
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Oncol., 02 November 2016
Sec. Women's Cancer
Volume 6 - 2016 | https://doi.org/10.3389/fonc.2016.00227
This article is part of the Research TopicReproductive history and breast cancer riskView all 7 articles
Postpartum breast cancers are a highly metastatic subset of young women’s breast cancers defined as breast cancers diagnosed in the postpartum period or within 5 years of last child birth. Women diagnosed with postpartum breast cancer are nearly twice as likely to develop metastasis and to die from breast cancer when compared with nulliparous women. Additionally, epidemiological studies utilizing multiple cohorts also suggest that nearly half of all breast cancers in women aged <45 qualify as postpartum cases. Understanding the biology that underlies this increased risk for metastasis and death may lead to identification of targeted interventions that will benefit the large number of young women with breast cancer who fall into this subset. Preclinical mouse models of postpartum breast cancer have revealed that breast tumor cells become more aggressive if they are present during the normal physiologic process of postpartum mammary gland involution in mice. As involution appears to be a period of lymphatic growth and remodeling, and human postpartum breast cancers have high peritumor lymphatic vessel density (LVD) and increased incidence of lymph node metastasis (1, 2), we propose that novel insight into is to be gained through the study of the biological mechanisms driving normal postpartum mammary lymphangiogenesis as well as in the microenvironment of postpartum tumors.
Postpartum breast cancer is an under-recognized and highly metastatic subset of young women’s breast cancer, which we define as breast cancers diagnosed within 5 years of a women’s most recent child birth (3, 4). The distinction of postpartum cases from the various interactions of breast cancer and pregnancy, or pregnancy-associated breast cancer (PABC), arose from epidemiologic studies indicating that women diagnosed with breast cancer in the postpartum years are nearly three times as likely to develop metastasis and to die from breast cancer in comparison with nulliparous women (3–6). Importantly, this research highlighted the need to clearly separate breast cancer cases as nulliparous, pregnant, or postpartum, as opposed to defining PABC as cancers diagnosed both during and in the 1–2 years after parturition as one entity, to avoid diluting the risk signal (5). Epidemiological studies utilizing multiple cohorts also identify that ~45% of all breast cancers in Caucasian women aged ≤45 are diagnosed within 5–6 years of childbirth (5). More recently, it was identified that a breast cancer diagnosis of up to 10 years postpartum confers an ongoing measure of increased risk for metastasis, which would represent 60% or more of all young onset diagnosis in the US (7). Ideally, understanding the biology that underlies this epidemiologic risk for metastasis and death will lead to identification of targeted interventions that will benefit the large number of young women with breast cancer who fall into this subset (8).
To define the mechanisms of increased risk for metastasis, preclinical mouse models of postpartum breast cancer have revealed that tumors become more aggressive if they are exposed to the normal physiologic process of postpartum mammary gland involution. The process of postpartum breast or mammary gland involution is when the mammary epithelium regresses from the lactational state, undergoes a period of significant tissue remodeling, and resets to the pre-pregnant state. Multiple hallmarks of cancer are identified as also being important aspects of the involution process (9–37). Moreover, increased tumor growth, invasion, and metastasis are all identified when either human breast cancer or murine mammary tumors cells are implanted into postpartum hosts during involution compared with nulliparous controls (1, 36, 38–40). Mechanisms underlying this aggressive tumor promotional phenotype of involution include the induction of immunosuppression and lymphangiogenesis in the tumor microenvironment. Focusing on lymphangiogenesis, involution appears to be a period of significant lymphatic growth and remodeling, and human postpartum breast cancers have high peritumor lymphatic vessel density (LVD) and increased incidence of lymph node metastasis (2, 38, 41). Thus, we believe lymphangiogensis is an important pathway in the metastasis of postpartum breast cancer. Deeper understanding of the biological mechanisms driving normal postpartum mammary lymphangiogenesis offers potential novel insight into tumor-associated lymphangiogenesis.
Lymphangiogenesis is the outgrowth of new lymphatic vessels, which is required for development of the immune system, fluid homeostasis, trafficking of lymphatic cells, normal wound healing, and tissue regeneration (42–47). Differential expression of lymphatic markers, which distinguish lymphatic vessels from blood vessels, has been described in detail over the past decade and has aided the field by allowing researchers to distinguish between newly formed neo- and mature lymphatics (43, 48–57). The adult lymphatic system consists of initial lymphatics, also known as lymphatic capillaries, which drain lymph fluid into pre-collecting lymphatics, followed by drainage into collecting lymphatics that then lead to the lymph node where foreign bodies can be trapped, immune reactions occur, and lymph fluid is concentrated (42, 44). While the lymphatic endothelial marker Lyve-1 is absent in the collecting lymphatics, it is highly expressed for the initial lymphatics or lymphatic capillaries (58). Furthermore, both the lymphatic capillaries and the collecting vessels exhibit high expression levels of Prox-1, VEGFR-3, and podoplanin (48–55, 59). These results suggest that Lyve-1 may be a marker that can be used to specifically measure new lymphatic formation or neo-lymphangiogenesis.
Neo-lymphangiogenesis occurs in adult tissues as an active normal response to infection, inflammation, and wound healing. Neo-lymphangiogenesis can be stimulated by the local production of the vascular endothelial growth factors VEGF-C, and -D within the damaged tissue and subsequent binding to VEGFR-2 and VEGFR-3 on nearby lymphatic endothelial cells (LECs), resulting in the expansion of lymphatics via sprouting from pre-existing lymphatic vasculature (50, 51, 59–69). Primary sources of VEGF-A, -C, and -D include fibroblasts, inflammatory cells, and macrophages (70–74). An alternative theory has emerged whereby bone marrow-derived cells, specifically macrophages, may also be recruited to contribute to lymphangiogenesis (75–77). In support of this theory, bone marrow transplanted from GFP+ mice into GFP− recipients revealed GFP+ cells localized and/or incorporated into new lymphatics during inflammation, and additional lineage tracing experiments support these findings (75, 76, 78). Furthermore, tissue-resident and bone marrow-derived macrophages express lymphatic markers, such as Lyve-1, Prox-1, and podoplanin (78–80), and the presence of macrophages at sites of neo-lymphangiogenesis during inflammation has been reported (78, 80). Thus, macrophages appear to be involved in neo-lymphangiogenesis. As macrophages are also an important part of the normal program of involution (19), we believe there is a role for macrophages in facilitating the neo-lymphangiogenesis seen during postpartum mammary involution.
Postpartum mammary involution has been extensively characterized using rodent mammary glands with more recent preliminary confirmation in human tissues (11, 12, 17, 19, 20, 26, 32, 35, 36, 40, 81–89). Postpartum mammary gland involution occurs in two distinct phases. During the first phase, which is reversible and lasts for 48 h (days 1–2 post weaning), apoptosis of the epithelium occurs and repopulation of the gland with adipocytes is observed. During the second phase (days 3–14), a remodeling program is initiated which results in additional cell death, increased expression of matrix remodeling proteases, degradation and remodeling of extracellular matrix components, and re-differentiation of adipocytes. Recently, we observed that neo- lymphangiogenesis occurs during postpartum mammary gland involution; however, the functional significance of these increased lymphatics has yet to be described (38, 41). Prior to our studies only a few reports had focused on mammary lymphatics, which are described below.
Expression of the VEGF family members has been characterized during the pregnancy/lactation/involution cycle, with a goal of understanding regulation of angiogenesis in the mammary gland. In these studies, VEGF-A expression was observed as increased during pregnancy and lactation where it likely drives angiogenesis and vascular permeability, which are important for milk production. In contrast, pro-lymphangiogenic VEGFC expression levels were overall lower over the course of pregnancy and lactation, remained extremely low during the first phase of involution (days 1 and 2), and then increased nearly twofold in the second phase (days 3 and 7); this provides evidence that pro-lymphangiogenic programs may be activated during postpartum mammary involution (90). Consistent with these findings, we have observed upregulation of pro-lymphangiogenic VEGF-C and VEGF-D mRNA expression, along with their receptors, VEGFR2/3, during postpartum involution in rat mammary tissues (38) (Figure 1A). Additional studies have utilized elegant high-resolution imaging of sectioned and/or whole mounted mouse mammary glands to better understand lymphatic development during the pregnancy/lactation/involution cycle of the mammary gland. These studies revealed that VEGF-C and -D are produced locally by the mammary epithelium and myo-epithelium and that Prox-1-positive lymphatic vessels were intimately associated with the mammary epithelium and the blood vasculature.
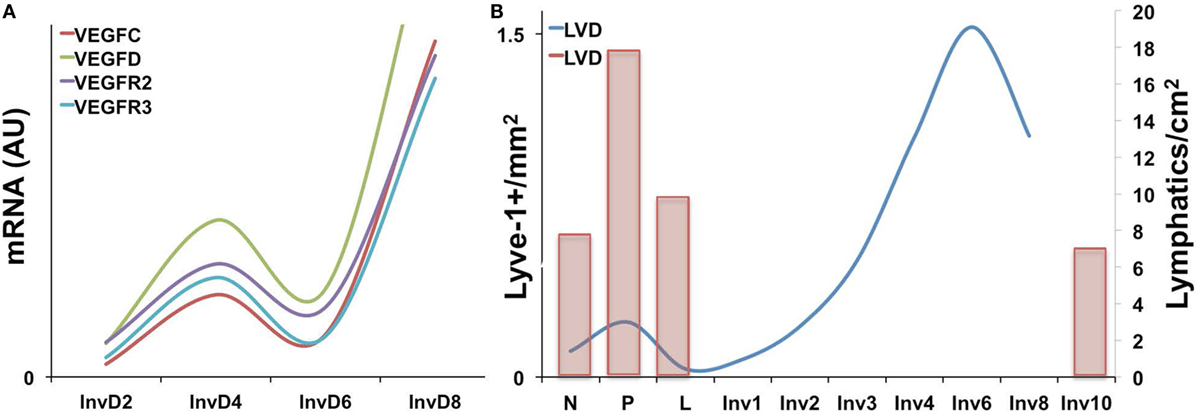
Figure 1. (A) Pro-lymphangiogenic growth factor gene expression, as measured by qPCR is increased during early and late involution in whole rat mammary tissues [adapted from Lyons et al. (38)]. (B) Lyve-1+ lymphatic vessels per area (left axis) is increased during pregnancy and again during involution in mouse mammary tissues with peak levels observed at day 6. (Right axis) A previous study showing a similar increase in Prox-1+ lymphatic vessels during pregnancy as well as levels at involution day 10 [adapted from Betterman et al. (91)].
These studies, by Betterman et al., also examined Prox-1-positive lymph vessels per area to determine density. Interestingly, they observed peak LVD during pregnancy, which decreased during lactation and involution (91). However, their analysis included only a single timepoint during involution, involution day 10, which is near the end of the involution process in mice. In contrast, while our results also reveal that the number of Lyve-1 positive vessels per area in rodent mammary glands similarly drops from pregnancy to lactation, we observe that this drop is accompanied by a subsequent rise in LVD during the early phases involution, which peaks at involution day 6 in mouse and at day 10 in rat mammary tissue (38, 41) (Figure 1B). These results suggest that neo-lymphangiogenesis occurs during the active phase of mammary remodeling in rodents. Importantly, we also analyzed podoplanin positive vessels in normal breast tissue from women who were biopsied within 10 years postpartum to determine whether the increase in lymph vessels is also evident and whether the increase persisted over time, as has been suggested by a gene signature that contains pro-angiogenic molecules angiopoietin and VEGF-A (9). The results from our study showed that women who were within 1 year of giving birth, and no longer lactating, had the highest LVD compared with never been pregnant (NBP) women. In addition, women between 3 and 10 years of giving birth also had elevated LVD compared with nulliparous suggesting that neo-lymphangiogenesis occurs during postpartum breast involution in women, and the resulting lymphatics may persist beyond the period of remodeling (38).
Consistent with a potential role for bone marrow-derived cells in neo-lymphangiogenesis, additional studies of postpartum mammary gland involution have revealed specific changes in immune cell populations, and regulation, during the involution process (12, 19, 20, 32, 36, 40, 81–83). Initial gene expression analyses during the pregnancy/lactation/involution cycle identified upregulation of genes important for acute inflammatory responses in the mammary epithelium during postpartum involution (12, 26). Specifically, Stat3 and NF-κB are primary mediators of involution in the mouse and are also known to be key mediators of acute-phase inflammatory response. Recently, and in support of these gene expression data, the postpartum mammary gland was shown to have a cascade of infiltrating immune cells, including T-cells, T regulatory cells, and dendritic cells during involution that mimic a wound-healing pattern (92). Furthermore, numerous studies have shown that macrophages are present during involution in mouse, rat, and human tissues and that macrophage ablation during the first phase of involution in mice blocks epithelial cell death and adipocyte repopulation. The details of these studies have been reviewed elsewhere (17, 20, 81). Importantly, we observe that macrophages and lymphatics may be similarly regulated during postpartum involution in mouse, rat, and human tissues (19, 38, 93) (Figure 2). We have also shown that macrophages present during postpartum involution in rodent and human tissues express markers of an M2-polarized phenotype, such as mannose receptor, arginase-1, and CD11b (19, 36, 81). CD11b+ macrophages produce pro-lymphangiogenic factors VEGF-C and -D (78, 80, 94). Moreover, subpopulations of CD11b+ positive macrophages express lymphatic endothelial markers Lyve-1 and VEGFR-3 (95, 96). Together, these findings indicate that the CD11b+ “involution macrophages” may either stimulate lymphangiogenesis through release of pro-lymphangiogenic cytokines or through expression of lymphatic markers and incorporation into existing lymphatics; evidence for both has been published in models of inflammation and cancer (68, 78, 80, 96, 97).
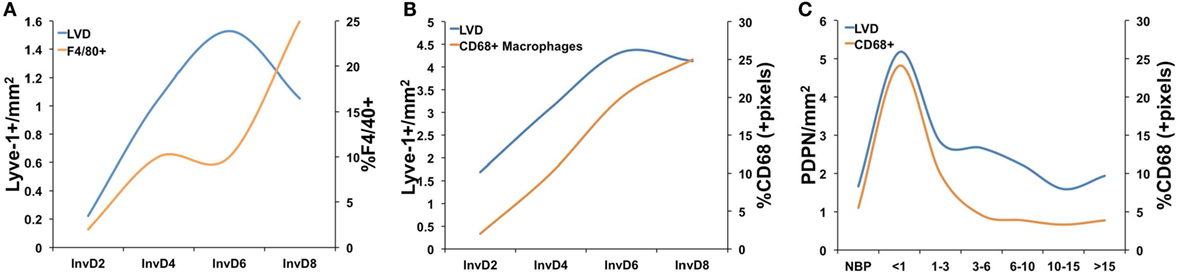
Figure 2. A comparison of lymphatic vessel density (LVD) and macrophage infiltration in (A) mouse mammary tissue where macrophages were measured as %F4/80+ cells from whole mammary tissue by flow cytometry [data adapted from Lyons et al. (38) and Martinson et al. (36)], (B) rat mammary tissue where macrophages were measured by quantitative IHC as %CD68+ cells/pixel [data adapted from Lyons et al. (38) and O’Brien et al. (19)], and (C) in normal human breast tissues from women who had never been pregnant (NBP), were no longer lactating, and <1, 1–3, 3–6, 6–10, 10–15, and >15 years since last childbirth where lymphatics were measured as number of podopanin+ vessels per area and CD68 by quantitative IHC [data adapted from Lyons et al. (38) and Jindal et al. (93)].
Our analysis of lymphangiogenesis during postpartum involution also revealed that administration of a selective COX-2 inhibitor, celecoxib (CXB), during postpartum involution reduced Lyve-1-positive LVD at involution day 4 (38). These results are consistent with previous observations in tumor models (98–102). While, the mechanism by which CXB blocked lymphangiogenesis was not directly revealed by these studies, our in vitro data suggested that a product of COX-2 activity, PGE2, acts directly on the lymphatic endothelium via the EP2 receptor. However, PGE2 can also stimulate macrophages to an M2 phenotype (103, 104); thus, it is also possible that CXB inhibits lymphangiogenesis through macrophage-dependent mechanisms. Understanding the mechanisms underlying “involution macrophage” contribution to lymphangiogenesis during normal mammary gland development could lead to insight into macrophage-mediated lymphangiogenesis during breast cancer. We postulate that postpartum involution is a developmental window that allows for studies of mechanisms driving lymphangiogenesis.
While expansion of the lymphatic vasculature has been linked to faster healing and greater ability to fight infection, lymphangiogenesis can also be pathologic. Pathologic lymphangiogenesis has been observed in graft-versus-host disease, in chronic inflammatory diseases (e.g., Rheumatoid arthritis and inflammatory bowel disease), and in the tumor microenvironment. Lymph node metastasis, lymphatic vessel presence at the tumor margin, and invasion of tumor cells into peritumor lymphatics are all poor prognostic factors for breast cancer patients (105). Further, increased LVD in the peritumor region correlates with increased metastasis in a number of human cancers, directly implicating new lymphatic vessel formation in tumor cell dissemination (106–108). A multitude of studies have examined mechanisms driving neo-lymphangiogenesis in the breast tumor environment, and VEGF-C, VEGF-D, macrophages, and COX-2/PGE2 have emerged as key players (51, 68, 72, 73, 80, 99–101, 109–118).
VEGF-C is secreted by macrophages and other lymphatic cells to stimulate lymphangiogenesis, but can also be secreted by tumor cells for the same purpose (51, 62, 72, 73, 94, 117, 119). Macrophages have also been shown to participate directly in lymphangiogenesis via inducing lymphatic vessel sprouting and incorporating into existing tumor-associated lymphatics (69, 80). COX-2 and its product PGE2 also promote lymphangiogenesis in the tumor microenvironment (38, 41, 98, 99, 101, 102, 116, 120, 121). Furthermore, VEGF-D promotes lymphatic vessel dilation through a COX-2-dependent mechanism. Dilation of pre-existing, peritumor, and intratumor lymphatics allows for the intravasation of tumor cells into the lymphatic vessels and subsequent transmigration to regional lymph nodes (109, 114, 119, 122). Together these results suggest there is a connection between COX-2-mediated lymphangiogenesis and lymphogenous tumor cell spread.
Postpartum breast cancers are nearly three times as likely to metastasize when compared with breast cancers in nulliparous women (5, 123, 124). Furthermore, we have shown that postpartum breast cancers have increased peritumor LVD and increased lymph node involvement (1). It is anticipated that postpartum breast tumors will utilize mechanisms similar to those observed during postpartum involution to induce lymphangiogenesis. The first, and most obvious mechanism, is upregulation of COX-2 in the mammary epithelium (125), which results in increased PGE2 production to increase lymphangiogenesis. Indeed, in animals treated with celecoxib (CXB) during postpartum involution, the resultant postpartum tumors exhibit lower levels of LVD compared with untreated controls. In addition, COX-2 in the tumor cell appears to be required as well since tumors with stable siRNA knockdown of COX-2 exhibit decreased tumor-associated LVD. Finally, if postpartum tumors are re-implanted in nulliparous hosts they maintain their ability to drive tumor-associated lymphangiogenesis (38). Of interest, these results suggest that involution-induced pro-lymphangiogenic programs persist in tumor cells long after the process of involution is complete. These results are supported by gene-expression studies indicating that there is an involution signature observed in breast tissue of parous women that persists for 10 years postpartum (9).
In addition to a COX-2-dependent mechanism, if “involution macrophages” promote lymphangiogenesis during normal involution then postpartum tumor-associated macrophages (TAMs) may also acquire and utilize similar mechanisms to mediate lymphangiogenesis in the postpartum tumor microenvironment. CD11b+ is expressed by “involution macrophages” and by TAMs (96). TAMs also predict poor prognosis of breast cancer (126), and an association between TAMs and LVD has been reported for pancreatic cancer (127). Furthermore, TAMs express VEGF-C and may promote metastasis via lymphangiogenesis (71–73, 126, 128). Thus, the CD11b+ macrophages present during postpartum involution may promote lymphangiogenesis in a manner similar to TAMs, and we have preclinical data indicating that CD11b+ macrophages are also increased in the tumor microenvironment of involution/postpartum tumors compared with nulliparous controls (36).
While it is not clear why the lymphatics are expanded during postpartum involution, it is clear that postpartum tumors hijack the lymphatic vessels in the postpartum gland to drive increased metastasis. In addition, the observed tumor-promoted neo-lymphangiogenesis offers a targetable mechanism to reduce cancer metastasis (123, 124, 129–131). Anti-lymphangiogenic therapies, such as anti-VEGFR2/3 antibodies and small molecule inhibitors that target VEGFR2/3, have been tested in clinical trials for multiple solid tumor types and have shown some successes and low toxicities (132–134). Since our studies suggest that COX-2 specific inhibitors may serve to reduce tumor-associated lymphangiogenesis, we suggest that identifying whether COX-2 inhibitors can be combined with current therapies, and/or with anti-lymphangiogenesis therapy, to reduce lymphogenous spread and metastatic recurrence should be explored for postpartum breast cancer patients.
TL was responsible for overseeing writing and editing of the manuscript, assigning contributions from VB and AE, and for preparation of the figures. VB and AE contributed to writing and editing the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This study was supported by NCI 1 R01 CA169175-01 and Robert F. and Patricia Young Connor Endowed Chair in Young Women’s Breast Cancer Research to VB. NCI R21CA185226-01, NIH 1KL2TR001080, and UL1 TR001082 to TL. TL was also supported by funding from the Cancer League of Colorado.
1. Lyons TR, O’Brien J, Borges VF, Conklin MW, Keely PJ, Eliceiri KW, et al. Postpartum mammary gland involution drives progression of ductal carcinoma in situ through collagen and COX-2. Nat Med (2011) 17:1109–15. doi:10.1038/nm.2416
2. Neill T, Zoeller J. Matrix biology highlights. Matrix Biol (2014) 40:1–4. doi:10.1016/j.matbio.2014.11.002
3. Borges VF, Schedin PJ. Pregnancy-associated breast cancer: an entity needing refinement of the definition. Cancer (2012) 118:3226–8. doi:10.1002/cncr.26643
4. Lyons TR, Schedin PJ, Borges VF. Pregnancy and breast cancer: when they collide. J Mammary Gland Biol Neoplasia (2009) 14:87–98. doi:10.1007/s10911-009-9119-7
5. Callihan EB, Gao D, Jindal S, Lyons TR, Manthey E, Edgerton S, et al. Postpartum diagnosis demonstrates a high risk for metastasis and merits an expanded definition of pregnancy-associated breast cancer. Breast Cancer Res Treat (2013) 138:549–59. doi:10.1007/s10549-013-2437-x
6. Liu Q, Wuu J, Lambe M, Hsieh SF, Ekbom A, Hsieh CC. Transient increase in breast cancer risk after giving birth: postpartum period with the highest risk (Sweden). Cancer Causes Control (2002) 13:299–305. doi:10.1023/A:1015287208222
7. Borges VF, Goddard E, Partridge AH, Schedin P. Abstract P6-08-08: postpartum breast cancer demonstrates increased liver and brain metastasis with a proposed role for postpartum involution. Cancer Res (2015). doi:10.1158/1538-7445.SABCS14-P6-08-08
8. Fornetti J, Martinson HA, Betts CB, Lyons TR, Jindal S, Guo Q, et al. Mammary gland involution as an immunotherapeutic target for postpartum breast cancer. J Mammary Gland Biol Neoplasia (2014) 19:213–28. doi:10.1007/s10911-014-9322-z
9. Asztalos S, Gann PH, Hayes MK, Nonn L, Beam CA, Dai Y, et al. Gene expression patterns in the human breast after pregnancy. Cancer Prev Res (Phila) (2010) 3:301–11. doi:10.1158/1940-6207.CAPR-09-0069
10. Atabai K, Sheppard D, Werb Z. Roles of the innate immune system in mammary gland remodeling during involution. J Mammary Gland Biol Neoplasia (2007) 12:37–45. doi:10.1007/s10911-007-9036-6
11. Clarkson RW, Heeley JL, Chapman R, Aillet F, Hay RT, Wyllie A, et al. NF-kappaB inhibits apoptosis in murine mammary epithelia. J Biol Chem (2000) 275:12737–42. doi:10.1074/jbc.275.17.12737
12. Clarkson RW, Wayland MT, Lee J, Freeman T, Watson CJ. Gene expression profiling of mammary gland development reveals putative roles for death receptors and immune mediators in post-lactational regression. Breast Cancer Res (2004) 6:R92–109. doi:10.1186/bcr754
13. Alexander CM, Selvarajan S, Mudgett J, Werb Z. Stromelysin-1 regulates adipogenesis during mammary gland involution. J Cell Biol (2001) 152:693–703. doi:10.1083/jcb.152.4.693
14. Djonov V, Andres AC, Ziemiecki A. Vascular remodelling during the normal and malignant life cycle of the mammary gland. Microsc Res Tech (2001) 52:182–9. doi:10.1002/1097-0029(20010115)52:2<182::AID-JEMT1004>3.0.CO;2-M
15. Green KA, Lund LR. ECM degrading proteases and tissue remodelling in the mammary gland. Bioessays (2005) 27:894–903. doi:10.1002/bies.20281
16. Hughes K, Wickenden JA, Allen JE, Watson CJ. Conditional deletion of Stat3 in mammary epithelium impairs the acute phase response and modulates immune cell numbers during post-lactational regression. J Pathol (2012) 227:106–17. doi:10.1002/path.3961
17. Monks J, Geske FJ, Lehman L, Fadok VA. Do inflammatory cells participate in mammary gland involution? J Mammary Gland Biol Neoplasia (2002) 7:163–76. doi:10.1023/A:1020351919634
18. Nickerson SC. Immunological aspects of mammary involution. J Dairy Sci (1989) 72:1665–78. doi:10.3168/jds.S0022-0302(89)79278-X
19. O’Brien J, Lyons T, Monks J, Lucia MS, Wilson RS, Hines L, et al. Alternatively activated macrophages and collagen remodeling characterize the postpartum involuting mammary gland across species. Am J Pathol (2010) 176:1241–55. doi:10.2353/ajpath.2010.090735
20. O’Brien J, Schedin P. Macrophages in breast cancer: do involution macrophages account for the poor prognosis of pregnancy-associated breast cancer? J Mammary Gland Biol Neoplasia (2009) 14:145–57. doi:10.1007/s10911-009-9118-8
21. Pensa S, Watson CJ, Poli V. Stat3 and the inflammation/acute phase response in involution and breast cancer. J Mammary Gland Biol Neoplasia (2009) 14:121–9. doi:10.1007/s10911-009-9124-x
22. Ramirez RA, Lee A, Schedin P, Russell JS, Masso-Welch PA. Alterations in mast cell frequency and relationship to angiogenesis in the rat mammary gland during windows of physiologic tissue remodeling. Dev Dyn (2012) 241:890–900. doi:10.1002/dvdy.23778
23. Schedin P, Mitrenga T, McDaniel S, Kaeck M. Mammary ECM composition and function are altered by reproductive state. Mol Carcinog (2004) 41:207–20. doi:10.1002/mc.20058
24. Schedin P, Strange R, Mitrenga T, Wolfe P, Kaeck M. Fibronectin fragments induce MMP activity in mouse mammary epithelial cells: evidence for a role in mammary tissue remodeling. J Cell Sci (2000) 113(Pt 5):795–806.
25. Stein T, Morris JS, Davies CR, Weber-Hall SJ, Duffy MA, Heath VJ, et al. Involution of the mouse mammary gland is associated with an immune cascade and an acute-phase response, involving LBP, CD14 and STAT3. Breast Cancer Res (2004) 6:R75–91. doi:10.1186/bcr894
26. Stein T, Salomonis N, Gusterson BA. Mammary gland involution as a multi-step process. J Mammary Gland Biol Neoplasia (2007) 12:25–35. doi:10.1007/s10911-007-9035-7
27. Stein T, Salomonis N, Nuyten DS, van de Vijver MJ, Gusterson BA. A mouse mammary gland involution mRNA signature identifies biological pathways potentially associated with breast cancer metastasis. J Mammary Gland Biol Neoplasia (2009) 14:99–116. doi:10.1007/s10911-009-9120-1
28. Strange R, Li F, Saurer S, Burkhardt A, Friis RR. Apoptotic cell death and tissue remodelling during mouse mammary gland involution. Development (1992) 115:49–58.
29. Talhouk RS, Bissell MJ, Werb Z. Coordinated expression of extracellular matrix-degrading proteinases and their inhibitors regulates mammary epithelial function during involution. J Cell Biol (1992) 118:1271–82. doi:10.1083/jcb.118.5.1271
30. Watson CJ. Post-lactational mammary gland regression: molecular basis and implications for breast cancer. Expert Rev Mol Med (2006) 8:1–15. doi:10.1017/S1462399406000196
31. Watson CJ. Involution: apoptosis and tissue remodelling that convert the mammary gland from milk factory to a quiescent organ. Breast Cancer Res (2006) 8:203. doi:10.1186/bcr1401
32. Watson CJ. Immune cell regulators in mouse mammary development and involution. J Anim Sci (2009) 87:35–42. doi:10.2527/jas.2008-1333
33. Werb Z, Ashkenas J, MacAuley A, Wiesen JF. Extracellular matrix remodeling as a regulator of stromal-epithelial interactions during mammary gland development, involution and carcinogenesis. Braz J Med Biol Res (1996) 29:1087–97.
34. Zhao L, Melenhorst JJ, Hennighausen L. Loss of interleukin 6 results in delayed mammary gland involution: a possible role for mitogen-activated protein kinase and not signal transducer and activator of transcription 3. Mol Endocrinol (2002) 16:2902–12. doi:10.1210/me.2001-0330
35. Kreuzaler PA, Staniszewska AD, Li W, Omidvar N, Kedjouar B, Turkson J, et al. Stat3 controls lysosomal-mediated cell death in vivo. Nat Cell Biol (2011) 13:303–9. doi:10.1038/ncb2171
36. Martinson HA, Jindal S, Durand-Rougely C, Borges VF, Schedin P. Wound healing-like immune program facilitates postpartum mammary gland involution and tumor progression. Int J Cancer (2015) 136:1803–13. doi:10.1002/ijc.29181
37. Vaught DB, Cook RS. Clearance of dying cells accelerates malignancy. Oncotarget (2015) 6:24590–1. doi:10.18632/oncotarget.5670
38. Lyons TR, Borges VF, Betts CB, Guo Q, Kapoor P, Martinson HA, et al. Cyclooxygenase-2-dependent lymphangiogenesis promotes nodal metastasis of postpartum breast cancer. J Clin Invest (2014) 124(9):3901–12. doi:10.1172/JCI73777
39. Gupta PB, Proia D, Cingoz O, Weremowicz J, Naber SP, Weinberg RA, et al. Systemic stromal effects of estrogen promote the growth of estrogen receptor-negative cancers. Cancer Res (2007) 67:2062–71. doi:10.1158/0008-5472.CAN-06-3895
40. Stanford JC, Young C, Hicks D, Owens P, Williams A, Vaught DB, et al. Efferocytosis produces a prometastatic landscape during postpartum mammary gland involution. J Clin Invest (2014) 124:4737–52. doi:10.1172/JCI76375
41. Swartz MA. Inflammatory lymphangiogenesis in postpartum breast tissue remodeling. J Clin Invest (2014) 124(9):1–4. doi:10.1172/JCI77765
42. Betterman K, Harvey N. The lymphatic vasculature: development and role in shaping immunity. Immunol Rev (2016) 27:276–92. doi:10.1111/imr.12413
43. Tammela T, Alitalo K. Lymphangiogenesis: molecular mechanisms and future promise. Cell (2010) 140:460–76. doi:10.1016/j.cell.2010.01.045
44. Butler MG, Isogai S, Weinstein BM. Lymphatic development. Birth Defects Res C Embryo Today (2009) 87:222–31. doi:10.1002/bdrc.20155
45. Schulte-Merker S, Sabine A, Petrova TV. Lymphatic vascular morphogenesis in development, physiology, and disease. J Cell Biol (2011) 193:607–18. doi:10.1083/jcb.201012094
46. Karpanen T, Makinen T. Regulation of lymphangiogenesis – from cell fate determination to vessel remodeling. Exp Cell Res (2006) 312:575–83. doi:10.1016/j.yexcr.2005.10.034
47. Karpanen T, Wirzenius M, Mäkinen T, Veikkola T, Haisma HJ, Achen MG, et al. Lymphangiogenic growth factor responsiveness is modulated by postnatal lymphatic vessel maturation. Am J Pathol (2006) 169:708–18. doi:10.2353/ajpath.2006.051200
48. Wigle JT, Harvey N, Detmar M, Lagutina I, Grosveld G, Gunn MD, et al. An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. EMBO J (2002) 21:1505–13. doi:10.1093/emboj/21.7.1505
49. Pan Y, Wang WD, Yago T. Transcriptional regulation of podoplanin expression by Prox1 in lymphatic endothelial cells. Microvasc Res (2014) 94:96–102. doi:10.1016/j.mvr.2014.05.006
50. Norrmén C, Tammela T, Petrova TV, Alitalo K. Biological basis of therapeutic lymphangiogenesis. Circulation (2011) 123:1335–51. doi:10.1161/CIRCULATIONAHA.107.704098
51. Karkkainen MJ, Haiko P, Sainio K, Partanen J, Taipale J, Petrova TV, et al. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat Immunol (2004) 5:74–80. doi:10.1038/ni1013
52. Breiteneder-Geleff S, Soleiman A, Kowalski H, Horvat R, Amann G, Kriehuber E, et al. Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillaries: podoplanin as a specific marker for lymphatic endothelium. Am J Pathol (1999) 154:385–94. doi:10.1016/S0002-9440(10)65285-6
53. Norrmén C, Ivanov KI, Cheng J, Zangger N, Delorenzi M, Jaquet M, et al. FOXC2 controls formation and maturation of lymphatic collecting vessels through cooperation with NFATc1. J Cell Biol (2009) 185:439–57. doi:10.1083/jcb.200901104
54. Petrova TV, Karpanen T, Norrmén C, Mellor R, Tamakoshi T, Finegold D, et al. Defective valves and abnormal mural cell recruitment underlie lymphatic vascular failure in lymphedema distichiasis. Nat Med (2004) 10:974–81. doi:10.1038/nm1094
55. Petrova TV, Mäkinen T, Mäkelä TP, Saarela J, Virtanen I, Ferrell RE, et al. Lymphatic endothelial reprogramming of vascular endothelial cells by the Prox-1 homeobox transcription factor. EMBO J (2002) 21:4593–9. doi:10.1093/emboj/cdf470
56. Johnson NC, Dillard ME, Baluk P, McDonald DM, Harvey NL, Frase SL, et al. Lymphatic endothelial cell identity is reversible and its maintenance requires Prox1 activity. Genes Dev (2008) 22:3282–91. doi:10.1101/gad.1727208
57. Shin JW. Prox1 promotes lineage-specific expression of fibroblast growth factor (FGF) receptor-3 in lymphatic endothelium: a role for FGF signaling in lymphangiogenesis. Mol Biol Cell (2005) 17:576–84. doi:10.1091/mbc.E05-04-0368
58. Podgrabinska S, Braun P, Velasco P, Kloos B, Pepper MS, Skobe M. Molecular characterization of lymphatic endothelial cells. Proc Natl Acad Sci U S A (2002) 99:16069–74. doi:10.1073/pnas.242401399
59. Klotz L, Norman S, Vieira JM, Masters M, Rohling M, Dubé KN, et al. Cardiac lymphatics are heterogeneous in origin and respond to injury. Nature (2015) 522:62–7. doi:10.1038/nature14483
60. Zheng W, Aspelund A, Alitalo K. Lymphangiogenic factors, mechanisms, and applications. J Clin Invest (2014) 124:878–87. doi:10.1172/JCI71603
61. Breier G. Lymphangiogenesis in regenerating tissue: is VEGF-C sufficient? Circ Res (2005) 96:1132–4. doi:10.1161/01.RES.0000170976.63688.ca
62. Achen MG, Jeltsch M, Kukk E, Mäkinen T, Vitali A, Wilks AF, et al. Vascular endothelial growth factor D (VEGF-D) is a ligand for the tyrosine kinases VEGF receptor 2 (Flk1) and VEGF receptor 3 (Flt4). Proc Natl Acad Sci U S A (1998) 95:548–53. doi:10.1073/pnas.95.2.548
63. Cao Y, Linden P, Farnebo J, Cao R, Eriksson A, Kumar V, et al. Vascular endothelial growth factor C induces angiogenesis in vivo. Proc Natl Acad Sci U S A (1998) 95:14389–94. doi:10.1073/pnas.95.24.14389
64. Cao Y, Zhong W. Tumor-derived lymphangiogenic factors and lymphatic metastasis. Biomed Pharmacother (2007) 61:534–9. doi:10.1016/j.biopha.2007.08.009
65. Cao Z, Shang B, Zhang G, Miele L, Sarkar FH, Wang Z, et al. Tumor cell-mediated neovascularization and lymphangiogenesis contrive tumor progression and cancer metastasis. Biochim Biophys Acta (2013) 1836:273–86. doi:10.1016/j.bbcan.2013.08.001
66. Cao R, Eriksson A, Kubo H, Alitalo K, Cao Y, Thyberg J, et al. Comparative evaluation of FGF-2-, VEGF-A-, and VEGF-C-induced angiogenesis, lymphangiogenesis, vascular fenestrations, and permeability. Circ Res (2004) 94:664–70. doi:10.1161/01.RES.0000118600.91698.BB
67. Cao Y. Opinion: emerging mechanisms of tumour lymphangiogenesis and lymphatic metastasis. Nat Rev Cancer (2005) 5:735–43. doi:10.1038/nrc1693
68. Hall KL, Volk-Draper LD, Flister MJ, Ran S. New model of macrophage acquisition of the lymphatic endothelial phenotype. PLoS One (2012) 7:e31794. doi:10.1371/journal.pone.0031794
69. Ran S, Montgomery KE. Macrophage-mediated lymphangiogenesis: the emerging role of macrophages as lymphatic endothelial progenitors. Cancers (Basel) (2012) 4:618–57. doi:10.3390/cancers4030618
70. Björndahl MA, Cao R, Burton JB, Brakenhielm E, Religa P, Galter D, et al. Vascular endothelial growth factor-a promotes peritumoral lymphangiogenesis and lymphatic metastasis. Cancer Res (2005) 65:9261–8. doi:10.1158/0008-5472.CAN-04-2345
71. Gallego E, Vicioso L, Alvarez M, Hierro I, Pérez-Villa L, Blanes A, et al. Stromal expression of vascular endothelial growth factor C is relevant to predict sentinel lymph node status in melanomas. Virchows Arch (2011) 458:621–30. doi:10.1007/s00428-011-1044-7
72. Schoppmann SF, Fenzl A, Nagy K, Unger S, Bayer G, Geleff S, et al. VEGF-C expressing tumor-associated macrophages in lymph node positive breast cancer: impact on lymphangiogenesis and survival. Surgery (2006) 139:839–46. doi:10.1016/j.surg.2005.12.008
73. Schoppmann SF, Birner P, Stöckl J, Kalt R, Ullrich R, Caucig C, et al. Tumor-associated macrophages express lymphatic endothelial growth factors and are related to peritumoral lymphangiogenesis. Am J Pathol (2002) 161:947–56. doi:10.1016/S0002-9440(10)64255-1
74. Skobe M, Hamberg LM, Hawighorst T, Schirner M, Wolf GL, Alitalo K, et al. Concurrent induction of lymphangiogenesis, angiogenesis, and macrophage recruitment by vascular endothelial growth factor-C in melanoma. Am J Pathol (2001) 159:893–903. doi:10.1016/S0002-9440(10)61765-8
75. Zumsteg A, Baeriswyl V, Imaizumi N, Schwendener R, Rüegg C, Christofori G. Myeloid cells contribute to tumor lymphangiogenesis. PLoS One (2009) 4:e7067. doi:10.1371/journal.pone.0007067
76. Religa P, Cao R, Bjorndahl M, Zhou Z, Zhu Z, Cao Y. Presence of bone marrow-derived circulating progenitor endothelial cells in the newly formed lymphatic vessels. Blood (2005) 106:4184–90. doi:10.1182/blood-2005-01-0226
77. Jiang S, Bailey AS, Goldman DC, Swain JR, Wong MH, Streeter PR, et al. Hematopoietic stem cells contribute to lymphatic endothelium. PLoS One (2008) 3:e3812. doi:10.1371/journal.pone.0003812
78. Maruyama K, Ii M, Cursiefen C, Jackson DG, Keino H, Tomita M, et al. Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. J Clin Invest (2005) 115:2363–72. doi:10.1172/JCI23874
79. Cursiefen C, Cao J, Chen L, Liu Y, Maruyama K, Jackson D, et al. Inhibition of hemangiogenesis and lymphangiogenesis after normal-risk corneal transplantation by neutralizing VEGF promotes graft survival. Invest Ophthalmol Vis Sci (2004) 45:2666–73. doi:10.1167/iovs.03-1380
80. Kerjaschki D. The crucial role of macrophages in lymphangiogenesis. J Clin Invest (2005) 115:2316–9. doi:10.1172/JCI26354
81. O’Brien J, Martinson H, Durand-Rougely C, Schedin P. Macrophages are crucial for epithelial cell death and adipocyte repopulation during mammary gland involution. Development (2012) 139:269–75. doi:10.1242/dev.071696
82. Alowami S, Troup S, Al-Haddad S, Kirkpatrick I, Watson PH. Mammographic density is related to stroma and stromal proteoglycan expression. Breast Cancer Res (2003) 5:R129–35. doi:10.1186/bcr622
83. Watson CJ, Oliver CH, Khaled WT. Cytokine signalling in mammary gland development. J Reprod Immunol (2011) 88(2):124–9. doi:10.1016/j.jri.2010.11.006
84. Walker NI, Bennett RE, Kerr JF. Cell death by apoptosis during involution of the lactating breast in mice and rats. Am J Anat (1989) 185:19–32. doi:10.1002/aja.1001850104
85. Richert MM, Schwertfeger KL, Ryder JW, Anderson SM. An atlas of mouse mammary gland development. J Mammary Gland Biol Neoplasia (2000) 5:227–41. doi:10.1023/A:1026499523505
86. Schwertfeger KL, Richert MM, Anderson SM. Mammary gland involution is delayed by activated Akt in transgenic mice. Mol Endocrinol (2001) 15:867–81. doi:10.1210/mend.15.6.0663
87. Monks J, Rosner D, Geske FJ, Lehman L, Hanson L, Neville MC, et al. Epithelial cells as phagocytes: apoptotic epithelial cells are engulfed by mammary alveolar epithelial cells and repress inflammatory mediator release. Cell Death Differ (2005) 12:107–14. doi:10.1038/sj.cdd.4401517
88. Rudolph MC, McManaman JL, Hunter L, Phang T, Neville MC. Functional development of the mammary gland: use of expression profiling and trajectory clustering to reveal changes in gene expression during pregnancy, lactation, and involution. J Mammary Gland Biol Neoplasia (2003) 8:287–307. doi:10.1023/B:JOMG.0000010030.73983.57
89. Monks J, Smith-Steinhart C, Kruk ER, Fadok VA, Henson PM. Epithelial cells remove apoptotic epithelial cells during post-lactation involution of the mouse mammary gland. Biol Reprod (2008) 78:586–94. doi:10.1095/biolreprod.107.065045
90. Pepper MS, Baetens D, Mandriota SJ, Di Sanza C, Oikemus S, Lane TF, et al. Regulation of VEGF and VEGF receptor expression in the rodent mammary gland during pregnancy, lactation, and involution. Dev Dyn (2000) 218:507–24. doi:10.1002/1097-0177(200007)218:3<507::AID-DVDY1012>3.0.CO;2-5
91. Betterman KL, Paquet-Fifield S, Asselin-Labat ML, Visvader JE, Butler LM, Stacker SA, et al. Remodeling of the lymphatic vasculature during mouse mammary gland morphogenesis is mediated via epithelial-derived lymphangiogenic stimuli. Am J Pathol (2012) 181:2225–38. doi:10.1016/j.ajpath.2012.08.035
92. Martinson H, Jindal S, Borges V, Schedin P. Immune cell influx during postpartum mammary gland involution reveals immunosuppression and tumor promotion. Cancer Res (2013):73.
93. Jindal S, Gao D, Bell P, Albrektsen G, Edgerton SM, Ambrosone CB, et al. Postpartum breast involution reveals regression of secretory lobules mediated by tissue-remodeling. Breast Cancer Res (2014) 16:R31. doi:10.1186/bcr3633
94. Kataru RP, Jung K, Jang C, Yang H, Schwendener RA, Baik JE, et al. Critical role of CD11b+ macrophages and VEGF in inflammatory lymphangiogenesis, antigen clearance, and inflammation resolution. Blood (2009) 113:5650–9. doi:10.1182/blood-2008-09-176776
95. Hamrah P, Chen L, Cursiefen C, Zhang Q, Joyce NC, Dana MR. Expression of vascular endothelial growth factor receptor-3 (VEGFR-3) on monocytic bone marrow-derived cells in the conjunctiva. Exp Eye Res (2004) 79:553–61. doi:10.1016/j.exer.2004.06.028
96. Xu H, Chen M, Reid DM, Forrester JV. LYVE-1-positive macrophages are present in normal murine eyes. Invest Ophthalmol Vis Sci (2007) 48:2162–71. doi:10.1167/iovs.06-0783
97. Qian B, Deng Y, Im JH, Muschel RJ, Zou Y, Li J, et al. A distinct macrophage population mediates metastatic breast cancer cell extravasation, establishment and growth. PLoS One (2009) 4:e6562. doi:10.1371/journal.pone.0006562
98. Barnes NL, Warnberg F, Farnie G, White D, Jiang W, Anderson E, et al. Cyclooxygenase-2 inhibition: effects on tumour growth, cell cycling and lymphangiogenesis in a xenograft model of breast cancer. Br J Cancer (2007) 96:575–82. doi:10.1038/sj.bjc.6603593
99. Timoshenko AV, Chakraborty C, Wagner GF, Lala PK. COX-2-mediated stimulation of the lymphangiogenic factor VEGF-C in human breast cancer. Br J Cancer (2006) 94:1154–63. doi:10.1038/sj.bjc.6603067
100. Xin X, Majumder M, Girish GV, Mohindra V, Maruyama T, Lala PK. Targeting COX-2 and EP4 to control tumor growth, angiogenesis, lymphangiogenesis and metastasis to the lungs and lymph nodes in a breast cancer model. Lab Invest (2012) 92:1115–28. doi:10.1038/labinvest.2012.90
101. Liu H, Yang Y, Xiao J, Lv Y, Liu Y, Yang H, et al. Inhibition of cyclooxygenase-2 suppresses lymph node metastasis via VEGF-C. Anat Rec (Hoboken) (2009) 292:1577–83. doi:10.1002/ar.20940
102. Bhattacharjee RN, Timoshenko AV, Cai J, Lala PK. Relationship between cyclooxygenase-2 and human epidermal growth factor receptor 2 in vascular endothelial growth factor C up-regulation and lymphangiogenesis in human breast cancer. Cancer Sci (2010) 101:2026–32. doi:10.1111/j.1349-7006.2010.01647.x
103. YlÖstalo JH, Bartosh TJ, Coble K, Prockop DJ. Human mesenchymal stem/stromal cells cultured as spheroids are self-activated to produce prostaglandin E2 that directs stimulated macrophages into an anti-inflammatory phenotype. Stem Cells (2012) 30:2283–96. doi:10.1002/stem.1191
104. Liu L, Ge D, Ma L, Mei J, Liu S, Zhang Q, et al. Interleukin-17 and prostaglandin E2 are involved in formation of an M2 macrophage-dominant microenvironment in lung cancer. J Thorac Oncol (2012) 7(7):1091–100. doi:10.1097/JTO.0b013e3182542752
105. Jain RK, Padera TP. Prevention and treatment of lymphatic metastasis by antilymphangiogenic therapy. J Natl Cancer Inst (2002) 94:785–7. doi:10.1093/jnci/94.11.785
106. Gudlaugsson E, Skaland I, Undersrud E, Janssen EA, Søiland H, Baak JP. D2-40/p63 defined lymph vessel invasion has additional prognostic value in highly proliferating operable node negative breast cancer patients. Mod Pathol (2011) 24:502–11. doi:10.1038/modpathol.2010.199
107. Schoppmann SF, Bayer G, Aumayr K, Taucher S, Geleff S, Rudas M, et al. Prognostic value of lymphangiogenesis and lymphovascular invasion in invasive breast cancer. Ann Surg (2004) 240:306–12. doi:10.1097/01.sla.0000133355.48672.22
108. Swartz MA, Skobe M. Lymphatic function, lymphangiogenesis, and cancer metastasis. Microsc Res Tech (2001) 55:92–9. doi:10.1002/jemt.1160
109. Achen MG, Stacker SA. Molecular control of lymphatic metastasis. Ann N Y Acad Sci (2008) 1131:225–34. doi:10.1196/annals.1413.020
110. Alitalo A, Detmar M. Interaction of tumor cells and lymphatic vessels in cancer progression. Oncogene (2012) 31:4499–508. doi:10.1038/onc.2011.602
111. Alitalo K, Carmeliet P. Molecular mechanisms of lymphangiogenesis in health and disease. Cancer Cell (2002) 1:219–27. doi:10.1016/S1535-6108(02)00051-X
112. Baldwin ME, Stacker SA, Achen MG. Molecular control of lymphangiogenesis. Bioessays (2002) 24:1030–40. doi:10.1002/bies.10173
113. Jussila L, Alitalo K. Vascular growth factors and lymphangiogenesis. Physiol Rev (2002) 82:673–700. doi:10.1152/physrev.00005.2002
114. Karnezis T, Shayan R, Caesar C, Roufail S, Harris NC, Ardipradja K, et al. VEGF-D promotes tumor metastasis by regulating prostaglandins produced by the collecting lymphatic endothelium. Cancer Cell (2012) 21:181–95. doi:10.1016/j.ccr.2011.12.026
115. Karpanen T, Egeblad M, Karkkainen MJ, Kubo H, Ylä-Herttuala S, Jäättelä M, et al. Vascular endothelial growth factor C promotes tumor lymphangiogenesis and intralymphatic tumor growth. Cancer Res (2001) 61:1786–90.
116. Liu H, Yang Y, Xiao J, Lv Y, Liu Y, Yang H, et al. COX-2-mediated regulation of VEGF-C in association with lymphangiogenesis and lymph node metastasis in lung cancer. Anat Rec (Hoboken) (2010) 293:1838–46. doi:10.1002/ar.21240
117. Skobe M, Hawighorst T, Jackson DG, Prevo R, Janes L, Velasco P, et al. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat Med (2001) 7:192–8. doi:10.1038/84643
118. Wang CA, Jedlicka P, Patrick AN, Micalizzi DS, Lemmer KC, Deitsch E, et al. SIX1 induces lymphangiogenesis and metastasis via upregulation of VEGF-C in mouse models of breast cancer. J Clin Invest (2012) 122:1895–906. doi:10.1172/JCI59858
119. Stacker SA, Caesar C, Baldwin ME, Thornton GE, Williams RA, Prevo R, et al. VEGF-D promotes the metastatic spread of tumor cells via the lymphatics. Nat Med (2001) 7:186–91. doi:10.1038/84635
120. Katoh H, Hosono K, Ito Y, Suzuki T, Ogawa Y, Kubo H, et al. COX-2 and prostaglandin EP3/EP4 signaling regulate the tumor stromal proangiogenic microenvironment via CXCL12-CXCR4 chemokine systems. Am J Pathol (2010) 176:1469–83. doi:10.2353/ajpath.2010.090607
121. Su JL, Shih JY, Yen ML, Jeng YM, Chang CC, Hsieh CY, et al. Cyclooxygenase-2 induces EP1- and HER-2/Neu-dependent vascular endothelial growth factor-C up-regulation: a novel mechanism of lymphangiogenesis in lung adenocarcinoma. Cancer Res (2004) 64:554–64. doi:10.1158/0008-5472.CAN-03-1301
122. Mäkinen T, Veikkola T, Mustjoki S, Karpanen T, Catimel B, Nice EC, et al. Isolated lymphatic endothelial cells transduce growth, survival and migratory signals via the VEGF-C/D receptor VEGFR-3. EMBO J (2001) 20:4762–73. doi:10.1093/emboj/20.17.4762
123. Hoshida T, Isaka N, Hagendoorn J, di Tomaso E, Chen YL, Pytowski B, et al. Imaging steps of lymphatic metastasis reveals that vascular endothelial growth factor-C increases metastasis by increasing delivery of cancer cells to lymph nodes: therapeutic implications. Cancer Res (2006) 66:8065–75. doi:10.1158/0008-5472.CAN-06-1392
124. Quagliata L, Klusmeier S, Cremers N, Pytowski B, Harvey A, Pettis RJ, et al. Inhibition of VEGFR-3 activation in tumor-draining lymph nodes suppresses the outgrowth of lymph node metastases in the MT-450 syngeneic rat breast cancer model. Clin Exp Metastasis (2014) 31:351–65. doi:10.1007/s10585-013-9633-2
125. Fornetti J, Jindal S, Middleton KA, Borges VF, Schedin P. Physiological COX-2 expression in breast epithelium associates with COX-2 levels in ductal carcinoma in situ and invasive breast cancer in young women. Am J Pathol (2014) 184:1219–29. doi:10.1016/j.ajpath.2013.12.026
126. Lewis C, Pollard J. Distinct role of macrophages in different tumor microenvironments. Cancer Res (2006) 66(2):605–12. doi:10.1158/0008-5472.CAN-05-4005
127. Kurahara H, Takao S, Maemura K, Mataki Y, Kuwahata T, Maeda K, et al. M2-polarized tumor-associated macrophage infiltration of regional lymph nodes is associated with nodal lymphangiogenesis and occult nodal involvement in pN0 pancreatic cancer. Pancreas (2013) 42:155–9. doi:10.1097/MPA.0b013e318254f2d1
128. Mantovani A, Romero P, Palucka AK, Marincola FM. Tumour immunity: effector response to tumour and role of the microenvironment. Lancet (2008) 371:771–83. doi:10.1016/S0140-6736(08)60241-X
129. Burton JB, Priceman SJ, Sung JL, Brakenhielm E, An DS, Pytowski B, et al. Suppression of prostate cancer nodal and systemic metastasis by blockade of the lymphangiogenic axis. Cancer Res (2008) 68:7828–37. doi:10.1158/0008-5472.CAN-08-1488
130. Pytowski B, Goldman J, Persaud K, Wu Y, Witte L, Hicklin DJ, et al. Complete and specific inhibition of adult lymphatic regeneration by a novel VEGFR-3 neutralizing antibody. J Natl Cancer Inst (2005) 97:14–21. doi:10.1093/jnci/dji003
131. Roberts N, Kloos B, Cassella M, Podgrabinska S, Persaud K, Wu Y, et al. Inhibition of VEGFR-3 activation with the antagonistic antibody more potently suppresses lymph node and distant metastases than inactivation of VEGFR-2. Cancer Res (2006) 66:2650–7. doi:10.1158/0008-5472.CAN-05-1843
132. Saif WM, Knost JA, Chiorean EG, Kambhampati SRP, Yu D, Pytowski B, et al. Phase I study of anti-VEGF receptor-3 (VEGFR-3) monoclonal antibody (Mab) LY3022856/IMC-3C5 (3C5). J Clin Oncol (2015) 33.
133. Mross K, Frost A, Scheulen ME, Krauss J, Strumberg D, Schultheiss B, et al. Phase I study of telatinib (BAY 57-9352): analysis of safety, pharmacokinetics, tumor efficacy, and biomarkers in patients with colorectal cancer. Vasc Cell (2011) 3:16. doi:10.1186/2045-824X-3-16
Keywords: breast cancer, lymphangiogenesis, lymphatic metastasis, macrophages, postpartum
Citation: Borges VF, Elder AM and Lyons TR (2016) Deciphering Pro-Lymphangiogenic Programs during Mammary Involution and Postpartum Breast Cancer. Front. Oncol. 6:227. doi: 10.3389/fonc.2016.00227
Received: 30 August 2016; Accepted: 10 October 2016;
Published: 02 November 2016
Edited by:
Kara Louise Britt, Peter MacCallum Cancer Centre, AustraliaReviewed by:
Takayuki Ueno, Kyorin University, JapanCopyright: © 2016 Borges, Elder and Lyons. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Traci R. Lyons, dHJhY2kubHlvbnNAdWNkZW52ZXIuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.