Results: Patients, 1,008, who underwent radical surgery, were enrolled. Our results showed that positive postoperative CEA and CEA increment were related to clinical stage, T stage, N stage, tumor differentiation, and lymphatic invasion (p < 0.05). Univariate and multivariable analysis results suggested that positive postoperative CEA and CEA increment were independent prognostic factors for PFS (HR = 3.149, 95% CI, 2.426–4.088, p = 0.000 for postoperative CEA; HR = 2.708, 95% CI, 2.106–3.482, p = 0.000 for CEA increment) and OS (HR = 3.414, 95% CI, 2.549–4.574, p = 0.000 for postoperative CEA; HR = 2.373, 95% CI, 1.783–3.157, p = 0.000 for CEA increment). The survival analyses revealed positive postoperative CEA, and CEA increment predicted worse prognosis. Furthermore, our results indicated that the 3- and 5-year PFS rates were 86.6 and 78.4% in group A, but decreased to 25.3 and 7.2% in group D (p < 0.001). Similarly, the 3- and 5-year OS rates for group A were 92.5 and 83.9%, much higher than group D (p < 0.001). In other words, patients with both postoperative CEA elevation and CEA increment had the worst prognosis.
Conclusions: Positive postoperative CEA and CEA increment were independent prognostic factors for stage II and III CRC. Additionally, postoperative CEA and CEA increment had significant impacts on PFS and OS of CRC.
Introduction
Colorectal cancer (CRC) is one of the most commonly diagnosed cancers worldwide with high morbidity and mortality rates (1). In recent years, although the treatment of colorectal cancer has been greatly developed, 5-year survival rate is only 67% for patients with rectal cancer, slightly higher than 64% with colon cancer (2). In China, incidence rate of CRC has been increasing year by year from 2000 to 2011 due to westernization of lifestyle (3).
The carcinoembryonic antigen (CEA) is mainly secreted by solid tumors. In CRC, CEA has always been recommended as a reliable tumor marker by the National Comprehensive Cancer Network (NCCN) and the American Society of Clinical Oncology (4). CEA plays an important role in diagnosis, postoperative recurrence, and metastasis, and the effect of chemotherapy of CRC (5–7). High levels of preoperative serum CEA always indicate worse prognosis and shorter progression-free survival time (PFS) in CRC (8, 9). Besides, postoperative CEA level is an independent prognosis index for CRC and its positivity reflects the probability of liver metastasis after surgery (10, 11). Increased postoperative CEA level at short intervals indicates the possibility of CRC recurrence and suggests that patients should be followed up more frequently (12). For metastasis CRC (mCRC), baseline level of CEA predicts the efficacy of some chemotherapy drugs and provides different information of overall survival time (13, 14). Several studies have also elucidated the effect of post/preoperative CEA ratio on the treatment of CRC, and post/preoperative CEA ratio <1 reveals a better prognosis than CEA ratio >1 for CRC (15, 16).
Generally speaking, the value of CEA in prognosis of CRC has well been demonstrated. However, few studies have systematically analyzed the significances of postoperative CEA level and post-preoperative CEA increment for the prognosis of stage II and III CRC after radical resection. Therefore, we conducted this multicenter retrospective clinical trial to analyze the importance of postoperative CEA and post-preoperative CEA increment in survival of CRC patients.
Methods
Data Collection
This study was a multicenter retrospective clinical study, and it was registered in the Chinese Clinical Trial Registry (Approval No. ChiCTR1800016906). Our study was also approved by the ethics committee of Shanghai Jiao Tong University Affiliated Sixth People's Hospital (Approval No. 2018-KY-031K). All of the patients are from Shanghai Jiao Tong University Affiliated Sixth People's Hospital, the Second Affiliated Hospital of Harbin Medical University, and the Sixth Affiliated Hospital of Sun Yat-sen University. All patients were pathologically diagnosed as stage II and III CRC from January 2009 to December 2015. Written informed consents were obtained from all patients in this study. The criteria for exclusion were as follows: (1) without available postoperative CEA value within 12 weeks after surgery; (2) loss to follow-up; (3) unsuitable pathological type; (4) without available preoperative CEA value.
The clinical characteristics of the patients, including gender, age, CEA value, and pathological reports, were all acquired from electronic patients' records and the departmental database. Pathological reports are detailed description of the surgical excised colorectal tissues, including T stage, N stage, pathological type, tumor differentiation, lymphatic invasion, and vascular invasion. Pathological stage was defined according to the 8th AJCC criterion for CRC. T stage meant the depth of primary tumor infiltration and N stage represented the number and extent of lymph node metastasis. Preoperative CEA value was tested within 1 week before surgery, and postoperative CEA value was gained within 12 weeks after surgery but before medical treatment. The value of CEA > 5 ng/ml is defined as positive (17). Patients were grouped as follows: (Group A) normal postoperative CEA (≤ 5 ng/ml) and without post-preoperative CEA increment; (Group B) normal postoperative CEA and with CEA increment; (Group C) positive postoperative CEA (>5 ng/ml) and without CEA increment; (Group D) positive postoperative CEA and with CEA increment. All patients were followed up according to current guidelines, including serum tumor markers, colonoscopy, chest X-ray, and CT (or MRI). Survival status and recurrence/metastasis status were updated by telephone, email, and medical history. Progression-free survival (PFS) was defined as the time from surgery to cancer metastasis or recurrence. Overall survival (OS) was defined as the time from surgery to death.
Statistical Analysis
All data in this study were analyzed by IBM SPSS STATISTICS 22.0 software and GraphPad Prism 6. Categorical variables were compared using Pearson chi-square test. Survival rates, 3 and 5 years, were assessed by the Kaplan–Meier method, and the log-rank test was used to compare the differences in survival rates among different groups. Cox regression was used to test the effect of various indicators on the prognosis of CRC and estimate hazard ratios (HRs) and 95% CIs. P-values were two-sided, with statistically significant differences at P < 0.05.
Results
Patients' Information
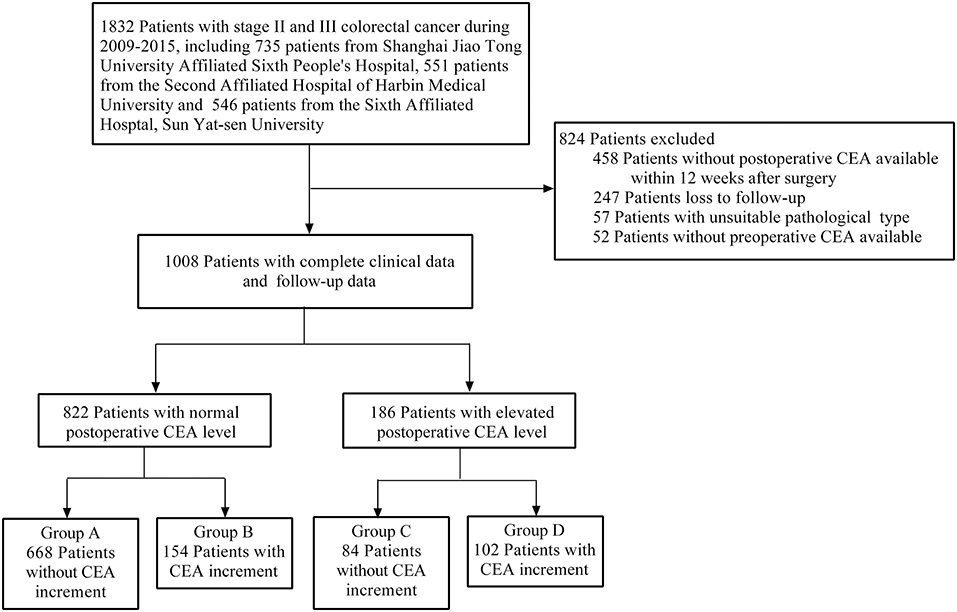
A total of 1,832 patients with stage II and III CRC were enrolled in our study. According to our inclusion and exclusion criteria, 1,008 patients with complete clinical and follow-up data eventually were included. Furthermore, based on our grouped rules, the final number of patients in each group was as follows: (A) 668 patients; (B) 154 patients; (C) 84 patients; (D) 102 patients (Figure 1).
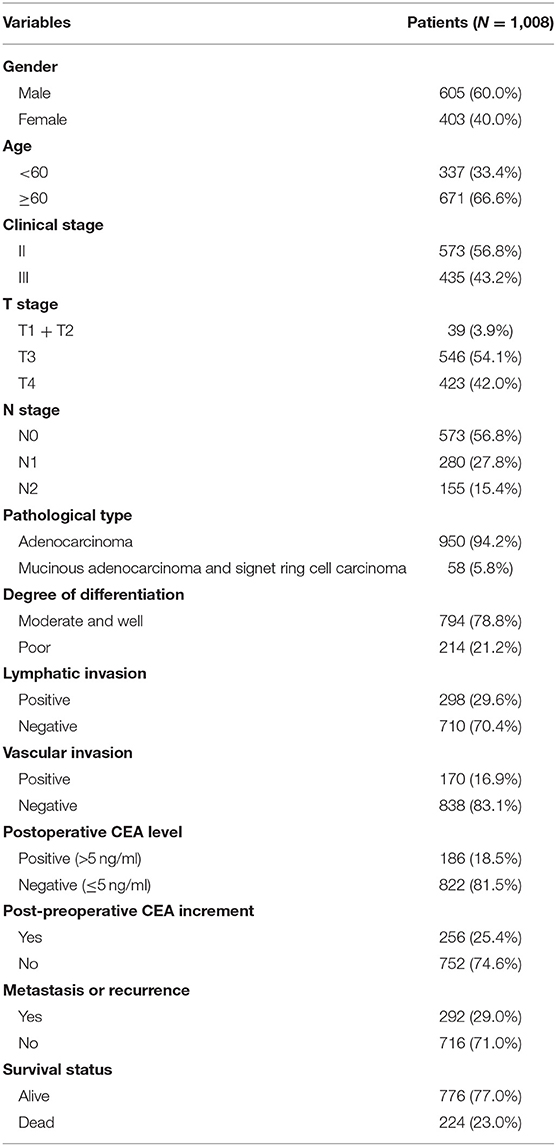
Our study included 605 males (60.0%) and 403 females (40.0%). The age of 671 patients (66.6%) was over 60. Patients, 573 (56.8%), were diagnosed as stage II CRC, slightly more than stage III CRC. Specifically, 546 patients (54.1%) had T3 stage, and 423 patients (42.0%) had T4 stage, while there were only 39 patients with T1 and T2 (3.9%). Patients, 435, had lymph node metastasis, including 280 cases (27.8%) with N1, and 155 cases (15.4%) with N2. The pathological type of 950 patients (94.2%) was adenocarcinoma. CRC tissues, 794 (78.8%), were well or moderately differentiated, while 214 tissues (21.2%) were poorly differentiated. According to our data, lymphatic invasion was observed in 298 patients (29.6%), and vascular invasion was found in 170 patients (16.9%). Only 186 patients (18.5%) had elevated postoperative CEA value even after radical surgery. Post-preoperative CEA increment was found in 256 patients (25.4%). The median follow-up time was 46 months. According to our follow-up data, 292 patients (29.0%) had recurrence or metastasis, and 224 patients (23.0%) died (Table 1).
Positive Postoperative CEA and Post-preoperative CEA Increment Were Associated With Several Clinicopathologic Characteristics
To investigate the correlation of postoperative CEA level and CEA increment with clinical and pathological parameters, we did the chi-square tests to analyze. Our results showed that positive postoperative CEA was related to clinical stage, T stage, N stage, tumor differentiation, lymphatic and vascular invasion (all values of p < 0.05) (Table 2). In contrast, there was no significant difference in gender, age, or pathological type (Table 2). Besides, our data also found that post-preoperative CEA increment was significantly different in terms of clinical stage, T stage, N stage, tumor differentiation, and lymphatic invasion (all values of p < 0.05) (Table 2). However, there was no significant difference in terms of gender, age, pathological type, and vascular invasion (Table 2). In addition, the high levels of postoperative CEA and CEA increment suggested recurrence or metastasis and poor prognosis of CRC (p < 0.05) (Table 2).
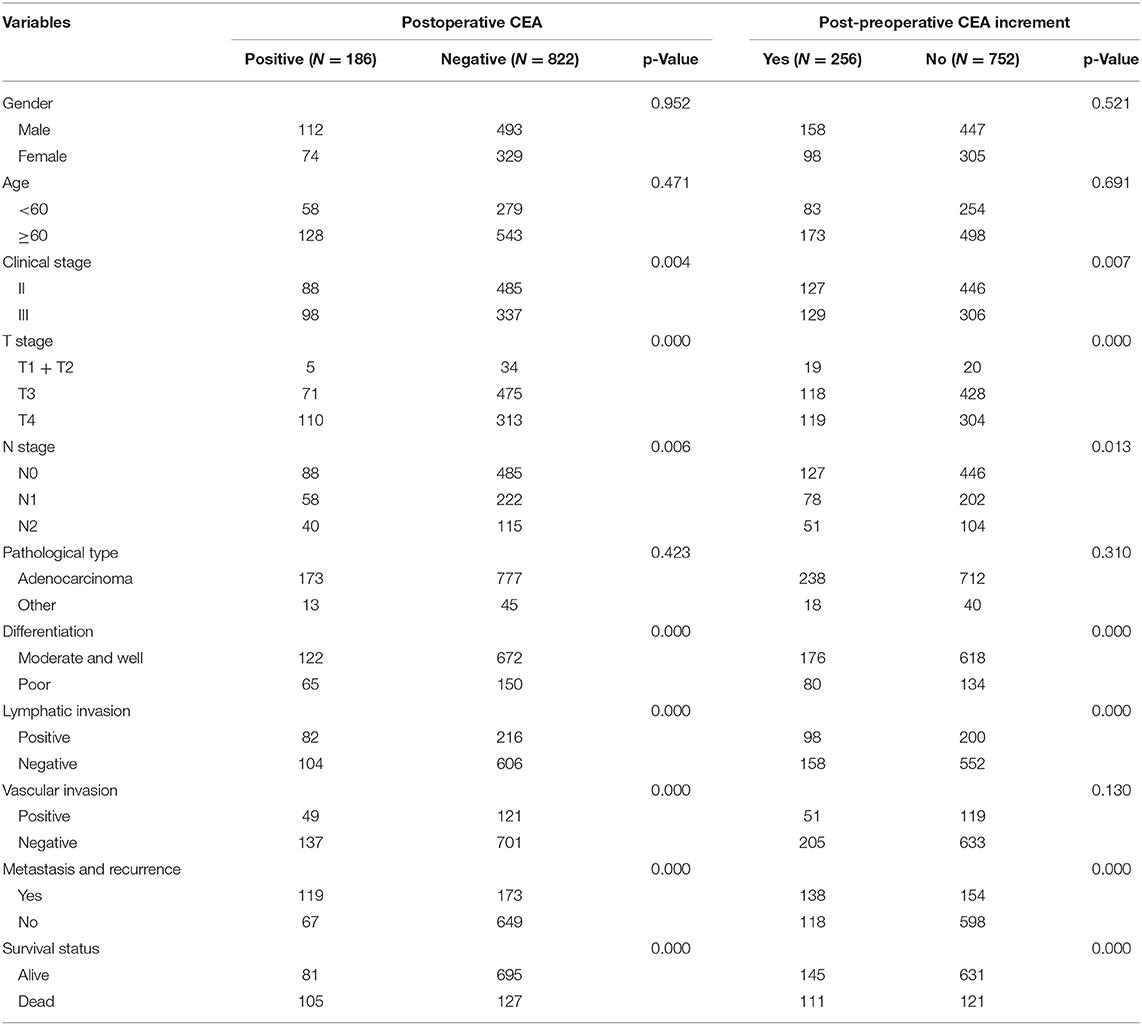
Table 2. The association of clinicopathologic characteristics with postoperative carcinoembryonic antigen (CEA) level and post-preoperative CEA increment.
Univariate and Multivariable Analyses Revealed That Positive Postoperative CEA and Post-preoperative CEA Increment Were Independent Prognostic Factors for CRC
To examine the relationship between clinical variables and progression-free survival (PFS) and overall survival (OS) in CRC patients, we performed univariate and multivariable Cox regression analyses. Thirty-nine patients with T1 and T2 in our study were all stage III CRC with lymph node metastasis and had poor prognosis, which would have a large bias in univariate and multivariable analyses. Thus, these patients were excluded in this part. Our univariate analysis results suggested that clinical stage, T stage, N stage, tumor differentiation, lymphatic invasion, vascular invasion, postoperative CEA level, and CEA increment were prognostic factors of PFS and OS (all values of p < 0.05) (Table 3). However, gender, age, and center had no significance for PFS and OS. Furthermore, multivariable analysis results indicated that positive postoperative CEA was an independent prognostic factor for PFS (HR = 3.149, 95% CI, 2.426–4.088, p = 0.000) (Table 3) and OS (HR = 3.414, 95% CI, 2.549–4.574, p = 0.000) (Table 3). Similarly, our results also found that CEA increment had significant impacts on PFS (HR = 2.708, 95% CI, 2.106–3.482, p = 0.000) (Table 3) and OS (HR = 2.373, 95% CI, 1.783–3.157, p = 0.000) (Table 3). In general, our results demonstrated that positive postoperative CEA and CEA increment had great significances to the prognosis of patients with stage II and III CRC.
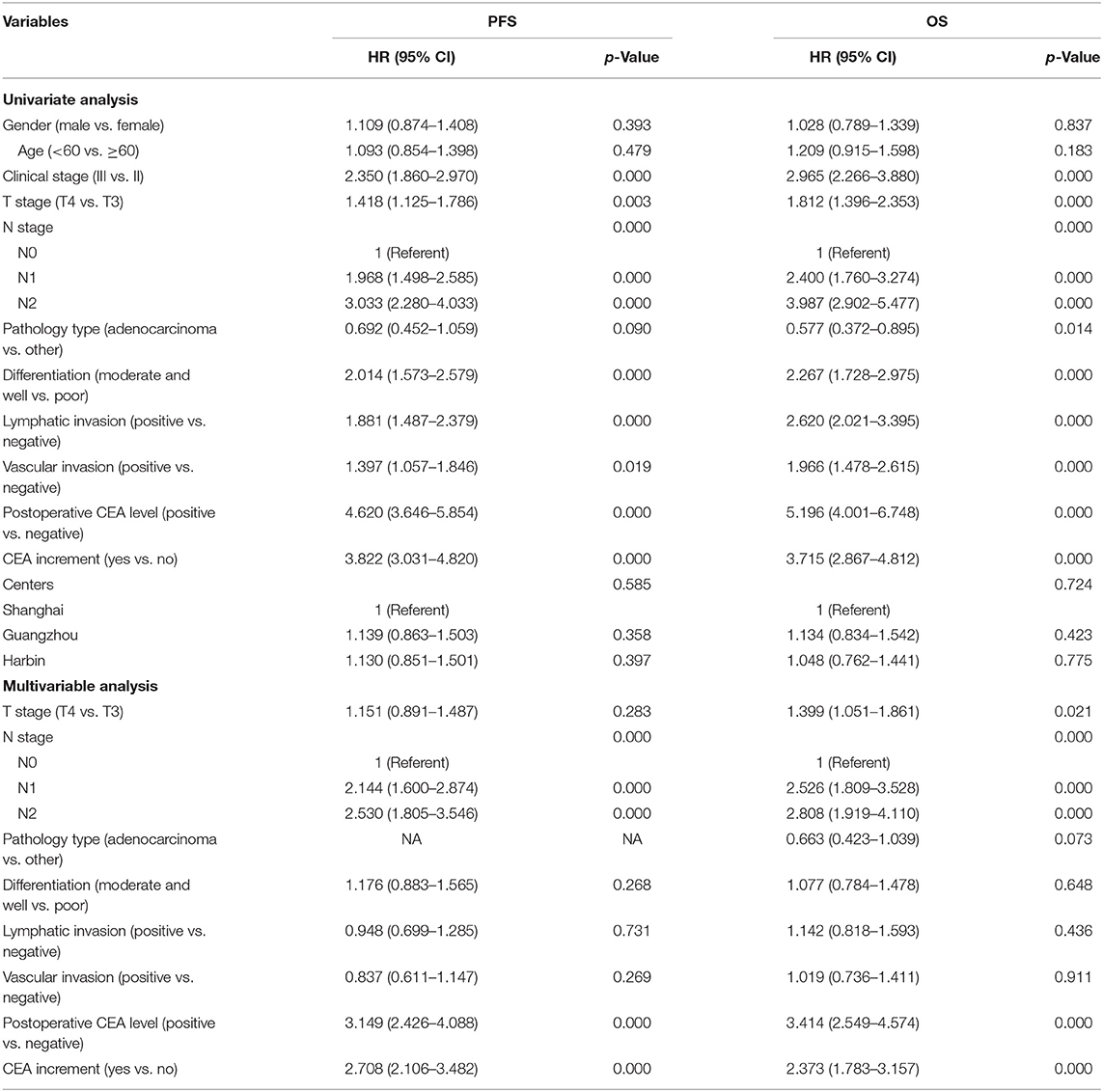
Table 3. Univariate and multivariable Cox regression analyses for progression-free survival time (PFS) and overall survival (OS) of colorectal cancer (CRC) patients (T1 and T2 were excluded).
Elevated Postoperative CEA and CEA Increment Predicted Worse Prognosis of Stage II and III CRC Patients
To assess the effects of positive postoperative CEA and CEA increment on the survival time of stage II and III CRC, Kaplan–Meier (K-M) survival curves were used according to our follow-up data. Our results showed that patients with positive postoperative CEA and positive increment had poor PFS and OS. The 3- and 5-year PFS rates for patients with negative postoperative CEA were 83.8 and 75.8%, much higher than patients with positive post-CEA (44.4% and 29.0%, p < 0.001) (Figure 2A). Similarly, the 3- and 5-year PFS rates were only 53.1 and 41.7% in patients with CEA increment, which was much worse than patients without increment (84.6 and 76.1%, p < 0.001) (Figure 2C). As for OS, we got same conclusions. The 3- and 5-year rates decreased from 90.3 and 81.7% in patients with negative postoperative CEA to 48.2 and 35.0% in patients with positive post-CEA (p < 0.001) (Figure 2B). For patients without CEA increment, the 3- and 5-year OS rates were 90.3 and 80.8%, better than patients with CEA increment (63.4 and 51.1%, p < 0.001) (Figure 2D).
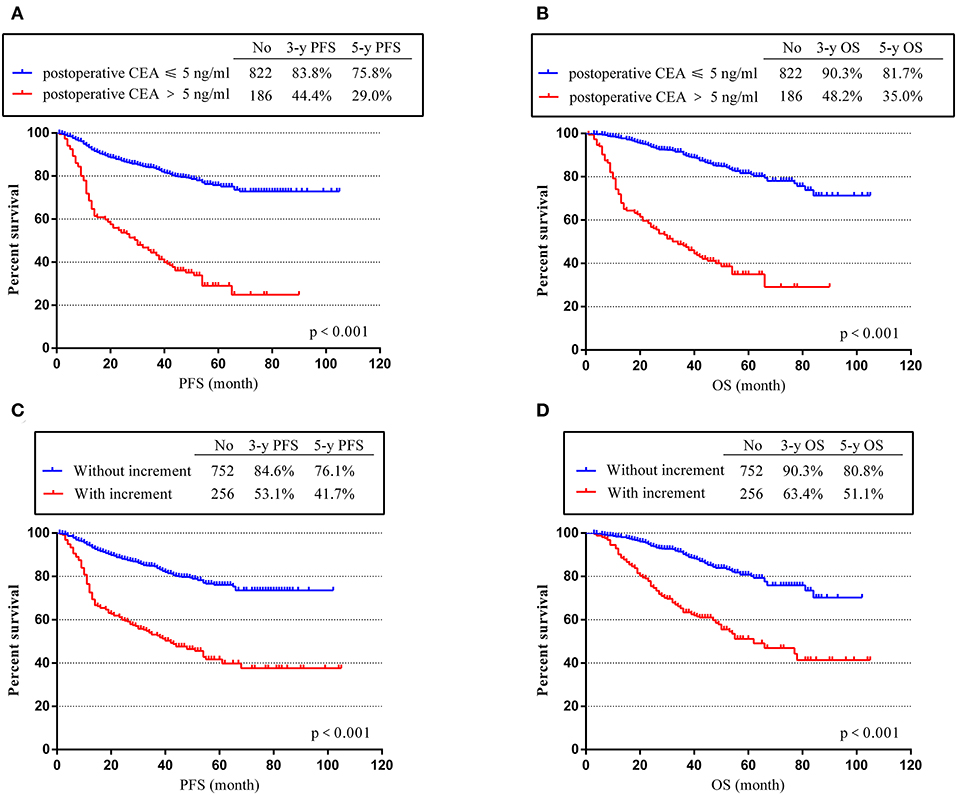
Figure 2. Progression-free survival time (PFS) and overall survival (OS) according to postoperative carcinoembryonic antigen (CEA) level and post-preoperative CEA increment. (A) K-M curves of PFS based on postoperative CEA level. (B) Kaplan–Meier (K-M) curves of OS based on postoperative CEA level. (C) K-M curves of PFS based on CEA increment. (D) K-M curves of OS based on CEA increment.
PFS and OS Differences Among Four Groups
As described in the Experimental section, we divided the patients into four groups (A, B, C, and D). As shown in Figure 3, group A had the best prognosis, while group D had the worst. The 3- and 5-year PFS rates decreased from 86.6 and 78.4% in group A to 25.3 and 7.2% in group D (p < 0.001) (Figure 3A). Consistent with the trend of PFS, the 3- and 5-year OS rates for group A were 92.5 and 83.9%, much higher than rates of group D (only 38.7 and 20.0%, p < 0.001) (Figure 3B). These results showed that patients in group D with positive postoperative CEA and CEA increment had the worst prognosis, while patients in group A with normal postoperative CEA and without CEA increment had the highest PFS and OS rates. For groups B and C, we could see that they had similar PFS, but the OS of group C was worse. This phenomenon suggested that elevated postoperative CEA may have more important effects on the prognosis of stage II and III CRC patients.
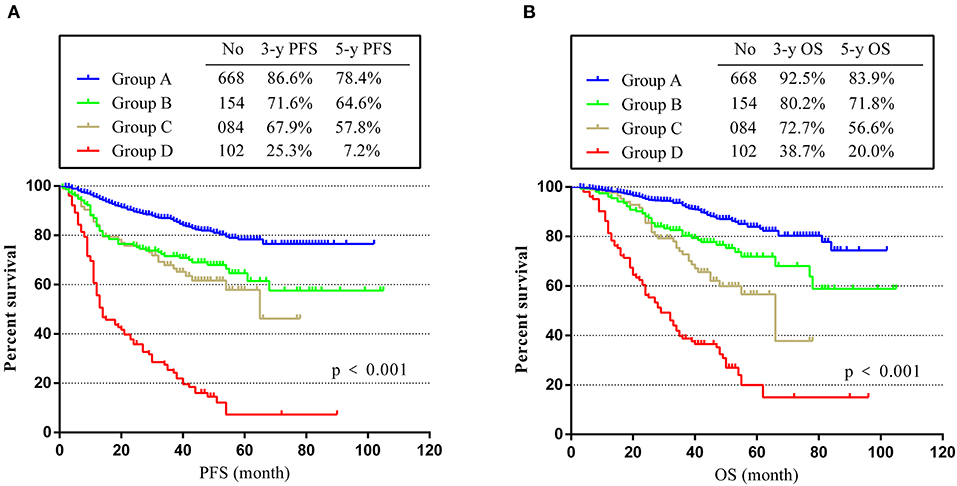
Figure 3. PFS and OS in different groups. (A) K-M curves of PFS in different groups. (B) K-M curves of OS in different groups.
Subgroup Analyses to Test the Effect of CEA Levels on Prognosis of CRC
To investigate the effect of perioperative abnormal CEA on the prognosis of CRC, we performed subgroup analyses. The subgroups were as follows: (Subgroup A) patients with positive preoperative CEA but normal postoperative CEA after radical surgery, n = 252; (Subgroup B) patients with normal preoperative CEA but positive postoperative CEA after radical surgery, n = 41; (Subgroup C) patients with positive preoperative CEA and positive postoperative CEA after radical surgery, n = 145. K-M curves illustrated that sub-B and sub-C had a worse PFS and OS than A, and sub-B was the worst (p < 0.001) (Figure 4). By comparing sub-A and sub-C, the results demonstrated that positive postoperative CEA patients had a poor prognosis even after radical resection. Furthermore, the survival of sub-B was worse than that of sub-C, indicating that patients with normal preoperative CEA but positive postoperative CEA had the worst prognosis in these subgroups.
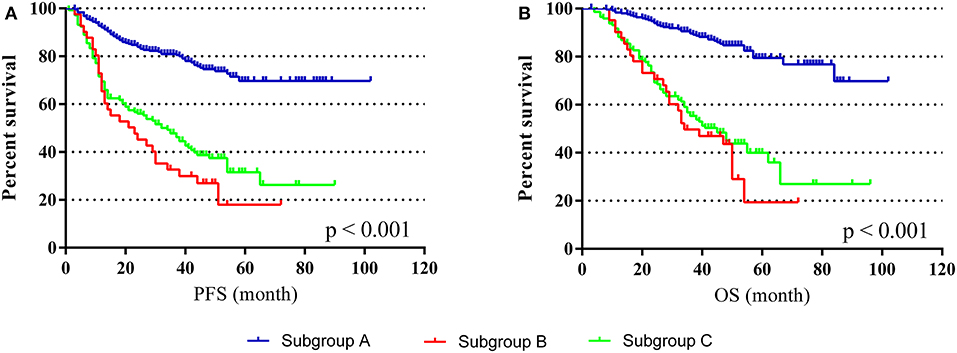
Figure 4. PFS and OS in different subgroups. (A) K-M curves of PFS in different subgroups. (B) K-M curves of OS in different subgroups.
Discussion
In our study, 1,008 patients with stage II and III CRC were enrolled. Our results suggested that positive postoperative CEA was associated with clinical stage, T stage, N stage, tumor differentiation, lymphatic and vascular invasion, while post-preoperative CEA increment was related to clinical stage, T stage, N stage, tumor differentiation, and lymphatic invasion. Besides, our multivariable analyses demonstrated that positive postoperative CEA and post-preoperative CEA increment were independent prognostic factors for CRC. Patients with elevated CEA level and CEA increment had shorter PFS and OS than patients with normal CEA and without increment. Furthermore, group D patients had the worst prognosis, and positive postoperative CEA had negative impacts on prognosis of CRC. Our subgroup analyses revealed high hazard of recurrence and poor survival in patients with perioperative CEA elevation, consistent with a recent study (18).
Similar to our study, the early postoperative CEA percent drop may be a helpful factor for the prognosis of colon cancer, but the influence of preoperative and postoperative CEA trends on survival has not well been demonstrated (19). Huang et al. conforms that CEA reduction ratio is a prognostic factor in rectal cancer patients who receive chemoradiotherapy and radical surgery (20). Serum CEA alone is less sensitive to detecting CRC recurrence, even though the threshold is low (21). Nevertheless, CT or CEA test each provides a reliable rate of recurrence with minimal follow-up after surgical treatment, and combining CEA and CT shows no advantage (22). Another study suggests that postoperative CEA limit is 15 ng/ml, with a high chance of recurrence after resection for colorectal liver metastasis (11). The same conclusion in a retrospective cohort analysis shows that patients with normal postoperative CEA have 14.9% higher 3-year RFS than patients with elevated post-operative CEA (17). Serum CEA is also correlated with RAS-mutant allele fraction (23).
According to current guidelines, patients undergoing radical surgery for stage II and III CRC need to test serum CEA every 3–6 months (24–27). However, these guidelines do not have individual follow-up and adjuvant therapy advice. Therefore, in clinical practice, should we refer to the levels of perioperative serum CEA when we give patients treatment recommendations? In addition, we also found that the number of preoperative elevated tumor markers also had important impact on the prognosis of CRC, including CEA, CA19-9, CA242, and CA125 (28). We believe that serum tumor markers have great value in CRC, but these markers have not been paid enough attention in the clinic. Thus, our study may provide some references for clinical workers in this field. Admittedly, there are some shortcomings in our research. First, this is a retrospective study, while prospective studies demonstrating the significance of CEA in CRC are more convincing. Second, our study included only one indicator, CEA. Other serum tumor markers were not analyzed. Finally, patients in our study are all Chinese.
In general, the treatment of stage II and III CRC after radical surgery still has some controversial problems (29, 30). Our study demonstrates the effects of postoperative CEA level and CEA increment on the prognosis of stage II and III CRC. Thus, our results will provide useful information for clinical references in the follow-up treatment of CRC patients.
Conclusion
Positive postoperative CEA and CEA increment are independent prognostic factors for stage II and III CRC. Patients with elevated postoperative CEA level and positive CEA increment have the worst PFS and OS compared to other groups. Our results may be helpful to the adjuvant treatment of stage II and III CRC after radical surgery.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by The ethics committee of Shanghai Jiao Tong University Affiliated Sixth People's Hospital. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
ZW conceived the project. WY, LY, and NS collected the clinical and follow-up data from Shanghai Jiao Tong University Affiliated Sixth People's Hospital. XW and ZC collected the clinical and follow-up data from the Sixth Affiliated Hospital of Sun Yat-sen University. GW and LX collected the clinical and follow-up data from the Second Affiliated Hospital of Harbin Medical University. WY analyzed all data and wrote the manuscript.
Funding
This work was supported by the National Key Clinical Discipline, Shanghai Municipal Education Commission-Gaofeng Clinical Medicine Grant Support (no. 20172023), Shanghai Science and Technology Commission Medical Project (no. 16411953200), Shanghai Pujiang Program (no. 16PJ1408200), Natural Science Foundation of Shanghai (no. 16ZR1449600), and National Natural Science Foundation of China (no. 81602689).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We sincerely thank the ethics committee and the electronic patients' record department.
References
1. Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet. (2019) 394:1467–80. doi: 10.1016/S0140-6736(19)32319-0
2. Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RGS, Barzi A, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. (2017) 67:177–93. doi: 10.3322/caac.21395
3. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. (2016) 66:115–32. doi: 10.3322/caac.21338
4. Locker GY, Hamilton S, Harris J, Jessup JM, Kemeny N, Macdonald JS, et al. ASCO 2006 update of recommendations for the use of tumor markers in gastrointestinal cancer. J Clin Oncol. (2006) 24:5313–27. doi: 10.1200/JCO.2006.08.2644
5. Huh JW, Oh BR, Kim HR, Kim YJ. Preoperative carcinoembryonic antigen level as an independent prognostic factor in potentially curative colon cancer. J Surg Oncol. (2010) 101:396–400. doi: 10.1002/jso.21495
6. Strimpakos AS, Cunningham D, Mikropoulos C, Petkar I, Barbachano Y, Chau I. The impact of carcinoembryonic antigen flare in patients with advanced colorectal cancer receiving first-line chemotherapy. Ann Oncol. (2010) 21:1013–9. doi: 10.1093/annonc/mdp449
7. Duffy MJ, Lamerz R, Haglund C, Nicolini A, Kalousova M, Holubec L, et al. Tumor markers in colorectal cancer, gastric cancer and gastrointestinal stromal cancers: European group on tumor markers 2014 guidelines update. Int J Cancer. (2014) 134:2513–22. doi: 10.1002/ijc.28384
8. Tarantino I, Warschkow R, Worni M, Merati-Kashani K, Koberle D, Schmied BM, et al. Elevated preoperative CEA is associated with worse survival in stage I-III rectal cancer patients. Br J Cancer. (2012) 107:266–74. doi: 10.1038/bjc.2012.267
9. Saito G, Sadahiro S, Okada K, Tanaka A, Suzuki T, Kamijo A. Relation between carcinoembryonic antigen levels in colon cancer tissue and serum carcinoembryonic antigen levels at initial surgery and recurrence. Oncology. (2016) 91:85–9. doi: 10.1159/000447062
10. Lin JK, Lin CC, Yang SH, Wang HS, Jiang JK, Lan YT, et al. Early postoperative CEA level is a better prognostic indicator than is preoperative CEA level in predicting prognosis of patients with curable colorectal cancer. Int J Colorectal Dis. (2011) 26:1135–41. doi: 10.1007/s00384-011-1209-5
11. Araujo RL, Gonen M, Allen P, DeMatteo R, Kingham P, Jarnagin W, et al. Positive postoperative CEA is a strong predictor of recurrence for patients after resection for colorectal liver metastases. Ann Surg Oncol. (2015) 22:3087–93. doi: 10.1245/s10434-014-4358-2
12. Bhatti I, Patel M, Dennison AR, Thomas MW, Garcea G. Utility of postoperative CEA for surveillance of recurrence after resection of primary colorectal cancer. Int J Surg. (2015) 16(Pt A):123–8. doi: 10.1016/j.ijsu.2015.03.002
13. Yoshino T, Obermannova R, Bodoky G, Garcia-Carbonero R, Ciuleanu T, Portnoy DC, et al. Baseline carcinoembryonic antigen as a predictive factor of ramucirumab efficacy in RAISE, a second-line metastatic colorectal carcinoma phase III trial. Eur J Cancer. (2017) 78:61–9. doi: 10.1016/j.ejca.2017.03.007
14. Thomsen M, Skovlund E, Sorbye H, Bolstad N, Nustad KJ, Glimelius B, et al. Prognostic role of carcinoembryonic antigen and carbohydrate antigen 19-9 in metastatic colorectal cancer: a BRAF-mutant subset with high CA 19-9 level and poor outcome. Br J Cancer. (2018) 118:1609–16. doi: 10.1038/s41416-018-0115-9
15. Yang KL, Yang SH, Liang WY, Kuo YJ, Lin JK, Lin TC, et al. Carcinoembryonic antigen (CEA) level, CEA ratio, and treatment outcome of rectal cancer patients receiving pre-operative chemoradiation and surgery. Radiat Oncol. (2013) 8:43–3. doi: 10.1186/1748-717X-8-43
16. Hotta T, Takifuji K, Yokoyama S, Matsuda K, Oku Y, Nasu T, et al. Impact of the post/preoperative serum CEA ratio on the survival of patients with rectal cancer. Surg Today. (2014) 44:2106–15. doi: 10.1007/s00595-014-0852-1
17. Konishi T, Shimada Y, Hsu M, Tufts L, Jimenez-Rodriguez R, Cercek A, et al. Association of preoperative and postoperative serum carcinoembryonic antigen and colon cancer outcome. JAMA Oncol. (2018) 4:309–15. doi: 10.1001/jamaoncol.2017.4420
18. Nakamura Y, Shida D, Tanabe T, Takamizawa Y, Imaizumi J, Ahiko Y, et al. Prognostic impact of preoperatively elevated and postoperatively normalized carcinoembryonic antigen levels following curative resection of stage I-III rectal cancer. Cancer Med. (2020) 9:653–62. doi: 10.1002/cam4.2758
19. Lee WS, Baek JH, Kim KK, Park YH. The prognostic significant of percentage drop in serum CEA post curative resection for colon cancer. Surg Oncol. (2012) 21:45–51. doi: 10.1016/j.suronc.2010.10.003
20. Huang CS, Lin JK, Wang LW, Liang WY, Lin CC, Lan YT, et al. Assessment of the value of carcinoembryonic antigen reduction ratio as a prognosis factor in rectal cancer. Am J Surg. (2014) 208:99–105. doi: 10.1016/j.amjsurg.2013.08.054
21. Nicholson BD, Shinkins B, Pathiraja I, Roberts NW, James TJ, Mallett S, et al. Blood CEA levels for detecting recurrent colorectal cancer. Cochrane Database Syst Rev. (2015) 2015:CD011134. doi: 10.1002/14651858.CD011134.pub2
22. Primrose JN, Perera R, Gray A, Rose P, Fuller A, Corkhill A, et al. Effect of 3 to 5 years of scheduled CEA and CT follow-up to detect recurrence of colorectal cancer: the FACS randomized clinical trial. JAMA. (2014) 311:263–70. doi: 10.1001/jama.2013.285718
23. Elez E, Chianese C, Sanz-Garcia E, Martinelli E, Noguerido A, Mancuso FM, et al. Impact of circulating tumor DNA mutant allele fraction on prognosis in RAS-mutant metastatic colorectal cancer. Mol Oncol. (2019) 13:1827–35. doi: 10.1002/1878-0261.12547
24. Labianca R, Nordlinger B, Beretta GD, Brouquet A, Cervantes A, Group EGW. Primary colon cancer: ESMO Clinical Practice Guidelines for diagnosis, adjuvant treatment and follow-up. Ann Oncol. (2010) 21 Suppl 5:v70–77. doi: 10.1093/annonc/mdq168
25. Glynne-Jones R, Wyrwicz L, Tiret E, Brown G, Rodel C, Cervantes A, et al. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2017) 28 (Suppl_4):iv22–40. doi: 10.1093/annonc/mdx224
26. Benson AB, Venook AP, Al-Hawary MM, Cederquist L, Chen YJ, Ciombor KK, et al. NCCN guidelines insights: colon cancer, Version 2.2018. J Natl Compr Canc Netw. (2018) 16:359–69. doi: 10.6004/jnccn.2018.0021
27. Benson AB, Venook AP, Al-Hawary MM, Cederquist L, Chen YJ, Ciombor KK, et al. Rectal Cancer, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. (2018) 16:874–901. doi: 10.6004/jnccn.2018.0061
28. You W, Sheng N, Yan L, Chen H, Gong J, He Z, et al. The difference in prognosis of stage II and III colorectal cancer based on preoperative serum tumor markers. J Cancer. (2019) 10:3757–66. doi: 10.7150/jca.31660
29. Lonardi S, Sobrero A, Rosati G, Di Bartolomeo M, Ronzoni M, Aprile G, et al. Phase III trial comparing 3-6 months of adjuvant FOLFOX4/XELOX in stage II-III colon cancer: safety and compliance in the TOSCA trial. Ann Oncol. (2016) 27:2074–81. doi: 10.1093/annonc/mdw404
30. Andre T, Vernerey D, Mineur L, Bennouna J, Desrame J, Faroux R, et al. Three versus 6 months of oxaliplatin-based adjuvant chemotherapy for patients with stage III colon cancer: disease-free survival results from a randomized, open-label, International Duration Evaluation of Adjuvant (IDEA) France, Phase III Trial. J Clin Oncol. (2018) 36:1469. doi: 10.1200/JCO.2017.76.0355
Keywords: colorectal cancer, carcinoembryonic antigen, prognosis, stage II, stage III
Citation: You W, Yan L, Cai Z, Xie L, Sheng N, Wang G, Wu X and Wang Z (2020) Clinical Significances of Positive Postoperative Serum CEA and Post-preoperative CEA Increment in Stage II and III Colorectal Cancer: A Multicenter Retrospective Study. Front. Oncol. 10:671. doi: 10.3389/fonc.2020.00671
Received: 10 December 2019; Accepted: 09 April 2020;
Published: 20 May 2020.
Edited by:
Mark Girgis, University of California, Los Angeles, United StatesReviewed by:
Francesco Cavallin, Independent Researcher, Solagna, ItalyJianbin Xiang, Fudan University, China
Copyright © 2020 You, Yan, Cai, Xie, Sheng, Wang, Wu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guiyu Wang, Z3VpeXdhbmcmI3gwMDA0MDsxNjMuY29t; Xiaojian Wu, d3V4amlhbiYjeDAwMDQwO21haWwuc3lzdS5lZHUuY24=; Zhigang Wang, c3VybGFiJiN4MDAwNDA7aG90bWFpbC5jb20=
†These authors have contributed equally to this work
 Weiqiang You1†
Weiqiang You1† Zerong Cai
Zerong Cai Zhigang Wang
Zhigang Wang