- 1Department of Radiation Oncology (MAASTRO Lab), GROW – School for Oncology and Developmental Biology, Maastricht University Medical Centre, Maastricht, Netherlands
- 2Department of Radiation Oncology, MAASTRO Clinic, Maastricht, Netherlands
Hypoxia is a characteristic of many solid tumors and an adverse prognostic factor for treatment outcome. Hypoxia increases the expression of carbonic anhydrase IX (CAIX), an enzyme that is predominantly found on tumor cells and is involved in maintaining the cellular pH balance. Many clinical studies investigated the prognostic value of CAIX expression, but most have been inconclusive, partly due to small numbers of patients included. The present meta-analysis was therefore performed utilizing the results of all clinical studies to determine the prognostic value of CAIX expression in solid tumors. Renal cell carcinoma was excluded from this meta-analysis due to an alternative mechanism of upregulation. 958 papers were identified from a literature search performed in PubMed and Embase. These papers were independently evaluated by two reviewers and 147 studies were included in the analysis. The meta-analysis revealed strong significant associations between CAIX expression and all endpoints: overall survival [hazard ratio (HR) = 1.76, 95% confidence interval (95%CI) 1.58–1.98], disease-free survival (HR = 1.87, 95%CI 1.62–2.16), locoregional control (HR = 1.54, 95%CI 1.22–1.93), disease-specific survival (HR = 1.78, 95%CI 1.41–2.25), metastasis-free survival (HR = 1.82, 95%CI 1.33–2.50), and progression-free survival (HR = 1.58, 95%CI 1.27–1.96). Subgroup analyses revealed similar associations in the majority of tumor sites and types. In conclusion, these results show that patients having tumors with high CAIX expression have higher risk of locoregional failure, disease progression, and higher risk to develop metastases, independent of tumor type or site. The results of this meta-analysis further support the development of a clinical test to determine patient prognosis based on CAIX expression and may have important implications for the development of new treatment strategies.
Introduction
Hypoxia is a characteristic of many different types of solid tumors and is caused by an inadequate vascular supply. Hypoxic areas are characterized by low oxygen concentrations, limited nutrient supply, and an acidic extracellular environment. Hypoxia is an independent prognostic factor of poor outcome in patients (1) and decreases the efficacy of standard treatment modalities, such as surgery, chemotherapy, and radiotherapy (2–4). Many strategies are therefore being investigated to measure tumor hypoxia to predict treatment outcome and to overcome or target tumor hypoxia with newly designed treatments (5–8).
Tumor cells have adopted several mechanisms to survive the hostile conditions during hypoxia, of which one is the hypoxia-inducible factor (HIF) pathway (9, 10). Upon hypoxic conditions, the expression of the dimeric zinc-containing glycoprotein carbonic anhydrase IX (CAIX) is enhanced as a consequence of HIF stabilization (11, 12). CAIX is important in maintaining the cellular pH regulation and is located on the cell membrane where it hydrolyzes carbon dioxide, produced as a waste product during glycolysis, to bicarbonate and a proton. The bicarbonate is transported intracellularly by different proteins (e.g., anion exchangers), thereby slightly increasing the intracellular pH to promote tumor cell proliferation. The protons in turn add to an acidic extracellular environment causing extracellular matrix degradation favoring invasion, migration, and subsequent metastasis formation (12). Hypoxia-induced CAIX expression, tumor-specific expression of CAIX, and its important role in maintaining the pH balance make CAIX a promising endogenous marker of tumor hypoxia and an attractive target for anti-cancer therapies with newly designed inhibitors (6, 11, 12).
Many clinical studies investigated the prognostic value of CAIX, and a recent meta-analysis of renal cell carcinoma (RCC) concluded that high CAIX expression was associated with a better overall survival (OS) (13). By contrast, a meta-analysis in head and neck cancer patients showed high CAIX expression was associated with a decrease in both OS and disease-free survival (DFS) (14). This discrepancy can be explained by the fact that RCCs are often characterized by an inactive mutant version of the Von Hippel–Lindau (VHL) protein preventing proteasomal degradation of CAIX upon normoxia and making its expression therefore independent of hypoxia (15, 16). To the best of our knowledge, a comprehensive meta-analysis of the association between CAIX expression and treatment outcome in other tumor types has not been performed. The aim of this meta-analysis of published clinical studies is therefore to elucidate the prognostic value of CAIX expression in all solid tumor types besides RCC. In addition, current analysis has included sensitivity and subgroup analysis to be able to determine if the prognostic value of CAIX expression varies in patients with different tumor types.
Methods
Literature Search
The research question of this meta-analysis was defined as follows: “what is the prognostic value of tumoral CAIX expression in patients with solid tumors?” From this research question, three distinctive keywords were identified, i.e., prognosis, CAIX, and tumor. Different formulations and truncations of the keywords were tested as free text searches to see if appropriate papers could be identified. The search algorithm was applied as a free text search and consisted of the combined mention of all three keywords, in any of the formulations or truncations (Data sheet 1 in Supplementary Material). The search for literature was performed on the 31st of August 2015 in both the PubMed and Embase databases. A total of 958 papers were identified from both databases (Figure 1).
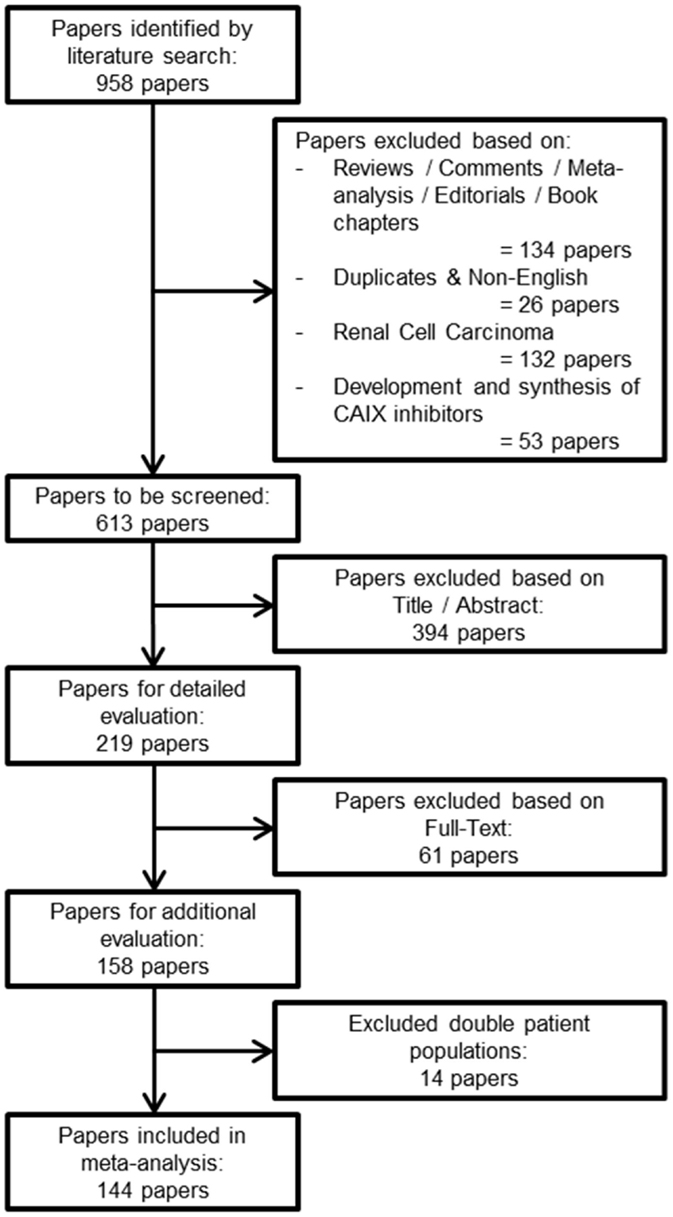
Figure 1. Flowchart of selecting articles describing the association between tumoral CAIX expression and prognosis.
Exclusion Criteria
From the total number of papers, 134 reviews, conference abstracts, commentaries, meta-analyses, editorials, or book chapters were excluded, as were 26 duplicates or non-English papers. From the remaining articles, 132 papers about RCC were excluded, since upregulation of CAIX in RCC is biologically different from other solid tumor types (15, 16). Furthermore, from our experience, we know that papers that describe solely the development and synthesis of CAIX inhibitors do not include patient data, and 53 papers were therefore also excluded. The total number of papers for further screening was thereby reduced to 613 (Figure 1).
Screening of Papers
Two researchers (SK and AY) screened the remaining papers independently. The first round of screening was based on the title and abstract, whereas the second round consisted of a detailed evaluation of the full-text. Papers were evaluated based on the predetermined inclusion criteria. First, only solid primary tumors of various types were included, thereby automatically excluding hematological cancer. Second, only immunohistochemical detection of CAIX was included, because mRNA upregulation of CA9 does not fully correlate with an increase in functional protein expression, possibly due to posttranscriptional processing and/or differences in stability (17–19). Third, all endpoints were included (see below) with a minimal median follow-up of 1 year. Fourth, all treatment modalities along with experimental treatments were included. Finally, we included every human patient population without making distinction based on tumor grades or stages. Discrepancies between the included papers by both reviewers were discussed and consensus was reached on all. An additional 14 papers were excluded because their patient populations were similar or overlapping with other papers. Among these repetitive studies, the paper that was included contained the most detailed information about the patient population. A total of 144 papers were included in the meta-analysis (Figure 1).
Data Extraction
Several different parameters, if reported, were extracted from each paper, i.e., the number and origin of patients, number of events, treatment modalities, tumor site, tumor stage, tumor type, group dichotomization, antibody supplier, expression pattern, cellular localization, and endpoints. The univariate hazard ratio (HR) was extracted to assess prognostic value of CAIX expression. When the univariate HR with corresponding 95% confidence interval (95%CI) was not reported, the method from Tierney et al. was used to estimate the HR (20). Multivariate HR was only included in the meta-analysis when the univariate HR was not reported or could not be estimated. When insufficient data were reported for estimating HR, the authors were contacted to obtain additional data.
Quality Assessment
The methodological quality of the included papers was evaluated with an adjusted version of the Newcastle–Ottawa scale (NOS) to better suit the study design of the included papers (Data sheet 2 in Supplementary Material). The method of scoring based on awarding stars in different categories remained, however, identical. The NOS was commended in the 2011 version of the Cochrane Collaboration handbook and is an easy method to evaluate the methodological quality of cohort studies (available at http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp) (21).
Statistical Analysis and Sensitivity Analysis
Distribution and frequencies of the extracted data parameters were analyzed using SPSS (version 22). Meta-analysis was performed using R statistical software with the Metafor Library (version 1.9-8) (22). Fixed-effect modeling was performed when no statistical significant heterogeneity between studies was observed. When the heterogeneity between studies was statistically significant, random-effects modeling was applied based on the DerSimonian and Laird method (23). The assigned weight of each study in the analysis was based on its inverse variance. The following endpoints have been addressed: OS, DFS, locoregional control (LC), disease-specific survival (DSS), metastasis-free survival (MFS), and progression-free survival (PFS). Sensitivity analysis was performed by analyzing subgroups of studies separately, e.g., per tumor organ site. Funnel plots were created to visualize possible publication bias or heterogeneity between studies. Asymmetric funnel plots and studies outside the funnel plot suggest heterogeneity between them and/or publication bias (21). p-Values <0.05 were considered as statistically significant.
Results
This meta-analysis encompassed a total number of 24,523 patients across 147 independent studies. Many studies included only a small number of patients (median per study 93, range 15–3630) with a median follow-up time between 12.6 months and 13.9 years and are often inconclusive, which underlines the need for a meta-analysis. All papers were published between 2001 and 2015 of which approximately 50% were published after 2010. The majority of the included studies treated patients with surgery alone (36.7%) or in combination with either chemotherapy (8.8%) or standard radiotherapy (8.8%), or the combination of all three modalities (23.1%). Single radiotherapy treatment or combined with chemotherapy was reported in 5.4 and 6.1% of the papers, respectively. In 4.8% of the studies, a form of experimental treatment was administered, including experimental radiotherapy (24–28), hormonal treatment (29), and VEGF-targeted therapy (30). Most of the studies reported on head and neck cancer patients (21.8%) followed by breast (16.3%) and brain cancer patients (10.2%). By contrast, cancers of the adrenal gland, the cartilage, and the penis were only described once.
Immunohistochemical staining of CAIX was predominantly performed using the M75 antibody (46.3%) targeting the proteoglycan domain of CAIX (31, 32). Other studies used anti-CAIX antibodies obtained from different suppliers. A membranous expression of CAIX was described in 46.3% of the studies, although cytoplasmatic staining or a combination of the two was also reported (4.8 and 17.7%, respectively). Nuclear staining was only reported in one paper, whereas the rest did not state the staining localization. Different quantification methods and thresholds have been applied to stratify patients into groups with low and high tumoral CAIX expression. Taken together, 33.9% of the total tumors were classified as expressing high levels of CAIX.
Overall, patients suffering from tumors with high CAIX expression had a worse treatment outcome (Figure 2). This association was strong and significant for all endpoints. The negative association of CAIX expression with outcome was dominant for DFS and weaker for LC. Systematic heterogeneity in the present meta-analysis as demonstrated by an asymmetric funnel plot (21) for most of the endpoints (Image 1 in Supplementary Material) can at least in part be attributed to the considerable variation in tumor types and sites across the studies. Therefore, in addition, subgroup analysis based on organ site of the tumor was performed. The results of the subgroup analysis demonstrate a significant prognostic value of CAIX in most of the cancer types investigated (see below).
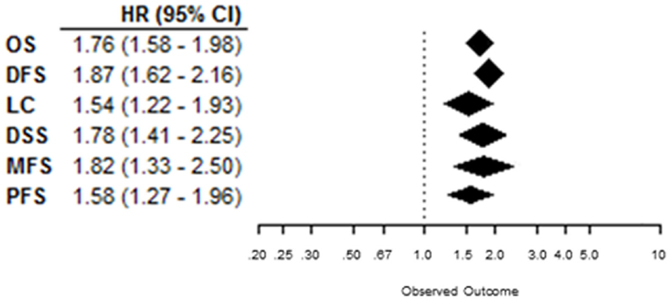
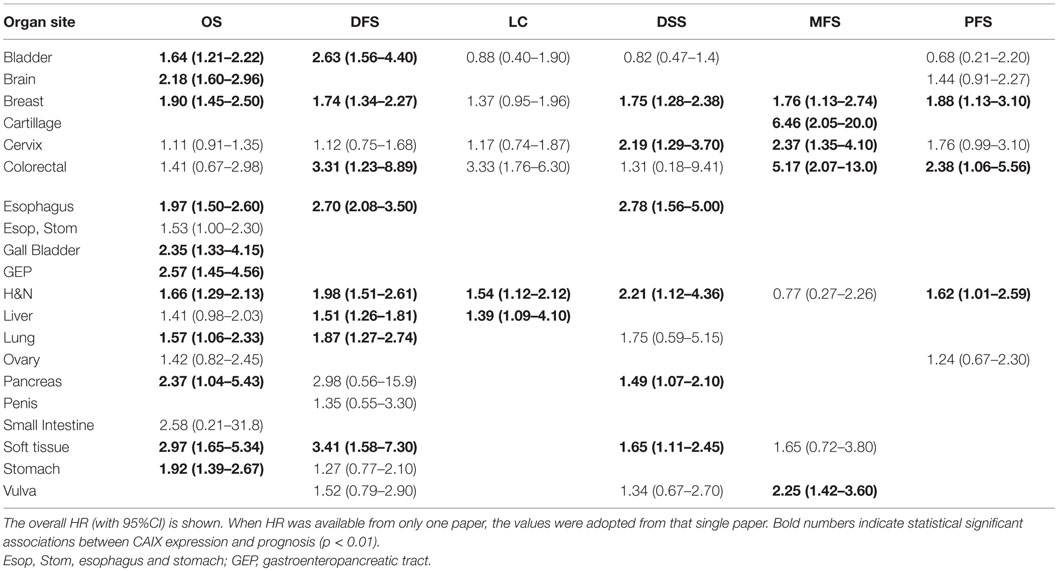
Figure 2. Summary plot of the overall HRs from each endpoint analyzed. Symbols represent the HR with 95%CI, and dashed line indicates no association between CAIX expression and prognosis.
Overall Survival
Effect of pretreatment expression of CAIX on OS could be evaluated in 104 studies. The complete data to estimate the HR could not be retrieved from 11 papers and were therefore not included in the analysis [Table 1 in Supplementary Material (33–43)]. Overall, high CAIX expression was associated with a worse OS (HR = 1.76, 95%CI 1.58–1.98, p < 0.0001, Figure 3). Subgroup analysis of the different organ sites revealed a similar significant association between tumoral CAIX expression and OS in 11 organ sites: bladder (HR = 1.64, 95%CI = 1.21–2.22), brain (HR = 2.18, 95%CI 1.60–2.96), breast (HR = 1.90, 95%CI = 1.45–2.50), esophagus (HR = 1.97, 95%CI 1.50–2.60), gall bladder (HR = 2.35, 95%CI 1.33–4.15), gastroenteropancreatic tract (HR = 2.57, 95%CI 1.45–4.56), head and neck (HR = 1.66, 95%CI 1.29–2.13), lung (HR = 1.57, 95%CI 1.06–2.33), pancreas (HR = 2.37, 95%CI 1.04–5.43), soft tissue (HR = 2.97, 95%CI 1.65–5.34), and the stomach (HR = 1.92, 95%CI 1.39–2.67). The other six organ sites show a similar trend with worse OS, albeit not statistically significant (Table 1). Similar results were often, but not always, observed for different tumor types per organ site (Table 2 in Supplementary Material). A hypoxia-associated perinecrotic staining pattern was reported in 16 of these studies, whereas a diffuse staining pattern was reported in 3 papers. Interestingly, both patterns of CAIX expression significantly associated with OS (perinecrotic: HR = 1.99, 95%CI 1.60–2.48; diffuse: HR = 1.77, 95%CI 1.22–2.56). These results suggest that the expression pattern of CAIX does not affect its prognostic value.
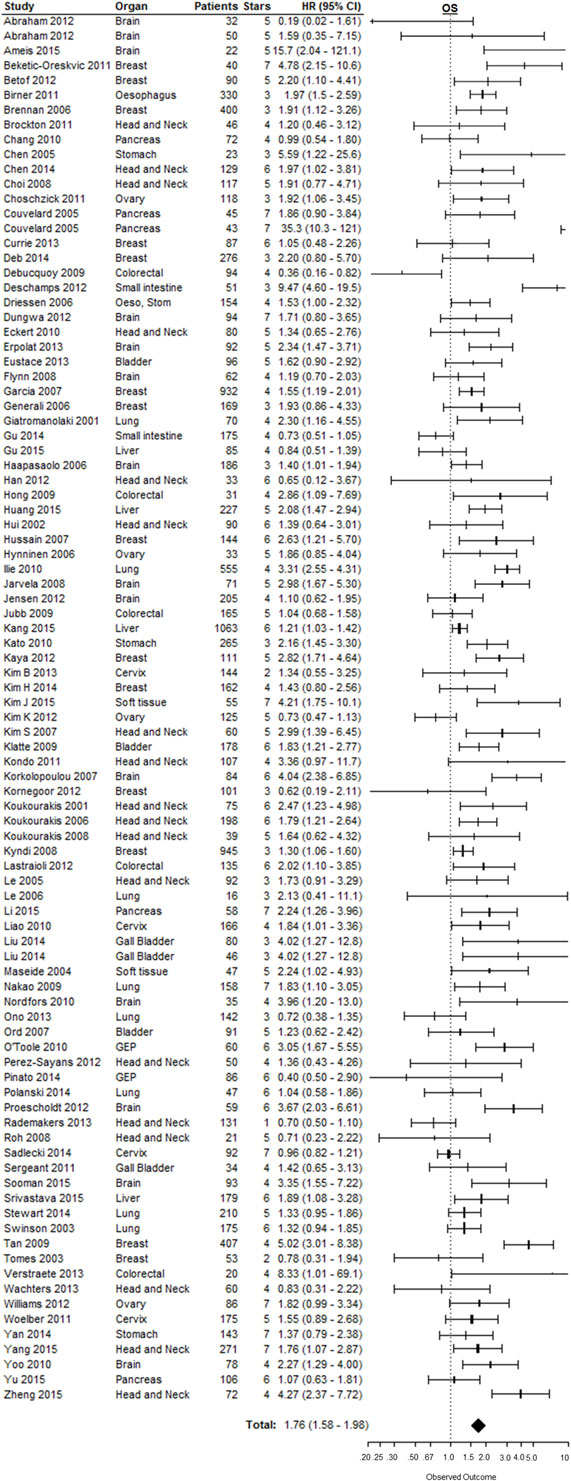
Figure 3. Forest plot of the papers describing the association between CAIX expression and OS. Horizontal bars represent HR with corresponding 95%CI. Symbol size represents the assigned weight of the study. The overall HR with 95%CI is visualized with the diamond shape. Dashed line indicates no association between CAIX and prognosis. Esop, Stom, esophagus and stomach; GEP, gastroenteropancreatic tract (25–27, 29, 30, 44–129).
Disease-Free Survival
A total of 40 from the selected 147 studies investigated the association between CAIX expression and DFS. Five studies could not be included in this analysis due to incomplete reporting [Table 1 in Supplementary Material (39, 40, 43, 124, 130)]. Based on 35 studies, high CAIX expression was statistically significantly associated with a decreased DFS (HR = 1.87, 95%CI 1.62–2.16, p < 0.001) (Figure 4). Subgroup analysis based on organ site of the tumor showed that high CAIX expression was significantly associated with shorter DFS in bladder (HR = 2.63, 95%CI 1.56–4.40), breast (HR = 1.74, 95%CI 1.34–2.27), colorectal (HR = 3.31, 95%CI 1.23–8.89), esophagus (HR = 2.70, 95%CI 2.08–3.50), head and neck (HR = 1.98, 95%CI 1.51–2.61), liver (HR = 1.51, 95%CI 1.26–1.81), lung (HR = 1.87, 95%CI 1.27–2.74), and soft tissue tumors (HR = 3.41, 95%CI 1.58–7.30). By contrast, no significant association with DFS was observed for tumors in the cervix (HR = 1.12, 95%CI 0.75–1.68), pancreas (HR = 2.98, 95%CI 0.56–15.9), penis (HR = 1.35, 95%CI 0.55–3.30), stomach (HR = 1.27, 95%CI 0.77–2.10), and vulva (HR = 1.52, 95%CI 0.79–2.90) (Table 1). A similar trend was observed for all different tumor types per organ sites (Table 2 in Supplementary Material).
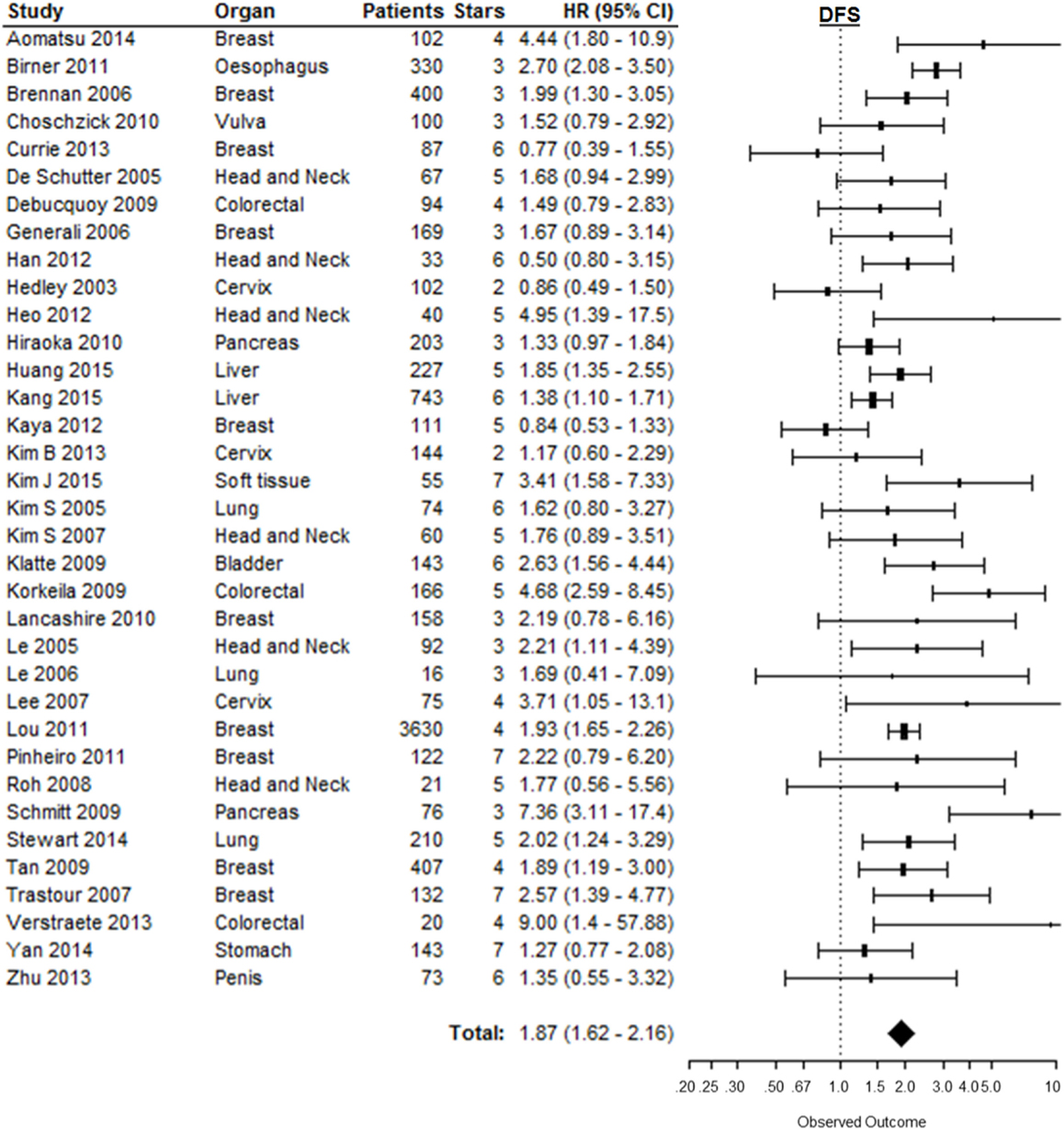
Figure 4. Forest plot of the papers describing the association between CAIX expression and DFS. Horizontal bars represent HR with corresponding 95%CI. Symbol size represents the assigned weight of the study. The overall HR with 95%CI is visualized with the diamond shape. Dashed line indicates no association between CAIX and prognosis (29, 33, 34, 48, 57, 59, 68, 73, 74, 82, 84, 85, 87, 89, 90, 97, 98, 112, 117, 119, 121, 125, 131–143).
Locoregional Control
The risk of locoregional relapse associated with CAIX expression was evaluated in 25 studies in 6 different organ sites. Figure 5 shows the overall LC outcome, which indicates that patients with high tumoral CAIX expression have a higher risk of locoregional recurrences than patients with low expression of CAIX in tumors (HR = 1.54, 95%CI 1.22–1.93, p = 0.0002). The negative association between high CAIX expression in tumors and worse LC remained significant in head and neck (HR = 1.54, 95%CI 1.12–2.12) and liver tumors (HR = 1.39, 95%CI 1.09–4.10) (Table 1). A similar association was observed in most of the tumor types per organ sites (Table 2 in Supplementary Material).
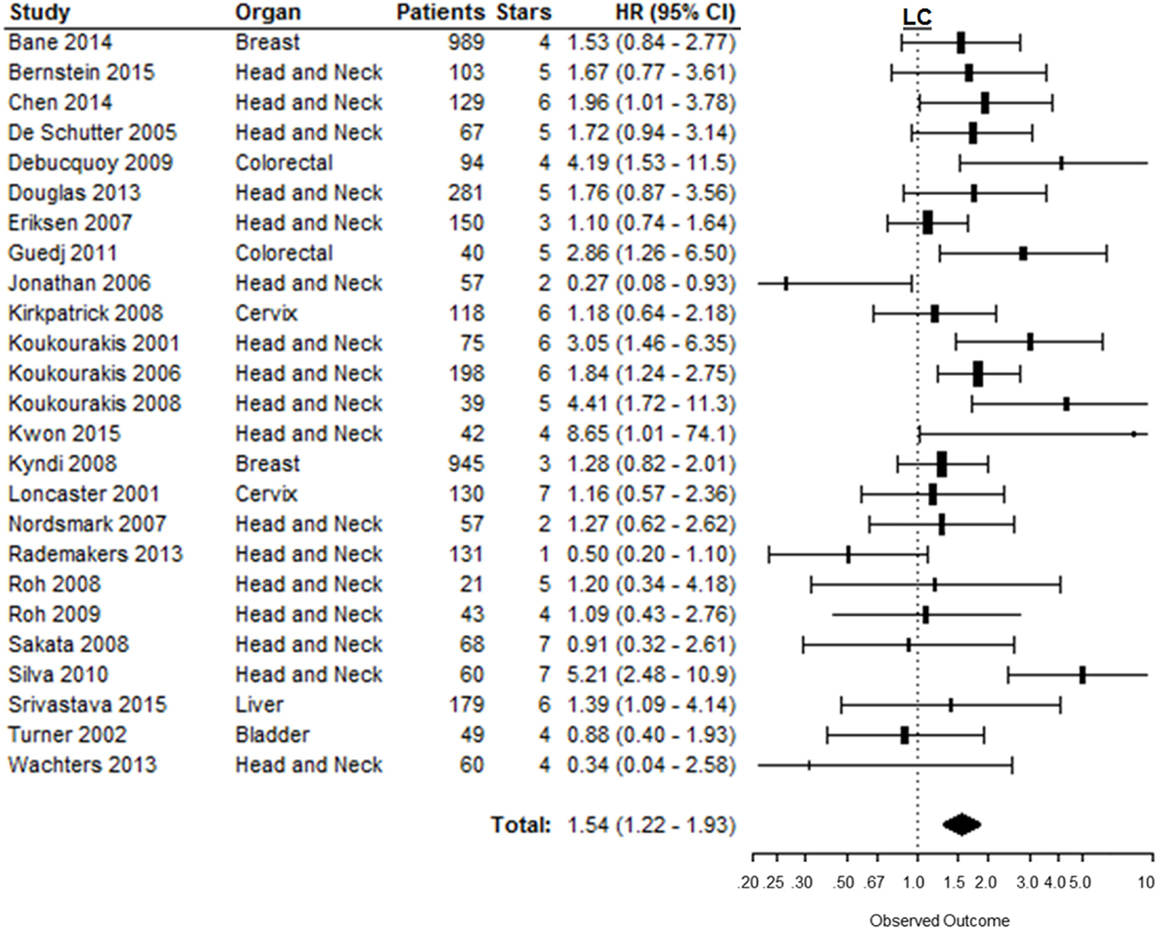
Figure 5. Forest plot of the papers describing the association between CAIX expression and LC. Horizontal bars represent HR with corresponding 95%CI. Symbol size represents the assigned weight of the study. The overall HR with 95%CI is visualized with the diamond shape. Dashed line indicates no association between CAIX and prognosis (24–28, 52, 59, 94, 95, 112, 116, 122, 133, 144–155).
Disease-Specific Survival
Disease-specific survival was reported in 23 studies, of which 1 study provided incomplete data to estimate the HR [Table 1 in Supplementary Material (43)]. In the remaining 22 studies, patients suffering from tumors with high CAIX expression had a significantly shorter DSS (HR = 1.78, 95%CI 1.41–2.25, p < 0.0001) (Figure 6). Subgroup analyses by organ site revealed significant associations between high CAIX expression and worse DSS in tumors of the breast (HR = 1.75, 95%CI 1.28–2.38), cervix (HR = 2.19, 95%CI 1.29–3.70), esophagus (HR = 2.78, 95%CI 1.56–5.00), head and neck (HR = 2.21, 95%CI 1.12–4.36), pancreas (HR = 1.49, 95%CI 1.07–2.10), and soft tissue (HR = 1.65, 95%CI 1.11–2.45) (Table 1). Subgroup analyses of the tumor types per organ sites revealed a worse DSS to be associated with high CAIX expression in the majority of tumor types (Table 2 in Supplementary Material).
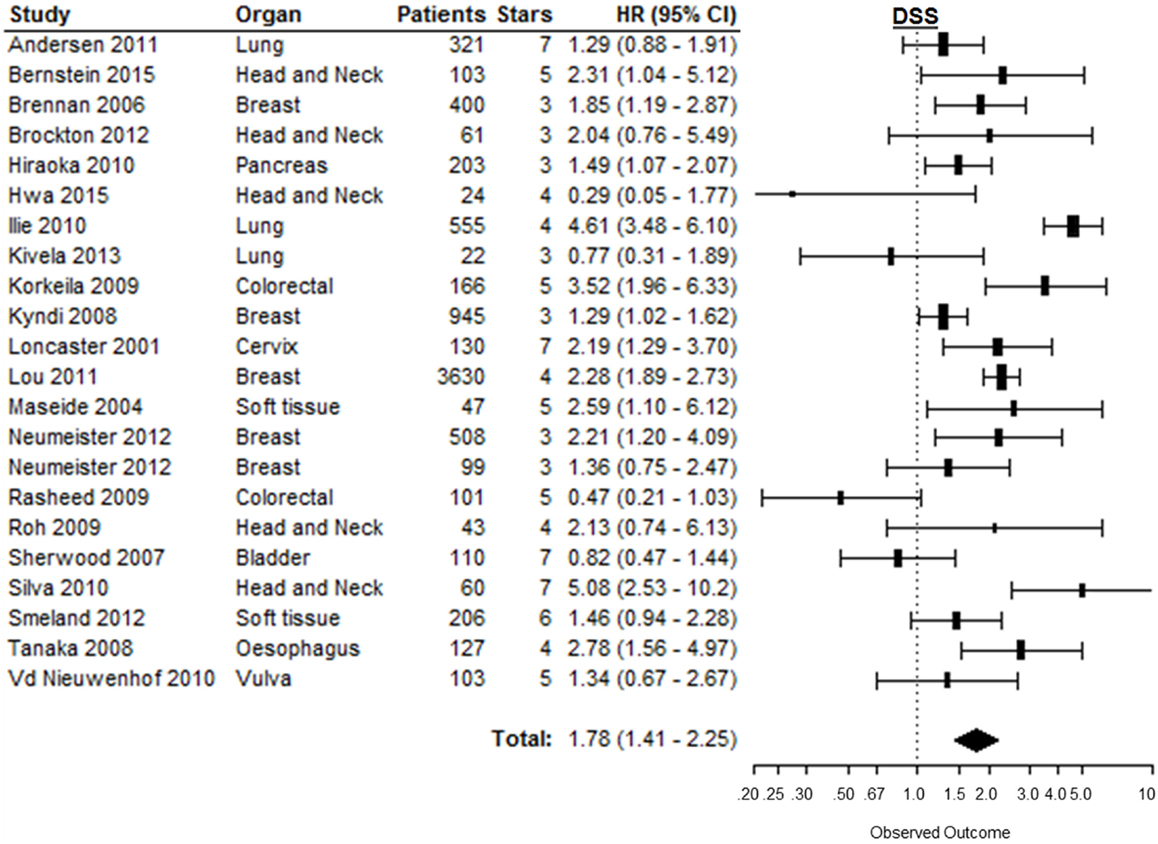
Figure 6. Forest plot of the papers describing the association between CAIX expression and DSS. Horizontal bars represent HR with corresponding 95%CI. Symbol size represents the assigned weight of the study. The overall HR with 95%CI is visualized with the diamond shape. Dashed line indicates no association between CAIX and prognosis (29, 78, 95, 102, 136, 138, 140, 144, 150, 152, 154, 156–165).
Metastasis-Free Survival
Metastasis-free survival was reported in 12 of the 147 included studies. Based on 11 of these studies, high CAIX expression was significantly associated with a shorter MFS (HR = 1.82, 95%CI 1.33–2.50, p = 0.0002) [Figure 7; Table 1 in Supplementary Material (166)]. Subgroup analyses of the different organ sites of the tumors, independent of tumor types, revealed high CAIX expression to be significantly associated with a worse MFS in most of the organ sites reported, i.e., breast (HR = 1.76, 95%CI 1.13–2.74), cartilage (HR = 6.46, 95%CI 2.05–20.0), cervix (HR = 2.37, 95%CI 1.35–4.10), colorectal (HR = 5.17, 95%CI 2.07–13.0), and vulva (HR = 2.25, 95%CI 1.42–3.60), but not in head and neck (HR = 0.77, 95%CI 0.27–2.26) and soft tissue cancers (HR = 1.65, 95%CI 0.72–3.80) (Table 1). Interestingly, one study reported a significant positive association between high CAIX expression and better MFS in squamous cell carcinoma of the head and neck (HR = 0.27, 95%CI 0.09–0.80) (Table 2 in Supplementary Material), which may be attributed to the hypoxia-modifying component of the treatment (24).
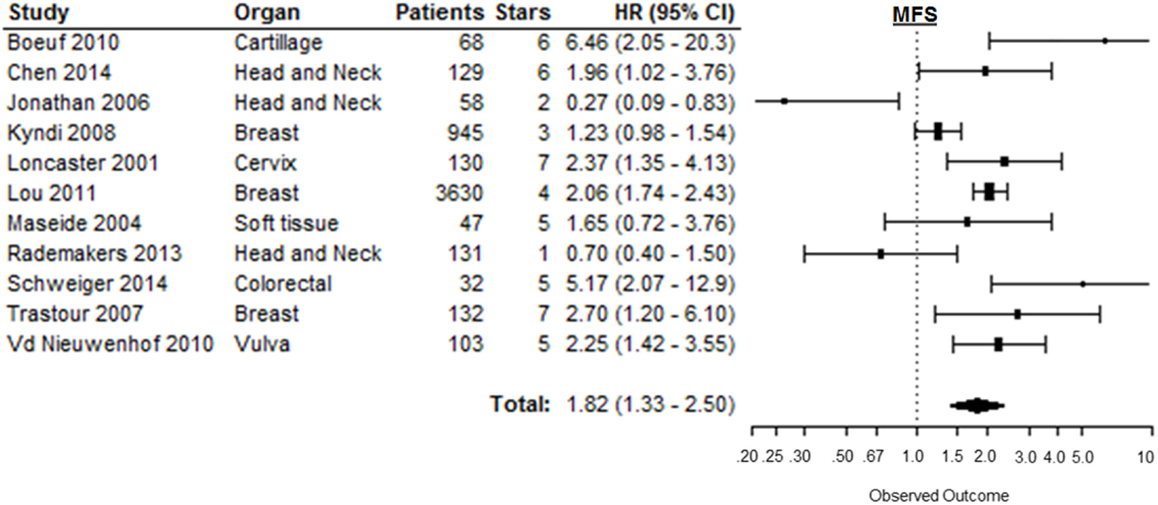
Figure 7. Forest plot of the papers describing the association between CAIX expression and MFS. Horizontal bars represent HR with corresponding 95%CI. Symbol size represents the assigned weight of the study. The overall HR with 95%CI is visualized with the diamond shape. Dashed line indicates no association between CAIX and prognosis (24, 27, 34, 52, 95, 102, 140, 34, 150, 165, 167, 168).
Progression-Free Survival
Eleven out of 12 studies could be included to estimate the risk of disease progression after treatment based on CAIX expression in tumors [Table 1 in Supplementary Material (169)]. Similar to the other endpoints, PFS was significantly shorter in patients with tumors expressing high levels of CAIX (HR = 1.58, 95%CI 1.27–1.96, p < 0.0001) (Figure 8). Subgroup analyses per organ site revealed that the association with PFS only remained statistically significant in breast (HR = 1.88, 95%CI 1.13–3.10), colorectal (HR = 2.38, 95%CI 1.06–5.56), and head and neck tumors (HR = 1.62, 95%CI 1.01–2.59) (Table 1). The subgroup analyses of tumor types per organ site showed similar associations between high CAIX expression and a worse PFS (Table 2 in Supplementary Material).
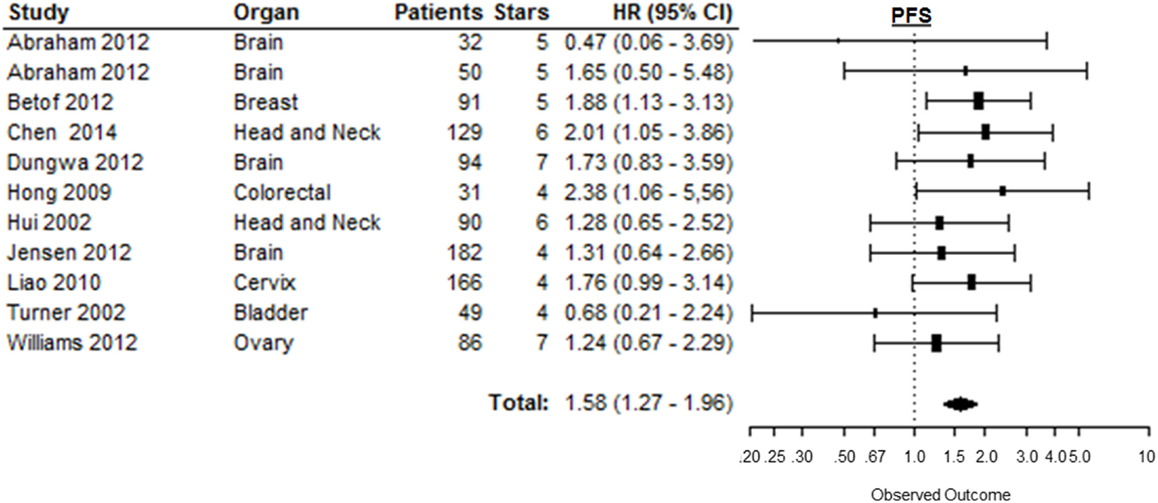
Figure 8. Forest plot of the papers describing the association between CAIX expression and PFS. Horizontal bars represent the HR with corresponding 95%CI. Symbol size represents the assigned weight of the study. The overall HR with 95%CI is visualized with the diamond shape. Dashed line indicates no association between CAIX and prognosis (30, 44, 47, 52, 62, 75, 80, 100, 123, 155).
High-Quality Papers
This meta-analysis used an adjusted version of the NOS to evaluate the quality of a study. The scores of this quality assessment ranged between 1 and 7 stars, i.e., the maximum, awarded per study. Approximately half of the studies (52.4%) were considered as high-quality studies, i.e., with a number of stars greater or equal to the median (5 stars). Meta-analysis of only the high-quality studies revealed significant prognostic values of CAIX expression for OS (HR = 1.81, 95%CI 1.57–2.09, n = 50), DFS (HR = 1.81, 95%CI 1.47–2.23, n = 18), DSS (HR = 1.71, 95%CI 1.16–2.51, n = 10), and PFS (HR = 1.59, 95%CI 1.21–2.07, n = 7). For both LC (HR = 1.90, 95%CI 1.58–2.30, n = 14) and MFS (HR = 2.47, 95%CI 1.92–3.19, n = 7), the association with CAIX expression became even stronger when only high-quality studies were included.
Discussion
Many clinical studies investigated the prognostic association of CAIX expression with treatment outcome. Most of these studies, however, include only limited numbers of patients and remain inconclusive. This current meta-analysis is the first complete overview of all reported clinical studies investigating the impact of pretreatment CAIX expression in solid tumors on prognosis. Overall, these results clearly show that high CAIX expression is an adverse prognostic marker in solid tumors, irrespectively of the endpoint evaluated, as summarized in Figure 2. A strong association between high CAIX expression and poor prognosis was also found in the majority of different tumor sites, supporting an important role of CAIX in disease progression and treatment resistance in many cancer types.
The papers included in the current meta-analysis were all published between 2001 and 2015, which is likely attributed to the identification of the hypoxic responsive element in the promotor region of ca9 in the end of 2000 (170). This study identified a direct link between CAIX expression and its hypoxic upregulation through HIF stabilization. This crucial finding encouraged research to evaluate CAIX as an endogenous marker of tumor hypoxia, a known biological factor of therapy resistance (2–4). Nevertheless, because alternative mechanisms can also regulate CAIX expression, e.g., via PI3K (171) or the unfolded protein response (10, 172), tumoral CAIX expression may not accurately identify hypoxic tumors. Apart from the hypoxia-associated mechanisms underlying resistance of tumor cells to several treatment modalities, CAIX can directly affect cancer prognosis as its main function is to maintain the balance between intracellular and extracellular pH, thereby generating an acidic extracellular microenvironment (11, 12). This is supported by data demonstrating that CAIX is involved in promoting tumorigenesis and leads to a more aggressive phenotype of tumor cells (173). This can partly be explained by the association between CAIX expression and the induction of tumor cell migration and invasion, which could be caused by the reduction in extracellular pH (174–176). In addition, cancer stem cell markers also appear to be enriched in the CAIX expressing population of tumor cells (57, 177). The important role of CAIX, either directly or indirectly, in cancer prognosis is also supported by the results of the current meta-analysis, which shows that tumors with high CAIX expression have higher risk of locoregional failure, disease progression, and higher risk to develop metastasis. Other proton exchangers and transporters have been shown preclinically and clinically to play an important role in the regulation of cellular pH homeostasis promoting survival and invasion as well as causing treatment resistance (178–180). Therefore, assessment of several major pH regulators in tumors prior and/or during therapy may represent a more powerful prognostic and predictive biomarker as well as important targets for new anti-cancer treatments, which warrants further investigations.
A meta-analysis usually overestimates its results because of selective reporting and publication bias (21). This meta-analysis identified a total of 147 studies reported in 144 papers of which 15 could not be included in final analysis because the HR could not be estimated due to incomplete reporting (33–43, 124, 130, 166, 169). Non-significant association between CAIX and outcome was found in these studies (Table 1 in Supplementary Material). Including these 15 papers in the analysis might therefore decrease the magnitude of the prognostic values of CAIX expression reported here. This overestimation can be further increased by publication bias, i.e., when negative associations are not published at all and can therefore not be identified and included in this meta-analysis. Nevertheless, since the prognostic value of CAIX expression was highly statistically significant, we believe that the possible effect of publication bias on this association is minimal.
The different staining and scoring methods used in the included papers to quantify CAIX expression might be an additional source of bias. Visual quantification was used in the majority of the reports and could either be based on staining intensity, the number of stained cells, or a combination of both. In addition, different thresholds have been used to dichotomize patients based on their CAIX expression. This discrepancy in methods is one of the reasons of significant heterogeneity between studies, which therefore requires the use of a random-effect model in the meta-analysis (181, 182). Additionally, tissue microarrays (TMAs) are used in the majority of included papers to visualize and quantify CAIX expression, even though TMAs may underestimate the actual expression levels of the protein (183). The use of TMAs might therefore bias the prognostic value of CAIX when CAIX expression levels are dichotomized erroneous. Furthermore, this meta-analysis is limited by difficulties in obtaining homogenous endpoints and by non-uniform observation times, although most of the data are based on reports with a median follow-up of more than 1 year.
To identify possible bias in a selected study, an adjusted version of the NOS was used, which is a quick and easy method to assess the quality of studies that has been commended in the Cochrane handbook (21). However, the validity and reproducibility of the NOS have been questioned because of the subjective interpretation of certain criteria, which require detailed guidelines to obtain a better inter-rater agreement (184–186). The test–retest reliability of the NOS is, however, better, which allows for a single reviewer to continuously use uniform criteria while rating papers (185). When only high-quality papers, i.e., those with minimal bias, were included in our analyses, there was no significant difference in the results as compared with all studies included.
It remains impossible to eliminate every source of bias in a meta-analysis. Nevertheless, the high statistical significance of the results presented here clearly show that CAIX expression is associated with worse prognosis in a global patient population and in the majority of tumor sites. These findings are similar to the results of the meta-analysis in head and neck cancer (14), but different from RCC (13) due to the alternative mechanism of CAIX upregulation in RCC (15, 16). New treatment options are currently being developed to specifically inhibit CAIX function (6, 187), of which one is currently in a Phase I clinical trial (NCT02215850). These types of compounds might prove to be beneficial for the specific treatment of tumors with high CAIX expression. The results of this meta-analysis further support the development of a clinical test to determine patient prognosis based on CAIX expression, although a standardized protocol remains to be developed and validated.
Author Contributions
Study was conceived and designed by SK, AY, RN, PL, and LD. Screening of papers and data extraction was performed by SK and AY. Statistical analyses were performed by RH. Writing of the first draft of the manuscript was performed by SK. AY, RH, RN, PL, and LD contributed to the writing of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
This study was performed with financial support from the EU 7th framework program METOXIA (ref. 2008-222741), NGI Pre-Seed grant (no. 93612005), and the Dutch Cancer Society (KWF UM 2011-5020 and KWF MAC 2013-6089).
Supplementary Material
The Supplementary Material for this article can be found online at http://journal.frontiersin.org/article/10.3389/fonc.2016.00069
Abbreviations
95%CI, 95% confidence interval; CAIX, carbonic anhydrase IX; DFS, disease-free survival; DSS, disease-specific survival; HIF, hypoxia-inducible factor; HR, hazard ratio; LC, locoregional control; MFS, metastasis-free survival; NOS, Newcastle–Ottawa scale; OS, overall survival; PFS, progression-free survival; RCC, renal cell carcinoma; TMAs, tissue microarrays; VHL, Von Hippel–Lindau protein.
References
1. Nordsmark M, Bentzen SM, Rudat V, Brizel D, Lartigau E, Stadler P, et al. Prognostic value of tumor oxygenation in 397 head and neck tumors after primary radiation therapy. An international multi-center study. Radiother Oncol (2005) 77:18–24. doi:10.1016/j.radonc.2005.06.038
2. Hockel M, Schlenger K, Aral B, Mitze M, Schaffer U, Vaupel P. Association between tumor hypoxia and malignant progression in advanced cancer of the uterine cervix. Cancer Res (1996) 56:4509–15.
3. Wojtkowiak JW, Verduzco D, Schramm KJ, Gillies RJ. Drug resistance and cellular adaptation to tumor acidic pH microenvironment. Mol Pharm (2011) 8:2032–8. doi:10.1021/mp200292c
4. Good JS, Harrington KJ. The hallmarks of cancer and the radiation oncologist: updating the 5Rs of radiobiology. Clin Oncol (2013) 25:569–77. doi:10.1016/j.clon.2013.06.009
5. Helbig L, Koi L, Bruchner K, Gurtner K, Hess-Stumpp H, Unterschemmann K, et al. BAY 87-2243, a novel inhibitor of hypoxia-induced gene activation, improves local tumor control after fractionated irradiation in a schedule-dependent manner in head and neck human xenografts. Radiat Oncol (2014) 9:207. doi:10.1186/1748-717X-9-207
6. Dubois LJ, Niemans R, van Kuijk SJ, Panth KM, Parvathaneni NK, Peeters SG, et al. New ways to image and target tumour hypoxia and its molecular responses. Radiother Oncol (2015) 116:352–7. doi:10.1016/j.radonc.2015.08.022
7. Peeters SG, Zegers CM, Biemans R, Lieuwes NG, van Stiphout RG, Yaromina A, et al. TH-302 in combination with radiotherapy enhances the therapeutic outcome and is associated with pretreatment [18F]HX4 hypoxia PET imaging. Clin Cancer Res (2015) 21:2984–92. doi:10.1158/1078-0432.CCR-15-0018
8. Pettersen EO, Ebbesen P, Gieling RG, Williams KJ, Dubois L, Lambin P, et al. Targeting tumour hypoxia to prevent cancer metastasis. From biology, biosensing and technology to drug development: the METOXIA consortium. J Enzyme Inhib Med Chem (2015) 30:689–721. doi:10.3109/14756366.2014.966704
9. Denko NC. Hypoxia, HIF1 and glucose metabolism in the solid tumour. Nat Rev Cancer (2008) 8:705–13. doi:10.1038/nrc2468
10. Wouters BG, Koritzinsky M. Hypoxia signalling through mTOR and the unfolded protein response in cancer. Nat Rev Cancer (2008) 8:851–64. doi:10.1038/nrc2501
11. Neri D, Supuran CT. Interfering with pH regulation in tumours as a therapeutic strategy. Nat Rev Drug Discov (2011) 10:767–77. doi:10.1038/nrd3554
12. Pastorek J, Pastorekova S. Hypoxia-induced carbonic anhydrase IX as a target for cancer therapy: from biology to clinical use. Semin Cancer Biol (2015) 31:52–64. doi:10.1016/j.semcancer.2014.08.002
13. Zhao Z, Liao G, Li Y, Zhou S, Zou H, Fernando S. Prognostic value of carbonic anhydrase IX immunohistochemical expression in renal cell carcinoma: a meta-analysis of the literature. PLoS One (2014) 9:e114096. doi:10.1371/journal.pone.0114096
14. Peridis S, Pilgrim G, Athanasopoulos I, Parpounas K. Carbonic anhydrase-9 expression in head and neck cancer: a meta-analysis. Eur Arch Otorhinolaryngol (2011) 268:661–70. doi:10.1007/s00405-011-1488-z
15. Gnarra JR, Tory K, Weng Y, Schmidt L, Wei MH, Li H, et al. Mutations of the VHL tumour suppressor gene in renal carcinoma. Nat Genet (1994) 7:85–90. doi:10.1038/ng0594-85
16. Oosterwijk-Wakka JC, Boerman OC, Mulders PF, Oosterwijk E. Application of monoclonal antibody G250 recognizing carbonic anhydrase IX in renal cell carcinoma. Int J Mol Sci (2013) 14:11402–23. doi:10.3390/ijms140611402
17. Rafajova M, Zatovicova M, Kettmann R, Pastorek J, Pastorekova S. Induction by hypoxia combined with low glucose or low bicarbonate and high posttranslational stability upon reoxygenation contribute to carbonic anhydrase IX expression in cancer cells. Int J Oncol (2004) 24:995–1004. doi:10.3892/ijo.24.4.995
18. Said HM, Staab A, Hagemann C, Vince GH, Katzer A, Flentje M, et al. Distinct patterns of hypoxic expression of carbonic anhydrase IX (CA IX) in human malignant glioma cell lines. J Neurooncol (2007) 81:27–38. doi:10.1007/s11060-006-9205-2
19. Tafreshi NK, Lloyd MC, Proemsey JB, Bui MM, Kim J, Gillies RJ, et al. Evaluation of CAIX and CAXII expression in breast cancer at varied O levels: CAIX is the superior surrogate imaging biomarker of tumor hypoxia. Mol Imaging Biol (2016) 18:219–31. doi:10.1007/s11307-015-0885-x
20. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials (2007) 8:16. doi:10.1186/1745-6215-8-16
21. Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 4.2.6. Chichester: John Wiley & Sons, Ltd (2006).
22. Viechtbauer W. Conducting meta-analyses in R with the metafor Package. J Stat Softw (2010) 36:1–48. doi:10.18637/jss.v036.i03
23. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials (1986) 7:177–88. doi:10.1016/0197-2456(86)90046-2
24. Jonathan RA, Wijffels KI, Peeters W, de Wilde PC, Marres HA, Merkx MA, et al. The prognostic value of endogenous hypoxia-related markers for head and neck squamous cell carcinomas treated with ARCON. Radiother Oncol (2006) 79:288–97. doi:10.1016/j.radonc.2006.04.008
25. Koukourakis MI, Bentzen SM, Giatromanolaki A, Wilson GD, Daley FM, Saunders MI, et al. Endogenous markers of two separate hypoxia response pathways (hypoxia inducible factor 2 alpha and carbonic anhydrase 9) are associated with radiotherapy failure in head and neck cancer patients recruited in the CHART randomized trial. J Clin Oncol (2006) 24:727–35. doi:10.1200/jco.2005.02.7474
26. Koukourakis MI, Giatromanolaki A, Danielidis V, Sivridis E. Hypoxia inducible factor (HIf1alpha and HIF2alpha) and carbonic anhydrase 9 (CA9) expression and response of head-neck cancer to hypofractionated and accelerated radiotherapy. Int J Radiat Biol (2008) 84:47–52. doi:10.1080/09553000701616114
27. Rademakers SE, Hoogsteen IJ, Rijken PF, Oosterwijk E, Terhaard CH, Doornaert PA, et al. Pattern of CAIX expression is prognostic for outcome and predicts response to ARCON in patients with laryngeal cancer treated in a phase III randomized trial. Radiother Oncol (2013) 108:517–22. doi:10.1016/j.radonc.2013.04.022
28. Bane AL, Whelan TJ, Pond GR, Parpia S, Gohla G, Fyles AW, et al. Tumor factors predictive of response to hypofractionated radiotherapy in a randomized trial following breast conserving therapy. Ann Oncol (2014) 25:992–8. doi:10.1093/annonc/mdu090
29. Brennan DJ, Jirstrom K, Kronblad A, Millikan RC, Landberg G, Duffy MJ, et al. CA IX is an independent prognostic marker in premenopausal breast cancer patients with one to three positive lymph nodes and a putative marker of radiation resistance. Clin Cancer Res (2006) 12:6421–31. doi:10.1158/1078-0432.ccr-06-0480
30. Hong YS, Cho HJ, Kim SY, Jung KH, Park JW, Choi HS, et al. Carbonic anhydrase 9 is a predictive marker of survival benefit from lower dose of bevacizumab in patients with previously treated metastatic colorectal cancer. BMC Cancer (2009) 9:246. doi:10.1186/1471-2407-9-246
31. Pastorekova S, Zavadova Z, Kostal M, Babusikova O, Zavada J. A novel quasi-viral agent, MaTu, is a two-component system. Virology (1992) 187:620–6. doi:10.1016/0042-6822(92)90464-Z
32. Zavada J, Zavadova Z, Pastorek J, Biesova Z, Jezek J, Velek J. Human tumour-associated cell adhesion protein MN/CA IX: identification of M75 epitope and of the region mediating cell adhesion. Br J Cancer (2000) 82:1808–13. doi:10.1054/bjoc.2000.1111
33. Lancashire LJ, Powe DG, Reis-Filho JS, Rakha E, Lemetre C, Weigelt B, et al. A validated gene expression profile for detecting clinical outcome in breast cancer using artificial neural networks. Breast Cancer Res Treat (2010) 120:83–93. doi:10.1007/s10549-009-0378-1
34. Trastour C, Benizri E, Ettore F, Ramaioli A, Chamorey E, Pouyssegur J, et al. HIF-1alpha and CA IX staining in invasive breast carcinomas: prognosis and treatment outcome. Int J Cancer (2007) 120:1451–8. doi:10.1002/ijc.22436
35. Blank A, Schmitt AM, Korpershoek E, van Nederveen F, Rudolph T, Weber N, et al. SDHB loss predicts malignancy in pheochromocytomas/sympathethic paragangliomas, but not through hypoxia signalling. Endocr Relat Cancer (2010) 17:919–28. doi:10.1677/erc-09-0316
36. Cleven AH, van Engeland M, Wouters BG, de Bruine AP. Stromal expression of hypoxia regulated proteins is an adverse prognostic factor in colorectal carcinomas. Cell Oncol (2007) 29:229–40.
37. Huber AR, Tan D, Sun J, Dean D, Wu T, Zhou Z. High expression of carbonic anhydrase IX is significantly associated with glandular lesions in gastroesophageal junction and with tumorigenesis markers BMI1, MCM4 and MCM7. BMC Gastroenterol (2015) 15:80. doi:10.1186/s12876-015-0310-6
38. Jung JH, Im S, Jung ES, Kang CS. Clinicopathological implications of the expression of hypoxia-related proteins in gastric cancer. Int J Med Sci (2013) 10:1217–23. doi:10.7150/ijms.6054
39. Lee-Kong SA, Ruby JA, Chessin DB, Pucciarelli S, Shia J, Riedel ER, et al. Hypoxia-related proteins in patients with rectal cancer undergoing neoadjuvant combined modality therapy. Dis Colon Rectum (2012) 55:990–5. doi:10.1097/DCR.0b013e31825bd80c
40. Noh S, Kim JY, Koo JS. Metabolic differences in estrogen receptor-negative breast cancer based on androgen receptor status. Tumour Biol (2014) 35:8179–92. doi:10.1007/s13277-014-2103-x
41. Preusser M, Wolfsberger S, Haberler C, Breitschopf H, Czech T, Slavc I, et al. Vascularization and expression of hypoxia-related tissue factors in intracranial ependymoma and their impact on patient survival. Acta Neuropathol (2005) 109:211–6. doi:10.1007/s00401-004-0938-8
42. Seeber LM, Horree N, van der Groep P, van der Wall E, Verheijen RH, van Diest PJ. Necrosis related HIF-1alpha expression predicts prognosis in patients with endometrioid endometrial carcinoma. BMC Cancer (2010) 10:307. doi:10.1186/1471-2407-10-307
43. Winter SC, Shah KA, Han C, Campo L, Turley H, Leek R, et al. The relation between hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha expression with anemia and outcome in surgically treated head and neck cancer. Cancer (2006) 107:757–66. doi:10.1002/cncr.21983
44. Abraham S, Hu N, Jensen R. Hypoxia-inducible factor-1-regulated protein expression and oligodendroglioma patient outcome: comparison with established biomarkers and preoperative UCSF low-grade scoring system. J Neurooncol (2012) 108:459–68. doi:10.1007/s11060-012-0839-y
45. Ameis HM, Drenckhan A, Freytag M, Izbicki JR, Supuran CT, Reinshagen K, et al. Carbonic anhydrase IX correlates with survival and is a potential therapeutic target for neuroblastoma. J Enzyme Inhib Med Chem (2015) 17:1–6. doi:10.3109/14756366.2015.1029471
46. Beketic-Oreskovic L, Ozretic P, Rabbani ZN, Jackson IL, Sarcevic B, Levanat S, et al. Prognostic significance of carbonic anhydrase IX (CA-IX), endoglin (CD105) and 8-hydroxy-2’-deoxyguanosine (8-OHdG) in breast cancer patients. Pathol Oncol Res (2011) 17:593–603. doi:10.1007/s12253-010-9355-6
47. Betof AS, Rabbani ZN, Hardee ME, Kim SJ, Broadwater G, Bentley RC, et al. Carbonic anhydrase IX is a predictive marker of doxorubicin resistance in early-stage breast cancer independent of HER2 and TOP2A amplification. Br J Cancer (2012) 106:916–22. doi:10.1038/bjc.2012.32
48. Birner P, Jesch B, Friedrich J, Riegler M, Zacherl J, Hejna M, et al. Carbonic anhydrase IX overexpression is associated with diminished prognosis in esophageal cancer and correlates with Her-2 expression. Ann Surg Oncol (2011) 18:3330–7. doi:10.1245/s10434-011-1730-3
49. Brockton N, Dort J, Lau H, Hao D, Brar S, Klimowicz A, et al. High stromal carbonic anhydrase IX expression is associated with decreased survival in P16-negative head-and-neck tumors. Int J Radiat Oncol Biol Phys (2011) 80:249–57. doi:10.1016/j.ijrobp.2010.11.059
50. Chang DT, Chapman CH, Norton JA, Visser B, Fisher GA, Kunz P, et al. Expression of p16(INK4A) but not hypoxia markers or poly adenosine diphosphate-ribose polymerase is associated with improved survival in patients with pancreatic adenocarcinoma. Cancer (2010) 116:5179–87. doi:10.1002/cncr.25481
51. Chen J, Rocken C, Hoffmann J, Kruger S, Lendeckel U, Rocco A, et al. Expression of carbonic anhydrase 9 at the invasion front of gastric cancers. Gut (2005) 54:920–7. doi:10.1136/gut.2004.047340
52. Chen Y, Li X, Wu S, Xu G, Zhou Y, Gong L, et al. Expression of HIF-1alpha and CAIX in nasopharyngeal carcinoma and their correlation with patients’ prognosis. Med Oncol (2014) 31:304. doi:10.1007/s12032-014-0304-1
53. Choi SW, Kim JY, Park JY, Cha IH, Kim J, Lee S. Expression of carbonic anhydrase IX is associated with postoperative recurrence and poor prognosis in surgically treated oral squamous cell carcinoma. Hum Pathol (2008) 39:1317–22. doi:10.1016/j.humpath.2007.10.026
54. Choschzick M, Oosterwijk E, Muller V, Woelber L, Simon R, Moch H, et al. Overexpression of carbonic anhydrase IX (CAIX) is an independent unfavorable prognostic marker in endometrioid ovarian cancer. Virchows Arch (2011) 459:193–200. doi:10.1007/s00428-011-1105-y
55. Couvelard A, O’Toole D, Leek R, Turley H, Sauvanet A, Degott C, et al. Expression of hypoxia-inducible factors is correlated with the presence of a fibrotic focus and angiogenesis in pancreatic ductal adenocarcinomas. Histopathology (2005) 46:668–76. doi:10.1111/j.1365-2559.2005.02160.x
56. Couvelard A, O’Toole D, Turley H, Leek R, Sauvanet A, Degott C, et al. Microvascular density and hypoxia-inducible factor pathway in pancreatic endocrine tumours: negative correlation of microvascular density and VEGF expression with tumour progression. Br J Cancer (2005) 92:94–101. doi:10.1038/sj.bjc.6602245
57. Currie MJ, Beardsley BE, Harris GC, Gunningham SP, Dachs GU, Dijkstra B, et al. Immunohistochemical analysis of cancer stem cell markers in invasive breast carcinoma and associated ductal carcinoma in situ: relationships with markers of tumor hypoxia and microvascularity. Hum Pathol (2013) 44:402–11. doi:10.1016/j.humpath.2012.06.004
58. Deb S, Johansson I, Byrne D, Nilsson C, Investigators k, Constable L, et al. Nuclear HIF1A expression is strongly prognostic in sporadic but not familial male breast cancer. Mod Pathol (2014) 27:1223–30. doi:10.1038/modpathol.2013.231
59. Debucquoy A, Goethals L, Libbrecht L, Perneel C, Geboes K, Ectors N, et al. Molecular and clinico-pathological markers in rectal cancer: a tissue micro-array study. Int J Colorectal Dis (2009) 24:129–38. doi:10.1007/s00384-008-0608-8
60. Deschamps L, Bacha D, Rebours V, Mebarki M, Bretagnol F, Panis Y, et al. The expression of the hypoxia markers CA9 and CXCR4 is correlated with survival in patients with neuroendocrine tumours of the ileum. Neuroendocrinology (2012) 95:214–22. doi:10.1159/000329873
61. Driessen A, Landuyt W, Pastorekova S, Moons J, Goethals L, Haustermans K, et al. Expression of carbonic anhydrase IX (CA IX), a hypoxia-related protein, rather than vascular-endothelial growth factor (VEGF), a pro-angiogenic factor, correlates with an extremely poor prognosis in esophageal and gastric adenocarcinomas. Ann Surg (2006) 243:334–40. doi:10.1097/01.sla.0000201452.09591.f3
62. Dungwa JV, Hunt LP, Ramani P. Carbonic anhydrase IX up-regulation is associated with adverse clinicopathologic and biologic factors in neuroblastomas. Hum Pathol (2012) 43:1651–60. doi:10.1016/j.humpath.2011.12.006
63. Eckert AW, Lautner MH, Schutze A, Bolte K, Bache M, Kappler M, et al. Co-expression of Hif1alpha and CAIX is associated with poor prognosis in oral squamous cell carcinoma patients. J Oral Pathol Med (2010) 39:313–7. doi:10.1111/j.1600-0714.2009.00829.x
64. Erpolat OP, Gocun PU, Akmansu M, Ozgun G, Akyol G. Hypoxia-related molecules HIF-1alpha, CA9, and osteopontin: predictors of survival in patients with high-grade glioma. Strahlenther Onkol (2013) 189:147–54. doi:10.1007/s00066-012-0262-5
65. Eustace A, Irlam JJ, Taylor J, Denley H, Agrawal S, Choudhury A, et al. Necrosis predicts benefit from hypoxia-modifying therapy in patients with high risk bladder cancer enrolled in a phase III randomised trial. Radiother Oncol (2013) 108:40–7. doi:10.1016/j.radonc.2013.05.017
66. Flynn JR, Wang L, Gillespie DL, Stoddard GJ, Reid JK, Owens J, et al. Hypoxia-regulated protein expression, patient characteristics, and preoperative imaging as predictors of survival in adults with glioblastoma multiforme. Cancer (2008) 113:1032–42. doi:10.1002/cncr.23678
67. Garcia S, Dales JP, Charafe-Jauffret E, Carpentier-Meunier S, Andrac-Meyer L, Jacquemier J, et al. Poor prognosis in breast carcinomas correlates with increased expression of targetable CD146 and c-Met and with proteomic basal-like phenotype. Hum Pathol (2007) 38:830–41. doi:10.1016/j.humpath.2006.11.015
68. Generali D, Fox SB, Berruti A, Brizzi MP, Campo L, Bonardi S, et al. Role of carbonic anhydrase IX expression in prediction of the efficacy and outcome of primary epirubicin/tamoxifen therapy for breast cancer. Endocr Relat Cancer (2006) 13:921–30. doi:10.1677/erc.1.01216
69. Giatromanolaki A, Koukourakis MI, Sivridis E, Pastorek J, Wykoff CC, Gatter KC, et al. Expression of hypoxia-inducible carbonic anhydrase-9 relates to angiogenic pathways and independently to poor outcome in non-small cell lung cancer. Cancer Res (2001) 61:7992–8.
70. Gu MJ, Kwon KW. Carbonic anhydrase IX expression is associated with favorable prognostic factors in small intestinal carcinoma. J Histochem Cytochem (2014) 62:205–10. doi:10.1369/0022155413512657
71. Gu M. CA9 overexpression is an independent favorable prognostic marker in intrahepatic cholangiocarcinoma. Int J Clin Exp Pathol (2015) 8:862–6.
72. Haapasalo JA, Nordfors KM, Hilvo M, Rantala IJ, Soini Y, Parkkila AK, et al. Expression of carbonic anhydrase IX in astrocytic tumors predicts poor prognosis. Clin Cancer Res (2006) 12:473–7. doi:10.1158/1078-0432.ccr-05-0848
73. Han MW, Lee HJ, Cho KJ, Kim JS, Roh JL, Choi SH, et al. Role of FDG-PET as a biological marker for predicting the hypoxic status of tongue cancer. Head Neck (2012) 34:1395–402. doi:10.1002/hed.21945
74. Huang WJ, Jeng YM, Lai HS, Fong IU, Sheu FY, Lai PL, et al. Expression of hypoxic marker carbonic anhydrase IX predicts poor prognosis in resectable hepatocellular carcinoma. PLoS One (2015) 10:e0119181. doi:10.1371/journal.pone.0119181
75. Hui EP, Chan AT, Pezzella F, Turley H, To KF, Poon TC, et al. Coexpression of hypoxia-inducible factors 1alpha and 2alpha, carbonic anhydrase IX, and vascular endothelial growth factor in nasopharyngeal carcinoma and relationship to survival. Clin Cancer Res (2002) 8:2595–604.
76. Hussain SA, Ganesan R, Reynolds G, Gross L, Stevens A, Pastorek J, et al. Hypoxia-regulated carbonic anhydrase IX expression is associated with poor survival in patients with invasive breast cancer. Br J Cancer (2007) 96:104–9. doi:10.1038/sj.bjc.6603530
77. Hynninen P, Vaskivuo L, Saarnio J, Haapasalo H, Kivela J, Pastorekova S, et al. Expression of transmembrane carbonic anhydrases IX and XII in ovarian tumours. Histopathology (2006) 49:594–602. doi:10.1111/j.1365-2559.2006.02523.x
78. Ilie M, Mazure NM, Hofman V, Ammadi RE, Ortholan C, Bonnetaud C, et al. High levels of carbonic anhydrase IX in tumour tissue and plasma are biomarkers of poor prognostic in patients with non-small cell lung cancer. Br J Cancer (2010) 102:1627–35. doi:10.1038/sj.bjc.6605690
79. Jarvela S, Parkkila S, Bragge H, Kahkonen M, Parkkila AK, Soini Y, et al. Carbonic anhydrase IX in oligodendroglial brain tumors. BMC Cancer (2008) 8:1. doi:10.1186/1471-2407-8-1
80. Jensen R, Lee J. Predicting outcomes of patients with intracranial meningiomas using molecular markers of hypoxia, vascularity, and proliferation. Neurosurgery (2012) 71:146–56. doi:10.1227/NEU.0b013e3182567886
81. Jubb AM, Turley H, Moeller HC, Steers G, Han C, Li JL, et al. Expression of delta-like ligand 4 (Dll4) and markers of hypoxia in colon cancer. Br J Cancer (2009) 101:1749–57. doi:10.1038/sj.bjc.6605368
82. Kang HJ, Kim IH, Sung CO, Shim JH, Yu E. Expression of carbonic anhydrase 9 is a novel prognostic marker in resectable hepatocellular carcinoma. Virchows Arch (2015) 466:403–13. doi:10.1007/s00428-014-1709-0
83. Kato Y, Yashiro M, Noda S, Kashiwagi S, Matsuoka J, Fuyuhiro Y, et al. Expression of a hypoxia-associated protein, carbonic anhydrase-9, correlates with malignant phenotypes of gastric carcinoma. Digestion (2010) 82:246–51. doi:10.1159/000297208
84. Kaya AO, Gunel N, Benekli M, Akyurek N, Buyukberber S, Tatli H, et al. Hypoxia inducible factor-1 alpha and carbonic anhydrase IX overexpression are associated with poor survival in breast cancer patients. J BUON (2012) 17:663–8.
85. Kim BW, Cho H, Chung JY, Conway C, Ylaya K, Kim JH, et al. Prognostic assessment of hypoxia and metabolic markers in cervical cancer using automated digital image analysis of immunohistochemistry. J Transl Med (2013) 11:185. doi:10.1186/1479-5876-11-185
86. Kim HM, Jung WH, Koo JS. Site-specific metabolic phenotypes in metastatic breast cancer. J Transl Med (2014) 12:354. doi:10.1186/s12967-014-0354-3
87. Kim JI, Choi KU, Lee IS, Choi YJ, Kim WT, Shin DH, et al. Expression of hypoxic markers and their prognostic significance in soft tissue sarcoma. Oncol Lett (2015) 9:1699–706. doi:10.3892/ol.2015.2914
88. Kim K, Park WY, Kim JY, Sol MY, Shin DH, Park do Y, et al. Prognostic relevance of the expression of CA IX, GLUT-1, and VEGF in ovarian epithelial cancers. Korean J Pathol (2012) 46:532–40. doi:10.4132/KoreanJPathol.2012.46.6.532
89. Kim SJ, Shin HJ, Jung KY, Baek SK, Shin BK, Choi J, et al. Prognostic value of carbonic anhydrase IX and Ki-67 expression in squamous cell carcinoma of the tongue. Jpn J Clin Oncol (2007) 37:812–9. doi:10.1093/jjco/hym121
90. Klatte T, Seligson DB, Rao JY, Yu H, de Martino M, Kawaoka K, et al. Carbonic anhydrase IX in bladder cancer: a diagnostic, prognostic, and therapeutic molecular marker. Cancer (2009) 115:1448–58. doi:10.1002/cncr.24163
91. Kondo Y, Yoshikawa K, Omura Y, Shinohara A, Kazaoka Y, Sano J, et al. Clinicopathological significance of carbonic anhydrase 9, glucose transporter-1, Ki-67 and p53 expression in oral squamous cell carcinoma. Oncol Rep (2011) 25:1227–33. doi:10.3892/or.2011.1216
92. Korkolopoulou P, Perdiki M, Thymara I, Boviatsis E, Agrogiannis G, Kotsiakis X, et al. Expression of hypoxia-related tissue factors in astrocytic gliomas. A multivariate survival study with emphasis upon carbonic anhydrase IX. Hum Pathol (2007) 38:629–38. doi:10.1016/j.humpath.2006.07.020
93. Kornegoor R, Verschuur-Maes AH, Buerger H, Hogenes MC, de Bruin PC, Oudejans JJ, et al. Fibrotic focus and hypoxia in male breast cancer. Mod Pathol (2012) 25:1397–404. doi:10.1038/modpathol.2012.101
94. Koukourakis MI, Giatromanolaki A, Sivridis E, Simopoulos K, Pastorek J, Wykoff CC, et al. Hypoxia-regulated carbonic anhydrase-9 (CA9) relates to poor vascularization and resistance of squamous cell head and neck cancer to chemoradiotherapy. Clin Cancer Res (2001) 7:3399–403.
95. Kyndi M, Sorensen FB, Knudsen H, Alsner J, Overgaard M, Nielsen HM, et al. Carbonic anhydrase IX and response to postmastectomy radiotherapy in high-risk breast cancer: a subgroup analysis of the DBCG82 b and c trials. Breast Cancer Res (2008) 10:R24. doi:10.1186/bcr1981
96. Lastraioli E, Bencini L, Bianchini E, Romoli MR, Crociani O, Giommoni E, et al. hERG1 channels and Glut-1 as independent prognostic indicators of worse outcome in stage I and II colorectal cancer: a pilot study. Transl Oncol (2012) 5:105–12. doi:10.1593/tlo.11250
97. Le QT, Shi G, Cao H, Nelson DW, Wang Y, Chen EY, et al. Galectin-1: a link between tumor hypoxia and tumor immune privilege. J Clin Oncol (2005) 23:8932–41. doi:10.1200/jco.2005.02.0206
98. Le QT, Chen E, Salim A, Cao H, Kong CS, Whyte R, et al. An evaluation of tumor oxygenation and gene expression in patients with early stage non-small cell lung cancers. Clin Cancer Res (2006) 12:1507–14. doi:10.1158/1078-0432.ccr-05-2049
99. Li Y, Dong M, Sheng W, Huang L. Roles of carbonic anhydrase IX in development of pancreatic cancer. Pathol Oncol Res (2015). doi:10.1007/s12253-015-9935-6
100. Liao SY, Darcy KM, Randall LM, Tian C, Monk BJ, Burger RA, et al. Prognostic relevance of carbonic anhydrase-IX in high-risk, early-stage cervical cancer: a gynecologic oncology group study. Gynecol Oncol (2010) 116:452–8. doi:10.1016/j.ygyno.2009.10.062
101. Liu Z, Yang Z, Jiang S, Zou Q, Yuan Y, Li J, et al. Paxillin and carbonic anhydrase IX are prognostic markers in gallbladder squamous cell/adenosquamous carcinomas and adenocarcinomas. Histopathology (2014) 64:921–34. doi:10.1111/his.12341
102. Maseide K, Kandel RA, Bell RS, Catton CN, O’Sullivan B, Wunder JS, et al. Carbonic anhydrase IX as a marker for poor prognosis in soft tissue sarcoma. Clin Cancer Res (2004) 10:4464–71. doi:10.1158/1078-0432.ccr-03-0541
103. Nakao M, Ishii G, Nagai K, Kawase A, Kenmotsu H, Kon-No H, et al. Prognostic significance of carbonic anhydrase IX expression by cancer-associated fibroblasts in lung adenocarcinoma. Cancer (2009) 115:2732–43. doi:10.1002/cncr.24303
104. Nordfors K, Haapasalo J, Korja M, Niemela A, Laine J, Parkkila AK, et al. The tumour-associated carbonic anhydrases CA II, CA IX and CA XII in a group of medulloblastomas and supratentorial primitive neuroectodermal tumours: an association of CA IX with poor prognosis. BMC Cancer (2010) 10:148. doi:10.1186/1471-2407-10-148
105. Ono S, Ishii G, Nagai K, Takuwa T, Yoshida J, Nishimura M, et al. Podoplanin-positive cancer-associated fibroblasts could have prognostic value independent of cancer cell phenotype in stage I lung squamous cell carcinoma: usefulness of combining analysis of both cancer cell phenotype and cancer-associated fibroblast phenotype. Chest (2013) 143:963–70. doi:10.1378/chest.12-0913
106. Ord JJ, Agrawal S, Thamboo TP, Roberts I, Campo L, Turley H, et al. An investigation into the prognostic significance of necrosis and hypoxia in high grade and invasive bladder cancer. J Urol (2007) 178:677–82. doi:10.1016/j.juro.2007.03.112
107. O’Toole D, Couvelard A, Rebours V, Zappa M, Hentic O, Hammel P, et al. Molecular markers associated with response to chemotherapy in gastro-entero-pancreatic neuroendocrine tumors. Endocr Relat Cancer (2010) 17:847–56. doi:10.1677/erc-09-0204
108. Perez-Sayans M, Suarez-Penaranda JM, Pilar GD, Supuran CT, Pastorekova S, Barros-Angueira F, et al. Expression of CA-IX is associated with advanced stage tumors and poor survival in oral squamous cell carcinoma patients. J Oral Pathol Med (2012) 41:667–74. doi:10.1111/j.1600-0714.2012.01147.x
109. Pinato DJ, Tan TM, Toussi ST, Ramachandran R, Martin N, Meeran K, et al. An expression signature of the angiogenic response in gastrointestinal neuroendocrine tumours: correlation with tumour phenotype and survival outcomes. Br J Cancer (2014) 110:115–22. doi:10.1038/bjc.2013.682
110. Polanski R, Hodgkinson CL, Fusi A, Nonaka D, Priest L, Kelly P, et al. Activity of the monocarboxylate transporter 1 inhibitor AZD3965 in small cell lung cancer. Clin Cancer Res (2014) 20:926–37. doi:10.1158/1078-0432.ccr-13-2270
111. Proescholdt MA, Merrill MJ, Stoerr EM, Lohmeier A, Pohl F, Brawanski A. Function of carbonic anhydrase IX in glioblastoma multiforme. Neuro Oncol (2012) 14:1357–66. doi:10.1093/neuonc/nos216
112. Roh JL, Cho KJ, Kwon GY, Choi SH, Nam SY, Kim SY. Prognostic values of pathologic findings and hypoxia markers in 21 patients with salivary duct carcinoma. J Surg Oncol (2008) 97:596–600. doi:10.1002/jso.21045
113. Sadlecki P, Bodnar M, Grabiec M, Marszalek A, Walentowicz P, Sokup A, et al. The role of hypoxia-inducible factor-1 alpha, glucose transporter-1, (GLUT-1) and carbon anhydrase IX in endometrial cancer patients. Biomed Res Int (2014) 2014:616850. doi:10.1155/2014/616850
114. Sergeant G, Lerut E, Ectors N, Hendrickx T, Aerts R, Topal B. The prognostic relevance of tumor hypoxia markers in resected carcinoma of the gallbladder. Eur J Surg Oncol (2011) 37:80–6. doi:10.1016/j.ejso.2010.10.007
115. Sooman L, Freyhult E, Jaiswal A, Navani S, Edqvist PH, Ponten F, et al. FGF2 as a potential prognostic biomarker for proneural glioma patients. Acta Oncol (2015) 54:385–94. doi:10.3109/0284186x.2014.951492
116. Srivastava S, Thakkar B, Yeoh KG, Ho KY, Teh M, Soong R, et al. Expression of proteins associated with hypoxia and Wnt pathway activation is of prognostic significance in hepatocellular carcinoma. Virchows Arch (2015) 466:541–8. doi:10.1007/s00428-015-1745-4
117. Stewart DJ, Nunez MI, Behrens C, Liu D, Lin YH, Lee JJ, et al. Membrane carbonic anhydrase IX expression and relapse risk in resected stage I-II non-small-cell lung cancer. J Thorac Oncol (2014) 9:675–84. doi:10.1097/jto.0000000000000148
118. Swinson DE, Cox G, O’Byrne KJ. Coexpression of epidermal growth factor receptor with related factors is associated with a poor prognosis in non-small-cell lung cancer. Br J Cancer (2004) 91:1301–7. doi:10.1038/sj.bjc.6602149
119. Tan EY, Yan M, Campo L, Han C, Takano E, Turley H, et al. The key hypoxia regulated gene CAIX is upregulated in basal-like breast tumours and is associated with resistance to chemotherapy. Br J Cancer (2009) 100:405–11. doi:10.1038/sj.bjc.6604844
120. Tomes L, Emberley E, Niu Y, Troup S, Pastorek J, Strange K, et al. Necrosis and hypoxia in invasive breast carcinoma. Breast Cancer Res Treat (2003) 81:61–9. doi:10.1023/a:1025476722493
121. Verstraete M, Debucquoy A, Devos E, Sagaert X, Penninckx F, Begg A, et al. Investigation of possible endogenous hypoxia markers in colorectal cancer. Int J Radiat Biol (2013) 89:9–15. doi:10.3109/09553002.2012.715789
122. Wachters JE, Schrijvers ML, Slagter-Menkema L, Mastik M, de Bock GH, Langendijk JA, et al. Prognostic significance of HIF-1a, CA-IX, and OPN in T1-T2 laryngeal carcinoma treated with radiotherapy. Laryngoscope (2013) 123:2154–60. doi:10.1002/lary.23831
123. Williams E, Martin S, Moss R, Durrant L, Deen S. Co-expression of VEGF and CA9 in ovarian high-grade serous carcinoma and relationship to survival. Virchows Arch (2012) 461:33–9. doi:10.1007/s00428-012-1252-9
124. Woelber L, Kress K, Kersten JF, Choschzick M, Kilic E, Herwig U, et al. Carbonic anhydrase IX in tumor tissue and sera of patients with primary cervical cancer. BMC Cancer (2011) 11:12. doi:10.1186/1471-2407-11-12
125. Yan P, Li YH, Tang ZJ, Shu X, Liu X. High monocarboxylate transporter 4 protein expression in stromal cells predicts adverse survival in gastric cancer. Asian Pac J Cancer Prev (2014) 15:8923–9. doi:10.7314/APJCP.2014.15.20.8923
126. Yang JS, Lin CW, Chuang CY, Su SC, Lin SH, Yang SF. Carbonic anhydrase IX overexpression regulates the migration and progression in oral squamous cell carcinoma. Tumour Biol (2015) 36(12):9517–24. doi:10.1007/s13277-015-3692-8
127. Yoo H, Sohn S, Nam BH, Min HS, Jung E, Shin SH, et al. The expressions of carbonic anhydrase 9 and vascular endothelial growth factor in astrocytic tumors predict a poor prognosis. Int J Mol Med (2010) 26:3–9. doi:10.3892/ijmm_00000427
128. Yu M, Zhou Q, Zhou Y, Fu Z, Tan L, Ye X, et al. Metabolic phenotypes in pancreatic cancer. PLoS One (2015) 10:e0115153. doi:10.1371/journal.pone.0115153
129. Zheng G, Peng C, Jia X, Gu Y, Zhang Z, Deng Y, et al. ZEB1 transcriptionally regulated carbonic anhydrase 9 mediates the chemoresistance of tongue cancer via maintaining intracellular pH. Mol Cancer (2015) 14:84. doi:10.1186/s12943-015-0357-6
130. Rajaganeshan R, Prasad R, Guillou PJ, Scott N, Poston G, Jayne DG. Expression patterns of hypoxic markers at the invasive margin of colorectal cancers and liver metastases. Eur J Surg Oncol (2009) 35:1286–94. doi:10.1016/j.ejso.2009.05.008
131. Aomatsu N, Yashiro M, Kashiwagi S, Kawajiri H, Takashima T, Ohsawa M, et al. Carbonic anhydrase 9 is associated with chemosensitivity and prognosis in breast cancer patients treated with taxane and anthracycline. BMC Cancer (2014) 14:400. doi:10.1186/1471-2407-14-400
132. Choschzick M, Woelber L, Hess S, zu Eulenburg C, Schwarz J, Simon R, et al. Overexpression of carbonic anhydrase IX (CAIX) in vulvar cancer is associated with tumor progression and development of locoregional lymph node metastases. Virchows Arch (2010) 456:483–90. doi:10.1007/s00428-010-0905-9
133. De Schutter H, Landuyt W, Verbeken E, Goethals L, Hermans R, Nuyts S. The prognostic value of the hypoxia markers CA IX and GLUT 1 and the cytokines VEGF and IL 6 in head and neck squamous cell carcinoma treated by radiotherapy ± chemotherapy. BMC Cancer (2005) 5:42. doi:10.1186/1471-2407-5-42
134. Hedley D, Pintilie M, Woo J, Morrison A, Birle D, Fyles A, et al. Carbonic anhydrase IX expression, hypoxia, and prognosis in patients with uterine cervical carcinomas. Clin Cancer Res (2003) 9:5666–74.
135. Heo K, Kim YH, Sung HJ, Li HY, Yoo CW, Kim JY, et al. Hypoxia-induced up-regulation of apelin is associated with a poor prognosis in oral squamous cell carcinoma patients. Oral Oncol (2012) 48:500–6. doi:10.1016/j.oraloncology.2011.12.015
136. Hiraoka N, Ino Y, Sekine S, Tsuda H, Shimada K, Kosuge T, et al. Tumour necrosis is a postoperative prognostic marker for pancreatic cancer patients with a high interobserver reproducibility in histological evaluation. Br J Cancer (2010) 103:1057–65. doi:10.1038/sj.bjc.6605854
137. Kim SJ, Rabbani ZN, Dewhirst MW, Vujaskovic Z, Vollmer RT, Schreiber EG, et al. Expression of HIF-1alpha, CA IX, VEGF, and MMP-9 in surgically resected non-small cell lung cancer. Lung Cancer (2005) 49:325–35. doi:10.1016/j.lungcan.2005.03.036
138. Korkeila E, Talvinen K, Jaakkola PM, Minn H, Syrjanen K, Sundstrom J, et al. Expression of carbonic anhydrase IX suggests poor outcome in rectal cancer. Br J Cancer (2009) 100:874–80. doi:10.1038/sj.bjc.6604949
139. Lee S, Shin HJ, Han IO, Hong EK, Park SY, Roh JW, et al. Tumor carbonic anhydrase 9 expression is associated with the presence of lymph node metastases in uterine cervical cancer. Cancer Sci (2007) 98:329–33. doi:10.1111/j.1349-7006.2007.00396.x
140. Lou Y, McDonald PC, Oloumi A, Chia S, Ostlund C, Ahmadi A, et al. Targeting tumor hypoxia: suppression of breast tumor growth and metastasis by novel carbonic anhydrase IX inhibitors. Cancer Res (2011) 71:3364–76. doi:10.1158/0008-5472.can-10-4261
141. Pinheiro C, Sousa B, Albergaria A, Paredes J, Dufloth R, Vieira D, et al. GLUT1 and CAIX expression profiles in breast cancer correlate with adverse prognostic factors and MCT1 overexpression. Histol Histopathol (2011) 26:1279–86.
142. Schmitt AM, Schmid S, Rudolph T, Anlauf M, Prinz C, Kloppel G, et al. VHL inactivation is an important pathway for the development of malignant sporadic pancreatic endocrine tumors. Endocr Relat Cancer (2009) 16:1219–27. doi:10.1677/erc-08-0297
143. Zhu Y, Zhou XY, Yao XD, Zhang SL, Dai B, Zhang HL, et al. Prognostic value of carbonic anhydrase IX expression in penile squamous cell carcinoma: a pilot study. Urol Oncol (2013) 31:706–11. doi:10.1016/j.urolonc.2011.04.011
144. Bernstein JM, Andrews TD, Slevin NJ, West CM, Homer JJ. Prognostic value of hypoxia-associated markers in advanced larynx and hypopharynx squamous cell carcinoma. Laryngoscope (2015) 125:E8–15. doi:10.1002/lary.24933
145. Douglas CM, Bernstein JM, Ormston VE, Hall RC, Merve A, Swindell R, et al. Lack of prognostic effect of carbonic anhydrase-9, hypoxia inducible factor-1alpha and bcl-2 in 286 patients with early squamous cell carcinoma of the glottic larynx treated with radiotherapy. Clin Oncol (R Coll Radiol) (2013) 25:59–65. doi:10.1016/j.clon.2012.07.004
146. Eriksen JG, Overgaard J; Danish Head and Neck Cancer Study Group (DAHANCA). Lack of prognostic and predictive value of CA IX in radiotherapy of squamous cell carcinoma of the head and neck with known modifiable hypoxia: an evaluation of the DAHANCA 5 study. Radiother Oncol (2007) 83:383–8. doi:10.1016/j.radonc.2007.05.009
147. Guedj N, Bretagnol F, Rautou PE, Deschamps L, Cazals-Hatem D, Bedossa P, et al. Predictors of tumor response after preoperative chemoradiotherapy for rectal adenocarcinomas. Hum Pathol (2011) 42:1702–9. doi:10.1016/j.humpath.2011.01.015
148. Kirkpatrick JP, Rabbani ZN, Bentley RC, Hardee ME, Karol S, Meyer J, et al. Elevated CAIX expression is associated with an increased risk of distant failure in early-stage cervical cancer. Biomark Insights (2008) 3:45–55.
149. Kwon OJ, Park JJ, Ko GH, Seo JH, Jeong BK, Kang KM, et al. HIF-1alpha and CA-IX as predictors of locoregional control for determining the optimal treatment modality for early-stage laryngeal carcinoma. Head Neck (2015) 37:505–10. doi:10.1002/hed.23620
150. Loncaster JA, Harris AL, Davidson SE, Logue JP, Hunter RD, Wycoff CC, et al. Carbonic anhydrase (CA IX) expression, a potential new intrinsic marker of hypoxia: correlations with tumor oxygen measurements and prognosis in locally advanced carcinoma of the cervix. Cancer Res (2001) 61:6394–9.
151. Nordsmark M, Eriksen JG, Gebski V, Alsner J, Horsman MR, Overgaard J. Differential risk assessments from five hypoxia specific assays: the basis for biologically adapted individualized radiotherapy in advanced head and neck cancer patients. Radiother Oncol (2007) 83:389–97. doi:10.1016/j.radonc.2007.04.021
152. Roh JL, Cho KJ, Kwon GY, Ryu CH, Chang HW, Choi SH, et al. The prognostic value of hypoxia markers in T2-staged oral tongue cancer. Oral Oncol (2009) 45:63–8. doi:10.1016/j.oraloncology.2008.03.017
153. Sakata K, Someya M, Nagakura H, Nakata K, Oouchi A, Takagi M, et al. Brachytherapy for oral tongue cancer: an analysis of treatment results with various biological markers. Jpn J Clin Oncol (2008) 38:402–7. doi:10.1093/jjco/hyn050
154. Silva P, Slevin NJ, Sloan P, Valentine H, Ryder D, Price P, et al. Use of multiple biological markers in radiotherapy-treated head and neck cancer. J Laryngol Otol (2010) 124:650–8. doi:10.1017/s0022215110000228
155. Turner KJ, Crew JP, Wykoff CC, Watson PH, Poulsom R, Pastorek J, et al. The hypoxia-inducible genes VEGF and CA9 are differentially regulated in superficial vs. invasive bladder cancer. Br J Cancer (2002) 86:1276–82. doi:10.1038/sj.bjc.6600215
156. Andersen S, Eilertsen M, Donnem T, Al-Shibli K, Al-Saad S, Busund LT, et al. Diverging prognostic impacts of hypoxic markers according to NSCLC histology. Lung Cancer (2011) 72:294–302. doi:10.1016/j.lungcan.2010.10.006
157. Brockton NT, Klimowicz AC, Bose P, Petrillo SK, Konno M, Rudmik L, et al. High stromal carbonic anhydrase IX expression is associated with nodal metastasis and decreased survival in patients with surgically-treated oral cavity squamous cell carcinoma. Oral Oncol (2012) 48:615–22. doi:10.1016/j.oraloncology.2012.01.018
158. Hwa JS, Kwon OJ, Park JJ, Woo SH, Kim JP, Ko GH, et al. The prognostic value of immunohistochemical markers for oral tongue squamous cell carcinoma. Eur Arch Otorhinolaryngol (2015) 272:2953–9. doi:10.1007/s00405-014-3254-5
159. Kivela AJ, Knuuttila A, Rasanen J, Sihvo E, Salmenkivi K, Saarnio J, et al. Carbonic anhydrase IX in malignant pleural mesotheliomas: a potential target for anti-cancer therapy. Bioorg Med Chem (2013) 21:1483–8. doi:10.1016/j.bmc.2012.09.018
160. Neumeister VM, Sullivan CA, Lindner R, Lezon-Geyda K, Li J, Zavada J, et al. Hypoxia-induced protein CAIX is associated with somatic loss of BRCA1 protein and pathway activity in triple negative breast cancer. Breast Cancer Res Treat (2012) 136:67–75. doi:10.1007/s10549-012-2232-0
161. Rasheed S, Harris AL, Tekkis PP, Turley H, Silver A, McDonald PJ, et al. Assessment of microvessel density and carbonic anhydrase-9 (CA-9) expression in rectal cancer. Pathol Res Pract (2009) 205:1–9. doi:10.1016/j.prp.2008.08.008
162. Sherwood BT, Colquhoun AJ, Richardson D, Bowman KJ, O’Byrne KJ, Kockelbergh RC, et al. Carbonic anhydrase IX expression and outcome after radiotherapy for muscle-invasive bladder cancer. Clin Oncol (R Coll Radiol) (2007) 19:777–83. doi:10.1016/j.clon.2007.06.014
163. Smeland E, Kilvaer TK, Sorbye S, Valkov A, Andersen S, Bremnes RM, et al. Prognostic impacts of hypoxic markers in soft tissue sarcoma. Sarcoma (2012) 2012:541650. doi:10.1155/2012/541650
164. Tanaka N, Kato H, Inose T, Kimura H, Faried A, Sohda M, et al. Expression of carbonic anhydrase 9, a potential intrinsic marker of hypoxia, is associated with poor prognosis in oesophageal squamous cell carcinoma. Br J Cancer (2008) 99:1468–75. doi:10.1038/sj.bjc.6604719
165. van de Nieuwenhof HP, de Hullu JA, Kaanders JH, Bulten J, Massuger LF, van Kempen LC. Hemoglobin level predicts outcome for vulvar cancer patients independent of GLUT-1 and CA-IX expression in tumor tissue. Virchows Arch (2010) 457:693–703. doi:10.1007/s00428-010-0981-x
166. Doyen J, Trastour C, Ettore F, Peyrottes I, Toussant N, Gal J, et al. Expression of the hypoxia-inducible monocarboxylate transporter MCT4 is increased in triple negative breast cancer and correlates independently with clinical outcome. Biochem Biophys Res Commun (2014) 451:54–61. doi:10.1016/j.bbrc.2014.07.050
167. Boeuf S, Bovee JV, Lehner B, Hogendoorn PC, Richter W. Correlation of hypoxic signalling to histological grade and outcome in cartilage tumours. Histopathology (2010) 56:641–51. doi:10.1111/j.1365-2559.2010.03528.x
168. Schweiger T, Kollmann D, Nikolowsky C, Traxler D, Guenova E, Lang G, et al. Carbonic anhydrase IX is associated with early pulmonary spreading of primary colorectal carcinoma and tobacco smoking. Eur J Cardiothorac Surg (2014) 46:92–9. doi:10.1093/ejcts/ezt542
169. Grigsby PW, Malyapa RS, Higashikubo R, Schwarz JK, Welch MJ, Huettner PC, et al. Comparison of molecular markers of hypoxia and imaging with (60)Cu-ATSM in cancer of the uterine cervix. Mol Imaging Biol (2007) 9:278–83. doi:10.1007/s11307-007-0095-2
170. Wykoff CC, Beasley NJ, Watson PH, Turner KJ, Pastorek J, Sibtain A, et al. Hypoxia-inducible expression of tumor-associated carbonic anhydrases. Cancer Res (2000) 60:7075–83.
171. Kaluz S, Kaluzova M, Chrastina A, Olive PL, Pastorekova S, Pastorek J, et al. Lowered oxygen tension induces expression of the hypoxia marker MN/carbonic anhydrase IX in the absence of hypoxia-inducible factor 1 alpha stabilization: a role for phosphatidylinositol 3’-kinase. Cancer Res (2002) 62:4469–77.
172. van den Beucken T, Ramaekers CH, Rouschop K, Koritzinsky M, Wouters BG. Deficient carbonic anhydrase 9 expression in UPR-impaired cells is associated with reduced survival in an acidic microenvironment. Radiother Oncol (2009) 92:437–42. doi:10.1016/j.radonc.2009.06.018
173. Lock FE, McDonald PC, Lou Y, Serrano I, Chafe SC, Ostlund C, et al. Targeting carbonic anhydrase IX depletes breast cancer stem cells within the hypoxic niche. Oncogene (2013) 32:5210–9. doi:10.1038/onc.2012.550
174. Svastova E, Witarski W, Csaderova L, Kosik I, Skvarkova L, Hulikova A, et al. Carbonic anhydrase IX interacts with bicarbonate transporters in lamellipodia and increases cell migration via its catalytic domain. J Biol Chem (2012) 287:3392–402. doi:10.1074/jbc.M111.286062
175. Csaderova L, Debreova M, Radvak P, Stano M, Vrestiakova M, Kopacek J, et al. The effect of carbonic anhydrase IX on focal contacts during cell spreading and migration. Front Physiol (2013) 4:271. doi:10.3389/fphys.2013.00271
176. Ward C, Meehan J, Mullen P, Supuran C, Dixon JM, Thomas JS, et al. Evaluation of carbonic anhydrase IX as a therapeutic target for inhibition of breast cancer invasion and metastasis using a series of in vitro breast cancer models. Oncotarget (2015) 6:24856–70. doi:10.18632/oncotarget.4498
177. Ledaki I, McIntyre A, Wigfield S, Buffa F, McGowan S, Baban D, et al. Carbonic anhydrase IX induction defines a heterogeneous cancer cell response to hypoxia and mediates stem cell-like properties and sensitivity to HDAC inhibition. Oncotarget (2015) 6:19413–27. doi:10.18632/oncotarget.4989
178. Papagerakis S, Bellile E, Peterson LA, Pliakas M, Balaskas K, Selman S, et al. Proton pump inhibitors and histamine 2 blockers are associated with improved overall survival in patients with head and neck squamous carcinoma. Cancer Prev Res (2014) 7:1258–69. doi:10.1158/1940-6207.CAPR-14-0002
179. Fais S. Evidence-based support for the use of proton pump inhibitors in cancer therapy. J Transl Med (2015) 13:368. doi:10.1186/s12967-015-0735-2
180. Spugnini EP, Sonveaux P, Stock C, Perez-Sayans M, De Milito A, Avnet S, et al. Proton channels and exchangers in cancer. Biochim Biophys Acta (2015) 1848:2715–26. doi:10.1016/j.bbamem.2014.10.015
181. Borenstein M, Hedges LV, Higgins JP, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods (2010) 1:97–111. doi:10.1002/jrsm.12
182. Liu Z, Yao Z, Li C, Liu X, Chen H, Gao C. A step-by-step guide to the systematic review and meta-analysis of diagnostic and prognostic test accuracy evaluations. Br J Cancer (2013) 108:2299–303. doi:10.1038/bjc.2013.185
183. Adams A, van Brussel AS, Vermeulen JF, Mali WP, van der Wall E, van Diest PJ, et al. The potential of hypoxia markers as target for breast molecular imaging – a systematic review and meta-analysis of human marker expression. BMC Cancer (2013) 13:538. doi:10.1186/1471-2407-13-538
184. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol (2010) 25:603–5. doi:10.1007/s10654-010-9491-z
185. Oremus M, Oremus C, Hall GB, McKinnon MC; ECT & Cognition Systematic Review Team. Inter-rater and test-retest reliability of quality assessments by novice student raters using the Jadad and Newcastle-Ottawa scales. BMJ Open (2012) 2:ii:e001368. doi:10.1136/bmjopen-2012-001368
186. Hartling L, Milne A, Hamm MP, Vandermeer B, Ansari M, Tsertsvadze A, et al. Testing the Newcastle Ottawa scale showed low reliability between individual reviewers. J Clin Epidemiol (2013) 66:982–93. doi:10.1016/j.jclinepi.2013.03.003
Keywords: cancer, carbonic anhydrase IX, hypoxia, meta-analysis, prognosis
Citation: van Kuijk SJA, Yaromina A, Houben R, Niemans R, Lambin P and Dubois LJ (2016) Prognostic Significance of Carbonic Anhydrase IX Expression in Cancer Patients: A Meta-Analysis. Front. Oncol. 6:69. doi: 10.3389/fonc.2016.00069
Received: 23 December 2015; Accepted: 08 March 2016;
Published: 29 March 2016
Edited by:
Christian Gomez, University of Mississippi Medical Center, USAReviewed by:
Arkaitz Carracedo, Center for Cooperative Research in Biosciences, SpainStefano Fais, Istituto Superiore di Sanità, Italy
Copyright: © 2016 van Kuijk, Yaromina, Houben, Niemans, Lambin and Dubois. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Simon J. A. van Kuijk, cy52YW5rdWlqa0BtYWFzdHJpY2h0dW5pdmVyc2l0eS5ubA==
†Simon J.A. van Kuijk and Ala Yaromina contributed equally.
‡Philippe Lambin and Ludwig J. Dubois contributed equally.
 Simon J. A. van Kuijk
Simon J. A. van Kuijk Ala Yaromina
Ala Yaromina Ruud Houben
Ruud Houben Raymon Niemans
Raymon Niemans Philippe Lambin
Philippe Lambin Ludwig J. Dubois
Ludwig J. Dubois