
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 20 March 2020
Sec. Women's Cancer
Volume 10 - 2020 | https://doi.org/10.3389/fonc.2020.00366
This article is part of the Research Topic Quality of Life in Breast Cancer Patients and Survivors View all 14 articles
 Jihyoun Lee1†
Jihyoun Lee1† Heba M. Alqudaihi2,3†
Heba M. Alqudaihi2,3† Michael Seungcheol Kang4
Michael Seungcheol Kang4 Jisun Kim3
Jisun Kim3 Jong Won Lee3
Jong Won Lee3 Beom Seok Ko3
Beom Seok Ko3 Byung Ho Son3
Byung Ho Son3 Sei Hyun Ahn3
Sei Hyun Ahn3 Jong Eun Lee5
Jong Eun Lee5 Sun Wook Han5
Sun Wook Han5 Zisun Kim6
Zisun Kim6 Sung Mo Hur6
Sung Mo Hur6 Ji Sung Lee7,8*
Ji Sung Lee7,8* Il Yong Chung3*
Il Yong Chung3*Background: Although international guidelines recommend bone screening for premenopausal breast cancer patients taking adjuvant tamoxifen, the effects of tamoxifen on osteoporosis and related risks remain controversial. The objective of this study was to investigate the incidence of and risk factors for osteoporosis and osteoporotic fractures in younger breast cancer patients.
Methods: A nationwide retrospective cohort study was conducted using South Korea Health Insurance Review and Assessment Service claims data. The rates of osteoporosis and osteoporotic fracture were calculated as incident cases per person-year and disease-free probability rates were analyzed with the Kaplan-Meier method. To identify risk factors for osteoporosis and osteoporotic fracture, a multivariable Cox proportional hazard regression model was applied.
Results: From January 2009 to December 2014, a total of 47,649 breast cancer patients were included. The incidence rates of osteoporosis and osteoporotic fracture were 23.59 and 2.40 per 1,000 person-years, respectively. In the overall population, tamoxifen was significantly associated with a decreased risk of osteoporosis and osteoporotic fractures 0.76). However, tamoxifen was not associated with the risk of osteoporosis (HR 1.24, CI 0.85–1.82) and osteoporotic fracture (HR 8.15, CI 0.36–186.70) in patients under age 40. In the 40–49 years subgroup, tamoxifen significantly decreased the risk of osteoporosis (HR 0.74, CI 0.65–0.84) and osteoporotic fracture (HR 0.49, CI 0.31–0.76).
Conclusions: Tamoxifen is not associated with an increased risk of osteoporosis and osteoporotic fracture in premenopausal breast cancer patients. Tailored screening strategies for breast cancer survivors with different osteoporosis risks are needed.
Precis: Tamoxifen is not associated with an increased risk of osteoporosis and osteoporotic fracture in premenopausal breast cancer patients. Tailored screening strategies for breast cancer survivors who are at different risks of developing osteoporosis are needed.
As the survival rate of breast cancer patients increases, optimal survivorship care has become an essential part of clinical practice (1, 2). One of the common long-term effects of breast cancer treatments is osteoporosis, with up to 80% of breast cancer patients experiencing bone loss (3, 4). Women with breast cancer, even in the absence of skeletal metastases, are known to have a higher incidence of fractures than women of the same age without breast cancer (5). Aromatase inhibitor (AI) is one of the well-known risk factors for osteoporosis in postmenopausal breast cancer patients (6, 7). Osteoporotic fractures impose an enormous health burden on individuals and take a substantial economic toll on society (8–10).
Tamoxifen is a known risk factor for osteoporosis in premenopausal breast cancer patients. In previous studies involving premenopausal breast cancer patients taking tamoxifen, bone mineral density decreased progressively over a 3-years follow-up period (11), and tamoxifen was associated with significant bone loss in patients who remained premenopausal after adjuvant chemotherapy (12). Based on these studies, the American Cancer Society/American Society of Clinical Oncology (ACS/ASCO) guidelines recommend bone screening every 2 years for premenopausal women receiving tamoxifen (13).
However, these previous studies have limitations. The sample sizes were small and only univariate analyses were performed to calculate the difference between the tamoxifen and placebo groups. Also, the primary outcome was the percent change in bone mineral density (BMD) rather than clinically meaningful outcomes such as osteoporosis or osteoporotic fractures, and the follow-up periods were short. For these reasons, the effect of tamoxifen on osteoporosis risks in premenopausal breast cancer patients remains controversial. Therefore, we conducted this nationwide retrospective cohort study using data from the Health Insurance Review and Assessment Service (HIRA), which archives data from nearly 98% of all citizens in South Korea (14). The objective of this study was to investigate the incidence of and risk factors for osteoporosis and osteoporotic fractures in breast cancer patients and to assess whether tamoxifen is a risk factor for osteoporosis and osteoporotic fractures in younger breast cancer patients.
The HIRA, a governmental organization in South Korea, assesses healthcare services and makes reimbursement decisions under the national healthcare insurance service. The HIRA collects nationwide claims data from healthcare providers (14). The HIRA data consists of six parts: (1) general information; (2) healthcare services; (3) diagnoses; (4) outpatient prescriptions; (5) medication file; and (6) provider information. The diagnostic information is based on the International Classification of Diseases 10th revision (ICD-10).
We selected the study period of January 2007 to December 2017 because of data availability. Newly diagnosed breast cancer was defined by the C50 code (invasive breast cancer) in combination with the specialized V193 claim code, which is an identifier for reimbursement of cancer patients (15). Because we considered a 2-years period before breast cancer diagnosis as a washout period to exclude prevalent breast cancer and any cancer, subjects were excluded from the study if they received a C code within that period. Patients who did not undergo breast cancer surgery and those with a history of in situ carcinoma, presumed metastatic breast cancer, preexisting or recent (within 1 year after breast cancer diagnosis) osteoporosis, previous rheumatoid arthritis, or long-term corticosteroid treatment (more than 90 days) were excluded. Male patients or subjects who did not have follow-up claims data after breast cancer diagnosis were also excluded.
From January 2009 to December 2014, a total of 191,942 patients received C50 and V193 codes in the HIRA database. We excluded 118,820 who had C codes (any cancer) within the washout period. We excluded 2,531 patients with a previous history of in situ carcinoma, 5,801 with metastatic or recurrent breast cancer, 8,433 with preexisting or recently diagnosed osteoporosis, 134 with previous rheumatoid arthritis, 2,046 with long-term corticosteroid treatment, and 6,336 who did not undergo breast cancer surgery. One hundred eighty-seven male patients and 4 patients who did not have follow-up data after breast cancer diagnosis were also excluded (Figure 1).
Patients' characteristics such as age, type of insurance (health insurance vs. medical aid), and the Charlson Comorbidity Index (CCI) based on ICD-10 codes were analyzed (16). We defined the treatment groups based on claims data within 1 year after breast cancer diagnosis. Radiation therapy (either left and/or right), chemotherapy, ovarian function suppression (OFS), trastuzumab, and endocrine treatment (tamoxifen or AI) were reviewed. Regardless of whether they may have subsequently switched anti-hormonal medications, the patients were allocated into treatment groups according to the initially prescribed endocrine therapy. We defined osteoporosis as the newly claimed osteoporosis codes (M80, M81, M82) in conjunction with at least one of osteoporosis medications (pamidronate, alendronate, ibandronate, risedronate, tibolone, dienogest, estradiol hemihydrate, estradiol valerate, estropipate, conjugated equine estrogens, medroxyprogesterone acetate). The development of osteoporosis was defined using the newly claimed diagnosis in conjunction with medications. Osteoporotic fracture was defined as fracture-related codes (M80, osteoporosis with pathological fracture; S22, fracture of rib, sternum, and thoracic spine; S32, fracture of lumbar spine and pelvis; S52, fracture of forearm; S62, fracture at wrist and hand; S72, fracture of femur) or treatment of fractures and osteoporosis within 6 months before or after the fracture.
The baseline characteristics of the included patients are presented as the number of patients (%) or mean ± SD. The incidence rates of osteoporosis and osteoporotic fracture were calculated by dividing the number of incident cases by the total follow-up period (person-years). The disease-free probability of osteoporosis and osteoporotic fracture were calculated by the Kaplan-Meier method, and the log-rank test was performed to confirm differences across risk factors.
For the identification of risk factors for osteoporosis and osteoporotic fracture, a multivariable Cox proportional hazard regression model was applied and adjusted hazard ratio (HR) and 95% confidence interval (CI) were estimated. Age, type of insurance, CCI, chemotherapy, endocrine therapy, OFS, radiotherapy, and trastuzumab were selected as covariates for regression models. Subgroup analyses were performed within age groups (<40, 40–49, 50–59, 60–69, and ≥70 years) to further clarify risk factors of osteoporosis and osteoporotic fracture.
Statistical analyses were performed with SAS software (version 9.4, SAS Institute, Cary, NC, USA). This study was approved by the Soonchunhyang University Seoul Hospital Institutional Review Board (IRB no. SCHUH 2018-11-011).
A total of 47,649 breast cancer survivors were included in this analysis. Among them, the proportion aged 40–49 years at the time of diagnosis was 42.04 % (Table 1). The proportion of breast cancer survivors who received any type of chemotherapy was 67.57% (n = 32,198). More than two-thirds of the survivors received endocrine treatment, and tamoxifen was the most frequently prescribed agent. OFS with goserelin or leuprolide was prescribed in 10.54% of the survivors. All the subjects underwent breast cancer surgeries, according to our operational definition.
During the study period, 5,955 osteoporosis events were observed in 252,396 person-years. The incidence rate of osteoporosis in breast cancer survivors was 23.59 per 1000 person-years (95% CI, 23.00–24.20). Osteoporotic fracture incidence was assessed as 2.40 per 1000 person-years (95% CI, 2.23–2.60), with 647 events occurring in 269,075 person-years (Table 2). Age at diagnosis and CCI were significantly associated with development of osteoporosis (p < 0.0001) and osteoporotic fracture (p < 0.0001) in the univariate analysis. The incidence rate of osteoporosis was highest in patients aged 70–79 years. The risk of osteoporotic fracture was highest in patients older than 80 years (17.16 per 1,000 person-years) followed by patients aged 70–79 years. Event-free probability of osteoporosis and osteoporotic fracture after 1 year following breast cancer diagnosis is presented in Supplementary Figure 1.
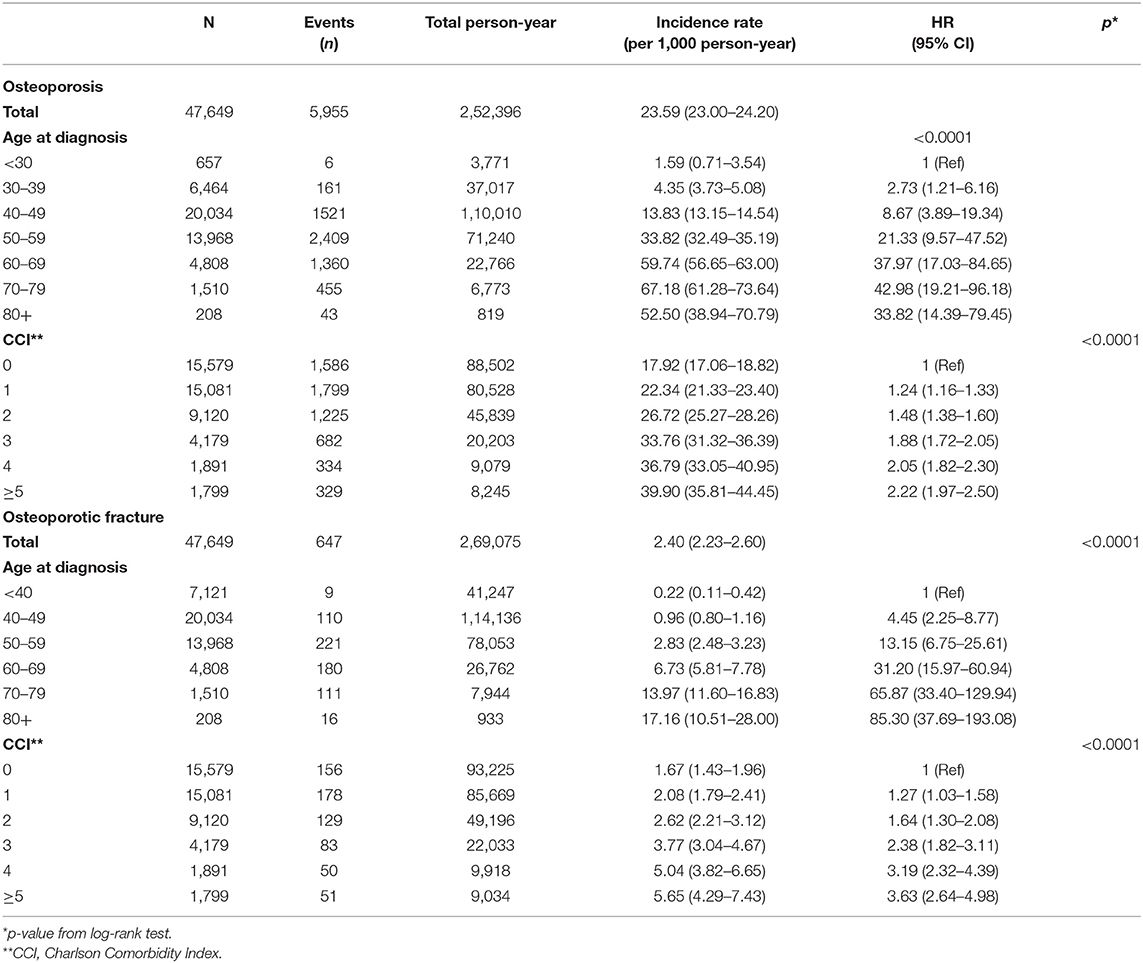
Table 2. Incidence rates of osteoporosis and osteoporotic fracture according to age at diagnosis and comorbidities.
Factors associated with the incidence of osteoporosis and osteoporotic fracture showed different patterns according to age subgroups (Table 3). In patients younger than 40 years, the use of OFS was significantly related to an increased incidence of osteoporosis. In the age 40–49 group, chemotherapy, AI and OFS were significantly associated with an increased risk of osteoporosis. In patients aged 50–69 years, AI significantly increased the risk of osteoporosis; in contrast, tamoxifen was associated with a decreased risk of osteoporosis.
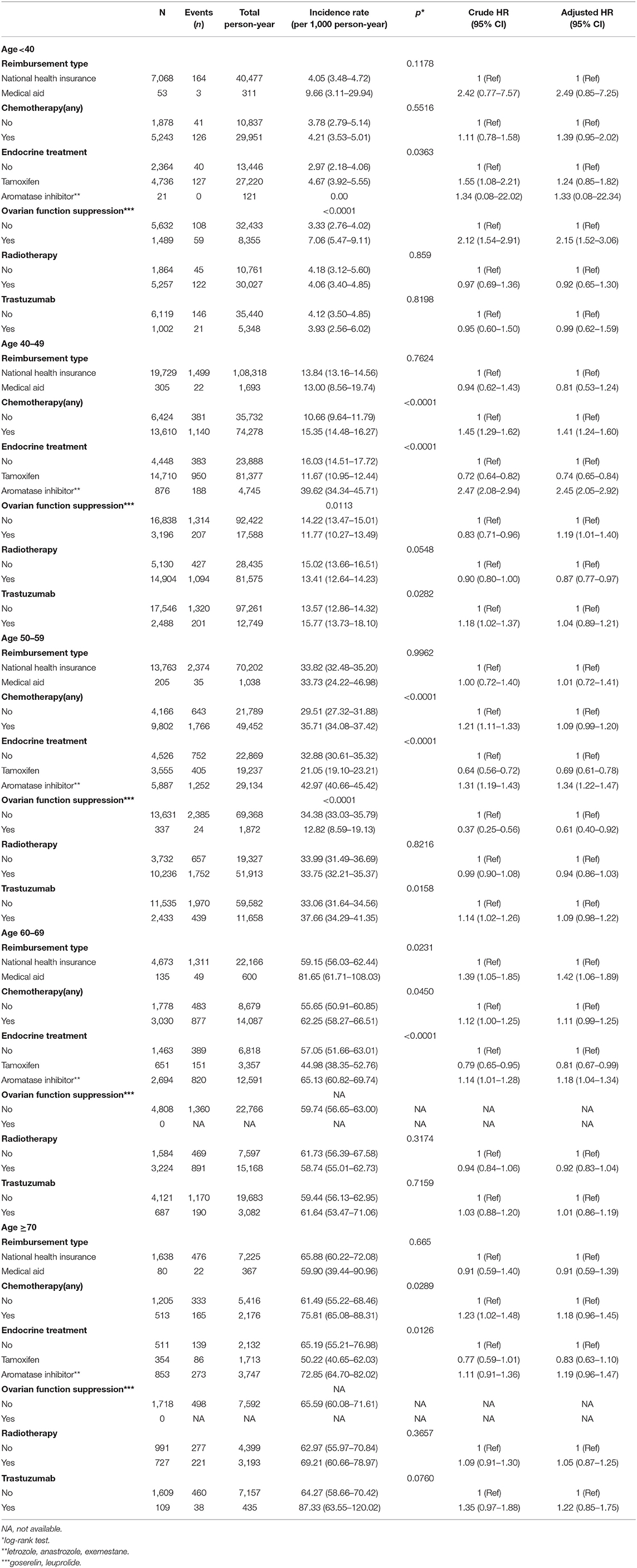
Table 3. Factors associated with osteoporosis according to age subgroups in univariate and multivariate analysis.
There were only 9 cases of osteoporotic fracture in 7,121 patients younger than 40 years, and OFS was not associated with an increased risk of osteoporotic fracture in these patients (Supplementary Table 1). An increased risk of osteoporotic fracture was significantly associated with chemotherapy (HR, 1.75; 95% CI, 1.07–2.88) and AI (HR, 2.35; 95% CI, 1.34–4.12) in the age 40–49 subgroup.
In the total population, tamoxifen was significantly associated with a decreased risk of osteoporosis and osteoporotic fracture (Figure 2). The risk of osteoporosis (HR, 1.24; CI, 0.85–1.82) and osteoporotic fracture (HR, 8.15; CI, 0.36–186.70) was not associated with tamoxifen in patients younger than 40 years (Table 3 and Supplementary Table 1). However, in the age 40–49 group, tamoxifen significantly decreased the risk of osteoporosis (HR, 0.74; CI, 0.65–0.84) and osteoporotic fracture (HR, 0.49; CI, 0.31–0.76).
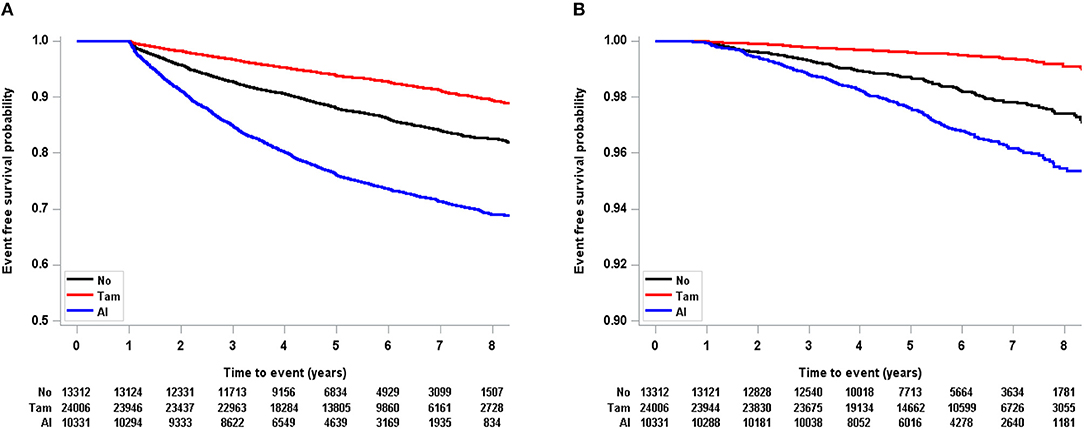
Figure 2. Kaplan-Meier event free probability according to endocrine treatment in total population (A: osteoporosis, B: osteoporotic fracture). Tam, tamoxifen; AI, aromatase inhibitor.
The results of this large retrospective study show that the use of adjuvant tamoxifen does not increase the risk of osteoporosis and osteoporotic fracture in younger breast cancer survivors. In patients younger than 40 years at the time of breast cancer diagnosis, adjuvant tamoxifen was not associated with the development of osteoporosis and osteoporotic fracture. Furthermore, tamoxifen significantly decreased the risk of osteoporosis and osteoporotic fracture in breast cancer patients aged 40–49 years at the time of diagnosis. OFS was significantly associated with an increased risk of osteoporosis in patients younger than 50 years. Chemotherapy and AI were significantly related to the risk of osteoporosis and osteoporotic fracture in patients between the ages of 40 and 49 years.
The results of this study differ from those of previous studies. Previous studies showed an association between tamoxifen use and bone loss in premenopausal patients. One previous study showed an annual loss of BMD of 1.44% in premenopausal breast cancer patients on tamoxifen (11). However, only 125 premenopausal breast cancer patients were enrolled in that study and treatment variables that can influence BMD were not statistically adjusted. Another study showed a 4.6% decrease in BMD at the 3-years follow-up evaluation in premenopausal patients taking tamoxifen after adjuvant chemotherapy. The number of patients enrolled in that study was also small, and only univariate analyses were conducted (12). Recently, researchers from Germany reported that tamoxifen increased the risk of fracture in premenopausal breast cancer patients compared to control patients without cancer (17). However, the selection criteria for the non-cancer control patients were not able to demonstrate the effect of tamoxifen on fracture because adjuvant treatments such as OFS and chemotherapy could not be statistically adjusted. To address these drawbacks, we conducted this nationwide retrospective cohort study.
To our knowledge, this is the largest retrospective cohort study of the effect of tamoxifen on bone health in younger breast cancer patients. Approximately 27,000 breast cancer patients younger than 50 years were enrolled, comprising 150,798 person-years. The results of this study indicate that tamoxifen does not increase the risk of osteoporosis and osteoporotic fracture in younger breast cancer survivors. These results contradict the ASCO recommendation that primary clinicians should refer premenopausal breast cancer survivors who are taking tamoxifen for repeat bone screening every 2 years (13).
One of the interesting findings of this study is that tamoxifen significantly decreased the risk of osteoporosis and osteoporotic fracture in breast cancer patients aged 40 to 49 years at the time of diagnosis. This can be explained by their perimenopausal status and long-term treatment with adjuvant tamoxifen. For patients with hormone receptor-positive breast cancers and lymph node metastasis, 10 years of adjuvant tamoxifen treatment are usually recommended (18). With the extended tamoxifen therapy, premenopausal women who are premenopausal at the time of breast cancer diagnosis might continue to take tamoxifen beyond the start of menopause, after which tamoxifen would show a protective effect on bone health.
In postmenopausal patients, AI increases the incidence of osteoporosis and osteoporotic fracture. The current study, in accordance with other reports (19, 20), demonstrated a marked increase in the risk of osteoporosis with AI, reflecting the near-complete estrogen depletion and subsequent disruption in bone homeostasis caused by these agents (21).
Multiple factors are related to the incidence of osteoporosis. The most common causes of bone loss in women are menopause and aging. Aging is associated with greater bone resorption and less bone formation, whereas menopause induces accelerated bone loss due to lowering levels of endogenous estrogen (22). In HIRA data study about the burden of osteoporosis in the general population, the prevalence of osteoporosis increased with age; the peak was at 70–79 years, with a rate of 5,253 diagnoses per 10,000 persons (23). Although we cannot directly compare this to the results of our study because of the different operational definitions, we similarly found an increasing incidence of osteoporosis in the older age group.
The limitations of this study should be noted. First, we were not able to perform survival analysis because the HIRA data is claims-based in accordance with the Personal Information Protection Act in Korea. Therefore, we could not assess the effect of osteoporosis and osteoporotic fracture on overall survival. Second, endocrine therapy treatment group allocation was based on claims data from the 1st year after diagnosis, and some patients may have subsequently switched from tamoxifen to AI. Third, as we defined osteoporosis as osteoporosis diagnosis codes in combination with osteoporosis medications, osteoporosis patients to whom osteoporosis medications were not prescribed due to other medical conditions were not included in this analysis. Lastly, due to the limitations of claims data, we were unable to gather information about diet, exercise, exposure to sunlight, and vitamin D supplementation, which are important factors for maintaining bone health.
The results of this study should be interpreted in the context of the study period. First, we did not analyze the use of denosumab which has been reimbursed for osteoporosis treatment from 2018 in South Korea. The resulting change in clinical practice could potentially affect the study outcomes. Second, after the practice-changing report from the Suppression of Ovarian Function Trial in 2015, adjuvant ovarian suppression in premenopausal breast cancer patients who remain premenopausal after chemotherapy, especially young patients, is recommended (24). Although OFS was not associated with a significantly increased risk of osteoporotic fracture in patients younger than 40 years in this study, OFS is now more often prescribed, possibly affecting the incidence of osteoporosis and osteoporotic fractures.
In conclusion, tamoxifen is not associated with an increased risk of osteoporosis and osteoporotic fracture in premenopausal breast cancer patients. Risk factors for osteoporosis and osteoporotic fractures vary according to patient age. Tailored screening strategies for breast cancer survivors who are at different risks of developing osteoporosis are needed.
The datasets generated for this study will not be made publicly available. The datasets are from the Korean National database which is not allowed to be extracted from the server.
The studies involving human participants were reviewed and approved by Soonchunhyang University Seoul Hospital Institutional Review Board (2018-11-011). Written informed consent from the participants' legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
JLe and HA: planned, wrote and revised the article. JK, MK, JWL, BK, BS, SA, JEL, SHa, ZK, and SHu: reviewed and edited the article. JSL: planned, analyzed data and statistics. IC: planned, wrote, revised, and supervised the concept of the article.
This study was supported by the Soonchunhyang University Research Fund.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Reviewer MI is currently organizing a Research Topic with one of the authors JK, and confirms the absence of any other collaboration.
We extend our gratitude to the Health Insurance Review and Assessment Service for providing nationwide data.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.00366/full#supplementary-material
Supplementary Figure 1. Kaplan-Meier event free probability in total population (A: osteoporosis, B: osteoporotic fracture).
Supplementary Table 1. Factors associated with osteoporotic fracture according to age subgroups in univariate and multivariate analysis.
1. Binbing Y, Tiwari RC, Feuer EJ. Estimating the personal cure rate of cancer patients using population-based grouped cancer survival data. Stat Methods Med Res. (2011) 20:261–74. doi: 10.1177/0962280209347046
2. Ahn SH, Park BW, Noh DY, Nam SJ, Lee ES, Lee MK, et al. Health-related quality of life in disease-free survivors of breast cancer with the general population. Ann Oncol. (2007) 18:173–82. doi: 10.1093/annonc/mdl333
3. Chen Z, Maricic M, Pettinger M, Ritenbaugh C, Lopez AM, Barad DH, et al. Osteoporosis and rate of bone loss among postmenopausal survivors of breast cancer. Cancer. (2005) 104:1520–30. doi: 10.1002/cncr.21335
4. Lindsey AM, Gross G, Twiss J, Waltman N, Ott C, Moore, et al. Postmenopausal survivors of breast cancer at risk for osteoporosis: nutritional intake and body size. Cancer Nurs. (2002) 25:50–6. doi: 10.1097/00002820-200202000-00010
5. Kanis JA, McCloskey EV, Powles T, Paterson AH, Ashley S, Spector, et al. A high incidence of vertebral fracture in women with breast cancer. Br J Cancer. (1999) 79:1179–81. doi: 10.1038/sj.bjc.6690188
6. Tremollieres F.A. Screening for osteoporosis after breast cancer: for whom, why and when. Maturitas. (2014) 79:343–8. doi: 10.1016/j.maturitas.2014.08.001
7. Eastell R, Adams J, Clack G, Howell A, Cuzick J, Mackey J, et al. Long-term effects of anastrozole on bone mineral density: 7-year results from the ATAC trial. Ann Oncol. (2011) 22:857–62. doi: 10.1093/annonc/mdq541
8. Hadji P, Aapro MS, Body JJ, Gnant M, Brandi ML, Reginster JY, et al. Management of aromatase inhibitor-associated bone loss (AIBL) in postmenopausal women with hormone sensitive breast cancer: joint position statement of the IOF, CABS, ECTS, IEG, ESCEO IMS, and SIOG. J Bone Oncol. (2017) 7:1–12. doi: 10.1016/j.jbo.2017.03.001
9. Body JJ. Increased fracture rate in women with breast cancer: a review of the hidden risk. BMC Cancer. (2011) 11:384. doi: 10.1186/1471-2407-11-384
10. Cosman F, de Beur SJ, LeBoff MS, Lewiecki EM, Tanner B, Randall S, et al. Clinician's guide to prevention and treatment of osteoporosis. Osteoporos Int. (2014) 25:2359–81. doi: 10.1007/s00198-014-2794-2
11. Powles TJ, Hickish T, Kanis JA, Tidy A, Ashley S. Effect of tamoxifen on bone mineral density measured by dual-energy x-ray absorptiometry in healthy premenopausal and postmenopausal women. J Clin Oncol. (1996) 14:78–84. doi: 10.1200/JCO.1996.14.1.78
12. Vehmanen L, Elomaa I, Blomqvist C, Saarto T. Tamoxifen treatment after adjuvant chemotherapy has opposite effects on bone mineral density in premenopausal patients depending on menstrual status. J Clin Oncol. (2006) 24:675–80. doi: 10.1200/JCO.2005.02.3515
13. Runowicz CD, Leach CR, Henry NL, Henry KS, Mackey HT, Cowens-Alvarado RL, et al. American cancer society/American society of clinical oncology breast cancer survivorship care guideline. J Clin Oncol. (2016) 34:611–35. doi: 10.1200/JCO.2015.64.3809
14. Kim JA, Yoon S, Kim LY, Kim DS. Towards actualizing the value potential of Korea health insurance review and assessment (HIRA) data as a resource for health research: strengths, limitations, applications, and strategies for optimal use of HIRA data. J Korean Med Sci. (2017) 32:718–28. doi: 10.3346/jkms.2017.32.5.718
15. Chung IY, Lee J, Park S, Lee JW, Youn HJ, Hong JH, et al. Nationwide analysis of treatment patterns for Korean breast cancer survivors using national health insurance service data. J Korean Med Sci. (2018) 33:e276. doi: 10.3346/jkms.2018.33.e276
16. Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. (2005) 43:1130–9. doi: 10.1097/01.mlr.0000182534.19832.83
17. Kyvernitakis I, Kostev K, Hadji P. The tamoxifen paradox-influence of adjuvant tamoxifen on fracture risk in pre- and postmenopausal women with breast cancer. Osteoporos Int. (2018) 29:2557–64. doi: 10.1007/s00198-018-4642-2
18. Davies C, Pan H, Godwin J, Gray R, Arriagada R, Raina V, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. (2013) 381:805–16. doi: 10.1016/S0140-6736(12)61963-1
19. Eastell R, Adams JE, Coleman RE, Howell A, Hannon RA, Cuzick J, et al. Effect of anastrozole on bone mineral density: 5-year results from the anastrozole, tamoxifen, alone or in combination trial 18233230. J Clin Oncol. (2008) 26:1051–7. doi: 10.1200/JCO.2007.11.0726
20. Eidtmann H, de Boer R, Bundred N, Llombart-Cussac A, Davidson N, Neven P, et al. Efficacy of zoledronic acid in postmenopausal women with early breast cancer receiving adjuvant letrozole: 36-month results of the ZO-FAST Study. Ann Oncol. (2010) 21:2188–94. doi: 10.1093/annonc/mdq217
21. Conte P, Frassoldati A. Aromatase inhibitors in the adjuvant treatment of postmenopausal women with early breast cancer: putting safety issuesinto perspective. Breast J. (2007) 13:28–35. doi: 10.1111/j.1524-4741.2006.00359.x
22. Demontiero O, Vidal C, Duque G. Aging and bone loss: new insights for the clinician. Ther Adv Musculoskelet Dis. (2012) 4:61–76. doi: 10.1177/1759720X11430858
23. Choi HJ, Shin CS, Ha YC, Jang S, Jang S, Park C, et al. Burden of osteoporosis in adults in Korea: a national health insurance database study. J Bone Miner Metab. (2012) 30:54–8. doi: 10.1007/s00774-011-0280-x
Keywords: breast neoplasms, survivorship, osteoporosis, bone fractures, tamoxifen
Citation: Lee J, Alqudaihi HM, Kang MS, Kim J, Lee JW, Ko BS, Son BH, Ahn SH, Lee JE, Han SW, Kim Z, Hur SM, Lee JS and Chung IY (2020) Effect of Tamoxifen on the Risk of Osteoporosis and Osteoporotic Fracture in Younger Breast Cancer Survivors: A Nationwide Study. Front. Oncol. 10:366. doi: 10.3389/fonc.2020.00366
Received: 22 August 2019; Accepted: 02 March 2020;
Published: 20 March 2020.
Edited by:
Sarah M. Temkin, Virginia Commonwealth University, United StatesReviewed by:
Matteo Lazzeroni, European Institute of Oncology, ItalyCopyright © 2020 Lee, Alqudaihi, Kang, Kim, Lee, Ko, Son, Ahn, Lee, Han, Kim, Hur, Lee and Chung. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ji Sung Lee, dG90b3JvOTZhQGdtYWlsLmNvbQ==; Il Yong Chung, ZG9vcmtlZXBlcjFAZ21haWwuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.