
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol. , 29 May 2019
Sec. Genitourinary Oncology
Volume 9 - 2019 | https://doi.org/10.3389/fonc.2019.00435
This article is part of the Research Topic Case Reports in Urothelial Cancer View all 7 articles
 Jyoti Sharma1,2
Jyoti Sharma1,2 Barnali Deb1,2
Barnali Deb1,2 Irene A. George1
Irene A. George1 Shruthi Kapil3
Shruthi Kapil3 Karunakaran Coral3
Karunakaran Coral3 Nandita Kakkar4
Nandita Kakkar4 Smita Pattanaik5
Smita Pattanaik5 Arup Kumar Mandal6
Arup Kumar Mandal6 Ravimohan S. Mavuduru6*
Ravimohan S. Mavuduru6* Prashant Kumar1,2*
Prashant Kumar1,2*Background: Urothelial carcinoma is the most common malignancy of the bladder and is primarily considered as a disease of the elderly. Studies that address bladder tumor occurrence in young age groups are rare.
Case Presentation: A 19-year-old male presented with a gross total painless hematuria. A histology after biopsy revealed a high-grade transitional cell carcinoma with lymph node metastasis. The patient succumbed to the disease on day 72 of the treatment. Here, we used whole-exome sequencing of a paired tumor-normal sample to identify the somatic mutations and the possible targets of treatment.
Result: We predicted eight potential driver mutations (TP53 p.V157L, RB1 c.1498+1G>T, MED23 p.L1127P, CTNND1 p.S713C, NSD1 p.P2212A, MED17 p.G556V, DPYD p.Q814K, and SPEN p.S1078*). In addition, we predicted deleterious mutations in genes involved in the ion channels (CACNA1S p.E1581K, CACNG1 p.P71T, CACNG8 p.G404W, GRIN2B p.A1096T, KCNC1 p.G16V, KCNH4 p.E874K, KCNK9 p.R131S, P2RX7 p.A296D, and SCN8A p.R558H).
Conclusions: Most likely, mutations in genes involved in ion channels may be responsible for the aggressive behavior of a tumor. Ion channels are the second largest class of drug targets, and may thus serve as a putative potential therapeutic target in advanced stage urothelial carcinoma.
Urothelial carcinoma (UC) originates in the inner lining of the bladder epithelium and accounts for more than 90% of bladder cancer (1). Management of this cancer largely depends on prevention of progression and early identification of patients in non-muscle invasive bladder cancer (NMIBC) stage. Low-risk NMIBCs are likely to develop and progress to high-risk muscle invasive bladder cancer (MIBC). About 75% of the cases are NMIBC which tend to reoccur frequently (5–25%) and progress to the more aggressive MIBC (2). The median age for the diagnosis of urothelial carcinoma is approximately 69 years in males and 71 years in females (3). It rarely occurs in young adults (<20 years of age) with a total incidence of <200 cases worldwide by 2017 (4). UC in these rare cases are pathologically unique and have molecular features with very few genetic and epigenetic events reported (5). A list of standard drugs is routinely used to treat UC. Systemic treatment for this cancer has been restricted to cisplatin-based chemotherapy with negligible advancement over the past decades (6). Regardless of the treatment by transurethral resection combined with intravesical chemotherapy, more than 50% recurrence has been observed, and eventually 10–20% of these tumors progress to muscle invasive stage (7). Molecular studies in young adults would identify several oncogenic targets that could hold the promise for therapy. Whole-exome sequencing has not been carried out in the reported pediatric cases (8–14) and would aid in the identification of such targets. Here, we used whole-exome sequencing to identify somatic mutations and possible targets for treatment of a 19-year-old male patient with metastatic urothelial transitional cell carcinoma.
A 19-year-old male presented with gross total painless hematuria of a 5 days duration. There was no past family history of cancer. General physical examination and systemic examination were normal. Blood workup showed anemia. Renal function and liver functions were within normal limits. An ultrasound showed a polypoidal mass attached to the anterior wall of the bladder of 7 × 5 cm in size, which was further confirmed by a contrast enhanced CT scan (CECT) of abdomen. There was no evidence of lymph node or visceral metastasis. Transurethral resection of the bladder mass was performed. The upper gastrointestinal tract (GI) and lower GI endoscopy was within normal limits. A bone scan did not show any skeletal metastasis. Thereafter, the patient underwent robot assisted partial cystectomy and bilateral lymph node dissection till aortic bifurcation. The histopathology was suggestive of a high-grade urothelial carcinoma with six out of seven nodes showing metastasis. Post operatively, the patient developed fever and intestinal obstruction, initially managed conservatively, however, the patient did not show improvement. A repeat CECT abdomen was done which showed soft tissue lesions in both lungs, with pleural effusion, multiple liver lesions and ascites, suggestive of disseminated metastasis. The patient's general condition deteriorated, and he subsequently succumbed to his disease. An overview of the medical disease history is illustrated in a timeline (Figure 1A). Photomicrographs of the tumor from the urinary bladder showed a high-grade urothelial carcinoma with plenty of large pleomorphic cells and infiltrating the detrusor muscle (Figure 1B).
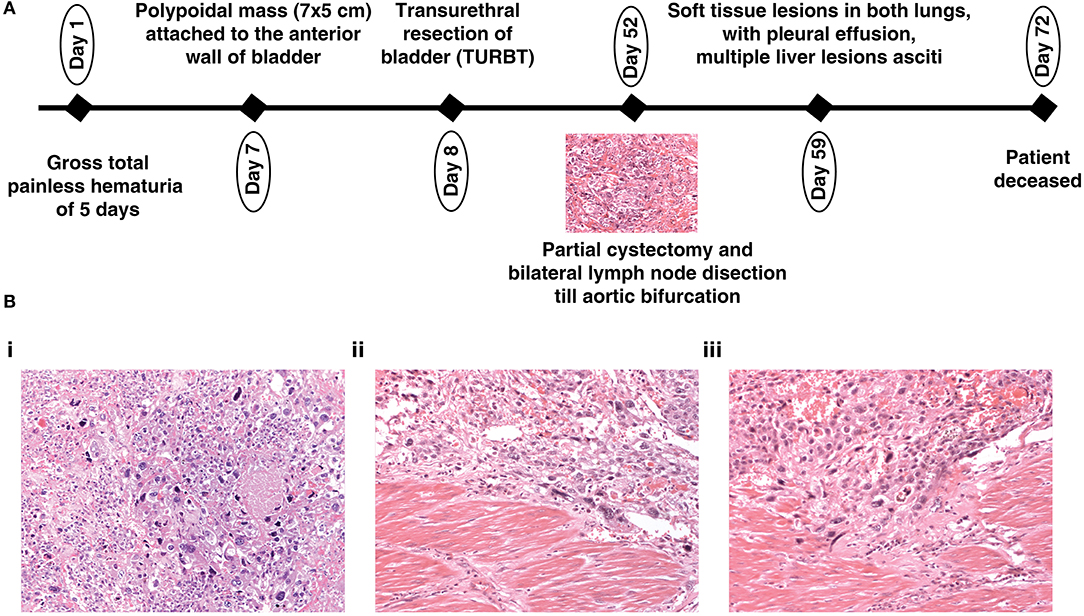
Figure 1. (A) Depiction of the history of a 19-year-old patient with urothelial carcinoma and (B) (20X magnification) Hematoxylin and Eosin stained section (i) showing a high-grade urothelial carcinoma with plenty of large pleomorphic cells; (ii,iii) showing a high-grade urothelial carcinoma infiltrating the detrusor muscle.
Whole-exome sequencing (WES) analysis of the paired tumor-normal sample from the patient was performed. A detailed description of the sequencing methods is provided in the Supplementary Material. WES data analysis revealed 558 exonic somatic mutations, of which 360 missense, 26 nonsense, 30 frameshift deletions/ insertions and, also 10 splice site mutations were annotated (Supplementary Figure 1). Thirty mutations are reported in COSMIC database (15) including in genes, such as TP53, ABL1, ARID5B, and P2RX7 (Supplementary Table 1). In addition, using Cancer Genome Interpreter (16), we predicted eight potential driver mutations among all the somatic mutations detected in this rare tumor. These predicted driver mutations including loss-of-function mutations in TP53, RB1, MED23, CTNND1 and activating mutations in NSD1and MED17 (Table 1). The TP53 p.V157L a known oncogenic mutation was identified as a recurrent hotspot in various cancer types (17). RB1 is involved in the regulation of the cell cycle checkpoint and DNA damage response. The RB1 c.1498+1G>T alteration is likely oncogenic. Mutations in RB1 is associated with poor overall survival in patients with urothelial carcinoma (18). Domain structures of these genes highlighting the predicted deleterious mutations were generated using MutationMapper (Supplementary Figure 2).
Given that the above predicted driver mutations are in the genes that are limited to already known/predicted cancer driver genes, we carried out a network analysis of 347 genes that harbor a missense mutation using the STRING database. An analysis of the enriched interaction network was performed against the whole genome genes and the enrichment of ion channel pathways was identified (Supplementary Figure 3). Ion channels play a pivotal role in regulating self-sufficiency in growth, insensitivity to anti-growth signals, evasion of apoptosis, limitless replication potential, sustained angiogenesis, tissue invasion and metastasis (19, 20). We identified somatic alterations in 22 genes involved in the ion channels. Table 2 shows the list of seventeen missense and one frameshift insertion somatic mutations in genes involved in the ion channels. The human genome encodes approximately 328 ion channel genes1 (Supplementary Table 2A). Mutated genes in this patient belong to 11 groups of ion channels (Supplementary Table 2B). We generated the ion channels interaction network of 141 genes (Supplementary Table 2C) comprising of 11 groups using STRING database. Interaction network shows the highly connected network of voltage-gated calcium, cation channels, voltage-gated potassium and voltage-gated sodium channels (Supplementary Figure 4A). Domain structures of nine genes highlighting the predicted deleterious somatic mutations are shown in Supplementary Figure 4B.
The incidence of UC has been rising with increased life expectancy. UC occurs mainly in older people however, young patients with UC are reported in rare cases. In this case report, we presented a study of a tumor which progressed aggressively, and the patient died on the 72 day of presentation. A typical tumor exhibits two to five driver genes (21), however our sequencing analysis of the primary tumor identified eight predicted somatic driver mutations as well as the predicted deleterious somatic mutations in genes involved in ion channels, such as CACNA1S, KCNK9, SCN8A, and P2RX7. Mostly, these somatic mutations were predicted by multiple tools (Supplementary Figure 5). A study by Biasiotta et al. have reported the significantly altered expression of CACNAD1, CATSPER, CATSPER2, KCNN1, KCNN4, TRPM2, TRPM4, TRPV4, and AQP3 in the bladder carcinoma (22). Several landmark studies have been performed to study the role of ion channels in the tumorigenesis. Jacquemet et al. have reported that CACNA1S promotes filopodia stability and maturation in breast cancer cell lines (23). Overexpression of KCNK9, a proto-oncogene has been reported in breast tumors (24). Carrithers and colleagues have reported that SCN8A contributes to cell invasion via podosome and invadopodia formation in macrophages derived from human monocytic leukemia and melanoma cancer cells (25).
Several studies provide evidence for the role of ion channels in carcinogenesis. However, limited studies have been conducted to observe the significance of ion channels as a potential therapeutic target. For example, inhibition of CACNA1S has been reported to block invasion in breast cancer cell lines (23) and pancreatic cancer (26). The blocking of voltage gated potassium channels in small cell lung cancer (27), melanoma cells (28), breast cancer cells (29), and prostate cancer cells (30) with therapeutic agents have also been reported to reduce the cell proliferation. Thus, a growing body of research demonstrates that ion channels could be potential therapeutic targets for UC. Currently, the large availability of pharmacological agents targeting the majority of ion channels: amlodipine and cilnidipine, calcium channel blockers in breast cancer (26); Iberiotoxin, charybdotoxin and clotrimazole, potassium channel blockers in breast and cervical cancers (31); tetrodotoxin, voltage gated sodium channels blocker in breast cancer (32) and others, offer a broad therapeutic avenue for anticancer therapy.
Our results underpin the value of WES in revealing the somatic mutations in the known cancerdriver genes and genes involved in ion channels in a patient. Ion channels could be further explored as a potential class of oncological targets for future therapeutics in advanced stage urothelial carcinoma.
This manuscript contains previously unpublished data. The name of the repository and accession number are not available.
Written informed consent was obtained from the parents of the participant for the publication of this case report. The study was approved by the ethics committee of the PGI under number PGI/IEC/2018/000874, dated: 01.06.2018.
PK conceptualized and designed the entire study. SP, AKM, and RSM carried out the sample collection from the patient. NK provided pathology images. SK and KC carried out the sequencing experiments. JS analyzed and interpreted the exome sequencing data. BD, IAG, RSM, JS and PK were involved in the preparation of the manuscript and the figures were prepared by BD and IAG.
This research was funded by Department of Science and Technology (DST), Ramanujan Fellowship, Government of India, grant number SB/S2/RJN-077/2015 and Bio-CARe by Department of Biotechnology (DBT), Government of India, grant number BT/PR19924/BIC/101/568/2016. We thank Sabarinathan Radhakrishnan for the critical evaluation of manuscript. PK is a recipient of the Ramanujan Fellowship awarded by Department of Science and Technology (DST), Government of India. JS is a recipient of Bio-CARe Women Scientists award by Department of Biotechnology (DBT), Government of India. BD is a recipient of INSPIRE Fellowship from Department of Science and Technology (DST), Government of India. IAG is a recipient of Junior Research Fellowship from Council of Scientific and Industrial Research (CSIR), Government of India.
SK and KC are employed by MedGenome Labs Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2019.00435/full#supplementary-material
1. Pasin E, Josephson DY, Mitra AP, Cote RJ, Stein JP. Superficial bladder cancer: an update on etiology, molecular development, classification, and natural history. Rev Urol. (2008) 10:31–43.
2. Kamat AM, Bagcioglu M, Huri E. What is new in non-muscle-invasive bladder cancer in 2016? Turk J Urol. (2017) 43:9–13. doi: 10.5152/tud.2017.60376
3. Lynch CF, Cohen MB. Urinary system. Cancer. (1995) 75(suppl. 1):316–29. doi: 10.1002/1097-0142(19950101)75:1+<316::AID-CNCR2820751314>3.0.CO;2-T
4. Shelmerdine SC, Lorenzo AJ, Gupta AA, Chavhan GB. Pearls and pitfalls in diagnosing pediatric urinary bladder masses. Radiographics. (2017) 37:1872–91. doi: 10.1148/rg.2017170031
5. Paner GP, Zehnder P, Amin AM, Husain AN, Desai MM. Urothelial neoplasms of the urinary bladder occurring in young adult and pediatric patients: a comprehensive review of literature with implications for patient management. Adv Anat Pathol. (2011) 18:79–89. doi: 10.1097/PAP.0b013e318204c0cf
6. Sharma J, Deb B, Kumar P. Developments in the area of bladder cancer genomics and its importance in the treatment selection. J Mol Oncol Res. (2018) 2:58–62.
7. Li F, Hong X, Hou L, Lin F, Chen P, Pang S, et al. A greater number of dissected lymph nodes is associated with more favorable outcomes in bladder cancer treated by radical cystectomy: a meta-analysis. Oncotarget. (2016) 7:61284–94. doi: 10.18632/oncotarget.11343
8. Neogi S, Kariholu PL, Dhakre G, Gupta V, Agarwal N, Bhadani P. Malignant urothelial carcinoma of urinary bladder in a young child: a rare case report. Urology. (2013) 81:888–90. doi: 10.1016/j.urology.2012.12.016
9. Sheehan L, Anwar A, Kommu S. Presentation of case: bladder cancer in an 18 year old female patient. Int J Surg Case Rep. (2015) 7C:42–6. doi: 10.1016/j.ijscr.2014.12.024
10. Rifat UN, Hamadalla NY, Chiad Safi KC, Al Habash SS, Mohammed M. Urothelial bladder tumour in childhood: a report of two cases and a review. Arab J Urol. (2015) 13:116–21. doi: 10.1016/j.aju.2014.11.002
11. Khan R, Ibrahim H, Tulpule S, Iroka N. Bladder cancer in a young patient: undiscovered risk factors. Oncol Lett. (2016) 11:3202–4. doi: 10.3892/ol.2016.4355
12. Kral M, Michalek J, Skarda J, Tichy T, Smakal O, Kodet R, et al. High-grade urothelial bladder cancer in children: a case report and critical analysis of the literature. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. (2016) 160:578–82. doi: 10.5507/bp.2016.045
13. Mau EE, Leonard MP. Urothelial carcinoma of the bladder in a pediatric patient. Can Urol Assoc J. (2016) 10:E268–9. doi: 10.5489/cuaj.3412
14. Hesse AN, Fabricius W, Thomas CA, Gaindh R, Christman R, Selvam P, et al. Genomic profiling of two histologically distinct rare urothelial cancers in a clinical setting to identify potential therapeutic options for treatment and management of disease. Case Rep Oncol. (2018) 11:196–205. doi: 10.1159/000487882
15. Forbes SA, Beare D, Bindal N, Bamford S, Ward S, Cole CG, et al. COSMIC: High-resolution cancer genetics using the catalogue of somatic mutations in cancer. Curr Protoc Hum Genet. (2016) 91:10.11.1–37. doi: 10.1002/cphg.21
16. Tamborero D, Rubio-Perez C, Deu-Pons J, Schroeder MP, Vivancos A, Rovira A, et al. Cancer Genome Interpreter annotates the biological and clinical relevance of tumor alterations. Genome Med. (2018) 10:25. doi: 10.1186/s13073-018-0531-8
17. Chang MT, Asthana S, Gao SP, Lee BH, Chapman JS, Kandoth C, et al. Identifying recurrent mutations in cancer reveals widespread lineage diversity and mutational specificity. Nat Biotechnol. (2016) 34:155–63. doi: 10.1038/nbt.3391
18. Yin M, Grivas P, Emamekhoo H, Mendiratta P, Ali S, Hsu J, et al. ATM/RB1 mutations predict shorter overall survival in urothelial cancer. Oncotarget. (2018) 9:16891–8. doi: 10.18632/oncotarget.24738
19. Phan NN, Wang CY, Chen CF, Sun Z, Lai MD, Lin YC. Voltage-gated calcium channels: novel targets for cancer therapy. Oncol Lett. (2017) 14:2059–74. doi: 10.3892/ol.2017.6457
20. Prevarskaya N, Skryma R, Shuba Y. Ion channels in cancer: are cancer hallmarks oncochannelopathies? Physiol Rev. (2018) 98:559–621. doi: 10.1152/physrev.00044.2016
21. Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA Jr, Kinzler KW. Cancer genome landscapes. Science. (2013) 339:1546–58. doi: 10.1126/science.1235122
22. Biasiotta A, D'Arcangelo D, Passarelli F, Nicodemi EM, Facchiano A. Ion channels expression and function are strongly modified in solid tumors and vascular malformations. J Transl Med. (2016) 14:285. doi: 10.1186/s12967-016-1038-y
23. Jacquemet G, Baghirov H, Georgiadou M, Sihto H, Peuhu E, Cettour-Janet P, et al. L-type calcium channels regulate filopodia stability and cancer cell invasion downstream of integrin signalling. Nat Commun. (2016) 7:13297. doi: 10.1038/ncomms13297
24. Mu D, Chen L, Zhang X, See LH, Koch CM, Yen C, et al. Genomic amplification and oncogenic properties of the KCNK9 potassium channel gene. Cancer Cell. (2003) 3:297–302. doi: 10.1016/S1535-6108(03)00054-0
25. Carrithers MD, Chatterjee G, Carrithers LM, Offoha R, Iheagwara U, Rahner C, et al. Regulation of podosome formation in macrophages by a splice variant of the sodium channel SCN8A. J Biol Chem. (2009) 284:8114–26. doi: 10.1074/jbc.M801892200
26. Min XJ, Li H, Hou SC, He W, Liu J, Hu B, et al. Dysfunction of volume-sensitive chloride channels contributes to cisplatin resistance in human lung adenocarcinoma cells. Exp Biol Med. (2011) 236:483–91. doi: 10.1258/ebm.2011.010297
27. Pancrazio JJ, Tabbara IA, Kim YI. Voltage-activated K+ conductance and cell proliferation in small-cell lung cancer. Anticancer Res. (1993) 13:1231–4.
28. Nilius B, Wohlrab W. Potassium channels and regulation of proliferation of human melanoma cells. J Physiol. (1992) 445:537–48. doi: 10.1113/jphysiol.1992.sp018938
29. Woodfork KA, Wonderlin WF, Peterson VA, Strobl JS. Inhibition of ATP-sensitive potassium channels causes reversible cell-cycle arrest of human breast cancer cells in tissue culture. J Cell Physiol. (1995) 162:163–71. doi: 10.1002/jcp.1041620202
30. Skryma RN, Prevarskaya NB, Dufy-Barbe L, Odessa MF, Audin J, Dufy B. Potassium conductance in the androgen-sensitive prostate cancer cell line, LNCaP: involvement in cell proliferation. Prostate. (1997) 33:112–22. doi: 10.1002/(SICI)1097-0045(19971001)33:2<112::AID-PROS5>3.0.CO;2-M
31. Kale VP, Amin SG, Pandey MK. Targeting ion channels for cancer therapy by repurposing the approved drugs. Biochim Biophys Acta. (2015) 1848:2747–55. doi: 10.1016/j.bbamem.2015.03.034
Keywords: NGS, bladder carcinoma, altered pathways, drugs, therapy
Citation: Sharma J, Deb B, George IA, Kapil S, Coral K, Kakkar N, Pattanaik S, Mandal AK, Mavuduru RS and Kumar P (2019) Somatic Mutations Profile of a Young Patient With Metastatic Urothelial Carcinoma Reveals Mutations in Genes Involved in Ion Channels. Front. Oncol. 9:435. doi: 10.3389/fonc.2019.00435
Received: 11 March 2019; Accepted: 07 May 2019;
Published: 29 May 2019.
Edited by:
Ja Hyeon Ku, Seoul National University, South KoreaReviewed by:
Sazzad Hassan, Indiana University Bloomington, United StatesCopyright © 2019 Sharma, Deb, George, Kapil, Coral, Kakkar, Pattanaik, Mandal, Mavuduru and Kumar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ravimohan S. Mavuduru, cmF2aXNtaTIwMDNAeWFob28uY29t; Prashant Kumar, cHJhc2hhbnRAaWJpb2luZm9ybWF0aWNzLm9yZw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.