- 1Department of Radiation Oncology, Xiamen Cancer Hospital, First Affiliated Hospital of Xiamen University, Xiamen, China
- 2State Key Laboratory of Oncology in South China, Department of Radiation Oncology, Sun Yat-sen University Cancer Center, Collaborative Innovation Center of Cancer Medicine, Guangzhou, China
Introduction: Distant metastasis remains the major cause of treatment failure in esophageal cancer, though there have been few large-scale studies of the patterns of distant metastasis in different histological types. We investigated the patterns of distant metastasis in esophageal adenocarcinoma (AC) and squamous cell carcinoma (SCC) using a population-based approach.
Methods: Patients with de novo stage IV esophageal cancer at diagnosis were identified using the Surveillance, Epidemiology, and End Results database. Multivariable logistic regression was performed to identify potential risk factors for site-specific distant metastasis to the distant lymph nodes, bone, liver, brain, and lung at diagnosis.
Results: We identified 1,470 patients with complete data for analysis including 1,096 (74.6%) patients with AC and 374 (25.4%) patients with SCC. A total of 2,243 sites of distant metastasis were observed, the liver was the most common site of distant metastasis (727, 32.4%), followed by the distant lymph nodes (637, 28.4%), lung (459, 20.5%), bone (344, 15.3%), and brain (76, 3.4%). Multivariable logistic regression showed that compared to patients with SCC, patients with AC were more likely to have metastasis to the brain (odds ratio [OR] 3.026, 95% confidence interval [CI] 1.441-6.357, p = 0.003) and liver (OR 1.848, 95% CI 1.394–2.451, p < 0.001), and less likely to have metastasis to the lung (OR 0.404, 95% CI 0.316–0.516, p < 0.001). Histological type had no effect on metastasis to the distant lymph nodes or bone.
Conclusions: Patients with esophageal AC are more likely to present with liver and brain metastases, and less likely to present with lung metastasis than patients with esophageal SCC.
Background
Esophageal cancer is one of the most common malignant neoplasms (1–3). Approximately half of patients have distant metastasis at initial diagnosis and more than one-third develop distant metastases after surgery or radiotherapy. Distant metastases mostly develop within 6 months of radical treatment, and median survival after diagnosis of distant metastasis is only 5 months (4–6). Therefore, distant metastasis remains the major cause of treatment failure and death in esophageal cancer.
Squamous cell carcinoma (SCC) is the predominant histological subtype of esophageal cancer in Asian countries, whereas the incidence of esophageal adenocarcinoma (AC) has been increasing in Western countries in recent decades. The etiology, clinical features, prognosis and potential treatment response of esophageal SCC and AC differ markedly (1–7). However, it is not known whether these different histological subtypes have distinct patterns of distant metastasis. In this study, we used the Surveillance, Epidemiology and End Results (SEER) database to compare the patterns of metastasis in de novo stage IV esophageal SCC and AC.
Materials and Methods
We included patients from the SEER database diagnosed with esophageal cancer between 2010 and 2014. The SEER program includes information on cancer incidence, treatment and mortality for approximately 30% of the US population, and is maintained by the National Cancer Institute1. Patients who met the following criteria were included: (1) esophageal SCC or AC with de novo stage IV disease at initial diagnosis; (2) information on age, gender, race/ethnicity, tumor location, histological subtype, tumor grade, tumor (T) stage, and regional lymph node status were available; (3) data on sites of synchronous metastatic lesions available, including distant lymph nodes, bone, liver, brain, and lung. Patients without positive histology were excluded. This study was approved by the institutional review boards of the First Affiliated Hospital of Xiamen University and Sun Yat-sen University Cancer Center.
The relationship between the clinicopathological features of the patients and the sites of distant metastasis were assessed via univariate analysis using the χ2 and Fisher's exact probability tests. Independent prognostic factors for the sites of distant metastasis were confirmed in multivariable logistic regression analysis. Risk factors that were statistically significant in univariate analysis were entered into the multivariable logistic regression model. All statistical tests were performed using SPSS (version 21.0; IBM Corporation, Armonk, NY, USA). A p-value less than 0.05 was considered statistically significant in all analyses.
Results
A total of 1,470 patients with complete data available for analysis, including 1,096 (74.6%) patients with esophageal AC and 374 (25.4%) patients with esophageal SCC, were identified. Table 1 lists the clinicopathological features of the patients. Median age at diagnosis was 63 years (range, 25–96 years). Most patients were ≥50 years-old (91.0%), male (84.0%), white race (84.6%), and had regional lymph node-positive disease (77.6%).
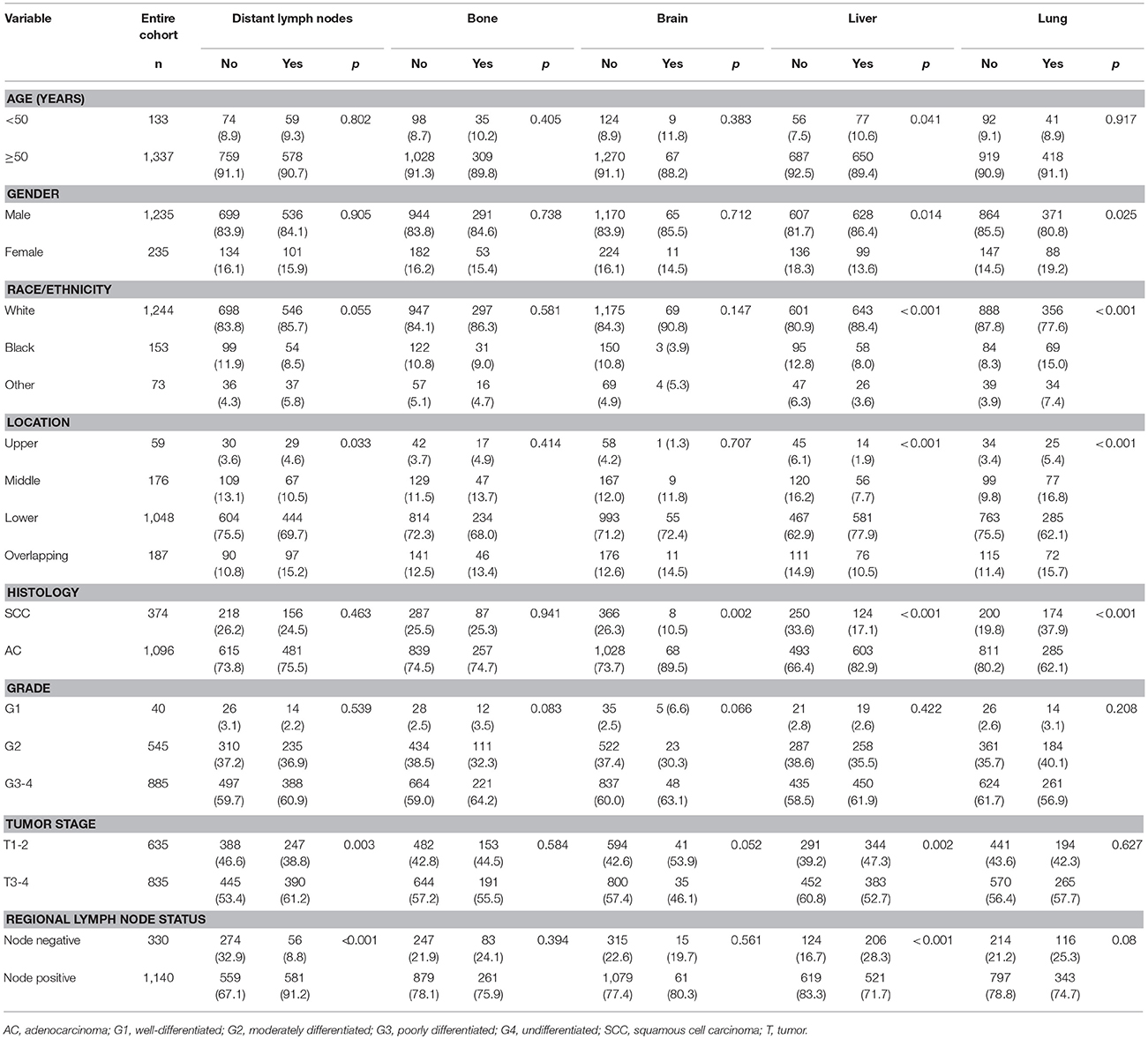
Table 1. Clinicopathological features and sites of distant metastases for the 1,470 patients with esophageal cancer.
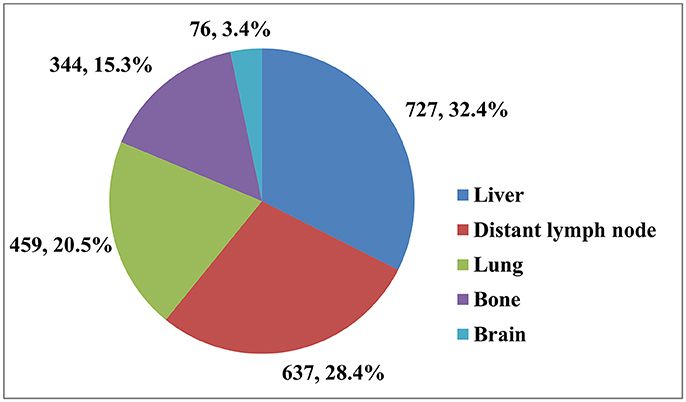
Table 1 shows the distribution of different sites of distant metastasis for the 1,470 patients. A total of 2,243 sites of distant metastasis were observed, the liver was the most common site (727, 32.4%), followed by the distant lymph nodes (637, 28.4%), lungs (459, 20.5%), bones (344, 15.3%), and brain (76, 3.4%; Figure 1). Overall, 888 (60.4%) of patients had a single site of distant metastasis, and 424 (28.8%), 128 (8.7%), 27 (1.8%), and 3 (0.2%) patients had two, three, four, and five sites, respectively.
In univariate analysis, patients with overlapping lesions (p = 0.033), advanced T stage (p = 0.003), and regional lymph node-positive disease (p < 0.001) were more likely to have distant lymph node metastasis. No risk factors were associated with bone metastasis. However, patients with esophageal AC were more likely to develop brain metastasis compared to patients with SCC: of the 76 patients with brain metastases, 89.5% (n = 68) had AC and only eight had SCC (10.5%; p = 0.002). Histological subtype (p < 0.001), age (p = 0.041), gender (p = 0.014), race/ethnicity (p < 0.001), tumor location (p < 0.002), T stage (p = 0.002), and regional lymph node status (p < 0.002) were significantly associated with liver metastasis. In addition, histological subtype (p < 0.002), gender (p = 0.025), race/ethnicity (p < 0.001), and tumor location (p < 0.001) were associated with lung metastasis (Table 1).
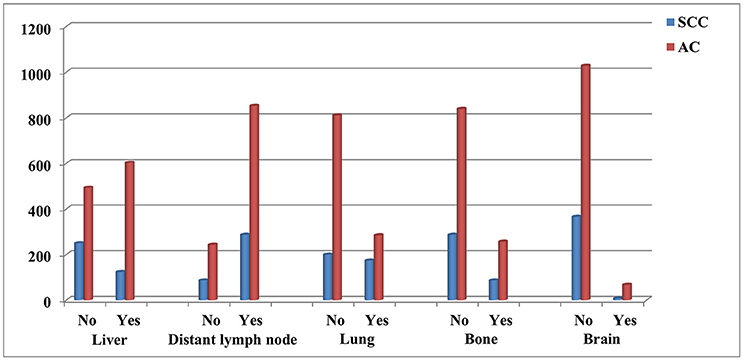
The significant risk factors in univariate analysis were entered into the multivariable logistic regression model (Table 2). Regional lymph node-positive disease (odds ratio [OR] 5.071, 95% confidence interval [CI] 3.717–6.918, p < 0.001) was an independent risk factor for distant lymph node metastasis. Specifically, patients with AC were more likely to have brain metastasis (OR 3.026, 95% CI 1.441–6.357, p = 0.003), liver metastasis (OR 1.848, 95% CI 1.394–2.451, p < 0.001) and less likely to have lung metastasis (OR 0.404, 95% CI 0.316–0.516, p < 0.001) than patients with SCC. Primary tumors located in the lower esophagus were an independent risk factors for liver metastasis in esophageal cancer compared to patients with tumor located in upper esophagus (OR 0.383, 95% CI 0.199–0.739, p = 0.004), middle esophagus (OR 0.488, 95% CI 0.337–0.705, p < 0.001), and overlapping tumors (OR 0.672, 95% CI 0.482–0.935, p = 0.018). In addition, regional lymph node status was also the risk factor of liver metastasis in esophageal cancer. Figure 2 shows the distribution of site-specific distant metastasis by histological subtype.
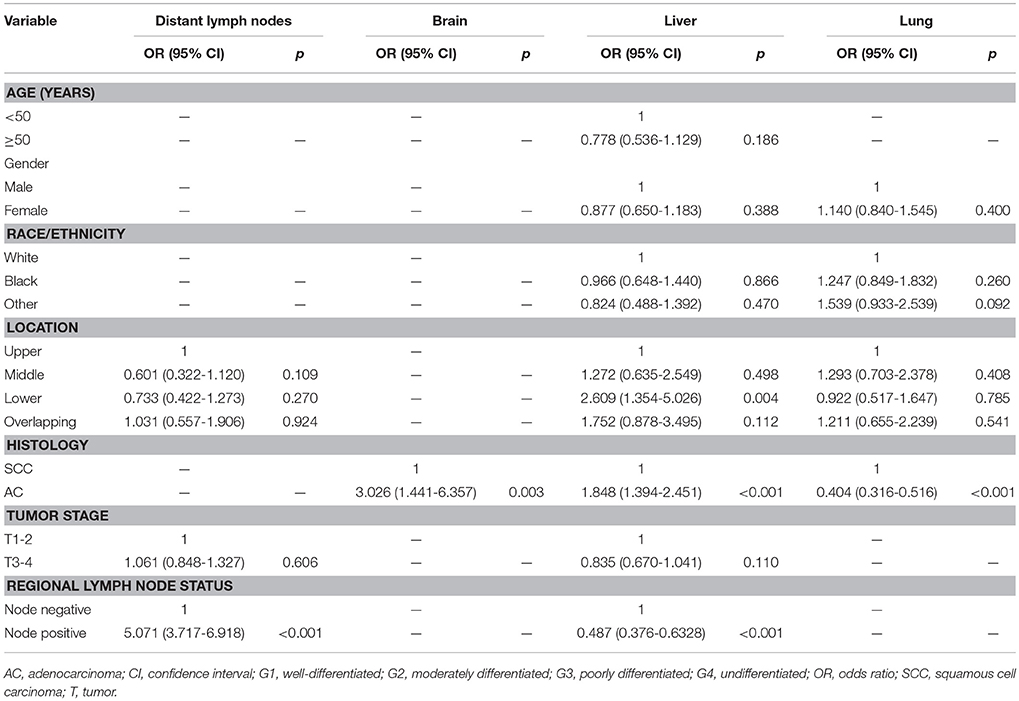
Table 2. Multivariable logistic regression of factors associated with site-specific distant metastases in esophageal cancer.
Another multivariable logistic regression model including all available variables was used to investigate the predict indicators independently associated with the histological subtype (Table 3). The results also indicated that there was an increasing incidence rate of AC subtype in patients with brain and liver metastases, younger age, male, white race, advanced T stage, and tumors located in lower esophagus compared with SCC subtype, while lung metastasis was more likely to develop in SCC subtype. The regional lymph node status, tumor grade, bone, and distant lymph node metastasis were not associated with the presence of histological subtype.
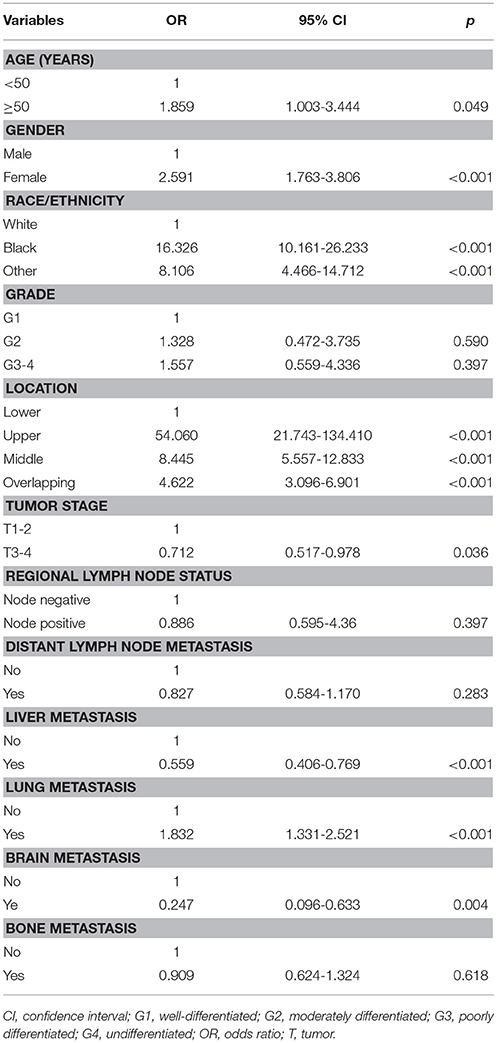
Table 3. Multivariable logistic regression of factors associated with the presence of histological subtype (adenocarcinoma as a reference).
Discussion
Due to the high frequency of distant metastasis in esophageal cancer, it is necessary to define the patterns of spread. This study reveals site-specific patterns of distant metastasis occur in the two major histological types of esophageal cancer, indicating a need for more rigorous, tailored pretreatment imaging evaluations in each histological subtype, especially for patients with advanced stage esophageal cancer.
Similarly to previous studies that included patients with distant disease at initial diagnosis or who developed distant metastasis during follow-up (8, 9), we found the liver was the most common site of distant metastasis in esophageal cancer overall, followed by the lymph nodes, lung, bone and brain. Up to now, the patterns of distant metastasis in SCC and AC have been poorly defined. Tustumi et al. found patients with AC were more likely to have liver metastasis than patients with SCC (13.7% vs. 24.5%, p = 0.002), with no significant difference in metastasis to lung (9.8% vs. 14.8%, p = 0.254), bone (11.8% vs. 8.4%, p = 0.416) or brain (4.9% vs. 2.5%, p = 0.524) (9). Quint et al. also reported AC had a higher incidence of liver metastasis than SCC (37.9% vs. 9%, p < 0.001), with no significant differences in lung, bone, and brain metastasis (10). However, these previous studies were limited by small sample sizes and a lack of population-based data. A previous SEER study included 9,934 stage I-IV esophageal cancer patients (3,242 patients with de novo stage IV esophageal cancer), and the results indicated that the SCC tumors had a higher rate of lung metastasis than AC subtype, while AC subtype had a higher rate of liver, bone, and brain metastases compared with SCC tumors (11). However, the variables included in the analysis were age, gender, race/ethnicity, histological subtype, and tumor grade. The risk factors including tumor location, tumor stage, and regional lymph node status were not included in the multiple linear regression models. In addition, they also not analysis the risk factors affecting the metastasis of distant lymph nodes. In our study, we only included patients with de novo stage IV esophageal SCC and AC, and our results found that liver metastases were more common in patients with esophageal AC. Moreover, patients with AC presented more often with brain metastasis, whereas patients with SCC had a higher frequency of lung metastasis. Although the lymph nodes and bone are also common sites of distant metastasis, we did not observe any difference in the frequencies of distant lymph node and bone metastasis between SCC and AC. Regional lymph node status was an independent risk factor for distant lymph node metastasis, though no risk factors were associated with bone metastasis. Overall, these findings support the idea that the different histological subtypes of primary esophageal cancer exhibit distinct patterns of distant metastasis.
The underlying mechanisms driving the varied patterns of distant metastasis between the two histological subtypes are somewhat unclear. Overall, 55.0% of patients with AC and 33.2% with SCC had liver metastasis at diagnosis. Multivariate analysis demonstrated a tumor located in the lower esophagus was an independent risk factor for liver metastasis. These findings suggest the higher incidence of liver metastasis in AC may be related to tumor location as well as histological type, as most cases of AC are located in the lower esophagus, whereas SCC is more evenly distributed throughout the middle and lower esophagus.
We also observed SCC had a significantly higher risk of lung metastasis. Previous studies reported the frequency of lung metastasis was not significantly different between SCC and AC (9, 10). In a study of 35 Chinese patients with SCC and distant metastasis, 22 (62.9%) had lung metastasis and two (5.8%) had liver metastasis (12). Several studies from Japan have also reported the lung is the most common site of distant metastasis in SCC (13–15).
The brain remains a rare site of metastasis (1-5%) in esophageal cancer (16). The increased of incidence brain metastasis in recent years could be attributable to more sensitive imaging modalities or improved overall survival. Smith et al. reported that seven of 53 patients with esophageal cancer (13%) developed brain metastasis during follow-up (17). However, few studies have investigated the frequency of brain metastasis in the different histological subtypes of esophageal cancer. Studies from western countries indicate that AC accounts for 68.8–90.9% of cases of brain metastasis compared to only 9.1–31.1% for SCC (18–21). However, studies in Asian countries, including China and Japan, indicate 82.1–90.9% of cases of brain metastasis occur in SCC compared to 9.1–17.9% for AC (22–24). In this population-based study of the SEER database, brain metastases were detected in 6.2% of patients with AC and only 2.1% of patients with SCC. Of the 76 patients with brain metastases, most (89.5%) had AC.
However, no previous studies reported a significant difference in the frequency of brain metastasis between histological subtypes, which may due to their small sample sizes, especially the numbers of patients with SCC (19, 21). Previous studies reported larger primary tumors and advanced clinical stage were associated with brain metastasis (19, 21, 25). In this study, histological subtype was an independent risk factor for brain metastasis: patients with esophageal AC had a higher risk of brain metastasis than those with SCC, which may reflect the differences in tumor biology between SCC and AC. In non-small cell lung carcinoma, the incidence of brain metastasis is at least two-fold higher for AC than SCC (26–29). Due to the large differences in the distribution of the histological subtypes of esophageal cancer between Asian and Western patients, it is difficult to draw a definitive conclusion on the association between brain metastasis and histological subtype. However, biomarkers could be potentially be used to identify patients at high risk of brain metastasis in the future. For example, approximately 19–43% of cases of esophageal AC overexpress human epidermal growth factor receptor 2 (HER2), which is significantly higher than the frequency in SCC; therefore, overexpression of HER2 may potentially be associated with an increased risk of brain metastasis in esophageal AC (30–34).
It is important to describe the limitations of this study. First, retrospective studies are inherently biased. Second, the SEER database only included data on five specific sites of distant metastasis at initial diagnosis, and we could not obtain further details on the occurrence and timing of secondary metastasis. In addition, we could only extract information on synchronous metastasis to the liver, lung, bone, and brain; however, a minority of patients will develop metachronous lesions. These limitations may have led to an underestimation of other sites of metastasis, but as we have noted, the four sites of metastasis assessed in this study account for approximately 90% of metastases in stage IV esophageal cancer (8–10). Moreover, the difference in the number of patients in each subtype may affect the results, given that the SCC subtype has only one third as many patients as the AC subtype. However, the results of our study were similar to the studies from the epidemic area of SCC such as China and Japan (12, 13, 15, 22–24).
Conclusion
In conclusion, our results suggest site-specific distant metastasis occurs in different histological subtypes of esophageal cancer. Patients with AC are more likely to develop synchronous liver and brain metastasis and less likely to develop lung metastasis than patients with SCC. Based on these differences, we suggest clinicians should take histological subtype into account when designing diagnostic and follow-up algorithms for esophageal cancer.
Ethics Statement
The study was approved by the ethics committee of the First Affiliated Hospital of Xiamen University and Sun Yat-sen University Cancer Center.
Author Contributions
S-GW, W-WZ, QL, and Z-YH are lead authors who participated in data collection, manuscript drafting, table/figure creation, and manuscript revision. W-WZ, F-YL, and J-YS aided in data collection. S-GW and W-WZ are senior authors who aided in drafting the manuscript and manuscript revision. QL and Z-YH is the corresponding author who initially developed the concept and drafted and revised the manuscript. All authors read and approved the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
1. ^Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 18 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2016 Sub (1973-2014 varying) - Linked To County Attributes - Total U.S., 1969-2015 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2017, based on the November 2016 submission.
References
1. Arnold M, Soerjomataram I, Ferlay J, Forman D. Global incidence of oesophageal cancer by histological subtype in 2012. Gut (2015) 64:381–7. doi: 10.1136/gutjnl-2014-308124
2. Rustgi AK, El-Serag HB. Esophageal carcinoma. N Engl J Med. (2014) 371:2499–509. doi: 10.1056/NEJMra1314530
3. Rubenstein JH, Shaheen NJ. Epidemiology, diagnosis, and management of esophageal adenocarcinoma. Gastroenterology (2015) 149:302–17.e1. doi: 10.1053/j.gastro.2015.04.053
4. Ichida H, Imamura H, Yoshimoto J, Sago H, Koriyama Y, Tsurumaru M, et al. Pattern of postoperative recurrence and hepatic and/or pulmonary resection for liver and/or lung metastases from esophageal carcinoma. World J Surg. (2013) 37:398–407. doi: 10.1007/s00268-012-1830-7
5. Shiozaki H, Sudo K, Xiao L, Wadhwa R, Elimova E, Hofstetter WL, et al. Distribution and timing of distant metastasis after local therapy in a large cohort of patients with esophageal and esophagogastric junction cancer. Oncology (2014) 86:336–9. doi: 10.1159/000360703
6. Robb WB, Messager M, Dahan L, Mornex F, Maillard E, D'Journo XB, et al. Patterns of recurrence in early-stage oesophageal cancer after chemoradiotherapy and surgery compared with surgery alone. Br J Surg. (2016) 103:117–25. doi: 10.1002/bjs.9959
7. Brown LM, Devesa SS. Epidemiologic trends in esophageal and gastric cancer in the United States. Surg Oncol Clin N Am. (2002) 11:235–56. doi: 10.1016/S1055-3207(02)00002-9
8. Chen MQ, Xu BH, Zhang YY. Analysis of prognostic factors for esophageal squamous cell carcinoma with distant organ metastasis at initial diagnosis. J Chin Med Assoc. (2014) 77:562–6. doi: 10.1016/j.jcma.2014.05.014
9. Tustumi F, Kimura CM, Takeda FR, Sallum RA, Ribeiro-Junior U, Cecconello I. Evaluation of lymphatic spread, visceral metastasis and tumoral local invasion in esophageal carcinomas. Arq Bras Cir Dig. (2016) 29:215–7. doi: 10.1590/0102-6720201600040001
10. Quint LE, Hepburn LM, Francis IR, Whyte RI, Orringer MB. Incidence and distribution of distant metastases from newly diagnosed esophageal carcinoma. Cancer (1995) 76:1120–5.
11. Ai D, Zhu H, Ren W, Chen Y, Liu Q, Deng J, et al. Patterns of distant organ metastases in esophageal cancer: a population-based study. J Thorac Dis. (2017) 9:3023–30. doi: 10.21037/jtd.2017.08.72
12. Wang Y, Wang L, Yang Q, Li J, He M, Yao J, et al. Patterns of recurrence in patients with stage pT3N0 thoracic esophageal squamous cellcarcinoma after two-field esophagectomy. [Article in Chinese] Zhonghua Zhong Liu Za Zhi (2016) 38:48–54. doi: 10.3760/cma.j.issn.0253-3766
13. Natsugoe S, Okumura H, Matsumoto M, Uchikado Y, Setoyama T, Uenosono Y, et al. The role of salvage surgery for recurrence of esophageal squamous cell cancer. Eur J Surg Oncol. (2006) 32:544–7. doi: 10.1016/j.ejso.2006.02.014
14. Sugiyama M, Morita M, Yoshida R, Ando K, Egashira A, Takefumi O, et al. Patterns and time of recurrence after complete resection of esophageal cancer. Surg Today (2012) 42:752–8. doi: 10.1007/s00595-012-0133-9
15. Ninomiya I, Okamoto K, Tsukada T, Kinoshita J, Oyama K, Fushida S, et al. Recurrence patterns and risk factors following thoracoscopic esophagectomy with radical lymph node dissection for thoracic esophageal squamous cell carcinoma. Mol Clin Oncol. (2016) 4:278–84. doi: 10.3892/mco.2015.688
16. Go PH, Klaassen Z, Meadows MC, Chamberlain RS. Gastrointestinal cancer and brain metastasis: a rare and ominous sign. Cancer (2011) 117:3630–40. doi: 10.1002/cncr.25940
17. Smith RS, Miller RC. Incidence of brain metastasis in patients with esophageal carcinoma. World J Gastroenterol. (2011) 17:2407–10. doi: 10.3748/wjg.v17.i19.2407
18. Rades D, Dziggel L, Bartscht T, Gliemroth J. Predicting overall survival in patients with brain metastases from esophageal cancer. Anticancer Res. (2014) 34:6763–5.
19. Weinberg JS, Suki D, Hanbali F, Cohen ZR, Lenzi R, Sawaya R. Metastasis of esophageal carcinoma to the brain. Cancer (2003) 98:1925–33. doi: 10.1002/cncr.11737
20. Kothari N, Mellon E, Hoffe SE, Frakes J, Shridhar R, Pimiento J, et al. Outcomes in patients with brain metastasis from esophageal carcinoma. J Gastrointest Oncol. (2016) 7:562–9. doi: 10.21037/jgo.2016.03.12
21. Gabrielsen TO, Eldevik OP, Orringer MB, Marshall BL. Esophageal carcinoma metastatic to the brain: clinical value and cost-effectiveness of routine enhanced head CT before esophagectomy. AJNR Am J Neuroradiol. (1995) 16:1915–21.
22. Feng W, Zhang P, Zheng X, Shan G, Chen M, Mao W. Neuroimaging and clinical characteristics of brain metastases from esophageal carcinoma in Chinese patients. J Cancer Res Ther. (2014) 10 (Suppl.):296–303. doi: 10.4103/0973-1482.151536
23. Song Z, Lin B, Shao L, Zhang Y. Brain metastases from esophageal cancer: clinical review of 26 cases. World Neurosurg. (2014) 81:131–5. doi: 10.1016/j.wneu.2013.02.058
24. Kanemoto A, Hashimoto T, Harada H, Asakura H, Ogawa H, Furutani K, et al. Occurrence and clinical features of brain metastasis after chemoradiotherapy for esophageal carcinoma. J Radiat Res. (2011) 52:509–15. doi: 10.1269/jrr.10184
25. Ogawa K, Toita T, Sueyama H, Fuwa N, Kakinohana Y, Kamata M, et al. Brain metastases from esophageal carcinoma: natural history, prognostic factors, and outcome. Cancer (2002) 94:759–64. doi: 10.1002/cncr.10271
26. Mujoomdar A, Austin JH, Malhotra R, Powell CA, Pearson GD, Shiau MC, et al. Clinical predictors of metastatic disease to the brain from non-small cell lung carcinoma: primary tumor size, cell type, and lymph node metastases. Radiology (2007) 242:882–8. doi: 10.1148/radiol.2423051707
27. Bajard A, Westeel V, Dubiez A, Jacoulet P, Pernet D, Dalphin JC, et al. Multivariate analysis of factors predictive of brain metastases in localised non-small cell lung carcinoma. Lung Cancer (2004) 45:317–23. doi: 10.1016/j.lungcan.2004.01.025
28. Figlin RA, Piantadosi S, Feld R; Lung Cancer Study Group. Intracranial recurrence of carcinoma after complete surgical resection of stage I, II, and III non-small-cell lung cancer. N Engl J Med. (1988) 318:1300–5.
29. Shi AA, Digumarthy SR, Temel JS, Halpern EF, Kuester LB, Aquino SL. Does initial staging or tumor histology better identify asymptomatic brain metastases in patients with non-small cell lung cancer? J Thorac Oncol. (2006) 1:205–10. doi: 10.1016/S1556-0864(15)31569-0
30. Safran H, Dipetrillo T, Akerman P, Ng T, Evans D, Steinhoff M, et al. Phase I/II study of trastuzumab, paclitaxel, cisplatin and radiation for locally advanced, HER2 overexpressing, esophageal adenocarcinoma. Int J Radiat Oncol Biol Phys. (2007) 67:405–9. doi: 10.1016/j.ijrobp.2006.08.076
31. Abu Hejleh T, Deyoung BR, Engelman E, Deutsch JM, Zimmerman B, et al. Relationship between HER-2 overexpression and brain metastasis in esophageal cancer patients. World J Gastrointest Oncol. (2012) 4:103–8. doi: 10.4251/wjgo.v4.i5.103
32. Preusser M, Berghoff AS, Ilhan-Mutlu A, Dinhof C, Magerle M, Marosi C, et al. Brain metastases of gastro-oesophageal cancer: evaluation of molecules with relevance for targeted therapies. Anticancer Res. (2013) 33:1065–71.
33. van Hagen P, Biermann K, Boers JE, Stoss O, Sleddens HF, van Lanschot JJ, et al. Human epidermal growth factor receptor 2 overexpression and amplification in endoscopic biopsies and resection specimens in esophageal and junctional adenocarcinoma. Dis Esophagus (2015) 28:380–5. doi: 10.1111/dote.12204
Keywords: esophageal cancer, metastasis, histological type, population-based cancer registry, epidemiology
Citation: Wu S-G, Zhang W-W, Sun J-Y, Li F-Y, Lin Q and He Z-Y (2018) Patterns of Distant Metastasis Between Histological Types in Esophageal Cancer. Front. Oncol. 8:302. doi: 10.3389/fonc.2018.00302
Received: 17 April 2018; Accepted: 18 July 2018;
Published: 08 August 2018.
Edited by:
Imtiaz Ahmad Siddiqui, University of Wisconsin-Madison, United StatesReviewed by:
Nidhi Jain, Cedars-Sinai Medical Center, United StatesIshaan Gupta, Weill Cornell Medicine, Cornell University, United States
Copyright © 2018 Wu, Zhang, Sun, Li, Lin and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qin Lin, bGlucWluOTUzMUAxMjYuY29t
Zhen-Yu He, aGV6aHlAc3lzdWNjLm9yZy5jbg==
†These authors have contributed equally to this work
 San-Gang Wu
San-Gang Wu Wen-Wen Zhang2†
Wen-Wen Zhang2† Zhen-Yu He
Zhen-Yu He