- 1Department of Radiation Oncology, Cancer Hospital, The First Affiliated Hospital of Xiamen University, Teaching Hospital of Fujian Medical University, Xiamen, China
- 2State Key Laboratory of Oncology in South China, Department of Radiation Oncology, Sun Yat-sen University Cancer Center, Collaborative Innovation Center of Cancer Medicine, Guangzhou, China
- 3Department of Obstetrics and Gynecology, The First Affiliated Hospital of Xiamen University, Teaching Hospital of Fujian Medical University, Xiamen, China
Introduction: Although advances in surgical and chemotherapeutic approaches have improved management of epithelial ovarian cancer (EOC) in recent decades. The mortality of EOC over time remains controversial. The aim of this study was to assess the survival trends of EOC according to period of diagnosis using real-world data.
Methods: Patients with EOC diagnosed from 1990 to 2014 were included from the Surveillance, Epidemiology, and End Results database. The Kaplan-Meier method and multivariate Cox regression models were used to evaluate the trends in survival over time.
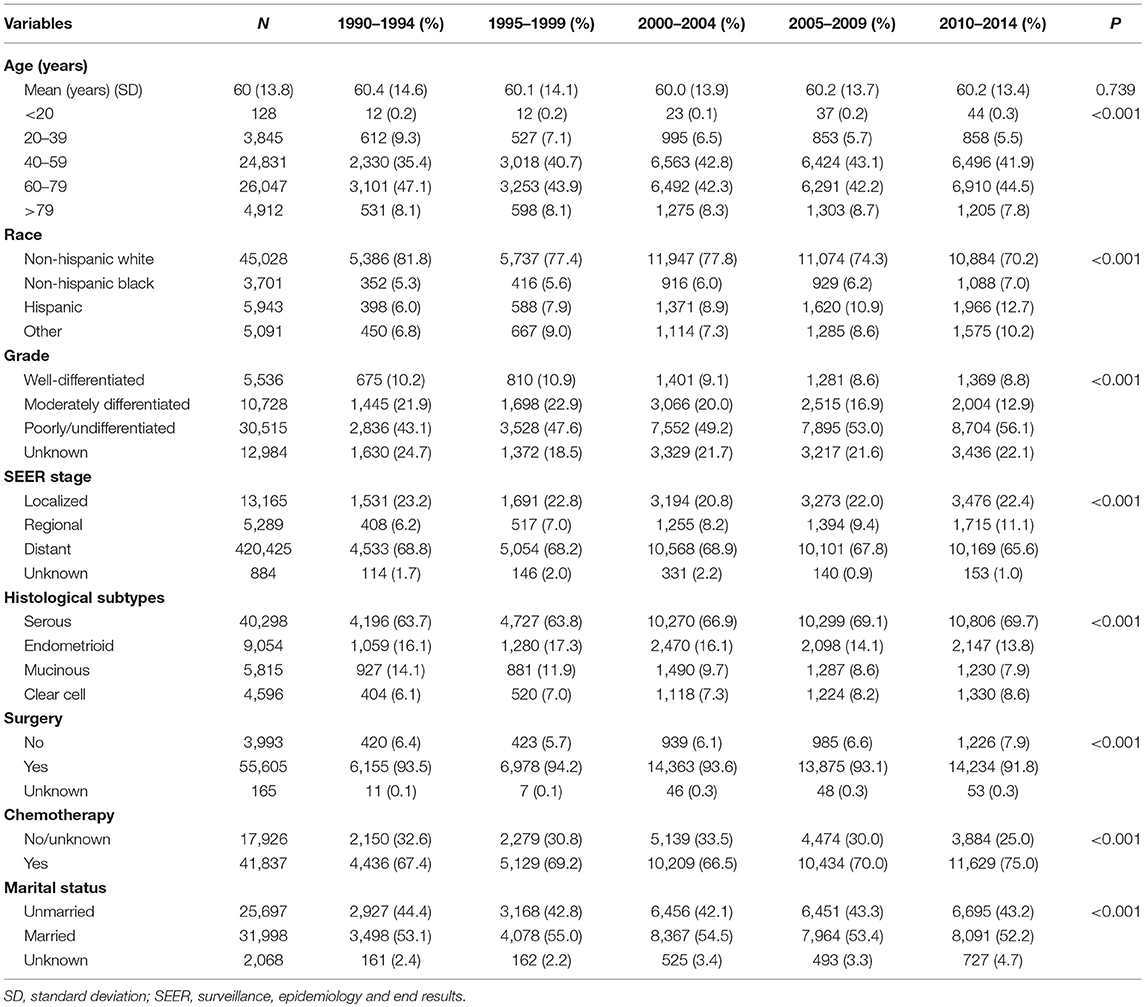
Results: We identified 59,763 patients diagnosed with EOC as follows: 6,586 (11.0%) in 1990–1994, 7,408 (12.4%) in 1995–1999, 15,348 (25.7%) in 2000–2004, 14,908 (24.9%) in 2005–2009, and 15,513 (26.0%) in 2010–2014. In the distant stage, the use of surgery decreased from 92.0% in 1990–1994 to 88.9% in 2010–2014. The use of chemotherapy increased from 67.4% in 1990–1994 to 75.0% in 2010–2014. The 5-year cause-specific survival (CSS) increased from 48.6% in 1990–1994 to 57.4% in 2010–2014 (P < 0.001). The 5-year overall survival (OS) increased from 42.7% in 1990–1994 to 51.7% in 2010–2014 (P < 0.001). The 5-year CSS and OS showed slight improvement in the localized stage (CSS, 91.9 vs. 93.1%; OS, 85.6 vs. 88.5%), and largely improved in the distant stage (CSS, 31.4 vs. 42.7%; OS, 26.7 vs. 37.4%) between 1990–1994 and 2010–2014. The multivariate analysis indicated that being diagnosed in the later years was related to better CSS and OS of EOC.
Conclusion: The trends in survival of EOC have improved over time, but net survival remains poor overall in distant-stage EOC.
Background
Approximately 238,719 new patients with ovarian cancer are diagnosed each year worldwide. Although the survival trends between 1995–1999 and 2010–2014 were relatively constant in most countries, 5-year survival was still <50% for women diagnosed in 2010–2014 (1). Approximately 22,240 newly diagnosed ovarian cancer and 14,070 ovarian cancer-related deaths are expected in the United States (US) in 2018 (2). Epithelial ovarian cancer (EOC) is the most common histological subtype of ovarian cancer, and 90% of patients are diagnosed with EOC. However, 80% of patients have been diagnosed with advanced stage disease (2) due to lack of specificity and obvious symptoms (3, 4). In addition, there are still lack of sensitive and specific screening techniques and biomarkers for ovarian cancer (3–6).
A previous Surveillance, Epidemiology and End Results (SEER) study including EOC patients between 1973 and 1997, the 5-year relative survival was gradually increased in patients with localized (84% in 1973–1979 to 92% in 1990–1997), regional (49% in 1973–1979 to 77% in 1990–1997) and distant (17% in 1973–1979 to 27% in 1990–1997) stage (7). The significantly increased the survival in regional stage may be due to the use of paclitaxel-containing chemotherapy in clinical practice. In a recent ovarian cancer statistics between 2007 to 2013 from US, the 5-year survival rate was 89 and 71% in stage I and II EOC patients, respectively, while it has significantly decreased to 41 and 20% in those with stage III and IV disease, respectively (2). Although the above three studies have different definitions of staging system, the ovarian cancer mortality was slowly decreasing in recent decades, which may be related to its unknown etiology, lack of effective diagnostic methods in the early disease process, and lack of effective treatment.
Advances in surgical and chemotherapeutic approaches have improved management of EOC in recent decades. The cytoreductive surgery (debulking) and paclitaxel-containing chemotherapy were introduced into the clinical practice of EOC in the late 1960s and early 1990s, respectively. In the current clinical practice, the standard treatment for early-stage EOC is complete surgical staging procedure including examination of the abdominal cavity, omentectomy, several prescribed biopsies, and thorough pelvic and para-aortic lymph node sampling. Debulking surgery in combination with neoadjuvant or adjuvant platinum/taxane-based chemotherapy has become the standard treatment for patients with advanced EOC (8, 9). The main goal of all these therapeutic changes is to improve the prognosis. However, the results of several population-based studies on survival trends over time in EOC are inconsistent (10–15), which may be due to the different period of diagnosis, heterogeneity of the population, and different treatment strategies. A SEER study included EOC patients between 1973 and 1997, reported a gradual increase in survival over time. However, the 5-year overall survival (OS) only increased slightly from 40% in 1980–1989 to 45% in 1990–1997 (7). In addition, another SEER study included Hispanic EOC patients during 1992–2013, and the results showed no significantly difference in survival outcomes among three periods of diagnosis 1992–1999, 2000–2006, and 2007–2013 (16). There are lacking studies regarding to the changes in the survival rates of EOC in the recent three decades over time. Due to improvements in diagnostic techniques, surgery, treatment, and individualized care, it is hypothesized that the survival rate of EOC may be further improved in recent decades. The purpose of the present study was to assess the survival trends of EOC between 1990 and 2014 using real-world data from SEER program.
Materials and Methods
Patients
Data on patients diagnosed with EOC from 1990 to 2014 were analyzed using the SEER 18 Regs Research Data of the National Cancer Institute (17). This cancer-registration database contains data on cancer incidence, demographic and clinicopathologic characteristics, first course of treatment, and survival of approximately 28% of the US population. Patients without a positive histology or with a prior malignancy diagnosis were excluded. The use of data from the SEER database was exempt from the approval process of the Institutional Review Board because its patient-related information is de-identified.
Measures
Demographic, clinicopathologic, treatment variables, and vital status were included as follows: age, year of diagnosis, race/ethnicity, tumor grade, SEER stage, histological subtypes, surgery, chemotherapy, and marital status at diagnosis. Survival trends were examined over five time periods: 1990–1994, 1996–1999, 2000–2004, 2005–2009, and 2010–2014. Tumor grade was classified as well-differentiated, moderately differentiated, poorly differentiated, undifferentiated, or unknown tumor grade. The histologic subtypes were classified as serous, mucinous, endometrioid, and clear-cell. The SEER staging system corresponds to the commonly used International Federation of Gynecology and Obstetrics (FIGO) staging system, as follows: localized (I-A, I-B, I-not otherwise specified [NOS]), regional (FIGO I-C, II-A, II-B, II-C, II-NOS), distant (FIGO III-A, III-B, III-C, III-NOS, IV) (18).
Statistical Analysis
The chi-square test and one-way analysis of variance were used to compare patients' demographic, clinicopathologic, and treatment variables over the five periods. Kaplan–Meier analyses for 5-year cause-specific survival (CSS) and OS were performed using the log-rank test. CSS was defined as the time from the date of the initial diagnosis to the date of the ovarian cancer-related death. OS was defined as the time from the date of diagnosis to the date of death or last follow-up. The Cox proportional hazard was used for multivariate analyses. We included the following variables in the multivariate Cox proportional hazard models: age, race/ethnicity, grade, SEER stage, histological subtypes, surgery, chemotherapy, marital status, and years of diagnosis. Trends in survival were analyzed separately for localized, regional, and distant stages. All analyses were performed using SPSS version 22.0 (IBM Corporation, Armonk, USA), and P < 0.05 was considered significant.
Results
Patient Characteristics
We identified 59,763 EOC patients in this study, including 6,586 (11.0%), 7,408 (12.4%), 15,348 (25.7%), 14,908 (24.9%), and 15,513 (26.0%) patients diagnosed in 1990–1994, 1995–1999, 2000–2004, 2005–2009, and 2010–2014, respectively (Table 1). Patients with poorly differentiated/undifferentiated disease increased from 43.1% in 1990–1994 to 56.1% in 2010–2014 (P < 0.001). Patients with regional stage disease increased from 6.2% in 1990–1994 to 11.1% in 2010–2014 (P < 0.001). Serous EOC was the most common histological subtype; the number of serous and clear-cell subtypes increased during the study period, whereas the number of endometrioid and mucinous subtypes decreased during the study period (P < 0.001).
Trends in Treatment
The proportion of patients who received surgical treatment decreased from 93.5% in 1990–1994 to 91.8% in 2010–2014 (P < 0.001). No significant difference was found over time in the proportion of patients in the localized (P = 0.140) and regional stages (P = 0.099) who received surgical treatment. However, the use of surgery decreased from 92.0% in 1990–1994 to 88.9% in 2010–2014 in the distant stage (P < 0.001). The use of chemotherapy increased from 67.4% in 1990–1994 to 75.0% in 2010–2014. The use of chemotherapy in the localized stage increased from 34.0% in 1990–1994 to 49.7% in 2010–2014 (P < 0.001). The use of chemotherapy in the regional stage increased from 70.6% in 1990–1994 to 78.5% in 2010–2014 (P < 0.001), and it increased in the distant stage from 78.7% in 1990–1994 to 83.5% in 2010–2014 (P < 0.001).
Survival Analysis
The median follow-up was 40 months (range = 0–311 months). A total of 36,031 patients died, including 28,852 patients from ovarian cancer. The 5-year CSS and OS were 53.7 and 48.0%, respectively, and the median CSS and OS were 72.0 and 56.0 months, respectively.
The 5-year CSS rates were 48.6, 51.6, 52.2, 55.1, and 57.4% for patients diagnosed between 1990–1994, 1995–1999, 2000–2004, 2005–2009, and 2010–2014, respectively (P < 0.001) (Figure 1A), and the 5-year OS rates were 42.7, 46.4, 46.8, 49.3, and 51.7%, respectively (P < 0.001) (Figure 1B). Figure 2 lists the 5-year CSS (Figure 2A) and OS (Figure 2B) by year of diagnosis from 1990 to 2010. There was a 9.6% and a 10.9% absolute increase in the CSS and OS from 1990 to 2010, respectively.
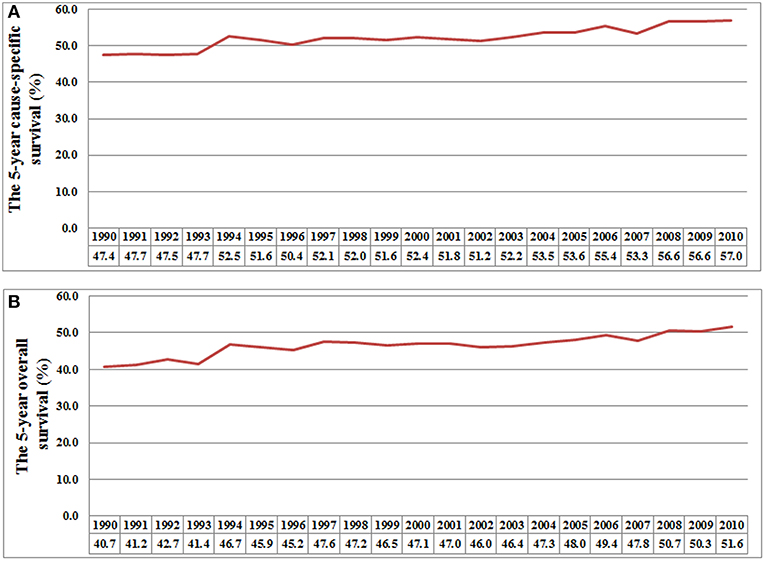
Figure 2. The 5-year cause-specific survival (A) and overall survival (B) by each year of diagnosis from 1990 to 2010.
Multivariate Analysis of Prognostic Factors
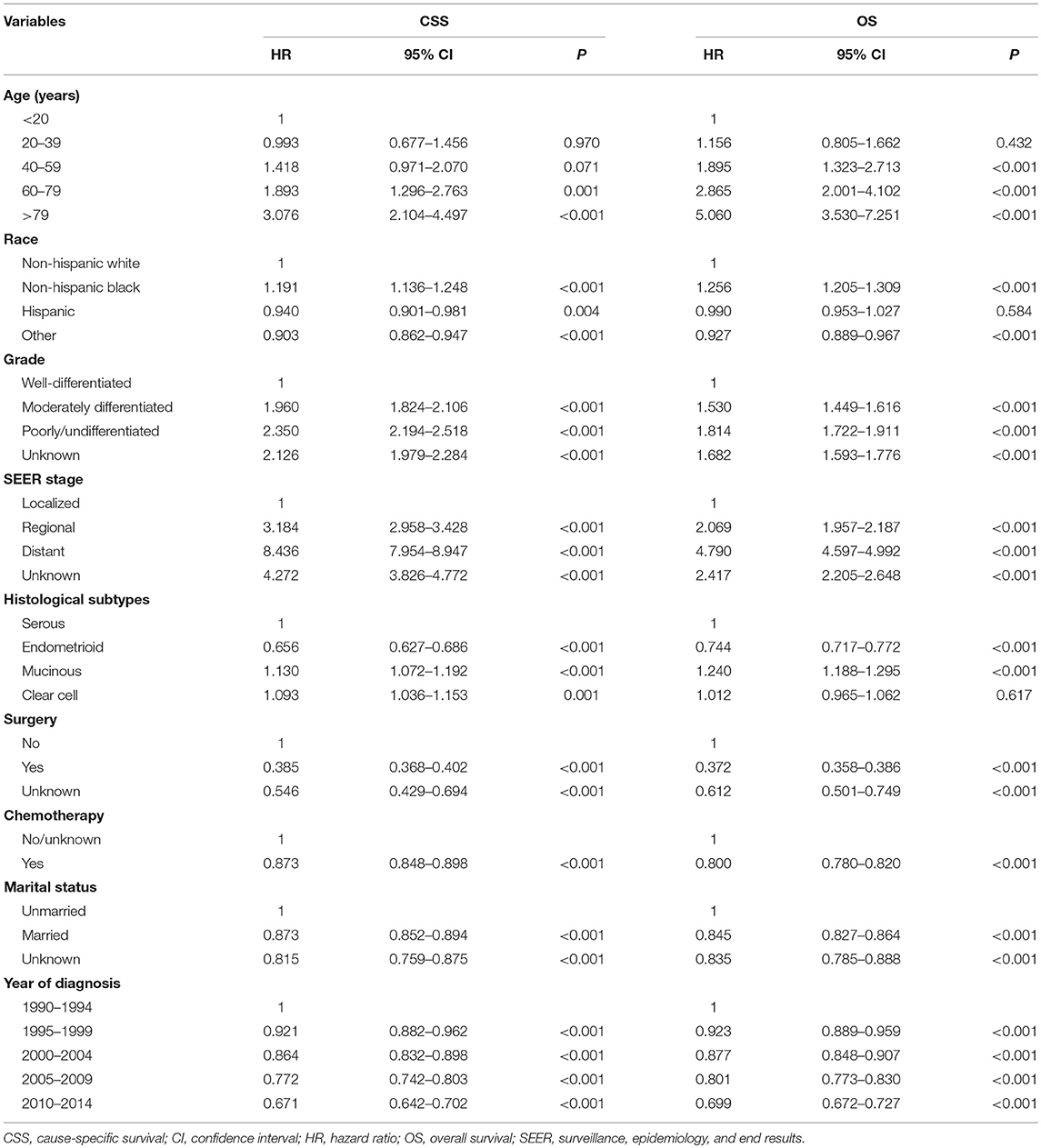
The results multivariate analysis showed that diagnoses in later years were associated with better CSS and OS (Table 2). Age, race/ethnicity, grade, SEER stage, histological subtypes, surgery, chemotherapy, and marital status were also the independent prognostic factors of CSS and OS.
When adjusted by age, race, grade, histological subtypes, surgery, chemotherapy, and marital status, patients in the localized stage diagnosed in 2005–2009 (CSS hazard ratio [HR], 0.828; 95% confidence interval [CI], 0.694–0.988, P = 0.036; OS HR, 0.876; 95% CI 0.779–0.986, P = 0.028) and 2010–2014 (CSS HR, 0.668; 95% CI 0.538–0.831, P < 0.001; OS HR, 0.783; 95% CI 0.670–0.914, P = 0.002) had a better CSS and OS than those diagnosed in 1990–1994. The CSS increased over time among patients in the regional stage, with patients diagnosed in 2005–2009 (HR, 0.745; 95% CI, 0.641–0.866, P < 0.001) and 2010–2014 (HR, 0.802; 95% CI, 0.680–0.947, P = 0.009) having better OS compared to those diagnosed in 1990–1994. Patients diagnosed in later years had a higher CSS and OS in the distant stage when compared to patients diagnosed in earlier years (Table 3).
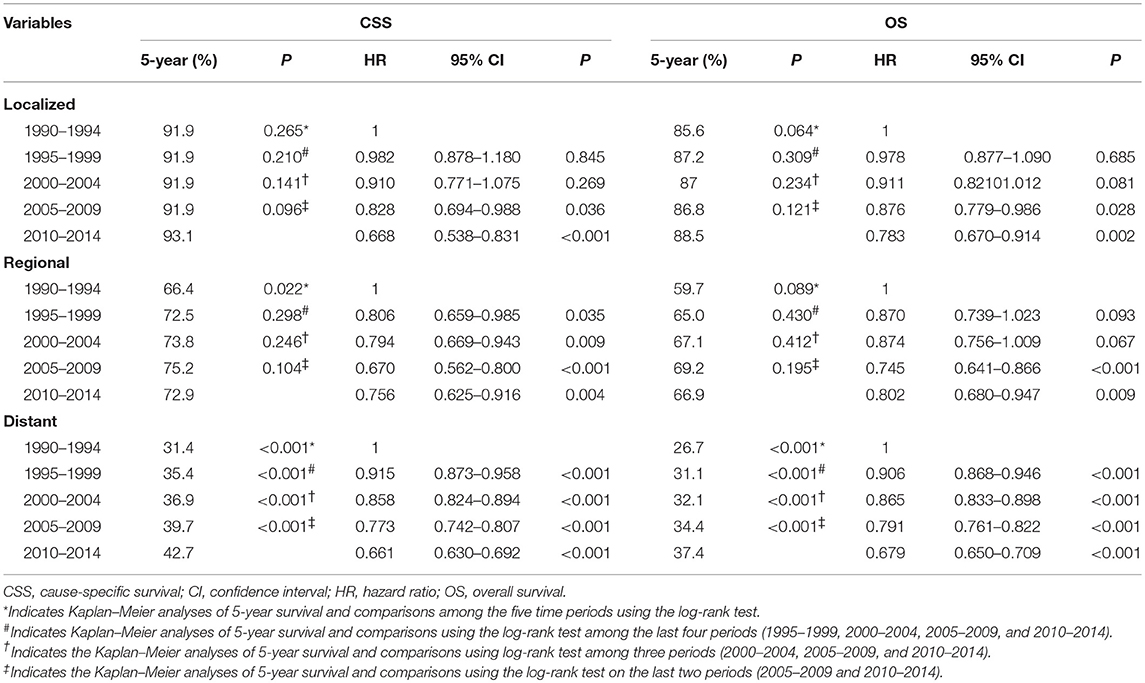
Table 3. The 5-year cause-specific survival, overall survival, and adjusted hazard ratios for epithelial ovarian cancer patients by period of diagnosis according to SEER stage.
The 5-year CSS and OS by period of diagnosis according to SEER stage are presented in Table 3. The period of diagnosis had no effect on the CSS and OS of patients in the localized stage (Figures 3A,D). The CSS significantly improved in the regional state after 1995–2014 compared to 1990–1994, while it did not differ significantly from 1995 to 2014. The OS in the regional stage during the study period was not significantly different (Figures 3B,E). However, the CSS and OS gradually increased from 1990 to 2014 in the distant stage (Figures 3C,F).
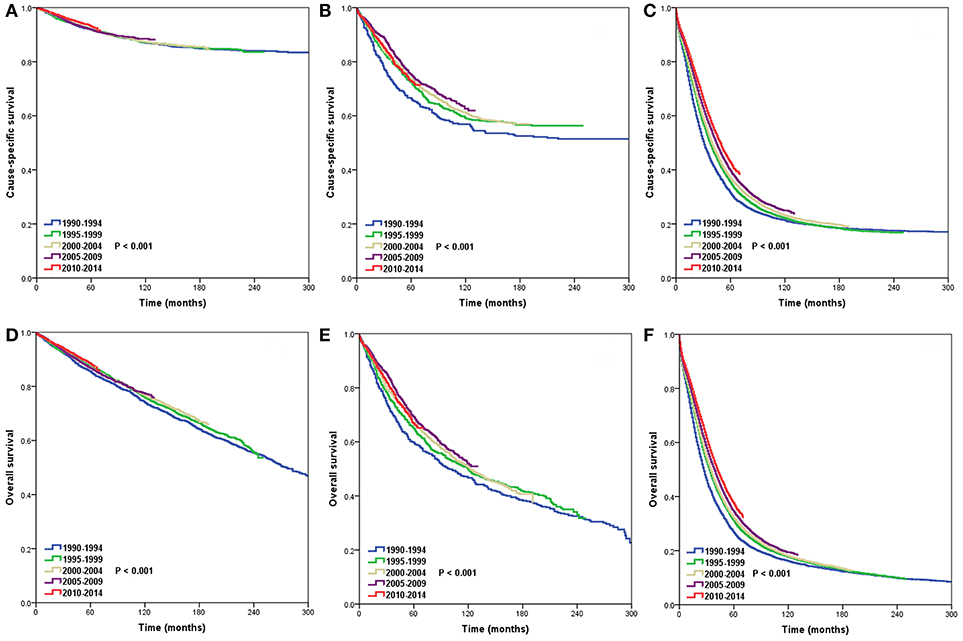
Figure 3. Trends in cause-specific survival (CSS) and overall survival (OS) in the localized (CSS, A; OS, D), regional (CSS, B; OS, E), and distant stages (CSS, C; OS, F) over time.
Discussion
The purpose of the present study was to assess trends in survival outcomes among EOC patients between 1990 and 2014. We found a 9.6% improvement in the 5-year CSS and a 10.9% improvement in the 5-year OS between 1990 and 2010, and survival showed the greatest increase among patients in the distant stage. The incidence of EOC has gradually decreased in recent decades (2). However, the absolute number of EOC cases in our study increased and was relatively stable after 2000, which may be related to changes in the histological diagnostic patterns of EOC (19). The increase in the number of cases classified as EOC may be due to better coding and recording of ovarian cancer morphology by cancer registries (20). We found approximately 25% of patients were diagnosed in the localized stage, and this proportion did not change over time, which may be related to the lack of reliable and effective screening methods to diagnosis early-stage EOC (3–6). Therefore, although the incidence of EOC is declining, there is a need to explore effective screening methods, including mathematical modeling, cancer-specific biomarkers, and real-time imaging to detect it earlier (21).
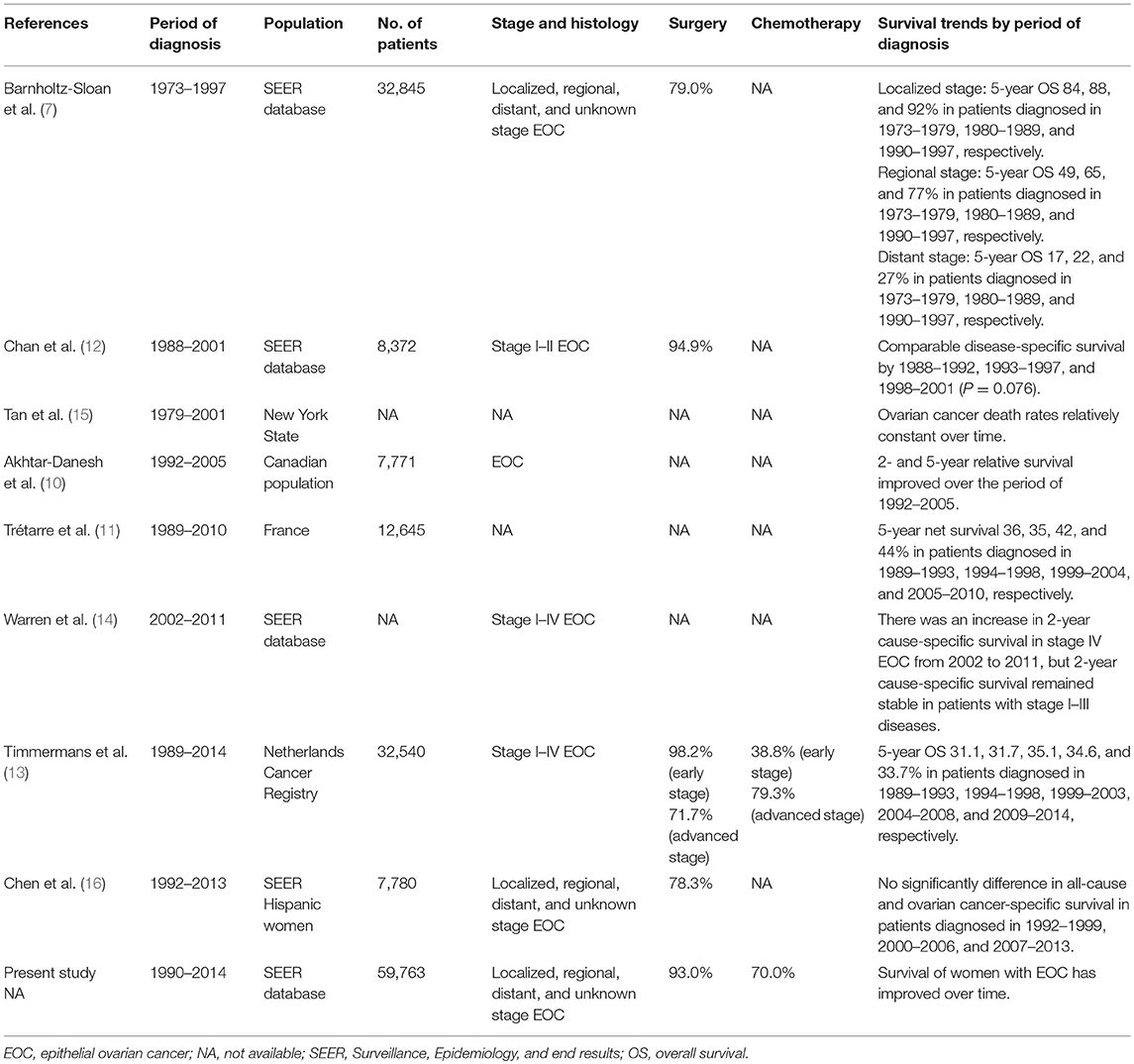
There were also several SEER studies attempted to answer similar questions, but the results were inconsistent (7, 12, 14, 16). A SEER study included patients from 1973 to 1997, and the results showed improved outcomes in localized, regional, and distant stage EOC over time (7). Two SEER studies included patients in later years (1988–2011 and 1992–2013) showed comparable outcomes during the periods of diagnosis (12, 16). However, a study only included patients with stage I-II disease (12), and another study only included Hispanic women (16), which could not represent the general population. A recent SEER study included patients from 2002 to 2011, and the results showed improved outcomes in patients with stage IV EOC, but not in patients with stage I–III disease (14). However, the information regarding sample of patients, surgery and chemotherapy were not included in this study. Although studies from Canadian and France population showed an improved outcome over time. A large cohort from Netherlands Cancer Registry indicated that long-term outcome has not improved of EOC in the last 25 years (13). As listed in Table 4, there were significantly differences in the inclusion criteria and patterns of treatment in the above-mentioned studies, which may be contributed to the inconsistent results. In addition, the diagnostic techniques, postoperative care, and palliative care may also have different effects on the survival outcome for patients.
Surgery remains the standard treatment for localized stage EOC. Our multivariate analysis found survival outcomes were better in 2005–2014 compared to 1990–1994. However, a slight improvement was found in CSS and OS; the 5-year CSS was 91.9% in 1990–1994 and in 2005–2009, and only a 1.2% absolute increase in OS was found between 1990–1994 and 2005–2009. A SEER study of patients with stages I-II EOC between 1988 and 2001 found the year of diagnosis was associated with better disease-specific survival in patients who received surgery that excluded lymphadenectomy, but it was not associated with better disease-specific survival in patients who received surgery that included lymphadenectomy (12). Therefore, the use of staging procedures may impact the role of period of diagnosis in EOC outcomes.
In our study, the CSS of patients diagnosed after 1995 significantly improved compared to those diagnosed in 1990–1994. This improvement might be related to the introduction of taxanes to clinical practice in EOC (22). Moreover, the proportion of optimal debulking surgeries for residual tumors ≤1 cm has increased in the past two decades, which may also account for improved outcomes in the regional disease stage between 1990–1994 and 1998–2014; it did not differ significantly between 1995 and 2015 (23, 24).
Our findings showed that the CSS and OS of patients in the distant stage improved over time. The reasons for improved survival may involve advances in chemotherapy, the introduction of carboplatin and paclitaxel for first-line treatment, the use of backup regimens for non-responsive tumors, and progress in developing treatments, such as PAPR-1 inhibitor and PD-L1 inhibitor (8, 25–29). In addition, there is also evidence of more accurate surgical staging procedures, more extensive tumor debulking surgery, and more accurate patient selection for secondary cytoreduction. Moreover, it is also possible the dissemination of evidence-based practices has had an impact on improving survival outcomes (8). Finally, significant improvements in survival outcomes may reflect the impact of new government-administrative strategies, such as having oncology multidisciplinary teams provide optimal treatment and care. Although gynecologic-oncologist consultations have increased in ovarian cancer over time, <40% of patients who saw a gynecologic oncologist received guidelines pertaining to surgery and chemotherapy (14). A recent review of outcomes of the management of ovarian cancer found gynecologic oncologists in specialized hospitals consistently performed staging and debulking surgery better (30). Therefore, collaboration among patients, gynecologic oncologists, and institutions is crucial.
This study has many strengths. The primary strength of this study is that we used high quality, nationwide, population-based data to gain greater insight into the survival patterns of EOC patients over a long period of time. In addition, a large sample of EOC patients was included, thus providing sufficient power to detect the smallest differences in relative survival for EOC patients.
This study also has several limitations. First, the major limitation of this study was the bias of observational studies and the unavailability of several variables including performance status, comorbidities, smoking status, and obesity, which may be the possible confounders for the modeling of outcome for EOC. In addition, the completeness of chemotherapy and surgery were also not recorded in the SEER database, which might have influenced the results. Second, schedules, agents, and the number of courses of chemotherapy, target therapy, interval debulking surgery, and the extent of residual tumors after cytoreductive surgery (available data since 2010) were not recorded in the SEER database. Third, the SEER program does not include treatment data beyond 4 months after diagnosis, and the treatment strategy after disease recurrence are also not recorded. Moreover, a high rate of under-reporting chemotherapy has been found in the SEER database (31). Finally, the median follow-up in patients diagnosed after 2010 was significantly shorted compared to patients who diagnosed before 2010. However, approximately two-thirds of patients have been diagnosed as distant stage, which was associated with poor prognosis. Therefore, although the follow-up time of our study needs to be further extended to confirm the results, we believe that our study is still representative to reflect survival trend of EOC over time.
Conclusion
In conclusion, our results indicate the survival of women with EOC has improved over time. This study indicates that advances in the management of patients with EOC have improved survival, but net survival remains generally poor in distant-stage EOC. There is a need to explore additional treatment strategies to improve the survival of patients with EOC.
Data Availability
Publicly available datasets were analyzed in this study. This data can be found here: www.seer.cancer.gov (access number: 11025-Nov2016).
Ethics Statement
This study was exempt from the approval processes of the Institutional Review Boards because the SEER database patient information is de-identified.
Author Contributions
JZ, S-GW, and W-WZ are lead authors who participated in data collection, manuscript drafting, table/figure creation, and manuscript revision. JW, Z-YH, and J-YS are senior authors who aided in drafting the manuscript and manuscript revision. W-WZ and JZ are the corresponding authors who initially developed the concept and drafted and revised the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by grants from the National Natural Science Foundation of China (No. 81802600) and the Science and Technology Planning Projects of Xiamen Science & Technology Bureau (No. 3502Z20184016).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. (2018) 391:1023–75. doi: 10.1016/S0140-6736(17)33326-3
2. Torre LA, Trabert B, DeSantis CE, Miller KD, Samimi G, Runowicz CD, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin. (2018) 68:284–96. doi: 10.3322/caac.21456
3. Bast RC Jr, Badgwell D, Lu Z, Marquez R, Rosen D, Liu J, et al. New tumor markers: CA125 and beyond. Int J Gynecol Cancer. (2005) 15(Suppl. 3):2741–81. doi: 10.1111/j.1525-1438.2005.00441.x
4. Badgwell D, Bast RC Jr. Early detection of ovarian cancer. Dis Markers. (2007) 23:397–410. doi: 10.1155/2007/309382
5. Rosenthal AN, Menon U, Jacobs IJ. Screening for ovarian cancer. Clin Obstet Gynecol. (2006) 49:433–47. doi: 10.1097/00003081-200609000-00004
6. Buys SS, Partridge E, Black A, Johnson CC, Lamerato L, Isaacs C, et al. Effect of screening on ovarian cancer mortality: the Prostate, Lung, Colorectal and Ovarian (PLCO) cancer screening randomized controlled trial. JAMA. (2011) 305:2295–303. doi: 10.1001/jama.2011.766
7. Barnholtz-Sloan JS, Schwartz AG, Qureshi F, Jacques S, Malone J, Munkarah AR. Ovarian cancer: changes in patterns at diagnosis and relative survival over the last three decades. Am J Obstet Gynecol. (2003) 189:1120–7. doi: 10.1067/S0002-9378(03)00579-9
8. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) -Ovarian Cancer Version 2. Fort Washington: National Comprehensive Cancer Network (2018). Available online at: https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf (accessed July 19, 2018).
9. Suh DH, Chang SJ, Song T, Lee S, Kang WD, Lee SJ, et al. Practice guidelines for management of ovarian cancer in Korea: a Korean Society of Gynecologic Oncology Consensus Statement. J Gynecol Oncol. (2018) 29:e56. doi: 10.3802/jgo.2018.29.e56
10. Akhtar-Danesh N, Elit L, Lytwyn A. Temporal trends in the relative survival among patients diagnosed with ovarian cancer in Canada 1992–2005: a population-based study. Gynecol Oncol. (2011) 123:192–5. doi: 10.1016/j.ygyno.2011.07.098
11. Trétarre B, Molinié F, Woronoff AS, Bossard N, Bessaoud F, Marrer E, et al. Ovarian cancer in France: trends in incidence, mortality and survival, 1980–2012. Gynecol Oncol. (2015) 139:324–9. doi: 10.1016/j.ygyno.2015.09.013
12. Chan J, Fuh K, Shin J, Cheung M, Powell C, Chen LM, et al. The treatment and outcomes of early-stage epithelial ovarian cancer: have we made any progress? Br J Cancer. (2008) 98:1191–6. doi: 10.1038/sj.bjc.6604299
13. Timmermans M, Sonke GS, Van de Vijver KK, van der Aa MA, Kruitwagen RFPM. No improvement in long-term survival for epithelial ovarian cancer patients: a population-based study between 1989 and 2014 in the Netherlands. Eur J Cancer. (2018) 88:31–7. doi: 10.1016/j.ejca.2017.10.030
14. Warren JL, Harlan LC, Trimble EL, Stevens J, Grimes M, Cronin KA. Trends in the receipt of guideline care and survival for women with ovarian cancer: a population-based study. Gynecol Oncol. (2017) 145:486–92. doi: 10.1016/j.ygyno.2017.03.016
15. Tan W, Stehman FB, Carter RL. Mortality rates due to gynecologic cancers in New York state by demographic factors and proximity to a Gynecologic Oncology Group member treatment center: 1979–2001. Gynecol Oncol. (2009) 114:346–52. doi: 10.1016/j.ygyno.2009.03.033
16. Chen C, Markossian TW, Silva A, Tarasenko YN. Epithelial ovarian cancer mortality among Hispanic women: sub-ethnic disparities and survival trend across time: an analysis of SEER 1992–2013. Cancer Epidemiol. (2018) 52:134–41. doi: 10.1016/j.canep.2017.12.003
17. Surveillance Epidemiology and, End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 18 Regs Custom Data (with additional treatment fields), Nov 2017 Sub (1973–2015 varying) - Linked To County Attributes - Total U.S., 1969–2016 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2018, based on the November 2017 submission.
18. Young JLJ, Roffers SD, Ries LAG, Fritz AG, Hurlburt AA (editors). SEER Summary Staging Manual - 2000: Codes and Coding Instructions. Bethesda, MD: National Institute of Health (2001). Available online at: https://seer.cancer.gov/tools/ssm/breast_femgen.pdf (accessed August 17, 2017).
19. Kurman RJ, Carcangiu ML, Herrington CS, Young RH. WHO Classification of Tumours of Female Reproductive Organs. 4th ed. Lyon: IARC (2014).
20. Chen VW, Ruiz B, Killeen JL, Coté TR, Wu XC, Correa CN. Pathology and classification of ovarian tumors. Cancer. (2003) 97:2631–42. doi: 10.1002/cncr.11345
21. Menon U, Griffin M, Gentry-Maharaj A. Ovarian cancer screening–current status, future directions. Gynecol Oncol. (2014) 132:490–5. doi: 10.1016/j.ygyno.2013.11.030
22. McGuire WP, Hoskins WJ, Brady MF, Kucera PR, Partridge EE, Look KY, et al. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med. (1996) 334:1–6. doi: 10.1056/NEJM199601043340101
23. Chi DS, Liao JB, Leon LF, Venkatraman ES, Hensley ML, Bhaskaran D, et al. Identification of prognostic factors in advanced epithelial ovarian carcinoma. Gynecol Oncol. (2001) 82:532–7. doi: 10.1006/gyno.2001.6328
24. van Altena AM, Karim-Kos HE, de Vries E, Kruitwagen RF, Massuger LF, Kiemeney LA. Trends in therapy and survival of advanced stage epithelial ovarian cancer patients in the Netherlands. Gynecol Oncol. (2012) 125:649–54. doi: 10.1016/j.ygyno.2012.02.033
25. Bristow RE, Tomacruz RS, Armstrong DK, Trimble EL, Montz FJ. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol. (2002) 20:1248–59. doi: 10.1200/JCO.2002.20.5.1248
26. Averette HE, Janicek MF, Menck HR. The National Cancer Data Base report on ovarian cancer. American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer. (1995) 76:1096–103. doi: 10.1002/1097-0142(19950915)76:6<1096::AID-CNCR2820760626>3.0.CO;2-4
27. Broekman K, Jalving M, van Tinteren H, Sessa C, Reyners A. Clinical benefit of controversial first line systemic therapies for advanced stage ovarian cancer – ESMO-MCBS scores. Cancer Treat Rev. (2018) 69:233–42. doi: 10.1016/j.ctrv.2018.06.008
28. Mirza MR, Monk BJ, Herrstedt J, Oza AM, Mahner S, Redondo A, et al. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. (2016) 375:2154–64. doi: 10.1056/NEJMoa1611310
29. Bellone S, Buza N, Choi J, Zammataro L, Gay L, Elvin J, et al. Exceptional response to pembrolizumab in a metastatic, chemotherapy/radiation-resistant ovarian cancer patient harboring a PD-L1-genetic rearrangement. Clin Cancer Res. (2018) 24:3282–91. doi: 10.1158/1078-0432.CCR-17-1805
30. Vernooij F, Heintz P, Witteveen E, van der Graaf Y. The outcomes of ovarian cancer treatment are better when provided by gynecologic oncologists and in specialized hospitals: a systematic review. Gynecol Oncol. (2007) 105:801–12. doi: 10.1016/j.ygyno.2007.02.030
Keywords: ovarian neoplasms, general surgery, prognosis, SEER, time
Citation: Wu S-G, Wang J, Sun J-Y, He Z-Y, Zhang W-W and Zhou J (2019) Real-World Impact of Survival by Period of Diagnosis in Epithelial Ovarian Cancer Between 1990 and 2014. Front. Oncol. 9:639. doi: 10.3389/fonc.2019.00639
Received: 28 February 2019; Accepted: 01 July 2019;
Published: 06 August 2019.
Edited by:
Ivan Garcia-Bassets, University of California, San Diego, United StatesReviewed by:
Saori Furuta, University of Toledo, United StatesPaul Terry, The University of Tennessee, Knoxville, United States
Copyright © 2019 Wu, Wang, Sun, He, Zhang and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wen-Wen Zhang, emhhbmd3d0BzeXN1Y2Mub3JnLmNu; Juan Zhou, anVhbnpob3UxMjM0NUAxNjMuY29t
†These authors have contributed equally to this work
 San-Gang Wu
San-Gang Wu Jun Wang
Jun Wang Jia-Yuan Sun2
Jia-Yuan Sun2 Zhen-Yu He
Zhen-Yu He Wen-Wen Zhang
Wen-Wen Zhang Juan Zhou
Juan Zhou