
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 10 September 2019
Sec. Hematologic Malignancies
Volume 9 - 2019 | https://doi.org/10.3389/fonc.2019.00892
This article is part of the Research Topic 50 Years of BMT: Conditioning Regimens and Early Complications After Transplantation View all 5 articles
 Andrei Colita1,2†
Andrei Colita1,2† Anca Colita3,4†
Anca Colita3,4† Horia Bumbea1,5†
Horia Bumbea1,5† Adina Croitoru4†
Adina Croitoru4† Carmen Orban4†
Carmen Orban4† Lavinia Eugenia Lipan4
Lavinia Eugenia Lipan4 Oana-Gabriela Craciun4
Oana-Gabriela Craciun4 Dan Soare1,5
Dan Soare1,5 Cecilia Ghimici1,2
Cecilia Ghimici1,2 Raluca Manolache1,2
Raluca Manolache1,2 Ionel Gelatu1,2
Ionel Gelatu1,2 Ana-Maria Vladareanu1
Ana-Maria Vladareanu1 Sergiu Pasca6,7
Sergiu Pasca6,7 Patric Teodorescu7,8
Patric Teodorescu7,8 Delia Dima7
Delia Dima7 Anca Lupu1,2
Anca Lupu1,2 Daniel Coriu1,9
Daniel Coriu1,9 Ciprian Tomuleasa6,7*
Ciprian Tomuleasa6,7* Alina Tanase4
Alina Tanase4High-dose chemotherapy (HDT) followed by autologous hematopoietic stem cell transplantation (ASCT) is widely used in patients with malignant lymphomas. In Europe over 8,000 ASCTs for lymphoma were performed out of a total of 40,000 transplants according to the European Bone Marrow Transplant (EBMT) activity survey in 2017. ASCT is considered the standard treatment for eligible patients failing to achieve remission after first line chemotherapy or patients with relapsed or refractory lymphomas, including classical Hodkin's lymphoma, diffuse large B-cell lymphoma, mantle cell lymphoma, and follicular lymphoma, as well as consolidation therapy in first remission in mantle cell lymphoma. BEAM (BCNU/carmustine, etoposide, cytarabine, and melphalan) is the most commonly used conditioning regimen for ASCT in patients with relapsed/refractory (R/R) lymphomas in Europe, whereas the CBV (cyclophosphamide, BCNU, and etoposide) regimen is also widely used in North America. Recently, concerns regarding BCNU toxicity as well as restricted availability of BCNU and melphalan has determined an increasing number of transplant centers to use alternative conditioning regimens. Currently, only a few comparative studies, most of them retrospective, between different conditioning protocols regarding efficacy and toxicity have been published. Thus, in the current manuscript, we report the experience of 2 transplant centers in ASCT in R/R lymphomas with three types of conditioning: BEAM, CLV (cyclophosphamide, lomustine, etoposide) and LEAM (lomustine, etoposide, cytarabine, and melphalan), with the aim to evaluate the results of alternative conditioning regimens using lomustine (LEAM and CLV) and compare them with the standard BEAM regarding early toxicity, engraftment, and transplant related mortality (TRM). All patients developed grade IV neutropenia, anemia with/without transfusion necessity. Severe thrombocytopenia with transfusion requirements is reported in most cases. Median time to platelet engraftment and neutrophil engraftment was 13 days (range) and 10 days (range), respectively. Gastrointestinal toxicity was the most common non-hematologic toxicity after all three conditioning regimens. Oral mucositis in various grades from I to IV was diagnosed in most cases. Other side effects include vomiting, diarrhea, colitis, and skin rash but with low severity grades. For the LEAM arm, one patient died after transplant, before engrafting, one patient didn't achieve platelet engraftment in day 100, one patient developed grade 3 upper gastrointestinal bleeding, one patient died (grade 5 toxicity) with acute renal failure, one patient developed hypoxic events up to grade 4 acute respiratory failure and one patient developed grade 3 itchy skin rash. For the CLV arm, one patient died after transplant, before engrafting, one patient developed grade 3 colitis, one patient with grade 3 hepatic cytolysis, one patient with cardiac toxicity followed by death (grade 5) caused by an acute myocardial infarction with ST elevation and one patient with pulmonary toxicity clinically manifested with grade 3 pleurisy. For the BEAM arm, one patient developed grade 3 cardiac toxicity with sinus bradycardia and afterwards grade 4 with acute pulmonary edema, three patients presented a grade 3 pruritic skin rash and two patients developed grade 3 seizures. In the present study we presented the differences that were observed between BEAM, LEAM, and CLV conditioning regimens offering clinical arguments for an SCT practitioner choice in the ideal situation, but also of choice for alternative regimens in the case that one regimen cannot be used.
In 2019, an estimated of approximately 82,000 lymphomas will be diagnosed in the Western world, with almost 21,000 deaths (1). Chemoimmunotherapy is the first line therapy of choice for most of these cases, especially for aggressive lymphomas, out of which up to 30% of all B-cell non-Hodgkin's lymphomas (NHL) don't achieve a complete remission (CR) with standard induction treatment, as is the case for R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) (2). The role of ASCT in the standard treatment of T cell lymphomas is less well defined than for relapsed B-cell lymphoma. A relapsed NHL is treated using salvage chemotherapy (ST) and HDT, followed by consolidation with an ASCT (3, 4).
For R/R lymphomas that undergo an ASCT, BEAM is a conditioning regimen option, being compared with LEAM (lomustine, etoposide, cytarabine, and melphalan) (5). The choice of conditioning chemotherapy has not been clarified by retrospective studies and there is no prospective data to support the use of one conditioning regimen over another. The replacement of BCNU with lomustine has been studied by Kothari et al. with no significant difference in transplant outcomes, although the number of patients included in this study was small. When comparing LEAM to CBV (cyclophosphamide, BCNU and etoposide) on 71 ASCT R/R lymphoma patients, dos Santos et al. have shown that mortality within the first 100 days was statistically favorable for LEAM when compared with CBV, suggesting less toxicity, but there was no statistically significant difference in PFS between the LEAM and CBV protocols. When the OS was evaluated, it was higher in the LEAM group (6). The number of patients was still limited. The CBV (cyclophosphamide, BCNU, etoposide) regimen is also widely used in North America (7). Over the last years, concerns regarding BCNU toxicity as well as restricted availability of BCNU and melphalan have determined an increasing number of transplant centers to use alternative conditioning regimens. Currently, there are only a few comparative studies, most of them retrospective, between different conditioning protocols regarding efficacy and toxicity (8).
As a limited number of studies have looked over the toxicity and efficacy of conditioning regimens on R/R lymphoma patients undergoing an ASCT, in the current manuscript we present a retrospective analysis on a large cohort (n = 222), comparing BEAM vs. LEAM vs. CLV (CBV with lomustine replacing BCNU).
The study was a retrospective analysis of the Romanian Society for Bone Marrow Transplantation. We identified 222 patients reported in the registry of the Fundeni Clinical Institute and the Coltea Clinical Hospital in Bucharest, Romania, as having undergone an ASCT for a R/R malignant lymphoma [both Hodgkin lymphomas (HL) as well as NHL] (Table 1). The inclusion criteria were date of ASCT between January 2012 and August 2017, diagnosis of HL, T-cell NHL, B-cell NHL and composite NHL, no previous stem cell procedures and conditioning with either LEAM, BEAM and CLV chemotherapy. We have included 34 patients from the pediatric ward and 188 patients from the adult ward. Functional imaging with 18-F-fluorodeoxyglucose (FDG)-positron emission tomography combined with computed tomography (PET-CT) was carried out in all cases, both before autologous SCT in order to optimize the outcome of the therapeutics with only patients in CR being eligible for SCT. PET-CT was also performed post-transplant, to evaluate the outcome of the therapy, according to previously published data (9–11).
Dosages for BEAM conditioning regimen were according to Caballero et al. (12), in LEAM, Lomustine 200 mg/m2 at day −7 replaced BCNU, whereas in CVL dosages were according to Majolno et al. with Lomustine 300 mg/m2 at day −6 replacing BCNU (Table 2) (13–15).
The objective was to assess the hematological and extra-hematological toxicities of the three conditioning regimens, the NRM and OS of the three patient cohorts. Standard conditioning therapies for ASCT in lymphoma are currently thus BCNU-based and BEAM is frequently used in Europe, while CBV is frequently used in North America. Shortage of BCNU is a world-wide problem caused by limited availability of the alcoholic solvent needed for its preparation, leading to the development of other conditioning regimens for ASCT in patients with lymphoma. Lomustine (CCNU) is a potential substitute of BCNU and determines the use of alternative regimens, as is the case for LEAM and CLV. Melphalan-related problems on market required the emergence of new conditioning regimens without melphalan (e.g., CLV, LACE (lomustine, cytarabine (Ara-C), cyclophosphamide, etoposide. The current manuscript presents retrospective data in the real-life setting. In Romania, BCNU was not available and thus it was replaced with CCNU, turning BEAM conditioning chemotherapy into LEAM conditioning. The same situation was reported when melphalan was absent and thus an alternative for conditioning was CLV.
Neutrophil engraftment was defined as the first of the three consecutive days post-transplant where absolute neutrophil count reached > 0.5 × 109/l and platelet count >20 × 109/l, unsupported by transfusion and G-CSF more than 5 days from the first increase in the investigated parameters.
Disease status was defined as follows: CR was defined as the disappearance of tumor masses and disease-related symptoms; partial response (PR) was considered when measurable lesions decreased by at least 50%; disease status at transplant was considered to be “chemosensitive” if at least PR was achieved following the last course of chemotherapy, otherwise it was considered as “chemo resistant.” CR/PR1 refers to transplants performed in first response; CR/PR > 1 refers to transplants performed beyond first response. NRM included all deaths without a previously registered episode of relapse or progression. Relapse was defined as the occurrence of new sites of disease after a CR lasting for 3 months or longer, whereas progression was considered when CR had not been achieved. OS was defined as the time from transplant to death from any cause. Good performance status (PS) was defined as Karnofsky score >80% or ECOG score 0–1.
Data analysis was performed using R 3.5.1. Categorical data was represented as percent (absolute value). Shapiro-Wilk test was used to determine the normality of the distribution. Mean and standard deviation were used for normally distributed samples. Median, quartile 1 and quartile 3 were used for non-normally distributed samples. ANOVA was used for comparing more than 2 normally distributed groups. Kruskal-Wallis was used for comparing more than 2 non-normally distributed groups. If Kruskal-Wallis test was significant, we used pairwise Wilcox test with Benjamini-Hochberg correction. Welch t-test was used for comparing 2 normally distributed groups. Mann-Whitney-Wilcoxon test was used for comparing 2 non-normally distributed groups. Fisher test was used to compare categories. Kaplan-Meier curves were used to represent survival differences between the three conditioning regimens. For the multivariate survival analysis, we used a Cox proportional hazards model in which we included the variables that presented statistical significance in the univariate Cox proportional hazards model. A p-value under 0.05 was considered significant.
For the univariate analysis for RFS at 100 days we included age over 50, male sex, Hodgkin lymphoma diagnosis, Stage 3 or 4 at diagnosis, the use of more than 2 previous chemotherapy lines, over 20 months between diagnosis and transplant, CR pre-transplant status, LEAM vs. BEAM conditioning, the use of red cell transfusion, mucositis grade 3 or 4 post-transplant, because none of them reached statistical significance, we did not perform the multivariate analysis. Because there were few deaths at 100 day a univariate cox proportional hazards analysis would yield aberrant results, thus it was not performed. For the univariate analysis for RFS at 2 years we included age over 50, male sex, Hodgkin lymphoma diagnosis, Stage 3 or 4 at diagnosis, the use of more than 2 previous chemotherapy lines, over 20 months between diagnosis and transplant, CR pre-transplant status, BEAM vs. LEAM, or CLV conditioning, the use of red cell transfusion, mucositis grade 3 or 4 post-transplant, because none of them reached statistical significance, we did not perform the multivariate analysis. For the univariate analysis for OS at 2 years we included age over 50, male sex, Hodgkin lymphoma diagnosis, Stage 3 or 4 at diagnosis, the use of more than 2 previous chemotherapy lines, over 20 months between diagnosis and transplant, CR pre-transplant status, BEAM vs. LEAM, or CLV conditioning, the use of red cell transfusion, mucositis grade 3 or 4 post-transplant, from these, Hodgkin Lymphoma diagnosis and the use of red cell transfusion were statistically significant and remained statistically significant in the multivariate Cox proportional hazards model.
The number of patients analyzed was 222 with age between 9 and 65 years old (median 36 years). There were 132 patients in BEAM group, 48 patients in LEAM group and 42 patients in CLV group.
All patients developed grade IV neutropenia, anemia with/without transfusion necessity, severe thrombocytopenia with transfusion requirements in most cases. Only 2 patients (0.9%) that received BEAM conditioning regimen didn't require platelet transfusions. Table 3 presents the data regarding the hematological toxicity. Median time to platelet engraftment and neutrophil engraftment was 13 days (range) and 10 days (range), respectively.
For the BEAM arm, two of the patients did not achieve platelet engraftment and died after transplant, whereas one patient didn't achieve neutrophil engraftment day 100 after transplant. Two patients developed as a complication hemophagocytic lymphohistiocytosis that led to delayed engraftment for platelets and neutrophils, until day 51, respectively, day 97 (Figure 1). For the LEAM arm, one patient died after transplant, before engrafting, and one patient didn't achieve platelet engraftment in day 100. For the CLV arm, one patient died after transplant, before engrafting.
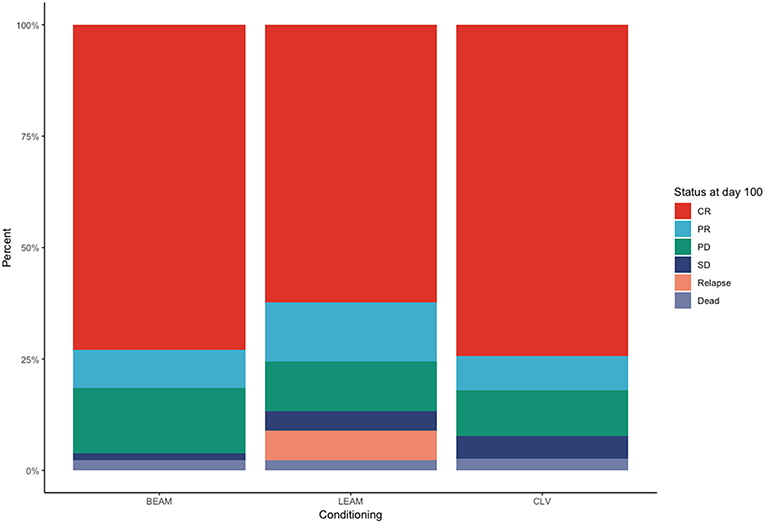
Figure 1. Status of the patients treated with the three conditioning chemotherapy regimens at day +100.
Gastrointestinal toxicity was the most common non-hematologic toxicity observed after all three conditioning regimens. Oral mucositis in various grades from I to IV was diagnosed in most cases. Other side effects include vomiting, diarrhea, colitis, and skin rash but with low severity grades (grade 1–2). Table 4 shows organ toxicity with clinical significance. From the BEAM arm, one patient developed grade 3 cardiac toxicity with sinus bradycardia and afterwards grade 4 with acute pulmonary edema, three patients presented a grade 3 pruritic skin rash and two patients developed grade 3 seizures. From the LEAM arm, one patient developed grade 3 upper gastrointestinal bleeding, one patient died (grade 5 toxicity) with acute renal failure, one patient developed hypoxic events up to grade 4 acute respiratory failure and one patient developed grade 3 itchy skin rash. From the CLV arm, one patient developed grade 3 colitis, one patient with grade 3 hepatic cytolysis, one patient with cardiac toxicity followed by death (grade 5) caused by an acute myocardial infarction with ST elevation and one patient with pulmonary toxicity clinically manifested with grade 3 pleurisy.
No statistical significance was observed in the univariate Cox proportional hazards model for relapse free survival until day 100. When considering RFS at 2 years (Figure 2), the only statistically significant difference was observed when comparing BEAM with LEAM or CLV (HR = 0.5, 95% CI 0.26–0.94, p = 0.031).
When considering OS at 2 years (Figure 3), the statistically significant difference in survival was observed when comparing red blood cell transfusion (HR = 3.7, 95% CI 1.3–11, p = 0.013) and the diagnosis of Hodgkin Lymphoma (HR = 0.28, 95% CI 0.1–0.78, p = 0.015). Both the diagnosis of Hodgkin Lymphoma (HR = 0.34, 95% CI 0.12–0.96, p = 0.041) and red cell transfusion (HR = 3.58, 95% CI 1.26–10.17, p = 0.017) remained statistically significant in the multivariate Cox proportional hazards model. The Cox proportional hazard analysis is presented in Tables 5A–C.
The TRM, hospitalization time and duration of antibiotics use are depicted in Table 6. For the BEAM arm, three patients died before day +100, all of them with sepsis, for the LEAM arm one patient died because of acute renal failure, whereas for the CLV arm, one died of acute myocardial infarction.
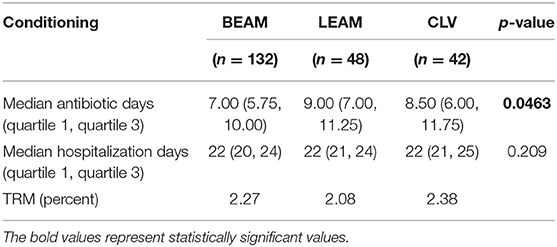
Table 6. TRM, hospitalization time and duration of antibiotics, for the three conditioning chemotherapy regimens.
In Europe over 8,000 ASCT for lymphomas were performed out of a total of 40,000 transplants according to the EBMT activity survey in 2017 (16). Retrospective and prospective studies have shown that PTCL patients who present response to initial chemotherapy and proceed to ASCT have a 4 years OS of 76–84% (5, 6). According to the MD Anderson Cancer Center (MDACC) experience, when using BEAM conditioning chemotherapy, both the OS and the PFS were higher in CR1. For CR2 and CR3, the OS were 59 and 53%. In Hodgkin's lymphoma, when comparing BEAM-based HDT to conventional chemotherapy as frontline treatment, patients with an advanced unfavorable HL that achieved CR or partial response (PR) after four courses of doxorubicin-containing regimens have a favorable outcome, with no benefit being shown from an early intensification with HDT and ASCT (17). Still, for relapsed/refractory (R/R) HL, ST followed by HDT and an ASCT is the standard of care, with a (18) F-fluordeoxyglucose (FDG) PET/CT intermediate negativity being the strongest predictor of clinical outcome (18). Choosing the best ST is difficult, being based on individual patient characteristics (19). ICE (ifosfamide, carboplatin, etoposide) is associated with 50% CR (20) and similar results are reported with DHAP and ESHAP (etoposide, methylprednisolone, high dose cytarabine and cisplatin), as well as with IGEV (ifosfamide, prednisolone, gemcitabine, and vinorelbine) (54% CR and 27% PR) (21–23).
Standard conditioning therapies for ASCT in lymphoma are currently thus BCNU-based, BEAM being the most commonly used conditioning regimen in Europe, while CBV is frequently used in North America. Shortage of BCNU is a world-wide problem determined by limited availability of the alcoholic solvent needed for its preparation, leading to the development of other conditioning regimens for ASCT in patients with lymphoma. Lomustine (CCNU) seemed the logical replacement of BCNU and resulted in the use of alternative regimens, as is the case for LEAM and CLV. Melphalan-related problems on market required the emergence of new conditioning regimens without melphalan (e.g., CLV, LACE (lomustine, cytarabine (Ara-C), cyclophosphamide, etoposide) (24–26). In our centers, we examined the toxicities of LEAM and CLV regimens, compared with standard BEAM.
Early data about high incidence of pulmonary toxicity (20–30%) associated to BCNU has driven the search for alternative regimens. Chao et al. have recommended a dose-escalation study with CCNU replacing BCNU in CBV regimen in 12 lymphoma patients and reported the maximum tolerated dose (MTD) of 15 mg/kg of CCNU orally (27, 28). At Stanford, a phase II study evaluating CLV vs. CBV conditioning in lymphoma patients reported the same efficacy but with higher pulmonary toxicity in the CLV regimen using 15 mg/kg CCNU (28), equivalent to more than 500 mg/m2. In a dose escalation prospective study performed on 14 patients, do Santos et al. have reported an MTD of 300 mg/m2 CCNU in combination with etoposide, cytarabine and melphalan as an alternative LEAM regimen (8). Most studies comparing the CCNU-based regimen with the standard BEAM used the dosage of 200 mg/m2 reporting similar efficacy and toxicity (5, 29, 30). Still, there are no randomized studies comparing standard BEAM conditioning with other preparative regimens for ASCT in lymphoma patients.
Our study compares engraftment, early toxicity and TRM, between the three conditioning regimens BEAM, LEAM and CLV for a cohort of 222 patients receiving ASCT over a period of 5 years. We did not find statistically significant differences between the three groups in parameters measuring immediate post-transplant outcome, as is the case for data regarding engraftment, therapy-related toxicity, antibiotic use, duration of hospitalization, 100 days TRM. The median time to neutrophil engraftment in our study was 10 days, comparable with data reported by Sharma et al. (14) and Kothari et al. (5). The median time for platelet engraftment in our study, of 13 days, was comparable with 12 days reported by Kothari et al. but shorter compared to the data, of 18.5 days, presented by Sharma et al. (Table 7). Still, when comparing our results with similar studies, major drawbacks are the differences in timepoints and demographic characteristics.
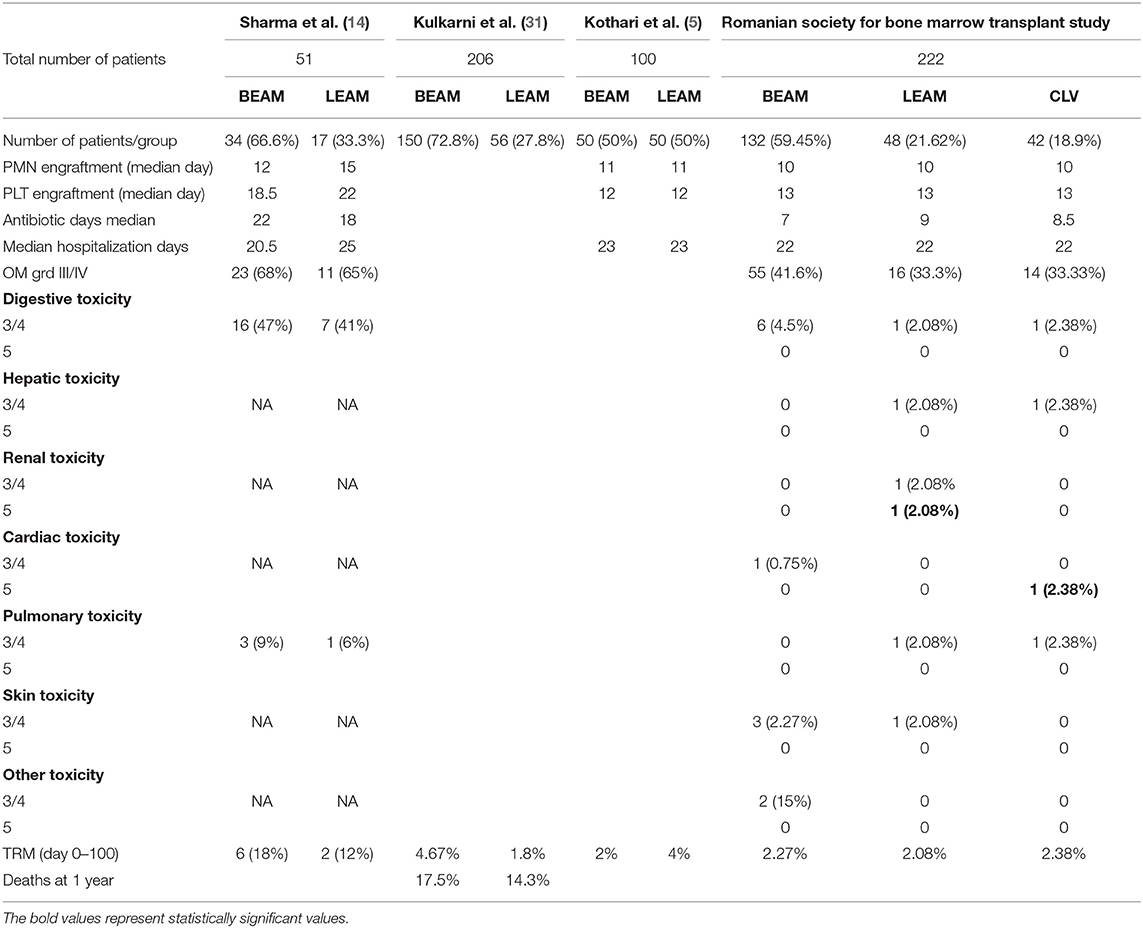
Table 7. Comparison of the toxicity results between previously published data and the Romanian cohort.
The median hospitalization period for patients in the 3 groups in our study was 22 days, similar to data reported by other studies (5, 14), but we observed a shorter duration of antibiotic treatment compared with the Sharma et al. study. The most frequent grade 3/4 non-hematologic toxicity was oral mucositis with a higher incidence of 41.6% in the BEAM group, compared to 33.3% in both the LEAM and CLV groups, but with lower incidence than in other studies in both CCNU and BCNU based conditioning reporting (reporting 2/3 of cases) (14, 32).
A major concern of BCNU treatment was pulmonary toxicity reported in earlier studies (33, 34). Regimens containing high doses of CCNU also led to an important incidence of interstitial pneumonitis (IP) in up to 63% of cases (28). The use of lower doses of CCNU was associated with lower incidence of IP. Sharma et al. (14) compared LEAM vs. BEAM and reported 6% and, respectively, 9% grade III and IV pulmonary toxicity in the 2 groups. In our study, there were no cases with grade III/IV pulmonary toxicity in the BEAM group and 1 case in each group receiving LEAM and CLV (2.08 and 2.38%, respectively).
In our study TRM was similar in the three groups, with 3 infection related deaths in the BEAM group (TRM 2.27%) and 1 toxicity related death in each of the other two groups (LEAM- TRM 2.08% and CLV- TRM 2.38%). Our data are comparable with those reported by Kulkarni et al. (31) and Kothary et al. (5) and are significantly lower than those presented by the Sharma et al. (14).
The choice of HDT regimen before an ASCT for both HL and NHL is guided by limited data. In a large retrospective analysis of almost 5,000 patients treated over a 13-year period between 1995 and 2003, the study coordinated by Pasquini et al. on behalf of the Center for International Blood and Marrow Research (CIBMTR) have compared the conditioning chemotherapy regimens BEAM (1730 patients) to cyclophosphamide plus BCNU plus etoposide (CBV) (1853 patients), busulfan plus cyclophosphamide (789 patients) and to TBI-based treatment (545 patients) (35–40). The 1-year incidence of IPS was 3 to 6% and was highest in recipients of CBV and TBI, compared with BEAM. One-year TRM was 4 to 8%, respectively, and was similar between regimens. For NHL cases, there was a significant interaction between histology, HDT regimen, and outcome. Compared with BEAM, CBV was associated with lower mortality in follicular lymphoma and CBV was associated with higher mortality in diffuse large B cell lymphoma. For patients with HL, CBV, busulfan plus cyclophosphamide and TBI were associated with higher mortality compared with BEAM. They concluded thus that the impact of specific AHCT regimen on post-transplantation survival is different depending on histology. Therefore, further studies are required to define the best regimen for specific diseases, our study bringing new information for transplant physicians for R/R lymphomas that undergo an autologous stem cell transplantation.
An autologous stem cell transplantation is the best therapeutic approach for a R/R lymphoma. Conditioning chemotherapy is of utmost importance, with LEAM, BEAM and CLV conditioning regimens being considered as viable alternatives. In this study we present the comparative analysis of a large cohort of patients with R/R lymphoma undergoing ASCT with BEAM/LEAM/CLV conditioning and we document the similar outcome regarding engraftment, early toxicity, and TRM. The data presented only analyses the clinical status of the patients in the first 100 days following the transplant and future studies should be carried out to compare the three conditioning regimens beyond day +100 after the transplant.
All datasets generated for this study are included in the manuscript/supplementary files.
The study was reviews and approved by the ethics committees of both the Fundeni Clinical Institute in Bucharest, as well as of the Coltea Hospital in Bucharest.
All authors have read and approved the manuscript and contributed to data gathering. SP did the statistics. HB, AncC, AndC, and CT wrote the manuscript. AT supervised the work.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. (2019) 69:7–34. doi: 10.3322/caac.21551
2. Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. (2002) 346:235–42. doi: 10.1056/NEJMoa011795
3. Brammer JE, Khouri I, Gaballa S, Anderlini P, Tomuleasa C, Ahmed S, et al. Outcomes of haploidentical stem cell transplantation for lymphoma with melphalan-based conditioning. Biol Blood Marrow Transplant. (2016) 22:493–8. doi: 10.1016/j.bbmt.2015.10.015
4. Gaballa S, Ge I, El Fakih R, Brammer JE, Kongtim P, Tomuleasa C, et al. Results of a 2-arm, phase 2 clinical trial using post-transplantation cyclophosphamide for the prevention of graft-versus-host disease in haploidentical donor and mismatched unrelated donor hematopoietic stem cell transplantation. Cancer. (2016) 122:3316–26. doi: 10.1002/cncr.30180
5. Kothari J, Foley M, Peggs KS, Mackenzie S, Thomson K, Morris E, et al. A retrospective comparison of toxicity and initial efficacy of two autologous stem cell transplant conditioning regimens for relapsed lymphoma: LEAM and BEAM. Bone Marrow Transplant. (2016) 51:1397–9. doi: 10.1038/bmt.2016.134
6. Dos Santos KB, Costa LJM, Bettarello G, da Matta Abreu M, Mayrink GTC, Mota MA, et al. LEAM versus CBV for conditioning in autologous hematopoietic stem cell transplantation for lymphoma. Bone Marrow Transplant. (2018) 54:625–8. doi: 10.1038/s41409–018-0349–4
7. Martínez AS. Classical Hodgkin's Lymphoma. In: Carreras E, Dufour C, Mohty M, Kröger N, editors. The EBMT Handbook. Hematopoietic Stem CellTransplantation and Cellular Therapies. Heidelberg: EBMT; Springer Open (2019). p. 653–62.
8. dos Santos KB, Costa LJ, Atalla A, Pereira J, Hallack-Neto AE. Lomustine use in combination with etoposide, cytarabine and melphalan in a brief conditioning regimen for auto-HSCT in patients with lymphoma: the optimal dose. Bone Marrow Transplant. (2014) 49:1239–40. doi: 10.1038/bmt.2014.121
9. Thanarajasingam G, Bennani-Baiti N, Thompson CA. PET-CT in staging, response evaluation, and surveillance of lymphoma. Curr Treat Options Oncol. (2016) 17:24. doi: 10.1007/s11864-016-0399-z
10. Tanase A, Tomuleasa C, Marculescu A, Bardas A, Colita A, Orban C, et al. Haploidentical donors: can faster transplantation be life-saving for patients with advanced disease? Acta Haematol. (2016) 135:211–6. doi: 10.1159/000443469
11. Tănase A, Tomuleasa C, Mărculescu A, Bardaş A, Coliţă A, Ciurea ŞO. First successful haploidentical stem cell transplantation in Romania. Rom J Intern Med. (2016) 54:194–200. doi: 10.1515/rjim-2016-0021
12. Caballero MD, Rubio V, Rifon J, Heras I, García-Sanz R, Vázquez L, et al. BEAM chemotherapy followed by autologous stem cell support in lymphoma patients: analysis of efficacy, toxicity and prognostic factors. Bone Marrow Transplant. (1997) 20:451–8. doi: 10.1038/sj.bmt.1700913
13. Reid RM, Baran A, Friedberg JW, Phillips GL, Liesveld JL, Becker MW, et al. Outpatient administration of BEAM conditioning prior to autologous stem cell transplantation for lymphoma is safe, feasible, and cost-effective. Cancer Med. (2016) 5:3059–67. doi: 10.1002/cam4.879
14. Sharma A, Kayal S, Iqbal S, Malik PS, Raina V. Comparison of BEAM vs. LEAM regimen in autologous transplant for lymphoma at AIIMS. Springerplus. (2013) 2:489. doi: 10.1186/2193-1801-2-489
15. Majolino I, Scimè R, Indovina A, Vasta S, Patti C, Gentile S, et al. Treatment of malignant lymphomas with very-high-dose CVB followed by transplantation of autologous blood stem cells collected after mobilizing chemotherapy. Haematologica. (1991) 76 (suppl. 1):66–71.
16. Passweg JR, Baldomero H, Bader P, Bonini C, Cesaro S, Dreger P, et al. Hematopoietic stem cell transplantation in Europe 2014: more than 40 000 transplants annually. Bone Marrow Transplant. (2016) 51:786–92. doi: 10.1038/bmt.2016.20
17. Federico M, Bellei M, Brice P, Brugiatelli M, Nagler A, Gisselbrecht C, et al. High-dose therapy and autologous stem-cell transplantation versus conventional therapy for patients with advanced Hodgkin's lymphoma responding to front-line therapy. J Clin Oncol. (2003) 21:2320–5. doi: 10.1200/JCO.2003.11.103
18. Moskowitz AJ, Yahalom J, Kewalramani T, Maragulia JC, Vanak JM, Zelenetz AD, et al. Pretransplantation functional imaging predicts outcome following autologous stem cell transplantation for relapsed and refractory Hodgkin lymphoma. Blood. (2010) 116:4934–7. doi: 10.1182/blood-2010-05-282756
19. von Tresckow B, Moskowitz CH. Treatment of relapsed and refractory Hodgkin Lymphoma. Semin Hematol. (2016) 53:180–5. doi: 10.1053/j.seminhematol.2016.05.010
20. Moskowitz CH, Nimer SD, Zelenetz AD, Trippett T, Hedrick EE, Filippa DA, et al. A 2-step comprehensive high-dose chemoradiotherapy second-line program for relapsed and refractory Hodgkin disease: analysis by intent to treat and development of a prognostic model. Blood. (2001) 97:616–23. doi: 10.1182/blood.V97.3.616
21. Santoro A, Magagnoli M, Spina M, Pinotti G, Siracusano L, Michieli M, et al. Ifosfamide, gemcitabine, and vinorelbine: a new induction regimen for refractory and relapsed Hodgkin's lymphoma. Haematologica. (2007) 92:35–41. doi: 10.3324/haematol.10661
22. Rancea M, von Tresckow B, Monsef I, Engert A, Skoetz N. High-dose chemotherapy followed by autologous stem cell transplantation for patients with relapsed or refractory Hodgkin lymphoma: a systematic review with meta-analysis. Crit Rev Oncol Hematol. (2014) 92:CD009411. doi: 10.1016/j.critrevonc.2014.04.003
23. Rancea M, Monsef I, von Tresckow B, Engert A, Skoetz N. High-dose chemotherapy followed by autologous stem cell transplantation for patients with relapsed/refractory Hodgkin lymphoma. Cochrane Database Syst Rev. (2013) 6:CD009411. doi: 10.1002/14651858.CD009411.pub2
24. Khattry N, Gupta A, Jain R, Gore A, Thippeswamy R, Jeevangi N, et al. LACE versus BEAM conditioning in relapsed and refractory lymphoma transplant: retrospective multicenter analysis of toxicity and efficacy. Int J Hematol. (2016) 103:292–8. doi: 10.1007/s12185-015-1927-5
25. Pavlu J, Auner HW, Ellis S, Szydlo RM, Giles C, Contento A, et al. LACE-conditioned autologous stem cell transplantation for relapsed or refractory diffuse large B-cell lymphoma: treatment outcome and risk factor analysis from a single centre. Hematol Oncol. (2011) 29:75–80. doi: 10.1002/hon.956
26. Scortechini I, Montanari M, Mancini G, Inglese E, Calandrelli M, Chiarucci M, et al. Conditioning regimen with BCNU, etoposide, cytarabine and melphalan plus amifostine for outpatient autologous stem cell transplant: feasibility and outcome in 97 patients with lymphoma. Leuk Lymphoma. (2014) 55:1657–60. doi: 10.3109/10428194.2013.842989
27. Chao NJ, Kastrissios H, Long GD, Negrin RS, Horning SJ, Wong RM, et al. A new preparatory regimen for autologous bone marrow transplantation for patients with lymphoma. Cancer. (1995) 75:1354–9. doi: 10.1002/1097-0142(19950315)75:6<1354::AID-CNCR2820750618>3.0.CO;2-M
28. Stuart MJ, Chao NS, Horning SJ, Wong RM, Negrin RS, Johnston LJ, et al. Efficacy and toxicity of a CCNU-containing high-dose chemotherapy regimen followed by autologous hematopoietic cell transplantation in relapsed or refractory Hodgkin's disease. Biol Blood Marrow Transplant. (2001) 7:552–60. doi: 10.1016/S1083-8791(01)70015-8
29. Ramzi M, Mohamadian M, Vojdani R, Dehghani M, Nourani H, Zakerinia M, et al. Autologous noncryopreserved hematopoietic stem cell transplant with CEAM as a modified conditioning regimen in patients with Hodgkin lymphoma: a single-center experience with a new protocol. Exp Clin Transplant. (2012) 10:163–7. doi: 10.6002/ect.2011.0092
30. Rotaru I, Ciurea T, Foarfă C, Tănase AD, Găman G. The diagnostic characteristics of a group of patients with primary gastric lymphoma: macroscopic, histopathological and immunohistochemical aspects. Rom J Morphol Embryol. (2012) 53:343–50.
31. Kulkarni S, Kaye D, Bloor A. Safety and Efficacy Of Lomustine (CCNU) Substituting Carmustine (BCNU) in conditioning for autologous haematopoietic stem cell transplantation in lymphoma. a retrospective analysis of two patient cohorts over a ten year period. Blood. (2013) 122:2127.
32. Wang EH, Chen YA, Corringham S, Bashey A, Holman P, Ball ED, et al. High-dose CEB vs BEAM with autologous stem cell transplant in lymphoma. Bone Marrow Transplant. (2004) 34:581–7. doi: 10.1038/sj.bmt.1704637
33. Cordonnier C, Vernant JP, Mital P, Lange F, Bernaudin JF, Rochant H. Pulmonary fibrosis subsequent to high doses of CCNU for chronic myeloid leukemia. Cancer. (1983) 51:1814–8. doi: 10.1002/1097-0142(19830515)51:10<1814::AID-CNCR2820511010>3.0.CO;2-Y
34. Reece DE, Barnett MJ, Connors JM, Fairey RN, Fay JW, Greer JP, et al. Intensive chemotherapy with cyclophosphamide, carmustine, and etoposide followed by autologous bone marrow transplantation for relapsed Hodgkin's disease. J Clin Oncol. (1991) 9:1871–9. doi: 10.1200/JCO.1991.9.10.1871
35. Chen YB, Lane AA, Logan B, Zhu X, Akpek G, Aljurf M, et al. Impact of conditioning regimen on outcomes for patients with lymphoma undergoing high-dose therapy with autologous hematopoietic cell transplantation. Biol Blood Marrow Transplant. (2015) 21:1046–53. doi: 10.1016/j.bbmt.2015.02.005
36. Constantinescu C, Bodolea C, Pasca S, Teodorescu P, Dima D, Rus I, et al. Clinical approach to the patient in critical state following immunotherapy and/or stem cell transplantation: guideline for the on-call physician. J Clin Med. (2019) 8:E884. doi: 10.3390/jcm8060884
37. Tomuleasa C, Selicean C, Cismas S, Jurj A, Marian M, Dima D, et al. Minimal residual disease in chronic lymphocytic leukemia: a consensus paper that presents the clinical impact of the presently available laboratory approaches. Crit Rev Clin Lab Sci. (2018) 55:329–45. doi: 10.1080/10408363.2018.1463508
38. Tomuleasa C, Fuji S, Berce C, Onaciu A, Chira S, Petrushev B, et al. Chimeric antigen receptor T-cells for the treatment of B-cell acute lymphoblastic leukemia. Front Immunol. (2018) 9:239. doi: 10.3389/fimmu.2018.00239
39. Qian L, Dima D, Berce C, Liu Y, Rus I, Raduly LZ, et al. Protein dysregulation in graft versus host disease. Oncotarget. (2017) 9:1483–91. doi: 10.18632/oncotarget.23276
Keywords: relapsed/refractory lymphoma, autologous stem cell transplantation, conditioning chemotherapy, retrospective analysis, real-life data
Citation: Colita A, Colita A, Bumbea H, Croitoru A, Orban C, Lipan LE, Craciun O-G, Soare D, Ghimici C, Manolache R, Gelatu I, Vladareanu A-M, Pasca S, Teodorescu P, Dima D, Lupu A, Coriu D, Tomuleasa C and Tanase A (2019) LEAM vs. BEAM vs. CLV Conditioning Regimen for Autologous Stem Cell Transplantation in Malignant Lymphomas. Retrospective Comparison of Toxicity and Efficacy on 222 Patients in the First 100 Days After Transplant, On Behalf of the Romanian Society for Bone Marrow Transplantation. Front. Oncol. 9:892. doi: 10.3389/fonc.2019.00892
Received: 26 May 2019; Accepted: 27 August 2019;
Published: 10 September 2019.
Edited by:
Alessandro Isidori, AORMN Hospital, ItalyReviewed by:
Anna Sureda, Catalan Institute of Oncology, SpainCopyright © 2019 Colita, Colita, Bumbea, Croitoru, Orban, Lipan, Craciun, Soare, Ghimici, Manolache, Gelatu, Vladareanu, Pasca, Teodorescu, Dima, Lupu, Coriu, Tomuleasa and Tanase. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ciprian Tomuleasa, Y2lwcmlhbi50b211bGVhc2FAdW1mY2x1ai5ybw==
†These authors have contributed equally to this work as first authors
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.