
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Oncol. , 13 June 2018
Sec. Molecular and Cellular Oncology
Volume 8 - 2018 | https://doi.org/10.3389/fonc.2018.00199
This article is part of the Research Topic Redox and Metabolic Circuits in Cancer View all 16 articles
 Sabrina Piras1†
Sabrina Piras1† Anna L. Furfaro1†
Anna L. Furfaro1† Rocco Caggiano2
Rocco Caggiano2 Lorenzo Brondolo1
Lorenzo Brondolo1 Silvano Garibaldi3
Silvano Garibaldi3 Caterina Ivaldo1
Caterina Ivaldo1 Umberto M. Marinari1
Umberto M. Marinari1 Maria A. Pronzato1
Maria A. Pronzato1 Raffaella Faraonio2,4*
Raffaella Faraonio2,4* Mariapaola Nitti1*
Mariapaola Nitti1*
Heme oxygenase 1 (HO-1) is crucially involved in cell adaptation to oxidative stress and has been demonstrated to play an important role in cancer progression and resistance to therapies. We recently highlighted that undifferentiated neuroblastoma (NB) cells are prone to counteract oxidative stress through the induction of HO-1. Conversely, differentiated NB cells were more sensitive to oxidative stress since HO-1 was scarcely upregulated. In this work, we investigated the role played by miR-494, which has been proved to be involved in cancer biology and in the modulation of oxidative stress, in the upregulation of HO-1. We showed that NB differentiation downregulates miR-494 level. In addition, endogenous miR-494 inhibition in undifferentiated cells impairs HO-1 induction in response to exposure to 500 µM H2O2, reducing the number of viable cells. The analysis of Bach1 expression did not reveal any significant modifications in any experimental conditions tested, proving that the impairment of HO-1 induction observed in cells treated with miR-494 inhibitor and exposed to H2O2 is independent from Bach1. Our results underline the role played by miR-494 in favoring HO-1 induction and cell adaptation to oxidative stress and contribute to the discovery of new potential pharmacological targets to improve anticancer therapies.
Heme oxygenase 1 (HO-1) is a 32-kDa inducible enzyme belonging to the HO system, which catalyzes the degradation of the iron-containing molecule heme, leading to the generation of free iron (Fe2+), carbon monoxide (CO), and biliverdin. Biliverdin reductase converts biliverdin into bilirubin (1) and ferritin quenches free iron (2). Overall, ferritin, CO, and bilirubin exert strong antioxidant, anti-apoptotic, and anti-inflammatory effects (3). Different activators are involved in HO-1 induction and the nuclear factor erythroid 2-related factor 2 (Nrf2) is considered the most important (4, 5). Moreover, Keap1 by favoring Nrf2 proteasomal degradation, and Bach1 by preventing Nrf2 binding to the promoter region of HO-1, work as HO-1 repressors (6–8).
A sustained HO-1 expression in cancer correlates with a high degree of malignancy (e.g., aggressiveness, metastatic, and angiogenetic potential), although the pro-tumorigenic role of HO-1 seems to be tumor specific and tissue specific (9, 10). In the treatment of highly aggressive neuroblastoma (NB), the upregulation of HO-1 limits the efficacy of bortezomib (11, 12) suggesting HO-1 inhibition may represent a molecular target in the clinical strategies against NB (13, 14).
By modulating the expression of many different proteins, microRNAs (miRs) supervise and integrate numerous signaling pathways and their involvement has been postulated in various physiological and pathophysiological processes, from differentiation to senescence or oncogenesis (15–17). Strong evidence supports the notion that miRs can behave as oncogenes or tumor suppressor genes (18), and given the important role of oxidative stress response in tumorigenesis, understanding miR regulation in this condition is of major interest. Since a role played by miR-494 in the modulation of oxidative stress has been demonstrated in other contexts (19, 20), but no studies have been conducted in NB cells so far. In this work, we aimed at investigating the functional role of miR-494 in NB cell response to oxidative stress, focusing on its involvement in HO-1 induction.
SH-SY5Y and SK-N-BE(2C) NB cells were cultured in RPMI 1640 medium (Euroclone, Italy) supplemented with 10% fetal bovine serum (Euroclone), 2 mM glutamine (Sigma-Aldrich, Italy), 1% amphotericin B (Sigma-Aldrich), and 1% penicillin/streptomycin (Sigma-Aldrich). Cells were differentiated by growth in the same medium supplemented with 10 µM all-trans retinoic acid (ATRA) (Sigma-Aldrich) for 4 days, up to 8 days. Differentiation was monitored by checking morphological changes such as neurite elongation and biochemical markers such as MAP2 and NeuroD1 expression (21, 22).
Total RNA was extracted using TRIZOL reagent (Life Technologies, Carlsbad, CA, USA) according to the manufacturer’s instructions. The cDNA templates for evaluation of mature miR levels were obtained from input RNAs (10 ng) using TaqMan™ Advanced miR cDNA Synthesis Kit (Thermo Fisher Scientific, USA, Cat. No. A28007) following the manufacturer’s protocol. Real-time quantitative PCR for hsa-miR-494, hsa-miR-128, hsa-miR-425-5p, and hsa-let7g-5p was performed in triplicate on diluted cDNA templates (1:10) by using the TaqMan® Advanced miR Assays (Thermo Fisher Scientific, Cat. No. A25576). hsa-miR-425-5p and hsa-let7g-5p were used as endogenous reference miRs. Relative quantification of miR expression levels was performed according to the ΔΔCt method.
SH-SY5Y cells were transiently transfected with 100 pmol of miR-494 inhibitor (miRCURY LNA miR inhibitor—hsa-miR-494-3p, QIAGEN, Hilder, Germany) and miR inhibitor Control (mirCURY LNA miR inhibitor Control—negative Control A, QIAGEN) by using Lipofectamine 2000 (Life Technologies) following the manufacturer’s instruction. 96 h after transfection, cells were treated with 500 µM H2O2 for further 6 h to assess Bach1 levels, or for further 24 h to assess HO-1 levels and cell viability.
Cell viability was evaluated by Trypan blue assay as previously described (12).
Evaluation of ROS was performed by using 2′,7′-dichlorofluorescein diacetate (DCFH-DA; Sigma-Aldrich) assay. After treatments, cells stained with 5 µM DCFH-DA for 30 min at 37°C were analyzed by FACS (Attune™ Acoustic Focusing Flow Cytometer, Thermo Fisher Scientific). Values are expressed as arbitrary units of fluorescence.
Total protein lysates, prepared by using RIPA buffer (13), were subjected to electrophoresis on SDS-polyacrylamide gel (Mini protean precast TGX gel, Bio-Rad, Milan, Italy) (22). Immunodetection was performed using mouse anti-hnRNPQ (1:1,000, Santa Cruz), rabbit anti-PTEN (1:1,000, Cell Signaling Technology, MA, USA), rabbit anti-Bach1 (1:4,000, Bethyl Lab, Montgomery, TX, USA), and rabbit anti-HO-1 (1:2,000, Origene, Herford, Germany) and specific secondary antibodies (GE Healthcare). The membranes were re-probed with the loading control antibodies, rabbit anti-GAPDH (1:1,000, Santa Cruz) or mouse anti-tubulin (1:2,000, AbCam). The bands were detected by means of an enhanced chemiluminescence system (GE Healthcare) and developed films analyzed using a specific software (GelDoc, Bio-Rad).
Statistical analysis of the differences among mean values ± SEM from three or more experiments was performed by using t-test to compare two groups or one-way ANOVA followed by Dunnett’s post-test to compare more groups.
The analysis of miR-494 expression, performed after 4-day exposure to 10 µM ATRA, showed a significant reduction in both SH-SY5Y and SK-N-BE(2C) cell lines (Figure 1A). SH-SY5Y cells increase the expression of differentiation markers already after 4-day exposure to ATRA, as widely proved (21–23). However, since SK-N-BE(2C) need more time to complete ATRA-induced differentiation (24), miR evaluation was also performed after 6- and 8-day exposure to 10 µM ATRA on SK-N-BE(2C) cells. In these conditions, only a small further decrease of miR-494 expression has been observed (Figure S1a in Supplementary Material). The expression of miR-128 has been also analyzed due to its involvement in stress response and differentiation (25, 26), but no changes were observed in both cell lines after differentiation (Figure 1B). The following experiments have been carried out on SH-SY5Y NB cells which strongly downregulated miR-494 (−10-folds vs undifferentiated) in the shortest experimental time (4 days).
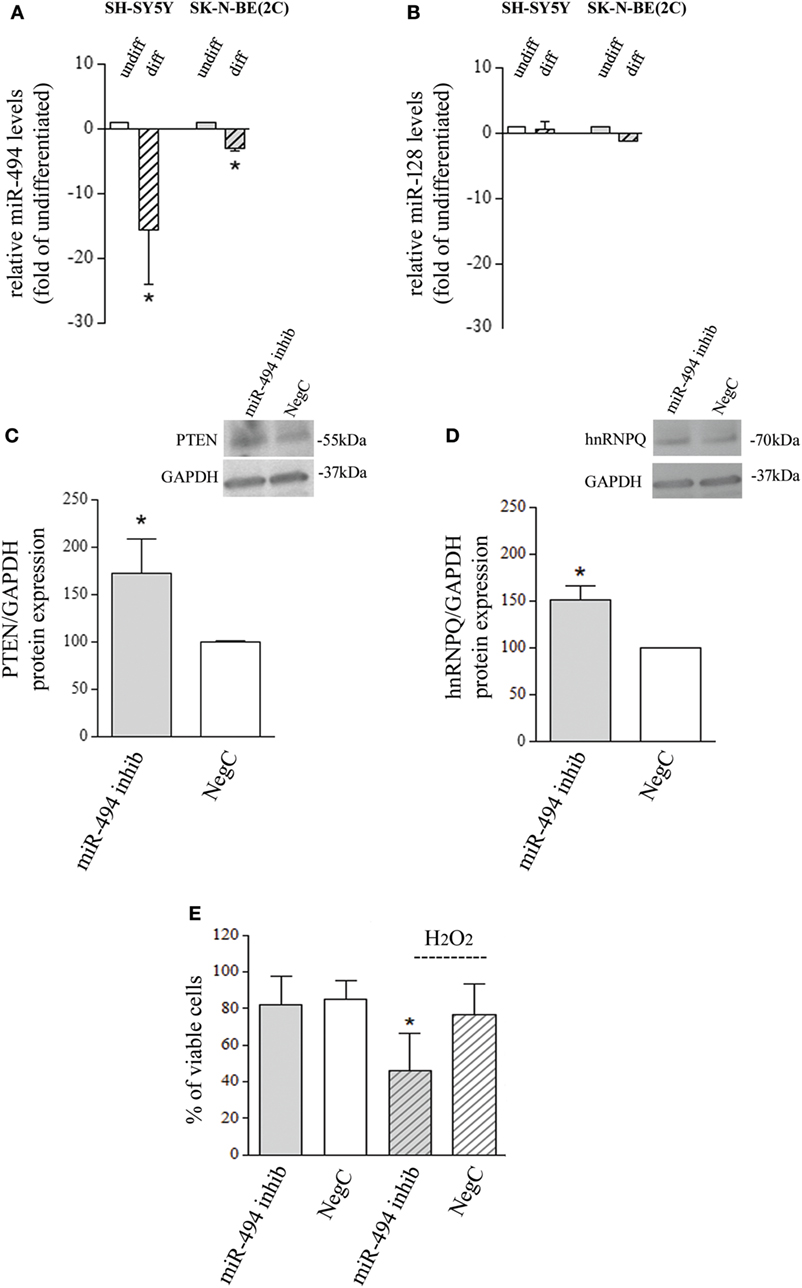
Figure 1. miR-494 downregulation occurs in neuroblastoma (NB) differentiation and modify cell response to H2O2. (A) Expression levels of mature miR-494 in undifferentiated or all-trans retinoic acid (ATRA)-differentiated SH-SY5Y and SK-N-BE(2C) NB cells. hsa-miR-425-5p and hsa-let7g-5p were used as endogenous reference miRs. Results are reported as relative to the values obtained in untreated control cells, which was set equal to 1. Statistical analysis: n = 3; *p < 0.05 vs undifferentiated. (B) Expression levels of mature miR-128 in undifferentiated or ATRA-differentiated SH-SY5Y and SK-N-BE(2C) NB cells. hsa-miR-425-5p and hsa-let7g-5p were used as endogenous reference miRs. Results are reported as relative to the values obtained in untreated control cells, which was set equal to 1. Statistical analysis: n = 3. No significant differences. (C) WB analysis of PTEN. GAPDH expression has been used as loading control. 40 µg of protein has been loaded. The bands show the most representative experiment. Statistical analysis: n = 3; *p < 0.05 vs NegC. (D) WB analysis of hnRNPQ. GAPDH expression has been used as loading control. 40 µg of protein has been loaded. The bands show the most representative experiment. Statistical analysis: n = 2; *p < 0.05 vs NegC. (E) Percentage of viable cells (Trypan blue analysis) after miR-494 inhibition and 24 h exposure to 500 µM H2O2. Statistical analysis: n = 4; *p < 0.05 vs NegC and miRNA 494 inhibitor.
To evaluate whether the reduction of endogenous miR-494 could modify NB cell sensitivity to oxidative stress, cells were transfected with a specific miR-494 inhibitor and then exposed to 500 µM H2O2. The effectiveness of miR-494 inhibition was checked by evaluating the protein levels of two miR-494 targets, namely, PTEN and hnRNPQ that resulted upregulated of about 50% (Figures 1C,D). The analysis of viable cells revealed no changes induced by miR-494 inhibition itself, in comparison to cells transfected with a NegC. Conversely, miR-494 inhibition significantly decreased the percentage of viable cells after the exposure to 500 µM H2O2 (Figure 1E). The analysis of markers of apoptosis such as BAX and PARP did not show any changes (Figures S1b and S1c in Supplementary Material) and this led us to rule out the occurrence of apoptosis. These results indicate that the expression of miR-494 in undifferentiated NB cells favors cell adaptation/response to oxidative stress.
To investigate whether a reduced expression of miR-494 influences oxidative stress response, NB cells transfected with miR-494 inhibitor or NegC were treated with H2O2 and ROS levels and HO-1 expression were evaluated. ROS levels were increased only in cells treated with miR-494 inhibitor and exposed to H2O2 (Figure 2A). In this experimental condition, no significant induction of HO-1 has been observed (Figure 2B). Conversely, in NB cells transfected with NegC, the exposure to H2O2 was able to significantly increase the expression of HO-1, and the level of ROS was not significantly modified.
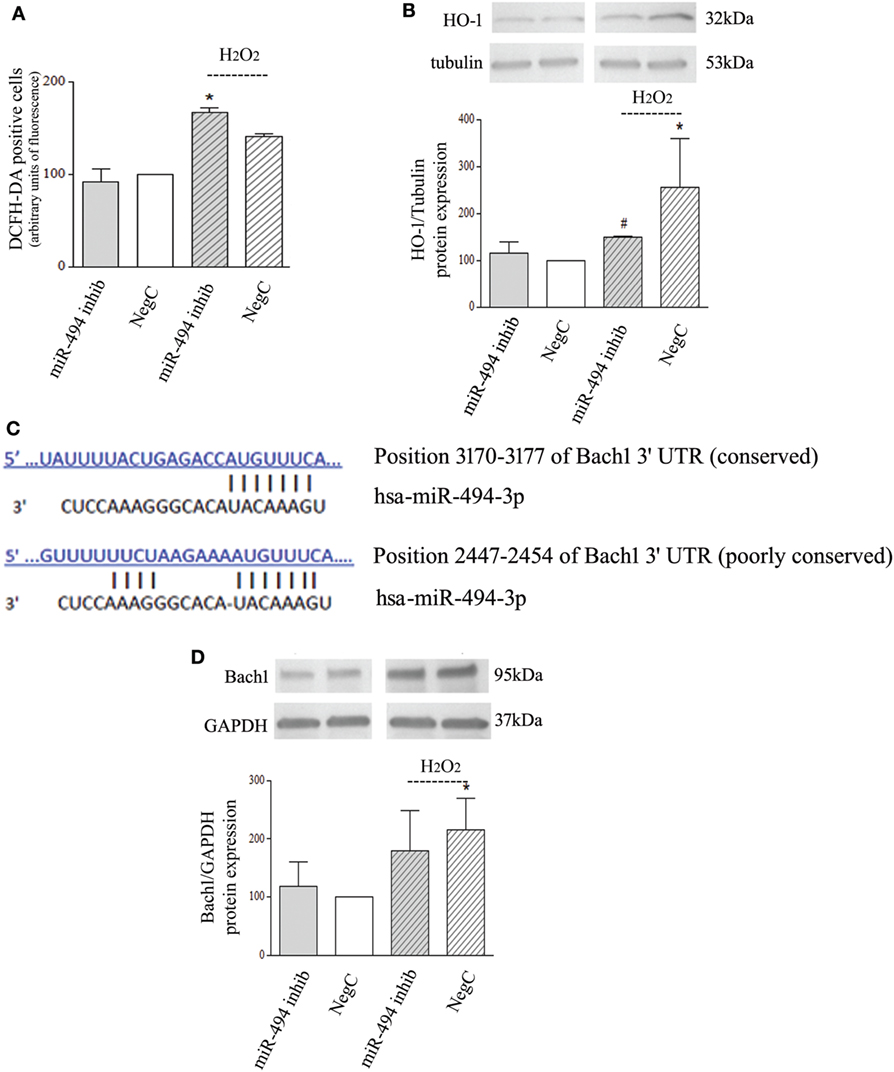
Figure 2. miR-494 inhibition impairs heme oxygenase 1 (HO-1) induction in response to H2O2 in Bach1-independent way. (A) Positivity to DCFH-DA has been measured by cytofluorimetric analyses after miR-494 inhibition and 6 h exposure to 500 µM H2O2. Statistical analysis: n = 2; *p < 0.05 vs NegC. (B) WB analysis of HO-1 expression. Tubulin expression has been used as loading control. 30 µg of protein has been loaded. The bands show the most representative experiment. Statistical analysis: n = 3; *p < 0.05 vs NegC. #p < 0.05 vs NegC + H2O2. (C) The human Bach1 3′UTR contains two seed sites for miR-494. The sequence alignments were predicted using TargetScan. (D) WB analysis of Bach1 expression. GAPDH expression has been used as loading control. 30 µg of protein has been loaded. The bands show the most representative experiment. Statistical analysis: n = 4; *p < 0.05 vs NegC.
The ubiquitination pattern was also analyzed but no changes were detected in any experimental conditions (Figure S1d in Supplementary Material).
In silico analyses predicted Bach1 as a target of miR-494 with two putative sites within its 3′UTR (Figure 2C). Thus, we checked the protein levels of Bach1. WB analysis showed that miR-494 inhibition did not modify Bach1 expression after H2O2 exposure (Figure 2D). Different Bach1 post-translational modifications have also been analyzed; ubiquitination and sumoylation were not detected and Bach1 acetylation was not modified in any experimental conditions (Figure S1e in Supplementary Material). These results show no involvement of Bach1 in the miR-494 dependent HO-1 regulation.
Furthermore, Keap1 levels have been also checked but no changes were detected in any experimental conditions (Figure S1f in Supplementary Material), proving no involvement of Nrf2 in this context.
In this work, we pointed out the involvement of miR-494 in the upregulation of HO-1 in NB cell response to oxidative stress. We took into consideration two miRs, such as miR-128 and miR-494. Indeed, miR-128 has been demonstrated to be involved in NB differentiation (26) and response to oxidative stress (25) but we did not observe any modification of miR-128 levels in the different experimental conditions we tested. Thus, we evaluated miR-494 which, from bioinformatics analyses, was predicted to have two putative binding sites on Bach1 3′UTR, the main repressor of HO-1 transcription.
In numerous contexts, miR-494 functions as tumor suppressor gene and has been linked to the induction of senescent phenotype in normal cells (19, 27) but in other contexts it correlates with tumor aggressiveness and progression (28). To the best of our knowledge, there has been no evidence of miR-494 expression in NB so far. We demonstrated that miR-494 is expressed in two undifferentiated NB cell lines and undergoes a significant downregulation after ATRA-induced differentiation. The reduction is dramatic for SH-SY5Y cells that easily differentiate in response to ATRA and minor but always significant in SK-N-BE(2C) which shown medium sensitivity to ATRA (24). There is only a paper in literature showing that the expression of miR-494 is upregulated by ATRA in the acute myeloid leukemia cell line HL-60 (29), and this lets us hypothesize that there may be a cell-type-specific regulation for miR-494. Thus, we further analyzed SH-SY5Y cells which, from our previous works, have been proved to increase their sensitivity to oxidative stress after differentiation (22, 30), investigating a possible correlation with the miR-494 downregulation. We observed that miR-494 inhibition in undifferentiated cells significantly reduced the number of viable cells after exposure to H2O2. The role of miR-494 in cell survival is controversial, depending on the cellular context where miR operates and on the accessibility of its targets. As also shown in our work, miR-494 inhibition is able to increase PTEN expression and, potentially, to antagonize the AKT survival pathway, as proved in other contexts (31, 32). However, the modulation of miR-494-PTEN signaling under stress condition has not yet been investigated.
Next, we provided evidence that endogenous miR-494 inhibition impairs HO-1 upregulation in response to oxidative stress, similar to what we have already shown in differentiated cells exposed to H2O2 (22). In addition, we showed that the lack of HO-1 upregulation correlates with higher ROS levels, highlighting the importance of HO-1 induction in quenching ROS. However, the analysis of Bach1 expression revealed that there are no significant modifications in the level of Bach1 in response to H2O2 in NB cells treated with miR-494 inhibitor compared with cells transfected with NegC. Moreover, there are no changes in Bach1 ubiquitination, sumoylation, and acetylation in any experimental conditions examined. Thus, miR-494 could contribute through a Bach1-independent mechanism to modulate HO-1 expression under stress response. It has been already demonstrated that HO-1 transcription can be controlled via Bach1 turnover in the absence or presence of oxidative stress and can also be insensitive to Bach1-mediated repression (33). Moreover, AKT-dependent HO-1 induction has been already proved (34), and miR-494-PTEN might crucially modulate it. To validate this hypothesis, further analyses are needed.
SP, ALF, SG, RF, and MN conceived and designed the experiments. SP, ALF, RC, LB, SG, and CI conducted the experiments. SP, ALF, SG, RF, and MN analyzed the results. UMM, MAP, RF, and MN contributed reagents/materials/analysis. SP, ALF, UMM, RF, and MN wrote the paper. All the authors reviewed the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This study was supported by grants from University of Genoa (to MN).
The Supplementary Material for this article can be found online at https://www.frontiersin.org/articles/10.3389/fonc.2018.00199/full#supplementary-material.
FIGURE S1. (A) Expression levels of mature miR-494 and miR-128 in undifferentiated and 6- or 8-day-differentiated SK-N-BE(2C) NB cells. hsa-miR-425-5p and hsa-let7g-5p have been used as endogenous reference miRs. Results are reported as relative to the values obtained in untreated undifferentiated cells which was set equal to 1. Statistical analysis: n = 3; *p < 0.05 vs undifferentiated. (B) WB analysis of BAX in SH-SY5Y cells treated with miR-494 inhibitor and exposed to 500mM H2O2, as indicated. GAPDH expression has been used as loading control. 10 mg of proteins was loaded. The blots show one representative experiment. Statistical analysis: n = 3; no significant differences. (C) WB analysis of PARP in SH-SY5Y cells treated with miR-494 inhibitor and exposed to 500mM H2O2, as indicated. GAPDH expression has been used as loading control. 50 mg of proteins was loaded. The blots show one representative experiment. Statistical analysis: n = 2; no significant differences. (D) WB analysis of ubiquitination in SH-SY5Y cells treated with miR-494 inhibitor and exposed to 500mM H2O2, as indicated. 20 mg of proteins was loaded. The blot shows one representative experiment. (E) Analysis of Bach1 post-translational modifications in SH-SY5Y cells treated with miR-494 inhibitor and exposed to 500mM H2O2 for 6 h. 300 mg of protein lysate was immunoprecipitated using anti Bach1 and loaded in electrophoresis (ip). An aliquot of supernatant collected after the first step of immunoprecipitation was loaded in electrophoresis (sn). WB detection was performed as indicated. The blots show the most representative experiment. (F) WB analysis of Keap1 in SH-SY5Y cells treated with miR-494 inhibitor and exposed to 500mM H2O2 as indicated. GAPDH expression has been used as loading control. 40 mg of proteins was loaded. The blots show one representative experiment. Statistical analysis: n = 3; no significant differences.
1. Maines MD. Heme oxygenase: function, multiplicity, regulatory mechanisms, and clinical applications. FASEB J (1988) 2:2557–68. doi:10.1096/fasebj.2.10.3290025
2. Cheng HT, Yen CJ, Chang CC, Huang KT, Chen KH, Zhang RY, et al. Ferritin heavy chain mediates the protective effect of heme oxygenase-1 against oxidative stress. Biochim Biophys Acta (2015) 1850:2506–17. doi:10.1016/j.bbagen.2015.09.018
3. Loboda A, Damulewicz M, Pyza E, Jozkowicz A, Dulak J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: an evolutionarily conserved mechanism. Cell Mol Life Sci (2016) 73:3221–47. doi:10.1007/s00018-016-2223-0
4. Hayes JD, Dinkova-Kostova AT. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem Sci (2014) 39:199–218. doi:10.1016/j.tibs.2014.02.002
5. Nguyen T, Nioi P, Pickett CB. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem (2009) 284:13291–5. doi:10.1074/jbc.R900010200
6. Itoh K, Mimura J, Yamamoto M. Discovery of the negative regulator of Nrf2, Keap1: a historical overview. Antioxid Redox Signal (2010) 13:1665–78. doi:10.1089/ars.2010.3222
7. Kobayashi M, Yamamoto M. Molecular mechanisms activating the Nrf2-Keap1 pathway of antioxidant gene regulation. Antioxid Redox Signal (2005) 7:385–94. doi:10.1089/ars.2005.7.385
8. Villeneuve NF, Lau A, Zhang DD. Regulation of the Nrf2-Keap1 antioxidant response by the ubiquitin proteasome system: an insight into cullin-ring ubiquitin ligases. Antioxid Redox Signal (2010) 13:1699–712. doi:10.1089/ars.2010.3211
9. Nitti M, Piras S, Marinari UM, Moretta L, Pronzato MA, Furfaro AL. HO-1 induction in cancer progression: a matter of cell adaptation. Antioxidants (Basel) (2017) 6:E29. doi:10.3390/antiox6020029
10. Was H, Dulak J, Jozkowicz A. Heme oxygenase-1 in tumor biology and therapy. Curr Drug Targets (2010) 11:1551–70. doi:10.2174/1389450111009011551
11. Furfaro AL, Piras S, Passalacqua M, Domenicotti C, Parodi A, Fenoglio D, et al. HO-1 up-regulation: a key point in high-risk neuroblastoma resistance to bortezomib. Biochim Biophys Acta (2014) 1842:613–22. doi:10.1016/j.bbadis.2013.12.008
12. Furfaro AL, Piras S, Domenicotti C, Fenoglio D, De Luigi A, Salmona M, et al. Role of Nrf2, HO-1 and GSH in neuroblastoma cell resistance to bortezomib. PLoS One (2016) 11:e0152465. doi:10.1371/journal.pone.0152465
13. Furfaro AL, Macay JR, Marengo B, Nitti M, Parodi A, Fenoglio D, et al. Resistance of neuroblastoma GI-ME-N cell line to glutathione depletion involves Nrf2 and heme oxygenase-1. Free Radic Biol Med (2012) 52:488–96. doi:10.1016/j.freeradbiomed.2011.11.007
14. Furfaro AL, Traverso N, Domenicotti C, Piras S, Moretta L, Marinari UM, et al. The Nrf2/HO-1 axis in cancer cell growth and chemoresistance. Oxid Med Cell Longev (2016) 2016:1958174. doi:10.1155/2016/1958174
15. Paul P, Chakraborty A, Sarkar D, Langthasa M, Rahman M, Bari M, et al. Interplay between miRNAs and human diseases. J Cell Physiol (2018) 233:2007–18. doi:10.1002/jcp.25854
16. Tüfekci KU, Oner MG, Meuwissen RLJ, Genç S. The role of microRNAs in human diseases. Methods Mol Biol (2014) 1107:33–50. doi:10.1007/978-1-62703-748-8_3
17. Tüfekci KU, Meuwissen RLJ, Genç S. The role of microRNAs in biological processes. Methods Mol Biol (2014) 1107:15–31. doi:10.1007/978-1-62703-748-8_2
18. Peng Y, Croce CM. The role of microRNAs in human cancer. Signal Transduct Target Ther (2016) 1:15004. doi:10.1038/sigtrans.2015.4
19. Faraonio R, Salerno P, Passaro F, Sedia C, Iaccio A, Bellelli R, et al. A set of miRNAs participates in the cellular senescence program in human diploid fibroblasts. Cell Death Differ (2012) 19:713–21. doi:10.1038/cdd.2011.143
20. Xiong R, Wang Z, Zhao Z, Li H, Chen W, Zhang B, et al. MicroRNA-494 reduces DJ-1 expression and exacerbates neurodegeneration. Neurobiol Aging (2014) 35:705–14. doi:10.1016/j.neurobiolaging.2013.09.027
21. Nitti M, Furfaro AL, Cevasco C, Traverso N, Marinari UM, Pronzato MA, et al. PKC delta and NADPH oxidase in retinoic acid-induced neuroblastoma cell differentiation. Cell Signal (2010) 22:828–35. doi:10.1016/j.cellsig.2010.01.007
22. Piras S, Furfaro AL, Brondolo L, Passalacqua M, Marinari UM, Pronzato MA, et al. Differentiation impairs Bach1 dependent HO-1 activation and increases sensitivity to oxidative stress in SH-SY5Y neuroblastoma cells. Sci Rep (2017) 7:7568. doi:10.1038/s41598-017-08095-7
23. Piras S, Furfaro AL, Piccini A, Passalacqua M, Borghi R, Carminati E, et al. Monomeric Abeta1-42 and RAGE: key players in neuronal differentiation. Neurobiol Aging (2014) 35:1301–8. doi:10.1016/j.neurobiolaging.2014.01.002
24. Yao P-L, Chen L, Dobrzański TP, Zhu B, Kang B-H, Müller R, et al. Peroxisome proliferator-activated receptor-β/δ inhibits human neuroblastoma cell tumorigenesis by inducing p53- and SOX2-mediated cell differentiation. Mol Carcinog (2017) 56:1472–83. doi:10.1002/mc.22607
25. Caggiano R, Cattaneo F, Moltedo O, Esposito G, Perrino C, Trimarco B, et al. miR-128 is implicated in stress responses by targeting MAFG in skeletal muscle cells. Oxid Med Cell Longev (2017) 2017:9308310. doi:10.1155/2017/9308310
26. Evangelisti C, Florian MC, Massimi I, Dominici C, Giannini G, Galardi S, et al. miR-128 up-regulation inhibits reelin and DCX expression and reduces neuroblastoma cell motility and invasiveness. FASEB J (2009) 23:4276–87. doi:10.1096/fj.09-134965
27. Comegna M, Succoio M, Napolitano M, Vitale M, D’Ambrosio C, Scaloni A, et al. Identification of miR-494 direct targets involved in senescence of human diploid fibroblasts. FASEB J (2014) 28:3720–33. doi:10.1096/fj.13-239129
28. Zhang Y, Guo L, Li Y, Feng G-H, Teng F, Li W, et al. MicroRNA-494 promotes cancer progression and targets adenomatous polyposis coli in colorectal cancer. Mol Cancer (2018) 17:1. doi:10.1186/s12943-017-0753-1
29. Jian P, Li ZW, Fang TY, Jian W, Zhuan Z, Mei LX, et al. Retinoic acid induces HL-60 cell differentiation via the upregulation of miR-663. J Hematol Oncol (2011) 4:20. doi:10.1186/1756-8722-4-20
30. Nitti M, Furfaro AL, Traverso N, Odetti P, Storace D, Cottalasso D, et al. PKC delta and NADPH oxidase in AGE-induced neuronal death. Neurosci Lett (2007) 416:261–5. doi:10.1016/j.neulet.2007.02.013
31. Li X-T, Wang H-Z, Wu Z-W, Yang T-Q, Zhao Z-H, Chen G-L, et al. miR-494-3p regulates cellular proliferation, invasion, migration, and apoptosis by PTEN/AKT signaling in human glioblastoma cells. Cell Mol Neurobiol (2015) 35:679–87. doi:10.1007/s10571-015-0163-0
32. Wang Y, Xu J, Gao G, Li J, Huang H, Jin H, et al. Tumor suppressor NFκB2 p100 interacts with ERK2 and stabilizes PTEN mRNA via inhibition of miR-494. Oncogene (2016) 35:4080–90. doi:10.1038/onc.2015.470
33. Tan M-KM, Lim H-J, Bennett EJ, Shi Y, Harper JW. Parallel SCF adaptor capture proteomics reveals a role for SCFFBXL17 in NRF2 activation via BACH1 repressor turnover. Mol Cell (2013) 52:9–24. doi:10.1016/j.molcel.2013.08.018
Keywords: heme oxygenase 1, neuroblastoma, miR-494, oxidative stress, Bach1
Citation: Piras S, Furfaro AL, Caggiano R, Brondolo L, Garibaldi S, Ivaldo C, Marinari UM, Pronzato MA, Faraonio R and Nitti M (2018) microRNA-494 Favors HO-1 Expression in Neuroblastoma Cells Exposed to Oxidative Stress in a Bach1-Independent Way. Front. Oncol. 8:199. doi: 10.3389/fonc.2018.00199
Received: 27 February 2018; Accepted: 17 May 2018;
Published: 13 June 2018
Edited by:
Giuseppe Filomeni, Danish Cancer Society, DenmarkReviewed by:
Luisa Rossi, Università di Roma Tor Vergata, ItalyCopyright: © 2018 Piras, Furfaro, Caggiano, Brondolo, Garibaldi, Ivaldo, Marinari, Pronzato, Faraonio and Nitti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Raffaella Faraonio, cmFmZmFlbGxhLmZhcmFvbmlvQHVuaW5hLml0;
Mariapaola Nitti, bWFyaWFwYW9sYS5uaXR0aUB1bmlnZS5pdA==
†These authors have contributed equally to this work.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.