
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 28 March 2019
Sec. Radiation Oncology
Volume 9 - 2019 | https://doi.org/10.3389/fonc.2019.00184
This article is part of the Research TopicRole of SRS/SBRT in Oligometastatic DiseaseView all 15 articles
 Geoffrey Martinage1
Geoffrey Martinage1 Julien Geffrelot2
Julien Geffrelot2 Dinu Stefan2
Dinu Stefan2 Emilie Bogart3
Emilie Bogart3 Erwan Rault4
Erwan Rault4 Nicolas Reyns5
Nicolas Reyns5 Evelyne Emery6
Evelyne Emery6 Samira Makhloufi-Martinage7
Samira Makhloufi-Martinage7 Raphaelle Mouttet-Audouard1
Raphaelle Mouttet-Audouard1 Laurent Basson1
Laurent Basson1 Xavier Mirabel1
Xavier Mirabel1 Eric Lartigau1,8
Eric Lartigau1,8 David Pasquier1,8*
David Pasquier1,8*Purpose: The aim of this study was to assess, in a large series, the efficacy and tolerance of post-operative adjuvant hypofractionated stereotactic radiation therapy (HFSRT) for brain metastases (BMs).
Materials and Methods: Between July 2012 and January 2017, 160 patients from 2 centers were operated for BM and treated by HFSRT. Patients had between 1 and 3 BMs, no brainstem lesions or carcinomatous meningitis. The primary endpoint was local control. Secondary endpoints were distant brain control, overall survival (OS) and tolerance to HFSRT.
Results: 73 patients (46%) presented with non-small cell lung cancer (NSCLC), 23 (14%) had melanoma and 21 (13%) breast cancer. Median age was 58 years (range, 22–83 years). BMs were synchronous in 50% of the cases. The most frequent prescription regimens were 24 Gy in 3 fractions (n = 52, 33%) and 30 Gy in 5 fractions (n = 37, 23%). Local control rates at 1 and 2 years were 88% [95%CI, 81–93%] and 81% [95%CI, 70–88%], respectively. Distant control rate at 1 year was 48% [95%CI, 81–93%]. In multivariate analysis, primary NSCLC was associated with a significant reduction in the risk of death compared to other primary sites (HR = 0.57, p = 0.007), the number of extra-cerebral metastatic sites (HR = 1.26, p = 0.003) and planning target volumes (HR = 1.15, p = 0.012) were associated with a lower OS. There was no prognostic factor of time to local progression. Median OS was 15.2 months [95%CI, 12.0–17.9 months] and the OS rate at 1 year was 58% [95% CI, 50–65%]. Salvage radiotherapy was administered to 72 patients (45%), of which 49 received new HFSRT. Ten (7%) patients presented late grade 2 and 4 (3%) patients late grade 3 toxicities. Thirteen (8.9%) patients developed radiation necrosis.
Conclusions: This large multicenter retrospective study shows that HFSRT allows for good local control of metastasectomy tumor beds and that this technique is well-tolerated by patients.
Brain metastases (BMs) are the most frequent brain tumors, and, throughout disease course, 20–40% of cancer patients will develop a BM (1). In subjects in good general health and presenting with a single BM, surgical resection has been shown to improve survival (2, 3). After surgery, adjuvant whole-brain radiotherapy (WBRT) allows to significantly reduce local and brain recurrence rates, as well as the risk of death from neurological cause (4, 5). Nevertheless, WBRT has not been shown to be beneficial in terms of overall survival (4–6) and the length of time in which patients remain functionally independent (4, 5). In addition it contributes, in the short term, to a poorer quality of life in patients (6) and causes acute toxicities including asthenia, alopecia, nausea, and a decline in learning and memory functions (7). Stereotactic radiosurgery (SRS) allows for good local control of the disease while avoiding the neurocognitive decline triggered by WBRT (8). Consequently, after resection of a BM, SRS and Hypofractionated stereotactic radiation therapy (HFSRT) are increasingly being used and could be considered as an alternative treatment standard to WBRT allowing to limit toxicity (7, 8).
To date, there is no consensus on the optimal dose, fractionation, or prescription regimens of HFSRT on the surgical cavity. Several prescription patterns are described in the literature, including schemas of 3 fractions with doses ranging from 7.7 to 11 Gy, (9–12) or schemas of 5 fractions (13, 14). Such heterogeneity in prescription doses prevents any direct comparison between studies. The largest phase III randomized study, comparing SRS to WBRT published by Brown et al. showed a longer cognitive-deterioration-free survival in patients assigned to SRS (median 37 months) than in patients assigned to WBRT (median 30 months) (p < 0.0001) (8). Overall survival (OS) was identical in the 2 arms, but local and distant brain control were lower in the SRS arm.
The aim of this study was to evaluate the efficacy and safety of post-operative HFSRT in resection cavity of secondary brain lesions in a large cohort of patients.
Between July 2012 and January 2017, patients treated with post-operative HFSRT to the resection cavity in two French centers were included. Data were retrospectively collected. Inclusion criteria were: adult patients, with 1 to 3 BMs, no previous radiotherapy treatment to the brain, treated by surgery for BM of a solid tumor and with anatomical pathology data, no brainstem lesion or carcinomatous meningitis, eligible to be treated by HFSRT as decided in a multidisciplinary meeting, with a life expectancy of more than 3 months, and not opposed to the use of their medical data for research and educational purposes.
Patients were immobilized using a thermoplastic mask system. Computed tomography (CT) scan and gadolinium contrast-enhanced magnetic resonance imaging (MRI) were used for treatment planning. Imaging was performed using millimetric slices and rigid registration. Target volumes and organs at risk were contoured on MRI and concordance with CT was controlled. Contouring software's used were Oncentra (version 4.3.0) and Multiplan (Accuray, version 3.2.0).
Target volumes were contoured using the surgical and anatomical pathology assessment of resection specimens. The clinical target volume (CTV) included the surgical cavity, contrast enhancement of tumor border and a 1–2 mm margin which delineates it on the CT scan and planning MRI. In the case of metastasis in contact with dura, the CTV included a larger margin (5–10 mm) beyond the area where there was contact before surgery. The gross tumor volume (GTV) was defined if macroscopic disease could be identified by nodular contrast enhancement by T1-Gadolinum MRI imaging. The planning target volume (PTV) was defined as CTV + 1 mm.
HFSRT treatment was delivered using a CyberKnife®-type robotic accelerator (Accuray, Inc., Sunnyvale, CA), using 6 MeV photon beams, in Centre Oscar Lambret in Lille and Centre François Baclesse in Caen. Dose was prescribed at the 80% isodose and patients were treated every 2 days.
Follow-up of patients included collection of clinical data and brain perfusion MRI at 2 months and then every 3 months after the end of irradiation during the first year, and every 4 to 6 months thereafter. Local recurrence was defined as the appearance or growth of nodules in the surgical cavity visible on a T1-gadolinium MRI sequence. OS was defined as the time from HFSRT until death from any cause. Time-to distant brain control (DBC) was defined as to the time from HFSRT until progression in the brain outside of the surgical cavity. Radiation necrosis was diagnosed based on clinical, morphological, and metabolic criteria, and was validated by experts. MR spectroscopic imaging and 18F-DOPA PET (Positron emission tomography) were used to support the diagnosis if needed.
Patient and disease characteristics were analyzed using descriptive statistics. Quantitative variables were expressed as median and range. Survival curves were estimated using the Kaplan Meier method. For time to progression, patients were censored at the date of last news or date of death from any cause. Time interval for overall survival was calculated from the date of HFSRT to the date of death from any cause. Patients alive were censored at the date of last news. Patients were censored at day 1 in case of missing information on the event.
After having checked the proportional hazard assumption (Schoenfeld residuals), prognostic factors of survival were identified using a univariate Cox regression model. Hazard Ratios (HR) and the 95% CI as well as the calculated probability (p-value) were presented for each model. In cases of non-proportional hazards, the “restricted mean survival time difference” (RMSTD) was used (15). Significant variables at p = 0.10 in the univariate model were included in the multivariate Cox stepwise backward model analysis. The following factors were analyzed: sex, age, primary disease, primary histology, RPA (recursive partitioning analysis) score, DS-GPA (Diagnostic-Specific Graded Prognostic Assessment) score, controlled primary tumor, location of BM, extracranial metastasis status, number of BM, time between primary tumor and BM diagnosis, partial resection and gross total resection, interval time between surgery and HFSRT, dose of HFSRT, salvage WBRT, SRS or HFSRT, pre- and post-operative volumes, conformity index, and homogeneity index.
The association of the radiation necrosis with the different factors was analyzed with the Fisher exact test for qualitative variables and with the Wilcoxon Mann-Whitney test for quantitative variables.
Statistical analyses were performed using Stata 13.1 (StataCorp. 2013 Stata Statistical Software: Release 11. College Station, TX: StataCorp LP) and significance level was set at a p-value of 0.05 for all analyses.
This was a retrospective study involving 160 patients and 167 surgical cavities. Patients were 76 women (47.5%) and 84 men. The median age at diagnosis of BM was 58 years (range, 22–83 years) (Table 1). Seventy-three patients (46%) presented with primary lung cancer, 23 patients (15%) with melanoma and 21 patients (13%) with breast cancer. The median time interval between the primary tumor diagnosis and BM surgery was 8.4 months (range, 0–148.6 months).
At the time of diagnosis, 115 patients (72%) had a single BM; 77 patients (50%) had symptoms of intracranial hypertension and 126 patients (81%) had neurological symptoms (Table 2). Seventy-eight patients (50%) presented with synchronous BM, 63 patients (40%) with a metachronous BM and a controlled primary tumor, and 16 patients (10%) with a metachronous and a non-controlled primary tumor. Pre-operative MRI revealed a median tumor size of 32 mm (range, 7–78 mm) and 75% of the cases (n = 124) were supratentorial. Planning MRI was performed in 151 patients (94%). The median surgery cavity size was 27 mm (range, 5–66 mm) and in 46 patients (30%) a nodular contrast enhancement by planning MRI led to the diagnosis of an early relapse in the surgical cavity. Most frequent prescription regimens were 24 Gy in 3 fractions (n = 52, 33%) and 30 Gy in 5 daily fractions (n = 37, 23%). Median CTV and PTV volumes were 10.6 mL (range, 0.9–98.8 mL) and 15.2 mL (range, 2.2–129.8 mL), respectively.
The median follow-up was 30.6 months. At the end of the follow-up, 23 local recurrence (14.4%) were observed. Local control rates at 6 months, 1 year and 2 years were 91% [95% CI, 85–95%], 88% [95% CI, 81–93%], and 81% [95% CI, 70–88%], respectively (Figure 1A). No factor appears to be prognostic of local control.
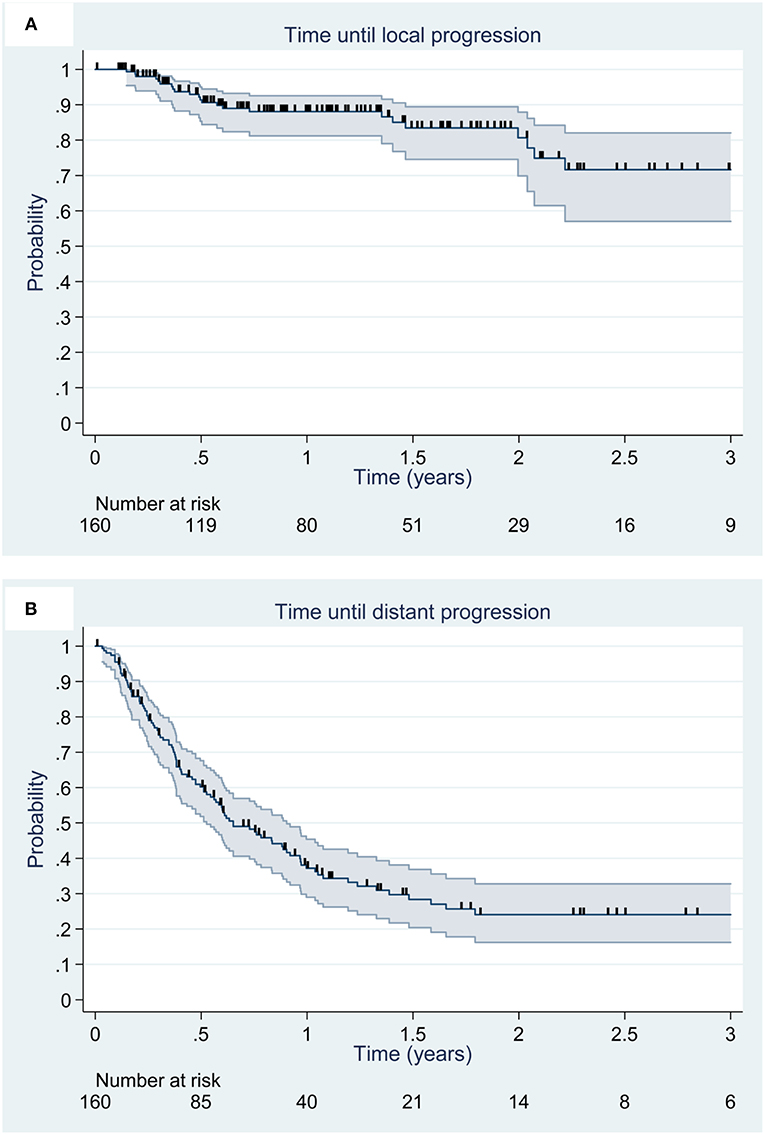
Figure 1. Kaplan Meier estimation (95% CI) of local (A) and distant (B) control. Deceased patients and patients alive at the date of last news were censored and are illustrated by vertical lines.
At the end of the follow-up, 86 patients (53.7%) presented with DBC. The median time to brain recurrence was 11.2 months (range, 8.4–18.0 months). DBC rates at 6 months, 1 and 2 years were 71% [95% CI, 63–78%], 48% [95% CI, 39–56%], and 34% [95% CI, 24–43%], respectively (Figure 1B). No factor appears to be prognostic of time to distant brain progression. The progression free leptomeningeal progression rate were 80% [95% CI, 73–86%] at 1 year and 72% [95% CI, 59–82%] at 3 years.
At the end of the follow-up, 113 deaths (70.6%) were observed, including 33 deaths (42%) due to disease brain progression. Median OS was 15.2 months [95% CI, 12.0–17.9 months], and 6 months, 1 and 2 year OS rates were 81% [95% CI, 74–86%], 58% [95% CI, 50–65%] and 32% [95% CI, 24–39%] (Figure 2).
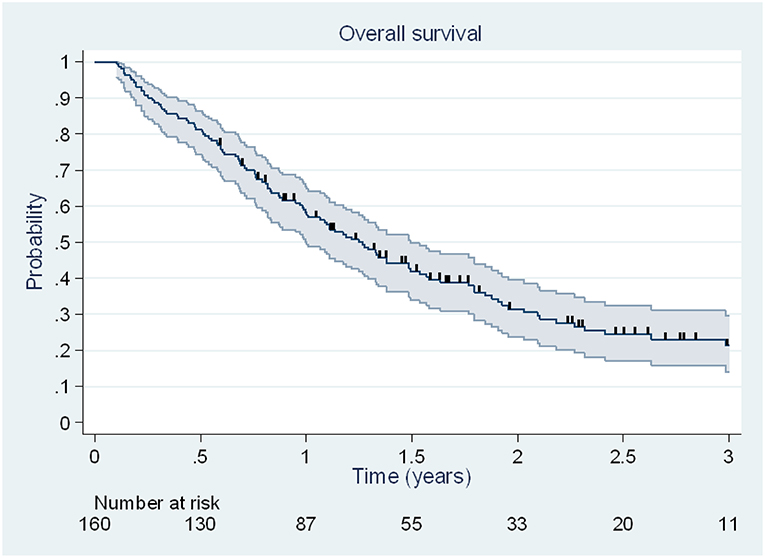
Figure 2. Kaplan Meier estimation (95% CI) of overall survival. Patients alive at the date of last news were censored and are illustrated by vertical lines.
In the univariate analysis, different prognostic factors appears to be associated with overall survival. Lung primary tumor was associated with a significant reduction of the risk of death compared to other primary tumors (HR = 0.65, [95% CI, 0.44–0.94], p = 0.023). The number of extra-cerebral metastatic sites (HR = 1.19, [95% CI, 1.02–1.39], p = 0.027), the number of BM (HR = 1.17, [95% CI, 1.00–1.35], p = 0.046), the absence of systemic control of the disease (HR = 1.39, p = 0.035) and larger PTV (HR = 1.12, [95% CI, 1.01–1.26], p = 0.040) were associated with a significant increase of the risk of death. In multivariate analysis, lung cancer (HR = 0.57, [95% CI, 0.38–0.86], p = 0.007), the number of extra-cerebral metastatic sites (HR = 1.26, [95% CI, 1.08–1.48], p = 0.003) and the larger PTV (HR = 1.15, [95% CI, 1.03–1.28], p = 0.012) were prognostic of OS (Table 3). The number of BM did not achieve significance with a HR = 1.16 (p = 0.055).
Among the 23 patients presenting a local recurrence, 7 were treated by stereotactic re-irradiation, 6 by a WBRT, and 9 did not receive additional irradiation (Table 4). Overall, 72 patients (45%) underwent another brain irradiation: 38 (24%) received exclusively SRT at a median delay of 7.3 months (range, 1.3–58 months) and 34 (21%) received WBRT at a median delay of 7 months (range, 1.8–33 months). Sixty-six patients (41.8%) presented new neurological sign related to disease progression, at a median delay of 6.3 months (range, 0.9–32.2 months) after the initial radiotherapy treatment.
According to the Common Terminology Criteria for Adverse Events (CTCAE) grading system, version 4.03, 5 patients (3.4%) presented an acute grade 2 toxicity and 1 patient (0.7%) presented an acute brain hemorrhage of grade 3. Ten patients (7.2%) developed late toxicity of grade 2 and 4 patients (2.7%) a late toxicity of grade 3 (two brain necroses, one seizure and one stroke). Radiation necrosis during follow-up occurred in 13 patients (8.9%). The stereotactic re-irradiation or WBRT was the only factor associated with an increased risk of developing a radiation necrosis (p < 0.001, Fisher exact test). Among patients that received HFSRT exclusively, the rate of radiation necrosis at the end of follow-up was 6.9% and among the 7 patients treated with stereotactic re-irradiation in the surgical cavity, 4 (57%) presented with a radiation necrosis.
This large series, the second largest after Keller et al.'s to our knowledge (12), shows that post-operative HFSRT to the surgical cavity of BM allows for a good local control with acceptable acute and late toxicity profiles.
Local control rates achieved in our series with HFSRT to the surgical cavity are comparable to those of the larger retrospective series. Keller et al. reported local control rates of 92.9% at 6 months, 88.2% at 1 year and 86.5% at 2 years in a series involving 181 patients and 189 surgical cavities (12) (Table 5). In this study, the prescribed dose was 3 × 11 Gy to the isocenter. Factors associated with a greater rate of local relapse in multivariate analysis were larger PTV (>24 mL), a greater GPA score and meningeal contact of the BM. Patel et al. and Mahajan et al. also demonstrated in their series that the tumor volume was predictive of local control (16, 19). The 1 year local control rate of 88% [95% CI, 81–93%] in our study is similar to the 85% revealed by the meta-analysis involving 629 patients treated by SRT to the surgical cavity (17).
The phase III study from Kocher et al. evaluated the combination of WBRT or SRS with surgery to treat 359 patients with 1 to 3 BMs (4). Of 160 patients treated with surgery, 79 patients were randomized to the observation arm and 81 to the adjuvant WBRT arm. The 2 years local control rate was 41% in the observation arm vs. 73% (p < 0.001) in the surgery and WBRT combination arm, close to the rates observed in our study. A randomized study, recently published by Brown et al., included 194 patients from 48 centers and compared radiosurgery and WBRT as adjuvant treatment (8). The surgical cavities had to be smaller than 5 cm. Patients treated by WBRT had 6 months and 1-year local control rates of 87.1 and 80.6%, respectively. In addition, this study showed weak local control rates in the radiosurgery arm (80.4% at 6 months and 60.5% at 1 year), well-below the local control rates obtained in the WBRT arm (p = 0.00068). The lower local control rate in this study could be explained by the weak dose delivered. Indeed, patients treated by SRS received 12 to 20 Gy in one fraction, while patients in the WBRT arm received 37.5 Gy in 15 fractions or 30 Gy in 10 fractions. Robbins et al. demonstrated in their study the use of radiosurgery to the surgical cavity as adjuvant therapy for resected BM that a marginal dose in SRS under 16 Gy was predictive of local control (20). Mahajan et al. reported in a phase III study the local control rates of 85% at 6 months and of 72% at 1 year after adjuvant SRS after surgery for patients with 1 to 3 BM (16).
In our study, 46 (30%) patients presented a nodular contrast enhancement by planning MRI, even though resection was macroscopically complete in 92% of the cases. This diagnosis is difficult, and because RANO and RECIST 1.1 criteria are not adapted in the post-surgery setting, radiologists used heterogeneous methods for diagnosis (21, 22). In the study from Jarvis et al. before post-operative radiosurgery, 12% of the patients presented a local recurrence at 1 month. The early local recurrence rate was 37.5% at 1 month in patients with a subtotal resection (23). In these studies, the median delay between surgery and radiotherapy was 4–7 weeks (11, 13, 18), but could range from 18 days (14, 24) to 4.5 months (25).
In our series, DBC rates are comparable to those reported in the literature after SRT (12). The phase III studies from Mahajan et al. and de Kocher et al. reported similar distant brain control rates, 43% at 1 year and 58% at 2 years, respectively in the observation arms (4, 16).
In this study, leptomeningeal disease seems more frequent (5, 12, 26). In Keller et al. study 89.4% [95% CI, 85.0–93.8%] and 88.9% [82.2–91.9%] of patients did not developed leptomeningeal disease at 1 and 2 year, respectively (12). In Atalar's study, 161 brain metastasis resection cavities treated from 1998 to 2011 with post-operative SRS were retrospectively reviewed. One and 2 year rates of leptomeningeal disease were 13%, but until 34% at 1 year for breast cancer (26). In our study, the rate of leptomeningeal disease may have been overestimated as we also reported very moderate leptomeningeal disease on MRI.
Median OS of patients in our study was 15.2 months [95% CI, 12.0–17.9 months] and it was comparable to that observed in other studies. In the meta-analysis by Gans et al. median OS was of 14 months; 12.2 and 17 months in the randomized studies by Brown et al. and Mahajan et al., respectively, and of 12.7 and 17.3 months in the large retrospective series by Ling et al. and Keller et al., respectively (8, 12, 16–18).
In our study, patients presenting a primary NSCLC had a lower risk of death, with an HR of 0.57 [95% CI, 0.38–0.86], (p = 0.007), with respect to patients presenting other primary tumors. The risk of death increased also with the number of extra-cerebral metastatic sites at the time of diagnosis (HR = 1.26 [95% CI, 1.08–1.48], p = 0.003) and with larger PTV (HR = 1.15 [95% CI, 1.03–1.28], p = 0.012).
In the meta-analysis by Gans et al. a higher prevalence of single metastases in the cohort was the only factor associated with higher OS (p < 0.02) (17). The study by Keller et al. reported in multivariate analysis, that a RPA score of 3 (p = 0.02), piecemeal resection (p = 0.017) and an increased number of BMs (p < 0.001) were independent prognostic factors for a lower OS. Patients with multiple BMs had a risk of death 2.4 times greater than patients with a solitary BM (p < 0.001) (12). Kocher et al. randomized phase III study evaluating the interest of adjuvant WBRT found in a multivariate analysis that the only factors with a significant impact on survival with Performance Status (PS) ≤ 2 were the initial PS (0 vs. 2, p = 0.004) and the presence of macroscopic tumor outside the brain (absent vs. present p = 0.001) (4).
Based on 7 randomized studies of the RTOG and 2,350 patients treated for BMs, Barnholtz-Sloan et al. developed a nomogram to estimate OS in patients with BM (27). The model revealed that the primary site was predictive of OS, with breast cancer and lung adenocarcinoma being associated with improved survival. Contrary to previous studies, in our series, the survival of patients with NSCLC could be improved with treatments including immunotherapy, targeted therapy and third generation tyrosine kinase inhibitors (28).
The tolerance was acceptable with 2.7 and 0.7% of patients presenting acute grade 2 and grade 3 toxicities, and 7.2 and 2.7% late grade 2 and 3 toxicities, respectively. These results are in line with the 10% toxicity rate after HFSRT revealed by the meta-analysis by Gans et al. (17). Risk of radiation necrosis has been shown to decrease with lower doses, greater number of fractions and smaller volume of the treated surgical cavity (29, 30). Eaton et al. demonstrated that for cavities bigger than 3 cm, treatment by radiosurgery was associated with a greater rate of radiation necrosis, with a HR = 3.81 [95% CI, 1.04–13.93], (p = 0.043) compared to treatment by HFSRT (29). The risk of radiation necrosis at 1 year was of 10.3% with HFSRT and of 19.2% after radiosurgery. In our study, 8.9% of patients presented a radiation necrosis during follow up and only re-irradiation was found to be predictive of radiation necrosis, possibly due to a lack of statistical power related to a low number of events. Median volumes of brain that received doses of 10 Gy (V10Gy), 12 Gy (V12Gy), and 21 Gy (V21Gy) were not found to influence radiation necrosis. In Minniti et al.'s study using a schema of 3 × 9 Gy, the rate of radiation necrosis was 7% at 1 year and 16% at 2 years (31). This study showed that V24Gy was the only factor associated with radiation necrosis with a 16.8 mL threshold (p = 0.03).
In order to standardize practice, Soliman et al. recently published CTV contouring guidelines for SRS of completely resected cavity BM defined by 10 experts based on 10 clinical cases (32). Our delineation practices are in line with these guidelines. Improvements in local control can be achieved by adding a 2 mm margin around the resection cavity (33). The choice of 1 mm to define PTV is arguable. In our study, this PTV allowed to compensate for repositioning errors. In other studies a margin between 0 and 4 mm was more frequently used. However, in Gans et al.'s meta-analysis the use of a margin to define PTV did not allow to improve local control or OS (17).
This is a retrospective study and several irradiation schemas were used. Nevertheless, the prescription regimen at the 80% isodose was homogeneous. For 55% of patients a dose of 8, 9 or 10 Gy in 3 fractions was prescribed.
This large retrospective multi-center study shows that, in our population of patients operated for BM, adjuvant treatment by HFSRT allows for good local control in the surgical cavity. This non-invasive technique was well-tolerated by patients. HFSRT is an efficient treatment option for patients with operated BM. The rate of distant recurrence and in particular leptomeningeal disease seems higher than the rate observed after WBRT. A close follow-up by MRI is necessary in patients with a high risk of intra-cerebral recurrence.
All datasets generated for this study are included in the manuscript and/or the supplementary files.
This study was carried out in accordance with the recommendations of local ethics committee and conducted in accordance with the Helsinki declaration with written informed consent from all subjects. All subjects gave written informed consent in accordance with the Declaration of Helsinki. The protocol was approved by the Centre O. Lambret ethics committee.
DP and GM contributed to the conception, design, and manuscript preparation. GM performed data acquisition. GM, JG, DS, EB, ER, NR, EE, SM-M, RM-A, LB, XM, EL, and DP performed data analysis and interpretation. EB carried out statistical analysis.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ms. Jone Iriondo for writing assistance.
HFSRT, Hypofractionated Stereotactic Radiation Therapy; BM, Brain Metastase; OS, Overall Survival; NSCLC, Non-Small Cell Lung Cancer; WBRT, Whole-Brain Radiotherapy; HFSRT, Hypofractionated Stereotactic Radiation Therapy; SRS, Stereotactic Radiosurgery; CT, Computed Tomography; MRI, Magnetic Resonance Imaging; CTV, Clinical Target Volume; GTV, Gross Tumor Volume; PTV, Planning Target Volume; DBC, Distant Brain Control; PET, Positron Emission Tomography; HR, Hazard Ratio; RMSTD, Restricted Mean Survival Time Difference; RPA, Recursive Partitioning Analysis; DS-GPA, Diagnostic-Specific Graded Prognostic Assessment; CTCAE, Common Terminology Criteria for Adverse Events.
1. Patchell RA. The management of brain metastases. Cancer Treat Rev. (2003) 29:533–40. doi: 10.1016/S0305-7372(03)00105-1
2. Patchell RA, Tibbs PA, Walsh JW, Dempsey RJ, Maruyama Y, Kryscio RJ, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. (1990) 322:494–500. doi: 10.1056/NEJM199002223220802
3. Vecht CJ, Haaxma-Reiche H, Noordijk EM, Padberg GW, Voormolen JH, Hoekstra FH, et al. Treatment of single brain metastasis: radiotherapy alone or combined with neurosurgery? Ann Neurol. (1993) 33:583–90. doi: 10.1002/ana.410330605
4. Kocher M, Soffietti R, Abacioglu U, Villà S, Fauchon F, Baumert BG, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol. (2011) 29:134–41. doi: 10.1200/JCO.2010.30.1655
5. Patchell RA, Tibbs PA, Regine WF, Dempsey RJ, Mohiuddin M, Kryscio RJ, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA. (1998) 280:1485–9. doi: 10.1001/jama.280.17.1485
6. Soffietti R, Kocher M, Abacioglu UM, Villa S, Fauchon F, Baumert BG, et al. A European Organisation for Research and Treatment of Cancer phase III trial of adjuvant whole-brain radiotherapy versus observation in patients with one to three brain metastases from solid tumors after surgical resection or radiosurgery: quality-of-life results. J Clin Oncol. (2013) 31:65–72. doi: 10.1200/JCO.2011.41.0639
7. Chang EL, Wefel JS, Hess KR, Allen PK, Lang FF, Kornguth DG, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. (2009) 10:1037–44. doi: 10.1016/S1470-2045(09)70263-3
8. Brown PD, Ballman KV, Cerhan JH, Anderson SK, Carrero XW, Whitton AC, et al. Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC·3): a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. (2017) 18:1049–60. doi: 10.1016/S1470-2045(17)30441-2
9. Wang C-C, Floyd SR, Chang C-H, Warnke PC, Chio CC, Kasper EM, et al. Cyberknife hypofractionated stereotactic radiosurgery (HSRS) of resection cavity after excision of large cerebral metastasis: efficacy and safety of an 800 cGy × 3 daily fractions regimen. J Neurooncol. (2012) 106:601–10. doi: 10.1007/s11060-011-0697-z
10. Minniti G, Esposito V, Clarke E, Scaringi C, Lanzetta G, Salvati M, et al. Multidose stereotactic radiosurgery (9 Gy × 3) of the postoperative resection cavity for treatment of large brain metastases. Int J Radiat Oncol Biol Phys. (2013) 86:623–9. doi: 10.1016/j.ijrobp.2013.03.037
11. Pessina F, Navarria P, Cozzi L, Ascolese AM, Maggi G, Riva M, et al. Outcome evaluation of oligometastatic patients treated with surgical resection followed by hypofractionated stereotactic radiosurgery (HSRS) on the tumor bed, for single, large brain metastases. PLoS ONE. (2016) 11:e0157869. doi: 10.1371/journal.pone.0157869
12. Keller A, Doré M, Cebula H, Thillays F, Proust F, Darié I, et al. Hypofractionated stereotactic radiation therapy to the resection bed for intracranial metastases. Int J Radiat Oncol Biol Phys. (2017) 99:1179–89. doi: 10.1016/j.ijrobp.2017.08.014
13. Ahmed KA, Freilich JM, Abuodeh Y, Figura N, Patel N, Sarangkasiri S, et al. Fractionated stereotactic radiotherapy to the post-operative cavity for radioresistant and radiosensitive brain metastases. J Neurooncol. (2014) 118:179–86. doi: 10.1007/s11060-014-1417-2
14. Al-Omair A, Soliman H, Xu W, Karotki A, Mainprize T, Phan N, et al. Hypofractionated stereotactic radiotherapy in five daily fractions for post-operative surgical cavities in brain metastases patients with and without prior whole brain radiation. Technol Cancer Res Treat. (2013) 12:493–9. doi: 10.7785/tcrt.2012.500336
15. Royston P, Parmar MKB. The use of restricted mean survival time to estimate the treatment effect in randomized clinical trials when the proportional hazards assumption is in doubt. Stat Med. (2011) 30:2409–21. doi: 10.1002/sim.4274
16. Mahajan A, Ahmed S, McAleer MF, Weinberg JS, Li J, Brown P, et al. Post-operative stereotactic radiosurgery versus observation for completely resected brain metastases: a single-centre, randomised, controlled, phase 3 trial. Lancet Oncol. (2017) 18:1040–8. doi: 10.1016/S1470-2045(17)30414-X
17. Gans JH, Raper DMS, Shah AH, Bregy A, Heros D, Lally BE, et al. The role of radiosurgery to the tumor bed after resection of brain metastases. Neurosurgery. (2013) 72:317–25; discussion 325–6. doi: 10.1227/NEU.0b013e31827fcd60
18. Ling DC, Vargo JA, Wegner RE, Flickinger JC, Burton SA, Engh J, et al. Postoperative stereotactic radiosurgery to the resection cavity for large brain metastases: clinical outcomes, predictors of intracranial failure, and implications for optimal patient selection. Neurosurgery. (2015) 76:150–6; discussion 156–7; quiz 157. doi: 10.1227/NEU.0000000000000584
19. Patel AJ, Suki D, Hatiboglu MA, Abouassi H, Shi W, Wildrick DM, et al. Factors influencing the risk of local recurrence after resection of a single brain metastasis. J Neurosurg. (2010) 113:181–9. doi: 10.3171/2009.11.JNS09659
20. Robbins JR, Ryu S, Kalkanis S, Cogan C, Rock J, Movsas B, et al. Radiosurgery to the surgical cavity as adjuvant therapy for resected brain metastasis. Neurosurgery. (2012) 71:937–43. doi: 10.1227/NEU.0b013e31826909f2
21. Alexander BM, Brown PD, Ahluwalia MS, Aoyama H, Baumert BG, Chang SM, et al. Clinical trial design for local therapies for brain metastases: a guideline by the Response Assessment in Neuro-Oncology Brain Metastases working group. Lancet Oncol. (2018) 19:e33–42. doi: 10.1016/S1470-2045(17)30692-7
22. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. (2009) 45:228–47. doi: 10.1016/j.ejca.2008.10.026
23. Jarvis LA, Simmons NE, Bellerive M, Erkmen K, Eskey CJ, Gladstone DJ, et al. Tumor bed dynamics after surgical resection of brain metastases: implications for postoperative radiosurgery. Int J Radiat Oncol Biol Phys. (2012) 84:943–8. doi: 10.1016/j.ijrobp.2012.01.067
24. Ogiwara H, Kalakota K, Rakhra SS, Helenowski IB, Marymont MH, Kalapurakal JA, et al. Intracranial relapse rates and patterns, and survival trends following post-resection cavity radiosurgery for patients with single intracranial metastases. J Neurooncol. (2012) 108:141–6. doi: 10.1007/s11060-012-0808-5
25. Prabhu R, Shu HK, Hadjipanayis C, Dhabaan A, Hall W, Raore B, et al. Current dosing paradigm for stereotactic radiosurgery alone after surgical resection of brain metastases needs to be optimized for improved local control. Int J Radiat Oncol Biol Phys. (2012) 83:e61–66. doi: 10.1016/j.ijrobp.2011.12.017
26. Atalar B, Modlin LA, Choi CYH, Adler JR, Gibbs IC, Chang SD, et al. Risk of leptomeningeal disease in patients treated with stereotactic radiosurgery targeting the postoperative resection cavity for brain metastases. Int J Radiat Oncol Biol Phys. (2013) 87:713–8. doi: 10.1016/j.ijrobp.2013.07.034
27. Barnholtz-Sloan JS, Yu C, Sloan AE, Vengoechea J, Wang M, Dignam JJ, et al. A nomogram for individualized estimation of survival among patients with brain metastasis. Neuro Oncol. (2012) 14:910–8. doi: 10.1093/neuonc/nos087
28. Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. (2015) 373:1627–39. doi: 10.1056/NEJMoa1507643
29. Eaton BR, LaRiviere MJ, La Riviere MJ, Kim S, Prabhu RS, Patel K, et al. Hypofractionated radiosurgery has a better safety profile than single fraction radiosurgery for large resected brain metastases. J Neurooncol. (2015) 123:103–11. doi: 10.1007/s11060-015-1767-4
30. Shaw E, Scott C, Souhami L, Dinapoli R, Kline R, Loeffler J, et al. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: final report of RTOG protocol 90-05. Int J Radiat Oncol Biol Phys. (2000) 47:291–8. doi: 10.1016/S0360-3016(99)00507-6
31. Minniti G, Scaringi C, Paolini S, Lanzetta G, Romano A, Cicone F, et al. Single-fraction versus multifraction (3 × 9 Gy) stereotactic radiosurgery for large (>2 cm) brain metastases: a comparative analysis of local control and risk of radiation-induced brain necrosis. Int J Radiat Oncol Biol Phys. (2016) 95:1142–8. doi: 10.1016/j.ijrobp.2016.03.013
32. Soliman H, Ruschin M, Angelov L, Brown PD, Chiang VLS, Kirkpatrick JP, et al. Consensus contouring guidelines for postoperative completely resected cavity stereotactic radiosurgery for brain metastases. Int J Radiat Oncol Biol Phys. (2018) 100:436–42. doi: 10.1016/j.ijrobp.2017.09.047
Keywords: radiotherapy, hypofractionated stereotactic radiation therapy, brain metastasis, surgery, Cyberknife
Citation: Martinage G, Geffrelot J, Stefan D, Bogart E, Rault E, Reyns N, Emery E, Makhloufi-Martinage S, Mouttet-Audouard R, Basson L, Mirabel X, Lartigau E and Pasquier D (2019) Efficacy and Tolerance of Post-operative Hypo-Fractionated Stereotactic Radiotherapy in a Large Series of Patients With Brain Metastases. Front. Oncol. 9:184. doi: 10.3389/fonc.2019.00184
Received: 16 November 2018; Accepted: 04 March 2019;
Published: 28 March 2019.
Edited by:
Christopher Schultz, Medical College of Wisconsin, United StatesReviewed by:
Michael Charles Repka, Winthrop University Hospital, United StatesCopyright © 2019 Martinage, Geffrelot, Stefan, Bogart, Rault, Reyns, Emery, Makhloufi-Martinage, Mouttet-Audouard, Basson, Mirabel, Lartigau and Pasquier. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: David Pasquier, ZC1wYXNxdWllckBvLWxhbWJyZXQuZnI=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.