- 1Department of Oncology, Shengjing Hospital of China Medical University, Shenyang, China
- 2Department of Pathology, Shengjing Hospital of China Medical University, Shenyang, China
Background: Patients with advanced gastric cancer, especially the HER2-positive type, have a poor prognosis; there is a paucity of effective anti-HER2 drug therapies in patients who develop resistance to trastuzumab.
Case presentation: We report the case of a 36-year-old male with HER2-positive gastric cancer with lung and liver metastases. The patient responded after treatment with trastuzumab combined with chemotherapy and attained a progression-free survival (PFS) of 17 months. Subsequently, the patient received apatinib that selectively inhibits the VEGFR2 and obtained an evident tumor response and a PFS of 8 months. When the disease progressed again, the regimen containing lapatinib failed. Then, the patient received treatment with nivolumab. However, he presented with hyper-progressive disease (HPD). Finally, he received a combination of capecitabine and pyrotinib, an irreversible dual TKI, acting on HER2 and EGFR. The tumor shrank markedly with this combination therapy. The mechanism of both HPD due to immunotherapy and the resistance to trastuzumab and lapatinib were investigated in this case. Loss of phosphatase and tensin homolog (PTEN) and new mutations of BRCA1 and KRAS were detected after resistance to trastuzumab and lapatinib.
Conclusions: For patients with HER2-positive advanced gastric cancer who have developed resistance to trastuzumab, pyrotinib is a promising new agent, which can be used as salvage therapy.
Introduction
In recent years, use of targeted therapy against human epidermal growth factor receptor-2 (HER2) and immunotherapy against programmed cell death protein-1 (PD-1) have resulted in progress in the treatment of advanced gastric cancer. Moreover, small molecule tyrosine kinase inhibitors (TKI) (e.g., regorafenib and apatinib) targeting vascular endothelial growth factor receptor (VEGFR) have been investigated for their role in gastric cancer. A phase III randomized controlled trial (RCT) has shown that apatinib as third-line treatment for advanced gastric cancer significantly improves the objective response rate (ORR) and overall survival (OS), as compared with placebo (1). Based on this, apatinib was approved by the China Food and Drug Administration (CFDA) as third-line treatment for advanced gastric cancer.
The proportion of HER2-positive gastric cancers varies widely from 7 to 34% (2, 3). Trastuzumab is effective in improving the survival of patients with HER2-positive gastric cancer (4). However, there is no standard of care for trastuzumab-resistant advanced gastric cancer (5). Pyrotinib is an irreversible TKI targeting both HER2 and epidermal growth factor receptor (EGFR). In a phase II RCT, compared with lapatinib plus capecitabine, pyrotinib plus capecitabine significantly improved ORR (78.5 vs. 57.1%) and prolonged progression-free survival (PFS) (18.1 vs. 7.0 months) in patients with HER2-positive advanced breast cancer. Moreover, patients were able to benefit from pyrotinib, regardless of whether trastuzumab was administered previously (6). Based on this trial, CFDA approved pyrotinib for the treatment of HER2-positive advanced breast cancer (7). Currently, it is unclear whether pyrotinib is effective in trastuzumab-resistant gastric cancer. Herein, we report a case with HER2-positive advanced gastric cancer, who had previously been treated with trastuzumab, benefiting from apatinib and pyrotinib.
Case Presentation
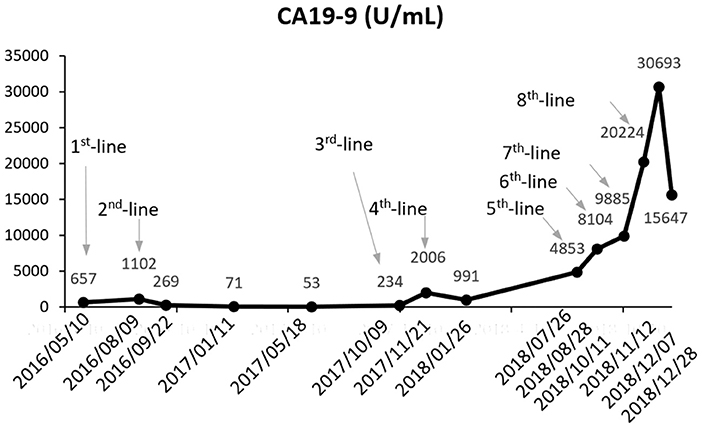
A 36-year-old male patient was clinically diagnosed with gastric cancer and underwent D2 radical gastrectomy in October 2015. The histopathological diagnosis was moderately to highly differentiated adenocarcinoma (intestinal type) with HER2 amplification (FISH); TNM staging (7th edition) was pT4aN2M0 (stage IIIB). Subsequently, the patient underwent six cycles of adjuvant chemotherapy (oxaliplatin and capecitabine) without trastuzumab. Figures 1–3 illustrate the entire treatment procedure and corresponding changes in the lesions on computed tomography (CT) scan and changes in CA19-9 levels.
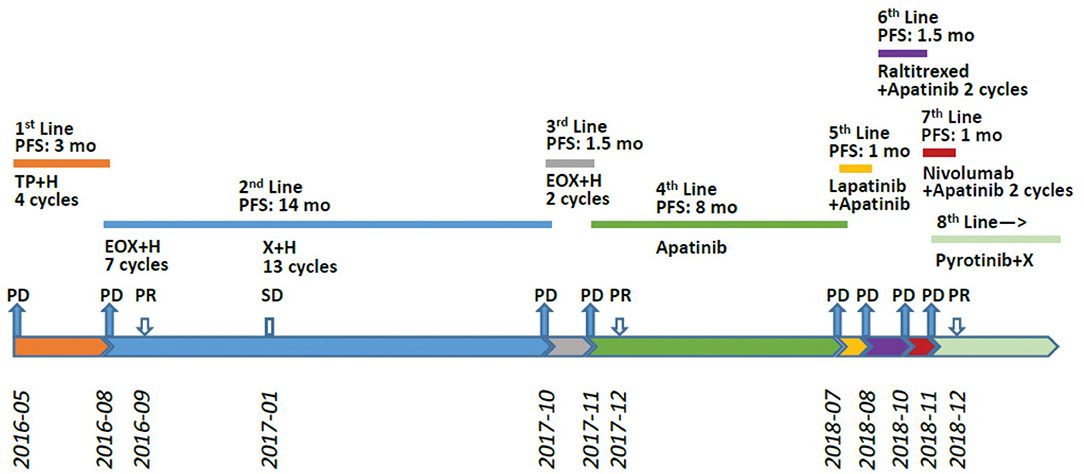
Figure 1. Treatment procedure after post-operative relapse. PFS, progression-free survival; H, trastuzumab; DP, docetaxel/cisplatin; X, xeloda (capecitabine); EOX, epirubicin/oxaliplatin/xeloda; PR, partial response; PD, progressive disease; mo, months.

Figure 2. Chest and abdominal computed tomography (CT) scans. (A) CT (2016-05) demonstrates multiple liver and lung metastases before first-line chemotherapy (DP: docetaxel and cisplatin) combined with trastuzumab (H). (B) Liver and lung metastases are reduced in size after second-line chemotherapy (EOX: epirubicin, oxaliplatin, and capecitabine) combined with trastuzumab. (C) CT (2017-11) demonstrates progressive disease (PD) in the lung and liver metastases when chemotherapy combined with trastuzumab failed. (D) CT (2017-12) reveals that a cavity is formed inside the pulmonary metastatic lesions, and the size of the liver metastases are reduced than before, after using fourth-line therapy (apatinib) for 1 month. (E) Lung and liver metastases are stable during apatinib treatment until 2018-07, when all the lesions progressed significantly. (F) After 4 weeks of oral lapatinib and apatinib treatment as fifth-line therapy, CT (2018-08) scans suggest new intrapulmonary nodules and intrahepatic metastases. (G) CT scans reveal hyper-progressive disease (HPD) and that the lesions are increased and enlarged significantly after treatment with nivolumab plus apatinib for 4 weeks. (H) CT scans (2018-12) show partial response (PR) in the hepatic and pulmonary metastatic lesions after using pyrotinib plus capecitabine for 1 month.
First- and Second-Line Chemotherapy Combined With Trastuzumab
In May 2016, a CT scan demonstrated multiple liver and lung metastases; he was then initially treated with docetaxel, cisplatin, and trastuzumab. After four cycles, a CT scan in August 2016 showed that the intrapulmonary nodules were stable; the liver lesions, however, had enlarged markedly. He received second-line chemotherapy with EOX (epirubicin, oxaliplatin, capecitabine) combined with trastuzumab. Chest and abdominal CT examinations performed after two cycles showed partial remission in both the lung and liver lesions. After seven cycles, the regimen was switched to trastuzumab plus capecitabine, as maintenance therapy, until April 2017, when an abdominal CT showed slowly progressive lesions in the liver. Subsequently, he underwent transarterial chemoembolization twice and simultaneously continued with trastuzumab plus capecitabine for two more cycles, until October 2017, when CT scans demonstrated progressive disease (PD) in both the lung and liver metastases. From October 2017, the patient was rechallenged with two cycles of trastuzumab plus EOX, but this regimen failed to demonstrate any efficacy.
Apatinib Monotherapy
From November 2017, the patient was treated with 750 mg of oral apatinib, once daily, as fourth-line therapy. One month later, CT showed that the intrahepatic lesions had markedly shrunken in size, and the intrapulmonary lesions showed either reduced density or cavity formation. During treatment with apatinib, the patient developed grade 2 adverse effects of leukopenia, hematuria, and anorexia, which subsided after symptomatic treatment.
Lapatinib Combined With Apatinib
On July 26, 2018, a follow-up CT scan revealed that the lung and liver metastatic lesions had progressed significantly. He presented with the symptoms of cough, fatigue, and anorexia. The ECOG (Eastern Cooperative Oncology Group) score decreased to 2, and the quality of life (QOL) score was 39. Re-biopsy of the progressive lesions was suggested, but the patient refused. A regimen of lapatinib (1.25 g, once daily) combined with continuous apatinib (500 mg, once daily) was initiated. After 4 weeks of oral lapatinib and apatinib treatment, CT scans revealed new mediastinal lymph node metastasis, intrapulmonary nodules, and intrahepatic metastasis. Cough, fatigue, and anorexia persisted. In September 2018, the patient received raltitrexed combined with apatinib. However, the treatment failed after two cycles.
Nivolumab Combined With Continuous Apatinib
In October 2018, a re-biopsy to the liver metastasis revealed a persistent histopathological diagnosis of adenocarcinoma. Subsequently, next generation sequencing (NGS) (543 genes; GeneCast Biotechnology Co. Ltd., Beijing, China) was performed. The detailed results before and after treatment with trastuzumab and lapatinib are listed in Table 1. Compared with the results before treatment with trastuzumab, NGS revealed a decreased gene copy number (GCN) of phosphatase and tensin homolog (PTEN), new mutations of BRCA1 and KRAS, and greater HER2 GCN. NGS also showed microsatellite stability (MSS) and moderate tumor mutational burden (TMB) (11.44/Megabase [Mb]). Immunohistochemistry (IHC) using both Ventana SP142 antibody and Dako 22c3 antibody was negative for PD-L1 expression. A regimen of nivolumab combined with continuous apatinib was given in October 2018. After 4 weeks, CT scans revealed that the tumors in the liver and lungs had increased and enlarged significantly. We calculated the tumor growth rate (TGR), before and during treatment, per month, and its variation (ΔTGR). The ΔTGR exceeded 50%, suggesting a hyper-progressive disease (HPD) and a poor prognosis (8).
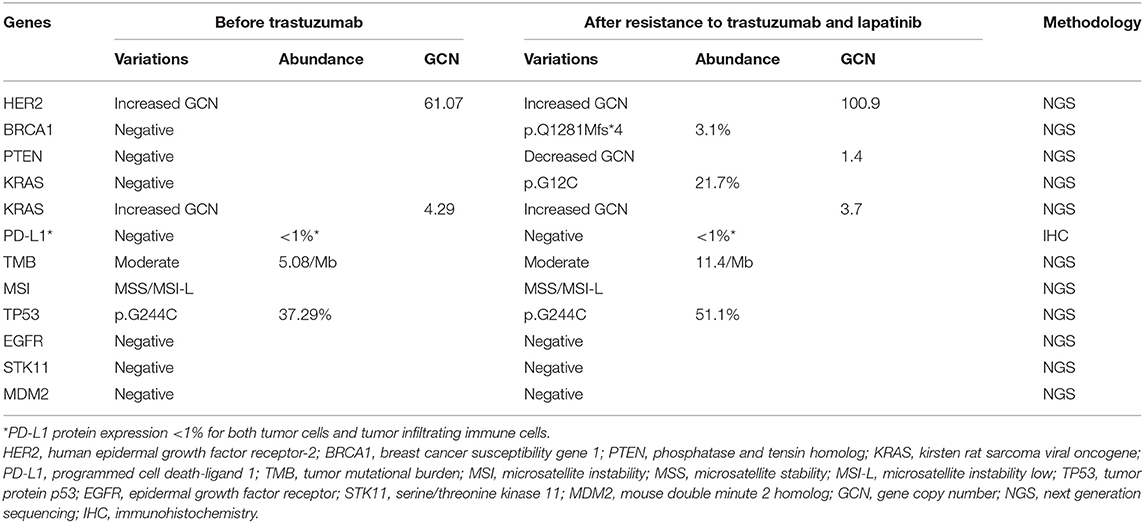
Table 1. Results detected before treatment with trastuzumab and after resistance to treatment with trastuzumab and lapatinib.
Pyrotinib Combined With Capecitabine
In November 2018, the patient was administered pyrotinib and capecitabine (pyrotinib 400 mg, once daily; capecitabine 1,000 mg/m2, bid, days 1–14, every 3 weeks). On the fifth day of treatment, the patient's dyspnea and cough gradually improved. After 2 weeks of treatment, the patient's dyspnea, dry cough, and fatigue completely subsided. After two cycles, chest and abdominal CT scans showed partial response (PR) in the pulmonary, mediastinal lymph node, hepatic, and peritoneal metastases. The patient tolerated treatment with pyrotinib well. Grade 1 diarrhea with no additional adverse events was observed and disappeared soon after symptomatic treatment. Until the submission of the case draft, the patient has survived for over 35 months since post-operative recurrence and now continues to receive the combination treatment of pyrotinib and capecitabine and the PFS is over 8 months.
Discussion
Studies have shown that HER2 protein plays a critical role in the tumorigenesis, progression, and metastasis of HER2-positive gastric cancer, and portends a poor prognosis (9–11). Trastuzumab is presently the only drug with definite clinical evidence and proven efficacy in treating HER2-positive gastric cancer. The ToGA trial confirmed that chemotherapy combined with trastuzumab significantly improved ORR and OS for advanced HER2-positive gastric cancer compared with chemotherapy alone (4). The case we present responded to first-line trastuzumab treatment and attained a PFS of 8 months. After progression, the patient received multi-line chemotherapy successively without achieving remission, until the anti-angiogenic drug apatinib achieved a PR and a PFS of 8 months. Although subsequent treatment with lapatinib and immunotherapy failed, pyrotinib achieved apparent clinical remission, suggesting its ability to overcome prior resistance to anti-HER2 therapy. This underscores the fact that the HER2 signaling pathway has always been crucial for driving tumor progression.
Other anti-HER2 agents (e.g., lapatinib, afatinib, neratinib, dacomitinib, pertuzumab, and ado-trastuzumab emtansine) have not been validated for the treatment of HER2-positive gastric cancer with progression on or after trastuzumab-based therapy. In this case, we attempted to explore the mechanisms of resistance to lapatinib and/or trastuzumab by NGS. We speculate that acquired PTEN deletion (decreased GCN) is perhaps the main cause of drug resistance. It has been reported that low levels of PTEN may contribute to resistance of breast cancer cells to trastuzumab both in vitro and in vivo. Activated HER2 and downstream PI3K-Akt signaling pathway can be negatively regulated by PTEN molecule. Constitutively activated Akt via PTEN deletion leads to trastuzumab resistance (12–14). A retrospective study that included 129 patients with HER2-positive gastric cancer treated with first-line trastuzumab-based regimens, indicates that patients with loss of PTEN expression have a significantly shortened median PFS (4.5 vs. 12.4 months) and OS (12.3 vs. 28.9 months) compared with those with intact PTEN expression (15). In our case, pyrotinib has been effective, although the patient demonstrated PTEN deletion and resistance to trastuzumab and lapatinib. The reason for this is worth exploring.
Both pyrotinib and lapatinib are small-molecule TKIs that inhibit EGFR and HER2; however, lapatinib is a reversible TKI, whereas pyrotinib is irreversible. Primary resistance to lapatinib may be due in part to its reversible drug activity and incomplete inhibition of downstream pathways. Pyrotinib directly acts on the tyrosine kinase domain of the HER2 pathway and completely blocks downstream pathways activated by homodimers or heterodimers of EGFR, HER2, and HER4 on the tumor cell membrane. Monoclonal antibodies such as trastuzumab and pertuzumab only act on the HER2 pathway (16). Preclinical studies that investigated the inhibitory activity of various anti-HER2 drugs in HER2-positive gastric cancer cell lines (NCI-N87) found the IC50 of lapatinib, neratinib, and pyrotinib to be 10, 0.6, and 1.0 nmol/L, respectively (17, 18), suggesting that the antitumor activity of lapatinib in HER2-positive gastric cancer is evidently weaker than that of irreversible TKI pyrotinib. A phase I study of pyrotinib in the treatment of HER2-positive gastric cancer (NCT03480256) is currently underway; however, no data have been reported as yet.
Of note, as the fourth-line therapy in this case, apatinib achieved PR and a PFS of 8 months. There is no clinical trial reporting the efficacy of apatinib in specific HER2-positive gastric cancer. Our case suggests that patients with HER2-positive gastric cancer can still benefit from apatinib after resistance to trastuzumab. Further clinical trials will be needed to verify and validate the efficacy of apatinib in advanced HER2-positive gastric cancer.
The results of NGS and IHC before treatment with the immune checkpoint inhibitor (ICI), nivolumab, suggest that some molecular biomarkers (e.g., PD-L1, TMB, MSI, TP53, KRAS, BRCA1, STK11, MDM2) may be related to the efficacy of ICI (Table 1). Retrospective data from clinical studies have shown that patients with lung adenocarcinoma harboring both TP53 and KRAS mutations have a significant survival benefit when treated with ICI (19, 20). Unfortunately, the patient developed HPD after two cycles of nivolumab. A randomized, placebo-controlled, phase III trial shows nivolumab can prolong median OS by 1 month (5.26 vs. 4.14 months) in PD-L1-positive (≥1%) advanced gastric cancer (21). However, patients with HER2-positive gastric cancer were excluded from enrolment in this trial; hence, it is unclear whether these HER2-positive patients were prone to developing HPD. A clinical study exploring the predictors of HPD after the use of nivolumab in advanced gastric cancer found that the frequencies of HPD in HER2-positive and negative gastric cancers are 23.4% (11/47) and 14.3% (2/14) (p = 0.71), respectively (22). This suggests that HER2-positive status may not be associated with HPD. Moreover, previous studies have shown that loss of PTEN promotes resistance to T cell–mediated immunotherapy (23, 24). In view of this, ICI may not be a good option for patients who are resistant to HER2 agents with loss of PTEN. Instead, pyrotinib could be proposed even in patients with loss of PTEN. For patients who are resistant to pyrotinib, agents acting on the PI3k/PTEN-Akt-mTOR pathway (e.g., everolimus), could be tried as follow-up combination treatment.
Conclusion
In our case, pyrotinib was administered to treat advanced HER2-positive gastric cancer after resistance to prior anti-HER2 therapy and the patient has achieved an evident treatment response. Pyrotinib is a promising novel agent, which can be used as a salvage therapeutic drug for anti-HER2-resistant gastric cancer. Further studies are necessary to validate this result.
Ethics Statement
Written informed consent was obtained from the patient for publication of this case report and any potentially identifying information and images.
Author Contributions
All authors have read and approved the final manuscript. C-BH designed the report. L-TH wrote the manuscript. C-BH and J-TM were responsible for the conception and revision of the manuscript. X-HL undertook the pathological diagnosis. C-BH, J-TM, L-TH, S-LZ, and LS carried out the clinical management of the patient. L-TH, S-LZ, and LS collected the patient's clinical data. WJ, J-ZZ, and Y-RW analyzed the data.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Qin S. Phase III study of apatinib in advanced gastric cancer: a randomized, double-blind, placebo-controlled trial. J Clin Oncol. (2014) 32:4003. doi: 10.1200/jco.2014.32.15_suppl.4003
2. Tanner M, Hollmen M, Junttila T, Kapanen AI, Tommola S, Soini Y, et al. Amplification of HER-2 in gastric carcinoma: association with Topoisomerase IIα gene amplification, intestinal type, poor prognosis and sensitivity to trastuzumab. Annal Oncol. (2005) 16:273–8. doi: 10.1093/annonc/mdi064
3. Jørgensen JT, Hersom M. HER2 as a prognostic marker in gastric cancer-a systematic analysis of data from the literature. J Cancer. (2012) 3:137–44. doi: 10.7150/jca.4090
4. Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-esophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. (2010) 376:687–97. doi: 10.1016/S0140-6736(10)61121-X
5. Jomrich G, Schoppmann SF. Targeting HER 2 and angiogenesis in gastric cancer. Expert Rev Anticancer Ther. (2016) 16:111–22. doi: 10.1586/14737140.2016.1121110
6. Xu B, Ma F, Ouyang Q, Li W, Jiang Z, Tong Z, et al. A randomized phase II trial of pyrotinib plus capecitabine versus lapatinib plus capecitabine in patients with HER2-positive metastatic breast cancer previously treated with taxanes, anthracyclines and/or trastuzumab. Cancer Res. (2018) 78:PD3–08. doi: 10.1158/1538-7445.SABCS17-PD3-08
7. De Leon J. China Approves Hengrui Breast Cancer Drug on Phase II Data. (2018). Available online at: https://www.biocentury.com/bc-extra/companynews/2018-08-22/china-approves-hengrui-breast-cancer-drugphase-ii-data (accessed September 25, 2018).
8. Ferrara R, Mezquita L, Texier M, Lahmar J, Audigier-Valette C, Tessonnier L, et al. Hyperprogressive disease in patients with advanced non–small cell lung cancer treated with PD-1/PD-L1 inhibitors or with single-agent chemotherapy. JAMA Oncol. (2018) 4:1543–52. doi: 10.1001/jamaoncol.2018.3676
9. Gravalos C, Jimeno A. HER2 in gastric cancer: a new prognostic factor and a novel therapeutic target. Annal Oncol. (2008) 19:1523–9. doi: 10.1093/annonc/mdn169
10. Chua TC, Merrett ND. Clinicopathologic factors associated with HER2-positive gastric cancer and its impact on survival outcomes—a systematic review. Int J Cancer. (2012) 130:2845–56. doi: 10.1002/ijc.26292
11. Gómez-Martin C, Garralda E, Echarri MJ, Ballesteros A, Arcediano A, Rodríguez-Peralto JL, et al. HER2/neu testing for anti-HER2-based therapies in patients with unresectable and/or metastatic gastric cancer. J Clin Pathol. (2012) 65:751–7. doi: 10.1136/jclinpath-2012-200774
12. Zhang X, Park JS, Park KH, Kim KH, Jung M, Chung HC, et al. PTEN deficiency as a predictive biomarker of resistance to HER2-targeted therapy in advanced gastric cancer. Oncology. (2015) 88:76–85. doi: 10.1159/000366426
13. Berns K, Horlings HM, Hennessy BT, Madiredjo M, Hijmans EM, Beelen K, et al. A functional genetic approach identifies the pi3k pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell. (2007) 12:395–402. doi: 10.1016/j.ccr.2007.08.030
14. Nagata Y, Lan KH, Zhou X, Tan M, Esteva FJ, Sahin AA, et al. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell. (2004) 6:117–27. doi: 10.1016/j.ccr.2004.06.022
15. Kim C, Lee CK, Chon HJ, Kim JH, Park HS, Heo SJ, et al. PTEN loss and level of HER2 amplification is associated with trastuzumab resistance and prognosis in HER2-positive gastric cancer. Oncotarget. (2017) 8:113494–501. doi: 10.18632/oncotarget.23054
16. Ma F, Li Q, Chen S, Zhu W, Fan Y, Wang J, et al. Phase I study and biomarker analysis of pyrotinib, a novel irreversible Pan-ErbB receptor tyrosine kinase inhibitor, in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol. (2017) 35:3105–12. doi: 10.1200/JCO.2016.69.6179
17. Li X, Yang C, Wan H, Zhang G, Feng J, Zhang L, et al. Discovery and development of pyrotinib: a novel irreversible EGFR/HER2 dual tyrosine kinase inhibitor with favorable safety profiles for the treatment of breast cancer. Eur J Pharm Sci. (2017) 110:51–61. doi: 10.1016/j.ejps.2017.01.021
18. Chan A, Delaloge S, Holmes FA, Moy B, Iwata H, Harvey VJ, et al. Neratinib after trastuzumab-based adjuvant therapy in patients with HER2-positive breast cancer (ExteNET): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. (2016) 17:367–77. doi: 10.1016/S1470-2045(15)00551-3
19. Dong ZY, Zhong WZ, Zhang XC, Su J, Xie Z, Liu SY, et al. Potential predictive value of TP53 and KRAS mutation status for response to PD-1 blockade immunotherapy in lung adenocarcinoma. Clin Cancer Res. (2017) 23:3012–24. doi: 10.1158/1078-0432.CCR-16-2554
20. Biton J, Mansuet-Lupo A, Pécuchet N, Alifano M7, Ouakrim H, Arrondeau J, et al. TP53, STK11, and EGFR mutations predict tumor immune profile and the response to Anti–PD-1 in lung adenocarcinoma. Clin Cancer Res. (2018) 24:5710–23. doi: 10.1158/1078-0432.CCR-18-0163
21. Kang YK, Boku N, Satoh T, Ryu MH1, Chao Y4, Kato K, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. (2017) 390:2461–71. doi: 10.1016/S0140-6736(17)31827-5
22. Sasaki A, Nakamura Y, Mishima S, Kawazoe A, Kuboki Y, Bando H, et al. Predictive factors for hyperprogressive disease during nivolumab as anti-PD1 treatment in patients with advanced gastric cancer. Gastric Cancer. (2019) 5:1–10. doi: 10.1007/s10120-018-00922-8
23. Peng W, Chen J Q, Liu C, Malu S, Creasy C, Tetzlaff MT, et al. Loss of PTEN promotes resistance to T cell–mediated immunotherapy. Cancer Discov. (2016) 6:202–16. doi: 10.1158/2159-8290.CD-15-0283
Keywords: apatinib, gastric cancer, HER2, nivolumab, pyrotinib
Citation: Huang L-T, Ma J-T, Zhang S-L, Li X-H, Sun L, Jing W, Zhao J-Z, Wang Y-R and Han C-B (2019) Durable Clinical Response to Pyrotinib After Resistance to Prior Anti-HER2 Therapy for HER2-Positive Advanced Gastric Cancer: A Case Report. Front. Oncol. 9:1453. doi: 10.3389/fonc.2019.01453
Received: 20 August 2019; Accepted: 05 December 2019;
Published: 20 December 2019.
Edited by:
Andrew Zloza, Rush University Medical Center, United StatesReviewed by:
Antonio Rozzi, Centre Hospitalier Régional Metz-Thionville, FranceGino B. Ferraro, Harvard Medical School, United States
Copyright © 2019 Huang, Ma, Zhang, Li, Sun, Jing, Zhao, Wang and Han. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cheng-Bo Han, hanchengbo@sj-hospital.org
 Le-Tian Huang
Le-Tian Huang Jie-Tao Ma1
Jie-Tao Ma1 Wei Jing
Wei Jing