
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Oncol., 10 December 2019
Sec. Genitourinary Oncology
Volume 9 - 2019 | https://doi.org/10.3389/fonc.2019.01378
This article is part of the Research TopicOptimizing Local Therapy for High-Risk Prostate Cancer: Evidence and Emerging OptionsView all 13 articles
For patients with unfavorable or high-risk prostate cancer, dose escalated radiation therapy leads to improved progression free survival but attempts to deliver increased dose by external beam radiation therapy (EBRT) alone can be limited by late toxicities to nearby genitourinary and gastrointestinal organs at risk. Brachytherapy is a method to deliver dose escalation in conjunction with EBRT with a potentially improved late toxicity profile and improved prostate cancer related outcomes. At least three randomized controlled trials have demonstrated improved biochemical control with the addition of either low-dose rate (LDR) or high-dose rate (HDR) brachytherapy to EBRT, although only ASCENDE-RT compared brachytherapy to dose-escalated EBRT but did report an over 50% improvement in biochemical failure with a LDR boost. Multiple single institution and comparative research series also support the use of a brachytherapy boost in the DE-EBRT era and demonstrate excellent prostate cancer specific outcomes. Despite improved oncologic outcomes with a brachytherapy boost in the high-risk setting, the utilization of both LDR, and HDR brachytherapy use is declining. The acute genitourinary toxicities when brachytherapy boost is combined with EBRT, particularly a LDR boost, are of concern in comparison to EBRT alone. HDR brachytherapy boost has many physical properties inherent to its rapid delivery of a large dose which may reduce acute toxicities and also appeal to the radiobiology of prostate cancer. We herein review the evidence for use of either LDR or HDR brachytherapy boost for high-risk prostate cancer and summarize comparisons between the two treatment modalities.
Nearly 180,000 new cases of prostate cancer are estimated to be diagnosed in 2019 (1, 2). For patients with high-risk prostate cancer, treatment options most often include surgery or a combination of androgen deprivation therapy (ADT) and radiation therapy. External beam radiation therapy (EBRT) is the most common method to deliver radiotherapy for localized prostate cancer.Multiple studies have demonstrated that dose-escalated external beam radiation therapy (DE-EBRT) improves local control, freedom from biochemical failure, freedom from distance metastases, and decreases the need for salvage therapy (3–6). DE-EBRT, however, has also been associated with increases in late genitourinary (GU) and gastrointestinal (GI) toxicities (3). In the NRG Oncology/RTOG 0126 randomized clinical trial, the 5-years rates of both GI and GU late toxicity were increased with dose escalation (3). Brachytherapy is a method to deliver high-dose radiotherapy and escalate the biologically equivalent dose (BED), either as monotherapy or in tandem with EBRT as a boost, which is highly conformal and can often provide sparing of the surrounding organs at risk that is often not achievable with EBRT. Both permanent seed low dose-rate (LDR) or high dose-rate (HDR) brachytherapy provide a highly conformal escalation of dose to the cancer and allow greater sparing of surrounding normal organs than that possible with any type of EBRT (7). The American Society of Clinical Oncology (ASCO)/Cancer Care Ontario (CCO) Joint Guideline Update published in 2017 explicitly states that for patients with high-risk prostate cancer receiving EBRT and androgen deprivation therapy (ADT), brachytherapy boost (either LDR or HDR) should be offered to eligible patients (8). This recommendation is largely based on the ASCENDE-RT trial which demonstrated a significant improvement in the rates of biochemical relapse for patients treated with a brachytherapy boost (9). In ASCENDE-RT, the brachytherapy boost was delivered using a permanent seed low-dose rate (LDR) implant. LDR brachytherapy is a proven method with decades of follow-up and endorsement by numerous expert consensus groups. Another method of brachytherapy, high-dose rate (HDR) brachytherapy, is an alternative to LDR which has many properties that may make it a superior alternative to LDR. In contrast to LDR, HDR is not a permanent implant and generally allows for more consistent dose coverage and relative lower dose to the rectum, bladder, and urethra (7, 10, 11). Both LDR and HDR boost are recommended in the ASCO/CCO Joint Guideline recommendations and the choice between the two is often determined by physician, hospital, patient, and disease characteristics. We herein report on the importance of the brachytherapy boost as well as compare and contrast the use of both LDR and HDR brachytherapy as a boost in high-risk prostate cancer, and summarize future directions using these treatment modalities.
LDR brachytherapy, commonly referred to as permanent prostate brachytherapy or seed implant, is a type of procedure in which implanted radioactive sources are permanently placed into the prostate. Defined by the International Commission on Radiologic Units and Measurements, LDR brachytherapy is the utilization of a radiation source with a dose-rate of <2 Gy per hour (12). Brachytherapy boost delivered with LDR has been a well-established treatment modality in the treatment of high-risk prostate cancer with numerous studies supporting its use and efficacy (13–15). Sathya et al. (16) conducted a randomized controlled trial comparing EBRT to 40 Gy in 20 fractions plus a temporary LDR brachytherapy boost with iridium-192–35 Gy vs. EBRT alone to 66 Gy in 33 fractions in patients with high-risk prostate cancer. No androgen deprivation therapy was given neoadjuvantly or concurrently in either arm. The primary outcome was biochemical or clinical failure. With a median follow-up of 8.2 years, 29% of patients in the EBRT plus temporary LDR brachytherapy boost arm failed vs. 61% in the EBRT alone arm (hazard ratio, 0.42; p = 0.0024) (16, 17). While the EBRT dose used in this study was low compared to modern standards, this trial laid the groundwork and confirmed the principal that brachytherapy in conjunction with moderate dose EBRT resulted in increased rates of biochemical control than that achieved with EBRT alone.
Recently, the highly anticipated results of a large randomized trial comparing the now standard dose-escalated EBRT to EBRT plus LDR brachytherapy boost were published. The ASCENDE-RT trial was a randomized Phase III study comparing EBRT alone (78 Gy/39 fractions) to EBRT (46 Gy/23 fractions) plus an LDR brachytherapy boost (115 Gy using 125I) in patients with intermediate or high-risk prostate cancer (9). Both arms included 12 months of androgen deprivation therapy. The trial included 398 patients and demonstrated a statistically significant improvement in biochemical progression-free survival (b-PFS) in favor of the brachytherapy boost arm, with 9-years b-PFS of 83 vs. 62% (18). At a median follow-up of 6.5 years, there was no statistical difference in 7-years overall survival although there was a trend toward improvement with the LDR boost (85.7 vs. 81.5%). Longer follow-up of the trial will be necessary to determine if the addition of the LDR-boost correlates to improved metastasis free, cause-specific, and overall survival with these results anticipated with median follow-up of 13 years. With regards to overall survival, Johnson et al. (19) identified patients from the National Cancer Database (NCDB) with unfavorable prostate cancer who were treated with either DE-EBRT or EBRT with a LDR boost. This study attempted to mirror enrollment criteria of ASCENDE-RT but allowed patients to have received ADT up to 8 months prior to definitive radiation therapy. They found that the LDR boost was associated with improved overall survival (7-years OS 82 vs. 73%; p <0.001) (19). The improved survival outcome persisted in multivariable analysis and with propensity score matching, although the study cannot fully account for selection bias in the choice of treatment.
HDR differs from LDR in that radiation sources with higher activity are temporarily inserted into the prostate gland using catheter needles and then removed after the prescribed dose has been delivered. The International Commission on Radiologic Units and Measurements defines HDR as a dose delivered at a rate >12 Gy/h, although in actuality this is usually much higher, often in excess of 1 Gy per minute (7, 12). With regards to radiation biology, the degree of dose escalation achievable with HDR brachytherapy, compared to other EBRT techniques and LDR, may be more effective in killing prostate cancer cells (7, 20, 21). The rapid dose delivery seen in HDR is considered to be selectively more damaging to cells with lower alpha/beta ratios, such as prostate cancer and late responding normal tissues (22–24). Radiobiologic models thus support current clinical evidence for equivalent outcomes with either LDR or HDR, with theoretical advantages to HDR brachytherapy (22).
In terms of HDR brachytherapy boost, there does exist at least one randomized trial although it predates DE-EBRT. Hoskin et al. (25) performed a randomized controlled trial of a HDR boost vs. EBRT alone in patients with mostly intermediate and high-risk disease (25). Patients were randomized to receive either EBRT alone (55 Gy/20 fractions) or EBRT (35.75 Gy/13 fractions) plus an HDR boost (17 Gy/2 fractions). Neoadjuvant androgen deprivation therapy was given in 76% of patients at the discretion of the treating physician. Men in the HDR boost cohort had a 31% decrease in the risk of local recurrence, and late genitourinary toxicities were similar in both arms (7, 25). Despite providing randomized evidence, criticisms of this trial include a low EBRT alone radiation dose (55 Gy/20 fractions) compared to current standards of 60 Gy in 20 fractions or 78–80 Gy in standard fractionation. A randomized feasibility study was conducted by Vigneault et al. to assess the ability to randomize patients between dose escalated image-guided radiation therapy (IGRT) (78 Gy/39 fractions or 60 Gy/20 fractions) and IGRT plus HDR brachytherapy boost (37.5 Gy/15 fractions + 15 Gy HDR boost) with good compliance although small numbers (57 patients randomized) (26). Rates of protocol deviations and acute toxicities were low in both arms, but no biochemical control rates are reported as data matures (26).
While no other prospective, randomized comparisons of DE-EBRT and HDR boost exist, multiple single institution reports have demonstrated favorable biochemical control rates similar to those in ASCENDE-RT with better toxicity profiles. Vigneault et al. (27) reported on a cohort of 832 men with intermediate and high-risk disease treated with a range of doses of HDR brachytherapy boost in combination with EBRT and found biochemical control of 95% with median follow-up of 66 months (27). In this trial, they reported that late grade 3 GU toxicity ranged from ~2–5% dependent on the dose level the patient was treated on (27). There were no grade 3 GI toxicities reported. Androgen suppression was used in 41.3% of patients in this study (4–6 months in intermediate cases and 18–36 months in high-risk cases). There was significant differences in the median follow-up between the different HDR dose levels which did not allow for valid comparison of biochemical control rates between the different groups. Martinez et al. reported on a dose escalation trial using a HDR brachytherapy boost and found very favorable 10-years PSA control approaching 81% in men receiving the escalated dose treatment (28). Of the over 470 patients treated with EBRT plus HDR, grade 3 genitourinary toxicity was extremely rare at <1% (28). Neoadjuvant and/or concurrent androgen suppression was used in 51.3% of the patients. Additional data is also emerging in support of an HDR boost in the high-risk setting. Kent et al. (29) recently published results of their single institution retrospective review of 46 Gy EBRT plus HDR boost (median boost 18 Gy/3 fractions) compared to EBRT alone (median 70 Gy). The 5, 10, and 15-years overall survival was higher at 92, 81, and 67%, respectively, for the EBRT plus HDR cohort, compared with 88, 71, and 53%, respectively, in the EBRT alone cohort (p <0.001) (29). The 5, 10, and 15-years cause specific survival was also higher in the HDR boost group with survival of 96, 93, and 87% (EBRT plus HDR) and 95, 88, and 79% (EBRT alone), respectively (p <0.037) (29). A limitation of this study is the heterogenous use of androgen suppression at the discretion of the treating physician and specifically an increased use of ADT in the EBRT plus HDR group. Also, a median dose of 70 Gy by standard fractionation in the external beam alone group is again lower than the current standard for dose escalated therapy. Numerous other single institution trials also support the use of HDR brachytherapy boost (11, 30–34) (Supplemental Methods).
Multiple studies have suggested that when used in the monotherapy setting for more favorable localized prostate cancer, both HDR and LDR brachytherapy have equivalent biochemical progression-free survival outcomes (35, 36). For high-risk patients, the 2017 American Society of Clinical Oncology (ASCO)/Cancer Care Ontario (CCO) brachytherapy guidelines state that men with high-risk prostate cancer should be offered either an LDR or HDR boost if choosing a definitive radiation management approach (8). The recommendation of a brachytherapy boost was largely based on the previously discussed three randomized controlled trials comparing EBRT alone to EBRT plus a brachytherapy boost and demonstrate improved disease free survival with the boost (Table 1). In each of these trials, however, a different modality/type of brachytherapy boost was used. Data comparing LDR and HDR head-to-head are much more limited in the boost setting.
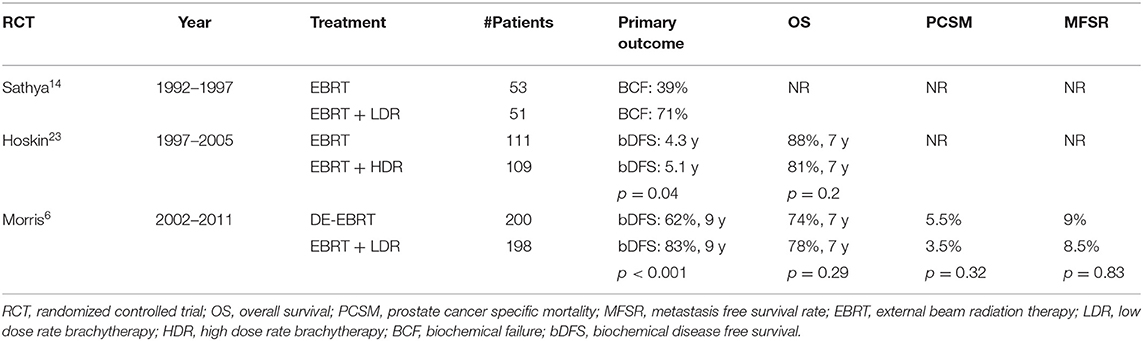
Table 1. Randomized controlled trials comparing external beam radiation therapy (EBRT) vs. EBRT plus brachytherapy boost.
For men with high-risk disease, Kishan et al. (37) reported on the differences in prostate cancer-specific mortality and distant metastasis in prostate cancer patients with high-risk disease treated with either surgery, EBRT with ADT, or EBRT plus either LDR or HDR brachytherapy with ADT in a large multi-institutional cohort (37). Androgen deprivation therapy was given in 89.5% of patients receiving EBRT alone and 92.4% of patients receiving EBRT plus brachytherapy boost (37). The duration of androgen suppression was significantly shorter in the EBRT plus brachytherapy arm (12 vs. 22 months EBRT alone; p <0.001) (37). Despite the difference in androgen suppression duration, this study found that among patients with Gleason 9–10 disease, treatment with EBRT plus brachytherapy and ADT was associated with significantly better prostate cancer-specific mortality and longer time to distant metastases compared to surgery or ADT and EBRT alone (37). They performed a cause-specific regression to determine an effect of LDR vs. HDR on clinical outcomes, including both prostate cancer-specific mortality and distant metastasis, and found no difference between the two techniques (37).
King et al. (38) used the National Cancer Database in an attempt to compare LDR vs. HDR boost with regards to overall survival outcomes. In their study, they estimated overall survival in patients with unfavorable prostate cancer treated with dose-escalated EBRT and EBRT followed by LDR boost vs. HDR boost (38). Patients included were diagnosed with NCCN intermediate or high-risk prostate cancer from a time period of 2004–2014. In their analysis of over 120,000 patients, HDR boost was associated with a similar overall survival compared to LDR boost using multivariable analysis [adjusted hazard ratio (AHR), 1.03 (0.96–1.11); p = 0.38]. Compared to dose-escalated EBRT, HDR boost was associated with significantly better overall survival [AHR, 1.36 (1.29–1.44); p <0.001] (38). Androgen deprivation therapy was given in 40.4% of patients with the HDR boost, 43.1% of patients with the LDR boost, and 49% of patients with DE-EBRT (p <0.001) (38).
RTOG P-0019 was a phase II study of EBRT combined with LDR brachytherapy boost (45 Gy/25 fractions + 108 Gy 125I boost) for intermediate risk prostate cancer with the primary goal to estimate the acute and late Grade 3-5 GU and GI toxicity (39). Short-term androgen suppression up to 6 months was allowed and 27% received ADT. A total of 138 patients from 28 institutions were enrolled on the study with acute toxicity evaluable in 131 patients (39). Acute Grade 3 GU toxicity was recorded in 7.6% of patients without any Grade 4 or 5 events (39). Six months after radiation therapy, ~63% of patients reported a higher International Prostate Symptom Score (IPSS) score compared to baseline (39). The 18-months estimate of both late Grade 3 GU and GI toxicity was 3.3% (39). With longer follow-up, increased rates of Grade 3 or greater GU/GI toxicity were reported, estimated at 15% (95% CI, 8–21%) at 48 months (40). CALGB 99809 was another multi-institutional trial designed to assess the toxicity and feasibility of EBRT plus LDR brachytherapy boost (45 Gy/25 fractions + 100 Gy 125I or 90 Gy 103Pd boost) combined with 6 months of ADT (41). Acute Grade 2 and 3 toxicity occurred in 25 and 7% of men and was most commonly urinary frequency/urgency (41). Late Grade 2 and 3 toxicity was observed in 20% and 2% of men, respectively (41). Differences between these two multi-institutional protocols included an expansion on the LDR boost clinical target volume (CTV) of 5 mm (0 mm posteriorly) in the RTOG trial compared to no expansion in the CALGB trial, which may contribute to the rates of late Grade 3 or greater toxicities.
In the randomized ASCENDE-RT trial, toxicity was increased in the brachytherapy group with the cumulative incidence of grade 3 GU events at 5 years of ~18% for the brachytherapy boost arm vs. 5% for the EBRT alone arm (p <0.001). There was also a trend toward increased gastrointestinal toxicity with the brachytherapy boost, 8 vs. 3% (p = 0.12) (42). However, at the 6-years follow-up time point, health-related quality of life was similar between the two groups in most domains with the exception that physical and urinary function scales were lower in the LDR arm (43). Regardless, the increased toxicity observed in the combined EBRT plus LDR boost arm ASCENDE-RT highlights the importance of careful patient selection and diligent treatment planning as well as early intervention with symptom management as needed for these patients. A detailed analysis of the treatment related morbidity from the trial is available (42).
HDR brachytherapy may be a method to overcome the acute toxicities seen with LDR given the physical properties of this treatment modality. With an LDR implant, the radiation dose is delivered over a time period of months compared to minutes with HDR. For this reason, LDR is associated with a more prolonged recovery period. A prospective non-randomized comparison of quality of life after LDR vs. HDR boost (combined with 4.5 weeks of EBRT) showed a return to baseline IPSS at 6 months with LDR compared to only 12 weeks with HDR (44). Another early analysis of a randomized controlled trial of HDR vs. LDR in the monotherapy setting suggests improved quality of life, shorter return time to baseline urinary function, and lower rates of acute urinary symptoms with HDR monotherapy (45). While the previous studies show very favorable toxicity profiles with HDR brachytherapy compared to LDR, HDR has been associated with non-insignificant rates of urethral stricture. In a study by Bece et al. reported in 2015, various doses and fractionations of HDR boost (19.5 Gy/3fractions; 17 Gy/2 fractions; 18 Gy/2 fractions; and 19 Gy/2 fractions) in combination with EBRT were used and overall 3 and 6-years stricture incidence were 7.8 and 15.3%, respectively (46). The HDR boost fractionation scheme evolved during their study and the most recent fractionation used (19 Gy/2 fractions) resulted in the lowest three-year stricture rate of 3.0% (46). Yaxley et al. retrospectively analyzed a series of 507 men consecutively treated with EBRT plus HDR brachytherapy with a median follow-up of 10.3 years and found that rates of urethral stricture can be significantly reduced with careful attention to dose heterogeneity constraints, imaging prior to second HDR fraction to control for needle displacement, and tighter apical (inferior) PTV margins during the EBRT (47). Prior to implementation of these “stricture prevention measures,” the rate of stricture was 13.6% and this rate dramatically fell to 4.2% using these planning considerations (47).
In terms of long-term toxicity, an investigation using the Surveillance, Epidemiology, and End Results Medicare database (SEER) did not show a statistically significant difference in Grade 3 genitourinary adverse events between LDR and HDR (48). The results of the BrachyQOL randomized controlled trial (NCT01936883) are highly anticipated as they will shed more definitive light on both the acute and late GU/GI side-effect profiles between LDR and HDR in the boost setting (49).
Despite potential improved outcomes with either LDR or HDR boost, the rates of brachytherapy boost utilization are declining (50, 51). Multiple reasons for the declining use of a boost have been reported including increase of prostatectomies for higher risk patients (52), increases in reimbursement for other EBRT techniques (53), decrease in brachytherapy training (54), and potential perception that brachytherapy is a procedure with excessive liability risk (51). In an analysis by Johnson et al. (19), the utilization of LDR brachytherapy boost dropped from ~29% in 2004 to 14% in 2012. Previous database-based studies also report the declining use of EBRT plus brachytherapy boost (52). The American Brachytherapy Society (ABS) has started a “300 in 10” initiative to increase the training of brachytherapists by assisting in the training of 30 oncologists per year over a 10-years period. Initiatives such as this are extremely important as a brachytherapy boost has the potential to improve prostate-specific survival outcomes when compared to EBRT alone.
While an optimal dose for LDR brachytherapy boost has been established, studies continue to determine the optimal HDR schedule and dose escalation continues to be investigated in the HDR setting. Also, limited data on prostate cancer specific survival outcomes between HDR and LDR exist. The British Columbia Cancer Agency is conducting a Phase III randomized controlled trial in patients with unfavorable intermediate risk and high-risk prostate cancer who will receive 46 Gy in 23 fractions of EBRT and then be randomized to either a LDR boost using 125I (115 Gy) or HDR boost using 192Ir (15Gy x 1). In addition to quality of life measures, a secondary outcome is PSA recurrence-free survival which will provide randomized head-to-head outcomes between LDR and HDR brachytherapy boost. Another unknown for both LDR and HDR boost in the high risk setting is defining the optimal planning target volume (PTV) to balance tumor coverage while minimizing toxicity. The differences in CTV to PTV expansion between RTOG 0019 and CALBG 99809 in the intermediate risk setting were 5 vs. 0 mm, respectively, and may have long term toxicity consequences. In high-risk prostate cancer, while the external beam target volumes should include any extracapsular extension and the at risk proximal seminal vesicles, some intuitions are including both the proximal seminal vesicles and extracapsular extension in the brachytherapy boost volume, but the clinical significance of such inclusion is unknown. Advanced computer planning and CT/MRI/Ultrasound-based planning with HDR brachytherapy may allow better and more reproducible coverage of the seminal vesicles and extracapsular extension compared to LDR given the inherent post-implant treatment planning capabilities with HDR. Additionally, as imaging technology continues to improve, small institutional trials are underway or have completed investigating focal brachytherapy boost to intraprostatic lesions using MRI-transrectal-ultrasound fusion (55). Lastly, SBRT continues to gain popularity given its shorter treatment course and less invasive nature, comparisons between SBRT and brachytherapy are emerging. Preliminary data from a reported literature search of 47 studies on PubMed and Embase (6 SBRT boost and 41 HDR boost), showed that a SBRT boost may be associated with higher acute Grade 2 genitourinary toxicity but lower late Grade 3 GU toxicity, and no difference was seen between the two by quality of life reports. Randomized trials between both LDR and HDR boost and SBRT boost are warranted and underway.
Both HDR and LDR brachytherapy provide a method of biologically equivalent dose escalation in patients with high-risk prostate cancer who are undergoing definitive intent radiation therapy. In combination with EBRT, brachytherapy is a modality to deliver highly conformal dose escalation while drastically sparing the rectum and bladder compared to EBRT alone. Two randomized controlled trials have shown improved biochemical control with EBRT plus brachytherapy boost but neither demonstrated a statistical difference in overall survival (16, 25). The LDR boost arm in the ASCENDE-RT trial demonstrated a significant improvement in biochemical progression-free survival and long term survival outcomes are eagerly anticipated (9). Despite the improvements in biochemical control with brachytherapy boost, trends in the use of brachytherapy continue to decline nationally, possibly secondary to concerns of acute genitourinary toxicity with LDR. Initiatives to increase brachytherapy use are currently underway, and HDR brachytherapy may be an opportunity to improve toxicity profiles while exploiting the radiobiology of prostate cancer in the boost setting.
BF-V, HG, SP, BB, and JM: literature search, writing, and final approval.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2019.01378/full#supplementary-material
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. (2019) 69:7–34. doi: 10.3322/caac.21551
2. Mohler JL, Antonarakis ES. NCCN guidelines updates: management of prostate cancer. J Natl Compr Canc Netw. (2019) 17:583–6. doi: 10.6004/jnccn.2019.5011
3. Michalski JM, Moughan J, Purdy J, Bosch W, Bruner DW, Bahary JP, et al. Effect of standard vs. dose-escalated radiation therapy for patients with intermediate-risk prostate cancer: the NRG oncology RTOG 0126 randomized clinical trial. JAMA Oncol. (2018) 4:e180039. doi: 10.1001/jamaoncol.2018.0039
4. Trada Y, Plank A, Martin J. Defining a dose-response relationship for prostate external beam radiotherapy. J Med Imaging Radiat Oncol. (2013) 57:237–46. doi: 10.1111/1754-9485.12008
5. Pryor D, Sidhom M, Arumugam S, Bucci J, Gallagher S, Smart J, et al. Phase 2 multicenter study of gantry-based stereotactic radiotherapy boost for intermediate and high risk prostate cancer (PROMETHEUS). Front Oncol. (2019) 9:217. doi: 10.3389/fonc.2019.00217
6. Zaorsky NG, Keith SW, Shaikh T, Nguyen PL, Horwitz EM, Dicker AP, et al. Impact of radiation therapy dose escalation on prostate cancer outcomes and toxicities. Am J Clin Oncol. (2018) 41:409–15. doi: 10.1097/COC.0000000000000285
7. Mendez LC, Morton GC. High dose-rate brachytherapy in the treatment of prostate cancer. Transl Androl Urol. (2018) 7:357–70. doi: 10.21037/tau.2017.12.08
8. Chin J, Rumble RB, Kollmeier M, Heath E, Efstathiou J, Dorff T, et al. Brachytherapy for patients with prostate cancer: American society of clinical oncology/cancer care ontario joint guideline update. J Clin Oncol. (2017) 35:1737–43. doi: 10.1200/JCO.2016.72.0466
9. Morris WJ, Tyldesley S, Rodda S, Halperin R, Pai H, McKenzie M, et al. Androgen suppression combined with elective nodal and dose escalated radiation therapy (the ASCENDE-RT Trial): an analysis of survival endpoints for a randomized trial comparing a low-dose-rate brachytherapy boost to a dose-escalated external beam boost for high- and intermediate-risk prostate cancer. Int J Radiat Oncol Biol Phys. (2017) 98:275–85. doi: 10.1016/j.ijrobp.2016.11.026
10. Wang Y, Sankreacha R, Al-Hebshi A, Loblaw A, Morton G. Comparative study of dosimetry between high-dose-rate and permanent prostate implant brachytherapies in patients with prostate adenocarcinoma. Brachytherapy. (2006) 5:251–5. doi: 10.1016/j.brachy.2006.08.006
11. Martinez AA, Demanes J, Vargas C, Schour L, Ghilezan M, Gustafson GS. High-dose-rate prostate brachytherapy: an excellent accelerated-hypofractionated treatment for favorable prostate cancer. Am J Clin Oncol. (2010) 33:481–8. doi: 10.1097/COC.0b013e3181b9cd2f
12. International Commission on Radiological Units and Measurements. Dose and Volume Specifications for Reporting Intracavitary Therapy in Gynecology. ICRU report 38 (ICRU).
13. Taira AV, Merrick GS, Butler WM, Galbreath RW, Lief J, Adamovich E, et al. Long-term outcome for clinically localized prostate cancer treated with permanent interstitial brachytherapy. Int J Radiat Oncol Biol Phys. (2011) 79:1336–42. doi: 10.1016/j.ijrobp.2010.01.005
14. Crook J, Borg J, Evans A, Toi A, Saibishkumar EP, Fung S, et al. 10-year experience with I-125 prostate brachytherapy at the Princess Margaret Hospital: results for 1,100 patients. Int J Radiat Oncol Biol Phys. (2011) 80:1323–9. doi: 10.1016/j.ijrobp.2010.04.038
15. Marshall RA, Buckstein M, Stone NN, Stock R. Treatment outcomes and morbidity following definitive brachytherapy with or without external beam radiation for the treatment of localized prostate cancer: 20-years experience at Mount Sinai Medical Center. Urol Oncol. (2014) 32:38 e1–7. doi: 10.1016/j.urolonc.2013.03.004
16. Sathya JR, Davis IR, Julian JA, Guo Q, Daya D, Dayes IS, et al. Randomized trial comparing iridium implant plus external-beam radiation therapy with external-beam radiation therapy alone in node-negative locally advanced cancer of the prostate. J Clin Oncol. (2005) 23:1192–9. doi: 10.1200/JCO.2005.06.154
17. Dayes IS, Parpia S, Gilbert J, Julian JA, Davis IR, Levine MN, et al. Long-term results of a randomized trial comparing iridium implant plus external beam radiation therapy with external beam radiation therapy alone in node-negative locally advanced cancer of the prostate. Int J Radiat Oncol Biol Phys. (2017) 99:90–3. doi: 10.1016/j.ijrobp.2017.05.013
18. Muralidhar V, Xiang M, Orio PF III, Martin NE, Beard CJ, Feng FY, et al. Brachytherapy boost and cancer-specific mortality in favorable high-risk versus other high-risk prostate cancer. J Contemp Brachytherapy. (2016) 8:1–6. doi: 10.5114/jcb.2016.58080
19. Johnson SB, Lester-Coll NH, Kelly JR, Kann BH, Yu JB, Nath SK. Brachytherapy boost utilization and survival in unfavorable-risk prostate cancer. Eur Urol. (2017) 72:738–44. doi: 10.1016/j.eururo.2017.06.020
20. Stish BJ, Davis BJ, Mynderse LA, McLaren RH, Deufel CL, Choo R. Low dose rate prostate brachytherapy. Transl Androl Urol. (2018) 7:341–56. doi: 10.21037/tau.2017.12.15
21. King CR, DiPetrillo TA, Wazer DE. Optimal radiotherapy for prostate cancer: predictions for conventional external beam, IMRT, and brachytherapy from radiobiologic models. Int J Radiat Oncol Biol Phys. (2000) 46:165–72. doi: 10.1016/S0360-3016(99)00406-X
22. King CR. LDR vs. HDR brachytherapy for localized prostate cancer: the view from radiobiological models. Brachytherapy. (2002) 1:219–26. doi: 10.1016/S1538-4721(02)00101-0
23. Brenner DJ, Hall EJ. Fractionation and protraction for radiotherapy of prostate carcinoma. Int J Radiat Oncol Biol Phys. (1999) 43:1095–101. doi: 10.1016/S0360-3016(98)00438-6
24. Brenner DJ, Martinez AA, Edmundson GK, Mitchell C, Thames HD, Armour EP. Direct evidence that prostate tumors show high sensitivity to fractionation (low alpha/beta ratio), similar to late-responding normal tissue. Int J Radiat Oncol Biol Phys. (2002) 52:6–13. doi: 10.1016/S0360-3016(01)02664-5
25. Hoskin PJ, Rojas AM, Bownes PJ, Lowe GJ, Ostler PJ, Bryant L. Randomised trial of external beam radiotherapy alone or combined with high-dose-rate brachytherapy boost for localised prostate cancer. Radiother Oncol. (2012) 103:217–22. doi: 10.1016/j.radonc.2012.01.007
26. Vigneault E, Morton G, Parulekar WR, Niazi TM, Springer CW, Barkati M, et al. Randomised phase II feasibility trial of image-guided external beam radiotherapy with or without high dose rate brachytherapy boost in men with intermediate-risk prostate cancer (CCTG PR15/NCT01982786). Clin Oncol. (2018) 30:527–33. doi: 10.1016/j.clon.2018.05.007
27. Vigneault E, Mbodji K, Magnan S, Despres P, Lavallee MC, Aubin S, et al. High-dose-rate brachytherapy boost for prostate cancer treatment: different combinations of hypofractionated regimens and clinical outcomes. Radiother Oncol. (2017) 124:49–55. doi: 10.1016/j.radonc.2017.06.012
28. Martinez AA, Gonzalez J, Ye H, Ghilezan M, Shetty S, Kernen K, et al. Dose escalation improves cancer-related events at 10 years for intermediate- and high-risk prostate cancer patients treated with hypofractionated high-dose-rate boost and external beam radiotherapy. Int J Radiat Oncol Biol Phys. (2011) 79:363–70. doi: 10.1016/j.ijrobp.2009.10.035
29. Kent AR, Matheson B, Millar JL. Improved survival for patients with prostate cancer receiving high-dose-rate brachytherapy boost to EBRT compared with EBRT alone. Brachytherapy. (2019) 18:313–21. doi: 10.1016/j.brachy.2019.01.013
30. Borghede G, Hedelin H, Holmang S, Johansson KA, Aldenborg F, Pettersson S, et al. Combined treatment with temporary short-term high dose rate iridium-192 brachytherapy and external beam radiotherapy for irradiation of localized prostatic carcinoma. Radiother Oncol. (1997) 44:237–44. doi: 10.1016/S0167-8140(97)00121-7
31. Martinez A, Gonzalez J, Spencer W, Gustafson G, Kestin L, Kearney D, et al. Conformal high dose rate brachytherapy improves biochemical control and cause specific survival in patients with prostate cancer and poor prognostic factors. J Urol. (2003) 169:974–9; discussion 9–80. doi: 10.1097/01.ju.0000052720.62999.a9
32. Galalae RM, Loch T, Riemer B, Rzehak P, Kuchler T, Kimmig B, et al. Health-related quality of life measurement in long-term survivors and outcome following radical radiotherapy for localized prostate cancer. Strahlenther Onkol. (2004) 180:582–9. doi: 10.1007/s00066-004-1254-x
33. Duchesne GM, Williams SG, Das R, Tai KH. Patterns of toxicity following high-dose-rate brachytherapy boost for prostate cancer: mature prospective phase I/II study results. Radiother Oncol. (2007) 84:128–34. doi: 10.1016/j.radonc.2007.05.019
34. Kalkner KM, Wahlgren T, Ryberg M, Cohn-Cedermark G, Castellanos E, Zimmerman R, et al. Clinical outcome in patients with prostate cancer treated with external beam radiotherapy and high dose-rate iridium 192 brachytherapy boost: a 6-year follow-up. Acta Oncol. (2007) 46:909–17. doi: 10.1080/02841860601156140
35. Shah C, Lanni TB Jr, Ghilezan MI, Gustafson GS, Marvin KS, Ye H, et al. Brachytherapy provides comparable outcomes and improved cost-effectiveness in the treatment of low/intermediate prostate cancer. Brachytherapy. (2012) 11:441–5. doi: 10.1016/j.brachy.2012.04.002
36. Grimm P, Billiet I, Bostwick D, Dicker AP, Frank S, Immerzeel J, et al. Comparative analysis of prostate-specific antigen free survival outcomes for patients with low, intermediate and high risk prostate cancer treatment by radical therapy. Results from the Prostate Cancer Results Study Group. BJU Int. (2012) 109 (Suppl. 1):22–9. doi: 10.1111/j.1464-410X.2011.10827.x
37. Kishan AU, Cook RR, Ciezki JP, Ross AE, Pomerantz MM, Nguyen PL, et al. Radical prostatectomy, external beam radiotherapy, or external beam radiotherapy with brachytherapy boost and disease progression and mortality in patients with gleason score 9-10 prostate cancer. JAMA. (2018) 319:896–905. doi: 10.1001/jama.2018.0587
38. King MT, Yang DD, Muralidhar V, Mahal B, Butler S, Devlin PM, et al. A comparative analysis of overall survival between high-dose-rate and low-dose-rate brachytherapy boosts for unfavorable-risk prostate cancer. Brachytherapy. (2019) 18:186–91. doi: 10.1016/j.brachy.2018.12.007
39. Lee WR, DeSilvio M, Lawton C, Gillin M, Morton G, Firat S, et al. A phase II study of external beam radiotherapy combined with permanent source brachytherapy for intermediate-risk, clinically localized adenocarcinoma of the prostate: preliminary results of RTOG P-0019. Int J Radiat Oncol Biol Phys. (2006) 64:804–9. doi: 10.1016/j.ijrobp.2005.09.002
40. Lee WR, Bae K, Lawton C, Gillin M, Morton G, Firat S, et al. Late toxicity and biochemical recurrence after external-beam radiotherapy combined with permanent-source prostate brachytherapy: analysis of Radiation Therapy Oncology Group study 0019. Cancer. (2007) 109:1506–12. doi: 10.1002/cncr.22560
41. Hurwitz MD, Halabi S, Archer L, McGinnis LS, Kuettel MR, DiBiase SJ, et al. Combination external beam radiation and brachytherapy boost with androgen deprivation for treatment of intermediate-risk prostate cancer: long-term results of CALGB 99809. Cancer. (2011) 117:5579–88. doi: 10.1002/cncr.26203
42. Rodda S, Tyldesley S, Morris WJ, Keyes M, Halperin R, Pai H, et al. ASCENDE-RT: an analysis of treatment-related morbidity for a randomized trial comparing a low-dose-rate brachytherapy boost with a dose-escalated external beam boost for high- and intermediate-risk prostate cancer. Int J Radiat Oncol Biol Phys. (2017) 98:286–95. doi: 10.1016/j.ijrobp.2017.01.008
43. Rodda S, Morris WJ, Hamm J, Duncan G. ASCENDE-RT: an analysis of health-related quality of life for a randomized trial comparing low-dose-rate brachytherapy boost with dose-escalated external beam boost for high- and intermediate-risk prostate cancer. Int J Radiat Oncol Biol Phys. (2017) 98:581–9. doi: 10.1016/j.ijrobp.2017.02.027
44. Rose TGE, Bachand F, Kim D. QOL comparison of acute side effects from a high dose rate vs. low dose rate brachytherapy boost combined with external beam radiotherapy. Brachytherapy. (2015) 14:S36. doi: 10.1016/j.brachy.2015.02.245
45. Hathout L, Mahmoud O, Barkati M, Despres P, Mbodjii K, Martin AG, et al. A phase II randomized pilot study comparing high-dose rate brachytherapy and low-dose rate brachytherapy as monotherapy in localized prostate cancer. Brachytherapy. (2018) 17:S56–S5. doi: 10.1016/j.brachy.2018.04.090
46. Bece A, Patanjali N, Jackson M, Whitaker M, Hruby G. High-dose-rate brachytherapy boost for prostate cancer: outcomes and genitourinary toxicity. Brachytherapy. (2015) 14:670–6. doi: 10.1016/j.brachy.2015.04.004
47. Yaxley JW, Lah K, Yaxley JP, Gardiner RA, Samaratunga H, MacKean J. Long-term outcomes of high-dose-rate brachytherapy for intermediate- and high-risk prostate cancer with a median follow-up of 10 years. BJU Int. (2017) 120:56–60. doi: 10.1111/bju.13659
48. Tward JD, Jarosek S, Chu H, Thorpe C, Shrieve DC, Elliott S. Time course and accumulated risk of severe urinary adverse events after high- versus low-dose-rate prostate brachytherapy with or without external beam radiation therapy. Int J Radiat Oncol Biol Phys. (2016) 95:1443–53. doi: 10.1016/j.ijrobp.2016.03.047
49. Improving quality of life after prostate brachytherapy: a comparison of HDR and LDR brachytherapy. https://clinicaltrials.gov/ct2/show/NCT01936883.
50. Barnes J, Kennedy WR, Fischer-Valuck BW, Baumann BC, Michalski JM, Gay HA. Treatment patterns of high-dose-rate and low-dose-rate brachytherapy as monotherapy for prostate cancer. J Contemp Brachyther. (2019) 11:320–8. doi: 10.5114/jcb.2019.86974
51. Petereit DG, Frank SJ, Viswanathan AN, Erickson B, Eifel P, Nguyen PL, et al. Brachytherapy: where has it gone? J Clin Oncol. (2015) 33:980–2. doi: 10.1200/JCO.2014.59.8128
52. Martin JM, Handorf EA, Kutikov A, Uzzo RG, Bekelman JE, Horwitz EM, et al. The rise and fall of prostate brachytherapy: use of brachytherapy for the treatment of localized prostate cancer in the National Cancer Data Base. Cancer. (2014) 120:2114–21. doi: 10.1002/cncr.28697
53. Mitchell JM. Urologists' use of intensity-modulated radiation therapy for prostate cancer. N Engl J Med. (2013) 369:1629–37. doi: 10.1056/NEJMsa1201141
54. Compton JJ, Gaspar LE, Shrieve DC, Wilson LD, Griem KL, Amdur RJ, et al. Resident-reported brachytherapy experience in ACGME-accredited radiation oncology training programs. Brachytherapy. (2013) 12:622–7. doi: 10.1016/j.brachy.2013.06.004
55. Gomez-Iturriaga A, Casquero F, Urresola A, Ezquerro A, Lopez JI, Espinosa JM, et al. Dose escalation to dominant intraprostatic lesions with MRI-transrectal ultrasound fusion High-Dose-Rate prostate brachytherapy. Prospective phase II trial. Radiother Oncol. (2016) 119:91–6. doi: 10.1016/j.radonc.2016.02.004
Keywords: HDR, LDR, brachytherapy, boost, high risk prostate, cancer
Citation: Fischer-Valuck BW, Gay HA, Patel S, Baumann BC and Michalski JM (2019) A Brief Review of Low-Dose Rate (LDR) and High-Dose Rate (HDR) Brachytherapy Boost for High-Risk Prostate. Front. Oncol. 9:1378. doi: 10.3389/fonc.2019.01378
Received: 26 September 2019; Accepted: 22 November 2019;
Published: 10 December 2019.
Edited by:
Timothy Showalter, University of Virginia, United StatesReviewed by:
Gerard Morton, University of Toronto, CanadaCopyright © 2019 Fischer-Valuck, Gay, Patel, Baumann and Michalski. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jeff M. Michalski, am1pY2hhbHNraUB3dXN0bC5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.