- Department of Dermatology, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan
Merkel cell carcinoma (MCC) is a rare but aggressive skin cancer with frequent metastasis and death. MCC has a mortality rate of 30%, making it more lethal than malignant melanoma, and incidence of MCC has increased almost fourfold over the past 20 years in the USA. MCC has long been considered to be an immunogenic cancer because it occurs more frequently in immunosuppressed patients from organ transplant and HIV infection than in those with immunocompetent. Chronic UV light exposure and clonal integration of Merkel cell polyomavirus (MCPyV) are two major causative factors of MCC. Approximately 80% of MCC are associated with MCPyV, and T cells specific for MCPyV oncoproteins are present in the blood and tumors of patients. Several studies have shown that a subset of MCCs express PD-1 on tumor-infiltrating lymphocytes and express PD-L1 on tumor cells, which suggests an endogenous tumor-reactive immune response that might be unleashed by anti-PD-1 or anti-PD-L1 drugs.
Background
Merkel cell carcinoma (MCC) is a rare but highly aggressive neuroendocrine skin cancer, which was described for the first time in 1972 as trabecular carcinoma of the skin (1). Based on the ultra-structural proof of neuroendocrine granules and the expression of CK20 and CD56 (2–4), Merkel cells were considered to be the source of MCC. However, the cells of origin of MCC remain a controversial issue. Recent studies have suggested the origin of MCC may reside in epidermal/dermal stem cells in the dermis (5) or in precursor B cells (6, 7). The incidence of MCC is rising steadily and more than one-third of patients die of MCC, making it twice as lethal as malignant melanoma (8). Risk factors for MCC include fair skin, chronic sun exposure, chronic immune suppression, and advanced age (9–12). In the USA, age-adjusted incidence increased from 0.15 to 0.44 per 100,000 from 1986 to 2004 (13). Consistent with other UV-related skin cancers, incidence rate of MCC in Queensland, Australia is higher than those in the rest of the world (age-adjusted incidence of 1.6 per 100,000) (14). The incidence of MCC in Asia is thought to be low, although no population-based data are available (15, 16). The majority of MCC is associated with Merkel cell polyomavirus (MCPyV), while the remaining is triggered by UV-mediated mutations (17, 18). MCPyV DNA integrates into the host genome of approximately up to 80% of MCCs in the northern hemisphere, whereas its presence is much lower in other geographic regions such as Australia (~30%) (17, 19). Since several lines of evidence indicate the outstanding immunogenicity of MCC, irrespective of MCPyV integration, immune modulating treatment strategies are particularly attractive. Promising results from immune checkpoint inhibitor therapy in first and second line are now available, which expands the treatment armamentarium for MCC patients.
Clinical and Histological Features
Merkel cell carcinoma presents as a firm, painless, rapidly enlarging, red-violet cutaneous nodule with a smooth surface. The most frequently affected site is the head and neck region (50%), followed by the trunk (30%) and the limbs (10%), although MCC may arise in any body site, including the mucosae (20–22). Heath et al. developed the AEIOU acronym to define the clinical features associated with MCC: asymptomatic/lack of tenderness, expanding rapidly, immune suppression, older than age 50, and UV-exposed site on a person with fair skin. In a study of 195 patients, 89% presented with three or more of the AEIOU characteristics (23). MCC originates in the dermis and only occasionally exhibits an epidermal involvement. Histopathological characteristics of MCC include a monotonous population of tumor cells with large prominent nuclei and scant cytoplasm (24). Immunohistochemically, MCC is positive for EMA, CK20 with a perinuclear dot staining pattern, and neuroendocrine markers including synaptophysin and chromogranin (3, 25–27). Metastatic pulmonary small cell carcinoma can be excluded when the tumor cells prove negative for TTF-1 (28). Unknown primary MCC, which usually presents clinically positive nodal disease with unidentified primary tumor, are likely to have a significantly improved survival compared to those with concurrent primary tumor (29–32). Recent reports showed that unknown primary MCC had higher tumor mutational burden and lower association with MCPyV than those with known primary (33), In addition, nodal tumors from unknown primary MCC contained abundant UV-signature mutations (33), suggesting underlying immunological mechanism between regression of primary tumor and better prognosis of unknown primary MCC.
Etiology
Like Kaposi’s sarcoma, immunocompromised patients with T-cell dysfunction are more likely to be affected by MCC. For example, patients with AIDS have an incidence rate that is 11–13 times greater compared with the general population (11), and solid organ transplant recipients are 5–10 times more likely to develop MCC (34, 35). Also, case reports have described spontaneous regression of MCC tumors after biopsy or an improvement in immune function, further indicating a link to the immune system (36–39). These data collectively suggested that MCC may be linked to a pathogen and in 2008, MCPyV was discovered, and it is now clear that this virus plays a key role in the majority of MCC cases (17).
Merkel cell polyomavirus is a member of the polyomavirus family comprised of non-enveloped, double-stranded circular DNA viruses. MCPyV-specific antibodies have been detected in 9% of children under 4 years of age, 35% of teenagers, and 80% of individuals 50 years or older (40), suggesting that it may be part of the cutaneous microbiome (41). Interestingly, despite this high prevalence, MCPyV has not been shown to cause any disease other than MCC (42). MCPyV-related oncogenesis requires integration of the viral genome into the host-genome and mutation of the large T (LT) antigen that is required for viral DNA replication (43). Indeed, MCPyV isolated from MCCs, in contrast with MCPyV from non-tumor sources, present mutations that are responsible for the premature truncation of the MCV LT helicase (43, 44). These mutations do not affect the Rb binding domain, but eliminate the capacity of the viral DNA to replicate. In this way, the virus loses its capability to replicate in MCC tumor cells, but continues to express motifs that may potentially lead to uncontrolled proliferation (43, 45). Prognostic significance of tumor viral status is still controversial, but the largest cohort study so far including 282 MCC cases (281 cases with available clinical data) showed that, relative to MCPyV-positive MCC patients, MCPyV-negative MCC patients had significantly increased risk of disease progression (hazard ratio = 1.77, 95% confidence interval = 1.20–2.62) and death from MCC (hazard ratio = 1.85, 95% confidence interval = 1.19–2.89) in a multivariate analysis including age, sex, and immunosuppression (46).
Merkel cell carcinoma development is also linked to exposure to UV radiation, and primary MCC lesions preferentially develop on sun-exposed skin (20, 21). The incidence of MCC was determined to be 100-fold greater in patients who underwent PUVA treatment (47). MCPyV-negative MCC is among the most mutated of all solid tumors, including melanoma (18, 48–50). These mutations are mostly UV-signature mutations, such as p53 and Rb, commonly resulting in loss of functional protein expression (18, 49). The high mutational burden in MCC correlates to frequent amino acid changes and large numbers of UV-induced neoantigens (49). Despite significant genetic differences, both MCPyV-positive and -negative MCC exhibit nuclear accumulation of oncogenic transcription factors such as NFAT, phosphorylated CREB, and phosphorylated STAT3, indicating commonly deregulated pathogenic mechanisms (50).
Treatment
For patients with locoregional MCC, wide excision and/or complete lymph node dissection and/or adjuvant radiation therapy is usually recommended (51). Sentinel lymph node biopsy should be considered for patients with clinically nodal negative patients, although its impact on overall survival is still unclear (51–53).
Although cytotoxic chemotherapy (carboplatin or cisplatin plus etoposide) has been commonly used to treat patients with advanced MCC, responses are rarely durable and few studies have shown a survival benefit (54–57). Early studies showed that levels of intratumoral CD8+ T cells serve as predictors of MCC-specific survival, with 100% survival reported for patients with the highest level of CD8+ infiltrate compared to 60% survival in those with little or no CD8+ infiltration (58, 59). Then MCPyV oncoprotein-specific cells were found to be present in MCC patient blood and enriched in their tumors (60), whose frequency appears to increase with tumor burden (61). Importantly, signs of dysfunction were evident in MCPyV-specific CD8+ T cells from patients, as they expressed both PD-1 and Tim3, suggesting functional exhaustion (61). MCPyV-negative MCC is also associated with high levels of T-cell infiltrates (18). Although both MCPyV-positive and -negative tumor cells express PD-L1, the expression levels of PD-L1 in virus-positive tumors seem to be higher than those in virus-negative tumors (18, 62). These findings, therefore, provide rationale for immunotherapy targeting the PD-1 pathway in advanced MCC.
A multicenter, phase 2, non-controlled clinical trial studied pembrolizumab (anti-PD-1 Ab) 2 mg/kg every 2 weeks in 26 patients with advanced MCC who had not received prior systemic therapy. The objective response rate (ORR) to pembrolizumab among the 25 patients with at least one evaluation during treatment was 56% including a 16% complete response (CR) rate. Of the 14 responsive patients, the response duration ranged from at least 2.2 months to at least 9.7 months. Overall, the trial had an estimated progression free survival (PFS) of 67% at 6 months. Pembrolizumab was effective in both MCPyV-positive and -negative tumors (ORR 62 and 44%, respectively, not significantly different) (63). The preliminary data from this trial led to pembrolizumab being listed as a treatment option for disseminated disease in the 2017 NCCN guidelines for MCC (64).
A multicenter, international, open-label, phase 2 clinical trial studied avelumab (anti-PD-L1 Ab) in 88 patients with distant metastatic disease who had previously received at least one line of chemotherapy. This trial found an ORR of 33% with a CR rate of 11%. At 6 months, PFS was 40% and the estimated PFS at 1 year was 30%. As with pembrolizumab, avelumab was found to be effective in both MCPyV-positive and -negative tumors (ORR 26 and 35%, respectively, not significantly different) (65). Based on these results, FDA granted an accelerated approval for avelumab as first-line treatment of patients with metastatic MCC in March 2017. In the avelumab trial, a trend toward a higher response rate was observed in patients with fewer lines of prior treatment, which along with the pembrolizumab data strongly suggest that immunotherapy targeting the PD-1 pathway should be considered for first-line treatment in patients with advanced MCC.
An international, single arm, open-label trial of nivolumab (anti-PD-1 Ab) 240 mg/body every 2 weeks included both patients who had and those who had not received prior chemotherapy (36 and 64%, respectively) is ongoing (NCT02488759; CheckMate358). In this study, 15 of 22 patients (68%) had objective responses, and PFS at 3 months was 82%. Responses occurred in 10 of 14 treatment-naive patients including 3 CR, in 5 of 8 patients including 5 partial responses with 1–2 prior systemic therapies (63%) (Table 1). Based on the preliminary data from this trial, nivolumab was listed along with avelumab and pembrolizumab as a treatment option for disseminated disease in the 2018 NCCN guidelines for MCC (51).
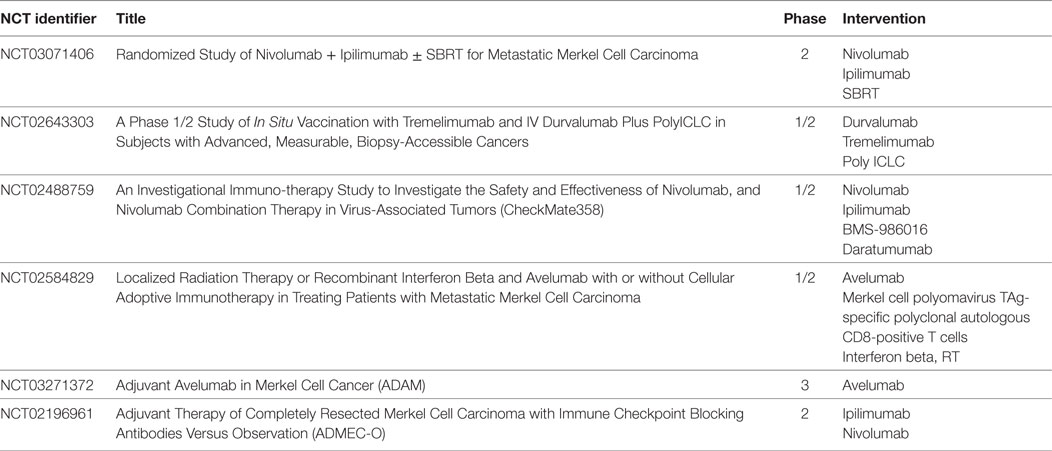
Table 1. Ongoing clinical trials in MCC (http://ClinicalTrials.gov).
Conclusion
Advanced MCC is generally considered to be sensitive to chemotherapy, but responses are transient, offering a median PFS of only 3 months (55). On the other hand, although no randomized trials compare chemotherapy with immunotherapy, data from treatment with immune checkpoint inhibitors are promising with responses both in MCPyV-positive and -negative MCC, although nearly half of patients do not derive durable benefit from these drugs. Now that avelumab has been approved for treatment of advanced MCC in the USA, EU, and Japan, the spectrum of current therapy for patients with MCC is changing. Several clinical trials of immune checkpoint inhibitors (anti-PD-1, PD-L1, and CTLA-4 Abs) administered as monotherapy or in combination with other agents or modalities are ongoing (Table 1) and may provide further treatment options for patients with advanced MCC in the near future.
Author Contributions
The author confirms being the sole contributor of this work and approved it for publication.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Toker C. Trabecular carcinoma of the skin. Arch Dermatol (1972) 105:107–10. doi:10.1001/archderm.1972.01620040075020
2. Tang CK, Toker C. Trabecular carcinoma of the skin: an ultrastructural study. Cancer (1978) 42:2311–21. doi:10.1002/1097-0142(197811)42:5<2311::AID-CNCR2820420531>3.0.CO;2-L
3. Moll R, Lowe A, Laufer J, Franke WW. Cytokeratin 20 in human carcinomas. A new histodiagnostic marker detected by monoclonal antibodies. Am J Pathol (1992) 140:427–47.
4. Gallego R, García-Caballero T, Fraga M, Beiras A, Forteza J. Neural cell adhesion molecule immunoreactivity in Merkel cells and Merkel cell tumours. Virchows Arch (1995) 426:317–21. doi:10.1007/BF00191370
5. Lemasson G, Coquart N, Lebonvallet N, Boulais N, Galibert MD, Marcorelles P, et al. Presence of putative stem cells in Merkel cell carcinomas. J Eur Acad Dermatol Venereol (2012) 26:789–95. doi:10.1111/j.1468-3083.2011.04132.x
6. Zur Hausen A, Rennspiess D, Winnepenninckx V, Speel EJ, Kurz AK. Early B-cell differentiation in Merkel cell carcinomas: clues to cellular ancestry. Cancer Res (2013) 73:4982–7. doi:10.1158/0008-5472.CAN-13-0616
7. Sauer CM, Haugg AM, Chteinberg E, Rennspiess D, Winnepenninckx V, Speel EJ, et al. Reviewing the current evidence supporting early B-cells as the cellular origin of Merkel cell carcinoma. Crit Rev Oncol Hematol (2017) 116:99–105. doi:10.1016/j.critrevonc.2017.05.009
8. Miller RW, Rabkin CS. Merkel cell carcinoma and melanoma: etiological similarities and differences. Cancer Epidemiol Biomarkers Prev (1999) 8:153–8.
9. Albores-Saavedra J, Batich K, Chable-Montero F, Sagy N, Schwartz AM, Henson DE. Merkel cell carcinoma demographics, morphology, and survival based on 3870 cases: a population based study. J Cutan Pathol (2010) 37:20–7. doi:10.1111/j.1600-0560.2009.01370.x
10. Grabowski J, Saltzstein SL, Sadler GR, Tahir Z, Blair S. A comparison of Merkel cell carcinoma and melanoma: results from the California Cancer Registry. Clin Med Oncol (2008) 2:327–33.
11. Engels EA, Frisch M, Goedert JJ, Biggar RJ, Miller RW. Merkel cell carcinoma and HIV infection. Lancet (2002) 359:497–8. doi:10.1016/S0140-6736(02)07668-7
12. Kaae J, Hansen AV, Biggar RJ, Boyd HA, Moore PS, Wohlfahrt J, et al. Merkel cell carcinoma: incidence, mortality, and risk of other cancers. J Natl Cancer Inst (2010) 102:793–801. doi:10.1093/jnci/djq120
14. Youlden DR, Soyer HP, Youl PH, Fritschi L, Baade PD. Incidence and survival for Merkel cell carcinoma in Queensland, Australia, 1993-2010. JAMA Dermatol (2014) 150:864–72. doi:10.1001/jamadermatol.2014.124
15. Chun SM, Yun SJ, Lee SC, Won YH, Lee JB. Merkel cell polyomavirus is frequently detected in Korean patients with Merkel cell carcinoma. Ann. Dermatol (2013) 25:203–7. doi:10.5021/ad.2013.25.2.203
16. Hattori T, Takeuchi Y, Takenouchi T, Hirofuji A, Tsuchida T, Kabumoto T, et al. The prevalence of Merkel cell polyomavirus in Japanese patients with Merkel cell carcinoma. J Dermatol Sci (2013) 70:99–107. doi:10.1016/j.jdermsci.2013.02.010
17. Feng H, Shuda M, Chang Y, Moore PS. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science (2008) 319:1096–100. doi:10.1126/science.1152586
18. Wong SQ, Waldeck K, Vergara IA, Schröder J, Madore J, Wilmott JS, et al. UV-associated mutations underlie the etiology of MCV-negative Merkel cell carcinomas. Cancer Res (2015) 75:5228–34. doi:10.1158/0008-5472.CAN-15-1877
19. Garneski KM, Warcola AH, Feng Q, Kiviat NB, Leonard JH, Nghiem P. Merkel cell polyomavirus is more frequently present in North American than Australian Merkel cell carcinoma tumors. J Invest Dermatol (2009) 129:246–8. doi:10.1038/jid.2008.229
20. Smiths VA, Camp ER, Lentsch EJ. Merkel cell carcinoma: identification of prognostic factors unique to tumors located in the head and neck based on analysis of SEER data. Laryngoscope (2012) 122:1283–90. doi:10.1002/lary.23222
21. Hussain SK, Sundquist J, Hemminki K. Incidence trends of squamous cell and rare skin cancers in the Swedish national cancer registry point to calendar year and age-dependent increases. J Invest Dermatol (2010) 130:1323–8. doi:10.1038/jid.2009.426
22. Nguyen AH, Tahseen AI, Vaudreuil AM, Caponetti GC, Huerter CJ. Clinical features and treatment of vulvar Merkel cell carcinoma: a systematic review. Gynecol Oncol Res Pract (2017) 4:2. doi:10.1186/s40661-017-0037-x
23. Heath M, Jaimes N, Lemos B, Mostaghimi A, Wang LC, Peñas PF, et al. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the AEIOU features. J Am Acad Dermatol (2008) 58:375–81. doi:10.1016/j.jaad.2007.11.020
24. Acebo E, Vidaurrazaga N, Varas C, Burgos-Bretones JJ, Díaz-Pérez JL. Merkel cell carcinoma: a clinicopathological study of 11 cases. J Eur Acad Dermatol Venereol (2005) 19:546–51. doi:10.1111/j.1468-3083.2005.01224.x
25. Chan JK, Suster S, Wenig BM, Tsang WY, Chan JB, Lau AL. Cytokeratin 20 immunoreactivity distinguishes Merkel cell (primary cutaneous neuroendocrine) carcinomas and salivary gland small cell carcinomas from small cell carcinomas of various sites. Am J Surg Pathol (1997) 21:226–34. doi:10.1097/00000478-199702000-00014
26. Visscher D, Cooper PH, Zarbo RJ, Crissman JD. Cutaneous neuroendocrine (Merkel cell) carcinoma: an immunophenotypic, clinicopathologic, and flow cytometric study. Mod Pathol (1989) 2:331–8.
27. Mount SL, Taatjes DJ. Neuroendocrine carcinoma of the skin (Merkel cell carcinoma). an immunoelectron-microscopic case study. Am J Dermatopathol (1994) 16:60–5. doi:10.1097/00000372-199402000-00012
28. Kaufmann O, Dietel M. Expression of thyroid transcription factor-1 in pulmonary and extrapulmonary small cell carcinomas and other neuroendocrine carcinomas of various primary sites. Histopathology (2000) 36:415–20. doi:10.1046/j.1365-2559.2000.00890.x
29. Harms KL, Healy MA, Nghiem P, Sober AJ, Johnson TM, Bichakjian CK, et al. Analysis of prognostic factors from 9387 Merkel cell carcinoma cases forms the basis for the new 8th edition AJCC staging system. Ann Surg Oncol (2016) 23:3564–71. doi:10.1245/s10434-016-5266-4
30. Pan Z, Chen YY, Wu X, Trisal V, Wilczynski SP, Weiss LM, et al. Merkel cell carcinoma of lymph node with unknown primary has a significantly lower association with Merkel cell polyomavirus than its cutaneous counterpart. Mod Pathol (2014) 27:1182–92. doi:10.1038/modpathol.2013.250
31. Chen KT, Papavasiliou P, Edwards K, Zhu F, Perlis C, Wu H, et al. A better prognosis for Merkel cell carcinoma of unknown primary origin. Am J Surg (2013) 206:752–7. doi:10.1016/j.amjsurg.2013.02.005
32. Foote M, Veness M, Zarate D, Poulsen M. Merkel cell carcinoma: the prognostic implications of an occult primary in stage IIIB (nodal) disease. J Am Acad Dermatol (2012) 67:395–9. doi:10.1016/j.jaad.2011.09.009
33. Vandeven N, Lewis CW, Makarov V, Riaz N, Paulson KG, Hippe D, et al. Merkel cell carcinoma patients presenting without a primary lesion have elevated markers of immunity, higher tumor mutation burden, and improved survival. Clin Cancer Res (2018) 24(4):963–71. doi:10.1158/1078-0432
34. Lanoy E, Costagliola D, Engels EA. Skin cancers associated with HIV infection and solid-organ transplantation among elderly adults. Int J Cancer (2010) 126:1724–31. doi:10.1002/ijc.24931
35. Clarke CA, Robbins HA, Tatalovich Z, Lynch CF, Pawlish KS, Finch JL, et al. Risk of Merkel cell carcinoma after solid organ transplantation. J Natl Cancer Inst (2015) 107. doi:10.1093/jnci/dju382
36. Wooff JC, Trites JR, Walsh NM, Bullock MJ. Complete spontaneous regression of metastatic Merkel cell carcinoma: a case report and review of the literature. Am J Dermatopathol (2010) 32:614–7. doi:10.1097/DAD.0b013e3181cd3158
37. Walsh NM. Complete spontaneous regression of Merkel cell carcinoma (1986-2016): a 30 year perspective. J Cutan Pathol (2016) 43:1150–4. doi:10.1111/cup.12812
38. Ahmadi Moghaddam P, Cornejo KM, Hutchinson L, Tomaszewicz K, Dresser K, Deng A, et al. Complete spontaneous regression of Merkel cell carcinoma after biopsy: a case report and review of the literature. Am J Dermatopathol (2016) 38:e154–8. doi:10.1097/DAD.0000000000000614
39. Burack J, Altschuler EL. Sustained remission of metastatic Merkel cell carcinoma with treatment of HIV infection. J R Soc Med (2003) 96:238–9. doi:10.1177/014107680309600512
40. Chang Y, Moore PS. Merkel cell carcinoma: a virus-induced human cancer. Annu Rev Pathol (2012) 7:123–44. doi:10.1146/annurev-pathol-011110-130227
41. Foulongne V, Kluger N, Dereure O, Mercier G, Molès JP, Guillot B, et al. Merkel cell polyomavirus in cutaneous swabs. Emerg Infect Dis (2013) 16:685–7. doi:10.3201/eid1604.091278
42. Spurgeon ME, Lambert PF. Merkel cell polyomavirus: a newly discovered human virus with oncogenic potential. Virology (2013) 435:118–30. doi:10.1016/j.virol.2012.09.029
43. Shuda M, Feng H, Kwun HJ, Rosen ST, Gjoerup O, Moore PS, et al. T antigen mutations are a human tumor-specific signature for Merkel cell polyomavirus. Proc Natl Acad Sci U S A (2008) 105:16272–7. doi:10.1073/pnas.0806526105
44. Duncavage EJ, Zehnbauer BA, Pfeifer JD. Prevalence of Merkel cell polyomavirus in Merkel cell carcinoma. Mod Pathol (2009) 22:516–21. doi:10.1038/modpathol.2009.3
45. Neumann F, Borchert S, Schmidt C, Reimer R, Hohenberg H, Fischer N, et al. Replication, gene expression and particle production by a consensus Merkel Cell Polyomavirus (MCPyV) genome. PLoS One (2011) 6:e29112. doi:10.1371/journal.pone.0029112
46. Moshiri AS, Doumani R, Yelistratova L, Blom A, Lachance K, Shinohara MM, et al. Polyomavirus-negative Merkel cell carcinoma: a more aggressive subtype based on analysis of 282 cases using multimodal tumor virus detection. J Invest Dermatol (2017) 137:819–27. doi:10.1016/j.jid.2016.10.028
47. Lunder EJ, Stern RS. Merkel-cell carcinomas in patients treated with methoxsalen and ultraviolet A radiation. N Engl J Med (1998) 339:1247–8. doi:10.1056/NEJM199810223391715
48. Harms PW, Vats P, Verhaegen ME, Robinson DR, Wu YM, Dhanasekaran SM, et al. The distinctive mutational spectra of polyomavirus-negative Merkel cell carcinoma. Cancer Res (2015) 75:3720–7. doi:10.1158/0008-5472.CAN-15-0702
49. Goh G, Walradt T, Markarov V, Blom A, Riaz N, Doumani R, et al. Mutational landscape of MCPyV-positive and MCPyV-negative Merkel cell carcinomas with implications for immunotherapy. Oncotarget (2016) 7:3403–15. doi:10.18632/oncotarget.6494
50. González-Vela MD, Curiel-Olmo S, Derdak S, Beltran S, Santibañez M, Martínez N, et al. Shared oncogenic pathways implicated in both virus-positive and UV-induced Merkel cell carcinomas. J Invest Dermatol (2017) 137:197–206. doi:10.1016/j.jid.2016.08.015
51. NCCN. NCCN Clinical Practice Guidelines in Oncology. Merkel Cell Carcinoma. Version 1. 2018 ed. Fort Washington, PA: National Comprehensive Cancer Network, Inc (2017).
52. Gupta SG, Wang LC, Peñas PF, Gellenthin M, Lee SJ, Nghiem P. Sentinel lymph node biopsy for evaluation and treatment of patients with Merkel cell carcinoma: the Dana-Farber experience and meta-analysis of the literature. Arch Dermatol (2006) 142:685–90. doi:10.1001/archderm.142.6.685
53. Fields RC, Busam KJ, Chou JF, Panageas KS, Pulitzer MP, Kraus DH, et al. Recurrence and survival in patients undergoing sentinel lymph node biopsy for Merkel cell carcinoma: analysis of 153 patients from a single institution. Ann Surg Oncol (2011) 18:2529–37. doi:10.1245/s10434-011-1662-y
54. Voog E, Biron P, Martin JP, Blay JY. Chemotherapy for patients with locally advanced or metastatic Merkel cell carcinoma. Cancer (1999) 85:2589–95. doi:10.1002/(SICI)1097-0142(19990615)85:12<2589::AID-CNCR15>3.0.CO;2-F
55. Tai PT, Yu E, Winquist E, Hammond A, Stitt L, Tonita J, et al. Chemotherapy in neuroendocrine/Merkel cell carcinoma of the skin: case series and review of 204 cases. J Clin Oncol (2000) 18:2493–9. doi:10.1200/JCO.2000.18.12.2493
56. Iyer JG, Blom A, Doumani R, Lewis C, Tarabadkar ES, Anderson A, et al. Response rates and durability of chemotherapy among 62 patients with metastatic Merkel cell carcinoma. Cancer Med (2016) 5:2294–301. doi:10.1002/cam4.815
57. Schadendorf D, Lebbé C, Zur Hausen A, Avril MF, Hariharan S, Bharmal M, et al. Merkel cell carcinoma: epidemiology, prognosis, therapy and unmet medical needs. Eur J Cancer (2017) 71:53–69. doi:10.1016/j.ejca.2016.10.022
58. Paulson KG, Iyer JG, Tegeder AR, Thibodeau R, Schelter J, Koba S, et al. Transcriptome-wide studies of Merkel cell carcinoma and validation of intratumoral CD8+ lymphocyte invasion as an independent predictor of survival. J Clin Oncol (2011) 29:1539–46. doi:10.1200/JCO.2010.30.6308
59. Paulson KG, Iyer JG, Simonson WT, Blom A, Thibodeau RM, Schmidt M, et al. CD8+ lymphocyte intratumoral infiltration as a stage-independent predictor of Merkel cell carcinoma survival: a population-based study. Am J Clin Pathol (2014) 142:452–8. doi:10.1309/AJCPIKDZM39CRPNC
60. Iyer JG, Afanasiev OK, McClurkan C, Paulson K, Nagase K, Jing L, et al. Merkel cell polyomavirus specific CD8(+) and CD4(+) T-cell responses identified in Merkel cell carcinomas and blood. Clin Cancer Res (2011) 17:6671–80. doi:10.1158/1078-0432.CCR-11-1513
61. Afanasiev OK, Yelistratova L, Miller N, Nagase K, Paulson K, Iyer JG, et al. Merkel polyomavirus specific T cells fluctuate with Merkel cell carcinoma burden and express therapeutically targetable PD-1 and Tim-3 exhaustion markers. Clin Cancer Res (2013) 19:5351–60. doi:10.1158/1078-0432.CCR-13-0035
62. Lipson EJ, Vincent JG, Loyo M, Kagohara LT, Luber BS, Wang H, et al. PD-L1 expression in the Merkel cell carcinoma microenvironment: association with inflammation, Merkel cell polyomavirus and overall survival. Cancer Immunol Res (2013) 1:54–63. doi:10.1158/2326-6066.CIR-13-0034
63. Nghiem PT, Bhatia S, Lipson EJ, Kudchadkar RR, Miller NJ, Annamalai L, et al. PD-1 blockade with pembrolizumab in advanced Merkel-cell carcinoma. N Engl J Med (2016) 374:2542–52. doi:10.1056/NEJMoa1603702
64. NCCN. NCCN Clinical Practice Guidelines in Oncology. Merkel Cell Carcinoma. Version 1. 2017 ed. Fort Washington, PA: National Comprehensive Cancer Network, Inc (2016).
Keywords: PD-1, PD-L1, Merkel cell carcinoma, Merkel cell polyomavirus, UV
Citation: Uchi H (2018) Merkel Cell Carcinoma: An Update and Immunotherapy. Front. Oncol. 8:48. doi: 10.3389/fonc.2018.00048
Received: 18 January 2018; Accepted: 19 February 2018;
Published: 06 March 2018
Edited by:
Atsushi Otsuka, Kyoto University, JapanCopyright: © 2018 Uchi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hiroshi Uchi, dWNoaWhpckBkZXJtYXRvbC5tZWQua3l1c2h1LXUuYWMuanA=
 Hiroshi Uchi
Hiroshi Uchi