- 1Division of Dermatology, University of Ottawa, Ottawa, ON, Canada
- 2Division of Dermatology, McGill University, Montréal, QC, Canada
The applications of disease cluster investigations in medicine have developed rather rapidly in recent decades. Analyzing the epidemiology of non-random aggregation of patients with a particular disease fostered identification of environmental and external exposures as disease triggers and promoters. Observation of patient clusters and their association with nearby exposures, such as Dr. John Snow's astute mapping analysis in the mid-1800's, which revealed proximity of cholera patients in London to a contaminated water pump infected with Vibrio cholerae, have paved the way for the field of epidemiology. This approach enabled the identification of triggers for many human diseases including infections and cancers. Cutaneous T-cell lymphomas (CTCL) represent a group of non-Hodgkin lymphomas that primarily affect the skin. The detailed pathogenesis by which CTCL develops remains largely unknown. Notably, non-random clustering of CTCL patients was reported in several areas worldwide and this rare malignancy was also described to affect multiple members of the same family. These observations indicate that external factors are possibly implicated in promoting CTCL lymphomagenesis. Here, we review the epidemiology of CTCL worldwide and the clinical characteristics of CTCL patients, as revealed by global epidemiological data. Further, we review the known risk factors including sex, age, race as well as environmental, infectious, iatrogenic and other exposures, that are implicated in CTCL lymphomagenesis and discuss conceivable mechanisms by which these factors may trigger this malignancy.
Cutaneous T-cell Lymphoma
Cutaneous T-cell lymphomas (CTCL) are a class of non-Hodgkin lymphomas. CTCL represents a heterogeneous group of lymphoproliferative disorders that are characterized by the infiltration of activated bystander and malignant CLA+ CCR4+ T cells into the skin (1, 2). Patients with CTCL, depending on the disease subtype, can present with a spectrum of clinical morphologies including erythematous, hyper- or hypo-pigmented patches with or without atrophy, or present with thickened plaques, that may mimic benign, inflammatory, or autoimmune disorders such as eczema, psoriasis, morphea, pityriasis lichenoides chronica, pityriasis rubra pilaris, drug eruptions, poikiloderma, panniculitis, vitiligo, and pigmented purpuric dermatoses. Thus, CTCL is considered a “great mimicker” in dermatology. In fact, CTCL is often challenging to diagnose, especially in early and erythrodermic stages, and on average it takes ~6 years to establish a definitive diagnosis since its initial presentation (3). As the malignancy progresses in a subset of patients, the disease can spread to lymph nodes and other organs. While mycosis fungoides (MF) and Sézary syndrome (SS) are the most commonly recognized subtypes, other variants of CTCL were documented by the 2016 World Health Organization classification of primary cutaneous lymphomas (4) and include angioimmunoblastic T-cell lymphoma, subcutaneous panniculitis-like T-cell lymphoma, adult T-cell leukemia/lymphoma (ATLL), mature T-cell lymphoma not otherwise specified (NOS), cluster of differentiation 30 positive (CD30+) T-cell lymphoproliferative disorders of the skin and extranodal natural killer (NK)/T-cell lymphoma, nasal type (ENKL). Despite the aforementioned variability in clinical presentation, the majority of MF lesions develop on the trunk and lower extremities often asymmetrically and follow a “1930's bathing suit” distribution on skin not commonly exposed to the sun.
Demographics and Clinical Characteristics of CTCL Patients
While CTCL has been shown to affect individuals of almost all ethnicities and age groups in both sexes, extensive epidemiological studies have consistently reported that CTCL preferentially affects patients of “typical” profiles and in classic body sites. Indeed, the mean age at the time of diagnosis is in the mid- to late-fifties. The mean age of diagnosis was reported in Canada as 59.4 years (5) and the median age of diagnosis in the United States, United Kingdom and Switzerland is in the range of 54–57.5 years, with marked differences between different ethnicities (6–9). In addition, more males are diagnosed with CTCL than females. In fact, the reported incidence-rate-ratio (IRR) of males-to-female patients in the United States during 2001–2005 and 2005–2009 years were 1.7:1 and 1.6:1, respectively (10, 11). The IRR of males-to-females in Canada during 1992–2010 was 1.4:1 (5). There are also important differences in CTCL incidence and prognosis between different ethnic backgrounds. The average age of CTCL diagnosis in African-Americans is significantly lower compared to Caucasians (6–9, 12). In one study that examined the clinical characteristics of 4,496 patients diagnosed with cutaneous lymphoma between 2004 and 2008 in the United States, the mean age at the time of diagnosis for Caucasian, African-American Asian/Pacific Islander, and Native American/other/unknown patients were 59.2, 51.5, 51.3, and 53.8 years, respectively (9). In fact, CTCL was reported to occur at a significantly higher rate in individuals of African-American descent, when compared to individuals of European or Asian origin. In a population-based study in the United States, the age-adjusted incidence rates of CTCL in 8 states from 2001 to 2005 were the highest among African-Americans (10.0/1,000,000 person-years), followed by non-Hispanic whites (8.1), Hispanic whites (5.1), and Asian/Pacific Islander (5.1) (10). Further, in these individuals, the disease was often associated with worse prognosis (9, 12).
In terms of subtype prevalence, MF, SS, CD30+ lymphoproliferative disorders and primary cutaneous peripheral T cell lymphomas not otherwise specified (PCTCL-NOS) are the most common and well-recognized variants of CTCL. Indeed, MF is the predominant subtype of CTCL and is corroborated by several studies showing that it accounts for 44–60% of all CTCL cases (1, 8).
Overall Incidence Patterns for CTCL
The incidence of CTCL worldwide has demonstrated a 2 to 3-fold increase during the last 25–30 years (9, 13), and ranged between ~5 and 11 cases per million individuals per year depending on the CTCL subtypes and the examined year range. In the United States, incidence rates of CTCL were on the rise from 1973 until 1998 and stabilized at 10.2 cases per million individuals per year (11). In Canada, we recently reported that the incidence rate of CTCL increased steadily during the 1990s and then stabilized at ~11 cases per million individuals per year, demonstrating geographical continuity between the two North American countries (5). The incidence rates of CTCL in other parts of the world including Norway, Wales, France, Kuwait, and Japan are summarized in Table 1.
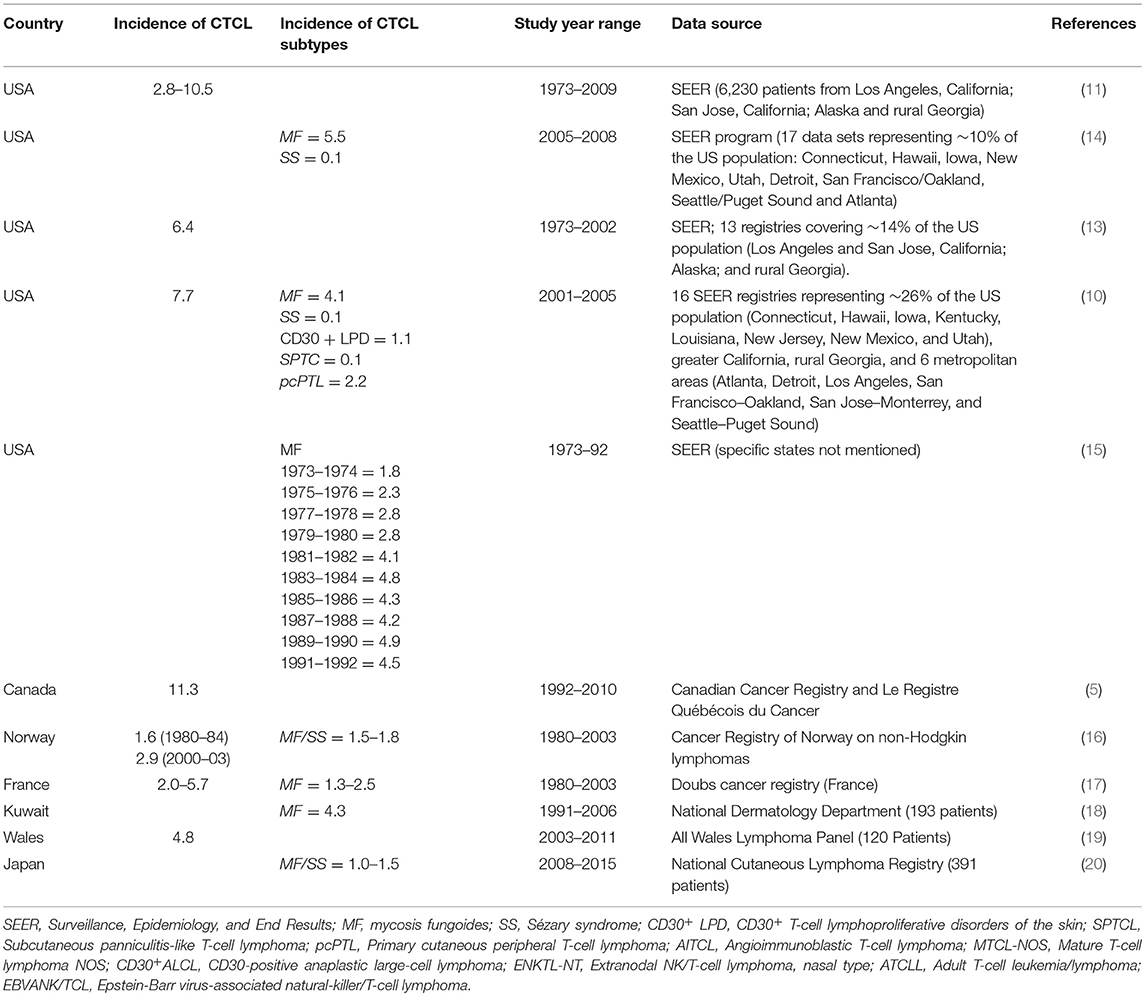
Table 1. Reported CTCL incidence rates worldwide (rates are denoted as cases per 1 million individuals per year).
Clustering of CTCL Patients
Despite the rarity of this malignancy, clustering of CTCL patients was reported in several regions around the world including the Västernorrland county of Sweden (21) as well as in Pittsburgh, Pennsylvania (22) and in Texas (23) in the United States. Based on the analysis of 1990 patients using two cancer registries (MD Anderson Cancer Center and Texas Cancer Registry), we previously reported geographic clustering of patients in several communities in the state of Texas including in cities of Katy, Beaumont, and Spring, where CTCL incidence rates were 3–20 times higher than the US national average (23, 24). Notably clustering of patients was observed in our recent study in Canada in the areas of heavy industrial presence (e.g., in Winnipeg, MB, Hamilton and Oakville, ON, etc.), while cities with limited industrial presence including Ottawa, Gatineau and Quebec City were relatively spared by CTCL (5). Similarly, Moreau et al. demonstrated CTCL case clustering in certain location of the Pittsburgh metropolitan area (22). Further, familial aggregation of this rare malignancy were reported among Jewish nationals in Israel (25). The non-random distribution of CTCL patients argues that external environmental, occupational, behavioral, and communicable triggers may indeed exist for this malignancy.
Environmental and External Risk Factors of CTCL
Analysis of the epidemiology and spatial distribution of diseases enabled the identification of occupational, communicable and environmental exposures as initiators/promoters of many malignancies, such as arsenic triggering cutaneous squamous cell carcinomas, benzene exposure contributing to the carcinogenesis of Acute Myelogenous Leukemia, and asbestos accounting for the majority of mesothelioma cases, reviewed in Ghazawi et al. (26, 27).
A number of external triggers/disease promoting factors such as hydrochlorothiazide diuretics, immunosuppression, as well as several putative bacterial and viral agents have been proposed for CTCL (28) and are summarized in Supplementary Table 1. Early evidence shows that air pollution and chemical exposure, including pesticides (e.g., Glyphosate in Roundup®, Monsanto Inc.) and detergents may increase one's risk of developing MF, SS, and other Non-Hodgkin Lymphomas (29, 30). Indeed, as mentioned above, our previous analysis on the distribution of CTCL patient clusters in Texas revealed that many of the patients were residing along the same streets/highways and/or rivers/streams. Similarly, analysis of CTCL distribution by postal codes in Canada identified several regions with elevated CTCL incidence rates that were located in the proximity to heavy industrial factories and major transportation hubs (5, 31). The same trend was observed by Moreau et al. in Pittsburgh, PA area (22). These combined results argue that common exposures may be promoting this cancer in certain communities. Furthermore, our study in Texas demonstrated that two densely populated adjacent zip codes located in a sunny desert climate near El Paso, Texas were completely spared by CTCL (23, 24).
In other population-based studies in the United States, low household income, types of owner-occupied housing units and foreign birth (although, the countries of birth were not detailed in the cited study) were correlated with increased incidence of CTCL (11). Incidence of CTCL has also been correlated with high physician density (correlation coefficient (r) = 0.6; p = 0.04) and high family income (r = 0.7; p = 0.01) (13). In addition, body mass index, tobacco use, personal history of eczema, family history of multiple myeloma, crop, and vegetable farming activities, painting, woodworking and carpentering occupations have all been linked to an increased risk of MF and SS. Alcohol use and sun exposure were also reported as exacerbating and protective lifestyle risk factors for MF, respectively (32). Regarding sun exposure being a protective factor, one plausible hypothesis is centered on low vitamin D levels in CTCL patients. A study by Talpur et al. reported that low vitamin D levels were present in 76.9% of the MF/SS patients, comparable to the levels in other cancer patients (75.2%) (33).
As mentioned earlier, iatrogenic immunosuppression with conventional systemic or newer biologic (i.e., anti TNF-α) therapies increases ones likelihood of developing MF/SS and other lymphomas (28). The use of hydrochlorothiazide was also evaluated in MF and SS patients with hypertension and was found to be a possible trigger of disease in a subset of patients with early MF (34). Although not proving causality, hydrochlorothiazide use has been linked to increased severity in MF and SS cases. The discontinuation of hydrochlorothiazide in these patients has led to the clearing or amelioration of their MF; when re-challenged with this medication, a subset of these patients had a reoccurrence of their MF lesions (34). Other medications that were proposed as possible triggers for MF include antihistamines, antiepileptics, antihypertensives, and serotonin reuptake inhibitors (28).
Familial clustering studies showed an increased incidence of CTCL by analyzing the allele frequency of HLA DQB1*03 in first-degree relatives (25). Furthermore, a few cases have reported that organ transplant recipients (albeit these patients are on immunosuppressive drugs) (35) and patients with HIV-related immunodeficiency had an increased risk of developing CTCL (36).
Based on current literature, infections may play more than one role in natural CTCL disease course. Specifically, some infections were proposed to trigger/promote the disease. At the same time, as the malignancy progresses to more advanced stages the host becomes susceptible to an increasing number of infections that ultimately lead to a demise of a patient. Several studies reported a significant incidence of skin infections in CTCL patients with an association between the pathogenic burden and disease severity (37, 38). Staphylococcus aureus, Mycobacterium leprae, Chlamydophila pneumoniae, and dermatophytes are among some of the infectious agents implicated as triggers/promoters of CTCL. Moreover, certain viruses investigated for their role in triggering CTCL include Human T-cell leukemia/lymphotropic virus type 1 (HTLV-1), which was consistently associated with Adult T-Cell Lymphoma (39), but not MF/SS (40–46). Further, HTLV-2 (41, 47), HIV (36, 48, 49), Epstein-Barr virus (50–61), Cytomegalovirus (62, 63), Human Herpesvirus (HHV)-6, HHV-7 (57, 64–66), HHV-8 (67) and even Polyomaviruses including Merkel cell polyomavirus (MCV) (68–70) were also proposed to play an important role in disease pathogenesis. However, some of these studies have yielded conflicting results, as highlighted by Mirvish et al. (71), and ultimately failed to report a clear explanation for CTCL pathogenesis (71).
How Could External Factors Promote or Trigger CTCL?
While the precise triggers are not yet identified/confirmed, and the mechanism of lymphomagenesis remains enigmatic, several studies have investigated a number of different hypotheses (72). Chromosomal instability as well as dysregulated expression of many genes such as cancer testis and meiomitosis genes, Suppressor of cytokine signaling 3 (SOCS3), B-Raf proto-oncogene, serine/threonine kinase (BRAF), Interleukin-2 receptor common gamma chain, Thymocyte selection-associated high-mobility group box (TOX), among others [reviewed in (72, 73)] were reported in CTCL patients. Aberrant expression of SOCS3, a regulator of the Jak-3/STAT disrupts the normal expression of several cytokines including IL-5, IL-10, IL-17A, and IL-17F and tumor suppressor microRNAs such as miR-22 further highlighting the important role of the cytokine milieu in disease pathogenesis (74). As disease progresses, an important switch from a Th1 to Th2 profile immune response is observed in patients with subsequent eosinophilia and superinfections with S. aureus (75, 76). On the other hand, a recent study by Fanok et al. demonstrated that T-cell receptor engagement is critical for malignant transformation of T lymphocytes in the setting of presumed bacterial trigger (77). S. aureus, being a common pathogen residing on the skin, is thought to promote malignant inflammation. Lack of Th1 immune response is a driving factor for the growth of S. aureus on the CTCL skin. Following this event, a Th2 immune-mediated response is precipitated by the downregulation of STAT4 and upregulation of STAT5 and STAT3 by oncomiRs (miR-155) making CTCL patients more susceptible to S. aureus colonization and prolonged antigenic stimulation (75, 76, 78, 79).
Further, a recent study described the mechanism by which S. aureus activates oncogenic STAT3 signaling in malignant T cells (80). Staphylococcal enterotoxin A (SEA) was shown to impact malignant T cells in an indirect mechanism by promoting infiltrating bystander non-malignant T cells to produce IL-2 and other regulatory cytokines, thus upregulating JAK3/STAT3 signaling and leading to proliferation of malignant T cells in the skin (28, 80). Finally, Staphylococcal toxins have been implicated in the activation of malignant T cells in SS patients by acting as superantigens and binding to a TCR-v β chain, leading to T cell activation and proliferation (81–83). Collectively, it is likely that patients' genetic profiles and skewed cytokine milieu in response to select infectious agents may predispose individuals to develop MF/SS.
Also, central to CTCL pathogenesis remains chronic and persistent antigen stimulation of skin-homing CD4+ memory T-cells by external factors. The resultant chronic inflammation drives the immune system toward the proliferation and expansion of specific T cell malignant clone(s) (84). This was reinforced by a collection of different observations. For instance, it was reported that one patient, who had implanted gravel in the hip from a traumatic injury developed a chronic plaque, which histologically appeared as parapsoriasis and progressed in 15 years to MF (85). The fact that, after about 10 years on average, some patients developed MF at the sites previously inoculated with foreign substances also supports the notion that CTCL may develop after chronic antigen stimulation (86). Consistent with these observations, it is well-established that implant associated-Anaplastic Lage Cell Lymphoma (ALCL), an indolent disease similar to primary cutaneous ALCL, can arise as a result of breast implants, tibial implants, dental implants, chest injection ports, gluteal implants, shoulder repairs, and a gastric band placement (87). It is now standard procedure to include a discussion of ALCL risk before any breast implant procedures and inform patients of a possible onset of ALCL within a median time frame of 8 to 10 years, albeit this disease affects 1 in 2,000 to 1 in 70,000 implant recipients (87). Notably, textured prostheses have higher risk of ALCL then smooth implants (87).
The reviewed implicated factors in Supplementary Table 1 clearly indicate that more than one antigen may stimulate CTCL. Additional evidence of this comes from studies that established a widespread repertoire of the stimulated CTCL clones (or single-cell heterogeneity in Sézary Syndrome) between different patients (88). Therefore, particular antigen engagement, coupled with the combination of aberrant cytokine milieu and chronicity of antigenic-stimulation, may collectively play critical roles in the development of cutaneous lymphoma.
Conclusions
Epidemiological studies have repeatedly helped identify definitive triggers for several diseases. As highlighted in this perspective report, previous studies strongly argue for the interplay between intrinsic factors and putative preventable extrinsic triggers/promoters for CTCL. Given the evidence of geographical regional clustering of CTCL patients, CTCL occurrence in unrelated family members and recent evidence implicating S. aureus in the pathogenesis/progression of CTCL, more research is needed to decipher the precise mechanism by which specific environmental exposures may be driving the pathogenesis of this malignancy. Hopefully, such knowledge of potential triggers and perpetuating factors for this cancer would enable us at some point to significantly decrease CTCL incidence. Therefore, it is important to take into consideration the effects of hydrochlorothiazide diuretics and immunosuppressive medications in patients with definite or suspected diagnosis of CTCL (e.g., recalcitrant eczematous patches/plaques that appear in a bathing-suit distribution) (89). Patients living near heavy industrial factories may consider an air filtration system, if feasible, to decrease their risk of developing a malignancy. Minimizing daily exposure to pesticides/herbicides containing chemicals that are listed as probable or possible (Groups 1-2B) carcinogens on the International Agency for Research on Cancer (IARC) database, such as glyphosate, is also important. Any traumatically implanted in the skin or subcutaneous tissue foreign objects (especially textured objects) should be surgically removed. Furthermore, given our further understanding of S. aureus in the pathogenesis of CTCL progression, clinicians may consider decolonizing patients using various techniques already used for patients with atopic dermatitis such as bleach baths as well as topical and systemic antibiotics. Similarly, patients with detectable dermatophytes may similarly benefit from anti-fungal treatments to avoid developing CTCL.
Author Contributions
FG, NA, ML, EN, SG, DS, and IL have contributed by participating in reviewing the literature on the topic, preparing the tables summarizing existing evidence, writing and critically reviewing the manuscript.
Funding
This work was supported by the Cole Foundation Grant to Dr. Litvinov, Canadian Dermatology Foundation research grants to Dr. Sasseville and Dr. Litvinov and the Fonds de la recherche en santé du Québec (FRSQ# 34753 and 36769) research grants to Dr. Litvinov. No funding bodies had any role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2019.00300/full#supplementary-material
References
1. Willemze R, Jaffe ES, Burg G, Cerroni L, Berti E, Swerdlow SH, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. (2005) 105:3768–85. doi: 10.1182/blood-2004-09-3502
2. Campbell JJ, Clark RA, Watanabe R, Kupper TS. Sezary syndrome and mycosis fungoides arise from distinct T-cell subsets: a biologic rationale for their distinct clinical behaviors. Blood. (2010) 116:767–71. doi: 10.1182/blood-2009-11-251926
3. Kirsch IR, Watanabe R, O'malley JT, Williamson DW, Scott LL, Elco CP, et al. TCR sequencing facilitates diagnosis and identifies mature T cells as the cell of origin in CTCL. Sci Transl Med. (2015) 7:308ra158. doi: 10.1126/scitranslmed.aaa9122
4. Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. (2016) 127:2375–90. doi: 10.1182/blood-2016-01-643569
5. Ghazawi FM, Netchiporouk E, Rahme E, Tsang M, Moreau L, Glassman S, et al. Comprehensive analysis of cutaneous T-cell lymphoma (CTCL) incidence and mortality in Canada reveals changing trends and geographic clustering for this malignancy. Cancer. (2017) 123:3550–67. doi: 10.1002/cncr.30758
6. Kim YH, Liu HL, Mraz-Gernhard S, Varghese A, Hoppe RT. Long-term outcome of 525 patients with mycosis fungoides and Sezary syndrome: clinical prognostic factors and risk for disease progression. Arch Dermatol. (2003) 139:857–66. doi: 10.1001/archderm.139.7.857
7. Agar NS, Wedgeworth E, Crichton S, Mitchell TJ, Cox M, Ferreira S, et al. Survival outcomes and prognostic factors in mycosis fungoides/Sezary syndrome: validation of the revised International Society for Cutaneous Lymphomas/European Organisation for Research and Treatment of Cancer staging proposal. J Clin Oncol. (2010) 28:4730–9. doi: 10.1200/JCO.2009.27.7665
8. Jenni D, Karpova MB, Seifert B, Golling P, Cozzio A, Kempf W, et al. Primary cutaneous lymphoma: two-decade comparison in a population of 263 cases from a Swiss tertiary referral centre. Br J Dermatol. (2011) 164:1071–7. doi: 10.1111/j.1365-2133.2010.10143.x
9. Wilson LD, Hinds GA, Yu JB. Age, race, sex, stage, and incidence of cutaneous lymphoma. Clin Lymphoma Myeloma Leuk. (2012) 12:291–6. doi: 10.1016/j.clml.2012.06.010
10. Bradford PT, Devesa SS, Anderson WF, Toro JR. Cutaneous lymphoma incidence patterns in the United States: a population-based study of 3884 cases. Blood. (2009) 113:5064–73. doi: 10.1182/blood-2008-10-184168
11. Korgavkar K, Xiong M, Weinstock M. Changing incidence trends of cutaneous T-cell lymphoma. JAMA Dermatol. (2013) 149:1295–9. doi: 10.1001/jamadermatol.2013.5526
12. Sun G, Berthelot C, Li Y, Glass DA 2nd, George D, Pandya A, Kurzrock R, et al. Poor prognosis in non-Caucasian patients with early-onset mycosis fungoides. J Am Acad Dermatol. (2009) 60:231–5. doi: 10.1016/j.jaad.2008.09.063
13. Criscione VD, Weinstock MA. Incidence of cutaneous T-cell lymphoma in the United States, 1973-2002. Arch Dermatol. (2007) 143:854–9. doi: 10.1001/archderm.143.7.854
14. Imam MH, Shenoy PJ, Flowers CR, Phillips A, Lechowicz MJ. Incidence and survival patterns of cutaneous T-cell lymphomas in the United States. Leuk Lymphoma. (2013) 54:752–9. doi: 10.3109/10428194.2012.729831
15. Weinstock MA, Gardstein B. Twenty-year trends in the reported incidence of mycosis fungoides and associated mortality. Am J Public Health. (1999) 89:1240–4. doi: 10.2105/AJPH.89.8.1240
16. Saunes M, Nilsen TI, Johannesen TB. Incidence of primary cutaneous T-cell lymphoma in Norway. Br J Dermatol. (2009) 160:376–9. doi: 10.1111/j.1365-2133.2008.08852.x
17. Riou-Gotta MO, Fournier E, Mermet I, Pelletier F, Humbert P, Danzon A, et al. Primary cutaneous lymphomas: a population-based descriptive study of 71 consecutive cases diagnosed between 1980 and 2003. Leuk Lymphoma. (2008) 49:1537–44. doi: 10.1080/10428190802136368
18. Alsaleh QA, Nanda A, Al-Ajmi H, Al-Sabah H, Elkashlan M, Al-Shemmari S, et al. Clinicoepidemiological features of mycosis fungoides in Kuwait, 1991-2006. Int J Dermatol. (2010) 49:1393–8. doi: 10.1111/j.1365-4632.2010.04567.x
19. Abbott RA, Aldridge C, Dojcinov S, Piguet V. Incidence of primary cutaneous T-cell lymphoma in wales. Br J Dermatol. (2013) 169:1366–7. doi: 10.1111/bjd.12516
20. Hamada T, Nomura H, Iwatsuki K. Regional incidences of adult T-cell leukemia/lymphoma with cutaneous involvement in Japan. J Dermatol. (2018) 45:58–63. doi: 10.1111/1346-8138.14100
21. Gip L, Nilsson E. Clustering of mycosis fungoides in the County of Vasternorrland. Lakartidningen. (1977) 74:1174–6.
22. Moreau JF, Buchanich JM, Geskin JZ, Akilov OE, Geskin LJ. Non-random geographic distribution of patients with cutaneous T-cell lymphoma in the greater pittsburgh area. Dermatol. Online J. (2014) 20. Retrieved from: https://escholarship.org/uc/item/4nw7592w
23. Litvinov IV, Tetzlaff MT, Rahme E, Habel Y, Risser DR, Gangar P, et al. Identification of geographic clustering and regions spared by cutaneous T-cell lymphoma in Texas using 2 distinct cancer registries. Cancer. (2015) 121:1993–2003. doi: 10.1002/cncr.29301
24. Litvinov IV, Tetzlaff MT, Rahme E, Jennings MA, Risser DR, Gangar P, et al. Demographic patterns of cutaneous T-cell lymphoma incidence in Texas based on two different cancer registries. Cancer Med. (2015) 4:1440–7. doi: 10.1002/cam4.472
25. Hodak E, Klein T, Gabay B, Ben-Amitai D, Bergman R, Gdalevich M, et al. Familial mycosis fungoides: report of 6 kindreds and a study of the HLA system. J Am Acad Dermatol. (2005) 52:393–402. doi: 10.1016/j.jaad.2003.12.052
26. Ghazawi FM, Glassman SJ, Sasseville D, Litvinov IV. Using patient registries to identify triggers of rare diseases. In Heston TF, editor. eHealth - Making Health Care Smarter. IntechOpen (2018). Available online at: https://www.intechopen.com/books/ehealth-making-health-care-smarter/using-patient-registries-to-identify-triggers-of-rare-diseases
27. Ghazawi FM, Le M, Cyr J, Netchiporouk E, Rahme E, Alakel A, et al. Analysis of acute myeloid leukemia incidence and geographic distribution in Canada from 1992 to 2010 reveals disease clusters in Sarnia and other industrial US border cities in Ontario. Cancer. (2019). doi: 10.1002/cncr.32034. [Epub ahead of print].
28. Litvinov IV, Shtreis A, Kobayashi K, Glassman S, Tsang M, Woetmann A, et al. Investigating potential exogenous tumor initiating and promoting factors for cutaneous T-cell lymphomas (CTCL), a rare skin malignancy. Oncoimmunology. (2016) 5:e1175799. doi: 10.1080/2162402X.2016.1175799
29. Fischmann AB, Bunn PA Jr, Guccion JG, Matthews MJ, Minna JD. Exposure to chemicals, physical agents, and biologic agents in mycosis fungoides and the Sezary syndrome. Cancer Treat Rep. (1979) 63:591–6.
30. Chang ET, Delzell E. Systematic review and meta-analysis of glyphosate exposure and risk of lymphohematopoietic cancers. J Environ Sci Health B. (2016) 51:402–34. doi: 10.1080/03601234.2016.1142748
31. Ghazawi FM, Netchiporouk E, Rahme E, Tsang M, Moreau L, Glassman S, et al. Distribution and clustering of cutaneous T-cell lymphoma (CTCL) cases in Canada during 1992 to 2010. J Cutan Med Surg. (2018) 22:154–65. doi: 10.1177/1203475417745825
32. Morales Suarez-Varela MM, Olsen J, Kaerlev L, Guenel P, Arveux P, Wingren G, et al. Are alcohol intake and smoking associated with mycosis fungoides? A European multicentre case-control study. Eur J Cancer. (2001) 37:392–7. doi: 10.1016/S0959-8049(00)00383-X
33. Talpur R, Cox KM, Hu M, Geddes ER, Parker MK, Yang BY, et al. Vitamin D deficiency in mycosis fungoides and Sezary syndrome patients is similar to other cancer patients. Clin Lymphoma Myeloma Leuk. (2014) 14:518–24. doi: 10.1016/j.clml.2014.06.023
34. Jahan-Tigh RR, Huen AO, Lee GL, Pozadzides JV, Liu P, Duvic M. Hydrochlorothiazide and cutaneous T cell lymphoma: prospective analysis and case series. Cancer. (2013) 119:825–31. doi: 10.1002/cncr.27740
35. Pomerantz RG, Campbell LS, Jukic DM, Geskin LJ. Posttransplant cutaneous T-cell lymphoma: case reports and review of the association of calcineurin inhibitor use with posttransplant lymphoproliferative disease risk. Arch Dermatol. (2010) 146:513–6. doi: 10.1001/archdermatol.2010.60
36. Biggar RJ, Engels EA, Frisch M, Goedert JJ. Risk of T-cell lymphomas in persons with AIDS. J Acquir Immune Defic Syndr. (2001) 26:371–6. doi: 10.1097/00126334-200104010-00015
37. Axelrod PI, Lorber B, Vonderheid EC. Infections complicating mycosis fungoides and Sezary syndrome. JAMA. (1992) 267:1354–8. doi: 10.1001/jama.1992.03480100060031
38. Bonin S, Tothova SM, Barbazza R, Brunetti D, Stanta G, Trevisan G. Evidence of multiple infectious agents in mycosis fungoides lesions. Exp Mol Pathol. (2010) 89:46–50. doi: 10.1016/j.yexmp.2010.05.001
39. Amar L, Le M, Ghazawi FM, Rahme E, Segal A, Netchiporouk E, et al. Human T-Cell Lymphotropic Virus-1 (HTLV-1) infection prevalence in Canada. Curr Oncol. (2019) 26:1–3. doi: 10.3747/co.26.4593
40. Hall WW, Liu CR, Schneewind O, Takahashi H, Kaplan MH, Roupe G, et al. Deleted HTLV-I provirus in blood and cutaneous lesions of patients with mycosis fungoides. Science. (1991) 253:317–20. doi: 10.1126/science.1857968
41. Zucker-Franklin D, Hooper WC, Evatt BL. Human lymphotropic retroviruses associated with mycosis fungoides: evidence that human T-cell lymphotropic virus type II (HTLV-II) as well as HTLV-I may play a role in the disease. Blood. (1992) 80:1537–45.
42. Pancake BA, Zucker-Franklin D, Coutavas EE. The cutaneous T cell lymphoma, mycosis fungoides, is a human T cell lymphotropic virus-associated disease. A study of 50 patients. J Clin Invest. (1995) 95:547–54. doi: 10.1172/JCI117697
43. Khan ZM, Sebenik M, Zucker-Franklin D. Localization of human T-cell lymphotropic virus-1 tax proviral sequences in skin biopsies of patients with mycosis fungoides by in situ polymerase chain reaction. J Invest Dermatol. (1996) 106:667–72. doi: 10.1111/1523-1747.ep12345488
44. Pancake BA, Wassef EH, Zucker-Franklin D. Demonstration of antibodies to human T-cell lymphotropic virus-I tax in patients with the cutaneous T-cell lymphoma, mycosis fungoides, who are seronegative for antibodies to the structural proteins of the virus. Blood. (1996) 88:3004–9.
45. Shohat M, Hodak E, Hannig H, Bodemer W, David M, Shohat B. Evidence for the cofactor role of human T-cell lymphotropic virus type 1 in mycosis fungoides and Sezary syndrome. Br J Dermatol. (1999) 141:44–9. doi: 10.1046/j.1365-2133.1999.02919.x
46. Netchiporouk E, Gantchev J, Tsang M, Thibault P, Watters AK, Hughes JM, et al. Analysis of CTCL cell lines reveals important differences between mycosis fungoides/Sezary syndrome vs. HTLV-1(+) leukemic cell lines. Oncotarget. (2017) 8:95981–98. doi: 10.18632/oncotarget.21619
47. Poiesz B, Dube D, Dube S, Love J, Papsidero L, Uner A, et al. HTLV-II-associated cutaneous T-cell lymphoma in a patient with HIV-1 infection. N Engl J Med. (2000) 342:930–6. doi: 10.1056/NEJM200003303421304
48. Bachelez H, Hadida F, Gorochov G. Massive infiltration of the skin by HIV-specific cytotoxic CD8+ T cells. N Engl J Med. (1996) 335:61–2. doi: 10.1056/NEJM199607043350118
49. Wilkins K, Turner R, Dolev JC, Leboit PE, Berger TG, Maurer TA. Cutaneous malignancy and human immunodeficiency virus disease. J Am Acad Dermatol. (2006) 54:189–206; quiz 207-10. doi: 10.1016/j.jaad.2004.11.060
50. Lee PY, Charley M, Tharp M, Jegasothy BV, Deng JS. Possible role of Epstein-Barr virus infection in cutaneous T-cell lymphomas. J Invest Dermatol. (1990) 95:309–12. doi: 10.1111/1523-1747.ep12485017
51. Borisch B, Boni J, Burki K, Laissue JA. Recurrent cutaneous anaplastic large cell (CD30+) lymphoma associated with Epstein-Barr virus. A case report with 9-year follow-up. Am J Surg Pathol. (1992) 16:796–801. doi: 10.1097/00000478-199208000-00009
52. Dreno B, Celerier P, Fleischmann M, Bureau B, Litoux P. Presence of Epstein-Barr virus in cutaneous lesions of mycosis fungoides and Sezary syndrome. Acta Derm Venereol. (1994) 74:355–7.
53. Cho KH, Kim CW, Lee DY, Sohn SJ, Kim DW, Chung JH. An Epstein-Barr virus-associated lymphoproliferative lesion of the skin presenting as recurrent necrotic papulovesicles of the face. Br J Dermatol. (1996) 134:791–6. doi: 10.1111/j.1365-2133.1996.tb06994.x
54. Mouly F, Baccard M, Rybojad M, Lebbe C, Morinet F, Morel P. Aggressive cutaneous T-cell lymphoma associated with the presence of Epstein-Barr virus. 2 cases. Ann Dermatol Venereol. (1996) 123:574–6.
55. Jumbou O, Mollat C, N'guyen JM, Billaudel S, Litoux P, Dreno B. Increased anti-Epstein-Barr virus antibodies in epidermotropic cutaneous T-cell lymphoma: a study of 64 patients. Br J Dermatol. (1997) 136:212–6. doi: 10.1111/j.1365-2133.1997.tb14898.x
56. Jumbou O, Huet S, Bureau B, Litoux P, Dreno B. Epstein-Barr virus research by in situ hybridization in 65 cutaneous T cell epidermotropic lymphomas. Ann Dermatol Venereol. (1998) 125:90–3.
57. Nagore E, Ledesma E, Collado C, Oliver V, Perez-Perez A, Aliaga A. Detection of Epstein-Barr virus and human herpesvirus 7 and 8 genomes in primary cutaneous T- and B-cell lymphomas. Br J Dermatol. (2000) 143:320–3. doi: 10.1046/j.1365-2133.2000.03657.x
58. Tournadre A, D'incan M, Dubost JJ, Franck F, Dechelotte P, Souteyrand P, et al. Cutaneous lymphoma associated with Epstein-Barr virus infection in 2 patients treated with methotrexate. Mayo Clin Proc. (2001) 76:845–8. doi: 10.1016/S0025-6196(11)63231-X
59. Noorali S, Yaqoob N, Nasir MI, Moatter T, Pervez S. Prevalence of mycosis fungoides and its association with EBV and HTLV-1 in Pakistanian patients. Pathol Oncol Res. (2002) 8:194–9. doi: 10.1007/BF03032394
60. Novelli M, Merlino C, Ponti R, Bergallo M, Quaglino P, Cambieri I, et al. Epstein-Barr virus in cutaneous T-cell lymphomas: evaluation of the viral presence and significance in skin and peripheral blood. J Invest Dermatol. (2009) 129:1556–61. doi: 10.1038/jid.2008.396
61. Park S, Lee DY, Kim WS, Ko YH. Primary cutaneous Epstein-Barr virus-associated T-cell lymphoproliferative disorder-2 cases with unusual, prolonged clinical course. Am J Dermatopathol. (2010) 32:832–6. doi: 10.1097/DAD.0b013e3181d68381
62. Herne KL, Talpur R, Breuer-Mcham J, Champlin R, Duvic M. Cytomegalovirus seropositivity is significantly associated with mycosis fungoides and Sezary syndrome. Blood. (2003) 101:2132–6. doi: 10.1182/blood-2002-07-2247
63. Ballanger F, Bressollette C, Volteau C, Planche L, Dreno B. Cytomegalovirus: its potential role in the development of cutaneous T-cell lymphoma. Exp Dermatol. (2009) 18:574–6. doi: 10.1111/j.1600-0625.2008.00817.x
64. Brice SL, Jester JD, Friednash M, Golitz LE, Leahy MA, Stockert SS, et al. Examination of cutaneous T-cell lymphoma for human herpesviruses by using the polymerase chain reaction. J Cutan Pathol. (1993) 20:304–7. doi: 10.1111/j.1600-0560.1993.tb01266.x
65. Erkek E, Senturk N, Dincer I, Olut AI, Kocagoz T, Bukulmez G, et al. Identification of herpes simplex virus DNA and lack of human herpesvirus-8 DNA in mycosis fungoides. Acta Derm Venereol. (2002) 82:214–6. doi: 10.1080/00015550260132569
66. Ponti R, Bergallo M, Costa C, Quaglino P, Fierro MT, Comessatti A, et al. Human herpesvirus 7 detection by quantitative real time polymerase chain reaction in primary cutaneous T-cell lymphomas and healthy subjects: lack of a pathogenic role. Br J Dermatol. (2008) 159:1131–7. doi: 10.1111/j.1365-2133.2008.08811.x
67. Kreuter A, Bischoff S, Skrygan M, Wieland U, Brockmeyer NH, Stucker M, et al. High association of human herpesvirus 8 in large-plaque parapsoriasis and mycosis fungoides. Arch Dermatol. (2008) 144:1011–6. doi: 10.1001/archderm.144.8.1011
68. Shuda M, Arora R, Kwun HJ, Feng H, Sarid R, Fernandez-Figueras MT, et al. Human Merkel cell polyomavirus infection I. MCV T antigen expression in Merkel cell carcinoma, lymphoid tissues and lymphoid tumors. Int J Cancer. (2009) 125:1243–9. doi: 10.1002/ijc.24510
69. Andres C, Puchta U, Sander CA, Ruzicka T, Flaig MJ. Prevalence of Merkel cell polyomavirus DNA in cutaneous lymphomas, pseudolymphomas, and inflammatory skin diseases. Am J Dermatopathol. (2010) 32:593–8. doi: 10.1097/DAD.0b013e3181ce8beb
70. Kreuter A, Silling S, Dewan M, Stucker M, Wieland U. Evaluation of 4 recently discovered human polyomaviruses in primary cutaneous B-cell and T-cell lymphoma. Arch Dermatol. (2011) 147:1449–51. doi: 10.1001/archdermatol.2011.330
71. Mirvish JJ, Pomerantz RG, Falo LD Jr, Geskin LJ. Role of infectious agents in cutaneous T-cell lymphoma: facts and controversies. Clin Dermatol. (2013) 31:423–31. doi: 10.1016/j.clindermatol.2013.01.009
72. Bagherani N, Smoller BR. (2016). An overview of cutaneous T cell lymphomas. F1000Res. 5:F1000 Faculty Rev-1882. doi: 10.12688/f1000research.8829.1
73. Tsang M, Gantchev J, Netchiporouk E, Moreau L, Ghazawi FM, Glassman S, et al. A study of meiomitosis and novel pathways of genomic instability in cutaneous T-cell lymphomas (CTCL). Oncotarget. (2018) 9:37647–61. doi: 10.18632/oncotarget.26479
74. Sibbesen NA, Kopp KL, Litvinov IV, Jonson L, Willerslev-Olsen A, Fredholm S, et al. Jak3, STAT3, and STAT5 inhibit expression of miR-22, a novel tumor suppressor microRNA, in cutaneous T-Cell lymphoma. Oncotarget. (2015) 6:20555–69. doi: 10.18632/oncotarget.4111
75. Litvinov IV, Cordeiro B, Fredholm S, Odum N, Zargham H, Huang Y, et al. Analysis of STAT4 expression in cutaneous T-cell lymphoma (CTCL) patients and patient-derived cell lines. Cell Cycle. (2014) 13:2975–82. doi: 10.4161/15384101.2014.947759
76. Netchiporouk E, Litvinov IV, Moreau L, Gilbert M, Sasseville D, Duvic M. Deregulation in STAT signaling is important for cutaneous T-cell lymphoma (CTCL) pathogenesis and cancer progression. Cell Cycle. (2014) 13:3331–5. doi: 10.4161/15384101.2014.965061
77. Fanok MH, Sun A, Fogli LK, Narendran V, Eckstein M, Kannan K, et al. Role of dysregulated cytokine signaling and bacterial triggers in the pathogenesis of cutaneous T-cell lymphoma. J Invest Dermatol. (2018) 138:1116–25. doi: 10.1016/j.jid.2017.10.028
78. Kopp KL, Ralfkiaer U, Gjerdrum LM, Helvad R, Pedersen IH, Litman T, et al. STAT5-mediated expression of oncogenic miR-155 in cutaneous T-cell lymphoma. Cell Cycle. (2013) 12:1939–47. doi: 10.4161/cc.24987
79. Litvinov IV, Pehr K, Sasseville D. Connecting the dots in cutaneous T cell lymphoma (CTCL): STAT5 regulates malignant T cell proliferation via miR-155. Cell Cycle. (2013) 12:2172–3. doi: 10.4161/cc.25550
80. Willerslev-Olsen A, Krejsgaard T, Lindahl LM, Litvinov IV, Fredholm S, Petersen DL, et al. Staphylococcal enterotoxin A (SEA) stimulates STAT3 activation and IL-17 expression in cutaneous T-cell lymphoma. Blood. (2016) 127:1287–96. doi: 10.1182/blood-2015-08-662353
81. Detmar M, Pauli G, Anagnostopoulos I, Wunderlich U, Herbst H, Garbe C, et al. A case of classical mycosis fungoides associated with human T-cell lymphotropic virus type I. Br J Dermatol. (1991) 124:198–202. doi: 10.1111/j.1365-2133.1991.tb00434.x
82. Tokura Y, Yagi H, Ohshima A, Kurokawa S, Wakita H, Yokote R, et al. Cutaneous colonization with staphylococci influences the disease activity of Sezary syndrome: a potential role for bacterial superantigens. Br J Dermatol. (1995) 133:6–12. doi: 10.1111/j.1365-2133.1995.tb02485.x
83. Jackow CM, Cather JC, Hearne V, Asano AT, Musser JM, Duvic M. Association of erythrodermic cutaneous T-cell lymphoma, superantigen-positive Staphylococcus aureus, and oligoclonal T-cell receptor V beta gene expansion. Blood. (1997) 89:32–40.
84. Tan RS, Butterworth CM, Mclaughlin H, Malka S, Samman PD. Mycosis fungoides–a disease of antigen persistence. Br J Dermatol. (1974) 91:607–16. doi: 10.1111/j.1365-2133.1974.tb12449.x
85. Duvic M. Current treatment of cutaneous T-cell lymphoma. Dermatol. Online J. (2001) 7. Retrieved from: https://escholarship.org/uc/item/3bs957xs
86. Paul LJ, Duvic M. Mycosis fungoides following skin trauma. J Am Acad Dermatol. (2012) 67:e148. doi: 10.1016/j.jaad.2011.11.953
87. Dixon JM, Clemens M. Breast implants and anaplastic large cell lymphoma. BMJ. (2018) 363:k5054. doi: 10.1136/bmj.k5054
88. Buus TB, Willerslev-Olsen A, Fredholm S, Blumel E, Nastasi C, Gluud M, et al. Single-cell heterogeneity in Sezary syndrome. Blood Adv. (2018) 2:2115–26. doi: 10.1182/bloodadvances.2018022608
Keywords: cutaneous T-cell lymphoma, CTCL, epidemiology, incidence, environmental risk factors
Citation: Ghazawi FM, Alghazawi N, Le M, Netchiporouk E, Glassman SJ, Sasseville D and Litvinov IV (2019) Environmental and Other Extrinsic Risk Factors Contributing to the Pathogenesis of Cutaneous T Cell Lymphoma (CTCL). Front. Oncol. 9:300. doi: 10.3389/fonc.2019.00300
Received: 23 January 2019; Accepted: 01 April 2019;
Published: 18 April 2019.
Edited by:
Catherine Grace Chung, The Ohio State University, United StatesReviewed by:
Himanshi Bhatia, Washington University in St. Louis, United StatesVemika Chandra, Children's Hospital of Philadelphia, United States
Copyright © 2019 Ghazawi, Alghazawi, Le, Netchiporouk, Glassman, Sasseville and Litvinov. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ivan V. Litvinov, aXZhbi5saXR2aW5vdkBtY2dpbGwuY2E=
 Feras M. Ghazawi
Feras M. Ghazawi Nebras Alghazawi
Nebras Alghazawi Michelle Le2
Michelle Le2 Elena Netchiporouk
Elena Netchiporouk Ivan V. Litvinov
Ivan V. Litvinov