- 1Department of Gastroenterology, Taihe Hospital, Hubei University of Medicine, Shiyan, China
- 2Department of Oncology, Taihe Hospital, Hubei University of Medicine, Shiyan, China
- 3Post Graduate Department, Hubei University of Medicine, Shiyan, China
- 4Department of Laboratory Medicine, Taihe Hospital, Hubei University of Medicine, Shiyan, China
Objective: Neutrophil lymphocyte ratio (NLR), Lymphocyte mononuclear cell ratio (LMR), and Platelet lymphocyte ratio (PLR) can be used as various prognostic factors for malignant tumors, but the value of prognosis for patients with adenocarcinoma of the esophagogastric junction (AEG) has not been determined. This study used meta-analysis to assess the value of these indicators in the evaluation of AEG prognosis.
Methods: Relevant literatures on the prognostic relationship between NLR, LMR, PLR, and AEG was retrieved from PubMed, Web of Science, Embase, Cochrane Library, Cochrane Central Register of Controlled Trials, Wanfang data, and Chinese National Knowledge Infrastructure. The search time from database establishment to June 30, 2019. The language is limited to English and Chinese. Data was analyzed using Stata 15.0 software.
Result: Six retrospective studies were included, five of them involved NLR and six of them involved PLR. No LMR literature that adequately satisfied the conditions was retrieved. Increased NLR was significantly associated with a significant reduction in overall survival (OS), cancer-specific survival (CSS), or disease specific survival (DSS) in patients with AEG [hazard ratio (HR) = 1.545, 95% CI: 1.096–2.179, P < 0.05]. Subgroup analysis showed that NLR had significant value in the prognosis of both Chinese and Non-Chinese patients (P = 0.009 vs. P = 0.000). NLR had significant prognostic value for ≥3 and <3 groups (P = 0.022 vs. P = 0.000). NLR has a significant prognostic value for samples ≥500 and <500 (P = 0.000 vs. P = 0.022). NLR and OS/CSS/DSS single factor meta-regression showed that regional NLR cut-off values and sample size may be the source of heterogeneity in AEG patients (all P < 0.05). There was no significant association between elevated PLR and OS in patients with AEG (HR = 1.117, 95% CI: 0.960–1.300, P > 0.05). PLR had no significant prognostic value for both Chinese and UK patients (P = 0.282 vs. P = 0.429). PLR had no significant prognostic value for ≥150 group and <150 group (P = 0.141 and P = 0.724). No significant prognostic value was found in either the 300 group and <300 group (P = 0.282 vs. P = 0.429).
Conclusion: Preoperative NLR rise was an adverse prognostic indicator of AEG. High-risk patients should be treated promptly. The results showed that PLR was not recommended as a prognostic indicator of AEG.
Introduction
Esophagogastric junction (EGJ) cancer mainly refers to cancer whose center of the malignant tumors is within 5 cm of the proximal and distal ends of EGJ, including EGJ distal esophageal adenocarcinoma, cardiac cancer, and proximal gastric cancer (1). In recent years, trend of EGJ cancer is increasing yearly in Europe, the United States, and many Asian countries (2–6) and has become a worldwide problem that seriously endangers human health (7). The adenocarcinoma of the esophagogastric junction (AEG), first proposed by Siewert (8) in 1999, is a tumor with unique clinicopathological features and biological behavior. AEG lymph node metastasis has a high incidence and a low long-term survival rate, affecting the prognosis of patients seriously (9, 10).
Effective markers are screened to identify high-risk patients and helpful for the individualized treatment and prognosis improvement of AEG. Xu et al. (11) reported that log odds of positive lymph nodes can predict the prognosis of patients with Siewert type II AEG. Felismino et al. (12) believes that the prognosis of locally advanced esophagogastric cancer can be determined by pathological staging and primary site. Kudou et al. (13) believes that postoperative sarcopenia can be used as a prognostic indicator for AEG, but these indicators are postoperative. Unfortunately, effective preoperative biomarkers are still lacking.
Laboratory indicators may be used as prognostic indicators for gastrointestinal tumors (14, 15), including: neutrophil to lymphocyte ratio (NLR), platelet to lymphocyte ratio (PLR), lymphocyte monocyte ratio (LMR), Glasgow prognostic score (GPS), and Prognostic nutritional index (PNI). Although some studies have reported the relationship between these indicators and the prognosis of patients with AEG, a consensus has not been reached. Urabe et al. (14) indicated that preoperative NLR and PLR is associated with OS and DFS in patients with AEG. Zhou et al. (16) postulated that preoperative LMR and PLR are very useful predictors for AEG surgery; but Zhang et al. (17) reported that preoperative NLR can be used as prognostic factor for Siewert type II/III AEG patients, but PLR value is limited. The present study aims to evaluate the value of NLR, LMR, and PLR in evaluating the prognosis of patients with AEG through systematic review and meta-analysis and to provide evidence-based supporting the use of these markers as prognostic indicators of AEG.
Materials and Methods
Literature Search Strategy
Search for the relationship between NLR, PLR, LMR, and AEG prognosis or clinicopathological features in databases such as PubMed, Web of Science, Embase, Cochrane Library, Cochrane Central Register of Controlled Trials, Wanfang data, and Chinese National Knowledge Infrastructure. The search time frame for database establishment was till June 30, 2019. Search terms included: “NLR,” “PLR,” “LMR,” “esophagogastric junction cancer,” and “AEG.” The language was limited to English and Chinese.
Inclusion and Exclusion Criteria
Inclusion criteria: (1) Patients confirmed pathologically as AEG; (2) Assessed preoperative NLR, PLR, or LMR overall survival (OS), disease-free survival (DFS), tumor-specific survival (relationship between cancer specific survival (CSS), or disease specific survival (DSS); (3) Reported hazard ratio (HR) and 95% confidence interval (CI) or indirect calculation HR and 95% CI. If HR could not be directly extracted from the literature, we calculated it by formula. Our calculation formula is b = In (HR), stderr = b/inverse-normal-distribution (P/2), 95% CI = exp (b ± 1.96 * stderr). (4) Full text in English or Chinese.
Exclusion criteria: (1) HR and 95% CI cannot be obtained directly or indirectly, NLR, PLR, or LMR have no clear cut-off point; (2) Non-research literature such as review, case report, conference summary, etc.; (3) Animal research or basic cell research; (4) Repeated published literature, research literature on the same cohort study subjects.
Test Screening and Data Extraction
We conducted this study in accordance with the systematic reporting and preliminary analysis items (18) (preferred reporting items for systematic reviews and meta analyses, PRISMA). Since all data are based on published literature, this study does not address any ethical issues. Two reviewers (Liu and Zhang) read the title and abstract independently in a double-blind manner, excluded non-compliant studies, read full-text documents that met the inclusion criteria, and cross-checked the included articles. In case of differences and discussions, an independent third party was asked to decide. Data extraction content was based on: title, author, region, publication time, sample size, critical value, HR, and 95% CI of OS, DFS, CSS, or DSS.
Quality Evaluation
The quality of the included studies was assessed using the Newcastle-Ottawa Quality Assessment Scale (NOS). NOS consists of three aspects: selection, comparability, and exposure or outcome; a total score of 9 points, and a total score of ≥6 in the study is considered high quality (19).
Statistical Methods
Meta-analysis was performed using Stata statistical software (Stata Corporation, version 15.0, College Station, TX, USA). HR and 95% CI were combined in the study to evaluate the value of NLR, PLR, and LMR in predicting the prognosis of patients with AEG. A meta-analysis forest map that plots the effect indicators. The Q-test and I2 of the chi-square test were used to evaluate the heterogeneity between studies. I2 and Q-tests were used to evaluate the heterogeneity of the included research questions. If I2 <50% and P-test of P > 0.1, it indicates that the studies are homogeneous and a fixed effect model is selected; P < 0.1, indicating that there is heterogeneity between studies, and a random effect model was selected (19, 20). To find the sources of heterogeneity, subgroup analyses were used to explore sources of heterogeneity. The publication bias was assessed by Egger test and Begg test. When P < 0.05, the difference was considered statistically significant and there was publication bias.
Results
Characteristics of the Included Literature
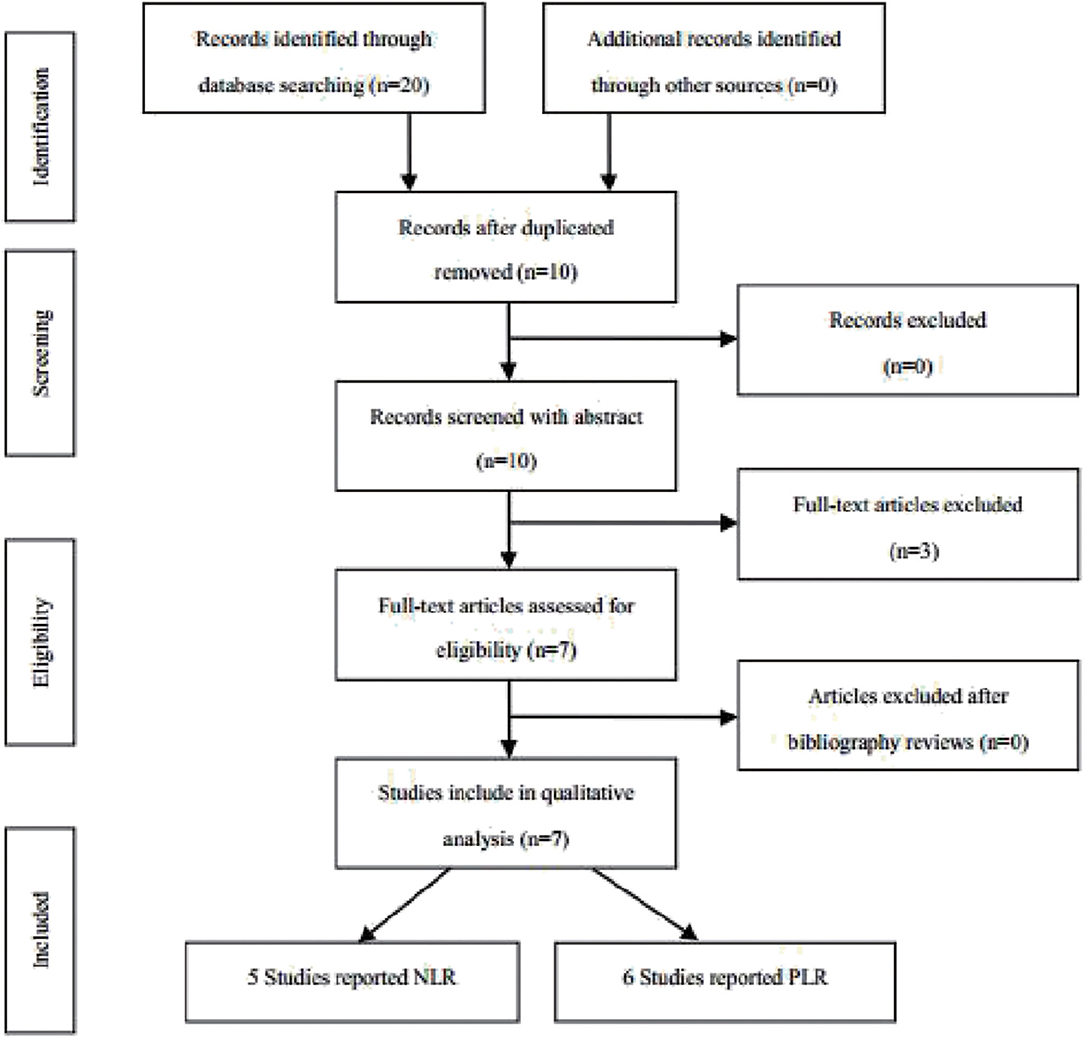
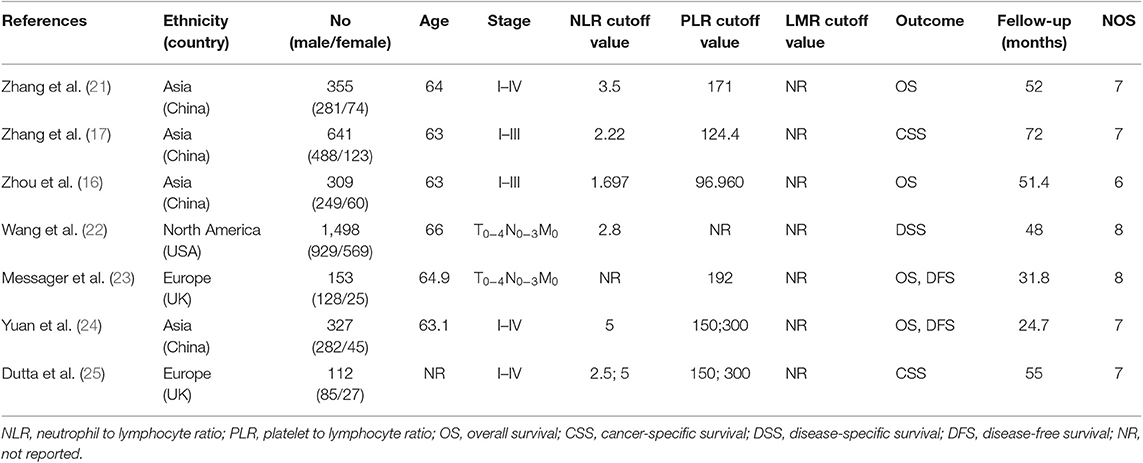
According to the above strategy, a total of 20 articles were screened and finally included in six articles (Figure 1), all of which were retrospective studies. Five of which involved NLR and six of which involved PLR. The information on the literature included in this study (Table 1).
Meta Analysis Results
The Prognostic Role of NLR in AEG
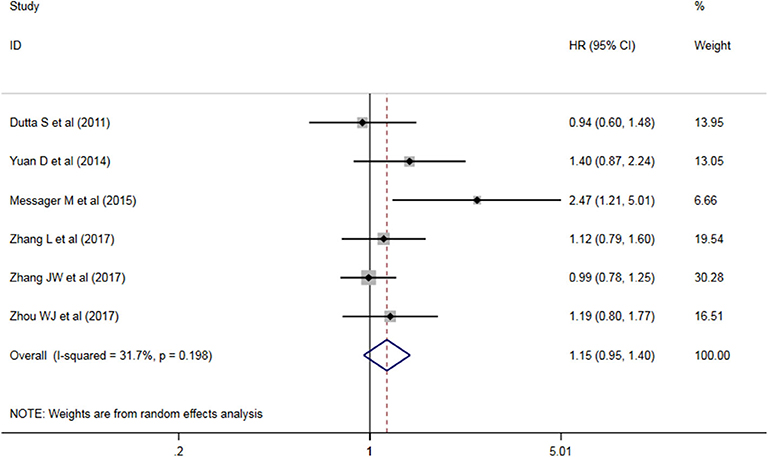
Five studies have analyzed the relationship between NLR and OS/CSS/DSS in patients with AEG, and a significant heterogeneity exists among these studies (P < 0.05, I2 = 91.6%), and random effects model was used. These results indicate that an increase in NLR decreases predicts OS/CSS/DSS shortening in patients with AEG (HR = 1.545, 95% CI: 1.96–2.179, P < 0.05, Figure 2).
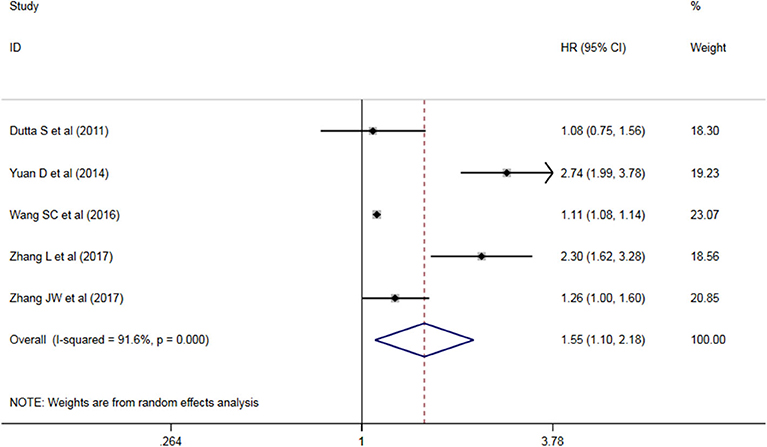
Figure 2. Forest map of the relationship between NLR and OS/CSS/DSS in patients with esophageo-gastric junction cancer.
The Prognostic Role of PLR in AEG
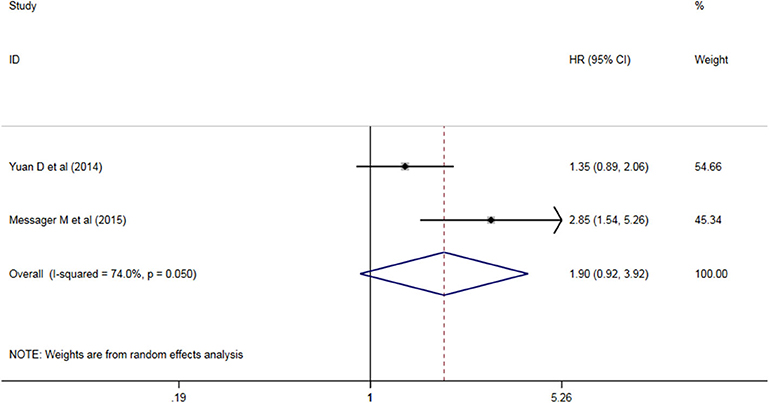
Six studies analyzed the relationship between PLR levels and OS in patients with AEG. There was no significant heterogeneity between the studies (P = 0.198, I2 = 31.7%), so a fixed effect model was used. The results showed that PLR was not suitable as an OS judgment index for AEG patients (HR = 1.117, 95% CI: 0.960–1.300, P > 0.05, Figure 3). Two studies analyzed the relationship between PLR levels and DFS in patients with AEG. There was significant heterogeneity between the studies (P = 0.050, I2 = 74.0%), and random effects model was used for analysis. The results showed that PLR was not suitable as a predictor of DFS in patients with AEG (HR = 1.90, 95% CI: 0.92–3.92, P > 0.05, Figure 4).
Subgroup Analysis Results
Heterogeneity Between NLR Studies
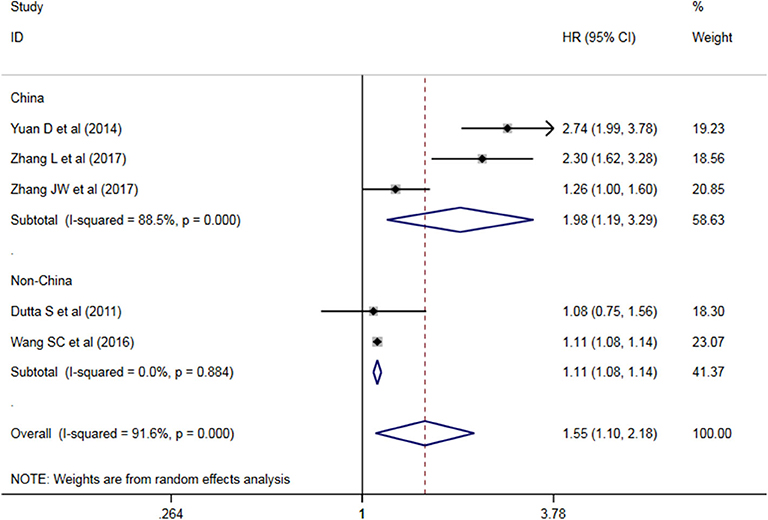
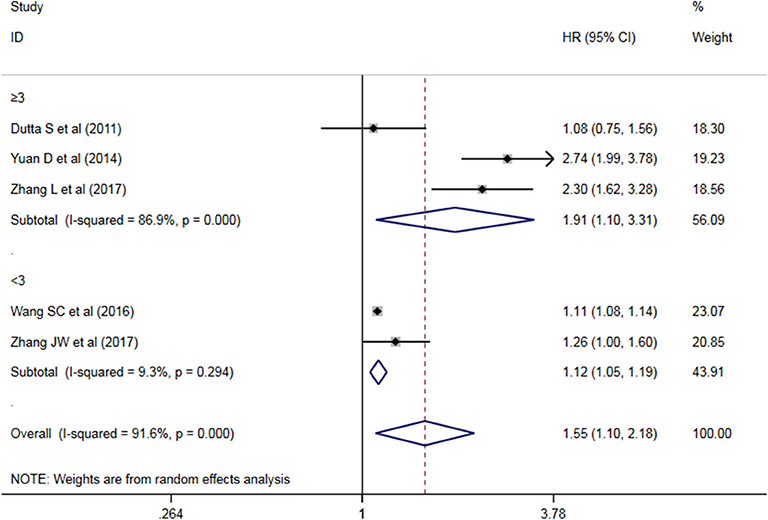
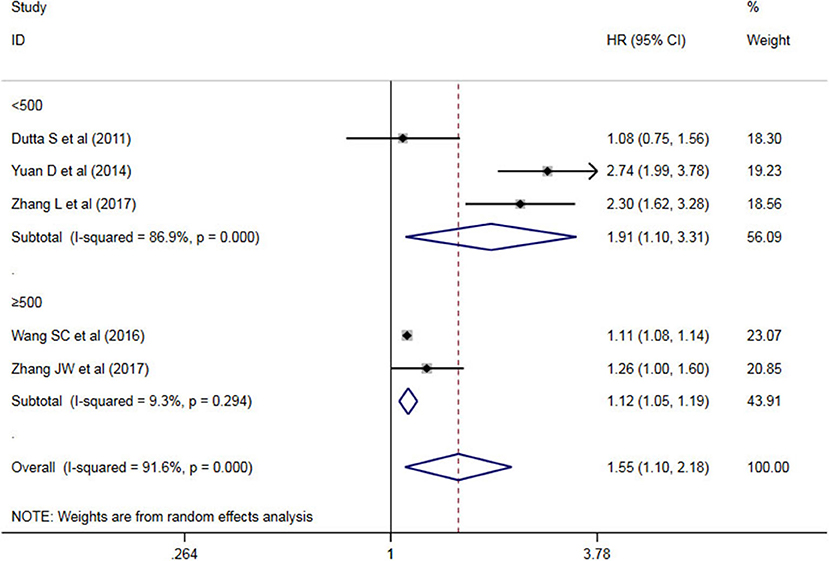
A subgroup analysis of heterogeneity sources between OS/CSS/DSS studies of NLR and AEG patients. NLR had significant value in the prognosis of patients in China and Non-China (P = 0.009 vs. P = 0.000, Figure 5); China group had significant heterogeneity (P = 0.000), but Non-China group did not have significant heterogeneity (P = 0.884). NLR had significant prognostic value for cutoff value ≥3 group and <3 group (P = 0.022 vs. P = 0.000, Figure 6); ≥3 group had significant heterogeneity (P = 0.000), <3 group was not significant heterogeneity (P = 0.294). NLR had significant prognostic value for samples ≥500 group and <500 group (P = 0.000 vs. P = 0.022, Figure 7), ≥500 group had no significant heterogeneity (P = 0.294), while <500 group had significant heterogeneity Sex (P = 0.000). The results are presented in Table 2.
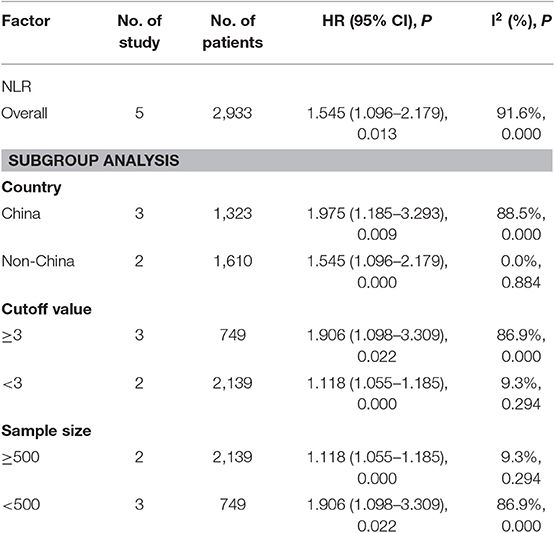
Table 2. Meta-analysis results of NLR and OS/CSS/DSS in patients with carcinoma of the esophagogastric junction.
Heterogeneity Between PLR Studies
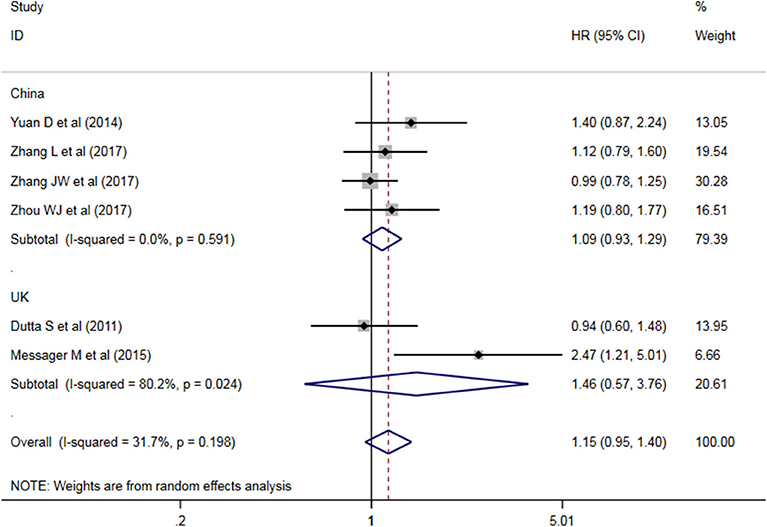
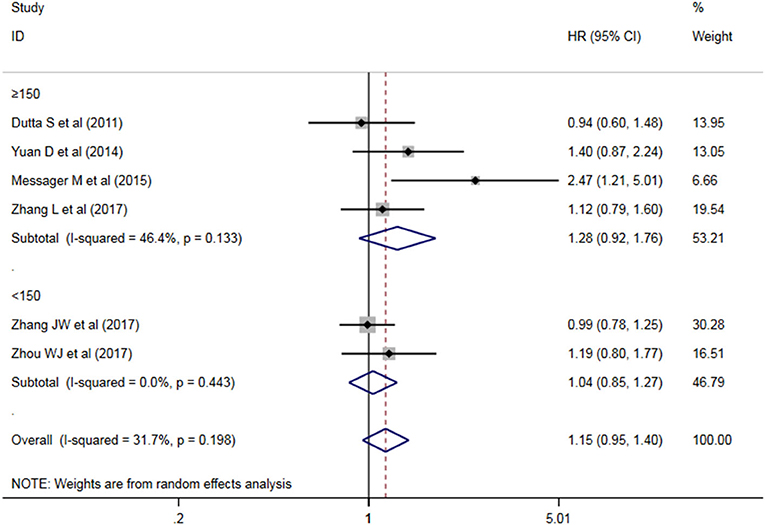
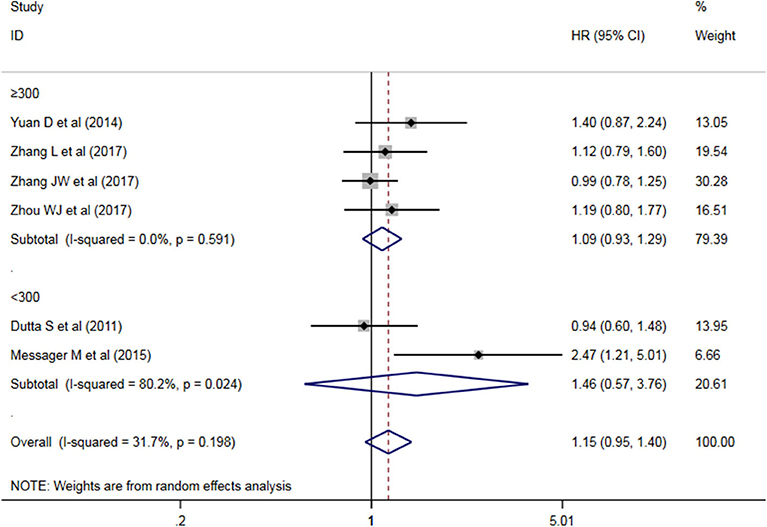
A subgroup analysis of heterogeneity sources between OS studies of PLR and AEG patients. There was no significant heterogeneity in the China group (P = 0.591) (Figure 8), and there was significant heterogeneity in the UK group (P = 0.024). PLR had no significant prognostic value for Cutoff value ≥150 group and <150 group (P = 0.141 vs. P = 0.724, Figure 9); there was no significant heterogeneity between the two groups (P = 0.133 and P = 0.443). PLR had no significant prognostic value for ≥300 group and <300 group (P =0.282 vs. P =0.429, Figure 10); ≥300 group had no significant heterogeneity (P = 0.591), <300 group was significantly heterogeneous Sex (P = 0.024). The results are presented in Table 3.
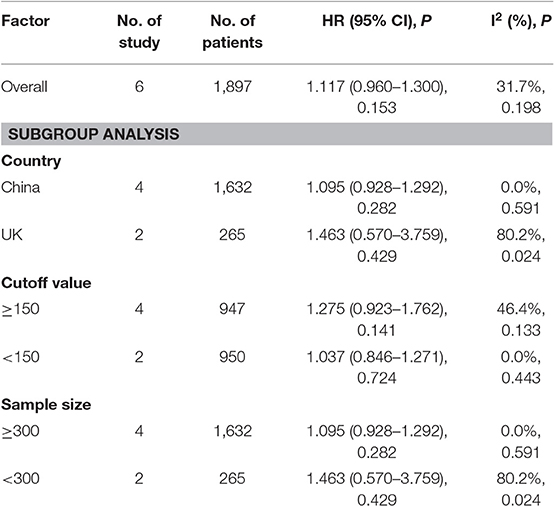
Table 3. Meta-analysis results of PLR and DFS in patients with carcinoma of the esophagogastric junction.
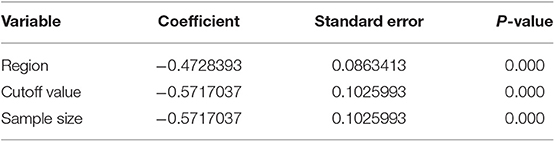
NLR shows a significant prognostic value in Chinese and non-Chinese patients. A significant heterogeneity was also observed in the Chinese group but not in the non-Chinese group. Meta-regression analysis was conducted to determine the factors that might cause heterogeneity. The variables included region and sample size. NLR and OS/CSS/DSS meta-regression analysis showed that the region might be the source of AEG heterogeneity.
Meta-Review Analysis
To find factors that may cause heterogeneity, we used meta-regression analysis, the variables included region, cut-off value, and sample size. The NLR and OS/CSS/DSS single factor meta-regression showed that the region, cut-off value, and sample size were all possible reason. It is the source of heterogeneity in patients with AEG (all P < 0.05, Table 4).
Risk of Bias
Begger's test and Egger's test were used to evaluate the published bias, and the results showed that the NLR published bias test (PBegg = 0.806 vs. PEgger = 0.141) revealed no significant bias.
Discussion
Infection may involve the entire process of tissue carcinogenesis, directly or indirectly affecting its development (26). Systemic inflammatory response is associated with the inhibition of apoptosis, angiogenesis and DNA damage, leading to tumor progression and metastasis (27). Although the mechanism between hematological parameters and tumors remains unclear, their correlation can be explained by infiltrating immune cells and inflammatory proteins (28). A tumor microenvironment contains many different mediators. Neutrophils promote tumor development (29), cytokine production, and provide a microenvironment for tumor survival. Neutrophils can promote the production of a variety of inflammatory cytokines, providing a good microenvironment for tumor survival and proliferation. On the contrary, lymphocytes play an important role in tumor-specific immune response.
As an independent factor, the effect of chronic inflammation on gastrointestinal cancer has been demonstrated (30). The level of NLR may reflect the inflammatory state of the body. Neutrophils can promote tumor growth and progression by increasing the concentration of some inflammatory substances, such as vascular endothelial growth factor, interleukin-6, and IL-1 (31, 32).
In addition, increased neutrophils inhibit the lysis activity of some cells, such as lymphocytes, natural killer cells and activated T lymphocytes. Lymphocytes play an immunity-related role in tumors. Cytokines released and their mediated cytotoxic by lymphocytes can inhibit cell proliferation and metastasis. However, cytokines produced by cells can lead to lymphocyte depletion and decrease the anti-tumor effect of lymphocytes. As a result, the risks of recurrence and metastasis are increased, leading to poor prognosis (33).
Relative lymphocyte reduction may reduce lymphocyte-mediated anti-tumor cellular immune responses. Platelet aggregation promotes adhesion and aggregation of circulating tumor cells, which enhances the ability of tumor cells to escape immune attack (34). In addition, activated platelets release more vascular endothelial growth factor and a variety of cytokines, thereby increasing the angiogenesis of tumor tissue and ultimately promoting its growth (35, 36). Therefore, in the current situation of the lack of more reliable tumor prognostic indicators, NLR and PLR may provide information on patient prognosis. It is currently known that NLR and/or PLR may be associated with a variety of tumor prognosis, including non-digestive tumor NSCLC (37), breast cancer (38), ovarian cancer (39), Hodgkin lymphoma (40), prostate cancer (41), cervical cancer (42), nasopharyngeal carcinoma (28), and tumors of the digestive tract such as esophageal squamous cell carcinoma (43, 44), gastric cancer (45), pancreatic cancer (46), and colorectal cancer (47).
As far as we know, our research involves the first meta-analysis of the value of the above indicators in the diagnosis of AEG. In this study, 2,933 and 1,897 patients with AEG were included to investigate the prognostic value of NLR and PLR for AEG. The meta-analysis showed that NLR can be used as a prognostic indicator for patients with AEG, but PLR may not be suitable for the OS and DFS of patients with AEG as indicators. Similarly, NLR has significant prognostic value in each subgroup, but PLR has no significant prognostic difference in each subgroup. NLR and OS/CSS/DSS univariate meta-regression showed that regions, NLR cutoffs, and sample sizes may be sources of heterogeneity in patients with AEG, while PLR and OS/CSS univariate metasindicate that the regions, cutoffs, and sample sizes are not possible sources of heterogeneity in patients with AEG. Despite great efforts to obtain relevant research, some data that are not published online are still not available. Hence, more studies should be included in the later stage to reduce heterogeneity.
This study has the following limitations. First, despite we were very cautious about the literature included to draw our conclusions, the number of included studies is not large and covers only Chinese and English literature. Second, the selected literatures are retrospective studies, lacking a prospective cohort study, may result in analytical bias. Third, other inflammatory conditions have not been discussed in the original study. Inflammatory control samples from patients without AEG are not included. Fourth, whether the stage of the tumor will affect the outcome is unknown. We also tried to further determine a possible effect through the subgroup analysis. However, on the basis of the available data, the subgroup analysis cannot be completed. Relative to more emergence, we can conduct additional analysis to further the value of NLR and PLR for AEG in different stages. Finally, meta-analysis is an observational study that may be limited by raw data bias and defects.
NLR is an inexpensive, easy-to-access, and multi-examination index. It is also an expected index for patients with AEG. This indicator helps identify high-risk patients and determine treatment plans. However, given the above limitations, NLR should be carefully used as a marker before it is recommended for clinical applications. The value of NLR in the prognosis of AEG should be verified before it is applied to clinical decision-making, and the value of PLR and LMR in AEG is worth further exploration.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.
Author Contributions
XL, QT, and SL conceived and designed this study. ZG and QZ searched and collected the data. XL and QZ contributed to data extraction and data analysis. QZ and BG performed the statistical analysis and interpretation of data. XL and FY wrote the manuscript. SP, ZG, and QT reviewed and revised the paper. XL and SL approved and submitted the final manuscript. All authors read, and approved the final manuscript and its submission.
Funding
This study was funded by the 2016 Joint Diagnostic Medicine Research Project of Taihe Hospital (2016JD02).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We sincerely thank all the authors and study participants for their support in this study.
References
1. Siewert JR, Stein HJ. Classification of adenocarcinoma of the oesophagogastric junction. Br J Surg. (1998) 85:1457–9. doi: 10.1046/j.1365-2168.1998.00940.x
2. Drahos J, Wu M, Anderson WF, Trivers KF, King J, Rosenberg PS, et al. Regional variations in esophageal cancer rates by census region in the United States, 1999–2008. PLoS ONE. (2013) 8:e67913. doi: 10.1371/journal.pone.0067913
3. Dubecz A, Solymosi N, Stadlhuber RJ, Schweigert M, Stein HJ, Peters JH. Does the incidence of adenocarcinoma of the esophagus and gastric cardia continue to rise in the twenty-first century?-a SEER database analysis. J Gastrointest Surg. (2014) 18:124–9. doi: 10.1007/s11605-013-2345-8
4. Hatta W, Tong D, Lee YY, Ichihara S, Uedo N, Gotoda T. Different time trend and management of esophagogastric junction adenocarcinoma in three Asian countries. Dig Endosc. (2017) 29(Suppl. 2):18–25. doi: 10.1111/den.12808
5. Liu K, Yang K, Zhang W, Chen X, Chen X, Zhang B, et al. Changes of esophagogastric junctional adenocarcinoma and gastroesophageal reflux disease among surgical patients during 1988–2012: a single-institution, high-volume experience in China. Ann Surg. (2016) 263:88–95. doi: 10.1097/SLA.0000000000001148
6. Thrift AP, Whiteman DC. The incidence of esophageal adenocarcinoma continues to rise: analysis of period and birth cohort effects on recent trends. Ann Oncol. (2012) 23:3155–62. doi: 10.1093/annonc/mds181
7. Zhou Y. Current status and challenges of clinical trial on adenocarcinoma of esophagogastric junction. Zhonghua Wei Chang Wai Ke Za Zhi. (2019) 22:112–8. doi: 10.3760/cma.j.issn.1671-0274.2019.02.003
8. Siewert JR. Adenocarcinoma of the esophago-gastric junction. Gastric Cancer. (1999) 2:87–8. doi: 10.1007/s101200050028
9. Kurokawa Y, Hiki N, Yoshikawa T, Kishi K, Ito Y, Ohi M, et al. Mediastinal lymph node metastasis and recurrence in adenocarcinoma of the esophagogastric junction. Surgery. (2015) 157:551–5. doi: 10.1016/j.surg.2014.08.099
10. Liu K, Zhang W, Chen X, Chen X, Yang K, Zhang B, et al. Comparison on clinicopathological features and prognosis between esophagogastric junctional adenocarcinoma (Siewert II/III Types) and distal gastric adenocarcinoma: retrospective cohort study, a single institution, high volume experience in China. Medicine. (2015) 94:e1386. doi: 10.1097/MD.0000000000001386
11. Xu J, Cao J, Wang L, Wang Z, Wang Y, Wu Y, et al. Prognostic performance of three lymph node staging schemes for patients with Siewert type II adenocarcinoma of esophagogastric junction. Sci Rep. (2017) 7:10123. doi: 10.1038/s41598-017-09625-z
12. Felismino TC, de Oliveira ACF, Alves ACF, da Costa WL Jr, Coimbra FJF, de Souza Beqnami MDF, et al. Primary tumor location is a predictor of poor prognosis in patients with locally advanced esophagogastric cancer treated with perioperative chemotherapy. J Gastrointest Cancer. (2019). doi: 10.1007/s12029-019-00258-1. [Epub ahead of print].
13. Kudou K, Saeki H, Nakashima Y, Sasaki S, Jogo T, Hirose K, et al. Postoperative development of sarcopenia is a strong predictor of a poor prognosis in patients with adenocarcinoma of the esophagogastric junction and upper gastric cancer. Am J Surg. (2019) 217:757–63. doi: 10.1016/j.amjsurg.2018.07.003
14. Urabe M, Yamashita H, Watanabe T, Seto Y. Comparison of prognostic abilities among preoperative laboratory data indices in patients with resectable gastric and esophagogastric junction adenocarcinoma. World J Surg. (2018) 42:185–94. doi: 10.1007/s00268-017-4146-9
15. Cui Y, Li J, Liu M, Shi Z, Fu Y, Cai L, et al. Value of Glasgow prognostic score in patients with adenocarcinoma of esophagogastric junction. Zhonghua Wei Chang Wai Ke Za Zhi. (2016) 19:54–7. doi: 10.3760/cma.j.issn.1671-0274.2016.01.012
16. Zhou WJ, Wu J, Li XD, Wang Q, Ni XF, Jiang JT, et al. Effect of preoperative monocyte-lymphocyte ratio on prognosis of patients with resectable esophagogastric junction cancer. Zhonghua Zhong Liu Za Zhi. (2017) 39:178–83. doi: 10.3760/cma.j.issn.0253-3766.2017.03.004
17. Zhang JW, Huang L, Xu AM. Preoperative monocyte-lymphocyte and neutrophil-lymphocyte but not platelet-lymphocyte ratios are predictive of clinical outcomes in resected patients with non-metastatic Siewert type II/III adenocarcinoma of esophagogastric junction: a prospective cohort study (the AMONP corhort). Oncotarget. (2017) 8:57516–27. doi: 10.18632/oncotarget.15497
18. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. (2009) 339:b2700. doi: 10.1136/bmj.b2700
19. Gao ZY, Liu XB, Yang FM, Liu L, Zhao JZ, Gao B, et al. Octamer binding transcription factor-4 expression is associated with cervical cancer malignancy and histological differentiation: a systematic review and meta-analysis. Biosci Rep. (2019) 39:BSR20182328. doi: 10.1042/BSR20182328
20. Liu XB, Gao ZY, Zhang QH, Jin S, Gao B, Yang GL, et al. Serum pepsinogen assay is not recommended for the diagnosis of esophageal squamous cell carcinoma: a systematic review and meta-analysis. Cancer Manag Res. (2019) 11:5643–54. doi: 10.2147/CMAR.S196760
21. Zhang L, Su Y, Chen Z, Wei Z, Han W, Xu A. The prognostic value of preoperative inflammation-based prognostic scores and nutritional status for overall survival in resected patients with nonmetastatic Siewert type II/III adenocarcinoma of esophagogastric junction. Medicine. (2017) 96:e7647. doi: 10.1097/MD.0000000000007647
22. Wang SC, Chou JF, Strong VE, Brennan MF, Capanu M, Coit DG. Pretreatment neutrophil to lymphocyte ratio independently predicts disease-specific survival in resectable gastroesophageal junction and gastric adenocarcinoma. Ann Surg. (2016) 263:292–7. doi: 10.1097/SLA.0000000000001189
23. Messager M, Neofytou K, Chaudry MA, Allum WH. Prognostic impact of preoperative platelets to lymphocytes ratio (PLR) on survival for oesophageal and junctional carcinoma treated with neoadjuvant chemotherapy: a retrospective monocentric study on 153 patients. Eur J Surg Oncol. (2015) 41:1316–23. doi: 10.1016/j.ejso.2015.06.007
24. Yuan D, Zhu K, Li K, Yan R, Jia Y, Dang C. The preoperative neutrophil-lymphocyte ratio predicts recurrence and survival among patients undergoing R0 resections of adenocarcinomas of the esophagogastric junction. J Surg Oncol. (2014) 110:333–40. doi: 10.1002/jso.23651
25. Dutta S, Crumley AB, Fullarton GM, Horgan PG, McMillan DC. Comparison of the prognostic value of tumour- and patient-related factors in patients undergoing potentially curative resection of oesophageal cancer. World J Surg. (2011) 35:1861–6. doi: 10.1007/s00268-011-1130-7
26. Lauritano D, Sbordone L, Nardone M, Iapichino A, Scapoli L, Carinci F. Focus on periodontal disease and colorectal carcinoma. Oral Implantol. (2017) 10:229–33. doi: 10.11138/orl/2017.10.3.229
27. Munn LL. Cancer and inflammation. Wiley Interdiscip Rev Syst Biol Med. (2017) 9. doi: 10.1002/wsbm.1370
28. Yang S, Zhao K, Ding X, Jiang H, Lu H. Prognostic significance of hematological markers for patients with nasopharyngeal carcinoma: a meta-analysis. J Cancer. (2019) 10:2568–77. doi: 10.7150/jca.26770
29. Coffelt SB, Wellenstein MD, de Visser KE. Neutrophils in cancer: neutral no more. Nat Rev Cancer. (2016) 16:431–46. doi: 10.1038/nrc.2016.52
30. Hofman PM. Pathobiology of the neutrophil-intestinal epithelial cell interaction: role in carcinogenesis. World J Gastroenterol. (2010) 16:5790–800. doi: 10.3748/wjg.v16.i46.5790
31. Calkins CM, Bensard DD, Shames BD, Pulido EJ, Abraham E, Fernandez N, et al. IL-1 regulates in vivo C-X-C chemokine induction and neutrophil sequestration following endotoxemia. J Endotoxin Res. (2002) 8:59–67. doi: 10.1177/09680519020080010601
32. Roxburgh CS, McMillan DC. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol. (2010) 6:149–63. doi: 10.2217/fon.09.136
33. Sconocchia G, Eppenberger S, Spagnoli GC, Tornillo L, Droeser R, Caratelli S, et al. NK cells and T cells cooperate during the clinical course of colorectal cancer. Oncoimmunology. (2014) 3:e952197. doi: 10.4161/21624011.2014.952197
34. Jurasz P, Alonso-Escolano D, Radomski MW. Platelet–cancer interactions: mechanisms and pharmacology of tumour cell-induced platelet aggregation. Br J Pharmacol. (2004) 143:819–26. doi: 10.1038/sj.bjp.0706013
35. Menter DG, Tucker SC, Kopetz S, Sood AK, Crissman JD, Honn KV. Platelets and cancer: a casual or causal relationship: revisited. Cancer Metastasis Rev. (2014) 33:231–69. doi: 10.1007/s10555-014-9498-0
36. Bambace NM, Holmes CE. The platelet contribution to cancer progression. J Thromb Haemost. (2011) 9:237–49. doi: 10.1111/j.1538-7836.2010.04131.x
37. Chen H, Xue H, Liu W, Wu F, Wang Y, Gao H. Meta-analysis of platelet lymphocyte ratio as a prognostic factor fornon-small cell lung cancer. Zhongguo Fei Ai Za Zhi. (2019) 22:289–98. doi: 10.3779/j.issn.1009-3419.2019.05.05
38. Guo W, Lu X, Liu Q, Zhang T, Li P, Qiao W, et al. Prognostic value of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio for breast cancer patients: an updated meta-analysis of 17079 individuals. Cancer Med. (2019) 8:4135–48. doi: 10.1002/cam4.2281
39. Lu C, Zhou L, Ouyang J, Yang H. Prognostic value of lymphocyte-to-monocyte ratio in ovarian cancer: a meta-analysis. Medicine. (2019) 98:e15876. doi: 10.1097/MD.0000000000015876
40. Lee SF, Ng TY, Spika D. Prognostic value of lymphocyte-monocyte ratio at diagnosis in Hodgkin lymphoma: a meta-analysis. BMC Cancer. (2019) 19:338. doi: 10.1186/s12885-019-5552-1
41. Peng H, Luo X. Prognostic significance of elevated pretreatment systemic inflammatory markers for patients with prostate cancer: a meta-analysis. Cancer Cell Int. (2019) 19:70. doi: 10.1186/s12935-019-0785-2
42. Yang L, Chen H. Establishing the prognostic value of platelet-to-lymphocyte ratio in cervical cancer: a systematic review and meta-analysis. Int J Gynecol Cancer. (2019) 29:683–90. doi: 10.1136/ijgc-2018-000090
43. Hu G, Liu G, Ma JY, Hu RJ. Lymphocyte-to-monocyte ratio in esophageal squamous cell carcinoma prognosis. Clin Chim Acta. (2018) 486:44–8. doi: 10.1016/j.cca.2018.07.029
44. Sun Y, Zhang L. The clinical use of pretreatment NLR, PLR, and LMR in patients with esophageal squamous cell carcinoma: evidence from a meta-analysis. Cancer Manag Res. (2018) 10:6167–79. doi: 10.2147/CMAR.S171035
45. Ma JY, Liu Q. Clinicopathological and prognostic significance of lymphocyte to monocyte ratio in patients with gastric cancer: a meta-analysis. Int J Surg. (2018) 50:67–71. doi: 10.1016/j.ijsu.2018.01.002
46. Zhou Y, Cheng S, Fathy AH, Qian H, Zhao Y. Prognostic value of platelet-to-lymphocyte ratio in pancreatic cancer: a comprehensive meta-analysis of 17 cohort studies. Onco Targets Ther. (2018) 11:1899–908. doi: 10.2147/OTT.S154162
Keywords: AEG, NLR, PLR, prognosis, meta-analysis
Citation: Liu X, Gao Z, Zhang Q, Pandey S, Gao B, Yang F, Tong Q and Li S (2020) Preoperative Neutrophil Lymphocyte Ratio Can Be Used as a Predictor of Prognosis in Patients With Adenocarcinoma of the Esophagogastric Junction: A Systematic Review and Meta Analysis. Front. Oncol. 10:178. doi: 10.3389/fonc.2020.00178
Received: 06 August 2019; Accepted: 03 February 2020;
Published: 21 February 2020.
Edited by:
Zexian Liu, Sun Yat-sen University Cancer Center (SYSUCC), ChinaReviewed by:
Bangshun He, Nanjing Medical University, ChinaHua You, Guangzhou Medical University, China
Yan Xia Li, Wuhan University, China
Copyright © 2020 Liu, Gao, Zhang, Pandey, Gao, Yang, Tong and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiang Tong, xinl2012@163.com; Sheng-bao Li, from2018@126.com
 Xiao-bo Liu
Xiao-bo Liu Zi-ye Gao2
Zi-ye Gao2 Sandeep Pandey
Sandeep Pandey Bo Gao
Bo Gao Qiang Tong
Qiang Tong Sheng-bao Li
Sheng-bao Li