
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol. , 02 May 2016
Sec. Women's Cancer
Volume 6 - 2016 | https://doi.org/10.3389/fonc.2016.00108
This article is part of the Research Topic Cancer Care Delivery and Women's Health View all 23 articles
High-grade serous carcinoma (HGSC) is the most common and aggressive histotype of epithelial ovarian cancer (EOC), and it is the predominant histotype associated with hereditary breast and ovarian cancer syndrome (HBOC). Mutations in BRCA1 and BRCA2 are responsible for most of the known causes of HBOC, while mutations in mismatch repair genes and several genes of moderate penetrance are responsible for the remaining known hereditary risk. Women with a history of familial ovarian cancer or with known germline mutations in highly penetrant genes are offered the option of risk-reducing surgery that involves the removal of the ovaries and fallopian tubes (salpingo-oophorectomy). Growing evidence now supports the fallopian tube epithelia as an etiological site for the development of HGSC and consequently, salpingectomy alone is emerging as a prophylactic option. This review discusses the site of origin of EOC, the rationale for risk-reducing salpingectomy in the high-risk population, and opportunities for salpingectomy in the low-risk population.
In 2015, approximately 22,000 women in the United States were diagnosed with ovarian cancer and 14,000 died from this devastating disease (1). Ovarian cancer is a heterogeneous disease that can be divided into three main types: sex cord stromal tumors, germ cell tumors, and epithelial ovarian cancer (EOC). EOC accounts for the vast majority of ovarian cancers and consists of different subtypes, namely, mucinous, endometrioid, clear cell, low-grade serous, and high-grade serous carcinoma (HGSC) (2). The various histotypes differ in epidemiology, etiology, and treatment. High-grade serous ovarian carcinoma is not only the most common subtype of EOC, accounting for 75% of cases, but also the most aggressive. Most women present at advanced stages (stage III or IV) at diagnosis, at which point the 5-year survival rate ranges between 20 and 40%. However, for patients with stage I disease, the 5-year survival rate exceeds 90% (3). Molecular and genetic data indicate that HGSC of the ovary may have a similar origin to HGSC of the fallopian tube and peritoneum, and therefore, it has been suggested that all the three be described collectively as HGSC (4). Of the patients diagnosed with a HGSC, 15–20% will have a known germline mutation in the highly penetrant homologous repair pathway genes, BRCA1 or BRCA2.
In the general population, the incidence of ovarian cancer is higher in white women than in women from other racial or ethnic groups, and survival rates at 12 years are better in Caucasian American women (38%) compared with African-American women (32%). Of interest, Hispanic women (43%) and Asian women (52%) have higher survival rates. It is estimated that about 1 in 500 Americans have a mutation in BRCA1 or BRCA2. The lifetime risk of developing ovarian cancer with germline mutations in BRCA1 and BRCA2 is 40–60 and 11–27%, respectively (5–9). The burden of hereditary breast and ovarian cancer syndrome (HBOC) was previously thought to be confined to white women, particularly those of Ashkenazi Jewish descent. However, recent studies of different immigrant populations in the United States and in their respective countries of origin have identified pockets of women who bare a similarly high genetic burden as the Ashkenazi Jewish population. Women of Bahamian heritage, for example, are estimated to have 27.1% of breast cancer cases due to BRCA mutations (10, 11). The ovarian cancer burden in these isolated high-risk populations is still unclear, but likely to be as high as those women of Ashkenazi descent. Other highly penetrant genes, such as PTEN and TP53, and moderately penetrant genes, such as PALB2, BRIP1, CHEK2, and ATM (12), are also associated with HBOC, albeit at lower frequencies than the prevalence of BRCA1 and BRCA2 mutations. Norquist et al. recently reported RAD51C, RAD51D, and BARD1 as additional genes mutated in the germline of invasive serous ovarian cancer patients (12, 13). These data suggest that despite the growing list of genes involved in ovarian cancer predisposition, 70–85% of the women diagnosed with HGSC have “sporadic” disease.
Ovarian cancer incidence and mortality among US women has declined in those aged 35–59 years due to earlier detection methods or changes in risk (3). Conversely, in Southern and Eastern Europe, there is a corresponding rise in incidence (14) as women reduce breastfeeding and have fewer children (decrease in parity), which are both known risk factors. A similar trend of increasing incidence is expected in low–middle income countries (14).
Screening methods with CA-125 and transvaginal ultrasound have proved mostly ineffective in decreasing mortality for sporadic HGSC and ovarian cancer in general (15, 16). Early detection has been and continues to be a challenge in ovarian cancer because the disease is habitually asymptomatic before peritoneal spread (17). However, with the identification of pockets of the population at high risk for HBOC, there is an opportunity to reduce the burden of disease through increased and targeted genetic testing as well as screening and prevention measures for ovarian cancer risk reduction.
Prior to the reported observation of in situ carcinoma in the distal end of the fallopian tube of women undergoing prophylactic surgery, the ovary was thought to be the etiological site of high-grade serous ovarian cancer. Now, there are two candidates for the cell of origin, namely, the fallopian tube epithelium (FTE) and the ovarian surface epithelium (OSE). Both share common mesodermal embryological origin and close anatomic proximity. The fallopian tube, along with the uterus, uterine endocervix, and superior aspect of the vagina are derived from an invagination of the celom known as the Mullerian or paramesonephric ducts. The OSE is derived from the mesothelial celomic epithelium that lines the primitive ovary (18).
The “incessant ovulation” hypothesis, proposed by Fathalla (19), suggested that continuous ovulatory cycles during the reproductive lifespan of a woman increase her risk of developing HGSC (19). He proposed that ovulation resulted in an increase in inflammation through which the secretion of cytokines, chemokines, bradykines, and hormones induce DNA damage via oxidative stress in the cortical inclusion cysts (CIC) observed in the ovary. These events, along with proliferation of the OSE, promote metaplastic changes leading to neoplastic transformation (2, 15, 19).
Xenografts of transformed OSE cell lines and genetic animal models have been used in an attempt to model HGSC in the absence of in situ pre-neoplastic lesions in the ovary. Genetic mouse models deleting BRCA1, Rb1, and TP53 genes from the OSE resulted in leiomyosarcomas (20) and not HGSC. In contrast, targeted deletion of these genes in the fallopian tube epithelia of mice has led to the development of tumors genomically and pathologically similar to HGSC (21). The somatic mutational spectrum found in lesions associated with the ovary proper and neoplastic lesions have been shown to have KRAS, BRAF, CTNNB1, ARID1A, PTEN, PPP2R1A, and PIK3CA (22). These tumors rarely have TP53 mutations, which suggest a distinct etiology and natural history of tumorigenesis from that of HGSC.
There is now substantial convincing clinical and molecular evidence in support of the FTE as the source of the cell of origin of low- and high-grade serous ovarian cancer (22). Experimental in vitro manipulation and transformation of human fallopian tube epithelial cells have demonstrated that these cells in a xenograft model can give rise to tumors, which resemble primary HGSC (23). Additionally, mouse models targeting BRCA and TP53 in fallopian tube epithelia develop HGSC (21).
A series of transcriptional studies by Tone et al. and George et al. have shown that the phenotypically normal fallopian tube epithelia from BRCA1 and BRCA2 mutation carriers show transcriptional differences when compared to epithelial cells with a normal BRCA genotype. These differences have been shown to impact different molecular pathways. Consequently, these pathways are implicated in tumor initiation, progression, and recurrence (24–26). As a result of these studies, the authors proposed that chronic inflammatory states through cyclical ovulation in the presence of a mutated BRCA allele could predispose the normal FTE to undergo neoplastic transformation, which may lead to serous carcinoma. This would primarily occur through deregulation of DNA damage response genes and synergistically through upregulation of cytokines, proinflammatory and proliferation genes.
The BRCA-associated carcinomas share some common genomic features such as frequent mutations of TP53 and copy number landscape features including Cyclin-E1 amplification and deletion of Rb1 (27). Altered BRCA function is not unique to hereditary HGSC but is prevalent via somatic mutations (6%) (28–31), promoter hypermethylation (13–31%) (28, 32–34), and other genetic or epigenetic alterations, predominantly in the homologous recombination (HR) pathway in HGSC. This has led to determining the “BRCAness” profile in patients (35, 36). Overall, these differences in morphologically normal epithelia from BRCA mutation carriers have shed light into the effects of heterozygosity and predisposition to the development of HGSC and, importantly, potential features that might be manifested in the STIC.
Detailed histopathological examination of tubal epithelia in BRCA mutation carriers undergoing risk-reducing surgery led to the discovery of putative cancer precursor lesions in the fallopian tube referred to as serous tubal intraepithelial carcinoma (STIC) (37–40). STIC was first reported by Piek et al., who described dysplastic epithelial changes in the fallopian tubes of women with a BRCA1 or BRCA2 mutation, who underwent risk-reducing salpingo-oophorectomies (RRSO) (38, 41). These lesions have distinct morphological features such as loss of polarity, epithelial tufting, and pleomorphic nuclei, and in addition, there is abnormal p53 expression and a high-proliferative index (refer to Lheureux et al. for commentary) (2, 42).
Since the discovery of the STIC, three possible pre-neoplastic lesions have been described, including the p53-signature, low grade serous tubal intraepithelial lesions (STIL), and secretory cell outgrowths (SCOUTS) (43). These lesions share a combination of phenotypic and/or genomic alterations with the cancer cells in HGSC. TP53 mutations, which are ubiquitous in HGSC, are usually concomitantly found in STIC and HGSC (2, 44). Over-expression of p16 has been documented in some STILS and over-expression of Pax8, Bcl-2, and loss of Pax2 expression has been observed in SCOUTS (45). However, none of these lesions are clinically actionable, as it is still unclear which of these lesions and/or combination of genomic alterations, has the pathogenic capacity to give rise to a carcinoma.
Many studies have now reported the incidence of non-invasive neoplastic lesions (STIC) in the distal end of the fallopian tube. It is estimated that occult invasive and STIC are identified in 0.9–8.5% of women undergoing RRSOs (2, 39, 40, 43, 46–63) (Table 1). The frequency of STIC lesions increases with age and is lower with oral contraceptive use (64). It is important to note that the large range in estimates of the prevalence of occult and STIC lesions is reflective of the variances in diagnostic methodologies used by different centers and study groups (42).
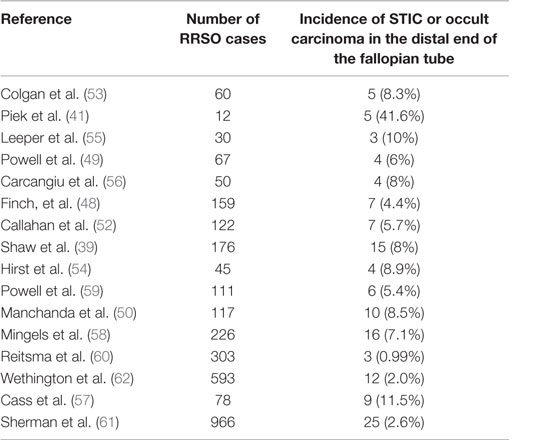
Table 1. Evidence of serous tubal intraepithelial carcinoma in risk-reducing salpingo-oophorectomy of asymptomatic women with known BRCA1 or BRCA2 mutations or strong family history of breast or ovarian cancer.
Powell and colleagues reported that in a long-term follow-up study of women diagnosed with non-invasive serous tubal epithelial carcinoma who underwent RRSO, 6% (1/17) recurred 43 months after risk-reducing surgery compared to 43% of women who had unsuspected invasive carcinoma at time of surgery (65). There is a continued need to understand the effects of inflammation and hormones on the fallopian tube epithelia, relating to latency and the preferential sites of seeding, are critical for addressing prevention and risk-reduction strategies in genetically high-risk populations.
Epidemiological data show that oral contraceptives, aspirin, and other non-steroidal anti-inflammatory drugs reduce the risk of ovarian cancer. In a meta-analysis as a primary prevention mechanism by Havrilesky et al., oral contraceptive pills use reduced ovarian cancer risk by 50% if used for more than 10 years (66). Recently, aspirin use was associated with a reduced risk of ovarian cancer, especially among daily users of low-dose aspirin (67). These observations highlight the relationship between ovulation and its inflammatory accompaniment with ovarian cancer development. Women identified at highest risk, that is, germline mutation carriers and/or strong family history of ovarian cancer, may benefit from use of these chemoprevention strategies.
Tubal ligation (tubal sterilization) has been shown to reduce ovarian cancer risk that theoretically is spread through retrograde menstrual flow (68–70). In particular, tubal ligation was associated with reduced risk of invasive ovarian cancer, with the greatest benefit seen in the endometrioid and clear cell subtypes (71). The mechanism of protection is through prevention of retrograde menstruation, and hence, a decrease in Fenton’s reaction (generates reactive oxidative species) in the environment of the fallopian tube as well as prevention of endometrial cells implanting in the ovary. Although tubal ligation appears to be protective for all histotypes of ovarian cancer, it is least effective in reducing risk for the most lethal subtype, HGSC (71).
As early as 2002, Rebbeck et al. suggested that bilateral prophylactic oophorectomies reduced the risk of ovarian and breast cancer in women with BRCA1 or BRCA2 mutations by as much as 96% (72). Olivier et al. demonstrated that risk-reducing salpingo-oophorectomy reduced the risk of ovarian, fallopian tube, and peritoneal papillary serous carcinoma in BRCA1 and BRCA2 mutation carriers (some women still developed peritoneal disease) (73).
As previously mentioned, there is clear evidence supporting the role of the fallopian tube as the etiological site of HGSC [and most likely low-grade serous carcinoma (22)]. For this reason, women with known risk for breast and ovarian cancer may undergo prophylactic surgical removal of the ovaries and fallopian tubes, a procedure known as RRSO. Current guidelines from the National Comprehensive Cancer Network and the Society of Gynecologic Oncologists suggest that RRSO to be completed by the post-child bearing period, the age of 35–40, or 10 years younger than a first-degree relative diagnosed with ovarian cancer (74). However, it is believed that the majority of these high-risk women do not undergo RRSO by age 40 (75). This modality of precision prevention involves risk stratification and risk reduction in patients carrying both highly penetrant (76) (BRCA1 and BRCA2) and moderate to lower penetrant genes such as PTEN, PALB2, CHEK2, ATM, and BRIP1. The removal of the fallopian tubes alone is referred to as risk-reducing salpingectomy (RRS). In young women identified with a BRCA1 or BRCA2 mutation, RRS is performed in an effort to reduce ovarian cancer risk while maintaining adequate hormonal levels to avoid the effects of early menopause. This latter approach, however, is not restricted to women at high risk for serous ovarian cancer, as it will also have a beneficial impact on reducing the risk of development of endometriosis-associated clear cell and endometrioid ovarian cancer (Figure 1).
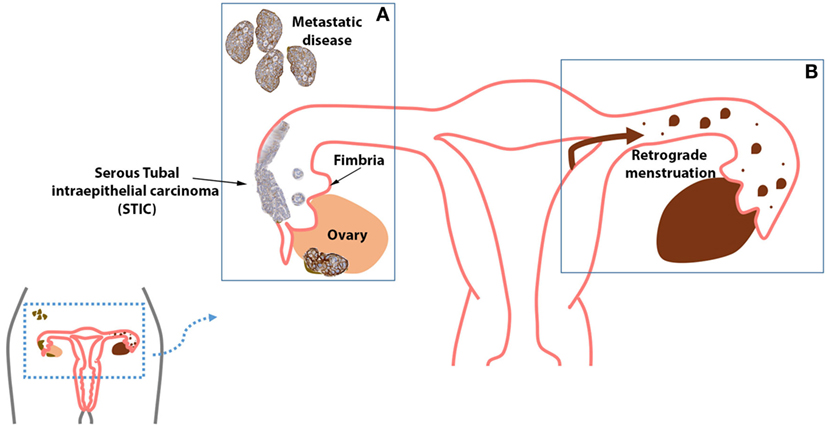
Figure 1. (A) High-grade serous carcinoma (HGSC) is the most common histologic type of cancer seen in the ovaries, fallopian tube, and peritoneum. (B) The fallopian tubes may act as a possible conduit for retrograde menstrual flow, which is theorized to induce the malignant transformation of cells via oxidative stress, inflammation, and hyperestrogenism. Further, endometriosis has been strongly linked with endometrioid ovarian carcinoma and clear cell ovarian carcinoma.
Opportunistic salpingectomy refers to removal of the fallopian tubes in women who are not at an increased risk of developing ovarian cancer. In 2010, a population-based and institution-wide study in British Columbia, Canada, was initiated whereby three recommendations to gynecological surgeons were made: (1) consider opportunistic salpingectomy during hysterectomy, (2) consider excisional bilateral salpingectomy rather than tubal ligation for sterilization, and (3) refer all HGSC patients for BRCA1/2 germline testing (77). Interim results on surgical outcomes revealed that the rates of hysterectomy with bilateral salpingectomy increased 3.5-fold compared to hysterectomy alone, and the rates of tubal ligation as a mode of surgical sterility decreased from 99.7% in 2009 to 66.7% in 2011, while the rate of bilateral salpingectomy concomitantly increased 111-fold compared to 2009 rates (77). The authors also reported that the length of hospitalization post-hysterectomy and bilateral salpingectomy was not longer than for hysterectomy alone and that there was no significant difference in the rate of blood transfusion or hospital readmission among these two groups. In addition, there was no significant difference in length of hospitalization or rate of transfusion for bilateral salpingectomy compared to tubal ligation.
The caveat to opportunistic salpingectomy is that even if implemented on a large scale, the true impact of ovarian cancer reduction will take years to be realized (77). It is also important to note that salpingectomy alone, unlike oophorectomy, does not reduce the risk of breast cancer by modulating levels of estrogen.
There is categorical evidence that RRSO reduces ovarian and breast cancer death and all-cause mortality (78, 79). There is currently no evidence that points to the outcome and impact of ovarian cancer risk reduction for two-stage procedure of salpingectomy followed by oophorectomy. In the United States, MD Anderson is conducting a clinical trial assessing prophylactic salpingectomy with delayed oophorectomy (80). A report from the Nurses’ Health Study concluded that compared with ovarian conservation, bilateral oophorectomy at the time of hysterectomy for benign disease was associated with a decreased risk of breast and ovarian cancer but an increased risk of all-cause mortality (81, 82); therefore, one can stipulate that salpingectomy alone may be sufficient in the genetically “low-risk” population, while the overall benefit versus harm of these approaches requires close attention in the genetically high-risk population. Specifically, oophorectomy offers protection against breast cancer even after menopause and improves survival in those with breast cancer (83). As these prevention modalities are implemented, it is important that the goal of decreasing the incidence and burden of ovarian cancer is not at the expense of worsening the incidence and mortality of breast cancer in women who are at increased risk due to co-morbidities.
SG wrote and conceptualized. RG researched references and made figure. BS wrote and conceptualized.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to thank the Sylvester Comprehensive Cancer Center for funding and the Foundation for Women’s Cancer – Belinda Sue/Mary-Jane Welker Fund for Ovarian Cancer Early Detection Research.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin (2015) 65(1):5–29. doi: 10.3322/caac.21254
2. George SH, Shaw P. BRCA and early events in the development of serous ovarian cancer. Front Oncol (2014) 4:5. doi:10.3389/fonc.2014.00005
3. Sopik V, Iqbal J, Rosen B, Narod SA. Why have ovarian cancer mortality rates declined? Part I. Incidence. Gynecol Oncol (2015) 138(3):741–9. doi:10.1016/j.ygyno.2015.06.017
4. Berek JS, Crum C, Friedlander M. Cancer of the ovary, fallopian tube, and peritoneum. Int J Gynaecol Obstet (2015) 131(Suppl 2):S111–22. doi:10.1016/j.ijgo.2015.06.007
5. Sogaard M, Kjaer SK, Gayther S. Ovarian cancer and genetic susceptibility in relation to the BRCA1 and BRCA2 genes. Occurrence, clinical importance and intervention. Acta Obstet Gynecol Scand (2006) 85(1):93–105. doi:10.1080/00016340500324621
6. Antoniou A, Pharoah PD, Narod S, Risch HA, Eyfjord JE, Hopper JL, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet (2003) 72(5):1117–30. doi:10.1086/375033
7. Foulkes WD, Narod SA. Ovarian cancer risk and family history. Lancet (1997) 349(9055):878. doi:10.1016/S0140-6736(05)61782-5
8. King MC, Marks JH, Mandell JB, New York Breast Cancer Study Group. Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science (2003) 302(5645):643–6. doi:10.1126/science.1088759
9. Easton DF, Ford D, Bishop DT. Breast and ovarian cancer incidence in BRCA1-mutation carriers. Breast Cancer Linkage Consortium. Am J Hum Genet (1995) 56(1):265–71.
10. Akbari MR, Donenberg T, Lunn J, Curling D, Turnquest T, Krill-Jackson E, et al. The spectrum of BRCA1 and BRCA2 mutations in breast cancer patients in the Bahamas. Clin Genet (2014) 85(1):64–7. doi:10.1111/cge.12132
11. Donenberg T, Lunn J, Curling D, Turnquest T, Krill-Jackson E, Royer R, et al. A high prevalence of BRCA1 mutations among breast cancer patients from the Bahamas. Breast Cancer Res Treat (2011) 125(2):591–6. doi:10.1007/s10549-010-1156-9
12. Walsh T, Casadei S, Lee MK, Pennil CC, Nord AS, Thornton AM, et al. Mutations in 12 genes for inherited ovarian, fallopian tube, and peritoneal carcinoma identified by massively parallel sequencing. Proc Natl Acad Sci U S A (2011) 108(44):18032–7. doi:10.1073/pnas.1115052108
13. Norquist BM, Harrell MI, Brady MF, et al. Inherited mutations in women with ovarian carcinoma. JAMA Oncol (2016) 2(4):482–90. doi:10.1001/jamaoncol.2015.5495
14. Chornokur G, Amankwah EK, Schildkraut JM, Phelan CM. Global ovarian cancer health disparities. Gynecol Oncol (2013) 129(1):258–64. doi:10.1016/j.ygyno.2012.12.016
15. Fathalla MF. Incessant ovulation and ovarian cancer – a hypothesis re-visited. Facts Views Vis Obgyn (2013) 5(4):292–7.
16. Moyer VA, Force USPST. Screening for ovarian cancer: U.S. Preventive Services Task Force reaffirmation recommendation statement. Ann Intern Med (2012) 157(12):900–4. doi:10.7326/0003-4819-157-11-201212040-00539
17. Bowtell DD, Böhm S, Ahmed AA, Aspuria PJ, Bast RC Jr, Beral V, et al. Rethinking ovarian cancer II: reducing mortality from high-grade serous ovarian cancer. Nat Rev Cancer (2015) 15(11):668–79. doi:10.1038/nrc4019
18. Dubeau L. The cell of origin of ovarian epithelial tumours. Lancet Oncol (2008) 9(12):1191–7. doi:10.1016/S1470-2045(08)70308-5
19. Fathalla MF. Incessant ovulation – a factor in ovarian neoplasia? Lancet (1971) 2(7716):163. doi:10.1016/S0140-6736(71)92335-X
20. Clark-Knowles KV, Senterman MK, Collins O, Vanderhyden BC. Conditional inactivation of Brca1, p53 and Rb in mouse ovaries results in the development of leiomyosarcomas. PLoS One (2009) 4(12):e8534. doi:10.1371/journal.pone.0008534
21. Perets R, Wyant GA, Muto KW, Bijron JG, Poole BB, Chin KT, et al. Transformation of the fallopian tube secretory epithelium leads to high-grade serous ovarian cancer in Brca;Tp53;Pten models. Cancer Cell (2013) 24(6):751–65. doi:10.1016/j.ccr.2013.10.013
22. Vang R, Shih IeM, Kurman RJ. Fallopian tube precursors of ovarian low- and high-grade serous neoplasms. Histopathology (2013) 62(1):44–58. doi:10.1111/his.12046
23. Karst AM, Levanon K, Drapkin R. Modeling high-grade serous ovarian carcinogenesis from the fallopian tube. Proc Natl Acad Sci U S A (2011) 108(18):7547–52. doi:10.1073/pnas.1017300108
24. George SH, Greenaway J, Milea A, Clary V, Shaw S, Sharma M, et al. Identification of abrogated pathways in fallopian tube epithelium from BRCA1 mutation carriers. J Pathol (2011) 225(1):106–17. doi:10.1002/path.2927
25. Tone AA, Begley H, Sharma M, Murphy J, Rosen B, Brown TJ, et al. Gene expression profiles of luteal phase fallopian tube epithelium from BRCA mutation carriers resemble high-grade serous carcinoma. Clin Cancer Res (2008) 14(13):4067–78. doi:10.1158/1078-0432.CCR-07-4959
26. Tone AA, Virtanen C, Shaw P, Brown TJ. Prolonged postovulatory proinflammatory signaling in the fallopian tube epithelium may be mediated through a BRCA1/DAB2 axis. Clin Cancer Res (2012) 18(16):4334–44. doi:10.1158/1078-0432.CCR-12-0199
27. Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature (2012) 490(7418):61–70.
28. TCGA. Integrated genomic analyses of ovarian carcinoma. Nature (2011) 474(7353):609–15. doi:10.1038/nature10166
29. Berchuck A, Heron KA, Carney ME, Lancaster JM, Fraser EG, Vinson VL, et al. Frequency of germline and somatic BRCA1 mutations in ovarian cancer. Clin Cancer Res (1998) 4(10):2433–7.
30. Foster KA, Harrington P, Kerr J, Russell P, DiCioccio RA, Scott IV, et al. Somatic and germline mutations of the BRCA2 gene in sporadic ovarian cancer. Cancer Res (1996) 56(16):3622–5.
31. Merajver SD, Pham TM, Caduff RF, Chen M, Poy EL, Cooney KA, et al. Somatic mutations in the BRCA1 gene in sporadic ovarian tumours. Nat Genet (1995) 9(4):439–43. doi:10.1038/ng0495-439
32. Esteller M, Silva JM, Dominguez G, Bonilla F, Matias-Guiu X, Lerma E, et al. Promoter hypermethylation and BRCA1 inactivation in sporadic breast and ovarian tumors. J Natl Cancer Inst (2000) 92(7):564–9. doi:10.1093/jnci/92.7.564
33. Chiang JW, Karlan BY, Cass L, Baldwin RL. BRCA1 promoter methylation predicts adverse ovarian cancer prognosis. Gynecol Oncol (2006) 101(3):403–10. doi:10.1016/j.ygyno.2005.10.034
34. Press JZ, De Luca A, Boyd N, Young S, Troussard A, Ridge Y, et al. Ovarian carcinomas with genetic and epigenetic BRCA1 loss have distinct molecular abnormalities. BMC Cancer (2008) 8:17. doi:10.1186/1471-2407-8-17
35. Turner N, Tutt A, Ashworth A. Hallmarks of ‘BRCAness’ in sporadic cancers. Nat Rev Cancer (2004) 4(10):814–9. doi:10.1038/nrc1457
36. Bast RC Jr, Mills GB. Personalizing therapy for ovarian cancer: BRCAness and beyond. J Clin Oncol (2010) 28(22):3545–8. doi:10.1200/JCO.2010.28.5791
37. Folkins AK, Jarboe EA, Saleemuddin A, Lee Y, Callahan MJ, Drapkin R, et al. A candidate precursor to pelvic serous cancer (p53 signature) and its prevalence in ovaries and fallopian tubes from women with BRCA mutations. Gynecol Oncol (2008) 109(2):168–73. doi:10.1016/j.ygyno.2008.01.012
38. Piek JM, van Diest PJ, Zweemer RP, Jansen JW, Poort-Keesom RJ, Menko FH, et al. Dysplastic changes in prophylactically removed fallopian tubes of women predisposed to developing ovarian cancer. J Pathol (2001) 195(4):451–6. doi:10.1002/path.1000
39. Shaw PA, Rouzbahman M, Pizer ES, Pintilie M, Begley H. Candidate serous cancer precursors in fallopian tube epithelium of BRCA1/2 mutation carriers. Mod Pathol (2009) 22(9):1133–8. doi:10.1038/modpathol.2009.89
40. Lee Y, Miron A, Drapkin R, Nucci MR, Medeiros F, Saleemuddin A, et al. A candidate precursor to serous carcinoma that originates in the distal fallopian tube. J Pathol (2007) 211(1):26–35. doi:10.1002/path.2091
41. Piek JM, Dorsman JC, Zweemer RP, Verheijen RH, van Diest PJ, Colgan TJ. Women harboring BRCA1/2 germline mutations are at risk for breast and female adnexal carcinoma. Int J Gynecol Pathol (2003) 22(3):315–6. doi:10.1097/01.PGP.0000079451.72325.63 author reply-6
42. Lheureux S, Shaw PA, Karakasis K, Oza AM. Cancer precursor lesions in the BRCA population at the time of prophylactic salpingo-oophorectomy: accuracy of assessment and potential surrogate marker for prevention. Gynecol Oncol (2015) 138(2):235–7. doi:10.1016/j.ygyno.2015.06.014
43. Chen EY, Mehra K, Mehrad M, Ning G, Miron A, Mutter GL, et al. Secretory cell outgrowth, PAX2 and serous carcinogenesis in the fallopian tube. J Pathol (2010) 222:110–6. doi:10.1002/path.2739
44. Kuhn E, Kurman RJ, Vang R, Sehdev AS, Han G, Soslow R, et al. TP53 mutations in serous tubal intraepithelial carcinoma and concurrent pelvic high-grade serous carcinoma – evidence supporting the clonal relationship of the two lesions. J Pathol (2012) 226(3):421–6. doi:10.1002/path.3023
45. Milea A, George SH, Matevski D, et al. Retinoblastoma pathway deregulatory mechanisms determine clinical outcome in high-grade serous ovarian carcinoma. Mod Pathol (2014) 27(7):991–1001. doi:10.1038/modpathol.2013.218
46. Lee Y, Medeiros F, Kindelberger D, Callahan MJ, Muto MG, Crum CP. Advances in the recognition of tubal intraepithelial carcinoma: applications to cancer screening and the pathogenesis of ovarian cancer. Adv Anat Pathol (2006) 13(1):1–7. doi:10.1097/01.pap.0000201826.46978.e5
47. Medeiros F, Muto MG, Lee Y, Elvin JA, Callahan MJ, Feltmate C, et al. The tubal fimbria is a preferred site for early adenocarcinoma in women with familial ovarian cancer syndrome. Am J Surg Pathol (2006) 30(2):230–6. doi:10.1097/01.pas.0000180854.28831.77
48. Finch A, Shaw P, Rosen B, Murphy J, Narod S, Colgan T. Clinical and pathologic findings of prophylactic salpingo-oophorectomies in 159 BRCA1 and BRCA2 carriers. Gynecol Oncol (2006) 100(1):58–64. doi:10.1016/j.ygyno.2005.06.065
49. Powell CB, Kenley E, Chen LM, Crawford B, McLennan J, Zaloudek C, et al. Risk-reducing salpingo-oophorectomy in BRCA mutation carriers: role of serial sectioning in the detection of occult malignancy. J Clin Oncol (2005) 23(1):127–32. doi:10.1200/JCO.2005.04.109
50. Manchanda R, Abdelraheim A, Johnson M, Rosenthal AN, Benjamin E, Brunell C, et al. Outcome of risk-reducing salpingo-oophorectomy in BRCA carriers and women of unknown mutation status. BJOG (2011) 118(7):814–24. doi:10.1111/j.1471-0528.2011.02920.x
51. Conner JR, Meserve E, Pizer E, Garber J, Roh M, Urban N, et al. Outcome of unexpected adnexal neoplasia discovered during risk reduction salpingo-oophorectomy in women with germ-line BRCA1 or BRCA2 mutations. Gynecol Oncol (2014) 132(2):280–6. doi:10.1016/j.ygyno.2013.12.009
52. Callahan MJ, Crum CP, Medeiros F, Kindelberger DW, Elvin JA, Garber JE, et al. Primary fallopian tube malignancies in BRCA-positive women undergoing surgery for ovarian cancer risk reduction. J Clin Oncol (2007) 25(25):3985–90. doi:10.1200/JCO.2007.12.2622
53. Colgan T, Murphy J, Cole D, Narod S, Rosen B. Occult carcinoma in prophylactic oophorectomy specimens: prevalence and association with BRCA germline mutation status. Am J Surg Pathol (2001) 25(10):1283–9. doi:10.1097/00000478-200110000-00009
54. Hirst JE, Gard GB, McIllroy K, Nevell D, Field M. High rates of occult fallopian tube cancer diagnosed at prophylactic bilateral salpingo-oophorectomy. Int J Gynecol Cancer (2009) 19(5):826–9. doi:10.1111/IGC.0b013e3181a1b5dc
55. Leeper K, Garcia R, Swisher E, Goff B, Greer B, Paley P. Pathologic findings in prophylactic oophorectomy specimens in high-risk women. Gynecol Oncol (2002) 87(1):52–6. doi:10.1006/gyno.2002.6779
56. Carcangiu ML, Peissel B, Pasini B, Spatti G, Radice P, Manoukian S. Incidental carcinomas in prophylactic specimens in BRCA1 and BRCA2 germ-line mutation carriers, with emphasis on fallopian tube lesions: report of 6 cases and review of the literature. Am J Surg Pathol (2006) 30(10):1222–30. doi:10.1097/01.pas.0000202161.80739.ac
57. Cass I, Walts AE, Barbuto D, Lester J, Karlan B. A cautious view of putative precursors of serous carcinomas in the fallopian tubes of BRCA mutation carriers. Gynecol Oncol (2014) 134(3):492–7. doi:10.1016/j.ygyno.2014.07.084
58. Mingels MJ, Roelofsen T, van der Laak JA, de Hullu JA, van Ham MA, Massuger LF, et al. Tubal epithelial lesions in salpingo-oophorectomy specimens of BRCA-mutation carriers and controls. Gynecol Oncol (2012) 127(1):88–93. doi:10.1016/j.ygyno.2012.06.015
59. Powell CB, Chen LM, McLennan J, Crawford B, Zaloudek C, Rabban JT, et al. Risk-reducing salpingo-oophorectomy (RRSO) in BRCA mutation carriers: experience with a consecutive series of 111 patients using a standardized surgical-pathological protocol. Int J Gynecol Cancer (2011) 21(5):846–51. doi:10.1097/IGC.0b013e31821bc7e3
60. Reitsma W, de Bock GH, Oosterwijk JC, Bart J, Hollema H, Mourits MJ. Support of the ‘fallopian tube hypothesis’ in a prospective series of risk-reducing salpingo-oophorectomy specimens. Eur J Cancer (2013) 49(1):132–41. doi:10.1016/j.ejca.2012.07.021
61. Sherman ME, Piedmonte M, Mai PL, Ioffe OB, Ronnett BM, Van Le L, et al. Pathologic findings at risk-reducing salpingo-oophorectomy: primary results from gynecologic oncology group trial GOG-0199. J Clin Oncol (2014) 32(29):3275–83. doi:10.1200/JCO.2013.54.1987
62. Wethington SL, Park KJ, Soslow RA, Kauff ND, Brown CL, Dao F, et al. Clinical outcome of isolated serous tubal intraepithelial carcinomas (STIC). Int J Gynecol Cancer (2013) 23(9):1603–11. doi:10.1097/IGC.0b013e3182a80ac8
63. Jarboe E, Folkins A, Drapkin R, Ince T, Agoston E, Crum C. Tubal and ovarian pathways to pelvic epithelial cancer: a pathological perspective. Histopathology (2008) 53:127–38. doi:10.1111/j.1365-2559.2007.02938.x
64. Vicus D, Shaw PA, Finch A, Rosen B, Murphy J, Armel S, et al. Risk factors for non-invasive lesions of the fallopian tube in BRCA mutation carriers. Gynecol Oncol (2010) 118(3):295–8. doi:10.1016/j.ygyno.2010.05.012
65. Powell CB, Swisher EM, Cass I, McLennan J, Norquist B, Garcia RL, et al. Long term follow up of BRCA1 and BRCA2 mutation carriers with unsuspected neoplasia identified at risk reducing salpingo-oophorectomy. Gynecol Oncol (2013) 129(2):364–71. doi:10.1016/j.ygyno.2013.01.029
66. Havrilesky LJ, Moorman PG, Lowery WJ, Gierisch JM, Coeytaux RR, Urrutia RP, et al. Oral contraceptive pills as primary prevention for ovarian cancer: a systematic review and meta-analysis. Obstet Gynecol (2013) 122(1):139–47. doi:10.1097/AOG.0b013e318291c235
67. Trabert B, Ness RB, Lo-Ciganic WH, Murphy MA, Goode EL, Poole EM, et al. Aspirin, nonaspirin nonsteroidal anti-inflammatory drug, and acetaminophen use and risk of invasive epithelial ovarian cancer: a pooled analysis in the Ovarian Cancer Association Consortium. J Natl Cancer Inst (2014) 106(2):djt431. doi:10.1093/jnci/djt431
68. Rice MS, Murphy MA, Tworoger SS. Tubal ligation, hysterectomy and ovarian cancer: a meta-analysis. J Ovarian Res (2012) 5(1):13. doi:10.1186/1757-2215-5-13
69. Cibula D, Widschwendter M, Majek O, Dusek L. Tubal ligation and the risk of ovarian cancer: review and meta-analysis. Hum Reprod Update (2011) 17(1):55–67. doi:10.1093/humupd/dmq030
70. Narod SA, Sun P, Ghadirian P, Lynch H, Isaacs C, Garber J, et al. Tubal ligation and risk of ovarian cancer in carriers of BRCA1 or BRCA2 mutations: a case-control study. Lancet (2001) 357(9267):1467–70. doi:10.1016/S0140-6736(00)04642-0
71. Sieh W, Salvador S, McGuire V, Weber RP, Terry KL, Rossing MA, et al. Tubal ligation and risk of ovarian cancer subtypes: a pooled analysis of case-control studies. Int J Epidemiol (2013) 42(2):579–89. doi:10.1093/ije/dyt042
72. Rebbeck TR, Lynch HT, Neuhausen SL, Narod SA, Van’t Veer L, Garber JE, et al. Prophylactic oophorectomy in carriers of BRCA1 or BRCA2 mutations. N Engl J Med (2002) 346(21):1616–22. doi:10.1056/NEJMoa012158
73. Olivier RI, van Beurden M, Lubsen MA, Rookus MA, Mooij TM, van de Vijver MJ, et al. Clinical outcome of prophylactic oophorectomy in BRCA1/BRCA2 mutation carriers and events during follow-up. Br J Cancer (2004) 90(8):1492–7. doi:10.1038/sj.bjc.6601692
74. Finch AP, Lubinski J, Møller P, Singer CF, Karlan B, Senter L, et al. Impact of oophorectomy on cancer incidence and mortality in women with a BRCA1 or BRCA2 mutation. J Clin Oncol (2014) 32(15):1547–53. doi:10.1200/JCO.2013.53.2820
75. Garcia C, Lyon L, Littell RD, Powell CB. Comparison of risk management strategies between women testing positive for a BRCA variant of unknown significance and women with known BRCA deleterious mutations. Genet Med (2014) 16(12):896–902. doi:10.1038/gim.2014.48
76. Kensler TW, Spira A, Garber JE, Szabo E, Lee JJ, Dong Z, et al. Transforming cancer prevention through precision medicine and immune-oncology. Cancer Prev Res (Phila) (2016) 9(1):2–10. doi:10.1158/1940-6207.CAPR-15-0406
77. McAlpine JN, Hanley GE, Woo MM, Tone AA, Rozenberg N, Swenerton KD, et al. Opportunistic salpingectomy: uptake, risks, and complications of a regional initiative for ovarian cancer prevention. Am J Obstet Gynecol (2014) 210(5):471.e1–11. doi:10.1016/j.ajog.2014.01.003
78. Domchek SM, Friebel TM, Singer CF, Evans DG, Lynch HT, Isaacs C, et al. Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA (2010) 304(9):967–75. doi:10.1001/jama.2010.1237
79. Rebbeck TR, Kauff ND, Domchek SM. Meta-analysis of risk reduction estimates associated with risk-reducing salpingo-oophorectomy in BRCA1 or BRCA2 mutation carriers. J Natl Cancer Inst (2009) 101(2):80–7. doi:10.1093/jnci/djn442
80. Holman LL, Friedman S, Daniels MS, Sun CC, Lu KH. Acceptability of prophylactic salpingectomy with delayed oophorectomy as risk-reducing surgery among BRCA mutation carriers. Gynecol Oncol (2014) 133(2):283–6. doi:10.1016/j.ygyno.2014.02.030
81. Parker WH, Feskanich D, Broder MS, Chang E, Shoupe D, Farquhar CM, et al. Long-term mortality associated with oophorectomy compared with ovarian conservation in the nurses’ health study. Obstet Gynecol (2013) 121(4):709–16. doi:10.1097/AOG.0b013e3182864350
82. Parker WH, Broder MS, Chang E, Feskanich D, Farquhar C, Liu Z, et al. Ovarian conservation at the time of hysterectomy and long-term health outcomes in the nurses’ health study. Obstet Gynecol (2009) 113(5):1027–37. doi:10.1097/AOG.0b013e3181a11c64
Keywords: salpingectomy, BRCA1, BRCA2, ovarian cancers, fallopian tubes
Citation: George SHL, Garcia R and Slomovitz BM (2016) Ovarian Cancer: The Fallopian Tube as the Site of Origin and Opportunities for Prevention. Front. Oncol. 6:108. doi: 10.3389/fonc.2016.00108
Received: 25 January 2016; Accepted: 18 April 2016;
Published: 02 May 2016
Edited by:
Sarah M. Temkin, National Cancer Institute, USAReviewed by:
Reuven Reich, Hebrew University of Jerusalem, IsraelCopyright: © 2016 George, Garcia and Slomovitz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sophia H. L. George, c29waGlhLmdlb3JnZUBtZWQubWlhbWkuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.