- 1Department of Pharmacy, The First Hospital of Jilin University, Changchun, China
- 2Jilin Provincial Key Laboratory on Molecular and Chemical Genetic, The Second Hospital of Jilin University, Changchun, China
- 3Department of Gastrointestinal Colorectal Surgery, China-Japan Union Hospital of Jilin University, Changchun, China
Natural compounds are highly effective anticancer chemotherapeutic agents, and the targets of plant-derived anticancer agents have been widely reported. In this review, we focus on the main signaling pathways of apoptosis, proliferation, invasion, and metastasis that are regulated by polyphenols, alkaloids, saponins, and polysaccharides. Alkaloids primarily affect apoptosis-related pathways, while polysaccharides primarily target pathways related to proliferation, invasion, and metastasis. Other compounds, such as flavonoids and saponins, affect all of these aspects. The association between compound structures and signaling pathways may play a critical role in drug discovery.
Introduction
In 2018, an estimated 9.6 million deaths were caused by cancer, and cancer is anticipated to be the leading cause of death worldwide in the twenty-first century (1). Therefore, cancer prevention remains an innovative area of anticancer research, in addition to cancer therapy. The mechanisms of aberrant signal transduction pathways in cancer and the impacts of these pathways on tumorigenesis, apoptosis, and metastasis have been increasingly revealed due to intensified study (2). Searching for targeted molecules that can regulate signal transduction has recently emerged as a globally popular research area in biomedicine.
Herbal medicines, such as Chinese medicines, are naturally exceptional at ameliorating many human diseases. Increasing numbers of new drugs with pharmacological activity have been discovered due to the modernization of herbal medicine. The anticancer agents vincristine, taxol, and vinblastine have been used for their anticancer effects in many countries (3). Moreover, other promising anticancer agents are available, including arteannuin (4), quercetin (5), and tetrandrine (6). Alkaloids and polyphenols are significantly dominant among cancer therapeutics (7, 8). Recently, the targets and mechanisms of plant-derived anticancer agents have been widely reported (9). In this review, we will focus on advances in knowledge about the signaling pathways affected by plant-derived natural products.
Polyphenols
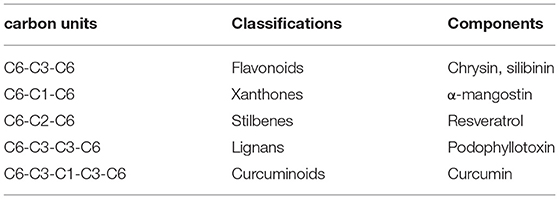
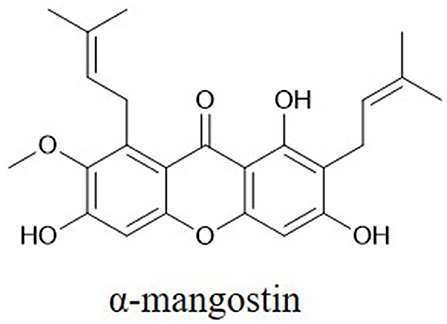
Polyphenols are particularly ubiquitous in vegetables, fruits, and other foods. Thousands of polyphenols have been identified (10), and these compounds have broad-spectrum pharmacological activities including anticancer effects. Polyphenols can be classified by their chemical structures into several classes such as flavonoids, xanthones, stilbenes, lignans, and curcuminoids (Table 1) (11–14). Many natural polyphenols have cytostatic and apoptotic properties because of their antioxidant characteristics (11). The anticancer effects of polyphenols depend not only on their chemical structure and concentration but also on the type of cancer. Lignans considered to be phytoestrogens are bioactive compounds exhibiting various anticancer properties, such as apoptosis induction and tumor growth reduction (15). Xanthones, such as α-mangostin, mediate cytotoxicity mainly via cell cycle arrest and reactive oxygen species (ROS)-induced apoptosis (16). The anticancer effects and molecular mechanisms of polyphenols are reported to be associated with their chemical constitution which is necessary for its anticancer activities, such as the C-3 prenylation of benzoxanthone-type prenylated flavonoids, C-1 hydroxy group and isoprenyl group at C-8 of prenylated xanthones, the C-2 carbonyl group, C-4 prenyl group and pyran ring connected at the C-2 and C-3 of caged xanthones (9). Anticarcinogenic activities of polyphenols include suppressing the proliferation, differentiation, metastasis, and angiogenesis of various kinds of cancer cells through inhibiting several kinases involved in signal transduction (17–20). Polyphenols can bind and cross cell membranes easily and trigger various pathways involving microRNAs (miRNAs), caspases, B cell lymphoma 2 (Bcl-2) family proteins, nuclear factor (NF)-κB, epidermal growth factor (EGF)/epidermal growth factor receptor (EGFR), phosphatidylinositol-3-kinase (PI3K)/Akt, mitogen-activated protein kinase (MAPK) (Table 2).
MicroRNAs
MicroRNAs (miRNAs) are small non-coding RNAs (NC-RNAs) and regulate gene expression via binding to 3′ untranslated regions (UTRs) of target mRNA (44). Approximate 1,500 miRNA have been identified in the human (45). Oncogenic miRNAs have been identified in many kinds of cancers such as miR-7-1, miR-21, miR-92, miR-122, miR-125b, miR-155, miR-330 (46). It is indicated miRNAs are critical in cancer cell proliferations, differentiation, apoptosis, and invasion through the regulation of oncogenic gene expression (47, 48). It is predicted a miRNA can recognize an average of 100–200 different mRNA targets (49, 50). For example, miR-155 modulates the expression of NF-κB and MAFK via regulation of BACH1 (BTB and CNC homology 1, basic leucine zipper transcription factor 1) and LDOC1 (leucine zipper, downregulated in cancer 1) which is critical to malignant transformation in leukemia, breast and lung cells (51–53). It is emphasized that miRNAs are novel therapeutic targets of polyphenols such as curcumin, resveratrol, genistein, EGCG and silibinin (45, 54–56).
Curcumin [(1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptane-3,5-dione] is a curcuminoid extracted from the rhizome of Curcuma longa Linn (57). It is demonstrated that 5–40 μM of curcumin has effects on a variety of miRNAs in different cancer cell lines such as miR-192-5b (58), miRNA-98 (59), miR-21 (60–62), miR-15a (63, 64), miR-101 (65, 66) in lung cancer, colorectal cancer, leukemia, colon cancer, and breast cancer to inhibit cell viability and metastasis, induce apoptosis.
According to quantitative reverse transcription-polymerase chain reaction (qRT-PCR) analysis, resveratrol (3,4′,5-trihydroxy-trans-stilbene) with dosage of 10–150 μM induces apoptosis and depresses cell proliferation, invasion via inhibition of NF-κB activity, Akt/Bcl-2 pathway, EZH2 pathway, STAT3 and COX-2 activity through upregulation of miR-34a (67), miR-326 (68), miR-200c (69), miR-137 (70), and miR-328 (71), and downregulation of miR-19 (72), miR-21 (73), miR-196b (74), miR-1290 (74), and miR-221 (75, 76).
Genistein (4′,5,7-trihydroxyisoflavone, Figure 1), found in soy products, has effects on miRNAs in various cancer cells (77). Breast cancer cell growth is inhibited by the induction of miR-23b and inhibition of miR-155 by 25–175 μM of genistein treatment (78, 79). Genistein inhibits the expression of miR-27a (80) and miR-223 (81) and induces the expression of let-7d (82) and miR-34a (83) which play an important role in pancreatic cancer cell growth and invasion. Genistein also exerts its anticancer activity via upregulation of miR-200c (84) and downregulation of miR-151 in prostate cancer (85).
The green tea extracts (–)-epigallocatechin (EGC) and (–)-epigallocatechin-3-gallate (EGCG) also targets oncogenic miRNAs including upregulation of miR-16, let-7a, and miR-221 and downregulation of miR-18a, miR34b, miR-193, miR-222, and miR-342 in human hepatocellular carcinoma cells (86). Expression of miR-548m and miR-720 are down-regulated in human breast cancer MCF-7 cells (87). miR-210 is up-regulated by EGCG in lung cancer cells which is associated with HIF-1α (hypoxia-inducible factor 1-alpha) (88). EGCG (40–60 μg/ml) suppresses cell growth of cervical carcinoma by regulation of miRNAs including up-regulation of miR-29, miR-29a, miR-203 and miR-210, and down-regulation of miR-125b, miR-203, miR-125b (89).
NF-κB Pathways
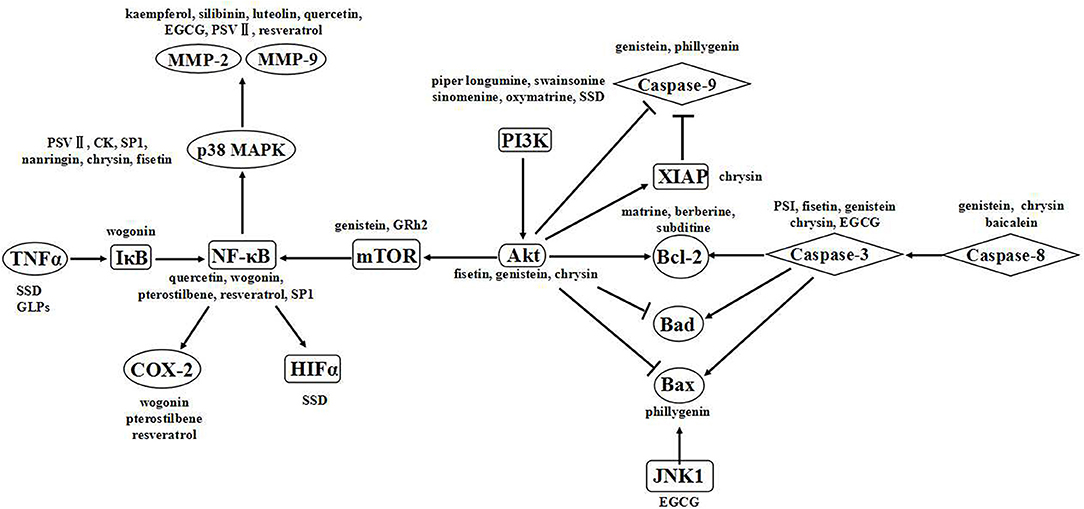
NF-κB can regulate the transcription of genes associated with the inflammatory response, cell death, and proliferation (90, 91). NF-κB pathways participating in the development of various cancers can be disrupted by polyphenols. The PI3K/Akt signaling pathway and MAPK signaling pathways are related to the activation of NF-κB in numerous tumor cell lines (92).
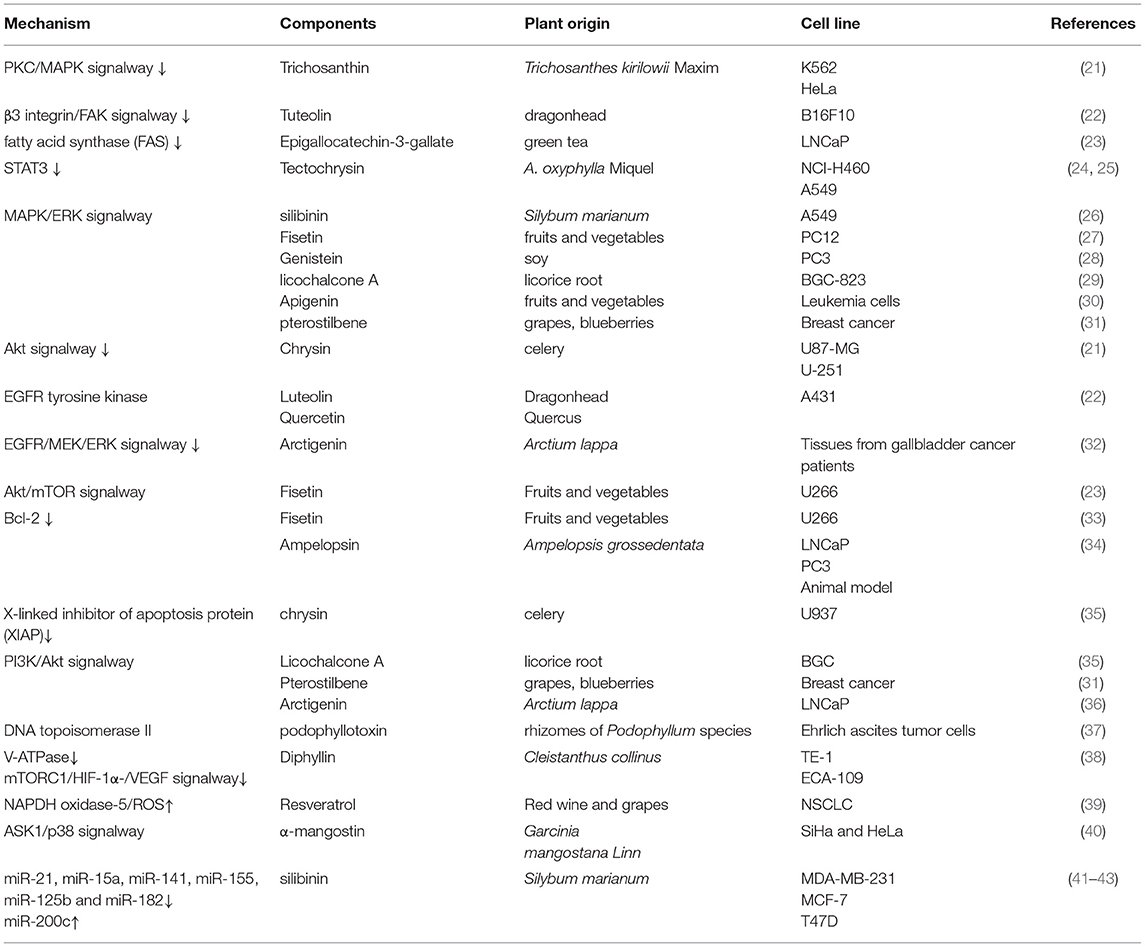
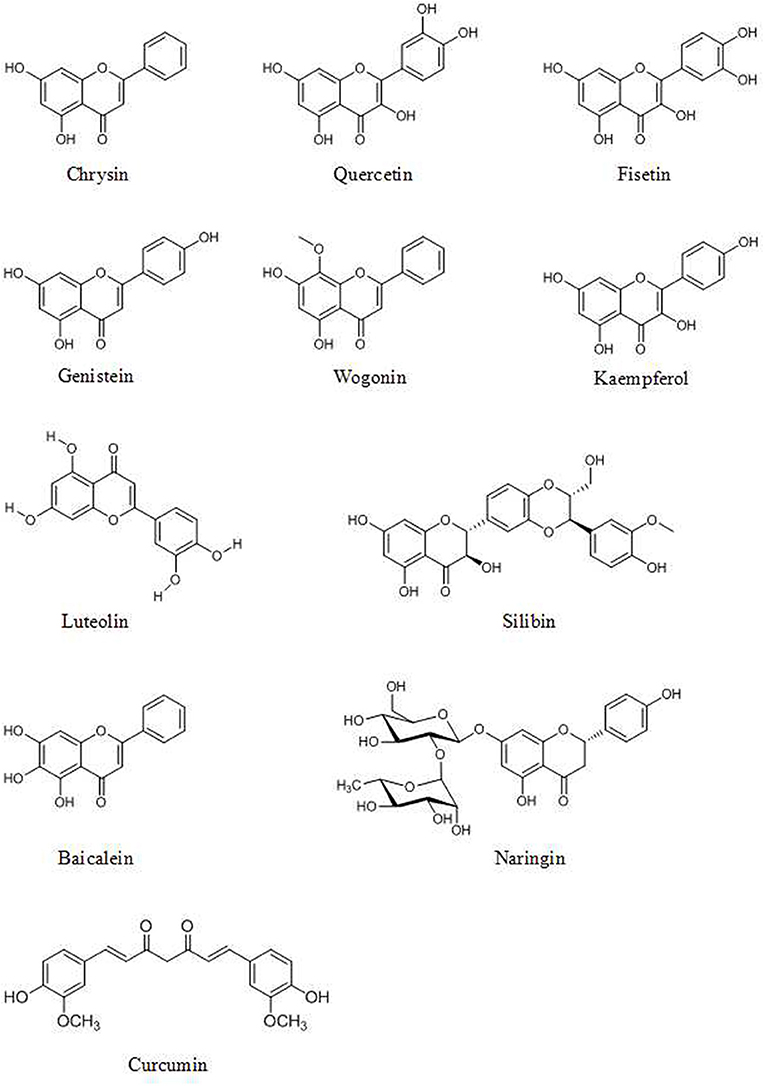
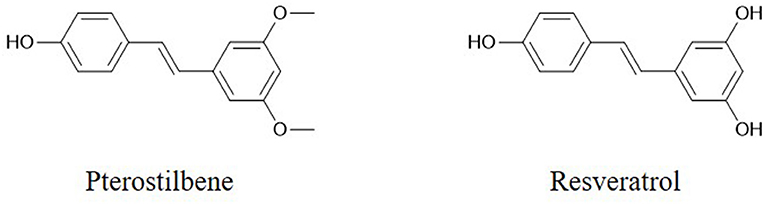
The flavonoid component chrysin (5,7-dihydroxyflavone, Figure 1) has been shown to suppress the growth of colon cancer cells via direct inhibition of NF-κB expression and activity, according to computational docking experiments (24). In addition, 30 μM chrysin activates NF-κB/p65 by inducing p38 MAPK signaling pathways in HeLa cells (33). Quercetin (Figure 1) has a potential role in inhibiting processes in human oral cancer cells through the NF-κB pathway (93). The results of Western blot and flow cytometric assays indicate that the flavonoid fisetin (3,3′,4′,7-tetrahydroxyflavone, Figure 1) effectively suppresses the apoptosis, metastasis, angiogenesis and invasion of cancer cells via ERK1/2-, Akt/NF-κB/mTOR- and p38 MAPK-dependent NF-κB signaling pathways (94, 95). Furthermore, fisetin is not cytotoxic to normal cells (94). Genistein has a potential role in inhibiting cell division and apoptosis via Akt and NF-κB (28). Wogonin (Figure 1), extracted from Scutellaria baicalensis Georgi, can decrease the phosphorylation levels of IκB and p65. Modulation of the NF-κB/Bcl-2 signaling pathway has been shown by Western blot analysis to play a critical role in both of the invasion and proliferation of hepatocellular carcinoma (HCC) in a dose-dependent manner (96). Wogonin is shown to decrease the protein and mRNA levels of cyclooxygenase (COX)-2 in skin fibroblast NIH/3T3 cells and in animal experiments (97). The stilbene pterostilbene (trans-3,5-dimethoxy-4-hydroxystilbene, Figure 2), the dimethylated analog of resveratrol, is a highly bioactive natural polyphenolic compound that is mainly found in grapes, blueberries, tomatoes, and other berries (98). According to the results of COX-2 activity assays and enzymatic immunoassays, both resveratrol (Figure 2) and pterostilbene cause COX-2 inactivation via the NF-κB signaling pathway (31, 99).
Matrix Metalloproteinase (MMP)-2 and MMP-9
The MMPs are a group of metal-dependent proteolytic enzymes that are involved in matrix remodeling and facilitate the migration of cancer cells through degradation of the extracellular matrix (100). MMP-2 and MMP-9 can degrade type IV collagen in the basement membrane and facilitate tumor cell metastasis (101).
Various polyphenols affect MMPs. Some, such as 5 μM resveratrol (102) and 75–100 μM kaempferol, inhibit the activity of MMPs (Figure 1) (103). Others decrease the expression of MMPs. The flavone luteolin (Figure 1) inhibits colon cancer metastasis by reducing the expression of MMP-2 and MMP-9 (104). The flavonolignan silibinin (C25H22O10, Figure 1), an active compound of Silybum marianum (L.) Gaertn, decreases the expression of MMP-2, MMP-3 and MMP-9 and increases the expression of TIMP-2 in prostate tumor tissue in transgenic adenocarcinoma of the mouse prostate (TRAMP) model mice and in vitro in various cancer cells (26, 104, 105). MMP-2 expression is downregulated in human prostate cancer cells by genistein treatment (28). In addition, treatment with 5 μM quercetin and chrysin decreases the expression of MMP-9 in A549 cells (106). Still other polyphenols affect both the activity and expression of MMPs. For example, naringin (4',5,7-trihydroxyflavanone 7-rhamnoglucoside, Figure 1) can inhibit the adhesion and invasion of human glioblastoma U87 cells and U251 cells via dose-dependent reductions in both the activity and expression of MMP-2 and MMP-9, according to zymograohy and Western blotting results, this effect is associated with the p38 MAPK signaling pathway (107, 108). EGCG (20 μM) reduce the activity of MMP-2 and MMP-9 in prostate cancer cells (109) and decrease the expression of MMP-9 in bladder cancer cells (110).
Caspases
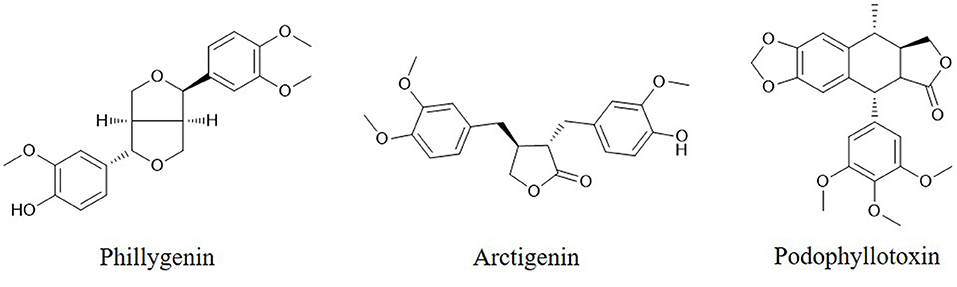
Caspases, which are activated by other caspases, are cysteinyl aspartate-specific proteases and are divided into two groups. One group comprises initiators (caspase-8, -9, and -10); the others, executioners (caspase-3, -6, and -7). Caspase -3 is considered the major downstream target of caspase-4, -8, and -9. Overexpression of caspases is a common alteration in cancer cells that can be exploited therapeutically. Activation of caspase-3 by fisetin treatment associated with induction of the proapoptotic proteins Bad, Bax, Bim, and inhibition of the antiapoptotic proteins Bcl-2 and Mcl-1(L) (35). Genistein has also been shown to increase the expression of caspase-3,-9 and Bax in vitro (28). Chrysin-induced apoptosis was associated with induction of caspase-3 and-8 and downregulation of phospholipase C-gamma-1 (PLC-gamma1) and XIAP. This finding suggests that the mechanism of apoptosis induced by chrysin is associated with Akt dephosphorylation in the PI3K signaling pathway (33). EGCG can induce apoptosis and reduce cancer cell proliferation by decreasing the mitochondrial membrane potential (ΔΨm) and stimulating caspase-3, -9 and c-Jun N-terminal kinase 1 (JNK1) expression in human glioblastoma T98G and U87MG cells but does not induce apoptosis in human normal astrocytes (111). The flavonoid baicalein (Figure 1), found in Scutellaria baicalensis Georgi, participates in apoptosis by increasing the expression of caspase-3 and -8 (112). The lignan phillygenin (Figure 3) induces apoptosis by increasing the mitochondrial membrane potential due to increased ROS levels in human esophageal cancer SH-1-V1 cells. Concurrent upregulation of Bax and cleaved caspase-3 and -9, along with dose-dependent downregulation of Bcl-2, was found by propidium iodide staining and Western blotting (15). The anticancer effects of arctigenin (Figure 3), the active component of Arctium lappa, are mainly directed toward cancer cell growth inhibition and apoptosis through the peroxisome proliferation-activated receptor α (PPARα)/gankyrin, Bax and caspase pathways (36). The xanthone α-mangostin (Figure 4) increases the activity of caspase-3 and causes late apoptosis in ovarian adenocarcinoma SKOV3 cells after 12 h and 72 h of treatment, respectively (113).
Alkaloids
Alkaloids are the secondary biologically active components found in many plants. Alkaloids have various biological activities that render them important sources for drug discovery. The presence of nitrogen in their molecular architecture is critical to the biological activity of this class of compounds. Many studies have shown that alkaloids inhibit the growth of human breast, liver, colon, prostate, and liver cancer cells (114).
Bcl-2 Protein Family
Bcl-2 proteins are divided into two groups. Bcl-2 and Bcl-xL are antiapoptotic proteins, while Bax and Bad are multidomain proapoptotic proteins. The balance of antiapoptotic proteins to proapoptotic proteins, for example, the ratio of Bax to Bcl-2 is crucial to the regulation of apoptotic pathways (115). The balance between Bcl-2 family proteins is a potential target of alkaloids for inducing cell death (116).
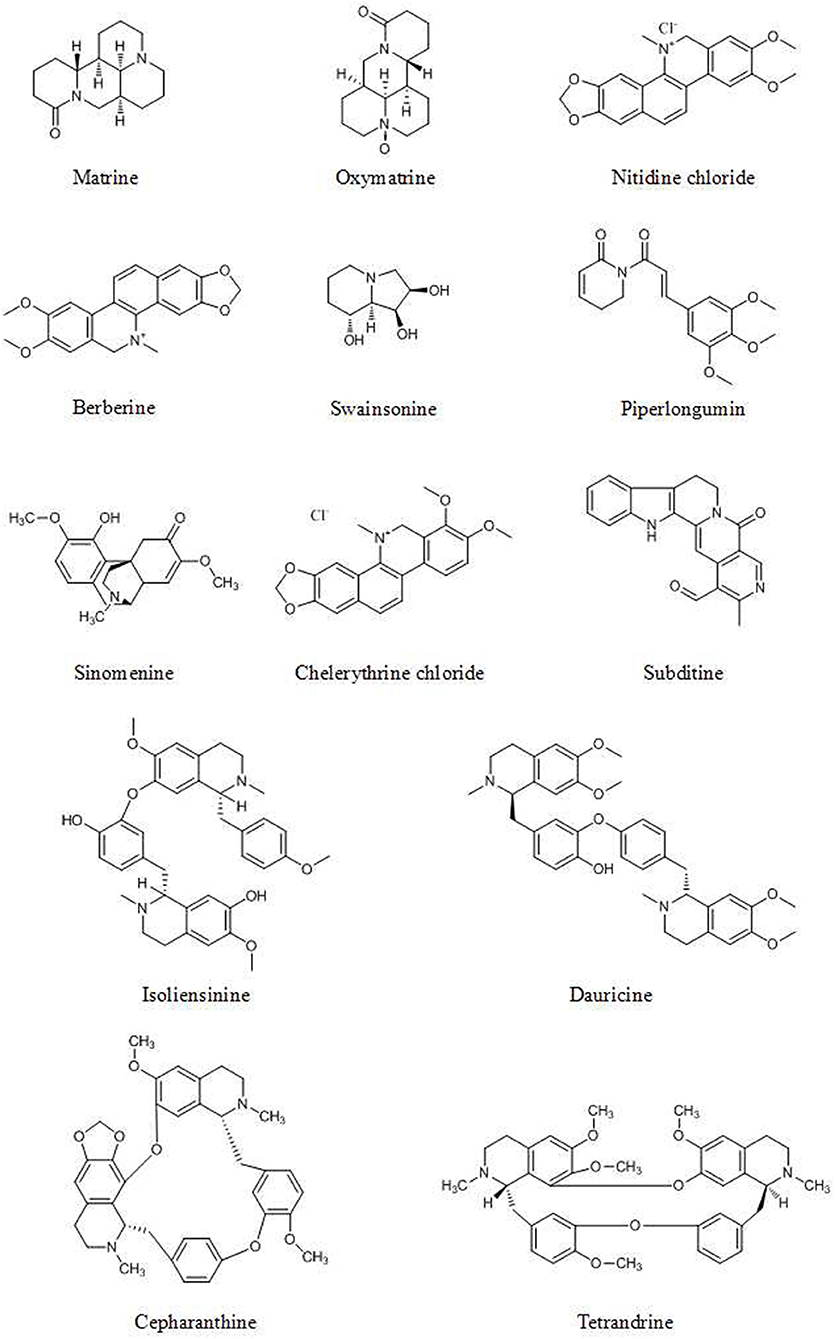
Oxymatrine (Figure 5), derived from Sophora flavescens Aiton, significantly increases p53 and Bax expression and decreases Bcl-2 expression dose-dependently, as evidenced by A Western blot assay, in osteosarcoma cancer cells via dephosphorylation of PI3K and Akt in the PI3K/Akt signaling pathway (117).
Treatment with crude alkaloid extractof Rhazya stricta (CAERS) induced apoptosis and suppressed the proliferation of HCT116 cells. Downregulation of Bcl-2, survivin, Bcl-X and XIAP expression and upregulation of Bad and Noxa expression were examined by qRT-PCR and Western blot analyses and coincided with the increase in the Bax/Bcl-2 ratio (118).
Various alkaloids induce apoptosis via an increase in the Bax/Bcl-2 ratio. Cancer cells treated with nitidine chloride (NC, Figure 5), matrine (Figure 5), berberine (Figure 5), and subditine (Figure 5) showed upregulation of Bax expression and downregulation of Bcl-2 expression (119–123).
PI3K/Akt/mTOR Signaling Pathway
Autophagy is a critical process for maintaining intracellular homeostasis. Generally, autophagy may play a critical role in cancer prevention (124). The PI3K/Akt/mTOR pathway is critical for autophagy induction and is a latent target in cancer therapeutics and control (101).
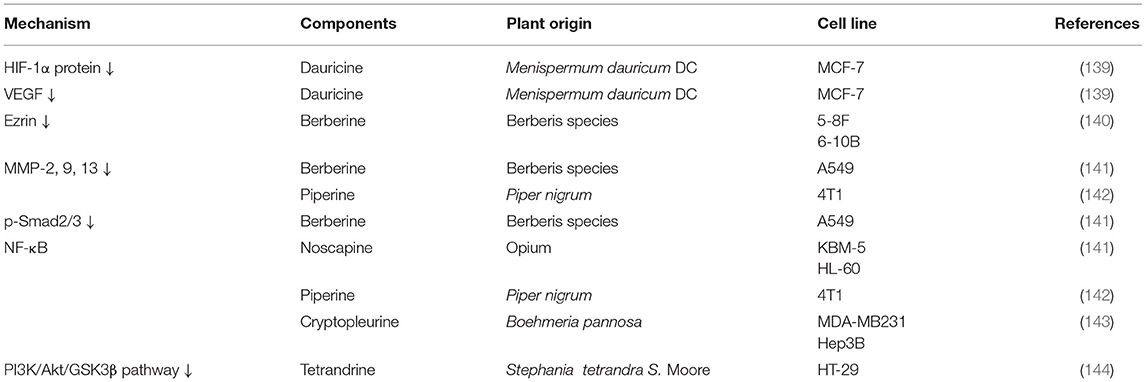
Piperlongumine (Figure 5) (125), swainsonine (Figure 5) (126), and sinomenine (Figure 5) (127) induce apoptosis and inhibit cancer cell growth through the PI3K/Akt/mTOR pathway, with decreased levels of p-Akt and p-mTOR, as evidenced by the results of Western blot analysis and immunofluorescence. Isoliensinine (Figure 5), matrine, dauricine (Figure 5), and cepharanthine (Figure 5) induce autophagy through the AMPK-TSC2-mTOR signaling pathway, with suppression of mTOR activity (128–130).
ERK Signaling Pathway
The MAPK/ERK pathway participates in multiple processes in cancer including growth, invasion, metastasis, angiogenesis, and inhibition of apoptosis (131, 132). Because of these multiaspect effects, the MAPK/ERK pathway plays a critical role in the promotion of cancer cell growth and the inhibition of apoptosis (133, 134).
β-carboline alkaloids extracted from the seeds of Peganum harmala inhibit the proliferation and induce the apoptosis of SGC-7901 cells, possibly because β-carboline alkaloids can disrupt the balance between PTEN and ERK, inhibit the MAPK/ERK signaling pathway and induce apoptosis in cancer cells (135). Berberine can suppress the senescence of human glioblastoma cells by inhibiting the EGFR/Raf/MEK/ERK pathway (136). Sinomenine, extracted from Sinomenium acutum, is reported to inhibit various types of cancer cells. Sinomenine hydrochloride (SH) increases the phosphorylation of ERK1/2, p38 and JNK but does not affect the total levels of the abovementioned cytokines (137). The benzo phenanthridine alkaloid chelerythrine chloride (CC, Figure 5) (5 and 10 μM) significantly enhances ERK1/2 phosphorylation and dose-dependently decreases Akt phosphorylation, as detected by Western blot analysis (138).
The other anticancer targets of alkaloids are summarized in Table 3.
Saponins
Saponins are valuable sources with minimal toxic effects and are found in many dietary plants. Saponins are composed of a triterpenoid or steroidal aglycone attached to one or more sugar chains (145). Saponins are divided into two types: triterpenoid saponins and steroidal saponins. Both types have various biological activities, such as anticancer and immunological adjuvant activities (146).
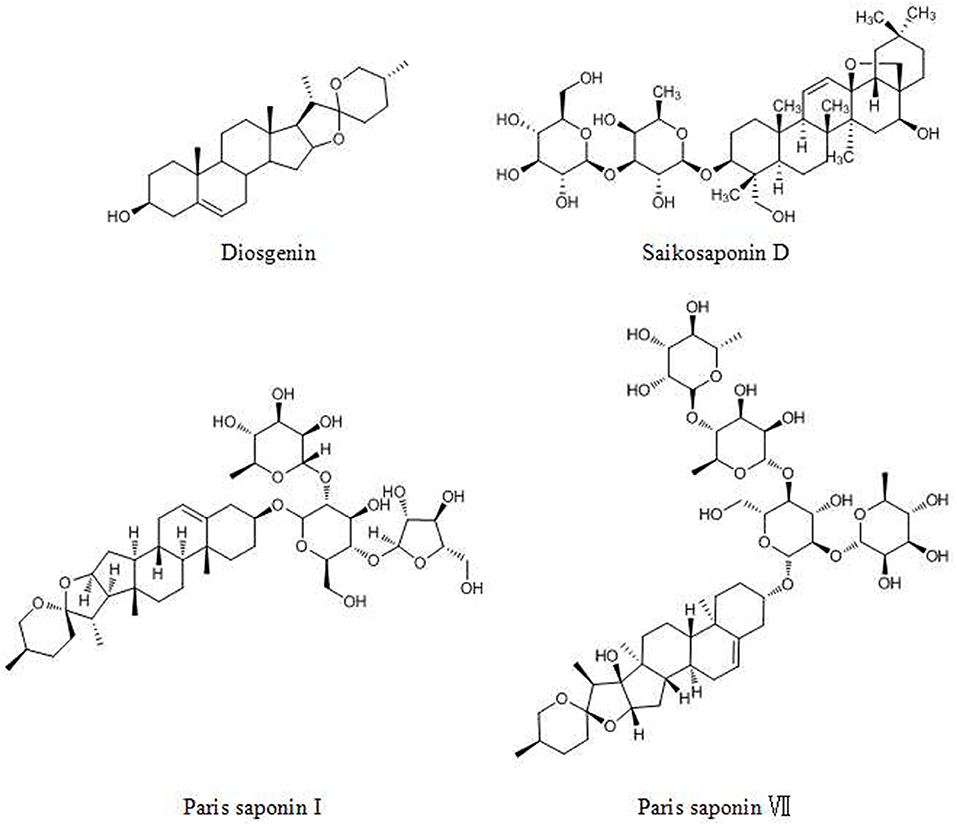
Diosgenin (DG, Figure 6), a steroidal saponin, has been shown to be an anticancer agent in many tumors. DG acts against cancers via the following pathways and mechanisms: (1) the STAT pathway, (2) activation of caspase-3 and p53, (3) activation of the TRAIL death receptor DR5 and (4) the Wnt-β-catenin pathway (147).
The steroidal saponin of Paris polyphylla (Chinese name: Chonglou) has long been used for lung cancer treatment (148). Paris saponin I (PSI, Figure 6) and Paris polyphylla steroidal saponins (PPSS) regulate the Bcl-2 family and caspase-3 and -8, inducing apoptosis (149). In addition, PSI and PPSS induce autophagy by the conversion of LC3 I to LC3 II and upregulation of Beclin 1 (150). Paris saponin VII (PS VII, Figure 6), extracted from Trillium tschonoskii Maxim, inhibits the migration and invasion of several types of cancer cells via the downregulation of MMP-2 and -9 expression and p38 MAPK phosphorylation in a dose- and time- dependent manner (151).
Saikosaponin D (SSD, Figure 6), prescribed for liver diseases, was reported to exhibit anticancer activities (152, 153). SSD effectively suppresses invasion, metastasis and angiogenesis via the downregulation of TNF-α mediated NF-κB signaling, affecting proteins such as MMP-9, VEGF, c-myc, cyclin D1, ICAM-1, and COX-2. In addition, SSD activates the Ca2+/calmodulin-dependent kinase/AMPK/mTOR pathway and attenuates STAT3/HIF-1 pathway signaling, which induces the apoptosis and inhibits the proliferation of cancer cells (154, 155).
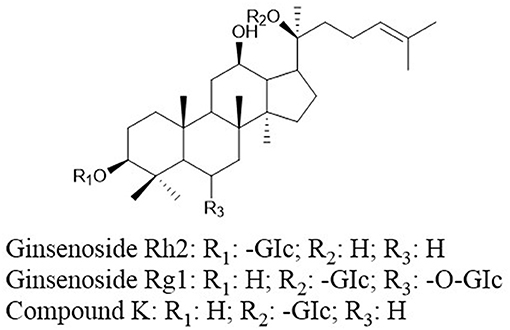
Ginsenosides (ginseng saponins) derived from ginseng were reported to exhibit anticancer effects. Ginsenoside Rh2 (GRh2, Figure 7) and ginsenoside Rg1 (Figure 7) induce apoptosis via activating extrinsic apoptosis pathways by p53-Fas-caspase-8 signaling and the EpoR-mediated JAK2/STAT5 signaling pathway, respectively (156, 157). Moreover, the expression of phosphoglucose isomerase/autocrine motility factor (PGI/AMF) enhances the anticancer effects of GRh2 by attenuating Akt/mTOR signaling (158). A metabolite of ginsenoside compound K (CK, 20-O-D-glucopyranosyl-20(S)-protopanaxadiol, Figure 7) can enhance apoptosis via the ROS-mediated p38 MAPK pathway (159).
Polysaccharides
Polysaccharides which are abundant in plants, possess anticancer activities, and are being used as immunopotentiators for cancer patients, thus they are relatively ideal anticancer agents (160).
Fucoidans, a class of fucose-enriched sulfated polysaccharides, primarily affect apoptosis-related pathways, as proven both in vivo and in vitro (161, 162). Apoptotic morphological changes result from the activation of caspases. Caspase-3 and-9 are activated by fucoidan from Ascophyllum nodosum (163) mainly composed of 52.1% fucose, 21.3% glucose, 19% sulfate content, and 16.5% xylose. And caspase-7 and -8 are regulated by a sulfated polysaccharide isolated from an enzymatic digestion of Ecklonia cava (164). Cell apoptosis induced by S-fucoidan from Cladosiphon okamuranus depends on caspase-3 and -7 (165). Other targets involved in apoptotic effects include Bax and Bcl-xL, ERKs, p38, and the PI3K/Akt signaling pathway (166). Fucoidan, from Cladosiphon novae-caledoniae Kylin, which is consisted of 73% fucose, 12% xylose and mannose, inhibits invasion and tubule formation via the suppression of MMP-2 and -9 activity and downregulation of VEGF expression in tumor cells (167).
The purified polysaccharide extracted from Caulerpa lentillifera, SP1, composed mainly of sulfated xylogalatan and galactose, showed potent immunostimulatory effects by activating macrophage cells through both the NF-κB and p38 MAPK signaling pathways (168). SP1 decreased the levels of IκBα and the NF-κB p65 subunit and increased p38 MAPK phosphorylation, as determined by Western blot assay.
Polysaccharides extracted from Phellinus linteus (PL) significantly inhibit cell proliferation by decreasing β-catenin and cyclin D1 expression in vitro. In addition, PL inhibits invasion and motility by directly reducing the activity of MMP-2 and -9, with no effect on the gene expression or secretion of MMPs, as indicated by RT-PCR and gelatin zymography (169).
The Radix astragali active extract Astragalus polysaccharide (APS) can enhance the immune response by promoting IL-2, IL-6, and TNF-α in H22 tumor-bearing mice. The effects on the immune response are involved in the inhibition of cancer. In addition to the immune response, the anticancer mechanism involves apoptosis, cell cycle arrest, Akt phosphorylation, Bcl-2 and Bax, caspase-3 and -9, p53 and PTEN (163, 170).
The polysaccharides obtained from enzymatic digestion by Celluclast enzyme digest (CCP) suppresses the activation of NF-κB p50 and p65 and the phosphorylation of p38 MAPK in macrophages (171).
Ganoderma lucidum (G. lucidum) polysaccharides (GLPs) can inhibit growth in many types of cancer by inducing apoptosis through FOXO3a-TNF-α-NF-κB signalway (172).
Conclusion
Natural compounds offer a great diversity of chemical structures that are likely important in cancer therapeutics (18). Many studies have shown that phytochemicals influence targets and signaling pathways involved in oncogenesis and tumor progression such as proliferation, invasion, metastasis and angiogenesis (173). Different components have various anticancer activities. (1) Alkaloids, with low bioavailability and poor water solubility, have difficulty to reaching the intended target. Moreover, the toxicity of alkaloids cannot be ignored, primarily target apoptosis-related pathways (174). (2) Flavonoids can affect the development of colon, lung, esophageal, stomach and endometrial cancer, with minimal acute toxic effects because of their poor water solubility accompanied by their rapid digestion (17, 175). Polyphenols primarily target pathways related to proliferation, apoptosis, invasion and metastasis. (3) Polysaccharides and saponins effectively modulate the immune response rather than directly inducing cell death. Polysaccharides primarily affect apoptosis-related pathways, while saponins affect apoptosis-related and invasion- and metastasis-related pathways (176). The anticancer effects of these compounds are associated with multiple targets (Figure 8) (176). Signaling pathways are believed to be associated with specific chemical structures, and this association is critical for continuing drug development.
Author Contributions
LS, WZ, and HZ wrote the article. QG, WY, BL, ZS, and SG provided critical advises of the article. ZS and RC provided final revision.
Funding
This study was supported by the Natural Science Foundation of China (NSFC) (81772291) and the Jilin Science and Technology Agency (20180519003JH and 20180414050GH).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
2. Lezhnina K, Kovalchuk O, Zhavoronkov AA, Korzinkin MB, Zabolotneva AA, Shegay PV, et al. Novel robust biomarkers for human bladder cancer based on activation of intracellular signaling pathways. Oncotarget. (2014) 5:9022–32. doi: 10.18632/oncotarget.2493
3. Batra P, Sharma AK. Anti-cancer potential of flavonoids: recent trends and future perspectives. 3 Biotech. (2013) 3:439–59. doi: 10.1007/s13205-013-0117-5
4. Li Y. Qinghaosu (artemisinin): chemistry and pharmacology. Acta Pharmacol Sin. (2012) 33:1141–6. doi: 10.1038/aps.2012.104
5. Kim GT, Lee SH, Kim JI, Kim YM. Quercetin regulates the sestrin 2-AMPK-p38 MAPK signaling pathway and induces apoptosis by increasing the generation of intracellular ROS in a p53-independent manner. Int J Mol Med. (2014) 33:863–9. doi: 10.3892/ijmm.2014.1658
6. Liu T, Men Q, Wu G, Yu C, Huang Z, Liu X, et al. Tetrandrine induces autophagy and differentiation by activating ROS and Notch1 signaling in leukemia cells. Oncotarget. (2015) 6:7992–8006. doi: 10.18632/oncotarget.3505
7. Cragg PJ, Sharma K. A Pillar[5]arenes: fascinating cyclophanes with a bright future. Chem Soc Rev. (2012) 41:597–607. doi: 10.1039/C1CS15164A
8. Newman DJ, Cragg GM. Natural products as sources of new drugs from 1981 to 2014. J Nat Prod. (2016) 79:629–61. doi: 10.1021/acs.jnatprod.5b01055
9. Ren Y, de Blanco EJC, Fuchs JR, Soejarto DD, Burdette JE, Swanson SM, et al. Potential anticancer agents characterized from selected tropical plants. J Nat Prod. (2019) 82:657–79. doi: 10.1021/acs.jnatprod.9b00018
10. Kim HP, Son KH, Chang HK, Kang SS. Anti-inflammatory plant flavonoids and cellular action mechanisms. J Pharmacol Sci. (2004) 96:229–45. doi: 10.1254/jphs.CRJ04003X
11. Focaccetti C, Izzi V, Benvenuto M, Fazi S, Ciuffa S, Giganti MG, et al. Polyphenols as immunomodulatory compounds in the tumor microenvironment: friends or foes? Int J Mol Sci. (2019) 20:E1714. doi: 10.3390/ijms20071714
12. Yi J, Li S, Wang C, Cao N, Qu H, Cheng C, et al. Potential applications of polyphenols on main ncRNAs regulations as novel therapeutic strategy for cancer. Biomed Pharmacother. (2019) 113:108703. doi: 10.1016/j.biopha.2019.108703
13. Kandaswami C, Lee LT, Lee PP, Hwang JJ, Ke FC, Huang YT, et al. The antitumor activities of flavonoids. In Vivo. (2005) 19:895–909.
14. Quideau S, Deffieux D, Douat-Casassus C, Pouységu L. Plant polyphenols: chemical properties, biological activities, and synthesis. Angew Chem Int Ed Engl. (2011) 50:586–621. doi: 10.1002/anie.201000044
15. He J, Wei W, Yang Q, Wang Y. Phillygenin exerts in vitro and in vivo antitumor effects in drug-resistant human esophageal cancer cells by inducing mitochondrial-mediated apoptosis, ROS generation, and inhibition of the nuclear factor kappa B NF-kB signalling Pathway. Med Sci Monit. (2019) 25:739–45. doi: 10.12659/MSM.913138
16. Zhang C, Yu G, Shen Y. The naturally occurring xanthone α-Mangostin induces ROS-mediated cytotoxicity in non-small scale lung cancer cells. Saudi J Biol Sci. (2018) 25:1090–5. doi: 10.1016/j.sjbs.2017.03.005
17. Kumar S, Pandey AK. Chemistry and biological activities of flavonoids: an overview. Sci World J. (2013) 2013:162750. doi: 10.1155/2013/162750
18. Sak K. Cytotoxicity of dietary flavonoids on different human cancer types. Pharmacogn Rev. (2014) 8:122–46. doi: 10.4103/0973-7847.134247
19. Kilani-Jaziri S, Frachet V, Bhouri W, Ghedira K, Chekir-Ghedira L, Ronot X. Flavones inhibit the proliferation of human tumor cancer cell lines by inducing apoptosis. Drug Chem Toxicol. (2012) 35:1–10. doi: 10.3109/01480545.2011.564180
20. Bulzomi P, Bolli A, Galluzzo P, Acconcia F, Ascenzi P, Marino M. The naringenin-induced proapoptotic effect in breast cancer cell lines holds out against a high nisphenol a background. IUBMB Life. (2012) 64:690–6. doi: 10.1002/iub.1049
21. Sha O, Niu J, Ng TB, Cho EY, Fu X, Jiang W. Anti-tumor action of trichosanthin, a type 1 ribosome-inactivating protein, employed in traditional Chinese medicine: a mini review. Cancer Chemother Pharmacol. (2013) 71:1387–93. doi: 10.1007/s00280-013-2096-y
22. Ruan JS, Liu YP, Zhang L, Yan LG, Fan FT, Shen CS, et al. Luteolin reduces the invasive potential of malignant melanoma cells by targeting β3 integrin and the epithelial-mesenchymal transition. Acta Pharmacol Sin. (2012) 33:1325–31. doi: 10.1038/aps.2012.93
23. Brusselmans K, de Schrijver E, Heyns W, Verhoeven G, Swinnen JV. Epigallocatechin-3-gallate is a potent natural inhibitor of fatty acid synthase in intact cells and selectively induces apoptosis in prostate cancer cells. Int J Cancer. (2003) 106:856–62. doi: 10.1002/ijc.11317
24. Park MH, Hong JE, Park ES, Yoon HS, Seo DW, Hyun BK, et al. Anticancer effect of tectochrysin in colon cancer cell via suppression of NF-kappaB activity and enhancement of death receptor expression. Mol Cancer. (2015) 14:124–36. doi: 10.1186/s12943-015-0377-2
25. Oh SB, Hwang CJ, Song SY, Jung YY, Yun HM, Sok CH, et al. Anti-cancer effect of tectochrysin in NSCLC cells through overexpression of death receptor and inactivation of STAT3. Cancer Lett. (2014) 353:95–103. doi: 10.1016/j.canlet.2014.07.007
26. Deep G, Agarwal R. Anti-metastatic efficacy of silibinin: molecular mechanisms and therapeutic potential against cancer. Cancer Metastasis Rev. (2010) 29:447–63. doi: 10.1007/s10555-010-9237-0
27. Feng Z, Hao W, Lin X, Fan D, Zhou J. Antitumor activity of total flavonoids from Tetrastigma hemsleyanum Diels et Gilg is associated with the inhibition of regulatory T cells in mice. Onco Targets Ther. (2014) 7:947–56. doi: 10.2147/OTT.S61794
28. Pavese JM, Farmer RL, Bergan RC. Inhibition of cancer cell invasion and metastasis by genistein. Cancer Metastasis Rev. (2010) 29:465–82. doi: 10.1007/s10555-010-9238-z
29. Hao W, Yuan X, Yu L, Gao C, Sun X, Wang D, et al. Licochalcone A-induced human gastric cancer BGC-823 cells apoptosis by regulating ROSmediated MAPKs and PI3K/AKT signaling pathways. Sci Rep. (2015) 5:10336. doi: 10.1038/srep10336
30. Vargo MA, Voss OH, Poustka F. Cardounel AJ, Grotewold E, Doseff AI. Apigenin-induced-apoptosis is mediated by the activation of PKCδ and caspases in leukemia cells. Biochem Pharmacol. (2006) 72:681–92. doi: 10.1016/j.bcp.2006.06.010
31. Chen RJ, Kuo HC, Cheng LH, Lee YH, Chang WT, Wang BJ, et al. Apoptotic and nonapoptotic activities of pterostilbene against cancer. Int J Mol Sci. (2018) 19:E287. doi: 10.3390/ijms19010287
32. Zhang M, Cai S, Zuo B, Gong W, Tang Z, Zhou D, et al. Arctigenin induced gallbladder cancer senescence through modulating epidermal growth factor receptor pathway. Tumour Biol. (2017) 39:1010428317698359. doi: 10.1177/1010428317698359
33. Khoo BY, Chua SL, Balaram P. Apoptotic effects of chrysin in human cancer cell lines. Int J Mol Sci. (2010) 11:2188–99. doi: 10.3390/ijms11052188
34. Huang YT, Hwang JJ, Lee PP, Ke FC, Huang JH, Huang CJ, et al. Effects of luteolin and quercetin, inhibitors of tyrosine kinase, on cell growth and metastasis-associated properties in A431 cells overexpressing epidermal growth factor recepto. Br J Pharmacol. (1999) 128:999–1010. doi: 10.1038/sj.bjp.0702879
35. Deeba NS, Adhami VM, Khan MI, Mukhtar H. Inhibition of Akt/mTOR signaling by the dietary flavonoid fisetin. Anticancer Agents Med Chem. (2013) 13:995–1001. doi: 10.2174/18715206113139990129
36. Sun Y, Tan YJ, Lu ZZ, Li BB, Sun CH, Li T, et al. Arctigenin inhibits liver cancer tumorigenesis by inhibiting gankyrin expression via C/EBPα and PPARα. Front Pharmacol. (2018) 9:268. doi: 10.3389/fphar.2018.00268
37. Yalowich JC, Goldman ID. Analysis of the inhibitory effects of VP-16-213 (etoposide) and podophyllotoxin on thymidine transport and metabolism in Ehrlich ascites tumor cells in vitro. Cancer Res. (1984) 44:984–9.
38. Chen H, Liu P, Zhang T, Gao Y, Zhang Y, Shen X, et al. Effects of diphyllin as a novel V-ATPase inhibitor on TE-1 and ECA-109 cells. Oncol Rep. (2018) 39:921–8. doi: 10.3892/or.2018.6191
39. Luo H, Yang A, Schulte BA, Wargovich MJ, Wang GY. Resveratrol induces premature senescence in lung cancer cells via ROS-mediated DNA damage. PLoS ONE. (2013) 8:e60065. doi: 10.1371/journal.pone.0060065
40. Lee CH, Ying TH, Chiou HL, Hsieh SC, Wen SH, Chou RH, et al. Alpha-mangostin induces apoptosis through activation of reactive oxygen species and ASK1/p38 signaling pathway in cervical cancer cells. Oncotarget. (2017) 8:47425–39. doi: 10.18632/oncotarget.17659
41. Hossainzadeh S, Ranji N, Naderi Sohi A, Najafi F. Silibinin encapsulation in polymersome: a promising anticancer nanoparticle for inducing apoptosis and decreasing the expression level of miR-125b/miR-182 in human breast cancer cells. J Cell Physiol. (2019) 234:22285–98. doi: 10.1002/jcp.28795
42. Yazdi Rouholamini SE, Moghassemi S, Maharat Z, Hakamivala A, Kashanian S, Omidfar K. Effect of silibinin-loaded nano-niosomal coated with trimethyl chitosan on miRNAs expression in 2D and 3D models of T47D breast cancer cell line. Artif Cells Nanomed Biotechnol. (2018) 46:524–35. doi: 10.1080/21691401.2017.1326928
43. Zadeh MM, Motamed N, Ranji N, Majidi M, Falahi F. Silibinin-induced apoptosis and downregulation of microRNA-21 and microRNA-155 in MCF-7 human breast cancer cells. J Breast Cancer. (2016) 19:45–52. doi: 10.4048/jbc.2016.19.1.45
44. Motaei J, Yaghmaie M, Ahmadvand M, Pashaiefar H, Kerachian MA. MicroRNAs as potential diagnostic, prognostic and predictive biomarkers for acute graft versus host disease. Biol Blood Marrow Transplant. (2019) S1083-8791(19)30518-X. doi: 10.1016/j.bbmt.2019.08.004
45. Lançon A, Kaminski J, Tili E, Michaille JJ, Latruffe N. Control of MicroRNA expression as a new way for resveratrol to deliver its beneficial effects. J Agric Food Chem. (2012) 60:8783–9. doi: 10.1021/jf301479v
46. Milenkovic D, Jude B, Morand C. miRNA as molecular target of polyphenols underlying their biological effects. Free Radic Biol Med. (2013) 64:40–51. doi: 10.1016/j.freeradbiomed.2013.05.046
47. Chen H, Liu RH. Potential mechanisms of action of dietary phytochemicals for cancer prevention by targeting cellular signaling transduction pathways. J Agric Food Chem. (2018) 66:3260–76. doi: 10.1021/acs.jafc.7b04975
48. Pandima Devi K, Rajavel T, Daglia M, Nabavi SF, Bishayee A, Nabavi SM. MicroRNAs in cancer management and their modulation by dietary agents. Semin Cancer Biol. (2017) 46:146–57. doi: 10.1016/j.semcancer.2017.02.001
49. Khan SI, Aumsuwan P, Khan IA, Walker LA, Dasmahapatra AK. Epigenetic events associated with breast cancer and their prevention by dietary components targeting the epigenome. Chem Res Toxicol. (2012) 25:61–73. doi: 10.1021/tx200378c
50. Lewis B P, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are mocroRNA targets. Cell. (2005) 120:15–17. doi: 10.1016/j.cell.2004.12.035
51. Karius T, Schnekenburger M, Dicato M, Diederich M. MicroRNAs in cancer management and their modulation by dietary agents. Biochem Pharmacol. (2012) 83:1591–601. doi: 10.1016/j.bcp.2012.02.004
52. Costinean S, Zanesi N, Pekarsky Y, Tili E, Volinia S, Heerema N, et al. Pre-B cell proliferation and lymphoblastic leukemia/high-grade lymphoma in E(mu)-miR155 transgenic mice. Proc Natl Acad Sci USA. (2006) 103:7024–9. doi: 10.1073/pnas.0602266103
53. Skalsky RL, Samols MA, Plaisance KB, Boss IW, Riva A, Lopez MC, et al. Kaposi's sarcoma-associated herpesvirus encodes an ortholog of miR-155. J Virol. (2007) 81:12836–45. doi: 10.1128/JVI.01804-07
54. Estrela JM, Mena S, Obrador E, Benlloch M, Castellano G, Salvador R, et al. Polyphenolic phytochemicals in cancer prevention and therapy: bioavailability versus bioefficacy. J Med Chem. (2017) 60:9413–36. doi: 10.1021/acs.jmedchem.6b01026
55. Mirzaei H, Masoudifar A, Sahebkar A, Zare N, Sadri Nahand J, Rashidi B, et al. MicroRNA: a novel target of curcumin in cancer therapy. J Cell Physiol. (2018) 233:3004–15. doi: 10.1002/jcp.26055
56. Jahanafrooz Z, Motamed N, Rinner B, Mokhtarzadeh A, Baradaran B. Silibinin to improve cancer therapeutic, as an apoptotic inducer, autophagy modulator, cell cycle inhibitor, and microRNAs regulator. Life Sci. (2018) 213:236–47. doi: 10.1016/j.lfs.2018.10.009
57. Liu Y, Sun H, Makabel B, Cui Q, Li J, Su C, et al. The targeting of non-coding RNAs by curcumin: facts and hopes for cancer therapy (Review). Oncol Rep. (2019) 42:20–34. doi: 10.3892/or.2019.7148
58. Jin H, Qiao F, Wang Y, Xu Y, Shang Y. Curcumin inhibits cell proliferation and induces apoptosis of human non-small cell lung cancer cells through the upregulation of miR-192-5p and suppression of PI3K/Akt signaling pathway. Oncol Rep. (2015) 34:2782–9. doi: 10.3892/or.2015.4258
59. Liu WL, Chang JM, Chong IW, Hung YL, Chen YH, Huang WT, et al. Curcumin inhibits lin-28A through the activation of miRNA-98 in the lung cancer cell line A549. Molecules. (2017) 22:E929. doi: 10.3390/molecules22060929
60. Zhang W, Bai W, Zhang W. miR-21 suppresses the anticancer activities of curcumin by targeting PTEN gene in human non-small cell lung cancer A549 cells. Clin Transl Oncol. (2014) 16:708–13. doi: 10.1007/s12094-013-1135-9
61. Mudduluru G, George-William JN, Muppala S, Asangani IA, Kumarswamy R, Nelson LD, et al. Curcumin regulates miR-21 expression and inhibits invasion and metastasis in colorectal cancer. Biosci Rep. (2011) 31:185–97. doi: 10.1042/BSR20100065
62. Taverna S, Fontana S, Monteleone F, Pucci M, Saieva L, De Caro V, et al. Curcumin modulates chronic myelogenous leukemia exosomes composition and affects angiogenic phenotype via exosomal miR-21. Oncotarget. (2016) 7:30420–39. doi: 10.18632/oncotarget.8483
63. Gao SM, Yang JJ, Chen CQ, Chen JJ, Ye LP, Wang LY, et al. Pure curcumin decreases the expression of WT1 by upregulation of miR-15a and miR-16-1 in leukemic cells. J Exp Clin Cancer Res. (2012) 31:27. doi: 10.1186/1756-9966-31-27
64. Yang J, Cao Y, Sun J, Zhang Y. Curcumin reduces the expression of Bcl-2 by upregulating miR-15a and miR-16 in MCF-7 cells. Med Oncol. (2010) 27:1114–8. doi: 10.1007/s12032-009-9344-3
65. Wu GQ, Chai KQ, Zhu XM, Jiang H, Wang X, Xue Q, et al. Anti-cancer effects of curcumin on lung cancer through the inhibition of EZH2 and NOTCH1. Oncotarget. (2016) 7:26535–50. doi: 10.18632/oncotarget.8532
66. Toden S, Okugawa Y, Jascur T, Wodarz D, Komarova NL, Buhrmann C, et al. Curcumin mediates chemosensitization to 5-fluorouracil through miRNA-induced suppression of epithelial-to-mesenchymal transition in chemoresistant colorectal cancer. Carcinogenesis. (2015) 36:355–67. doi: 10.1093/carcin/bgv006
67. Wang H, Feng H, Zhang Y. Resveratrol inhibits hypoxiainduced glioma cell migration and invasion by the p-STAT3/miR-34a axis. Neoplasma. (2016) 63:532–9. doi: 10.4149/neo_2016_406
68. Wu H, Wang Y, Wu C, Yang P, Li H, Li Z. Resveratrol induces cancer cell apoptosis through MiR-326/PKM2-mediated ER stress and mitochondrial fission. J Agric Food Chem. (2016) 64:9356–67. doi: 10.1021/acs.jafc.6b04549
69. Yang S, Li W, Sun H, Wu B, Ji F, Sun T, et al. Resveratrol elicits anti-colorectal cancer effect by activating miR-34c-KITLG in vitro and in vivo. BMC Cancer. (2015) 15:969. doi: 10.1186/s12885-015-1958-6
70. Ren X, Bai X, Zhang X, Li Z, Tang L, Zhao X, et al. Quantitative nuclear proteomics identifies that miR-137-mediated EZH2 reduction regulates resveratrol-induced apoptosis of neuroblastoma cells. Mol Cell Proteom. (2015) 14:316–28. doi: 10.1074/mcp.M114.041905
71. Yang SF, Lee WJ, Tan P, Tang CH, Hsiao M, Hsieh FK, et al. Upregulation of miR-328 and inhibition of CREBDNA-binding activity are critical for resveratrol-mediated suppression of matrix metalloproteinase-2 and subsequent metastatic ability in human osteosarcomas. Oncotarget. (2015) 6:2736–53. doi: 10.18632/oncotarget.3088
72. Wang G, Dai F, Yu K, Jia Z, Zhang A, Huang Q, et al. Resveratrol inhibits glioma cell growth via targeting oncogenic microRNAs and multiple signaling pathways. Int J Oncol. (2015) 46:1739–47. doi: 10.3892/ijo.2015.2863
73. Li H, Jia Z, Li A, Jenkins G, Yang X, Hu J, et al. Resveratrol repressed viability of U251 cells by miR-21 inhibiting of NF-kappaB pathway. Mol Cell Biochem. (2013) 382:137–43. doi: 10.1007/s11010-013-1728-1
74. Zhou W, Wang S, Ying Y, Zhou R, Mao P. miR-196b/ miR-1290 participate in the antitumor effect of resveratrol via regulation of IGFBP3 expression in acute lymphoblastic leukemia. Oncol Rep. (2017) 37:1075–83. doi: 10.3892/or.2016.5321
75. Wang M, Jiang S, Yu F, Zhou L, Wang K. Noncoding RNAs as molecular targets of resveratrol underlying its anticancer effects. J Agric Food Chem. (2019) 67:4709–19. doi: 10.1021/acs.jafc.9b01667
76. Wu F, Cui L. Resveratrol suppresses melanoma by inhibiting NF-kappaB/miR-221 and inducing TFG expression. Arch Dermatol Res. (2017) 309:823–31. doi: 10.1007/s00403-017-1784-6
77. Gerhäuser C. Epigenetics, Plant (Poly)phenolics, and Cancer Prevention. In A Romani, V Lattanzio, S Quideau, editors. Recent Advances in Polyphenol Research, Vol. 4. New Jersey, NJ: Wiley Blackwell Press (2014). p. 181–3.
78. Avci CB, Susluer SY, Caglar HO, Balci T, Aygunes D, Dodurga Y, et al. Genistein-induced mir-23b expression inhibits the growth of breast cancer cells. Contemp Oncol. (2015) 19:32–5. doi: 10.5114/wo.2014.44121
79. de la Parra C, Castillo-Pichardo L, Cruz-Collazo A, Cubano L, Redis R, Calin GA, et al. Soy isoflavone genistein-mediated downregulation of miR-155 contributes to the anticancer effects of genistein. Nutr Cancer. (2016) 68:154–64. doi: 10.1080/01635581.2016.1115104
80. Xia J, Cheng L, Mei C, Ma J, Shi Y, Zeng F, et al. Genistein inhibits cell growth and invasion through regulation of miR-27a in pancreatic cancer cells. Curr Pharm Des. (2014) 20:5348–53. doi: 10.2174/1381612820666140128215756
81. Ma J, Cheng L, Liu H, Zhang J, Shi Y, Zeng F, et al. Genistein down-regulates miR-223 expression in pancreatic cancer cells. Curr Drug Targets. (2013) 14:1150–6. doi: 10.2174/13894501113149990187
82. Asama H, Suzuki R, Hikichi T, Takagi T, Masamune A, Ohira H. MicroRNA let-7d targets thrombospondin-1 and inhibits the activation of human pancreatic stellate cells. Pancreatology. (2019) 19:196–203. doi: 10.1016/j.pan.2018.10.012
83. Xia J, Duan Q, Ahmad A, Bao B, Banerjee S, Shi Y, et al. Genistein inhibits cell growth and induces apoptosis through up-regulation of miR-34a in pancreatic cancer cells. Curr Drug Targets. (2012) 13:1750–6. doi: 10.2174/138945012804545597
84. Lynch SM, O'Neill KM, McKenna MM, Walsh CP, McKenna DJ. Regulation of miR-200c and miR-141 by methylation in prostate cancer. Prostate. (2016) 76:1146–59. doi: 10.1002/pros.23201
85. Chiyomaru T, Yamamura S, Zaman MS, Majid S, Deng G, Shahryari V, et al. Genistein suppresses prostate cancer growth through inhibition of oncogenic microRNA-151. PLoS ONE. (2012) 7:e43812. doi: 10.1371/journal.pone.0043812
86. Tsang WP, Kwok TT. Epigallocatechin gallate up-regulation of miR-16 and induction of apoptosis in human cancer cells. J Nutr Biochem. (2010) 21:140–6. doi: 10.1016/j.jnutbio.2008.12.003
87. Fix LN, Shah M, Efferth T, Farwell MA, Zhang B. MicroRNA expression profile of MCF-7 human breast cancer cells and the effect of green tea polyphenon-60. Cancer Genomics Proteom. (2010) 7:261–77.
88. Wang H, Bian S, Yang CS. Green tea polyphenol EGCG suppresses lung cancer cell growth through upregulating miR-210 expression caused by stabilizing HIF-1α. Carcinogenesis. (2011) 32:1881–9. doi: 10.1093/carcin/bgr218
89. Zhu Y, Huang Y, Liu M, Yan Q, Zhao W, Yang P, et al. Epigallocatechin gallate inhibits cell growth and regulates miRNA expression in cervical carcinoma cell lines infected with different high-risk human papillomavirus subtypes. Exp Ther Med. (2019) 17:1742–8. doi: 10.3892/etm.2018.7131
90. Altundal EM, Kasac T, YJlmaz AM, Karademir B, Koçtürk S, Taga Y, et al. Quercetin-induced cell death in human papillary thyroid cancer (B-CPAP). Cells J Thyroid Res. (2015) 2016:1–10. doi: 10.1155/2016/9843675
91. Baruah MM, Khandwekar AP, Sharma N. Quercetin modulates Wnt signaling components in prostate cancer cell line by inhibiting cell viability, migration, and metastases. Tumour Biol. (2016) 37:14025–34. doi: 10.1007/s13277-016-5277-6
92. Chao W, Deng JS, Li PY, Liang YC, Huang GJ. 3,4-Dihydroxybenzalactone suppresses human non-small cell lung carcinoma cells metastasis via suppression of epithelial to mesenchymal transition, ROS-mediated PI3K/AKT/MAPK/MMP and NFκB signaling pathways. Molecules. (2017) 22:537–51. doi: 10.3390/molecules22040537
93. Lai WW, Hsu SC, Chueh FS, Chen YY, Yang JS, Lin JP, et al. Quercetin inhibits migration and invasion of SAS human oral cancer cells through inhibition of NF-kappaB and matrix metalloproteinase-2/-9 signaling pathways. Anticancer Res. (2013) 33:1941–50.
94. Zhang XJ, Jia SS. Fisetin inhibits laryngeal carcinoma through regulation of AKT/NF-κB/mTOR and ERK1/2 signaling pathways. Biomed Pharmacother. (2016) 20:1164–74. doi: 10.1016/j.biopha.2016.08.035
95. Chou RH, Hsieh SC, Yu YL, Huang MH, Huang YC, Hsieh YH. Fisetin inhibits migration and invasion of human cervical cancer cells by down-regulating urokinase plasminogen activator expression through suppressing the p38 MAPK-dependent NF-κB signaling pathway. PLoS ONE. (2013) 8:e71983. doi: 10.1371/journal.pone.0071983
96. Liu X, Tian S, Liu M, Jian L, Zhao L. Wogonin inhibits the proliferation and invasion, and induces the apoptosis of HepG2 and Bel7402 HCC cells through NF-κB/Bcl-2, EGFR and EGFR downstream ERK/AKT signaling. Int J Mol Med. (2016) 38:1250–6. doi: 10.3892/ijmm.2016.2700
97. Li-Weber M. New therapeutic aspects of flavones: the anticancer properties of Scutellaria and its main active constituents Wogonin, Baicalein and Baicalin. Cancer Treat Rev. (2009) 35:57–68. doi: 10.1016/j.ctrv.2008.09.005
98. Zhang L, Wen X, Li M, Li S, Zhao H. Targeting cancer stem cells and signaling pathways by resveratrol and pterostilbene. Biofactors. (2018) 44:61–8. doi: 10.1002/biof.1398
99. Cichocki M, Paluszczak J, Szaefer H, Piechowiak A, Rimando AM, Baer-Dubowska W. Pterostilbene is equally potent as resveratrol in inhibiting 12-O-tetradecanoylphorbol-13-acetate activated NFkappaB, AP-1, COX-2, and iNOS in mouse epidermis. Mol Nutr Food Res. (2008) 52:S62–70. doi: 10.1002/mnfr.200700466
100. Balli U, Cetinkaya BO, Keles GC, Keles ZP, Guler S, Sogut MU, et al. Assessment of MMP-1, MMP-8 and TIMP-2 in experimental periodontitis treated with kaempferol. J Periodontal Implant Sci. (2016) 46:84–95. doi: 10.5051/jpis.2016.46.2.84
101. Chen MH, Cui SX, Cheng YN, Sun LR, Li QB, Xu WF, et al. Galloyl cyclic-imide derivative CH1104I inhibits tumor invasion through suppressing matrix metalloproteinase activity. Anticancer Drugs. (2008) 19:957–65. doi: 10.1097/CAD.0b013e328313e15b
102. Buhrmann C, Yazdi M, Popper B, Shayan P, Goel A, Aggarwal BB, et al. Resveratrol chemosensitizes TNF-β-induced survival of 5-FU-treated colorectal cancer cells. Nutrients. (2018) 10:E888. doi: 10.3390/nu10070888
103. Hung TW, Chen PN, Wu HC, Wu SW, Tsai PY, Hsieh YS, et al. Kaempferol inhibits the invasion and migration of renal cancer cells through the downregulation of AKT and FAK pathways. Int J Med Sci. (2017) 14:984–93. doi: 10.7150/ijms.20336
104. Guo YF, Xu NN, Sun W, Zhao Y, Li CY, Guo MY. Luteolin reduces inflammation in Staphylococcus aureus-induced mastitis by inhibiting NF-kB activation and MMPs expression. Oncotarget. (2017) 8:28481–93. doi: 10.18632/oncotarget.16092
105. Raina K, Rajamanickam S, Singh RP, Deep G, Chittezhath M, Agarwal R. Stage-specific inhibitory effects and associated mechanisms of silibinin on tumor progression and metastasis in transgenic adenocarcinoma of the mouse prostate model. Cancer Res. (2008) 68:6822–30. doi: 10.1158/0008-5472.CAN-08-1332
106. Wu TC, Chan ST, Chang CN, Yu PS, Chuang CH, Yeh SL. Quercetin and chrysin inhibit nickel-induced invasion and migration by downregulation of TLR4/NF-κB signaling in A549 cells. Chem Biol Interact. (2018) 292:101–9. doi: 10.1016/j.cbi.2018.07.010
107. Aroui S, Aouey B, Chtourou Y, Meunier AC, Fetoui H, Kenani A. Naringin suppresses cell metastasis and the expression of matrix metalloproteinases (MMP-2 and MMP-9) via the inhibition of ERK-P38-JNK signaling pathway in human glioblastoma. Chem Biol Interac. (2016) 244:195–203. doi: 10.1016/j.cbi.2015.12.011
108. Aroui S, Najlaoui F, Chtourou Y, Meunier AC, Laajimi A, Kenani A, et al. Naringin inhibits the invasion and migration of human glioblastoma cell via downregulation of MMP-2 and MMP-9 expression and inactivation of p38 signaling pathway. Tumour Biol. (2016) 37:3831–9. doi: 10.1007/s13277-015-4230-4
109. Deb G, Shankar E, Thakur VS, Ponsky LE, Bodner DR, Fu P, et al. Green tea-induced epigenetic reactivation of tissue inhibitor of matrix metalloproteinase-3 suppresses prostate cancer progression through histone-modifying enzymes. Mol Carcinog. (2019) 58:1194–207. doi: 10.1002/mc.23003
110. Luo KW, Wei Chen, Lung WY, Wei XY, Cheng BH, Cai ZM, Huang WR. EGCG inhibited bladder cancer SW780 cell proliferation and migration both in vitro and in vivo via down-regulation of NF-κB and MMP-9. J Nutr Biochem. (2017) 41:56–64. doi: 10.1016/j.jnutbio.2016.12.004
111. Das A, Banik NL, Ray SK. Flavonoids activated caspases for apoptosis in human glioblastoma T98G and U87MG cells but not in human normal astrocytes. Cancer. (2010) 116:164–76. doi: 10.1002/cncr.24699
112. Choi EO, Park C, Hwang HJ, Hong SH, Kim GY, Cho EJ, et al. Baicalein induces apoptosis via ROS-dependent activation of caspases in human bladder cancer 5637 cells. Int J Oncol. (2016) 49:1009–18. doi: 10.3892/ijo.2016.3606
113. Ittiudomrak T, Puthong S, Roytrakul S, Chanchao C. α-mangostin and apigenin induced cell cycle arrest and programmed cell death in SKOV-3 ovarian cancer cells. Toxicol Res. (2019) 35:167–79. doi: 10.5487/TR.2019.35.2.167
114. Lu MK, Shih YW, Chang Chien TT, Fang LH, Huang HC, Chen PS. α-Solanine inhibits human melanoma cell migration and invasion by reducing matrix metalloproteinase-2/9 activities. Biol Pharm Bull. (2010) 33:1685–91. doi: 10.1248/bpb.33.1685
115. Zhai H, Hu S, Liu T, Wang F, Wang X, Wu G, et al. Nitidine chloride inhibits proliferation and induces apoptosis in colorectal cancer cells by suppressing the ERK signaling pathway. Mol Med Rep. (2016) 13:2536–42. doi: 10.3892/mmr.2016.4827
116. Xu H, Zhao X, Liu X, Xu P, Zhang K, Lin X. Antitumor effects of traditional Chinese medicine targeting the cellular apoptotic pathway. Drug Des Devel Ther. (2015) 9:2735–44. doi: 10.2147/DDDT.S80902
117. Wu C, Huang W, Guo Y, Xia P, Sun X, Pan X, et al. Oxymatrine inhibits the proliferation of prostate cancer cells in vitro and in vivo. Mol Med Rep. (2015) 11:4129–34. doi: 10.3892/mmr.2015.3338
118. Elkady AI, Hussein RA, Abu-Zinadah OA. Differential control of growth, apoptotic activity and gene expression in human colon cancer cells by extracts derived from medicinal herbs, Rhazya stricta and Zingiber officinale and their combination. World J Gastroenterol. (2014) 20:15275–88. doi: 10.3748/wjg.v20.i41.15275
119. Fang Z, Tang Y, Jiao W, Xing Z, Guo Z, Wang W, et al. Nitidine chloride induces apoptosis and inhibits tumor cell proliferation via suppressing ERK signaling pathway in renal cancer. Food Chem Toxicol. (2014) 66:210–6. doi: 10.1016/j.fct.2014.01.049
120. Liu Y, Xu Y, Ji W, Li X, Sun B, Gao Q, et la. Antitumor activities of matrine and oxymatrine: literature review. Tumour Biol. (2014) 35:5111–9. doi: 10.1007/s13277-014-1680-z
121. Li H, Li X, Bai M, Suo Y, Zhang G, Cao X. Matrine inhibited proliferation and increased apoptosis in human breast cancer MCF-7 cells via upregulation of Bax and downregulation of Bcl-2. Int J Clin Exp Pathol. (2015) 8:14793–9.
122. Li DX, Zhang J, Zhang Y, Zhao PW, Yang LM. Inhibitory effect of berberine on human skin squamous cell carcinoma A431 cells. Genet Mol Res. (2015) 14:53–68. doi: 10.4238/2015.September.8.17
123. Liew SY, Looi CY, Paydar M, Cheah FK, Leong KH, Wong WF, et al. Subditine, a new monoterpenoid indole alkaloid from bark of nauclea subdita (Korth.) steud. induces apoptosis in human prostate cancer cells. PLoS ONE. (2014) 9:e87286. doi: 10.1371/journal.pone.0087286
124. Xu DW, Zhang GQ, Wang ZW, Xu XY, Tong XL. Autophagy in tumorigenesis and cancer treatment. Asian Pac J Cancer. (2015) 16:2167–75. doi: 10.7314/APJCP.2015.16.6.2167
125. Wang F, Mao Y, You Q, Hua D, Cai D. Piperlongumine induces apoptosis and autophagy in human lung cancer cells through inhibition of PI3K/Akt/mTOR pathway. Int J Immunopathol Pharmacol. (2015) 28:362–73. doi: 10.1177/0394632015598849
126. Sun L, Jin X, Xie L, Xu G, Cui Y, Chen Z. Swainsonine represses glioma cell proliferation, migration and invasion by reduction of miR-92a expression. BMC Cancer. (2019) 19:247. doi: 10.1186/s12885-019-5425-7
127. Sun Z, Zheng L, Liu X, Xing W, Liu X. Sinomenine inhibits the growth of melanoma by enhancement of autophagy via PI3K/AKT/mTOR inhibition. Drug Des Devel Ther. (2018) 12:2413–21. doi: 10.2147/DDDT.S155798
128. Niu H, Zhang Y, Wu B, Zhang Y, Jiang H, He P. Matrine induces the apoptosis of lung cancer cells through downregulation of inhibitor of apoptosis proteins and the Akt signaling pathway. Oncol Rep. (2014) 32:1087–93. doi: 10.3892/or.2014.3273
129. Mizushima N, Klionsky DJ. Protein turnover via autophagy: implications for metabolism. Annu Rev Nutr. (2007) 27:19–40. doi: 10.1146/annurev.nutr.27.061406.093749
130. Shaw RJ. LKB1 and AMP-activated protein kinase control of mTOR signalling and growth. Acta Physiol. (2009) 196:65–80. doi: 10.1111/j.1748-1716.2009.01972.x
131. Khan M, Yu B, Rasul A, Al Shawi A, Yi F, Yang H, et al. Jaceosidin induces apoptosis in U87 glioblastoma cells through G2/M Phase arrest. Evid Based Complement Alternat Med. (2012) 2012:703034. doi: 10.1155/2012/703034
132. Thompson N, Lyons J. Recent progress in targeting the Raf/MEK/ERK pathway with inhibitors in cancer drug discovery. Curr Opin Pharmacol. (2005) 5:350–6. doi: 10.1016/j.coph.2005.04.007
133. Santarpia L, Lippman SM, El-Naggar AK. Targeting the MAPK- RAS- RAF signaling pathway in cancer therapy. Expert Opin Ther Target. (2012) 16:103–19. doi: 10.1517/14728222.2011.645805
134. Khan M, Maryam A, Qazi JI, Ma T. Targeting apoptosis and multiple signaling pathways with icariside II in cancer cells. Int J Biol Sci. (2015) 11:1100–13. doi: 10.7150/ijbs.11595
135. Fan Y, Patima A, Chen Y, Zeng F, He W, Luo L, et al. Cytotoxic effects of β-carboline alkaloids on human gastric cancer SGC-7901 cells. Int J Clin Exp Med. (2015) 8:12977–82.
136. Liu Q, Xu X, Zhao M, Wei Z, Li X, Zhang X, et al. Berberine induces senescence of human glioblastoma cells by downregulating the EGFR-MEK-ERK signaling pathway. Mol Cancer Ther. (2015) 14:355–63. doi: 10.1158/1535-7163.MCT-14-0634
137. Li X, Wang K, Ren Y, Zhang L, Tang XJ, Zhang HM, et al. MAPK signaling mediates sinomenine hydrochlorideinduced human breast cancer cell death via both reactive oxygen species-dependent and -independent pathways: an in vitro and in vivo study. Cell Death Dis. (2014) 5:e1356. doi: 10.1038/cddis.2014.321
138. Chen XM, Zhang M, Fan PL, Qin YH, Zhao HW. Chelerythrine chloride induces apoptosis in renal cancer HEK-293 and SW-839 cell lines. Oncol Lett. (2016) 11:3917–24. doi: 10.3892/ol.2016.4520
139. Tang XD, Zhou X, Zhou KY. Dauricine inhibits insulin-like growth factor-I-induced hypoxia inducible factor 1α protein accumulation and vascular endothelial growth factor expression in human breast cancer cells. Acta Pharmacol Sin. (2009) 30:605–16. doi: 10.1038/aps.2009.8
140. Tang F, Wang D, Duan C, Huang D, Wu Y, Chen Y, et al. Berberine inhibits metastasis of nasopharyngeal carcinoma 5-8F cells by targeting Rho kinase-mediated Ezrin phosphorylation at threonine 567. J Biol Chem. (2009) 284:27456–66. doi: 10.1074/jbc.M109.033795
141. Sung B, Ahn KS, Aggarwal BB. Noscapine, a benzylisoquinoline alkaloid, sensitizes leukemic cells to chemotherapeutic agents and cytokines by modulating the NF-κB signaling pathway. Cancer Res. (2010) 70:3259–68. doi: 10.1158/0008-5472.CAN-09-4230
142. Lai LH, Fu QH, Liu Y, Jiang K, Guo QM, Chen QY, et al. Piperine suppresses tumor growth and metastasis in vitro and in vivo in a 4T1 murine breast cancer model. Acta Pharmacol Sin. (2012) 33:523–30. doi: 10.1038/aps.2011.209
143. Jin HR, Jin SZ, Cai XF, Li D, Wu X, Nan JX, et al. Cryptopleurine targets NF-κB pathway, leading to inhibition of gene products associated with cell survival, proliferation, invasion, and angiogenesis. PLoS ONE. (2012) 7:e40355. doi: 10.1371/journal.pone.0040355
144. Ma JW, Zhang Y, Ye JC, Li R, Wen YL, Huang JX, et al. Tetrandrine exerts a radiosensitization effect on human glioma through inhibiting proliferation by attenuating ERK phosphorylation. Biomol Ther. (2017) 25:186–93. doi: 10.4062/biomolther.2016.044
145. Rajput ZI, Hu SH, Xiao CW, Arijo AG. Adjuvant effects of saponins on animal immune responses. Zhejiang Univ Sci B. (2007) 8:153–61. doi: 10.1631/jzus.2007.B0153
146. Xu XH, Li T, Fong CM, Chen X, Chen XJ, Wang YT, et al. Saponins from chinese medicines as anticancer agents. Molecules. (2016) 21:1326–53. doi: 10.3390/molecules21101326
147. Bhuvanalakshmi G, Basappa Kanchugarakoppal S, Rangappa Dharmarajan A, Sethi G, Kumar AP, Warrier S. Breast cancer stem-like cells are inhibited by diosgenin, a steroidal saponin, by the attenuation of the Wnt b-catenin signaling via the Wnt antagonist secreted frizzled related protein-4. Front Pharmacol. (2017) 8:124. doi: 10.3389/fphar.2017.00124
148. Yan LL, Zhang YJ, Gao WY, Man SL, Wang Y. In vitro and in vivo anticancer activity of steroid saponins of Paris polyphylla var. yunnanensis. Exp Oncol. (2009) 31:27–32.
149. Song S, Du L, Jiang H, Zhu X, Li J, Xu J. Paris saponin I sensitizes gastric cancer cell lines to cisplatin via cell cycle arrest and apoptosis. Med Sci Monit. (2016) 22:3798–803. doi: 10.12659/MSM.898232
150. He H, Sun YP, Zheng L, Yue ZG. Steroidal saponins from paris polyphylla induce apoptotic cell death and autophagy in A549 human lung cancer cells. Asian Pac J Cancer Prev. (2015) 16:1169–73. doi: 10.7314/APJCP.2015.16.3.1169
151. Cheng G, Gao F, Sun X, Bi H, Zhu Y. Paris saponin VII suppresses osteosarcoma cell migration and invasion by inhibiting MMP-2/9 production via the p38 MAPK signaling pathway. Mol Med Rep. (2016) 14:3199–205. doi: 10.3892/mmr.2016.5663
152. Liu A, Tanaka N, Sun L, Guo B, Kim JH, Krausz KW, et al. Saikosaponin d protects against acetaminophen-induced hepatotoxicity by inhibiting NFκB and STAT3 signaling. Chem Biol Interact. (2014) 223:80–6. doi: 10.1016/j.cbi.2014.09.012
153. Tundis R, Bonesi M, Deguin B, Loizzo MR, Menichini F, Conforti F, et al. Cytotoxic activity and inhibitory effect on nitric oxide production of triterpene saponins from the roots of Physospermum verticillatum (Waldst & Kit) (Apiaceae). Bioorg Med Chem. (2009) 17:4542–7. doi: 10.1016/j.bmc.2009.05.006
154. He S, Lu G, Hou H, Zhao Z, Zhu Z, Lu X, et al. Saikosaponin-d suppresses the expression of cyclooxygenase2 through the phosphosignal transducer and activator of transcription 3/hypoxiainducible factor1α pathway in hepatocellular carcinoma cells. Mol Med Rep. (2014) 10:2556–62. doi: 10.3892/mmr.2014.2574
155. Wong VK, Li T, Law BY, Ma ED, Yip NC, Michelangeli F, et al. Saikosaponin-d, a novel serca inhibitor, induces autophagic cell death in apoptosis-defective cells. Cell Death Dis. (2013) 4:e720. doi: 10.1038/cddis.2013.217
156. Guo XX, Li Y, Sun C, Jiang D, Lin YJ, Jin FX, et al. p53-dependent Fas expression is critical for Ginsenoside Rh2 triggered caspase-8 activation in HeLa cells. Protein Cell. (2014) 5:224–34. doi: 10.1007/s13238-014-0027-2
157. You ZM, Zhao L, Xia J, Wei Q, Liu YM, Liu XY, et al. Down-regulation of phosphoglucose isomerase/autocrine motility factor enhances gensenoside Rh2 pharmacological action on leukemia KG1α cells. Asian Pac J Cancer Prev. (2014) 15:1099–104. doi: 10.7314/APJCP.2014.15.3.1099
158. Li J, Wei Q, Zuo GW, Xia J, You ZM, Li CL, et al. Ginsenoside Rg1 induces apoptosis through inhibition of the EpoR-mediated JAK2/STAT5 signalling pathway in the TF-1/Epo human leukemia cell line. Asian Pac J Cancer Prev. (2014) 15:2453–9. doi: 10.7314/APJCP.2014.15.6.2453
159. Wang H, Jiang D, Liu J, Ye S, Xiao S, Wang W, et al. Compound K induces apoptosis of bladder cancer T24 cells via reactive oxygen species-mediated p38 MAPK pathway. Cancer Biother Radiopharm. (2013) 28:607–15. doi: 10.1089/cbr.2012.1468
160. Sun DL, Xie HB, Xia YZ. A study on the inhibitory effect of polysaccharides from radix ranunculus ternati on human breast cancer MCF-7 cell lines. Afr J Tradit Complement Altern Med. (2013) 10:439–43. doi: 10.4314/ajtcam.v10i6.6
161. Croci DO, Cumashi A, Ushakova NA, Preobrazhenskaya ME, Piccoli A, Totani L, et al. Fucans, but not fucomannoglucuronans, determine the biological activities of sulfated polysaccharides from laminaria saccharina brown seaweed. PLoS ONE. (2011) 6:e17283. doi: 10.1371/journal.pone.0017283
162. Jiao G, Yu G, Zhang J, Ewart HS. Chemical structures and bioactivities of sulfated polysaccharides from marine algae. Mar Drugs. (2011) 9:196–223. doi: 10.3390/md9020196
163. Foley SA, Szegezdi E, Mulloy B, Samali A, Tuohy MG. An unfractionated fucoidan from Ascophyllum nodosum: Extraction, characterization, and apoptotic effects in vitro. J Nat Prod. (2011) 74:1851–61. doi: 10.1021/np200124m
164. Athukorala Y, Ahn GN, Jee YH, Kim GY, Kim SH, Ha JH, et al. Antiproliferative activity of sulfated polysaccharide isolated from an enzymatic digest of Ecklonia cava on the U-937 cell line. J Appl Phycol. (2009) 21:307–14. doi: 10.1007/s10811-008-9368-7
165. de Jesus Raposo MF, de Morais AM, de Morais RM. Marine polysaccharides from algae with potential biomedical applications. Mar Drugs. (2015) 13:2967–3028. doi: 10.3390/md13052967
166. Hyun JH, Kim SC, Kang JI, Kim MK, Boo HJ, Kwon JM, et al. Apoptosis inducing activity of fucoidan in HCT-15 colon carcinoma cells. Biol Phar Bull. (2009) 32:1760–4. doi: 10.1248/bpb.32.1760
167. Ye J, Li Y, Teruya K, Katakura Y, Ichikawa A, Eto H, et al. Enzyme-digested fucoidan extracts derived from seaweed mozuku of cladosiphon novae-caledoniae kylin inhibit invasion and angiogenesis of tumor cells. Cytotechnology. (2005) 47:117–26. doi: 10.1007/s10616-005-3761-8
168. Maeda R, Ida T, Inara H, Sakamoto T. Immunostimulatory activity of polysaccharides isolated from Caulerpa lentillifera on macrophage cells. Biosci Biotechnol Biochem. (2012)76:501–5. doi: 10.1271/bbb.110813
169. Song KS, Li G, Kim JS, Jing K, Kim TD, Kim JP, et al. Protein-bound polysaccharide from Phellinus linteus inhibits tumor growth, invasion, and angiogenesis and alters Wnt/β-catenin in SW480 human colon cancer cells. BMC Cancer. (2011) 11:307. doi: 10.1186/1471-2407-11-307
170. Lai X, Xia W, Wei J, Ding X. Therapeutic effect of astragalus polysaccharides on hepatocellular carcinoma H22-bearing mice. Dose Response. (2017) 15:1559325816685182. doi: 10.1177/1559325816685182
171. Sanjeewa KK, Fernando IP, Kim EA, Ahn G, Jee Y, Jeon YJ. Anti-inflammatory activity of a sulfated polysaccharide isolated from an enzymatic digest of brown seaweed Sargassum horneri in RAW 264.7 cells. Nutr Res Pract. (2017) 11:3–10. doi: 10.4162/nrp.2017.11.1.3
172. Na K, Li K, Sang T, Wu K, Wang Y, Wang X. Anticarcinogenic effects of water extract of sporoderm-broken spores of Ganoderma lucidum on colorectal cancer in vitro and in vivo. Int J Oncol. (2017) 50:1541–54. doi: 10.3892/ijo.2017.3939
173. Liu J, Wang S, Zhang Y, Fan HT, Lin HS. Traditional Chinese medicine and cancer: History, present situation, and development. Thoracic Cancer. (2015) 6:561–9. doi: 10.1111/1759-7714.12270
174. Lu JJ, Bao JL, Chen XP, Huang M, Wang YT. Alkaloids isolated from natural herbs as the anticancer agents. Evid-Based Complement Altern Med. (2012) 2012:485042. doi: 10.1155/2012/485042
175. Koen B, Ruth V, Guido V, Johannes VS. Induction of cancer cell apoptosis by flavonoids is associated with their ability to inhibit fatty acid synthase activity. J Biol Chem. (2005) 280:5636–45. doi: 10.1074/jbc.M408177200
Keywords: natural active compounds, signaling pathway, cancer, polyphenol, alkaloid, saponin, polysaccharide
Citation: Sun L, Zhou W, Zhang H, Guo Q, Yang W, Li B, Sun Z, Gao S and Cui R (2019) Modulation of Multiple Signaling Pathways of the Plant-Derived Natural Products in Cancer. Front. Oncol. 9:1153. doi: 10.3389/fonc.2019.01153
Received: 20 May 2019; Accepted: 16 October 2019;
Published: 08 November 2019.
Edited by:
Huizi Jin, Shanghai Jiao Tong University, ChinaReviewed by:
Reik Löser, Helmholtz-Gemeinschaft Deutscher Forschungszentren (HZ), GermanyYu Zhangy, Northeast Normal University, China
Copyright © 2019 Sun, Zhou, Zhang, Guo, Yang, Li, Sun, Gao and Cui. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhi-hui Sun, c3VuemhpaHVpNzc3N0AxMjYuY29t; Shuo-hui Gao, Z2Fvc2h1b2h1aUBmb3htYWlsLmNvbQ==; Ran-ji Cui, Y3VpcmFuamlAamx1LmVkdS5jbg==
†These authors have contributed equally to this work
 Li-rui Sun
Li-rui Sun Wei Zhou1†
Wei Zhou1† Hong-mei Zhang
Hong-mei Zhang Ran-ji Cui
Ran-ji Cui