- 1Department of Surgery, Erasmus MC University Medical Center, Rotterdam, Netherlands
- 2Department of Medicine, Memorial Sloan Kettering Cancer Center, New York, NY, United States
- 3David M. Rubenstein Center for Pancreatic Cancer Research, New York, NY, United States
Approximately 20% of pancreatic ductal adenocarcinoma (PDAC) patients have (borderline) resectable pancreatic cancer [(B)RPC] at diagnosis. Upfront resection with adjuvant chemotherapy has long been the standard of care for these patients. However, although surgical quality has improved, still about 50% of patients never receive adjuvant treatment. Therefore, recent developments have focused on a neoadjuvant approach. Directly comparing results from neoadjuvant and adjuvant regimens is challenging due to differences in patient populations that influence outcomes. Neoadjuvant trials include all patients who have (B)RPC on imaging, while adjuvant-only trials include patients who underwent a complete resection and recovered to a good performance status without any evidence of residual disease. Guidelines recommend neoadjuvant treatment for BRPC patients mainly to improve negative resection margin (R0) rates. For resectable PDAC, upfront resection is still considered the standard of care. However, theoretical advantages of neoadjuvant treatment, including the increased R0 resection rate, early delivery of systemic therapy to all patients, directly addressing occult metastatic disease, and improved patient selection for resection, may also apply to these patients. A systematic review by intention-to-treat showed a superior median overall survival (OS) for any neoadjuvant approach (19 months) compared to upfront surgery (15 months) in (B)RPC patients. A neoadjuvant approach was recently supported by three randomized controlled trials (RCTs). For resectable PDAC, neoadjuvant treatment was superior in a Japanese RCT of neoadjuvant gemcitabine with S-1 vs. upfront surgery, with adjuvant S-1 in both arms (median OS: 37 vs. 27 months, p = 0.015). A Korean trial of neoadjuvant gemcitabine-based chemoradiotherapy vs. upfront resection in BRPC patients was terminated early due to superiority of the neoadjuvant group (median OS: 21 vs. 12 months, p = 0.028; R0 resection: 52 vs. 26%, p = 0.004). The PREOPANC-1 trial for (B)RPC patients also showed favorable outcome for neoadjuvant gemcitabine-based chemoradiotherapy vs. upfront surgery (median OS: 17 vs. 14 months, p = 0.07; R0 resection: 63 vs. 31%, p < 0.001). FOLFIRINOX is likely a better neoadjuvant regimen, because of superiority compared to gemcitabine in both the metastatic and adjuvant setting. Currently, five RCTs evaluating neoadjuvant modified or fulldose FOLFIRINOX are accruing patients.
Introduction
Pancreatic ductal adenocarcinoma (PDAC) accounts for 3% of all new cancer diagnoses, and incidence rates continue to slowly increase. In contrast to the decreasing cancer-related death rates for many other solid organ malignancies, PDAC survival has not shown much improvement over the last decades (1). As a consequence, PDAC is expected to be the second leading cause of cancer-related death in the United States by 2030 (2). An important explanation for the high mortality rate compared to other solid tumors, is that the majority of patients are diagnosed with metastatic disease (40%) or locally advanced disease (40%). For metastatic PDAC, palliative treatment using multi-agent chemotherapy such as a combination of 5-FU, oxaliplatin, and irinotecan (FOLFIRINOX) or gemcitabine with nab-paclitaxel is the standard of care based on randomized controlled trials (RCTs) (3, 4). These therapies have been shown to increase life expectancy with 2–4 months. For locally advanced pancreatic cancer (LAPC), no RCT has been completed, but based on a patient-level meta-analysis and the survival benefit in metastatic PDAC, FOLFIRINOX, and gemcitabine with nab-paclitaxel are the standard initial treatments (5). Following induction chemotherapy, some patients will also receive chemoradiation and about 20% of LAPC patients undergoes surgical resection. The remaining 20% of PDAC patients have (borderline) resectable pancreatic cancer [(B)RPC] at diagnosis.
Resection remains the only curative-intent treatment. However, even curative-intent surgery typically does not overcome the aggressive biology, resulting in recurrent disease within 2 years after resection in the vast majority of patients (6). Studies focusing on recurrence patterns have demonstrated that the initial recurrence in 76% of patients was systemic (7, 8). Therefore, also (B)RPC could be approached as a systemic disease, irrespective of apparent non-metastatic disease on imaging (9).
The objective of this paper is two-fold. First, we aim to give a general overview of the current treatment strategies for (B)RPC patients, to discuss the rationale for neoadjuvant and adjuvant therapy, and to consider the challenges when comparing these treatment approaches. Second, we aim to summarize the currently available evidence for neoadjuvant treatment with a special focus on neoadjuvant FOLFIRINOX, including published and ongoing phase II-III trials for neoadjuvant treatment.
Methods
To identify relevant studies for neoadjuvant treatment, a comprehensive search of Clinicaltrials, Embase, and MEDLINE was performed. Search terms included “neoadjuvant,” “FOLFIRINOX,” “folinic acid,” “fluorouracil,” “irinotecan,” “oxaliplatin,” “pancreas cancer,” “drug combination,” and relevant variants thereof. Only articles written in English were assessed. Articles were selected based on relevance for our objectives, considering methodological quality, study type, number of included patients, and additional value to current knowledge. A selection was made for prospective studies with restriction to phase II and III trials and publication dates from 2006 to 2019. Furthermore, references of included articles were assessed for additional relevant literature.
Disease Staging
Non-metastatic pancreatic cancer is subdivided into resectable PDAC, BRPC, and LAPC. Historically however, BRPC was not recognized as a unique disease stage. In 2001, a first definition of marginally resectable tumors was proposed (10). The term “borderline resectable” was thereafter introduced by the 2006 National Comprehensive Cancer Network (NCCN) guidelines for tumors at risk for margin-positive resection when treated with upfront surgery, and adopted by other guidelines. The critical aspects that need to be evaluated are the contact of the tumor with the superior mesenteric vein or portal vein complex (SMV-PVC) as venous structures, and the superior mesenteric artery (SMA), common hepatic artery (CHA), and celiac artery (CA) as major surrounding arteries. Over time, several criteria have been proposed to define resectability status, summarized in Table 1.
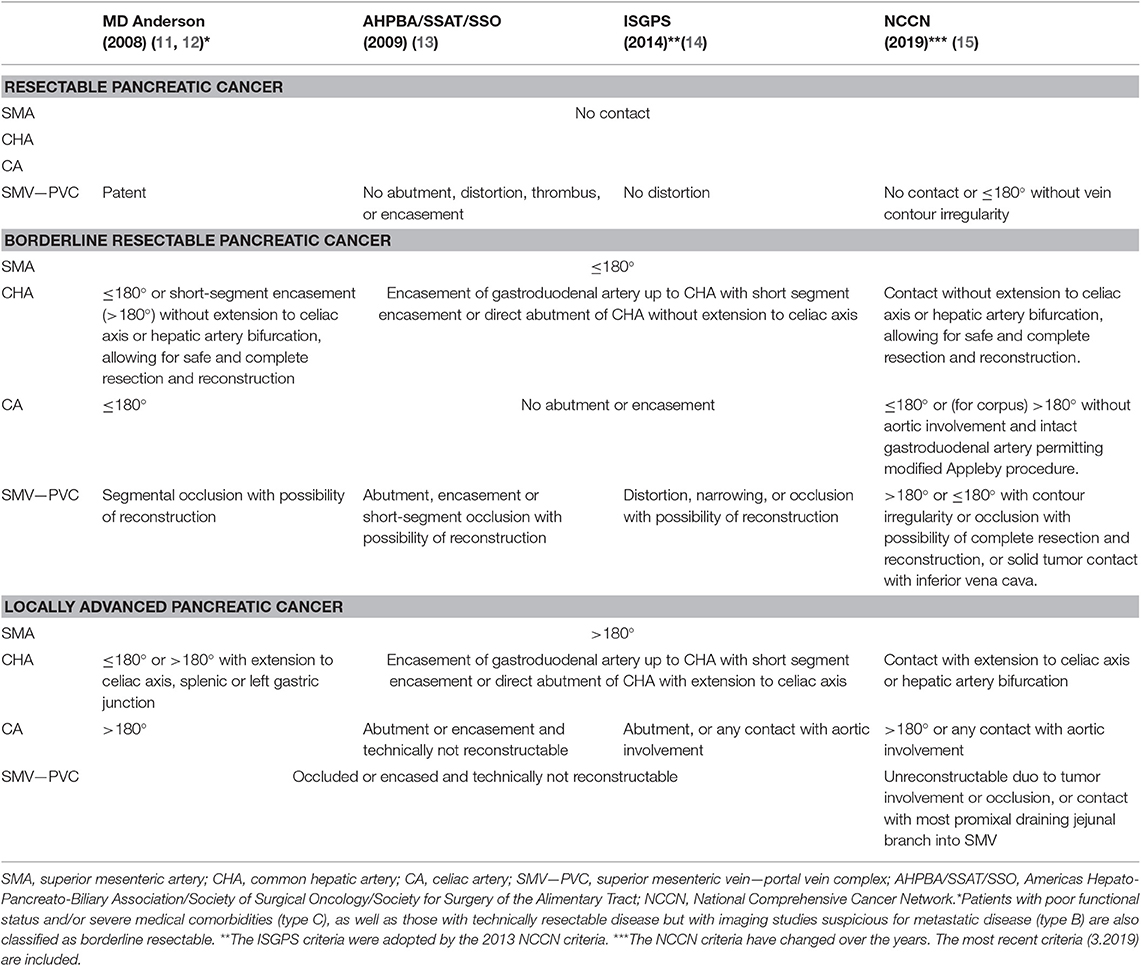
Table 1. Comparison of imaging-based criteria distinguishing resectable, borderline resectable, and locally advanced pancreatic cancer.
Commonly used criteria include the NCCN guidelines (15, 16), MD Anderson Cancer Center (MDACC) guidelines (11, 12), the AHPBA/SSAT/SSO expert consensus guidelines (13), and the International Study Group of Pancreatic Surgery (ISGPS) criteria (14). The 2013 NCCN guidelines adopted the ISGPS criteria, and minor modifications were made in the following NCCN guidelines. The AHPBA/SSAT/SSO guidelines require less vascular abutment to classify patients as BRPC compared to the NCCN and MDACC guidelines. For example, tumors with any SMV-PVC abutment are BRPC in the AHPBA/SSAT/SSO guidelines. In contrast, the other two guidelines require venous occlusion (MDACC) or vein contour irregularity (NCCN), regardless of the extent of abutment of the tumor with the SMV-PVC.
Several factors associated with these criteria have complicated comparison of study outcomes. First, no uniformly accepted set of criteria exists. Second, the NCCN guidelines have been modified several times. Third, most guidelines include ambiguous terms to define the resectability stages, including “abutment, impingement, involvement, and encasement.” The classifications are based on apparent contact on imaging of tumor and blood vessel. The actual presence of tumor cells surrounding the vessels (or invading the vessel wall) is rarely known before pathological examination of the resected specimen. However, patients with extensive apparent contact on imaging often undergo a surgically incomplete (R1) resection, suggesting imaging is indeed a good predictor of the presence of tumor cells surrounding and/or invading the vessel wall. Lack of international agreement on the definition of an R0 resection (i.e., >1 vs. >0 mm) and standardized protocols for pathological examination (i.e., axial slicing vs. bivalving) may explain variation in published R0 resection rates (17, 18). At a consensus meeting in 2016, it has been proposed to add biological and functional risk factors to the resectability criteria. Biological factors include elevated Carbohydrate Antigen (CA) 19.9 levels above 500 units/mL, regional lymph node metastases, and suspicion of distant metastases without the possibility for pathological proof. The functional factors include performance status and comorbidity (19). These biological and functional factors have also been implemented in the NCCN 2018 and American Society of Clinical Oncology (ASCO) 2019 guidelines, further decreasing the number of patients classified as resectable PDAC (20, 21). Similarly, within the MDACC guidelines, three sub-types of BRPC are distinguished; based on local tumor-artery contact (type A), based on tumor marker levels or imaging suggestive of metastatic disease but lacking pathological proof (type B), or based on marginal performance status prior to treatment (type C) (11, 12).
Adjuvant Treatment—Practice Changing Trials
Upfront surgery followed by adjuvant chemotherapy has long been the standard of care for patients with potentially resectable PDAC. Initial adjuvant treatment strategies included both chemotherapy and radiotherapy. In 2004, the long-term results from the ESPAC-1 (European Study Group for Pancreatic Cancer) trial were published (22). This multicenter European collaboration used a 2 × 2 factorial design to compare adjuvant 5-FU-based chemoradiotherapy alone (arm A, n = 73), adjuvant 5-FU based chemoradiotherapy followed by 5-FU (arm B, n = 72), adjuvant 5-FU alone (arm C, n = 75), and observation alone (arm D, n = 69). The trial was not powered for a direct comparison of the four groups, yet survival was longer in patients who received chemotherapy compared to patients who did not [median OS: 20 vs. 16 months, hazard ratio (HR) 0.71, p = 0.009]. Furthermore, comparison of patients with or without chemoradiotherapy showed inferior median OS for patients who received chemoradiotherapy (median OS: 16 vs. 18 months, HR 1.28, 95% CI: 0.99–1.66, p = 0.05). The CONKO-001 (Charité Onkologie 001) trial found that adjuvant gemcitabine was superior to observation alone with a 5-year survival rate of 21 vs. 10% (p = 0.01) (6). In 2017, the ESPAC-4 trial included 730 patients comparing gemcitabine (n = 366) to gemcitabine plus capecitabine (n = 364) (23). Median OS was 26 months with gemcitabine alone and 28 months with gemcitabine plus capecitabine (HR 0.82, 95% CI: 0.68–0.98, p = 0.032). In 2018, the results of the PRODIGE 24/CCTG PA.6 trial comparing adjuvant gemcitabine to modified FOLFIRINOX (mFOLFIRINOX) exceeded expectations (24). The median OS was 54 months with mFOLFIRINOX compared to 35 months with gemcitabine (stratified HR 0.64, 95% CI: 0.48–0.86, p = 0.003). mFOLFIRINOX is currently the best adjuvant treatment for patients with a good performance score.
Neoadjuvant Treatment—Rationale
The strategy of chemotherapy following surgery has several drawbacks. First, approximately 20% of patients with (B)RPC on imaging will never undergo a resection because of occult metastatic or locally irresectable disease (25). More advanced disease is often diagnosed at exploratory laparotomy, which has considerable morbidity and mortality, and the majority of these patients will not receive any palliative chemotherapy. Even after successful resection, only about 55% of patients are able to receive adjuvant chemotherapy due to postoperative complications, clinical deterioration, or early progressive disease (26–29). Especially those patients not able to receive adjuvant chemotherapy have very poor prognosis. The CONKO-001 RCT reported that about 50% of patients in the observation arm (i.e., without adjuvant chemotherapy) hadrecurrent disease or died within 6 months after surgery; the median DFS was only 6.7 months after surgery without adjuvant chemotherapy (6). In an attempt to overcome some of these drawbacks, there is an ongoing paradigm shift toward a neoadjuvant approach. This is supported by promising results in other malignancies such as breast cancer, rectal cancer, and esophagogastric cancer (30–32). Theoretical advantages of a neoadjuvant approach are numerous. First, a much larger population can benefit from effective systemic treatment. Second, neoadjuvant systemic therapy directly addresses radiographically occult metastatic disease. Third, delaying surgery during neoadjuvant treatment allows for restaging prior to surgery. This provides improved patient selection by identifying those individuals who have responded to neoadjuvant treatment and may benefit from a resection, whilst preventing futile surgery in patients with rapidly progressive disease. Furthermore, several studies have shown that complication rates, including postoperative pancreatic fistula and postpancreatectomy hemorrhage, are lower following neoadjuvant treatment (33–36). Lastly, neoadjuvant treatment may reduce tumor volume, with increased likelihood of a margin negative (R0) resection (25, 37).
Conversely, the neoadjuvant approach has some potential drawbacks. First, patients might have progressive disease during neoadjuvant treatment, precluding curative-intent resection. However, it is unlikely that patients with progressive disease during chemotherapy would have been cured with upfront resection, since cure is exceedingly rare with a 10-year OS of only 4% after surgery (38). Furthermore, since patients with progression during neoadjuvant treatment do not seem to respond to chemotherapy, it is likely that these patients would not have responded to adjuvant chemotherapy either, increasing their risk of early recurrent or metastatic disease following surgery. Thus, rather than a missed opportunity of cure, it is more likely that these patients have been spared futile surgery. Another potential drawback is the risk of deterioration during neoadjuvant treatment. Chemotherapy may reduce the patients' performance status and quality of life because of toxicities. More specifically, FOLFIRINOX is known for its gastrointestinal complications, increased risk of infections, fatigue, and sensory peripheral neuropathy (24). Fortunately, it is rare that patients become unfit for surgery due to chemotherapy, and no deaths have been attributed to FOLFIRINOX in two systematic reviews (5, 39). A final potential drawback is that biliary drainage is required before chemotherapy in patients with obstructive jaundice. Biliary drainage is associated with mainly infectious complications (40), but this can be avoided with upfront surgery.
Comparing Adjuvant With Neoadjuvant Trials
The PRODIGE 24/CCTG PA.6 trial showed a median survival of almost 5 years for patients with resectable PDAC treated with upfront resection and adjuvant mFOLFIRINOX: a survival estimate far superior than previously reported for other treatments (24). However, these results apply only to a highly selected subset of patients. Only patients with favorable tumor biology and good performance status after a complete curative-intent resection are eligible for adjuvant trials. Several hurdles need to be taken by patients with resectable PDAC on imaging. A small percentage of patients become unfit for surgery in the preoperative phase due to stent-related complications causing clinical deterioration. In the operative phase, a resection is not performed in about 20% of patients who are found to have occult metastatic or locally irresectable disease. Next, patients need to recover sufficiently within 12 weeks after surgery to receive adjuvant chemotherapy. In large cohorts, only about 50% of patients received adjuvant gemcitabine after a complete resection (26–29). For adjuvant mFOLFIRINOX, patients need to have an even better World Health Organization (WHO) performance status of 0 or 1. Lastly, for the PRODIGE 24/CCTG PA.6 trial, patients were ineligible if the CA 19.9 level was above 180 U/mL before start of chemotherapy or in the event of early postoperative disease recurrence on imaging. We estimate that on a nationwide level only about 25% of patients with (B)RPC on imaging could become eligible for adjuvant mFOLFIRINOX. This also explains the low accrual rate of the PRODIGE 24/CCTG PA.6 trial of only 1–2 patients on average per center per year.
Patients do not need to overcome most of these hurdles for inclusion in a neoadjuvant trial. Most patients presenting in the clinic with (B)RPC on imaging are eligible for neoadjuvant trials after adequate biliary drainage. Thus, direct comparison of outcomes of neoadjuvant and adjuvant trials is biased, because neoadjuvant trials can include almost all patients whilst for adjuvant trials only the 25% of patients with the best tumor biology and performance status can be included.
Neoadjuvant Treatment—Systematic Reviews and Meta-Analyses
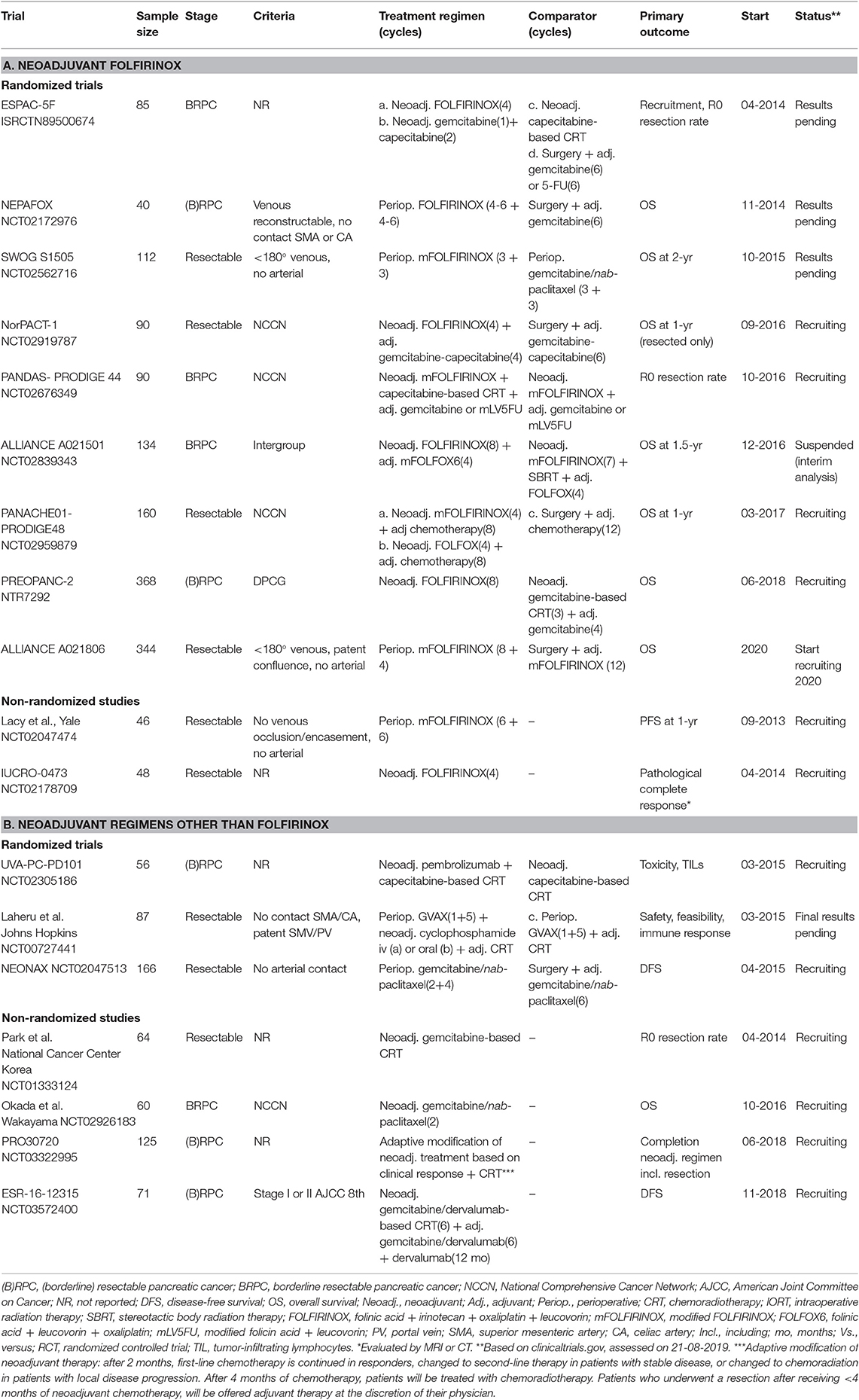
One of the first studies describing neoadjuvant treatment for pancreatic cancer was published in 1980 (41). Over time, different single-agent or multi-agent chemotherapy regimens were used, including 5-FU, gemcitabine, mitomycin C, and platinum compounds. Three large meta-analyses have been published for non-metastatic PDAC patients describing outcomes after preoperative treatment (irrespective of the regimen used) compared to upfront surgery (Table 2) (25, 37, 42). The first meta-analysis by Gillen et al. included 111 studies published from 1980 to 2009. Chemotherapy regimens were mainly gemcitabine or 5-FU based, and 94% of studies used chemoradiotherapy (42). This meta-analysis showed that 33% of patients initially staged as unresectable pancreatic cancer (BRPC and LAPC) were able to undergo a resection after preoperative treatment. Furthermore, estimated survival following resection and R0 resection rates for patients with initially unresectable PDAC were comparable to patients with resectable PDAC (Median OS: 23 vs. 21 months; R0 resection: 82 vs. 79%). A second meta-analysis by Dhir et al. provided an update of the literature published since 2009, which marks the endorsement of the AHPBA/SSAT/SSO consensus criteria, as well as the introduction of newer preoperative regimens (37). In this meta-analysis of 96 studies, the median OS after neoadjuvant treatment for resectable PDAC and BRPC was similar (18 vs. 19 months). Furthermore, the R0 resection rate of 85% was much higher than previously reported in the setting of upfront resection. The third meta-analysis by Versteijne et al. included only studies that did not exclude patients who didn't undergo resection after neoadjuvant treatment or patients who didn't undergo adjuvant chemotherapy after resection (25). These criteria allowed for intention-to-treat analysis of the survival outcomes. Reporting by intention-to-treat reflects actual clinical practice and outcomes, because it allows for non-compliance and protocol deviations, increasing the generalizability of the results (43). This reduces potential bias of the treatment effect, because the study population is not limited to patients that received planned treatment such as surgery or adjuvant chemotherapy. Without the intention-to-treat analysis, a selection of patients with better outcomes due to immortal time bias is likely to occur (44). This meta-analysis of 38 studies comprising 3843 (B)RPC patients found superior survival following any neoadjuvant treatment compared to upfront resection (weighted median OS: 19 vs. 15 months). Only a negligible number of patients received neoadjuvant FOLFIRINOX. The resection rate was higher with upfront surgery (66 vs. 81%, p < 0.001), but the R0 resection rate was better after neoadjuvant treatment (87 vs. 67%, p < 0.001).
Following the ACCORD-11/PRODIGE-4 trial for metastatic PDAC by Conroy et al. in 2011, FOLFIRINOX emerged as a potential preoperative treatment for non-metastatic PDAC (3). No RCT has been performed for neoadjuvant FOLFIRINOX in the setting of (B)RPC. The best available estimate for the outcomes of patients treated with neoadjuvant FOLFIRINOX comes from a patient-level meta-analysis by Janssen et al. that included 283 BRPC patients and showed a median OS of 22.2 months (39). The pooled resection rate was 68%, with an R0 resection rate of 84%.
Neoadjuvant Treatment—Large Retrospective Series
In addition to these meta-analyses, two large retrospective studies investigated the neoadjuvant approach (45, 46). The largest retrospective study used data from the National Cancer Database (NCDB) including patients with clinical stage I and II resected PDAC (45). A propensity score matched analysis was conducted comparing outcomes for patients who received neoadjuvant treatment before resection (n = 2005) to patients who underwent upfront resection (n = 6015). The neoadjuvant patients had a longer median OS compared to patients who underwent upfront resection (26 vs. 21 months, adjusted HR 0.72, 95% CI: 0.68–0.78, p < 0.01). Moreover, compared with a subgroup of patients who received adjuvant therapy after upfront resection, the neoadjuvant group still had better survival (26 vs. 23 months, adjusted HR 0.83, 95% CI: 0.73–0.89, p < 0.01). Second, a large observational cohort study from Verona Hospital included all consecutive BRPC (n = 267) and LAPC (n = 413) patients (46). Of all patients with newly diagnosed BRPC or LAPC, 7% received only supportive care owing to clinical deterioration. FOLFIRINOX (46%) and gemcitabine with nab-paclitaxel (22%) were the most commonly used regimens, and additional radiotherapy was applied in 23% of patients. Resection rate was 24% for BRPC patients, with an R0 resection rate of 58% for all patients combined. No differences were found in R0 resection rates between BRPC and LAPC patients and chemotherapy regimens used.
Published Neoadjuvant FOLFIRINOX Trials (Phase II and III)
Three non-randomized small (<50 patients) phase II studies on neoadjuvant FOLFIRINOX for (B)RPC have been published to date (Table 3A) (47–49). In 2016, the first prospective multicenter trial was published (ALLIANCE A021101), including 22 BRPC patients who received preoperative mFOLFIRINOX (4 cycles) followed by capecitabine-based chemoradiotherapy (50.4 Gy in 28 fractions) (47). This study demonstrated the feasibility of recruiting patients in a multi-institutional neoadjuvant FOLFIRINOX study. Fifteen patients (68%) completed the neoadjuvant treatment and underwent a resection, with an R0 resection rate of 93%. The median OS was 22 months. In 2018, a similar study was published to determine the tolerability and efficacy of four cycles of mFOLFIRINOX both pre- and post-operative in resectable PDAC (48). Twenty-one patients were included, of whom 81% underwent a resection with an R0 resection rate of 94%. Following resection, 82% of patients completed 4 cycles of adjuvant mFOLFIRINOX. The largest study was a single-arm phase II clinical trial conducted at the Massachusetts General Hospital (49). In this study, 48 BRPC patients were treated with 8 cycles of neoadjuvant FOLFIRINOX followed by individualized chemoradiotherapy. In patients with resolution of vascular involvement, FOLFIRINOX was followed by short-course capecitabine-based chemoradiotherapy (25 Gy in 5 fractions), whilst patients with persistent vascular involvement were treated with long-course chemoradiotherapy (50.4 0Gy in 28 fractions). Forty-four patients (92%) proceeded to chemoradiotherapy, of whom 27 (56%) received short-course chemoradiotherapy and 17 (35%) received long-course chemoradiotherapy. Surgical resection was performed in 32 (67%) patients, of whom 31 (97%) had an R0 resection. After a median follow-up of 18 months, median OS was 38 months, with a 2-year OS of 56% (NCT0591733).
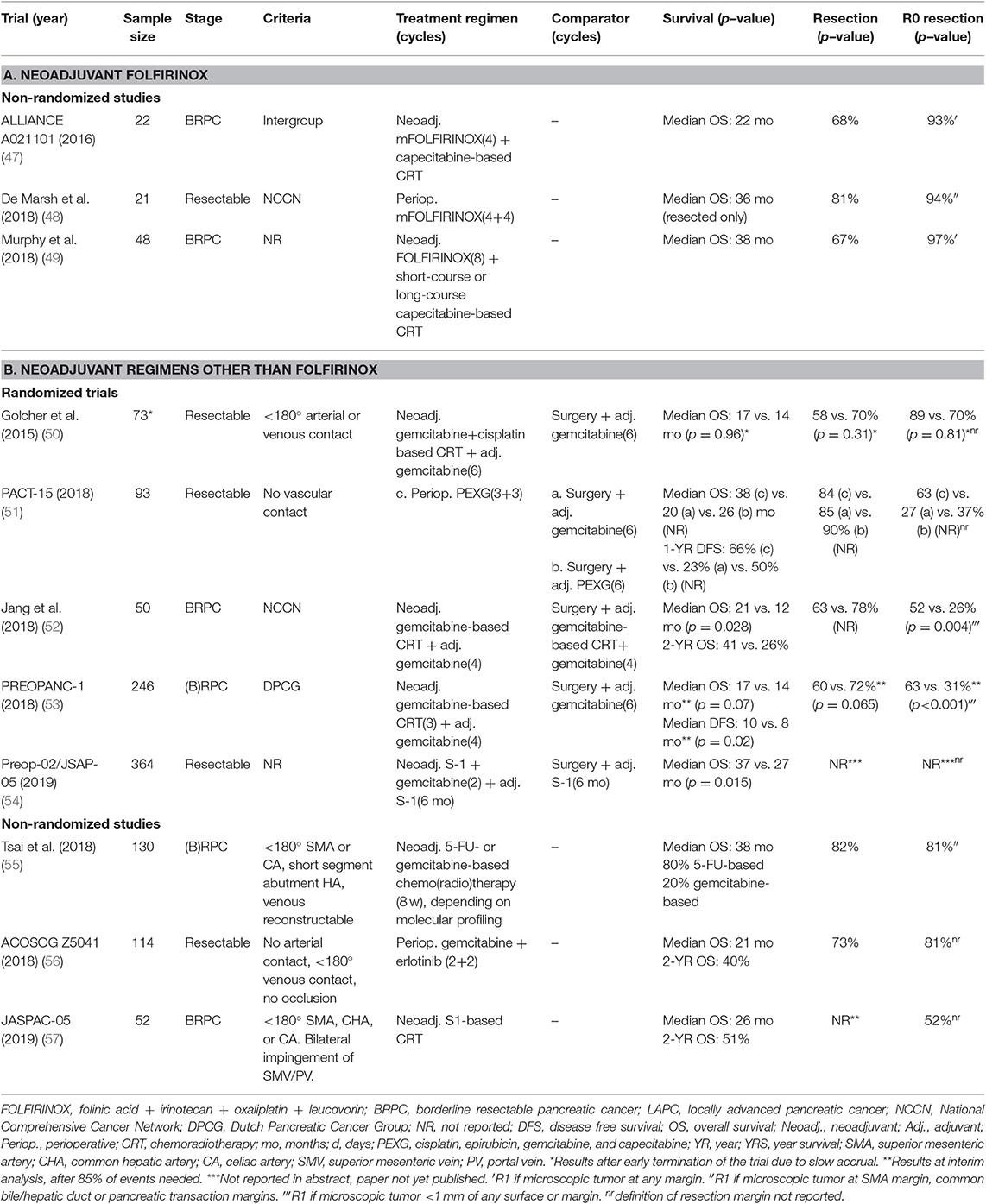
Table 3. Recently published neoadjuvant trials in (borderline) resectable pancreatic cancer from 2016 to 2019.
Although, the three studies slightly differ in the treatment regimen and sequence, neoadjuvant (m)FOLFIRINOX treatment with or without chemoradiotherapy is feasible with high R0 resection rates. The survival estimates are promising, but need confirmation in larger RCT's.
Published Neoadjuvant Trials—Regimens Other Than FOLFIRINOX (Phase II and III)
A number of phase II–III trials have been conducted using other neoadjuvant regimens, yet several of these RCTs were terminated early due to slow accrual. This emphasizes the difficulties in conducting large neoadjuvant RCTs in pancreatic cancer. Table 3B shows eight published studies on neoadjuvant regimens other than FOLFIRINOX. Three RCTs have been published on neoadjuvant gemcitabine-based chemoradiotherapy vs. upfront surgery for patients with (B)RPC (50, 52, 53). The study by Golcher et al. was terminated early due to slow accrual after inclusion of 73 (29%) patients (50). They concluded that neoadjuvant chemoradiation is safe with respect to toxicity, postioperative morbidity, and mortality, but no difference in OS could be demonstrated (median OS: 17 vs. 14 months, p = 0.96). In the Korean randomized phase II-III trial, BRPC patients were randomly assigned to receive gemcitabine-based chemoradiotherapy (45 Gy in 25 fractions and 9 Gy in 5 fractions) (arm A) or upfront surgery followed by chemoradiotherapy following the same protocol as the neoadjuvant group (arm B) (52). Both groups received 4 cycles of gemcitabine as maintenance chemotherapy after completion of initial treatment. After inclusion of 50 patients, interim-analysis showed superior median OS (21 vs. 12 months, HR = 1.97, 95% CI: 1.07–3.62, p = 0.028), better 2-year survival rate (41 vs. 26%), and a superior R0 resection rate (52 vs. 26%, p = 0.004) in the neoadjuvant group compared to upfront surgery. Consequently, the study was discontinued due to superiority and lack of equipoise (NCT01458717). At ASCO 2018, the Dutch phase III PREOPANC-1 trial presented preliminary results, after inclusion of 246 (B)RPC patients who were randomly allocated to neoadjuvant gemcitabine-based chemoradiotherapy followed by a resection and adjuvant 4 cycles of gemcitabine (arm A), or upfront surgery followed by 6 cycles of gemcitabine (arm B) (53). After 85% of events needed, the interim analysis showed superior R0 resection rate (63 vs. 31%, p < 0.001) and superior DFS (10 vs. 8 months, p = 0.02) in the neoadjuvant group, but a difference in OS could not be demonstrated (17 vs. 14 months, HR = 0.74, p = 0.07). To allow for comparison with adjuvant trials, a subgroup analysis was performed of patients who received at least one cycle of adjuvant chemotherapy, showing a median OS of 42 months in the neoadjuvant group and 19 months in the upfront surgery group (p = 0.006). Final results are awaited soon. The PACT-15 trial was an Italian multicenter phase II trial, in which 93 resectable PDAC patients were randomly assigned (1:1:1) to receive adjuvant gemcitabine (arm A), adjuvant PEXG (cisplatin, epirubicin, gemcitabine, and capecitabine) (arm B), or 3 cycles of PEXG pre- and postoperative (arm C) (51). Median OS was 20 months in arm A, 26 months in arm B, and 38 months in arm C (p-value not reported). Three non-randomized studies on regimens other than FOLFIRINOX have been published (55–57). The phase II trial from Tsai et al. used molecular profiling of pretreatment EUS-FNA guided tumor biopsies using 6 biomarkers to guide neoadjuvant therapy in 130 (B)RPC patients (55). Eighty percent of patients received 5-FU based treatment whilst 20% received gemcitabine-based treatment. The median OS was 38 months, with a 5-year survival of 34%, a resection rate of 82%, and an R0 resection rate of 81%. The ACOSOG Z5401 single-arm phase II trial was a study of neoadjuvant gemcitabine plus erlotinib for resectable PDAC. (56) This study demonstrated a favorable 2-year OS for 114 evaluable patients of 40% (95% CI: 31–49%), with a median OS of 21 months. At the 2019 ASCO congress, final results of two Japanese trial were presented. The JASPAC-05 study was a multicenter, single-arm, phase II of neoadjuvant S-1 based chemoradiotherapy (57). Fifty-two BRPC patients were included, and 50 (96%) patients completed the neoadjuvant treatment. The 2-year OS was 51%, with a median OS of 26 months, and an R0 resection rate of 52%. The phase II-III Preop-02/JSAP-05 trial was a large collaboration study of 57 centers in which 364 patients with resectable PDAC were randomized to either neoadjuvant gemcitabine and S-1 chemotherapy (2 cycles) or upfront surgery, both followed by 6 months of adjuvant S-1 (54). This study also showed superior survival following neoadjuvant treatment, with a median OS of 37 vs. 27 months (HR = 0.72, 95% CI: 0.55–0.94, p = 0.015). No differences were found regarding the resection rate, R0 resection rate, and postoperative morbidity. Although S-1 is only used as standard-of-care in East Asia, the study does provide additional proof of the superiority of neoadjuvant therapy over upfront resection for patients with resectable PDAC.
In summary, although based on only three RCTs, a neoadjuvant approach seems to be consistently superior to upfront resection for R0 resection rates, at least equal or superior for DFS, and at least equal or superior for OS in both BRPC and resectable PDAC patients. The results of the R0 resection rates were notable, with a two-fold increase in two out of the three evaluable RCTs. However, it remains unclear whether superior R0 resection rate is an appropriate intermediate outcome for OS in the neoadjuvant setting. The results of ongoing larger RCTs may further clarify the survival benefit of neoadjuvant treatment as opposed to upfront resection for (B)RPC patients.
Standard of Care—Current Guidelines
The NCCN guideline, ASCO Clinical Practice Guideline, and European Society for Medical Oncology (ESMO) Clinical Practice Guideline are commonly used guidelines for pancreatic cancer treatment (15, 21, 58, 59). Due to the lack of large RCTs for neoadjuvant treatment of PDAC, most recommendations in these guidelines are based on systematic reviews of cohort studies, providing Oxford Levels of Evidence category 2A (60).
The 2019 NCCN guidelines (15) recommend upfront surgery followed by adjuvant treatment for resectable PDAC, but advise to consider neoadjuvant treatment in patients with high-risk features, preferably in the setting of a clinical trial. High-risk features include imaging findings suspicious of advanced or metastatic disease, significantly elevated Carcinogen Antigen (CA) 19-9, large primary tumors or regional lymph nodes, excessive weight loss, and notable pain. The adjuvant treatment of first choice is mFOLFIRINOX. For BRPC patients, neoadjuvant treatment is recommended, with therapeutic options including FOLFIRINOX or gemcitabine/nab-paclitaxel, both with or without subsequent chemoradiotherapy. The 2019 ASCO Clinical Practice Guideline (21) recommends primary surgical resection for patients without any radiographic evidence of metastatic disease, with no interface between the primary tumor and surrounding mesenteric vasculature, CA 19.9 level suggestive of potentially curable disease, and a performance status and comorbidity profile appropriate for major abdominal surgery. However, neoadjuvant therapy can also be offered as an alternative strategy for patients with resectable PDAC. For patients who do not meet all of these criteria, the ASCO guideline recommends neoadjuvant therapy. No specific neoadjuvant treatment regimen is recommended. Options for consideration include FOLFIRINOX or gemcitabine/nab-paclitaxel ± subsequent chemoradiotherapy. In the adjuvant setting, mFOLFIRINOX is recommended as treatment of first choice. In case of concern for toxicity and tolerance, doublet therapy with gemcitabine and capecitabine, or monotherapy with either gemcitabine or fluorouracil (5-FU) can be offered. Following neoadjuvant therapy, patients may be candidates for additional chemotherapy following surgery, depending on their performance status and initial response to the neoadjuvant treatment. The ASCO guideline recommends a total of 6 months of chemotherapy, considering both neoadjuvant and adjuvant treatment. Adjuvant chemoradiotherapy may be offered to patients who underwent primary resection with microscopically positive margins (R1) and/or node-positive disease after completion of systemic adjuvant chemotherapy. The 2019 ESMO guideline (58, 59) recommends adjuvant mFOLFIRINOX as first therapeutic option in selected and fit individuals with resectable tumors. For patients with age >70 years, WHO performance status 2, or patients who have any contraindication for FOLFIRINOX, doublet therapy with gemcitabine-capecitabine can be offered as alternative. Gemcitabine monotherapy should be used only in frail patients. For BRPC patients, neoadjuvant treatment with gemcitabine or FOLFIRINOX followed by chemoradiotherapy and surgery is recommended.
Ongoing Neoadjuvant FOLFIRINOX Trials (Phase II and III)
The optimal chemotherapy regimen in the neoadjuvant setting, the number of cycles pre- and postoperatively, the additional benefit of (chemo)radiotherapy, and the timing of surgery after neoadjuvant treatment still need to be further investigated. Several ongoing phase II and III trials are investigating these aspects of neoadjuvant treatment regimens in patients with (B)RPC. Table 4A presents selected ongoing trials including neoadjuvant FOLFIRINOX, and Table 4B shows ongoing trials for neoadjuvant regimens other than FOLFIRINOX.
Of the nine RCTs, two originate from France: the PANDAS-PRODIGE44 trial for BRPC patients, and the PANACHE01-PRODIGE48 trial for resectable PDAC. In the PANDAS-PRODIGE44 trial, 90 BRPC patients will receive neoadjuvant mFOLFIRINOX with (arm A) or without capecitabine-based chemoradiotherapy (arm B), both followed by surgery and adjuvant gemcitabine or modified LV5FU (NCT02676349). This study uses R0 resection rate as primary endpoint. The PANACHE01-PRODIGE48 is a three-arm trial with 2:2:1 allocation to 4 cycles of neoadjuvant mFOLFIRINOX (arm A) or FOLFOX (arm B), both followed by 8 cycles of adjuvant chemotherapy, or upfront surgery followed by 12 cycles of adjuvant chemotherapy (arm C) (NCT02959879) (61). The choice of adjuvant chemotherapy regimen will be left to the medical teams, according to guidelines during the recruitment period. The trial will include 160 resectable PDAC patients, and the primary endpoint is 1-year OS. The SWOG S1505 trial is a randomized phase II study for patients with resectable PDAC designed to determine the most promising perioperative regimen for a larger phase III trial (NCT02562716). This study has completed accrual and randomized 147 patients to either 3 cycles of perioperative mFOLFIRINOX (arm A) or perioperative gemcitabine with nab-paclitaxel (arm B). The primary outcome is 2-year OS, and results are anticipated in 2020. The ALLIANCE A021501 was initially designed to evaluate the additional value of hypofractionated radiation therapy to systemic therapy as neoadjuvant treatment for BRPC of the pancreatic head, with 18-month OS rate as primary outcome(NCT02839343). (62) The initial design of this study was to randomize 134 patients to receive 8 cycles of mFOLFIRINOX (arm A), or 7 cycles of mFOLFIRINOX followed by either hypofractionated stereotactic body radiation therapy (SBRT, 33 Gy in 5 fractions) or hypofractionated image guided radiation therapy (HIGRT, 25 Gy in 5 fractions) (arm B). Following surgery, all patients were scheduled for 4 cycles of adjuvant modified FOLFOX6 (mFOLFOX6). However, an interim analysis of the R0 resection rate was conducted after accrual of 30 patients, after which the radiotherapy arm (B) was suspended due to futility. The NorPACT-1 trial is a multicenter trial for patients with resectable PDAC of the pancreatic head, in which patients are randomized in a 3:2 ratio to receive 4 cycles of neoadjuvant FOLFIRINOX and adjuvant 4 cycles of gemcitabine-capecitabine (arm A), or upfront surgery followed by 6 cycles of adjuvant gemcitabine-capecitabine (arm B) (NCT02919787) (63). The sample size is 90 patients, and the primary endpoint is 1-year OS for those patients who ultimately undergo a resection. The PREOPANC-2 trial is a multicenter study performed by the Dutch Pancreatic Cancer Group (DPCG) (NTR7292) (64). In this study, 368 (B)RPC patients will be randomized to receive 8 cycles of neoadjuvant FOLFIRINOX (arm A) or 3 cycles of neoadjuvant gemcitabine-based chemoradiotherapy with adjuvant 4 cycles of gemcitabine, with median OS as primary endpoint. Last, the ALLIANCE A021806 trial will compare 8 cycles of neoadjuvant and 4 cycles of adjuvant mFOLFIRINOX to all 12 cycles adjuvant mFOLFIRINOX for resectable PDAC. This trial will start recruiting patients by the beginning of 2020 and will include 344 patients using median OS as primary endpoint. The remaining three studies investigate neoadjuvant FOLFIRINOX with a sample size of <50 patients, thereby limiting potential impact on future guidelines [NCT02047474, NCT02178709, NCT02172976 (NEPAFOX)].
Ongoing Neoadjuvant Trials—Regimens Other Than FOLFIRINOX (Phase II and III)
At least three ongoing randomized phase II-III trials (NCT02305186, NCT00727441, NCT02047513) and four ongoing single-arm phase II trials are investigating neoadjuvant regimens other than FOLFIRINOX (NCT01333124, NCT02926183, NCT03322995, NCT03572400) (Table 4B). The three-arm trial from Johns Hopkins aims to study the feasibility and toxicity of perioperative GVAX vaccine therapy ± cyclophosphamide (oral or intravenous) in addition to standard adjuvant chemoradiotherapy for resectable PDAC (NCT00727441). This study is awaiting final results. In the randomized NEONAX trial, 166 patients with resectable PDAC are randomized to receive 6 cycles of gemcitabine with nab-paclitaxel perioperative (2 neoadjuvant, 4 adjuvant) (arm A), or all cycles adjuvant (arm B) (65). In the PRO30720 study, the neoadjuvant regimen depends on the response on CT or MRI scan, tumor marker levels, and performance status assessment (NCT03322995). Sample size is 125 (B)RPC patients, who will all start with 2 months of neoadjuvant chemotherapy. Subsequent treatment depends on the response and may include a therapy switch to an alternative chemotherapy regimen or chemoradiotherapy. With this adaptive design, the feasibility of personalized treatment will be evaluated. The other ongoing trials comprise a variety of interventions including chemoradiotherapy (NCT02305186, NCT01333124, doublet chemotherapy (NCT02926183), and a combination of chemotherapy and immunotherapy (NCT03572400).
Most ongoing studies of both neoadjuvant FOLFIRINOX and other neoadjuvant regimens are underpowered to detect a clinically relevant difference (e.g., 3 or 6 months) in OS. Some studies are hypothesis-generating in their selection of intermediate outcome, such as R0 resection or treatment completion rates. Other studies do have survival as primary outcome, but have a sample size that is too small to detect a clinically relevant survival difference of 3 or 6 months. Assuming an alpha error of 0.05 and a power of 80%, a sample size exceeding 300 patients is needed to detect a difference in median OS of 6 months. An explanation for inadequate sample size is often a concern for feasibility. The PREOPANC-2 trial appears to be the only RCT that may be adequately powered to assess whether neoadjuvant FOLFIRINOX is superior to other regimens. Furthermore, the ALLIANCE A021806 is the only adequately powered RCT comparing perioperative (8 + 4 cycles) mFOLFIRINOX with adjuvant mFOLFIRINOX (12 cycles).
Conclusion
Selection bias hampers comparing survival outcomes between neoadjuvant and adjuvant trials. Patients in neoadjuvant trials may have occult metastatic disease at surgery or may not fully recover from surgery; patients in adjuvant trials were selected after overcoming these hurdles. Only a direct comparison in an RCT will avoid this inevitable selection bias. Despite the limited number of published RCTs comparing a neoadjuvant approach to upfront surgery, patients with resectabel PDAC and BRPC seem to consistently benefit from a neoadjuvant approach with regards to the R0 resection rate. Furthermore, the DFS and OS were at least equal or superior with a neoadjuvant approach compared to upfront surgery. The currently published RCTs supporting neoadjuvant treatment over upfront resection included mostly single-agent based regimens. The multi-agent regimen FOLFIRINOX has considerable toxicity requiring a good performance status. FOLFIRINOX has already proven superior to gemcitabine in the metastatic and adjuvant setting. Ongoing RCTs will investigate whether FOLFIRINOX is indeed the superior regimen in the neoadjuvant setting. Likely, neoadjuvant FOLFIRINOX may further improve the outcomes of this vulnerable patient group. In addition, future RCTs should study the optimal number of neoadjuvant cycles, the value of additional neoadjuvant chemoradiotherapy, the optimal patient selection for surgical resection, and the need for subsequent adjuvant chemotherapy. For patients with a good performance status, we advocate patient participation in one of the large ongoing RCTs evaluating the potential benefit of neoadjuvant FOLFIRINOX for (B)RPC patients.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest
EO'R received funding for the Memorial Sloan-Kettering Cancer Center for pancreatic cancer research in general from the following companies: Genentech, Roche, BMS, Halozyme, Celgene, ActaBiologica, OncoMed, Parker Institute, AstraZenica, Silenseed. Consulting/Advisory: Cytomx, BioLineRx, Targovax, Halozyme, Celgene, Bayer, Polaris. EO'R receives personal fees/consulting/advisory funds from Cytomx, BioLineRx, Targovax, Halozyme, Celgene, Bayer, Polaris.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor declared a past co-authorship with one of the authors BK.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. (2019) 69:7–34. doi: 10.3322/caac.21551
2. Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. (2014) 74:2913–21. doi: 10.1158/0008-5472.CAN-14-0155
3. Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. (2011) 364:1817–25. doi: 10.1056/NEJMoa1011923
4. Goldstein D, El-Maraghi RH, Hammel P, Heinemann V, Kunzmann V, Sastre J, et al. nab-Paclitaxel plus gemcitabine for metastatic pancreatic cancer: long-term survival from a phase III trial. J Natl Cancer Inst. (2015) 107:dju413. doi: 10.1093/jnci/dju413
5. Suker M, Beumer BR, Sadot E, Marthey L, Faris JE, Mellon EA, et al. FOLFIRINOX for locally advanced pancreatic cancer: a systematic review and patient-level meta-analysis. Lancet Oncol. (2016) 17:801–10. doi: 10.1016/S1470-2045(16)00172-8
6. Oettle H, Neuhaus P, Hochhaus A, Hartmann JT, Gellert K, Ridwelski K, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA. (2013) 310:1473–81. doi: 10.1001/jama.2013.279201
7. Gnerlich JL, Luka SR, Deshpande AD, Dubray BJ, Weir JS, Carpenter DH, et al. Microscopic margins and patterns of treatment failure in resected pancreatic adenocarcinoma. Arch Surg. (2012) 147:753–60. doi: 10.1001/archsurg.2012.1126
8. Hishinuma S, Ogata Y, Tomikawa M, Ozawa I, Hirabayashi K, Igarashi S. Patterns of recurrence after curative resection of pancreatic cancer, based on autopsy findings. J Gastrointest Surg. (2006) 10:511–8. doi: 10.1016/j.gassur.2005.09.016
9. Sohal DP, Walsh RM, Ramanathan RK, Khorana AA. Pancreatic adenocarcinoma: treating a systemic disease with systemic therapy. J Natl Cancer Inst. (2014) 106:dju011. doi: 10.1093/jnci/dju011
10. Mehta VK, Fisher G, Ford JA, Poen JC, Vierra MA, Oberhelman H, et al. Preoperative chemoradiation for marginally resectable adenocarcinoma of the pancreas. J Gastrointest Surg. (2001) 5:27–35. doi: 10.1016/S1091-255X(01)80010-X
11. Varadhachary GR, Tamm EP, Abbruzzese JL, Xiong HQ, Crane CH, Wang H, et al. Borderline resectable pancreatic cancer: definitions, management, and role of preoperative therapy. Ann Surg Oncol. (2006) 13:1035–46. doi: 10.1245/ASO.2006.08.011
12. Katz MH, Pisters PW, Evans DB, Sun CC, Lee JE, Fleming JB, et al. Borderline resectable pancreatic cancer: the importance of this emerging stage of disease. J Am Coll Surg. (2008) 206:833–46; discussion: 846–8. doi: 10.1016/j.jamcollsurg.2007.12.020
13. Callery MP, Chang KJ, Fishman EK, Talamonti MS, William Traverso L, et al. Pretreatment assessment of resectable and borderline resectable pancreatic cancer: expert consensus statement. Ann Surg Oncol. (2009) 16:1727–33. doi: 10.1245/s10434-009-0408-6
14. Bockhorn M, Uzunoglu FG, Adham M, Imrie C, Milicevic M, Sandberg AA, et al. Borderline resectable pancreatic cancer: a consensus statement by the International Study Group of Pancreatic Surgery (ISGPS). Surgery. (2014) 155:977–88. doi: 10.1016/j.surg.2014.02.001
15. Tempero MA. NCCN guidelines updates: pancreatic cancer. J Natl Compr Canc Netw. (2019) 17:603–5. doi: 10.6004/jnccn.2018.0043
16. Tempero MA, Malafa MP, Al-Hawary M, Asbun H, Bain A, Behrman SW, et al. Pancreatic adenocarcinoma, version 2.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2017) 15:1028–61. doi: 10.6004/jnccn.2017.0131.
17. College of American Pathologists (CAP). Protocol for the Examination of Specimens From Patients With Carcinoma of the Pancreas Version: PancreasExocrine 4.0.0.1 Protocol. Available online at: https://documents.cap.org/protocols/cp-pancreas-exocrine-17protocol-4001.pdf
18. The Royal College of Pathologists. Dataset for the Histopathological Reporting of Carcinomas of the Pancreas, Ampulla of Vater and Common Bile Duct. Available online at: https://www.rcpath.org/uploads/assets/34910231-c106–4629-a2de9e9ae6f87ac1/g091-pancreasdataset-mar17.pdf
19. Isaji S, Mizuno S, Windsor JA, Bassi C, Fernández-Del Castillo C, Hackert T, et al. International consensus on definition and criteria of borderline resectable pancreatic ductal adenocarcinoma 2017. Pancreatology. (2018) 18:2–11. doi: 10.1016/j.pan.2017.11.011
20. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology. Pancreatic adenocarcinoma. NCCN Guidelines version 2 (2018). Available online at: https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf (accessed February 10, 2019).
21. Khorana AA, McKernin SE, Berlin J, Hong TS, Maitra A, Moravek C, et al. Potentially curable pancreatic adenocarcinoma: ASCO clinical practice guideline update. J Clin Oncol. (2019) 37:2082–8. doi: 10.1200/JCO.19.00946
22. Neoptolemos JP, Stocken DD, Friess H, Bassi C, Dunn JA, Hickey H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. (2004) 350:1200–10. doi: 10.1056/NEJMoa032295
23. Neoptolemos JP, Palmer DH, Ghaneh P, Psarelli EE, Valle JW, Halloran CM, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet. (2017) 389:1011–24. doi: 10.1016/S0140-6736(16)32409-6
24. Conroy T, Hammel P, Hebbar M, Ben Abdelghani M, Wei AC, Raoul JL, et al. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med. (2018) 379:2395–406. doi: 10.1056/NEJMoa1809775
25. Versteijne E, Vogel JA, Besselink MG, Busch ORC, Wilmink JW, Daams JG, et al. Meta-analysis comparing upfront surgery with neoadjuvant treatment in patients with resectable or borderline resectable pancreatic cancer. Br J Surg. (2018) 105:946–58. doi: 10.1002/bjs.10870
26. Mayo SC, Gilson MM, Herman JM, Cameron JL, Nathan H, Edil BH, et al. Management of patients with pancreatic adenocarcinoma: national trends in patient selection, operative management, and use of adjuvant therapy. J Am Coll Surg. (2012) 214:33–45. doi: 10.1016/j.jamcollsurg.2011.09.022
27. IKNL. Report on Pancreatic and Periampullary Carcinoma in the Netherlands. (2014). Available online at: https://www.iknl.nl/docs/default-source/KIB-rapportages/portfolio_kib_pancreas-en-periamplullair-carcinoom.pdf
28. Merkow RP, Bilimoria KY, Tomlinson JS, Paruch JL, Fleming JB, Talamonti MS, et al. Postoperative complications reduce adjuvant chemotherapy use in resectable pancreatic cancer. Ann Surg. (2014) 260:372–7. doi: 10.1097/SLA.0000000000000378
29. Nussbaum DP, Adam MA, Youngwirth LM, Ganapathi AM, Roman SA, Tyler DS, et al. Minimally invasive pancreaticoduodenectomy does not improve use or time to initiation of adjuvant chemotherapy for patients with pancreatic adenocarcinoma. Ann Surg Oncol. (2016) 23:1026–33. doi: 10.1245/s10434-015-4937-x
30. Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. (2004) 351:1731–40. doi: 10.1056/NEJMoa040694
31. Rastogi P, Anderson SJ, Bear HD, Geyer CE, Kahlenberg MS, Robidoux A, et al. Preoperative chemotherapy: updates of national surgical adjuvant breast and bowel project protocols B-18 and B-27. J Clin Oncol. (2008) 26:778–85. doi: 10.1200/JCO.2007.15.0235
32. van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. (2012) 366:2074–84. doi: 10.1056/NEJMoa1112088
33. Marchegiani G, Andrianello S, Nessi C, Sandini M, Maggino L, Malleo G, et al. Neoadjuvant therapy versus upfront resection for pancreatic cancer: the actual spectrum and clinical burden of postoperative complications. Ann Surg Oncol. (2018) 25:626–37. doi: 10.1245/s10434-017-6281-9
34. Cheng TY, Sheth K, White RR, Ueno T, Hung CF, Clary BM, et al. Effect of neoadjuvant chemoradiation on operative mortality and morbidity for pancreaticoduodenectomy. Ann Surg Oncol. (2006) 13:66–74. doi: 10.1245/ASO.2006.02.003
35. Yamada S, Takami H, Sonohara F, Hayashi M, Fujii T, Kodera Y. Effects of duration of initial treatment on postoperative complications in pancreatic cancer. J Hepatobiliary Pancreat Sci. (2019) 26:235–241. doi: 10.1002/jhbp.622
36. Ferrone CR, Marchegiani G, Hong TS, Ryan DP, Deshpande V, McDonnell EI, et al. Radiological and surgical implications of neoadjuvant treatment with FOLFIRINOX for locally advanced and borderline resectable pancreatic cancer. Ann Surg. (2015) 261:12–7. doi: 10.1097/SLA.0000000000000867
37. Dhir M, Malhotra GK, Sohal DPS, Hein NA, Smith LM, O'Reilly EM, et al. Neoadjuvant treatment of pancreatic adenocarcinoma: a systematic review and meta-analysis of 5520 patients. World J Surg Oncol. (2017) 15:183. doi: 10.1186/s12957-017-1240-2
38. Paniccia A, Hosokawa P, Henderson W, Schulick RD, Edil BH, McCarter MD, et al. Characteristics of 10-year survivors of pancreatic ductal adenocarcinoma. JAMA Surg. (2015) 150:701–10. doi: 10.1001/jamasurg.2015.0668
39. Janssen QP, Buettner S, Suker M, Beumer BR, Addeo P, Bachellier P, et al. Neoadjuvant FOLFIRINOX in patients with borderline resectable pancreatic cancer: a systematic review and patient-level meta-analysis. J Natl Cancer Inst. (2019) 111:782–94. doi: 10.1093/jnci/djz073
40. van der Gaag NA, Rauws EA, van Eijck CH, Bruno MJ, van der Harst E, Kubben FJ, et al. Preoperative biliary drainage for cancer of the head of the pancreas. N Engl J Med. (2010) 362:129–37. doi: 10.1056/NEJMoa0903230
41. Pilepich MV, Miller HH. Preoperative irradiation in carcinoma of the pancreas. Cancer. (1980) 46:1945–9. doi: 10.1002/1097-0142(19801101)46:9<1945::AID-CNCR2820460908>3.0.CO;2-X
42. Gillen S, Schuster T, Meyer Zum Büschenfelde C, Friess H, Kleeff J. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med. (2010) 7:e1000267. doi: 10.1371/journal.pmed.1000267
43. Gupta SK. Intention-to-treat concept: a review. Perspect Clin Res. (2011) 2:109–12. doi: 10.4103/2229-3485.83221
44. Giobbie-Hurder A, Gelber RD, Regan MM. Challenges of guarantee-time bias. J Clin Oncol. (2013) 31:2963–9. doi: 10.1200/JCO.2013.49.5283
45. Mokdad AA, Minter RM, Zhu H, Augustine MM, Porembka MR, Wang SC, et al. Neoadjuvant therapy followed by resection versus upfront resection for resectable pancreatic cancer: a propensity score matched analysis. J Clin Oncol. (2017) 35:515–22. doi: 10.1200/JCO.2016.68.5081
46. Maggino L, Malleo G, Marchegiani G, Viviani E, Nessi C, Ciprani D, et al. Outcomes of primary chemotherapy for borderline resectable and locally advanced pancreatic ductal adenocarcinoma. JAMA Surg. (2019) 154:932–42. doi: 10.1001/jamasurg.2019.2277
47. Katz MH, Shi Q, Ahmad SA, Herman JM, Marsh Rde W, Collisson E, et al. Preoperative modified FOLFIRINOX treatment followed by capecitabine-based chemoradiation for borderline resectable pancreatic cancer: alliance for clinical trials in oncology trial A021101. JAMA Surg. (2016) 151:e161137. doi: 10.1001/jamasurg.2016.1137
48. de W Marsh R, Talamonti MS, Baker MS, Posner M, Roggin K, Matthews J, et al. Primary systemic therapy in resectable pancreatic ductal adenocarcinoma using mFOLFIRINOX: a pilot study. J Surg Oncol. (2018) 117:354–62. doi: 10.1002/jso.24872
49. Murphy JE, Wo JY, Ryan DP, Jiang W, Yeap BY, Drapek LC, et al. Total neoadjuvant therapy with FOLFIRINOX followed by individualized chemoradiotherapy for borderline resectable pancreatic adenocarcinoma: a phase 2 clinical trial. JAMA Oncol. (2018) 4:963–9. doi: 10.1001/jamaoncol.2018.0329
50. Golcher H, Brunner TB, Witzigmann H, Marti L, Bechstein WO, Bruns C, et al. Neoadjuvant chemoradiation therapy with gemcitabine/cisplatin and surgery versus immediate surgery in resectable pancreatic cancer: results of the first prospective randomized phase II trial. Strahlenther Onkol. (2015) 191:7–16. doi: 10.1007/s00066-014-0737-7
51. Reni M, Balzano G, Zanon S, Zerbi A, Rimassa L, Castoldi R, et al. Safety and efficacy of preoperative or postoperative chemotherapy for resectable pancreatic adenocarcinoma (PACT-15): a randomised, open-label, phase 2–3 trial. Lancet Gastroenterol Hepatol. (2018) 3:413–23. doi: 10.1016/S2468-1253(18)30081-5
52. Jang JY, Han Y, Lee H, Kim SW, Kwon W, Lee KH, et al. Oncological benefits of neoadjuvant chemoradiation with gemcitabine versus upfront surgery in patients with borderline resectable pancreatic cancer: a prospective, randomized, open-label, multicenter phase 2/3 trial. Ann Surg. (2018) 268:215–22. doi: 10.1097/SLA.0000000000002705
53. van Tienhoven G, Versteijne E, Suker M, Groothuis KBC, Busch OR, Bonsing BA, et al. Preoperative chemoradiotherapy vs immediate surgery for resectable and borderline resectable pancreatic cancer (PREOPANC-1): a randomized, controlled, multicenter phase III trial. J Clin Oncol. (2018) 36:LBA4002. doi: 10.1200/JCO.2018.36.18_suppl.LBA4002
54. Motoi F, Kosuge T, Ueno H, Yamaue H, Satoi S, Sho M, et al. Randomized phase II/III trial of neoadjuvant chemotherapy with gemcitabine and S-1 versus upfront surgery for resectable pancreatic cancer (Prep-02/JSAP05). Jpn J Clin Oncol. (2019) 49:190–4. doi: 10.1093/jjco/hyy190
55. Tsai S, Christians KK, George B, Ritch PS, Dua K, Khan A, et al. A phase II clinical trial of molecular profiled neoadjuvant therapy for localized pancreatic ductal adenocarcinoma. Ann Surg. (2018) 268:610–9. doi: 10.1097/SLA.0000000000002957
56. Wei AC, Ou FS, Shi Q, Carrero X, O'Reilly EM, Meyerhardt J, et al. Perioperative Gemcitabine + Erlotinib Plus pancreaticoduodenectomy for resectable pancreatic adenocarcinoma: ACOSOG Z5041 (Alliance) phase II Trial. Ann Surg Oncol. (2019) 26:4489–97. doi: 10.1245/s10434-019-07685-1
57. Takahashi S, ohno I, Ikeda M, Konishi M, Kobayashi T, Akimoto T. for the JASPAC Group. Final results of JASPAC05: phase II trial of neoadjuvant S-1 and concurrent radiotherapy followed by surgery in borderline resectable pancreatic cancer. J Clin Oncol. 37:4127. doi: 10.1200/JCO.2019.37.15_suppl.4127
58. Ducreux M, Cuhna AS, Caramella C, Hollebecque A, Burtin P, Goéré D, et al. Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2015) 26(Suppl. 5):v56–68. doi: 10.1093/annonc/mdv295
59. Pentheroudakis G. Recent eUpdates to the ESMO Clinical Practice Guidelines on hepatocellular carcinoma, cancer of the pancreas, soft tissue and visceral sarcomas, cancer of the prostate and gastric cancer. Ann Oncol. (2019) 30:1395–7. doi: 10.1093/annonc/mdz180
60. Oxford Centre for Evidence-Based Medicine. The Oxford Levels of Evidence 2. Available online at: https://www.cebm.net/index.aspx?o=5653.
61. Schwarz L, Vernerey D, Bachet JB, Tuech JJ, Portales F, Michel P, et al. Resectable pancreatic adenocarcinoma neo-adjuvant FOLF(IRIN)OX-based chemotherapy - a multicenter, non-comparative, randomized, phase II trial (PANACHE01-PRODIGE48 study). BMC Cancer. (2018) 18:762. doi: 10.1186/s12885-018-4663-4
62. Katz MHG, Ou FS, Herman JM, Ahmad SA, Wolpin B, Marsh R, et al. Alliance for clinical trials in oncology (ALLIANCE) trial A021501: preoperative extended chemotherapy vs. chemotherapy plus hypofractionated radiation therapy for borderline resectable adenocarcinoma of the head of the pancreas. BMC Cancer. (2017) 17:505. doi: 10.1186/s12885-017-3441-z
63. Labori KJ, Lassen K, Hoem D, Grønbech JE, Søreide JA, Mortensen K, et al. Neoadjuvant chemotherapy versus surgery first for resectable pancreatic cancer (Norwegian Pancreatic Cancer Trial - 1 (NorPACT-1) - study protocol for a national multicentre randomized controlled trial. BMC Surg. (2017) 17:94. doi: 10.1186/s12893-017-0291-1
64. Janssen Q, Besselink MG, Wilmink JW, van Tienhoven G, Homs M, Groot Koerkamp B, on behalf of the Dutch Pancreatic Cancer Group. The (cost)effectiveness of neoadjuvant FOLFIRINOX vs neoadjuvant gemcitabine-based chemoradiotherapy and adjuvant gemcitabine for (borderline) resectable pancreatic cancer: the PREOPANC-2 study. Available online at: https://www.trialregister.nl/trial/7094. In: 13th IHPBA World Congress. Geneva.
65. Uhl W, Ettrich TJ, Reinacher-Schick AC, Algül H, Friess H, Kornmann M, et al. NEONAX trial: Neoadjuvant plus adjuvant or only adjuvant nab-paclitaxel plus gemcitabine for resectable pancreatic cancer, a phase II study of the AIO pancreatic cancer group (AIO-PAK-0313)—safety interim analysis. In: 2019 ASCO Annual Meeting. Chicago, IL.
Keywords: pancreatic cancer, neoadjuvant, FOLFIRINOX, borderline resectable, pancreatic ductal adenocarcinoma, resectable, ongoing trials, evidence
Citation: Janssen QP, O'Reilly EM, van Eijck CHJ and Groot Koerkamp B (2020) Neoadjuvant Treatment in Patients With Resectable and Borderline Resectable Pancreatic Cancer. Front. Oncol. 10:41. doi: 10.3389/fonc.2020.00041
Received: 10 September 2019; Accepted: 10 January 2020;
Published: 31 January 2020.
Edited by:
Francesco Giovinazzo, Queen Elizabeth Hospital Birmingham, United KingdomReviewed by:
Hanna Sternby, Lund University, SwedenJorg Kleeff, Martin Luther University of Halle-Wittenberg, Germany
Copyright © 2020 Janssen, O'Reilly, van Eijck and Groot Koerkamp. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bas Groot Koerkamp, Yi5ncm9vdGtvZXJrYW1wQGVyYXNtdXNtYy5ubA==
 Quisette P. Janssen
Quisette P. Janssen Eileen M. O'Reilly
Eileen M. O'Reilly Casper H. J. van Eijck1
Casper H. J. van Eijck1 Bas Groot Koerkamp
Bas Groot Koerkamp