- 1College of Medicine, Qatar University, Doha, Qatar
- 2Biomedical Research Centre, Qatar University, Doha, Qatar
- 3Oncology Department, McGill University, Montreal, Quebec, Canada
Oncoviruses are implicated in around 20% of all human cancers including both solid and non-solid malignancies. Epstein–Barr virus (EBV) and human papillomaviruses (HPVs) are the most common oncoviruses worldwide. Currently, it is well established that onco-proteins of EBV (LMP1, LMP2A, and EBNA1) and high-risk HPVs (E5 and E6/E7) play an important role in the initiation and/or progression of several human carcinomas, including cervical, oral, and breast. More significantly, it has been recently pointed out that viral onco-proteins of EBV and high-risk HPVs can be co-present and consequently cooperate to initiate and/or amplify epithelial–mesenchymal transition (EMT), which is the hallmark of cancer progression and metastasis. This could occur by β-catenin, JAK/STAT/SRC, PI3k/Akt/mTOR, and/or RAS/MEK/ERK signaling pathways, which onco-proteins of EBV and HPVs share. This review presents the most recent advances related to EBV and high-risk HPVs onco-proteins interactions and their roles in the progression of human carcinomas especially oral and breast via the initiation of EMT.
Introduction
Today, it is well-established that lifestyle, gene alteration in addition to infections from microorganisms are important risk factors for human oncogenesis. Accordingly, it was revealed that more than 50% of malignancy cases are associated with preventable origins, including oncoviruses infections (1). Globally, cancer cases associated with infections is around 20%; as roughly, two million of new malignancies reported in humans are linked with pathogens; among them 1.6 million occur in developing countries. More than two-thirds of malignancy cases are associated with well-characterized oncogenic viruses, including Epstein–Barr virus (EBV) and human papillomaviruses (HPVs) (2, 3).
Carcinogenic properties of oncoviruses are determined based on their capability to provoke cellular transformation and consequently tumor development; an effect that is attributed to genetic deregulation of infected cells leading to alteration of their normal functions. For instance, it is well-established that EBV and high-risk HPVs onco-proteins can take over intracellular and extracellular signaling pathways, provoke genomic instability, increase the life-span of infected cells (by inhibiting apoptosis), and destabilize cell senescence process, resulting in uncontrolled cell proliferation (3). These elements are important biological features of carcinogenesis (4), which can be provoked following infection by oncoviruses, including EBV and high-risk HPVs.
On the other hand, it has been established that the epithelial–mesenchymal transition (EMT) event is an important physiological procedure in the development of metastatic cancer (5). Likewise, it has been pointed out that onco-proteins of EBV (LMP1, LMP2A, and EBNA1) and high-risk HPVs (E5 and E6/E7), can enhance cancer progression of human carcinomas via the initiation of EMT (6, 7). Meanwhile, it is important to highlight that EBV and high-risk HPVs can be co-present in certain types of human malignancies especially oral and breast cancer (8, 9); consequently, onco-proteins of these viruses can cooperate to increase invasive ability of such cancers via the “amplification” of EMT. This review consolidates the existing evidence on putative effects of the co-presence of EBV and high-risk HPVs and their association with EMT and human carcinomas, especially oral and breast, in order to explain the conceptual framework for the impact of co-viral infection in cancer progression.
EBV and EMT in Human Carcinomas
Epstein–Barr virus is a very common human gammaherpesvirus, as roughly more than 90% of the adult population is infected by this virus at one point of their life (10). Acute infection with EBV can cause infectious mononucleosis (glandular fever), and its latent state can evolve to yield several B-cell lymphomas, oral cancers (especially nasopharyngeal carcinomas: NPC), gastric cancer, and other malignancies (11, 12). EBV-infected cells express six EBV nuclear antigens (EBNA1, -2, -3A, -3B, -3C, and -LP) in addition to three latent membrane proteins (LMP1, -2A, and -2B), and multiple non-coding RNAs (EBERs and miRNAs) (13–15).
The expression patterns of these genes define the types of cancers correlated with EBV (11, 12). For example, type II latency which is associated with LMP1, -2A, and EBNA1 gene expressions is linked with Hodgkin’s lymphoma and nasopharyngeal as well as other carcinomas, including gastric and probably breast (16–18). Thus, LMP1 is regarded as the main EBV-encoded oncogenic protein as it induces a multitude of effects promoting cell growth, protecting cells from apoptosis, enhancing cell motility, and stimulating angiogenesis; additionally, it is frequently expressed in EBV-associated human oral carcinomas (18, 19). Several recent studies including two from our lab revealed that EBV is present in around 40% of human breast cancer samples and its presence is associated with more aggressive phenotypes (9, 20–26).
Regarding the interaction between EBV onco-proteins and EMT, it has been revealed that LMP1 can trigger multiple signaling pathways, including NF-κB, PI3K/Akt, and MAPK, all of which are actively involved in the induction of EMT (7, 27, 28). Accumulating evidence has shown that LMP1 can downregulate E-cadherin expression (27, 29) by inducing a transcriptional repression complex composed of DNA methyltransferase and histone deacetylase, which is located on the E-cadherin gene promoter (CDH1 gene). LMP1 can also stimulate the exchange from E-cadherin to N-cadherin; and enhance the association of β-catenin with N-cadherin (30). Furthermore, LMP1 stimulates the expression of metalloproteinase 9 and regulates the transcription factors TWIST, SNAIL, and β-catenin (28, 31, 32).
On the other hand, LMP2A is another onco-protein of EBV and is overexpressed in the vast majority of EBV-associated carcinomas, especially NPC (33). It has been shown that LMP2A augments the invasive/migratory ability and incites changes in EMT-like cellular biomarkers (34); additionally, the same authors pointed out that LMP2A can induce EMT initiation by activating the 4EBP1–eIF4E axis thereby enhancing the expression of metastatic tumor antigen-1 by targeting the rapamycin (mTOR) pathway.
EBNA-1 onco-protein of EBV has a multifunctional role as a virus-related protein. EBNA-1 is overexpressed in NPC, inducing higher invasion and metastatic ability, as well as influencing EMT biomarkers (35, 36); EBNA1 regulates EMT through the de-regulation of SLUG, SNAIL, TCF8/ZEB1, vimentin, occludins-1, as well as E-cadherin, which are important genes associated with EMT (36).
Finally, it is important to underline that miRNAs, as posttranscriptional regulators, are integrated into the EBV-regulated EMT program and consequently cancer progression (7, 37). So far, a total of 25 EBV miRNA precursors with 44 mature miRNAs have been classified and mapped to the BHRF1 and BART regions (4 and 40 miRNAs, respectively) of the EBV DNA (38). miR-BART9 is overexpressed in NPC and has been found to stimulate its metastatic ability by targeting E-cadherin and inducing a mesenchymal phenotype and biomarkers (39). Recently, it has been reported that targeting PTEN 3′UTR, miR-BART7-3p downregulates epithelial biomarkers, and persuades mesenchymal features via PI3K/Akt/GSK-3 signaling pathways; this can lead to a high expression and nuclear accumulation of Snail and β-catenin in NPC and associates positively with lymph node metastasis (40).
Aga et al. (41) reported that treatment of EBV-negative cells with LMP1-exosomes increases migration and invasiveness of NP cell lines, which correlates with phenotypes associated with EMT. He et al. (42) pointed out that miR-BART6-3p, which is an EBV-encoded microRNA, inhibits EBV-associated cancer cell migration and invasion of NPC and gastric cancer cells by reversing the EMT event. On the other hand, a recent investigation by Zuo et al. (43) revealed that cadherin 6 is upregulated in LMP1-positive NPC tissues, which is identified as a target of the epithelium-specific miR-203. While, cadherin 6 activation in turn can induce EMT and promote metastasis in NPC. Moreover, it has been recently indicated that the most abundant miRNAs of EBV, in gastric cancer, are Bart4, Bart11, Bart2, Bart6, Bart9, and Bart18. Among them, Bart9 displays the same sequence as hsa miR-200a and miR-141; while, BART9 knockdown can enhance E-cadherin expression in EBV-positive gastric cancer cells (44). Taken together it implicates EBV infection in EMT initiation and consequently cancer progression, especially oral, via its onco-proteins and non-coding RNAs; however, we believe that more investigations are necessary to understand the role of all EBV onco-proteins and miRNA in the initiation of EMT in human carcinomas, especially breast.
High-Risk HPVs and EMT in Human Malignancies
HPVs are small, double-stranded DNA viruses that mostly infect cutaneous and mucosal epithelial tissues of the anogenital tract. Over 150 different types of HPV have been identified so far, one-third of which infect epithelial cells in the genital area (6). HPVs are classified as high or low risk, where high-risk types are linked with cancer development, while low-risk types are generally self-limiting and do not cause cancer. In contrast, infections with high-risk HPVs (type 16, 18, 31, 33, 35, 39, 45, 51, 52, 55, 56, 58, 59, 68, 73, 82, and 83) are correlated mainly with cervical cancers, as approximately 96% of these malignancies are positive for high-risk HPVs (45–49). More specifically, high-risk HPV early proteins, or onco-proteins, including the E5, E6, and E7 increase cellular alterations that can possibly lead to HPV-induced carcinogenesis (6, 50, 51). In this regard, earlier studies demonstrated that the E5 onco-protein could affect cellular transformation and consequently lead to carcinogenesis via its interaction with EGF-R1 signaling pathways (MAP kinase and PI3K/Akt) and pro-apoptotic proteins (52–54).
E6 and E7 of high-risk HPVs are assumed to work together, as they are both expressed from bicistronic mRNA (55) and initiated from the viral early promoter (p97). E6 and E7 both have functions that affect cell cycle progression due to their association with cell cycle controllers (50, 56, 57).
The viral E7 onco-protein causes an unscheduled S-phase entry which is complemented by the role of E6 that prevents the induction of apoptosis (58). Alternatively, it has been shown that the interaction of E6 with p53 leads to the inactivation of p53-mediated growth suppression and/or apoptosis (59). Also, E6 can associate with other pro-apoptotic proteins, including Bak and Bax (60–62). Nevertheless, the E6 onco-protein of high-risk HPVs can enhance cell proliferation independently of E7 through its C-terminal PDZ-ligand domain (63), which mediates suprabasal cell proliferation (64, 65) and may lead to cancer progression by disrupting normal cell–cell adhesion. On the other hand, several investigations have documented the correlation between E7 with members of the pocket protein family, such as pRb. This connection prevents S-phase entry by displacing E2F family of transcription factors (56), irrespective of the presence of external growth factors, causing the expression of DNA replication proteins (55, 66).
Concerning the role of high-risk HPVs in cancer progression and EMT, it is well-known that onco-proteins of these viruses are consistently expressed in infected carcinoma cells (67); this could form an important element to initiate cellular transformation and, therefore, tumor formation of certain types of malignancies via their involvement in the EMT process (68, 69). For instance, E6/E7 onco-proteins of HPV type 16 activate Jagged1, which can be associated with the induction of PI3K-mediated EMT. In addition, E6/E7 apparently incite FGF-induced EMT in cervical oncogenesis (70). In parallel, it has been shown that E6/E7 onco-proteins suppress the expression of E-cadherin in cervical cancer cells triggered by FGF stimulation, and consequently increase the invasiveness of cancer cells (70). On the other hand, it was reported that E6/E7 can induce EMT via PI3K/AKT and/or MEK/ERK in primary human keratinocytes (71); also, E6/E7 promote EMT via the activation of its transcriptional factors especially ZEB1 and ZEB2 (72).
In our laboratory, we have generated a novel model to explore the interaction outcome between E6/E7 onco-proteins of high-risk HPV and HER-2/ErbB-2 receptor in human head and neck (HN) carcinogenesis; this model was developed since ~25–30% of human HN cancers are positive for HPVs and express/overexpress HER-2 (73). Using this model, we reported that E6/E7 onco-proteins of HPV type 16 cooperate with HER-2 receptors to provoke cell transformation of human normal oral epithelial cells; this is accompanied by a delocalization of β-catenin from the undercoat membrane to the nucleus in these cells. The E6/E7/HER-2 cooperation also induces morphologic changes from a cobblestone-shaped epithelial to the spindle-shaped mesenchyme form, which enhances cell invasion and metastatic ability of these transformed cells. Additionally, our studies revealed that cyclin D1 is the main target of E6/E7/HER-2 interaction via the alteration of β-catenin’s role from a cell–cell adhesion protein to a transcriptional controller (73). Also, we have shown that cyclins D1, D2, and D3 are crucial for cell transformation provoked by E6/E7/HER-2 cooperation in our cell models and mouse normal embryonic fibroblast cells (74, 75). Last, our data pointed out that the cooperation outcome of E6/E7 and HER-2 takes place via β-catenin activation through pp60 (c-Src) phosphorylation (76).
Likewise, in oral cancer samples, it has been shown that E-cadherin is downregulated in HPV-positive samples in comparison with HPV-negative ones, while, vimentin expression remained unaltered (69); herein, it is important to highlight that both E-cadherin and vimentin are important biomarkers of EMT (5). In addition, Wakisaka et al. (77) reported that HPV-positivity is associated with EMT phenotype of oropharyngeal carcinomas and lymph node metastasis. Additionally, in tonsillar carcinoma cases, HPV-positivity is correlated with downregulation of E-cadherin and nuclear translocation of β-catenin indicating a more aggressive phenotype and risk of metastasis (78).
Next, to identify the role of high-risk HPVs infection in human cancer progression, we assessed the outcome of E6/E7 onco-proteins of HPV 16 in two non-invasive human breast cancer cell lines, MCF7 and BT20. Our data showed that E6/E7 of HPV 16 provoke cell invasive and metastatic capabilities of both cell lines (79). This is associated with an upregulation of Id-1, a family member of helix-loop-helix transcription factors, which is an important regulator of invasion and metastasis of breast cancer (80, 81). We further established that E6/E7 onco-proteins can enhance Id-1 promoter activity in both cancer cell lines. Our study on tissue samples indicated that HPV type 16 presence is significantly higher in invasive breast carcinomas in comparison with ductal carcinoma in in situ and normal breast tissues. Furthermore, our results displayed that Id-1 upregulation is associated with the presence of high-risk HPVs in human invasive and metastatic breast cancer tissues from Canadian and Syrian women (79, 82, 83). Herein, we would like to mention that the presence of high-risk HPVs in human breast cancers varies from 2 to 83% (please refer to the next section).
Concerning the role of E5 onco-protein of high-risk HPV and cancer progression, it is important to highlight that there are few investigations related to this critical topic (84, 85). However, it is evident that E5 can enhance cancer progression alone via its interaction with EGF-R1 signaling pathways (MAP kinase and PI3K/Akt) or via switching FGFR2b to FGFR2c (52–54, 85). In addition, it has been recently proposed that E5 of high-risk HPVs can cooperate with E6/E7 onco-proteins to enhance cancer progression via EMT (6).
Finally, it is important to highlight that recent studies have identified and validated HPV-encoded miRNAs (86). Accordingly, Liu et al. (87) reported that miR-375 deregulation can affect cell invasion ability of E6/E7-expressing cervical cancer cells via the modulation of EMT. Moreover, it has been revealed that E6 of HPV 16 can promote EMT and invasion in cervical cancer via the repression of miR-218 (88). On the other hand, it has been pointed out that E6 deregulate miR-34a, in HN cancer cells, which is an important controller of EMT and, therefore, cancer progression (89). Thus, it is evident that HPV-miRNA can play an important role in the regulation of cell invasion and metastasis via EMT.
Altogether these findings support the idea that high-risk HPVs can enhance cancer progression via the initiation of EMT.
EBV/HPVs Interaction and EMT in Human Cancer
It is evident that some organs and tissues can be co-infected with more than one species of viruses, including EBV and HPVs. Accordingly, in 2009, we hypothesized that human oral normal epithelial cells, particularly nasopharyngeal tissues, are prone to persistent HPVs and EBV co-infections; hence, high-risk HPVs and EBV co-infections could have a major role in the initiation and/or progression of oral cancer (8). Several investigations have explored this avenue and showed a co-presence of EBV and HPV in different types of carcinomas, including cervical, oral (nasopharyngeal), and breast cancer, as well as other malignancies (90–93). Herein, we must underline that EBV or high-risk HPVs infection alone is not enough to initiate cellular transformation of normal epithelial cells; the infected cells must endure additional genetic changes, and/or co-infection with more than one type of oncovirus to reach a full transformation (9, 73–75). Thus, we have generated a new model to study the cooperation effect between high-risk HPVs and HER-2 receptor in HN oncogenesis; as approximately 25 and 30% of HN cancers overexpress HER-2 and are positive for high-risk HPVs (73). As we mentioned above, we found that E6/E7 onco-proteins of HPV 16 cooperate with HER-2 to provoke cellular transformation and initiate EMT of human normal oral epithelial cells (73, 74).
In order to further explore the prevalence of EBV and high-risk HPVs in human HN cancers including oral malignancy in the Syrian population, we examined the presence of these viruses in a cohort of 80 oral cancer tissue samples from Syria using immunohistochemistry and Tissue Microarray methodologies. Our data revealed that 43% of these cancers are positives for high-risk HPVs (48, 49, 83). Genotyping investigation of high-risk HPVs showed that HPV types 16, 18, 31, 33, and 35 are the most frequent HPV types in HN cancers in Syria (94). The co-presence of EBV and high-risk HPVs in these samples is currently under investigation. In parallel, and in collaboration with our colleagues (Drs. Alaoui-Jamali and da Silva from McGill University), we are exploring the co-prevalence of EBV and high-risk HPVs in Canadian oral cancer samples. While, presently, there are no studies vis-à-vis the mechanisms of EBV and HPVs onco-proteins interactions in human oncogenesis; however, we believe that EMT initiation and amplification is the main target of EBV/HPVs interaction in human carcinogenesis (Figure 1). Thus, in our laboratory, we are presently exploring this important topic using both human normal oral and mammary epithelial cells as well as cancer cells.
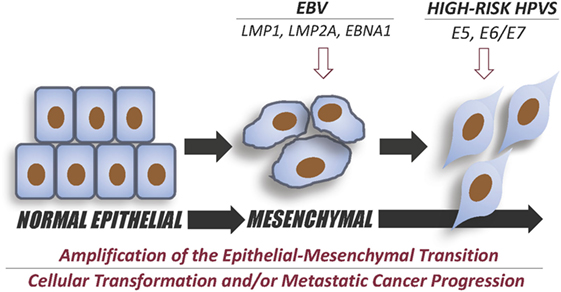
Figure 1. Diagram summary of the effect of Epstein–Barr virus EBV and high-risk human papillomaviruses (HPV) co-infection in the initiation and intensification of epithelial–mesenchymal transition (EMT) event. EBV onco-proteins, LMP1, LMP2A, and/or EBNA1 can initiate EMT and, therefore, cancer progression; while, co-expressing E5 and/or E6/E7 of high-risk HPV onco-proteins could enhance the progression of EMT leading to a more aggressive metastatic cancer, as previously reported by Al Moustafa et al. (9) and Jiang et al. (95).
Meanwhile, few studies have correlated the presence of EBV with HPV in human oral squamous cell carcinomas (SCCs). For instance, in oropharyngeal cancer, the presence of EBV and HPV viruses together in approximately 15–20% of oral SCCs (91, 96).
Likewise, Jiang et al. (89), found that 75% of tonsillar carcinomas and 90% of tongue SCCs are HPV-positive. However, EBV alone was found in 42 and 80% of tonsillar and tongue SCCs, respectively. In parallel, EBV and HPV co-infection was observed in 25% of tonsillar and 70% of tongue SCCs (95). Herein, it is important to emphasize that the presence of EBV or HPVs in NPC and/or oral SCCs is correlated with an overall better survival compared to EBV or HPVs-negative cancers (97–99). This may be attributed to the possible role of onco-proteins of these viruses, especially in the case of HPVs, in making cancer cells more sensitive to chemotherapy (100). However, it has been demonstrated that EBV and HPV co-infections can enhance invasiveness ability of human oral cancer (89) and breast cancer, as described in the next paragraph.
Concerning EBV and HPVs in human breast cancer, it has been shown that around 30–50% of breast malignancy cases are positive for EBV (21–25). In contrast, few studies were unable to detect EBV in human breast carcinomas (101, 102). In our lab, we explored the prevalence and role of EBV infections in human breast carcinogenesis; our investigation showed that around 52% of our samples are positive for EBV. We also noted that the presence of EBV is associated with invasive breast cancer phenotype in more than 60% of the examined cases (26).
Earlier studies showed that HPVs could be found in 2–86% of human breast cancer cases (24, 82, 92, 103–105). However, a small number of investigations could not find HPVs in breast cancer and normal mammary tissues (106–108). In this regard, E6/E7 onco-proteins of HPVs have been identified in breast cancer (109); though, there is a low level of transcription of these onco-proteins (110). Meanwhile, it has been reported that the presence of HPVs in breast cancer is associated with more aggressive phenotype (9, 82, 103, 111).
Previous studies predicted that oncoviruses, including EBV and high-risk HPVs, can be co-present in human breast cancer and consequently can play critical roles in the initiation and/or progression of this cancer (24, 92, 112, 113). To explore the co-presence and cooperation effect of EBV and high-risk HPVs in human breast cancer, we investigated their co-presence in breast cancer samples from Syria. We found that 32% of our samples are positive for both high-risk HPVs and EBV. Additionally, we examined the association between the co-existence of these viruses and cancer phenotype. Our data pointed out that the co-presence of EBV and HPVs is linked with high-grade invasive ductal carcinomas and lymph node involvement (9).
On the other hand, we would like to mention that, in this current issue, Vranic et al. (114) as well as de Lima et al. (90) reviewed the prevalence and role of EBV/HPVs co-infection in human cervical cancer. They pointed out that ~29% of human cervical carcinomas are positive for both EBV and HPVs, which is associated with an invasive cancer phenotype.
Overall, several studies as well as ours clearly indicate that oncoviruses, including EBV and high-risk HPVs, can be found in several human carcinomas, such as oral, breast, and cervical. We believe that their co-infection can have critical roles in the development of these malignancies and their progression; especially, since EBV and HPVs onco-proteins share several signaling pathways, such as β-catenin, JAK/STAT/SRC, PI3k/Akt/mTOR, and/or RAS/MEK/ERK, which can enhance cancer metastatic progression via the amplification of EMT (Figure 2). Thus, we think that the activation of these four pathways together could be the main mechanism behind the amplification of EMT (Table 1). Meanwhile, it is important to emphasize that co-infection of EBV and HPVs as well as other human viruses, such as herpes simplex virus 1 and 2, human cytomegalovirus, BK virus, JC virus, and adeno-associated virus, could also play a significant role in the development and/or progression of certain types of human carcinomas; this could involve other “unknown” mechanisms related to these co-infections (99). Nevertheless, it is important to highlight that there are no mechanistic studies regarding the role of EBV/HPV viral co-infection and the EMT event.
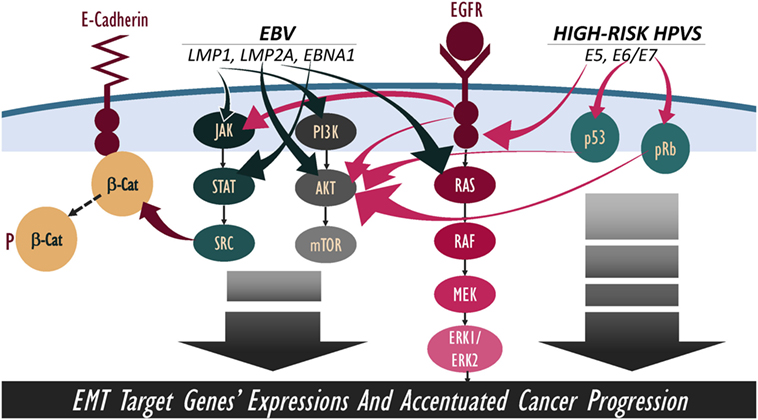
Figure 2. Schematic outline showing potential cooperation between Epstein–Barr virus (EBV) and high-risk human papillomaviruses (HPVs) onco-proteins in the amplification of epithelial–mesenchymal transition (EMT) event. We note that EBV and high-risk HPVs onco-proteins share various downstream-signaling pathways, including β-catenin, JAK/STAT/SRC, PI3k/Akt/mTOR, and RAS/MEK/ERK; thus, pathways’ crosstalk of EBV/HPVs onco-proteins can lead to a more hostile metastatic cancer.
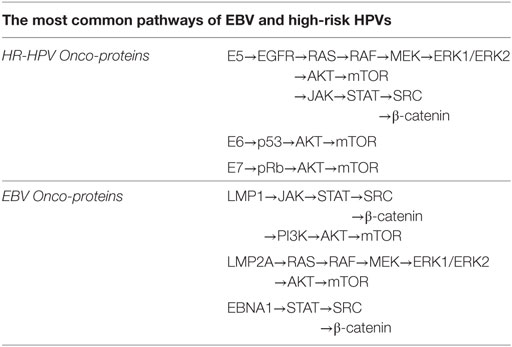
Table 1. Epstein–Barr virus (EBV) and high-risk human papillomaviruses (HPVs) onco-proteins interactions can occur via β-catenin, JAK/STAT/SRC, PI3k/Akt/mTOR, and/or RAS/MEK/ERK signaling pathways and probably other paths.
Conclusion and Future Objectives
This review presented evidence that oncoviruses co-infection, including EBV and high-risk HPVs, are important factors in human oncogenesis, thus, it is clear that they can enhance the progression of human carcinomas via the initiation of EMT which could occur by β-catenin, JAK/STAT/SRC, PI3k/Akt/mTOR, and/or RAS/MEK/ERK pathways. Therefore, further studies are necessary to identify the exact signaling pathways of EBV/HPVs onco-proteins’ interactions with the EMT event, given that no studies are currently available on this topic. Meanwhile, we assume that generating new in vitro and, in vivo models, as in cell lines and animal ones are important to determine the exact roles of these oncoviruses together and to discern their functions in the initiation and/or progression of oncogenesis; this could provide new targets to manage the malignancies associated with these oncoviruses and their co-incidence.
Alternatively, and regarding the prevention of oncoviruses-associated cancers, we believe that the elimination of some known risk factors related to lifestyle can reduce the development of these malignancies and metastases; especially, since it has been pointed out that oncoviruses co-infection could play an important role in the progression of these cancers. Meanwhile, prevention of EBV and HPV co-infections using the upcoming and/or presently available vaccines, respectively (115–117), could significantly decrease the rate of EBV and HPVs-associated malignancies and their progression to invasive forms that are responsible for most cancer-related deaths.
Author Contributions
FC, HA-F, SV, and SA edited the paper. A-EM wrote the paper from conception to its finalized form.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Mrs. A. Kassab for her critical reading of the manuscript. Our lab work is supported by Qatar University and GCC grant #2017-002 QU/KU.
Funding
This review presents the most recent advances related to EBV and high-risk HPVs onco-proteins interactions and their roles in the progression of human carcinomas especially oral and breast via the initiation of EMT.
References
1. Colditz GA, Wolin KY, Gehlert S. Applying what we know to accelerate cancer prevention. Sci Transl Med (2012) 4(127):127rv4. doi:10.1126/scitranslmed.3003218
2. de Martel C, Ferlay J, Franceschi S, Vignat J, Bray F, Forman D, et al. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol (2012) 13(6):607–15. doi:10.1016/S1470-2045(12)70137-7
3. Elgui de Oliveira D, Müller-Coan BG, Pagano JS. Viral carcinogenesis beyond malignant transformation: EBV in the progression of human cancers. Trends Microbiol (2016) 24(8):649–64. doi:10.1016/j.tim.2016.03.008
4. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell (2011) 144(5):646–74. doi:10.1016/j.cell.2011.02.013
5. Al Moustafa AE, Achkhar A, Yasmeen A. EGF-receptor signaling and epithelial-mesenchymal transition in human carcinomas. Front Biosci (Schol Ed) (2012) 4:671–84. doi:10.2741/s292
6. Al Moustafa AE. E5 and E6/E7 of high-risk HPVs cooperate to enhance cancer progression through EMT initiation. Cell Adh Migr (2015) 9(5):392–3. doi:10.1080/19336918.2015.1042197
7. Chen X, Bode AM, Dong Z, Cao Y. The epithelial-mesenchymal transition (EMT) is regulated by oncoviruses in cancer. FASEB J (2016) 30(9):3001–10. doi:10.1096/fj.201600388R
8. Al Moustafa AE, Chen D, Ghabreau L, Akil N. Association between human papillomavirus and Epstein-Barr virus infections in human oral carcinogenesis. Med Hypotheses (2009) 73(2):184–6. doi:10.1016/j.mehy.2009.02.025
9. Al Moustafa AE, Al-Antary N, Aboulkassim T, Akil N, Batist G, Yasmeen A. Co-prevalence of Epstein-Barr virus and high-risk human papillomaviruses in Syrian women with breast cancer. Hum Vaccin Immunother (2016) 12(7):1936–9. doi:10.1080/21645515.2016.1139255
10. Niedobitek G, Meru N, Delecluse HJ. Epstein-Barr virus infection and human malignancies. Int J Exp Pathol (2001) 82(3):149–70. doi:10.1111/j.1365-2613.2001.iep190.x
11. Thompson MP, Kurzrock R. Epstein-Barr virus and cancer. Clin Cancer Res (2004) 10(3):803–21. doi:10.1158/1078-0432.CCR-0670-3
12. Münz C, Moormann A. Immune escape by Epstein-Barr virus associated malignancies. Semin Cancer Biol (2008) 18(6):381–7. doi:10.1016/j.semcancer.2008.10.002
13. Middeldorp JM, Brink AA, Van Den Brule AJ, Meijer CJ. Pathogenic roles for Epstein-Barr virus (EBV) gene products in EBV-associated proliferative disorders. Crit Rev Oncol Hematol (2003) 45:1–36. doi:10.1016/S1040-8428(02)00078-1
14. Young LS, Rickinson AB. Epstein-Barr virus: 40 years on. Nat Rev Cancer (2004) 4(10):757–68. doi:10.1038/nrc1452
15. Murata T, Tsurumi T. Switching of EBV cycles between latent and lytic states. Rev Med Virol (2014) 24(3):142–53. doi:10.1002/rmv.1780
16. Michelow P, Wright C, Pantanowitz L. A review of the cytomorphology of Epstein-Barr virus-associated malignancies. Acta Cytol (2012) 56(1):1–14. doi:10.1159/000334235
17. Amarante MK, Watanabe MA. The possible involvement of virus in breast cancer. J Cancer Res Clin Oncol (2009) 135(3):329–37. doi:10.1007/s00432-008-0511-2
18. Dawson CW, Port RJ, Young LS. The role of the EBV-encoded latent membrane proteins LMP1 and LMP2 in the pathogenesis of nasopharyngeal carcinoma (NPC). Semin Cancer Biol (2012) 22(2):144–53. doi:10.1016/j.semcancer.2012.01.004
19. Morris MA, Dawson CW, Young LS. Role of the Epstein-Barr virus-encoded latent membrane protein-1, LMP1, in the pathogenesis of nasopharyngeal carcinoma. Future Oncol (2009) 5(6):811–25. doi:10.2217/fon.09.53
20. Kalkan A, Ozdarendeli A, Bulut Y, Yekeler H, Cobanoglu B, Doymaz MZ. Investigation of Epstein-Barr virus DNA in formalin-fixed and paraffin-embedded breast cancer tissues. Med Princ Pract (2005) 14:268–71. doi:10.1159/000085748
21. Fawzy S, Sallam M, Awad NM. Detection of Epstein-Barr virus in breast carcinoma in Egyptian women. Clin Biochem (2008) 41(7–8):486–92. doi:10.1016/j.clinbiochem.2007.12.017
22. Lorenzetti MA, De Matteo E, Gass H, Martinez Vazquez P, Lara J, Gonzalez P, et al. Characterization of Epstein Barr virus latency pattern in Argentine breast carcinoma. PLoS One (2010) 5:e13603. doi:10.1371/journal.pone.0013603
23. Hachana M, Amara K, Ziadi S, Romdhane E, Gacem RB, Trimeche M. Investigation of Epstein-Barr virus in breast carcinomas in Tunisia. Pathol Res Pract (2011) 207:695–700. doi:10.1016/j.prp.2011.09.007
24. Glenn WK, Heng B, Delprado W, Iacopetta B, Whitaker NJ, Lawson JS. Epstein-Barr virus, human papillomavirus and mouse mammary tumour virus as multiple viruses in breast cancer. PLoS One (2012) 7(11):e48788. doi:10.1371/journal.pone.0048788
25. Zekri AR, Bahnassy AA, Mohamed WS, El-Kassem FA, El-Khalidi SJ, Hafez MM, et al. Epstein-Barr virus and breast cancer: epidemiological and molecular study on Egyptian and Iraqi women. J Egypt Natl Canc Inst (2012) 24:123–31. doi:10.1016/j.jnci.2012.06.001
26. Aboulkassim T, Yasmeen A, Akil N, Batist G, Al Moustafa AE. Incidence of Epstein-Barr virus in Syrian women with breast cancer: a tissue microarray study. Hum Vaccin Immunother (2015) 11(4):951–5. doi:10.1080/21645515.2015.1009342
27. Tsai CL, Li HP, Lu YJ, Hsueh C, Liang Y, Chen CL, et al. Activation of DNA methyltransferase 1 by EBV LMP1 involves c-Jun NH(2)-terminal kinase signaling. Cancer Res (2006) 66(24):11668–76. doi:10.1158/0008-5472.CAN-06-2194
28. Cyprian FS, Al-Antary N, Al Moustafa AE. HER-2/Epstein-Barr virus crosstalk in human gastric carcinogenesis: a novel concept of oncogene/oncovirus interaction. Cell Adh Migr (2018) 12(1):1–4. doi:10.1080/19336918.2017.1330244
29. Jeon YK, Lee BY, Kim JE, Lee SS, Kim CW. Molecular characterization of Epstein-Barr virus and oncoprotein expression in nasopharyngeal carcinoma in Korea. Head Neck (2004) 26(7):573–83. doi:10.1002/hed.10370
30. Shair KH, Schnegg CI, Raab-Traub N. Epstein-Barr virus latent membrane protein-1 effects on junctional plakoglobin and induction of a cadherin switch. Cancer Res (2009) 69(14):5734–42. doi:10.1158/0008-5472.CAN-09-0468
31. Horikawa T, Yang J, Kondo S, Yoshizaki T, Joab I, Furukawa M, et al. Twist and epithelial-mesenchymal transition are induced by the EBV oncoprotein latent membrane protein 1 and are associated with metastatic nasopharyngeal carcinoma. Cancer Res (2007) 67(5):1970–8. doi:10.1158/0008-5472.CAN-06-3933
32. Horikawa T, Yoshizaki T, Kondo S, Furukawa M, Kaizaki Y, Pagano JS. Epstein-Barr virus latent membrane protein 1 induces snail and epithelial-mesenchymal transition in metastatic nasopharyngeal carcinoma. Br J Cancer (2011) 104(7):1160–7. doi:10.1038/bjc.2011.38
33. Kong QL, Hu LJ, Cao JY, Huang YJ, Xu LH, Liang Y, et al. Epstein-Barr virus-encoded LMP2A induces an epithelial-mesenchymal transition and increases the number of side population stem-like cancer cells in nasopharyngeal carcinoma. PLoS Pathog (2010) 6(6):e1000940. doi:10.1371/journal.ppat.1000940
34. Lin Z, Wan X, Jiang R, Deng L, Gao Y, Tang J, et al. Epstein-Barr virus-encoded latent membrane protein 2A promotes the epithelial-mesenchymal transition in nasopharyngeal carcinoma via metastatic tumor antigen 1 and mechanistic target of rapamycin signaling induction. J Virol (2014) 88(20):11872–85. doi:10.1128/JVI.01867-14
35. Wang L, Tian WD, Xu X, Nie B, Lu J, Liu X, et al. Epstein-Barr virus nuclear antigen 1 (EBNA1) protein induction of epithelial-mesenchymal transition in nasopharyngeal carcinoma cells. Cancer (2014) 120(3):363–72. doi:10.1002/cncr.28418
36. Gaur N, Gandhi J, Robertson ES, Verma SC, Kaul R. Epstein-Barr virus latent antigens EBNA3C and EBNA1 modulate epithelial to mesenchymal transition of cancer cells associated with tumor metastasis. Tumour Biol (2015) 36(4):3051–60. doi:10.1007/s13277-014-2941-6
37. Yoshizaki T, Kondo S, Wakisaka N, Murono S, Endo K, Sugimoto H, et al. Pathogenic role of Epstein-Barr virus latent membrane protein-1 in the development of nasopharyngeal carcinoma. Cancer Lett (2013) 337(1):1–7. doi:10.1016/j.canlet.2013.05.018
38. Skalsky RL, Cullen BR. EBV noncoding RNAs. Curr Top Microbiol Immunol (2015) 391:181–217. doi:10.1007/978-3-319-22834-1_6
39. Hsu CY, Yi YH, Chang KP, Chang YS, Chen SJ, Chen HC. The Epstein-Barr virus-encoded microRNA miR-BART9 promotes tumor metastasis by targeting E-cadherin in nasopharyngeal carcinoma. PLoS Pathog (2014) 10(2):e1003974. doi:10.1371/journal.ppat.1003974
40. Cai LM, Lyu XM, Luo WR, Cui XF, Ye YF, Yuan CC, et al. EBV-miR-BART7-3p promotes the EMT and metastasis of nasopharyngeal carcinoma cells by suppressing the tumor suppressor PTEN. Oncogene (2015) 34(17):2156–66. doi:10.1038/onc.2014.341
41. Aga M, Bentz GL, Raffa S, Torrisi MR, Kondo S, Wakisaka N, et al. Exosomal HIF1α supports invasive potential of nasopharyngeal carcinoma-associated LMP1-positive exosomes. Oncogene (2014) 33(37):4613–22. doi:10.1038/onc.2014.66
42. He B, Li W, Wu Y, Wei F, Gong Z, Bo H, et al. Epstein-Barr virus-encoded miR-BART6-3p inhibits cancer cell metastasis and invasion by targeting long non-coding RNA LOC553103. Cell Death Dis (2016) 7(9):e2353. doi:10.1038/cddis.2016.253
43. Zuo LL, Zhang J, Liu LZ, Zhou Q, Du SJ, Xin SY, et al. Cadherin 6 is activated by Epstein-Barr virus LMP1 to mediate EMT and metastasis as an interplay node of multiple pathways in nasopharyngeal carcinoma. Oncogenesis (2017) 6(12):402. doi:10.1038/s41389-017-0005-7
44. Tsai CY, Liu YY, Liu KH, Hsu JT, Chen TC, Chiu CT, et al. Comprehensive profiling of virus microRNAs of Epstein-Barr virus-associated gastric carcinoma: highlighting the interactions of ebv-Bart9 and host tumor cells. J Gastroenterol Hepatol (2017) 32(1):82–91. doi:10.1111/jgh.13432
45. Zuna RE, Allen RA, Moore WE, Mattu R, Dunn ST. Comparison of human papillomavirus genotypes in high-grade squamous intraepithelial lesions and invasive cervical carcinoma: evidence for differences in biologic potential of precursor lesions. Mod Pathol (2004) 17(11):1314–22. doi:10.1038/modpathol.3800223
46. Castellsagué X, Díaz M, Vaccarella S, de Sanjosé S, Muñoz N, Herrero R, et al. Intrauterine device use, cervical infection with human papillomavirus, and risk of cervical cancer: a pooled analysis of 26 epidemiological studies. Lancet Oncol (2011) 12(11):1023–31. doi:10.1016/S1470-2045(11)70223-6
47. Smith JS, Lindsay L, Hoots B, Keys J, Franceschi S, Winer R, et al. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: a meta-analysis update. Int J Cancer (2007) 121(3):621–32. doi:10.1002/ijc.22527
48. Al Moustafa AE, Al-Awadhi R, Missaoui N, Adam I, Durusoy R, Ghabreau L, et al. Human papillomaviruses-related cancers. Presence and prevention strategies in the Middle East and North African regions. Hum Vaccin Immunother (2014) 10(7):1812–21. doi:10.4161/hv.28742
49. Al Moustafa AE, Ghabreau L, Akil N, Rastam S, Alachkar A, Yasmeen A. High-risk HPVs and human carcinomas in the Syrian population. Front Oncol (2014) 4:68. doi:10.3389/fonc.2014.00068
50. Grm HS, Massimi P, Gammoh N, Banks L. Crosstalk between the human papillomavirus E2 transcriptional activator and the E6 oncoprotein. Oncogene (2005) 24(33):5149–64. doi:10.1038/sj.onc.1208701
51. Yuan CH, Filippova M, Duerksen-Hughes P. Modulation of apoptotic pathways by human papillomaviruses (HPV): mechanisms and implications for therapy. Viruses (2012) 4(12):3831–50. doi:10.3390/v4123831
52. Kim SH, Juhnn YS, Kang S, Park SW, Sung MW, Bang YJ, et al. Human papillomavirus 16 E5 up-regulates the expression of vascular endothelial growth factor through the activation of epidermal growth factor receptor, MEK/ERK1,2 and PI3K/Akt. Cell Mol Life Sci (2006) 63(7–8):930–8. doi:10.1007/s00018-005-5561-x
53. Suprynowicz FA, Disbrow GL, Krawczyk E, Simic V, Lantzky K, Schlegel R. HPV-16 E5 oncoprotein upregulates lipid raft components caveolin-1 and ganglioside GM1 at the plasma membrane of cervical cells. Oncogene (2008) 27(8):1071–8. doi:10.1038/sj.onc.1210725
54. Oh JM, Kim SH, Cho EA, Song YS, Kim WH, Juhnn YS. Human papillomavirus type 16 E5 protein inhibits hydrogen-peroxide-induced apoptosis by stimulating ubiquitin-proteasome-mediated degradation of Bax in human cervical cancer cells. Carcinogenesis (2010) 31(3):402–10. doi:10.1093/carcin/bgp318
55. Stacey SN, Jordan D, Williamson AJ, Brown M, Coote JH, Arrand JR. Leaky scanning is the predominant mechanism for translation of human papillomavirus type 16 E7 oncoprotein from E6/E7 bicistronic mRNA. J Virol (2000) 74(16):7284–97. doi:10.1128/JVI.74.16.7284-7297.2000
56. Münger K, Basile JR, Duensing S, Eichten A, Gonzalez SL, Grace M, et al. Biological activities and molecular targets of the human papillomavirus E7 oncoprotein. Oncogene (2001) 20(54):7888–98. doi:10.1038/sj.onc.1204860
57. Odone A, Visciarelli S, Lalic T, Pezzetti F, Spagnoli F, Pasquarella C, et al. Human papillomavirus-associated cancers: a survey on otorhinolaryngologists’ knowledge and attitudes on prevention. Acta Otorhinolaryngol Ital (2015) 35(6):379–85. doi:10.14639/0392-100X-621
58. Ghittoni R, Accardi R, Hasan U, Gheit T, Sylla B, Tommasino M. The biological properties of E6 and E7 oncoproteins from human papillomaviruses. Virus Genes (2010) 40(1):1–13. doi:10.1007/s11262-009-0412-8
59. Doorbar J. The papillomavirus life cycle. J Clin Virol (2005) 32(Suppl 1):S7–15. doi:10.1016/j.jcv.2004.12.006
60. Thomas M, Banks L. Inhibition of Bak-induced apoptosis by HPV-18 E6. Oncogene (1998) 17(23):2943–54. doi:10.1038/sj.onc.1202223
61. Magal SS, Jackman A, Ish-Shalom S, Botzer LE, Gonen P, Schlegel R, et al. Downregulation of Bax mRNA expression and protein stability by the E6 protein of human papillomavirus 16. J Gen Virol (2005) 86(Pt 3):611–21. doi:10.1099/vir.0.80453-0
62. Altamura G, Corteggio A, Pacini L, Conte A, Pierantoni GM, Tommasino M, et al. Transforming properties of Felis catus papillomavirus type 2 E6 and E7 putative oncogenes in vitro and their transcriptional activity in feline squamous cell carcinoma in vivo. Virology (2016) 496:1–8. doi:10.1016/j.virol.2016.05.017
63. Thomas M, Laura R, Hepner K, Guccione E, Sawyers C, Lasky L, et al. Oncogenic human papillomavirus E6 proteins target the MAGI-2 and MAGI-3 proteins for degradation. Oncogene (2002) 21(33):5088–96. doi:10.1038/sj.onc.1205668
64. Nguyen MM, Nguyen ML, Caruana G, Bernstein A, Lambert PF, Griep AE. Requirement of PDZ-containing proteins for cell cycle regulation and differentiation in the mouse lens epithelium. Mol Cell Biol (2003) 23(24):8970–81. doi:10.1128/MCB.23.24.8970-8981.2003
65. Nguyen ML, Nguyen MM, Lee D, Griep AE, Lambert PF. The PDZ ligand domain of the human papillomavirus type 16 E6 protein is required for E6’s induction of epithelial hyperplasia in vivo. J Virol (2003) 77(12):6957–64. doi:10.1128/JVI.77.12.6957-6964.2003
66. Doorbar J. Latent papillomavirus infections and their regulation. Curr Opin Virol (2013) 3(4):416–21. doi:10.1016/j.coviro.2013.06.003
67. McLaughlin-Drubin ME, Munger K. Viruses associated with human cancer. Biochim Biophys Acta (2008) 1782(3):127–50. doi:10.1016/j.bbadis.2007.12.005
68. Chamulitrat W, Sattayakhom A, Herold-Mende C, Stremmel W. Human papillomavirus 16 E6/E7-immortalized human gingival keratinocytes with epithelial mesenchymal transition acquire increased expression of cIAP-1, Bclx and p27(Kip1). Exp Dermatol (2009) 18(12):1067–9. doi:10.1111/j.1600-0625.2009.00888.x
69. Hatakeyama H, Mizumachi T, Sakashita T, Kano S, Homma A, Fukuda S. Epithelial-mesenchymal transition in human papillomavirus-positive and -negative oropharyngeal squamous cell carcinoma. Oncol Rep (2014) 32(6):2673–9. doi:10.3892/or.2014.3509
70. Cheng YM, Chou CY, Hsu YC, Chen MJ, Wing LY. The role of human papillomavirus type 16 E6/E7 oncoproteins in cervical epithelial-mesenchymal transition and carcinogenesis. Oncol Lett (2012) 3(3):667–71. doi:10.3892/ol.2011.512
71. Hochmann J, Sobrinho JS, Villa LL, Sichero L. The Asian-American variant of human papillomavirus type 16 exhibits higher activation of MAPK and PI3K/AKT signaling pathways, transformation, migration and invasion of primary human keratinocytes. Virology (2016) 492:145–54. doi:10.1016/j.virol.2016.02.015
72. Jung YS, Kato I, Kim HR. A novel function of HPV16-E6/E7 in epithelial-mesenchymal transition. Biochem Biophys Res Commun (2013) 435(3):339–44. doi:10.1016/j.bbrc.2013.04.060
73. Al Moustafa AE, Foulkes WD, Benlimame N, Wong A, Yen L, Bergeron J, et al. E6/E7 proteins of HPV type 16 and ErbB-2 cooperate to induce neoplastic transformation of primary normal oral epithelial cells. Oncogene (2004) 23(2):350–8. doi:10.1038/sj.onc.1207148
74. Al Moustafa AE, Foulkes WD, Wong A, Jallal H, Batist G, Yu Q, et al. Cyclin D1 is essential for neoplastic transformation induced by both E6/E7 and E6/E7/ErbB-2 cooperation in normal cells. Oncogene (2004) 23(30):5252–6. doi:10.1038/sj.onc.1207679
75. Yasmeen A, Hosein AN, Yu Q, Al Moustafa AE. Critical role for D-type cyclins in cellular transformation induced by E6/E7 of human papillomavirus type 16 and E6/E7/ErbB-2 cooperation. Cancer Sci (2007) 98(7):973–7. doi:10.1111/j.1349-7006.2007.00504.x
76. Al Moustafa AE, Kassab A, Darnel A, Yasmeen A. High-risk HPV/ErbB-2 interaction on E-cadherin/catenin regulation in human carcinogenesis. Curr Pharm Des (2008) 14(22):2159–72. doi:10.2174/138161208785740216
77. Wakisaka N, Yoshida S, Kondo S, Kita M, Sawada-Kitamura S, Endo K, et al. Induction of epithelial-mesenchymal transition and loss of podoplanin expression are associated with progression of lymph node metastases in human papillomavirus-related oropharyngeal carcinoma. Histopathology (2015) 66(6):771–80. doi:10.1111/his.12496
78. Stenner M, Yosef B, Huebbers CU, Preuss SF, Dienes HP, Speel EJ, et al. Nuclear translocation of β-catenin and decreased expression of epithelial cadherin in human papillomavirus-positive tonsillar cancer: an early event in human papillomavirus-related tumour progression? Histopathology (2011) 58(7):1117–26. doi:10.1111/j.1365-2559.2011.03805.x
79. Yasmeen A, Bismar TA, Kandouz M, Foulkes WD, Desprez PY, Al Moustafa AE. E6/E7 of HPV type 16 promotes cell invasion and metastasis of human breast cancer cells. Cell Cycle (2007) 6(16):2038–42. doi:10.4161/cc.6.16.4555
80. Fong S, Itahana Y, Sumida T, Singh J, Coppe JP, Liu Y, et al. Id-1 as a molecular target in therapy for breast cancer cell invasion and metastasis. Proc Natl Acad Sci U S A (2003) 100(23):13543–8. doi:10.1073/pnas.2230238100
81. Sikder HA, Devlin MK, Dunlap S, Ryu B, Alani RM. Id proteins in cell growth and tumorigenesis. Cancer Cell (2003) 3(6):525–30. doi:10.1016/S1535-6108(03)00141-7
82. Akil N, Yasmeen A, Kassab A, Ghabreau L, Darnel AD, Al Moustafa AE. High-risk human papillomavirus infections in breast cancer in Syrian women and their association with Id-1 expression: a tissue microarray study. Br J Cancer (2008) 99(3):404–7. doi:10.1038/sj.bjc.6604503
83. Al Moustafa AE, Yasmeen A, Ghabreau L, Akil N. Does the Syrian population have to wait for the new generation of human papillomaviruses vaccine?Hum Vaccin Immunother (2012) 8(12):1867–8. doi:10.4161/hv.21973
84. Boulenouar S, Weyn C, Van Noppen M, Moussa Ali M, Favre M, Delvenne PO, et al. Effects of HPV-16 E5, E6 and E7 proteins on survival, adhesion, migration and invasion of trophoblastic cells. Carcinogenesis (2010) 31(3):473–80. doi:10.1093/carcin/bgp281
85. Ranieri D, Belleudi F, Magenta A, Torrisi MR. HPV16 E5 expression induces switching from FGFR2b to FGFR2c and epithelial-mesenchymal transition. Int J Cancer (2015) 137(1):61–72. doi:10.1002/ijc.29373
86. Shishodia G, Verma G, Das BC, Bharti AC. miRNA as viral transcription tuners in HPV-mediated cervical carcinogenesis. Front Biosci (Schol Ed) (2018) 10:21–47. doi:10.2741/s499
87. Liu S, Song L, Yao H, Zhang L, Xu D, Gao F, et al. miR-375 is epigenetically downregulated by HPV-16 E6 mediated DNMT1 upregulation and modulates EMT of cervical cancer cells by suppressing lncRNA MALAT1. PLoS One (2016) 11(9):e0163460. doi:10.1371/journal.pone.0163460
88. Jiang Z, Song Q, Zeng R, Li J, Li J, Lin X, et al. MicroRNA-218 inhibits EMT, migration and invasion by targeting SFMBT1 and DCUN1D1 in cervical cancer. Oncotarget (2016) 7(29):45622–36. doi:10.18632/oncotarget.9850
89. Sun Z, Hu W, Xu J, Kaufmann AM, Albers AE. MicroRNA-34a regulates epithelial-mesenchymal transition and cancer stem cell phenotype of head and neck squamous cell carcinoma in vitro. Int J Oncol (2015) 47(4):1339–50. doi:10.3892/ijo.2015.3142
90. de Lima MAP, Neto PJN, Lima LPM, Gonçalves Júnior J, Teixeira Junior AG, Teodoro IPP, et al. Association between Epstein-Barr virus (EBV) and cervical carcinoma: a meta-analysis. Gynecol Oncol (2018) 148(2):317–28. doi:10.1016/j.ygyno.2017.10.005
91. Polz-Gruszka D, Morshed K, Stec A, Polz-Dacewicz M. Prevalence of human papillomavirus (HPV) and Epstein-Barr virus (EBV) in oral and oropharyngeal squamous cell carcinoma in south-eastern Poland. Infect Agent Cancer (2015) 10:37. doi:10.1186/s13027-015-0031-z
92. Lawson JS, Salmons B, Glenn WK. Oncogenic viruses and breast cancer: mouse mammary tumor virus (MMTV), bovine leukemia virus (BLV), human papilloma virus (HPV), and Epstein-Barr virus (EBV). Front Oncol (2018) 8:1. doi:10.3389/fonc.2018.00001
93. Mirzaei H, Goudarzi H, Eslami G, Faghihloo E. Role of viruses in gastrointestinal cancer. J Cell Physiol (2018) 233(5):4000–14. doi:10.1002/jcp.26194
94. Al Moustafa A-E, Cyprian FS, Al-Antary N, Yasmeen A. High-risk human papillomaviruses and Epstein-Barr virus presence and crosstalk in human oral carcinogenesis. In: Al Moustafa A-E, editor. Development Oral Cancer: Risk Factors & Prevention Strategies. Doha: Springer (2017). p. 83–94.
95. Jiang R, Ekshyyan O, Moore-Medlin T, Rong X, Nathan S, Gu X, et al. Association between human papilloma virus/Epstein-Barr virus coinfection and oral carcinogenesis. J Oral Pathol Med (2015) 44(1):28–36. doi:10.1111/jop.12221
96. Jalouli J, Jalouli MM, Sapkota D, Ibrahim SO, Larsson PA, Sand L. Human papilloma virus, herpes simplex virus and epstein barr virus in oral squamous cell carcinoma from eight different countries. Anticancer Res (2012) 32(2):571–80.
97. Dogan S, Hedberg ML, Ferris RL, Rath TJ, Assaad AM, Chiosea SI. Human papillomavirus and Epstein-Barr virus in nasopharyngeal carcinoma in a low-incidence population. Head Neck (2014) 36(4):511–6. doi:10.1002/hed.23318
98. Stenmark MH, McHugh JB, Schipper M, Walline HM, Komarck C, Feng FY, et al. Nonendemic HPV-positive nasopharyngeal carcinoma: association with poor prognosis. Int J Radiat Oncol Biol Phys (2014) 88(3):580–8. doi:10.1016/j.ijrobp.2013.11.246
99. Guidry JT, Scott RS. The interaction between human papillomavirus and other viruses. Virus Res (2017) 231:139–47. doi:10.1016/j.virusres.2016.11.002
100. Hoffmann M, Quabius ES, Tribius S, Gebhardt S, Görögh T, Hedderich J, et al. Influence of HPV-status on survival of patients with tonsillar carcinomas (TSCC) treated by CO2-laser surgery plus risk adapted therapy – a 10 year retrospective single centre study. Cancer Lett (2018) 413:59–68. doi:10.1016/j.canlet.2017.10.045
101. Deshpande CG, Badve S, Kidwai N, Longnecker R. Lack of expression of the Epstein-Barr virus (EBV) gene products, EBERs, EBNA1, LMP1, and LMP2A, in breast cancer cells. Lab Invest (2002) 82(9):1193–9. doi:10.1097/01.LAB.0000029150.90532.24
102. Kadivar M, Monabati A, Joulaee A, Hosseini N. Epstein-Barr virus and breast cancer: lack of evidence for an association in Iranian women. Pathol Oncol Res (2011) 17(3):489–92. doi:10.1007/s12253-010-9325-z
103. de Villiers EM, Sandstrom RE, zur Hausen H, Buck CE. Presence of papillomavirus sequences in condylomatous lesions of the mamillae and in invasive carcinoma of the breast. Breast Cancer Res (2005) 7(1):R1–11. doi:10.1186/bcr940
104. Kan CY, Iacopetta BJ, Lawson JS, Whitaker NJ. Identification of human papillomavirus DNA gene sequences in human breast cancer. Br J Cancer (2005) 93(8):946–8. doi:10.1038/sj.bjc.6602778
105. Antonsson A, Spurr TP, Chen AC, Francis GD, McMillan NA, Saunders NA, et al. High prevalence of human papillomaviruses in fresh frozen breast cancer samples. J Med Virol (2011) 83(12):2157–63. doi:10.1002/jmv.22223
106. Lindel K, Forster A, Altermatt HJ, Greiner R, Gruber G. Breast cancer and human papillomavirus (HPV) infection: no evidence of a viral etiology in a group of Swiss women. Breast (2007) 16(2):172–7. doi:10.1016/j.breast.2006.09.001
107. Hachana M, Ziadi S, Amara K, Toumi I, Korbi S, Trimeche M. No evidence of human papillomavirus DNA in breast carcinoma in Tunisian patients. Breast (2010) 19(6):541–4. doi:10.1016/j.breast.2010.05.007
108. Gannon OM, Antonsson A, Bennett IC, Saunders NA. Viral infections and breast cancer – a current perspective. Cancer Lett (2018) 420:182–9. doi:10.1016/j.canlet.2018.01.076
109. Heng B, Glenn WK, Ye Y, Tran B, Delprado W, Lutze-Mann L, et al. Human papilloma virus is associated with breast cancer. Br J Cancer (2009) 101(8):1345–50. doi:10.1038/sj.bjc.6605282
110. Gannon OM, Antonsson A, Milevskiy M, Brown MA, Saunders NA, Bennett IC. No association between HPV positive breast cancer and expression of human papilloma viral transcripts. Sci Rep (2015) 5:18081. doi:10.1038/srep18081
111. Wang YW, Zhang K, Zhao S, Lv Y, Zhu J, Liu H, et al. HPV status and its correlation with BCL2, p21, p53, Rb, and survivin expression in breast cancer in a Chinese population. Biomed Res Int (2017) 2017:6315392. doi:10.1155/2017/6315392
112. Naushad W, Surriya O, Sadia H. Prevalence of EBV, HPV and MMTV in Pakistani breast cancer patients: a possible etiological role of viruses in breast cancer. Infect Genet Evol (2017) 54:230–7. doi:10.1016/j.meegid.2017.07.010
113. Aguayo F, Khan N, Koriyama C, González C, Ampuero S, Padilla O, et al. Human papillomavirus and Epstein-Barr virus infections in breast cancer from Chile. Infect Agent Cancer (2011) 6(1):7. doi:10.1186/1750-9378-6-7
114. Vranic S, Cyprian FS, Akhtar S, Al Moustafa A-E. The role of epstein–barr virus in cervical cancer: a brief update. Front Oncol (2018) 8:113. doi:10.3389/fonc.2018.00113
115. Rajčáni J, Bánáti F, Szenthe K, Szathmary S. The potential of currently unavailable herpes virus vaccines. Expert Rev Vaccines (2018) 9:1–10. doi:10.1080/14760584.2018.1425620
116. Luxembourg A, Moeller E. 9-Valent human papillomavirus vaccine: a review of the clinical development program. Expert Rev Vaccines (2017) 16(11):1119–39. doi:10.1080/14760584.2017.1383158
Keywords: Epstein–Barr virus, high-risk human papillomaviruses, onco-proteins, epithelial–mesenchymal transition, cancer progression
Citation: Cyprian FS, Al-Farsi HF, Vranic S, Akhtar S and Al Moustafa A-E (2018) Epstein–Barr Virus and Human Papillomaviruses Interactions and Their Roles in the Initiation of Epithelial–Mesenchymal Transition and Cancer Progression. Front. Oncol. 8:111. doi: 10.3389/fonc.2018.00111
Received: 14 February 2018; Accepted: 29 March 2018;
Published: 01 May 2018
Edited by:
Imtiaz Ahmad Siddiqui, University of Wisconsin-Madison, United StatesReviewed by:
Yonggang Pei, University of Pennsylvania, United StatesTarique Khan, Buck Institute for Research on Aging, United States
Ankita Arora, University of Colorado Denver, United States
Copyright: © 2018 Cyprian, Al-Farsi, Vranic, Akhtar and Al Moustafa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ala-Eddin Al Moustafa, YWFsbW91c3RhZmFAcXUuZWR1LnFh, YWxhLWVkZGluLmFsbW91c3RhZmFAbWNnaWxsLmNh
 Farhan S. Cyprian
Farhan S. Cyprian Halema F. Al-Farsi
Halema F. Al-Farsi Semir Vranic
Semir Vranic Saghir Akhtar
Saghir Akhtar Ala-Eddin Al Moustafa
Ala-Eddin Al Moustafa