
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 03 April 2020
Sec. Radiation Oncology
Volume 10 - 2020 | https://doi.org/10.3389/fonc.2020.00469
This article is part of the Research TopicModeling for Prediction of Radiation-Induced Toxicity to Improve Therapeutic Ratio in the Modern Radiation Therapy EraView all 35 articles
Purpose: Late gastrointestinal (GI) toxicity after radiotherapy for prostate cancer may have significant impact on the cancer survivor's quality of life. To date, little is known about local dose-effects after modern radiotherapy including hypofractionation. In the current study we related the local spatial distribution of radiation dose in the rectum to late patient-reported gastrointestinal (GI) toxicities for conventionally fractionated (CF) and hypofractionated (HF) modern radiotherapy in the randomized HYPRO trial.
Material and Methods: Patients treated to 78 Gy in 2 Gy fractions (n = 298) or 64.6 Gy in 3.4 Gy fractions (n = 295) with available late toxicity questionnaires (n ≥ 2 within 1–5 years post-treatment) and available 3D planning data were eligible for this study. The majority received intensity modulated radiotherapy (IMRT). We calculated two types of dose surface maps: (1) the total delineated rectum with its central axis scaled to unity, and (2) the delineated rectum with a length of 7 cm along its central axis aligned on the prostate's half-height point (prostate-half). For each patient-reported GI symptom, dose difference maps were constructed by subtracting average co-registered EQD2 (equivalent dose in 2 Gy) dose maps of patients with and without the symptom of interest, separately for HF and CF. P-values were derived from permutation tests. We evaluated patient-reported moderate to severe GI symptoms.
Results: Observed incidences of rectal bleeding and increased stool frequency were significantly higher in the HF group. For rectal bleeding (p = 0.016), mucus discharge (p = 0.015), and fecal incontinence (p = 0.001), significant local dose-effects were observed in HF patients but not in CF patients. For rectal pain, similar local dose-effects (p < 0.05) were observed in both groups. No significant local dose-effects were observed for increased stool frequency. Total rectum mapping vs. prostate-half mapping showed similar results.
Conclusion: We demonstrated significant local dose-effect relationships for patient-reported late GI toxicity in patients treated with modern RT. HF patients were at higher risk for increased stool frequency and rectal bleeding, and showed the most pronounced local dose-effects in intermediate-high dose regions. These findings suggest that improvement of current treatment optimization protocols could lead to clinical benefit, in particular for HF treatment.
Irradiation of tumors in the pelvic area through external beam radiotherapy comes inevitably with dose delivery to nearby organs at risk, such as the rectum. The potential permanent impact of late gastrointestinal (GI) toxicity after radiotherapy may have significant impact on the cancer survivor's quality of life (1). Preventing chronic late GI toxicity is therefore critical. For this purpose understanding how we should distribute radiation dose to surrounding normal tissues while keeping toxicity risks as low as possible is critical.
The QUANTEC project (quantitative analysis of normal tissue effects in the clinic) previously summarized the available clinical data and models on acute and late radiation-induced toxicities with the goal to improve patient care by providing useful tools (2). These models were mainly derived from traditionally fractionated 3D conformal radiotherapy (3DCRT). Shortcomings and open issues of the available models have broadly been recognized, including the uncertainty of fractionation effects, a lack of reliable models for modern radiotherapy with IMRT dose distributions and image-guidance, and a lack of knowledge concerning spatial effects (3). This causes a number of deficits in current strategies of treatment planning optimization in the current era of IMRT, image-guidance, and hypofractionated treatment.
With respect to the inhomogeneous dose distributions in the rectum, achieved with either radiation technique or fractionation schedule, we can theoretically translate physical dose distributions into (radio)biological dose parameters using mathematical models derived from radiobiology (4, 5). Altered fractionation schedules in recent hypofractionation trials in prostate cancer are based on such models (6–9). However, to achieve reliable biological NTCP models for late GI toxicity after modern RT, we first have to gain insight into local dose-effects and (hypo)fractionation effects in real patient populations rather than depending solely on theoretical models.
Historically, dose-response for normal tissues were evaluated taking dose-volume distributions to a whole single organ into consideration. It is nowadays recognized that function and radiosensitivity may vary within an organ, and that dose-shapes might be relevant. Therefore, local spatial dose evaluations beyond the boundaries of delineations and dose-volume may enhance our understanding of mechanisms causing radiation-induced damage (10). In particular voxel-based dose mapping procedures have been introduced to take into account the spatial dose distribution by co-registering dose distributions to a region of interest, often using a template patient. For hollow organs such as the rectum, a spatial 2D dose distribution of the rectal wall (i.e., virtual unfolding of the rectum to a 2D structure) is considered reasonably sufficient for this purpose (11–18). Evaluation of local rectal and anal dose distributions in relation to acute and late gastrointestinal toxicity endpoints by means of dose mapping have been previously performed by several research groups. This concerned mainly patients treated with conventional fractionation schedules, identifying local dose effects for various endpoints including rectal bleeding, fecal leakage, and increased stool frequency (11–18).
In the current study we explored local rectal dose distributions and their relation to GI toxicity endpoints, for both hypofractionated (HF) and conventionally fractionated (CF) treatment, using toxicity data and planning data from the HYPRO trial. In this trial patients were randomized between conventional and hypofractionated treatment, delivered with modern radiotherapy techniques including IMRT, image-guidance, and online prostate position verification.
The dataset of a recent Dutch randomized clinical trial (HYPRO) was analyzed in which patients were randomized to 78 Gy in conventional 2 Gy fractions (CF) or 64.4 Gy in hypofractionated 3.4 Gy fractions (HF) (19). Selected patients were eligible for the current study in case both late toxicity data (n ≥ 2 questionnaires within the period 1–5 year post-treatment (N = 633, Table 2) and 3D planning data were available (which were not available for 40 patients), leaving 593 patients for the current study. Because planning of patient visits may vary from the study schedule, we accepted questionnaires up to 5.5 year post-treatment.
Based on an estimated α/β for prostate cancer of 1.5 Gy, the EQD2 was 90.4 Gy for HF vs. 78.0 Gy for CF. For normal rectal tissue with an estimated α/β of 3 Gy, the EQD2 was 82.7 Gy for HF vs. 78.0 for CF. The clinical target volume was the prostate with or without the seminal vesicles (SV): based on the estimated risk of SV involvement according Partin tables (20), a SV dose of 0 Gy, 72.2 Gy, or 78 Gy was planned (19). The outer contours of the rectum were delineated on the planning CT scan from the anal verge to the bottom of the sacro-iliac joints. The HYPRO protocol prescribed that the rectal volume receiving 83% of the prescribed dose should be below 50% for the total rectal volume or below 60% for the rectal wall. Further treatment optimization was performed in accordance with local protocols at each participating center. The applied treatment technique for 99% of the patients was image-guided IMRT with daily online positioning of the prostate. For this purpose, cone beam CT was used in 23% and portal imaging devices was used in 77% of the cases. A small proportion was treated with 3DCRT (1%). One center applied a rectal balloon, which pushes the posterior rectal wall out of the intermediate dose region (21). Further details of treatment planning have been previously reported (6, 19). CF patients received 5 fractions per week, and HF patients 3 fractions per week with 1 day intervals (Monday, Wednesday, Friday).
The patient-reported GI symptoms were extracted from a patient-reported symptom list (questionnaire) distributed in the HYPRO trial at the late time points of 6 months, and yearly between 1 and 5 year (22). Evaluated GI symptoms were: rectal bleeding, fecal incontinence, pain/cramps with stools, mucus discharge (all had to be reported as moderate or severe to be scored), and increased stool frequency ≥ 4 per day. We identified from all available questionnaires the maximum score for each toxicity endpoint of interest.
For the rectal wall the dose surface mapping was based on a central axis which was first computed as the maximum of a Euclidean distance transform. The average length of the delineated rectum along the central axis was 14.9 cm for both HF and CF. The intersections of equidistant slices perpendicular to this axis with the delineated rectum surfaces provided the corresponding locations between patients. We calculated two types of dose surface maps: (1) “total rectum mapping”: the delineated rectum with its central axis scaled to unity, and (2) “prostate half mapping”: the delineated rectum next to the prostate with a length of 7 cm along the central axis (plus 4 cm in cranial direction and minus 3 cm in caudal direction, measured from the half-height position of the prostate). These cutoffs were chosen to cover the dose range in the rectum of about 50–100% of the prescribed dose.
To correct a patient averaged dose-surface map for fractionation effects using the linear-quadratic model (i.e., equivalent dose in 2 Gy: EQD2), we applied a chosen α/β ratio of 3 Gy to the dose distribution of each patient. The resolution of the dose maps was chosen to effectively slightly exceed a 2 mm dose grid resolution. In the circumferential direction 90 pixels were taken, i.e., every 4 degrees. In the axis direction of rectum maps 100 pixels were taken, which would effectively cover a 15 cm long rectum at 1.5 mm resolution. As a final step, resulting dose-surface maps of individual patients (physical and biological) could be averaged and subtracted for each identified toxicity endpoint (yes vs. no). Further details have been previously reported (14).
Distributions of baseline characteristics within the HF and CF group were calculated and tested for differences applying a Chisquare test for the ordinal and binary variables, and a T-test for age. Associations between clinical covariates and toxicity endpoints were tested univariate using binary logistic regression. For each evaluated GI symptom, dose difference maps were constructed by subtracting average EQD2 dose maps of patients with and without the toxicity of interest, separately for HF and CF. For the calculation of a p-value for each dose difference map, we used a permutation approach, randomly re-shuffling the patients among the subgroups (23). For the determination of significant differences within a dose-difference map, we calculated and evaluated the false discovery rates “q” as a realistic estimate of the local p-values, which is a practical and powerful approach to tackle the multiple testing issue (24, 25).
The baseline characteristics of the selected study patients are summarized in Table 1 which shows that distribution of the characteristics are similar for HF and CF except for a history of TURp which was more common in the CF group (11 vs. 7%, p = 0.07, Table 1). A history of TURp was however not associated with any of the evaluated moderate to severe GI symptoms.
In Table 2, the observed incidences of the late GI toxicities of interest are summarized per treatment group, both for all patients in the HYPRO trial who filled out ≥2 late questionnaires (N = 633) and for the selected group with available CT scans and dose distributions (N = 593). These are the result of accumulation over all available questionnaires between Year 1 and Year 5, taking maximum scores. The table shows that the selected population with available dose information, was a non-biased and representative selection of the patient group that filled out late questionnaires.
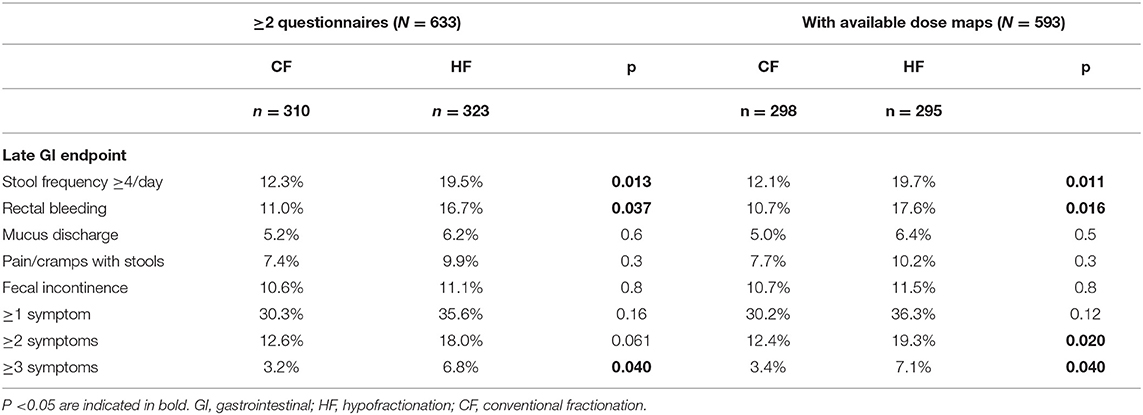
Table 2. Incidence of late gastrointestinal toxicity endpoint (evaluated by the patient as “moderate—severe”) on questionnaires in the period 1–5 year post-treatment.
About 35% of CF and HF patients experienced one or more moderate to severe late symptoms after modern RT, accumulated over the evaluated late period (Table 2). Compared to CF, significantly higher incidences after HF treatment were observed for the late endpoints of rectal bleeding and increased stool frequency. More HF patients experienced multiple moderate to severe GI symptoms.
Among the late GI endpoints of study, all endpoints showed significant correlations with the other ones (i.e., if a patient reported 1 symptom it was likely that he also reports one or more of the other symptoms). Highest correlations were observed between fecal incontinence-increased stool frequency, and rectal bleeding-mucus discharge (p < 0.001).
The results of assessing the associations between baseline covariates and the toxicity endpoints of interest are summarized in Table 3. Rectal incontinence was significantly associated with diabetes and age. Rectal bleeding and mucus discharge were significantly associated with T stage.
Figure 1 shows the average EQD2 dose-surface maps and local standard deviations for both types of mapping and for both groups (CF and HF). Comparing the EQD2 dose distributions of CF and HF, we observed that the high-dose area is darker red for HF which can be explained by the somewhat higher prescription dose in EQD2 for HF (82.7 Gy vs. 78 Gy). Furthermore, the rectal surface receiving dose levels in the range of ≥ 1–≥ 65 Gy EQD2 look very similar for HF and CF, whereas the rectal surface receiving dose levels in the range of > 65–≥ 80 Gy EQD2 were on average different with larger surfaces for HF. From previous calculations of “traditional” whole organ dose-surface histograms (DSH), it is known that indeed the average DSH of HF vs. CF only show a slightly unfavorable dose level in the range of > 65–≥ 80 Gy EQD2 (supporting DSH figure in Supplementary File). Furthermore, local standard deviations were larger for HF. The rectum adjacent to the prostate, as shown on the prostate-half maps, received dose levels in the range of 20–80 Gy, with the largest standard deviations (i.e., variation between patients) at the cranial and caudal side. The total rectum maps show dose levels in the range of 0–80 Gy, with 0–10 Gy in the most caudal 15% (the anal canal region) and the most cranial part close to the rectosigmoid region.
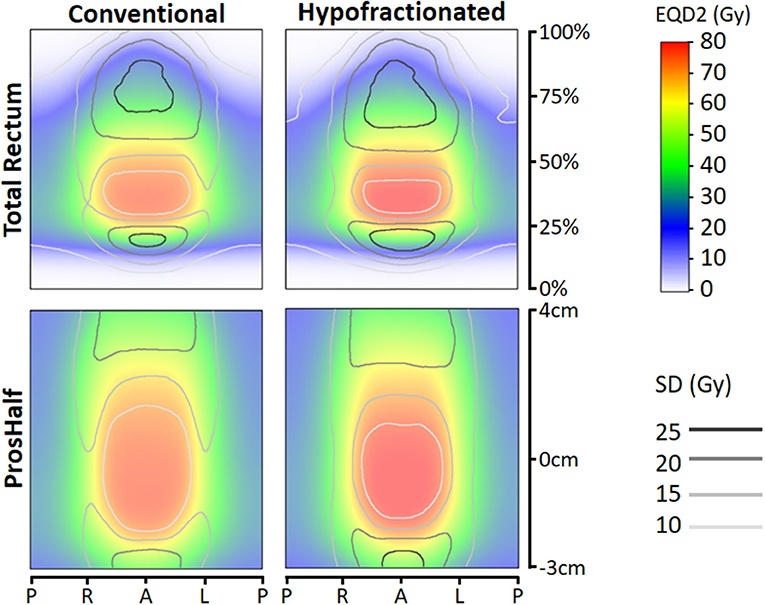
Figure 1. Total rectum (upper panes) and prostate-half (lower panes) mean dose-surface maps with distance along central axis (vertical) against location along circumference axis (horizontal). Left panes represent mean dose-surface maps of conventionally fractionated patients, right panes for hypofractionated patients. EQD2 = equivalent dose for 2 Gy fractions with α/β=3 Gy. Abbreviations: P, posterior; R, right; L, left; A, anterior; SD, standard deviation.
For each toxicity endpoint, four dose difference maps were constructed: total rectum mapping and prostate-half mapping, and for each type of mapping the HF and CF version (Figures 2, 3). In general, one or more significant dose difference maps were obtained for all GI endpoints except for increased stool frequency (lowest observed p = 0.086). All dose-difference maps were also generated with physical dose instead of EQD2 dose, to check whether this might change results. Since they were very similar to the EQD2 versions, we report here only results based on EQD2 dose maps.
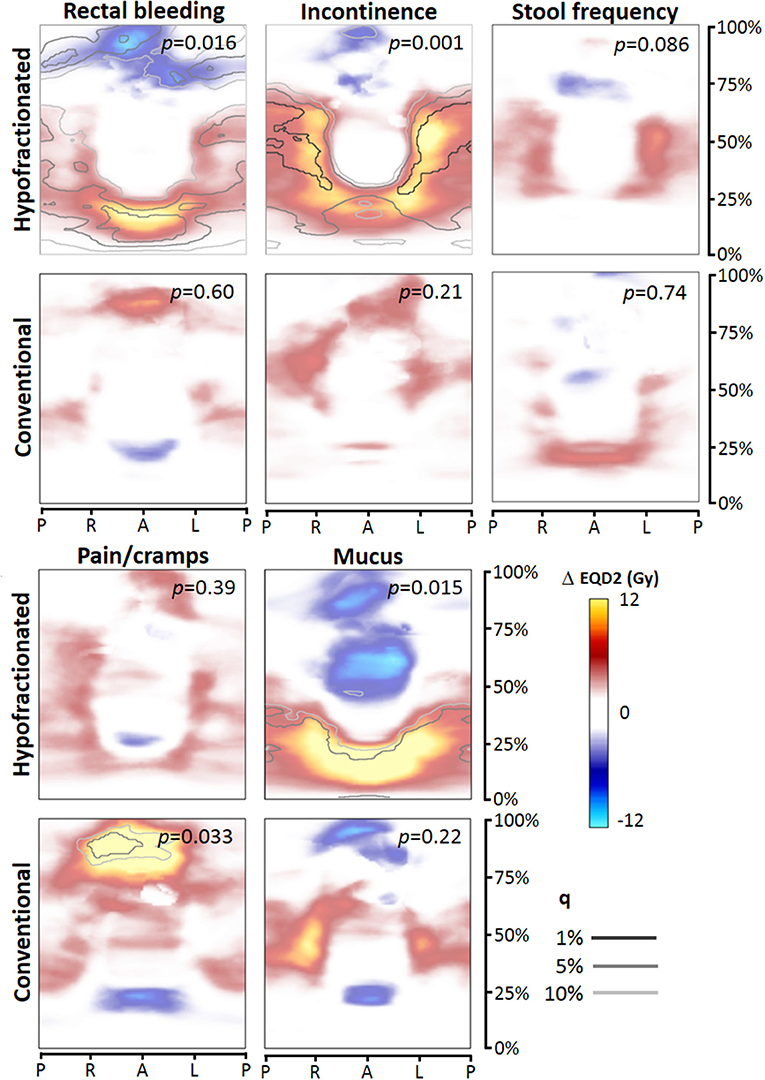
Figure 2. Dose difference maps (ΔEQD2) based on total rectum dose mapping, for the toxicity endpoints (moderate to severe), for the hypofractionated and conventional group separately. EQD2 = equivalent dose for 2 Gy fractions with α/β = 3 Gy, q = false discovery rate. Abbreviations: P, posterior; R, right; L, left; A, anterior.
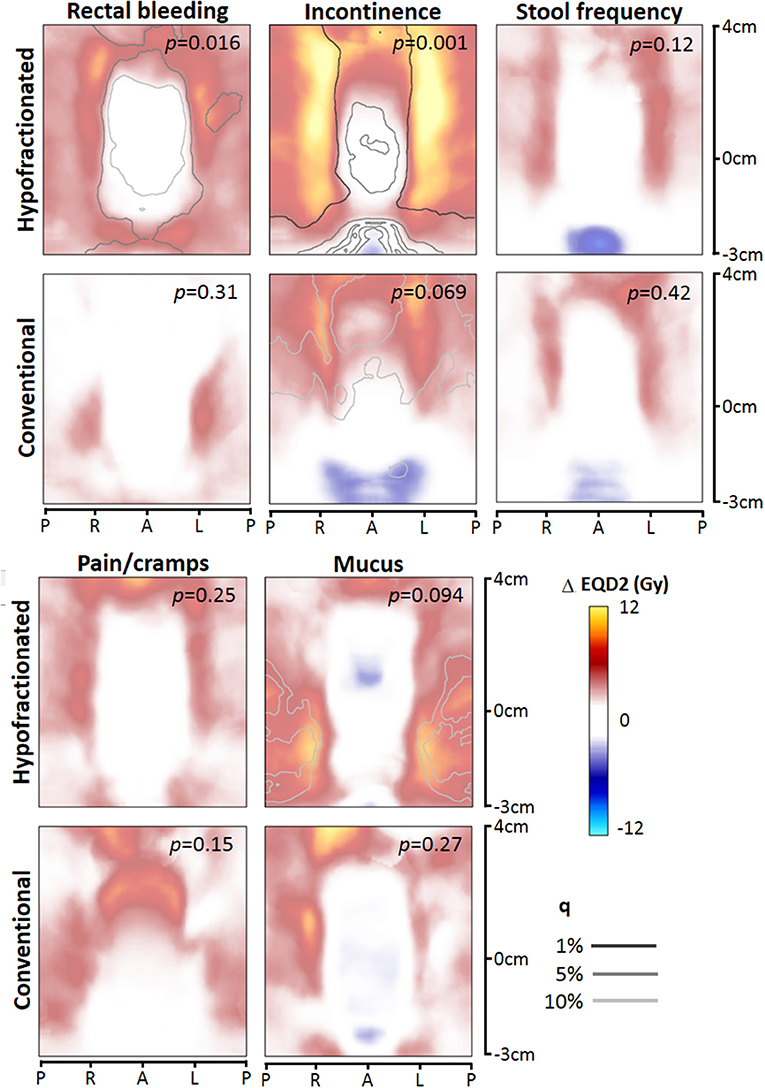
Figure 3. Dose difference maps (ΔEQD2) based on prostate-half height dose mapping, for the toxicity endpoints (moderate to severe), for the hypofractionated and conventional group separately. EQD2 = equivalent dose for 2 Gy fractions with α/β = 3 Gy, q = false discovery rate. Abbreviations: P, posterior; R, right; L, left; A, anterior.
For rectal bleeding, large local dose differences (p = 0.016) up to ≥10 Gy were observed between patients with and without this complaint (Figures 2, 3), but only for HF patients. Remarkably, the prostate-half mapping (Figure 3) indicates significant differences in the region next to the prostate, whereas the total rectum mapping (Figure 3) indicates local dose-effects at a more cranial part of the rectum. Both locations are regions were on average ≈60 Gy (EQD2) is received by the rectal tissue (Figure 1).
For the late endpoint fecal incontinence, highly significant local dose-effects were found for the region receiving intermediate to high dose, i.e., in the neighborhood of the prostate (Figures 2, 3), but again only for HF patients. For mucus discharge, we also observed local dose-effects for HF patients only, which were identified by the total rectum mapping (Figure 2). Pain/cramps with stools was associated with local dose distributions in CF patients; for HF patients no such effect was observed (Figure 2).
We explored local dose-effect relationships for GI toxicity in a study population treated with both conventionally fractionated and hypofractionated modern radiotherapy. Since both patient groups were treated within the same randomized trial, this is a unique dataset to study hypofractionation effects on rectal toxicity with a perfect internal reference group of CF patients. We observed significant local dose-effect relations for all studied GI endpoints, except for increased stool frequency. For the endpoints rectal bleeding, pain/cramps, and mucus discharge, we observed differences between HF and CF in the patterns and level of significance of local dose-effects, whereas for pain or cramps with stools, observed patterns and levels of significance were similar.
We evaluated two types of dose mapping. The “total rectum” mapping is more accurate in matching specific anatomical sub-locations within the rectum between different patients an also covers the most cranial and caudal part of the rectum, whereas the “prostate-half” mapping is more accurate in matching the intermediate-high dose areas behind the prostate from one patient to another. The identified local dose-effects for both types of mapping were similar with comparable p-values. Theoretically, we expected that the prostate-half mapping would be more accurate in identifying risks associated with high-dose regions close to the prostate and is therefore of added value to the total rectum mapping which covers the whole anorectal tract, which was demonstrated in a previous study (14). However, we could not confirm this in the current study.
In the current study we used patient-reported toxicity from a prospective setting, accumulating the incidence over available questionnaires between year 1 and 5. As a result, 30% (CF) and 36% (HF) reported ≥1 moderate to severe complaint within this period. Previously, we reported that at 36 months of follow-up, 36% (CF), and 38% (HF) had a clinically relevant deterioration on the gastrointestinal subscale of the Prostate Cancer 25 Quality of Life module (26), which is in fair agreement with the current findings based on the symptom questionnaire. As discussed in this previous paper (26), reported toxicity incidences and differences between CF and HF in the HYPRO trial are unfavorable compared to the CHHIP trial (7), which may have been caused by differences in target definition (for most HYPRO patients inclusion of the seminal vesicles), different patient population (HYPRO patients were mainly high-risk patients), and especially by a greater difference in EQD2 dose levels (with an α/β of 3 for normal tissue): 78 Gy (CF) vs. 82.7 Gy (HF) for the HYPRO trial, and 74 Gy (CF) vs. 72 Gy (HF – 20 × 3) for the CHHIP trial.
As reported by several previous studies, prospective registration translates in general into relatively high incidences of toxicity when compared to studies where only physician-reported toxicities are used, as we also previously demonstrated for the HYPRO trial (19). When we compare our patient-reported rates of rectal bleeding and fecal incontinence with the recent study of Onjukka et al. who also used patient-reported late toxicity in a modern IMRT setting with mainly conventional fractionation and partly mild hypofractionation, the reported rates are very similar for both endpoints: about 10% in both studies (18).
We found that patient-reported GI toxicity incidences were higher for HF compared to CF with respect to the endpoints ≥3 symptoms, stool frequency, and rectal bleeding. Furthermore, we demonstrated that after converting both the HF and CF dose maps with the linear-quadratic model (with α/β of 3 Gy) to EQD2, we obtained very different dose-difference maps (Figures 2, 3) where we would expect similar local dose-effects. This suggests that just by calculating EQD2 for a HF schedule, this might not completely capture the biological effect of a HF treatment. There are several differences between the HF and CF group which might have contributed to the observations of both higher incidences and different dose-difference maps: (a) applied dose constraints were based on earlier studies with CF; (b) the rectum dose for HF was on average somewhat higher because of the higher EQD2 prescription dose of 82.7 Gy with α/β = 3; (c) local dose variations (standard deviations) were larger for the HF group; and (d) HF was delivered three times a week with 3.4 Gy fractions instead of 5 times a week 2 Gy fractions.
The symptom rectal bleeding was highly correlated with mucus discharge, which can be expected since both symptoms are the result of a radiation proctitis. In the literature, the endpoint of rectal bleeding has been extensively studied and modeled since it is regarded as a dose-limiting late toxicity (3). We observed a significantly higher incidence of patient-reported moderate to severe late rectal bleeding for HF compared to CF (17.6% vs. 10.7%). We previously reported the EORTC/RTOG grade ≥2 incidence of rectal bleeding (requiring clotting time), which was also higher for HF patients (5 vs. 2%, p = 0.11). (22). For rectal bleeding pronounced local dose-effects were observed in the dose-difference maps in the moderate to high-dose rectal regions close to the prostate, but only for HF patients. The location is in general in concordance with the literature based on conventional treatment, were high-dose regions above ≈60–70 Gy are found to be relevant for rectal bleeding. Applied dose constraints in the clinic are based on these published models (3). In the HYPRO trial, rectal volumes receiving ≥83% of the prescribed dose (i.e., ≥65 Gy for CF and ≥54 Gy for HF) had to be limited at treatment optimization to ≤ 50%. Our results suggest that for HF this planning criterion was suboptimal, resulting in increased risks of rectal bleeding. However, this observation might also be in part related to the higher EQD2 prescription dose of 82.7 Gy.
We observed similar fecal incontinence rates between CF and HF, but higher rates of increased stool frequency for HF (Table 2). At the same time, these complaints were highly correlated. In a recent study of Cicchetti et al. (27), comparing CF with mild HF (2.25–2.75 Gy per fraction), higher levels of fecal incontinence were observed for mild HF compared to CF. For the endpoints increased stool frequency and fecal incontinence, dose to other neighboring structures, such as pelvic floor muscles and nerves, might be relevant as well, as reported in several studies (28, 29). However, in other studies, similar rates of fecal incontinence were observed between 3DCRT and IMRT groups whereas the latter was associated with largely reduced dose levels to the anal canal region (27, 30) which is in the same region as the pelvic floor muscles.
As previously published, the results of the HYPRO trial were negative with respect to its hypothesis, i.e., non-inferiority with respect to Grade ≥2 toxicity and superiority with respect to freedom from failure could both not be demonstrated for the HF arm (6). Therefore, is this hypofractionation schedule of 19 times 3.4 Gy not recommendable or acceptable for clinical practice. However, for studying hypofractionation effects and dose-effect relationships these data are very useful. In current clinical practice, the hypofractionation schedule of the CCHIP trial (7) and the Widmark trial (9) have been adopted by centers worldwide, in which hypofractionated treatment is distributed over several weeks of treatment with intervals >24 h between fractions, similar to the HYPRO trial. To understand more about fractionation effects and effects of intervals between fractions on late (permanent) damage to normal tissues, additional modeling of the dose and outcome data from hypofractionation trials is essential. Recently, Wilkins et al. reported on dose-effect analyses from the CCHIP trial, derived from both conventional whole organ evaluation and from spatial dose mapping, aiming at formulating novel dose constraints for mild hypofractionation regimens in 3 Gy fractions (31). They report that different rectal dose constraints were obtained for different GI symptoms. In their study, spatial dose metrics did not improve prediction compared to dose-volume information.
Data from the hypofractionated trial arm of the HYPRO trial have been used for toxicity modeling using dose-volume data and additional features derived from texture analysis (32). They reported models for the GI symptoms of fecal incontinence and rectal modeling including clinical factors, dose-volume factors, and derived texture features. From other phase III randomized hypofractionation trials (6–9, 33), there are to our knowledge no publications yet on additional dose-effect modeling.
It is nowadays broadly recognized that incorporating spatial local dose information from voxel-based organ-at-risk calculations, in contrast to whole organ evaluations, has the potential to improve NTCP models and therefore improve the quality of derived planning constraints (10). Several studies have demonstrated that spatial local dose metrics are suitable for NTCP modeling of rectal toxicity compared to traditional dose-surface (DSH) and dose-volume histograms (DVH) (12–18). Recently, Casares et al. (34) reported on the superiority of spatial metric by comparing NTCP models; they concluded that predictability of patient-reported GI toxicity increased using spatial metrics compared to DSH/DVH metrics. The HYPRO data set is a very suitable dataset for bioeffect modeling of toxicity with the goal to obtain meaningful NTCP models and related dose constraints for optimized treatment planning with modern techniques including hypofractionation. An essential question to answer prior to the modeling is: how to summarize the inhomogeneous dose distributions into meaningful dose parameters for subsequent modeling. The dose maps resulting from this study clearly show that especially intermediate-high dose areas in the rectum are associated with a number of GI symptoms, especially for HF treatment. As previously described by Bentzen et al. (4), true equieffective dose levels (with the same bioeffect) result in identical toxicity risks. Further modeling of the HYPRO data, by constructing NTCP models based on calculated EQD2 dose for each group, may demonstrate whether the calculated EQD2 levels are equieffective or whether other biological factors have to be taken into account to calculate the true biological equieffective dose. Furthermore, relevant clinical covariates have to be incorporated into such models as well to improve the predictive power of such models. As shown in Table 3, for the endpoint fecal leakage (age and diabetes) and for the endpoints rectal bleeding and mucus (T stage) predictive clinical covariates were identified. Our ultimate goal is to use the current findings to develop a biological NTCP model that correctly incorporates fractionation effects, modeling the GI toxicity as a function of biological dose. This could then theoretically be applied to all types of dose distributions including different fractionation schedules.
In conclusion, we demonstrated significant local dose-effect relationships for patient-reported late GI toxicity in patients treated with modern RT. HF patients were at higher risk for increased stool frequency and rectal bleeding, and showed the most pronounced local dose-effects in intermediate-high dose regions. These findings suggest that improvement of current treatment optimization protocols could lead to clinical benefit, in particular for HF treatment.
The datasets used in this study are available on request. The corresponding author can be contacted for this purpose.
This trial was approved by the Medical Ethics Committee of the Erasmus Medical Center in Rotterdam, the Netherlands (06-045). The patients/participants provided their written informed consent to participate in this study.
MW and WH contributed to the study design (patient selection, endpoint definitions, dose mapping procedures, statistical analyses) and writing of the drafts of the manuscript. LI and FP contributed to patient inclusion and follow-up. MW and BH contributed to data collection of dosimetric data. All authors contributed to critical reading, revision of the manuscript, and approval of the submitted version.
The HYPRO trial was financially supported by the Dutch Cancer Society (Grant No. CKTO 2006-08) which is a non-profit organization.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.00469/full#supplementary-material
1. Donovan JL, Hamdy FC, Lane JA, Mason M, Metcalfe C, Walsh E, et al. Patient reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med. (2016) 375:1425–37. doi: 10.1056/NEJMoa1606221
2. Marks LB, Yorke ED, Jackson A, Ten Haken RK, Constine LS, Eisbruch A, et al. Use of normal tissue complication probability models in the clinic. Int J Radiat Oncol Biol Phys. (2010) 76(Suppl. 3):S10–9. doi: 10.1016/j.ijrobp.2009.07.1754
3. Landoni V, Fiorino C, Cozzarini C, Sanguinet G, Valdagni R, Rancati T. Predicting toxicity in radiotherapy for prostate cancer. Phys Med. (2016) 32:521–32. doi: 10.1016/j.ejmp.2016.03.003
4. Bentzen SM, Dörr W, Gahbauer R, Howell RW, Joiner MC, Jones B, et al. Bioeffect modeling and equieffective dose concepts in radiation oncology—terminology, quantities and units. Radiother Oncol. (2012) 105:266–8. doi: 10.1016/j.radonc.2012.10.006
5. Fowler JF. The radiobiology of prostate cancer including new aspects of fractionated radiotherapy. Acta Oncol. (2005) 44:265–76. doi: 10.1080/02841860410002824
6. Incrocci L, Wortel RC, Alemayehu WG, Aluwini S, Schimmel E, Krol S, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with localised prostate cancer (HYPRO): final efficacy results from a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. (2016) 17:1061–69. doi: 10.1016/S1470-2045(16)30070-5
7. Dearnaley D, Syndikus I, Mossop H, Khoo V, Birtle A, Bloomfield D, et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. (2016) 17:1047–60. doi: 10.1016/S1470-2045(16)30102-4
8. Lee WR, Dignam JJ, Amin MB, Bruner DW, Low D, Swanson GP, et al. Randomized phase III noninferiority study comparing two radiotherapy fractionation schedules in patients with low-risk prostate cancer. J Clin Oncol. (2016) 34:2325–32. doi: 10.1200/JCO.2016.67.0448
9. Widmark A, Gunnlaugsson A, Beckman L, Thellenberg-Karlsson C, Hoyer M, Lagerlund M, et al. Ultra-hypofractionated versus conventionally fractionated radiotherapy for prostate cancer: 5-year outcomes of the HYPO-RT-PC randomised, non-inferiority, phase 3 trial. Lancet. (2019) 394:385–95. doi: 10.1016/S0140-6736(19)31131-6
10. Palma G, Monti S, Cella L. Voxel-based analysis in radiation oncology: a methodological cookbook. Phys Med. (2020) 69:192–204. doi: 10.1016/j.ejmp.2019.12.013
11. Heemsbergen WD, Hoogeman MS, Hart GA, Lebesque JV, Koper PC. Gastrointestinal toxicity and its relation to dose distributions in the anorectal region of prostate cancer patients treated with radiotherapy. Int J Radiat Oncol Biol Phys. (2005) 61:1011–8. doi: 10.1016/j.ijrobp.2004.07.724
12. Buettner F, Gulliford SL, Webb S, Sydes MR, Dearnaley DP, Partridge M. Assessing correlations between the spatial distribution of the dose to the rectal wall and late rectal toxicity after prostate radiotherapy: an analysis of data from the MRC RT01 trial (ISRCTN 47772397). Phys Med Biol. (2009) 54:6535–48. doi: 10.1088/0031-9155/54/21/006
13. Buettner F, Gulliford SL, Webb S, Sydes MR, Dearnaley DP, Partridge M. The dose-response of the anal sphincter region–an analysis of data from the MRC RT01 trial. Radiother Oncol. (2012) 103:347–52. doi: 10.1016/j.radonc.2012.03.002
14. Wortel RC, Witte MG, van der Heide UA, Pos FJ, Lebesque JV, van Herk M, et al. Dose-surface maps identifying local dose-effects for acute gastrointestinal toxicity after radiotherapy for prostate cancer. Radiother Oncol. (2015) 117:515–20. doi: 10.1016/j.radonc.2015.10.020
15. Acosta O, Drean G, Ospina JD, Simon A, Haigron P, Lafond C, de Crevoisier R. Voxel-based population analysis for correlating local dose and rectal toxicity in prostate cancer radiotherapy. Phys Med Biol. (2013) 58:2581–95. doi: 10.1088/0031-9155/58/8/2581
16. Munbodh R, Jackson A, Bauer J, Schmidtlein CR, Zelefsky MJ. Dosimetric and anatomic indicators of late rectal toxicity after high-dose intensity modulated radiation therapy for prostate cancer. Med Phys. (2008) 35:2137–50. doi: 10.1118/1.2907707
17. Moulton CR, House MJ, Lye V, Tang CI, Krawiec M, Joseph DJ, et al. Spatial features of dose-surface maps from deformably-registered plans correlate with late gastrointestinal complications. Phys Med Biol. (2017) 62:4118–39. doi: 10.1088/1361-6560/aa663d
18. Onjukka E, Fiorino C, Cicchetti A, Palorini F, Improta I, Gagliardi G, et al. Patterns in ano-rectal dose maps and the risk of late toxicity after prostate IMRT. Acta Oncol. (2019) 58:1757–64. doi: 10.1080/0284186X.2019.1635267
19. Aluwini S, Pos F, Schimmel E, van Lin E, Krol S, van der Toorn PP, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with prostate cancer (HYPRO): acute toxicity results from a randomised non-inferiority phase 3 trial. Lancet Oncol. (2015) 16:274–83. doi: 10.1016/S1470-2045(14)70482-6
20. Partin W, Mangold LA, Lamm DM, Walsh PC, Epstein JI, Pearson JD. Contemporary update of prostate cancer staging nomograms (partin tables) for the new millennium. Urology. (2001) 58:843–8. doi: 10.1016/s0090-4295(01)01441-8
21. Wortel RC, Heemsbergen WD, Smeenk RJ, Witte MG, Krol SDG, Pos FJ, et al. Local protocol variations for image guided radiation therapy in the multicenter dutch hypofractionation (HYPRO) trial: impact of rectal balloon and mri delineation on anorectal dose and gastrointestinal toxicity levels. Int J Radiat Oncol Biol Phys. (2017) 99:1243–52. doi: 10.1016/j.ijrobp.2017.07.044
22. Aluwini S, Pos F, Schimmel E, Krol S, van der Toorn PP, de Jager H, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with prostate cancer (HYPRO): late toxicity results from a randomised, non-inferiority, phase 3 trial. Lancet Oncol. (2016) 17:464–74. doi: 10.1016/S1470-2045(15)00567-7
23. Chen C, Witte M, Heemsbergen W, van Herk M, Multiple comparisons permutation test for image based data mining in radiotherapy. Radiat Oncol. (2013) 8:293. doi: 10.1186/1748-717X-8-293
24. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc B. (1995) 57:289–300.
25. Dréan G, Acosta O, Ospina JD, Fargeas A, Lafond C, Corrégé G, et al. Identification of a rectal subregion highly predictive of rectal bleeding in prostate cancer IMRT. Radiother Oncol. (2016) 119:388–97. doi: 10.1016/j.radonc.2016.04.023
26. Wortel RC, Oomen-de Hoop E, Heemsbergen WD, Pos FJ, Incrocci L. Moderate hypofractionation in intermediate- and high-risk, localized prostate cancer: health-related quality of life from the randomized, phase 3 hypro trial. Int J Radiat Oncol Biol Phys. (2019) 103:823–33. doi: 10.1016/j.ijrobp.2018.11.020
27. Cicchetti A, Avuzzi B, Palorini F, Ballarini F, Stucchi C, Fellin G, et al. Predicting late fecal incontinence risk after radiation therapy for prostate cancer: new insights from external independent validation. Int J Radiat Oncol Biol Phys. (2018) 102:127–36. doi: 10.1016/j.ijrobp.2018.05.013
28. Schaake W, van der Schaaf A, van Dijk LV, Bongaerts AH, van den Bergh AC, Langendijk JA. Normal tissue complication probability (NTCP) models for late rectal bleeding, stool frequency and fecal incontinence after radiotherapy in prostate cancer patients. Radiother Oncol. (2016) 119:381–7. doi: 10.1016/j.radonc.2016.04.005
29. Smeenk RJ, Hoffmann AL, Hopman WP, van Lin EN, Kaanders JH. Dose-effect relationships for individual pelvic floor muscles and anorectal complaints after prostate radiotherapy. Int J Radiat Oncol Biol Phys. (2012) 83:636–44. doi: 10.1016/j.ijrobp.2011.08.007
30. Wortel RC, Incrocci L, Pos FJ, van der Heide UA, Lebesque JV, Aluwini S, et al. Late side effects after image guided intensity modulated radiation therapy compared to 3D-conformal radiation therapy for prostate cancer: results from 2 prospective cohorts. Int J Radiat Oncol Biol Phys. (2016) 95:680–9. doi: 10.1016/j.ijrobp.2016.01.031
31. Wilkins A, Naismith O, Brand D, Fernandez K, Hall E, Dearnaley D, et al. Derivation of dose/volume constraints for the anorectum from clinician- and patient-reported outcomes in the chhip trial of radiation therapy fractionation. Int J Radiat Oncol Biol Phys. (2020) 106:928. doi: 10.1016/j.ijrobp.2020.01.003
32. Rossi L, Bijman R, Schillemans W, Aluwini S, Cavedon C, Witte M, et al. Texture analysis of 3D dose distributions for predictive modelling of toxicity rates in radiotherapy. Radiother Oncol. (2018) 129:548–53. doi: 10.1016/j.radonc.2018.07.027
33. Catton CN, Lukka H, Gu CS, Martin JM, Supiot S, Chung PWM, et al. Randomized trial of a hypofractionated radiation regimen for the treatment of localized prostate cancer. J Clin Oncol. (2017) 35:1884–90. doi: 10.1200/JCO.2016.71.7397
Keywords: prostate cancer, hypofractionation, gastrointestinal toxicity, dose-surface maps, radiotherapy, NTCP
Citation: Heemsbergen WD, Incrocci L, Pos FJ, Heijmen BJM and Witte MG (2020) Local Dose Effects for Late Gastrointestinal Toxicity After Hypofractionated and Conventionally Fractionated Modern Radiotherapy for Prostate Cancer in the HYPRO Trial. Front. Oncol. 10:469. doi: 10.3389/fonc.2020.00469
Received: 16 December 2019; Accepted: 16 March 2020;
Published: 03 April 2020.
Edited by:
Tiziana Rancati, National Cancer Institute Foundation (IRCCS), ItalyReviewed by:
Laura Cella, Italian National Research Council, ItalyCopyright © 2020 Heemsbergen, Incrocci, Pos, Heijmen and Witte. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wilma D. Heemsbergen, dy5oZWVtc2JlcmdlbkBlcmFzbXVzbWMubmw=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.