- 1Department of Otolaryngology, Eye Ear Nose and Throat Hospital, Fudan University, Shanghai, China
- 2Shanghai Key Clinical Disciplines of Otorhinolaryngology, Shanghai, China
- 3Department of Clinical Laboratory, Eye & ENT Hospital, Shanghai Medical College, Fudan University, Shanghai, China
Objective: High levels of red cell distribution width (RDW) may be associated with adverse outcomes in patients with cancer. The purpose of the present study was to investigate the prognostic impact of pretreatment RDW levels on overall survival (OS), cancer-specific survival (CSS), and disease-free survival (DFS) in a large cohort of male laryngeal squamous cell cancer (LSCC) patients.
Methods: A total of 809 LSCC patients who were treated between 2007 and 2011 at the Eye & ENT Hospital of Fudan University were enrolled and evaluated retrospectively. OS, CSS, and DFS were analyzed using the Kaplan–Meier method. To evaluate the prognostic significance of RDW levels, univariate, and multivariate Cox analyses were applied.
Results: Higher pretreatment RDW levels were significantly associated with high death events, red blood cell count, hemoglobin, radiotherapy, operation therapy, and advanced tumor stage (p < 0.05). From the univariate analysis, we observed that the higher (13.2–13.5%) and the highest (>13.5%) quartiles of RDW level were consistent factors for poor OS, CSS, and DFS in LSCC patients. In the multivariate analysis, after adjusting for confounding factors, the higher and highest quartiles of RDW levels were identified as independent prognostic factors in male LSCC patients.
Conclusion: Higher pretreatment RDW levels were demonstrated to be associated with poor clinical outcome in male LSCC patients and might be novel markers for patient stratification in LSCC management.
Introduction
Laryngeal cancer is one of the most common cancers of the head and neck, of which the estimated crude incidence and mortality rates in China were 1.86/100,000 and 1.01/100,000, respectively (1). This disease has a male predominance with a male-to-female ratio of 20 to 30:1 in China (2). The most commonly observed histological type of laryngeal cancer is laryngeal squamous cell cancer (LSCC), accounting for 95% of cases involving the stratified squamous epithelial lining of the larynx (3, 4). Despite improvements in diagnosis and treatment in the last four decades, there was an absence of a significant change in the 5-year overall survival (OS) rate for larynx cancer patients (5). Several prognostic factors have been identified to predict prognosis in LSCC patients, such as tumor size, histological subtype or grade, vascular invasion, and lymph node metastases. However, the majority of these factors can only be assessed after surgery.
Although “omics”-based technology has enhanced our perception of possible risk factors, prognoses and/or responses to treatment biomarkers, the validation of novel molecular biomarkers is associated with high costs, time-consuming procedures, and laboratory efforts. Therefore, a simple, rapid, reliable, and cheap pretreatment prognostic marker for LSCC patients is desired. In recent years, many studies have presented red cell distribution width (RDW) as a strong and independent risk factor for death (6, 7). RDW reflects impaired erythropoiesis and abnormal red blood cell survival, while the heterogeneity of red blood cell size correlates with inflammation and undernutrition status (8–10). Recent studies have also shown that RDW could be a prognostic factor in several carcinomas, including ovarian carcinoma (11), lung carcinoma (12), gastric carcinoma (13), and laryngeal carcinoma (14). In a retrospective, observational cohort study including 654 patients with epithelial ovarian cancer, enhanced RDW was found to be significantly associated with poorer overall survival (OS) (11). Bozkurt et al. (14) reported that laryngeal cancer patients (n = 132) with high RDW at diagnosis were at a higher risk of locoregional recurrence (hazard ratio [HR] = 5.818, 95% confidence interval (95% CI) 1.25–26.97; p = 0.024). Therefore, this study aims to evaluate the prognostic significance of pretreatment RDW levels on disease-free survival (DFS), OS and cancer-specific survival (CSS) in a large cohort of 809 LSCC patients.
Materials and Methods
Study Population
This study was conducted in accordance with the Helsinki Declaration and was approved by the committee of the Eye & ENT Hospital of Fudan University, Shanghai, China. Written informed consent was obtained and approved for all patients. The patient information was anonymized and deidentified prior to analysis. The patients were recruited from the Department of Otolaryngology-Head and Neck Surgery at the Eye & ENT Hospital of Fudan University using a primary cohort of consecutive patients who underwent partial or total laryngectomy between January 1, 2007 and December 31, 2011 as their first curative treatment option. All patients were followed up through telephone messages, and outpatient records were obtained every 3 months during the first 2 years and every 6 months thereafter until death events occurred. Survival status, disease progression, and period of death or metastasis were recorded. The last follow-up date was September 30, 2016. DFS was recorded from the date of laryngectomy to the date of recurrence within the follow-up period. CSS was recorded from the date of surgery until death because of intercurrent disease within the follow-up period. OS was recorded from the date of surgery until death.
Inclusion Criteria
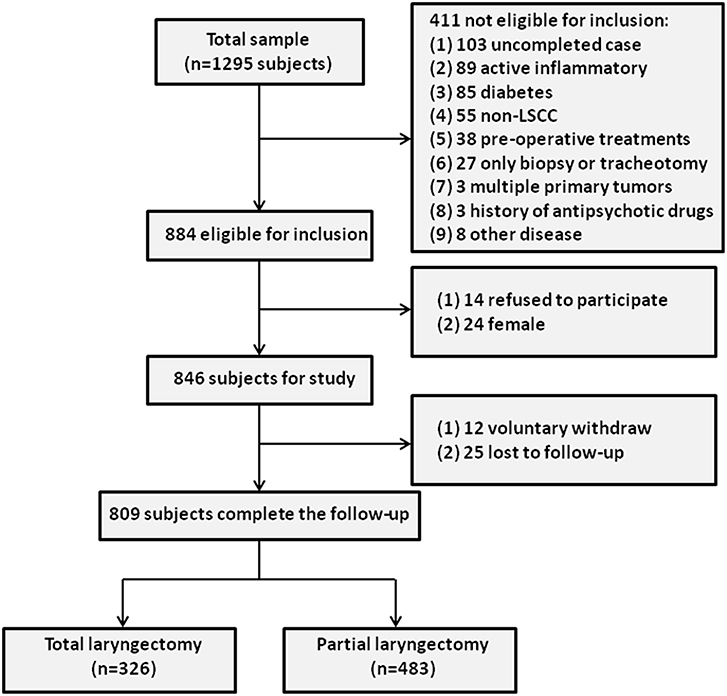
To select the LSCC group, 1295 LSCC patients who visited the Department of Otolaryngology-Head and Neck Surgery at the Eye & ENT Hospital of Fudan University between January 1, 2007 and December 31, 2011 were enrolled. A total of 1,244 subjects completed follow-up, of whom 360 patients were later excluded from the study based on the inclusion criteria. Furthermore, 24 female LSCC subjects were excluded, and 14 LSCC subjects refused to participate in this study. During the follow-up period, 37 LSCC patients (voluntary withdrawal = 12, no response = 25) were excluded, leaving a final sample of 809 LSCC patients. The study cohort flow diagram is shown in Figure 1.
The inclusion criteria and selection process for the included LSCC patients were as follows (15): (1) All patients had histologically proven squamous cell carcinoma, confirmed by pathology and classified under the seventh edition of the TNM-UICC/AJCC stage classification; (2) The patients were a minimum of 18 years old; (3) Blood samples were collected before the patient pretreatment; (4) complete clinical, laboratory, imaging, and follow-up data were collected; (5) LSCC subjects were selected from inpatients; and (6) the subjects were free of systemic diseases (self-reported), such as acute infectious diseases, active inflammation (WBC>10*109/l or CRP>10 mg/l),autoimmune disease, and other cancers.
Data Collection
Clinical and demographic information were obtained from the medical data platform of the Eye & ENT Hospital by two independent investigators. Thereafter, all data were collated by the two investigators together. During this process, the two independent investigators consulted the medical data platform to resolve any possible bias. These data included the following demographic information: age, sex, body mass index, drinking habits, smoking habits, blood pressure, white blood cell levels, red blood cell count, red blood cell distribution width levels, hemoglobin levels, creatinine levels, nitrogen levels, aspartate transaminase levels, and alanine aminotransferase levels. The following clinical information was also collected: medical history, date of diagnosis, tumor subsite, tumor size, local, and regional extension category of the primary tumor, clinical stage, differentiation grade, prior surgical therapy, and level of neck dissection. The follow-up data included date of primary resection, date and type of relapse, date of diagnosis of metastatic disease, and date of death.
Blood samples for routine blood examination were collected via standard venipuncture of the veins in the antecubital fossae (anterior elbow veins). White blood cell levels, red blood cell count, red blood cell distribution width levels, and hemoglobin levels were measured with the Mindray BC-5500 (Shenzhen, China) automatic blood counting system within 0.5 h after blood collection. Blood samples for biochemical measurements were also collected. Serum levels of creatinine, nitrogen, aspartate transaminase, and alanine aminotransferase were measured using a commercially available kit (Roche Diagnostics GmbH, Mannheim, Germany).
Statistical Analysis
All analyses were performed using SPSS 13.0 software (SPSS Inc., Chicago, IL). Normality was assessed using the Kolmogorov–Smirnoff test. RDW was divided into quartiles in order to fully cover the non-linearity. The association between the RDW levels and clinicopathological parameters was evaluated by one-way ANOVA and a chi-square test. Univariate Cox regression analyses were performed to determine the association between RDW on OS, DFS, and CSS. After application of the univariate Cox regression analysis, a multivariate Cox regression analysis (adjusted for covariates) was used to analyze the association between RDW and the OS, DFS, and CSS of LSCC. The patient clinical end points were calculated using the Kaplan–Meier method and compared by the log-rank test. Hazard ratios (HR) estimated from the Cox analysis were reported as relative risks with corresponding 95% confidence intervals (CI). A two-sided p < 0.05 was considered statistically significant.
Results
A total of 809 male LSCC subjects were eligible in this study. During a mean longitudinal follow-up period of 72.9 months (from a range of 3 to 116 months) after the surgery, 186 deaths events occurred (23.0%). The mean age of the participants was 60.6 years. Only 30.28% of the participants reported never smoking, and 62.1% reported never drinking. The baseline characteristics and clinicopathological features of the study subjects are shown in Table 1.
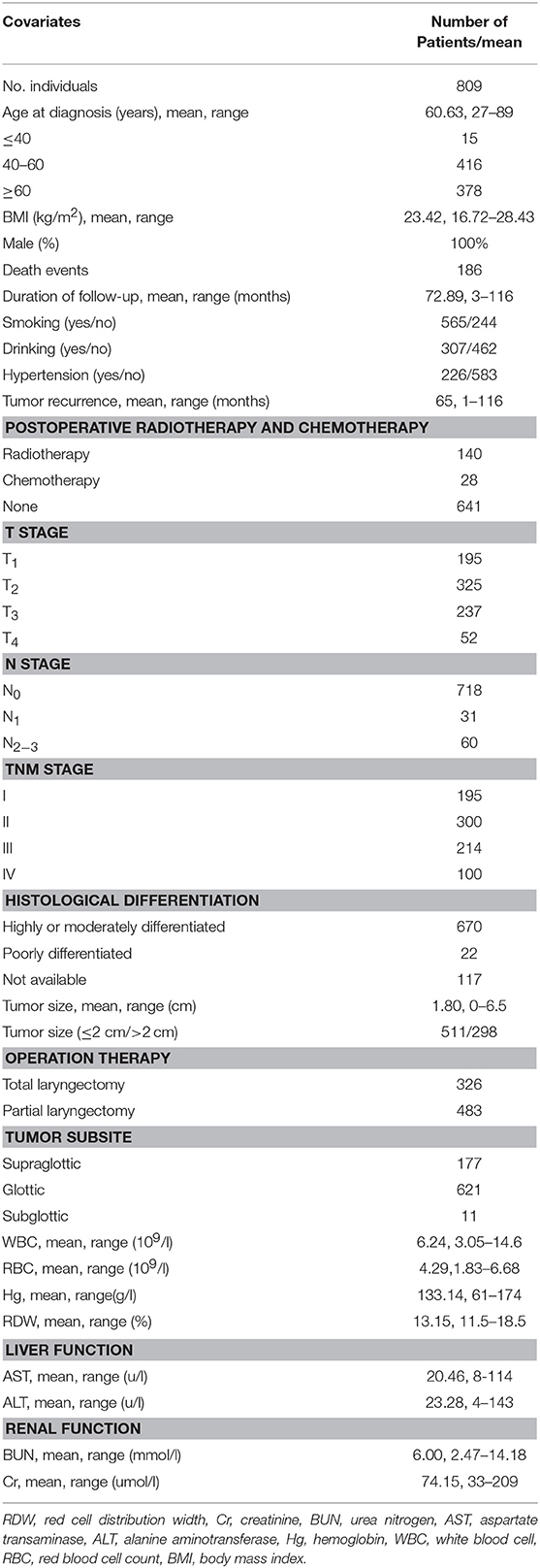
Table 1. Baseline demographic and lifestyle characteristics of laryngeal squamous cell cancer patients.
To test whether high RDW levels influence the clinical outcome of LSCC patients, we first subdivided the LSCC patients into four groups according to their RDW quartiles. The RDW quartiles were divided into the lowest quartile (RDW ≤ 12.9%), the lower quartile (12.9% < RDW ≤ 13.2%), the higher quartile (13.2% < RDW ≤ 13.5%), and the highest quartile (>13.5%). Rising levels of RDW were significantly correlated with higher death events, radiotherapy, reduced red blood cell count, reduced hemoglobin, long duration of follow-up, drinking habit, operation therapy, and advanced tumor stage (p < 0.05), whereas no association with age, smoking habits, hypertension, chemotherapy, tumor histological grade could be found (p > 0.05) (Table 2).
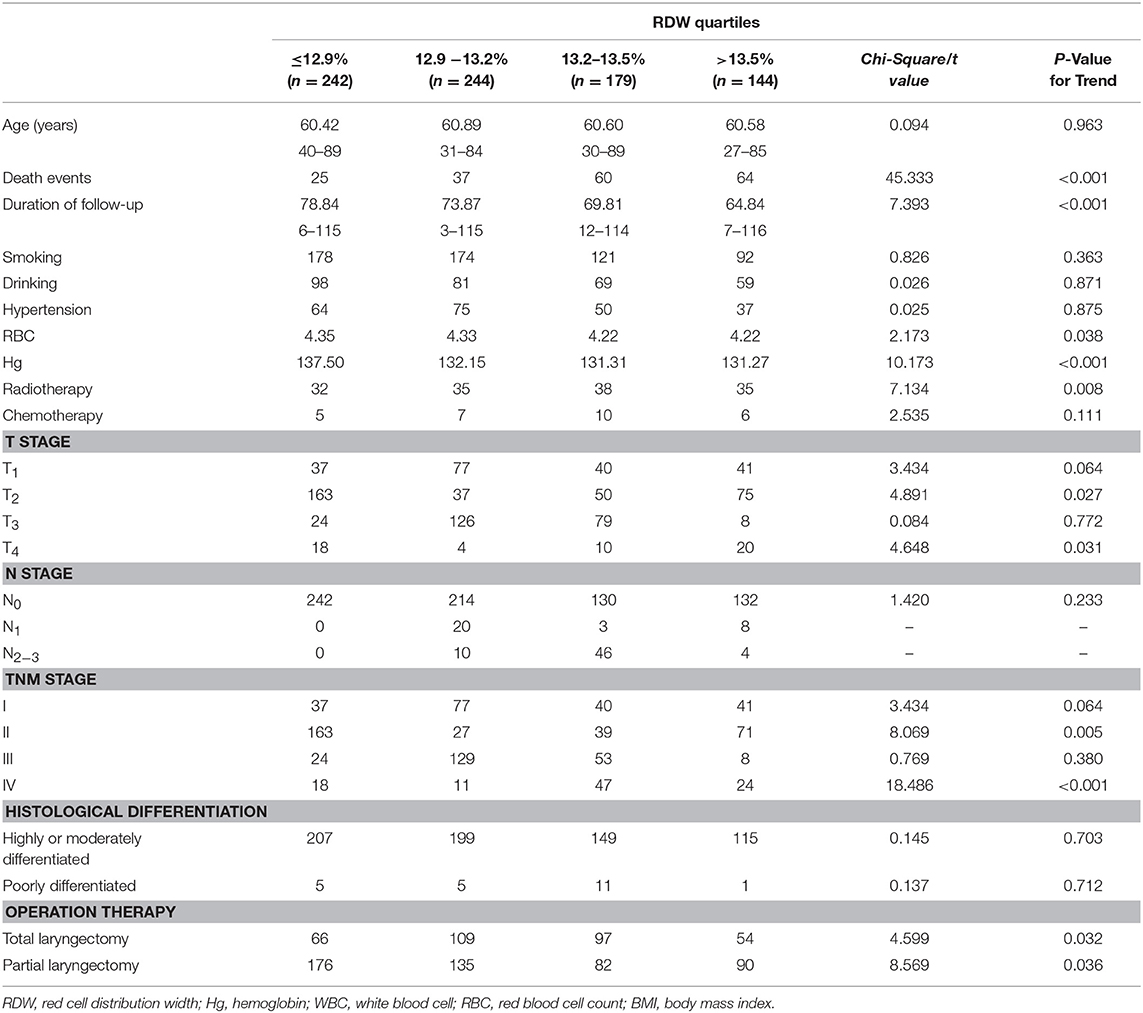
Table 2. The relation between clinicopathological parameters and pre-treatment red cell distribution width levels of patients with laryngeal squamous cell cancer.
Pearson correlation analyses were performed to identify the associations between RDW and hemoglobin. A significant negative correlation was found between the RDW and hemoglobin (r = −0.186, p < 0.001).
To investigate whether RDW level could be associated with the clinical outcome of LSCC patients, univariate, and multivariate Cox proportional models for OS, CSS, and DFS were calculated. Univariate analysis identified high RDW as a poor prognostic factor for OS (p < 0.001), CSS (p < 0.001), and DFS (p < 0.001) in this study cohort (Table 3).
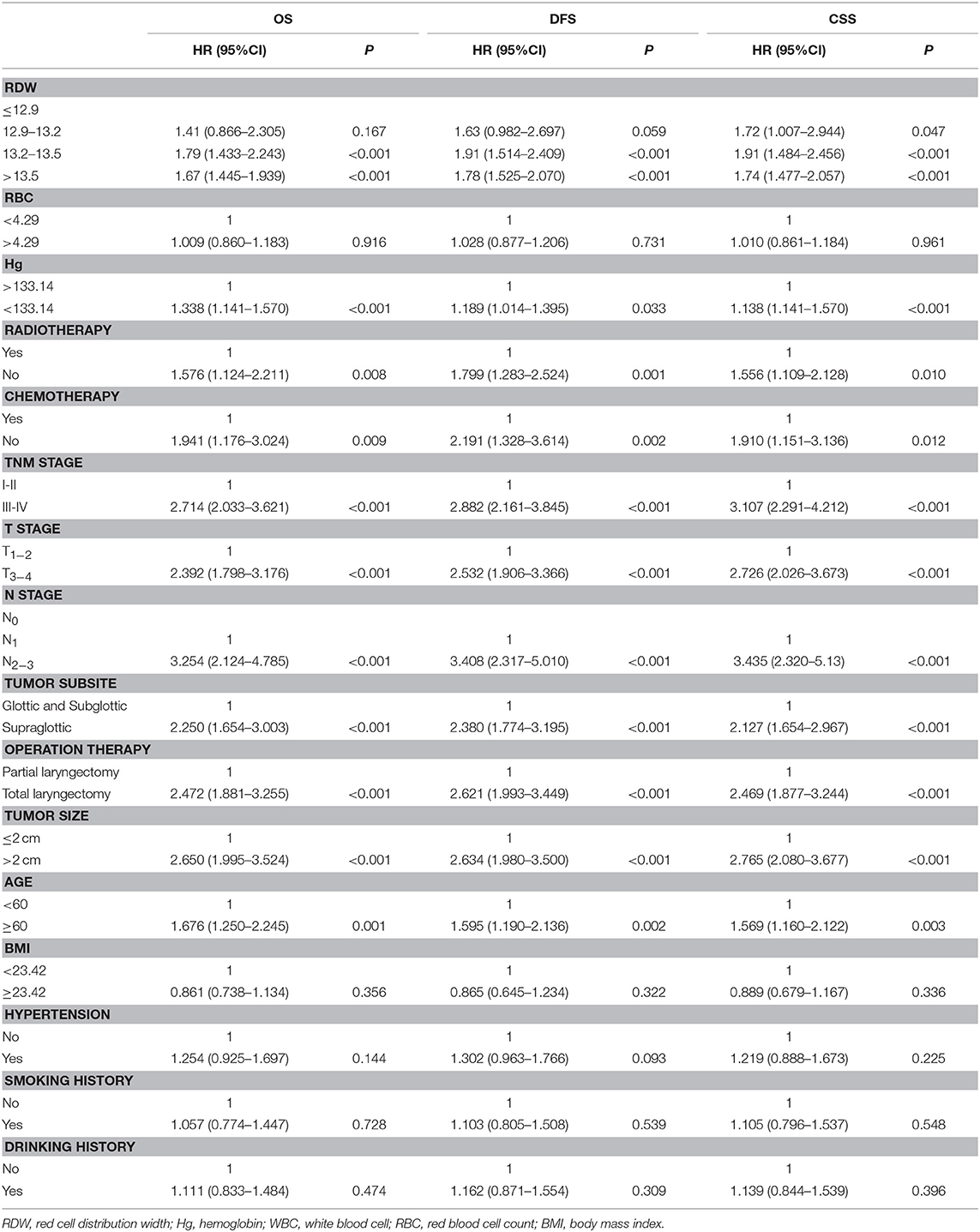
Table 3. Univariate Cox regression analysis for overall survival, disease-free survival and cancer specific survival in patients with laryngeal squamous cell cancer.
To determine the independent prognostic value of the RDW levels for OS, CSS, and DFS, a multivariate analysis using a Cox proportional hazard model was performed. After adjustments for age, body mass index, smoking, drinking, hypertension, white blood cell, hemoglobin, red blood cell count, creatinine, nitrogen, aspartate transaminase, alanine aminotransferase, tumor size, histological grade, tumor stage, tumor subsite and operation therapy, we identified the RDW level within the higher quartile (HR = 1.445, 95% CI = 1.721–2.894, p < 0.001) and the highest quartile (HR = 2.047, 95% CI = 1.587–2.641, p < 0.001) as independent prognostic factors for OS (Table 4); the RDW level within the higher quartile (HR = 1.339, 95% CI = 1.105–2.774, p < 0.001) and the highest quartile (HR = 2.043, 95% CI = 1.584–2.635, p < 0.001) as independent prognostic factors for CSS (Table 4); and the RDW level within the higher quartile (HR = 1.539, 95% CI = 1.787–2.010, p < 0.001) and the highest quartile (HR = 2.089, 95% CI = 1.622–2.691, P < 0.001) as independent prognostic factors for DFS (Table 4).
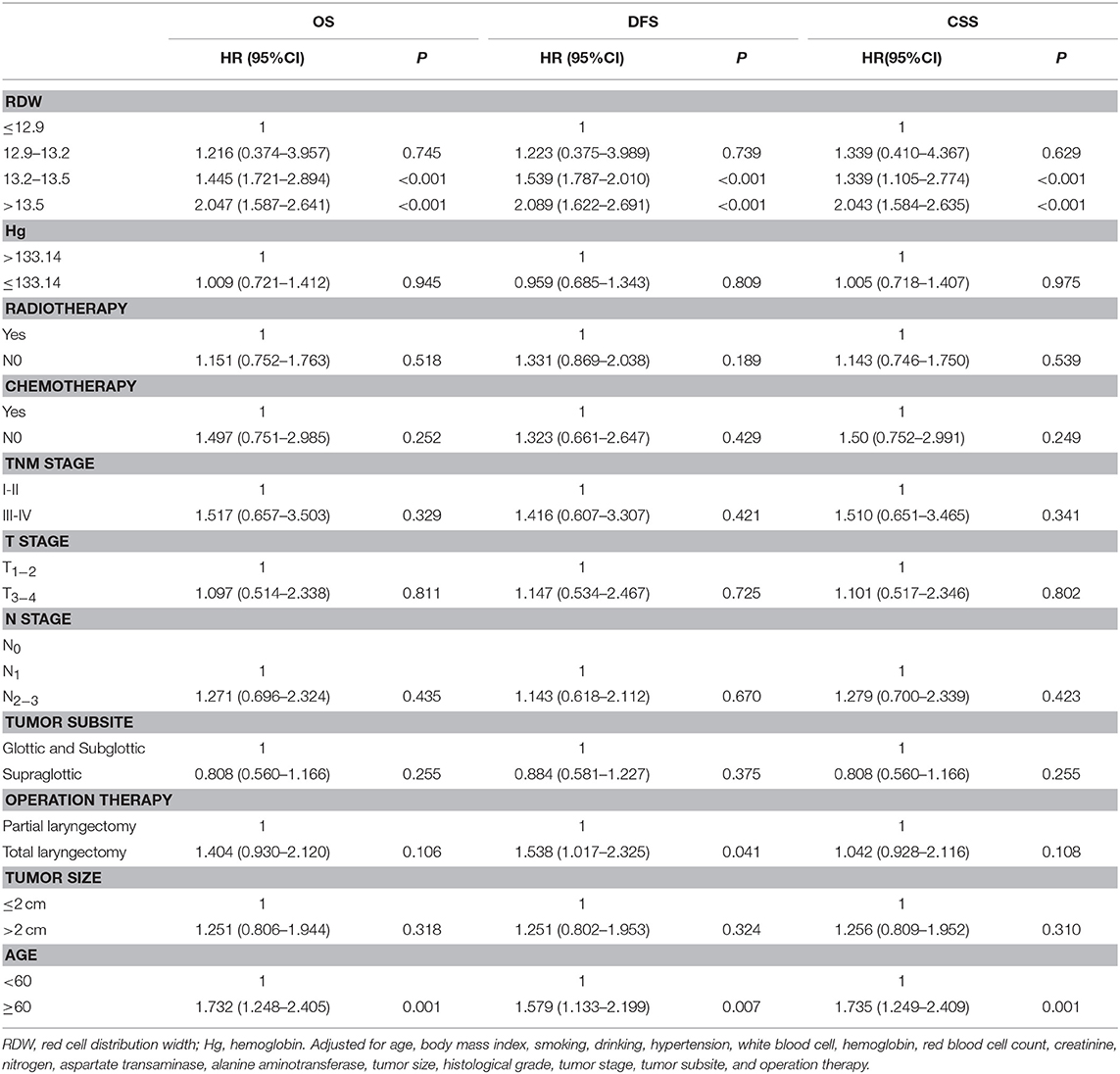
Table 4. Multivariate Cox regression analysis for overall survival, disease-free survival and cancer specific survival in patients with laryngeal squamous cell cancer.
Kaplan–Meier curves for OS, CSS, and DFS, which comprise groups according to quartiles of the RDW levels, are shown in Figure 2. The pairwise log-rank test indicates significant differences between the highest quartile (RDW > 13.5%) compared with the lowest quartile (RDW ≤ 12.9%), the lower quartile (13.2% ≥ RDW > 12.9%), and the higher quartile (13.5% ≥ RDW > 13.2%). The 5-year OS rates for patients in the RDW quartiles were as follows: the lowest quartile (89.3%), the lower quartile (84.6%), the higher quartile (78.3%), and the highest quartile (57.1%) (p < 0.001, Figure 2). The 5-year CSS rates for patients in the RDW quartiles were: the lowest quartile (91.0%), the lower quartile (85.75%), the higher quartile (80.08%), and the highest quartile (61.3%) (P < 0.001, Figure 2). The 5-year DFS rate for patients in the RDW quartiles were: the lowest quartile (89.3%), the lower quartile (83.5%), the higher quartile (72.0%), and the highest quartile (52.5%) (p < 0.001, Figure 2). Kaplan–Meier curves for OS, CSS, and DFS reveals that a high RDW level could be a risk factor consistent with poor prognosis in LSCC patients (p < 0.001, log-rank test).
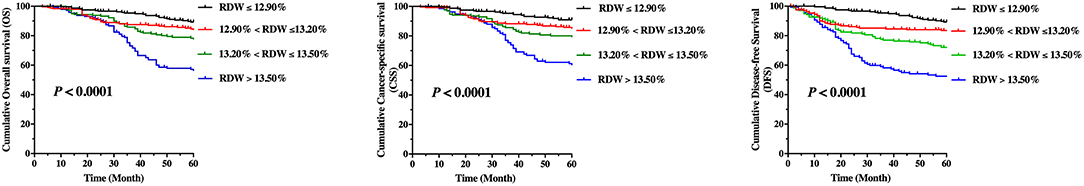
Figure 2. Kaplan–Meier curve stratified by red cell distribution width according to quartiles regarding overall survival (OS), cancer-specific survival, and disease-free survival (DFS) for patients with laryngeal squamous cell cancer.
Discussion
In this large-scale, retrospective study, we demonstrated that high RDW levels at the time of pretreatment were associated with adverse OS, CSS, and DFS in LSCC patients. Our multivariate analyses have shown that elevated pretreatment RDW is a risk factor, independent from other parameters, including age, smoking, drinking, TNM stage, tumor location, and surgery methods, and could be a useful hematological marker for poor prognosis prediction.
Inflammation and oxidative damage foster multiple cancer hallmark functions, including sustaining proliferative signaling, evading growth suppressors, resisting cell death, enabling replicative immortality, inducing angiogenesis, and activating invasion and metastasis, thus impacting patient survival (16). Elevated RDW has been reported to be a biomarker of inflammation and oxidative stress (17, 18). However, the prognostic significance of RDW in LSCC remains unknown.
Many efforts have been made to investigate the relationship between RDW and prognosis in various types of cancer. In other cancer studies, rich RDW levels have already been reported to be associated with poor prognosis and recurrence. For example, a meta-analysis of six studies predominantly conducted in a retrospective design showed that elevated RDW was significantly associated with worse OS/CSS of esophageal cancer patients when RDW > 13% (19). Moreover, a meta-analysis of eight cohort studies showed that elevated RDW may be an indicator of poor prognosis in upper aerodigestive tract cancers (20). Albayrak et al. (21) performed a case-control study and showed that higher RDW was associated with an increased risk of prostate cancer progression. Życzkowski et al. (22) retrospectively evaluated 434 patients and showed that RDW might be an easily obtainable prognostic marker in renal cell carcinoma patients treated with nephrectomy.
Only five studies have examined the relationship of RDW with cancer in the head and neck region, of which one study was for the thyroid cancer (23), two were for cancers of the oral cavity (24, 25) and two were for laryngeal cancers (14, 26). For thyroid cancer, Aktas et al. (23) reported that the level of RDW was significantly higher compared with that in normal subjects. Regarding oral cancer, the previously reported data were conflicting. Ge et al. (25) reported, in 236 patients, that an elevated preoperative RDW (≥15%) during diagnosis may independently predict poorer OS in patients with oral squamous cell carcinoma, while Tangthongkum et al. (24) found that RDW has no prognostic value on any outcome in patients with oral cancer. For laryngeal cancer, Kara et al. (26) reported in a smaller (n = 81) study and Bozkurt et al. (14) reported in 132 patients that RDW was an independent prognostic factor of disease survival. Moreover, in a smaller study, including 205 patients with head and neck cancer, Tham et al. (27) reported that a low hemoglobin/RDW ratio was associated with poorer event-free survival (HR = 2.02, p = 0.017). However, many of these studies included rather small numbers of investigated cases and differed in terms of inclusion criteria and clinical end points. In our study, we validated the prognostic impact of RDW levels on OS, CSS, and DFS as the end points and clearly demonstrated that the pretreatment RDW level was independently associated with OS, CSS, and DFS in a large cohort of 809 LSCC patients.
The possible role of RDW in the development of cancers has not been previously elucidated in experimental settings. Several factors could explain the prognostic value of RDW. The accepted explanation is that a higher tumor stage can result in a greater extent of systemic inflammation by the secretion of cytokines and the release of tumor-degradation products (28), which then decreases the lifetime of red blood cells (29) and increases the RDW level. Moreover, patients with advanced stage cancers might change their dietary habits or eat less due to problems with swallowing, which might lead to malnutrition and thereafter, a decreased volume of red blood cells, especially RDW levels, before their operations (30).
We must admit that several limitations in our study are as follows: RDW is associated with systematic inflammation but is not a specific marker of inflammation. Although we excluded interference factors, such as acute infectious diseases, and system diseases, chronic subclinical inflammation in LSCC subjects is still difficult to detect through medical screenings. Thus, there is a possibility to influence the RDW level. Furthermore, nutritional issue may be a confounding factor, but we were unable to evaluate this feature in our study. Additionally, our study is limited by its retrospective nature: the data were collected at a single-center in a retrospective study with a homogeneous group of single genders, which might limit the generalizability of the results.
In conclusion, excluding human bias, a high level of pretreatment RDW may be a poor prognostic factor for survival in male LSCC patients. Further studies should attempt to illuminate the mechanism and confirm its role in different population groups.
Ethics Statement
This study was conducted in accordance with the Helsinki Declaration and was approved by the committee of the Eye & ENT Hospital of Fudan University, Shanghai, China. Written informed consent was obtained and approved for all patients.
Author Contributions
HG, C-YH, and LZ designed the study. HG, C-YH, MZ, LT, and LZ contributed to the patient recruitment and collected the data. H-CL and C-YH performed the statistical analysis. H-CL and C-YH wrote the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by the Science and Technology Commission of Shanghai Municipality, China [Grant Nos. 12J1402100 and 16411950101] and the Shanghai Shen Kang Hospital Development Center [Grant No. SHDC12015114].
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Liu Y, Zhao Q, Ding G, Zhu Y, Li W, Chen W. Incidence and mortality of laryngeal cancer in China, 2008–2012. Chin J Cancer Res Chung-Kuo Yen Cheng Yen Chiu. (2018) 30:299–306. doi: 10.21147/j.issn.1000-9604.2018.03.02
2. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. (2016) 66:115–32. doi: 10.3322/caac.21338
3. Tamaki A, Miles BA, Lango M, Kowalski L, Zender CA. AHNS series: do you know your guidelines? Review of current knowledge on laryngeal cancer. Head Neck. (2018) 40:170–81. doi: 10.1002/hed.24862
4. Xie YC, Liu WP, Jiang YM, Zhao Y, Tang QL, Liang CY, et al. [A pathologic analysis on 9,666 cases of tumors of nose, pharynx and throat]. Zhonghua Er Bi Yan Hou Ke Za Zhi. (2003) 38:217–20. doi: 10.3760/j.issn:1673-0860.2003.03.015
5. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. (2016) 66:7–30. doi: 10.3322/caac.21332
6. Perlstein TS, Weuve J, Pfeffer MA, Beckman JA. Red blood cell distribution width and mortality risk in a community-based prospective cohort. Arch Intern Med. (2009) 169:588–94. doi: 10.1001/archinternmed.2009.55
7. Patel KV, Semba RD, Ferrucci L, Newman AB, Fried LP, Wallace RB, et al. Red cell distribution width and mortality in older adults: a meta-analysis. J Gerontol A Biol Sci Med Sci. (2010) 65:258–65. doi: 10.1093/gerona/glp163
8. Lippi G, Targher G, Montagnana M, Salvagno GL, Zoppini G, Guidi GC. Relation between red blood cell distribution width and inflammatory biomarkers in a large cohort of unselected outpatients. Arch Pathol Lab Med. (2009) 133:628–32. doi: 10.1043/1543-2165-133.4.628
9. Salvagno GL, Sanchis-Gomar F, Picanza A, Lippi G. Red blood cell distribution width: a simple parameter with multiple clinical applications. Crit Rev Clin Lab Sci. (2015) 52:86–105. doi: 10.3109/10408363.2014.992064
10. Celik A, Aydin N, Ozcirpici B, Saricicek E, Sezen H, Okumus M, et al. Elevated red blood cell distribution width and inflammation in printing workers. Med Sci Monit. (2013) 19:1001–5. doi: 10.12659/MSM.889694
11. Li Z, Hong N, Robertson M, Wang C, Jiang G. Preoperative red cell distribution width and neutrophil-to-lymphocyte ratio predict survival in patients with epithelial ovarian cancer. Sci Rep. (2017) 7:43001. doi: 10.1038/srep43001
12. Ichinose J, Murakawa T, Kawashima M, Nagayama K, Nitadori JI, Anraku M, et al. Prognostic significance of red cell distribution width in elderly patients undergoing resection for non-small cell lung cancer. J Thorac Dis. (2016) 8:3658–66. doi: 10.21037/jtd.2016.12.44
13. Yazici P, Demir U, Bozkurt E, Isil GR, Mihmanli M. The role of red cell distribution width in the prognosis of patients with gastric cancer. Cancer Biomark Sect Dis Markers. (2017) 18:19–25. doi: 10.3233/CBM-160668
14. Bozkurt G, Korkut AY, Soytaş P, Dizdar SK, Erol ZN. The role of red cell distribution width in the locoregional recurrence of laryngeal cancer. Braz J Otorhinolaryngol. (2018). doi: 10.1016/j.bjorl.2018.03.004. [Epub ahead of print].
15. Hsueh C, Tao L, Zhang M, Cao W, Gong H, Zhou J, et al. The prognostic value of preoperative neutrophils, platelets, lymphocytes, monocytes, and calculated ratios in patients with laryngeal squamous cell cancer. Oncotarget. (2017) 8:60514–27. doi: 10.18632/oncotarget.16234
16. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. (2011) 144:646–74. doi: 10.1016/j.cell.2011.02.013
17. Zhao Z, Liu T, Li J, Yang W, Liu E, Li G. Elevated red cell distribution width level is associated with oxidative stress and inflammation in a canine model of rapid atrial pacing. Int J Cardiol. (2014) 174:174–6. doi: 10.1016/j.ijcard.2014.03.189
18. Semba RD, Patel KV, Ferrucci L, Sun K, Roy CN, Guralnik JM, et al. Serum antioxidants and inflammation predict red cell distribution width in older women: the Women's Health and Aging Study I. Clin Nutr Edinb Scotl. (2010) 29:600–4. doi: 10.1016/j.clnu.2010.03.001
19. Xu WY, Yang XB, Wang WQ, Bai Y, Long JY, Lin JZ, et al. Prognostic impact of the red cell distribution width in esophageal cancer patients: a systematic review and meta-analysis. World J Gastroenterol. (2018) 24:2120–9. doi: 10.3748/wjg.v24.i19.2120
20. Tham T, Bardash Y, Teegala S, Herman WS, Costantino PD. The red cell distribution width as a prognostic indicator in upper aerodigestive tract (UADT) cancer: a systematic review and meta-analysis. Am J Otolaryngol. (2018) 39:453–8. doi: 10.1016/j.amjoto.2018.04.013
21. Albayrak S, Zengin K, Tanik S, Bakirtas H, Imamoglu A, Gurdal M. Red cell distribution width as a predictor of prostate cancer progression. Asian Pac J Cancer Prev APJCP. (2014) 15:7781–4. doi: 10.7314/APJCP.2014.15.18.7781
22. Życzkowski M, Rajwa P, Gabrys E, Jakubowska K, Jantos E, Paradysz A. The relationship between red cell distribution width and cancer-specific survival in patients with renal cell carcinoma treated with partial and radical nephrectomy. Clin Genitourin Cancer. (2018) 16:e677–83. doi: 10.1016/j.clgc.2017.12.003
23. Aktas G, Sit M, Karagoz I, Erkus E, Ozer B, Kocak MZ, et al. Could red cell distribution width be a marker of thyroid cancer? J Coll Phys Surg–Pak JCPSP. (2017) 27:556–8.
24. Tangthongkum M, Tiyanuchit S, Kirtsreesakul V, Supanimitjaroenporn P, Sinkitjaroenchai W. Platelet to lymphocyte ratio and red cell distribution width as prognostic factors for survival and recurrence in patients with oral cancer. Eur Arch Oto-Rhino-Laryngol. (2017) 274:3985–92. doi: 10.1007/s00405-017-4734-1
25. Ge W, Xie J, Chang L. Elevated red blood cell distribution width predicts poor prognosis in patients with oral squamous cell carcinoma. Cancer Manag Res. (2018) 10:3611–8. doi: 10.2147/CMAR.S176200
26. Kara M, Uysal S, Altinişik U, Cevizci S, Güçlü O, Dereköy FS. The pre-treatment neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and red cell distribution width predict prognosis in patients with laryngeal carcinoma. Eur Arch Oto-Rhino-Laryngol. (2017) 274:535–42. doi: 10.1007/s00405-016-4250-8
27. Tham T, Olson C, Wotman M, Teegala S, Khaymovich J, Coury J, et al. Evaluation of the prognostic utility of the hemoglobin-to-red cell distribution width ratio in head and neck cancer. Eur Arch Oto-Rhino-Laryngol. (2018) 275:2869–78. doi: 10.1007/s00405-018-5144-8
28. Szkandera J, Stotz M, Absenger G, Stojakovic T, Samonigg H, Kornprat P, et al. Validation of C-reactive protein levels as a prognostic indicator for survival in a large cohort of pancreatic cancer patients. Br J Cancer. (2014) 110:183–8. doi: 10.1038/bjc.2013.701
29. Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. (2005) 352:1011–23. doi: 10.1056/NEJMra041809
Keywords: laryngeal squamous cell cancer, male, red cell distribution width, prognosis, biomarker
Citation: Hsueh C-A, Lau H-C, Li S, Tao L, Zhang M, Gong H and Zhou L (2019) Pretreatment Level of Red Cell Distribution Width as a Prognostic Indicator for Survival in a Large Cohort Study of Male Laryngeal Squamous Carcinoma. Front. Oncol. 9:271. doi: 10.3389/fonc.2019.00271
Received: 12 December 2018; Accepted: 25 March 2019;
Published: 16 April 2019.
Edited by:
Jordi Giralt, Vall d'Hebron University Hospital, SpainReviewed by:
Sandro J. Stoeckli, Kantonsspital St. Gallen, SwitzerlandXavier León, Hospital de la Santa Creu i Sant Pau, Spain
Copyright © 2019 Hsueh, Lau, Li, Tao, Zhang, Gong and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongli Gong, gonghlent@126.com
Liang Zhou, zhoulent@126.com
†These authors have contributed equally to this work
 Chi-Yao Hsueh
Chi-Yao Hsueh Hui-Ching Lau
Hui-Ching Lau Shengjie Li
Shengjie Li Lei Tao
Lei Tao Ming Zhang
Ming Zhang Hongli Gong
Hongli Gong Liang Zhou
Liang Zhou