
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol., 31 January 2020
Sec. Cancer Molecular Targets and Therapeutics
Volume 10 - 2020 | https://doi.org/10.3389/fonc.2020.00013
This article is part of the Research TopicEmerging Translational Opportunities in Comparative Oncology with Companion Canine CancersView all 14 articles
Osteosarcoma is a malignant primary tumor of bone, arising from transformed progenitor cells with osteoblastic differentiation and osteoid production. While categorized as a rare tumor, most patients diagnosed with osteosarcoma are adolescents in their second decade of life and underscores the potential for life changing consequences in this vulnerable population. In the setting of localized disease, conventional treatment for osteosarcoma affords a cure rate approaching 70%; however, survival for patients suffering from metastatic disease remain disappointing with only 20% of individuals being alive past 5 years post-diagnosis. In patients with incurable disease, pulmonary metastases remain the leading cause for osteosarcoma-associated mortality; yet identifying new strategies for combating metastatic progression remains at a scientific and clinical impasse, with no significant advancements for the past four decades. While there is resonating clinical urgency for newer and more effective treatment options for managing osteosarcoma metastases, the discovery of druggable targets and development of innovative therapies for inhibiting metastatic progression will require a deeper and more detailed understanding of osteosarcoma metastasis biology. Toward the goal of illuminating the processes involved in cancer metastasis, a convergent science approach inclusive of diverse disciplines spanning the biology and physical science domains can offer novel and synergistic perspectives, inventive, and sophisticated model systems, and disruptive experimental approaches that can accelerate the discovery and characterization of key processes operative during metastatic progression. Through the lens of trans-disciplinary research, the field of comparative oncology is uniquely positioned to advance new discoveries in metastasis biology toward impactful clinical translation through the inclusion of pet dogs diagnosed with metastatic osteosarcoma. Given the spontaneous course of osteosarcoma development in the context of real-time tumor microenvironmental cues and immune mechanisms, pet dogs are distinctively valuable in translational modeling given their faithful recapitulation of metastatic disease progression as occurs in humans. Pet dogs can be leveraged for the exploration of novel therapies that exploit tumor cell vulnerabilities, perturb local microenvironmental cues, and amplify immunologic recognition. In this capacity, pet dogs can serve as valuable corroborative models for realizing the science and best clinical practices necessary for understanding and combating osteosarcoma metastases.
Since the institution of chemotherapy in the 1960s, relapse-free survival for osteosarcoma (OS) patients with localized disease has dramatically improved. The current standard of care involves surgical resection of the primary tumor and multi-agent chemotherapy (both in the neoadjuvant and adjuvant setting) which can result in 5-year survival rates up to 70% for patients with localized disease (1). For those patients who present with distant metastases (usually in the lung), outcomes are much poorer with a survival rate of about 20% (2). The negative prognoses associated with macroscopic disseminated OS burdens is not unique, but rather holds true for many types of cancers that metastasize (3); and underscores the broader need in combating metastatic progression across diverse solid tumor histologies. For OS patients, major hurdles that reduce overall survival include relapse, which occurs in 1/3 of patients with localized disease (4) and in the majority (~75%) of patients presenting with systemic disease (5); and the development of chemo-resistance (6). Since overall survival rates have plateaued with multi-agent chemotherapy (7), there remains an impetus to discover and clinically deploy alternative non-cytotoxin based anti-metastatic therapeutics that inhibit lung metastasis progression and may lead to improved patient outcomes. Several investigators in the metastasis research community have advocated the idea that delaying or inhibiting metastatic progression (particularly the early stages of lung colonization) should be the most clinical and biologic relevant metric rather than the cytoreduction of the primary tumor in the evaluation of new drugs (3, 8, 9). The merit of this proposed paradigm shift in therapeutic assessment is supported by historical clinical data that micrometastases in the lung are already present in OS patients with localized tumors and that adjuvant chemotherapy has been shown to improve relapse-free survival (10). Additionally, preclinical effectiveness of molecularly-targeted therapy for targeting early stages of lung colonization or micrometastases have been shown previously (11, 12) (also see Table 2), and justify the exploration of precision medicine approaches for improving survival outcomes. To accelerate discovery to impact, the rational development of anti-metastatic therapeutics requires a convergent science approach including (1) a better understanding of OS metastasis biology in relation to the lung microenvironment and (2) the availability of engineered and natural model systems that most faithfully recapitulate the complexities of metastatic progression. Through transdisciplinary collaborative research, it is envisioned that novel and effective anti-metastatic therapeutics can be identified and translated to extend the lives of patients with OS by eradicating or thwarting the progression of subclinical micrometastatic disease that persisted following standard multi-agent chemotherapy.
The metastatic process, or more commonly referred to as the metastatic cascade, describes the progressive steps of tumor cell dissemination from the primary tumor, transit within the blood vasculature, and the establishment of clinically detectable pulmonary metastases (Figure 1). Since each step of the metastatic cascade is rate limiting, metastasis is considered to be a very inefficient process (30–32). The initial stages of metastasis involve the acquisition of an invasive phenotype and migration away from the primary tumor site (step 1, Figures 1A,B). Several studies have shown that OS cells secrete proteolytic enzymes such as matrix metalloproteinases (MMPs) and cathepsins which causes the degradation of local tissue extracellular matrix (ECM) and basement membranes (33). Modulation of TIMP3, MMP1, MMP3, MMP11 have been shown to influence in vitro invasiveness of OS cells, and enhance in vivo tumorigenicity (34–36). OS cell interactions with local stromal cells such as mesenchymal stem cells (37) and endothelial cells (38, 39), have been found to be pro-tumorigenic, whereas interactions with natural killer cells (40) or primed dendritic cells (41), were shown to have anti-tumor effects.
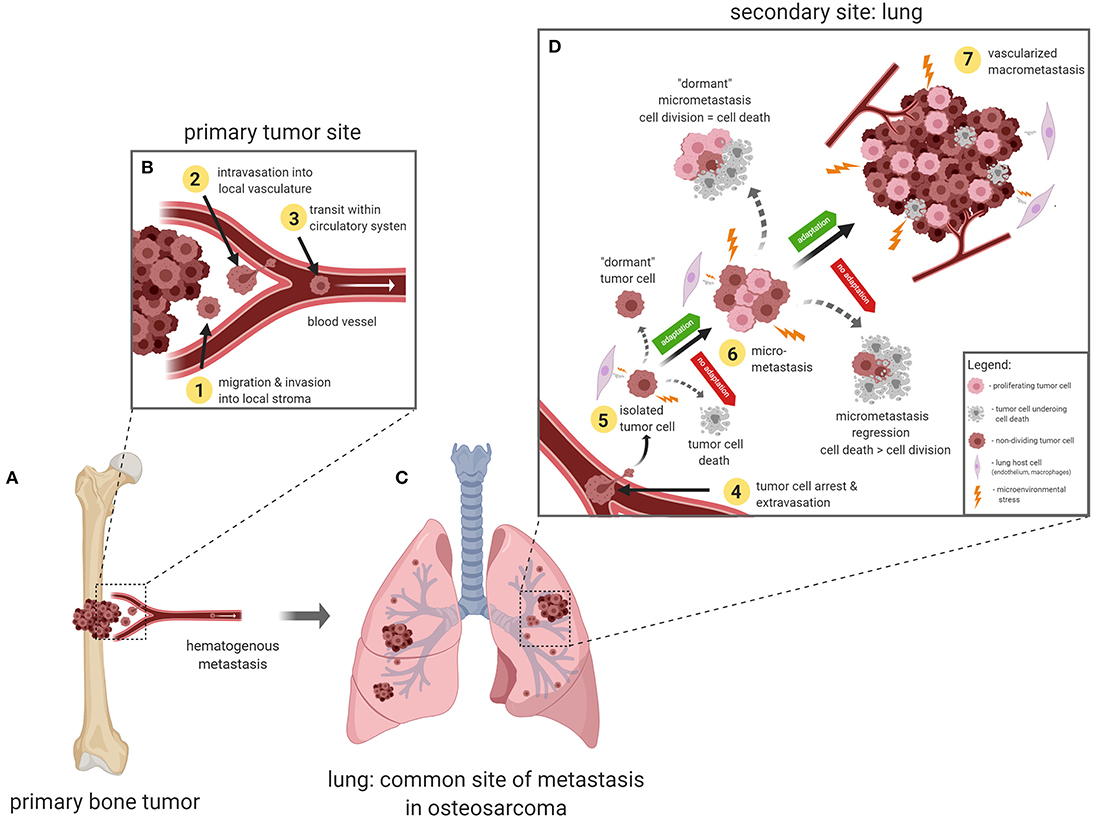
Figure 1. The metastatic cascade in osteosarcoma. (A) Primary OS tumor, usually in the long bones. (B) Tumor cells acquire an invasive phenotype and migrate away from the primary tumor and invade into surrounding tissues (step 1). Tumor cells interact with the basement membrane and endothelial cells to intravasate into the blood microvasculature (step 2) and travel in the circulation (step 3). (C) Upon arrival at the secondary site (lung), tumor cells arrest via size restriction or adhesion interactions with the pulmonary microvascular endothelial cells (step 4). (D) Once tumor cells extravasate out of the blood vessels, they must be able to adapt and survive in the lung microenvironment (step 5). At this vulnerable stage, tumor cells can undergo a number of fates which include- enter cellular dormancy, die off, or if the stresses of the lung microenvironment can be successfully managed, tumor cells can proliferate into multi-cellular micrometastases (step 6). Micrometastases can enter into a state of “angiogenic dormancy” and remain the same size, or regress if cell death is greater than proliferation, or recruit local blood vessels and form a vascularized secondary tumor (step 7).
Once tumor cells reach the local microvasculature, intravasation, or entry into blood vessels, is the next step in the metastatic cascade (step 2, Figures 1A,B). Entry into the local microvasculature requires OS cell interaction with endothelial cells. Several in vitro models exist to study tumor cell interactions with endothelial cells (42), with the simplest system being the co-culturing of tumor cells onto a monolayer of endothelial cells. Research from several groups have utilized this in vitro co-culture method and have shown that RUNX and osteopontin (43), uPAR (14), and αvβ3 (44) influence the physical interactions between OS cells and endothelial cells. More importantly, several of these studies have shown that interfering with these OS cell-endothelial interactions were found to inhibit metastasis formation in vivo (14, 43).
Once within the blood stream, OS cells must be able to resist anoikis, a specialized form of apoptosis induced by the disruption of cell-matrix interactions, as first described by Frisch and Francis (45). A number of key regulators of anoikis have been characterized since its initial discovery (e.g., Mcl-1, Cav-1, Bcl-xL, c-FLIP) (46) and several of these genes have been linked to metastatic capacity in breast cancer cells (47) and OS cells (24, 48, 49).
Another type of stress OS cells encounter within the circulation is the physical hemodynamic forces of blood flow. Observations on the hemodynamic destruction of tumor cells were initially made by Weiss and Dimitrov (50). The major physical stressor in the blood circulation is fluid shear stress (FSS), which is defined as the frictional forces between moving layers, and is measured in Newtons per meter squared (N/m2) or Dynes per centimeter squared (Dyn/cm2) (51). FSS in the blood circulation ranges from 1 to 30 Dyn/cm2 depending on the anatomical location (52). Lien and colleagues have demonstrated that OS cells (MG63) were found to have higher levels of apoptosis when exposed to FSS ranging from 0.5 to 12 Dyn/cm2 when compared to control static cells in an in vitro flow chamber (53). The authors also demonstrated that the level of OS apoptosis correlated with increasing times of exposure of various FSS conditions. It would be interesting to assess whether MG63.3 cells, a highly metastatic variant of MG63 cells, characterized by Ren et al. (54), exhibit some level of resistance to FSS-induced apoptosis.
If OS cells can resist anoikis and adapt to damaging FSS in the blood circulation, arrest, and survival in the lung microvasculature presents the next significant challenge to metastatic OS cells. Several studies using the experimental metastasis model (tail vein injection of tumor cells) have demonstrated that the majority of tumor cells that arrive in the lung do not survive, and only a small subset of the initial population (1–6%) were able to successfully establish metastases (31, 32). These studies have carefully analyzed tumor cell fate over time and concluded that metastatic colonization of the lung is a non-linear process where tumor cells can undergo any number of fates, as illustrated in Figure 1D. Newly arrested tumor cells can either: (1) enter a dormant, viable but non-dividing state, as observed in several lung metastasis studies (32, 55, 56); (2) proliferate into a pre-angiogenic micrometastasis, or (3) undergo apoptotic cell death (57, 58). Micrometastases, in turn, can also undergo a number of fates which include: (1) enter a state “angiogenic dormancy” where tumor cell proliferation is balanced with cell death (59), (2) proliferate into a vascularized macrometastatic lesion (60), or (3) regress if tumor cell death is greater than cell proliferation. The ability to adapt quickly to a harsh new microenvironment is a prerequisite for metastatic cancer cell survival and proliferation in the lung. Stress adaptation pathways depend on the nature of the particular stress encountered, and several research groups have begun to shed light on this aspect of metastasis biology.
Redox stress is a major microenvironmental stressor that contributes to tumor cell clearance in the lung since several studies have provided microscopic imaging evidence and “omic” data supporting this notion. Qiu et al. (58) have shown that the physical arrest of murine melanoma cells in the lung stimulates the local microvascular endothelial cells to release a burst of nitric oxide (NO), which was cytotoxic to tumor cells. Inhibition of NO release by L-NAME (a nitric oxide synthase inhibitor) treatment or the use of endothelial nitric oxide synthase knock-out mice resulted in higher lung tumor burden. Piskounova et al. (61) have shown that metastatic melanoma cells adapt to the redox stress in the lung by upregulating the NADPH-generating enzyme ALDH1L2; and targeting shRNAs against ALDH1L2 resulted in lower lung tumor burden (61). NADPH is important in maintaining redox homeostasis (62), and a recent study by Basnet et al. (63) have shown that micrometastases of breast cancer cells in the mouse lung have elevated transcript and protein levels of antioxidant genes (e.g., NRF2 and GPX1). The notion that ROS can negatively regulate metastasis formation is somewhat controversial since other studies seem to suggest the opposite (64–66). These discrepancies may be due to cell type-specific responses, or the particular dose of ROS exposure. Low, sublethal concentrations of ROS can turn on antioxidant responses, whereas high concentrations of ROS can cause irreversible damage to proteins, lipids and DNA with consequent cell death.
Another type of cellular stress closely linked to redox stress is endoplasmic reticulum (ER) stress. Protein folding processes within the ER are exquisitely sensitive to perturbations in cellular redox state, Ca2+ concentration within ER lumen, and ATP supply (67). Redox stress can alter the oxidative protein folding environment of the ER lumen, which results in the accumulation of unfolded proteins, a condition known as ER stress (68). The unfolded protein response (UPR) is activated by various sensors on the ER membrane, and an adaptive transcriptional program is activated to increase the chaperone capacity of the ER and increase ER-associated degradation pathways to compensate for the sudden load of unfolded proteins (67). The UPR has been found to be dysregulated in many types of cancer, including OS (69, 70). Several highly metastatic human OS cell lines were found to upregulate the ER chaperone protein GRP78 at higher levels compared to their low metastatic counterparts during ER stress (12), and shRNAs and IT-139, a small molecule inhibitor of GRP78 under clinical investigation (71), were found to reduce lung metastatic burden. Translocation of the transcription factor ATF6α to the nucleus is also part of ER stress response, and human OS cells were found to have elevated levels of nuclear ATF6α compared to osteoblast controls under ER stress conditions (72). Downstream targets of ATF6α such as GRP78, PDI, and ERO1β where found to confer chemotherapy resistance in OS cells, and down-modulation of ATF6α resulted in increased sensitivity to cisplatin. Moreover, elevated levels of ATF6α in patient samples was predictive or poorer overall survival and poorer response to chemotherapy (72). Several groups have also found UPR-related pathways to be dysregulated in OS (73–75).
Although the microenvironmental stressors discussed above can contribute to tumor cell clearance in the lung, these observations do not explain the apparent “organotropism” of OS cells for the lung. Why do metastases in OS preferentially occur in the lung? The answer, in part, may be due to mechanical restriction of disseminated tumor cells in the lung microvasculature. Human alveolar capillaries range from 5 to 8 μm in diameter (76), whereas the average diameter of osteoblastic osteosarcoma cells ranges from 10 to 19 μm (estimated from histology micrographs) (77). Circulating tumor cells often arrest via size restriction within the first microvascular capillary bed they encounter, and video microscopic evidence from animal studies suggest that organs such as the lung and liver are efficient at “filtering” out circulating tumor cells from the blood (78). OS cell “organotropism” for the lung can also be explained by the concept of the “pre-metastatic niche” (PMN), in which growth factors from the primary tumor “prime” downstream metastatic sites for tumor cell engraftment (79–81). As to whether PMN contributes to lung colonization in OS, Murgai et al. (82) found that metastatic OS cells secret exosomes containing cytokines that can induce lung perivascular cells to secrete fibronectin. The same authors also demonstrated that fibronectin promoted tumor cell adhesion, migration, and proliferation in vitro. Additionally, Macklin et al. (83) also found that highly metastatic OS cells are capable of secreting extracellular vesicles that were preferentially retained in the lung, but not liver. More definitive studies will be needed to define OS-specific changes in the lung during PMN formation, and whether or not modulation of OS-specific PMN can influence the formation of lung metastasis.
Since lung metastasis progression involves complex 3 dimensional (3D) interactions between OS cells, ECM, and lung parenchymal cells, model systems that can maintain or partially recapitulate some aspects of these 3D interactions will allow researchers to interpret metastatic OS cell responses to gene therapy or pharmacologic intervention in a relevant microenvironmental context. Indeed, several studies have demonstrated that tumor cell response to therapeutics differ when comparing 2D vs. 3D growth conditions (84–86). To this end, several microscope-based models exist that permit researchers to directly visualize and study metastatic cancer cell behavior in a 3D microenvironment. Such models are described below, and the benefits and limitations of each model are discussed.
The chick chorioallantoic membrane (CAM) is a highly vascularized membrane that primarily functions as a gas-exchange organ for the developing embryo (87). The CAM is commonly studied in a shell-less format (ex ovo), where xenograft human tumor fragments or a tumor cell suspension (Figure 2A) can be placed onto the CAM or injected into blood vessels of the CAM. The CAM has proven to be a useful model in studying tumor angiogenesis (90, 91), tumor cell migration and invasion (92), intravasation into blood vessels (93), tumor cells in transit within the vasculature, extravasation out from blood vessels (Figure 2B) (94–98), and the outgrowth of patient derived xenografts (99). In OS research, the CAM model has been used to study tumor growth of a variety of OS cell lines (100), angiogenesis (101), and metastasis to distant sites (102). The main benefits of using the chick CAM model include: (1) amenable to in vivo imaging, (2) relatively inexpensive, and (3) can be used for high-throughput screening of targeted therapies. Disadvantages of the CAM model include: (1) short observation times (days), (2) inability to study tumor cell interactions with the immune system since the chick CAM is immunodeficient until developmental day 18 (87), and (3) fewer antibodies available for host chicken antigens (103). The chick CAM model is applicable to the study of tumor cell invasion (step 1, Figure 1B), interactions between endothelial cells during intravasation, transit within blood vessels, and extravasation (steps 2, 3, and 4, Figures 1B,D) since these steps are readily observable at the surface of the chick CAM. Tumor colonization of distant sites in the chick CAM are not accessible for imaging, and thus harvesting the organs for histology, or polymerase chain reaction assays for tumor specific DNA sequences are required. To study lung colonization in OS, other microscope-based models that can examine lung tissue would be more appropriate.
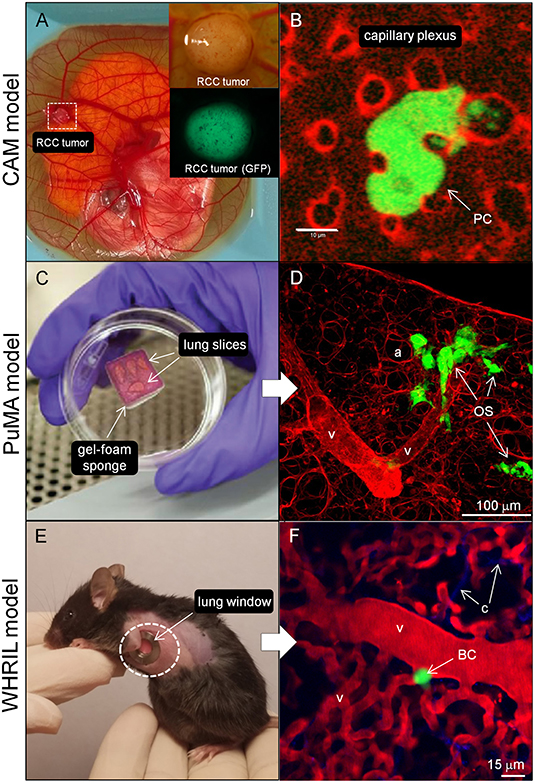
Figure 2. Imaging models to study the metastatic cascade in cancer. (A) The CAM model (whole mount image) showing the chick embryo and highly vascularized CAM. A small renal cell carcinoma (RCC) explant can be seen growing in the dashed white box. Zoomed image of a different established GFP-expressing RCC tumor where the entire tumor, associated vasculature, and corresponding fluorescence image (below) are shown (Image courtesy of Matthew Lowerison and Pengfei Song, UIUC). (B) High magnification, single cell imaging of a GFP-labeled prostatic carcinoma cell (PC) migrating through capillary plexus (labeled with rhodamine-lectin) and forming invadopodia (yellow arrowheads) into the lumen of 2 adjacent capillaries in the CAM model (Image courtesy of Fabrice Lucien and Yohan Kim, Mayo Clinic). (C) The PuMA is an ex vivo lung explant model where tumor cells in viable lung tissue is maintained in cell culture. The lung slices are kept at an air-liquid interface on top of a gelatin sponge. (D) Shows the lung parenchyma (stained red with DAR4M) and eGFP-expressing MG63 OS cells (OS) interacting with vessel-like structures (v). Scalebar = 100 μm. See Lizardo and Sorensen (88) for methods. (E) The WHRIL model allows for the direct visualize of lung tissue through a window in the mouse chest cavity (89) as shown with the dashed white circle. (F) Fluorescent micrograph showing the lung microvasculature (v) (labeled red with tetramethylrhodamine) and GFP-expressing breast cancer cell (BC). Blue fibers represent second harmonic imaging of connective tissue (c) fibers. Scalebar = 15 μm (Images courtesy of David R. Entenberg, Albert Einstein College of Medicine). UIUC, University of Illinois at Urbana-Champaign.
A technical advance that addresses the need to directly visualize and characterize the growth of metastatic OS cells in the lung microenvironment is called the pulmonary metastasis assay (PuMA), first developed by Mendoza et al. (104), and further refined by others (88, 105). The PuMA is an ex vivo, lung tissue explant model where fluorescently labeled tumor cells in viable lung tissue (Figures 2C,D) can be maintained in vitro for up to 21 days of observation. High and low metastatic pairs of human and mouse OS cell lines, whose in vivo metastatic phenotypes were characterized elsewhere (54), retain their metastatic propensities in the ex vivo PuMA model. Such observations suggest that despite the lack of blood flow, certain cellular, and extracellular features of lung tissue still exert “microenvironmental pressures” that are not conducive to the growth of low metastatic tumor cells, but still permit the growth of highly metastatic tumor cells. Indeed, the histology and microarchitecture of PuMA tissue sections are virtually indistinguishable from that of in vivo lungs (104). The PuMA model has been used to assess how gene modulation or drug treatment affects metastatic OS growth in lung tissue (12, 21–23, 25, 106). The PuMA model has several advantages which include: (1) the ability to directly study metastatic OS cells at both the cellular and subcellular level while in a relevant 3D microenvironment, (2) amenable to molecular imaging (gene or signaling pathways) by labeling tumor cells with fluorescent dyes, fluorescent protein reporter or protein fusion constructs, and (3) the PuMA model has recently been adapted to a 96-well plate format for a high-throughput drug screen (20). For image analysis, proprietary software is not needed, and analysis can be done with publicly available software packages such as ImageJ. Image processing can be expedited through automation as described by Young et al. (105). One major drawback of the PuMA model is the limited number of cell lines that are compatible with the assay. For tumor cell lines that have not been previously published to work within the PuMA model, researchers must empirically determine whether their metastatic tumor cell line of interest is compatible with the B-media used in the PuMA model, and whether their cell line can grow into progressively larger lesions over time. Secondly, the length of observation in the PuMA model is limited to 21 days post-injection of tumor cells. Beyond 21 days, the PuMA lung tissue becomes devoid of lung parenchymal cells, leaving only connective tissue. The PuMA model is ideal in studying tumor cell arrest in the lung microvasculature, extravasation, interactions with the lung parenchyma, and the formation of micrometastases (steps 4, 5, and 6, Figure 1D). If a researcher's investigations require an intact microcirculation, then an intravital (within a living subject) imaging model of the lung would be more appropriate.
Direct observation of labeled tumor cells in an intact lung perfusion model have been described previously (58, 107); however this method is an ex vivo perfusion model where the lungs were removed en bloc and imaged on an inverted microscope. Intravital imaging of the microcirculation of various organs (such as lung or liver) was reported by Varghese et al. (108), where an acute preparation of the organ of interest was stably imaged on an inverted microscope for 4–6 h in anesthetized mice. While innovative at the time, this technique was prone to motion artifact from physiologic processes such as breathing or heart beating, and necessitated movement artifact compensation through a post-processing image stabilization algorithm. More recently, imaging of labeled tumor cells in the lung of a live, free breathing mice was recently described by Entenberg et al. (89). In this intravital video microscopy model, called Window for High-Resolution Imaging in the Lung (WHRIL), a small circular window is implanted in the chest cavity of the mouse (Figure 2E) and permits serial imaging of the same area of the lung for a period of up to 2 weeks (protocol allowance). Using the WHRIL model, the authors were able to image tumor cells within the lung microvasculature (Figure 2F), tumor cell extravasation, cell division, and formation of micrometastases (89). The WHRIL model has capacity to thoroughly characterize the effects of targeted anti-metastatic therapeutics on pulmonary micrometastases and established metastases in a preclinical setting. Using fluorescent reporter genes or functional dyes, in combination with WHRIL model, would permit researchers to assess the effects of gene modulation or targeted therapies on metastatic OS cell biology in the lung, in real-time. The advantages of the WHRIL model include the unprecedented ability to study metastatic OS cells at the cellular, subcellular, and molecular level in live, free-breathing mice. Secondly, serial imaging can be performed to assess the effects of therapy over progressive (albeit limited) time points. One major drawback of this technique is the limited depth of imaging, which in turn is dependent on the type of microscope (single photon vs. multi-photon imaging) and the type of fluorophore used (109). Regular epifluorescence imaging would be limited to an imaging depth of 200 μm due to light scattering. In contrast, using a multi-photon confocal microscope and tumor cells labeled with near-infrared fluorophores (emission wavelengths between 650 and 900 nm) would push the imaging depth toward 700 μm (110). The WHRIL model can be implanted at a timepoint corresponding to tumor cell arrest, extravasation, colonization of extravascular lung tissue, formation of micrometastases, and vascularized macrometastases (steps 4, 5, 6, and 7, Figure 1D).
Based upon the complexity of metastatic biology, scientific discoveries that lead to new and effective therapies for OS metastases are expected to be derived through experimental models which most faithfully recapitulate the biology and key regulatory pathways involved in the genesis and metastatic progression of OS. Furthermore, models that accurately reproduce the natural progression of spontaneous micrometastases in the absence of a primary tumor are necessary to investigate activities of novel anti-metastatic therapeutics, as this clinical setting is the most pressing scenario in which humans diagnosed with OS require advances in treatment. Although an ideal animal model of OS has yet to be universally recognized or accepted, the most desirable model characteristics should include spontaneous primary bone tumor and pulmonary metastases development within an immunocompetent host.
In whole organisms, such as humans and dogs, successful metastasis occurs only when cancer cells, singly or in groups, become able to dehisce from not only the surrounding normal tissues, but also from malignantly transformed neighboring cells within the primary tumor. To be successful in seeding distant sites, these metastatic precursors must acquire the ability to invade through the tissue matrix, intravasate into the circulation, arrest within the target tissue, extravasate, survive within each of these diverse and heterotypic environments, and then proliferate within the target organ in ways that recapitulate the primary solid tumor (111). Doing so requires the acquisition of myriad behaviors not typical of the cells of origin, and these transformed phenotypes can arise from abnormal activation of cell-autonomous pathways that endow tumor cells with, for example, resistance to apoptosis (112) or the ability to affect unusually high levels of capped mRNA translation (22). Beyond these shifts in behavior that represent intrinsic properties of the malignant cells themselves, disseminated tumor cells often acquire additional malignancy-associated behaviors from interactions with the normal tissues that surround them within the metastatic niche (113). Interestingly, these interactions need not require close contact between the effector cells and the responder cells—they can occur at long distances, even being initiated by cells located within the primary tumor (114).
These complex interactions between malignant tumors and host cells and tissues make the study of metastasis difficult outside of whole organisms. As the laboratory workhorse for most biological systems, murine models have become those that researchers most often use for exploration into the mechanisms of OS metastasis (115). Murine models of metastasis are diverse and can facilitate the study of biology and therapeutic development through manipulation of the host (using genetically engineered mice, or GEMs), manipulation of the tumor cells themselves (using cell lines, xenografts, or allogeneic transplants), or both. Murine systems allow researchers to study elements critical to oncogenesis, as is evident in the multiple GEM models of spontaneous OS (116–118)—even facilitating whole-genome forward genetic screens into mechanisms of oncogenesis and metastasis (119). The use of immunodeficient mice has facilitated a recent explosion in the generation of patient-derived xenograft (PDX) models (120, 121) and their use in OS research (122), including orthotopic models of spontaneous metastasis which mimic the care patients receive through the implementation of hindlimb amputations (123, 124).
Generally, mouse models can be divided into three classes: (1) those that spontaneously develop primary tumors and subsequently develop metastasis, (2) those that are implanted orthotopically (usually into a leg bone) with spontaneous distant metastases (Figures 3A–C), and (3) those where tumor cells are inoculated directly into the circulation (often called experimental metastasis, Figures 3D,E) (115). Each of these approaches can ask different experimental questions, and each has unique strengths and weaknesses that should be recognized when interpreting results and formulating conclusions. Advantages and disadvantages associated with these models are summarized in Table 1.
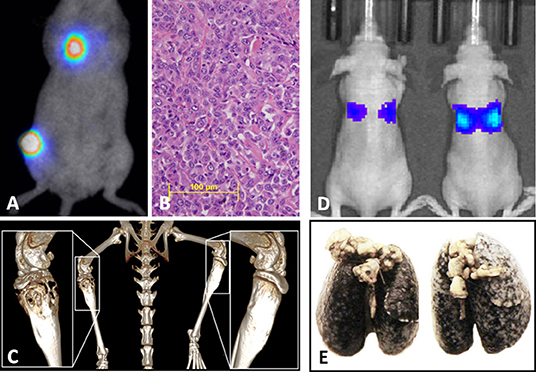
Figure 3. (A) Syngeneic orthotopic mouse model of primary bone OS (K7M3) with concurrent spontaneous pulmonary metastases development visualized by bioluminescent imaging, (B) with corollary histology of established pulmonary metastatic lesions and (C) micro CT images of the OS primary lesion showing profound osteolysis and contralateral unaffected tibia. (D) Bioluminescent imaging of an experimental metastases model in athymic nude mice following tail vein injection with the Abrams (canine OS) luciferase cell line demonstrating correlation between luminescent signal and (E) gross macroscopic tumor burdens.
Traditionally, the oncogenic transformation and malignant behaviors of cancer cells have been ascribed to perturbations involving multiple and interactive molecular factors rooted in genetic alterations and dysregulated biochemical signaling. While many aspects of cancer cell phenotype, including metastasis, can be adequately characterized and studied through the singular lens of biology, there is overwhelming evidence that mechanical forces exerted by and upon cancer cells, surrounding stromal elements, and ECM are integrally linked with oncologic activities, including cancer cell invasion and metastasis (51, 125). Living cells are capable of sensing mechanical stimuli (tensile, compressive, and shear forces), termed mechanotransduction, through specialized cellular structures including focal adhesions and stretch-gated ion channels (126, 127), which result in activation of gene and signaling pathways that regulate cellular behaviors. The realization that mechanical cues, in concert with biologic context, contribute collaboratively to diverse cancer processes has spurred rapid advancements in studying cancer metastasis through the deliberate inclusion of physical science, tumor bioengineering, and microfabrication approaches.
While a preponderance of cancer investigations includes studies based on two-dimensional (2D) cell models, such experimental methods that rely upon cancer cells grown in monolayer do not recapitulate the true interactions between cells-cells and cells-extracellular matrices encountered during solid tumor formation, evolution, and metastatic progression. The bidirectional interactions of cancer cells with the tumor microenvironment generates biological complexity, which can be more thoroughly studied through three-dimensional (3D) modeling strategies that include biomimetic engineered tumor models. Through the purposeful design of various mechanical platforms, it is now possible to ask and answer specific questions regarding how cancer cells respond to highly tunable variables including matrix stiffness, interfacial geometry, cell curvature, and other mechanotransduction gradients (128–131). By virtue of precise and reproducible fabrication techniques for generating engineered biomimetics, cancer cell reactivity in response to individual or collective stimuli can be investigated under controllable and quantitative experimental conditions. While providing unique opportunity to study cancer biology, awareness for the strengths and limitations of diverse mechanobiology platforms for elucidating cancer-associated processes is required to ensure their suitable applications. Given their capacity for high throughput data generation, bioengineered 3D cellular platforms are expected to complement existing biologic model systems for rapidly advancing the current state of knowledge regarding cancer metastasis. Several 3D in vitro biomimetic platforms currently used in cancer research are summarized, and their suitability for studying unique aspects related to OS metastasis are highlighted.
Tumor cell masses naturally grow in 3D and cellular behaviors are dependent upon multiple biochemical and mechanical cues heterogeneously distributed throughout the microenvironment (i.e., hypoxia and intercellular forces, respectively). Compared to conventional 2D cell culture methods, spherical 3D tumor models are superior for recapitulating the spatial cellular and biochemical heterogeneity of solid tumors. Tumor spheroids are cancer cell aggregates ranging in size from 20 to 1,000 μm in diameter and can be formed through various techniques, with the easiest method reliant upon cell buoyancy (132). Additionally, allowing cells to aggregate by gravity (hanging drop method) or culturing cancer cells on non-adherent or cell-repulsive substrates are alternative strategies for reproducible spheroid formation (133). The simplest spheroid models focus on single cell populations which can self-aggregate and produce endogenous ECM, thereby recapitulating homotypic cell-cell, as well as heterotypic cell-ECM interactions operative during solid tumor formation. The generation of more biologically complex suspension models can be achieved through multicellular spheroids whereby diverse cell populations (cancer, stromal, immune) are intermixed to create more realistic physiologic cues and cellular interactions produced within the naturally occurring tumor microenvironment. Collectively, advantages of tumor spheroid models include high-throughput analysis (Figure 4A) and capacity for rapid scale up, while limitations of scaffold-free 3D spheroids include difficulty in studying more complex and dynamic processes such as angiogenesis, invasion, and metastasis. Based upon these characteristics and limitations, scaffold-free spheroid models are well-suited for preclinical anti-cancer drug screening, characterizing diffusion kinetics and drug resistance mechanisms, and unicellular responses including migration, spreading, ECM deposition, and soluble mediator secretions (133, 134).
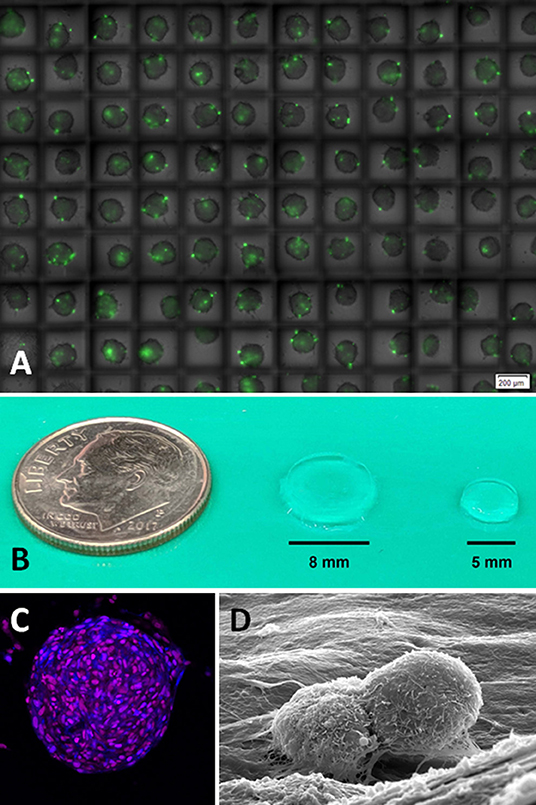
Figure 4. (A) Glioblastoma spheroids in high throughput high-density hanging drop culture on a microchip. Method allows for the rapid screening of novel therapeutic agents in cancer cells growing in 3D whereby diffusional gradients and cell-cell interactions are more accurately recapitulated than 2D cell culture conditions (monolayer). Green dye (Celltox™ Promega) shows cell death after 24 h of culture (Image courtesy of Anurup Ganguli and Rashid Bashir, UIUC). (B) Relative size of hydrogel scaffolds for the study of (C) 3D glioblastoma spheroids by confocal fluorescent microscopy and associated (D) homotypic (cell-cell) and heterotypic (cell-ECM) interactions by scanning electron microscopy (Images courtesy of Emily Chen and Brendan Harley, UIUC). UIUC, University of Illinois at Urbana-Champaign.
Specific for OS, 3D culture systems with spheroids have been utilized for the past 2 decades for studying the effects of the tumor microenvironment on various aspects of OS biology and has been thoroughly summarized by De Luca et al. (135). Derived from these multiple investigations and relevant to therapeutic strategies specifically for OS metastasis, OS spheroids have shed illumination on drug resistance mechanisms to conventional chemotherapeutics (136–142), the maintenance of cancer stem cells and tumor-initiating cells (139, 143, 144), impact of ECM stiffness and composition on metastatic phenotype (145, 146), cues that promote vasculogenic mimicry (147, 148), and metastasis favoring pathways including the roles of specific transcription factors (NF-κB) (149, 150) and miRNAs (151). In addition, the feasibility of generating co-culture bicellular spheroids through the combination of HUVEC and MG-63 cells for the study of VEGF-mediated angiogenesis has recently been described (152).
The ECM is critical in shaping tumor biologic responses through mechanotransducive mechanisms, and the investigation of cancer cells embedded within scaffold-based constructs that vary in chemical composition, shape, density, structure, and porosity allows for researchers to dissect differential mechanotransductive contributions for the induction of diverse malignant phenotypes and cellular processes displayed by cancer cells. Scaffolds can be constructed from either natural or synthetic polymers, with both sharing conserved properties of biocompatibility and promotion of cellular adhesion. Natural scaffold materials are typical ECM proteins while synthetic scaffolds are derived from tunable and crosslinking materials including polyethylene glycol (PEG) and polylactide-co-glycolide acid (PLGA), as well as porous ceramic biomaterials such as bioactive hydroxyapatite and tricalcium phosphate.
Hydrogel scaffolds (Figure 4B), composed of natural or synthetic polymers, are widely used for studying biologic responses of cancer cells, as a gel medium mimics the natural in vivo microenvironment of nascent tumor mass growth in 3D (Figure 4C), whereby cell-to-cell and cell-to-matrix interactions are preserved for directing phenotypic behaviors including proliferation, migration, chemoresistance, and angiogenesis (153, 154). The most common biocompatible polymeric hydrogel materials include collagen type I, Matrigel, and alginate; and these natural materials facilitate cancer cell attachment through heterotypic interactions via integrin receptors and ECM which regulate cell survival, growth, and differentiation (Figure 4D). In addition to natural biomolecules, synthetic constituents used for hydrogel formulation can include polyethylene glycol, polylactic acid, polyglycolic acid. By virtue of their chemistry, synthetic hydrogels have the advantage of being chemically tunable (stiffness, porosity, adhesion ligand density) via synthesis or crosslinking (155), and can recapitulate spatiotemporal changes in matrix heterogeneity encountered within the tumor microenvironment. Increasing sophistication of hydrogel-based models can be achieved through a combination of chemical engineering and biologic layering, including the construction of soluble mediator (growth factors, chemokines, peptidyl signaling molecules) gradients or combinatorial co-culturing of cancer cells with stromal cells including endothelial cells, fibroblasts, and immune cells. While hydrogels have been explored as a controlled drug release scaffold strategies for OS therapy (156–158), the study 3D scaffold tumor models for unraveling OS biology and metastasis remains limited, with some investigations describing differences in behavioral phenotype of malignant OS cells compared to non-transformed osteoblasts based upon matrix rigidity and elasticity (159, 160). In addition to hydrogel scaffolds, chitosan, silk, and synthetic polymers have served as adhesive constructs for 3D OS modeling and have illuminated mechanisms behind viral permissiveness (161), hypoxia-induced angiogenic mediator secretions (162), drug resistance (163), and maintenance of stem cell phenotype (164).
While 3D spheroids with or without scaffolds provide valuable information on cell-cell and cell-ECM interactions, the static nature of nutrient and metabolic waste transport under typical 3D culture systems does not accurately replicate spatiotemporal diffusional gradients naturally formed from lymphatic or blood vessel formation within solid tumors. Microfluidic systems are precisely fabricated from molds and made of materials that are biocompatible, oxygen permeable, and tunable (stiffness, temperature, shear flow pressure, molecular gradients). Structurally, microfluidic systems can be fabricated to include diverse shapes on a micro- or nanoscale including channels and chambers with highly precise diameters, shapes, and flow control rates. When combined with 3D cell culture systems such as spheroids, microfluidic platforms can recapitulate diverse complex processes representing different stages of the cancer progression including tumor-vascular interface responses, diffusional effects of biomolecules on cell populations, and pathologic cancer processes including invasion, angiogenesis, and metastasis (165–170). Recently, specific metastasis-on-a-chip platforms have been fabricated allowing for real time tracking of fluorescently labeled cancer cells and their heterotypic interactions with both ECM and normal resident cells along the full continuum of the metastatic cascade (171, 172). Kong and colleagues recently reported the construction and use of a microfluidic platform for studying the organotropism of cancer cell metastasis and demonstrated the correlative value of their microfluidic system with athymic nude mice models for the evaluation of small molecule inhibiting anti-metastatic strategies (173). Specific for OS, 3D microfluidic platforms have been used to study OS cell adhesive properties under various physiologic conditions (pH, temperature, shear flow) (174), cell morphology in response to gradient molecules (175), and drug screening of nanoparticle encapsulated chemotherapeutics (176).
Conventional OS models for studying experimental therapies most frequently are reliant upon xenogeneic and syngeneic transplant models conducted in mice, however, the inclusion of complementary model systems (CAM, PuMA, WHRIL, engineered 3D biomimetics) have gained wider appeal and scientific acceptance for improving predictive modeling of cancer biology and metastasis. While xenogeneic models, including patient derived xenografts, may provide information pertaining to the sensitivity of human OS tissues or cell lines to specific therapeutics, tumor-host interactions (especially immunobiologic responses) are poorly recapitulated in comparison to what occurs in people who develop OS spontaneously. Although syngeneic models more accurately represent immunologic tumor-host responses than xenogeneic systems, the process of tumor formation and spontaneous metastasis in any transplant model remains artificial, likely underestimating the complexity for how OS naturally progresses in an immunocompetent host. To accurately identify and expedite the clinical translation of novel therapeutics to people with metastatic OS, the evaluation of experimental strategies, in particular immune-based, should be conducted in the most highly relevant and immunocompetent tumor model.
Besides people, canines are the only other large mammal that spontaneously develops OS with substantive frequency. Canine appendicular OS is the most common primary bone tumor in dogs of large to giant skeletal size, and has been estimated to affect at least 10,000 pet dogs every year in North America (58), which is 10 times greater than the number of pediatric OS patients diagnosed annually in the United States. The clinical presentation, biologic behavior, natural disease progression, and genetic signature of OS in dogs is similar to people (177–179), and collectively emphasizes the comparative relevance of dogs to serve as a model system for both discovery and therapeutic investigations (180–184). This modeling strategy has been advocated by leaders in the field of OS basic science and clinical research, and ascribes value on the inclusion of pet dogs with OS as a distinctively informative model system for prioritizing novel therapeutic agents that target metastatic progression (8).
The current standard of care for human patients diagnosed with OS remains largely unchanged from that first used in the early 1980s (185), being neoadjuvant and adjuvant MAP chemotherapy (methotrexate, doxorubicin, cisplatin) together with aggressive local control by surgical excision (186). Building on techniques pioneered in pet dogs with OS (187, 188), most human patients diagnosed today benefit from limb salvage reconstructive techniques that preserve limb function. With these standard of care therapies, outcomes for patients with localized disease increased markedly, such that up to 60% of patients experience “cure” (5-year event-free survival) (189). However, these outcomes have changed little over the last four decades (190).
The factor that most strongly influences outcomes in human patients is the presence or absence of metastatic lesions, usually of the lung parenchyma. Patients who develop lung metastases, whether at diagnosis or years after completing therapy, face a dismal prognosis, with fewer than one in five patients surviving more than 5 years beyond this event (189, 191). Multiple efforts to improve this outcome through intensification of systemic therapy or the introduction of novel regimens have not succeeded.
Patients with both resectable and unresectable metastatic disease at relapse are usually offered systemic therapy, most commonly with high dose ifosfamide (192) or multi-tyrosine kinase inhibitors (193). Although these therapies do little to effect long-term outcomes, they can facilitate short-term disease control and prolong survival. While radiation has a relatively minor role in the curative care of patients with either localized or metastatic disease, modern techniques can be extremely helpful in the palliative setting, providing excellent disease and symptom control (194). The only intervention proven to offer hope for long-term “cure” of disease in patients with metastases remains surgical excision of all macrometastases, and several studies suggest that up to 30% of patients who achieve complete surgical remission will survive disease-free beyond 5 years (195–197).
As mentioned previously, each step of the metastatic cascade is a rate limiting step. For example, if a new drug can prevent OS cells from leaving the primary tumor, invading local tissue, or entering local blood vessels, then the metastatic cascade is stopped in its tracks. Indeed, every step of the metastatic cascade harbors several druggable targets in various types of cancer, as summarized in Table 2. In the clinical setting, however, it is presumed that patients with localized tumors already have subclinical micrometastatic disease in the lung. Thus, targeted therapies that act within the microenvironment of the primary tumor may not necessarily be effective on tumor cells have already spread to the lung since adaptation strategies depend on the particular microenvironment the tumor cells reside. In this scenario, therapeutic strategies that target the processes involved in lung colonization and micrometastases formation would be expected to be most effective in delaying metastatic progression. Further basic research into the molecular pathways underpinning OS lung colonization, micrometastases formation, and the establishment of macrometastases is needed to uncover more actionable targets.
Successful dissemination and colonization of distant tissues by a tumor cell requires navigating a gauntlet of interactions with normal cells and associated tissues (114). Each interaction can either help or hurt that cancer cell's chance of survival. The striking tropism that OS displays for lung tissues suggests that tumor cells elicit or receive signals from cells within the lung metastatic niche that facilitate their survival. Several emerging studies have defined characteristics of that environment that might support tumor growth, many of which constitute targetable vulnerabilities, including pathways that promote dormancy, alter susceptibility to chemotherapy, facilitate metastatic outgrowth, and the maintenance of stemness.
Stromal elements produced by both host cells and tumor cells may play a particular role in the survival of metastasis-initiating cells and in the maintenance of their stem-like features. Zhang and colleagues recently showed that FGF signaling within the metastatic environment triggers a fibrogenic program within disseminated tumor cells that promotes their stemness and survival (198). Signals transduced by way of mTOR complex 1 initiate this program, although the subsequent production of fibronectin by OS cells can then maintain this stem-like state independent of host signals, including FGF. These studies stop short of testing the therapeutic potential of targeting these pathways, but existing agents should facilitate future assessment of their capacity to affect disease progression, most likely in preventing emergence of late metastases.
The laboratories of Roberts and Cam have identified targetable bi-directional signaling between OS cells and lung epithelial cells that appears critical for metastatic colonization. Using a combination of human tissues, xenografts, syngeneic mouse models, and canine models of disease, they have shown how a ΔNp63/IL6/CXCL8 signaling axis mediates tumor-host signaling events critical to the metastatic process. In their model, tumor cells primed by aberrant expression of ΔNp63 (112) (an alternative isoform of the p53 family member TP63) respond to signals from lung epithelial cells by producing high levels of IL6 and CXCL8 (199). Disruption of these cytokine/chemokine signals effectively reduced metastasis formation. Indeed, more than 80% of mice treated with inhibitors of both IL6 and CXCL8 signaling survived long term, while 100% of mice bearing the same tumors succumbed to metastatic disease (26). Interestingly, this antimetastatic effect was only achieved with combination therapy. Mice treated with one or the other inhibitor showed only modest inhibition of metastasis, suggesting some signaling pathway redundancies that remains undefined. Unfortunately, inhibitors that proved effective in their models are unlikely to be developed clinically. Work aimed at identifying critical signaling nodes up- or down- stream of these pathways may identify targets that are more effective and druggable with small molecule inhibitors well-suited for clinical implementation.
Signals that facilitate tumor cell survival within the metastatic niche can emerge from either lung-resident cells or from cells that invade that niche, often in response to tumor-derived signals. For example, Baglio and colleagues have shown that TGFβ expressed on the surface of extracellular vesicles from OS cells can also elicit production of large amounts of IL6 by mesenchymal stem cells (200). The release of this cytokine into the metastatic niche triggers activation of STAT3 within the tumor cells, which promoted proliferation of those metastatic cells in their models. In evaluating the therapeutic relevance of this phenomenon, they showed that the administration of anti-IL6 antibodies reduced the number of metastatic lesions that formed in their animals (200).
Some tumor-host interactions prove detrimental to the survival of disseminated tumor cells. Kleinerman's group has made a series of observations that suggest most disseminated tumor cells that reach the lung will be eliminated through activation of a suicide signal when the FAS receptor expressed on the surface of the tumor cells engages FAS ligand, which is expressed constitutively within the lung (201). This phenomenon results in the selection of a subpopulation of tumor cells that are FAS-negative (202). Interestingly, they have shown that FAS downregulation within this subset of malignant cells can be reversed, as exposure to inhaled gemcitabine drives re-expression of the FAS receptor, engaging the death-inducing signaling complex and triggering apoptosis (203). Such therapies have yet to be tested clinically in pediatric OS patients but seem viable. As proof-of-concept, a study by Rodriguez and colleagues demonstrated that pet dogs with macrometastatic pulmonary OS receiving treatment with aerosolized gemcitabine did result in the upregulation of FAS receptor and markers of cell death by OS cells within pulmonary metastatic lesions (204).
Searching for epigenetic changes that facilitate metastatic colonization of lung tissue by OS cells, Morrow and colleagues recently identified genetic loci that acquire enhancer activity in cells with high metastatic potential (21). Among genes regulated by these metastatic variant enhancer loci, Factor 3 (F3, a gene which can activate blood clotting) demonstrated particular importance for metastasis when evaluated functionally. Disruption of F3 production by OS cells significantly impeded metastatic colonization efficiency in animal models but did not affect primary tumor growth (21). Interestingly, the importance of blood clotting for lung colonization in OS may have been suggested in previous work, lending credence to these findings (205, 206). While this target has not been evaluated in a therapeutic setting, F3 signaling (which triggers both clotting and intracellular signal cascades) should be targetable using existing, FDA-approved drugs (207).
The unique metabolic demands of the primary tumor vs. metastasis are reflective of their different microenvironments (cellular and extracellular components), nutritional availabilities, and level of oxygenation. A rapidly growing primary tumor mass requires a constant supply of energy (ATP) and biomacromolecules (lipids, carbohydrates, and proteins) (208). During glycolysis in normal cells, ATP is obtained from glucose via the oxidation of its carbon bonds through mitochondrial respiration, a process which also requires oxygen. However in cancer cells, the glycolytic intermediate pyruvate is shuttled away from the tricarboxylic acid cycle, and is fermented into lactic acid, even in the presence or absence of oxygen—a phenomenon called the Warburg effect; and several theories on how the Warburg effect might benefit proliferating cancer cells has been discussed elsewhere (209). Not surprisingly, subversion of the Warburg effect has been observed in several OS cell lines such as LM7 and 143B (210). Furthermore in a preclinical mouse model, Hua and colleagues demonstrated LM8 tumor-bearing mice had elevated levels of serum pyruvic acid and lactic acid compared to healthy controls, which suggested that proliferating OS tumor cells were highly glycolytic. The serum from tumor-bearing mice also had higher levels of intermediate metabolites of the tricarboxylic acid cycle compared to healthy controls, further underscoring the higher energy demands of proliferating OS cells within localized and metastatic sites (211). Interestingly, the majority of circulating metabolites in serum were lowest at initial primary tumor formation (week 1) and again at metastatic progression (week 4) following LM8 inoculation, which could suggest that similar global metabolic transformation mechanisms were shared by OS cells during incipient primary tumor growth and distant metastases development. Mechanistically, Hua and colleagues suggested that the unexpected lower metabolic profile in tumor-bearing mice identified at week 4 (metastatic progression) may be due to tumor microenvironmental hypoxia that restricted OS cell growth and reduced cellular metabolism, although this possibility wasn't confirmed in their study (211).
Surviving in the lung microenvironment presents a unique set of metabolic challenges that are distinct from the primary tumor. As mentioned previously, redox stress appears to be a major microenvironmental stressor in the lung. Reactive oxidative species (ROS) and reactive nitrogen species (RNS) produced by the lung parenchyma can affect tumor cell mitochondrial function in a number of ways (58, 212). For example, it is generally known that excess ROS, such as superoxide (O2−), can modify mitochondrial DNA, which in turn, can negatively affect the electron transport chain (ETC), mitochondrial membrane potential, and ATP production (213). Prolonged exposure to RNS such NO− can irreversibly inhibit complex I of the ETC (214). Peroxynitrite (ONOO−), another potent RNS, can inhibit multiple enzymes in the mitochondria such as complexes I–IV, as well as aconitase of the tricarboxylic acid cycle (214). Metabolic adaptation to such oxidative stress would be a pro-survival phenotype that would be selected for during the colonization process, and not surprisingly, anti-oxidant responses which consists of either the upregulation of redox-related enzymes or altered glutathione (GSH) metabolism have been observed in metastatic breast (63), melanoma (61), and osteosarcoma (21, 215). For example, Ren and colleagues have found that metabolites in the GSH metabolic pathway were found to be significantly altered in highly metastatic OS cells compared to their clonally related, low metastatic counterparts (215). Shuttling of metabolites into the GSH pathway is important for producing GSH, which in turn, react with and neutralize ROS and RNS to form less reactive intermediates (216, 217). Other metabolic pathways that were found to be altered in highly metastatic OS cells include arginine, inositol, and lipid metabolic pathways. The previously mentioned study by Hua and colleagues found that serum metabolites of lipid metabolism were found to be elevated in mice with lung metastases compared to tumor-bearing mice with no metastases (211). These observations noted by Hua and colleagues in a mouse model of OS are congruent with global lipidomic studies identifying differences between metastatic (143B) and non-metastatic (HOS) human OS cell lines (218), as well as the recognized importance of lipid metabolism in cancer metastases (219). Collectively, derived from preclinical studies inclusive of cell lines and murine models of cancer, evidence supports altered lipid metabolism being important for metastasis progression; where increased lipid production may address the heightened demand for membrane synthesis during cell growth and organelle biogenesis. As such, targeting unique metabolic demands of metastasis offers a new avenue of anti-metastatic therapy. Indeed, Ren and colleagues demonstrated that targeting the inositol metabolism of metastatic OS cells prevented their growth in the lung microenvironment (215). Further studies are needed to elucidate whether other metabolic susceptibilities exist in metastatic OS, and whether these metabolic susceptibilities can be exploited for new therapeutics.
Recently, immunotherapy has been heralded as a breakthrough for the management of diverse liquid and solid tumors, and its ascension as a major therapeutic pillar is underscored by a rapidly increasing number of FDA approved immune-based treatments for cancers that are resistant to conventional modalities. The anticancer activities of immunotherapies can be ascribed to the cooperative effector functions exerted by both the innate and adaptive immune arms, and while immunotherapy is highly effective for certain solid tumors like melanoma, renal cell carcinoma, and others, its promise for benefiting patients diagnosed with metastatic OS remains largely disappointing to date (220–223). Paradoxically, there is convincing evidence that OS can be recognized by trafficking immunocytes, yet successful exploitation of immunotherapeutic strategies remains elusive. To accelerate the clinical deployment of effective antitumor immune approaches for combating OS, recent scientific investigations have focused on characterizing the quantity, phenotype, dynamics, and functional nature of immune cells that infiltrate into primary and metastatic OS lesions, and these collective findings have been recently and thoroughly summarized (223, 224).
By way of detailed analyses, several innate and adaptive immunocytes have been identified to putatively participate in the initiation or suppression of anti-OS immune responses and include a plethora of diverse myeloid and lymphoid cell types. Of the various immunocytes identified within the OS microenvironment, both innate affector and adaptive effector populations have been characterized, and include antigen-presenting cells (macrophages/dendritic cells) and T lymphocytes, respectively. Within primary OS lesions, tumor-associated macrophages (TAMs) that can be distinguished via genomic signatures, cell surface markers, and functional activities (inflammation vs. immunosuppression) have received considerable attention for their prognostic value and functional role in OS metastasis (225–230). Most, but not all, investigations have identified that increases in TAMs (quantity) or macrophage infiltrate profiles (quality) favoring a M1-subtype polarization (INOS+; pro-inflammatory) rather than a M2-subtype profile (CD163+; immunosuppressive) are associated with better overall survival in OS patients. Incongruent findings among studies regarding the role of TAMs in OS biology could be related to the inherent limitations of single timepoint tissue assessments which fail to capture the dynamic nature of immune cell infiltration within the tumor microenvironment. Nonetheless, the majority of histologic findings provide supportive justification to therapeutically manipulate TAMs profiles within OS lesions that have potential to either favor immune activation (Mifamurtide) (231) or inhibit M2-macrophage polarization (ATRA) (232, 233), for the intended purpose of inhibiting metastatic progression.
Complementing the participatory role of TAMs, several studies have focused on characterizing tumor infiltrating lymphocytes (TILs) and their contribution to metastasis immunobiology. Analyses of TILs within the OS microenvironment have shown that both effector and suppressor T lymphocyte (CD3+) phenotypes participate in shaping immunosurveillance of OS lesions (229, 230, 234–236). Furthermore, several studies suggest that the density (number) or phenotype (activated or exhausted) of effector TILs within OS primary tumors correlate with prognosis. With regards to TILs density, recent studies have demonstrated that increases in the absolute number of CD8+ TILs or the ratio of CD8+/Foxp3+ TILs significantly correlate with improved overall survival (230, 234). Provocatively, the functional relevance of TILs and operative checkpoint blockade mechanisms might be especially important for metastases, as some studies have found the density of TILs to be enriched in metastatic lesions compared to primary tumors (236, 237). Despite the presence of TILs within OS lesions, several studies suggest that the activity of effector TILs might be attenuated, as supported by the expression of exhaustion markers (PD-1, CTLA-4, Tim3) by TILs and/or tumoral microenvironmental expression of PD-L1 (227–229, 235–237). Collectively, these detailed studies strongly suggest that OS lesions can be effectively infiltrated by T lymphocytes, and that therapeutic modulation of checkpoint blockade strategies could improve TILs effector capabilities.
With a basal understanding for the collection of immunocytes that are present within primary tumor and metastatic OS lesions, rational design of immunotherapeutic interventions can be constructed. Through these concerted efforts, the scientific and clinical oncology community can continue to forge toward understanding fundamental anticancer immune mechanisms and improving treatment outcomes in patients with OS metastases through diverse immunologic strategies, either singly or in combination with conventional therapies (radiochemotherapy).
Immunomodulatory agents modify immune responses by amplifying the recognition of cancer cells (immunostimulation) or by attenuating the immunosuppressive activities exerted by cancer cells within the local tumor microenvironment. The innate arm of the immune system comprised of natural killer cells, macrophages, dendritic cells, and primordial T cell subsets (natural killer and γδ) are predominant effector targets of immunomodulatory strategies. The clinical significance of immunomodulatory interventions relevant to sarcomas was noted over a century ago, when William Coley in 1891 reported objective responses in a small minority (10%) of patients with non-resectable sarcomas (bone and soft tissue) treated with heat-inactivated Streptococcus pyogenes and Serratia marcescens injections, termed Coley's toxin (238). The potent anticancer activities induced by bacterial products noted by Coley have been corroborated in both canine and human OS patients that develop surgical site infections (188, 239, 240), and mechanistically these favorable immunologic effects have been attributed to toll-like receptor activation with consequent amplified macrophage and natural killer cell effector functions in mouse models of OS (241).
The clinical translation of immunomodulatory agents which stimulate the innate immune arm for improving outcomes in OS patients remain limited, but include liposome-encapsulated muramyl tripeptide phosphatidylethanolamine (L-MTP-PE) and cytokine-based therapies. Based upon its mechanism in vitro and in preclinical investigations for activating monocytes and macrophage to a tumoricidal state (242, 243), as well as its unique evaluation singly or in combination with cisplatin in pet dogs with OS (243, 244), clinical investigations of MTP-based strategies have been conducted prospectively by the Children's Oncology Group consortium. In a seminal study by Meyers and colleagues, the addition of MTP to a MAP (methotrexate, doxorubicin, cisplatin) backbone in patients with localized OS significantly improved 6-year overall survival rate from 70 to 78% (245). Additionally in the setting of metastatic and/or recurrent OS, the 5-year event free survival rate of patients receiving chemotherapy alone (26%) vs. chemotherapy with L-MTP-PE (42%) appeared favorable (246), further supporting the clinical benefit of this immunomodulatory strategy for delaying the natural progression of OS pulmonary metastases. Complementing the mechanism of L-MTP-PE, exogenous cytokine therapies have also produced marginal improvements in patients diagnosed with OS. In particular, INF-α-2b and IL-2 have been evaluated in the adjuvant setting with either chemotherapy or other immune-based strategies. Recently, the 3-year event free survival benefit derived from adjuvant pegylated INF-α-2b with MAP has been described in a large consortium trial (EURAMOS-1) (247). While early results have not demonstrated significant improvements in event free survival between MAP alone (81%) vs. MAP with adjuvant pegylated INF-α-2b (84%), long term follow up remains active and will ultimately determine if adjuvant pegylated INF-α-2b has any definitive immune activating role for improving the control of OS micrometastases.
In the setting of macroscopic OS metastases, the tolerability and potential benefit exerted by exogenous IL-2 has been explored. In one study, Meazza and colleagues reported the outcomes of 35 pediatric OS patients with macroscopic OS treated with surgery and combinatorial chemoimmunotherapy comprised of IL-2, MAP, ifosfamide, and lymphokine-activated killer (LAK) cell infusion. While the study was not designed to determine the immunobiologic benefit derived from IL-2 and LAK cell infusion, adverse effects associated with IL-2 therapy were tolerable (grade I and II) with most common side effects being fever, flu-like symptoms, hypotension, and cytokine release syndrome (248). In a different study, Schwinger and colleagues reported the tolerability and activity of single-agent, high-dose IV IL-2 therapy in 10 pediatric patients, in which 4 adolescents had metastatic OS (249). While 2 of 4 OS patients achieved complete remission for 14 and 42 months in duration, systemic toxicity associated with high-dose IV IL-2 therapy was significant with 60–100% of treated patients experience some form of grade III or IV clinical toxicity (fatigue, anorexia, or diarrhea). Despite the high level of toxicity, this study clearly demonstrated the potential for IL-2 to amplify anticancer immune responses sufficient to regress macroscopic OS burdens. In attempts to reduce the toxicity associated with systemic IL-2, yet maintain favorable anticancer immune activities within the anatomic site of metastases (lungs), two significant studies have been conducted in pet dogs with pulmonary metastatic OS, which leverage innovative drug delivery or site-specific gene transducing strategies. Khanna and colleagues evaluated the feasibility and activity of aerosolizing liposome encapsulating IL-2 in pet dogs and demonstrated that robust anticancer immune effects could be induced within the pulmonary parenchyma sufficient to cytoreduce macroscopic OS burdens (2 of 4, CR) without significant toxicity (250). A complementary study reported by Dow and colleagues investigated the activity of intravenously administered liposome-DNA complexes (LDC) encoding the IL-2 gene in dogs with macroscopic OS metastases (251). Infusions of LDC was well-tolerated, generated systemic immune activation, and transgene IL-2 expression within the lung parenchyma. Furthermore, objective cytoreductive activities (2 PR, 1 CR) were achieved in three of 20 dogs treated.
The engineering of monoclonal antibodies to enhance the immune system's attack on cancer cells has dramatically expanded therapeutic options for multiple hematopoietic and solid tumor histologies, and currently over a dozen antibodies have received FDA approval for treating different cancers (252). Upon binding to their cognate epitope, monoclonal antibodies exert anticancer activities through various methods including direct cell killing, immune-mediated cell killing (phagocytosis, complement activation, or antibody-dependent cellular cytotoxicity), or disruption of the tumor microenvironment through vascular and stromal cell ablation. While widely instituted and capable of dramatically improving survival outcomes in patients diagnosed with specific forms of hematopoietic cancers, the clinical impact of monoclonal antibodies remains more limited for solid tumors, and almost non-existent in aggressive sarcomas. In part, the restricted application of monoclonal antibodies for OS metastases therapy is driven by the limited expression of extracellular membrane epitopes, as underscored in the study reported by Ebb and colleagues whereby adjuvant trastuzumab (HER2 targeting antibody) combined with chemotherapy failed to improve outcomes in patients diagnosed with metastatic OS (253). Nonetheless, several OS expressing epitopes remain a focus of interest for improving the management of OS metastases, and include GD2, GPNMB, and RANKL (254–257). In particular, GD2 as a surface epitope on OS cells continues to be an actively explored target with several ongoing clinical trials evaluating various anti-GD2 antibody strategies.
In addition to monoclonal antibodies that target OS cells, considerable focus has been on manipulating tumor-specific or tumor microenvironmental (TME) cues with antibody strategies, specifically enhancing the quantity and quality of intratumoral infiltration with immune cell populations through blockade of checkpoint signaling (258). Checkpoint blocking antibodies have been a breakthrough for the management of diverse cancer types including melanoma, non-small cell lung cancer, renal cell carcinoma, bladder cancer, and head and neck cancers. For these collective solid tumors, blockade of immune checkpoints, including CTLA-4, PD-1, and PD-L1, with antibodies have provided life-saving anticancer activities to a subset of patients who would otherwise experience disease progression and death. While several pieces of basic and clinical evidence support the potential benefit of checkpoint inhibition for the treatment of OS metastases including mutational burden, neoantigen load, overexpression of PD-L1 by OS cells and tissue samples, and preclinical activity of checkpoint inhibition in mouse models of metastatic OS (259–262), the clinical activity of single- or combined- checkpoint blockade in treating patients with advanced OS have been largely unfavorable, with objective response rates (CR or PR) ranging from 0 to 5% (NCT011445379, Ipilimumab; NCT02500797 [Alliance A091401], Nivolumab + Ipilimumab; NCT02301039 [SARC028], Pembrolizumab) (263). Given these initial disappointing results, combination therapies have been proposed whereby small molecule agents (Nab-rapamycin, apatinib, axitinib) that target multiple signaling pathways (mTOR, VEGFR1, VEGFR2, PDGFRβ, c-kit) should be combined with checkpoint blockade antibodies in hopes of improving response rates (264).
Tumor vaccines are an active form of immunotherapy in which robust adaptive immunity is developed and cytotoxic T lymphocytes are generated for recognizing and killing cancer cells. The induction of antitumor responses against known or unidentified tumor antigens can be achieved through vaccine strategies inclusive of whole cells, lysates, proteins, DNA, RNA, or peptides. For vaccine strategies to be effective, dendritic cells must present tumor peptides within major histocompatibility complexes, as well as costimulatory signals, to fully activate the effector functions of cytotoxic T lymphocytes. Given the key role of dendritic cells for initiating the activation and proliferation of cytotoxic T lymphocytes with anticancer effector functions, dendritic cell vaccines have been explored in patients with metastatic OS through the conductance of two recent clinical studies (265, 266). Miwa and colleagues evaluated 14 patients with recurrent and/or metastatic OS who were treated with 6 weekly subcutaneous vaccinations with autologous dendritic cells pulsed with autologous tumor lysate, TNFα, and OK-432 (265). While treated patients did demonstrate systemic immune activation represented by increases in circulating INFγ and IL-12, none of the OS patients achieved an objective response to dendritic vaccination alone. These negative findings were consistent with an earlier study in which Himoudi and colleagues evaluated 13 patients with relapsing OS treated with intradermal vaccines of autologous dendritic cells matured with autologous tumor lysate and keyhole limpet hemocyanin (266). While the vaccination protocol was well-tolerated, tumor-specific immune activation assessed by the identification of INFγ secreting T cells (ELISPOT) was only observed in 3 patients, and none of the OS patients experience any measurable reduction in macroscopic tumor burden. In light of these initial disappointing studies with dendritic cell vaccination in OS patients, ongoing efforts are focusing on innovative combinatorial strategies to boost OS cell antigen expression, as well as attenuate the localized immunosuppression associated with the tumor microenvironment.
Recently, a vaccine strategy based upon the intravenous infusion of a genetically modified, live attenuated Listeria monocytogenes which expresses three immunodominant epitopes of HER2 has been evaluated for in patients with HER2-expressing solid tumors (NCT02386501). This vaccine construct (ADXS31-164) was granted Fast Track designation by the FDA for treatment of patients with newly diagnosed, non-metastatic, surgically-resectable OS, and patients will be treated with this vaccine strategy through the Children's Oncology Group consortium. As a predecessor to the pediatric trial, ADXS31-164 was piloted in pet dogs with OS and provided valuable translational information. Mason and colleagues demonstrated that treatment with ADXS31-164 induced HER2-specific immunity in 15/18 dogs and resulted in a significant increase in median disease-free interval (615 days) and median survival (956 days) when compared to a historical control group. Overall survival rates at 1, 2, and 3 years for dogs treated with ADXS31-164 were 78, 61, and 50%, respectively (267).
Adoptive cellular therapies involve the direct administration of either innate (natural killer cells) or adaptive (cytotoxic T lymphocytes) immune effector cell populations that have been genetically manipulated. The infusion of cellular therapies, specifically cytotoxic T lymphocytes into tumor-bearing patients, circumvents the reliance of vaccine strategies to successfully active a large population of tumor-specific effector lymphocytes in the recipient host. For T-cell based strategies, adoptive cell therapies rely upon the genetic engineering of T lymphocytes to either express a known T cell receptor (transgenic TCR) or chimeric antigen receptor (CAR), with both strategies resulting in the production of T cells with defined specificity, which can be MHC-restricted (transgenic TCR) or -unrestricted (CAR). While CAR technology has clinically impacted the management of hematopoietic cancers such as acute lymphoblastic leukemia and diffuse large B-cell lymphoma, the application of CARs for effectively treating OS metastases remains incompletely defined. Recently, the results of a phase I/II clinical study evaluating the tolerability and activity of HER2-CAR T cells were reported by Ahmed and colleagues. In this study, 16 patients with recurrent and/or metastatic OS were treated with escalating intravenous doses of T cells expressing an HER2-specific CAR with CD28ζ signaling domain (268). Treatment with HER2-CAR T cells was tolerable and induced systemic inflammatory responses represented by elevations of circulating IL-8. Additionally, following infusion persistence of HER2-CAR T cells in circulation could be demonstrate, as well as their trafficking to tumor sites. However, the clinical impact of HER2-CAR T cells was marginal, with three OS patients experiencing stable disease lasting 12-15 weeks, and the remainder of patients having disease progression. While initial results with CAR T cells have yet to meaningfully impact outcomes in metastatic OS patients, strong enthusiasm exists for the continued exploration of additional adoptive cell therapies that include anti-GD2 CAR T cells, natural killer cells, transgenic TCR cells, and γδ T cells.
The development and clinical assessment of adoptive cell therapies have been piloted in canine OS, providing valuable preclinical data regarding feasibility and activity. For CAR T cells, HER2 has also been explored as a target for canine OS, and Mata and colleagues reported the successful development of HER2-CAR T cells that killed HER2+ canine OS cell lines in an antigen dependent manner (269). Additionally, combining radiation and immunotherapy has been recently explored in a first-in-dog trial of autologous natural killer (NK) adoptive cell therapy (270). In this study, OS-bearing dogs were treated with a coarsely fractionated radiation protocol consisting of 9 Gy once weekly for 4 treatments, with NK cells being harvested and expanded ex vivo, and then delivered back to dogs by intratumoral injection following the completion of radiation therapy. Of the 10 dogs treated, 5 remained metastasis-free at 6 months, and one had regression of a suspicious pulmonary nodule detected at the time of diagnosis. While preliminary in nature, these studies in canine OS provide the underpinnings to prospectively evaluate different combinatorial strategies inclusive of adoptive cell therapies for combating OS metastatic progression.
In order for animal models of human pathologies to be useful and informative, it is necessary for the experimental system to be readily available and accurately recapitulate the natural course of disease. For OS, where the genetic underpinnings are chaotic and the etiopathogenesis remains elusive, the creation of experimental model systems becomes fundamentally difficult, and potentially flawed; as the blueprint of models are derived from and constructed upon current understandings of disease processes. Pet dogs that spontaneously develop OS have potential to serve as excellent naturally-occurring models for diverse comparative pathologies, including OS. Through collaborative research efforts, canine OS can be uniquely positioned in the scientific discovery pathway for understanding metastasis biology, which can drive and accelerate the identification of new promising anti-metastatic therapies. Several characteristics of canine OS are noteworthy and with the continued inclusion and future innovative addition of pet dogs as comparative OS models, it would be expected that significant progress will be made toward improving the outcomes of patients diagnosed with OS metastases.
Pediatric OS, by definition is categorized as a rare tumor (<15 cases per 100,000 people per year). For localized OS, surgery and MAP chemotherapy in pediatric patients produces 5-year event free survival in the majority (60–70%) of patients treated. In this good responder population with favorable histologic Huvos grade, the opportunity to clinically evaluate and realize the anti-metastatic activity of novel therapies is narrow and temporally protracted. While patients with recurrent and/or metastatic OS can be readily included for investigating novel therapies, biologic responses to experimental agents might differ between macro- and microscopic disease settings, and confound the accurate identification of new agents with reproducible anti-metastatic properties.
Canine OS is the most common primary bone tumor in large and giant breed dogs, and has been estimated to affect at least 10,000 pet dogs every year in North America (271), which is a log order greater than the number of pediatric OS patients diagnosed annually in the United States (800–1,000 new cases/year). Conventional treatment with surgery and chemotherapy improves outcomes in affected dogs, producing a median survival time of ~9 months (272). However, even in treated dogs, death as a result of metastatic progression occurs in 85–90% of patients within 2-years of diagnosis. While these statistics for canine OS are sobering, when viewed through the lens of comparative oncology, pet dogs offer an unprecedented opportunity to be included in the evaluation and translation of new anti-metastatic agents. Ultimately, through their purposeful inclusion in drug assessment, pet dogs can accelerate the identification of new agents which hold promise to improve long term outcomes in both humans and canines diagnosed with metastatic OS.
Despite their accepted research value, some limitations of murine models remain irreconcilable including the >103 difference in anatomic size between mice (20 g) and humans (70 kg). Specifically for the evaluation of novel drugs or drug delivery strategies for combating OS metastatic progression, differences in anatomic size and corresponding dimensions of metastatic lesions can strongly bias treatment outcomes simply as a function of tumor volume and interstitial pressures, as therapeutics released into the pulmonary parenchyma are still governed by the physical laws of diffusion prior to reaching their intended targets, being OS metastatic foci. Fick's law of diffusion states that the rate of movement (mass flux) can be modeled mathematically by the following equation:
Where J represents mass flux, D represents molecular diffusivity of a specific therapeutic agent within a microenvironment, ΔC represents the change in concentration gradients, and Δx represents distance of diffusion. Although several determinants influence mass flux J (representing the movement of therapeutic agents), diffusional distances within a chosen experimental system directly affects the movement rates of therapeutics, thereby having the potential to either positively (small anatomic size and limited diffusion distances) or negatively (large anatomic size and expansive diffusion distances) bias treatment outcomes. As such, drugs that demonstrate potent activity in murine models of OS metastasis can be partially attributed to the diminutive diffusion distances required to be traversed by therapeutic agents. However, when translated to larger mammals including human or canine OS patients, where diffusion distances are log orders greater, achieving therapeutic response becomes more difficult (158), if not impossible as governed by the laws of diffusion. Governed by physical laws of diffusion, studies investigating novel therapies for OS metastases would be most predictive when evaluated in tumor model systems which most closely approximate anatomic sizes at both the organism (human) and target (OS metastases) levels. Under these assumptions, dogs with OS can serve as excellent comparative models for pediatric OS given comparable size in body mass and metastatic tumor burdens (Figures 5A–D) as occurs in both adolescents and giant breed dogs.
Figure 5. Radiologic assessment and relevance of comparable anatomic size and metabolic activity of OS tumors arising in pet dogs. (A) Early detection of emerging pulmonary metastatic lesion (red arrowhead) with (B) subsequent rapid macroscopic growth (red arrowhead) over a period of 8 weeks documented by serial CT imaging. Metabolic activity of (C) primary bone OS (Image courtesy of Kim Selting, UIUC) and (D) pulmonary metastases (Image courtesy of Lynn Griffin, Colorado State University) using PET/CT imaging in pet dogs with OS. UIUC, University of Illinois at Urbana-Champaign.
The natural development of cancer is dynamic, giving rise to heterogenous cell populations that in aggregate form solid tumors. Critically contributing to tumor mass evolution are stromal cells within the tumor microenvironment. While specific cell populations like fibroblasts and endothelial cells within the microenvironment can be modeled reasonably well in more sophisticated experimental systems, including mouse models and engineered biomimetics, it remains challenging to recapitulate the dynamic and heterogeneous processes of immune surveillance and editing through existing model systems. With the overwhelming focus on expanding immunotherapeutic strategies for treating various forms of cancer, the inclusion of a model system that firmly mimics immune interactions between cancer and immune effector cells remains of highest priority.
Given the abundance of scientific and clinical evidence supporting OS to be immunogenic in humans and dogs (273, 274), recent investigations have forged new ground which clearly demonstrate the conserved immunologic signatures and phenotypes shared between canine and human OS (275); and these correlative investigations underscore the unique and high valued information that might be gleaned from pet dogs in regards to systemic and immune microenvironment signatures that can be targeted and manipulated to thwart OS progression and metastasis.
A few salient examples of conserved immune targets include Foxp3 regulatory T cells, tumor-infiltrating macrophages, and tumoral PD-L1 expressions. In dogs with OS, the participation and prognostic value of regulatory T cells in systemic and tumor microenvironmental immunosuppression has been characterized. Biller and colleagues reported that a decreased CD8+/Treg ratio was associated with significantly shorter survival times in dogs with OS (276, 277), and these observations in dogs corroborate findings identified in human OS patient samples for the prognostic value of effector/suppressor T cell ratios in predicting long-term outcomes (234). Recently, the significance of tumor-infiltrating macrophages within primary OS lesions have been studied in both dogs and humans. Withers and colleagues characterized the innate and adaptive immune infiltrates within primary OS lesions from 30 dogs and correlated these findings with disease-free interval (278). In this study, the magnitude (dichotomous cutoff of 4.7%) of tumor infiltrating macrophages identified within the primary tumor correlated with survival time, suggesting that macrophages may play an integral role for inhibiting OS metastatic progression. These findings in pet dogs closely mirror some investigations in human OS patients, whereby tumor-infiltrating macrophages were also associated with reduced metastasis and improved survival in patients with high-grade OS (226). Lastly, PD-L1 expressions, which serve as a therapeutic target for checkpoint inhibitor strategies, have been studied in both canine and human OS samples. In canine OS primary samples, Maekawa and colleagues characterized PD-L1 expression across various canine tumor histologies and identified seven out of 10 OS samples to stain positively for PD-L1 (279). Comparatively, the expression of PD-L1 in human OS tissue samples appears more restricted (229), with potential enriched expressions in relapsed or metastatic lesions compared to primary tumors (236, 237). In aggregate, the immune signatures shared between canine and human OS are highly comparable, and provides a rational scientific foundation to leverage pet dogs for evaluating novel immune-based therapies against OS metastases.
Compared to the state of metastasis research in other types of cancer such as breast (~7,703 entries on PubMed) and prostate (~2,653 entries), basic metastasis research in OS (~860 entries) is taking its first furtive steps. Discovering new and critical processes that contribute to OS metastasis biology will pave the way for the development of novel anti-metastatic therapies that can be integrated in the current standard of care. Paradigm shifts in animal research should include a focus on lung colonization, clearing or halting the progression of micrometastases, and shrinkage of established metastases as being translational targets, as recognized and supported by leaders in the OS field (3, 8). Tumor cell dormancy in OS is also an area of much needed research since dormant tumor cells are thought of as a reservoir of future tumor recurrence (280, 281). Funding initiatives that focus on metastasis biology discovery, “omic” approaches in finding actionable targets involved in the metastatic cascade, or re-purposing of existing clinical drugs and assessing their anti-metastatic activity is sorely needed. Such experimental approaches require investment in more animal work, specialized equipment, and skilled personnel. Lastly, shifting the research landscape to include more metastasis-focused initiatives requires a voice from patient advocates, who are an integral part of research funding.
Fueled by increasing commitments of resources, veterinary oncology collaborative groups have rapidly become more organized, more agile, and more capable of efficiently conducting high-value pilot studies, complex biology studies, and large clinical trials in client-owned pets that develop cancer. The Comparative Oncology Trials Consortium, headquartered at NIH, has undertaken several initiatives to broaden the comparative biology of human and canine cancers and to improve the efficiency with which they can generate pharmacokinetic, pharmacodynamic, and initial efficacy data in canine clinical investigations that can guide human trial development. Recent initiatives arising from the NIH Cancer Moonshot program have recognized opportunities for incorporating canine clinical trials into the evaluation of immune-oncology approaches by funding several large grants and spawning new comparative immuno-oncology consortia.
Intelligent use of the data arising from this work should improve the likelihood of good outcomes in human clinical trials. Coordination across canine clinical consortia, basic scientists, and industry has become increasingly important. The ability to share data and to plan collaboratively has been enhanced by integration of veterinary oncologists into pediatric clinical trials groups and vice-versa. Efforts led by a handful of philanthropic groups to intensify dialog between these stakeholders have met with increasing interest and engagement. These achievements to date represent only initial forays into truly integrated drug development and science—with opportunity far surpassing the current actuality.
With continued and growing interest of the scientific community for including spontaneous tumor models to accelerate novel drug development, comparative oncology centers of excellence must keep pace and commensurately grow to meet the expectations of an ever increasing desire for executing high-value clinical trials in an agile and efficient manner. To achieve these deliverables, existing comparative oncology centers must expand capacity, and new centers must develop. Incentivization for such development can be achieved through different mechanisms including active participation in Clinical and Translational Science Awards (CTSA) program and integration with existing Basic or Comprehensive Cancer Centers.
Central coordination and unification across participating comparative oncology centers should be strongly advocated, thereby increasing the efficiency and impact of ongoing and future prospective clinical trials. Regional consortia operating in silos will not likely maximize collaborative efforts, and have potential to dilute limited shared resources and manpower. Visionary and inclusive leadership in this arena is necessary, and will ensure that all participating teams can be maximally and directionally aligned for the greatest translational impact as possible.
Cancer metastasis remains the leading cause of mortality for people afflicted with diverse solid tumor histologies. While tremendous advances in therapy have been achieved over the past decade for some tumor types, the management and outcomes of aggressive sarcoma metastases, including OS, remains almost at a standstill. To impact the lives of patients suffering from OS metastases, it will be necessary to deepen our fundamental understanding of OS metastasis and its specific vulnerabilities at both the cellular and microenvironmental levels. Additionally, the translation of new and promising therapeutic discoveries must be evaluated using complementary model systems that faithfully recapitulate natural metastatic disease progression in people. Given the conserved biology of OS in humans and dogs, unique opportunities exist for human and comparative oncology researchers to engage in translationally impactful collaborations, which uniquely include pet dogs with OS to expand the understanding of metastasis biology and clinically realize the activity of novel investigational therapeutics that target OS metastatic progression.
All animal experiments were done with ethics approval of the local Animal Care Committees.
TF, RR, and ML: project conception and manuscript authorship.
A portion of results in this review article were supported by funding from the following agencies: TF: NIH R01-CA120439, Morris Animal Foundation D09CA-083, D14CA-035, and D19CA-064. RR: supported by NIH K08-CA201638 and St. Baldrick's Scholar award. ML: supported by the BC Cancer Foundation.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Fluorescent images of tumor spheroids were generated using the Core Facilities at the Carl R. Woese Institute for Genomic Biology. Bioluminescent imaging of mouse OS models and micro-CT scans, as well as scanning electron images of homotypic and heterotypic interactions of tumor cells were generated in the Beckman Institute Imaging Technology Group and Biomedical Imaging Center at the University of Illinois at Urbana-Champaign. Development and maintenance of GBM PDX models was supported by Mayo Clinic, the Mayo SPORE in Brain Cancer, and the Mayo Clinic Brain Tumor Patient-Derived xenograft national resource.
1. Lindsey BA, Markel JE, Kleinerman ES. Osteosarcoma overview. Rheumatol Therapy. (2017) 4:25–43. doi: 10.1007/s40744-016-0050-2
2. Geller DS, Gorlick R. Osteosarcoma: a review of diagnosis, management, and treatment strategies. Clin Adv Hematol Oncol. (2010) 8:705–18.
4. Fuchs N, Bielack SS, Epler D, Bieling P, Delling G, Korholz D, et al. Long-term results of the co-operative German-Austrian-Swiss osteosarcoma study group's protocol COSS-86 of intensive multidrug chemotherapy and surgery for osteosarcoma of the limbs. Ann Oncol. (1998) 9:893–9. doi: 10.1023/A:1008391103132
5. Marina NM, Pratt CB, Rao BN, Shema SJ, Meyer WH. Improved prognosis of children with osteosarcoma metastatic to the lung(s) at the time of diagnosis. Cancer. (1992) 70:2722–7.
6. He H, Ni J, Huang J. Molecular mechanisms of chemoresistance in osteosarcoma (Review). Oncol Lett. (2014) 7:1352–62. doi: 10.3892/ol.2014.1935
7. Longhi A, Errani C, De Paolis M, Mercuri M, Bacci G. Primary bone osteosarcoma in the pediatric age: state of the art. Cancer Treat Rev. (2006) 32:423–36. doi: 10.1016/j.ctrv.2006.05.005
8. Khanna C, Fan TM, Gorlick R, Helman LJ, Kleinerman ES, Adamson PC, et al. Toward a drug development path that targets metastatic progression in osteosarcoma. Clin Cancer Res. (2014) 20:4200–9. doi: 10.1158/1078-0432.CCR-13-2574
9. Anderson RL, Balasas T, Callaghan J, Coombes RC, Evans J, Hall JA, et al. A framework for the development of effective anti-metastatic agents. Nat Rev Clin Oncol. (2019) 16:185–204. doi: 10.1038/s41571-018-0134-8
10. Link MP, Goorin AM, Miser AW, Green AA, Pratt CB, Belasco JB, et al. The effect of adjuvant chemotherapy on relapse-free survival in patients with osteosarcoma of the extremity. New Engl J Med. (1986) 314:1600–6. doi: 10.1056/NEJM198606193142502
11. Miao YR, Eckhardt BL, Cao Y, Pasqualini R, Argani P, Arap W, et al. Inhibition of established micrometastases by targeted drug delivery via cell surface-associated GRP78. Clin Cancer Res. (2013) 19:2107–16. doi: 10.1158/1078-0432.CCR-12-2991
12. Lizardo MM, Morrow JJ, Miller TE, Hong ES, Ren L, Mendoza A, et al. Upregulation of glucose-regulated protein 78 in metastatic cancer cells is necessary for lung metastasis progression. Neoplasia. (2016) 18:699–710. doi: 10.1016/j.neo.2016.09.001
13. Chen L, Bi S, Hou J, Zhao Z, Wang C, Xie S. Targeting p21-activated kinase 1 inhibits growth and metastasis via Raf1/MEK1/ERK signaling in esophageal squamous cell carcinoma cells. Cell Commun Signal. (2019) 17:31. doi: 10.1186/s12964-019-0343-5
14. Ingangi V, Bifulco K, Yousif AM, Ragone C, Motti ML, Rea D, et al. The urokinase receptor-derived cyclic peptide [SRSRY] suppresses neovascularization and intravasation of osteosarcoma and chondrosarcoma cells. Oncotarget. (2016) 7:54474–87. doi: 10.18632/oncotarget.9976
15. D'Amato NC, Rogers TJ, Gordon MA, Greene LI, Cochrane DR, Spoelstra NS, et al. A TDO2-AhR signaling axis facilitates anoikis resistance and metastasis in triple-negative breast cancer. Cancer Res. (2015) 75:4651–64. doi: 10.1158/0008-5472.CAN-15-2011
16. Zhang Y, Yang M, Ji Q, Fan D, Peng H, Yang C, et al. Anoikis induction and metastasis suppression by a new integrin αvβ3 inhibitor in human melanoma cell line M21. Invest New Drugs. (2011) 29:666–73. doi: 10.1007/s10637-010-9616-y
17. Wieland E, Rodriguez-Vita J, Liebler SS, Mogler C, Moll I, Herberich SE, et al. Endothelial notch1 activity facilitates metastasis. Cancer Cell. (2017) 31:355–67. doi: 10.1016/j.ccell.2017.01.007
18. Yao H, Veine DM, Livant DL. Therapeutic inhibition of breast cancer bone metastasis progression and lung colonization: breaking the vicious cycle by targeting alpha5beta1 integrin. Breast Cancer Res Treat. (2016) 157:489–501. doi: 10.1007/s10549-016-3844-6
19. Roblek M, Calin M, Schlesinger M, Stan D, Zeisig R, Simionescu M, et al. Targeted delivery of CCR2 antagonist to activated pulmonary endothelium prevents metastasis. J Control Release. (2015) 220(Pt A):341–7. doi: 10.1016/j.jconrel.2015.10.055
20. Bayles I, Krajewska M, Pontius WD, Saiakhova A, Morrow JJ, Bartels C, et al. Ex vivo screen identifies CDK12 as a metastatic vulnerability in osteosarcoma. J Clin Invest. (2019) 129:4377–92. doi: 10.1172/JCI127718
21. Morrow JJ, Bayles I, Funnell APW, Miller TE, Saiakhova A, Lizardo MM, et al. Positively selected enhancer elements endow osteosarcoma cells with metastatic competence. Nat Med. (2018) 24:176–85. doi: 10.1038/nm.4475
22. Morrow JJ, Mendoza A, Koyen A, Lizardo MM, Ren L, Waybright TJ, et al. mTOR inhibition mitigates enhanced mRNA translation associated with the metastatic phenotype of osteosarcoma cells in vivo. Clin Cancer Res. (2016) 22:6129–41. doi: 10.1158/1078-0432.CCR-16-0326
23. Bulut G, Hong SH, Chen K, Beauchamp EM, Rahim S, Kosturko GW, et al. Small molecule inhibitors of ezrin inhibit the invasive phenotype of osteosarcoma cells. Oncogene. (2012) 31:269–81. doi: 10.1038/onc.2011.245
24. Rao-Bindal K, Koshkina NV, Stewart J, Kleinerman ES. The histone deacetylase inhibitor, MS-275 (entinostat), downregulates c-FLIP, sensitizes osteosarcoma cells to FasL, and induces the regression of osteosarcoma lung metastases. Curr Cancer Drug Targets. (2013) 13:411–22. doi: 10.2174/1568009611313040005
25. Hong SH, Ren L, Mendoza A, Eleswarapu A, Khanna C. Apoptosis resistance and PKC signaling: distinguishing features of high and low metastatic cells. Neoplasia. (2012) 14:249–58. doi: 10.1593/neo.111498
26. Gross AC, Cam H, Phelps DA, Saraf AJ, Bid HK, Cam M, et al. IL-6 and CXCL8 mediate osteosarcoma-lung interactions critical to metastasis. JCI Insight. (2018) 3:e99791. doi: 10.1172/jci.insight.99791
27. Ohs I, Ducimetiere L, Marinho J, Kulig P, Becher B, Tugues S. Restoration of natural killer cell antimetastatic activity by IL12 and checkpoint blockade. Cancer Res. (2017) 77:7059–71. doi: 10.1158/0008-5472.CAN-17-1032
28. Dhupkar P, Gordon N, Stewart J, Kleinerman ES. Anti-PD-1 therapy redirects macrophages from an M2 to an M1 phenotype inducing regression of OS lung metastases. Cancer Med. (2018) 7:2654–64. doi: 10.1002/cam4.1518
29. Botham RC, Roth HS, Book AP, Roady PJ, Fan TM, Hergenrother PJ. Small-molecule procaspase-3 activation sensitizes cancer to treatment with diverse chemotherapeutics. ACS Cent Sci. (2016) 2:545–59. doi: 10.1021/acscentsci.6b00165
30. Weiss L. Metastatic inefficiency. Adv Cancer Res. (1990) 54:159–211. doi: 10.1016/S0065-230X(08)60811-8
31. Fidler IJ. Metastasis: quantitative analysis of distribution and fate of tumor emboli labeled with 125 I-5-iodo-2'-deoxyuridine. J Natl Cancer Inst. (1970) 45:773–82.
32. Cameron MD, Schmidt EE, Kerkvliet N, Nadkarni KV, Morris VL, Groom AC, et al. Temporal progression of metastasis in lung: cell survival, dormancy, and location dependence of metastatic inefficiency. Cancer Res. (2000) 60:2541–6.
33. Kane SE, Gottesman MM. The role of cathepsin L in malignant transformation. Semin Cancer Biol. (1990) 1:127–36.
34. Waresijiang N, Sun J, Abuduaini R, Jiang T, Zhou W, Yuan H. The downregulation of miR125a5p functions as a tumor suppressor by directly targeting MMP11 in osteosarcoma. Mol Med Rep. (2016) 13:4859–64. doi: 10.3892/mmr.2016.5141
35. Chen CL, Zhang L, Jiao YR, Zhou Y, Ge QF, Li PC, et al. miR-134 inhibits osteosarcoma cell invasion and metastasis through targeting MMP1 and MMP3 in vitro and in vivo. FEBS Lett. (2019) 593:1089–101. doi: 10.1002/1873-3468.13387
36. Han XG, Li Y, Mo HM, Li K, Lin D, Zhao CQ, et al. TIMP3 regulates osteosarcoma cell migration, invasion, and chemotherapeutic resistances. Tumour Biol. (2016) 37:8857–67. doi: 10.1007/s13277-015-4757-4
37. Xu WT, Bian ZY, Fan QM, Li G, Tang TT. Human mesenchymal stem cells (hMSCs) target osteosarcoma and promote its growth and pulmonary metastasis. Cancer Lett. (2009) 281:32–41. doi: 10.1016/j.canlet.2009.02.022
38. Liao YY, Tsai HC, Chou PY, Wang SW, Chen HT, Lin YM, et al. CCL3 promotes angiogenesis by dysregulation of miR-374b/ VEGF-A axis in human osteosarcoma cells. Oncotarget. (2016) 7:4310–25. doi: 10.18632/oncotarget.6708
39. Zeng C, Wen M, Liu X. Fibroblast activation protein in osteosarcoma cells promotes angiogenesis via AKT and ERK signaling pathways. Oncol Lett. (2018) 15:6029–35. doi: 10.3892/ol.2018.8027
40. Fernandez L, Valentin J, Zalacain M, Leung W, Patino-Garcia A, Perez-Martinez A. Activated and expanded natural killer cells target osteosarcoma tumor initiating cells in an NKG2D-NKG2DL dependent manner. Cancer Lett. (2015) 368:54–63. doi: 10.1016/j.canlet.2015.07.042
41. Kawano M, Tanaka K, Itonaga I, Iwasaki T, Miyazaki M, Ikeda S, et al. Dendritic cells combined with anti-GITR antibody produce antitumor effects in osteosarcoma. Oncol Rep. (2015) 34:1995–2001. doi: 10.3892/or.2015.4161
42. van Moorst M, Dass CR. Methods for co-culturing tumour and endothelial cells: systems and their applications. J Pharm Pharmacol. (2011) 63:1513–21. doi: 10.1111/j.2042-7158.2011.01352.x
43. Villanueva F, Araya H, Briceno P, Varela N, Stevenson A, Jerez S, et al. The cancer-related transcription factor RUNX2 modulates expression and secretion of the matricellular protein osteopontin in osteosarcoma cells to promote adhesion to endothelial pulmonary cells and lung metastasis. J Cell Physiol. (2019) 234:13659–79. doi: 10.1002/jcp.28046
44. Duan X, Jia SF, Zhou Z, Langley RR, Bolontrade MF, Kleinerman ES. Association of alphavbeta3 integrin expression with the metastatic potential and migratory and chemotactic ability of human osteosarcoma cells. Clin Exp Metastasis. (2004) 21:747–53. doi: 10.1007/s10585-005-0599-6
45. Frisch SM, Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol. (1994) 124:619–26. doi: 10.1083/jcb.124.4.619
46. Tan K, Goldstein D, Crowe P, Yang JL. Uncovering a key to the process of metastasis in human cancers: a review of critical regulators of anoikis. J Cancer Res Clin Oncol. (2013) 139:1795–805. doi: 10.1007/s00432-013-1482-5
47. Young AI, Law AM, Castillo L, Chong S, Cullen HD, Koehler M, et al. MCL-1 inhibition provides a new way to suppress breast cancer metastasis and increase sensitivity to dasatinib. Breast Cancer Res. (2016) 18:125. doi: 10.1186/s13058-016-0781-6
48. Sun T, Zhong X, Song H, Liu J, Li J, Leung F, et al. Anoikis resistant mediated by FASN promoted growth and metastasis of osteosarcoma. Cell Death Dis. (2019) 10:298. doi: 10.1038/s41419-019-1532-2
49. Zhao GS, Zhang Q, Cao Y, Wang Y, Lv YF, Zhang ZS, et al. High expression of ID1 facilitates metastasis in human osteosarcoma by regulating the sensitivity of anoikis via PI3K/AKT depended suppression of the intrinsic apoptotic signaling pathway. Am J Transl Res. (2019) 11:2117–39.
50. Weiss L, Dimitrov DS. A fluid mechanical analysis of the velocity, adhesion, and destruction of cancer cells in capillaries during metastasis. Cell Biophys. (1984) 6:9–22. doi: 10.1007/BF02788577
51. Wirtz D, Konstantopoulos K, Searson PC. The physics of cancer: the role of physical interactions and mechanical forces in metastasis. Nat Rev Cancer. (2011) 11:512–22. doi: 10.1038/nrc3080
52. Huang Q, Hu X, He W, Zhao Y, Hao S, Wu Q, et al. Fluid shear stress and tumor metastasis. Am J Cancer Res. (2018) 8:763–77.
53. Lien SC, Chang SF, Lee PL, Wei SY, Chang MD, Chang JY, et al. Mechanical regulation of cancer cell apoptosis and autophagy: roles of bone morphogenetic protein receptor, Smad1/5, and p38 MAPK. Biochim Biophys Acta. (2013) 1833:3124–33. doi: 10.1016/j.bbamcr.2013.08.023
54. Ren L, Mendoza A, Zhu J, Briggs JW, Halsey C, Hong ES, et al. Characterization of the metastatic phenotype of a panel of established osteosarcoma cells. Oncotarget. (2015) 6:29469–81. doi: 10.18632/oncotarget.5177
55. Goodison S, Kawai K, Hihara J, Jiang P, Yang M, Urquidi V, et al. Prolonged dormancy and site-specific growth potential of cancer cells spontaneously disseminated from nonmetastatic breast tumors as revealed by labeling with green fluorescent protein. Clin Cancer Res. (2003) 9(10 Pt 1):3808–14.
56. Suzuki M, Mose ES, Montel V, Tarin D. Dormant cancer cells retrieved from metastasis-free organs regain tumorigenic and metastatic potency. Am J Pathol. (2006) 169:673–81. doi: 10.2353/ajpath.2006.060053
57. Wong CW, Lee A, Shientag L, Yu J, Dong Y, Kao G, et al. Apoptosis: an early event in metastatic inefficiency. Cancer Res. (2001) 61:333–8.
58. Qiu H, Orr FW, Jensen D, Wang HH, McIntosh AR, Hasinoff BB, et al. Arrest of B16 melanoma cells in the mouse pulmonary microcirculation induces endothelial nitric oxide synthase-dependent nitric oxide release that is cytotoxic to the tumor cells. Am J Pathol. (2003) 162:403–12. doi: 10.1016/S0002-9440(10)63835-7
59. Holmgren L, O'Reilly MS, Folkman J. Dormancy of micrometastases: balanced proliferation and apoptosis in the presence of angiogenesis suppression. Nat Med. (1995) 1:149–53. doi: 10.1038/nm0295-149
60. Almog N, Ma L, Raychowdhury R, Schwager C, Erber R, Short S, et al. Transcriptional switch of dormant tumors to fast-growing angiogenic phenotype. Cancer Res. (2009) 69:836–44. doi: 10.1158/0008-5472.CAN-08-2590
61. Piskounova E, Agathocleous M, Murphy MM, Hu Z, Huddlestun SE, Zhao Z, et al. Oxidative stress inhibits distant metastasis by human melanoma cells. Nature. (2015) 527:186–91. doi: 10.1038/nature15726
62. Xiao W, Wang RS, Handy DE, Loscalzo J. NAD(H) and NADP(H) redox couples and cellular energy metabolism. Antioxid Redox Signal. (2018) 28:251–72. doi: 10.1089/ars.2017.7216
63. Basnet H, Tian L, Ganesh K, Huang YH, Macalinao DG, Brogi E, et al. Flura-seq identifies organ-specific metabolic adaptations during early metastatic colonization. Elife. (2019) 8:e43627. doi: 10.7554/eLife.43627.037
64. Ferraro D, Corso S, Fasano E, Panieri E, Santangelo R, Borrello S, et al. Pro-metastatic signaling by c-Met through RAC-1 and reactive oxygen species (ROS). Oncogene. (2006) 25:3689–98. doi: 10.1038/sj.onc.1209409
65. Ishikawa K, Takenaga K, Akimoto M, Koshikawa N, Yamaguchi A, Imanishi H, et al. ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis. Science. (2008) 320:661–4. doi: 10.1126/science.1156906
66. Gill JG, Piskounova E, Morrison SJ. Cancer, oxidative stress, and metastasis. Cold Spring Harb Symp Quant Biol. (2016) 81:163–75. doi: 10.1101/sqb.2016.81.030791
67. Szegezdi E, Logue SE, Gorman AM, Samali A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. (2006) 7:880–5. doi: 10.1038/sj.embor.7400779
68. Townsend DM. S-glutathionylation: indicator of cell stress and regulator of the unfolded protein response. Mol Interv. (2007) 7:313–24. doi: 10.1124/mi.7.6.7
69. Ojha R, Amaravadi RK. Targeting the unfolded protein response in cancer. Pharmacol Res. (2017) 120:258–66. doi: 10.1016/j.phrs.2017.04.003
70. Chaiyawat P, Sungngam P, Teeyakasem P, Sirikaew N, Klangjorhor J, Settakorn J, et al. Protein profiling of osteosarcoma tissue and soft callus unveils activation of the unfolded protein response pathway. Int J Oncol. (2019) 54:1704–18. doi: 10.3892/ijo.2019.4737
71. Burris HA, Bakewell S, Bendell JC, Infante J, Jones SF, Spigel DR, et al. Safety and activity of IT-139, a ruthenium-based compound, in patients with advanced solid tumours: a first-in-human, open-label, dose-escalation phase I study with expansion cohort. ESMO Open. (2016) 1:e000154. doi: 10.1136/esmoopen-2016-000154
72. Yarapureddy S, Abril J, Foote J, Kumar S, Asad O, Sharath V, et al. ATF6alpha activation enhances survival against chemotherapy and serves as a prognostic indicator in osteosarcoma. Neoplasia. (2019) 21:516–32. doi: 10.1016/j.neo.2019.02.004
73. Zhang R, Thamm DH, Misra V. The effect of Zhangfei/CREBZF on cell growth, differentiation, apoptosis, migration, and the unfolded protein response in several canine osteosarcoma cell lines. BMC Vet Res. (2015) 11:22. doi: 10.1186/s12917-015-0331-y
74. Yan M, Ni J, Song D, Ding M, Huang J. Activation of unfolded protein response protects osteosarcoma cells from cisplatin-induced apoptosis through NF-kappaB pathway. Int J Clin Exp Pathol. (2015) 8:10204–15.
75. Asling J, Morrison J, Mutsaers AJ. Targeting HSP70 and GRP78 in canine osteosarcoma cells in combination with doxorubicin chemotherapy. Cell Stress Chaperon. (2016) 21:1065–76. doi: 10.1007/s12192-016-0730-4
76. Weibel ER. Lung morphometry: the link between structure and function. Cell Tissue Res. (2017) 367:413–26. doi: 10.1007/s00441-016-2541-4
77. Huret J-L, Ahmad M, Arsaban M, Bernheim A, Cigna J, Desangles F, et al. Atlas of genetics and cytogenetics in oncology and haematology in 2013. Nucleic Acids Res. (2012) 41:D920–D4. doi: 10.1093/nar/gks1082
78. Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. (2002) 2:563–72. doi: 10.1038/nrc865
79. Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. (2005) 438:820–7. doi: 10.1038/nature04186
80. Hiratsuka S, Watanabe A, Aburatani H, Maru Y. Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat Cell Biol. (2006) 8:1369–75. doi: 10.1038/ncb1507
81. Psaila B, Lyden D. The metastatic niche: adapting the foreign soil. Nat Rev Cancer. (2009) 9:285–93. doi: 10.1038/nrc2621
82. Murgai M, Ju W, Eason M, Kline J, Beury DW, Kaczanowska S, et al. KLF4-dependent perivascular cell plasticity mediates pre-metastatic niche formation and metastasis. Nat Med. (2017) 23:1176–90. doi: 10.1038/nm.4400
83. Macklin R, Wang H, Loo D, Martin S, Cumming A, Cai N, et al. Extracellular vesicles secreted by highly metastatic clonal variants of osteosarcoma preferentially localize to the lungs and induce metastatic behaviour in poorly metastatic clones. Oncotarget. (2016) 7:43570–87. doi: 10.18632/oncotarget.9781
84. Ekert JE, Johnson K, Strake B, Pardinas J, Jarantow S, Perkinson R, et al. Three-dimensional lung tumor microenvironment modulates therapeutic compound responsiveness in vitro – implication for drug development. PLoS ONE. (2014) 9:e92248. doi: 10.1371/journal.pone.0092248
85. Luca AC, Mersch S, Deenen R, Schmidt S, Messner I, Schäfer K-L, et al. Impact of the 3D microenvironment on phenotype, gene expression, and EGFR inhibition of colorectal cancer cell lines. PLoS ONE. (2013) 8:e59689. doi: 10.1371/journal.pone.0059689
86. Pickl M, Ries CH. Comparison of 3D and 2D tumor models reveals enhanced HER2 activation in 3D associated with an increased response to trastuzumab. Oncogene. (2008) 28:461. doi: 10.1038/onc.2008.394
87. Ribatti D. The chick embryo chorioallantoic membrane (CAM) assay. Reprod Toxicol. (2017) 70:97–101. doi: 10.1016/j.reprotox.2016.11.004
88. Lizardo MM, Sorensen PH. Practical considerations in studying metastatic lung colonization in osteosarcoma using the pulmonary metastasis assay. J Vis Exp. (2018) 133:1–14. doi: 10.3791/56332
89. Entenberg D, Voiculescu S, Guo P, Borriello L, Wang Y, Karagiannis GS, et al. A permanent window for the murine lung enables high-resolution imaging of cancer metastasis. Nat Methods. (2018) 15:73–80. doi: 10.1038/nmeth.4511
90. Mangir N, Raza A, Haycock JW, Chapple C, Macneil S. An improved in vivo methodology to visualise tumour induced changes in vasculature using the chick chorionic allantoic membrane assay. In Vivo. (2018) 32:461–72. doi: 10.21873/invivo.11262
91. Deryugina EI. Chorioallantoic membrane microtumor model to study the mechanisms of tumor angiogenesis, vascular permeability, and tumor cell intravasation. Methods Mol Biol. (2016) 1430:283–98. doi: 10.1007/978-1-4939-3628-1_19
92. Armstrong PB, Quigley JP, Sidebottom E. Transepithelial invasion and intramesenchymal infiltration of the chick embryo chorioallantois by tumor cell lines. Cancer Res. (1982) 42:1826–37.
93. Zhang Y, Thayele Purayil H, Black JB, Fetto F, Lynch LD, Masannat JN, et al. Prostaglandin E2 receptor 4 mediates renal cell carcinoma intravasation and metastasis. Cancer Lett. (2017) 391:50–8. doi: 10.1016/j.canlet.2017.01.007
94. Koop S, Khokha R, Schmidt EE, MacDonald IC, Morris VL, Chambers AF, et al. Overexpression of metalloproteinase inhibitor in B16F10 cells does not affect extravasation but reduces tumor growth. Cancer Res. (1994) 54:4791–7.
95. Deryugina EI, Quigley JP. Chick embryo chorioallantoic membrane model systems to study and visualize human tumor cell metastasis. Histochem Cell Biol. (2008) 130:1119–30. doi: 10.1007/s00418-008-0536-2
96. Kim Y, Williams KC, Gavin CT, Jardine E, Chambers AF, Leong HS. Quantification of cancer cell extravasation in vivo. Nat Prot. (2016) 11:937–48. doi: 10.1038/nprot.2016.050
97. Leong HS, Robertson AE, Stoletov K, Leith SJ, Chin CA, Chien AE, et al. Invadopodia are required for cancer cell extravasation and are a therapeutic target for metastasis. Cell Rep. (2014) 8:1558–70. doi: 10.1016/j.celrep.2014.07.050
98. Williams KC, Cepeda MA, Javed S, Searle K, Parkins KM, Makela AV, et al. Invadopodia are chemosensing protrusions that guide cancer cell extravasation to promote brain tropism in metastasis. Oncogene. (2019) 38:3598–615. doi: 10.1038/s41388-018-0667-4
99. DeBord LC, Pathak RR, Villaneuva M, Liu HC, Harrington DA, Yu W, et al. The chick chorioallantoic membrane (CAM) as a versatile patient-derived xenograft (PDX) platform for precision medicine and preclinical research. Am J Cancer Res. (2018) 8:1642–60.
100. Kunz P, Schenker A, Sahr H, Lehner B, Fellenberg J. Optimization of the chicken chorioallantoic membrane assay as reliable in vivo model for the analysis of osteosarcoma. PLoS One. (2019) 14:e0215312. doi: 10.1371/journal.pone.0215312
101. Manjunathan R, Ragunathan M. Chicken chorioallantoic membrane as a reliable model to evaluate osteosarcoma-an experimental approach using SaOS2 cell line. Biol Proc Online. (2015) 17:10. doi: 10.1186/s12575-015-0022-x
102. Haeckel C, Krueger S, Roessner A. Antisense inhibition of urokinase: effect on malignancy in a human osteosarcoma cell line. Int J Cancer. (1998) 77:153–60. doi: 10.1002/(SICI)1097-0215(19980703)77:1<153::AID-IJC23>3.0.CO;2-E
103. Lokman NA, Elder AS, Ricciardelli C, Oehler MK. Chick chorioallantoic membrane (CAM) assay as an in vivo model to study the effect of newly identified molecules on ovarian cancer invasion and metastasis. Int J Mol Sci. (2012) 13:9959–70. doi: 10.3390/ijms13089959
104. Mendoza A, Hong SH, Osborne T, Khan MA, Campbell K, Briggs J, et al. Modeling metastasis biology and therapy in real time in the mouse lung. J Clin Invest. (2010) 120:2979–88. doi: 10.1172/JCI40252
105. Young ED, Strom K, Tsue AF, Usset JL, MacPherson S, McGuire JT, et al. Automated quantitative image analysis for ex vivo metastasis assays reveals differing lung composition requirements for metastasis suppression by KISS1. Clin Exp Metastasis. (2018) 35:77–86. doi: 10.1007/s10585-018-9882-1
106. Nomura M, Rainusso N, Lee YC, Dawson B, Coarfa C, Han R, et al. Tegavivint and the beta-catenin/ALDH axis in chemotherapy-resistant and metastatic osteosarcoma. J Natl Cancer Inst. (2019) 111:1216–27. doi: 10.1093/jnci/djz026
107. Al-Mehdi AB, Tozawa K, Fisher AB, Shientag L, Lee A, Muschel RJ. Intravascular origin of metastasis from the proliferation of endothelium-attached tumor cells: a new model for metastasis. Nat Med. (2000) 6:100–2. doi: 10.1038/71429
108. Varghese HJ, MacKenzie LT, Groom AC, Ellis CG, Chambers AF, MacDonald IC. Mapping of the functional microcirculation in vital organs using contrast-enhanced in vivo video microscopy. Am J Physiol Heart Circ Physiol. (2005) 288:H185–93. doi: 10.1152/ajpheart.01022.2003
109. James ML, Gambhir SS. A molecular imaging primer: modalities, imaging agents, and applications. Physiol Rev. (2012) 92:897–965. doi: 10.1152/physrev.00049.2010
110. Weissleder R. Scaling down imaging: molecular mapping of cancer in mice. Nat Rev Cancer. (2002) 2:11–8. doi: 10.1038/nrc701
111. Vanharanta S, Massague J. Origins of metastatic traits. Cancer Cell. (2013) 24:410–21. doi: 10.1016/j.ccr.2013.09.007
112. Cam M, Gardner HL, Roberts RD, Fenger JM, Guttridge DC, London CA, et al. DeltaNp63 mediates cellular survival and metastasis in canine osteosarcoma. Oncotarget. (2016) 7:48533–46. doi: 10.18632/oncotarget.10406
113. Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. (2012) 21:309–22. doi: 10.1016/j.ccr.2012.02.022
114. Liu Y, Cao X. Characteristics and significance of the pre-metastatic niche. Cancer Cell. (2016) 30:668–81. doi: 10.1016/j.ccell.2016.09.011
115. Khanna C, Hunter K. Modeling metastasis in vivo. Carcinogenesis. (2005) 26:513–23. doi: 10.1093/carcin/bgh261
116. Guijarro MV, Ghivizzani SC, Gibbs CP. Animal models in osteosarcoma. Front Oncol. (2014) 4:189. doi: 10.3389/fonc.2014.00189
117. Jones KB. Osteosarcomagenesis: modeling cancer initiation in the mouse. Sarcoma. (2011) 2011:694136. doi: 10.1155/2011/694136
118. Gupte A, Baker EK, Wan S-S, Stewart E, Loh A, Shelat AA, et al. Systematic screening identifies dual PI3K and mTOR inhibition as a conserved therapeutic vulnerability in osteosarcoma. Clin Cancer Res. (2015) 21:3216–29. doi: 10.1158/1078-0432.CCR-14-3026
119. Moriarity BS, Otto GM, Rahrmann EP, Rathe SK, Wolf NK, Weg MT, et al. A Sleeping Beauty forward genetic screen identifies new genes and pathways driving osteosarcoma development and metastasis. Nat Genet. (2015) 47:615–24. doi: 10.1038/ng.3293
120. Stewart E, Federico S, Karlstrom A, Shelat A, Sablauer A, Pappo A, et al. The childhood solid tumor network: a new resource for the developmental biology and oncology research communities. Dev Biol. (2016) 411:287–93. doi: 10.1016/j.ydbio.2015.03.001
121. Stewart E, Federico SM, Chen X, Shelat AA, Bradley C, Gordon B, et al. Orthotopic patient-derived xenografts of paediatric solid tumours. Nature. (2017) 549:96–100. doi: 10.1038/nature23647
122. Sayles LC, Breese MR, Koehne AL, Leung SG, Lee AG, Liu HY, et al. Genome-informed targeted therapy for osteosarcoma. Cancer Discov. (2019) 9:46–63. doi: 10.1158/2159-8290.CD-17-1152
123. Goldstein SD, Hayashi M, Albert CM, Jackson KW, Loeb DM. An orthotopic xenograft model with survival hindlimb amputation allows investigation of the effect of tumor microenvironment on sarcoma metastasis. Clin Exp Metastasis. (2015) 32:703–15. doi: 10.1007/s10585-015-9738-x
124. Goldstein SD, Trucco M, Bautista Guzman W, Hayashi M, Loeb DM. A monoclonal antibody against the Wnt signaling inhibitor dickkopf-1 inhibits osteosarcoma metastasis in a preclinical model. Oncotarget. (2016) 7:21114–23. doi: 10.18632/oncotarget.8522
125. Kumar S, Weaver VM. Mechanics, malignancy, and metastasis: the force journey of a tumor cell. Cancer Metastasis Rev. (2009) 28:113–27. doi: 10.1007/s10555-008-9173-4
126. Martino F, Perestrelo AR, Vinarsky V, Pagliari S, Forte G. Cellular mechanotransduction: from tension to function. Front Physiol. (2018) 9:824. doi: 10.3389/fphys.2018.00824
127. Martinac B. Mechanosensitive ion channels: molecules of mechanotransduction. J Cell Sci. (2004) 117(Pt 12):2449–60. doi: 10.1242/jcs.01232
128. Lee J, Abdeen AA, Wycislo KL, Fan TM, Kilian KA. Interfacial geometry dictates cancer cell tumorigenicity. Nat Mater. (2016) 15:856–62. doi: 10.1038/nmat4610
129. Wei SC, Fattet L, Tsai JH, Guo Y, Pai VH, Majeski HE, et al. Matrix stiffness drives epithelial-mesenchymal transition and tumour metastasis through a TWIST1-G3BP2 mechanotransduction pathway. Nat Cell Biol. (2015) 17:678–88. doi: 10.1038/ncb3157
130. Najafi M, Farhood B, Mortezaee K. Extracellular matrix (ECM) stiffness and degradation as cancer drivers. J Cell Biochem. (2019) 120:2782–90. doi: 10.1002/jcb.27681
131. Zhang D, Lee J, Sun MB, Pei Y, Chu J, Gillette MU, et al. Combinatorial discovery of defined substrates that promote a stem cell state in malignant melanoma. ACS Cent Sci. (2017) 3:381–93. doi: 10.1021/acscentsci.6b00329
132. Foty R. A simple hanging drop cell culture protocol for generation of 3D spheroids. J Vis Exp. (2011) 51:1–4. doi: 10.3791/2720
133. Sant S, Johnston PA. The production of 3D tumor spheroids for cancer drug discovery. Drug Discov Today Technol. (2017) 23:27–36. doi: 10.1016/j.ddtec.2017.03.002
134. Perche F, Torchilin VP. Cancer cell spheroids as a model to evaluate chemotherapy protocols. Cancer Biol Ther. (2012) 13:1205–13. doi: 10.4161/cbt.21353
135. De Luca A, Raimondi L, Salamanna F, Carina V, Costa V, Bellavia D, et al. Relevance of 3d culture systems to study osteosarcoma environment. J Exp Clin Cancer Res. (2018) 37:2. doi: 10.1186/s13046-017-0663-5
136. Baek N, Seo OW, Lee J, Hulme J, An SS. Real-time monitoring of cisplatin cytotoxicity on three-dimensional spheroid tumor cells. Drug Des Devel Ther. (2016) 10:2155–65. doi: 10.2147/DDDT.S108004
137. Baek N, Seo OW, Kim M, Hulme J, An SS. Monitoring the effects of doxorubicin on 3D-spheroid tumor cells in real-time. Onco Targets Ther. (2016) 9:7207–18. doi: 10.2147/OTT.S112566
138. Charoen KM, Fallica B, Colson YL, Zaman MH, Grinstaff MW. Embedded multicellular spheroids as a biomimetic 3D cancer model for evaluating drug and drug-device combinations. Biomaterials. (2014) 35:2264–71. doi: 10.1016/j.biomaterials.2013.11.038
139. Martins-Neves SR, Lopes AO, do Carmo A, Paiva AA, Simoes PC, Abrunhosa AJ, et al. Therapeutic implications of an enriched cancer stem-like cell population in a human osteosarcoma cell line. BMC Cancer. (2012) 12:139. doi: 10.1186/1471-2407-12-139
140. Leon IE, Cadavid-Vargas JF, Resasco A, Maschi F, Ayala MA, Carbone C, et al. In vitro and in vivo antitumor effects of the VO-chrysin complex on a new three-dimensional osteosarcoma spheroids model and a xenograft tumor in mice. J Biol Inorg Chem. (2016) 21:1009–20. doi: 10.1007/s00775-016-1397-0
141. Fujii H, Honoki K, Tsujiuchi T, Kido A, Yoshitani K, Takakura Y. Sphere-forming stem-like cell populations with drug resistance in human sarcoma cell lines. Int J Oncol. (2009) 34:1381–6.
142. Fallica B, Maffei JS, Villa S, Makin G, Zaman M. Alteration of cellular behavior and response to PI3K pathway inhibition by culture in 3D collagen gels. PLoS ONE. (2012) 7:e48024. doi: 10.1371/journal.pone.0048024
143. Pang LY, Gatenby EL, Kamida A, Whitelaw BA, Hupp TR, Argyle DJ. Global gene expression analysis of canine osteosarcoma stem cells reveals a novel role for COX-2 in tumour initiation. PLoS ONE. (2014) 9:e83144. doi: 10.1371/journal.pone.0083144
144. Schoeftner S, Scarola M, Comisso E, Schneider C, Benetti R. An Oct4-pRb axis, controlled by MiR-335, integrates stem cell self-renewal and cell cycle control. Stem Cells. (2013) 31:717–28. doi: 10.1002/stem.1315
145. Tanaka T, Yui Y, Naka N, Wakamatsu T, Yoshioka K, Araki N, et al. Dynamic analysis of lung metastasis by mouse osteosarcoma LM8: VEGF is a candidate for anti-metastasis therapy. Clin Exp Metastasis. (2013) 30:369–79. doi: 10.1007/s10585-012-9543-8
146. Elenjord R, Allen JB, Johansen HT, Kildalsen H, Svineng G, Maelandsmo GM, et al. Collagen I regulates matrix metalloproteinase-2 activation in osteosarcoma cells independent of S100A4. FEBS J. (2009) 276:5275–86. doi: 10.1111/j.1742-4658.2009.07223.x
147. Zhang LZ, Mei J, Qian ZK, Cai XS, Jiang Y, Huang WD. The role of VE-cadherin in osteosarcoma cells. Pathol Oncol Res. (2010) 16:111–7. doi: 10.1007/s12253-009-9198-1
148. Fu D, He X, Yang S, Xu W, Lin T, Feng X. Zoledronic acid inhibits vasculogenic mimicry in murine osteosarcoma cell line in vitro. BMC Musculoskelet Disord. (2011) 12:146. doi: 10.1186/1471-2474-12-146
149. Suzuki Y, Nishida Y, Naruse T, Gemba T, Ishiguro N. Pericellular matrix formation alters the efficiency of intracellular uptake of oligonucleotides in osteosarcoma cells. J Surg Res. (2009) 152:148–56. doi: 10.1016/j.jss.2008.02.037
150. Nishimura A, Akeda K, Matsubara T, Kusuzaki K, Matsumine A, Masuda K, et al. Transfection of NF-kappaB decoy oligodeoxynucleotide suppresses pulmonary metastasis by murine osteosarcoma. Cancer Gene Ther. (2011) 18:250–9. doi: 10.1038/cgt.2010.75
151. Guo X, Yu L, Zhang Z, Dai G, Gao T, Guo W. miR-335 negatively regulates osteosarcoma stem cell-like properties by targeting POU5F1. Cancer Cell Int. (2017) 17:29. doi: 10.1186/s12935-017-0398-6
152. Chaddad H, Kuchler-Bopp S, Fuhrmann G, Gegout H, Ubeaud-Sequier G, Schwinte P, et al. Combining 2D angiogenesis and 3D osteosarcoma microtissues to improve vascularization. Exp Cell Res. (2017) 360:138–45. doi: 10.1016/j.yexcr.2017.08.035
153. Park KM, Lewis D, Gerecht S. Bioinspired hydrogels to engineer cancer microenvironments. Annu Rev Biomed Eng. (2017) 19:109–33. doi: 10.1146/annurev-bioeng-071516-044619
154. Geckil H, Xu F, Zhang XH, Moon S, Demirci U. Engineering hydrogels as extracellular matrix mimics. Nanomedicine. (2010) 5:469–84. doi: 10.2217/nnm.10.12
155. Zhang YS, Khademhosseini A. Advances in engineering hydrogels. Science. (2017) 356:aaf3627. doi: 10.1126/science.aaf3627
156. Ali Gumustas S, Isyar M, Topuk S, Yilmaz I, Oznam K, Onay T, et al. Systematic evaluation of drug-loaded hydrogels for application in osteosarcoma treatment. Curr Pharm Biotechnol. (2016) 17:866–72. doi: 10.2174/1389201017666160519113104
157. Ma H, He C, Cheng Y, Yang Z, Zang J, Liu J, et al. Localized co-delivery of doxorubicin, cisplatin, and methotrexate by thermosensitive hydrogels for enhanced osteosarcoma treatment. ACS Appl Mater Interfaces. (2015) 7:27040–8. doi: 10.1021/acsami.5b09112
158. Yin Q, Tang L, Cai K, Tong R, Sternberg R, Yang X, et al. Pamidronate functionalized nanoconjugates for targeted therapy of focal skeletal malignant osteolysis. Proc Natl Acad Sci USA. (2016) 113:E4601–9. doi: 10.1073/pnas.1603316113
159. Jiang T, Zhao J, Yu S, Mao Z, Gao C, Zhu Y, et al. Untangling the response of bone tumor cells and bone forming cells to matrix stiffness and adhesion ligand density by means of hydrogels. Biomaterials. (2019) 188:130–43. doi: 10.1016/j.biomaterials.2018.10.015
160. Jiang T, Xu G, Chen X, Huang X, Zhao J, Zheng L. Impact of Hydrogel Elasticity and Adherence on Osteosarcoma Cells and Osteoblasts. Adv Healthc Mater. (2019) 8:e1801587. doi: 10.1002/adhm.201801587
161. Dey S, Laredj L, Damjanovic K, Muller M, Beard P. Growth of osteosarcoma cells in a three-dimensional bone-like matrix alters their susceptibility to adeno-associated virus. J Gen Virol. (2014) 95(Pt 7):1539–43. doi: 10.1099/vir.0.061945-0
162. Tan PH, Chia SS, Toh SL, Goh JC, Nathan SS. The dominant role of IL-8 as an angiogenic driver in a three-dimensional physiological tumor construct for drug testing. Tissue Eng Part A. (2014) 20:1758–66. doi: 10.1089/ten.tea.2013.0245
163. Bai C, Yang M, Fan Z, Li S, Gao T, Fang Z. Associations of chemo- and radio-resistant phenotypes with the gap junction, adhesion and extracellular matrix in a three-dimensional culture model of soft sarcoma. J Exp Clin Cancer Res. (2015) 34:58. doi: 10.1186/s13046-015-0175-0
164. Jabbari E, Sarvestani SK, Daneshian L, Moeinzadeh S. Optimum 3D matrix stiffness for maintenance of cancer stem cells is dependent on tissue origin of cancer cells. PLoS ONE. (2015) 10:e0132377. doi: 10.1371/journal.pone.0132377
165. Shang M, Soon RH, Lim CT, Khoo BL, Han J. Microfluidic modelling of the tumor microenvironment for anti-cancer drug development. Lab Chip. (2019) 19:369–86. doi: 10.1039/C8LC00970H
166. Turetta M, Ben FD, Brisotto G, Biscontin E, Bulfoni M, Cesselli D, et al. Emerging technologies for cancer research: towards personalized medicine with microfluidic platforms and 3D tumor models. Curr Med Chem. (2018) 25:4616–37. doi: 10.2174/0929867325666180605122633
167. Samatov TR, Shkurnikov MU, Tonevitskaya SA, Tonevitsky AG. Modelling the metastatic cascade by in vitro microfluidic platforms. Prog Histochem Cytochem. (2015) 49:21–9. doi: 10.1016/j.proghi.2015.01.001
168. Chaw KC, Manimaran M, Tay EH, Swaminathan S. Multi-step microfluidic device for studying cancer metastasis. Lab Chip. (2007) 7:1041–7. doi: 10.1039/b707399m
169. Ma YHV, Middleton K, You LD, Sun Y. A review of microfluidic approaches for investigating cancer extravasation during metastasis. Microsyst Nanoeng. (2018) 4:17104. doi: 10.1038/micronano.2017.104
170. Sontheimer-Phelps A, Hassell BA, Ingber DE. Modelling cancer in microfluidic human organs-on-chips. Nat Rev Cancer. (2019) 19:65–81. doi: 10.1038/s41568-018-0104-6
171. Skardal A, Devarasetty M, Forsythe S, Atala A, Soker S. A reductionist metastasis-on-a-chip platform for in vitro tumor progression modeling and drug screening. Biotechnol Bioeng. (2016) 113:2020–32. doi: 10.1002/bit.25950
172. Ruzycka M, Cimpan MR, Rios-Mondragon I, Grudzinski IP. Microfluidics for studying metastatic patterns of lung cancer. J Nanobiotechnol. (2019) 17:71. doi: 10.1186/s12951-019-0492-0
173. Kong J, Luo Y, Jin D, An F, Zhang W, Liu L, et al. A novel microfluidic model can mimic organ-specific metastasis of circulating tumor cells. Oncotarget. (2016) 7:78421–32. doi: 10.18632/oncotarget.9382
174. Stamp MEM, Jotten A, Kudella PW, Breyer D, Strobl FG, Geislinger TM, et al. Exploring the limits of cell adhesion under shear stress within physiological conditions and beyond on a chip. Diagnostics. (2016) 6:E38. doi: 10.3390/diagnostics6040038
175. Barata D, Spennati G, Correia C, Ribeiro N, Harink B, van Blitterswijk C, et al. Development of a shear stress-free microfluidic gradient generator capable of quantitatively analyzing single-cell morphology. Biomed Microdevices. (2017) 19:81. doi: 10.1007/s10544-017-0222-z
176. Mitxelena-Iribarren O, Zabalo J, Arana S, Mujika M. Improved microfluidic platform for simultaneous multiple drug screening towards personalized treatment. Biosens Bioelectron. (2019) 123:237–43. doi: 10.1016/j.bios.2018.09.001
177. Withrow SJ, Powers BE, Straw RC, Wilkins RM. Comparative aspects of osteosarcoma. Dog versus man. Clin Orthop Relat Res. (1991) 270:159–68. doi: 10.1097/00003086-199109000-00023
178. Paoloni M, Davis S, Lana S, Withrow S, Sangiorgi L, Picci P, et al. Canine tumor cross-species genomics uncovers targets linked to osteosarcoma progression. BMC Genomics. (2009) 10:625. doi: 10.1186/1471-2164-10-625
179. Mueller F, Fuchs B, Kaser-Hotz B. Comparative biology of human and canine osteosarcoma. Anticancer Res. (2007) 27:155–64.
180. Fenger JM, London CA, Kisseberth WC. Canine osteosarcoma: a naturally occurring disease to inform pediatric oncology. ILAR J. (2014) 55:69–85. doi: 10.1093/ilar/ilu009
181. Rankin KS, Starkey M, Lunec J, Gerrand CH, Murphy S, Biswas S. Of dogs and men: comparative biology as a tool for the discovery of novel biomarkers and drug development targets in osteosarcoma. Pediatr Blood Cancer. (2012) 58:327–33. doi: 10.1002/pbc.23341
182. Withrow SJ, Wilkins RM. Cross talk from pets to people: translational osteosarcoma treatments. ILAR J. (2010) 51:208–13. doi: 10.1093/ilar.51.3.208
183. Saraf AJ, Fenger JM, Roberts RD. Osteosarcoma: accelerating progress makes for a hopeful future. Front Oncol. (2018) 8:4. doi: 10.3389/fonc.2018.00004
184. LeBlanc AK, Breen M, Choyke P, Dewhirst M, Fan TM, Gustafson DL, et al. Perspectives from man's best friend: national academy of medicine's workshop on comparative oncology. Sci Transl Med. (2016) 8:324ps5. doi: 10.1126/scitranslmed.aaf0746
185. Winkler K, Beron G, Kotz R, Salzer-Kuntschik M, Beck J, Beck W, et al. Adjuvant chemotherapy in osteosarcoma - effects of cisplatinum, BCD, and fibroblast interferon in sequential combination with HD-MTX and adriamycin. Preliminary results of the COSS 80 study. J Cancer Res Clin Oncol. (1983) 106:1–7. doi: 10.1007/BF00625042
186. Roberts RD, Wedekind MF, Setty BA. Chemotherapy regimens for patients with newly diagnosed malignant bone tumors. In: Cripe TP, Yeager ND, editors. Malignant Pediatric Bone Tumors - Treatment & Management. Cham: Springer US (2015) 83–108. doi: 10.1007/978-3-319-18099-1_6
187. Kuntz CA, Asselin TL, Dernell WS, Powers BE, Straw RC, Withrow SJ. Limb salvage surgery for osteosarcoma of the proximal humerus: outcome in 17 dogs. Vet Surg. (1998) 27:417–22. doi: 10.1111/j.1532-950X.1998.tb00150.x
188. Liptak JM, Dernell WS, Ehrhart N, Lafferty MH, Monteith GJ, Withrow SJ. Cortical allograft and endoprosthesis for limb-sparing surgery in dogs with distal radial osteosarcoma: a prospective clinical comparison of two different limb-sparing techniques. Vet Surg. (2006) 35:518–33. doi: 10.1111/j.1532-950X.2006.00185.x
189. Marina NM, Smeland S, Bielack SS, Bernstein M, Jovic G, Krailo MD, et al. Comparison of MAPIE versus MAP in patients with a poor response to preoperative chemotherapy for newly diagnosed high-grade osteosarcoma (EURAMOS-1): an open-label, international, randomised controlled trial. Lancet Oncol. (2016) 17:1396–408. doi: 10.1016/S1470-2045(16)30214-5
190. Allison DC, Carney SC, Ahlmann ER, Hendifar A, Chawla S, Fedenko A, et al. A meta-analysis of osteosarcoma outcomes in the modern medical era. Sarcoma. (2012) 2012:704872. doi: 10.1155/2012/704872
191. Aljubran AH, Griffin A, Pintilie M, Blackstein M. Osteosarcoma in adolescents and adults: survival analysis with and without lung metastases. Ann Oncol. (2009) 20:1136–41. doi: 10.1093/annonc/mdn731
192. Berrak SGLN, Pearson M, Berberoglu S, Lhan NER, Jaffe N. High-dose ifosfamide in relapsed pediatric osteosarcoma: therapeutic effects and renal toxicity. Pediatr Blood Cancer. (2005) 44:215–9. doi: 10.1002/pbc.20228
193. Duffaud F, Mir O, Boudou-Rouquette P, Piperno-Neumann S, Penel N, Bompas E, et al. Efficacy and safety of regorafenib in adult patients with metastatic osteosarcoma: a non-comparative, randomised, double-blind, placebo-controlled, phase 2 study. Lancet Oncol. (2019) 20:120–33. doi: 10.1016/S1470-2045(18)30742-3
194. Chen EL, Yoo CH, Gutkin PM, Merriott DJ, Avedian RS, Steffner RJ, et al. Outcomes for pediatric patients with osteosarcoma treated with palliative radiotherapy. Pediatr Blood Cancer. (2019) 67:e27967. doi: 10.1002/pbc.27967
195. Chou AJ, Merola PR, Wexler LH, Gorlick RG, Vyas YM, Healey JH, et al. Treatment of osteosarcoma at first recurrence after contemporary therapy: the Memorial Sloan-Kettering Cancer Center experience. Cancer. (2005) 104:2214–21. doi: 10.1002/cncr.21417
196. Kempf-Bielack B, Bielack SS, Jurgens H, Branscheid D, Berdel WE, Exner GU, et al. Osteosarcoma relapse after combined modality therapy: an analysis of unselected patients in the Cooperative Osteosarcoma Study Group (COSS). J Clin Oncol. (2005) 23:559–68. doi: 10.1200/JCO.2005.04.063
197. Bacci G, Briccoli A, Longhi A, Ferrari S, Mercuri M, Faggioli F, et al. Treatment and outcome of recurrent osteosarcoma: experience at Rizzoli in 235 patients initially treated with neoadjuvant chemotherapy. Acta Oncol. (2005) 44:748–55. doi: 10.1080/02841860500327503
198. Zhang W, Ding M-L, Zhang J-N, Qiu J-R, Shen Y-H, Ding X-Y, et al. mTORC1 Maintains the tumorigenicity of SSEA-4+ high-grade osteosarcoma. Sci Rep. (2015) 5:9604. doi: 10.1038/srep09604
199. Bid HK, Roberts RD, Cam M, Audino A, Kurmasheva RT, Lin J, et al. DeltaNp63 promotes pediatric neuroblastoma and osteosarcoma by regulating tumor angiogenesis. Cancer Res. (2014) 74:320–9. doi: 10.1158/0008-5472.CAN-13-0894
200. Baglio SR, Lagerweij T, Perez-Lanzon M, Ho XD, Leveille N, Melo SA, et al. Blocking tumor-educated MSC paracrine activity halts osteosarcoma progression. Clin Cancer Res. (2017) 23:3721–33. doi: 10.1158/1078-0432.CCR-16-2726
201. Lafleur EA. Increased Fas expression reduces the metastatic potential of human osteosarcoma cells. Clin Cancer Res. (2004) 10:8114–9. doi: 10.1158/1078-0432.CCR-04-0353
202. Koshkina NV, Khanna C, Mendoza A, Guan H, DeLauter L, Kleinerman ES. Fas-negative osteosarcoma tumor cells are selected during metastasis to the lungs: the role of the Fas pathway in the metastatic process of osteosarcoma. Mol Cancer Res. (2007) 5:991–9. doi: 10.1158/1541-7786.MCR-07-0007
203. Gordon N, Koshkina NV, Jia SF, Khanna C, Mendoza A, Worth LL, et al. Corruption of the Fas pathway delays the pulmonary clearance of murine osteosarcoma cells, enhances their metastatic potential, and reduces the effect of aerosol gemcitabine. Clin Cancer Res. (2007) 13:4503–10. doi: 10.1158/1078-0432.CCR-07-0313
204. Rodriguez CO Jr, Crabbs TA, Wilson DW, Cannan VA, Skorupski KA, Gordon N, et al. Aerosol gemcitabine: preclinical safety and in vivo antitumor activity in osteosarcoma-bearing dogs. J Aerosol Med Pulm Drug Deliv. (2010) 23:197–206. doi: 10.1089/jamp.2009.0773
205. Hernández-Rodríguez NA, Correa E, Sotelo R, Gómez-Ruiz C, Contreras-Paredes A, Green L. Thrombin is present in the lungs of patients with primary extremity osteosarcoma and pulmonary metastases. Int J Biol Markers. (2002) 17:189–95. doi: 10.1177/172460080201700308
206. Tieken C, Verboom MC, Ruf W, Gelderblom H, Bovée JVMG, Reitsma PH, et al. Tissue factor associates with survival and regulates tumour progression in osteosarcoma. Thromb Haemost. (2016) 115:1025–33. doi: 10.1160/TH15-07-0541
207. Van Den Berg YW, Osanto S, Reitsma PH, Versteeg HH. The relationship between tissue factor and cancer progression: insights from bench and bedside. Blood. (2012) 119:924–32. doi: 10.1182/blood-2011-06-317685
208. Lehuede C, Dupuy F, Rabinovitch R, Jones RG, Siegel PM. Metabolic plasticity as a determinant of tumor growth and metastasis. Cancer Res. (2016) 76:5201–8. doi: 10.1158/0008-5472.CAN-16-0266
209. Liberti MV, Locasale JW. The Warburg effect: how does it benefit cancer cells? Trends Biochem Sci. (2016) 41:211–8. doi: 10.1016/j.tibs.2015.12.001
210. Giang AH, Raymond T, Brookes P, de Mesy Bentley K, Schwarz E, O'Keefe R, et al. Mitochondrial dysfunction and permeability transition in osteosarcoma cells showing the Warburg effect. J Biol Chem. (2013) 288:33303–11. doi: 10.1074/jbc.M113.507129
211. Hua Y, Qiu Y, Zhao A, Wang X, Chen T, Zhang Z, et al. Dynamic metabolic transformation in tumor invasion and metastasis in mice with LM-8 osteosarcoma cell transplantation. J Proteome Res. (2011) 10:3513–21. doi: 10.1021/pr200147g
212. Connelly L, Barham W, Onishko HM, Chen L, Sherrill TP, Zabuawala T, et al. NF-kappaB activation within macrophages leads to an anti-tumor phenotype in a mammary tumor lung metastasis model. Breast Cancer Res. (2011) 13:R83. doi: 10.1186/bcr2935
213. Orrenius S, Gogvadze V, Zhivotovsky B. Mitochondrial oxidative stress: implications for cell death. Annu Rev Pharmacol Toxicol. (2007) 47:143–83. doi: 10.1146/annurev.pharmtox.47.120505.105122
214. Poderoso JJ, Helfenberger K, Poderoso C. The effect of nitric oxide on mitochondrial respiration. Nitric Oxide. (2019) 88:61–72. doi: 10.1016/j.niox.2019.04.005
215. Ren L, Hong ES, Mendoza A, Issaq S, Tran Hoang C, Lizardo M, et al. Metabolomics uncovers a link between inositol metabolism and osteosarcoma metastasis. Oncotarget. (2017) 8:38541–53. doi: 10.18632/oncotarget.15872
216. Winterbourn CC. Revisiting the reactions of superoxide with glutathione and other thiols. Arch Biochem Biophys. (2016) 595:68–71. doi: 10.1016/j.abb.2015.11.028
217. Ridnour LA, Thomas DD, Mancardi D, Espey MG, Miranda KM, Paolocci N, et al. The chemistry of nitrosative stress induced by nitric oxide and reactive nitrogen oxide species. Putting perspective on stressful biological situations. Biol Chem. (2004) 385:1–10. doi: 10.1515/BC.2004.001
218. Roy J, Dibaeinia P, Fan TM, Sinha S, Das A. Global analysis of osteosarcoma lipidomes reveal altered lipid profiles in metastatic versus nonmetastatic cells. J Lipid Res. (2019) 60:375–87. doi: 10.1194/jlr.M088559
219. Luo X, Cheng C, Tan Z, Li N, Tang M, Yang L, et al. Emerging roles of lipid metabolism in cancer metastasis. Mol Cancer. (2017) 16:76. doi: 10.1186/s12943-017-0646-3
220. Miwa S, Shirai T, Yamamoto N, Hayashi K, Takeuchi A, Igarashi K, et al. Current and emerging targets in immunotherapy for osteosarcoma. J Oncol. (2019) 2019:7035045. doi: 10.1155/2019/7035045
221. Dyson KA, Stover BD, Grippin A, Mendez-Gomez HR, Lagmay J, Mitchell DA, et al. Emerging trends in immunotherapy for pediatric sarcomas. J Hematol Oncol. (2019) 12:78. doi: 10.1186/s13045-019-0756-z
222. Wedekind MF, Wagner LM, Cripe TP. Immunotherapy for osteosarcoma: where do we go from here? Pediatr Blood Cancer. (2018) 65:e27227. doi: 10.1002/pbc.27227
223. Wang Z, Wang Z, Li B, Wang S, Chen T, Ye Z. Innate immune cells: a potential and promising cell population for treating osteosarcoma. Front Immunol. (2019) 10:1114. doi: 10.3389/fimmu.2019.01114
224. Heymann MF, Lezot F, Heymann D. The contribution of immune infiltrates and the local microenvironment in the pathogenesis of osteosarcoma. Cell Immunol. (2019) 343:103711. doi: 10.1016/j.cellimm.2017.10.011
225. Dumars C, Ngyuen JM, Gaultier A, Lanel R, Corradini N, Gouin F, et al. Dysregulation of macrophage polarization is associated with the metastatic process in osteosarcoma. Oncotarget. (2016) 7:78343–54. doi: 10.18632/oncotarget.13055
226. Buddingh EP, Kuijjer ML, Duim RA, Burger H, Agelopoulos K, Myklebost O, et al. Tumor-infiltrating macrophages are associated with metastasis suppression in high-grade osteosarcoma: a rationale for treatment with macrophage activating agents. Clin Cancer Res. (2011) 17:2110–9. doi: 10.1158/1078-0432.CCR-10-2047
227. Han Q, Shi H, Liu F. CD163(+) M2-type tumor-associated macrophage support the suppression of tumor-infiltrating T cells in osteosarcoma. Int Immunopharmacol. (2016) 34:101–6. doi: 10.1016/j.intimp.2016.01.023
228. Hingorani P, Maas ML, Gustafson MP, Dickman P, Adams RH, Watanabe M, et al. Increased CTLA-4(+) T cells and an increased ratio of monocytes with loss of class II (CD14(+) HLA-DR(lo/neg)) found in aggressive pediatric sarcoma patients. J Immunother Cancer. (2015) 3:35. doi: 10.1186/s40425-015-0082-0
229. Koirala P, Roth ME, Gill J, Piperdi S, Chinai JM, Geller DS, et al. Immune infiltration and PD-L1 expression in the tumor microenvironment are prognostic in osteosarcoma. Sci Rep. (2016) 6:30093. doi: 10.1038/srep30093
230. Gomez-Brouchet A, Illac C, Gilhodes J, Bouvier C, Aubert S, Guinebretiere JM, et al. CD163-positive tumor-associated macrophages and CD8-positive cytotoxic lymphocytes are powerful diagnostic markers for the therapeutic stratification of osteosarcoma patients: an immunohistochemical analysis of the biopsies fromthe French OS2006 phase 3 trial. Oncoimmunology. (2017) 6:e1331193. doi: 10.1080/2162402X.2017.1331193
231. Pahl JH, Kwappenberg KM, Varypataki EM, Santos SJ, Kuijjer ML, Mohamed S, et al. Macrophages inhibit human osteosarcoma cell growth after activation with the bacterial cell wall derivative liposomal muramyl tripeptide in combination with interferon-gamma. J Exp Clin Cancer Res. (2014) 33:27. doi: 10.1186/1756-9966-33-27
232. Shao XJ, Xiang SF, Chen YQ, Zhang N, Cao J, Zhu H, et al. Inhibition of M2-like macrophages by all-trans retinoic acid prevents cancer initiation and stemness in osteosarcoma cells. Acta Pharmacol Sin. (2019) 40:1343–50. doi: 10.1038/s41401-019-0262-4
233. Zhou Q, Xian M, Xiang S, Xiang D, Shao X, Wang J, et al. All-trans retinoic acid prevents osteosarcoma metastasis by inhibiting M2 polarization of tumor-associated macrophages. Cancer Immunol Res. (2017) 5:547–59. doi: 10.1158/2326-6066.CIR-16-0259
234. Fritzsching B, Fellenberg J, Moskovszky L, Sapi Z, Krenacs T, Machado I, et al. CD8(+)/FOXP3(+)-ratio in osteosarcoma microenvironment separates survivors from non-survivors: a multicenter validated retrospective study. Oncoimmunology. (2015) 4:e990800. doi: 10.4161/2162402X.2014.990800
235. Li X, Chen Y, Liu X, Zhang J, He X, Teng G, et al. Tim3/Gal9 interactions between T cells and monocytes result in an immunosuppressive feedback loop that inhibits Th1 responses in osteosarcoma patients. Int Immunopharmacol. (2017) 44:153–9. doi: 10.1016/j.intimp.2017.01.006
236. Sundara YT, Kostine M, Cleven AH, Bovee JV, Schilham MW, Cleton-Jansen AM. Increased PD-L1 and T-cell infiltration in the presence of HLA class I expression in metastatic high-grade osteosarcoma: a rationale for T-cell-based immunotherapy. Cancer Immunol Immunother. (2017) 66:119–28. doi: 10.1007/s00262-016-1925-3
237. Lussier DM, O'Neill L, Nieves LM, McAfee MS, Holechek SA, Collins AW, et al. Enhanced T-cell immunity to osteosarcoma through antibody blockade of PD-1/PD-L1 interactions. J Immunother. (2015) 38:96–106. doi: 10.1097/CJI.0000000000000065
238. Coley WB. The treatment of sarcoma of the long bones. Ann Surg. (1933) 97:434–60. doi: 10.1097/00000658-193303000-00010
239. Lascelles BD, Dernell WS, Correa MT, Lafferty M, Devitt CM, Kuntz CA, et al. Improved survival associated with postoperative wound infection in dogs treated with limb-salvage surgery for osteosarcoma. Ann Surg Oncol. (2005) 12:1073–83. doi: 10.1245/ASO.2005.01.011
240. Jeys LM, Grimer RJ, Carter SR, Tillman RM, Abudu A. Post operative infection and increased survival in osteosarcoma patients: are they associated? Ann Surg Oncol. (2007) 14:2887–95. doi: 10.1245/s10434-007-9483-8
241. Sottnik JL, U'Ren LW, Thamm DH, Withrow SJ, Dow SW. Chronic bacterial osteomyelitis suppression of tumor growth requires innate immune responses. Cancer Immunol Immunother. (2010) 59:367–78. doi: 10.1007/s00262-009-0755-y
242. Fidler IJ, Sone S, Fogler WE, Barnes ZL. Eradication of spontaneous metastases and activation of alveolar macrophages by intravenous injection of liposomes containing muramyl dipeptide. Proc Natl Acad Sci USA. (1981) 78:1680–4. doi: 10.1073/pnas.78.3.1680
243. MacEwen EG, Kurzman ID, Rosenthal RC, Smith BW, Manley PA, Roush JK, et al. Therapy for osteosarcoma in dogs with intravenous injection of liposome-encapsulated muramyl tripeptide. J Natl Cancer Inst. (1989) 81:935–8. doi: 10.1093/jnci/81.12.935
244. Kurzman ID, MacEwen EG, Rosenthal RC, Fox LE, Keller ET, Helfand SC, et al. Adjuvant therapy for osteosarcoma in dogs: results of randomized clinical trials using combined liposome-encapsulated muramyl tripeptide and cisplatin. Clin Cancer Res. (1995) 1:1595–601.
245. Meyers PA, Schwartz CL, Krailo MD, Healey JH, Bernstein ML, Betcher D, et al. Osteosarcoma: the addition of muramyl tripeptide to chemotherapy improves overall survival–a report from the Children's Oncology Group. J Clin Oncol. (2008) 26:633–8. doi: 10.1200/JCO.2008.14.0095
246. Chou AJ, Kleinerman ES, Krailo MD, Chen Z, Betcher DL, Healey JH, et al. Addition of muramyl tripeptide to chemotherapy for patients with newly diagnosed metastatic osteosarcoma: a report from the Children's Oncology Group. Cancer. (2009) 115:5339–48. doi: 10.1002/cncr.24566
247. Bielack SS, Smeland S, Whelan JS, Marina N, Jovic G, Hook JM, et al. Methotrexate, doxorubicin, and cisplatin (MAP) plus maintenance pegylated interferon Alfa-2b versus MAP alone in patients with resectable high-grade osteosarcoma and good histologic response to preoperative MAP: first results of the EURAMOS-1 good response randomized controlled trial. J Clin Oncol. (2015) 33:2279–87. doi: 10.1200/JCO.2014.60.0734
248. Meazza C, Cefalo G, Massimino M, Daolio P, Pastorino U, Scanagatta P, et al. Primary metastatic osteosarcoma: results of a prospective study in children given chemotherapy and interleukin-2. Med Oncol. (2017) 34:191. doi: 10.1007/s12032-017-1052-9
249. Schwinger W, Klass V, Benesch M, Lackner H, Dornbusch HJ, Sovinz P, et al. Feasibility of high-dose interleukin-2 in heavily pretreated pediatric cancer patients. Ann Oncol. (2005) 16:1199–206. doi: 10.1093/annonc/mdi226
250. Khanna C, Anderson PM, Hasz DE, Katsanis E, Neville M, Klausner JS. Interleukin-2 liposome inhalation therapy is safe and effective for dogs with spontaneous pulmonary metastases. Cancer. (1997) 79:1409–21. doi: 10.1002/(SICI)1097-0142(19970401)79:7<1409::AID-CNCR19>3.0.CO;2-3
251. Dow S, Elmslie R, Kurzman I, MacEwen G, Pericle F, Liggitt D. Phase I study of liposome-DNA complexes encoding the interleukin-2 gene in dogs with osteosarcoma lung metastases. Hum Gene Ther. (2005) 16:937–46. doi: 10.1089/hum.2005.16.937
252. Chiavenna SM, Jaworski JP, Vendrell A. State of the art in anti-cancer mAbs. J Biomed Sci. (2017) 24:15. doi: 10.1186/s12929-016-0311-y
253. Ebb D, Meyers P, Grier H, Bernstein M, Gorlick R, Lipshultz SE, et al. Phase II trial of trastuzumab in combination with cytotoxic chemotherapy for treatment of metastatic osteosarcoma with human epidermal growth factor receptor 2 overexpression: a report from the children's oncology group. J Clin Oncol. (2012) 30:2545–51. doi: 10.1200/JCO.2011.37.4546
254. Weigel B, Malempati S, Reid JM, Voss SD, Cho SY, Chen HX, et al. Phase 2 trial of cixutumumab in children, adolescents, and young adults with refractory solid tumors: a report from the Children's Oncology Group. Pediatr Blood Cancer. (2014) 61:452–6. doi: 10.1002/pbc.24605
255. Roth M, Barris DM, Piperdi S, Kuo V, Everts S, Geller D, et al. Targeting glycoprotein NMB with antibody-drug conjugate, glembatumumab vedotin, for the treatment of osteosarcoma. Pediatr Blood Cancer. (2016) 63:32–8. doi: 10.1002/pbc.25688
256. Trinidad EM, Gonzalez-Suarez E. RANKL inhibitors for osteosarcoma treatment: hope and caution. Ann Transl Med. (2016) 4:534. doi: 10.21037/atm.2016.12.10
257. Roth M, Linkowski M, Tarim J, Piperdi S, Sowers R, Geller D, et al. Ganglioside GD2 as a therapeutic target for antibody-mediated therapy in patients with osteosarcoma. Cancer. (2014) 120:548–54. doi: 10.1002/cncr.28461
258. Galon J, Angell HK, Bedognetti D, Marincola FM. The continuum of cancer immunosurveillance: prognostic, predictive, and mechanistic signatures. Immunity. (2013) 39:11–26. doi: 10.1016/j.immuni.2013.07.008
259. Lussier DM, Johnson JL, Hingorani P, Blattman JN. Combination immunotherapy with alpha-CTLA-4 and alpha-PD-L1 antibody blockade prevents immune escape and leads to complete control of metastatic osteosarcoma. J Immunother Cancer. (2015) 3:21. doi: 10.1186/s40425-015-0067-z
260. Perry JA, Kiezun A, Tonzi P, Van Allen EM, Carter SL, Baca SC, et al. Complementary genomic approaches highlight the PI3K/mTOR pathway as a common vulnerability in osteosarcoma. Proc Natl Acad Sci USA. (2014) 111:E5564–73. doi: 10.1073/pnas.1419260111
261. Chen X, Bahrami A, Pappo A, Easton J, Dalton J, Hedlund E, et al. Recurrent somatic structural variations contribute to tumorigenesis in pediatric osteosarcoma. Cell Rep. (2014) 7:104–12. doi: 10.1016/j.celrep.2014.03.003
262. Wang D, Niu X, Wang Z, Song CL, Huang Z, Chen KN, et al. Multiregion sequencing reveals the genetic heterogeneity and evolutionary history of osteosarcoma and matched pulmonary metastases. Cancer Res. (2019) 79:7–20. doi: 10.1158/0008-5472.CAN-18-1086
263. Tawbi HA, Burgess M, Bolejack V, Van Tine BA, Schuetze SM, Hu J, et al. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): a multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol. (2017) 18:1493–501. doi: 10.1016/S1470-2045(17)30624-1
264. Thanindratarn P, Dean DC, Nelson SD, Hornicek FJ, Duan Z. Advances in immune checkpoint inhibitors for bone sarcoma therapy. J Bone Oncol. (2019) 15:100221. doi: 10.1016/j.jbo.2019.100221
265. Miwa S, Nishida H, Tanzawa Y, Takeuchi A, Hayashi K, Yamamoto N, et al. Phase 1/2 study of immunotherapy with dendritic cells pulsed with autologous tumor lysate in patients with refractory bone and soft tissue sarcoma. Cancer. (2017) 123:1576–84. doi: 10.1002/cncr.30606
266. Himoudi N, Wallace R, Parsley KL, Gilmour K, Barrie AU, Howe K, et al. Lack of T-cell responses following autologous tumour lysate pulsed dendritic cell vaccination, in patients with relapsed osteosarcoma. Clin Transl Oncol. (2012) 14:271–9. doi: 10.1007/s12094-012-0795-1
267. Mason NJ, Gnanandarajah JS, Engiles JB, Gray F, Laughlin D, Gaurnier-Hausser A, et al. Immunotherapy with a HER2-targeting listeria induces HER2-specific immunity and demonstrates potential therapeutic effects in a phase I trial in canine osteosarcoma. Clin Cancer Res. (2016) 22:4380–90. doi: 10.1158/1078-0432.CCR-16-0088
268. Ahmed N, Brawley VS, Hegde M, Robertson C, Ghazi A, Gerken C, et al. Human epidermal growth factor receptor 2 (HER2) -specific chimeric antigen receptor-modified T cells for the immunotherapy of HER2-positive sarcoma. J Clin Oncol. (2015) 33:1688–96. doi: 10.1200/JCO.2014.58.0225
269. Mata M, Vera JF, Gerken C, Rooney CM, Miller T, Pfent C, et al. Toward immunotherapy with redirected T cells in a large animal model: ex vivo activation, expansion, and genetic modification of canine T cells. J Immunother. (2014) 37:407–15. doi: 10.1097/CJI.0000000000000052
270. Canter RJ, Grossenbacher SK, Foltz JA, Sturgill IR, Park JS, Luna JI, et al. Radiotherapy enhances natural killer cell cytotoxicity and localization in pre-clinical canine sarcomas and first-in-dog clinical trial. J Immunother Cancer. (2017) 5:98. doi: 10.1186/s40425-017-0305-7
271. Chun R, de Lorimier LP. Update on the biology and management of canine osteosarcoma. Vet Clin North Am Small Anim Pract. (2003) 33:491–516, vi. doi: 10.1016/S0195-5616(03)00021-4
272. Selmic LE, Burton JH, Thamm DH, Withrow SJ, Lana SE. Comparison of carboplatin and doxorubicin-based chemotherapy protocols in 470 dogs after amputation for treatment of appendicular osteosarcoma. J Vet Intern Med. (2014) 28:554–63. doi: 10.1111/jvim.12313
273. Roberts SS, Chou AJ, Cheung NK. Immunotherapy of Childhood Sarcomas. Front Oncol. (2015) 5:181. doi: 10.3389/fonc.2015.00181
274. Wycislo KL, Fan TM. The immunotherapy of canine osteosarcoma: a historical and systematic review. J Vet Intern Med. (2015) 29:759–69. doi: 10.1111/jvim.12603
275. Scott MC, Temiz NA, Sarver AE, LaRue RS, Rathe SK, Varshney J, et al. Comparative transcriptome analysis quantifies immune cell transcript levels, metastatic progression, and survival in osteosarcoma. Cancer Res. (2018) 78:326–37. doi: 10.1158/0008-5472.CAN-17-0576
276. Biller BJ, Guth A, Burton JH, Dow SW. Decreased ratio of CD8+ T cells to regulatory T cells associated with decreased survival in dogs with osteosarcoma. J Vet Intern Med. (2010) 24:1118–23. doi: 10.1111/j.1939-1676.2010.0557.x
277. Biller BJ, Elmslie RE, Burnett RC, Avery AC, Dow SW. Use of FoxP3 expression to identify regulatory T cells in healthy dogs and dogs with cancer. Vet Immunol Immunopathol. (2007) 116:69–78. doi: 10.1016/j.vetimm.2006.12.002
278. Withers SS, Skorupski KA, York D, Choi JW, Woolard KD, Laufer-Amorim R, et al. Association of macrophage and lymphocyte infiltration with outcome in canine osteosarcoma. Vet Comp Oncol. (2019) 17:49–60. doi: 10.1111/vco.12444
279. Maekawa N, Konnai S, Okagawa T, Nishimori A, Ikebuchi R, Izumi Y, et al. Immunohistochemical analysis of PD-L1 expression in canine malignant cancers and PD-1 expression on lymphocytes in canine oral melanoma. PLoS ONE. (2016) 11:e0157176. doi: 10.1371/journal.pone.0157176
280. Goss PE, Chambers AF. Does tumour dormancy offer a therapeutic target? Nat Rev Cancer. (2010) 10:871–7. doi: 10.1038/nrc2933
Keywords: comparative oncology, metastasis biology, experimental models, translational therapeutics, canine cancer
Citation: Fan TM, Roberts RD and Lizardo MM (2020) Understanding and Modeling Metastasis Biology to Improve Therapeutic Strategies for Combating Osteosarcoma Progression. Front. Oncol. 10:13. doi: 10.3389/fonc.2020.00013
Received: 01 October 2019; Accepted: 07 January 2020;
Published: 31 January 2020.
Edited by:
Rodney L. Page, Colorado State University, United StatesReviewed by:
Christine F. Brainson, University of Kentucky, United StatesCopyright © 2020 Fan, Roberts and Lizardo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Timothy M. Fan, dC1mYW5AaWxsaW5vaXMuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.