- 1Breast Cancer Unit, Kyoto University Hospital Breast Surgery, Graduate School of Medicine, Kyoto University, Kyoto, Japan
- 2Department of Breast Surgical Oncology, Hakuaikai Social Medical Cooperation, Kagoshima, Japan
- 3Department of Surgery, Brigham and Women’s Hospital, Boston, MA, United States
- 4Department of Radiation Oncology, Dana-Farber Cancer Institute, Boston, MA, United States
The prevalence of ductal carcinoma in situ (DCIS) of the breast has increased substantially after the introduction of breast cancer screening programs, although the clinical effects of early DCIS detection and treatment remain unclear. The standard treatment for DCIS has involved local breast-conserving surgery (BCS) followed by radiotherapy (RT) or total mastectomy with/without endocrine therapy, and the choice of local treatment is not usually based on clinicopathologic or biological factors. However, we have investigated the effectiveness of local treatment using breast surgery and RT using Surveillance, Epidemiology, and End Results data, and found that the effectiveness of breast surgery was modified by the nuclear grade. Furthermore, breast cancer-specific survival was identical between patients with low-grade DCIS who did and did not undergo surgery. Moreover, we found that RT after BCS for DCIS was only associated with a survival benefit among patients with risk factors for local recurrence, such as nuclear grade, age, and tumor size. Ongoing clinical trials and translational research have attempted to develop a treatment strategy that prevents the overdiagnosis and overtreatment of low-risk DCIS, as well as a biology-based treatment strategy for using targeted therapy. Therefore, to develop a tailored treatment strategy for DCIS, we need to identify molecular and biological classifications based on the results from translational research, national databases, and clinical trials.
Introduction of Breast Screening Programs and the Increased Prevalence of Ductal Carcinoma In Situ (DCIS)
Ductal carcinoma in situ of the breast is an abnormal proliferation of epithelial cells within the breast ducts. The implementation of breast screening programs from the 1980s helped improve the prognosis of women who were diagnosed with breast cancer, although the incidences of DCIS, early breast cancer, and slow-growing breast cancer have also increased dramatically (1–4). For example, the incidence of DCIS increased from 1.87 cases per 100,000 population during the 1970s to 32.5 cases per 100,000 population during 2004, and the incidence has currently reached a plateau (5, 6). A systematic review of the incidences before and after the implementation of breast cancer screening programs in five countries also revealed a dramatic increase in the incidence of breast cancer (7). The incidence in the United Kingdom increased approximately 40% above the estimated natural increase. Thus, if early-stage breast cancers are detected during breast cancer screening, it would be logical to assume that the incidence of advanced breast cancer should decrease. However, there has been no relative decrease in the incidence of advanced breast cancer, which suggests that there is overdiagnosis of slow-growing lesions that may not require treatment (1, 8–10). Although 3–40% of the lesions detected by screening are assumed to be overdiagnosis in several studies, we have not confirmed it yet and this is an area of some uncertainty (11, 12).
Pathological Diagnosis and Molecular Classification of DCIS
Several retrospective studies have investigated the natural course of DCIS in the absence of curative treatment, and found progression to invasive breast cancer in 25–50% of cases during follow-ups of 15–25 years (13, 14). Thus, it is important to be aware that not all DCIS will become invasive breast cancer that can metastasize to other organs. Furthermore, a study of 6,900 slides from breast biopsies revealed variability in the pathological diagnoses (15), with 69.6% of pathologists providing diagnoses of DCIS and 18.5% of pathologists providing diagnoses of benign tissue or atypia. Therefore, as there is a broad biological spectrum of breast lesions that ranges from benign to invasive ductal carcinoma, it is important for physicians to consider the diagnostic gray area in clinical decision-making.
Silverstein et al. have demonstrated that the Van Nuys score based on nuclear grade and comedo necrosis is associated with local recurrence after breast-conserving surgery (BCS) for DCIS. This score is relatively reproducible to be implemented in clinical practice (16). The fourth edition of the World Health Organization’s Classification of Tumors of the Breast (2012) classified tumors based on nuclear grade, and DCIS has come to be classified as a low-grade, intermediate-grade, or high-grade lesion. Ozanne et al. have estimated that the cumulative rates of progression from DCIS to invasive cancer during a 10-year period are 60% for high-grade DCIS (patients who are <45 years old and have lesions that are >1 cm) and 16% for low-grade DCIS (patients who are >45 years old and have lesions that are >2.5 cm) (17). After local therapy for DCIS, nuclear grade has been shown to predict ipsilateral breast cancer recurrence in a randomized clinical trial and meta-analysis (18–20). Furthermore, comprehensive investigation of DCIS gene expressions revealed that low-grade DCIS and atypical ductal hyperplasia share a common chromosomal abnormality, while high-grade DCIS exhibits molecular profiles that are indistinguishable from invasive breast cancer (21, 22). Changing the terminology for low-grade DCIS currently referred to as “carcinoma” will allow physicians to shift medicolegal notions and perceived risk to reflect the evolving understanding of biology (3). Although several studies have attempted to create a molecular classification of DCIS cases, the gene expression profile for predicting progression to invasive breast cancer has not been clarified (23, 24). Therefore, we also need to develop a clinically useful classification system or a new treatment strategy for the lesions that are diagnosed as DCIS.
Surgery for DCIS Based on the Biology
The standard local therapy for DCIS is BCS followed by radiotherapy (RT) or total mastectomy. However, local treatments have not usually been individualized based on the likelihood of progression to invasive breast cancer and distant metastasis. Therefore, we performed a retrospective cohort study to investigate the effectiveness of surgery for DCIS based on nuclear grade using Surveillance, Epidemiology, and End Results data (25). We used a method of propensity score weighting to adjust covariates that influence the prognosis of patients between surgery and non-surgery groups. Among the 57,222 eligible women with DCIS and a pathologically confirmed nuclear grade, we identified 1,169 women who did not undergo surgery for DCIS at the diagnosis. This decision was motivated by their physician not recommending surgery (46.8%), their physician not recommending surgery because of contraindications (1.7%), patient refusal despite a physician’s recommendation (9.8%), and unknown reasons despite a physician’s recommendation (40.9%). We observed a better breast cancer-specific survival (BCSS) among patients who underwent surgery for high-grade DCIS, compared to patients with high-grade DCIS who did not undergo surgery at a median follow-up of 72 months from diagnosis. However, the BCSS rates were identical for patients with low-grade DCIS who did and did not undergo surgery (Figure 1). Among patients with low-grade DCIS, the weighted 10-year BCSS rates were 98.6% after surgery and 98.8% among patients who did not undergo surgery. Thus, it may be prudent to reconsider the necessity of surgical treatment after a diagnosis of low-grade DCIS. Several randomized controlled trials (RCTs), such as the COMET (NCT02926911) and LORIS trials, are currently investigating the feasibility and non-inferiority of active surveillance with or without endocrine therapy for managing low-risk DCIS (26).
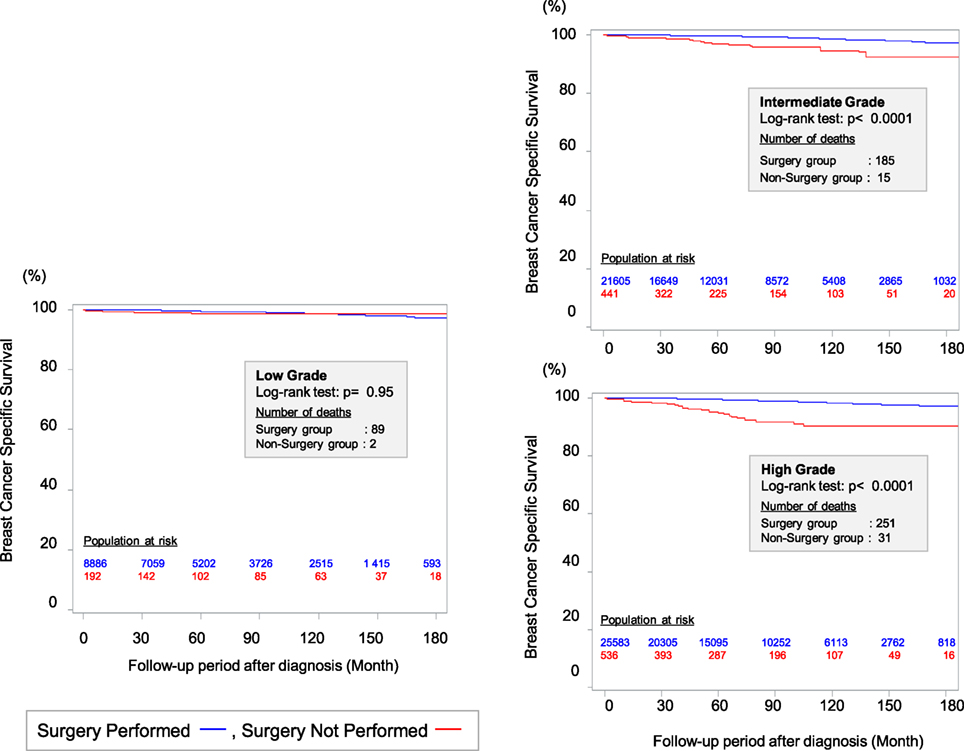
Figure 1. Kaplan–Meier curves for breast cancer-specific survival between surgery group and non-surgery group among patients weighted by inverse propensity score. Sagara et al. (25)
RT for DCIS Based on Risk Factors and Gene Expressions
A meta-analysis of four RCTs (n = 3,729) revealed that RT after BCS for DCIS provided a decreased risk of local recurrence (hazard ratio: 0.46), although RT did not improve the BCSS (27). However, the limited number of deaths caused by breast cancer (n = 96) may have limited the power of the survival analysis. Interestingly, the benefit of RT (reducing local recurrence without a survival benefit) is balanced by several drawbacks, including adverse events, cost, and a prolonged treatment period. Therefore, there is substantial physician- and center-specific variability in the decision to perform or omit RT after BCS for DCIS.
We hypothesized that RT would provide a survival benefit to patients with risk factors for local recurrence, such as young age, large tumor size, and higher nuclear grade. Thus, we performed a cohort study using Surveillance, Epidemiology, and End Results data (1988–2007), and evaluated the efficacy of RT among 32,144 eligible women with DCIS and pathological data regarding tumor size and nuclear grade (28). We used the prognostic score that was proposed by Smith et al. to stratify the DCIS cases according to their risk of recurrence (29), which is associated with patient age, tumor size, and nuclear grade (higher scores are associated with local recurrence). The results confirmed that the prognostic score predicted both local recurrence and the survival benefit from RT among patients with the risk factors for local recurrence (Figure 2). Thus, the prognostic score could predict the risk of local recurrence and possible benefit of RT among patients who undergo BCS for DCIS.
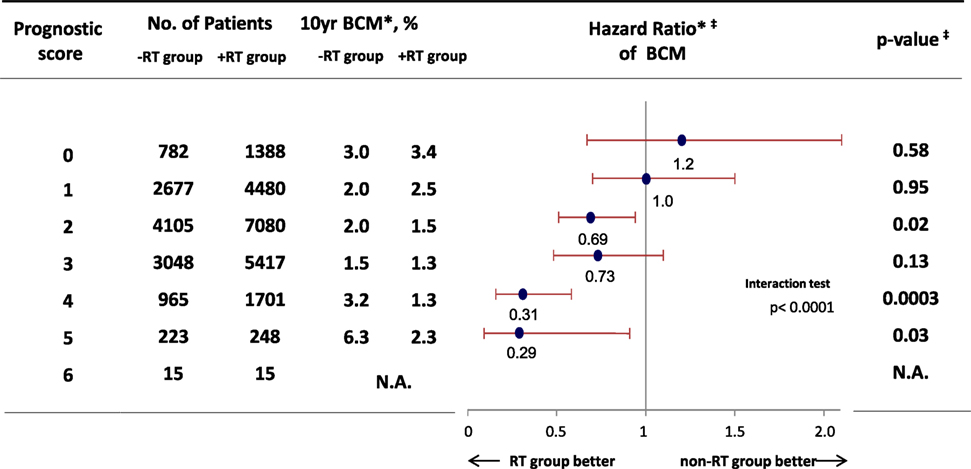
Figure 2. Hazard ratio comparing BCM among patients who received breast-conserving surgery for ductal carcinoma in situ between RT group and non-RT group. *Weighted by inverse propensity score. ‡Multivariate analysis adjusted by age of patients, year of diagnosis, race, tumor size, nuclear grade, and marital status. Abbreviation: RT, radiotherapy; BCM, breast cancer mortality. Sagara et al. (28).
The Oncotype Dx DCIS assay evaluates 12 genes to predict the risk of local recurrence after BCS for DCIS and has been validated in the Eastern Cooperative Oncology Group (ECOG) 5194 study and a study of a population-based cancer registry (30, 31). In the ECOG study, about 30% of the patients received tamoxifen. The DCIS score independently predicted the risk of recurrence after only BCS for DCIS, with 10-year ipsilateral breast event rates of 10.6% in the low-risk group, 26.7% in the intermediate-risk group, and 25.9% in the high-risk group (log rank p = 0.006). In a previous report from the ECOG study, the 7-year ipsilateral breast event rate was 10.5% for low- or intermediate-grade DCIS (32). Thus, the ipsilateral breast event rates appear to be similar between the low-risk group based on the DCIS score and low-grade DCIS cases. Modern clinical study of Radiation Therapy Oncology Group 9804 showed that BCS with RT have single digit local recurrence rates of only 0.9% at median 7.2-years follow-up period (33). Further studies are needed to confirm the incremental benefit that is provided by examining the patient’s genetic profile, compared to the classic clinicopathologic factors.
Role of Systematic Therapy for DCIS
Adjuvant endocrine therapy can reduce the risks of ipsilateral recurrence and contralateral breast cancer after BCS for hormone receptor-positive DCIS. Meta-analysis of two RCTs revealed that the risks of ipsilateral and contralateral breast cancers were decreased by approximately 50% after BCS followed by adjuvant tamoxifen for DCIS. The absolute 10-year reduction was 6.5% for all new breast events after tamoxifen treatment, and the number needed to treat was 15 for preventing one recurrent event (34, 35). Based on these results, the use of endocrine therapy has increased steadily (36, 37). A large RCT (IBIS-II) has recently compared the risks of local recurrence after BCS for DCIS between groups that received an antiestrogen agent (tamoxifen) or an aromatase inhibitor (anastrozole) (38). As both treatments provided similar efficacies, a 5-year adjuvant treatment using tamoxifen is still considered the standard endocrine therapy for DCIS after BCS.
Several studies are currently evaluating the efficacies of systemic therapies that target the underlying biology of DCIS. For example, the Cancer and Leukemia Group B 40903 trial is evaluating neoadjuvant endocrine therapy using letrozole for hormone receptor-positive DCIS (NCT01439711). That study may provide further information regarding the mechanism of endocrine therapy and suitable biomarkers. As retrospective studies have demonstrated that recurrence is more common in human epidermal growth factor receptor 2 (HER2)-positive DCIS, compared to other DCIS subtypes (39, 40), the use of anti-HER2 therapy has been suggested for HER2-positive DCIS. A small prospective phase II single-arm study has also demonstrated that lapatinib (a tyrosine kinase inhibitor) interrupts the HER2/neu and epidermal growth factor receptor pathways, and significantly decreases the expressions of pHER2 and pERK1 in patients with HER2-positive DCIS. However, it did not alter the expression of Ki-67 (a proliferation marker) (41). The National Surgical Adjuvant Breast Project B-43 trial is a large phase III RCT (target recruitment: 2,000 patients) that is evaluating the addition of trastuzumab to standard treatment using surgery and RT (NCT00769379) (42). However, the benefit of adding targeted therapy for disease with favorable prognosis should outweigh the high costs (43).
Conclusion and Perspective
Previous epidemiological studies have highlighted the issue of overdiagnosis and overtreatment of lesions that are detected during breast cancer screening. Furthermore, clinicians are confronted by a broad histological spectrum that ranges from normal tissue to invasive ductal carcinoma. Therefore, the ongoing clinical trials are needed to clarify the optimal management of DCIS. One strategy is active surveillance for low-risk DCIS, which is unlikely to develop into life-threatening disease, and systemic therapy may also be used to target the underlying biology of DCIS. Based on the steady increase in the use of adjuvant therapy, clinicopathologic factors and molecular profiles are needed to guide treatment based on the possibility of progression to invasive ductal carcinoma. Nevertheless, it may be prudent to de-escalate the comprehensive treatment for DCIS in select cases based on the tumor’s biology, which may reduce the overdiagnosis and overtreatment of lesions that are detected by breast cancer screening programs. While we wait for better molecular or other tests to risk-stratify DCIS, clinical decisions with the patients must be guided by information regarding the treatment’s benefits (reduced risk of local recurrence and increased survival) and drawbacks (e.g., comorbidities and cost).
Author Contributions
All the authors contributed to manuscript writing and final approval of manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Welch HG, Prorok PC, O’Malley AJ, Kramer BS. Breast-cancer tumor size, overdiagnosis, and mammography screening effectiveness. N Engl J Med (2016) 375(15):1438–47. doi:10.1056/NEJMoa1600249
2. Virnig BA, Shamliyan T, Tuttle TM, Kane RL, Wilt TJ. Diagnosis and management of ductal carcinoma in situ (DCIS). Evid Rep Technol Assess (Full Rep) (2009) 185(185):1–549.
3. Esserman LJ, Thompson IM, Reid B, Nelson P, Ransohoff DF, Welch HG, et al. Addressing overdiagnosis and overtreatment in cancer: a prescription for change. Lancet Oncol (2014) 15(6):e234–42. doi:10.1016/S1470-2045(13)70598-9
4. Esserman LJ, Shieh Y, Rutgers EJT, Knauer M, Retèl VP, Mook S, et al. Impact of mammographic screening on the detection of good and poor prognosis breast cancers. Breast Cancer Res Treat (2011) 130:725–34. doi:10.1007/s10549-011-1748-z
5. Virnig BA, Tuttle TM, Shamliyan T, Kane RL. Ductal carcinoma in situ of the breast: a systematic review of incidence, treatment, and outcomes. J Natl Cancer Inst (2010) 102:170–8. doi:10.1093/jnci/djp482
6. Allegra CJ, Aberle DR, Ganschow P, Hahn SM, Lee CN, Millon-Underwood S, et al. National Institutes of Health State-of-the-Science Conference statement: diagnosis and management of ductal carcinoma in situ September 22-24, 2009. J Natl Cancer Inst (2010) 102(3):161–9. doi:10.1093/jnci/djp485
7. Jørgensen KJ, Gøtzsche PC. Overdiagnosis in publicly organised mammography screening programmes: systematic review of incidence trends. BMJ (2009) 339:b2587. doi:10.1136/bmj.b2587
8. Bleyer A, Welch HG. Effect of three decades of screening mammography on breast-cancer incidence. N Engl J Med (2012) 367:1998–2005. doi:10.1056/NEJMoa1206809
9. Esserman L, Shieh Y, Thompson I. Rethinking screening for breast cancer and prostate cancer. JAMA (2009) 302:1685–92. doi:10.1001/jama.2009.1498
10. Iwase M, Tsunoda H, Nakayama K, Morishita E, Hayashi N, Suzuki K, et al. Overcalling low-risk findings: grouped amorphous calcifications found at screening mammography associated with minimal cancer risk. Breast Cancer (2017) 24(4):579–84. doi:10.1007/s12282-016-0742-z
11. Kopans DB. More misinformation on breast cancer screening. Gland Surg (2017) 6(1):125–9. doi:10.21037/gs.2016.12.15
12. Etzioni R, Gulati R, Mallinger L, Mandelblatt J. Influence of study features and methods on overdiagnosis estimates in breast and prostate cancer screening. Ann Intern Med (2013) 158(11):831–8. doi:10.7326/0003-4819-158-11-201306040-00008
13. Page DL, Dupont WD, Rogers LW, Jensen RA, Schuyler PA. Continued local recurrence of carcinoma 15–25 years after a diagnosis of low grade ductal carcinoma in situ of the breast treated only by biopsy. Cancer (1995) 76(7):1197–200. doi:10.1002/1097-0142(19951001)76:7<1197::AID-CNCR2820760715>3.0.CO;2-0
14. Page DL, Dupont WD, Rogers LW, Landenberger M. Intraductal carcinoma of the breast: follow-up after biopsy only. Cancer (1982) 49:751–8. doi:10.1002/1097-0142(19820215)49:4<751::AID-CNCR2820490426>3.0.CO;2-Y
15. Elmore JG, Nelson HD, Pepe MS, Longton GM, Tosteson ANA, Geller B, et al. Variability in pathologists’ interpretations of individual breast biopsy slides: a population perspective. Ann Intern Med (2016) 164:649–55. doi:10.7326/M15-0964
16. Poller DN, Barth A, Slamon DJ, Silverstein MJ, Gierson ED, Coburn WJ, et al. Prognostic classification of breast ductal carcinoma-in-situ. Lancet (1995) 345(8958):1154–7. doi:10.1016/S0140-6736(95)90982-6
17. Ozanne EM, Shieh Y, Barnes J, Bouzan C, Hwang ES, Esserman LJ. Characterizing the impact of 25 years of DCIS treatment. Breast Cancer Res Treat (2011) 129:165–73. doi:10.1007/s10549-011-1430-5
18. Benson JR, Wishart GC. Predictors of recurrence for ductal carcinoma in situ after breast-conserving surgery. Lancet Oncol (2013) 14(9):e348-57. doi:10.1016/S1470-2045(13)70135-9
19. Wapnir IL, Dignam JJ, Fisher B, Mamounas EP, Anderson SJ, Julian TB, et al. Long-term outcomes of invasive ipsilateral breast tumor recurrences after lumpectomy in NSABP B-17 and B-24 randomized clinical trials for DCIS. J Natl Cancer Inst (2011) 103:478–88. doi:10.1093/jnci/djr027
20. Wang S-Y, Shamliyan T, Virnig BA, Kane R. Tumor characteristics as predictors of local recurrence after treatment of ductal carcinoma in situ: a meta-analysis. Breast Cancer Res Treat (2011) 127:1–14. doi:10.1007/s10549-011-1387-4
21. Sgroi DC. Preinvasive breast cancer. Annu Rev Pathol (2010) 5:193–221. doi:10.1146/annurev.pathol.4.110807.092306
22. Abba MC, Gong T, Lu Y, Lee J, Zhong Y, Lacunza E, et al. A molecular portrait of high-grade ductal carcinoma in situ. Cancer Res (2015) 75(18):3980–90. doi:10.1158/0008-5472.CAN-15-0506
23. Polyak K. Molecular markers for the diagnosis and management of ductal carcinoma in situ. J Natl Cancer Inst Monogr (2010) 41:210–3. doi:10.1093/jncimonographs/lgq019
24. Clark SE, Warwick J, Carpenter R, Bowen RL, Duffy SW, Jones JL. Molecular subtyping of DCIS: heterogeneity of breast cancer reflected in pre-invasive disease. Br J Cancer (2011) 104(1):120–7. doi:10.1038/sj.bjc.6606021
25. Sagara Y, Mallory MA, Wong S, Aydogan F, DeSantis S, Barry WT, et al. Survival benefit of breast surgery for low-grade ductal carcinoma in situ: a population-based cohort study. JAMA Surg (2015) 150(8):739–45. doi:10.1001/jamasurg.2015.0876
26. Francis A, Thomas J, Fallowfield L, Wallis M, Bartlett JMS, Brookes C, et al. Addressing overtreatment of screen detected DCIS; the LORIS trial. Eur J Cancer (2014) 51(16):2296–303. doi:10.1016/j.ejca.2015.07.017
27. Correa C, McGale P, Taylor C, Wang Y, Clarke M, Davies C, et al. Overview of the randomized trials of radiotherapy in ductal carcinoma in situ of the breast. J Natl Cancer Inst Monogr (2010) 41:162–77. doi:10.1093/jncimonographs/lgq039
28. Sagara Y, Freedman RA, Vaz-Luis I, Mallory MA, Wong SM, Aydogan F, et al. Patient prognostic score and associations with survival improvement offered by radiotherapy after breast-conserving surgery for ductal carcinoma in situ: a population-based longitudinal cohort study. J Clin Oncol (2016) 34(11):1190–6. doi:10.1200/JCO.2015.65.1869
29. Smith GL, Smith BD, Haffty BG. Rationalization and regionalization of treatment for ductal carcinoma in situ of the breast. Int J Radiat Oncol Biol Phys (2006) 65(5):1397–403. doi:10.1016/j.ijrobp.2006.03.009
30. Rakovitch E, Nofech-Mozes S, Hanna W, Baehner FL, Saskin R, Butler SM, et al. A population-based validation study of the DCIS Score predicting recurrence risk in individuals treated by breast-conserving surgery alone. Breast Cancer Res Treat (2015) 152(2):389–98. doi:10.1007/s10549-015-3464-6
31. Solin LJ, Gray R, Baehner FL, Butler SM, Hughes LL, Yoshizawa C, et al. A multigene expression assay to predict local recurrence risk for ductal carcinoma in situ of the breast. J Natl Cancer Inst (2013) 105(10):701–10. doi:10.1093/jnci/djt067
32. Hughes LL, Wang M, Page DL, Gray R, Solin LJ, Davidson NE, et al. Local excision alone without irradiation for ductal carcinoma in situ of the breast: a trial of the Eastern Cooperative Oncology Group. J Clin Oncol (2009) 27(32):5319–24. doi:10.1200/JCO.2009.21.8560
33. McCormick B, Winter K, Hudis C, Kuerer HM, Rakovitch E, Smith BL, et al. RTOG 9804: a prospective randomized trial for good-risk ductal carcinoma in situ comparing radiotherapy with observation. J Clin Oncol (2015) 33(7):709–16. doi:10.1200/JCO.2014.57.9029
34. Staley H, McCallum I, Bruce J. Postoperative tamoxifen for ductal carcinoma in situ. Cochrane Database Syst Rev (2012) 10:CD007847. doi:10.1002/14651858.CD007847
35. Cuzick J, Sestak I, Pinder SE, Ellis IO, Forsyth S, Bundred NJ, et al. Effect of tamoxifen and radiotherapy in women with locally excised ductal carcinoma in situ: long-term results from the UK/ANZ DCIS trial. Lancet Oncol (2011) 12(1):21–9. doi:10.1016/S1470-2045(10)70266-7
36. Lo AC, Truong PT, Wai ES, Nichol A, Weir L, Speers C, et al. Population-based analysis of the impact and generalizability of the NSABP-B24 study on endocrine therapy for patients with ductal carcinoma in situ of the breast. Ann Oncol (2015) 26(9):1898–903. doi:10.1093/annonc/mdv251
37. Nichols HB, Bowles EJA, Islam J, Madziwa L, Sturmer T, Tran D-T, et al. Tamoxifen initiation after ductal carcinoma in situ. Oncologist (2016) 21(2):134–40. doi:10.1634/theoncologist.2015-0310
38. Forbes JF, Sestak I, Howell A, Bonanni B, Bundred N, Levy C, et al. Anastrozole versus tamoxifen for the prevention of locoregional and contralateral breast cancer in postmenopausal women with locally excised ductal carcinoma in situ (IBIS-II DCIS): a double-blind, randomised controlled trial. Lancet (2016) 387(10021):866–73. doi:10.1016/S0140-6736(15)01129-0
39. Williams KE, Barnes NLP, Cramer A, Johnson R, Cheema K, Morris J, et al. Molecular phenotypes of DCIS predict overall and invasive recurrence. Ann Oncol (2015) 26(5):1019–25. doi:10.1093/annonc/mdv062
40. Curigliano G, Disalvatore D, Esposito A, Pruneri G, Lazzeroni M, Guerrieri-Gonzaga A, et al. Risk of subsequent in situ and invasive breast cancer in human epidermal growth factor receptor 2-positive ductal carcinoma in situ. Ann Oncol (2015) 26(4):682–7. doi:10.1093/annonc/mdv013
41. Estévez LG, Suarez-Gauthier A, García E, Miró C, Calvo I, Fernández-Abad M, et al. Molecular effects of lapatinib in patients with HER2 positive ductal carcinoma in situ. Breast Cancer Res (2014) 16(4):R76. doi:10.1186/bcr3695
42. Siziopikou KP, Anderson SJ, Cobleigh MA, Julian TB, Arthur DW, Zheng P, et al. Preliminary results of centralized HER2 testing in ductal carcinoma in situ (DCIS): NSABP B-43. Breast Cancer Res Treat (2013) 142:415–21. doi:10.1007/s10549-013-2755-z
Keywords: ductal carcinoma in situ, surgery, radiotherapy, hormonal therapy, adjuvant therapy
Citation: Sagara Y, Julia W, Golshan M and Toi M (2017) Paradigm Shift toward Reducing Overtreatment of Ductal Carcinoma In Situ of Breast. Front. Oncol. 7:192. doi: 10.3389/fonc.2017.00192
Received: 26 May 2017; Accepted: 11 August 2017;
Published: 28 August 2017
Edited by:
José-Bines, Instituto Nacional de Câncer, BrazilReviewed by:
Anees Chagpar, Yale School of Medicine, United StatesChristos Markopoulos, University of Athens, Greece
Copyright: © 2017 Sagara, Julia, Golshan and Toi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Masakazu Toi, dG9pJiN4MDAwNDA7a3VocC5reW90by11LmFjLmpw
 Yasuaki Sagara
Yasuaki Sagara Wong Julia4
Wong Julia4 Masakazu Toi
Masakazu Toi