
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 23 September 2019
Sec. Thoracic Oncology
Volume 9 - 2019 | https://doi.org/10.3389/fonc.2019.00880
This article is part of the Research Topic Emerging Biomarkers for NSCLC: Recent Advances in Diagnosis and Therapy View all 20 articles
Patients' clinical factors and genetics factors such as anaplastic lymphoma kinase (ALK) fusion variants and BIM (Bcl-2-like 11) polymorphism were reported to be associated with clinical outcome in crizotinib-treated advanced non-small cell lung cancer (NSCLC). However, the results were still controversial. We analyzed outcome of 54 patients with known ALK fusion variants who received crizotinib for advanced NSCLC. Thirty of them had successful BIM polymorphism analysis and 6 (20%) had a BIM deletion. Multivariate Cox regression analysis found that previous anticancer therapy [adjusted hazard ratio (aHR) 1.35, 95% confidence interval (CI), 1.04–1.76 for each additional line of therapy, p = 0.025] and Eastern Cooperative Oncology Group (ECOG) performance status ≥2 (aHR 8.35, 95% CI, 1.52–45.94, p = 0.015) were independent factors for progression-free survival (PFS). Only ECOG performance status ≥2 (aHR 7.20, 95% CI, 1.27–40.79, p = 0.026) was an independent factor for overall survival (OS). Neither ALK fusion variants nor the presence of a BIM deletion was associated with crizotinib PFS or OS. After adjusting with clinical factors, different ALK variants and BIM polymorphism might not be independent factors for crizotinib PFS or OS in advanced NSCLC with ALK rearrangement.
In 2007, the echinoderm microtubule-associated protein-like 4 (EML4)–anaplastic lymphoma kinase (ALK) gene rearrangement was first discovered as a driver oncogene for non-small cell lung cancer (NSCLC) (1). Inversion in chromosome 2p fused the N-terminal domain of EML4 to the intracellular kinase domain of ALK, causing constitutive activation of tyrosine kinase, leading to uncontrolled cell growth and proliferation. During the following 10 years, targeting ALK with tyrosine kinase inhibitors (TKIs) has achieved great success. The first-generation ALK TKI crizotinib had better progression-free survival (PFS) (10.9 vs. 7.0 months) and a better overall response rate (ORR) (74 vs. 45%) than chemotherapy in treating naïve ALK rearranged {ALK positive [ALK(+)]} NSCLC in the PROFILE 1014 study (2). Crizotinib has been approved by the US Food and Drug Administration (US FDA) as first-line treatment for ALK(+) advanced NSCLC (3). The second-generation ALK TKIs alectinib (CH5424802/ RO5424802) and ceritinib (LDK378) also showed promising activity in controlling ALK(+) NSCLC in phase 3 trials (4, 5). Moreover, in ALK TKI-naïve patients treated with brigatinib, a next-generation ALK TKI, the PFS was longer than patients treated with crizotinib (6). The development of ALK TKI for use against ALK(+) NSCLC is one of the best stories in the history of developing anticancer therapy.
However, most patients still experienced disease progression after ALK TKI treatment. The latest released data for East Asian patients in PROFILE 1029 revealed that median PFS was 11.1 (95% confidence interval, CI: 8.3–12.6) months for first-line crizotinib-treated advanced ALK(+) NSCLC patients (7). Several factors were reported to be associated with crizotinib PFS, but the two main groups were clinical factors and genetic factors. Traditional clinical factors such as the patient's performance status (8, 9) and brain metastasis (10–12) prior to crizotinib treatment were reported to influence crizotinib PFS. Among the genetic factors, one of the most common was ALK fusion variants. In the preclinical data, different ALK fusion variants were associated with crizotinib sensitivity. ALK fusion variant 2 had lower crizotinib IC50 than variant 3. Longer ALK fusion variants were the most unstable and were supposed to be more sensitive to crizotinib than shorter ALK fusion variants (13). The presence of a tandem atypical beta-propeller in the EML protein (TAPE) domain was reported to influence the stability of EML4–ALK protein (14); “short variants,” such as variants 3a/b and 5a/b, lack a TAPE domain (15) and might be less responsive to crizotinib than the longer TAPE-containing variants, such as variant 1 and variant 2 (16). A circular RNA F-circEA found in only variant 3 was reported to promote cancer cell migration and proliferation (17). However, in spite of the supposed mechanism, the real-world data were conflicting. ALK variant 1 (18), variant 2 (19), and variants other than variant 3 (16, 20) were reported to have a better crizotinib PFS, but there were also several reports indicating that all variants had a similar outcome (21, 22). In fact, the largest cohort to date reported there was no crizotinib PFS difference between variant 1 and variant 3 (23). Whether or not different EML4–ALK fusion variants influence crizotinib PFS remains controversial.
Another interesting genetic factor was Bcl-2-like 11 (BIM). BIM is a pro-apoptotic member of the B-cell CLL/lymphoma 2 (BCL2) family of proteins, discovered in Asia only. Its upregulation is required for TKIs to induce apoptosis in kinase-driven cancers (24). The BIM deletion polymorphism was reported to be associated with primary resistance to or a short PFS with epidermal growth factor receptor (EGFR) TKI in advanced EGFR-mutant NSCLC (24, 25). Another report indicated that BIM deletion was related to a poor crizotinib response in advanced ALK(+) NSCLC (26). However, in our previous study, we could not find a relationship between the BIM deletion polymorphism and primary EGFR TKI resistance among our 327 Taiwanese patients, while 52 (16%) of them were positive for BIM deletion (27). In this study, we aimed to analyze the association of clinical factors and genetic factors, including ALK fusion variants and BIM polymorphism, with crizotinib PFS and overall survival (OS) in advanced EML4–ALK(+) NSCLC patients.
This study retrospectively enrolled patients receiving crizotinib for EML4–ALK rearrangement stage IV or postoperative recurrent (advanced) NSCLC between December, 31, 2010, and December, 31, 2017, at the National Taiwan University Hospital. Only patients with data on EML4–ALK variants using reverse transcription polymerase chain reaction (RT-PCR) were included. Patients who stopped crizotinib within 30 days due to intolerable side effects were excluded. Patients' baseline characteristics, including age, gender, smoking status, previous anticancer therapy, Eastern Cooperative Oncology Group (ECOG) performance status (28), prior brain metastasis, EML4–ALK variants, and status of BIM polymorphism, were checked. The patients were treated and followed up based on the clinician's decision. A blinded chest physician who was not involved in patient management and did not know the laboratory data on EML4–ALK variants and BIM polymorphism retrospectively reviewed the chart and images to determine disease progression according to RECIST criteria version 1.1 (29). PFS was defined as the duration from the first dose of crizotinib to disease progression or death during treatment. OS was defined as the duration from the first dose of crizotinib to the patient's death. Each patient's best overall response, PFS, and OS were recorded. This study was approved by the Institutional Review Board of National Taiwan University Hospital. Written informed consent was obtained from all patients before checking their cancer specimens for molecular studies. All methods were performed in accordance with the relevant guidelines and regulations.
Using immunohistochemistry (IHC) stain, we checked the patients' cancer specimens for ALK using Ventana ALK (D5F3) antibody. We further analyzed cancer specimens for EML4–ALK variants using RNA RT-PCR, as previously described (30). In brief, RNA extracted from patients' tissue specimens were collected for RT-PCR amplification by a OneStep RT-PCR Kit (Qiagen) using the following primers: 5′-TGGCTGATGTTTTGAGGCGT-3′ (forward, on exon 2 of EML4), 5′-AGAGCCCACACCTGGGAAAG-3′ (forward, on exon 13 of EML4), 5′-CCACACAGACGGGAATGAAC-3′ (forward, on exon 18 of EML4), and 5′-AGCAAAGCAGTAGTTGGGGT-3′ (reverse, on exon 20 of ALK). The PCR conditions were as follows: 50°C for 30 min, 95°C for 15 min (94°C for 50 s, 60°C for 50 s, 72°C for 60 s) × 40 cycles, and 72°C for 10 min. RT-PCR amplicons were purified and sequenced with Sanger sequencing in both sense and antisense directions. Because the length of the ALK fusion protein may contribute to its stability (13) and probably crizotinib PFS, we also separated different ALK fusion variants into a long group and a short group. Short ALK fusion variants were defined as variants that do not have the TAPE main, i.e., variant 3a, variant 3b, variant 5a, and variant 5b. Long ALK fusion variants were defined as EML4–ALK fusion variants that contain the TAPE, i.e., all variants other than variant 3a, 3b, 5a, or 5b (15, 22).
We checked patient cancer specimens with known EML4–ALK fusion variants for further BIM polymorphism analysis, as previously described (24). Cancer DNA was extracted from cancer specimens using the QIAamp DNA Mini Kit (Qiagen). PCR reactions were done to determine the presence of wild-type or deletion alleles using high-fidelity JumpStart™ REDAccuTaq® LA DNA Polymerase (Sigma) with the following conditions: 96°C for 30 s (94°C for 15 s, 60°C for 60 s, 68°C for 10 min) × 30 cycles, and 68°C for 20 min. The forward primer was 5′-AATACCACAGAGGCCCACAG-3′ and the reverse primer was 5′-GCCTGAAGGTGCTGAGAAAG-3′. The PCR products for the deletion (1,323 bp) and the wild-type (4,226 bp) alleles were applied on a 1% agarose gel and were sequenced.
Continuous variables were reported as median with interquartile range (IQR). Categorical data were compared using the chi-square test. PFS and OS were plotted using the Kaplan–Meier method and compared by log-rank test. A Cox proportional hazard model was used for univariate and multivariate analysis for crizotinib PFS and OS. Variables with p < 0.2 in the univariate analysis and clinically important variables such as ALK variant type, BIM deletion, and brain metastasis prior to crizotinib were forced into the final model. Statistical significance was set at p < 0.05. All statistical analyses were performed using the Statistical Package for the Social Sciences, version 18.0K (SPSS, Inc., Chicago, IL, USA). The data cutoff date was September 23, 2018.
A total of 104 ALK IHC(+) patients received crizotinib for advanced NSCLC during the study period. Fifty-five patients had known EML–ALK fusion variants, as determined by RT-PCR. One patient who received crizotinib for <30 days because of side effects was excluded. A total of 54 patients with known EML4–ALK fusion variants were included in the study. Because of the overlapping enrollment interval, 13 of the 54 patients were included in another published article (31). Thirty of the total 54 patients had adequate tissue for BIM polymorphism analysis.
Twenty-three patients had ALK variant 1; six patients had ALK variant 2; 18 patients had ALK variant 3a/b; and seven patients had other ALK variants (two with variant 5, one with variant V5a, two with variant 6, one with variant 8, and one with variant 1 plus insertion of 117 base pairs). The median follow-up time of the cohort was 13.8 (IQR, 7.4–25.4) months. Most patients had received prior anticancer therapy (median, 2 lines of prior anticancer therapy before crizotinib, range, 0–12) and three patients had received crizotinib as first-line therapy. The crizotinib response rate and the median follow-up time did not differ between the different ALK variant groups (Table 1). Patients with variant 2 had better ECOG performance status (0 or 1) (Table 1). Patients with long ALK variants were younger than patients with short ALK variants (p = 0.03) (Supplementary Table 1). In patients with long ALK variants, the baseline characteristics were not different significantly between variant 2 and other long ALK variants (Supplementary Table 2).
BIM deletion polymorphism was found in 20% (6/30) of the patients. There was no significant difference in demographic data between patients with deletion polymorphism and wild type (Supplementary Table 3).
The median crizotinib PFS was 7.3 [95% confidence interval (CI), 4.2–10.4] months in this cohort. The median PFS did not differ significantly among the four ALK variant groups [variant 1, 6.1 (95% CI, 1.6–10.6) months; variant 2, 11.0 (95% CI, 0–22.1) months; variant 3, 7.3 (95% CI, 3.6–10.9) months; other variants, 5.5 (95%, 3.1–8.0) months, p = 0.33 by log-rank test, Figure 1A]. The median PFS also did not differ significantly between variant 2 and all other variants [variant 2, 11.0 (95% CI, 0–22.1) months; all other variants, 6.1 (95% CI, 2.7–9.5) months, p = 0.21 by log-rank test, Figure 1B], between long ALK variants and short variants [long ALK variants, 6.1 (95% CI 2.3–9.8) months; short ALK variants, 8.2 (95% CI, 3.7–12.7) months, p = 0.97 by log-rank test, Figure 1C], and between BIM deletions and not [BIM deletion, 5.5 (95% CI 0–26.6) months; wild-type BIM, 8.6 (95% CI, 3.5–13.7) months, p = 0.57 by log-rank test, Figure 1D]. Multivariate analysis found that ECOG performance status ≥2 [adjusted hazard ratio (aHR) 8.35, 95% CI, 1.52–45.94, p = 0.015] and previous anticancer therapy (aHR 1.35, 95% CI, 1.04–1.76 for each additional line of therapy, p = 0.025) were independent factors for crizotinib PFS (Table 2). However, EML4–ALK fusion variants and BIM deletion were not independent factors for crizotinib PFS. ALK variant 1, variant 2, and variant 3a/b had nearly equal aHR (1.00 as the reference, 0.99 and 1.30, respectively). BIM deletion had a nearly neutral aHR 0.88, as well.
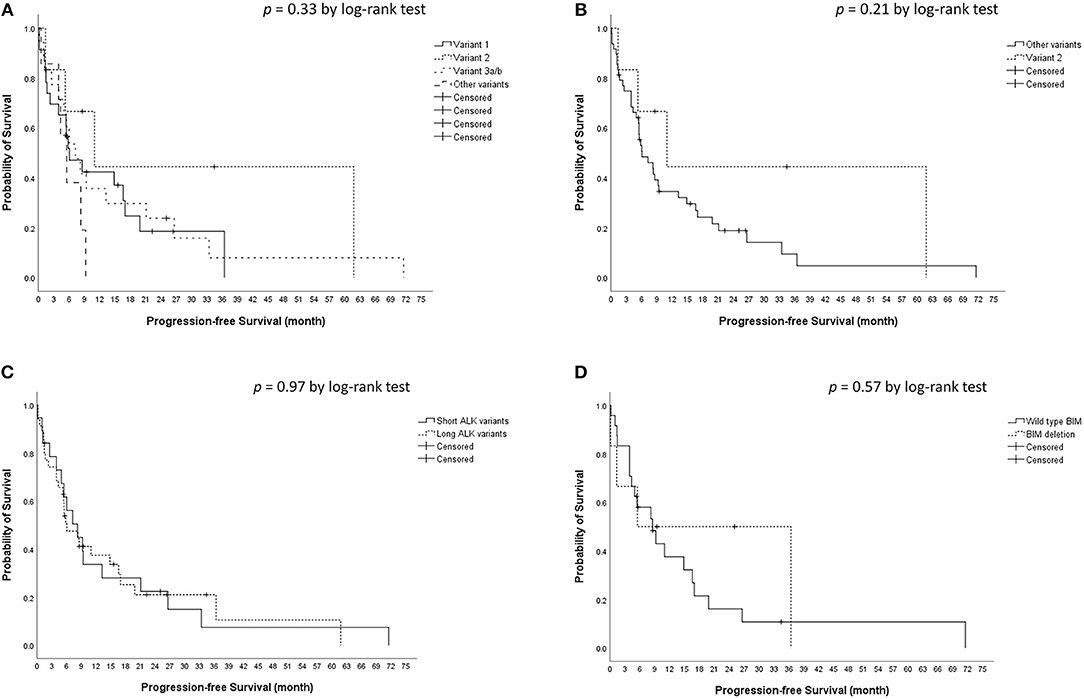
Figure 1. Kaplan–Meier analyses for progression-free survival (PFS). (A) PFS among different ALK fusion variants. (B) PFS between ALK fusion variant 2 and other fusion variants. (C) PFS between short (variant 3a/b and 5a/b) and long (all other variants) ALK fusion variants. (D) PFS between BIM deletion and wild type BIM.
The median OS was 22.0 [95% confidence interval (CI), 15.3–28.7) months in the cohort. The median OS did not differ significantly among the four ALK variant groups [variant 1, 16.1 (95% CI, 10.6–21.5) months; variant 2, not reached; variant 3, 25.1 (95% CI, 5.4–44.7) months; other variants, 10.3 (95%, 7.7–12.9) months, p = 0.45 by log-rank test, Figure 2A]. The median OS also did not differ significantly between variant 2 and all other variants [variant 2, not reached; all other variants, 19.7 (95% CI, 11.9–27.4) months, p = 0.21 by log-rank test, Figure 2B], between long ALK variants and short variants [long ALK variants, 18.5 (95% CI 10.3–26.8) months; short ALK variants, 25.1 (95% CI, 9.2–40.9) months, p = 0.85 by log-rank test, Figure 2C], and between BIM deletion and not [BIM deletion, 25.1 (95% CI 0–71.6) months; wild-type BIM, 22.0 (95% CI, 11.1–32.9) months, p = 0.57 by log-rank test, Figure 2D]. Multivariate analysis found that ECOG performance status ≥2 (aHR 7.20, 95% CI, 1.27–40.79, p = 0.026) was an independent factor for OS (Table 3), while ALK fusion variants and BIM deletion were not.
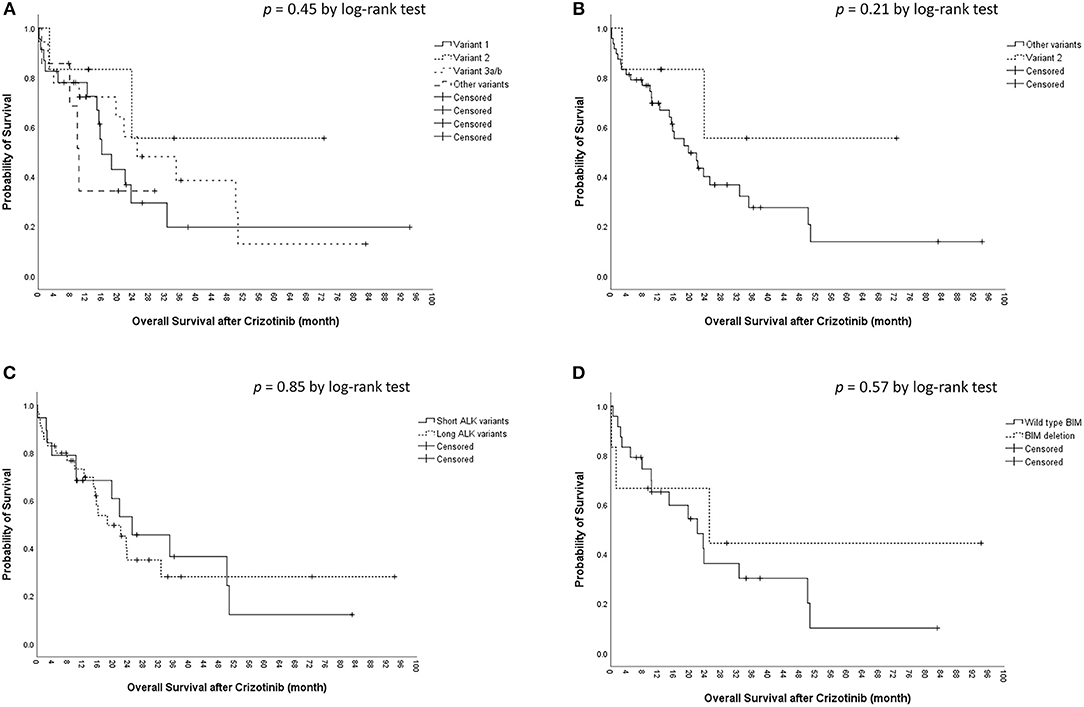
Figure 2. Kaplan–Meier analyses for overall survival (OS). (A) OS among different ALK fusion variants. (B) OS between ALK fusion variant 2 and other fusion variants. (C) OS between short (variant 3a/b and 5a/b) and long (all other variants) ALK fusion variants. (D) OS between BIM deletion and wild-type BIM.
We found that clinical factors such as prior anticancer therapy and ECOG performance status were independent factors for crizotinib PFS in advanced NSCLC bearing EML4–ALK fusion, while ALK fusion variants and BIM polymorphism were not. In this cohort with mainly previously treated patients, for each additional line of anti-cancer therapy, the adjusted HR was 1.42 (95% CI, 1.06–1.86). With regard to the first-line PROFILE 1014 (2) and second-line PROFILE 1007 (32) phase 3 trials for ALK(+) NSCLC, the median crizotinib PFS in the first-line trial seemed to be better (10.9 vs. 7.0 months). Zhou et al. reported on 73 ALK(+) NSCLC patients that received first-line crizotinib, pemetrexed/platinum, or non-pemetrexed chemotherapy/platinum. Poor ECOG performance status and crizotinib after non-pemetrexed chemotherapy were two independent factors for poor crozitinib PFS in multivariate analysis (33). Lin et al. reported on 94 advanced ALK(+) NSCLC patients and found that crizotinib had a better PFS in first-line use than in second-line use (median PFS 10.5 vs. 8.3 months, p = 0.020) (21). Unlike EGFR-TKIs, whose performance was the same in first-line or second-line treatment for advanced EGFR-mutant NSCLC (34), crizotinib had a tendency to do better in first-line use. Poor ECOG performance prior to crizotinib therapy was another independent factor for crizotinib PFS and OS. Poor performance status is a traditional negative prognostic marker among oncology patients (35). It was also found in crizotinib-treated advanced ALK(+) NSCLC patients in previous reports (8, 9, 33, 36).
Different EML4–ALK fusion variants were reported to influence crizotinib efficacy, but results from different reports are conflicting. The results of the current study and of previous reports regarding EML4–ALK fusion variants and crizotinib PFS are summarized in Table 4 (16, 18–23, 37). All studies were conducted in a single center except for the report by Mitiushkina et al., which included three different hospitals in St. Petersburg, Russia (22). Yoshida et al. first reported that ALK fusion variant 1 had better crizotinib PFS in 35 Japanese patients (18). This was the first clinical report on the influence of different ALK fusion variants on crizotinib PFS. The patient numbers were relatively small and it only included first-line crizotinib-treated patients. Moreover, Lin et al. reported that 55 patients with variant 1 and variant 3 received first-line crizotinib, and the PFS was similar (23). Li et al. (19), Woo et al. (16), and Christopoulos et al. (20) reported responsiveness of patients from China, South Korea, and Germany to crizotinib in 2018, and the results were similar. Although Li et al. concluded that variant 2 had better crizotinib PFS, there was still a tendency for non-variant 3a/b patients to have a longer crizotinib PFS (median, 18.4 vs. 13.1 months, p = 0.24), which was consistent with the findings reported by Woo et al. and Christopoulos et al. The three studies had a similar characteristic: the majority of patients had variant 3a/b. However, while variant 3 had disadvantages in both PFS and OS as reported by Christopoulos et al. (20), the OS was almost the same (p = 0.96), as reported by Woo et al. (16). On the other hand, the largest cohort to date by Lin et al. showed that there was no difference between variant 1 and variant 3, in patients treated with both first-line ALK TKI as crizotinib and first-line crizotinib (23). Lei et al. (21), Cha et al. (37), Mitiushkina et al. (22), and our study found no difference between different ALK fusion variants. In the five studies from the United States, China, South Korea, Russia, and Taiwan, the majority of patients had variant 1. Both positive and negative reports included Caucasian and Asian patients, so race may not have contributed to the differences in results. Is it possible that the composite of variants in study cohorts had some influence on the results? The patient percentages of variant 3 in the five studies, which did not find differences between variants, were 48%, 30%, 19%, 25%, and 33%, respectively. In fact, patients with variant 3 were the second largest group among the cohorts, which does not lend support to the hypothesis of smaller patient numbers leading to an overestimation of PFS for variant 3 in the studies. One of the possible explanations may be the use of multivariate analysis. Only studies by Yoshida et al. and Li et al., and our study used a multivariate analysis to determine the independent factors for crizotinib PFS. Although the clinicopathologic characteristics seemed to be similar between the two analyzed groups (such as variant 3a/b or non-variant 3a/b), multivariate analysis that includes clinically relevant variables may still be a better method to find independent factors. Different patient groupings may influence crizotinib PFS if they are not adjusted appropriately. This may partly explain the discordance of OS data between the reports from Woo et al. and Christopoulos et al. In Christopoulos's cohort, variant 3a/b patients had more initial metastatic sites, either thoracic or extra-thoracic, and fewer patients with variant 3a/b had cancer recurrence from an early-stage cancer rather than initial stage IV (20). More metastatic sites and less cancer recurrence from early-stage NSCLC had survival disadvantages (38, 39), and might have contributed to shorter PFS and OS in variant 3a/b patients in the cohort. On the other hand, in our cohort, although patients with variant 2 tended to have a longer PFS, they might also have clinical advantages (tended to be younger, never-smokers, with better baseline performance status, and with less initial brain metastasis) (Table 1). The PFS between variant 1, variant 2, and variant 3a/b were almost equal after multivariate analysis (aHR, 1.00 as reference, 0.99, 1.30, respectively, Table 2). We hypothesize that although different ALK fusion variants might contribute to different crizotinib PFS, the impact may not be significant after adjusting for clinical factors.
In this study, there were 30 patients with enough tissue for BIM analysis and six were positive for BIM deletion (20%). The prevalence rate was consistent with previous reports (11–19%) (24–27). The BIM deletion polymorphism was not associated with a difference in crizotinib PFS (Figure 1D) or OS (Figure 2D). Using the multivariate Cox proportional hazard model, BIM deletion was also not related to differences in PFS or OS (Tables 2, 3). BIM deletion was associated with shorter PFS in 47 ALK(+) NSCLC patients receiving crizotinib (26). BIM polymorphism was also reported to be associated with primary resistance or short PFS with EGFR TKIs (24, 25). However, Lee et al. checked 193 patients who received EGFR TKI for EGFR-mutant NSCLC and there was also no difference in EGFR TKI PFS between patients with and those without a BIM deletion (40). The result was similar to our previous analysis (27). Although BIM is a pro-apoptotic protein and may be related to TKI-induced cancer cell death, lung cancer cells may not be totally dependent on this pathway, and the concentration of BIM protein may also matter. Furthermore, the BIM deletion polymorphism is found only in Asians, and not in Caucasians (24). If the BIM deletion polymorphism was associated with shorter PFS, the effectiveness of crizotinib among Asians would be worse than in Western countries, but this is not true. Whether or not a simple BIM gene deletion influences TKI efficacy in NSCLC patients remains questionable.
There were several limitations to this study. First, it was a retrospective cohort study in a single center, as in previous reports. Because of the rarity of ALK(+) NSCLC, the patient number was still limited. The BIM deletion polymorphism in ALK(+) NSCLC patients, which is found in only 10–20% of ALK(+) patients, is even rarer. This may also be the reason that different reports have had different findings to date. As a result of limited patient numbers, the resistance mechanisms could not be addressed. Further larger multicenter or international prospective cohorts are warranted. Second, this was a cohort with mainly previously treated patients. Our results may not be generalizable to patients receiving first-line crizotinib therapy. Because reimbursement of crizotinib as first-line therapy was not approved by Taiwan's National Health Insurance until November 1, 2017, only three of 54 patients in our cohort used crizotinib as first-line therapy. However, although the U.S. FDA approved first-line crizotinib therapy, almost other studies also included mixed-line therapy with crizotinib, and purely first-line crizotinib data were rare (Table 4). Third, we used RT-PCR to determine ALK fusion variants. As in prior reports, not all ALK fusion variants could be detected. With the development of next-generation sequencing, more ALK fusion variants can be found, and the entire picture of ALK fusion lung cancer will become clearer.
In conclusion, clinical factors such as more prior anticancer therapies and ECOG performance status ≥2 were associated with a poorer crizotinib outcome. Different ALK variants and the BIM polymorphism were not independent factors for crizotinib PFS or OS in this study.
The datasets that were analyzed during the current study are available from the corresponding author on reasonable request.
This study was reviewed and approved by the Institutional Review Board of National Taiwan University Hospital. Written informed consent was obtained from all patients before checking their cancer specimens for molecular studies. All methods were performed in accordance with the relevant guidelines and regulations.
An earlier version of this study was presented as a poster presentation in the Asian Pacific Society of Respirology 2018 Congress.
Y-TL participated in the study design, review and collection of patients' data, statistical analysis, and drafting of the manuscript. Y-NL participated in collection of patients' data, analyses of ALK and BIM, and revision of the manuscript. J-YS designed the study, interpreted the data, and reviewed and revised the manuscript.
Y-TL has received speaking honoraria from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Pfizer, Roche, and TTY Biopharm; and travel expense from Pfizer. J-YS has received personal fees for advisory boards from AstraZeneca, Roche, Boehringer Ingelheim, Eli Lilly, Merck Sharp & Dohme, Ono Pharmaceutical, Chugai Pharmaceutical, AbbVie, and Bristol-Myers Squibb; speaking honoraria from AstraZeneca, Roche, Boehringer Ingelheim, Eli Lilly, Pfizer, Novartis, Merck Sharp & Dohme, Ono Pharmaceutical, Chugai Pharmaceutical, AbbVie, and Bristol-Myers Squibb; and travel expense from Roche, Boehringer Ingelheim, Pfizer, Merck Sharp & Dohme, Chugai Pharmaceutical, and Bristol-Myers Squibb.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2019.00880/full#supplementary-material
1. Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. (2007) 448:561–6. doi: 10.1038/nature05945
2. Solomon BJ, Mok T, Kim DW, Wu YL, Nakagawa K, Mekhail T, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. (2014) 371:2167–77. doi: 10.1056/NEJMoa1408440
3. Malik SM, Maher VE, Bijwaard KE, Becker RL, Zhang L, Tang SW, et al. U.S. Food and Drug Administration approval: Crizotinib for treatment of advanced or metastatic non-small cell lung cancer that is anaplastic lymphoma kinase positive. Clin Cancer Res. (2014) 20:2029–34. doi: 10.1158/1078-0432.CCR-13-3077
4. Peters S, Camidge DR, Shaw AT, Gadgeel S, Ahn JS, Kim DW, et al. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med. (2017) 377:829–38. doi: 10.1056/NEJMoa1704795
5. Soria JC, Tan DSW, Chiari R, Wu YL, Paz-Ares L, Wolf J, et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet. (2017) 389:917–29. doi: 10.1016/S0140-6736(17)30123-X
6. Camidge DR, Kim HR, Ahn MJ, Yang JC, Han JY, Lee JS, et al. Brigatinib versus crizotinib in ALK-positive non-small-cell lung cancer. N Engl J Med. (2018) 379:2027–39. doi: 10.1056/NEJMoa1810171
7. Wu YL, Lu S, Lu Y, Zhou J, Shi YK, Sriuranpong V, et al. Results of PROFILE 1029, a Phase III comparison of first-line crizotinib versus chemotherapy in East Asian patients with ALK-positive advanced non-small cell lung cancer. J Thorac Oncol. (2018) 13:1539–48. doi: 10.1016/j.jtho.2018.06.012
8. Ock CY, Yoo SH, Keam B, Kim M, Kim TM, Jeon YK, et al. Clinical factors affecting progression-free survival with crizotinib in ALK-positive non-small cell lung cancer. Korean J Intern Med. (2019) 34:1116–1124. doi: 10.3904/kjim.2018.011
9. Cao Y, Xiao G, Qiu X, Ye S, Lin T. Efficacy and safety of crizotinib among Chinese EML4-ALK-positive, advanced-stage non-small cell lung cancer patients. PLoS ONE. (2014) 9:e114008. doi: 10.1371/journal.pone.0114008
10. Xing P, Wang S, Hao X, Zhang T, Li J. Clinical data from the real world: efficacy of crizotinib in Chinese patients with advanced ALK-rearranged non-small cell lung cancer and brain metastases. Oncotarget. (2016) 7:84666–74. doi: 10.18632/oncotarget.13179
11. Yoshida T, Oya Y, Tanaka K, Shimizu J, Horio Y, Kuroda H, et al. Clinical impact of crizotinib on central nervous system progression in ALK-positive non-small lung cancer. Lung Cancer. (2016) 97:43–7. doi: 10.1016/j.lungcan.2016.04.006
12. Lei YY, Yang JJ, Zhong WZ, Chen HJ, Yan HH, Han JF, et al. Clinical efficacy of crizotinib in Chinese patients with ALK-positive non-small-cell lung cancer with brain metastases. J Thorac Dis. (2015) 7:1181–8. doi: 10.3978/j.issn.2072-1439.2015.06.04
13. Heuckmann JM, Balke-Want H, Malchers F, Peifer M, Sos ML, Koker M, et al. Differential protein stability and ALK inhibitor sensitivity of EML4-ALK fusion variants. Clin Cancer Res. (2012) 18:4682–90. doi: 10.1158/1078-0432.CCR-11-3260
14. Richards MW, Law EW, Rennalls LP, Busacca S, O'Regan L, Fry AM, et al. Crystal structure of EML1 reveals the basis for Hsp90 dependence of oncogenic EML4-ALK by disruption of an atypical beta-propeller domain. Proc Natl Acad Sci USA. (2014) 111:5195–200. doi: 10.1073/pnas.1322892111
15. Bayliss R, Choi J, Fennell DA, Fry AM, Richards MW. Molecular mechanisms that underpin EML4-ALK driven cancers and their response to targeted drugs. Cell Mol Life Sci. (2016) 73:1209–24. doi: 10.1007/s00018-015-2117-6
16. Woo CG, Seo S, Kim SW, Jang SJ, Park KS, Song JY, et al. Differential protein stability and clinical responses of EML4-ALK fusion variants to various ALK inhibitors in advanced ALK-rearranged non-small cell lung cancer. Ann Oncol. (2017) 28:791–7. doi: 10.1093/annonc/mdw693
17. Tan S, Gou Q, Pu W, Guo C, Yang Y, Wu K, et al. Circular RNA F-circEA produced from EML4-ALK fusion gene as a novel liquid biopsy biomarker for non-small cell lung cancer. Cell Res. (2018) 28:693–5. doi: 10.1038/s41422-018-0033-7
18. Yoshida T, Oya Y, Tanaka K, Shimizu J, Horio Y, Kuroda H, et al. Differential crizotinib response duration among ALK fusion variants in ALK-positive non-small-cell lung cancer. J Clin Oncol. (2016) 34:3383–9. doi: 10.1200/JCO.2015.65.8732
19. Li Y, Zhang T, Zhang J, Li W, Yuan P, Xing P, et al. Response to crizotinib in advanced ALK-rearranged non-small cell lung cancers with different ALK-fusion variants. Lung Cancer. (2018) 118:128–33. doi: 10.1016/j.lungcan.2018.01.026
20. Christopoulos P, Endris V, Bozorgmehr F, Elsayed M, Kirchner M, Ristau J, et al. EML4-ALK fusion variant V3 is a high-risk feature conferring accelerated metastatic spread, early treatment failure and worse overall survival in ALK(+) non-small cell lung cancer. Int J Cancer. (2018) 142:2589–98. doi: 10.1002/ijc.31275
21. Lei YY, Yang JJ, Zhang XC, Zhong WZ, Zhou Q, Tu HY, et al. Anaplastic lymphoma kinase variants and the percentage of ALK-positive tumor cells and the efficacy of crizotinib in advanced NSCLC. Clin Lung Cancer. (2016) 17:223–31. doi: 10.1016/j.cllc.2015.09.002
22. Mitiushkina NV, Tiurin VI, Iyevleva AG, Kholmatov MM, Filippova EA, Moiseyenko FV, et al. Variability in lung cancer response to ALK inhibitors cannot be explained by the diversity of ALK fusion variants. Biochimie. (2018) 154:19–24. doi: 10.1016/j.biochi.2018.07.018
23. Lin JJ, Zhu VW, Yoda S, Yeap BY, Schrock AB, Dagogo-Jack I, et al. Impact of EML4-ALK variant on resistance mechanisms and clinical outcomes in ALK-positive lung cancer. J Clin Oncol. (2018) 36:1199–206. doi: 10.1200/JCO.2017.76.2294
24. Ng KP, Hillmer AM, Chuah CT, Juan WC, Ko TK, Teo AS, et al. A common BIM deletion polymorphism mediates intrinsic resistance and inferior responses to tyrosine kinase inhibitors in cancer. Nat Med. (2012) 18:521–8. doi: 10.1038/nm.2713
25. Isobe K, Hata Y, Tochigi N, Kaburaki K, Kobayashi H, Makino T, et al. Clinical significance of BIM deletion polymorphism in non-small-cell lung cancer with epidermal growth factor receptor mutation. J Thorac Oncol. (2014) 9:483–7. doi: 10.1097/JTO.0000000000000125
26. Zhang L, Jiang T, Li X, Wang Y, Zhao C, Zhao S, et al. Clinical features of Bim deletion polymorphism and its relation with crizotinib primary resistance in Chinese patients with ALK/ROS1 fusion-positive non-small cell lung cancer. Cancer. (2017) 123:2927–35. doi: 10.1002/cncr.30677
27. Wu SG, Liu YN, Yu CJ, Yang PC, Shih JY. Association of BIM deletion polymorphism with intrinsic resistance to EGFR tyrosine kinase inhibitors in patients with lung adenocarcinoma. JAMA Oncol. (2016) 2:826–8. doi: 10.1001/jamaoncol.2016.0016
28. Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. (1982) 5:649–55. doi: 10.1097/00000421-198212000-00014
29. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. (2009) 45:228–47. doi: 10.1016/j.ejca.2008.10.026
30. Lin YT, Yu CJ, Yang JC, Shih JY. Anaplastic lymphoma kinase (ALK) kinase domain mutation following ALK inhibitor(s) failure in advanced ALK positive non-small-cell lung cancer: analysis and literature review. Clin. Lung Cancer. (2016) 17:e77–94. doi: 10.1016/j.cllc.2016.03.005
31. Lin YT, Chen CY, Shih JY. Real-world crizotinib use for anaplastic lymphoma kinase (ALK)-positive advanced non-small cell lung cancer under first-year national health insurance coverage in Taiwan. Thorac Med. (2018) 33:1–13.
32. Shaw AT, Kim DW, Nakagawa K, Seto T, Crino L, Ahn MJ, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. (2013) 368:2385–94. doi: 10.1056/NEJMoa1214886
33. Zhou J, Zheng J, Zhang X, Zhao J, Zhu Y, Shen Q, et al. Crizotinib in patients with anaplastic lymphoma kinase-positive advanced non-small cell lung cancer versus chemotherapy as a first-line treatment. BMC Cancer. (2018) 18:10. doi: 10.1186/s12885-017-3720-8
34. Wu JY, Yu CJ, Yang CH, Wu SG, Chiu YH, Gow CH, et al. First- or second-line therapy with gefitinib produces equal survival in non-small cell lung cancer. Am J Respir Crit Care Med. (2008) 178:847–53. doi: 10.1164/rccm.200803-389OC
35. Stanley KE. Prognostic factors for survival in patients with inoperable lung cancer. J Natl Cancer Inst. (1980) 65:25–32.
36. Noronha V, Ramaswamy A, Patil VM, Joshi A, Chougule A, Kane S, et al. ALK positive lung cancer: clinical profile, practice and outcomes in a developing country. PLoS ONE. (2016) 11:e0160752. doi: 10.1371/journal.pone.0160752
37. Cha YJ, Kim HR, Shim HS. Clinical outcomes in ALK-rearranged lung adenocarcinomas according to ALK fusion variants. J Transl Med. (2016) 14:296. doi: 10.1186/s12967-016-1061-z
38. Bates JE, Milano MT. Prognostic significance of sites of extrathoracic metastasis in patients with non-small cell lung cancer. J Thorac Dis. (2017) 9:1903–10. doi: 10.21037/jtd.2017.06.117
39. Ko R, Kenmotsu H, Hisamatsu Y, Akamatsu H, Omori S, Nakashima K, et al. The effect of gefitinib in patients with postoperative recurrent non-small cell lung cancer harboring mutations of the epidermal growth factor receptor. Int J Clin Oncol. (2015) 20:668–73. doi: 10.1007/s10147-014-0761-8
40. Lee JK, Shin JY, Kim S, Lee S, Park C, Kim JY, et al. Primary resistance to epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) in patients with non-small-cell lung cancer harboring TKI-sensitive EGFR mutations: an exploratory study. Ann Oncol. (2013) 24:2080–7. doi: 10.1093/annonc/mdt127
Keywords: non-small cell lung cancer, ALK, ALK variant, BIM, crizotinib
Citation: Lin Y-T, Liu Y-N and Shih J-Y (2019) The Impact of Clinical Factors, ALK Fusion Variants, and BIM Polymorphism on Crizotinib-Treated Advanced EML4–ALK Rearranged Non-small Cell Lung Cancer. Front. Oncol. 9:880. doi: 10.3389/fonc.2019.00880
Received: 03 June 2019; Accepted: 27 August 2019;
Published: 23 September 2019.
Edited by:
Umberto Malapelle, University of Naples Federico II, ItalyReviewed by:
Timothy F. Burns, University of Pittsburgh, United StatesCopyright © 2019 Lin, Liu and Shih. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jin-Yuan Shih, anlzaGloQG50dS5lZHUudHc=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.