
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 05 March 2020
Sec. Cancer Molecular Targets and Therapeutics
Volume 10 - 2020 | https://doi.org/10.3389/fonc.2020.00224
 Chunhua Ma1†
Chunhua Ma1† Juncheng Zhang2,3†
Juncheng Zhang2,3† Dongjiang Tang2,3
Dongjiang Tang2,3 Xin Ye2,3
Xin Ye2,3 Jing Li1
Jing Li1 Ning Mu1
Ning Mu1 Zhi Li4
Zhi Li4 Renzhong Liu4
Renzhong Liu4 Liang Xiang2,3
Liang Xiang2,3 Chuoji Huang2,3*†
Chuoji Huang2,3*† Rong Jiang1*†
Rong Jiang1*†Background: The significance of uncommon epidermal growth factor receptor (EGFR) mutations in patients with non-small cell lung cancer (NSCLC) and brain metastasis (BM) remains unclear. Cerebrospinal fluid (CSF) liquid biopsy is a novel tool for assessing EGFR mutations in BM. This study aimed to evaluate the EGFR mutations in patients with NSCLC and newly diagnosed BM and to examine the effect of EGFR tyrosine kinase inhibitors (TKI) on BM harboring CSF-tested uncommon EGFR mutations.
Methods: This was a prospective study of 21 patients with NSCLC and BM diagnosed between 04/2018 and 01/2019. CSF was obtained to detect the BM EGFR mutations by next-generation sequencing. BM characteristics at magnetic resonance imaging (MRI) and EGFR-TKI response were examined.
Results: Of 21 patients with NSCLC, 10 (47.6%) had leptomeningeal metastasis (LM), while 11 (52.4%) had brain parenchymal metastasis (BPM); 13 (61.9%) had confirmed EGFR mutation-positive primary tumors. The uncommon mutation rate in CSF ctDNA was 33.3% (7/21). Among those with EGFR mutation-positive primary tumors, the rate of uncommon EGFR mutations in CSF was 53.8% (7/13). Uncommon EGFR mutations were more common in patients with LM than in patients with PBM (6/11, 54.5% vs. 1/10, 10%), and included G719A, L861Q, L703P, and G575R. TKI was effective for four patients with BMs harboring uncommon EGFR mutations.
Conclusion: In patients with NSCLC and LM, the rate of uncommon EGFR mutation was high. The BMs with uncommon EGFR mutations seem to respond to EGFR-TKI treatment. CSF liquid biopsy could reveal the EGFR genetic profile of the BM and help guide treatment using small-molecule TKI.
Brain metastases (BM) occurs in 30–50% of patients with non-small cell lung cancer (NSCLC) during the course of their disease (1). About 50% of the BMs are diagnosed at presentation of NSCLC, with 50–60% as the only site of distant metastasis (1). Patients with NSCLC and BMs have a poor prognosis, and the median survival is only 1–2 months (2, 3). BMs include parenchymal BMs (PBMs) and leptomeningeal metastases (LMs). LMs are less common than PBMs, with an occurrence rate of 3.4–3.8% in NSCLC, but their prognosis is worse (4, 5).
The management of BMs from NSCLC mostly includes surgery and radiation therapy; chemotherapy is seldom applied, and targeted drugs could be more effective than chemotherapy (6). In NSCLC, the targeted therapies mainly include tyrosine kinase inhibitors (TKI). TKIs have replaced chemotherapy because of better responses and survival rates (7–9). Recently developed EGFR-TKIs, e.g., osimertinib, specifically address the challenges of acquired drug resistance and low blood-brain barrier (BBB) permeability of first and second-generation TKIs, demonstrating efficacy in the CNS (10). Nevertheless, only NSCLC cells harboring epidermal growth factor receptor (EGFR) sensitizing mutations will respond to EGFR TKIs (1). Activating mutations in EGFR are found in 20–40% of NSCLC, with exon 19 deletions (45%) and exon 21 L858R mutations (40–45%) as the most common mutations (10). In NSCLC patients with BMs, the prevalence of EGFR mutations has been reported to be 39–63% in Asians (11, 12) and 2–40% in North American and European populations (13, 14). A retrospective study in China showed that the rate of uncommon mutations [i.e., mutations other than 19Del and L858R (15)] was high, with 12% of 1,837 Chinese patients with NSCLC EGFR mutations having non-classical mutations such as exon 20 insertion (30%), G719X mutation (21%), L858R complex mutation (17%; complex mutation defined as more than one EGFR mutation within a tumor sample) and T790M complex mutation (14%) (16). Importantly, different EGFR mutations respond differently to TKI therapy, and the impact of the uncommon mutations found in Asian patients is unknown (17, 18). Clinical studies so far have focused on the TKI treatment of NSCLC BMs with sensitizing mutations. Gefitinib is indicated in the treatment of EGFR-positive NSCLC BM and erlotinib as the second-line treatment for BM from asymptomatic NSCLC (1). The BRAIN trial (CTONG1201) showed that icotinib significantly improved the progression-free survival (PFS) and intracranial objective response rate (ORR) of patients with EGFR mutation and BMs (19). The ongoing APOLLO trial (ClinicalTrials.org #NCT02972333) is examining the efficiency and safety of osimertinib EGFR TKI in the treatment of EGFR mutated patients with BMs. Based on the post hoc analysis of the LUX-Lung 2/3/6 trials (9, 20, 21), the treatment indication for afatinib has been expanded to the first-line treatment of metastatic NSCLC with non-resistant EGFR mutation including L861Q/G719X/S768I. Afatinib is able to cross the BBB in sufficient amounts to induce anti-tumor actions (22, 23).
Several studies showed that EGFR mutation patterns in NSCLC primary lesions and metastases in various body locations are not consistent with that found in the BMs (24–26), possibly because of the specific events required for cancer cell migration to and survival in the brain. Indeed, a primary tumor is composed of various clones (27, 28) and not all of them will have the abilities to spread in circulation, cross the BBB, survive in the brain microenvironment, and invade the brain tissue (1, 29). These abilities call for specific sets of factors and mutations and therefore the actual tumor mutation status of BMs may differ from the estimation using primary tumor tissue or peripheral blood (12, 30). Indeed, a discordance rate of 16–32% for EGFR mutation status (depending on assay sensitivity for mutational analysis) between the primary site and BMs has been previously reported (12). Recent studies indicated that cerebrospinal fluid (CSF) ctDNA from BMs were present in CSF and that clinically actionable EGFR mutations were also more frequently detected in CSF ctDNA than in plasma in patients with BMs (31). Therefore, there is a possibility that BMs harboring rare mutations (e.g., L861Q, G719X, and S768I) not found in the primary lesion or metastases in other body locations will respond to EGFR-TKIs that are effective against lesions harboring those rare mutations, e.g., afatinib (9, 20, 21).
Therefore, EGFR-TKI can be used for the management of BMs from NSCLC, but the significance of uncommon EGFR mutations on the development and treatment response of BMs is still unclear. There are no studies on the significance of uncommon EGFR mutations in patients with BMs from NSCLC. We hypothesized that EGFR-TKIs could be effective against BMs with uncommon EGFR mutations, as evaluated by CSF ctDNA. The objectives of the present study were: (1) to evaluate the EGFR mutations in patients with NSCLC and newly diagnosed BMs; and (2) to examine the effect of EGFR-TKI on BMs harboring uncommon EGFR mutations.
This was a prospective study of 21 consecutive patients with NSCLC and BMs diagnosed between April 2018 and January 2019. The study was approved by the ethics committee of Tianjin Huanhu Hospital. All patients provided written informed consent prior to any study procedure. The inclusion criteria were: (1) NSCLC confirmed by histopathological examination; (2) new diagnosis of BMs by MRI and CSF cytological test with ThinPrep [a liquid-based cytology test applied in the diagnosis of LM (29)]; and (3) no prior treatment against BMs.
Demographics, clinical data, pathological data, imaging data, and tumor markers [carcinoembryonic antigen (CEA)] were obtained routinely. The EGFR mutation status of the primary site was obtained from previous medical records.
CSF samples were obtained from all 21 patients by lumbar puncture and placed in SanMed fixative solution, a patented cell preservation solution (Zhuhau SanMed Diagnostics Inc.), for transport and storage. Total DNA was extracted from CSF using the QiAamp Circumstance Nucleic Acid kit (#55114, Qiagen, Venlo, The Netherlands) according to the manufacturer's instructions. The reference library was constructed using the Ion AmpliSeq Library Kit 2.0 and the Ion AmpliSeq Cancer HosSpot Panel v2 (#55114 and #4475346, Thermo Fisher Scientific, Waltham, MA, USA) and the Ion Library TaqMan Quantitation kit (#4468802, Thermo Fisher Scientific, Waltham, MA, USA), according to the manufacturer's instructions. Details on next-generation sequencing are provided in Supplementary File 1.
Due to the relatively small sample size, only descriptive statistics were used. Data are presented as numbers and percentages.
Among the 21 patients with NSCLC, there were 10 (47.6%) males and 11 (52.4%) females. The mean age was 59.7 ± 9.9 years. Ten (47.6%) patients had LMs, while 11 (52.4%) had PBMs. Thirteen (61.9%) patients had primary tumors confirmed with EGFR mutation.
The uncommon mutation-positive rate in CSF ctDNA from all study subjects was 33.3% (7/21) (Figure 1). Among the patients with primary tumors with EGFR mutation, the rate of uncommon mutations was 53.8% (7/13). Six of these seven patients were treated with TKI and showed disease progression in the brain during the course of treatment.
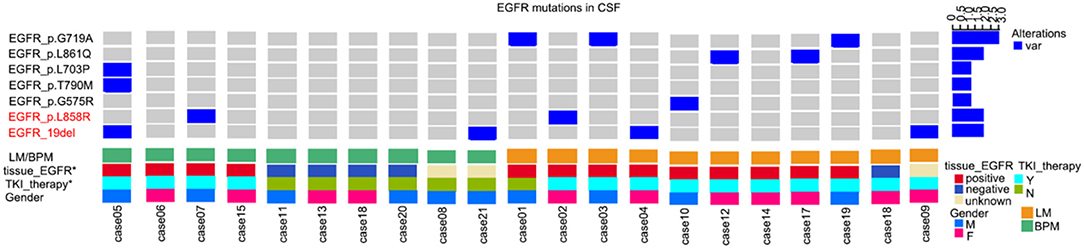
Figure 1. Uncommon mutations in the epidermal growth factor receptor (EGFR) gene from cerebrospinal fluid (CSF) circulating tumor DNA (ctDNA) from patients with non-small cell lung cancer (NSCLC). BPM, brain parenchymal metastases; LM, leptomeningeal metastases; TKI, tyrosine kinase inhibitor.
Compared with wild type EGFR, patients with primary tumors with EGFR mutation were more likely to display an uncommon EGFR mutation in CSF ctDNA (7/13, 50% vs. 0/5, 0%). Uncommon mutations were also more common in patients with LM than in patients with PBM (6/11, 54.5% vs. 1/10, 10%).
For the seven patients with uncommon EGFR mutations in CSF ctDNA (regardless of EGFR mutations status in brain/lung tissues), TKI was effective in four cases (57.1%), as shown by MRI and CEA levels.
Case 01 was a male of 34 years of age, with lung adenocarcinoma and with a history of smoking, but quitted 10 years ago (Figure 2). In April 2018, LM was diagnosed, and the EGFR p.G719A mutation was detected in CSF ctDNA (55.6%). The CSF CEA level was 9,470 ng/ml. The patient started afatinib treatment in May 2018, and achieved a partial response by July 2018, with a CSF CEA level of 2,111 ng/ml. The response was maintained in November 2018, with a CSF CEA level of 1,590 ng/ml and CSF EGFR p.G719A mutation at 23.1%.
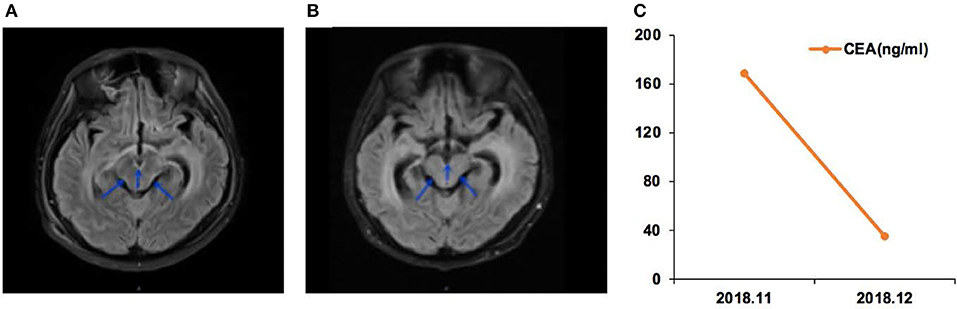
Figure 2. Case 01 was a male of 34 years of age, with lung adenocarcinoma and with a history of smoking, but quitted 10 years ago. (A) T2 FLAIR enhanced magnetic resonance imaging (MRI) showed abnormal high signal in the medulla, oblongata, pon, and ventral and dorsal midbrain, suggesting leptomeningeal metastases (LMs). (B) T2 FLAIR enhanced MRI during afatinib treatment showed that the abnormal high signal in the medulla, oblongata, and ventral and dorsal midbrain was lower than before treatment. (C) Carcinoembryonic antigen (CEA) levels before and after afatinib treatment.
Case 05 was a male of 71 years of age, with lung adenocarcinoma but without smoking history (Figure 3). The EGFR 19Del mutation was detected in the primary tumor. He received oral icotinib for 8 months before being admitted to the hospital for dizziness and episodes of loss of consciousness and was diagnosed with PBM. In December 2018, the EGFR p.L703P (2.0%) and EGFR p.T790M (2.1%) mutations, and the EGFR 19Del (86.0%) were detected in CSF ctDNA. The CSF CEA level was 96.1 ng/ml. The patient started osimertinib (80 mg qd) treatment, and the neurological symptoms were alleviated. In January 2019, the CSF CEA level was 8.7 ng/ml.
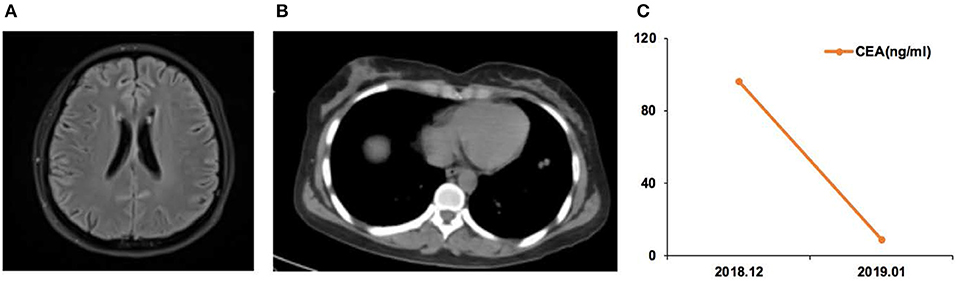
Figure 3. Case 05 was a male of 71 years of age, with lung adenocarcinoma but without smoking history. (A) Cerebellar vermis, bilateral cerebral hemispheres, and pia meninges shoed abnormal enhancement on magnetic resonance imaging. Leptomeningeal metastasis (LM) was considered. (B) Chest computed tomography revealing the primary lung lesion. (C) Carcinoembryonic antigen (CEA) levels before and after osimertinib treatment.
Case 12 was a female of 57 years of age, with lung adenocarcinoma but without smoking history (Figure 4). She was diagnosed with LM in September 2018. CSF ctDNA analysis revealed the EGFR p.L861Q (46.5%) mutation, and the CSF CEA level was 786.9 ng/ml. She started afatinib treatment. In December 2018, the CSF CEA level was 98.1 ng/ml.
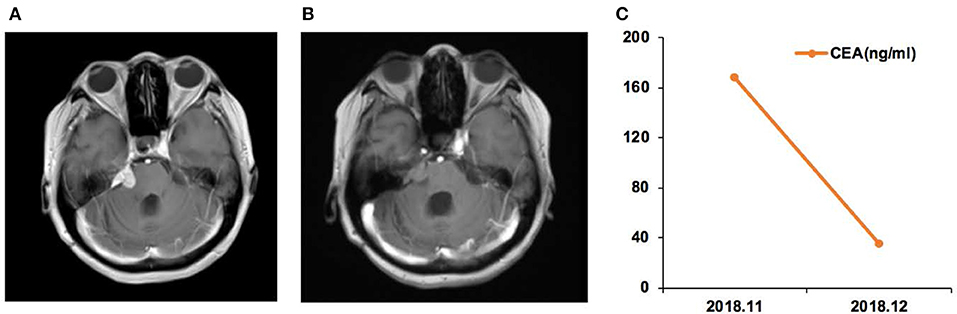
Figure 4. Case 12 was a female of 57 years of age, with lung adenocarcinoma but without smoking history. (A) In September 2018, the right cerebellopontine angle area, the edge of the tetras, and the lateral edge of the right arm were abnormally enhanced on magnetic resonance imaging. (B) In December, the enhancement intensity was decreased on the right side, and her condition was improved. (C) Carcinoembryonic antigen (CEA) levels before and after afatinib treatment.
Case 17 was a female of 65 years of age, with lung adenocarcinoma but without smoking history (Figure 5). In November 2018, CSF ctDNA analysis revealed EGFR p.L861Q (62.6%) and TP53 p.C135F (95.5%) mutations, and the CSF CEA level was 168.3 ng/ml. The patient started afatinib treatment. In December 2018, the CSF CEA level was 35.4 ng/ml.
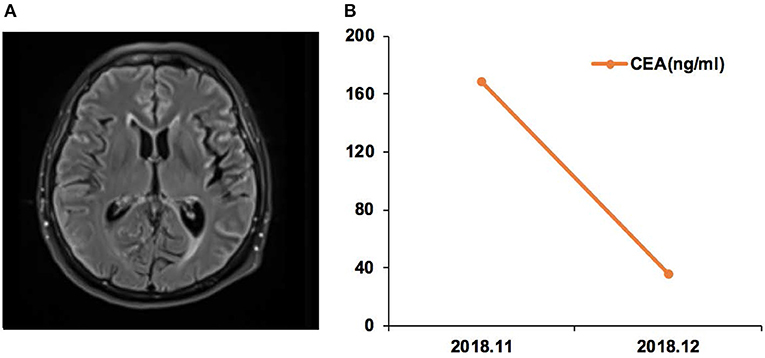
Figure 5. Case 17 was a female of 65 years of age, with lung adenocarcinoma but without smoking history. (A) Magnetic resonance imaging (MRI) of the brain. (B) Carcinoembryonic antigen (CEA) levels before and after afatinib treatment.
The rate of uncommon EGFR mutations in Asian patients with NSCLC is high, comprising 11.9% of all cases in a previous report (31). There were rare previous studies on the significance of EGFR uncommon mutations in patients with NSCLC and BMs. There is a possibility that BMs harboring rare mutations not found in other body locations will respond to EGFR-TKIs (9, 20, 21). Therefore, the aim of this study was to evaluate the EGFR mutations in patients with NSCLC and newly diagnosed BMs and examine the effect of EGFR TKI on BMs harboring uncommon EGFR mutations. The results showed that the rate of uncommon EGFR mutation in patients with NSCLC and BMs was high. The BMs with uncommon EGFR mutations seemed to respond to EGFR TKI treatment. Taken together, CSF liquid biopsy could reveal the EGFR genetic profile of the BM and help guide treatment using small-molecule TKI. These results do not imply that metastases in other body locations will answer or not to the BM-guided therapy, but since survival to BMs is short (2, 3), tailoring EGFR-TKI treatment specifically to the BMs might has a higher likelihood of prolonging survival in those patients.
In this study, the frequency of uncommon EGFR mutations was high, with these mutations detected in the CSF ctDNA in 33.3% (7/21) patients (considered to be from the BMs). These rates are higher than the 12% previously reported in patients with NSCLC but not necessarily with BM in China (16). This discrepancy might be due to the small sample size (selection bias) and the different testing methods. On the other hand, EGFR mutations have been reported to be more frequent in patients with NSCLC and BM (32). The exact role of uncommon EGFR mutations in BM development requires further research.
A primary tumor is a mosaic of various clones that evolved from the original tumor cell(s) (27, 28). Unlike cytotoxic chemotherapies that target all fast-growing cells, targeted treatments target specific cells within the tumor, raising the possibility of selecting resistant or unaffected clones, which can be responsible for relapse and metastasis (33, 34). BMs show significant molecular divergence with the primary tumor and with extracranial metastases (30, 31, 35–39). The process of BM development from the primary tumor necessitates specific steps, including crossing the BBB, surviving in the brain microenvironment, and invading the brain tissue, all of which requiring specific sets of biological aspects (1). The development of BMs in lung cancer patients who received an anti-EGFR treatment may be due to the TKI effectively killing the cancer cells with the exon 19 deletion or the L858R mutation, but the effect of the TKI could be insufficient on the cells with uncommon mutation, therefore increasing the possibility of these cells contributing to BM development. Indeed, it has been shown that mutations such as exon 20 insertions, L861Q, S768I, and G718X have inferior response to first- generation EGFR TKIs (40). In the present study, six of the seven patients with BMs harboring uncommon EGFR mutations had received adjuvant EGFR TKI, supporting the hypothesis of clone selection by EGFR TKI. Nevertheless, additional studies are necessary to examine this point since erlotinib has been shown to reduce the risk of BMs from NSCLC (41).
A number of studies indicated the efficacy of EGFR TKI treatment against NSCLC BMs (1, 26, 42). The results from the LUX-Lung 2/3/6 trials (9, 20, 21) indicate that afatinib can be used as first-line treatment of metastatic NSCLC with non-resistant EGFR mutation including L861Q/G719X/S768I. Of particular interest, afatinib is able to cross the BBB in sufficient amounts to induce anti-tumor actions (22, 23). In the present study, three patients with uncommon EGFR mutations responded well to afatinib, as shown by MRI and CEA levels. A good response was also observed with Osimertinib. Additional studies are necessary to determine the best treatment approaches for BMs harboring uncommon mutations, particularly in the context that the frequency of those mutations is high in Asia (16).
Obtaining genetic material from BMs is complicated because surgical resection and biopsy are often impossible or not indicated due to the patient's condition. The BBB prevents ctDNA from brain lesions to pass into the blood circulation and vice versa; therefore, the ctDNA found in CSF by liquid biopsy will reflect the status of the BMs (38, 43–46). Hence, a liquid biopsy of CSF in patients with NSCLC and BMs could provide the actual intracranial situation, helping to guide patient management. New technologies such as next-generation sequencing will allow personalized medicine to reach its full potential (38, 44).
It is well-known that LMs are less common than PBM, but their prognosis is poorer (4, 5). In the present study, the frequencies of LMs and PBMs were similar, hinting toward some possible selection bias. Nevertheless, an important result is that the frequency of uncommon EGFR mutation was higher in LMs than in PBMs. This could explain, at least in part, the poorer prognosis of LMs. The association of uncommon EGFR mutation and LM will have to be examined in future studies.
The present study had limitations. Because uncommon mutations are rarely diagnosed, the sample size was relatively small, and the study was performed in a single center. In addition, follow-up was short. Furthermore, no post-treatment radiological data were available in some cases after patient improvement and discharge, especially non-residents. Moreover, CEA assessment is not widely accepted as a response marker. Finally, patients were administered various TKIs that had different BBB penetration rates.
EGFR TKI could be effective against uncommon EGFR mutations in NSCLC BMs. Molecular testing of CSF could be helpful in guiding treatment and tracking treatment response. Uncommon mutation might be considered as participating in the process of brain metastases of NSCLC.
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
The studies involving human participants were reviewed and approved by the ethics committee of Tianjin Huanhu Hospital. The patients/participants provided their written informed consent to participate in this study.
CM, CH, RJ, and JZ conceived and coordinated the study, designed, performed, analyzed the experiments, and wrote the paper. JL, NM, ZL, and LX carried out the data collection, data analysis, and revised the paper. All authors reviewed the results and approved the final version of the manuscript.
This study was funded by the Joint Research Center of Liquid Biopsy in Guangdong, Hong Kong, and Macao, Zhuhai, China.
JZ, CH, DT, XY, and LX were employed by Zuhai SanMed Biotech Ltd. RL and ZL were employed by Zuhai Livzon Gene Diagnostics Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors acknowledge the help of the bio-information team at Livzongene LLC for their experimental work.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.00224/full#supplementary-material
Supplementary File 1. Details of next-generation sequencing.
BBB, blood-brain barrier; BM, brain metastases; CEA, carcinoembryonic antigen; CSF, cerebrospinal fluid; EGFR, epidermal growth factor receptor; LMs, leptomeningeal metastases; NSCLC, non-small cell lung cancer; ORR, objective response rate; PBMs, parenchymal BMs; PFS, progression-free survival; TKI, tyrosine kinase inhibitors.
1. Preusser M, Winkler F, Valiente M, Manegold C, Moyal E, Widhalm G, et al. Recent advances in the biology and treatment of brain metastases of non-small cell lung cancer: summary of a multidisciplinary roundtable discussion. ESMO Open. (2018) 3:e000262. doi: 10.1136/esmoopen-2017-000262
2. Ali A, Goffin JR, Arnold A, Ellis PM. Survival of patients with non-small-cell lung cancer after a diagnosis of brain metastases. Curr Oncol. (2013) 20:e300–6. doi: 10.3747/co.20.1481
3. Galluzzi S, Payne PM. Brain metastases from primary bronchial carcinoma: a statistical study of 741 necropsies. Br J Cancer. (1956) 10:408–14. doi: 10.1038/bjc.1956.47
4. Eichler AF, Loeffler JS. Multidisciplinary management of brain metastases. Oncologist. (2007) 12:884–98. doi: 10.1634/theoncologist.12-7-884
5. Cheng H, Perez-Soler R. Leptomeningeal metastases in non-small-cell lung cancer. Lancet Oncol. (2018) 19:e43–55. doi: 10.1016/S1470-2045(17)30689-7
6. Soffietti R, Abacioglu U, Baumert B, Combs SE, Kinhult S, Kros JM, et al. Diagnosis and treatment of brain metastases from solid tumors: guidelines from the European Association of Neuro-Oncology (EANO). Neuro Oncol. (2017) 19:162–74. doi: 10.1093/neuonc/now241
7. Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. (2009) 361:947–57. doi: 10.1056/NEJMoa0810699
8. Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. (2012) 13:239–46. doi: 10.1016/S1470-2045(11)70393-X
9. Sequist LV, Yang JC, Yamamoto N, O'Byrne K, Hirsh V, Mok T, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. (2013) 31:3327–34. doi: 10.1200/JCO.2012.44.2806
10. Syrigos KN, Georgoulias V, Zarogoulidis K, Makrantonakis P, Charpidou A, Christodoulou C. Epidemiological characteristics, EGFR status and management patterns of advanced non-small cell lung cancer patients: the greek REASON observational registry study. Anticancer Res. (2018) 38:3735–44. doi: 10.21873/anticanres.12654
11. Matsumoto S, Takahashi K, Iwakawa R, Matsuno Y, Nakanishi Y, Kohno T, et al. Frequent EGFR mutations in brain metastases of lung adenocarcinoma. Int J Cancer. (2006) 119:1491–4. doi: 10.1002/ijc.21940
12. Gow CH, Chang YL, Hsu YC, Tsai MF, Wu CT, Yu CJ, et al. Comparison of epidermal growth factor receptor mutations between primary and corresponding metastatic tumors in tyrosine kinase inhibitor-naive non-small-cell lung cancer. Ann Oncol. (2009) 20:696–702. ndoi: 10.1093/annonc/mdn679
13. Sun M, Behrens C, Feng L, Ozburn N, Tang X, Yin G, et al. HER family receptor abnormalities in lung cancer brain metastases and corresponding primary tumors. Clin Cancer Res. (2009) 15:4829–37. doi: 10.1158/1078-0432.CCR-08-2921
14. Daniele L, Cassoni P, Bacillo E, Cappia S, Righi L, Volante M, et al. Epidermal growth factor receptor gene in primary tumor and metastatic sites from non-small cell lung cancer. J Thorac Oncol. (2009) 4:684–8. doi: 10.1097/JTO.0b013e3181a52359
15. da Cunha Santos G, Shepherd FA, Tsao MS. EGFR mutations and lung cancer. Annu Rev Pathol. (2011) 6:49–69. doi: 10.1146/annurev-pathol-011110-130206
16. Tu HY, Ke EE, Yang JJ, Sun YL, Yan HH, Zheng MY, et al. A comprehensive review of uncommon EGFR mutations in patients with non-small cell lung cancer. Lung Cancer. (2017) 114:96–102. doi: 10.1016/j.lungcan.2017.11.005
17. Nan X, Xie C, Yu X, Liu J. EGFR TKI as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer. Oncotarget. (2017) 8:75712–26. doi: 10.18632/oncotarget.20095
18. Zhang YL, Yuan JQ, Wang KF, Fu XH, Han XR, Threapleton D, et al. The prevalence of EGFR mutation in patients with non-small cell lung cancer: a systematic review and meta-analysis. Oncotarget. (2016) 7:78985–93. doi: 10.18632/oncotarget.12587
19. Wu YL, Yang JJ, Zhou C, Feng J, Lu S, Song Y, et al. PL03.05: BRAIN: A Phase III trial comparing WBI and chemotherapy with icotinib in NSCLC with brain metastases harboring EGFR mutations (CTONG 1201). J Thor Oncol. (2017) 12:S6. doi: 10.1016/j.jtho.2016.11.007
20. Yang JC, Shih JY, Su WC, Hsia TC, Tsai CM, Ou SH, et al. Afatinib for patients with lung adenocarcinoma and epidermal growth factor receptor mutations (LUX-Lung 2): a phase 2 trial. Lancet Oncol. (2012) 13:539–48. doi: 10.1016/S1470-2045(12)70086-4
21. Wu YL, Zhou C, Hu CP, Feng J, Lu S, Huang Y, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol. (2014) 15:213–22. doi: 10.1016/S1470-2045(13)70604-1
22. Hoffknecht P, Tufman A, Wehler T, Pelzer T, Wiewrodt R, Schutz M, et al. Efficacy of the irreversible ErbB family blocker afatinib in epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI)-pretreated non-small-cell lung cancer patients with brain metastases or leptomeningeal disease. J Thorac Oncol. (2015) 10:156–63. doi: 10.1097/JTO.0000000000000380
23. Schuler M, Wu YL, Hirsh V, O'Byrne K, Yamamoto N, Mok T, et al. First-Line afatinib versus chemotherapy in patients with non-small cell lung cancer and common epidermal growth factor receptor gene mutations and brain metastases. J Thorac Oncol. (2016) 11:380–90. doi: 10.1016/j.jtho.2015.11.014
24. Burel-Vandenbos F, Ambrosetti D, Coutts M, Pedeutour F. EGFR mutation status in brain metastases of non-small cell lung carcinoma. J Neurooncol. (2013) 111:1–10. doi: 10.1007/s11060-012-0990-5
25. Hata A, Katakami N, Yoshioka H, Takeshita J, Tanaka K, Nanjo S, et al. Rebiopsy of non-small cell lung cancer patients with acquired resistance to epidermal growth factor receptor-tyrosine kinase inhibitor: Comparison between T790M mutation-positive and mutation-negative populations. Cancer. (2013) 119:4325–32. doi: 10.1002/cncr.28364
26. Kelly WJ, Shah NJ, Subramaniam DS. Management of brain metastases in epidermal growth factor receptor mutant non-small-cell lung cancer. Front Oncol. (2018) 8:208. doi: 10.3389/fonc.2018.00208
27. Jia Q, Wu W, Wang Y, Alexander PB, Sun C, Gong Z, et al. Local mutational diversity drives intratumoral immune heterogeneity in non-small cell lung cancer. Nat Commun. (2018) 9:5361. doi: 10.1038/s41467-018-07767-w
28. McGranahan N, Swanton C. Clonal heterogeneity and tumor evolution: past, present, and the future. Cell. (2017) 168:613–28. doi: 10.1016/j.cell.2017.01.018
29. Winkler F. Hostile takeover: how tumours hijack pre-existing vascular environments to thrive. J Pathol. (2017) 242:267–72. doi: 10.1002/path.4904
30. Brastianos PK, Carter SL, Santagata S, Cahill DP, Taylor-Weiner A, Jones RT, et al. Genomic characterization of brain metastases reveals branched evolution and potential therapeutic targets. Cancer Discov. (2015) 5:1164–77. doi: 10.1158/2159-8290.CD-15-0369
31. De Mattos-Arruda L, Mayor R, Ng CKY, Weigelt B, Martinez-Ricarte F, Torrejon D, et al. Cerebrospinal fluid-derived circulating tumour DNA better represents the genomic alterations of brain tumours than plasma. Nat Commun. (2015) 6:8839. doi: 10.1038/ncomms9839
32. Bhatt VR, Kedia S, Kessinger A, Ganti AK. Brain metastasis in patients with non-small-cell lung cancer and epidermal growth factor receptor mutations. J Clin Oncol. (2013) 31:3162–4. doi: 10.1200/JCO.2013.49.8915
33. Gambara G, Gaebler M, Keilholz U, Regenbrecht CRA, Silvestri A. From chemotherapy to combined targeted therapeutics: in vitro and in vivo models to decipher intra-tumor heterogeneity. Front Pharmacol. (2018) 9:77. doi: 10.3389/fphar.2018.00077
34. Amirouchene-Angelozzi N, Swanton C, Bardelli A. Tumor evolution as a therapeutic target. Cancer Discov. (2017) 7. doi: 10.1158/2159-8290.CD-17-0343
35. Paik PK, Shen R, Won H, Rekhtman N, Wang L, Sima CS, et al. Next-Generation sequencing of stage iv squamous cell lung cancers reveals an association of pi3k aberrations and evidence of clonal heterogeneity in patients with brain metastases. Cancer Discov. (2015) 5:610–21. doi: 10.1158/2159-8290.CD-14-1129
36. Saunus JM, Quinn MC, Patch AM, Pearson JV, Bailey PJ, Nones K, et al. Integrated genomic and transcriptomic analysis of human brain metastases identifies alterations of potential clinical significance. J Pathol. (2015) 237:363–78. doi: 10.1002/path.4583
37. Lee JY, Park K, Lim SH, Kim HS, Yoo KH, Jung KS, et al. Mutational profiling of brain metastasis from breast cancer: matched pair analysis of targeted sequencing between brain metastasis and primary breast cancer. Oncotarget. (2015) 6:43731–42. doi: 10.18632/oncotarget.6192
38. Pentsova EI, Shah RH, Tang J, Boire A, You D, Briggs S, et al. Evaluating cancer of the central nervous system through next-generation sequencing of cerebrospinal fluid. J Clin Oncol. (2016) 34:2404–15. doi: 10.1200/JCO.2016.66.6487
39. Priedigkeit N, Hartmaier RJ, Chen Y, Vareslija D, Basudan A, Watters RJ, et al. Intrinsic subtype switching and acquired ERBB2/HER2 amplifications and mutations in breast cancer brain metastases. JAMA Oncol. (2017) 3:666–71. doi: 10.1001/jamaoncol.2016.5630
40. O'Kane GM, Bradbury PA, Feld R, Leighl NB, Liu G, Pisters KM, et al. Uncommon EGFR mutations in advanced non-small cell lung cancer. Lung Cancer. (2017) 109:137–44. doi: 10.1016/j.lungcan.2017.04.016
41. Liu J, Xing L, Meng X, Yue J, Meng X, Xie P, et al. Risk of brain metastasis reduced after erlotinib treatment in advanced pulmonary adenocarcinoma patients with sensitive EGFR mutation. Onco Targets Ther. (2016) 9:671–9. doi: 10.2147/OTT.S100105
42. Remon J, Besse B. Brain metastases in oncogene-addicted non-small cell lung cancer patients: incidence and treatment. Front Oncol. (2018) 8:88. doi: 10.3389/fonc.2018.00088
43. Pan C, Diplas BH, Chen X, Wu Y, Xiao X, Jiang L, et al. Molecular profiling of tumors of the brainstem by sequencing of CSF-derived circulating tumor DNA. Acta Neuropathol. (2019) 137:297–306. doi: 10.1007/s00401-018-1936-6
44. Garinet S, Laurent-Puig P, Blons H, Oudart JB. Current and future molecular testing in NSCLC, what can we expect from new sequencing technologies? J Clin Med. (2018) 7:144. doi: 10.3390/jcm7060144
45. Boire A, Brandsma D, Brastianos PK, Le Rhun E, Ahluwalia M, Junk L, et al. Liquid biopsy in central nervous system metastases: a RANO review and proposals for clinical applications. Neuro Oncol. (2019) 21:571–84. doi: 10.1093/neuonc/noz012
Keywords: non-small cell lung cancer, brain metastasis, tyrosine kinase inhibitors, epidermal growth factor receptor, mutation
Citation: Ma C, Zhang J, Tang D, Ye X, Li J, Mu N, Li Z, Liu R, Xiang L, Huang C and Jiang R (2020) Tyrosine Kinase Inhibitors Could Be Effective Against Non-small Cell Lung Cancer Brain Metastases Harboring Uncommon EGFR Mutations. Front. Oncol. 10:224. doi: 10.3389/fonc.2020.00224
Received: 30 July 2019; Accepted: 10 February 2020;
Published: 05 March 2020.
Edited by:
Timothy F. Burns, University of Pittsburgh, United StatesReviewed by:
Hatim Husain, University of California, San Diego, United StatesCopyright © 2020 Ma, Zhang, Tang, Ye, Li, Mu, Li, Liu, Xiang, Huang and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rong Jiang, amlhbmdyb25nMTk5OUAxMjYuY29t; Chuoji Huang, aHVhbmdjaHVvamlAZm94bWFpbC5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.