- 1Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangdong Key Laboratory of Nasopharyngeal Carcinoma Diagnosis and Therapy, Guangzhou, China
- 2Department of Nasopharyngeal Carcinoma, Sun Yat-sen University Cancer Center, Guangzhou, China
Objective: To compare the survival outcomes brought by different radiation dose schedules to bone lesions and different chemotherapy regimens in bone metastatic nasopharyngeal carcinoma (NPC).
Background: The current treatment strategy for bone metastatic NPC patients was empirically given and poorly studied before. It is of necessity to optimize the treatment for bone metastasis to enhance the therapeutic effect and increase the proportion of long-term survived patients.
Methods: Three hundred patients who received chemoradiotherapy from 2002 to 2018 were involved in the study. Demographics, laboratory results, and detailed treatment plans were recorded. Radiotherapy plans were classified into three categories based on the intensity, and the survival analysis was performed using log-rank test. Multivariable analysis was made by the Cox proportional regression model.
Results: Patients who received 60–75 Gy/30–35 fractions of radiation to the metastatic bones had significantly longer bone relapse-free survival (BRFS) (HR, 0.53, 95% CI, 0.37–0.78, P = 0.003), overall survival (OS) (HR, 0.63, 95% CI, 0.46–0.84, P = 0.007), and progression-free survival (PFS) (HR, 0.80, 95% CI, 0.67–0.95, P = 0.041). The administration of paclitaxel, cisplatin and 5-fluorouracil regimen was also associated with better BRFS (HR, 0.27, 95% CI, 0.10–0.75, P = 0.007), PFS (HR, 0.60, 95% CI, 0.42–0.87, P = 0.007), and OS with borderline significance (HR, 0.54, 95% CI, 0.29–1.03, P = 0.058). In multivariable analysis, the post-treatment EBV DNA level and radical radiation dose were proved as independent prognostic factors for both BRFS and OS.
Conclusions: Radiotherapy to metastatic bones with palliative dose prescription should not be considered in bone metastatic NPC patients. TPF chemotherapy regimen might help to improve the survivals in NPC patients but failed to be an independent protective factor.
Introduction
Nasopharyngeal carcinoma (NPC), which stems from the epithelium of nasopharynx, is a special subtype of head and neck cancers. Characterized by the poor differentiated nature, NPC is highly sensitive to chemoradiotherapy and excellent locoregional control rates can often be achieved in non-metastatic NPC. Nonetheless, distant metastasis is the major threat and cause of death faced by all NPC patients. It's reported that nearly 10% newly-diagnosed patients presented with synchronous distant metastasis (1, 2) and approximately 20–30% patients developed metastasis after primary treatment (3–5). Bone metastasis (BM), especially axial bone, is the mostly frequently invaded organ with a proportion of over 50% (6–8) among all metastatic sites. Previous studies have shown diverse survival outcomes in this population with the median overall survival (OS) ranging from 20.3 to 36.9 months (9–13). Meanwhile, there are evidences favoring the long-term survival in some subgroups such as those with oligometastasis (11, 14), low level of pretreatment alkaline phosphatase (ALP) and Epstein-barr virus (EBV) DNA (12). However, the current treatment strategy for metastatic patients is mainly based on palliative chemotherapy, let alone the treating principle for BM, which is poorly understood. Therefore, it is of necessity to optimize the treatment of BM with different strategies considered to enhance effectiveness and increase the proportion of long-term survived patients.
Radiotherapy (RT) to metastatic bones is widely applied to relieve pain, prevent skeleton-related events (SRE) and improve quality of life among various cancer types. Accumulated evidences have pointed out the survival benefit brought by local radiotherapy to BM plus chemotherapy in NPC patients. In Liang et al.'s study (15), a significantly higher 3 year OS was found in the group receiving local treatment to metastatic sites (48.8 vs. 33.8%, P = 0.001), and similar results were also observed in Shen et al.'s (10) and He et al.'s study (12). Although several studies suggested the potential value of local radiotherapy to BM, no general consensus exists concerning the best candidates and the appropriate radiotherapy regimens (16). From single fraction, hypofracionation to normofractionation, radiotherapy regimens were empirically given without the underpinning of evidence, thus the optimal RT dose fractionation schedule for metastatic bones in NPC should be addressed. Similar to the situation of local therapy, the appropriate chemotherapy regimen among all platinum-based regimens for bone metastatic patients was little studied and also worth exploring.
Herein, we investigated the real-world therapeutic strategy for bone metastatic NPC patients who received chemoradiotherapy, and a retrospective cohort study was performed with an attempt to find out the optimal chemoradiation plan and yield insight into future studies to establish specific guidelines.
Methods
Patients
Patients treated in Sun Yat-sen University Cancer Center from January 2002 to December 2018 were consecutively evaluated for their eligibility. The diagnosis of BM was determined by at least one of the following examinations including computed tomography (CT) with contrast, magnetic resonance imaging (MRI) with contrast, positron emission tomography-computed tomography (PET/CT) and histologically proven metastatic lesion. The inclusion criteria were: (1) Patients were previously or concurrently diagnosed as NPC with pathological evidence. (2) Patients who had secondary BM received radical radiotherapy to the nasopharynx as an initial treatment. (3) Radiotherapy to the BM was performed. (4) Karnofsky performance status (KPS) ≥70. Patients were excluded if any of the following condition was met: (1) Radiotherapy was stopped halfway for any reason. (2) Coexistence of a second malignancy. (3) Incomplete medical records. (4) Patients who showed no evidence of progression were lost to follow up within 3 months after the BM-directed treatment. This study was approved by the Sun Yat-sen University Cancer Center Clinical Research Ethics Committee.
Treatment
All patients received multi-modality treatment including radiotherapy and chemotherapy. Chemotherapy was administered every 3 weeks for at least 4–6 cycles before the radiotherapy. Chemotherapy regimens included TP, paclitaxel plus cisplatin; PF, cisplatin plus 5-fluorouracil; GP, gemcitabine plus cisplatin; and TPF, paclitaxel plus cisplatin and 5-fluorouracil. Carboplatin and nedaplatin were also applicated as substitutes for cisplatin. If several regimens were applied, the regimen with which patients achieved major response was recorded. The adopted radiotherapy techniques for BM ranged from 2-dimentional (2D-RT) or 3-dimentional radiotherapy (3D-RT), intensity modulated radiotherapy (IMRT), volumetric modulated arc therapy (VMAT) to tomotherapy (TOMO). The dose-fractionation patterns were heterogeneous among patients from single fractionation schedule to radical dose regimen. In addition, all initially diagnosed patients received radiotherapy to the nasopharynx and neck. The prescribed dose to the gross tumor volume was 66–70 Gy and 60 Gy to the clinical target volume. The zoledronic acid was given to some patients with a dosage of 4 mg every 3–4 weeks. Epidermal growth factor receptor (EGFR) inhibitors such as cetuximab and nimotuzumab, immune checkpoint inhibitors including antibodies of CTLA-4, programmed cell death receptor (PD-1) and its ligand (PD-L1) and anti-angiogenic agents like endostar and apatinib were also considered as supplement to promote the therapeutic effect.
Data Collection
Demographics, laboratory results and detailed treatment plans of all patients were recorded. If multiple BM relapses occurred, the information collected and evaluation was subjected to the first episode. The pre-treatment levels of ALP, lactic dehydrogenase (LDH), and EBV DNA were measured at the time when the BM was detected. The plasma EBV DNA test was not performed in patients treated before 2007. The osteolytic lesion was defined when the BM appeared as disrupted trabecular structure or area of faint density, while the osteogenic lesion was distinguished by sclerotic change or enhanced density. The follow-up information of all patients was collected. The primary endpoint was in-situ bone relapse-free survival (BRFS), which referred to the interval from the diagnosis of BM to the in-situ relapse of the radiated bones. The second endpoints measured overall survival (OS) and progression-free survival (PFS), which, respectively referred to the interval from the diagnosis of BM to death caused by any reason or any tumor progression. Patients were censored at the date of last follow-up.
Statistical Analysis
Continuous variables were dichotomized to categorical variables before analysis. The cut-off value of LDH was categorized using the upper normal limit (250 U/L), and the cut-off values of ALP and EBV DNA were, respectively determined as 110 U/L and 4,000 copies/ml based on previous literatures (2, 12, 17). The Chi-square test was adopted to calculate the correlations between variables. Survival curves were plotted with the Kaplan-meier method, and the survival outcomes were compared by the log-rank test. The univariable and multivariable analysis were conducted using the Cox proportional hazard model. All statistical analyses were carried out with the use of SPSS, version 26.0 (SPSS Inc., Chicago, IL, USA). A 2-tailed P-value of <0.05 was considered statistically significant.
Results
Patient Characteristics
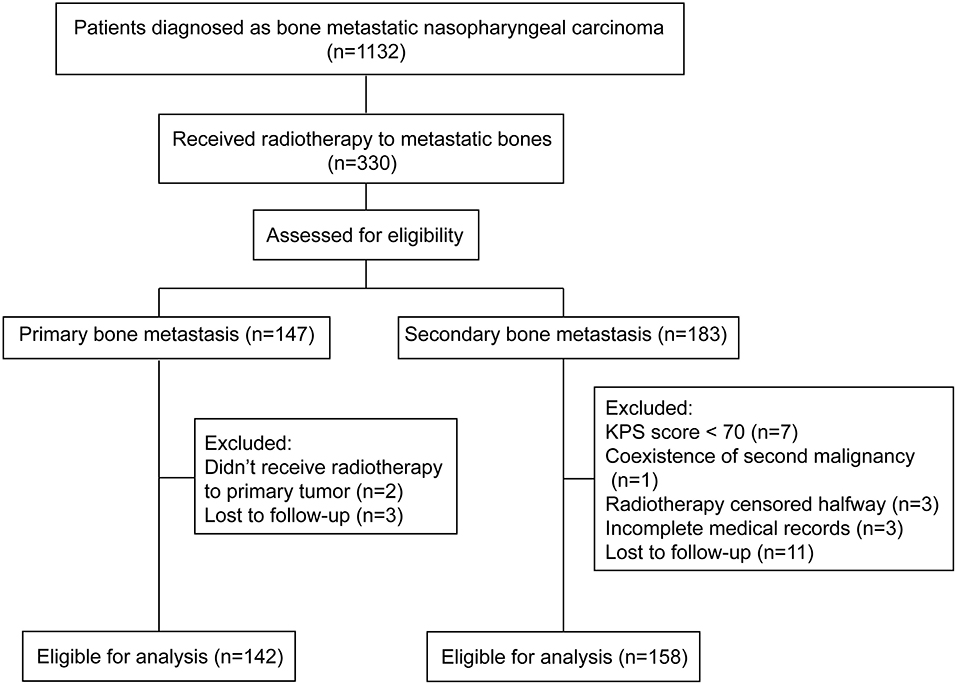
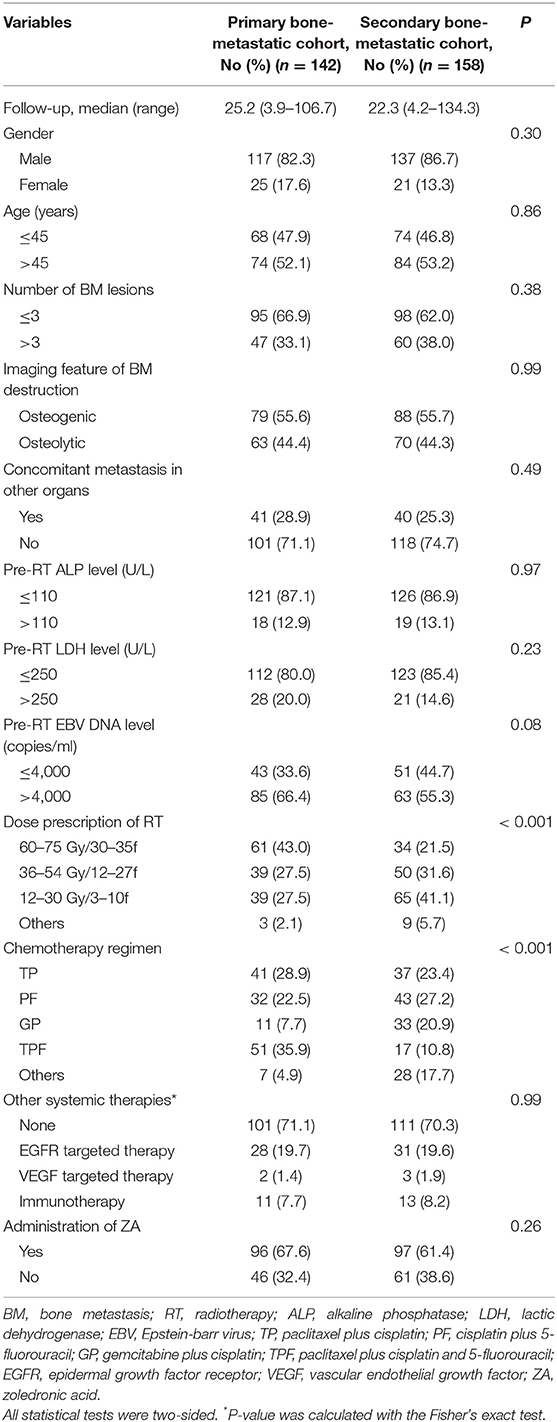
Clinical data of all patients who were diagnosed with bone metastatic nasopharyngeal carcinoma in Sun Yat-sen University Cancer Center from January 2002 to December 2018 were evaluated. After the inclusion and exclusion procedure, 300 patients were eligible for analysis (Figure 1). The baseline characteristics of all patients were listed in Table 1. In brief, 142 (47.3%) patients primarily presented with bone metastasis and 158 (52.7%) were found after radical treatment. Eighty one (27.0%) patients were accompanied by concomitant metastasis of other organs. In patients with bone-only metastasis, 73.1% of them had ≤3 metastatic bones involved. The pre-treatment plasma EBV DNA level was tested in 242 patients. Two hundred and thirteen (69.4%) patients showed an EBV DNA level above 4,000 copies/ml with the median level of 10,375 copies/ml. The median follow-up time was 23.5 months (IQR, 14.7–38.5 months). The 3 year BRFS, OS and PFS rates were 84.0, 80.7, and 42.0%, respectively. No one died from radiation or chemotherapy related adverse effects. One hundred and eighty eight (62.7%) patients had disease progression and 74 patients died during the follow-up. Among patients who developed progression, 48 (25.5%) patients had bone relapse in-situ with or without invasions to other bones or organs.
Radiotherapy
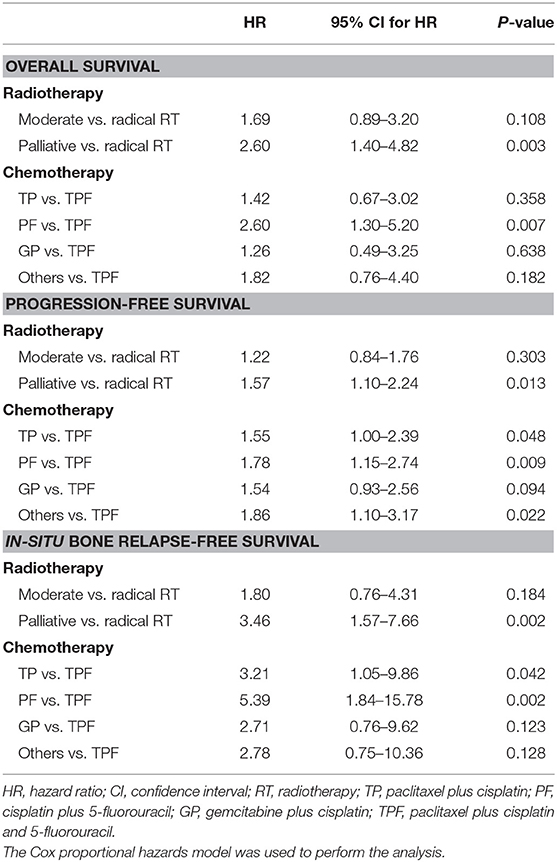
All patients accepted multimodality systemic treatment and radiotherapy was prescribed to the BM. The efficacy of different radiotherapy plans and chemotherapy plans were compared in Table 2. Radiotherapy was given to all metastatic bones in 208 (69.3%) patients while others had radiotherapy for palliative pain alleviation. Radiotherapy plans were classified into three categories based on the intensity. 60–75 Gy using 30–35 fractions was considered radical dose prescription. Meanwhile, 36–54 Gy with 12–27 fractions and 12–30 Gy with 3–10 fractions were, respectively categorized into moderate and palliative dose prescription. There were accordingly 95 (31.7%), 89 (29.7%), and 104 (34.7%) patients receiving the above-mentioned plans. The hypofractionated and single fractionated regimens were seldomly used. As the radiotherapy plans for 12 patients failed to be classified, those plans were listed separately in the Supplementary Table 1. The survival results were compared among the three groups, patients given radical radiation dose were associated with significantly higher BRFS (91.6 vs. 84.3 vs. 75.0%, HR, 0.53, 95% CI, 0.37–0.78, P = 0.003), OS (84.2 vs. 69.7 vs. 70.2%, HR, 0.63, 95% CI, 0.46–0.84, P = 0.007), and PFS (43.2 vs. 33.7% vs. 31.7, HR, 0.80, 95% CI, 0.67–0.95, P = 0.041) (Figure 2). In addition, a strong correlation existed between the option of radiotherapy plans and the incidence of post-treatment EBV DNA levels dropping to zero (HR, 0.58, 95% CI, 0.40–0.83, P = 0.004). Concerning the effect of fractionated dose on survival, it was found that a fractionated dose of ≤ 2 Gy helped to extend the BRFS (88.5 vs. 81.3%, HR, 0.49, 95% CI, 0.26–0.94, P = 0.026), but brought no benefit to the OS and PFS.
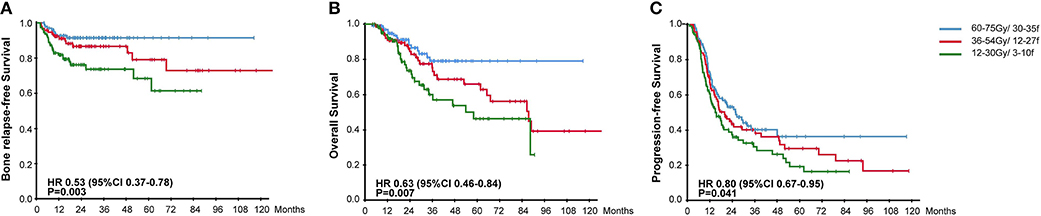
Figure 2. Kaplan-Meier Curves for (A) Bone Relapse Free Survival, (B) Overall Survival, (C) Progression Free Survival, among groups receiving different radiotherapy plans.
To further explore the effectiveness of radiotherapy plans on different types of BM, we separated patients according to the imaging features of BM destruction (osteogenic or osteolytic) and the absence/presence of soft tissue involvement. For patients with osteogenic BM, radical radiation plan significantly reduced the occurrence of in-situ relapse (96.6 vs. 83.3 vs. 76.4%, HR, 0.41, 95% CI, 0.23–0.74, P = 0.007). But in the meantime, radical radiotherapy failed to bring BRFS benefits to patients with osteolytic lesions or with soft tissue involvement (Figure 3).
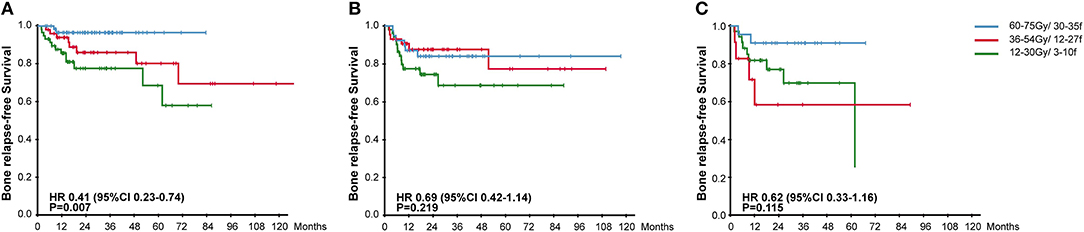
Figure 3. Kaplan-Meier Curves for Bone Relapse Free Survival among groups receiving different radiotherapy plans in patients with osteogenic lesions (A), patients with osteolytic lesions (B), and patients with soft tissue involvement (C).
Systemic Therapy
Most of patients (91.7%) received platinum-based chemotherapy. The number and proportion of patients receiving TP, PF, GP, and TPF were 78 (25.4%), 75 (24.4%), 44 (14.3%), and 68 (22.1%), respectively. EGFR inhibitors such as cetuximab and nimotuzumab were administered to 59 (19.7%) patients, immune checkpoint inhibitors including CTLA-4, PD-1, and PD-L1 antibodies were given to 24 (8.0%) patients and anti-angiogenic agents to five (1.7%) patients. Furthermore, zoledronic acid was administered to 193 (64.3%) patients.
The efficacy of different chemotherapy regimens was compared and no significant difference apropos BRFS, OS, and PFS was found among TP, PF, and GP regimens. However, distinct BRFS (94.1 vs. 81.0%, HR, 0.27, 95% CI, 0.10–0.75, P = 0.007) and PFS (50.0 vs. 33.6%, HR, 0.60, 95% CI, 0.42–0.87, P = 0.007) benefits were achieved with the administration of TPF regimen compared to other chemotherapy plans (Figure 4). An improved OS on the boundary of significance was also observed (83.8 vs. 72.8%, HR, 0.54, 95% CI, 0.29–1.03, P = 0.058). As it was found that TPF was mostly administered to newly-diagnosed patients (Chi-square test, P < 0.001), we further explored the efficacy of TPF in newly-diagnosed cohort, and patients using the TPF regimen tended to exhibit better OS, PFS in contrast to other chemotherapy regimens (Supplementary Figure 1). The addition of targeted agents or immunotherapy helped to prolong the OS (88.6 vs. 69.8%, HR, 0.42, 95% CI, 0.22–0.82, P = 0.009), but didn't show superiority in terms of PFS and BRFS (Supplementary Figure 2). On the other hand, the application of zoledronic failed to bring any survival benefit to neither osteogenic nor osteolytic BM (data not shown).
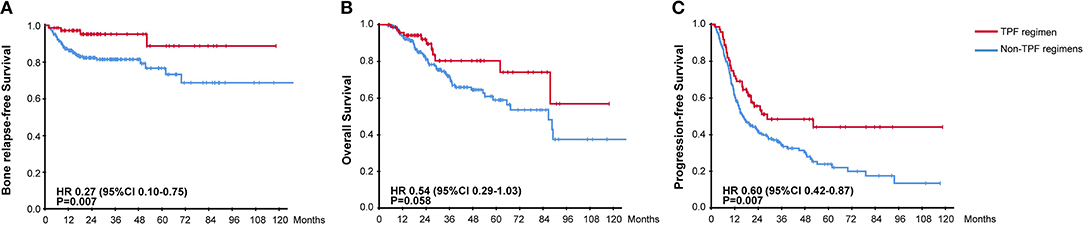
Figure 4. Kaplan-Meier Curves for (A) Bone Relapse Free Survival, (B) Overall Survival, (C) Progression Free Survival, between groups receiving or not receiving TPF chemotherapy regimen.
To evaluate the combined effectiveness of chemoradiotherapy, we divided patients into 4 groups based on the aboved results. As the survival curves showed in Figure 5, the combination of radical radiotherapy and TPF regimen gave rise to the significantly improved BRFS, OS, and PFS. Meanwhile, the survival results were similar between patients who either received radical radiated dose or TPF regimen, followed by those who received neither of them.
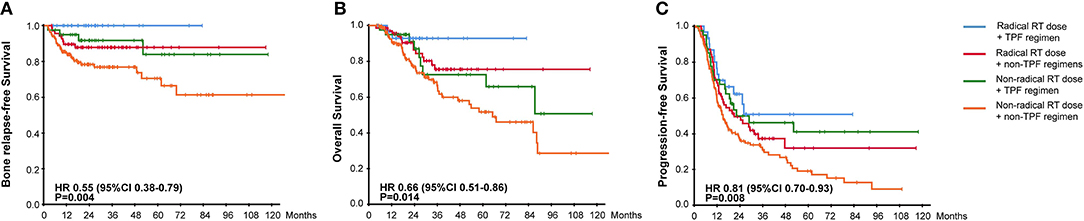
Figure 5. Kaplan-Meier Curves for (A) Bone Relapse Free Survival, (B) Overall Survival, (C) Progression Free Survival, among groups with different chemoradiotherapy.
Univariable and Multivariable Analysis
Univariable analysis was performed first to screen out potential prognostic factors for BRFS and OS, and the multivariable analysis was made thereafter (Table 3). Compared with pretreatment EBV DNA level, whether EBV DNA could drop down to zero after RT offered more prognostic value, and stayed significant in the multivariable analysis for both BRFS and OS. It also indicated that the pre-treatment level of ALP served as an independent risk factor in the multivariable model for OS (HR, 3.50, 95% CI, 1.58–7.77, P = 0.002). With regard to the treatment strategy, the administration of TPF regimen failed to be a prognostic variant, but radiotherapy to BM with radical dose was proved as an independent protective factor for both BRFS (HR, 0.60, 95% CI, 0.36–0.98, P = 0.039) and OS (HR, 0.59, 95% CI, 0.35–0.98, P = 0.039).
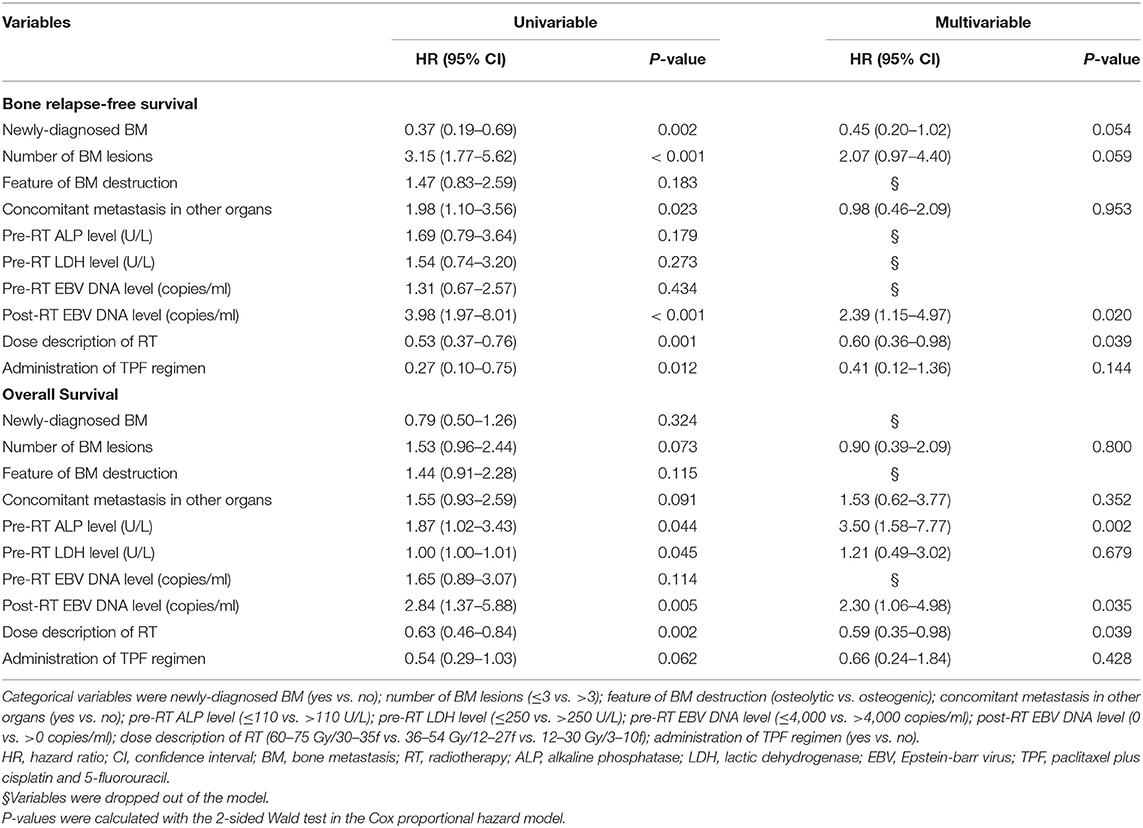
Table 3. Univariable and multivariable analysis of prognostic factors for bone relapse-free survival and overall survival.
Discussion
As good results have been achieved in the treatment of locoregionally advanced nasopharyngeal carcinoma, more attention should be paid to the management of metastatic NPC. The present study is the first one to explore the combinatorial treatment strategy for bone metastatic patients, and found that the intensity of dose prescription to the BM was strongly correlated to the BRFS, OS, and PFS. Meanwhile, the survival results in terms of BRFS, OS and PFS favored the administration of TPF regimen. Patients benefited most from the combination of radical radiation dose and TPF chemotherapy. Furthermore, radical radiation dose to the BM was proved to be an independent protective factor.
The appropriate dose prescription and fractionation to the BM has been discussed in many cancers. Although multiple studies demonstrated the equivalent effect of short fractionation schedules (including single fraction) on pain palliation, the situation might be different in treating NPC patients. As our results showed that 170 (56.7%) patients had asymptomatic BM, relieving the pain was not the major concern for giving radiotherapy. Besides, many patients presented with oligometastasis, and longer survival durations were often observed among them, thus the short fractionation schedules, which sometimes required the need for retreatment (18), were less adequate. Our results suggested the use of intensive dose prescription, which was consistent with the results in hepatocellular cancer (HCC), renal and prostate cancers (19–22). Kim et al. (20) selected HCC patients who were followed up for at least 1 year and a positive dose-response relationship was observed. In Koga et al.'s study (21), the intensive local therapy was beneficial not only for patients with solitary BM but also for multiple BMs. In our study, apart from reducing the chance of in-situ recurrence, the radical dose prescription significantly improved the OS and PFS. In summary, patients' predicted life expectancy and treatment goals should be considered when giving local radiotherapy. For patients with a chance of long-term survival, radical radiotherapy to the BM was preferred.
With respect to the optimum chemotherapy regimens for metastatic NPC, it had been inconclusive and the PF regimen was the most empirically-used regimen for over decades (23). Zhang et al. (24) carried out the first phase III trial to compare the efficacy of GP with PF regimen, and the results indicated that the GP regimen significantly prolonged the PFS in recurrent or metastatic NPC. However, conclusions in relative retrospective studies were slightly different. Jiang et al. (25) made comparisons among five cisplatin-based chemotherapy in metastatic NPC, and found significantly higher response rates were associated with the administration of GP and TPF regimens. Nonetheless, no difference was observed in OS and PFS, which might result from the less cycles of GP and TPF given to patients. In a meta-analysis which included 27 studies (26), triplet regimen demonstrated best short-term efficacy while TP regimen was associated with the highest 1 year OS. In the present study, TPF regimen was found superior to other regimens in terms of PFS and BRFS. However, a larger proportion of initially diagnosed patients receiving TPF regimen should be noticed. In multivariable analysis, the administration of TPF failed to be an independent protective factor. In that case, further prospective studies are still warranted to compare the effectiveness among chemotherapy regimens, especially between GP and TPF regimens. What's more, the administration of targeted therapy or immunotherapy shouldn't be neglected. Although the PFS rates were comparable between patients with or without targeted or immunotherapy, patients achieved significantly longer OS with the help of targeted or immunotherapy, which indicated the potential of them to prolong the survival after disease progression.
The study had several limitations. Primarily, because of the retrospective nature, selection bias may exist as clinicians were more inclined to give aggressive combinatorial treatment to patients with less metastatic burdens and satisfactory performance status. Secondly, given the scarcity of bone metastatic patients receiving local radiotherapy, we had to include patients with metastasis to other organs to guarantee the sample size for analysis, which might be a confounding variable while calculating PFS and OS. Furthermore, as the data was the representative of treating experience from single institute where the short-course hypofractionated (including single fraction) radiotherapy plans were seldomly used, the true efficacy of them in NPC patients remained unknown. Therefore, future studies which focus on the multi-modality treatment for bone metastatic NPC patients are in urgent need.
In conclusion, this study showed that the intensity of radiation dose to the BM was strongly associated with the BRFS, OS, and PFS, and it remained independently protective in multivariable analysis. The administration of TPF regimen might help to improve OS, PFS and BRFS compared with other platinum-based regimens, and patients benefited most from the combination of radical local radiation and TPF chemotherapy.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
This study was approved by the Sun Yat-sen University Cancer Center Clinical Research Ethics Committee.
Author Contributions
H-QM and L-QT: study concepts. X-YL, J-JY, and G-DJ: study design. X-SS, S-LL, and S-SG: data acquisition. L-TL: quality control of data and algorithms. X-YL, X-SS, and G-DJ: data analysis and interpretation. X-SS and X-YL: statistical analysis. X-YL, RS, and D-HL: manuscript preparation. X-SS, S-LL, and H-YM: manuscript editing. Q-YC and LG: manuscript review.
Funding
This work was supported by grants from the National Key R&D Program of China (2017YFC1309003 and 2017YFC0908500), the National Natural Science Foundation of China (Nos. 81425018, 81672868, 81602371, and 81802775), the Sci-Tech Project Foundation of Guangzhou City (201707020039), the Sun Yat-sen University Clinical Research 5010 Program, the Special Support Plan of Guangdong Province (No. 2014TX01R145), the Natural Science Foundation of Guangdong Province (Nos. 2017A030312003 and 2018A0303131004), the Natural Science Foundation of Guangdong Province for Distinguished Young Scholar (No. 2018B03036001) Sci-Tech Project Foundation of Guangdong Province (No. 2014A020212103), the Health & Medical Collaborative Innovation Project of Guangzhou City (Nos. 201400000001 and 201803040003), Pearl River S&T Nova Program of Guangzhou (No. 201806010135), the Planned Science and Technology Project of Guangdong Province (2019B020230002), the National Science & Technology Pillar Program during the Twelfth Five-year Plan Period (No. 2014BAI09B10), the PhD Start-up Fund of Natural Science Foundation of Guangdong Province (2016A030310221), the cultivation foundation for the junior teachers in Sun Yat-sen University (16ykpy28), and the Fundamental Research Funds for the Central Universities.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer XL declared a shared affiliation, with no collaboration, with the authors to the handling editor at time of review.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.00378/full#supplementary-material
Supplementary Figure 1. Kaplan-Meier Curves for (A) Bone Relapse Free Survival, (B) Overall Survival, (C) Progression Free Survival, between groups receiving or not receiving TPF chemotherapy regimen in newly-diagnosed patients.
Supplementary Figure 2. Kaplan-Meier Curves for (A) Bone Relapse Free Survival, (B) Overall Survival, (C) Progression Free Survival, between groups receiving or not receiving targeted therapy or immunotherapy.
Supplementary Table 1. Radiotherapy dose fractionation schedule for each case scenario.
References
1. Chen YP, Chan ATC, Le QT, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet. (2019) 394:64–80. doi: 10.1016/S0140-6736(19)30956-0
2. Tang LQ, Chen QY, Fan W, Liu H, Zhang L, Guo L, et al. Prospective study of tailoring whole-body dual-modality [18F] fluorodeoxyglucose positron emission tomography/computed tomography with plasma Epstein-Barr virus DNA for detecting distant metastasis in endemic nasopharyngeal carcinoma at initial staging. J Clin Oncol. (2013) 31:2861–9. doi: 10.1200/JCO.2012.46.0816
3. Sun X, Su S, Chen C, Han F, Zhao C, Xiao W, et al. Long-term outcomes of intensity-modulated radiotherapy for 868 patients with nasopharyngeal carcinoma: an analysis of survival and treatment toxicities. Radiother Oncol. (2014) 110:398–403. doi: 10.1016/j.radonc.2013.10.020
4. Razak AR, Siu LL, Liu FF, Ito E, O'Sullivan B, Chan K. Nasopharyngeal carcinoma: the next challenges. Eur J Cancer. (2010) 46:1967–78. doi: 10.1016/j.ejca.2010.04.004
5. Chan OS, Ngan RK. Individualized treatment in stage IVC nasopharyngeal carcinoma. Oral Oncol. (2014) 50:791–7. doi: 10.1016/j.oraloncology.2014.01.004
6. Zou X, You R, Liu H, He YX, Xie GF, Xie ZH, et al. Establishment and validation of M1 stage subdivisions for de novo metastatic nasopharyngeal carcinoma to better predict prognosis and guide treatment. Eur J Cancer. (2017) 77:117–26. doi: 10.1016/j.ejca.2017.02.029
7. Ong YK, Heng DM, Chung B, Leong SS, Wee J, Fong KW, et al. Design of a prognostic index score for metastatic nasopharyngeal carcinoma. Eur J Cancer. (2003) 39:1535–41. doi: 10.1016/S0959-8049(03)00310-1
8. Sun XS, Liu LT, Liu SL, Guo SS, Wen YF, Xie HJ, et al. Identifying optimal candidates for local treatment of the primary tumor among patients with de novo metastatic nasopharyngeal carcinoma: a retrospective cohort study based on Epstein-Barr virus DNA level and tumor response to palliative chemotherapy. BMC Cancer. (2019) 19:92. doi: 10.1186/s12885-019-5281-5
9. Cao X, Han Y, He L, Xiang J, Wen Z. Risk subset of the survival for nasopharyngeal carcinoma patients with bone metastases: who will benefit from combined treatment? Oral Oncol. (2011) 47:747–52. doi: 10.1016/j.oraloncology.2011.05.010
10. Shen L, Dong J, Li S, Wang Y, Dong A, Shu W, et al. M1 stage subdivision and treatment outcome of patients with bone-only metastasis of nasopharyngeal carcinoma. Oncologist. (2015) 20:291–8. doi: 10.1634/theoncologist.2014-0206
11. Shen L, Li W, Wang S, Xie G, Zeng Q, Chen C, et al. Image-based multilevel subdivision of M1 category in TNM staging system for metastatic nasopharyngeal carcinoma. Radiology. (2016) 280:805–14. doi: 10.1148/radiol.2016151344
12. He S, Wang Y, Peng H, Yang L, Chen H, Liang S, et al. Pretreatment alkaline phosphatase and Epstein-Barr Virus DNA predict poor prognosis and response to salvage radiotherapy in patients with nasopharyngeal carcinoma and metachronous bone-only metastasis. J Cancer. (2017) 8:417–24. doi: 10.7150/jca.17310
13. Chen C, Wu JB, Jiang H, Gao J, Chen JX, Pan CC, et al. A prognostic score for nasopharyngeal carcinoma with bone metastasis: development and validation from multicenter. J Cancer. (2018) 9:797–806. doi: 10.7150/jca.22663
14. Fandi A, Bachouchi M, Azli N, Taamma A, Boussen H, Wibault P, et al. Long-term disease-free survivors in metastatic undifferentiated carcinoma of nasopharyngeal type. J Clin Oncol. (2000) 18:1324–30. doi: 10.1200/JCO.2000.18.6.1324
15. Liang YJ, Sun XS, Yang ZC, Tang QN, Guo SS, Liu LT, et al. Effect of local treatment for metastasis and its sequence with chemotherapy on prognosis of post-treatment metastatic nasopharyngeal carcinoma patients. Oral Oncol. (2019) 92:40–5. doi: 10.1016/j.oraloncology.2019.03.015
16. Adelstein D, Gillison ML, Pfister DG, Spencer S, Adkins D, Brizel DM, et al. NCCN guidelines insights: head and neck cancers, version 2.2017. J Natl Compr Cancer Netw. (2017) 15:761–70. doi: 10.6004/jnccn.2017.0101
17. Lu T, Guo Q, Cui X, Chen Z, Lin S, Xu L, et al. Prognostic evaluation of nasopharyngeal carcinoma with bone-only metastasis after therapy. Yonsei Med J. (2016) 57:840–5. doi: 10.3349/ymj.2016.57.4.840
18. Hartsell WF, Scott CB, Bruner DW, Scarantino CW, Ivker RA, Roach M, et al. Randomized trial of short- versus long-course radiotherapy for palliation of painful bone metastases. J Natl Cancer Inst. (2005) 97:798–804. doi: 10.1093/jnci/dji139
19. Kim T, Cha HJ, Kim JW, Seong J, Lee IJ. High dose and compartmental target volume may improve patient outcome after radiotherapy for pelvic bone metastases from hepatocellular carcinoma. Oncotarget. (2016) 7:53921–9. doi: 10.18632/oncotarget.9767
20. Jung IH, Yoon SM, Kwak J, Park JH, Song SY, Lee SW, et al. High-dose radiotherapy is associated with better local control of bone metastasis from hepatocellular carcinoma. Oncotarget. (2017) 8:15182–92. doi: 10.18632/oncotarget.14858
21. Fukushima H, Hozumi T, Goto T, Nihei K, Karasawa K, Nakanishi Y, et al. Prognostic significance of intensive local therapy to bone lesions in renal cell carcinoma patients with bone metastasis. Clin Exp Metastasis. (2016) 33:699–705. doi: 10.1007/s10585-016-9805-y
22. Kountouri M, Zilli T, Rouzaud M, Dubouloz A, Linero D, Escudé L, et al. Moderate hypofractionated protracted radiation therapy and dose escalation for prostate cancer: do dose and overall treatment time matter? Int J Radiat Oncol Biol Phys. (2016) 94:272–9. doi: 10.1016/j.ijrobp.2015.10.055
23. Wang TL, Tan YO. Cisplatin and 5-fluorouracil continuous infusion for metastatic nasopharyngeal carcinoma. Ann Acad Med Singapore. (1991) 20:601–3.
24. Zhang L, Huang Y, Hong S, Yang Y, Yu G, Jia J, et al. Gemcitabine plus cisplatin versus fluorouracil plus cisplatin in recurrent or metastatic nasopharyngeal carcinoma: a multicentre, randomised, open-label, phase 3 trial. Lancet. (2016) 388:1883–92. doi: 10.1016/S0140-6736(16)31388-5
25. Jin Y, Shi YX, Cai XY, Xia XY, Cai YC, Cao Y, et al. Comparison of five cisplatin-based regimens frequently used as the first-line protocols in metastatic nasopharyngeal carcinoma. J Cancer Res Clin Oncol. (2012) 138:1717–25. doi: 10.1007/s00432-012-1219-x
Keywords: nasopharyngeal cancer, radiotherapy, bone metastases, chemotherapy, palliaitve care
Citation: Li X-Y, Jia G-D, Sun X-S, Guo S-S, Liu L-T, Liu S-L, Yan J-J, Luo D-H, Sun R, Guo L, Mo H-Y, Tang L-Q, Chen Q-Y and Mai H-Q (2020) Intensive Local Radiotherapy Is Associated With Better Local Control and Prolonged Survival in Bone-Metastatic Nasopharyngeal Carcinoma Patients. Front. Oncol. 10:378. doi: 10.3389/fonc.2020.00378
Received: 13 January 2020; Accepted: 04 March 2020;
Published: 20 March 2020.
Edited by:
Jordi Giralt, Vall d'Hebron University Hospital, SpainReviewed by:
Fan Zhang, Fifth Affiliated Hospital of Sun Yat-sen University, ChinaXing Lv, Sun Yat-sen University, China
Copyright © 2020 Li, Jia, Sun, Guo, Liu, Liu, Yan, Luo, Sun, Guo, Mo, Tang, Chen and Mai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lin-Quan Tang, dGFuZ2xxJiN4MDAwNDA7c3lzdWNjLm9yZy5jbg==; Qiu-Yan Chen, Y2hlbnF5JiN4MDAwNDA7c3lzdWNjLm9yZy5jbg==; Hai-Qiang Mai, bWFpaHEmI3gwMDA0MDtzeXN1Y2Mub3JnLmNu
†These authors have contributed equally to this work
‡These authors have contributed equally to this work and share senior authorship
 Xiao-Yun Li1,2†
Xiao-Yun Li1,2† Li-Ting Liu
Li-Ting Liu Hai-Qiang Mai
Hai-Qiang Mai