
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 06 June 2019
Sec. Women's Cancer
Volume 9 - 2019 | https://doi.org/10.3389/fonc.2019.00452
This article is part of the Research Topic New Approaches to Classification and Diagnostic Prediction of Breast Cancers View all 18 articles
 Maria Vittoria Dieci1,2*†
Maria Vittoria Dieci1,2*† Vassilena Tsvetkova1,3†
Vassilena Tsvetkova1,3† Gaia Griguolo2
Gaia Griguolo2 Federica Miglietta1
Federica Miglietta1 Mara Mantiero1
Mara Mantiero1 Giulia Tasca1,2
Giulia Tasca1,2 Enrico Cumerlato1
Enrico Cumerlato1 Carlo Alberto Giorgi2
Carlo Alberto Giorgi2 Tommaso Giarratano2
Tommaso Giarratano2 Giovanni Faggioni2
Giovanni Faggioni2 Cristina Falci2
Cristina Falci2 Grazia Vernaci1
Grazia Vernaci1 Alice Menichetti1
Alice Menichetti1 Eleonora Mioranza2
Eleonora Mioranza2 Elisabetta Di Liso2
Elisabetta Di Liso2 Simona Frezzini1
Simona Frezzini1 Tania Saibene4
Tania Saibene4 Enrico Orvieto5
Enrico Orvieto5 Valentina Guarneri1,2
Valentina Guarneri1,2Background: We evaluated immunohistochemical AR expression and correlation with prognosis in a large series of homogeneously treated patients with primary TNBC.
Material and Methods: Patients diagnosed with stage I-III TNBC between 2000 and 2015 at Istituto Oncologico Veneto who received treatment with surgery and neoadjuvant and/or adjuvant chemotherapy were included. Whole tissue slides were stained for AR. AR-positive expression was defined as >1% of positively stained tumor cells. Distant-disease-free survival (DDFS) was calculated from diagnosis to distant relapse or death. Late-DDFS was calculated from the landmark of 3 years after diagnosis until distant relapse or death.
Results: We included 263 primary TNBC patients. Mean AR expression was 14% (range 0–100%), and 29.7% (n = 78) of patients were AR+. AR+ vs. AR- cases presented more frequently older age (p < 0.001), non-ductal histology (p < 0.001), G1-G2 (p = 0.003), lower Ki67 (p < 0.001) and lower TILs (p = 0.008). At a median follow up of 81 months, 23.6% of patients experienced a DDFS event: 33.3% of AR+ and 19.5% of AR- patients (p = 0.015). 5 years DDFS rates were 67.2% and 80.6% for AR+ and AR- patients (HR = 1.82 95%CI 1.10–3.02, p = 0.020). AR maintained an independent prognostic role beyond stage, but when TILs were added to the model only stage and TILs were independent prognostic factors. AR was the only factor significantly associated with late-DDFS: 16.4% of AR+ and 3.4% of AR- patients experienced a DDFS after the landmark of 3 years after diagnosis (p = 0.001). Late-DDFS rates at 5 years from the 3-year landmark were 75.8% for AR+ and 95.2% for AR- patients (log-rank p < 0.001; HR = 5.67, 95%CI 1.90–16.94, p = 0.002).
Conclusions: AR expression is associated with worse outcome for patients with TNBC. In particular, AR+ TNBC patients are at increased risk of late DDFS events. These results reinforce the rationale of AR targeting in AR+ TNBC.
Triple negative breast cancer (TNBC) represents the most lethal breast cancer subtype, accounting for around 15% of all breast cancer diagnoses and being associated with an increased risk of relapse at distant sites, mostly occurring within the first 3 years from diagnosis (1). It is defined by the absence of expression of estrogen and progesterone receptors and lack of HER-2 overexpression/amplification. To date, chemotherapy remains the mainstay of systemic treatment for TNBC, since no relevant druggable targets have been identified (2).
In recent years, the application of genomic profiling techniques has allowed to dissect the heterogeneity of TNBC. At least four main TNBC subtypes have been defined (3, 4), including the luminal androgen receptor (LAR) class, which is enriched for hormonally regulated pathways and is dependent on AR signaling. The LAR subtype accounts for approximately 10–15% of TNBC and LAR cell lines have shown sensitivity to AR-antagonists (3, 4).
AR is found to be expressed by immunohistochemistry in 60–80% of breast cancers, less frequently in estrogen receptor-negative as compared to estrogen-receptor positive tumors (5). In TNBC series, the rate of AR-positive cases is generally 20–40% (5–8), with few studies showing rates up to 60% (9). Preclinical evidence shows that the AR effect depends on tumor subtype: in estrogen receptor-positive cancer cells AR activity is able to inhibit tumor growth (10), whereas in TNBC AR seems to retain an oncogenic effect (11, 12). With regards to the prognostic role of AR expression in patients cohorts, available evidence supports an association between AR expression and favorable prognosis for estrogen receptor-positive tumors (5, 13). In TNBC, data are more conflicting, with some studies showing a favorable prognosis associated with AR expression, some showing null results and others showing an association between AR expression and unfavorable outcome (5). Different methods of AR assessment and scoring, heterogeneity in patients cohorts and short follow up may have yielded to these contrasting results.
In this study, we evaluated AR expression by immunohistochemistry and its correlation with distant disease-free survival in a large cohort of patients with non-metastatic TNBC homogeneously treated with surgery and systemic chemotherapy.
We included 263 patients with non-metastatic TNBC (estrogen receptor and progesterone receptor <10%, HER2 0/1+ by immunohistochemistry and/or FISH non amplified) diagnosed from March 2000 to December 2015 at IRCCS Istituto Oncologico Veneto (Padova, Italy) who received treatment with surgery and neoadjuvant and/or adjuvant chemotherapy. Clinicopathological characteristics as well as treatment and follow up data were collected in a dedicated database. The study protocol was approved by the Ethical Committee of the Istituto Ocologico Veneto IRCCS (Padova, Italy). Written informed consent was obtained from patients.
AR expression was evaluated on the following FFPE primary tumor samples for main analyses: surgical sample for patients treated with primary surgery followed by adjuvant chemotherapy and diagnostic core-biopsy for patients treated with neoadjuvant chemotherapy followed by surgery.
In case of patients treated with neoadjuvant chemotherapy showing residual invasive breast cancer at the examination of the surgical sample, the FFPE surgical tumor block was also retrieved in order to conduct exploratory analysis of changes in AR expression from pre- to post-neoadjuvant chemotherapy.
AR nuclear staining was evaluated on whole sections by immunohistochemistry with the Dako AR441 antibody. AR was scored by a dedicated pathologist, blinded for clinical data, and was considered positive in case of staining in at least 1% of tumor cells, consistently with most recent studies (8, 9).
Tumor infiltrating lymphocytes (TILs) were evaluated according to consensus guidelines on hematoxylin and eosin-stained slides (14).
Statistical analysis was carried out using IBM SPSS (version 24) software.
Descriptive statistics were performed for patient demographics and clinical characteristics. For continuous variables, median and quartiles were computed. The χ2 test or the Mann-Whitney non-parametric test were used to study association between variables, according to their nature (categorical or continuous). The Wilcoxon signed-rank test was used to study the changes in AR expression before and after neoadjuvant chemotherapy in the subset of patients who received this type of treatment and showing residual invasive disease on the surgical sample.
Distant-disease free survival (DDFS) was defined as the time from diagnosis to relapse at a distant site or death from any cause, whichever first. Late-DDFS analysis were performed from the landmark of 3 years after diagnosis until relapse at a distant site or death from any cause, whichever first. In late-DDFS analysis, patients with an event or censored before the landmark point were excluded. The landmark for late-DDFS was defined based on the pattern of relapse for TNBC that shows a peak in the hazard rate of recurrence in the first 3 years after diagnosis (15). Overall survival (OS) was defined as the time from diagnosis to death from any cause. The Kaplan–Meier method was used to estimate survival curves, the log-rank test was used to test difference between groups. Univariate and multivariate Cox regression models were used to calculate HR and 95% CI. All reported p-values are two-sided, and significance level was set at p < 0.05.
Mean AR expression level was 14% (range 0–100%). Of 263 TNBC patients, 29.7% (N = 78) showed a positive AR expression. Images of representative slides are shown in Figure 1. Clinicopathological characteristics according to AR status are reported in Table 1. AR expression was significantly associated with older age (p = 0.002), non-ductal histology (p < 0.001), Grade 1–2 tumors (p = 0.003), lower Ki67 (p < 0.001), lower TILs (p = 0.008). There was no difference in stage and treatment received according to AR. Considering neoadjuvant and adjuvant therapy combined, 73% of patients received both an anthracycline and a taxane as part of chemotherapy treatment.
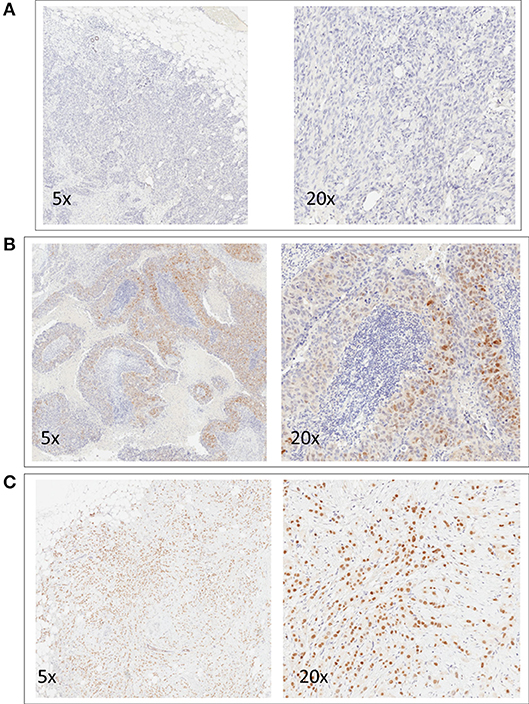
Figure 1. Representative images of immunohistochemical nuclear staining for AR. For each case, two images at different magnification are shown (5x and 20x). One negative case (A) and two positive cases (B,C) are shown.
At a median follow up of 81 months (95% CI 74–87), 62 patients have experienced a DDFS event (23.6%). Type of DDFS event was: distant relapse in 56 patients (90%) and death in 6 patients (10%, two deaths occurred in patients with unresectable chest locoregional recurrence and 4 patients died without known breast cancer relapse). The rate of events was higher in AR+ as compared to AR- patients (33.3 and 19.5%, respectively).
As shown in Figure 2A, Patients with AR+ tumor showed worse DDFS as compared to AR- patients: 5 years DDFS rates were 67.2 and 80.6%, respectively (log-rank p = 0.018). The HR for DDFS for the comparison of AR+ vs. AR- groups was 1.82 (95%CI 1.10-3.02, p = 0.020).
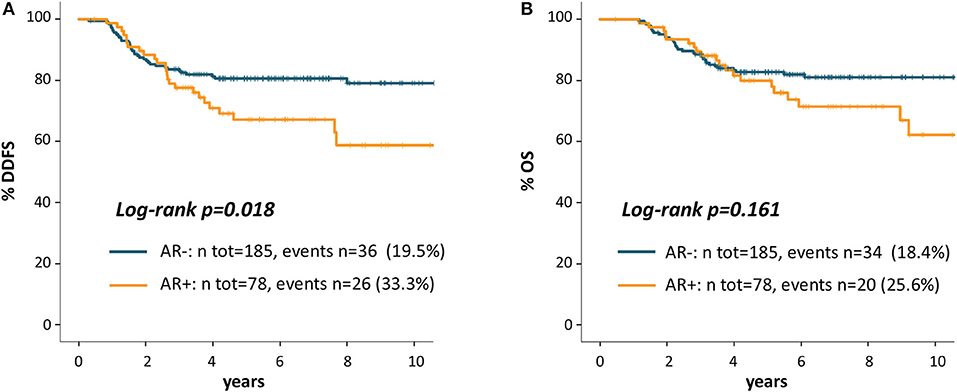
Figure 2. Kaplan Meier curves for distant disease-free survival (A) and overall survival (B) according to AR.
Figure 2B shows OS Kaplan-Meier curves: 5 years OS rate was 79.9% for AR+ and 82.7% for AR- patients (log-rank p = 0.161). The HR for OS for the comparison of AR+ vs. AR- patients was 1.48 (95% CI 0.85-2.58, p = 0.163).
Univariate and multivariate cox models for DDFS are reported in Table 2.
In addition to AR, the other factors that were associated in univariate analysis with DDFS, were stage (Stage II-III vs. I, p = 0.024) and TILs (considered as continuous variable for each 1% increment, p = 0.005). In multivariate analysis including AR and Stage, both factors maintained an independent prognostic role (AR+ vs. AR-: HR = 1.74, 95%CI 1.05-2.88, p = 0.032; Stage II-III vs. I: HR 3.05, 95%CI 1.83-5.08, p < 0.001). When TILs were added to the multivariate model, only stage and TILs maintained an independent prognostic value. The HR for the association between AR status and DDFS in multivariate models including the three variables was 1.57 (95% CI 0.94-2.61, p = 0.084).
Since Kaplan Meier curves showed that the prognostic effect of AR on DDFS appeared driven by the occurrence of late recurrences in AR+ patients, we performed a landmark survival analysis for late-DDFS to study the association between AR and late outcome. This analysis included 203 patients who were DDFS-free at 3 years from initial diagnosis and were not censored before the landmark point: n = 55 (27%) were AR+ and n = 148 (73%) were AR-. At a median follow up of 47 months (95% CI 41-53) n = 14 DDFS events have occurred. The rate of event was higher in AR+ (9/55, 16.4%) vs. AR- patients (5/148, 3.4%). Type of DDFS event included: 10 distant relapses and 4 deaths (1 in a patient with unresectable locoregional breast recurrence and 3 in patients without prior known breast cancer relapse). AR+ patients showed more frequently distant relapses (n = 8 of 9 total events, 89%) as compared to AR- patients (n = 2 of 5 total events, 40%).
Kaplan Meier curves in Figure 3 shows that patients with AR+ tumor experienced a significantly worse late outcome as compared to AR- patients: late-DDFS rate at 5 years from the 3-years landmark were 75.8% for AR+ patients and 95.2% for AR- patients (log-rank p < 0.001). Univariate late-DDFS cox analysis for the comparison of AR+ vs. AR- patients showed HR = 5.67 (95% CI 1.90-16.94, p = 0.002). No other factor showed a significant association with late-DDFS including: age (HR = 1.02, 95% CI 0.98-1.06, p = 0.377), histologic Grade (Grade 3 vs. 1-2 HR = 1.96, 95% CI 0.25-15.56, p = 0.524), stage (stage II-III vs. I, HR = 0.96, 95% CI 0.32-2.87, p = 0.943) and TILs (HR = 0.98, 95% CI 0.95-1.01, p = 0.178). However, number of events was low.
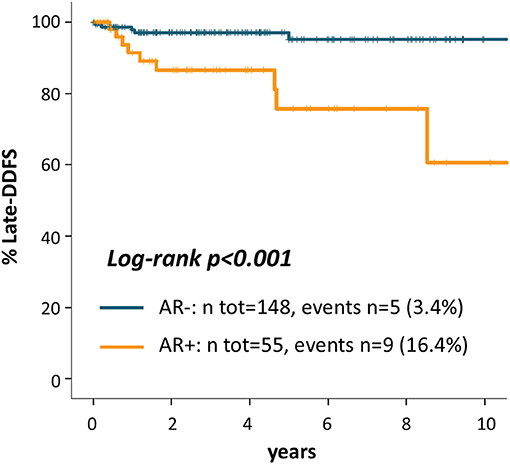
Figure 3. Kaplan Meier curves for late-distant disease-free survival from the landmark of 3 years after diagnosis according to AR.
A list of cases with DDFS event and matched clinicopathological features is provided as Table 3. Moreover, exploratory additional survival analyses according to a cut-off of >10% of AR expression are reported in Supplementary Figure 1.
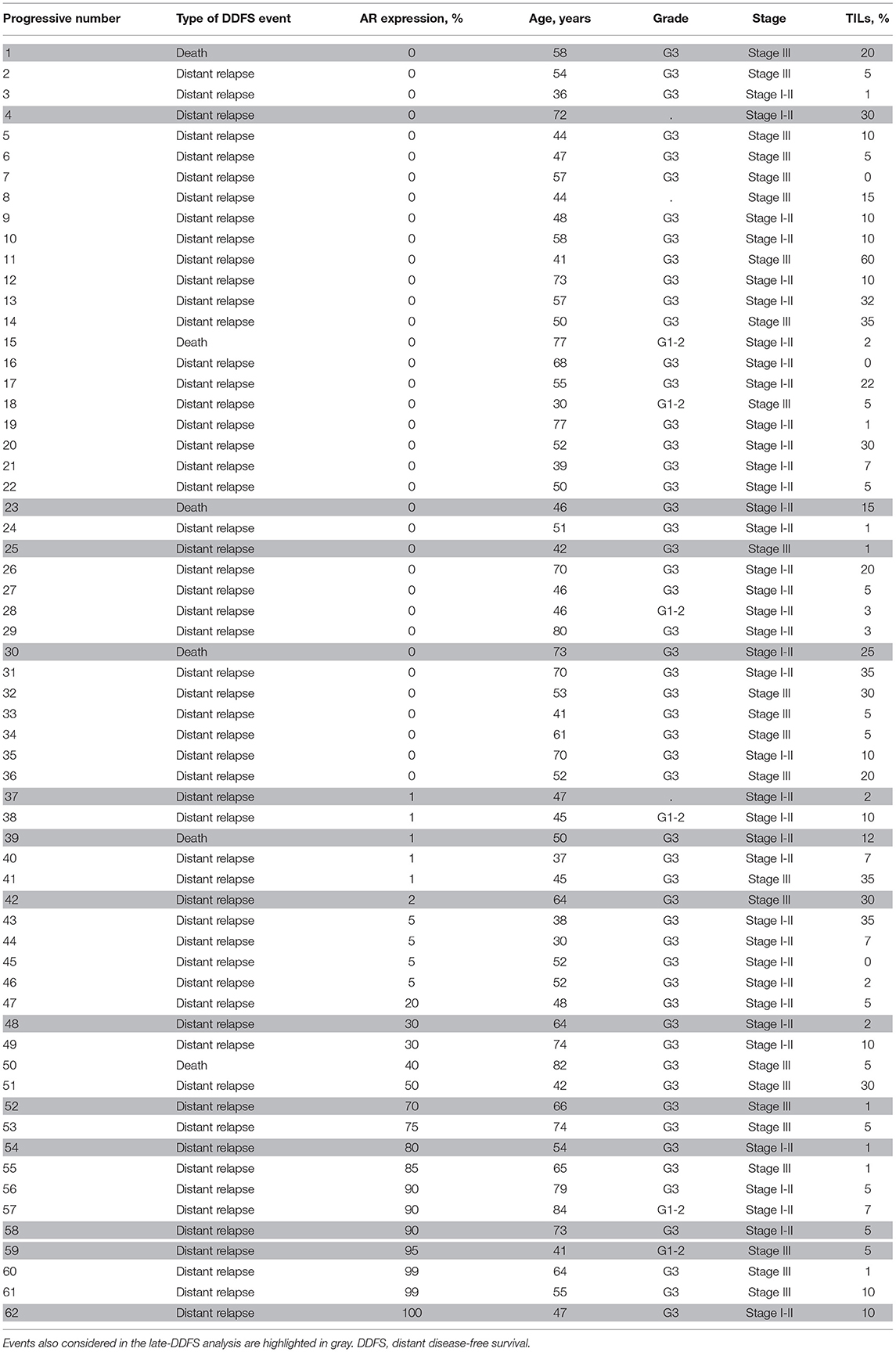
Table 3. List of cases with a DDFS event and matched clinicopathological features entered in univariate and multivariable cox regression models.
Of the 108 patients who received neoadjuvant chemotherapy, information on pathological response was available for 107 cases. A pathological complete response (pCR), defined as the absence of invasive cancer cells in the breast and axillary lymphnodes on the surgical specimen, was observed in 28% of cases (n = 30). The rate of pCR was similar in AR+ and AR- patients: 25.9 and 28.8%, respectively (p = 0.778). Tumor tissue sample from the surgical specimen obtained after neoadjuvant chemotherapy was available for AR evaluation for n = 60 patients without pCR (patients' flow diagram provided in Supplementary Figure 2). AR expression showed a non-significant decrease after neoadjuvant chemotherapy: mean 13% on the diagnostic core-biopsy and 10% on the paired surgical specimen (Wilcoxon signed-rank test p = 0.172). All those cases that were AR- on the diagnostic core-biopsy were also AR- on the surgical specimen (n = 43), whereas 41% of the 17 initially AR+ cases lost AR expression after neoadjuvant chemotherapy (χ2 p < 0.001).
In this study we showed that AR expression is associated with worse DDFS in TNBC patients treated with surgery and systemic chemotherapy. Although AR did not retain an independent prognostic value for DDFS in multivariate analysis in the total follow-up period, we found that AR expression was the only factor that resulted in a significant increase in the risk of late-DDFS event. Of note, the vast majority of events were distant relapses or deaths in patients with unresectable locoregional recurrences. Therefore, the potential confounding effect of deaths of unknown cause or not related to breast cancer (which may be relevant in studies with long-term follow up) is very limited.
We found that 30% of TNBC cases were classified as AR+, which is in line with a number of other studies (5–8). The correlation of AR+ status with other clinicopathologic characteristics such as older age, non-ductal histology, lower histologic grade, lower ki67 and lower TILs, is also consistent with other studies assessing AR by immunohistochemistry or evaluating the LAR molecular subtype (4, 9, 12, 16).
The available evidence on the prognostic role of AR for patients with early TNBC is conflicting. A recent metanalysis reported that AR expression significantly predicts for a better survival in TNBC (HR for DFS = 0.64, 95%CI 0.51-0.81 and HR for OS = 0.64, 95%CI 0.49-0.88) (13). Multivariate analysis was not available. It has to be noted that this was a study-level and not a patient-level metanalysis, including studies that were heterogeneous for methods of AR scoring, clinical cohorts characteristics, treatment and length of follow up. At least two other retrospective studies were issued after the publication of this metanalysis, reporting no association of AR with prognosis in TNBC (sample size of n = 130 and n = 182, respectively) (17, 18). In addition, two other larger studies have recently demonstrated an unfavorable prognosis for AR+ TNBC patients (8, 9). In both these studies the Dako AR441 antibody was used and the definition of AR+ in immunohistochemistry was based on the >1% cut-off, consistently with the methods applied in our analysis. Data from the TNBC subset of the prospective Nurses' Health Studies cohorts (n = 581) have reported, over a median follow up of 16.5 years, a significantly unfavorable breast cancer-specific survival in multivariable models for AR+ vs. AR- patients (8). In this study the prognostic impact of AR was evident in years 0–7 after diagnosis with an HR of 1.59 (95%CI 1.07–2.37) that maintained a similar value even >7 years after diagnosis, although not reaching statistical significance in this period (HR = 1.41, 95%CI 0.84–2.36). When looking at survival curves in this study, they result very similar to the ones reported in our analysis, with a separation of the curves for AR+ and AR- patients that starts around 3 years after diagnosis, supporting our findings of AR+ tumors being associated with an increased risk of late relapses. In another retrospective series of more than 300 TNBC (9), the significant association between AR+ and worse outcome was further refined by the combined evaluation of AR and forkhead-box A1 (FOXA1), a protein required for AR transcriptional activity (19). Indeed, patients with AR+/FOXA1+ TNBC showed a worse overall survival as compared to other patients in multivariable model (HR = 1.57, 95%CI 1.01-2.45) (9). Again, survival curves started to separate at around 3 years after diagnosis.
Although AR expression by itself can only be considered as a suboptimal surrogate of the molecular LAR TNBC subtype (20), our results, together with the ones by Kensler et al. and Guiu et al. are consistent with findings suggesting the association of LAR subtype with poor prognosis in TNBC (16). Potential biological reasons for this association may include: the proposed oncogenic role of AR in TNBC (11, 12) and a distinct genomic landscape including an enrichment in somatic PIK3CA and AKT1 mutations (9, 16). Moreover, AR+/LAR TNBC are associated with lower TILs (4), as also shown in our study. In particular, in our work, this correlation might explain the lack of independent prognostic role of AR for DDFS in the total follow-up period when both TILs and stage are added to the multivariate model.
Anti-androgen therapies are under investigation for breast cancer in different settings (12) and phase II studies in metastatic TNBC AR+ patients have already obtained encouraging results (21–23). If further validated by other studies, our results showing that TNBC AR+ patients are at increased risk of late DDFS event may be useful in planning the future development of antiandrogen adjuvant therapies in TNBC.
With regards to the subset of patients treated with neoadjuvant chemotherapy, we did not observe different rates of pCR according to AR, however sample size was limited. The majority of data indicate that TNBC with a positive AR expression or owing to the LAR subtype achieve lower rates of pCR as compared to other TNBC patients (4, 24–26), although other studies showed conflicting results (6). The achievement of pCR is associated with long-term outcome in TNBC. Whether and to which extent the less likelihood of pCR for AR+/LAR TNBC contributes to the long-term outcome of these patients is not clear at this time (25, 26). Moreover, interpretation of results from different studies is limited by the lack of concordance between the evaluation of AR expression by immunohistochemistry and the LAR classification by gene expression. The evaluation of combined chemotherapy and antiandrogen therapy is ongoing in the neoadjuvant setting (NCT02689427).
Our study has strengths, including: the large sample size, the homogeneous treatment received by patients which is consistent with contemporary standards (all patients treated with chemotherapy and surgery, the vast majority received both an anthracycline and a taxane), the methods for AR assessment in line with the most recent studies and the length of follow up (median 81 months), allowing to uncover the impact of AR on late outcome.
Limitations of our study include the retrospective nature and the low number of events in late-DDFS analysis that imposes caution in results interpretation and further validation in additional studies.
In conclusion, our results show that the evaluation of AR in TNBC is able to identify a subgroup of patients at worse prognosis, especially for the occurrence of late events. Further validation in other studies is warranted. These data support the rationale for the ongoing evaluation of antiandrogen therapies in TNBC.
MD planned and coordinated the manuscript. VT performed the pathology assessment. VG supervised the whole writing of the manuscript. Each author participated for appropriate portions of the content. All the authors conceived the review and approved of the final analysis and results.
MD has received fees from EliLilly for consultancy role and participation on advisory boards; fees from Genomic Health for consultancy role; fees from Celgene for participation on advisory boards. VG has received honoraria from EliLilly and Roche for participation on advisory boards, and honoraria from AstraZeneca and Novartis.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2019.00452/full#supplementary-material
1. Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. (2007) 13:4429–34. doi: 10.1158/1078-0432.CCR-06-3045
2. Bianchini G, Balko JM, Mayer IA, Sanders ME, Gianni L. Triple-negative breast cancer: challenges and opportunities of a heterogeneous disease. Nat Rev Clin Oncol. (2016) 13:674–90. doi: 10.1038/nrclinonc.2016.66
3. Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. (2011) 121:2750–67. doi: 10.1172/JCI45014
4. Lehmann BD, Jovanović B, Chen X, Estrada MV, Johnson KN, Shyr Y, et al. Refinement of triple-negative breast cancer molecular subtypes: implications for neoadjuvant chemotherapy selection. PLoS ONE. (2016) 11:e0157368. doi: 10.1371/journal.pone.0157368
5. Ricciardelli C, Bianco-Miotto T, Jindal S, Butler LM, Leung S, McNeil CM, et al. The magnitude of androgen receptor positivity in breast cancer is critical for reliable prediction of disease outcome. Clin Cancer Res. (2018) 24:2328–41. doi: 10.1158/1078-0432.CCR-17-1199
6. Loibl S, Müller BM, von Minckwitz G, Schwabe M, Roller M, Darb-Esfahani S, et al. Androgen receptor expression in primary breast cancer and its predictive and prognostic value in patients treated with neoadjuvant chemotherapy. Breast Cancer Res Treat. (2011) 130:477–87. doi: 10.1007/s10549-011-1715-8
7. Wang C, Pan B, Zhu H, Zhou Y, Mao F, Lin Y, et al. Prognostic value of androgen receptor in triple negative breast cancer: a meta-analysis. Oncotarget. (2016) 7:46482–91. doi: 10.18632/oncotarget.10208
8. Kensler KH, Poole ME, Heng YJ, Collins LC, Glass B, Beck AH, et al. Androgen receptor expression and breast cancer survival: results from the Nurses' Health Studies. J Natl Cancer Inst. (2018). doi: 10.1093/jnci/djy173. [Epub ahead of print].
9. Guiu S, Mollevi C, Charon-Barra C, Boissière F, Crapez E, Chartron E, et al. Prognostic value of androgen receptor and FOXA1 co-expression in non-metastatic triple negative breast cancer and correlation with other biomarkers. Br J Cancer. (2018) 119:76–9. doi: 10.1038/s41416-018-0142-6
10. Peters AA, Buchanan G, Ricciardelli C, Bianco-Miotto T, Centenera MM, Harris JM, et al. Androgen receptor inhibits estrogen receptor-alpha activity and is prognostic in breast cancer. Cancer Res. (2009) 69:6131–40. doi: 10.1158/0008-5472.CAN-09-0452
11. Doane AS, Danso M, Lal P, Donaton M, Zhang L, Hudis C, et al. An estrogen receptor-negative breast cancer subset characterized by a hormonally regulated transcriptional program and response to androgen. Oncogene. (2006) 25:3994–4008. doi: 10.1038/sj.onc.1209415
12. Gerratana L, Basile D, Buono G, De Placido S, Giuliano M, Minichillo S, et al. Androgen receptor in triple negative breast cancer: a potential target for the targetless subtype. Cancer Treat Rev. (2018) 68:102–10. doi: 10.1016/j.ctrv.2018.06.005
13. Bozovic-Spasojevic I, Zardavas D, Brohée S, Ameye L, Fumagalli D, Ades F, et al. The prognostic role of androgen receptor in patients with early-stage breast cancer: a meta-analysis of clinical and gene expression data. Clin Cancer Res. (2017) 23:2702–12. doi: 10.1158/1078-0432.CCR-16-0979
14. Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol. (2015) 26:259–71. doi: 10.1093/annonc/mdu450
15. Cossetti RJ, Tyldesley SK, Speers CH, Zheng Y, Gelmon KA. Comparison of breast cancer recurrence and outcome patterns between patients treated from 1986 to 1992 and from 2004 to 2008. J Clin Oncol. (2015) 33:65–73. doi: 10.1200/JCO.2014.57.2461
16. Bareche Y, Venet D, Ignatiadis M, Aftimos P, Piccart M, Rothe F, et al. Unravelling triple-negative breast cancer molecular heterogeneity using an integrative multiomic analysis. Ann Oncol. (2018) 29:895–902. doi: 10.1093/annonc/mdy024
17. Kraby MR, Valla M, Opdahl S, Haugen OA, Sawicka JE, Engstrøm MJ, et al. The prognostic value of androgen receptors in breast cancer subtypes. Breast Cancer Res Treat. (2018) 172:283–96. doi: 10.1007/s10549-018-4904-x
18. Aleskandarany MA, Abduljabbar R, Ashankyty I, Elmouna A, Jerjees D, Ali S, et al. Prognostic significance of androgen receptor expression in invasive breast cancer: transcriptomic and protein expression analysis. Breast Cancer Res Treat. (2016) 159:215–27. doi: 10.1007/s10549-016-3934-5
19. Robinson JL, Macarthur S, Ross-Innes CS, Tilley WD, Neal DE, Mills IG, et al. Androgen receptor driven transcription in molecular apocrine breast cancer is mediated by FoxA1. EMBO J. (2011) 30:3019–27. doi: 10.1038/emboj.2011.216
20. Traina T, Miller K, Yardley D, O'Shaughnessy J, Cortes J, Awada A, et al. In: results from a phase 2 study of enzalutamide (ENZA), an androgen receptor (AR) inhibitor, in advanced AR+ triple-negative breast cancer (TNBC). J Clin Oncol. (2015) 33:1003. doi: 10.1200/jco.2015.33.15_suppl.1003
21. Gucalp A, Tolaney S, Isakoff SJ, Ingle JN, Liu MC, Carey LA, et al. Phase II trial of bicalutamide in patients with androgen receptor-positive, estrogen receptor-negative metastatic breast cancer. Clin Cancer Res. (2013) 19:5505–12. doi: 10.1158/1078-0432.CCR-12-3327
22. Traina TA, Miller K, Yardley DA, Eakle J, Schwartzberg LS, O'Shaughnessy J, et al. Enzalutamide for the treatment of androgen receptor-expressing triple-negative breast cancer. J Clin Oncol. (2018) 36:884–90. doi: 10.1200/JCO.2016.71.3495
23. Bonnefoi H, Grellety T, Tredan O, Saghatchian M, Dalenc F, Mailliez A, et al. A phase II trial of abiraterone acetate plus prednisone in patients with triple-negative androgen receptor positive locally advanced or metastatic breast cancer (UCBG 12–1). Ann Oncol. (2016) 27:812–8. doi: 10.1093/annonc/mdw067
24. Echavarria I, López-Tarruella S, Picornell A, García-Saenz JÁ, Jerez Y, Hoadley K, et al. Pathological response in a triple-negative breast cancer cohort treated with neoadjuvant carboplatin and docetaxel according to Lehmann's refined classification. Clin Cancer Res. (2018) 24:1845–52. doi: 10.1158/1078-0432.CCR-17-1912
25. Jovanović B, Mayer IA, Mayer EL, Abramson VG, Bardia A, Sanders ME, et al. A randomized phase II neoadjuvant study of cisplatin, paclitaxel with or without everolimus in patients with stage II/III triple-negative breast cancer (TNBC): responses and long-term outcome correlated with increased frequency of DNA damage response gene mutations, TNBC subtype, AR status, and Ki67. Clin Cancer Res. (2017) 23:4035–45. doi: 10.1158/1078-0432.CCR-16-3055
Keywords: androgen receptor, triple negative, early breast cancer, androgen receptor, triple negative, early breast cancer, prognosis, late outcome
Citation: Dieci MV, Tsvetkova V, Griguolo G, Miglietta F, Mantiero M, Tasca G, Cumerlato E, Giorgi CA, Giarratano T, Faggioni G, Falci C, Vernaci G, Menichetti A, Mioranza E, Di Liso E, Frezzini S, Saibene T, Orvieto E and Guarneri V (2019) Androgen Receptor Expression and Association With Distant Disease-Free Survival in Triple Negative Breast Cancer: Analysis of 263 Patients Treated With Standard Therapy for Stage I-III Disease. Front. Oncol. 9:452. doi: 10.3389/fonc.2019.00452
Received: 24 January 2019; Accepted: 13 May 2019;
Published: 06 June 2019.
Edited by:
Mothaffar Rimawi, Baylor College of Medicine, United StatesReviewed by:
Rachelle Johnson, Vanderbilt University Medical Center, United StatesCopyright © 2019 Dieci, Tsvetkova, Griguolo, Miglietta, Mantiero, Tasca, Cumerlato, Giorgi, Giarratano, Faggioni, Falci, Vernaci, Menichetti, Mioranza, Di Liso, Frezzini, Saibene, Orvieto and Guarneri. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maria Vittoria Dieci, bWFyaWF2aXR0b3JpYS5kaWVjaUB1bmlwZC5pdA==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.