- 1Osteoncology and Rare Tumors Center, Istituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori (IRST) IRCCS, Meldola, Italy
- 2Unit of Biostatistics and Clinical Trials, Istituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori (IRST) IRCCS, Meldola, Italy
- 3Nuclear Medicine Unit, Istituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori (IRST) IRCCS, Meldola, Italy
Background: Several phase-II trials have been designed to evaluate tyrosine kinase inhibitors (TKIs), in particular, pazopanib in neuroendocrine neoplasia (NEN), but its efficacy has not yet been demonstrated in a randomised-controlled Phase III trial. A systematic review of the published clinical trials of metastatic NEN patients could reduce the possible bias of single phase II studies. The present systematic review focuses on the efficacy and safety of pazopanib in patients with metastatic and locally advanced NEN.
Methods: A systematic search in the major databases Medline/PubMed, Cochrane and Embase and in supplementary material from important international Meetings was performed to identify publications on pazopanib for the treatment of neuroendocrine neoplasia. English language was defined as a restriction. Four authors of the present review independently performed the study selection, assessed the risk of bias and extracted study data. Four published clinical trials and 2 abstracts were identified. One trial was excluded because the topic was Von-Hippel Landau disease and one abstract was eliminated because of the lack of information on meeting proceedings.
Results: In all of the trials pazopanib was orally administered at a dose of 800 mg daily continuously with a 28-day cycle. The intention-to-treat population for efficacy was composed of 230 patients with a median age of 62 years. The partial response rate was 10.7% (95% confidence interval 2.6–20.5). The rate for stable disease was 79.6% (range: 61.7–92.1%) with a disease control rate (DCR) of 90.3%. Progressive disease was reported in 9.7% (range 5.2–17.6) of patients. No complete responses were observed. Median progression-free survival was 11.6 months (95% CI: 9.2–13.9). Overall survival from all the trials was 24.6 (95% CI: 18.7–40.8) months. Severe adverse events (grade III–IV) included hypertension 31%, 16% increase in AST/ALT, diarrhoea 10% and fatigue 10%.
Conclusions: Pazopanib monotherapy achieved a DCR of 90.3% in patients with locally advanced and/or metastatic neuroendocrine neoplasia, with an overall response rate comparable to other TKIs and mTOR inhibitors and a safety profile similar to that of drugs of the same class.
Introduction
Rationale
Lung and gastroenteropancreatic (GEP) neuroendocrine tumours (NETs) are a heterogeneous group of malignancies derived from neuroendocrine cell compartments in various organs (1). A significant increase in the incidence of NETs over time has been reported ranging from 2.5 to 5 cases per 100,000 in Caucasian population (2–5). In unresectable or metastatic NETs, systemic treatment options are limited but in recent years there has been a renewed interest in expanding the therapeutic armamentarium (6). In particular, whilst in GEP-NETs the activity and safety of several compounds has been explored, in lung NETs only few drugs have been tested and the choice of treatment is often based on GEP-NET studies (7, 8).
NETs have been identified as hypervascular tumours. Vascular endothelial growth factor (VEGF) and VEGF receptors (VEGFRs) are usually overexpressed and are associated with poor prognosis (9). However, a modest clinical activity with bevacizumab, a monoclonal antibody targeting VEGF, has been observed in advanced neuroendocrine tumours in phase II studies (10, 11). In a phase III trial, sunitinib showed a superior efficacy to placebo in terms of progression-free survival (PFS) (11.4 vs. 5.5 months) leading to FDA and EMA approval for use in patients with advanced pancreatic NETs (pNETs) (12).
Pazopanib is an oral multitargeted tyrosine kinase inhibitor acting through VEGFR types 1–3, fibroblast-derived growth factor receptors (FGFR 1, 3, and 4), platelet-derived growth factor receptors α and β, and stem-cell factor receptor (c-Kit) (13, 14). Studies in vitro have shown that pazopanib inhibits ligand-induced autophosphorylation of VEGFR-2 PDGF-induced phosphorylation of c-Kit and PDGFRβ and VEGF-induced proliferation (13). In vivo pazopanib is known to inhibit FGF- and VEGF-induced angiogenesis in mouse models and has shown antitumour activity in different human models of solid tumours (15).
In one phase I trial, a patient with unknown primary neuroendocrine tumour obtained a partial response (PR) from treatment with pazopanib (16). Nevertheless, there are limited and non-conclusive data on the efficacy of tyrosine kinase inhibitors (TKIs) in both pNETs and non-pNETs, especially in those originating from the colorectum and small intestine where the incidence of the disease is high (6, 17).
Objectives
The aim of this systematic review was to evaluate the published studies assessing the activity and safety of pazopanib in patients with metastatic NEN (mNEN).
Research Questions
- Activity of pazopanib in patients with mNEN
- Safety of pazopanib in patients with mNEN
- Role of pazopanib in the therapeutic scenario of mNEN.
Methods
Study Design
We report the results of a phase II systematic review and meta-analysis on the activity and safety of pazopanib in patients with mNEN. This study was performed according to PRISMA guidelines (18, 19)(see Supplementary Materials). The quality of included studies was assessed using the Downs and Black checklist (D&B checklist), which is appropriate for both randomised and non-randomised clinical trials. This checklist consists of 27 items distributed between five subscales. The total maximum score is 32. A study scoring 16 or more is ranked as a high quality study (20).
Participants, Interventions, Comparator
We included all articles with prospective data on mNEN in adult patients treated with pazopanib. All of the studies included were in the English language.
Systematic Review Protocol
We developed a protocol that had pre-specified objectives, eligibility criteria, data of interest, search strategy, and analysis plan. The present systematic review was registered in the PROSPERO database.
Data Source Study Section and Data Extraction
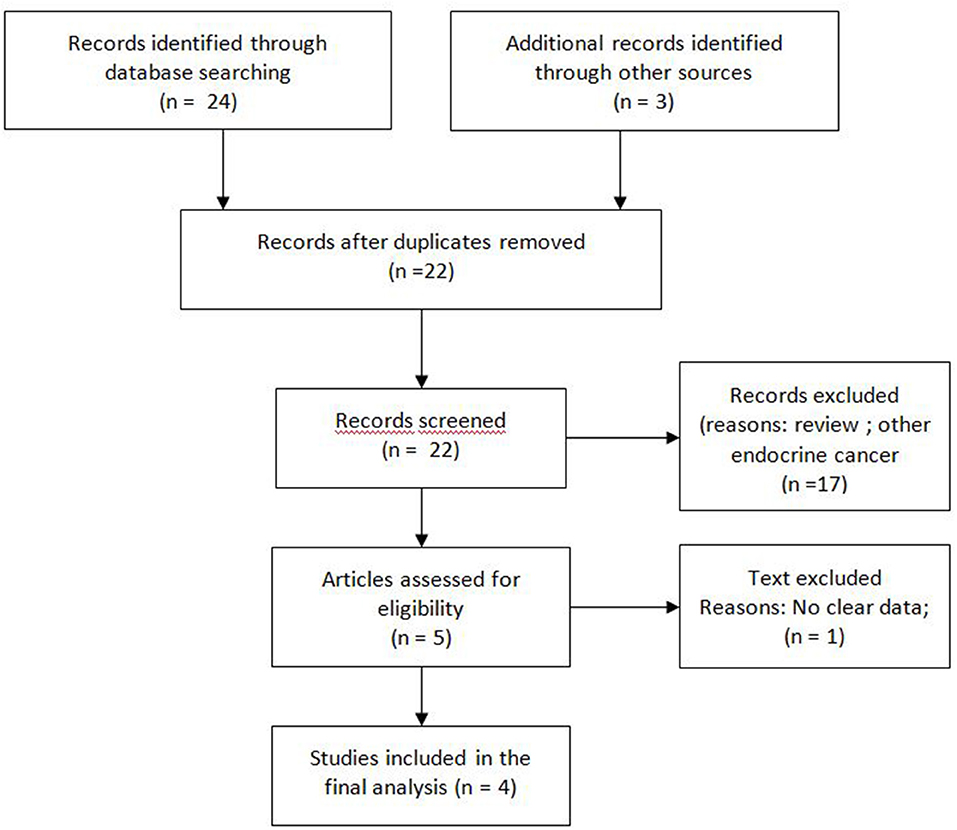
A search of the major databases Medline/PubMed, Cochrane and Embase was performed to identify publications on pazopanib for the treatment of neuroendocrine neoplasia (21). Search terms used included “pazopanib” and/or “neuroendocrine.” A supplementary search of congress abstracts published between 2014 and 2019 was also carried out for the annual meetings of the American Society of Clinical Oncology (ASCO), ASCO Gastrointestinal Symposium (ASCO-GI), and European Society for Medical Oncology (ESMO). A manual search of the references of retrieved articles for additional relevant publications was also performed. References from systematic reviews and meta-analyses were screened to ensure search sensitivity (Figure 1).
Two authors independently conducted a preliminary screening of reports by reading titles and abstracts. Duplicate publications were removed. All identified citations were reviewed and those considered unrelated were excluded. The full texts of potentially relevant articles were then downloaded for the second round of screening. When disagreement existed, two authors discussed with a third reviewer to reach a final decision. Data from included studies describing the population treated as well as treatment efficacy and toxicity parameters were extracted and pooled.
For each study, the following data were collected and tabularised for the analysis: year of publication, name of the first author, area of study; study design; baseline characteristics of patients included; intervention including regimens, dosages and cycles; outcomes including overall response rate (ORR), disease control rate (DCR), progression-free survival (PFS) and overall survival (OS); toxicities including those of a haematological and non-haematological nature.
Statistical Analysis
For survival primary endpoints, meta-analyses usually deal with hazard ratios which can only be obtained when the experimental treatment is compared to a control treatment. However, single-arm exploratory phase II studies aimed at estimating the survival curve are far from rare, especially in the area of rare tumours. In this scenario, the PFS and OS curves are usually summarised by medians and accompanied by their 95% confidence interval (95% CI), as is the case of the present review. Following the method used by McGrath et al., pooled estimates were obtained as the median of the study-specific PFS and OS medians (22), whereas the corresponding 95%CIs were obtained as the quantiles of the k observed study medians, with zα the α quantile of the standard normal distribution.
Heterogeneity between the median PFS and OS of studies was evaluated using the I2 index that quantifies values higher than 50%, indicating sizable heterogeneity. Furthermore, the Cochran Q-test was used to infer the null hypothesis between study homogeneity at a significance level α = 0.10.
All of the statistical analyses were performed with the statistical language R version 3.6.1. The metamedian package was used to compute the pooled estimates and their 95% CIs, while the ad hoc code was used to compute the I2 index and infer homogeneity via the Cochran Q-test.
Results
Study Selection and Characteristics
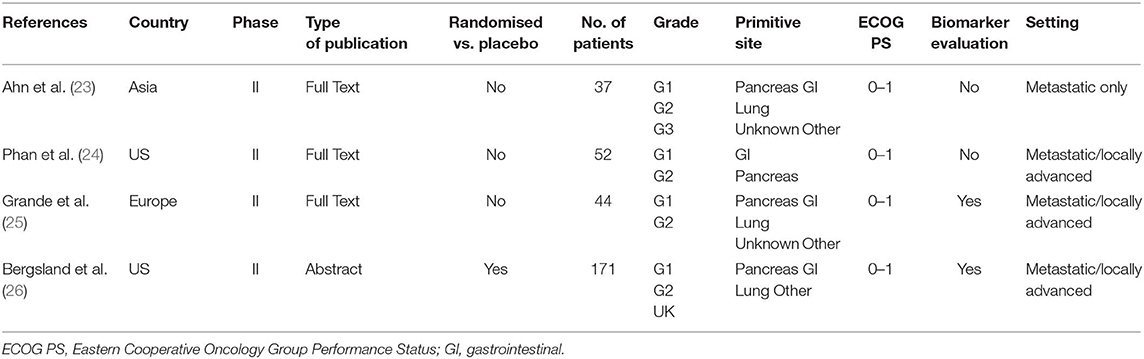
The systematic search of the literature identified four studies meeting selection criteria (Figure 1): three peer-reviewed journal publications [(23–25) and one conference abstract/poster (24)]. Briefly, one randomised and three non-randomised prospective phase II studies included a total of 304 patients of whom 74 were treated with placebo. Three studies were multicentric and only one was monocentric (23). Two studies had an independent review (23, 26). All the studies were of high quality according to the D&B checklist. Patient number, tumour histology (grade and primitive site), Eastern Cooperative Oncology Group Performance Status (ECOG PS) and other characteristics of each study are shown in Table 1.
Summary of Findings
Population Characteristics
A total of 304 patients were included in the selected trials. Progressive disease during other previous treatment was found at the time of enrolment in 283 (93.1%) patients. Previous therapies included somatostatin analogues (SSA) in 177 (58.2%) patients, other TKIs in 16 (5.2%), everolimus in 25 (8.2%), both TKI and everolimus in 8 (2.6%), chemotherapy in 56 (18.4%), hepatic locoregional treatment in 38 (12.5%) and other non-specified treatments in 19 (6.2%). One hundred fourteen (37.5%) patients had tumours of gastrointestinal (GI) origin, while the remaining (190, 62.5%) had NEN of lung, pancreatic and unknown origin. The majority of patients (76.3%) had grade 1 or 2 NEN and 15 (5%) had grade 3 NEN. Tumour grade was unknown in 58 (18.7%) patients. Seventy patients had a functioning tumour (23%). SSAs were administered together with pazopanib in 230 (75.6%) patients.
Clinical Outcomes
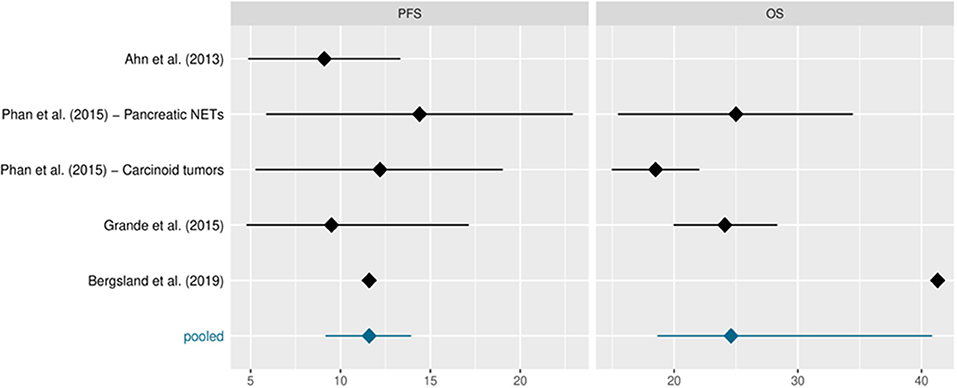
The intention-to-treat population treated with pazopanib comprised 230 patients, excluding 74 patients in the Bergsland study who were treated with placebo. Table 2 shows the study sample sizes or those of the various study arms when reported in the protocol. Median PFS and OS, reported in months, are also included along with their 95%CIs, whenever available. The data derive from single-arm phase II studies, with the exception of Bergsland et al.'s study (26) which was a phase II randomised controlled trial (for the purposes of this review we only considered the experimental pazopanib arm). Phan et al. (24) reported distinct median PFS and OS for patients with pNETs and carcinoid tumours, respectively. Ahn et al. (23) did not evaluate OS and therefore the pooled median was based on the remaining values. Bergsland et al. (26) did not report 95%CIs for PFS or OS. A response to pazopanib was reported in 186 patients. The studies registered stable disease (SD) in 148 (79.5%; range: 95% CI 61.7–92.1%) patients, partial response (PR) in 20 (10.7%; 95% CI, range 2.6–20.5%) and progressive disease (PD) in 18 (9.7%; 95% CI range: 5.8%−17.6%). No complete responses were observed. The DCR was 90.3%. Median PFS and OS from all trials was 11.6 (95% CI: 9.2, 13.9) and 24.6 (95% CI: 18.7, 40.8) months, respectively (Figure 2 and Table 2).
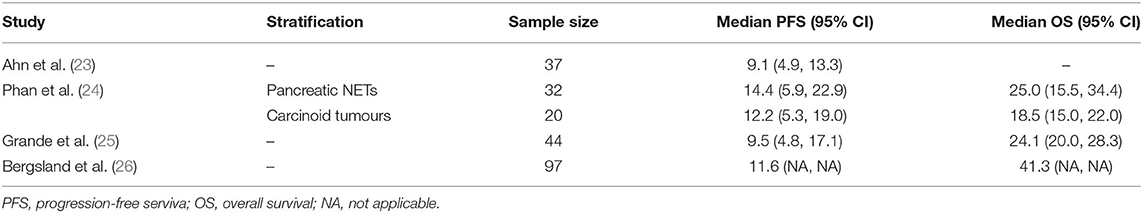
Table 2. Sample sizes and median PFS and OS in months along with their 95% confidence intervals (CIs).
Side-Effects
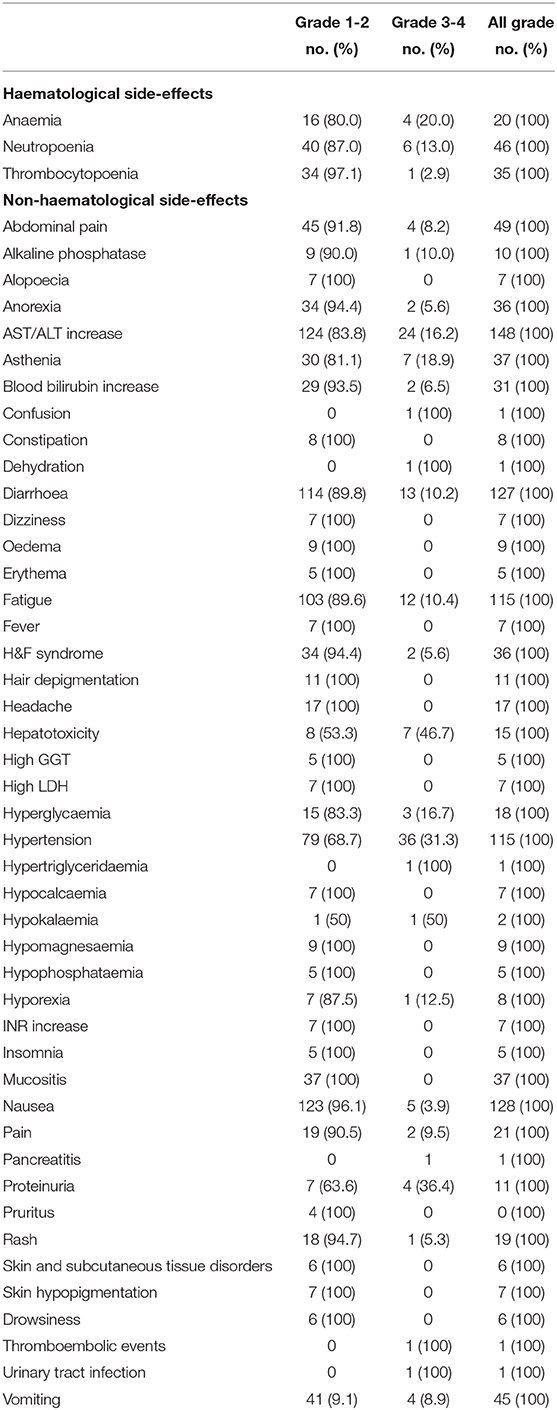
Safety outcomes are presented in Table 3. The rate of G1-G4 toxicities experienced was 70%. The most frequent adverse events were fatigue (65%), hypertension (50%), neutropoenia (26.5%), mucositis (16%), H&F syndrome (15.6%), thrombocytopoenia (15.2%), anaemia (9.1%) and proteinuria (4.7%). The rate of grade (G)3-4 toxicity was 45.2%. The most frequent G3-G4 adverse event was hypertension (15.6%).
Risk of Bias
The studies included in this systematic review were phase II studies. The fact that we included the survival estimates of the pazopanib arm in Bergsland et al.'s study (26) eliminates the potential drawbacks of considering trials with different designs. Similarly, the study by Phan et al. (24) reported distinct median PFS and OS for both pNET and carcinoid tumour arms. We considered these values in the meta-analysis because they came from different studies. The relative similarity between median survival estimates, especially for PFS, partially safeguarded against extreme results.
Discussion
Summary of Main Findings
Phase II trials provide a valuable insight into diseases, treatment efficacy and safety, especially in settings where is it difficult to carry out large randomised phase III clinical studies i.e., in the area of rare tumours. In a phase II setting, surrogate endpoints are usually taken into consideration as an early sign of drug activity and can facilitate the decision-making about whether to proceed with phase III testing. Sunitinib is still the only approved TKI for the treatment of advanced pNETs, showing a clear impact in terms of PFS and ORR. However, despite an initial benefit, sunitinib inevitably loses its effectiveness because of the activation of downstream pathways that induce resistance, leading to increased invasiveness and metastasis (27, 28). Peptide radionuclide receptor therapy (PRRT), chemotherapy and everolimus are other therapeutic options, but patients progressing on these treatments are left with few, if any, alternatives (29).
To the best of our knowledge, the present systematic review is the first to assess phase II literature on the effectiveness of pazopanib in NEN. Pazopanib achieved a DCR of 91.3% and a median PFS and OS of 11.6 and 24.6 months, respectively, superior to results of other targeted therapies in the same setting (DCR ranging from 72 to 84% and median PFS of 11–12.6 months) (12, 30–32). Of note, although half of the patients were pretreated, the pazopanib activity was maintained. Furthermore, the addition of SSAs would appear to promote a synergistic effect, increasing the DCR in this patient subgroup. A recently published network meta-analysis supports this hypothesis of the additional effect of the SSA combination with other therapies (33).
Recently, some phase II trials have been carried out to obtain a breakthrough therapy designation from the regulatory authorities for tumours whose therapeutic armamentarium is limited (34, 35). However, the interpretation of data from phase II trials has faced difficulties because of the lack of a control group, hampering direct and scientifically robust comparisons, and small patient samples. The added value of a phase II systematic review and meta-analysis could help to overcome the problem of sample size for patients treated in single trials and amplify the efficacy data of a drug evaluated prospectively in small studies.
Safety profile is also crucial factor. The results of the present review indicate that pazopanib carries a substantial risk of adverse events that can affect patient quality of life. However, the incidence of G3-G4 toxicities reported in the largest and most recent trial was 15% lower than that of previous studies. These data suggest an increasing familiarity with pazopanib over time due to its ł widespread use, and a better management of it side-effects. Overall, given that pazopanib seems to have a disease control rather than curative effect in NENs, quality of life should be take in consideration in future prospective studies.
Limitations
This study has some limitations. We conducted a comprehensive literature search with a sensitive search algorithm and an extensive manual search of reference lists and conference proceedings. However, we were unable to obtain additional unpublished data and are aware that a substantial amount of information is not available to the public. Another limitation is the low number of phase II clinical trials with different types of study design and populations included. Despite this, we believe that our results could provide important indications for the design of future dedicated clinical trials on NETs to underline the importance of head-to-head comparisons and the correct patient setting. Furthermore, the addition of SSAs to experimental drugs could be taken into consideration when designing dedicated trials on NETs.
Conclusions
Overall, our current pooled analyses of data on pazopanib in phase II studies are essentially consistent with the data available for other approved drugs. Surprisingly, although pazopanib was one of the first and most widely studied TKIs in neuroendocrine tumours, it has not moved to phase III. For this reason and because of the rarity of the disease, we decided to further investigate pazopanib activity in terms of DCR and mPFS. The clinical information available supports the use of pazopanib for the treatment of metastatic neuroendocrine tumours of different origin, especially those of the gastrointestinal tract.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Author Contributions
AB, CL, and TI conceived the idea for the study and drafted the article. GD, VF, CC, and FR were responsible for data acquisition. SN, SS, AV, and AD performed the meta-analysis and co-drafted the manuscript. CS, GM, and LM assessed the quality of the manuscript independently through the Downs and Black checklist. All authors read and approved the present version of the paper for submission.
Acknowledgements
The authors thank Gráinne Tierney and Cristiano Verna for editorial assistance.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.00414/full#supplementary-material
References
1. Kulke MH, Shah MH, Benson AB III, Bergsland E, Berlin JD, Blaszkowsky LS, et al. Neuroendocrine tumors, version 1.2015. J Natl Compr Canc Netw. (2015) 13:78–108. doi: 10.6004/jnccn.2015.0011
2. Fraenkel M, Kim MK, Faggiano A, Valk GD. Epidemiology of gastroenteropancreatic neuroendocrine tumours. Best Pract Res Clin Gastroenterol. (2012) 26:691–703. doi: 10.1016/j.bpg.2013.01.006
3. Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. (2017) 3:1335–42. doi: 10.1001/jamaoncol.2017.0589
4. Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, et al. One hundred years after “carcinoid”: Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. (2008) 26:3063–72. doi: 10.1200/JCO.2007.15.4377
5. Modlin IM, Champaneria MC, Chan AK, Kidd M. A threedecade analysis of 3,911 small intestinal neuroendocrine tumors: The rapid pace of no progress. Am J Gastroenterol. (2007) 102:1464–73. doi: 10.1111/j.1572-0241.2007.01185.x
6. Grillo F, Florio T, Ferraù F, Kara E, Fanciulli G, Faggiano A, et al. Emerging multitarget tyrosine kinase inhibitors in the treatment of neuroendocrine neoplasms. Endocr Relat Cancer. (2018) 25:R453–66. doi: 10.1530/ERC-17-0531
7. Yao JC, Fazio N, Singh S, Buzzoni R, Carnaghi C, Wolin E, et al. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a randomised, placebo-controlled, phase 3 study. Lancet. (2016) 387:968–77. doi: 10.1016/S0140-6736(15)00817-X
8. Bongiovanni A, Recine F, Riva N, Foca F, Liverani C, Mercatali L, et al. Outcome analysis of first-line somatostatin analog treatment in metastatic pulmonary neuroendocrine tumors and prognostic significance of 18FDG-PET/CT. Clin Lung Cancer. (2017) 18:415–20. doi: 10.1016/j.cllc.2016.11.004
9. Terris B, Scoazec JY, Rubbia L, Bregeaud L, Pepper MS, Ruszniewski P, et al. Expression of vascular endothelial growth factor in digestive neuroendocrine tumours. Histopathology. (1998) 32:133–8. doi: 10.1046/j.1365-2559.1998.00321.x
10. Yao JC, Phan A, Hoff PM, Chen HX, Charnsangavej C, Yeung SC, et al. Targeting vascular endothelial growth factor in advanced carcinoid tumor: a random assignment phase II study of depot octreotide with bevacizumab and pegylated interferon alpha-2b. J Clin Oncol. (2008) 26:1316–23. doi: 10.1200/JCO.2007.13.6374
11. Chan JA, Stuart K, Earle CC, Clark JW, Bhargava P, Miksad R, et al. Prospective study of bevacizumab plus temozolomide in patients with advanced neuroendocrine tumors. J Clin Oncol. (2012) 30:2963–8. doi: 10.1200/JCO.2011.40.3147
12. Raymond E, Dahan L, Raoul JL, Bang YJ, Borbath I, Lombard-Bohas C, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. (2011) 364:501–13. doi: 10.1056/NEJMoa1003825
13. Kumar R, Knick VB, Rudolph SK, Johnson JH, Crosby RM, Crouthamel MC, et al. Pharmacokinetic-pharmacodynamic correlation from mouse to human with pazopanib, a multikinase angiogenesis inhibitor with potent antitumor and antiangiogenic activity. Mol Cancer Ther. (2007) 6:2012–21. doi: 10.1158/1535-7163.MCT-07-0193
14. Hamberg P, Verweij J, Sleijfer S. (Pre-)clinical pharmacology and activity of pazopanib, a novel multikinase angiogenesis inhibitor. Oncologist. (2010) 15:539–47. doi: 10.1634/theoncologist.2009-0274
15. Li H, Wozniak A, Sciot R, Cornillie J, Wellens J, Van Looy T, et al. Pazopanib, a receptor tyrosine kinase inhibitor, suppresses tumor growth through angiogenesis in dedifferentiated liposarcoma xenograft models. Transl Oncol. (2014) 7:665–71. doi: 10.1016/j.tranon.2014.09.007
16. Hurwitz HI, Dowlati A, Saini S, Savage S, Suttle AB, Gibson DM, et al. Phase I trial of pazopanib in patients with advanced cancer. Clin Cancer Res. (2009) 15:4220–7. doi: 10.1158/1078-0432.CCR-08-2740
17. Kwekkeboom DJ. Pazopanib: a new drug for pancreatic neuroendocrine tumours. Lancet Oncol. (2015) 16:606–7. doi: 10.1016/S1470-2045(15)70200-7
18. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. (2009) 151:264–9, W64. doi: 10.7326/0003-4819-151-4-200908180-00135
19. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. (2009) 151:W65–94. doi: 10.7326/0003-4819-151-4-200908180-00136
20. Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. (1998) 52:377–84. doi: 10.1136/jech.52.6.377
21. Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration (2018). Available online at: http://handbook.cochrane.org (accessed January 8, 2019).
22. McGrath S, Zhao X, Qin ZZ, Steele R, Benedetti A. One-sample aggregate data meta-analysis of medians. Stat Med. (2019) 38:969–84. doi: 10.1002/sim.8013
23. Ahn HK, Choi JY, Kim KM, Kim H, Choi SH, Park SH, et al. Phase II study of pazopanib monotherapy in metastatic gastroenteropancreatic neuroendocrine tumours. Br J Cancer. (2013) 109:1414–9. doi: 10.1038/bjc.2013.470
24. Phan AT, Halperin DM, Chan JA, Fogelman DR, Hess KR, Malinowski P, et al. Pazopanib and depot octreotide in advanced, well-differentiated neuroendocrine tumours: a multicentre, single-group, phase 2 study. Lancet Oncol. (2015) 16:695–703. doi: 10.1016/S1470-2045(15)70136-1
25. Grande E, Capdevila J, Castellano D, Teulé A, Durán I, Fuster J, et al. Pazopanib in pretreated advanced neuroendocrine tumors: a phase II, open-label trial of the Spanish Task Force Group for Neuroendocrine Tumors (GETNE). Ann Oncol. (2015) 26:1987–93. doi: 10.1093/annonc/mdv252
26. Bergsland EK, Mahoney MR, Timothy AR, Hall N, Kumthekar P, Maitland ML, et al. Prospective randomized phase II trial of pazopanib versus placebo in patients with progressive carcinoid tumors (CARC) (Alliance A021202). J Clin Oncol. (2019) 37(15 Suppl):4005. doi: 10.1200/JCO.2019.37.15_suppl.4005
27. Faivre S, Delbaldo C, Vera K, Robert C, Lozahic S, Lassau N, et al. Safety, pharmacokinetic, and antitumor activity of SU11248, anovel oral multitarget tyrosine kinase inhibitor, in patients with cancer. J Clin Oncol. (2006) 24:25–35. doi: 10.1200/JCO.2005.02.2194
28. Casanovas O, Hicklin DJ, Bergers G, Hanahan D. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell. (2005) 8:299–309. doi: 10.1016/j.ccr.2005.09.005
29. Perez K, Chan J. Treatment of gastroenteropancreatic neuroendocrine tumors. Surg Pathol Clin. (2019) 12:1045–53. doi: 10.1016/j.path.2019.08.011
30. Yao JC, Pavel M, Lombard-Bohas C, Van Cutsem E, Voi M, Brandt U, et al. Everolimus for the treatment of advanced pancreatic neuroendocrine tumors: overall survival and circulating biomarkers from the randomized, Phase III RADIANT-3 Study. J Clin Oncol. (2016) 34:3906–13. doi: 10.1200/JCO.2016.68.0702
31. Faivre S, Niccoli P, Castellano D, Valle JW, Hammel P, Raoul JL, et al. Sunitinib in pancreatic neuroendocrine tumors: updated progression-free survival and final overall survival from a phase III randomized study. Ann Oncol. (2017) 28:339–43. doi: 10.1093/annonc/mdw561
32. Pavel ME, Hainsworth JD, Baudin E, Peeters M, Hörsch D, Winkler RE, et al. Everolimus plus octreotide long-acting repeatable for the treatment of advanced neuroendocrine tumours associated with carcinoid syndrome (RADIANT-2): a randomised, placebo-controlled, phase 3 study. Lancet. (2011) 378:2005–12. doi: 10.1016/S0140-6736(11)61742-X
33. Kaderli RM, Spanjol M, Kollár A, Bütikofer L, Gloy V, Dumont RA, et al. Therapeutic options for neuroendocrine tumors: a systematic review and network meta-analysis. JAMA Oncol. (2019) 5:480–9. doi: 10.1001/jamaoncol.2018.6720
34. Peters S, Gettinger S, Johnson ML, Jänne PA, Garassino MC, Christoph D, et al. Phase II trial of atezolizumab as first-line or subsequent therapy for patients with programmed death-ligand 1-selected advanced non-small-cell lung cancer (BIRCH). J Clin Oncol. (2017) 35:2781–9. doi: 10.1200/JCO.2016.71.9476
35. Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. (2016) 387:1837–46. doi: 10.1016/S0140-6736(16)00587-0
Keywords: pazopanib, neuroendocrine neoplasia, neuroendocrine tumours, review, carcinoid
Citation: Bongiovanni A, Liverani C, Recine F, Fausti V, Mercatali L, Vagheggini A, Spadazzi C, Miserocchi G, Cocchi C, Di Menna G, De Vita A, Severi S, Nicolini S and Ibrahim T (2020) Phase-II Trials of Pazopanib in Metastatic Neuroendocrine Neoplasia (mNEN): A Systematic Review and Meta-Analysis. Front. Oncol. 10:414. doi: 10.3389/fonc.2020.00414
Received: 23 October 2019; Accepted: 10 March 2020;
Published: 07 April 2020.
Edited by:
Olivier Feron, Université Catholique de Louvain, BelgiumReviewed by:
Loredana Bergandi, University of Turin, ItalyNaveen Yarasi, Compass Health Network, United States
Copyright © 2020 Bongiovanni, Liverani, Recine, Fausti, Mercatali, Vagheggini, Spadazzi, Miserocchi, Cocchi, Di Menna, De Vita, Severi, Nicolini and Ibrahim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alberto Bongiovanni, YWxiZXJ0by5ib25naW92YW5uaUBpcnN0LmVtci5pdA==
 Alberto Bongiovanni
Alberto Bongiovanni Chiara Liverani
Chiara Liverani Federica Recine
Federica Recine Valentina Fausti
Valentina Fausti Laura Mercatali1
Laura Mercatali1 Alessandro De Vita
Alessandro De Vita Stefano Severi
Stefano Severi Toni Ibrahim
Toni Ibrahim