- 1Division of Oncology, S.Orsola-Malpighi Hospital, Bologna, Italy
- 2Oncology Unit, Macerata Hospital, Macerata, Italy
- 3Section of Pathological Anatomy, School of Medicine, United Hospital, Polytechnic University of the Marche Region, Ancona, Italy
- 4Department of Pathology and Surgery, Faculty of Medicine, Cordoba, Spain
- 5Department of Pathology and Laboratory Medicine, Indiana University School of Medicine, Indianapolis, IN, United States
Management of localized and advanced prostate cancer benefits from several therapeutic options with a surprising improvement in terms of clinical outcome. The selection of patients more likely to benefit from a specific approach still remains a key issue as well as the early identification of patients with aggressive disease which could benefit from a more aggressive treatment strategy. The lack of reliable bio-marker in castration resistant setting able to monitor response to treatment and early inform about tumor progression is an emerging issue. Accordingly, circulating DNA and circulating tumor cells appears a promising and attractive approach despite to date practical applications of these techniques are few and not validated. The aim of this review of the literature is to explore current knowledge on liquid biopsy in prostate cancer focusing on possible future applications.
Introduction
Prostate Cancer (PCa) represents the most common adult malignancies ranking as one of the major cause of cancer related death in men (1). Management of the disease accounts various options in both localized and advanced stages. Each options are generally evaluated according to different variables related to patients (performance status, comorbidities, disease related symptoms, and patients' preferences) and tumor features (biological aggressiveness and site and number of metastases). Thus, the management of localized stages could range from a first instance no invasive approach (watchful waiting or active surveillance approach) to a radical approach by surgery, external radiation treatment, a combination of both of them (radiation treatment in case of positive surgical margins) or also brachytherapy (which consists on the prostate implantation of sealed radiotherapy sources) with or without an adjuvant androgen deprivation therapy (ADT) (2–8).
Similarly, advanced stages of the disease count different therapeutic options. As first approach ADT represents the cornerstone of advanced prostate cancer due to the high sensitivity of tumor cells to hormone deprivation. The addiction of further treatment including anti-androgens abiraterone acetate or docetaxel can improve the outcome of patients with metastatic castration sensitive prostate cancer (mCSPC) (9–15).
After a first period of hormone deprivation sensitivity, tumor cells develop several mechanisms which lead to overcome the hormone inhibition leading to metastatic castration resistant prostate cancer (mCRPC). In this setting, several different agents have demonstrated to be effective treatment: new hormonal agents (abiraterone, enzalutamide, apalutamide), chemotherapy (docetaxel, cabazitaxel), radiometabolic drugs (Radium 223), and Sipuleucel-T immunotherapy (16–25).
Rational for Liquid Biopsy in Prostate Cancer
The availability of several active therapeutic options has led to different emerging needs in clinical practice requiring the development of reliable markers able to monitor response to treatment and help clinicians to select patients more likely to benefit from one approach rather than another.
Prostate-Specific Antigen (PSA) represents a reliable and useful biomarker adopted for early detection and early diagnosis of disease recurrence progression. However, it does not give information about biological features of the disease and it loses its predictive rule in mCRPC setting (26).
Liquid biopsy is an emerging technique which purposes is the detection of tumor cells/tumor DNA from patients' peripheral blood.
There are several issues which make the development of liquid biopsies in prostate cancer an attractive approach: (1) the low invasiveness; (2) the early detection of more aggressive tumors since early phases;(3) the early diagnosis of residual tumors or micro-metastases after surgery. (4) the monitoring of tumor response/progression to systemic treatment in advanced setting of the disease and especially in mCRPC; (5) the prediction of tumor sensitivity/resistance to systemic treatments; (6) the acquisition of an accurate genetic assessment of the disease focusing on key alterations which are related to tumor resistance. In particular, several genomic alterations seem to be attractive target due to their correlation to treatment resistance and/or sensitivity to specific treatments (27–30). Some of the more attractive targets are:
- Phosphate and tensin homolog (PTEN) loss. PTEN loss results in PI3K/AKT activation which has been associated to worst survival due to higher tumor proliferation and resistance to hormonal treatment. The inhibition of the PI3K/AKT/mTOR pathway could be an interesting target in this subgroup of patients which could be associated to an Androgen Receptor (AR) inhibition (31, 32).
- MYC amplification is generally acquired in metastatic phases of the disease and is correlated to poor prognosis and higher Gleason score. Furthermore, more than one evidences seem to correlate the combination of MYC amplification and PTEN loos to worst prognosis and increase risk of tumor related death (33, 34).
- Androgen Receptor (AR) mutations and in particular AR splice variant 7 (AR-V7) is known to be related to resistance to hormonal treatments including also new hormonal agents abiraterone and enzalutamide (35).
- TMPRSS2-ERG gene fusion leads to ETS-related gene (ERG) and steroidogenic enzyme AKR1C3 co-overexpression which promotes AR signaling and represents a promising target in prostate cancer (36, 37).
- DNA repair genes deficiency and in particular genes related to the identification of single strand breaks (such as PARP1 and PARP2) as well as the identification of the alterations of non-homologous recombination system genes (such as BRCA1, BRCA2, PALBB2, MRE11, Check2, RAD51, XRCC2/3) appears an attractive approach for two reason. First, tumors with repair genes deficiency are related to more aggressive features and poorer survival. Second, therapeutic implications related to these genomic assessments involve a possible sensitivity to platinum cytotoxic therapy. The development of PARP inhibitors represents another possible target for the management of advanced prostate cancer which has already been evaluated in small trials and is currently under clinical investigation (38–43).
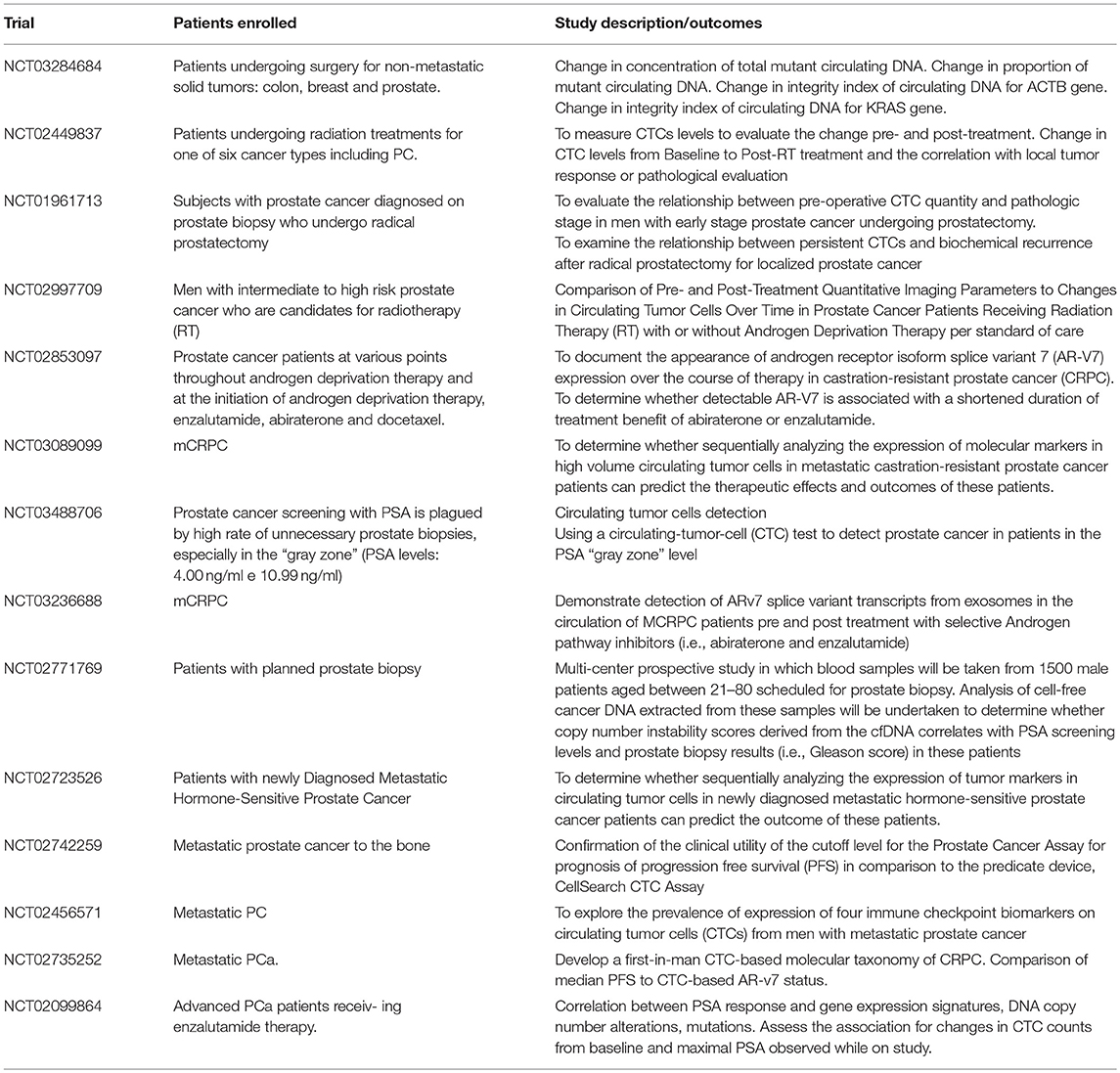
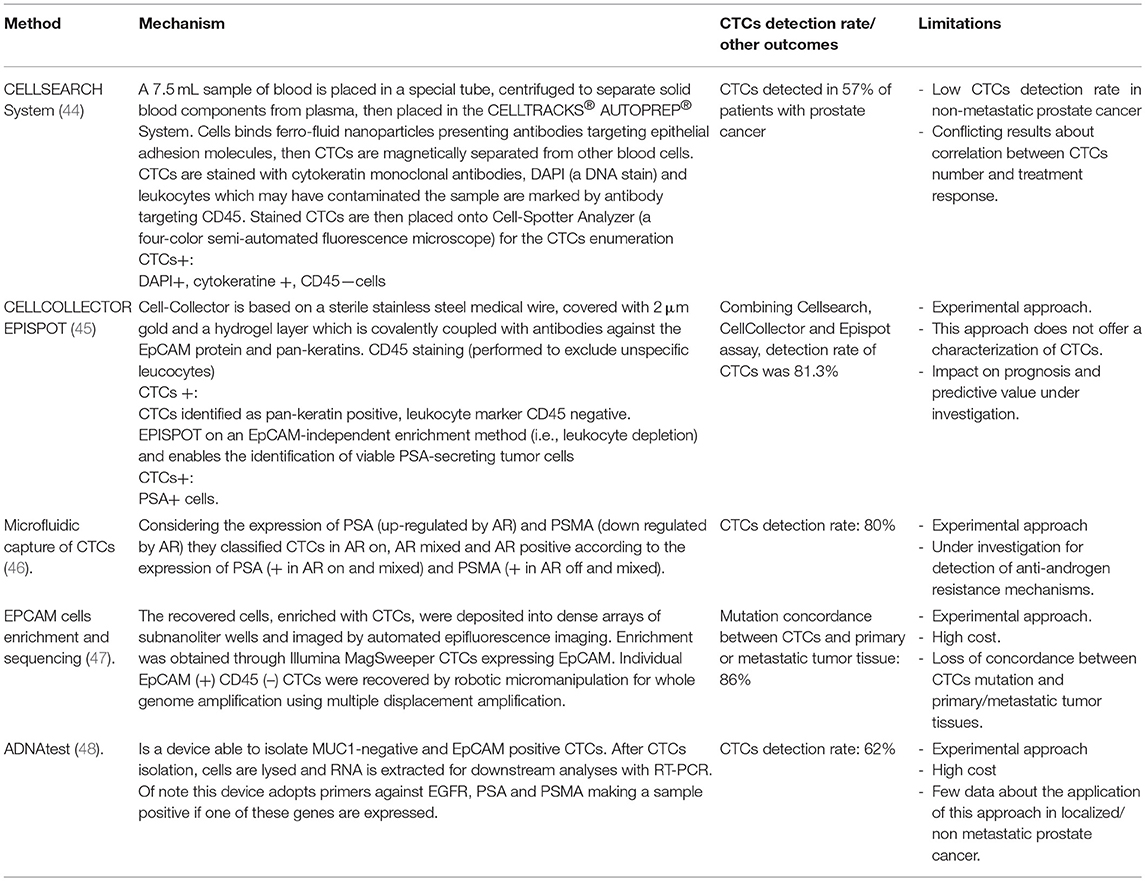
Due to these issues, the development of reliable techniques able to perform liquid biopsy appears a promising and suggestive approach (Table 1). Here we performed a review of the main techniques adopted or under investigation focusing our attention on approaches based on circulating tumor cell (CTC) and circulating DNA (ct-DNA) detection. Table 2 summarizes the current methods available for CTC detection as well as the percentage of detection (See also below).
Circulating Tumor Cells in Prostate Cancer
To date, the CellSearch system assay is the only FDA approved method for the detection of CTCs in prostate cancer (Table 2). This device consists of different components including a CellPrep system which is a semi-automated sample preparation system and a CellSearch Epithelial Cell Kit. This last component involves ferro-fluids coated with epithelial cell-specific EpCAM antibodies and a mixture of antibodies directed against cytokeratins 8, 18, 19, CD45 conjugated to allophycocyanin and DAPI (nuclear dye 4', 6 diamidino-2-phenylindole for fluorescent cells label). After an incubation period in which CTC are isolated from peripheral blood and enriched in EpCAM composed ferro-fluids the MagNest Cell Presentation Device (a device composed of a chamber with two magnets) orients labeled cells for analysis in a CellSpotter Analyzer (a four-color semi-automated fluorescence microscope) for the CTCs enumeration (44).
Initial studies carried out on patients with different solid tumor demonstrated a promising activity with this method and regarding patients with PCa detection of CTCs was possible in 57% of patients (44).
Further study aimed to investigate the clinical value of CTCs detected by CellSearch assay showed that CTCs baseline levels were an independent prognostic factor for overall survival (OS) (49).
In 2008, de Bono et al. identified a correlation between CTCs number and median overall survival. In this study carried out on 231mCRPC two distinct subgroup of patients were identified: one (Unfavorable group) which showed a CTCs number of 5 or more and the other (favorable) with < 5 CTCs per 7.5 mL of blood. Overall survival was significantly better in favorable group (21.7 vs. 11.5 months). Moreover, patients who presented a significant decrease of CTCs number during or after treatment (moving from unfavorable to favorable groups) significantly improved their survival compared to patients who continued to present a CTCs number of 5 or more CTCs. According to the results of de Bono et al, a meta-analysis of 10 studies confirmed the prognostic rule of CTCs in patients with prostate cancer (50).
Furthermore, pre-planned analyses of large phase III trials: SWOG 20421 (docetaxel with or without atrasentain in mCRPC patients), COU-AA-301 (in which a score composed by LDH levels and CTCs divided patients in 3 different subgroups with favorable, intermediate and poor prognosis) and AFFIRM (enzalutamide in patients with mCRPC progressed to chemotherapy) confirmed the prognostic rule of CTCs as independent factor related to OS (51–53).
Unfortunately, none of these studies demonstrated an association between CTCs number and response to treatment and so the role of CTCs in this setting still remains unclear. Moreover, another possible issue which could partially explain the failure of this approach in clinical practice is the low detection rate of CTCs in non metastatic patients which ranges only from 5 to 27% (54). To avoid this problem, Kuske et al combined three different methods for the detection of CTCs before and after prostatectomy in non metastatic patients with PC, CellSearch system assay, CellCollector (a system capturing EpCAM-positive CTCs by an antibody-coated needle introduced in arm vein) and EPISPOT (a system able to enrich CTCs by negative depletion of leukocytes and detects circulating prostate cancer cells thanks to their active secretion of PSA) (45). CTCs were detected in 37, 54.9 and 58.7% of patients using CellSearch, CellCollector, and EPISPOT, respectively. The cumulative positivity rate of the three CTC assay was 81.3% and despite it is not a validated approach, it represents an attractive early method able to estimate the risk of tumor recurrence or persistence after surgery.
A combined analysis of COU-AA-301 and IMMC-38 trials showed that an increase of 30% in CTCs count from baseline was independently associated to worst OS in patients treated with abiraterone and chemotherapy (53). To sustain the correlation between CTCs count changes and survival, another analysis performed on 119 patients with CRPC treated at the Royal Marsden Hospital suggested that a decrease of 30% in CTC counts from baseline was associated to improved survival (55).
The only CTCs enumeration resulted in an independent prognostic factor with an unclear role in terms of early diagnosis of disease recurrence/persistence after surgery as well as a predictive response factor. Another interesting approach consists in a characterization of CTCs resulting in a genetic assessment and in a detection of target altered pathways.
AR protein has been extensively investigated in prostate cancer CTCs. Through a FISH based assay AR gene amplification detection in CTCs was possible in 40% of cases, a percentage comparable to the AR amplification described in bone metastases biopsy analyses (47). Further investigations demonstrated that patients with higher cytoplasm expression of AR resulting in a reduction of nuclear translocation was significantly associated to better response to docetaxel (56). By a microfluidic capture of CTCs Miyamoto et al. evaluated dynamic changes in CTCs AR expression. In particular, considering the expression of PSA (up-regulated by AR) and PSMA (down regulated by AR) they classified CTCs in AR on, AR mixed and AR positive according to the expression of PSA (+ in AR on and mixed) and PSMA (+ in AR off and mixed). Moreover, Authors identified that AR status changed from “on” to “off” during ADT while patients treated with abiraterone acetate with an increase of AR-on CTCs or baseline level of AR-mixed more than 10% were significantly associated to worse overall survival (46). The technology developed by Myamoto et al was also adopted for the detection of anti-androgen resistance mechanisms in CTCs demonstrating higher activation of Wnt signaling and considerable heterogeneity in signaling pathways, expression of AR gene mutations and splicing variants (57).
Due to the important role of AR-V7 in mCRPC (35), several studies have focused on the detection of this splice variants on CTCs. An EpCAM assay demonstrated that CTCs-ARV7+ detection was associated to resistance to enzalutamide and abiraterone but no to docetaxel and cabazitaxel and that the detection of these CTCs was independently associated to worse clinical outcome compared to patients with CTCs-ARV7- cells (58–60). Other studies modified the CTCs and ARV7 detection method in order to evaluate the AR-V7 cellular localization (61) and the presence of other splice variants of AR (62). Particularly, not only AR-V7 but also other slice variants of the AR protein were significantly associated to worse progression free survival. Moreover 6 of 17 poor responders to treatment were AR-V7 negative, but carried other AR perturbations (62).
About other pathways detected in CTCs, the PTEN loss assessed by FISH and Epic Sciences test (an assay which adopted a fibreoptic array scanning techniques for the detection of DAPI, CD45, cytokeratins stained cells) has been associated to worse clinical outcomes (63, 64) while the detection of TMPRSS2-ERG fusion gene performed by microfluidic device and by the use of a RT-PCR analysis failed to show a predictive response value to abiraterone acetate in mCRPC patients (47).
Next Generation Sequencing (NGS) involves a series of different techniques able to perform a whole genome sequencing of tumor cells. The possibility to obtain a complete genomic assessment from CTCs appears a novel and promising approach investigated in different studies.
In 2014, Lohr et al evaluated a method able to perform a CTCs isolation, enrichment (throuEp-CAM expressing CTCs), genomic amplification and sequencing in metastatic PC (65). They demonstrated that a complete mapping of the standard exome was possible in CTCs. NGS analysis of CTCs and tumor sample of a single patient with advanced prostate cancer showed a concordance of 86% from the mutations isolated in CTCs and genomic anomalies identified in primary or metastatic tumors (66). Despite NGS performed to CTCs represents an attractive approach, to date no validated or prospective studies have been carried out and so this method is still under investigation.
Another interesting issue is the detection of whole blood RNA, without enriching for CTC. In 2012, Ross et al assessed a whole blood RNA transcript based model as prognostic factor in patients with PC. After the analysis of blood collected from 62 men with mCRPC, they identified a six gene model (genes considered were: ABL2, SEMA4D, ITGAL, C1QA, TIMP1, CDKN1A which are genes involved mainly in immunity regulation) able to divide patients in two risk groups with different mOS (67). In the same year, Olmos et al carried out a validation study of a nine-gene signature as prognostic factor (68). Design of the study consisted in a derivation set in which patients with mCRPC and patients in Active Surveillance were used as case and control groups respectively. After genomic assessment 94 patients were divided in four distinct prognostic groups. Thus nine altered genes HMBS, TMCC2, SLC4A1, STOM, GABARAPL2, RIOK3, TERF2IP, TFDP1) isolated in prognostic groups with worst survival (composed of only mCRPC patients) were validated in a validation set of patients with mCRPC. More recently, an assessment of 5 key genes (KLK3, KLK2, HOXB13, GRHL2, FOXA1) obtained after reverse transcription polymerase chain reaction (RT-PCR) demonstrated to be a reliable prognostic marker compared to CellSearch system count (48). Isolation of two or more of the selected genes were possible in 53% (51 /97) patients with mCRPC. AdnaTest is a technique adopted for CTCs enrichment and consists of a device able to isolate MUC1-negative and EpCAM positive CTCs. After CTCs isolation, cells are lysed and RNA is extracted for downstream analyses with RT-PCR. Of note this device adopts primers against EGFR, PSA, and PSMA making a sample positive if one of these genes are expressed. Sensitivity of KLK2, KLK3, HOXB13, GRHL2, and FOXA1 genes detection by this method is similar to DDPCR (direct detection PCR) and both of these techniques showed a higher sensitivity compared to CellSearch system (69).
Concerning the several devices utilized for CTCs detection, enrichment and evaluation, only CellSearch has been approved from FDA. However, despite a large range of potential applications (such as diagnosis, evaluation of treatment response, early detection of tumor relapse, and progression) CTCs detection by CellSearch is not commonly adopted in clinical practice. This mainly due to a low sensitivity of the method as well as a conflicting relationship between CTCs and treatment response evaluation. Several other approaches are under investigation. It is likely that CTCs evaluation will be an important factor able to improve our decisions in clinical practice (48, 69).
Circulating DNA in Prostate Cancer
The evidence that cell-free DNA could be detected in peripheral blood is a well known issue, and its application in clinical practice has been investigated only in last years. Regarding cancer patients, the unique composition of tumors' ctDNA presenting several genomic mutations (especially single base-pair substitution) which are not detectable in ctDNA originating from normal cells make tumor ctDNA an ideal markers of the disease. Moreover, the possible correlation between mutations detected on ctDNA and genomic mutations of primary or metastatic tumors make cDNA a unique markers able to provide key information by a no-invasive approach.
Regarding PC, ctDNA could be detected in peripheral blood and detection of known driver aberrations can be obtained in more than 97% of cases. Moreover, changings in ctDNA genomic mutations could be detected by repeated analyses of ctDNA with high grade of concordance with genomic assessment of primary tumors or metastases (70, 71).
The quantitative assessment of ctDNA has been related to prognosis of patients with PC in different studies (72, 73). In particular, Romanel A et al exanimated AR status of mCRPC patients starting abiraterone acetate. They detected a 45% of patients (tot number 97) with AR point mutations (T878A/L702H) before the first administration of abiraterone who showed a significant worse overall survival (73). Similarly, other studies confirmed the prognostic role of AR genomic alterations as prognostic markers raising the acquisition of ctDNA examination as a possible to monitor response to hormonal agents and to achieve an early diagnosis of progressive disease (74–77).
As known, mutation in DNA repair genes is acquiring an increasing interest in PC due to the association by these mutation and more aggressive tumor features and to the possible benefit derived from a PARP targeted treatment. Mutations in repair genes are common in prostate cancer. In a DNA assessment of 692 men with metastatic PC a total of 84 germiline DNA repair gene mutations (BRCA2, ATM, CHEK2, BRCA1, RAD51D, and PALB2) were found in 82 men (78). This study demonstrated that incidence of germline mutations of DNA-repair gene were common (as detected in 11.8% of all patients) in metastatic patients regardless to age and family history of prostate cancer.
PARP inhibition is one of the important strategy currently under investigation in patients with metastatic prostate cancer. In a phase II trial 50 mCRPC patients received the PARP inhibitor Olaparib (41). 17 (33%) patients showed an objective response while NGS sequencing showed that 16 patients presented homozygous delections, deleterious mutations, or both in DNA repair genes (BRCA1/2, ATM, Fanconi's anemia genes and CHECK2). A subsequent analysis of tumors DNA highlighted that patients with an overall reduction of 50% or more of ctDNA were associatied with better OS and PFS (79). In ASCO 2018, Clarke et al presented the results of a phase II study comparing the administration of Abiraterone with Olaparib or placebo in 171 patients with mCRPC (80). This trial met its primary endopoint showing a better radiological PFS in patients receiving olaparib. Of note Authors researched homologous recombination repair mutations by a NGS approach in tumor samples and plasma. Sequencing was possible on 91 of 136 patients and positive results (defined as discovery mutated patients) was obtained in 13 patients. By germline analysis and tumor sample analyses detection of homologous recombination repair mutations were identified in 3 patients (38 tumor samples analyzed of 68 total samples) and 7 patients (by a germline analysis of 102 patients). Results of this study raising the dosage and analyses of cDNA as possible approach for the detection of keys DNA-repair gene mutations. Other larger prospective trials are needed to explore the role of ctDNA in this setting.
Another promising target gene is represented by PTEN loss which has show to be a predictive biomarker of response to treatments targeting PI3K/AKT pathway. Hyper-activation of the PI3K/Akt/mTOR resulting from PTEN loss is related to decreased AR transcription output and stability and vice versa. The addiction of ipatasertib (an Akt inhibitor) to abiraterone acetate increased radiological PFS of patients with mCRPC and PTEN loss previously treated with docetaxel based therapy and progressed during at last one previous hormonal therapy (81).
Extracellular vesicles are membrane-enclosed structures that are released from all cells in the body. These vesicles contain several substances such as proteins, lipids, RNA, and DNA and are considered a very promising tumor-related biomarkers. Recently, it has been demonstrated that large extracellular vesicles isolated from plasma of patients with prostate cancer cells are an important source of chromosomal DNA which reflects faithfully genetic aberration of the cell of origin, including copy number variations of genes frequently altered in metastatic prostate cancer (such as MYC and PTEN) (82). The study of extracellular vesicles represents a novel and promising approach for biomarkers development in prostate cancer however further studies are needed to explore the effective value of this method.
Conclusion
The surprising potential of CTCs or tumors' ctDNA detection, characterization and genomic assessment have start a revolution which probably will give important results in next years. Despite to date application of these techniques are few probably that better knowledge of genomic anomalies of PC and their correlation with the clinical course of the disease as well as their potential relationship with specific targeted treatments will increase the attention on this issue.
Author Contributions
RM and FM: conception and design; VD and LG: drafting the manuscript; MSa and AC: review of the literature; LC, MSc, and AL-B: critical revision of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics 2018. CA Cancer J Clin. (2018) 68:7–30. doi: 10.3322/caac.21442
2. Wilt TJ, Jones KM, Barry MJ, Andriole GL, Culkin D, Wheeler T, et al. Follow-up of prostatectomy versus observation for early prostate cancer. N Engl J Med. (2017) 377:132–42. doi: 10.1056/NEJMoa1615869
3. Bill-Axelson A, Holmberg L, Garmo H, Rider JR, Taari K, Busch C, et al. Radical prostatectomy or watchful waiting in early prostate cancer. N Engl J Med. (2014) 370:932–42. doi: 10.1056/NEJMoa1311593
4. Parker C. Active surveillance: towards a new paradigm in the management of early prostate cancer. Lancet Oncol. (2004) 5:101–6. doi: 10.1016/S1470-204501384-1
5. Hamdy FC, Donovan JL, Lane JA, Mason M, Metcalfe C, Holding P, et al. 10-Year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. (2016) 375:1415–24. doi: 10.1056/NEJMoa1606220
6. Donovan JL, Hamdy FC, Lane JA, Mason M, Metcalfe C, Walsh E, et al. Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med. (2016) 375:1425–37. doi: 10.1056/NEJMoa1606221
7. Mason MD, Parulekar WR, Sydes MR, Brundage M, Kirkbride P, Gospodarowicz M, et al. Final report of the intergroup randomized study of combined androgen-deprivation therapy plus radiotherapy versus androgen-deprivation therapy alone in locally advanced prostate cancer. J Clin Oncol. (2015) 33:2143–50. doi: 10.1200/JCO.2014.57.7510
8. Messing EM, Manola J, Yao J, Kiernan M, Crawford D, Wilding G, et al. Immediate versus deferred androgen deprivation treatment in patients with node-positive prostate cancer after radical prostatectomy and pelvic lymphadenectomy. Lancet Oncol. (2006) 7:472–9. doi: 10.1016/S1470-204570700-8
9. Schmitt B, Bennett C, Seidenfeld J, Samson D, Wilt T. Maximal androgen blockade for advanced prostate cancer. Cochrane Database Syst Rev. (2000) 22:CD001526. doi: 10.1016/j.beem.2008.01.004
10. Samson DJ, Seidenfeld J, Schmitt B, Hasselblad V, Albertsen PC, Bennett CL, et al. Systematic review and meta-analysis of monotherapy compared with combined androgen blockade for patients with advanced prostate carcinoma. Cancer (2002) 95:361–76. doi: 10.1002/cncr.10647
11. Gravis G, Boher JM, Joly F, Soulie M, Albiges L, Priou F, et al. Androgen Deprivation Therapy (ADT) plus docetaxel versus ADT alone in metastatic non castrate prostate cancer: impact of metastatic burden and long-term survival analysis of the randomized phase 3 GETUG-AFU15 Trial. Eur Urol. (2016) 70:256–62. doi: 10.1016/j.eururo.2015.11.005
12. James ND, Sydes MR, Clarke NW, Mason MD, Dearnaley DP, Spears MR, et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet (2016) 387:1163–77. doi: 10.1016/S0140-673601037-5
13. Kyriakopoulos CE, Chen YH, Carducci MA, Liu G, Jarrard DF, Hahn NM, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer: long-term survival analysis of the randomized phase III E3805 CHAARTED trial. J Clin Oncol. (2018) 36:1080–7. doi: 10.1200/JCO.2017.75.3657
14. James ND, de Bono JS, Spears MR, Clarke NW, Mason MD, Dearnaley DP, et al. Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med. (2017) 377:338–51. doi: 10.1056/NEJMoa1702900
15. Fizazi K, Tran N, Fein L, Matsubara N, Rodriguez-Antolin A, Alekseev BY, et al. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med. (2017) 377:352–60. doi: 10.1056/NEJMoa1704174
16. Tannock IF, de Wit R, Berry WR, Horti J, Pluzanka A, Chi KN. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. (2004) 351:1502–12. doi: 10.1056/NEJMoa040720
17. Petrylak DP, Tangen CM, Hussain MH, Lara PN, Jones JA, Taplin ME. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. (2004) 351:1513–20. doi: 10.1056/NEJMoa041318
18. De Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. (2011) 364:1995–2005. doi: 10.1056/NEJMoa1014618
19. Ryan CJ, Smith MR, de Bono JS, Molina A, Logothetis CJ, de Souza P, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. (2013) 368:138–48. doi: 10.1056/NEJMoa1209096
20. Beer TM, Armstrong AJ, Rathkopf DE, Loriot Y, Sternberg CN, Higano CS, et al. Enzalutamide in metastatic prostate cancer be- fore chemotherapy. N Engl J Med. (2014) 371:424–33. doi: 10.1056/NEJMoa1405095
21. Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. (2012) 367:1187–97. doi: 10.1056/NEJMoa1207506
22. Smith MR, Saad F, Chowdhury S, Oudard S, Hadaschik BA, Graff JN, et al. Apalutamide treatment and metastasis-free survival in prostate cancer. N Engl J Med. (2018) 378:1408–18. doi: 10.1056/NEJMoa1715546
23. De Bono JS, Oudard S, Ozguroglu M, Hansen S, Machiels JP, Kocak I, et al. Prednisone plus cabazitaxel or mitoxantrone for me- tastatic castration-resistant prostate cancer progressing after do- cetaxel treatment: a randomised open-label trial. Lancet (2010) 376:1147–54. doi: 10.1016/S0140-673661389-X
24. Parker C, Nilsson S, Heinrich D, Helle SI, O'Sullivan JM, Fosså SD, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. (2013) 369:213–23. doi: 10.1056/NEJMoa1213755
25. Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. (2010) 363:411–22. doi: 10.1056/NEJMoa1001294
26. Scher HI, Morris MJ, Larson S, Heller G. Validation and clinical utility of prostate cancer biomarkers. Nat Rev Clin Oncol. (2013) 10:225–34. doi: 10.1038/nrclinonc.2013.30
27. Cancer Genome Atlas Research Network. The molecular taxonomy of primary prostate cancer. Cell (2015) 163:1011–25. doi: 10.1016/j.cell.2015.10.025
28. Robinson D, Van Allen EM, Wu YM, Schultz N, Lonigro RJ, Mosquera JM, et al. Integrative clinical genomics of advanced prostate cancer. Cell (2015)161:1215–28. doi: 10.1016/j.cell.2015.05.001
29. Massari F, Di Nunno V, Comito F, Cubelli M, Ciccarese C, Iacovelli R, et al. Circulating tumor cells in genitourinary tumors. Ther Adv Urol. (2017) 10:65–77. doi: 10.1177/1756287217742564
30. Ciccarese C, Montironi R, Fiorentino M, Martignoni G, Brunelli M, Iacovelli R, et al. Circulating tumor cells: a reliable biomarker for prostate cancer treatment assessment? Curr Drug Metab. (2017) 18:692–9. doi: 10.2174/1389200218666170518163549
31. Mulholland DJ, Tran LM, Li Y, Cai H, Morim A, Wang S, et al. Cell autonomous role of PTEN in regulating castration-resistant prostate cancer growth. Cancer Cell (2011) 19:792–804. doi: 10.1016/j.ccr.2011.05.006
32. De Bono JS, De Giorgi U, Massard C, Bracarda S, Nava Rodrigues D, Kocak I, et al. PTEN loss as a predictive biomarker for the Akt inhibitor ipatasertib combined with abiraterone acetate in patients with metastatic castration-resistant prostate cancer (mCRPC). Ann Oncol. (2016) 27(Suppl. 6):vi243–65. doi: 10.1093/annonc/mdw372.02
33. Anderson PD, McKissic SA, Logan M, Roh M, Franco OE, Wang J, et al. Nkx3.1 and Myc crossregulate shared target genes in mouse and human prostate tumorigenesis. J Clin Invest. (2012) 122:1907–19. doi: 10.1172/JCI58540
34. Kirschner AN, Wang J, van der Meer R, Anderson PD, Franco-Coronel OE, Kushner MH, et al. PIM kinase inhibitor AZD1208 for treatment of MYC-driven prostate cancer. J Natl Cancer Inst. (2014) 107:dju407. doi: 10.1093/jnci/dju407
35. Antonarakis ES, Lu C, Wang H, Luber B, Nakazawa M, Roeser JC, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med. (2014) 371:1028–38. doi: 10.1056/NEJMoa1315815
36. Stone L. Prostate cancer: mastering transcription: TMPRSS2-ERG and the cis-regulatory landscape. Nat Rev Urol. (2017) 14:579. doi: 10.1038/nrurol.2017.141
37. Brenner JC, Ateeq B, Li Y, Yocum AK, Cao Q, Asangani IA, et al. Mechanistic rationale for inhibition of poly(ADP-ribose) polymerase in ETS gene fusion- positive prostate cancer. Cancer Cell (2011) 19:664–78. doi: 10.1016/j.ccr.2011.04.010
38. Aparicio AM, Harzstark AL, Corn PG, Wen S, Araujo JC, Tu SM, et al. Platinum- based chemotherapy for variant castrate-resistant prostate cancer. Clin Cancer Res. (2013) 19:3621–30. doi: 10.1158/1078-0432.CCR-12-3791
39. Sternberg CN, Petrylak DP, Sartor O, Witjes JA, Demkow T, Ferrero JM, et al. Multinational, double-blind, phase III study of prednisone and either satraplatin or placebo in patients with castrate-refractory prostate cancer progressing after prior chemotherapy: the SPARC trial. J Clin Oncol. (2009) 27:5431–8. doi: 10.1200/JCO.2008.20.1228
40. Cerrato A, Morra F, Celetti A. Use of poly ADP ribose polymerase [PARP] inhibitors in cancer cells bearing DDR defects: the rationale for their inclusion in the clinic. J Exp Clin Cancer Res. (2016) 35:179. doi: 10.1186/s13046-016-0456-2
41. Mateo J, Carreira S, Sandhu S, Miranda S, Mossop H, Perez-Lopez R, et al. DNA- repair defects and olaparib in metastatic prostate cancer. N Engl J Med. (2015) 373:1697–708. doi: 10.1056/NEJMoa1506859
42. Schiewer MJ, Goodwin JF, Han S, Brenner JC, Augello MA, Dean JL, et al. Dual roles of PARP-1 promote cancer growth and progression. Cancer Discov. (2012) 2:1134–49. doi: 10.1158/2159-8290.CD-12-0120
43. Ciccarese C, Massari F, Iacovelli R, Fiorentino M, Montironi R, Di Nunno V, et al. Prostate cancer heterogeneity: discovering novel molecular targets for therapy. Cancer Treat Rev. (2017) 54:68–73. doi: 10.1016/j.ctrv.2017.02.001
44. Allard WJ, Matera J, Miller MC, Repollet M, Connely MC, Rao C, et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res. (2004) 10: 6897–904. doi: 10.1158/1078-0432.CCR-04-0378
45. Kuske A, Gorges TM, Tennstedt P, Tiebel AK, Pompe R, Preißer F, et al. Improved detection of circulating tumor cells in non- metastatic high-risk prostate cancer patients. Sci Rep. (2016) 6:39736. doi: 10.1038/srep39736
46. Miyamoto DT, Lee RJ, Stott SL, Ting DT, Wittner BS, Ulman M, et al. Androgen receptor signaling in circulating tumor cells as a marker of hormon- ally responsive prostate cancer. Cancer Discov. (2012) 2:995–1003. doi: 10.1158/2159-8290.CD-12-0222
47. Leversha MA, Han J, Asgari Z, Danila DC, Lin O, Gonzalez-Espinoza R, et al. Fluorescence in situ hybridization analysis of circulating tumor cells in metastatic prostate cancer. Clin. Cancer Res. (2009) 15:2091–7. doi: 10.1158/1078-0432.CCR-08-2036
48. Danila DC, Anand A, Schultz N, Heller G, Wan M, Sung CC, et al. Analytic and clinical validation of a prostate cancer-enhanced mes- senger RNA detection assay in whole blood as a prognostic bio- marker for survival. Eur Urol. (2014) 65:1191–7. doi: 10.1016/j.eururo.2013.07.006
49. Danila DC, Heller G, Gignac GA, Gonzalez-Espinoza R, Anand A, Tanaka E, et al. Circulating tumor cell number and prognosis in progressive castration-resistant prostate cancer. Clin Cancer Res. (2007) 13:7053–8. doi: 10.1158/1078-0432.CCR-07-1506
50. Zheng Y, Zhang C, Wu J, Cheng G, Yang H, Hua L, et al. Prognostic value of circulating tumor cells in castration resistant prostate cancer: a meta-analysis. Urol J. (2016) 13:2881–8. doi: 10.22037/uj.v13i6.3592
51. Goldkorn A, Ely B, Quinn DI, Tangen CM, Fink LM, Xu T, et al. Circulating tumor cell counts are prognostic of overall survival in SWOG S0421: a phase III trial of docetaxel with or without atrasentan for metastatic castration-resistant prostate cancer. J Clin Oncol. (2014) 32: 1136–42. doi: 10.1200/JCO.2013.51.7417
52. Fleisher M, Danila DC, Fizazi K Hirmand M, Selby B, Phung D, et al. Circulating tumor cell (CTC) enumeration in men with metastatic castration-resistant prostate cancer (mCRPC) treated with enzalutamide post- chemotherapy (phase 3 AFFIRM study). J Clin Oncol. (2015) 33:5035. doi: 10.1200/jco.2015.33.15_suppl.5035
53. Lorente D, Olmos D, Mateo J, Dolling D, Bianchini D, Seed G, et al. Circulating tumor cell increase as a biomarker of disease progression in metastatic castration-resistant prostate cancer patients with low baseline CTC counts. Ann Oncol. (2018) 29:1554–60. doi: 10.1093/annonc/mdy172
54. Thalgott M, Rack B, Horn T, Heck MM, Eiber M, Kübler H, et al. Detection of circulating tumor cells in locally advanced high-risk prostate cancer during neoadjuvant chemotherapy and radical prostatectomy. Anticancer Res. (2015) 35:5679–85.
55. Olmos D, Arkenau HT, Ang JE, Ledaki I, Attard G, Carden CP, et al. Circulating tumour cell (CTC) counts as intermediate end points in castration-resistant prostate cancer (CRPC): a single- centre experience. Ann Oncol. (2009) 20:27–33. doi: 10.1093/annonc/mdn544
56. Darshan MS, Loftus MS, Thadani-Mulero M, Levy BP, Escuin D, Zhou XK, et al. Taxane-induced blockade to nuclear accumulation of the androgen receptor predicts clinical responses in metastatic prostate cancer. Cancer Res. (2011) 71:6019–29. doi: 10.1158/0008-5472.CAN-11-1417
57. Miyamoto DT, Zheng Y, Wittner BS, Lee RJ, Zhu H, Broderick KT, et al. RNA-Seq of single prostate CTCs implicates noncanonical Wnt signaling in antiandro- gen resistance. Science (2015) 349:351–6. doi: 10.1126/science.aab0917
58. Antonarakis ES, Lu C, Luber B, Wang H, Chen Y, Nakazawa M, et al. Androgen receptor splice variant 7 and efficacy of taxane chemotherapy in patients with metastatic castration-resistant prostate cancer. JAMA Oncol. (2015) 1:582–91. doi: 10.1001/jamaoncol.2015.1341
59. Onstenk W, Sieuwerts AM, Kraan J, Van M, Nieuweboer AJ, Mathijssen RH, et al. Efficacy of cabazitaxel in castration- resistant prostate cancer is independent of the presence of AR-V7 in circulating tumor cells. Eur Urol. (2015) 68:939–45. doi: 10.1016/j.eururo.2015.07.007
60. Antonarakis ES., Lu C, Luber B, Wang H, Chen Y, Zhu Y, et al. Clinical significance of androgen receptor splice variant-7 mRNA detection in circulating tumor cells of men with metastatic castration-resistant prostate cancer treated with first- and second- line abiraterone and enzalutamide. J Clin Oncol. (2017) 35:2149–56. doi: 10.1200/JCO.2016.70.1961
61. Scher HI, Lu D, Schreiber NA, Louw J, Graf RP, Vargas HA, et al. Association of AR-V7 on circulating tumor cells as a treatment-specific biomarker with outcomes and survival in castration- resistant prostate cancer. JAMA Oncol. (2016) 2:1441–9. doi: 10.1001/jamaoncol.2016.1828
62. De Laere B, van Dam PJ, Whitington T, Mayrhofer M, Diaz EH, Van den Eynden G, et al. Comprehensive profiling of the androgen receptor in liquid biopsies from castration-resistant prostate cancer reveals novel intra-AR structural variation and splice variant expression patterns. Eur Urol. (2017);72:192–200. doi: 10.1016/j.eururo.2017.01.011
63. Werner SL, Graf RP, Landers M, Valenta DT, Schroeder M, et al. Analytical validation and capabilities of the epic CTC platform: enrichment-free circulating tumour cell detection and characterization. J Circ Biomark. (2015) 4:3. doi: 10.5772/60725
64. Punnoose EA, Ferraldeschi R, Szafer-Glusman E, Tucker EK, Mohan S, Flohr P, et al. PTEN loss in circulating tumour cells correlates with PTEN loss in fresh tumour tissue from castration-resistant prostate cancer patients. Br J Cancer (2015) 113:1225–33. doi: 10.1038/bjc.2015.332
65. Lohr JG, Adalsteinsson VA, Cibulskis K, Choudhury AD, Rosenberg M, Cruz-Gordillo P, et al. Whole-exome sequencing of circulating tumor cells provides a window into metastatic pros- tate cancer. Nat Biotechnol. (2014) 32:479–84. doi: 10.1038/nbt.2892
66. Jiang R, Lu YT, Ho H, Li B, Chen JF, Lin M, et al. A comparison of isolated circulating tumor cells and tissue biopsies using whole- genome sequencing in prostate cancer. Oncotarget (2015) 6:44781–93. doi: 10.18632/oncotarget.6330
67. Ross RW, Galsky MD, Scher HI, Magidson J, Wassmann K, Lee GS, et al. A whole-blood RNA transcript-based prognostic model in men with castration-resistant prostate cancer: a prospective study. Lancet Oncol. (2012) 13:1105–13. doi: 10.1016/S1470-204570263-2
68. Olmos D, Brewer D, Clark J, Danila DC, Parker C, Attard G, et al. Prognostic value of blood mRNA expression signatures in castration-resistant prostate cancer: a prospective, two-stage study. Lancet Oncol. (2012) 13:1114–24. doi: 10.1016/S1470-204570372-8
69. Danila DC, Samoila A, Patel C, Schreiber N, Herkal A, Anand A, et al. Clinical validity of detecting circulating tumor cells by AdnaTest assay compared with direct detection of tumor mRNA in stabilized whole blood, as a biomarker predicting overall survival for metastatic castration-resistant prostate cancer patients. Cancer J. (2016) 22:315–20. doi: 10.1097/PPO.0000000000000220
70. Ulz P, Belic J, Graf R, Auer M, Lafer I, Fischereder K, et al. Whole- genome plasma sequencing reveals focal amplifications as a driving force in metastatic prostate cancer. Nat Commun. (2016) 7:12008. doi: 10.1038/ncomms12008
71. Wyatt AW, Annala M, Aggarwal R, Beja K, Feng F, Youngren J, et al. Concordance of circulating tumor DNA and matched meta- static tissue biopsy in prostate cancer. J Natl Cancer Inst. (2018) 110:78–86. doi: 10.1093/jnci/djx118
72. Carreira S, Romanel A, Goodall J, Grist E, Ferraldeschi R, Miranda S, et al. Tumor clone dynamics in lethal prostate cancer. Sci Transl Med. (2014); 6:254ra125. doi: 10.1126/scitranslmed.3009448
73. Romanel A, Gasi Tandefelt D, Conteduca V, Jayaram A, Casiraghi N, Wetterskog D, et al. Plasma AR and abiraterone-resistant prostate cancer. Sci Transl Med. (2015) 7:312re10. doi: 10.1126/scitranslmed.aac9511
74. Conteduca V, Wetterskog D, Sharabiani MTA, Grande E, Fernandez-Perez MP, Jayaram A, et al. Androgen receptor gene status in plasma DNA associates with worse outcome on enzalutamide or abiraterone for castration-resistant prostate cancer: a multi-institution correlative biomarker study. Ann Oncol. (2017) 28:1508–16. doi: 10.1093/annonc/mdx155
75. Lallous N, Volik SV, Awrey S, Leblanc E, Tse R, Murillo J, et al. Functional analysis of androgen receptor mutations that confer anti- androgen resistance identified in circulating cell-free DNA from prostate cancer patients. Genome Biol. (2016) 17:10. doi: 10.1186/s13059-015-0864-1
76. Wyatt AW, Azad AA, Volik SV, Annala M, Beja K, McConeghy B, et al. Genomic alterations in cell-free DNA and enzalutamide resistance in castration-resistant prostate cancer. JAMA Oncol. (2016) 2:1598–606. doi: 10.1001/jamaoncol.2016.0494
77. Azad AA, Volik SV, Wyatt AW, Haegert A, Le Bihan S, Bell RH, et al. Androgen receptor gene aberrations in circulating cell-free DNA: biomarkers of therapeutic resistance in castration-resistant prostate cancer. Clin Cancer Res. (2015) 21:2315–24. doi: 10.1158/1078-0432.CCR-14-2666
78. Pritchard CC, Mateo J, Walsh MF, De Sarkar N, Abida W, Beltran H, et al. Inherited DNA-repair gene mutations in men with meta- static prostate cancer. N Engl J Med. (2016) 375:443–53. doi: 10.1056/NEJMoa1603144
79. Goodall J, Mateo J, Yuan W, Mossop H, Porta N, Miranda S, et al. Circulating cell-free DNA to guide prostate cancer treatment with PARP inhibition. Cancer Discov. (2017) 7:1006–17. doi: 10.1158/2159-8290.CD-17-0261
80. Clarke N, Wiechno P, Alekseev B, Sala N, Jones R, Kocak I. Olaparib combined with abiraterone in patients with metastatic castration-resistant prostate cancer: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. (2018) 19:975–86. doi: 10.1016/S1470-2045(18)30365-6
81. de Bono JS, De Giorgi U, Nava Rodrigues D, Massard C, Bracarda S, Font A, et al. Randomized phase II study of Akt blockade with or without Ipatasertib in Abiraterone-treated patients with metastatic prostate cancer with and without PTEN loss. Clin Cancer Res. (2018). doi: 10.1158/1078-0432.CCR-18-0981. [Epub ahead of print].
Keywords: prostate cancer, metastatic castration resistant prostate cancer, CTCs, liquid biopsy, circulating DNA
Citation: Di Nunno V, Gatto L, Santoni M, Cimadamore A, Lopez-Beltran A, Cheng L, Scarpelli M, Montironi R and Massari F (2018) Recent Advances in Liquid Biopsy in Patients With Castration Resistant Prostate Cancer. Front. Oncol. 8:397. doi: 10.3389/fonc.2018.00397
Received: 11 July 2018; Accepted: 03 September 2018;
Published: 24 September 2018.
Edited by:
Masaki Shiota, Kyushu University, JapanReviewed by:
Takeshi Yuasa, Japanese Foundation For Cancer Research, JapanDaniel C. Danila, Memorial Sloan Kettering Cancer Center, United States
Copyright © 2018 Di Nunno, Gatto, Santoni, Cimadamore, Lopez-Beltran, Cheng, Scarpelli, Montironi and Massari. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alessia Cimadamore, YWxlc3NpYWNpbWFkYW1vcmVAZ21haWwuY29t
Francesco Massari, Zm1hc3Nhcmk3OUBnbWFpbC5jb20=
 Vincenzo Di Nunno
Vincenzo Di Nunno Lidia Gatto1
Lidia Gatto1 Matteo Santoni
Matteo Santoni Alessia Cimadamore
Alessia Cimadamore Antonio Lopez-Beltran
Antonio Lopez-Beltran Liang Cheng
Liang Cheng Rodolfo Montironi
Rodolfo Montironi Francesco Massari
Francesco Massari