- Surgical Health Outcomes and Research Enterprise (SHORE), Division of Colorectal Surgery, Department of Surgery, University of Rochester Medical Center, Rochester, NY, United States
Rectal cancer surgery has seen significant improvement in recent years. This has been possible in part due to focus on surgeon education and training, specific surgical quality metrics, and longitudinal tracking of data through the use of registries. In countries that have implemented such efforts, data has shown significant improvement in outcomes. However, there continues to be significant variation in rectal cancer outcomes and practices worldwide. Just within the United States, county level mortality rates from rectal cancer range from 8–15 per 100,000 to 38–59 per 100,000. In order to continue to improve rectal cancer patient outcomes, there needs to be evidence based guidelines and standards centered around the framework of structure, process, and outcomes. In addition, there must be a feedback system by which programs can continually assess their performance. Obtaining evidence for specific standards and measures can be challenging and requires analyzing available data and literature, some of which may be conflicting. This article evaluates the evolution of metrics and standards used for quality improvement in rectal cancer and ongoing efforts to further improve patient outcomes.
Introduction
Rectal cancer continues to be a public health concern with 40,000 newly diagnosed cases annually in the United States (US) (1). Worldwide, colorectal cancer is the third most commonly diagnosed cancer and the fourth leading cause of death with 1.4 million new cases and almost 700,000 deaths in 2012 (2). Rectal cancer outcomes depend heavily upon the quality of surgical resection. Thanks to a concerted effort to improve rectal cancer resections, spearheaded by the concept of total mesorectal excision (TME) pioneered by MacFarlane et al. (3), there have been great strides made in the improvement of rectal cancer outcomes worldwide. However, there continues to be substantial variation in the outcome of rectal cancer patients. Therefore, the question remains of how to continue the trend of improving outcomes and decreasing variation.
The consensus is that the road to improving outcomes involves the capture of quality measurements. This has led to considerable debate and discussion about which quality measures best reflect surgical quality for rectal cancer. The National Quality Forum established four criteria for assessment of a measure for endorsement (4):
(1) Importance: A measure must be important to measure and report where the most impact can be made on healthcare quality.
(2) Usability and Relevance: A measure must be understandable and useful by intended users to improve quality.
(3) Scientifically Acceptable: A measure must produce consistent and credible results.
(4) Feasibility: The measure must be driven by data that is readily available and can be retrieved.
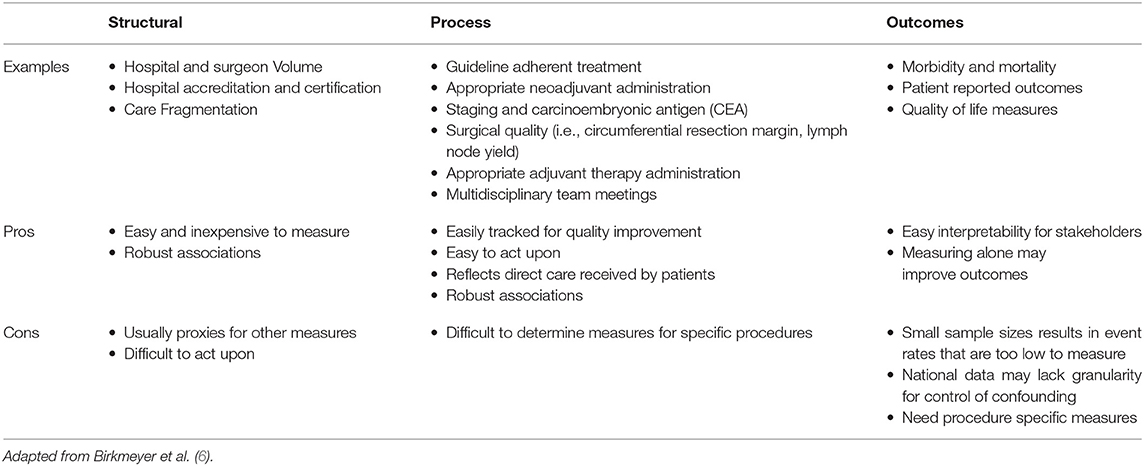
Quality measures can be categorized as either involving structure, process, or outcomes, based on the Donabedian classification [(5); Table 1]. Structural measures are the organizational features associated with delivery of care. Process measures refer to the care directly received by patients. Outcomes refer to results of treatment. Many single measures within the realm of these categories have been investigated for rectal cancer, but there is increasing evidence that for meaningful long term improvement, we need clinically relevant quality measures that span multiple phases of care (7). To achieve this, the National Accreditation Program for Rectal Cancer (NAPRC) was developed through a collaboration between the Optimizing the Surgical Treatment of Rectal Cancer (OSTRiCh) Consortium and the Commission on Cancer (CoC) of the American College of Surgeons (8). NAPRC proposes a set of standards pertaining to program structure and process of patient care, as well as performance indicators for the evaluation of outcomes. These standards were based on data supporting measures that can individually be classified in one of Donabedian's categories.
Quality Measures
One of the driving factors for development of rectal cancer quality measures in the United States has been the data and experience published out of Europe. Efforts made by Norway included the establishment of national consensus for rectal cancer management through the development of guidelines for chemotherapy/radiation therapy, preoperative imaging and staging, pathologic examination, and multidisciplinary workshops held to educate providers on TME surgery technique. A Norwegian Rectal Cancer Registry was established in order to monitor outcomes and feedback outcomes to institutions. With these efforts, they were able to improve 5-year relative survival by 9% and decrease 5-year local recurrence by 9.5% over the course of 10 years (9). In the United Kingdom (UK), the Calman-Hine Report had shown that the UK lagged behind other European countries significantly in rectal cancer outcomes (10). In order to close this gap, the UK formulated the National Health Services Cancer Plan, which emphasized centers of excellence, defining protocols for specific phases of care, and collecting and monitoring outcomes. Through this concerted effort, they were able to improve 5-year overall survival from 48.8 to 61% over the course of 14 years (11).
Compared to European data, the US was found to lag behind in several metrics. Based on National Cancer Database (NCDB) data, the positive circumferential margin rate in the US was 17.2%, compared to 11% in the UK (12, 13). Rectal cancer patients in the US also had higher rates of colostomies compared to many European countries (14, 15). In addition, without any accreditation system, large numbers of rectal cancer patients were being treated a low volume centers (16).
From a structural standpoint, one of the major factors that has been found to impact rectal cancer outcomes is volume. Robust volume-outcome relationships have been consistently found in many complex oncologic surgical procedures (17–19). Aquina et al. found that in New York State, there has been a natural migration of rectal cancer resections to high volume providers and facilities. Higher rectal cancer resection volume has been shown to be associated with decreased rates of colostomy formation and 30-day mortality (20). National US data has shown similar results with high volume centers having higher rates of guideline adherent treatment and providing improved long-term overall survival compared to their low volume counterparts (21). Internationally, studies have also shown high volume centers to have improved outcomes including lower rates of positive circumferential resection margins, improved rates of adequate lymph node yield, lower operative mortality, and improved long-term survival (22–24). Another factor related to healthcare infrastructure that has recently been shown to have an impact on rectal cancer patient outcomes is care fragmentation. Justiniano et al. showed that rectal cancer resection patients who required readmission had a 2-fold decrease in survival if patients were readmitted to a different surgeon than the index surgeon, regardless of whether it was the index facility (25). Finally, one method used to improve quality and outcomes from a structural standpoint is accreditation. Two well-known accreditation programs for facilities providing cancer treatment are the American College of Surgeon's Commission on Cancer (CoC) and the National Cancer Institute's NCI-designation. CoC-approved centers typically offer more cancer-related services such as chemotherapy and radiation, and they also performed more cancer operations per year compared to non-CoC centers (26). Colorectal cancer patients treated at NCI-designated centers have previously been shown to have a 16% improvement in overall mortality compared to patients treated at non-NCI-designated centers (27). However, while the evidence for structural measures tends to be very robust, structural measures such as volume and care fragmentation are difficult to act upon without significant cost and time. In addition, it is difficult to determine whether structural measures are proxies for other measures that may be more readily acted upon.
Several process measures have been implicated in rectal cancer outcomes. Due to the complexity of rectal cancer treatment, including neoadjuvant and adjuvant therapy for locally advanced disease, there are many opportunities for improvements in process. US National data shows that compliance with National Comprehensive Cancer Network (NCCN) recommendation for neoadjuvant chemotherapy/radiation and adjuvant chemotherapy for locally advanced rectal cancer is poor. Only approximately 30% of locally advanced rectal cancers in the US received neoadjuvant therapy (28). Among those patients, only 32% of patients received the recommended sandwich therapy with adjuvant chemotherapy (29). In both cases, receipt of appropriate chemotherapy and radiation has been associated with improved survival. European data has also revealed issues with poor compliance to guidelines in colorectal cancer, though compliance appears to be better than the US. For example, Glynne-Jones et al. and Heins et al. found that 80–90% of T2 rectal cancer patients received neoadjuvant radiation therapy (30, 31). Another important aspect of rectal cancer care process is staging. Staging directly impacts the therapy that patients ultimately receive. Modern standard of care is to evaluate staging with MRI. In an analysis of national staging data for rectal cancer, there was evidence that a large number of patients (24%) were initially under-staged, which resulted in higher rates of positive circumferential resection margin (CRM) and worse 5-year overall survival (32). Another increasingly recognized process that appear to impact outcomes for colorectal cancer patients is multidisciplinary team meetings (MDT), particularly for advanced disease. Previous studies have shown that among advanced colorectal cancer patients, MDTs decreased the hazard to death by 35% (33). The benefit of process measures is that similar to structural measures, there tends to be robust evidence supporting their effectiveness. In addition, these measures are very actionable and easily tracked for quality improvement. The main weakness of process measures, particularly when applied to specific procedures, is that the measures may be too broad and therefore, may not result in any improved outcomes despite improved compliance.
Outcomes measurement is an obvious road to quality improvement and therefore has been used for years with the most common being the ACS National Surgical Quality Improvement Program (NSQIP). Most registries capture outcomes measures in one form or another. For example, NCDB and the Surveillance, Epidemiology, and End Results (SEER) program tracks patient's vital status. The most basic outcomes involve survival, either overall or disease specific. Beyond survival and recurrence, operative quality measures are also of significant interest. While there has been significant progress made in chemotherapeutics and optimizing radiation therapy, surgery remains the cornerstone of treatment for rectal cancer, and therefore is a crucial contributor to process measures. We showed with NCDB data that in the US, there was a 3.5-fold difference in the rate of an adequate lymph node yield of 12 among individual hospitals performing proctectomies for rectal cancer. This was again independently associated with increased 5-year mortality (34). Rickles et al. had found a positive CRM rate of 17.2% in the US. Interestingly, facility location was a predictor of CRM positivity, which indicates significant geographical variation in the quality of surgery (13). Another growing field of interest is the impact of surgical complications on long-term outcomes for cancer patients (35, 36). Wang et al. found in their meta-analysis that anastomotic leak after proctectomy for rectal cancer was associated with a 71% increase in the risk of local recurrence, a 67% increase in the risk of overall mortality, and a 30% increase in the risk of cancer-specific mortality (36). Outcomes measures are highly advantageous because of easy interpretability, which may improve buy-in from stakeholders. The largest weakness of outcomes measures is that the event rate of some measures may be too low to detect significant differences (6). Many investigators, including our group, have combated this by analyzing large national and statewide datasets, or even combining procedures that may be interpreted as having similar complexity, such as pancreaticoduodenectomies and esophagectomies. This obviously leads to a couple of issues. Large datasets likely lack enough granularity to control for all confounders, leading to difficulty in result interpretation. And aggregating other organ systems rely on assumptions that may or may not be true, again, leading to difficulty interpreting results. Another issue with outcomes measurement is whether the appropriate outcome is being measured. Traditionally, short-term perioperative mortality has been measured at the 30-day mark. However, there is evidence that by only assessing 30-day outcomes there is a significant burden of post-operative mortality that is being missed that could potentially be intervened upon. Byrne et al. found in their analysis of colorectal surgical patients that by examining 90-day mortality as opposed to 30-day mortality, they were able to find twice the number of hospitals that were high outliers for mortality (37). This study highlights the importance of choosing the right outcome measure in order to maximize quality improvement initiatives.
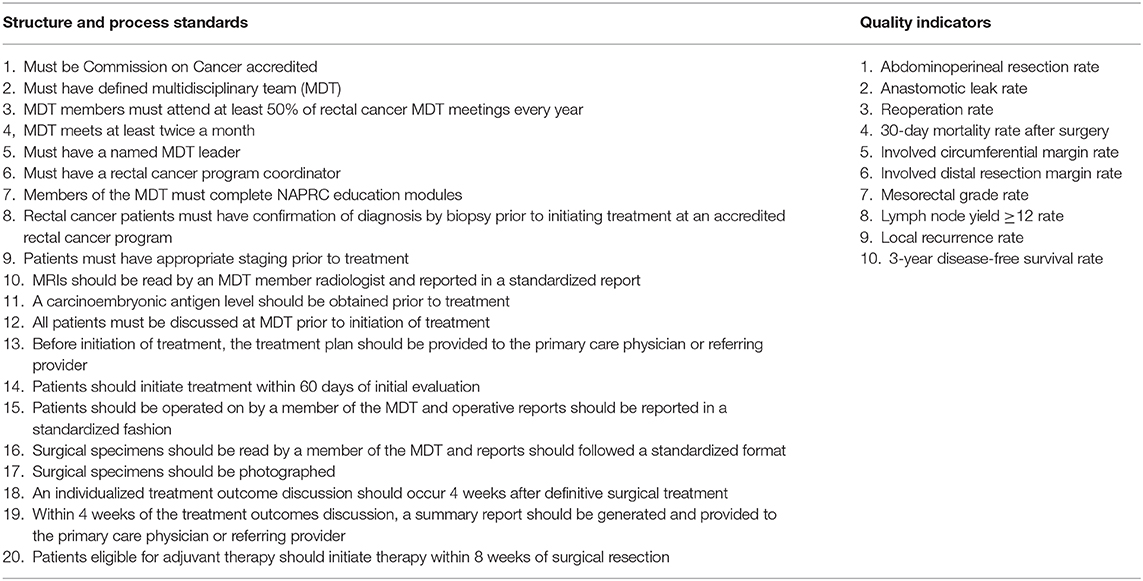
As discussed above, each individual measure has inherent weaknesses and strengths. Taken in isolation, individual measures are likely to miss opportunities for more significant improvement and synergies that may help decrease the overall cost of quality improvement. In many ways, NAPRC helps to bridge the gap between individual measures and having an evidence based quality improvement system that is able to evaluate the full spectrum of rectal cancer care delivery. The use of an accreditation system has been used to great effect by other countries such as Germany, where an accreditation system for colorectal cancer centers has existed since 2006 (38). NAPRC includes a set of 20 structure and process standards that include the development and maintenance of a multidisciplinary team, timing of treatment, standardized imaging and pathologic assessment, and a system of data submission and review. There are an additional 10 quality indicators, or outcomes measures, including many surgical quality indicators, which are meant to be collected and reported back to institutions for guidance of quality improvement [(39); Table 2]. There is ongoing work to help capture more measures nationally in order to better assess the impact of NAPRC, but based on currently available measures, Brady et al. found that only 28.1% of rectal cancer patients who underwent resection achieved all process measures and 56.3% achieved all outcomes measures (40). At the hospital level, Antunez et al. found that only 2.9% of hospitals in the US met all process measures. While the study was unable to find a direct impact on survival based on adherence to the available process measures, they did find that hospitals least likely to receive NAPRC accreditation were more likely to serve patients of lower socioeconomic position and have worse survival outcomes (41). In addition, New York state data shows that a model of centralization that limits proctectomies to only high volume centers could have a substantial impact on the travel distance of rectal cancer patients, particularly those in rural communities (21). This data hints at what is potentially a major gap in the current state of rectal cancer quality improvement: patient-centered outcomes.
Many quality measures revolve around surgical measures because surgical measures tend to be easier to measure and there are robust administrative datasets that already collect the data necessary to monitor surgical measures. However, there are several aspects of rectal cancer care that lack meaningful quality measures. While we appreciate the important of multidisciplinary team meetings for rectal cancer patients, there are no measures for the quality of this process. This lack of assessment is a crucial gap as treatment decisions made during MDT meetings have significant impact on downstream outcomes. We also place significant emphasis on improving what we are doing already within the phases of rectal cancer care. However, a major piece that is missed if we only look at what we do is how patients feel about the impact of their treatments. While there are some measures available for capturing patient perspective, such as the Medicare Outcomes Survey and the Hospital Consumer Assessment of Healthcare Providers and Systems Survey (HCAHPS), they are not sensitive enough to provide data for surgical quality assessment. To address this gap, there are ongoing initiatives to develop validated questionnaires and surveys that measure many aspects of health such as severity of symptoms, health care experience, and quality of life (42). These measure are known as patient reported outcomes (PROs). PROs allow for early detection of distress in a patient, provides a valuable opportunity for patients to be heard, and allows for interventions to be directed toward specific symptoms (43). Thanks to new psychometric techniques and electronic platforms, much of this data can now be easily collected. To utilize this data, the ACS started a multiphase pilot study to assess the feasibility of collecting this data within the ACS NSQIP platform. The first phase assessed feasibility in breast, colorectal, endocrine, hepato-pancreato-biliary, thoracic, trauma, and general surgery patients (42). While previous discussions of measures falling within the Donabedian classification are more about standardization of care, PROs is an opportunity for more individualized care for cancer patients (44).
Summary
There has been significant development in the improvement of rectal cancer quality measures. While the basis of quality measures can be categorized into structural, process, and outcomes measures, it is unlikely any one measure from any of these categories can singlehandedly improve the quality of rectal cancer care. Each measure has certain advantages and disadvantages. NAPRC is a more comprehensive program that includes measures from all phases of rectal cancer care to maximize quality improvement. Central to NAPRC, and the success of national programs instituted by other countries, is the ability of the program to track and feedback performance measures to institutions in order to facilitate continued advancement. The ultimate impact of NAPRC on rectal cancer care in the US remains to be seen, but what is becoming clear is that NAPRC alone will not be enough. With an increasing emphasis on patient centered care and outcomes, we can no longer only focus on what we, as providers do, but also what patients want and how they experience what we do to them. PROs is a strong step toward integrating this group of quality measures to our existing framework. The one certainty is that the development of a successful system for consistent quality improvement is crucial for the care of our rectal cancer patients.
Author Contributions
ZX and FF contributed to the writing and editing of this manuscript.
Conflict of Interest
FF receives royalties from UpToDate, Inc.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer KM declared a shared affiliation, with no collaboration, with the author FF to the handling editor at the time of review.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. (2016) 66:7–30. doi: 10.3322/caac.21332
2. Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. (2017) 66:683–91. doi: 10.1136/gutjnl-2015-310912
3. MacFarlane J, Ryall R, Heald R. Mesorectal excision for rectal cancer. Lancet. (1993) 341:457–60. doi: 10.1016/0140-6736(93)90207-W
5. Donabedian A. The quality of care: how can it be assessed? JAMA. (1988) 260:1743–8. doi: 10.1001/jama.260.12.1743
6. Birkmeyer JD, Dimick JB, Birkmeyer NJO. Measuring the quality of surgical care: structure, process, or outcomes? J Am College Surgeons. (2004) 198:626–32. doi: 10.1016/j.jamcollsurg.2003.11.017
7. Damle A, Alavi K. Objective assessment of quality measurement and improvement. Clin Colon Rectal Surg. (2014) 27:19–25. doi: 10.1055/s-0034-1366915
8. Monson JRT, Dietz DW, Boughey JC, You YN. Improving rectal cancer outcomes through advocacy, education, and research: the OSTRiCh consortium and the new NAPRC. Bull Am Coll Surg. (2016) 101:45–6. Available online at: https://bulletin.facs.org/2016/11/improving-rectal-cancer-outcomes-through-advocacy-education-and-research-the-ostrich-consortium-and-the-new-naprc/
9. Guren MG, Kørner H, Pfeffer F, Myklebust TÅ, Eriksen MT, Edna TH, et al. Nationwide improvement of rectal cancer treatment outcomes in Norway, 1993–2010. Acta Oncol. (2015) 54:1714–22. doi: 10.3109/0284186X.2015.1034876
10. Calman K, Hine D. A Policy Framework for Commissioning Cancer Services. A report by the Expert Advisory Group on Cancer to the Chief Medical Officers of England and Wales (1995).
11. Walters S, Benitez-Majano S, Muller P, Coleman MP, Allemani C, Butler J, et al. Is England closing the international gap in cancer survival? Br J Cancer. (2015) 113:848–60. doi: 10.1038/bjc.2015.265
12. Tilney HS, Heriot AG, Purkayastha S, Antoniou A, Aylin P, Darzi AW, et al. A national perspective on the decline of abdominoperineal resection for rectal cancer. Ann Surg. (2008) 247:77–84. doi: 10.1097/SLA.0b013e31816076c3
13. Rickles AS, Dietz DW, Chang GJ, Wexner SD, Berho ME, Remzi FH, et al. High rate of positive circumferential resection margins following rectal cancer surgery: a call to action. Ann Surg. (2015) 262:891–8. doi: 10.1097/SLA.0000000000001391
14. Quirke P, Steele R, Monson J, Grieve R, Khanna S, Couture J, et al. Effect of the plane of surgery achieved on local recurrence in patients with operable rectal cancer: a prospective study using data from the MRC CR07 and NCIC-CTG CO16 randomised clinical trial. Lancet. (2009) 373:821–8. doi: 10.1016/S0140-6736(09)60485-2
15. Ricciardi R, Roberts PL, Read TE, Baxter NN, Marcello PW, Schoetz DJ. Presence of specialty surgeons reduces the likelihood of colostomy after proctectomy for rectal cancer. Dis Colon Rectum. (2011) 54:207–13. doi: 10.1007/DCR.0b013e3181fb8903
16. Baek JH, Alrubaie A, Guzman EA, Choi SK, Anderson C, Mills S, et al. The association of hospital volume with rectal cancer surgery outcomes. Int J Colorectal Dis. (2013) 28:191–6. doi: 10.1007/s00384-012-1536-1
17. Lidsky ME, Sun Z, Nussbaum DP, Adam MA, Speicher PJ, Blazer DG. Going the extra mile: improved survival for pancreatic cancer patients traveling to high-volume centers. Ann Surg. (2016) 266:333–8. doi: 10.1097/SLA.0000000000001924
18. Schrag D, Panageas KS, Riedel E, Cramer LD, Guillem JG, Bach PB, et al. Hospital and surgeon procedure volume as predictors of outcome following rectal cancer resection. Ann Surg. (2002) 236:583–92. doi: 10.1097/00000658-200211000-00008
19. Speicher PJ, Englum BR, Ganapathi AM, Wang X, Hartwig MG, D'Amico TA, et al. Traveling to a high-volume center is associated with improved survival for patients with esophageal cancer. Ann Surg. (2017) 265:743–9. doi: 10.1097/SLA.0000000000001702
20. Aquina CT, Probst CP, Becerra AZ, Iannuzzi JC, Kelly KN, Hensley BJ, et al. High volume improves outcomes: the argument for centralization of rectal cancer surgery. Surgery. (2016) 159:736–48. doi: 10.1016/j.surg.2015.09.021
21. Xu Z, Becerra AZ, Justiniano CF, Boodry CI, Aquina CT, Swanger AA, et al. Is the distance worth it? Patients with rectal cancer traveling to high-volume centers experience improved outcomes. Dis Colon Rectum. (2017) 60:1250–9. doi: 10.1097/DCR.0000000000000924
22. Liu CJ, Chou YJ, Teng CJ, Lin CC, Lee YT, Hu YW, et al. Association of surgeon volume and hospital volume with the outcome of patients receiving definitive surgery for colorectal cancer: a nationwide population-based study. Cancer. (2015) 121:2782–90. doi: 10.1002/cncr.29356
23. McColl RJ, McGahan CE, Cai E, Olson R, Cheung WY, Raval MJ, et al. Impact of hospital volume on quality indicators for rectal cancer surgery in British Columbia, Canada. Am J Surg. (2017) 213:388–94. doi: 10.1016/j.amjsurg.2016.07.007
24. Gietelink L, Henneman D, van Leersum NJ, de Noo M, Manusama E, Tanis PJ, et al. The influence of hospital volume on circumferential resection margin involvement: results of the Dutch surgical colorectal audit. Ann Surg. (2015) 263:745–50 doi: 10.1097/SLA.0000000000001009
25. Justiniano CF, Xu Z, Becerra AZ, Aquina CT, Boodry CI, Swanger A, et al. Long-term deleterious impact of surgeon care fragmentation after colorectal surgery on survival: continuity of care continues to count. Dis Colon Rectum. (2017) 60:1147–54. doi: 10.1097/DCR.0000000000000919
26. Bilimoria KY, Bentrem DJ, Stewart AK, Winchester DP, Ko CY. Comparison of commission on cancer–approved and –nonapproved hospitals in the United States: implications for studies that use the national cancer data base. J Clin Oncol. (2009) 27:4177–81. doi: 10.1200/JCO.2008.21.7018
27. Paulson EC, Mitra N, Sonnad S, Armstrong K, Wirtalla C, Kelz RR, et al. National cancer institute designation predicts improved outcomes in colorectal cancer surgery. Ann Surg. (2008) 248:675–86. doi: 10.1097/SLA.0b013e318187a757
28. Monson JR, Probst CP, Wexner SD, Remzi FH, Fleshman JW, Garcia-Aguilar J, et al. Failure of evidence-based cancer care in the United States: the association between rectal cancer treatment, cancer center volume, and geography. Ann Surg. (2014) 260:625–32. doi: 10.1097/SLA.0000000000000928
29. Xu Z, Mohile SG, Tejani MA, Becerra AZ, Probst CP, Aquina CT, et al. Poor compliance with adjuvant chemotherapy use associated with poorer survival in patients with rectal cancer: an NCDB analysis. Cancer. (2017) 123:52–61. doi: 10.1002/cncr.30261
30. Glynne-Jones R, Wyrwicz L, Tiret E, Brown G, Rödel C, Cervantes A, et al. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2017) 28(Suppl. 4):iv22–40. doi: 10.1093/annonc/mdx224
31. Heins MJ, de Jong JD, Spronk I, Ho VKY, Brink M, Korevaar JC. Adherence to cancer treatment guidelines: influence of general and cancer-specific guideline characteristics. Eur J Public Health. (2016) 27:616–20. doi: 10.1093/eurpub/ckw234
32. Becerra AZ, Wexner SD, Dietz DW, Xu Z, Aquina CT, Justiniano CF, et al. Nationwide heterogeneity in hospital-specific probabilities of rectal cancer understating and its effects on outcomes. Ann Surg Oncol. (2018) 25:2332–9. doi: 10.1245/s10434-018-6530-6
33. Munro A, Brown M, Niblock P, Steele R, Carey F. Do Multidisciplinary Team (MDT) processes influence survival in patients with colorectal cancer? A population-based experience. BMC Cancer. (2015) 15:686. doi: 10.1186/s12885-015-1683-1
34. Xu Z, Berho ME, Becerra AZ, Aquina CT, Hensley BJ, Arsalanizadeh R, et al. Lymph node yield is an independent predictor of survival in rectal cancer regardless of receipt of neoadjuvant therapy. J Clin Pathol. (2017) 70:584–92. doi: 10.1136/jclinpath-2016-203995
35. Aquina C, Wexner SD, Dietz DW, Xu Z, Aquina CT, Justiniano CF, et al. The impact of age on complications, survival, and cause of death following colon cancer surgery. J Clin Oncol. (2016) 34:10012. doi: 10.1200/JCO.2016.34.15_suppl.10012
36. Wang S, Liu J, Wang S, Zhao H, Ge S, Wang W. Adverse effects of anastomotic leakage on local recurrence and survival after curative anterior resection for rectal cancer: a systematic review and meta-analysis. World J Surg. (2017) 41:277–84. doi: 10.1007/s00268-016-3761-1
37. Byrne BE, Mamidanna R, Vincent CA, Faiz O. Population-based cohort study comparing 30- and 90-day institutional mortality rates after colorectal surgery: postoperative mortality rates at 30 and 90 days. Br J Surg. (2013) 100:1810–7. doi: 10.1002/bjs.9318
38. Kowalski C, Graeven U, von Kalle C, Lang H, Beckmann MW, Blohmer JU, et al. Shifting cancer care towards multidisciplinarity: the cancer center certification program of the German cancer society. BMC Cancer. (2017) 17:850. doi: 10.1186/s12885-017-3824-1
39. Commission on Cancer. The National Accreditation Program for Rectal Cancer Standards Manual 2017 Edition (2017).
40. Brady JT, Xu Z, Scarberry KB, Saad A, Fleming FJ, Remzi FH, et al. Evaluating the current status of rectal cancer care in the US: where we stand at the start of the commission on cancer's National Accreditation Program for Rectal Cancer. J Am College of Surgeons. (2018) 226:881–90. doi: 10.1016/j.jamcollsurg.2018.01.057
41. Antunez AG, Kanters AE, Regenbogen SE. Evaluation of access to hospitals most ready to achieve national accreditation for rectal cancer treatment. JAMA Surg. (2019) 154:516–23. doi: 10.1001/jamasurg.2018.5521
42. Liu JB, Pusic AL, Matroniano A, Aryal R, Willarson PB, Hall BL, et al. First report of a multiphase pilot to measure patient-reported outcomes in the American College of Surgeons National Surgical Quality Improvement Program. Jt Comm J Qual Patient Saf. (2019) 45:319–28. doi: 10.1016/j.jcjq.2018.09.003
43. Van Cutsem E, De Gramont A, Henning G, Rougier P, Bonnetain F, Seufferlein T. Improving outcomes in patients with CRC: the role of patient reported outcomes—an ESDO report. Cancers. (2017) 9:59. doi: 10.3390/cancers9060059
Keywords: colorectal cancer, colorectal neoplasms, quality improvement, surgical outcomes, patient-reported outcomes
Citation: Xu Z and Fleming FJ (2020) Quality Assurance, Metrics, and Improving Standards in Rectal Cancer Surgery in the United States. Front. Oncol. 10:655. doi: 10.3389/fonc.2020.00655
Received: 09 January 2020; Accepted: 08 April 2020;
Published: 29 April 2020.
Edited by:
Des Winter, St. Vincent's University Hospital, IrelandReviewed by:
Kellie L. Mathis, Mayo Clinic, United StatesCarl Brown, University of British Columbia, Canada
Eric Rullier, Centre Hospitalier Universitaire de Bordeaux, France
Copyright © 2020 Xu and Fleming. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhaomin Xu, emhhb21pbl94dUB1cm1jLnJvY2hlc3Rlci5lZHU=
 Zhaomin Xu
Zhaomin Xu Fergal J. Fleming
Fergal J. Fleming