
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
METHODS article
Front. Oncol. , 20 March 2019
Sec. Cancer Genetics
Volume 9 - 2019 | https://doi.org/10.3389/fonc.2019.00119
 Rebecca F. Halperin1*
Rebecca F. Halperin1* Winnie S. Liang2
Winnie S. Liang2 Sidharth Kulkarni1
Sidharth Kulkarni1 Erica E. Tassone2
Erica E. Tassone2 Jonathan Adkins2
Jonathan Adkins2 Daniel Enriquez2
Daniel Enriquez2 Nhan L. Tran3
Nhan L. Tran3 Nicole C. Hank4
Nicole C. Hank4 James Newell5
James Newell5 Chinnappa Kodira6,7
Chinnappa Kodira6,7 Ronald Korn4,5
Ronald Korn4,5 Michael E. Berens8
Michael E. Berens8 Seungchan Kim9
Seungchan Kim9 Sara A. Byron2
Sara A. Byron2Archival tumor samples represent a rich resource of annotated specimens for translational genomics research. However, standard variant calling approaches require a matched normal sample from the same individual, which is often not available in the retrospective setting, making it difficult to distinguish between true somatic variants and individual-specific germline variants. Archival sections often contain adjacent normal tissue, but this tissue can include infiltrating tumor cells. As existing comparative somatic variant callers are designed to exclude variants present in the normal sample, a novel approach is required to leverage adjacent normal tissue with infiltrating tumor cells for somatic variant calling. Here we present lumosVar 2.0, a software package designed to jointly analyze multiple samples from the same patient, built upon our previous single sample tumor only variant caller lumosVar 1.0. The approach assumes that the allelic fraction of somatic variants and germline variants follow different patterns as tumor content and copy number state change. lumosVar 2.0 estimates allele specific copy number and tumor sample fractions from the data, and uses a to model to determine expected allelic fractions for somatic and germline variants and to classify variants accordingly. To evaluate the utility of lumosVar 2.0 to jointly call somatic variants with tumor and adjacent normal samples, we used a glioblastoma dataset with matched high and low tumor content and germline whole exome sequencing data (for true somatic variants) available for each patient. Both sensitivity and positive predictive value were improved when analyzing the high tumor and low tumor samples jointly compared to analyzing the samples individually or in-silico pooling of the two samples. Finally, we applied this approach to a set of breast and prostate archival tumor samples for which tumor blocks containing adjacent normal tissue were available for sequencing. Joint analysis using lumosVar 2.0 detected several variants, including known cancer hotspot mutations that were not detected by standard somatic variant calling tools using the adjacent tissue as presumed normal reference. Together, these results demonstrate the utility of leveraging paired tissue samples to improve somatic variant calling when a constitutional sample is not available.
Somatic mutations often drive cancer initiation and progression. The identification of somatic mutations through next generation sequencing has enabled the identification of cancer driver events in individual patient tumor samples (1–4). There is also ongoing effort to discover new cancer driver mutations, particularly in non-coding regions (5). Although sequencing of tumor-associated cancer gene panels and exomes is starting to be adopted in clinical practice to personalize therapy, there is much to learn about how mutation status correlates with response to therapy. Clinically annotated archival tissue collections represent a rich resource for identifying new driver mutations and clarifying how genomic features relate to clinical outcomes (6, 7). There are a number of sophisticated approaches for distinguishing driver from passenger mutations, but they all require accurate variant calls as inputs (8). In order to accurately distinguish somatic from germline variant, it is important to have a matched constitutional sample. However, most archival collections do not contain blood samples or other normal tissue samples from locations distant to the tumor to use as a constitutional reference. Here we present a novel approach to get more accurate somatic variant calls from archival samples.
Often, histologically normal tissue is available alongside the tumor biopsy or resection. For instance, surgeons typically remove a margin of adjacent normal tissue when resecting a tumor. This normal tissue can be leveraged for DNA sequencing to identify germline variants. However, histologically normal tissue may still have detectable molecular alterations for a variety of reasons. For example, it is difficult to know if the adjacent normal tissue is truly free of infiltrating tumor cells. Contamination of the adjacent normal tissue with the tumor tissue during processing could also confound interpretation of the results (9). Also, even without infiltrating tumor cells, the adjacent tissue may contain somatic mutations. Field cancerization, where molecular alterations are observed in tissue adjacent to the overt cancer, is thought to be an important risk factor for multifocal and recurrent disease (10). This phenomenon has been observed in many cancer types including breast (11) and prostate (12). Even healthy individuals have somatic mutations in normal tissues, and the mutation patterns tend to be similar to those of the cancers arising from that tissue type (13). Clonal hematopoiesis represents a well-documented example of somatic mutations in a normal tissue. There even appears to be positive selection for cancer driver mutations in normal skin (14). Therefore, it is important to consider potential sources of somatic variant contamination when normal tumor-adjacent tissue is used to identify tumor specific somatic variants.
When tumor-only sequencing data is available, researchers have developed various analytic strategies to distinguish germline and somatic variants. One obvious first step to identify somatic variants in tumor-only sequencing data is to filter out the germline variants found in population databases. Jones et al. showed that filtering alone is not sufficient, as each individual typically has an average of 249 private germline variants not found in the population databases that would be incorrectly classified as somatic in tumor-only sequencing (15). The number of private germline variants will vary based on the individual's ancestry. The private variant rate in a population depends both on how well-represented the population is in large scale sequencing projects, as well as the extent to which the population has undergone a recent expansion adding to the diversity of variants (16). More recently, Kalatskaya et al. published a machine-learning approach (ISOWN) to classify somatic and germline variants from tumor-only sequencing data (17). Their approach requires a large training set, and performs best when the training and test datasets are from the same cancer type and patient cohort. In the case of rare cancer types and case studies, obtaining such training sets may not be practical. The variant allele fraction, which is the fraction of reads supporting the mutated allele at a given locus, can also help to distinguish somatic from germline variants in impure tumors; the somatic variants should only be present in the tumor cells, leading to a low variant allele fraction, while the germline variants would be present in both the tumor and normal cells in the sample, leading to a variant allele fraction close to 0.5 for heterozygous variants. We, along with several other groups, have previously described methods to use the variant allele fractions to distinguish somatic and germline variants including somVarIUS (18), PureCN (19), and lumosVar 1.0. SomVarIUS assumes that variants with similar allele fractions to common germline variants within a copy number segment are germline and those with significantly different allelic fractions are somatic. Both PureCN and lumosVar 1.0 are conceptually similar in that they explicitly model integer copy number states and used the expected allelic fractions of somatic and germline variants to calculate likelihoods that variants are somatic or germline, though they differ in many of the model details. PureCN explicitly models tumor purity using the copy number and germline variant allele fractions, treating sub-clonal copy number alterations as an exception to the model. The lumosVar 1.0 model finds groups of both copy number alterations and somatic mutations that appear to occur in the same fraction of cells in the sample, thus treating sub-clonal variants more explicitly and allowing somatic mutations to inform the estimate of tumor purity. PureCN requires mutation calls as input and only removes variants that do not appear diploid in a set of unmatched normal, while lumosVar 1.0 does its own variant calling and quality filtering, taking into account both unmatched normal data and the tumor itself. A major limitation that all of these approaches have in common is that some combinations of copy number alterations and tumor purity can lead to considerable overlap in the expected somatic and germline variant allele fractions and greatly reduce the power to detect somatic variants (Figure 1). Thus, there is a need for new bioinformatics methods to call germline and somatic variants from tumor samples with high sensitivity and precision, even in the absence of a germline sample.
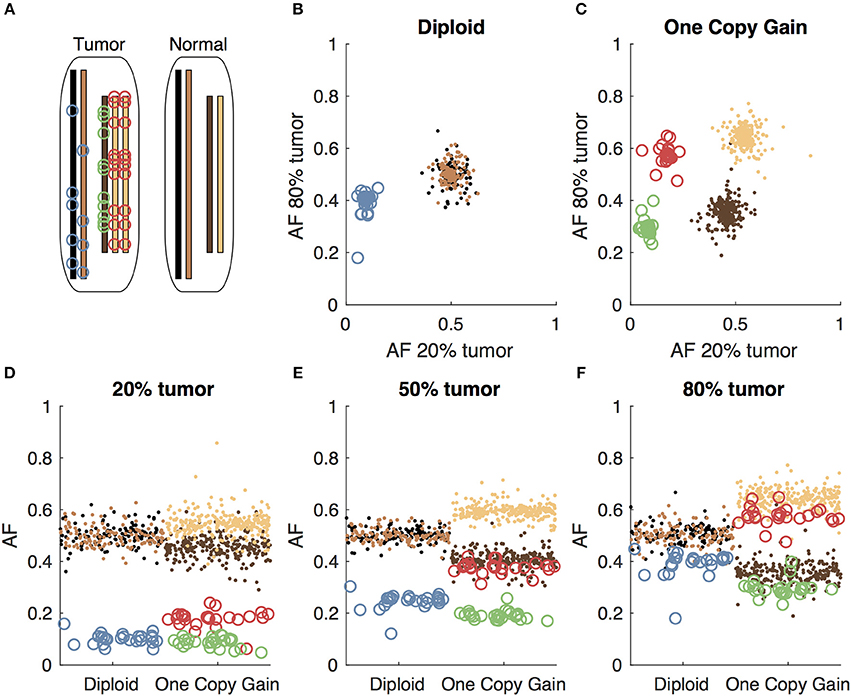
Figure 1. Somatic and germline variant allelic fractions example. (A) Two chromosomes are illustrated for this example. Both chromosomes are present in the diploid state in the normal cell. In the tumor cell, one chromosome is in the diploid state, and the other shows one-copy gain. Blue circles represent somatic variants on the diploid chromosome, green and red circles represent somatic variants on the minor and major alleles of the gained chromosome, respectively. Simulated allelic fractions of germline variants (brown/tan) and somatic variants are plotted for a simulated 20% tumor (D), 50% tumor (E) and 80% tumor (F) by chromosome position. In the 50% tumor example, somatic variants could easily be distinguished from germline on the diploid chromosome, but on the copy number gain chromosome, the allelic fractions of the somatic variants on the major allele overlap with the germline variants. By using both the 20 and 80% tumor samples, the somatic variants can be separated from the germline variants by allelic fraction on both the diploid chromosome (B) and the copy number gain chromosome (C).
Here, we present a new bioinformatics approach (lumosVar 2.0) that leverages adjacent normal tissue from tumor biopsies and permits somatic mutations to be present in the adjacent tissue. Similar to our previous approach (lumosVar 1.0—single sample-based variant caller) (16), we model allelic copy number to determine the expected allelic fractions for somatic and germline variants as well as incorporate population database frequencies to call variants as somatic or germline. We have extended the approach to find the joint probability of somatic and germline mutations across multiple samples from the same patient. We hypothesize that the patterns of allelic fractions across samples of different purities will be more informative than any individual sample in distinguishing somatic from germline variants (Figure 1). To test this hypothesis, we compare two approaches (1) jointly calling variants using two samples of different purities (joint approach) and (2) pooling the two samples resulting in one sample with twice the sequencing depth and the average of the purities (pooled approach). First, we used simulations to systematically evaluate the effects of tumor purity and copy number states for the two approaches. Next, we looked at a set of glioblastoma (GBM) patient samples where we have sequencing data for contrast-enhancing region (CE, high fraction of tumor cells) and non-enhancing region (NE, low fraction of tumor cells) biopsies, as well as sequencing data from peripheral blood samples to establish true somatic calls. Finally, we applied our method to an archival cohort of breast and prostate samples where FFPE sections from the tumor biopsies or resections were the only tissues available.
We used simulated read count data to systematically determine how the purity of the two tumor samples, the copy number, and the read depth affect our ability to detect somatic variants. The simulations were performed as previously described (16), where the total read depths were drawn from a log normal distribution and the number of reads supporting the variant were drawn from a binomial distribution with a probability of success of the expected allelic fraction given the tumor purity and copy number state. We simulated 1,000 somatic variants and 10,000 germline heterozygous variants for each coverage level and copy number state. To evaluate how the joint calling approach compares to the single sample approach, we added the read depths of each pair of simulated tumor samples used jointly.
A set of previously collected and de-identified whole exome data from seven recurrent GBM patients was used to evaluate the approach (Table 1). Each patient dataset contained exome sequencing data for CE biopsies (high tumor content), NE biopsies (low tumor content), and peripheral blood (germline). The acquisition and sequencing of these samples was performed following IRB approval and patient informed consent, as previously described (20). The consensus of three comparative somatic variant callers [seurat (21), strelka (22), and mutect (23)] using the CE samples as tumor and the peripheral blood as normal was used to define the true somatic variants. Variants called by only one of the three somatic variant callers were not counted as true positives or true negatives in the evaluation. Since lumosVar 2.0 could call variants that were only found in the NE samples, but that was not the goal of the evaluation, we also ran the three paired somatic variant callers using the NE samples as the tumor and the blood as the normal, and excluded variants detected in only the NE samples from the evaluation. In order to evaluate the benefit of jointly calling high tumor and low tumor content samples compared to our prior single sample tumor only approach, we merged the bam files from the CE and NE samples, and also called variants on these merged bams using lumosVar 2.0. We call the lumosVar 2.0 analysis of the merged CE and NE bams the pooled approach. In order to compare our results to what one would expect from using germline snp databases to classify variants as somatic or germline, we used dbSNPv149 (24), after excluding snps where the allele of origin was annotated as somatic. We determined the number of likely germline false positives based on the number of heterozygous variants called by GATK HaplotypeCaller (25) that were not found in the somatic excluded dbSNP set, and the number of somatic false negatives based on the number of true somatic variants found in the somatic excluded dbSNP set.
De-identified FFPE tissue sections, clinical data, and pathology data were acquired for 20 breast cancer patients and 20 prostate cancer patients from HonorHealth Scottsdale Shea Medical Center, in accordance with local institutional review boards and in compliance with the Health Insurance Portability and Accountability Act (HIPAA) (Table 1). Prostate cancer specimens were collected under IRB approved protocol with 45 CFR 46.111 (d) exemption; breast cancer specimens were collected under IRB approved protocol including patient informed consent per institutional policy and procedures. Retrospective analysis was performed using archival samples from treatment-naïve, invasive breast carcinomas or treatment-naïve prostate adenocarcinomas.
Breast tumors were collected following routine clinical lumpectomy or mastectomy, from women diagnosed with ER-positive, invasive mammary carcinoma between 2010 and 2016 at HonorHealth Scottsdale. Median age of diagnosis was 65 years and ranged from 39 to 86 years. All tumors were classified by pathology as estrogen receptor-positive. Nineteen of the twenty tumors were classified as HER2-negative. The breast tumor cohort spanned AJCC stages (IA-IV). Prostate tumors were collected following radical prostatectomy for men diagnosed with prostate adenocarcinoma between 2012 and 2016 at HonorHealth Scottsdale. Median age of diagnosis was 67 years, ranging from 57 to 74 years. Eighteen of the twenty tumors had a Gleason score of seven or greater. ER/PR/HER2 status (breast tumors), Gleason score (prostate tumors), histological type, tumor stage, treatment history, and clinical outcome, including progression-free survival and overall survival, were collected from medical records and the de-identified data was provided for this study. Pathology review (JN) identified a tissue block with high tumor content and a tissue block with a region considered to have low tumor content for each patient. Five 10-micron sections were provided for each sample (5 high tumor content; 5 low tumor content). The Qiagen GeneRead FFPE DNA Kit (cat# 180134) was used to isolate DNA from FFPE breast and prostate cancer tumor specimens (N = 80) following the manufacturer's protocol.
Exome libraries were constructed from 200 ng of DNA (DIN = 3–5) using KAPA Biosystems' Hyper Prep Kit (cat#KK8504) and the same bait set that was used in the evaluation dataset, following the manufacturer's protocols. The bait set included Agilent's SureSelectXT V5 baits plus custom content including copy number probes distributed across the entire genome, along with additional probes targeting tumor suppressor genes and genes involved in common cancer translocations to enable structural analysis. Libraries were equimolarly pooled, quantitated, and sequenced by synthesis on the Illumina HiSeq 4000 for paired 82 bp reads.
Two other variant calling approaches were applied to the archival samples for comparison. The first approach, called unmatched plus filtering (UPF) used the high tumor content samples as the tumor and an unmatched normal (GM12878) as the normal in the paired somatic variant callers (mutect, strelka, and seurat). dbSNP and COSMIC were used to classify variants as somatic or germline. Variants that were called by at least two of the three paired somatic variant callers (or two out of two for indels, since mutect does not call indels) and were not present in dbSNP or were present in dbSNP and also present in COSMIC were considered somatic calls in the UPF approach. In the second approach, called adjacent normal as reference (ANR), the high tumor content sample was used as the tumor sample and the adjacent normal sample was used as the normal in the paired somatic variant callers (mutect, strelka, and seurat). Variants called by at least two out of the three paired somatic variant callers were considered somatic calls in the ANR approach.
We previously created a single-sample strategy (lumosVar 1.0) to call somatic variants in impure tumor samples based on the differences in allelic frequency between the somatic and germline variants (16). Here, we describe an extension of lumosVar 1.0 to jointly analyze multiple samples from the same patient. The lumosVar 2.0 analysis has seven main steps (Figure 2). First, a set of unmatched control samples is analyzed for position quality scores and average read depth, as previously described (16). Second, read counts and quality metrics are extracted from the tumor bams. Third, quality scores are calculated for each candidate variant position. Fourth, segmentation is performed to define regions that have similar tumor/normal read depth ratios and B-allele fractions. The fifth step involves finding the most likely allele-specific copy number state for each segment. The sixth step involves classifying each candidate variant position as somatic, germline heterozygous, or homozygous. The final step entails optimization of the model parameters. The caller iterates between steps five, six, and seven until the solution converges. Model input parameters and notation are shown in Tables 2, 3.
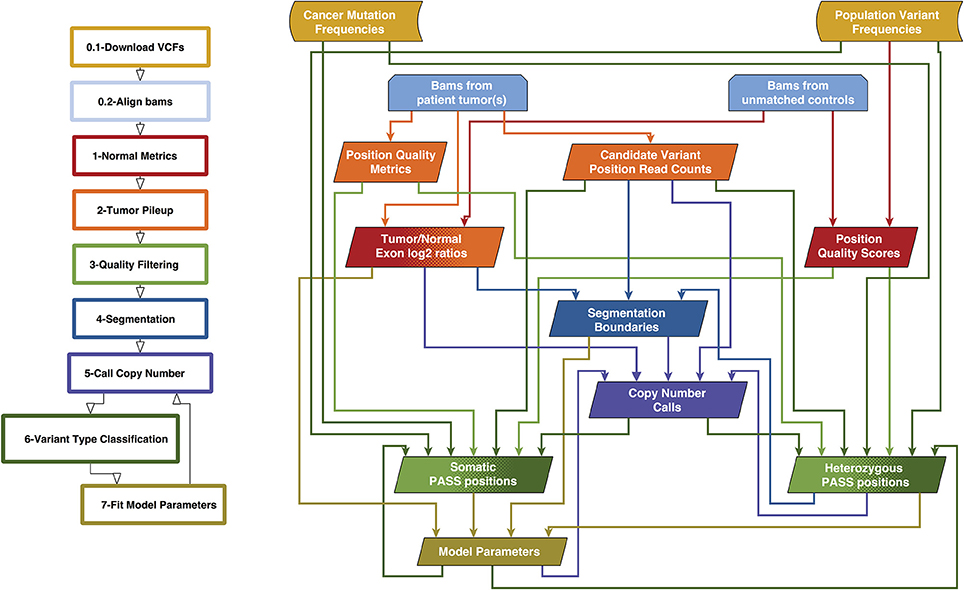
Figure 2. Overview of lumosVar 2.0 analysis. The flow-chart on the left show the main steps in the analysis. Steps 0.1 and 0.2 are data preparation, and steps 1–7 are performed by lumosVar 2.0. The graph on the right illustrates the main inputs and outputs of each step. The color of the arrows coming from each box indicates the steps where that data is used as input, and the color of each box indicates the step where the data is generated.
We used 16 quality metrics in a quadratic discriminant model to determine the posterior probability that each position belongs to a PASS group, as was previously used in lumosVar 1.0. The same quality metrics and thresholds are used in lumosVar 2.0 as were used in lumosVar 1.0 to assign candidate variant positions to PASS and REJECT training groups (16). As previously, here we also fit the model separately on candidate indel and point mutation positions. Since the quality metrics for the B allele are not relevant for homozygous positions, after the joint variant calling is performed as described below, we repeat the quality classification step fitting homozygous positions separately after setting all the quality metrics to only use the “A” allele and setting the difference metrics to zero. The model is fit independently on each sample, and then we calculate a trust score by taking the geometric mean of the posterior probability of belonging to the PASS group weighted by the number of reads supporting the variant across the samples. Candidate positions with a trust score greater than a threshold (Tpass) are considered for classification as somatic or germline variants as described in the joint somatic variant calling section below.
Prior to fitting the copy number model, segmentation is performed. We use the circular binary segmentation algorithm implemented in the Matlab Bioinformatics toolbox to segment both the tumor to normal read depth log2 ratio of each exon and the B-allele frequencies of common heterozygous variants. Segmentation is performed independently on each sample. We combine all of the segmentation boundaries from all of the samples for both the read depth log2 ratio and B-allele frequency segmentation, and then remove non-significant segments as follows. A two-sample t-test is used to compare each pair of adjacent segments for both the read depth log2 ratios and the B-allele fractions for each sample. For each segmentation boundary, the geometric mean is used to combine the p-values across the samples and data types. The segmentation boundary with the highest geometric mean p-value is removed, and the t-tests are then performed on the newly merged segment with its neighbors. This process is continued until of the segmentation boundaries have geometric mean p-values less than the segmentation significance threshold (α seg).
We use an expectation maximization approach to fit the model parameters and call variants. In the initial iteration, heuristics are used to find reasonable values of the model parameters. In the expectation step, the model parameters are used to identify somatic and germline heterozygous variant positions. Identifying these variant positions involves finding the copy number states of each segment, joint variant type classification, and variant quality filtering, all as described below. Using those variant positions, values of the model parameters that maximize the likelihood of the data are found.
The parameter f is a matrix with the number of rows corresponding to the number of clonal variant groups (K) and the number of columns corresponding the number of samples (J). The initial value of f is set such that there is a main clonal variant group that has a high sample fraction in all samples, there are J clonal variant groups that are clonal in each sample and low in the other samples, and there are another J clonal variant groups that are sub-clonal in each sample and very low in the other samples. The centering and spread parameters (C and W) are both vectors of length J, and their initial values are determined as previously described (16).
The copy number state of each segment (g) may be described by the total copy number (Ng), the minor allele copy number (Mg), and the index of clonal variant group of the segment (kg). The values of Ng, Mg, and kg are found that maximize a sum of log likelihoods for the segment (SLLg).
This sum includes the likelihoods of the exon mean read counts (Lxj), heterozygous variant read counts (Lyj), number of common germline variant positions that would be called germline heterozygous (LYdg) or somatic (LZdg), as well as the prior probabilities of the copy number states (π (N), π (M)) and sample fraction difference .
The likelihood calculations are defined below.
The values of f, W, and C are found that maximize the sum of segment log likelihoods (SLLg) and somatic variant log-likelihoods (Lz). Since the number of clonal variant groups (K) changes the degrees of freedom of the model, as f is a J by K matrix, we include a penalty term for increasing K.
In order to more efficiently search the parameter space, we use the parameter values from the previous EM iteration (or the initial values in the first iteration) as the starting point for the parameter optimization. Since the heuristic used to find the initial value of C may be incorrect, particularly for higher ploidy genomes, other values of the centering parameter are also tested, and the best one is used as a starting point for optimization of all parameters. In order to find a reasonable starting point for adding an additional clonal variant group, we use the previous f matrix and test a set of random values for the additional column. We use the best one as the starting point for optimizing all of the parameters. If the maximum likelihood score improves, the procedure is repeated with an additional clonal variant group. This process continues until adding a clonal variant group fails to improve the likelihood score. Since adding a clonal variant group may make a previous clonal variant group less important to the model, we also test removing each clonal variant group, and then do another round of optimization of all parameters. If this results in a new maximum, then the procedure will be repeated removing another clonal variant group. Once removing clonal variant groups no longer improves the model, the procedure returns to re-centering. The re-centering, adding clonal variant groups, and removing clonal variant groups is repeated until there are ξ consecutive iterations with no new maximum found.
As in lumosVar 1.0, the likelihood of the exon mean read depths are modeled as a Poisson distribution, and the somatic and germline heterozygous read counts are modeled as beta binomial distributions.
The expected germline heterozygous variant allele fraction is determined as follows.
lumosVar 2.0 considers three possible scenarios when finding the expected somatic variant allele fraction: (1) variant is in the same clonal variant group as the copy number alteration effecting the segment and is on the minor allele (kzj ≡ kgj∧Az ≡ 1), (2) variant is in the same clonal variant group as the copy number alteration effecting the segment and is on the major allele (kzj ≡ kgj ∧ Az ≡ 2), or (3) variant is in a non-copy number altered clonal variant group (kzj ≠ kgj).
The maximum likelihood is used to determine the clonal variant group assignment and allele of each somatic variant.
The probability of detecting a heterozygous variant in each segment is calculated based on the cumulative probability of observing at least the minimum number of reads required to be considered a candidate variant position (RB−min), given the mean read depth in the segment (RT) and the expected allele fraction of a heterozygous variant in that segment ().
In order to determine if parameter values would result in reasonable variant counts, the variant type classification is performed at common germline variant positions. The likelihood of detecting fewer than the observed number of heterozygous variants in a segment (Ydg) is modeled as the cumulative probability from a binomial distribution with Ydg successes, the number of bases examined in the segment (ηdg), and Phet probability of success.
In order to penalize models that would result in germline variants being called somatic, we then determine the likelihood of finding that many or more somatic variants in germline variant positions based on the cumulative probability from a binomial distribution with Zdg somatic variants detected of ηdg database variant positions tested, with a probability of success of ρSNV.
In lumosVar 1.0, we set a prior distribution on f in order to favor models where the sample fractions are close to what is expected. In lumosVar 2.0, we set a prior distribution on the difference in f across the samples to favor models where the sample fractions differ as much or more than the expected sample fractions. The mean difference is found for prior tumor sample fractions (f π) as follows:
The mean difference of the sample fractions for each clone is found similarly.
The prior probability that the sample fractions for each clone have a mean difference as much as or greater than observed is calculated from a beta distribution with a mode of the difference in the prior tumor sample fractions.
The probability of observing the read counts in each sample (k) given that the variant is somatic (P (Dk|S)), germline heterozygous (P (Dk|GAB)), germline homozygous (P (Dk|GAA)), or another genotype (P (Dk|O)) are calculated as previously described. The prior probabilities are also calculated as previously described (16). The product of the conditional probabilities across the set of samples gives the joint probability of each variant type, given all the samples' data, as we assume that the read counts for each sample are independent. The posterior probability that a position has a somatic variant given all the samples' data is calculated as shown below.
A custom pileup engine was written in C using htslib (https://github.com/tgen/gvm). The pileup engine extracts the mean exon read depths and calculates the quality scores form the unmatched control bams, as well as extracts the read counts and quality metrics from the tumor bams. The rest of the lumosVar 2.0 analysis was written in Matlab (https://github.com/tgen/lumosVar2). A precompiled binary is provided which enables users to run lumosVar 2.0 without a Matlab license.
Simulations were performed to determine how the tumor purity and copy number states affect the power to detect somatic variants in the joint approach, and how the power compares to the pooled approach. As previously shown, the pooled single sample approach performs best with a sample of intermediate tumor purity for variants in diploid regions, but copy number variation leads to situations where the expected somatic and germline allele fractions are very similar, making it difficult to classify somatic variants using a single sample (16). From the simulation results, we can see that the joint approach mitigates this limitation, and only provides poor detection when both samples fall into a range where the expected somatic and germline allele fractions are very similar (Figure 3). The joint approach generally only requires low-to-moderate coverage when one sample has low tumor content and the other sample has moderate-to-high tumor content.
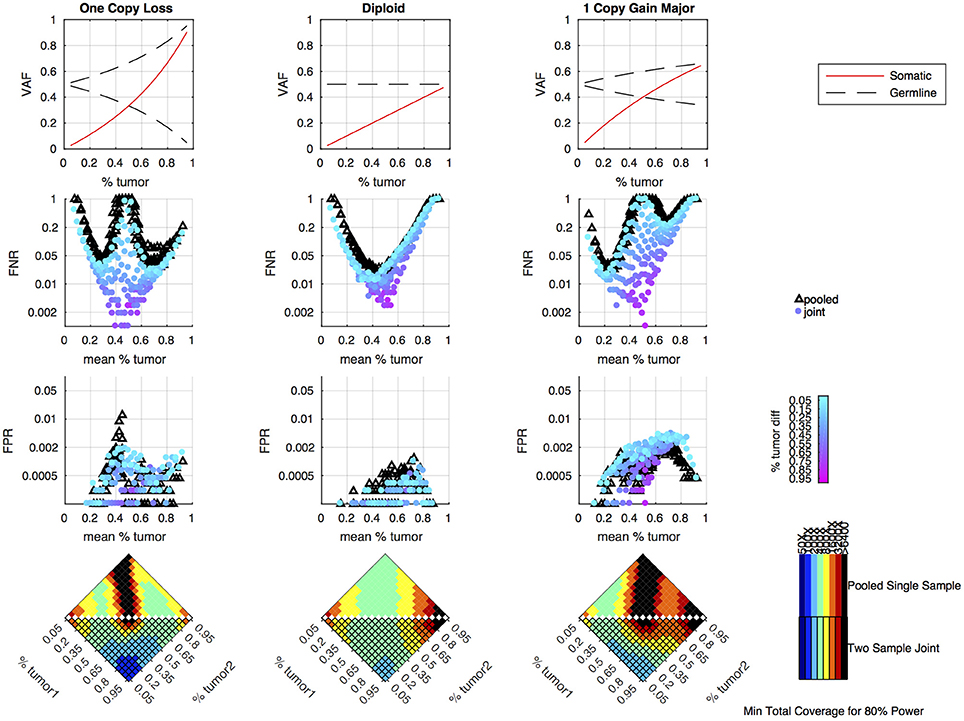
Figure 3. Simulation results comparing pooled and joint approaches. Top row of graphs shows the expected allele frequency of somatic (red) and germline variants (black) by tumor content (x-axis) for different copy number states. The middle two rows of graphs are based on simulation results using a mean coverage of 200X per sample (400X pooled). They show the false negative rate (FNR—simulated somatic variants not called somatic) and false positive rate (FPR—simulated germline heterozygous variants falsely called somatic) plotted by mean tumor content for the pooled (black triangles) and joint (colored circles) approaches. For the joint approach, the color of the circles represents the difference in tumor content between the two samples analyzed jointly. The bottom set of graphs shows the coverage required to detect at least 80% of the simulated somatic variants using two samples of different tumor content (shown on the x and y axis) using a joint approach (lower triangle of each heatmap) or using a single-sample approach on a merged sample with a tumor content that is the average of the two samples and coverage that is the sum of the two samples (upper triangle of heatmap). The color indicates the mean target coverage in the pooled approach, or the sum of the mean target coverage in the two-sample joint approach. Black squares indicate that <80% of the somatic variants were detected at the highest coverage simulated (6400X).
While the single sample version of lumosVar also assigned somatic mutations and copy number alterations to clonal variant groups, these clonal variant groups become much more informative when looking at more than one patient sample. An example patient's results are shown in Figure 4. There are three clonal variant groups found in this patient, one that appears clonal in both samples (blue), one that appears sub-clonal in the enhancing biopsy and not detected in the non-enhancing biopsy (red), and one that appears clonal in the non-enhancing biopsy and sub-clonal in the enhancing biopsy (green). From these clonal variant groups, we can infer that the blue and red variants are likely found in the same cells because their sample fractions in the CE sample would add up to >100%. However, it is not possible to definitively determine from these data whether the blue and green variants are found in the same cells. The blue variants may be “trunk” mutations found in all of the tumor cells, which would imply that roughly 65% of the cells in the NE sample, and 20% of the cells in the CE sample are normal cells. It is also possible that blue and green variants are found in different sets of tumor cells, implying that roughly 35% of cells in the NE sample, and 5% of the cells in the CE sample are normal cells, highlighting the difficulty of inferring clonality and tumor evolution from a small number of tumor samples. This patient also illustrates why the joint calling approach is advantageous to detect somatic variants if the germline was not available. With only the enhancing sample, the blue variants would be difficult to differentiate from the germline variants. If the non-enhancing sample were used as a reference in standard paired somatic variant calling, only the red variants would likely be detected.
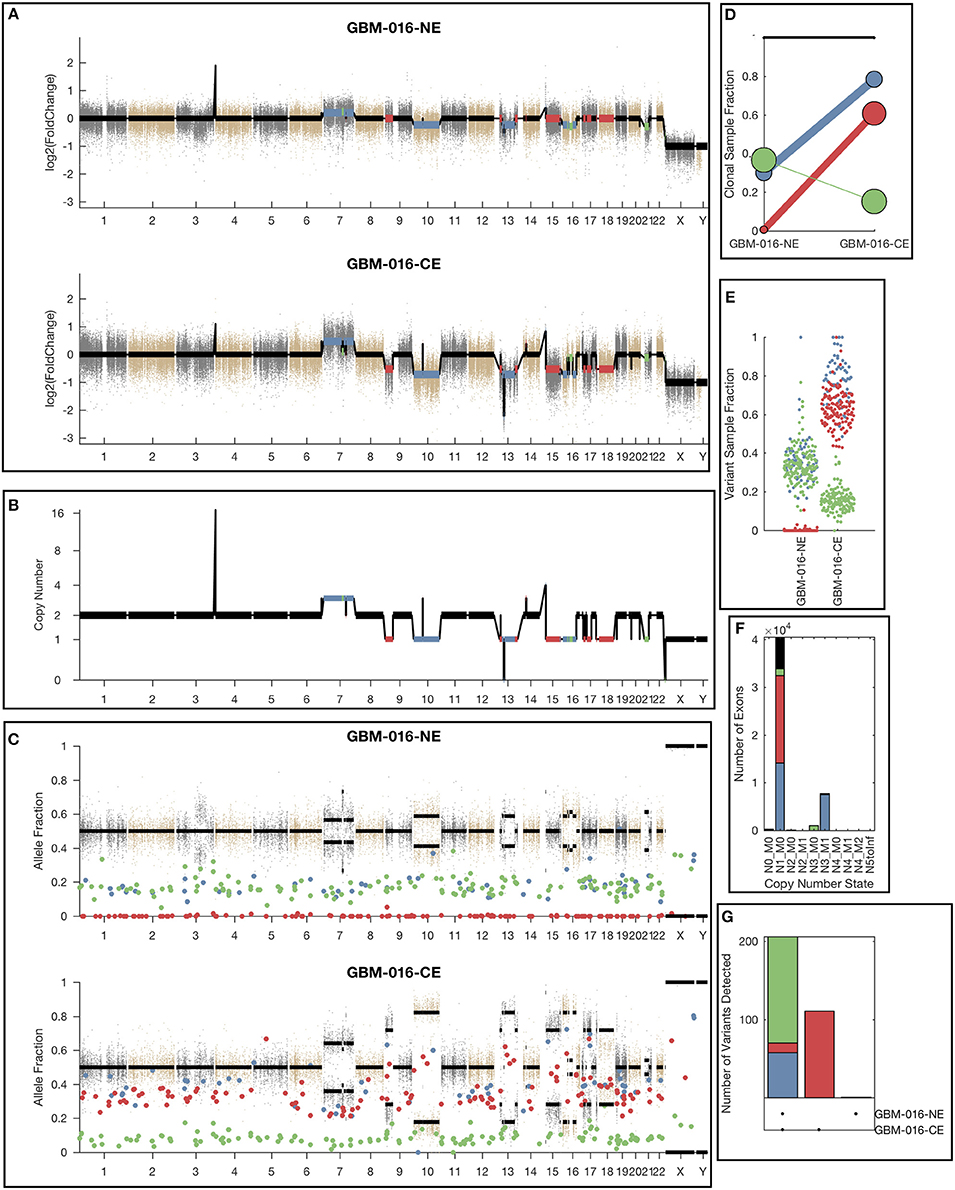
Figure 4. Example lumosVar 2.0 output. (A) Log2 fold change of the mean exon read depths compared to the unmatched controls. (B) The estimated integer copy number states are plotted for each genomic segment by chromosome position. (C) The variant allele fractions are plotted by chromosome position. The gray and brown dots represent variants called as germline heterozygous by lumosVar 2.0 and the large colored dots represent variants called somatic by lumosVar 2.0. (D) Summary of the clonal variant group patterns. The thickness of the lines represents the proportion of copy number events assigned to each group and the size of each circle is proportional to the number mutations assigned to each group. (E) Sample fraction (estimated proportion of cells in the sample containing the variant) distribution of somatic mutations. (F) Number of exons determined to be in each copy number state, excluding diploid. (G) Number of somatic mutations detected in both samples (left bar), enhancing only (middle bar), and non-enhancing only (right bar). On all plots, the colors indicate the clonal variant group.
To evaluate lumosVar 2.0 on real data, recurrent glioblastoma patients that had whole exome sequencing data available for two samples of different tumor contents (from contrast enhancing and non-enhancing biopsies), as well as germline sequencing data (from peripheral blood), were identified (Table 1). Three variant calling approaches were compared: (1) A filtering approach, where heterozygous germline variants not found in dbSNP were considered false positives, and somatic variants found in dbSNP were considered false negatives, (2) a pooled approach where the data for the high tumor content and low tumor content samples are combined in-silico, and (3) joint analysis of the paired high tumor content and low tumor content samples. Both the pooled and joint approaches use the lumosVar 2.0 software for variant calling. We find that the filtering approach consistently has better sensitivity, but much lower precision, and lower F1 scores (harmonic mean of sensitivity and precision) than both the pooled and joint analyses (Table 4). This is consistent with our previous findings that private germline variants result in a high number of false positives using a filtering approach (16). In most of the samples, we find modest improvements in sensitivity, precision, and F1 scores in the joint approach compared to the pooled. From the simulations, we would have expected to see similar precision and more consistent improvements in sensitivity. In order to more carefully evaluate where the joint approach and pooled approach are performing differently in detecting variants, we examined the sample fractions of variants that are true positives, false positives, and false negatives in each approach (Figure 5). We find that the pooled approach has more false positive variants that have similar allelic fractions in the CE and NE biopsies. We hypothesize that these variants have unexpected allelic fractions due to mapping noise or copy number call errors that would not be modeled in the simulations. The joint approach is better at avoiding these calls, as the allelic fractions do not fit the patterns of the clonal variant groups found in the patient. However, the joint approach also misses some true somatic variants that do not fit the patterns of clonal variant groups found in the patient, such as a set of lower sample fraction variants in GBM-003. GBM-014 is the only patient where the pooled approach outperforms the joint approach. This patient also appears to have the smallest difference in tumor content between the two biopsies as well as the most complex copy number profile of this set of patients (Supplemental Figure 1), both factors that likely contribute to the poor performance.
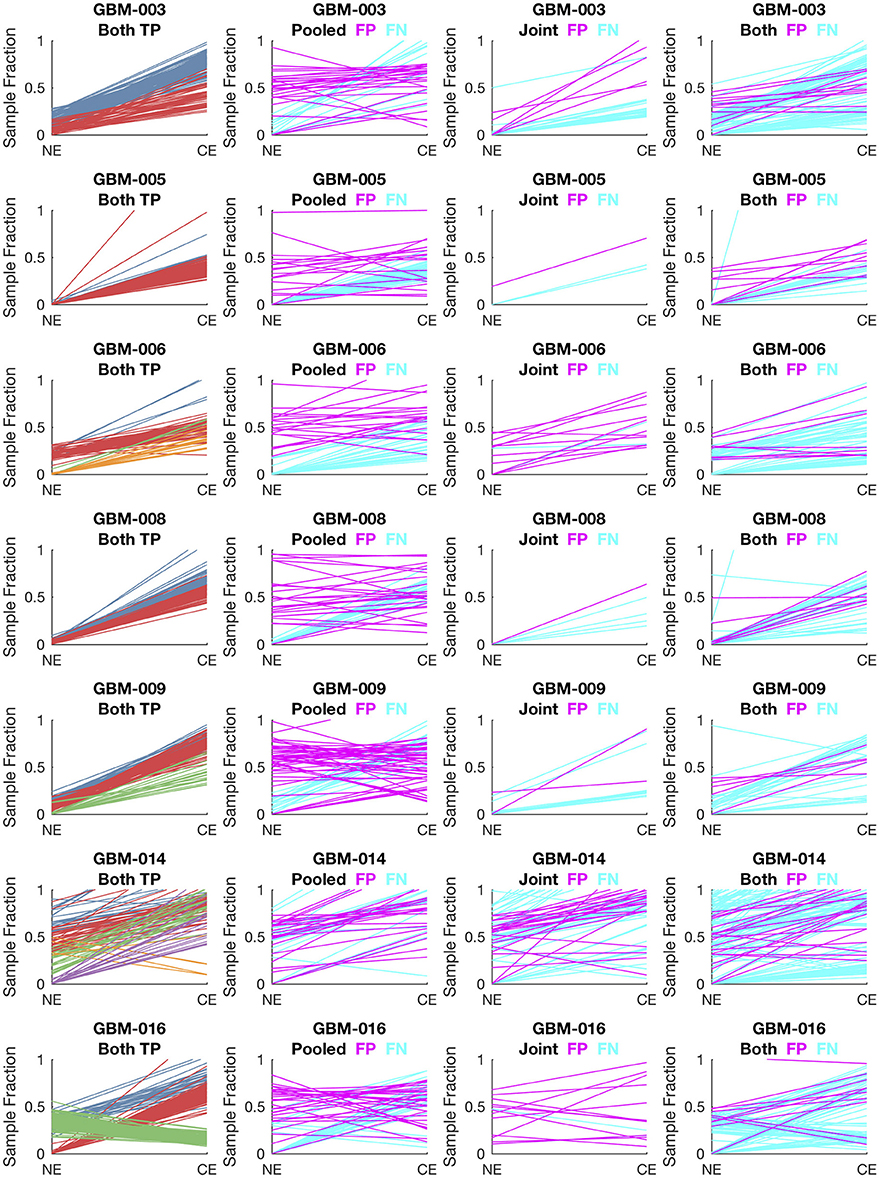
Figure 5. Comparison of variants called in pooled vs. joint approach. The first column of graphs shows the estimated sample fractions of true somatic variants that were detected by both the pooled and joint approaches. The variants are colored by clonal variant groups. The other three columns show the sample fractions of variants that were called incorrectly only in the pooled approach (column 2), only in the joint approach (column 3), or incorrectly in both approaches (column 4). False positives variants are shown in magenta and false negatives in cyan.
We applied our methods to archival breast cancer and prostate samples, where only FFPE tissue sections from biopsies or surgical tumor resections were available. For eight of the breast cancer patients, whole slides with adjacent histologically normal tissue were not available or did not have sufficient DNA yield, so adjacent normal areas were macro-dissected from tumor-containing slides. For the remaining patients, DNA was isolated from whole slides from additional FFPE blocks containing adjacent histologically normal tissue (Table 1). For two breast cancer cases (BHH02, BHH27), the additional “low tumor” blocks were from the contralateral breast following double mastectomy, though BHH02 was one of the eight patients that required macro-dissections of the tumor-containing slide to get sufficient DNA for the adjacent normal sequencing. Where macro-dissection was used to obtain the normal tissue samples, most of the somatic variants called were detected in the adjacent normal sample (median of 98%). For the patients where adjacent histologically normal tissue was obtained from separate slides, most patients still had some somatic variants detected in the normal tissue (median 35%–Figure 6).
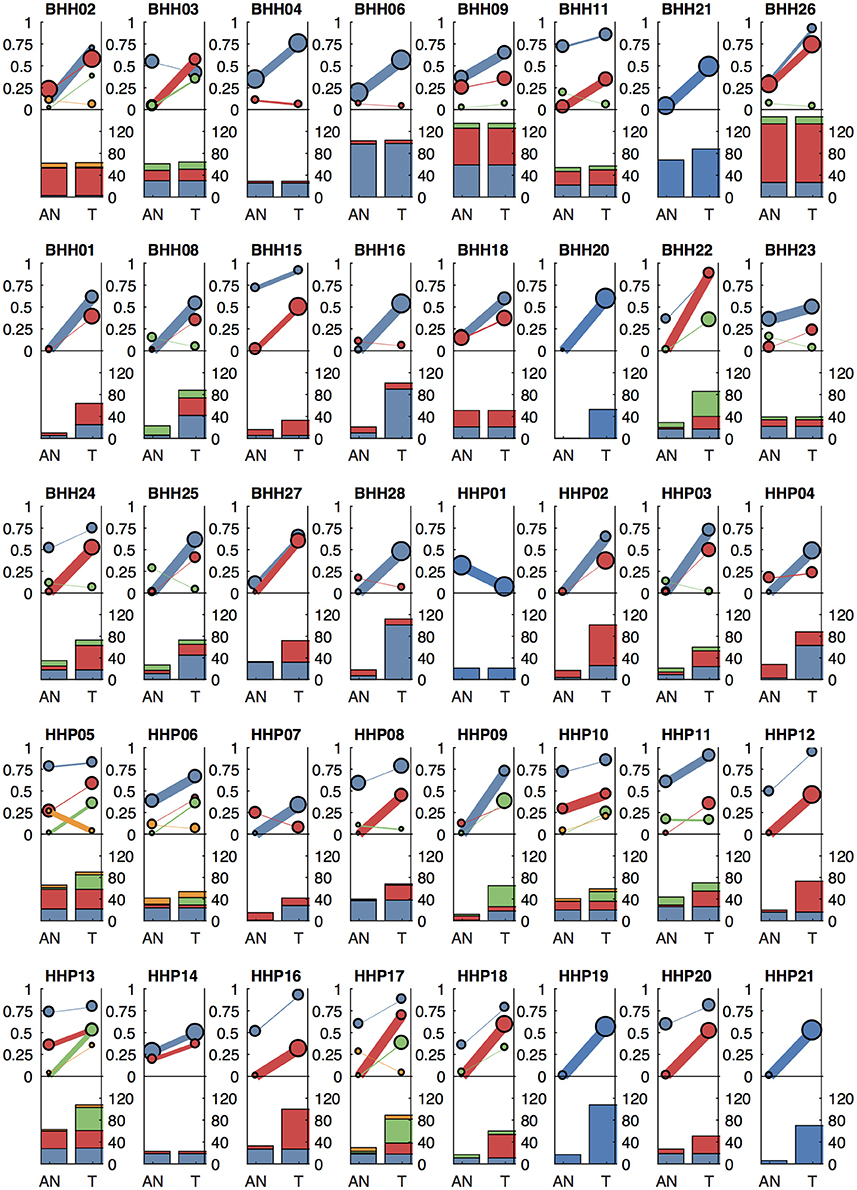
Figure 6. Clonal patterns and variant counts detected by lumosVar 2.0 in the archival dataset. The top half of each plot shows the summary of the clonal variant group patterns for each patient. Each line represents a clonal variant group and the thickness of the lines represents the proportion of copy number events assigned to each group and the size of each circle is proportional to the number mutations assigned to each group. The bottom half of each plot shows the number of somatic variants detected in the adjacent normal (AN) and tumor (T) samples, with the colors corresponding the clonal variant groups. The 8 patients in the top row had the adjacent normal tissue macrodissected from tumor containing slides and these patients typically have similar number of variants detected in the tumor and adjacent normal.
We also analyzed the archival tissue using two additional approaches: (1) a filtering strategy where standard somatic variant calling tools were used against an unmatched reference (GM12878), and variants found in dbSNP were excluded as likely germline, referred to as the unmatched plus filtering approach (UPF), and (2) a strategy that used the tumor adjacent normal sample as the normal reference in standard somatic variant calling tools, referred to as the adjacent normal as reference approach (ANR). In both cases the same three paired somatic variant calling tools (mutect, seurat, and strelka) were used and variants were considered positive if they were called by at least two callers. While the adjacent normal tissue was selected based on histology, we do not expect it to be free of molecular alterations due to potential contamination, field cancerization, or tissue specific mutational processes. We include the ANR strategy for comparison, as it is a commonly used strategy when other constitutional tissue is not available (9). Using the UPF strategy, we found that most of the variants called using the filtering strategy have variant allele fractions around 50% in both the low- and high-tumor-content samples, suggesting that most are private germline variants. Using the ANR approach, we only identified variants with allele fractions in the adjacent normal sample that were at or very close to zero. The variants called by lumosVar 2.0 generally have higher allele fractions in the tumor samples and low allele fractions in the adjacent normal samples, as expected (Figure 7).
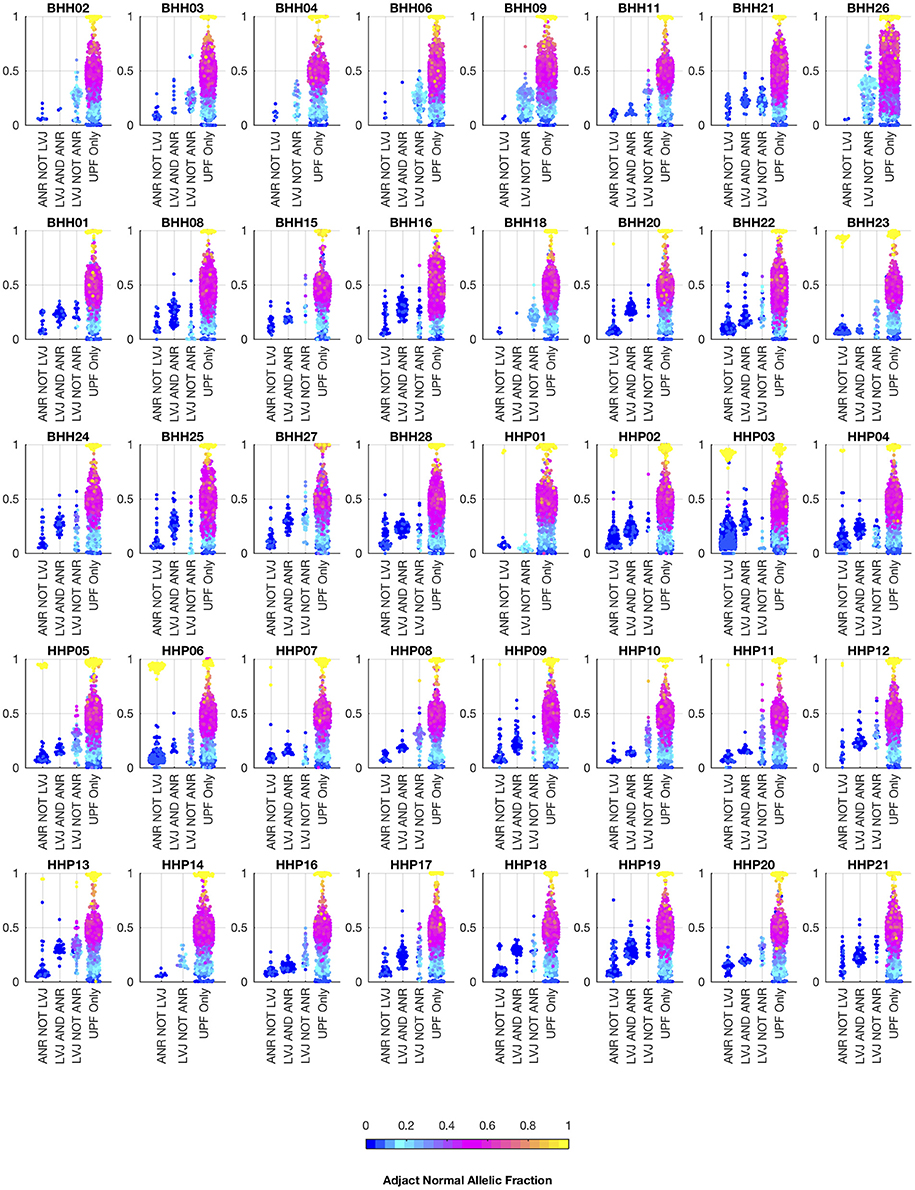
Figure 7. Comparison of allelic fractions of variants in archival dataset by calling method. For each of the breast and prostate patients, the allele fractions in the tumor sample are plotted for the variants detected in each of the three approaches. The color of each point indicates the allele fraction of the variant in the adjacent normal sample. Most of the variants detected in the adjacent normal as reference approach, but not lumosVar 2.0 joint analysis (ANR NOT LVJ), have low allele fractions in both the tumor and the adjacent normal. The variants detected by lumosVar 2.0 joint analysis, but not adjacent normal as reference approach (LVJ NOT ANR) typically have higher allele fractions in the tumor, and lower allele fractions in the adjacent normal, though lumosVar 2.0 joint analysis also detects some variants that are lower allele fraction in the tumor and higher allele fraction in the adjacent normal in a few patients such as HPP01. The variants only called in the unmatched filtering (UPF only) approach have similar allele fractions in the tumor and adjacent normal samples. The 8 patients in the top row had the adjacent normal tissue macrodissected from tumor containing slides and these patients typically have more variants detected by lumosVar 2.0 joint analysis and not ANR compared to the remaining patients whose adjacent normal sample was procured from separate slides.
In order to compare the ability of the three approaches to detect likely drivers, mutations called by any of the three approaches were compared against the Cancer Hotspots database, which reports recurrent mutations in 11,119 tumor samples (Cancer Hotspots1). A total of 28 hotspot mutations were called including eight mutations with in vitro or in vivo validation (level-3), two mutations detected in the Cancer Hotspots dataset that were previously reported (level-2), and eighteen mutations that were novel in the Cancer Hotspots dataset (level-1). Of the ten level-3 and level-2 mutations, all were called in the UPF approach, lumosVar 2.0 joint analysis called eight, and only six were called in the ANR approach (Table 5). The two level-2 and level-3 mutations missed by lumosVar 2.0 had low allele fractions in the tumor sample (5–6%) and were not detected in the adjacent tissue, while the four level-3 hotspots variants missed by the ANR approach had moderate allele fraction in the tumor (17–35%) and low allele fractions in the adjacent tissue (2–16%). Seventeen of the eighteen level-1 hotspots were called only in the UPF approach, and these tended to have similar allele fractions in the tumor and adjacent normal samples. These include the same APOBR mutation called in 13 patients, and the same DHRS4 mutation called in four patients (Supplemental Table 1). Putative mutations that are common within a dataset, but not known to be common in cancer, are suggestive of alignment artifacts (26). Both the UPF approach and lumosVar 2.0 detected the eighteenth level-1 hotspot which was a CDH3 truncating mutations with high allele fractions in the tumor and low in the adjacent normal.
Detecting somatic mutations when a normal tissue sample is not available remains a challenging problem. We present a method that leverages tumor-adjacent normal tissue, and is robust to significant levels of tumor contamination. Simulation studies suggest that a multi-sample approach should be more powerful than a single-sample approach, even if there is a small difference in tumor content between the two samples. Evaluation of a set of GBM samples with low tumor content (from NE biopsies) and high tumor content (from CE resections) further demonstrates the sensitivity and precision of the joint approach. Practical application of this approach to a set of FFPE breast and prostate samples shows the feasibility of this approach with typical archival samples.
While the approach described here represents an improvement over other tumor only somatic variant calling approaches, we believe it is best to sequence a true constitutional sample when feasible as the sensitivity of our approach is still limited compared to standard paired somatic variant calling. However, there are many open questions in precision oncology that can only be answered by collecting large amounts of patient genomic data linked to treatment response and clinical outcomes. For example, many factors may contribute to patient response to a targeted therapy, including the presence of other aberrations affecting the same pathway, aberrations affecting alternative pathways, and sub-clonal resistance mutations. Banks of archival samples show great potential to accelerate research predicting treatment response, as medium- and long-term outcomes may already be known. The approach outlined here should enable researchers to use archival samples more effectively, as accurately calling somatic variants is the first step in any analysis to answer such critical questions.
A complex relationship exists between tumor heterogeneity and clinical outcome, with moderately heterogeneous tumors having worse outcomes than both more homogenous tumors and more heterogeneous tumors (27, 28). Measuring the overall level of heterogeneity, in addition to detecting the clonal prevalence of individual variants, can provide insight into susceptibility and resistance to targeted therapies (29). lumosVar 2.0's ability to jointly analyze multiple samples from the same patient and integrate copy number and mutation data should be useful even when a matched normal sample is available to track mutations across longitudinal sample collections and spatially diverse samples, to gain insight into the tumor's evolution. Future work will further evaluate and benchmark lumosVar 2.0's clonal variant group analysis.
Compared to the single sample lumosVar 1.0 analysis, the joint approach requires lower total sequencing coverage to obtain the same sensitivity. Based on the simulation studies, we find that if the adjacent normal tissue has <25% tumor cell contamination, and the tumor sample has at least 55% percent tumor cells, then only 200X total coverage (100X for each sample) is required to detect 80% of the somatic variants that are in all of the tumor cells. However, higher coverage would be desirable in order to detect low abundance sub-clonal variants. We have shown that lumosVar 2.0 works best with a high tumor content and low tumor content sample from the same patient. These may not always be available such as with fine needle biopsies, or with brain tumors or metastases where resection of adjacent normal tissue would be avoided. However, we believe that the breast and prostate tumor blocks used in this study represent fairly typically archival samples, demonstrating the utility of the approach. Due to the large difference in prior probabilities of homozygous reference vs. somatic variants in this model, lumosVar 2.0 tends to be less sensitive to low abundance variants compared to other somatic variant callers. lumosVar 2.0 also has more stringent quality filtering than most paired somatic variant callers because the same artifacts often appear in the tumor and germline sample, so paired callers can use the presence in the germline to eliminate those artifacts. The probability that a variant is somatic or germline is calculated assuming that the allele specific copy number of the position is known with certainty, while there is clearly uncertainty in both setting the segmentation boundaries assigning both the copy number state of a given segment. Inspection of incorrectly classified variants suggests that segmentation boundary placement is a major source of error. We believe that a more sophisticated segmentation algorithm that is able to capture the uncertainty of segmentation boundary placement would yield the largest improvements in performance. We also recognize that an underlying assumption of our copy number model, that at most one copy number altered state may occur in a given segment across the patient samples, is an oversimplification, and a more realistic copy number model may improve both the copy number and variant calling results.
Though it may seem surprising that somatic variants were detected in histologically normal tumor adjacent tissue, previous studies have identified DNA, epigenetic, and gene expression alterations in tumor adjacent tissue (30). The theory of field cancerization proposes that epigenetic changes in the adjacent tissue creates a permissive environment for malignant transformation and sometimes can lead to multifocal disease and/or clonally independent recurrence. The sequencing of DNA from tumor adjacent tissue could serve a dual purpose in helping to identify somatic mutations when another source of normal tissue is not available, as well as helping to better understand the phenomenon of field cancerization.
The glioblastoma evaluation dataset is being submitted to dbGap under the accession phs001460.v1.p1 (https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs001460.v1.p1).
This study was carried out in accordance with the recommendations of HHS regulations, 45 CFR part 46 and HonorHealth with written informed consent from all breast cancer participants with waiver of consent for prostate cancer participants. All breast cancer participants gave written informed consent in accordance with the Declaration of Helsinki. The protocol was approved by the Western Institutional Review Board (WIRB protocol #20161603).
RH designed and implemented the lumosVar 2.0 software. SiK designed and implemented the custom pileup engine. RH, ET, JA, WL, NH, JN, CK, RK, MB, and SB were involved in designing and generating data for the breast and prostate study. RH, NT, MB, and SB were involved in the analysis and interpretation of the GBM study. DE assisted with data analysis. SeK aided in mathematical formulations. RH, SeK, and SB drafted and edited the manuscript. All authors have read and approved the manuscript.
RK and NH were employed by company Imaging Endpoints.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to thank Nicholas Schork, David Craig, Jessica Aldrich, Austin Christofferson, and Jonathan Keats for helpful discussion. We also thank the Ben and Catherine Ivy Foundation and GE Global Research for funding for this study. Texas A&M System Chancellor's Research Initiative for the Center for Computational Systems Biology at the Prairie View A&M University also provided funding to SeK. A pre-print of this manuscript has been deposited in the bioRxiv (31).
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2019.00119/full#supplementary-material
Supplemental Figure 1. lumosVar 2.0 output for GBM-014. Plots are analogous to Figure 4.
Supplemental Table 1. Level One Hotspot Variants.
1. ^Available at: http://cancerhotspots.org/#/home [Accessed December 15, 2017]
1. Allen EMV, Wagle N, Stojanov P, Perrin DL, Cibulskis K, Marlow S, et al. Whole-exome sequencing and clinical interpretation of FFPE tumor samples to guide precision cancer medicine. Nat Med. (2014) 20:682–8. doi: 10.1038/nm.3559
2. Bailey MH, Tokheim C, Porta-Pardo E, Sengupta S, Bertrand D, Weerasinghe A, et al. Comprehensive characterization of cancer driver genes and mutations. Cell. (2018) 173:371–85.e18. doi: 10.1016/j.cell.2018.02.060
3. Cheng DT, Mitchell TN, Zehir A, Shah RH, Benayed R, Syed A, et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): a hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J Mol Diagn. (2015) 17:251–64. doi: 10.1016/j.jmoldx.2014.12.006
4. Frampton GM, Fichtenholtz A, Otto GA, Wang K, Downing SR, He J, et al. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat Biotechnol. (2013) 31:1023–31. doi: 10.1038/nbt.2696
5. Khurana E, Fu Y, Chakravarty D, Demichelis F, Rubin MA, Gerstein M. Role of non-coding sequence variants in cancer. Nat Rev Genet. (2016) 17:93–108. doi: 10.1038/nrg.2015.17
6. Marrone M, Schilsky RL, Liu G, Khoury MJ, Freedman AN. Opportunities for translational epidemiology: the important role of observational studies to advance precision oncology. Cancer Epidemiol Prev Biomark. (2015) 24:484–9. doi: 10.1158/1055-9965.EPI-14-1086
7. Waldron L, Ogino S, Hoshida Y, Shima K, Reed AEM, Simpson PT, et al. Expression profiling of archival tumors for long-term health studies. Clin Cancer Res. (2012) 18:6136–46. doi: 10.1158/1078-0432.CCR-12-1915
8. Cheng F, Zhao J, Zhao Z. Advances in computational approaches for prioritizing driver mutations and significantly mutated genes in cancer genomes. Brief Bioinform. (2016) 17:642–56. doi: 10.1093/bib/bbv068
9. Wei L, Papanicolau-Sengos A, Liu S, Wang J, Conroy JM, Glenn ST, et al. Pitfalls of improperly procured adjacent non-neoplastic tissue for somatic mutation analysis using next-generation sequencing. BMC Med Genomics. (2016) 9:64. doi: 10.1186/s12920-016-0226-1
10. Dotto GP. Multifocal epithelial tumors and field cancerization: stroma as a primary determinant. J Clin Invest. (2014) 124:1446–53. doi: 10.1172/JCI72589
11. Heaphy CM, Griffith JK, Bisoffi M. Mammary field cancerization: molecular evidence and clinical importance. Breast Cancer Res Treat. (2009) 118:229–39. doi: 10.1007/s10549-009-0504-0
12. Nonn L, Ananthanarayanan V, Gann PH. Evidence for field cancerization of the prostate. Prostate. (2009) 69:1470–9. doi: 10.1002/pros.20983
13. Hoang ML, Kinde I, Tomasetti C, McMahon KW, Rosenquist TA, Grollman AP, et al. Genome-wide quantification of rare somatic mutations in normal human tissues using massively parallel sequencing. Proc Natl Acad Sci USA. (2016) 113:9846–51. doi: 10.1073/pnas.1607794113
14. Martincorena I, Roshan A, Gerstung M, Ellis P, Loo PV, McLaren S, et al. High burden and pervasive positive selection of somatic mutations in normal human skin. Science. (2015) 348:880–6. doi: 10.1126/science.aaa6806
15. Jones S, Anagnostou V, Lytle K, Parpart-Li S, Nesselbush M, Riley DR, et al. Personalized genomic analyses for cancer mutation discovery and interpretation. Sci Transl Med. (2015) 7:283ra53. doi: 10.1126/scitranslmed.aaa7161
16. Halperin RF, Carpten JD, Manojlovic Z, Aldrich J, Keats J, Byron S, et al. A method to reduce ancestry related germline false positives in tumor only somatic variant calling. BMC Med Genomics. (2017) 10:61. doi: 10.1186/s12920-017-0296-8
17. Kalatskaya I, Trinh QM, Spears M, McPherson JD, Bartlett JMS, Stein L. ISOWN: accurate somatic mutation identification in the absence of normal tissue controls. Genome Med. (2017) 9:59. doi: 10.1186/s13073-017-0446-9
18. Smith KS, Yadav VK, Pei S, Pollyea DA, Jordan CT, De S. SomVarIUS: somatic variant identification from unpaired tissue samples. Bioinformatics. (2015) 32:808–13. doi: 10.1093/bioinformatics/btv685
19. Riester M, Singh AP, Brannon AR, Yu K, Campbell CD, Chiang DY, et al. PureCN: copy number calling and SNV classification using targeted short read sequencing. Source Code Biol Med. (2016) 11:13. doi: 10.1186/s13029-016-0060-z
20. Byron SA, Tran NL, Halperin RF, Phillips JJ, Kuhn JG, de Groot JF, et al. Prospective feasibility trial for genomics-informed treatment in recurrent and progressive glioblastoma. Clin Cancer Res. (2017) 24:295–305. doi: 10.1158/1078-0432.CCR-17-0963
21. Christoforides A, Carpten JD, Weiss GJ, Demeure MJ, Hoff DDV, Craig DW. Identification of somatic mutations in cancer through Bayesian-based analysis of sequenced genome pairs. BMC Genomics. (2013) 14:302. doi: 10.1186/1471-2164-14-302
22. Saunders CT, Wong WSW, Swamy S, Becq J, Murray LJ, Cheetham RK. Strelka: accurate somatic small-variant calling from sequenced tumor–normal sample pairs. Bioinformatics. (2012) 28:1811–7. doi: 10.1093/bioinformatics/bts271
23. Cibulskis K, Lawrence MS, Carter SL, Sivachenko A, Jaffe D, Sougnez C, et al. Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nat Biotechnol. (2013) 31:213–9. doi: 10.1038/nbt.2514
24. Sherry ST, Ward MH, Kholodov M, Baker J, Phan L, Smigielski EM, et al. (2001). dbSNP: the NCBI database of genetic variation. Nucleic Acids Res. 29, 308–311.
25. DePristo MA, Banks E, Poplin R, Garimella KV, Maguire JR, Hartl C, et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet. (2011) 43:491–8. doi: 10.1038/ng.806
26. Teer JK, Zhang Y, Chen L, Welsh EA, Cress WD, Eschrich SA, et al. Evaluating somatic tumor mutation detection without matched normal samples. Hum Genomics. (2017) 11:22. doi: 10.1186/s40246-017-0118-2
27. Andor N, Graham TA, Jansen M, Xia LC, Aktipis CA, Petritsch C, et al. Pan-cancer analysis of the extent and consequences of intratumor heterogeneity. Nat Med. (2015) 22:105–13. doi: 10.1038/nm.3984
28. Andor N, Maley CC, Ji HP. Genomic instability in cancer: teetering on the limit of tolerance. Cancer Res. (2017) 77:2179–85. doi: 10.1158/0008-5472.CAN-16-1553
29. Saunders NA, Simpson F, Thompson EW, Hill MM, Endo-Munoz L, Leggatt G, et al. Role of intratumoural heterogeneity in cancer drug resistance: molecular and clinical perspectives. EMBO Mol Med. (2012) 4:675–84. doi: 10.1002/emmm.201101131
30. Troester MA, Hoadley KA, D'Arcy M, Cherniack AD, Stewart C, Koboldt DC, et al. DNA defects, epigenetics, and gene expression in cancer-adjacent breast: a study from The Cancer Genome Atlas. NPJ Breast Cancer. (2016) 2:16007. doi: 10.1038/npjbcancer.2016.7
Keywords: cancer genomics, somatic variant calling, next generation sequencing, tumor-only sequencing, tumor exome sequencing, cancer hotspot mutations
Citation: Halperin RF, Liang WS, Kulkarni S, Tassone EE, Adkins J, Enriquez D, Tran NL, Hank NC, Newell J, Kodira C, Korn R, Berens ME, Kim S and Byron SA (2019) Leveraging Spatial Variation in Tumor Purity for Improved Somatic Variant Calling of Archival Tumor Only Samples. Front. Oncol. 9:119. doi: 10.3389/fonc.2019.00119
Received: 16 July 2018; Accepted: 11 February 2019;
Published: 20 March 2019.
Edited by:
Sven Bilke, National Cancer Institute (NCI), United StatesReviewed by:
Parvin Mehdipour, Tehran University of Medical Sciences, IranCopyright © 2019 Halperin, Liang, Kulkarni, Tassone, Adkins, Enriquez, Tran, Hank, Newell, Kodira, Korn, Berens, Kim and Byron. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rebecca F. Halperin, cmhhbHBlcmluQHRnZW4ub3Jn
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.