- 1Lung Cancer Center & Institute, West China Hospital, Sichuan University, Chengdu, China
- 2Department of Oncology, Chengdu First People's Hospital, Chengdu, China
- 3Cancer Center, West China Hospital, Sichuan University, Chengdu, China
Background: Ki-67 is a widely used marker of tumor proliferation, but the prognostic value of ki-67 in triple-negative breast cancer (TNBC) has not been comprehensively reviewed. This meta-analysis was conducted to evaluate the association between ki-67 expression and survival of patients with resected TNBC.
Materials and Methods: Relevant studies, evaluating the prognostic impact of pretreatment ki-67 in resected TNBC patients, were identified from PubMed, Embase, Web of Science, China National Knowledge Infrastructure, and Cochrane Library until March 14, 2019. Hazard ratios (HRs) with 95% confidence intervals (CI) were calculated as effect values for disease-free survival (DFS) and overall survival (OS).
Results: In present meta-analysis, 35 studies with 7,716 enrolled patients were eligible for inclusion. Pooled results showed that a high ki-67 expression was significantly associated with poor DFS (HR = 1.73, 95% CI: 1.45–2.07, p < 0.001) and poor OS (HR = 1.65, 95% CI: 1.27–2.14, p < 0.001) in resected TNBC. In the subgroup analysis, when a cutoff of Ki-67 staining ≥40% was applied, the pooled HR for DFS and OS was 2.30 (95% CI 1.54–3.44, p < 0.001) and 2.95 (95% CI 1.67–5.19, p < 0.001), respectively.
Conclusion: A high Ki-67 expression is a poor prognostic factor of resected TNBC. The cut-off of ki-67 ≥40% is associated with a greater risk of recurrence and death compared with lower expression rates, despite the Ki-67 threshold with the greatest prognostic significance is as yet unknown.
Introduction
Breast cancer is one of the most frequently diagnosed cancers and the leading cause of cancer morbidity in women worldwide. It affected more than 1.6 million individuals in 2012 and constituted ~15% of all cancer-related deaths among females (1). Triple-negative breast cancer (TNBC) is a subtype of breast cancer and accounts for about 12 to 17% of all breast cancers (2). Due to lacking the expression of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor type 2 (HER2) in tumor cells, patients with TNBC are neither sensitive to endocrine therapy nor therapies targeted to HER2 (3). TNBC is usually a high-grade invasive ductal carcinoma without a special pathological type, and it is also a heterogeneous disease because some of these patients are obviously sensitive to chemotherapy with likelihood to achieve a favorable prognosis (4, 5). Thus, sufficient and valid prognostic factors of TNBC should be identified.
Ki-67, a non-histone nuclear protein, is present in the cell nucleus during all of the active phases of the cell cycle (G1, S, G2, and mitosis) but absent in quiescent cells (G0), which makes it a widely used biomarker of tumor proliferation and a crucial element of pathological assessment (6, 7). The prognostic significance of Ki-67 has been extensively evaluated in various malignancies, including breast cancer. Ki-67 is established as a vital factor in the distinction between luminal A and luminal B breast cancer subtypes by the 2011 and 2013 St. Gallen International Breast Cancer Conference (8, 9). Unlike its role in luminal diseases whose low Ki-67 expression achieves an enhanced prognosis after standard systematic treatments, the prognostic value of Ki-67 in TNBC is still unclear and no consensus has been reached (10). Therefore, this study focused on the assessment of the prognostic value of Ki-67 in resected TNBC patients.
Methods
Our meta-analysis was conducted in line with the “Preferred Reporting Items for Systematic Reviews and Meta-Analyses” (PRISMA) statement (11).
Search Strategy
A comprehensive electronic search of PubMed, Embase, Web of Science, China National Knowledge Infrastructure, and Cochrane Library was conducted without language restriction to identify all relevant full-length studies on the prognostic role of Ki-67 in patients with TNBC. To retrieve data as much as possible, we expand the search scope by using the keywords as follow: (“Ki-67” or “mib-1” or “proliferative marker”) and (“breast cancer” or “breast tumor” or “breast carcinoma” or “breast neoplasm”). The beginning date was not limited, and the search was up to March 14, 2019. References cited in eligible studies were also searched manually to obtain additional pertinent articles.
Study Selection
The inclusion criteria were as follows: (1) studies or subsets in studies investigating the association between Ki-67 and prognosis in resected TNBC who has received neo-adjuvant or adjuvant treatment; (2) studies have adequate data for calculation including the hazard ratio (HR) and its corresponding 95% confidence interval (CI), and (3) the threshold value of Ki-67 was determined by pretreatment biopsy specimen.
The exclusion criteria were as follows: (1) non-original research articles with limited data, such as reviews, letters, comments, conference abstracts, or case reports; (2) studies without adequate survival or recurrence data for further calculation; (3) studies involving metastatic diseases; (4) overlapping or duplicate data; and (5) studies with a sample size of <30 analyzable cases.
Data Extraction and Quality Assessment
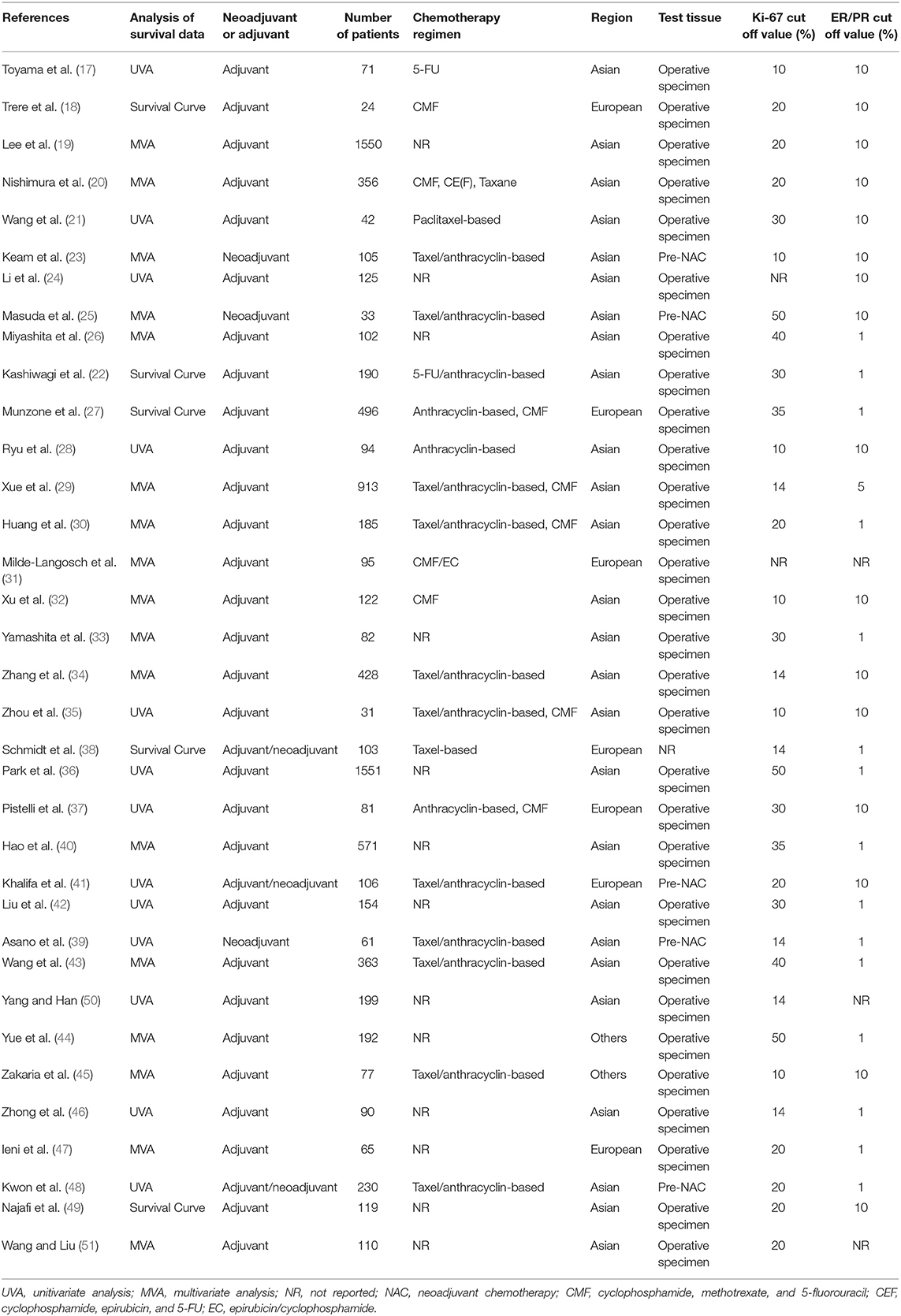
The following data was extracted: first author's name, year of publication, country, study design and sample size, demographic characteristics (e.g., age, gender, and geographical background), cut-off value of Ki-67 expression, percentage of positive lymph nodes, treatment, and the HR with 95% CI of disease-free survival (DFS) and overall survival (OS). Multivariate outcomes were preferred when multivariate and univariate analyses performed simultaneously.
Newcastle–Ottawa Scale (NOS) was used to examine the qualities of the included studies (12). This evaluation tool covered the selection, comparability, and clinical outcomes, and studies were considered to be of high quality when they scored 6 or more.
Statistical Analysis
Prognostic outcomes, including DFS and OS, were the primary endpoints of this study. DFS was defined as the interval period from the date of operation to the first observation of recurrence or the last follow-up without evidence of recurrence. OS was defined as the time from the first diagnosis of primary breast cancer to the time of death from any cause. HRs with 95% CIs for prognostic outcomes were extracted for further calculation. For those that were indirectly given in publications, published data and figures from original papers were extracted to calculate the corresponding HRs by utilizing the methods described by Tierney et al. (13).
Cochrane's Q (P < 0.1 was considered significant) and Higgins's I2 (I2 > 50% was considered substantially heterogeneous) statistic tests were used to evaluate the heterogeneity among the eligible studies (14). A fixed-effect model would be preferred in the analyses to acquire precise results if the heterogeneities were insignificant. Otherwise, a random-effect model should be utilized (15). Subgroup analyses were also conducted to investigate the role of Ki-67 in specific populations and the potential source of heterogeneity. Publication bias was assessed with a funnel plot via Egger's and Begg's tests, and results were considered insignificant when P > 0.1 (16). Sensitivity analysis was performed to explore the influence of individual studies on the summarized results.
Kaplan–Meier curves were recognized by Engauge Digitizer 4.1 (free software downloaded for http://getdata-graph-digitizer.com/). All tests were two sided, and P < 0.05 indicated statistical significance. Data analyses were performed with Stata 12.0 (StataCorp LP, TX, USA).
Results
Selection of Studies
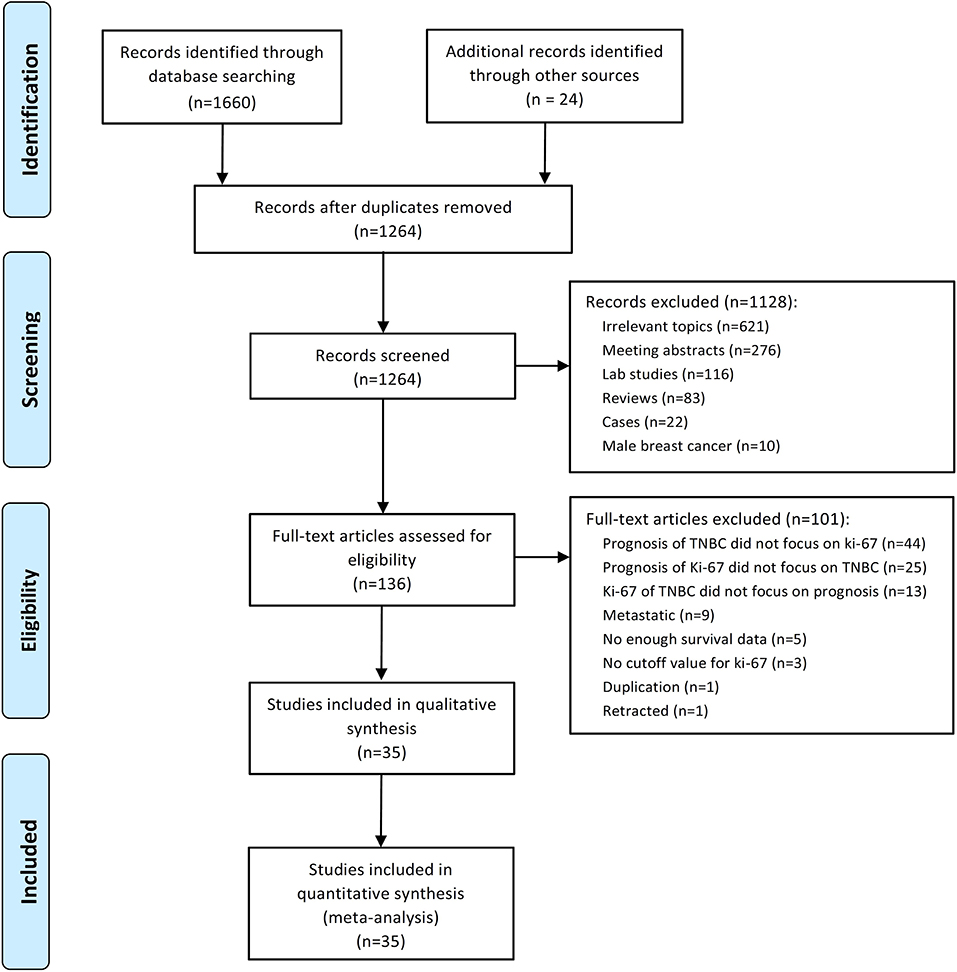
A total of 1,684 potential studies were identified by the search algorithm. After duplicates were removed, abstracts of the 1,264 remaining studies were reviewed. Of these studies, 1,128 were excluded, and 136 potentially relevant studies were selected for further examination. A total of 101 studies were excluded because the prognosis of TNBC did not focus on Ki-67 (n = 44); the prognosis of Ki-67 did not highlight TNBC (n = 25); and Ki-67 of TNBC did not cover prognosis (n = 13), metastatic disease (n = 9), insufficient survival data (n = 5), no cutoff for Ki-67 (n = 3), duplication (n = 1), and retracted study (n = 1). Finally, 35 studies regarding the prognostic role of Ki-67 in TNBC subjected to neo-adjuvant or adjuvant chemotherapy were eligible for this meta-analysis (17–51). The flow diagram of studies selection was summarized in Figure 1.
Study Characteristics
A total of 7,716 patients with TNBC were enrolled in the 35 included studies for analyses. The patients' median age ranged from 50 to 60 years, and the median follow-up varied from 11 to 112 months. The cutoff of Ki-67 was 10%−50%. The article quality assessed by NOS was 6–9, and 80% of the included studies had a quality of 7–9. None of these studies included patients who underwent surgery alone without neoadjuvant or adjuvant treatment. Table 1 summarizes the main characteristics of the included studies.
Relationship Between Ki-67 Expression and Prognosis
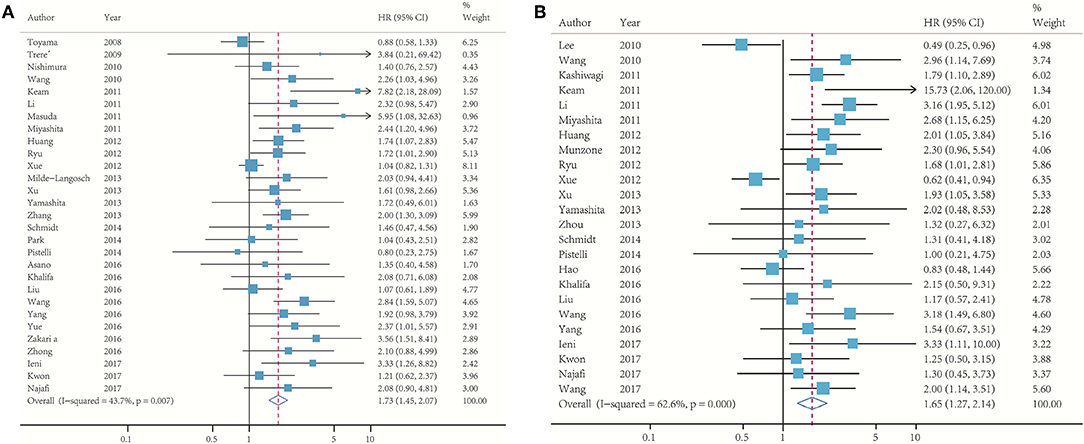
In Figure 2, 29 studies reported the association between Ki-67 and DFS, whereas 24 determined the OS. The pooled HR of DFS comparing the high Ki-67 expression level to the low was 1.73 (95% CI: 1.45–2.07; p < 0.001; Figure 2A). No significant heterogeneity (I2 = 43.7%) was found, and the fixed effect model was used. The pooled HR of OS was 1.65 (95% CI: 1.27–2.14; p < 0.001; Figure 2B), and moderate heterogeneity (I2 = 62.6%) existed among these studies.
Subgroup Analyses
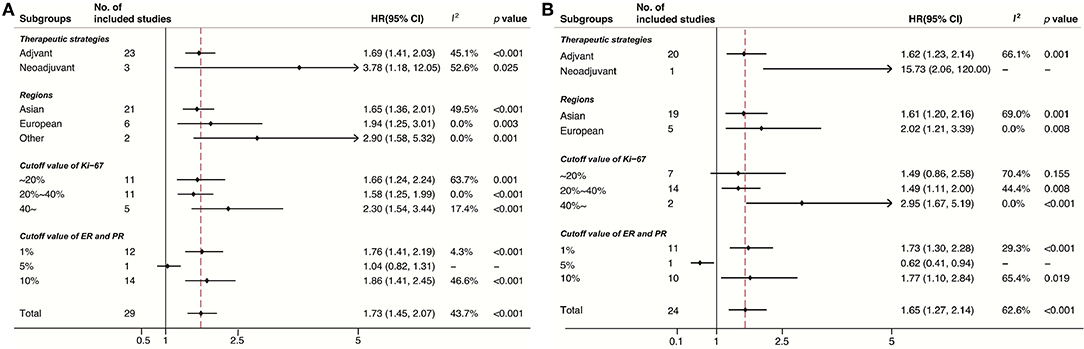
Subgroup analyses were conducted in accordance with Ki-67 cutoffs, positive ER/PR expression thresholds (1% or 10%), treatment strategies (neo-adjuvant or adjuvant), and geographic regions (Europe, Asian, or other regions). Despite the limited number of studies in some subgroups, the results of DFS (Figure 3A) and OS (Figure 3B) stratified by these factors were consistent. Noticeably, the pooled HR for DFS and OS was 2.30 (95% CI 1.54–3.44, p < 0.001) and 2.95 (95% CI 1.67–5.19, p < 0.001), respectively, under the circumstance of a cutoff of Ki-67 staining ≥40%.
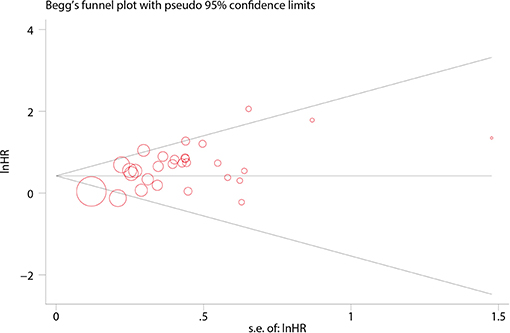
Publication Bias
In Begg's plots of publication bias, p-value was 0.209 (Figure 4), implying that publication bias did not exist in the present meta-analysis.
Discussion
TNBC has a worse prognosis than other phenotypes of breast cancer because of its aggressive biology and insensitivity to targeted therapy (52). Biomarkers useful in the selection of appropriate treatment strategies and the prediction of prognosis should be identified.
Previous studies demonstrated the prognostic role of Ki-67, as a critical biomarker of cell proliferation, in various malignancies that originate from organs and tissues, such as prostate, stomach, esophagus, cervix, and breast. A high expression level of Ki-67 protein was accompanied with poor prognostic outcomes (53). Several meta-analyses have shown that a high Ki-67 expression level is associated with the likelihood of achieving a pathological complete response (pCR) after patients with TNBC receive neo-adjuvant chemotherapy (NAC), and these patients may have favorable outcomes. Nevertheless, most of these studies included small sample sizes and contained diverse cut-offs of Ki-67 (54, 55).
In this meta-analysis, data were pooled to assess the prognostic value of Ki-67 in patients who suffered from resected TNBC and received neo-adjuvant or adjuvant chemotherapy. The results showed that patients with a high Ki-67 expression substantially had worse DFS and OS than their counterparts regardless of treatment strategies, study regions, Ki-67 cutoffs, or ER/PR thresholds.
Despite the consistency obtained in our study, the optimized cutoff of Ki-67 is still under deliberation (56). Some investigators suggested that Ki-67 should be used as a continuous marker to fully reflect the biological behavior of tumor proliferation and simultaneously resolve the cutoff issue; however, confronting diverse therapeutic strategies is impractical for clinical decision making (7). A previous meta-analysis indicated that a 25% cutoff of Ki-67 is adequate to distinguish patients with breast cancer at different risks of death (57). The cutoff selection of Ki-67 may be apparent if this parameter is considered within each subtype, and a 14% cutoff for the classification of luminal A and luminal B cancers was proposed in the 2011 St. Gallen Consensus (9). Considering that the baseline Ki-67 values of TNBC are usually higher than those of luminal diseases, Leskandarany et al. reported that the optimized Ki-67 cutoff within a TNBC subgroup population is 70% as determined by X-tile (58). Different Ki-67 values were selected as a cut-point in our included studies, and the threshold of Ki-67 varied between 10 and 50%. The subgroup analysis based on the Ki-67 cutoff indicated that the prediction was significant in all of the subgroups expect one subgroup (Ki-67 < 20%). This finding might indicate that further prospective studies should be performed to optimize the cutoff of Ki-67 in TNBC.
Baseline Ki-67 confirms the high chemosensitivity of highly proliferating TNBC after patients receive NAC, TNBC with a high Ki-67 expression likely has a high rate of pCR, which predicts favorable outcomes (59). However, studies have shown that TNBC with a high Ki-67 expression is associated with a poor prognosis because of rapid recurrence within 3 years despite a high pCR rate. A Korean study has demonstrated that a high Ki-67 expression (≥10%) is significantly associated with poor relapse-free survival and OS in preoperative TNBC despite a high pCR rate (26). Our subgroup analyses showed that a high Ki-67 expression is an adverse prognostic factor of DFS and OS both in the two groups of patients treated with adjuvant or neo-adjuvant therapy. Keam et al. reported that patients who suffer from TNBC and receive neo-adjuvant therapy with a high Ki-67 expression have a pattern of early recurrence. By contrast, the low-Ki-67-expressing subgroup did not have any pattern, indicating that a high Ki-67 expression, which indicated a high proliferation potential, might result in early recurrence. This phenomenon might partly explain why a high Ki-67 expression remained an adverse prognostic factor in the neo-adjuvant subgroup (23).
The American Society of Clinical Oncology and the College of American Pathologists Guideline Recommendations indicated that the cutoff for positive ER or PR should be ≥1% of immunoreactive tumor cell nuclei in 2010, and the previous threshold was >10%. Hence, a subgroup analysis classified by ER cut-off was performed. The results showed that a high Ki-67 expression was an adverse prognostic factor of all the subgroups, indicating that Ki-67 might be a prognostic factor of patients whose ER expression ranged from 2 to 10. Another study showed that defining triple-negative breast cancer as HER2-negative breast cancer with <10% rather than <1% of ER and progesterone receptor expression because HER2-negative primary breast cancer with ER < 10% clinically behaves like TNBC in terms of survival outcomes (60). This phenomenon might partly explain why Ki-67 was a poor prognostic factor of this patient subgroup.
Subgroup analyses on regions where these studies were conducted yielded the following classifications: Europe, Asia, and others. The results showed that a high Ki-67 expression was consistently an adverse prognostic factor of DFS and OS in these three subgroups. Moreover, the pooled data showed that TNBC was more likely to recur in Europe than in Asia. However, only eight studies were from Europe, while 27 studies were from Asia. Therefore, these findings should be carefully considered, and further studies should be performed to verify these results.
Notably, our study has a few limitations. First, due to linguistic constraints, we included studies written in English and Chinese only, hence publications in other languages could have been omitted. Second, we failed to perform subgroup analyses on other parameters, such as age or tumor stage, because of insufficient background information and thus might cause heterogeneity in the pooled results. Other clinical heterogeneities among studies, such as different NAC and adjuvant regimens, were not analyzed.
In conclusion, this study demonstrated that higher Ki-67 expression is a poorer prognostic factor of resected TNBC. The cut-off of ki-67 ≥40% is associated with a greater risk of recurrence and death compared with lower expression rates, despite the Ki-67 threshold with the greatest prognostic significance is as yet unknown.
Data Availability Statement
All datasets generated for this study are included in the manuscript/supplementary files.
Author Contributions
QW, WLi, and QZ contributed to the conception and design of this research. QW, GM, YD, WLu, YZ, WLi, and QZ contributed to the drafting of the article and final approval of the submitted version, and contributed to data analyses and the interpretation and completion of the figures and tables. All authors read and approved the final manuscript.
Funding
This study was supported by grants from the National Natural Science Foundation of China (81572288). The sponsor had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. (2015) 65:87–108. doi: 10.3322/caac.21262
2. Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. (2010) 363:1938–48. doi: 10.1056/NEJMra1001389
3. Penault-Llorca F, Viale G. Pathological and molecular diagnosis of triple-negative breast cancer: a clinical perspective. Ann Oncol. (2012) 23:vi19–22. doi: 10.1093/annonc/mds190
4. Weigelt B, Pusztai L, Ashworth A, Reis-Filho JS. Challenges translating breast cancer gene signatures into the clinic. Nat Rev Clin Oncol. (2011) 9:58–64. doi: 10.1038/nrclinonc.2011.125
5. Chen X, Li J, Gray WH, Lehmann BD, Bauer JA, Shyr Y, et al. TNBCtype: a subtyping tool for triple-negative breast cancer. Cancer Inform. (2012) 11:147–56. doi: 10.4137/CIN.S9983
6. Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. (2000) 182:311–22. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9
7. Denkert C, Budczies J, von Minckwitz G, Wienert S, Loibl S, Klauschen F. Strategies for developing Ki67 as a useful biomarker in breast cancer. Breast. (2015) 24:S67–72. doi: 10.1016/j.breast.2015.07.017
8. Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thürlimann B, et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol. (2013) 24:2206–23. doi: 10.1093/annonc/mdt303
9. Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thürlimann B, Senn HJ, et al. Strategies for subtypes–dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. (2011) 22:1736–47. doi: 10.1093/annonc/mdr304
10. Simon RM, Paik S, Hayes DF. Use of archived specimens in evaluation of prognostic and predictive biomarkers. J Natl Cancer Inst. (2009) 101:1446–52. doi: 10.1093/jnci/djp335
11. Knobloch K, Yoon U, Vogt PM. Preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement and publication bias. J Craniomaxfac Surg. (2011) 39:91–2. doi: 10.1016/j.jcms.2010.11.001
12. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
13. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. (2007) 8:16. doi: 10.1186/1745-6215-8-16
14. Higgins J, Thompson S, Deeks J, Altman D. Statistical heterogeneity in systematic reviews of clinical trials: a critical appraisal of guidelines and practice. J Health Serv Res Policy. (2002) 7:51–61. doi: 10.1258/1355819021927674
15. Kontopantelis E, Reeves D. Performance of statistical methods for meta-analysis when true study effects are non-normally distributed: a simulation study. Stat Methods Med Res. (2012) 21:409–26. doi: 10.1177/0962280210392008
16. Song F, Gilbody S. Bias in meta-analysis detected by a simple, graphical test. Increase in studies of publication bias coincided with increasing use of meta-analysis. BMJ (Clinical research ed.). (1998) 316:471. doi: 10.1136/bmj.316.7129.469
17. Toyama T, Yamashita H, Kondo N, Okuda K, Takahashi S, Sasaki H, et al. Frequently increased epidermal growth factor receptor (EGFR) copy numbers and decreased BRCA1 mRNA expression in Japanese triple-negative breast cancers. BMC Cancer. (2008) 8:309. doi: 10.1186/1471-2407-8-309
18. Treré D, Brighenti E, Donati G, Ceccarelli C, Santini D, Taffurelli M, et al. High prevalence of retinoblastoma protein loss in triple-negative breast cancers and its association with a good prognosis in patients treated with adjuvant chemotherapy. Ann Oncol. (2009) 20:1818–23. doi: 10.1093/annonc/mdp209
19. Lee JA, Kim KI, Bae JW, Jung YH, An H, Lee ES, et al. Triple negative breast cancer in Korea-distinct biology with different impact of prognostic factors on survival. Breast Cancer Res Treat. (2010) 123:177–87. doi: 10.1007/s10549-010-0998-5
20. Nishimura R, Osako T, Okumura Y, Hayashi M, Toyozumi Y, Arima N. Ki-67 as a prognostic marker according to breast cancer subtype and a predictor of recurrence time in primary breast cancer. Exp Ther Med. (2010) 1:747–54. doi: 10.3892/etm.2010.133
21. Wang J, Liu Y, Ji R, Gu Q, Zhao X, Liu Y, et al. Prognostic value of the X-linked inhibitor of apoptosis protein for invasive ductal breast cancer with triple-negative phenotype. Hum Pathol. (2010) 41:1186–95. doi: 10.1016/j.humpath.2010.01.013
22. Kashiwagi S, Yashiro M, Takashima T, Aomatsu N, Ikeda K, Ogawa Y, et al. Advantages of adjuvant chemotherapy for patients with triple-negative breast cancer at Stage II: usefulness of prognostic markers E-cadherin and Ki67. Breast Cancer Res. (2011) 13:R122. doi: 10.1186/bcr3068
23. Keam B, Im SA, Lee KH, Han SW, Oh DY, Kim JH, et al. Ki-67 can be used for further classification of triple negative breast cancer into two subtypes with different response and prognosis. Breast Cancer Res. (2011) 13:R22. doi: 10.1186/bcr2834
24. Li C, Li R, Song H, Wang D, Feng T, Yu X, et al. Significance of AEG-1 expression in correlation with VEGF, microvessel density and clinicopathological characteristics in triple-negative breast cancer. J Surg Oncol. (2011) 103:184–92. doi: 10.1002/jso.21788
25. Masuda H, Masuda N, Kodama Y, Ogawa M, Karita M, Yamamura J, et al. Predictive factors for the effectiveness of neoadjuvant chemotherapy and prognosis in triple-negative breast cancer patients. Cancer Chemother Pharmacol. (2011) 67:911–7. doi: 10.1007/s00280-010-1371-4
26. Miyashita M, Ishida T, Ishida K, Tamaki K, Amari M, Watanabe M, et al. Histopathological subclassification of triple negative breast cancer using prognostic scoring system: five variables as candidates. Virchows Archiv Int J Pathol. (2011) 458:65–72. doi: 10.1007/s00428-010-1009-2
27. Munzone E, Botteri E, Sciandivasci A, Curigliano G, Nolè F, Mastropasqua M, et al. Prognostic value of Ki-67 labeling index in patients with node-negative, triple-negative breast cancer. Breast Cancer Res Treat. (2012) 134:277–82. doi: 10.1007/s10549-012-2040-6
28. Ryu DW, Lee CH. Outcome of triple-negative breast cancer in patients with or without markers regulating cell cycle and cell death. J Korean Surg Soc. (2012) 83:187–95. doi: 10.4174/jkss.2012.83.4.187
29. Xue C, Wang X, Peng R, Shi Y, Qin T, Liu D, et al. Distribution, clinicopathologic features and survival of breast cancer subtypes in Southern China. Cancer Sci. (2012) 103:1679–87. doi: 10.1111/j.1349-7006.2012.02339.x
30. Huang L, Liu Z, Chen S, Liu Y, Shao Z. A prognostic model for triple-negative breast cancer patients based on node status, cathepsin-D and Ki-67 index. PLoS ONE. (2013) 8:e83081. doi: 10.1371/journal.pone.0083081
31. Milde-Langosch K, Karn T, Müller V, Witzel I, Rody A, Schmidt M, et al. Validity of the proliferation markers Ki67, TOP2A, and RacGAP1 in molecular subgroups of breast cancer. Breast Cancer Res Treat. (2013) 137:57–67. doi: 10.1007/s10549-012-2296-x
32. Xu J, Wu X, Zhou WH, Liu AW, Wu JB, Deng JY, et al. Aurora-A identifies early recurrence and poor prognosis and promises a potential therapeutic target in triple negative breast cancer. PLoS ONE. (2013) 8:e56919. doi: 10.1371/journal.pone.0056919
33. Yamashita N, Tokunaga E, Kitao H, Hisamatsu Y, Taketani K, Akiyoshi S, et al. Vimentin as a poor prognostic factor for triple-negative breast cancer. J Cancer Res Clin Oncol. (2013) 139:739–46. doi: 10.1007/s00432-013-1376-6
34. Zhang J, Wang Y, Yin Q, Zhang W, Zhang T, Niu Y. An associated classification of triple negative breast cancer: the risk of relapse and the response to chemotherapy. Int J Clin Exp Pathol. (2013) 6:1380–91.
35. Zhou L, Li K, Luo Y, Tian L, Wang M, Li C, et al. Novel prognostic markers for patients with triple-negative breast cancer. Hum Pathol. (2013) 44:2180–7. doi: 10.1016/j.humpath.2013.03.021
36. Park HS, Jang MH, Kim EJ, Kim HJ, Lee HJ, Kim YJ, et al. High EGFR gene copy number predicts poor outcome in triple-negative breast cancer. Mod Pathol. (2014) 27:1212–22. doi: 10.1038/modpathol.2013.251
37. Pistelli M, Caramanti M, Biscotti T, Santinelli A, Pagliacci A, De Lisa M, et al. Androgen receptor expression in early triple-negative breast cancer: clinical significance and prognostic associations. Cancers. (2014) 6:1351–62. doi: 10.3390/cancers6031351
38. Schmidt G, Meyberg-Solomayer G, Gerlinger C, Juhasz-Böss I, Herr D, Rody A, et al. Identification of prognostic different subgroups in triple negative breast cancer by Her2-neu protein expression. Archiv Gynecol Obstet. (2014) 290:1221–9. doi: 10.1007/s00404-014-3331-4
39. Asano Y, Kashiwagi S, Goto W, Kurata K, Noda S, Takashima T, et al. Tumour-infiltrating CD8 to FOXP3 lymphocyte ratio in predicting treatment responses to neoadjuvant chemotherapy of aggressive breast cancer. Br J Surg. (2016) 103:845–54. doi: 10.1002/bjs.10127
40. Hao S, He ZX, Yu KD, Yang WT, Shao ZM. New insights into the prognostic value of Ki-67 labeling index in patients with triple-negative breast cancer. Oncotarget. (2016) 7:24824–31. doi: 10.18632/oncotarget.8531
41. Khalifa J, Duprez-Paumier R, Filleron T, Lacroix Triki M, Jouve E, Dalenc F, et al. Outcome of pN0 triple-negative breast cancer with or without lymph node irradiation: a single institution experience. Breast J. (2016) 22:510–9. doi: 10.1111/tbj.12626
42. Liu YX, Wang KR, Xing H, Zhai XJ, Wang LP, Wang W. Attempt towards a novel classification of triple-negative breast cancer using immunohistochemical markers. Oncol Lett. (2016) 12:1240–56. doi: 10.3892/ol.2016.4778
43. Wang W, Wu J, Zhang P, Fei X, Zong Y, Chen X, et al. Prognostic and predictive value of Ki-67 in triple-negative breast cancer. Oncotarget. (2016) 7:31079–87. doi: 10.18632/oncotarget.9075
44. Yue Y, Astvatsaturyan K, Cui X, Zhang X, Fraass B, Bose S. Stratification of prognosis of triple-negative breast cancer patients using combinatorial biomarkers. PLoS ONE. (2016) 11:e0149661. doi: 10.1371/journal.pone.0149661
45. Zakaria F, El-Mashad N, Mohamed D. Androgen receptor expression as a prognostic and predictive marker in triple-negative breast cancer patients. Alexandria J Med. (2016) 52:131–40. doi: 10.1016/j.ajme.2015.06.002
46. Zhong Z, Shan M, Wang J, Liu T, Shi Q, Pang D. Decreased Wnt5a expression is a poor prognostic factor in triple-negative breast cancer. Med Sci Monitor. (2016) 22:1–7. doi: 10.12659/MSM.894821
47. Ieni A, Barresi V, Licata L, Cardia R, Fazzari C, Nuciforo G, et al. Immunoexpression of lactoferrin in triple-negative breast cancer patients: a proposal to select a less aggressive subgroup. Oncol Lett. (2017) 13:3205–9. doi: 10.3892/ol.2017.5859
48. Kwon J, Eom KY, Koo TR, Kim BH, Kang E, Kim SW, et al. A prognostic model for patients with triple-negative breast cancer: importance of the modified nottingham prognostic index and age. J Breast Cancer. (2017) 20:65–73. doi: 10.4048/jbc.2017.20.1.65
49. Najafi S, Mozaffari HR, Sadeghi M. Clinicopathological features of non-metastatic triple negative breast cancer. Iran J Blood Cancer. (2017) 9:18–23. doi: 10.1007/978-3-319-69980-6_2
50. Yang M, Han X. Nomogram model based on biomarkers for predicting the prognosis of triple negative breast cancer. Chin J Cancer Prev Treat. (2016) 23:988–995.
51. Wang C, Liu R. Expression of CDl47 in triple-negative breast cancer and its association With the prognosis. J Shanghai Jiao Tong Univ. (2017) 37:55–59.
52. Yao H, He G, Yan S, Chen C, Song L, Rosol TJ, et al. Triple-negative breast cancer: is there a treatment on the horizon? Oncotarget. (2017) 8:1913–24. doi: 10.18632/oncotarget.12284
53. Yerushalmi R, Woods R, Ravdin PM, Hayes MM, Gelmon KA. Ki67 in breast cancer: prognostic and predictive potential. Lancet Oncol. (2010) 11:174–83. doi: 10.1016/S1470-2045(09)70262-1
54. Chen X, He C, Han D, Zhou M, Wang Q, Tian J, et al. The predictive value of Ki-67 before neoadjuvant chemotherapy for breast cancer: a systematic review and meta-analysis. Fut Oncol. (2017) 13:843–57. doi: 10.2217/fon-2016-0420
55. Tian M, Zhong Y, Zhou F, Xie C, Zhou Y, Liao Z. Effect of neoadjuvant chemotherapy in patients with triple-negative breast cancer: a meta-analysis. Oncol Lett. (2015) 9:2825–32. doi: 10.3892/ol.2015.3072
56. Dowsett M, Nielsen TO, A'Hern R, Bartlett J, Coombes RC, Cuzick J, et al. Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in Breast Cancer working group. J Natl Cancer Inst. (2011) 103:1656–64. doi: 10.1093/jnci/djr393
57. Petrelli F, Viale G, Cabiddu M, Barni S. Prognostic value of different cut-off levels of Ki-67 in breast cancer: a systematic review and meta-analysis of 64,196 patients. Breast Cancer Res Treat. (2015) 153:477–91. doi: 10.1007/s10549-015-3559-0
58. Aleskandarany MA, Green AR, Benhasouna AA, Barros FF, Neal K, Reis-Filho JS, et al. Prognostic value of proliferation assay in the luminal, HER2-positive, and triple-negative biologic classes of breast cancer. Breast Cancer Res. (2012) 14:R3. doi: 10.1186/bcr3084
59. Gamucci T, Pizzuti L, Sperduti I, Mentuccia L, Vaccaro A, Moscetti L, et al. Neoadjuvant chemotherapy in triple-negative breastcancer: a multicentric retrospective observational study in real-life setting. J Cell Physiol. (2018) 233:2313–23. doi: 10.1002/jcp.26103
Keywords: Ki-67, triple-negative breast cancer, TNBC, prognosis, meta-analysis
Citation: Wu Q, Ma G, Deng Y, Luo W, Zhao Y, Li W and Zhou Q (2019) Prognostic Value of Ki-67 in Patients With Resected Triple-Negative Breast Cancer: A Meta-Analysis. Front. Oncol. 9:1068. doi: 10.3389/fonc.2019.01068
Received: 09 May 2019; Accepted: 30 September 2019;
Published: 17 October 2019.
Edited by:
Mothaffar Rimawi, Baylor College of Medicine, United StatesReviewed by:
Yoichi Naito, National Cancer Center Hospital East, JapanXiaosong Chen, Shanghai Jiao Tong University, China
Copyright © 2019 Wu, Ma, Deng, Luo, Zhao, Li and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wen Li, bGl3ZW5zY0AxNjMuY29t; Qinghua Zhou, emhvdXFoMTM1QDE2My5jb20=
†These authors have contributed equally to this work
 Qiang Wu1†
Qiang Wu1† Guangzhi Ma
Guangzhi Ma Wen Li
Wen Li