- 1School of Medical and Molecular Biosciences, University of Technology Sydney, Sydney, NSW, Australia
- 2Bill Walsh Translational Cancer Research Laboratory, Kolling Institute, Northern Sydney Local Health District, St. Leonards, NSW, Australia
- 3Sydney Medical School Northern, University of Sydney, Sydney, NSW, Australia
Epithelial ovarian cancer is the fifth leading cause of cancer-related deaths in women and the most lethal gynecological malignancy. Extracellular matrix (ECM) is an integral component of both the normal and tumor microenvironment. ECM composition varies between tissues and is crucial for maintaining normal function and homeostasis. Dysregulation and aberrant deposition or loss of ECM components is implicated in ovarian cancer progression. The mechanisms by which tumor cells induce ECM remodeling to promote a malignant phenotype are yet to be elucidated. A thorough understanding of the role of the ECM in ovarian cancer is needed for the development of effective biomarkers and new therapies.
Introduction
Epithelial ovarian cancer (EOC) is currently the most lethal gynecological malignancy affecting women and the fifth leading cause of cancer-related deaths in the United States (1). Early diagnosis of EOC grants a favorable prognosis and an average 5-year survival rate of 92%. However, due to the lack of available screening tests (2), diagnosis of patients is predominantly made at an advanced stage, reducing the average 5-year survival rate to only 27% (3). Standard treatment has not significantly improved for decades. The tumor microenvironment is gaining recognition in facilitating cancer progression, playing an essential role in mediating the growth, invasion, and metastasis of malignant tumors and therefore represents an attractive therapeutic target in solid tumors, including EOC. The tumor microenvironment consists of a variety of cell types including fibroblasts, immune cells, and endothelial cells, as well as non-cellular components such as the extracellular matrix (ECM), ECM remodeling enzymes [e.g., matrix metalloproteinases (MMPs), tissue inhibitors of metalloproteinases (TIMPs), and lysyl oxidases (LOXs)], and growth factors (e.g., VEGF, TGF-β, and PDGF). All these components work to create a microenvironment permissive for tumor cell growth, migration, and invasion. This review will focus on our current understanding of the roles that the ECM and ECM remodeling enzymes play in EOC progression, with specific emphasis placed on the individual key factors in the ECM known to date.
ECM Remodeling Promotes Ovarian Cancer Progression
The ECM is constructed from cellular secretions and is a critical regulator of normal tissue development and function (4). It is a dynamic, non-cellular structure existing within all tissues, which not only serves as a physical support for cells, but also has a unique role in tissue homeostasis (5). These diverse functions of the ECM are conferred through its complex organization, composition, and its continuous remodeling. The constituents of the ECM in different tissues vary, imparting a unique ability to accommodate the specific needs required by different tissues (6). This is facilitated by the chemical and physical interactions between the resident cells and the continuously changing microenvironment (7). The ECM is composed of two main types of macromolecules: fibrous proteins and proteoglycans (8).
An increasing number of studies have proposed an essential role for the ECM in tumor progression, with dysregulation of the ECM implicated in cancer and characterized by extensive modification of its structure and composition. The secretion and/or inhibition of various ECM components and the subsequent remodeling by tumor cells creates a protumorigenic microenvironment which ultimately assists in tumor cell survival while disregarding the normal physiological function of the tissue (9). Stiffness and atypical ECM deposition are recognized in various cancers (10), with ECM alteration necessary for tumor initiation, progression, and intraperitoneal dissemination in EOC (11). Figure 1 provides a schematic representation of the ECM components involved in EOC.
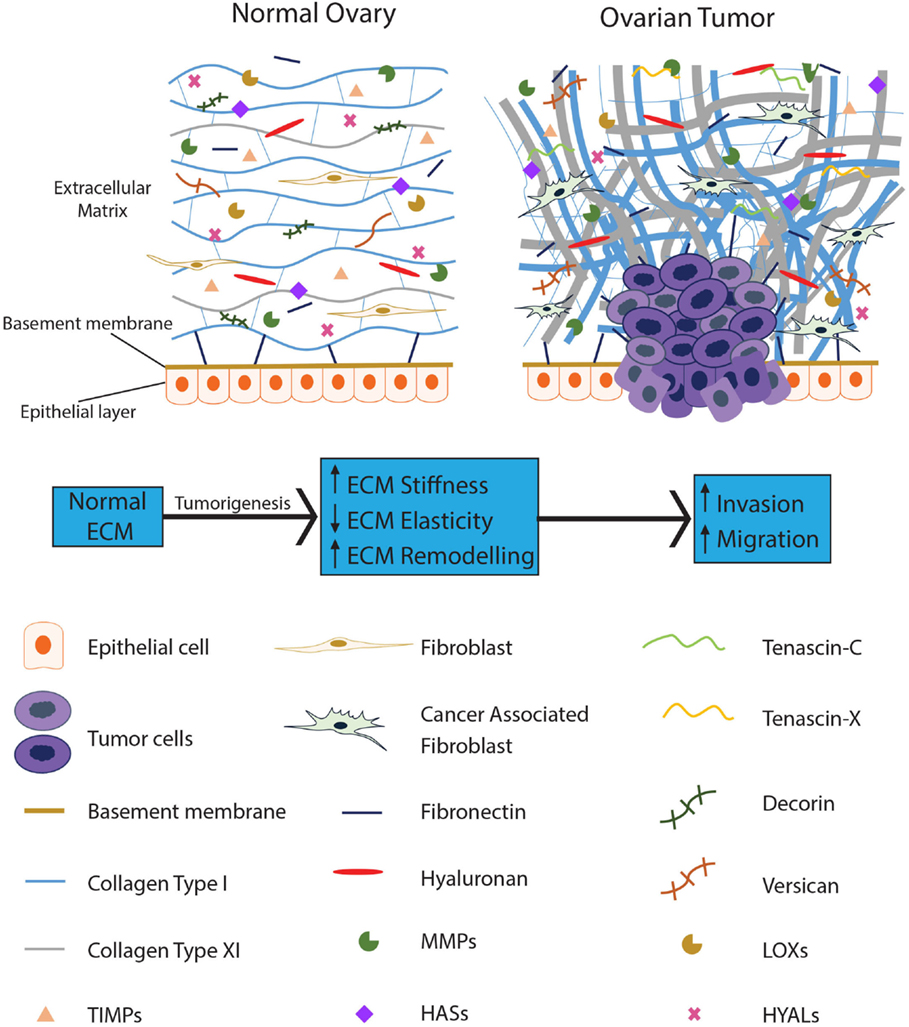
Figure 1. The ECM becomes dysregulated during ovarian tumorigenesis and contributes to tumor progression. The normal ovarian ECM consists of a highly ordered arrangement of collagen fibers, with hyaluronan interspersed throughout, regulating the distribution of the collagen in the ECM. Several proteoglycans, such as decorin and versican, are present to provide pressure and hydration to the tissue. In EOC, stromal fibroblasts are activated; collagen becomes progressively remodeled into short thick fibrils, randomly orientated into tracks at angles tending toward perpendicular rather than parallel to the epithelial boundary. In addition, versican, fibronectin, tenascin-C, and tenascin-X are upregulated with the loss of decorin. Reduced levels of HYALs lead to accumulation of hyaluronan; upregulation of LOXs leads to increased crosslinking of the ECM proteins resulting in increased stiffness of the ECM. MMPs are overexpressed in the EOC ECM, actively remodeling the ECM to promote tumor progression while TIMPs are unable to restrain these enzymes from dysregulating the ECM.
Fibrous Proteins
Fibrous proteins are major components of the ECM that provide tensile strength, elasticity, and structure to tissues. Many of these proteins become dysregulated in solid tumors and contribute to tumor growth and metastasis. Listed below are the fibrous proteins involved in EOC development and progression. Table 1 provides a summary of the ECM fibrous proteins reviewed here and their potential roles in EOC.
Collagens
Collagens are the most abundant fibrous proteins of the ECM. They associate with other collagens and interact with extracellular proteins, glycosaminoglycans and nucleic acids (7). Currently, there are 28 types of collagen identified which are divided into three major sub-groups: fibrillar, non-fibril forming, and fibril-associated collagens (51–53). Fibrillar collagens are the predominant sub-group present in the ECM, and their unique functions are governed by their conformation and structure, allowing them to form highly organized fibrils (6, 51). Particular focus will be placed on this specific sub-family of collagens. Fibrillar collagens are comprised of three α chains which assemble intracellularly into a triple helix that is secreted into the ECM as a procollagen molecule. Cleavage by metalloproteinases present in the ECM convert procollagen into collagen (6).
Insight into collagen morphology and organization and the resultant fibril composition and structure in the ECM are crucial for understanding how structural modifications are associated not only with the normal physiology of healthy tissue, but also with malignant processes linked to cancer progression (54). In normal tissue including normal ovary, collagen is organized as thin, long wavy fibrils, parallel to the epithelial boundary and providing elasticity to the ECM (Figure 1). In contrast, collagen remodeling in tumor stroma results in thicker and shorter fibrils, bunched into tracts at angles tending toward perpendicular to the epithelial boundary (Figure 1). Collagen tracts perpendicular to the epithelial boundary (also known as Tumor-Associated Collagen Signature (TACS)-3) are found in EOC (55). TACS-3 may facilitate entry into the stroma with invasive foci observed at these sites in mammary tumors (56). TACS-3 is also associated with a loss of elasticity and increase in stiffness of the ECM. These findings illustrate that ECM remodeling occurs in tumors and that fibrillar collagen contributes to this remodeling affecting the function of the resultant ECM.
Collagen-rich ECM was originally postulated to regulate normal tissue architecture and act as a physical barrier to tumor cell migration. However, it was shown that collagen-dense ECM induced by tumor cells essentially increased invasiveness and promoted tumor progression rather than inhibiting it (57). Increased risk of breast cancer has been associated with excessive collagen deposition and crosslinking (57, 58). Elevated collagen deposition and remodeling compromises drug delivery (59) and has been observed to be linked to cisplastin resistance in EOC (60–62). Collagen composition of the tumor ECM is crucial in mediating EOC progression and contributes to the poor response of ovarian cancer patients to chemotherapy, further emphasizing the importance of the ECM as an active participant in tumor progression.
Collagen I
Fibrillar type I collagen is the most abundant structural component of the ovarian ECM. Several in vitro studies have established the importance of type I collagen in EOC adhesion and migration, successfully demonstrating that collagen I enhanced migration of multiple EOC cell lines (14, 15), and primary EOC cells and spheroids preferentially and strongly adhered to type I collagen (12, 13). Collagen I has been demonstrated to have a novel role in inducing chemoresistance by upregulating tau (22), a microtubule-associated protein, which has been associated with paclitaxel resistance in EOC (63, 64). Flate and Stalvey (16) presented a study which implicates type I collagen as a steering mechanism for selected EOC cell lines in vitro and indicated that the migration of EOC cells induced by type I collagen was partially due to increased directionality. The promigratory cues which type I collagen confers on EOC cells highlight the multiple ways in which collagen can facilitate cancer cell migration. Thus, not only does collagen have a physical role in cancer progression, but it also has a potential role as a chemoattractant and may have an underlying role in chemoresistance. However, further studies are needed to consolidate these findings.
Collagen XI
Collagen XI is a minor fibrillary collagen predominantly found in cartilage with low or absent expression in most tissues (65–68). Hence stromal changes of collagen XI α-1 (COL11A1) expression are regarded as markers of cancer initiation and progression (20). High COL11A1 expression is associated with poor overall survival, poor clinical outcome and is a predictor of EOC recurrence correlating with the stage of disease (17, 20, 21). Increased gene expression of COL11A1 was observed in all EOC patients during tumor progression and was greatly increased in metastases (18). Varying mRNA and protein expression levels of COL11A1 at different stages and sites of the tumor suggests COL11A1 as a potential biomarker, with the highest COL11A1 levels detected in late stage disease (recurrent metastases) and lowest levels in earlier stage disease (primary ovarian tumors) (19). Though COL11A1 is clearly associated with cancer progression and metastasis, there are a limited number of studies detailing the role and mechanism of COL11A1 overexpression in metastasis. With limited biomarkers available for EOC, COL11A1 has potential as a clinical screening tool and prognostic marker.
Fibronectin
Fibronectin is implicated in cell growth, migration, and differentiation in processes including wound healing, embryonic development, and tumorigenesis (69, 70). Fibronectin plays a significant role in tumor progression, promoting metastasis, angiogenesis (71), and inhibiting apoptosis (72). Fibronectin expression is observed in the submesothelial basement membrane (BM) of metastatic omental tumors, ECM (23), and ascites (24). It is an indicator of poor prognosis in invasive EOC (25) and has been shown to mediate EOC cell migration and invasion (26) through the upregulation of the FAK/PI3K/Akt pathway (15). EOC cell motility and early metastatic competence is stimulated through the release of fibronectin from peritoneal mesothelial cells (23, 27). The protumorigenic role of fibronectin is further illustrated by Kenny et al. (23), who showed a significant reduction in the invasive and metastatic ability of EOC cells when fibronectin was knocked out from the peritoneal microenvironment. Another study by Kenny et al. (28) demonstrated that adhesion of EOC cells to the peritoneal surface was enhanced by MMP2 cleavage of fibronectin into small fragments. These studies have established fibronectin as a critical promoter of EOC migration and invasion. With its strong correlation with EOC progression, fibronectin presents a favorable target in cancer treatment.
Tenascin
There are four large extracellular glycoproteins which constitute the tenascin family: –C, –X, –R, and –W (73). Tenascins have roles in cell adhesion and proliferation. In certain cell types, they act as antiproliferative agents, while in other cell types, they act to promote adhesion and migration (74).
Tenascin-C
Tenascin-C (TNC) is an important tissue remodeling glycoprotein which contributes to tumorigenesis and metastasis by promoting proliferation, invasion, and angiogenesis (29, 75). TNC is either absent or present in minute amounts in healthy, developed tissues and significantly increased in pathological conditions, such as cancer (75). High TNC expression has been demonstrated in solid tumors, including breast, pancreas, prostate, brain, and ovary. High TNC expression correlated with poor survival in lung, glioma, breast, and colon cancers (76). In EOC, TNC levels were significantly higher than in non-cancer controls (75) and increased with increasing grade and stage, with malignant tumors displaying the highest expression (30). A subsequent study by the same investigators demonstrated a 100-fold increase in ovarian fibroblast media compared to media derived from EOC cell lines, suggesting that TNC is predominately secreted by fibroblasts (29). This study also indicated a potential role of TNC in invasion, demonstrating increased adhesion and migration in vitro.
The consistent finding of increasing TNC levels with increasing tumor stage for several cancer types suggests a potential biomarker role for TNC. However, a study by Didem et al. (75) determined that serum TNC levels had no prognostic value in EOC, with no correlation between high serum TNC levels and any prognostic factors, including tumor stage and grade, response to chemotherapy or survival, although patients with high TNC levels were observed to have poorer overall survival (75). This study only investigated serum TNC levels as a prognostic marker. It did not examine TNC levels in the immediate ECM of the ovarian tumor. There are limited studies available which examine TNC in EOC tumor tissue specifically, and further studies are required to establish its potential role in EOC progression.
Tenascin-X
Tenascin-X (TNX) is the largest member of the tenascin family, and during development TNX is widely expressed (77). TNX levels are significantly elevated in EOC compared to healthy tissues, normal ovaries, and benign tumors (31). Levels in ascites from EOC patients correlated with serum CA-125, implying that TNX secretion may be coordinated with the release of CA-125 (31), and may prove useful as a potential biomarker to complement CA-125. However, like TNC, there are limited studies available implicating TNX in EOC development (30).
Elastin
Elastin is a strong and insoluble biopolymer which constitutes ~90% of elastic fibers and is responsible for the resilience and elastic recoil of elastic vertebrate tissues (78). It has an extremely low turnover rate (79) and is formed through the crosslinking of its soluble precursor, tropoelastin, by LOX (80). Elastin has to some degree been associated with tumor growth and progression in other tumor types (81–83). Only one study is available to our knowledge which associates elastin with EOC. This study by Stewart et al. (84) evaluated the value of elastin staining in grading peritoneal implants associated with borderline serous EOC and demonstrated potential value in confirming superficial distribution of non-invasive peritoneal implants. However, this study did not specifically look at elastin levels in primary EOC tumors, and no studies are available at present which detail the potential role of elastin in EOC development and progression.
Laminin
The laminin family of glycoproteins consists of 12 unique heterotrimers and is the major non-collagen structural component of the BM (85). In addition to its structural functions, laminin also regulates cell adhesion and migration, demonstrating a role in tissue homeostasis and morphogenesis (86–89). This is partially mediated by the interactions between laminin and other ECM molecules, such as collagen type IV, fibronectin, and heparin sulfate proteoglycans (90). The role of laminin in the BM of EOC has been well studied; however, its potential role in the ECM has not been thoroughly examined. Laminin was observed to be absent around microinvasive cells and also in tumors of low malignant potential in the early stages of invasion (32). Studies show elevated laminin levels in the ascites from EOC patients compared to normal peritoneal fluid; however, there are conflicting results as to whether this corresponds to an increase in serum laminin levels (33, 34). Though there are strong indications of the possible tumor-promoting roles of laminin in EOC, further studies are needed to establish this association and its potential value as a biomarker or therapeutic target.
Proteoglycans
Proteoglycans are dispersed throughout ECM and act to provide compressive resistance and hydration to the tissue (6, 8). The two major ECM proteoglycan families consist of those containing leucine-rich repeats and hyalectans. Table 1 provides a summary of proteoglycans reviewed in this section and their potential roles in EOC.
Small Leucine-Rich Repeat Proteoglycans
Leucine-rich repeat (LRR) proteoglycans are the most abundant and also the largest class of proteoglycans in the ECM. They have various functions, combining roles as signaling molecules and structural components during tissue remodeling in cancer and inflammation. LRR proteoglycans are regulated by the TGF-β and Wnt signaling pathways and interact with a range of Toll-like receptors, receptor tyrosine kinases, and growth factors to regulate homeostatic processes, such as apoptosis, migration, proliferation, angiogenesis, differentiation, and survival (91–96). They are also involved with regulating fibrillary collagen assembly, degradation, and organization (97–103). Of the LRR proteoglycan family members, only decorin and lumican have to date been shown to play roles in the ovarian tumor ECM.
Decorin
Decorin, a fundamental component of the ECM, binds to collagen and facilitates tissue scaffolding (104). However, its expression in cancer, including EOC, is generally reduced or undetectable (35–38, 105–107). Decorin-induced growth suppression was observed in a study by Merle et al. (108), highlighting the importance of decorin in possibly inhibiting tumor growth. It was proposed that decorin was able to interfere with the interactions between the resident cells and the ECM, by inhibiting fibronectin binding and integrin interaction. Nash et al. (109) and Teicher et al. (110) demonstrated the synergistic effects of decorin with cisplastin and carboplatin in inhibiting the growth of breast and EOC. Decorin can inhibit tumor growth by suppressing TGF-β (105, 111) and directly interacting with the epidermal growth factor receptor and ERBB2 (112–115). The direct interaction with these receptors diminishes receptor-mediated intracellular signaling and induces apoptosis (116, 117). These studies suggest that decorin plays a major role in controlling tumor growth and its subsequent downregulation is associated with EOC development, indicating that possible therapies involving the restoration of decorin expression in the tumor stroma, coupled with chemotherapy, could potentially retard the growth of EOC.
Lumican
Lumican, another LRR proteoglycan, is involved in the regulation of collagen fibrillogenesis, migration, invasion, angiogenesis, and apoptosis (104, 118–120). Varying levels have been reported in the stroma of different tumor types (121–126). In breast and pancreatic cancers, high stromal lumican was associated with advanced cancer stage, invasion, and poor survival (127, 128), whereas a negative correlation was found between tumor grade and expression in neuroendocrine tumors of the colon (129). In support of this, lumican has been shown to inhibit tumor growth and progression in lung cancer and melanoma (92). To date, a single study has examined lumican expression in EOC, demonstrating reduced stromal expression and suggesting a possible role in cancer aggression (39). Given the varying clinical associations with lumican in several cancer types, more research is needed to determine the precise role of lumican in EOC.
Versican
In the healthy ovary, versican (VCAN) is tightly regulated and acts as an important ECM proteoglycan present in the granulosa cells of growing follicles, to aid in the expansion of the cumulus oophorus in the preovulatory stage (130). Normal processes, such as wound healing (131), follicle growth (130), and inflammation (132), induce VCAN expression. Many malignant tumors have elevated levels of VCAN (133–140). Elevated VCAN levels were also observed in the ECM of EOC and correlated with increased hyaluronan (HA) levels, suggesting that they may form a supportive partnership to assist in EOC survival and spread (40, 41). In vitro studies have demonstrated the production of VCAN by malignant cells; however, the source of VCAN in tumors remains to be elucidated, with no clear answer on the in vivo source of VCAN, which may also include stromal cells (137, 141–143). Upregulation of VCAN in EOC correlates with a poor disease outcome; however, the significance of VCAN as a prognostic marker is debatable, with HA, its binding partner, presenting greater value as a prognostic marker (42). The same study showed that despite a strong association with the apparent initiation and development of EOC, VCAN was not an independent indicator for patient survival.
Perlecan
Perlecan is a core heparan sulfate proteoglycan of the ECM and BMs of normal tissues and blood vessels (8, 144, 145). Perlecan is stored in abundant quantities in the BM. Degradation of the BM by MMPs during tumor invasion causes the release of perlecan into the ECM, and increased expression is reported in various cancer cell lines and tumors (146–148). Perlecan has been suggested to have various roles in tumor progression, by regulating the cell’s response to mitogenic and angiogenic growth factors, and mediating adhesion and migration (149, 150). Its inhibition is reported to impede tumor growth and invasion (144, 151). A study by Davies et al. (43) observed heterogeneous perlecan expression in ovarian tumors compared to normal ovary. Perlecan expression was observed in the BM, the stroma, the internal elastic lamina of blood vessels, and the submesothelial layer of the normal ovary. Perlecan was also present in the BM of benign and borderline tumors but absent in the BM of malignant tumors, enhancing invasive potential. Loss of perlecan was not observed in the stroma and BMs of blood vessels. Further studies are needed to determine its potential role in EOC progression.
Hyaluronan
Hyaluronan is strongly implicated in cell proliferation, migration, wound healing, and inflammation (152). HA binds and interacts closely with fibronectin during matrix construction (152–154) and regulates the distribution of collagen fibrils (155). HA can interact directly with cells by binding to cell surface receptors and constructing a protective coat (156–158). It also has a role in the distribution of proteoglycans in the ECM through non-covalent interactions (159–161). Changes in HA content and size are associated with tissue remodeling and pathological processes, such as tumor progression (162–166). Studies from several cancer types have recognized the elevation of HA in serum (167–169), and recently a study by Wu et al. (170) demonstrated the novel use of serum HA in differentiating non-metastatic from metastatic breast cancer, which suggests HA as a potential biomarker. Elevated HA levels were observed in and associated with tumor aggression in breast, lung, prostate, colorectal, and bladder cancer (163).
Epithelial ovarian cancer grade and metastasis are correlated with increasing HA levels, with Hiltunen et al. (44) demonstrating a 100-fold increase in HA expression in grade three EOC. Many cancers, including ovarian, are enveloped in a HA rich ECM (11, 42, 44). HA upregulation has been implicated as a strong, independent prognostic factor for EOC (42) and is positively correlated with invasion and metastasis (42, 45). Adhesion of ovarian cancer cells to the peritoneum is facilitated by interactions between HA and its major surface receptor CD44 (45–47, 171). HA has been shown to reduce the ability of chemotherapeutic drugs to induce cell death in several cancers (172–175). Ricciardelli et al. (48) demonstrated chemotherapy-induced HA production facilitates chemoresistance and EOC cell survival through a HA-CD44-mediated pathway. HA-chemotherapy conjugates were successful in increasing the efficacy of standard chemotherapy in EOC patients by CD44-mediated uptake of the chemotherapy (49, 50). Hence, HA is a promising therapeutic target, with HA inhibition potentially suppressing adhesion of EOC cells to the peritoneum, which is the preferential place for EOC metastasis. Conversely, HA can be utilized to enhance the cytotoxic effects of chemotherapy.
Enzymes
Abnormal expression and deposition of ECM components and alteration to its structure are implicated in malignancies such as EOC. Remodeling of the ECM in healthy tissues through chemical modification, synthesis, degradation, and reassembly are tightly controlled processes induced by cells in homeostasis (176). Crosstalk between the ECM and cancer cells causes the alteration of ECM structure and composition, resulting in the dysregulation of this tightly controlled system (5). Cleavage of ECM components by proteases and the subsequent remodeling of the ECM is implicated in EOC progression, where the degradation of the BM and ECM is necessary for the invasion and metastasis of EOC cells (177, 178). Malignant cells produce a wide range of ECM-degrading proteases implicated in ECM dysregulation and cancer progression. Inhibiting their activity may be of potential therapeutic value. Table 2 provides a summary of the ECM remodeling enzymes reviewed here and their potential roles in EOC.
Matrix Metalloproteinases
Matrix metalloproteinases are a family of extracellular proteins comprising >20 zinc metalloproteases which play major roles in tissue repair and remodeling in response to injury (209). In normal conditions, the activity of MMPs is low. However, in response to cellular and matrix interactions, growth factors, hormones, and inflammatory cytokines released during remodeling or repair processes and in inflamed or diseased tissues, MMP activity increases (5, 210). MMPs remodel the ECM and contribute to the tumor microenvironment by promoting tumor growth, metastasis, and angiogenesis (211, 212). Through these activities, MMPs promote cancer progression and correlate with poor patient prognosis (213).
MMP2 and MMP9
Matrix metalloproteinase 2 and MMP9 have been implicated to contribute to the malignant potential of tumor cells, due to their ability to degrade a major component of the BM, collagen type IV (214). MMP2 and MMP9 activity varies between normal ovaries and malignant ovarian tumors. MMP2 was observed to be prevalent in normal ovaries, while MMP9 was predominant in malignant tissues (215). EOC aggressiveness has been linked to MMP2 and MMP9 expression (179–181). Several studies have demonstrated the secretion of MMP2 and MMP9 from EOC cell lines in vitro and in ascites from advanced EOC patients. In vitro expression of MMP2 and -9 also correlated with the invasiveness of the EOC cell lines (182–185, 216). Schmalfeldt et al. (180) confirmed these studies, by not only demonstrating elevated levels of MMP2 and -9, but also identified higher total MMP2 and MMP9 activity in the metastatic site, compared to the primary site, suggesting their likely role in the progression from a benign state to an advanced stage. MMP2 expression correlated with clinical stage (186) and promoted metastasis along with MMP9 (187).
Sillanpää et al. (188) evaluated the prognostic significance of MMP9 in EOC, where high levels of epithelial MMP9 were associated with a better 10-year disease-related survival (DRS), while high stromal levels of MMP9 were associated with worse survival. This was supported by a study by Ozalp et al. (179). This however, has been contradicted by several other studies (189–192) which associated high epithelial and stromal MMP9 with shorter disease-specific survival and metastasis.
MMP7
Matrix metalloproteinase 7 is involved in the proteolysis of several ECM substrates, growth factors, and cellular receptors including collagens, proteoglycans, insulin-like growth factor-binding protein, heparin-binding epidermal growth factor, E-cadherin, and tumor necrosis factor-alpha precursor (193, 217). Overexpression of MMP7 in EOC has been demonstrated in several studies (193, 195, 196). Wang et al. (193) showed that overexpression of MMP7 promoted the invasion of EOC cells in vitro and likewise, suppression of MMP7 inhibited migration and invasion (194). These findings are in contrast to the study by Brun et al. (181) who showed that epithelial MMP7 expression was lower in malignant serous tumors, compared to its benign or borderline counterparts, while there was no difference observed among the mucinous tumors. Shigemasa et al. also supported this finding in mucinous ovarian tumors (195). MMP7 was observed to be an independent prognostic factor, with higher MMP7 expression in ovarian tumor cells correlating with good clinical and survival parameters (196). These discrepancies highlight the need to elucidate the functional role of MMP7 in EOC, where the grade and subtype of the tumor may result in these contrasting findings.
While MMP2, -7, and -9 have been implicated to play a role in EOC, there are discrepancies correlating the expression of these MMPs with the prognosis and certain clinicopathological features of EOC. This suggests that the functions of MMPs in EOC may be dependent on their epithelial or stromal associations in conjunction with the grade of the tumor and possibly the surrounding stroma. This highlights the complexity of MMPs and their roles in EOC progression and emphasizes the need for additional studies to provide an explanation for these differences. Nonetheless, MMPs have potential as therapeutic targets due to their indisputable activity during EOC progression. MMP2, -7, and -9 expression are elevated in EOC regardless of the grade; therefore, inhibition of MMPs may decrease the aggressiveness of EOC and aid in preventing invasion and metastasis.
Tissue Inhibitors of Metalloproteinases
Tissue inhibitors of metalloproteinases are endogenous inhibitors of major ECM remodeling proteinases, such as MMPs, and subsequently they play a crucial role in regulating ECM composition and function. TIMPs also have multiple functions in regulating cell proliferation, migration, invasion, apoptosis, and angiogenesis (218). There are four TIMP paralogs: TIMP1–4 (219). TIMP1 is the most widely distributed TIMP and has the ability to inhibit all active forms of MMP (220). TIMP2 is most selective for MMP2 (221). Simultaneous increase of MMP2 and decrease of TIMP2 levels were observed in malignant tumors compared to benign tumors and the normal ovary, with this imbalance indicative of the importance of MMP2–TIMP2 levels in promoting invasion (222). In contrast, TIMP1 levels were increased in malignant tumors compared to the normal ovary (222–224). Okamoto et al. (222) observed that despite the synchronous increase of MMP9 and TIMP1 in malignant tumors, the degree of increase of MMP9 was much greater than TIMP1, suggesting that TIMP1 has limited ability in compensating for an increase in MMP9. A study by Kikkawa et al. (225) also reported elevated TIMP1 and MMP9 levels in EOC samples compared to normal ovary. Given their role as inhibitors of MMPs, the relative levels of each family of enzymes are important when considering their function in EOC. MMPs and TIMPs have a dynamic relationship not just in normal ovaries, but also in EOC, where the expression of TIMPs adjusts with the relative levels of MMPs present to ultimately promote tumor progression.
Lysyl Oxidase
The LOX family comprises five members: LOX and four LOX-like isoenzymes LOXL1–4. LOX is a copper-dependent amine oxidase secreted by fibroblasts and, together with the other family members, has an important role in remodeling the ECM by regulating collagen and elastin crosslinking and therefore contributes to the strength and structure of many tissues (226–228). LOX and LOXL2 are heavily implicated in cancer progression (229, 230).
Lysyl Oxidase
The importance of the LOX family in ECM remodeling during normal physiological processes has been established in a variety of tissues. In the ovary, LOX is activated during ovulation, following follicle rupture and is critical in collagen synthesis and reassembly in the ovarian follicle (231). Several studies have demonstrated the expression of LOX in granulosa cells (232–234), and its expression and activity is tightly controlled by follicle-stimulating hormone during follicle development (232, 234). Wang et al. (197) initially showed that a single nucleotide polymorphism of the LOX gene, G473A, correlated with advanced stages and increased susceptibility to EOC in a Chinese population. This finding was supported by Wu et al. (198). In hypoxic conditions, hypoxia inducible factor-1α (HIF-1α) induced LOX expression and facilitated tumor migration, invasion, and metastasis in a range of cancers (229). LOX and HIF-1α overexpression were observed in hypoxic EOC cells, with expression levels correlating significantly with metastasis and tumor stage in EOC (199). HIF-1α upregulation induced LOX transcription through the accumulation of reactive oxygen species, subsequently repressing E-cadherin. Loss of E-cadherin was observed to promote EOC cell migration in vitro and tumor growth in vivo, while also correlating with tumor stage, differentiation, metastasis, and a poorer 5-year survival rate (200).
LOXL2
LOXL2 plays a similar role to LOX in crosslinking collagen and elastin in the ECM, contributing to the stability and strength of the tissue (226). LOXL2 overexpression has been linked to the aggressiveness of breast (235), skin (236), and colon cancers (237). Specific upregulation of the LOXL2 protein is found in EOC endothelial cells, where inhibition of LOXL2 reduced endothelial cell concentration within the tumor (201). A study by Zaffryar-Eilot et al. (202) confirmed the direct role of LOXL2 in angiogenesis, with LOXL2 inhibition decreasing microvessel density for the normalization of tumor-associated vasculature resulting in reduced tumor hypoxia with better response to therapy (238).
LOXL4
LOXL4 is expressed in head and neck squamous cell carcinoma and gastric cancer cell lines, and upregulation of LOXL4 significantly correlates with tumor stage and lymph node metastases (239–241). LOXL4 promotes proliferation, migration, and invasion in gastric cancer cell lines in vitro (241). A study by Sebban et al. (203) demonstrated a tumor suppressive effect in EOC in vivo, while also indicating a contrasting role of LOXL4 splice variants in vitro, with the variants enhancing tumor progression and metastatic potential. This study demonstrates the paradoxical roles of LOXL4 and its alternatively spliced isoforms. Specific variants of LOXL4 could be promising as a prognostic marker and a potential therapeutic target.
To our knowledge, only LOX, LOXL2, and LOXL4 have been studied in EOC. Aberrant LOX, LOXL2, and LOXL4 expression are implicated in dysregulating the ECM and inducing a malignant phenotype and promoting tumor progression in EOC. However, additional studies are needed to elucidate further potential roles of these LOX family members in EOC, where a wide range of studies have demonstrated a strong association between these enzymes and several tumorigenic pathways in a variety of other cancers (230). Due to the protumorigenic role of LOX, LOXL2, and LOXL4, inhibition of these LOX family members in conjunction with chemotherapy could potentially enhance its antitumorigenic effect and result in a better prognosis.
Hyaluronan Synthases
Hyaluronan synthases (HASs) are integral plasma membrane proteins which synthesize HA (242, 243). Three isoenzymes of HAS with differing enzymatic activities have been identified in humans: HAS1, HAS2, and HAS3 (244). Overexpression of HAS is implicated to promote growth and metastasis in a variety of cancers through excessive production of HA (245–249). In one study of ovarian cancer by Yabushita et al., HAS1-negative tumors were associated with increased overall survival and lower microvessel density relative to HAS1-positive tumors. No relationships were found between the levels of HAS2–3 and tumor stage, survival, chemotherapy, or microvessel density (204). Weiss et al. (206) compared the expression of HAS1–3 mRNA in serous EOC between effusions, primary carcinomas, and solid metastases, with differential HAS overexpression observed in each region. HAS1, HAS2, and HAS3 were overexpressed in effusions, solid metastases, and primary carcinomas, respectively. High HAS1 expression in EOC effusions correlated with shorter survival in agreement with the results of Yabushita et al. (204). However, a study by Nykopp et al. (205) demonstrated barely detectable HAS1 in EOC and no consistent increase in the HAS2 and HAS3 expression. These result suggest unique roles for each HAS isoenzyme during different stages of EOC progression, although no clear associations have been identified to date.
Hyaluronidases
Hyaluronan synthesis by HASs is opposed by the enzymatic action of hyaluronidases (HYALs), which degrade HA. The family of HYALs consists of six members (250), of which HYAL1 and HYAL2 are particularly well characterized (205). HYAL1 and HYAL2 are the main members responsible for HA turnover, with these two enzymes bearing several physiological and pathological roles, such as wound healing and inflammation (250, 251). High molecular weight HA is antiangiogenic; HYALs cleave HA into low molecular weight HA fragments which may promote angiogenesis, subsequently enhancing tumor growth (252). While chromosomal loss at the locus encoding HYAL1–3 (3p21.3) is common in both tumor and stromal tissue from EOC patients, this allelic loss is not associated with increased tissue HA levels (207). HYAL activity and expression were reported to be reduced and differentially expressed in malignant EOC compared to its benign and normal counterparts (44, 206). HYAL1 was absent in all serous EOC samples (206); however, expression of HYAL1 in EOC is subtype specific, with clear cell and mucinous EOC showing elevated levels of HYAL1 compared to serous and endometrioid EOC (208). Comparing transcript levels in serous EOC between primary tumors, solid metastases, and effusions, HYAL2 splice variant, HYAL2-var2, was significantly overexpressed in solid metastases, and HYAL3 var1–3 was significantly underexpressed in solid metastases. A positive correlation was identified between HYAL3 levels in effusions and paclitaxel treatment (206).
Regulation of HA synthesis and degradation is mediated by HASs and HYALs, respectively. As described above, HA accumulation is associated with the aggressiveness of EOC and has been demonstrated to promote EOC progression. The displacement of this otherwise delicate equilibrium of controlled HA synthesis and degradation by HASs and HYALs in EOC has major implications on the subsequent structure and function of the ECM and therefore may represent promising targets for cancer treatment.
Discussion
The ECM is a dynamic structure. It is crucial in regulating specific function, development, and homeostasis, achieved by organizing and regulating the plethora of ECM components unique to each differentiated tissue (4, 7, 176, 253). Remodeling of the ECM in EOC is thought to promote tumor progression. The interactions between the ECM and the resident cells are tightly regulated, and disruption of the ECM has severe consequences as described in this review. It is evident that the ECM in EOC remains relatively unexplored, with the mechanisms involved in tumor progression yet to be fully elucidated. To fully understand how alterations to the ECM influence tumorigenesis, it is essential to investigate not only how the ECM interacts with tumor cells, but also how the ECM components interact with each other.
The constituents of the ECM offer potential biomarkers and therapeutic targets, where the manipulation of the ECM composition may complement current chemotherapeutic treatment. Enzymes involved in ECM remodeling and elevated in EOC, such as MMPs and LOXs, which have been shown in preclinical models to promote tumor progression in other cancers, could also be a potential therapeutic targets in EOC. Though CA-125 is clinically approved to be used as a serum tumor biomarker for ovarian cancer, Moss et al. (254) demonstrated its poor sensitivity and specificity, with a high false positive rate. Other than CA-125, there are currently no reliable biomarkers for the staging and prognosis of ovarian cancer. With low overall survival rates for patients diagnosed with advanced disease, a sensitive and specific diagnostic biomarker of early stage EOC is needed. Tenascins have a limited presence in healthy tissues, hence could potentially serve as biomarkers for early diagnosis of EOC.
Though the identification of individual ECM components allows us to understand their basic functions in EOC, the ECM must be considered not just as its individual elements, but as a collective entity. EOC progression is multifactorial and influenced by an altered ECM. The ECM constituents described in this review reflect the complexity of the EOC microenvironment which is an evolving area of research. Advances in understanding how the ECM contributes to EOC pathogenesis and progression will assist in the development of better treatments for EOC.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by a Cancer Institute NSW Fellowship (to EC), Cure Cancer Australia Foundation (EC), Cancer Australia (EC) and the Bill Walsh Cancer Research Fund (VH).
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin (2015) 65(1):5–29. doi: 10.3322/caac.21254
2. Kurman RJ, Visvanathan K, Roden R, Wu TC, Shih IeM. Early detection and treatment of ovarian cancer: shifting from early stage to minimal volume of disease based on a new model of carcinogenesis. Am J Obstet Gynecol (2008) 198(4):351–6. doi:10.1016/j.ajog.2008.01.005
3. Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin (2014) 64(1):9–29. doi:10.3322/caac.21208
4. Mecham RP. Overview of extracellular matrix. Curr Proto Cell Biol. (2014). doi:10.1002/0471143030.cb1001s57.
5. Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol (2014) 15(12):786–801. doi:10.1038/nrm3904
6. Mouw JK, Ou G, Weaver VM. Extracellular matrix assembly: a multiscale deconstruction. Nat Rev Mol Cell Biol (2014) 15(12):771–85. doi:10.1038/nrm3902
7. Naba A, Clauser KR, Hoersch S, Liu H, Carr SA, Hynes RO. The matrisome: in silico definition and in vivo characterization by proteomics of normal and tumor extracellular matrices. Mol Cell Proteomics (2012) 11(4):M111.014647. doi:10.1074/mcp.M111.014647
8. Hynes RO, Naba A. Overview of the matrisome – an inventory of extracellular matrix constituents and functions. Cold Spring Harb Perspect Biol (2012) 4(1):a004903. doi:10.1101/cshperspect.a004903
9. Zigrino P, Löffek S, Mauch C. Tumor-stroma interactions: their role in the control of tumor cell invasion. Biochimie (2005) 87(3–4):321–8. doi:10.1016/j.biochi.2004.10.025
10. Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. J Cell Sci (2010) 123(Pt 24):4195–200. doi:10.1242/jcs.023820
11. Ween MP, Oehler MK, Ricciardelli C. Role of versican, hyaluronan and CD44 in ovarian cancer metastasis. Int J Mol Sci (2011) 12(2):1009–29. doi:10.3390/ijms12021009
12. Moser TL, Pizzo SV, Bafetti LM, Fishman DA, Stack MS. Evidence for preferential adhesion of ovarian epithelial carcinoma cells to type I collagen mediated by the alpha2beta1 integrin. Int J Cancer (1996) 67(5):695–701. doi:10.1002/(SICI)1097-0215(19960904)67:5<695::AID-IJC18>3.0.CO;2-4
13. Burleson KM, Casey RC, Skubitz KM, Pambuccian SE, Oegema TR Jr, Skubitz AP. Ovarian carcinoma ascites spheroids adhere to extracellular matrix components and mesothelial cell monolayers. Gynecol Oncol (2004) 93(1):170–81. doi:10.1016/j.ygyno.2003.12.034
14. Shen Y, Shen R, Ge L, Zhu Q, Li F. Fibrillar type I collagen matrices enhance metastasis/invasion of ovarian epithelial cancer via beta1 integrin and PTEN signals. Int J Gynecol Cancer (2012) 22(8):1316–24. doi:10.1097/IGC.0b013e318263ef34
15. Ahmed N, Riley C, Rice G, Quinn M. Role of integrin receptors for fibronectin, collagen and laminin in the regulation of ovarian carcinoma functions in response to a matrix microenvironment. Clin Exp Metastasis (2005) 22(5):391–402. doi:10.1007/s10585-005-1262-y
16. Flate E, Stalvey JR. Motility of select ovarian cancer cell lines: effect of extra-cellular matrix proteins and the involvement of PAK2. Int J Oncol (2014) 45(4):1401–11. doi:10.3892/ijo.2014.2553
17. Wu YH, Chang TH, Huang YF, Huang HD, Chou CY. COL11A1 promotes tumor progression and predicts poor clinical outcome in ovarian cancer. Oncogene (2014) 33(26):3432–40. doi:10.1038/onc.2013.307
18. Cheon DJ, Tong Y, Sim MS, Dering J, Berel D, Cui X, et al. A collagen-remodeling gene signature regulated by TGF-beta signaling is associated with metastasis and poor survival in serous ovarian cancer. Clin Cancer Res (2014) 20(3):711–23. doi:10.1158/1078-0432.CCR-13-1256
19. Tothill RW, Tinker AV, George J, Brown R, Fox SB, Lade S, et al. Novel molecular subtypes of serous and endometrioid ovarian cancer linked to clinical outcome. Clin Cancer Res (2008) 14(16):5198–208. doi:10.1158/1078-0432.CCR-08-0196
20. Raglow Z, Thomas SM. Tumor matrix protein collagen XIα1 in cancer. Cancer Lett (2015) 357(2):448–53. doi:10.1016/j.canlet.2014.12.011
21. Teng PN, Wang G, Hood BL, Conrads KA, Hamilton CA, Maxwell GL, et al. Identification of candidate circulating cisplatin-resistant biomarkers from epithelial ovarian carcinoma cell secretomes. Br J Cancer (2014) 110(1):123–32. doi:10.1038/bjc.2013.687
22. Gurler H, Yu Y, Choi J, Kajdacsy-Balla AA, Barbolina MV. Three-dimensional collagen type I matrix up-regulates nuclear isoforms of the microtubule associated protein tau implicated in resistance to paclitaxel therapy in ovarian carcinoma. Int J Mol Sci (2015) 16(2):3419. doi:10.3390/ijms16023419
23. Kenny HA, Chiang CY, White EA, Schryver EM, Habis M, Romero IL, et al. Mesothelial cells promote early ovarian cancer metastasis through fibronectin secretion. J Clin Invest (2014) 124(10):4614–28. doi:10.1172/JCI74778
24. Wilhelm O, Hafter R, Coppenrath E, Pflanz MA, Schmitt M, Babic R, et al. Fibrin-fibronectin compounds in human ovarian tumor ascites and their possible relation to the tumor stroma. Cancer Res (1988) 48(12):3507–14.
25. Franke FE, Von Georgi R, Zygmunt M, Münstedt K. Association between fibronectin expression and prognosis in ovarian carcinoma. Anticancer Res (2003) 23(5b):4261–7.
26. Lou X, Han X, Jin C, Tian W, Yu W, Ding D, et al. SOX2 targets fibronectin 1 to promote cell migration and invasion in ovarian cancer: new molecular leads for therapeutic intervention. OMICS (2013) 17(10):510–8. doi:10.1089/omi.2013.0058
27. Rieppi M, Vergani V, Gatto C, Zanetta G, Allavena P, Taraboletti G, et al. Mesothelial cells induce the motility of human ovarian carcinoma cells. Int J Cancer (1999) 80(2):303–7. doi:10.1002/(SICI)1097-0215(19990118)80:2<303::AID-IJC21>3.3.CO;2-N
28. Kenny HA, Kaur S, Coussens LM, Lengyel E. The initial steps of ovarian cancer cell metastasis are mediated by MMP-2 cleavage of vitronectin and fibronectin. J Clin Invest (2008) 118(4):1367–79. doi:10.1172/JCI33775
29. Wilson KE, Bartlett JM, Miller EP, Smyth JF, Mullen P, Miller WR, et al. Regulation and function of the extracellular matrix protein tenascin-C in ovarian cancer cell lines. Br J Cancer (1999) 80(5–6):685–92. doi:10.1038/sj.bjc.6690410
30. Wilson KE, Langdon SP, Lessells AM, Miller WR. Expression of the extracellular matrix protein tenascin in malignant and benign ovarian tumours. Br J Cancer (1996) 74(7):999–1004. doi:10.1038/bjc.1996.480
31. Kramer M, Pierredon S, Ribaux P, Tille JC, Petignat P, Cohen M. Secretome identifies tenascin-X as a potent marker of ovarian cancer. Biomed Res Int (2015) 2015:9. doi:10.1155/2015/208017
32. Campo E, Merino MJ, Tavassoli FA, Charonis AS, Stetler-Stevenson WG, Liotta LA. Evaluation of basement membrane components and the 72 kDa type IV collagenase in serous tumors of the ovary. Am J Surg Pathol (1992) 16(5):500–7. doi:10.1097/00000478-199205000-00009
33. Byers LJ, Osborne JL, Carson LF, Carter JR, Haney AF, Weinberg JB, et al. Increased levels of laminin in ascitic fluid of patients with ovarian cancer. Cancer Lett (1995) 88(1):67–72. doi:10.1016/0304-3835(94)03625-S
34. Chu Y, Yang Y, Lin M, Wang Z. Detection of laminin in serum and ascites from patients with epithelial ovarian tumor. J Huazhong Univ Sci Technolog Med Sci (2002) 22(1):58–9. doi:10.1007/BF02904790
35. Nash MA, Deavers MT, Freedman RS. The expression of decorin in human ovarian tumors. Clin Cancer Res (2002) 8(6):1754–60.
36. Shridhar V, Lee J, Pandita A, Iturria S, Avula R, Staub J, et al. Genetic analysis of early- versus late-stage ovarian tumors. Cancer Res (2001) 61(15):5895–904.
37. Grisaru D, Hauspy J, Prasad M, Albert M, Murphy KJ, Covens A, et al. Microarray expression identification of differentially expressed genes in serous epithelial ovarian cancer compared with bulk normal ovarian tissue and ovarian surface scrapings. Oncol Rep (2007) 18(6):1347–56. doi:10.3892/or.18.6.1347
38. Grazio D, Pichler I, Fuchsberger C, Zolezzi F, Guarnieri P, Heidegger H, et al. Differential gene expression analysis of ovarian cancer in a population isolate. Eur J Gynaecol Oncol (2008) 29(4):357–63.
39. Amankwah EK, Wang Q, Schildkraut JM, Tsai YY, Ramus SJ, Fridley BL, et al. Polymorphisms in stromal genes and susceptibility to serous epithelial ovarian cancer: a report from the ovarian cancer association consortium. PLoS One (2011) 6(5):e19642. doi:10.1371/journal.pone.0019642
40. Voutilainen K, Anttila M, Sillanpää S, Tammi R, Tammi M, Saarikoski S, et al. Versican in epithelial ovarian cancer: relation to hyaluronan, clinicopathologic factors and prognosis. Int J Cancer (2003) 107(3):359–64. doi:10.1002/ijc.11423
41. Ghosh S, Albitar L, LeBaron R, Welch WR, Samimi G, Birrer MJ, et al. Up-regulation of stromal versican expression in advanced stage serous ovarian cancer. Gynecol Oncol (2010) 119(1):114–20. doi:10.1016/j.ygyno.2010.05.029
42. Anttila MA, Tammi RH, Tammi MI, Syrjänen KJ, Saarikoski SV, Kosma VM. High levels of stromal hyaluronan predict poor disease outcome in epithelial ovarian cancer. Cancer Res (2000) 60(1):150–5.
43. Davies EJ, Blackhall FH, Shanks JH, David G, McGown AT, Swindell R, et al. Distribution and clinical significance of heparan sulfate proteoglycans in ovarian cancer. Clin Cancer Res (2004) 10(15):5178–86. doi:10.1158/1078-0432.CCR-03-0103
44. Hiltunen EL, Anttila M, Kultti A, Ropponen K, Penttinen J, Yliskoski M, et al. Elevated hyaluronan concentration without hyaluronidase activation in malignant epithelial ovarian tumors. Cancer Res (2002) 62(22):6410–3.
45. Yeo TK, Nagy JA, Yeo KT, Dvorak HF, Toole BP. Increased hyaluronan at sites of attachment to mesentery by CD44-positive mouse ovarian and breast tumor cells. Am J Pathol (1996) 148(6):1733–40.
46. Catterall JB, Jones LM, Turner GA. Membrane protein glycosylation and CD44 content in the adhesion of human ovarian cancer cells to hyaluronan. Clin Exp Metastasis (1999) 17(7):583–91. doi:10.1023/A:1006756518500
47. Casey RC, Skubitz AP. CD44 and beta1 integrins mediate ovarian carcinoma cell migration toward extracellular matrix proteins. Clin Exp Metastasis (2000) 18(1):67–75. doi:10.1023/A:1026519016213
48. Ricciardelli C, Ween MP, Lokman NA, Tan IA, Pyragius CE, Oehler MK. Chemotherapy-induced hyaluronan production: a novel chemoresistance mechanism in ovarian cancer. BMC Cancer (2013) 13:476. doi:10.1186/1471-2407-13-476
49. Auzenne E, Ghosh SC, Khodadadian M, Rivera B, Farquhar D, Price RE, et al. Hyaluronic acid-paclitaxel: antitumor efficacy against CD44(+) human ovarian carcinoma xenografts. Neoplasia (2007) 9(6):479–86. doi:10.1593/neo.07229
50. Banzato A, Bobisse S, Rondina M, Renier D, Bettella F, Esposito G, et al. A paclitaxel-hyaluronan bioconjugate targeting ovarian cancer affords a potent in vivo therapeutic activity. Clin Cancer Res (2008) 14(11):3598–606. doi:10.1158/1078-0432.CCR-07-2019
51. Carter EM, Raggio CL. Genetic and orthopedic aspects of collagen disorders. Curr Opin Pediatr (2009) 21(1):46–54. doi:10.1097/MOP.0b013e32832185c5
52. Grassel S, Bauer RJ. Collagen XVI in health and disease. Matrix Biol (2013) 32(2):64–73. doi:10.1016/j.matbio.2012.11.001
53. Ricard-Blum S. The collagen family. Cold Spring Harb Perspect Biol (2011) 3(1):a004978. doi:10.1101/cshperspect.a004978
54. Zoumi A, Yeh A, Tromberg BJ. Imaging cells and extracellular matrix in vivo by using second-harmonic generation and two-photon excited fluorescence. Proc Natl Acad Sci USA (2002) 99(17):11014–9. doi:10.1073/pnas.172368799
55. Adur J, Pelegati VB, de Thomaz AA, Baratti MO, Andrade LA, Carvalho HF, et al. Second harmonic generation microscopy as a powerful diagnostic imaging modality for human ovarian cancer. J Biophotonics (2014) 7(1–2):37–48. doi:10.1002/jbio.201200108
56. Provenzano PP, Eliceiri KW, Campbell JM, Inman DR, White JG, Keely PJ. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med (2006) 4(1):38. doi:10.1186/1741-7015-4-38
57. Provenzano PP, Inman DR, Eliceiri KW, Knittel JG, Yan L, Rueden CT, et al. Collagen density promotes mammary tumor initiation and progression. BMC Med (2008) 6:11. doi:10.1186/1741-7015-6-11
58. Ursin G, Hovanessian-Larsen L, Parisky YR, Pike MC, Wu AH. Greatly increased occurrence of breast cancers in areas of mammographically dense tissue. Breast Cancer Res (2005) 7(5):R605–8. doi:10.1186/bcr1260
59. Egeblad M, Nakasone ES, Werb Z. Tumors as organs: complex tissues that interface with the entire organism. Dev Cell (2010) 18(6):884–901. doi:10.1016/j.devcel.2010.05.012
60. Helleman J, Jansen MP, Span PN, van Staveren IL, Massuger LF, Meijer-van Gelder ME, et al. Molecular profiling of platinum resistant ovarian cancer. Int J Cancer (2006) 118(8):1963–71. doi:10.1002/ijc.21599
61. Jazaeri AA, Awtrey CS, Chandramouli GV, Chuang YE, Khan J, Sotiriou C, et al. Gene expression profiles associated with response to chemotherapy in epithelial ovarian cancers. Clin Cancer Res (2005) 11(17):6300–10. doi:10.1158/1078-0432.CCR-04-2682
62. Sherman-Baust CA, Weeraratna AT, Rangel LB, Pizer ES, Cho KR, Schwartz DR, et al. Remodeling of the extracellular matrix through overexpression of collagen VI contributes to cisplatin resistance in ovarian cancer cells. Cancer Cell (2003) 3(4):377–86. doi:10.1016/S1535-6108(03)00058-8
63. Smoter M, Bodnar L, Grala B, Stec R, Zieniuk K, Kozlowski W, et al. Tau protein as a potential predictive marker in epithelial ovarian cancer patients treated with paclitaxel/platinum first-line chemotherapy. J Exp Clin Cancer Res (2013) 32:25. doi:10.1186/1756-9966-32-25
64. Kavallaris M, Kuo DY, Burkhart CA, Regl DL, Norris MD, Haber M, et al. Taxol-resistant epithelial ovarian tumors are associated with altered expression of specific beta-tubulin isotypes. J Clin Invest (1997) 100(5):1282–93. doi:10.1172/JCI119642
65. Yoshioka H, Iyama K, Inoguchi K, Khaleduzzaman M, Ninomiya Y, Ramirez F. Developmental pattern of expression of the mouse alpha 1 (XI) collagen gene (Col11a1). Dev Dyn (1995) 204(1):41–7. doi:10.1002/aja.1002040106
66. Fischer H, Stenling R, Rubio C, Lindblom A. Colorectal carcinogenesis is associated with stromal expression of COL11A1 and COL5A2. Carcinogenesis (2001) 22(6):875–8. doi:10.1093/carcin/22.6.875
67. Fischer H, Salahshor S, Stenling R, Björk J, Lindmark G, Iselius L, et al. COL11A1 in FAP polyps and in sporadic colorectal tumors. BMC Cancer (2001) 1:17. doi:10.1186/1471-2407-1-17
68. Imamura Y, Scott IC, Greenspan DS. The pro-alpha3(V) collagen chain. Complete primary structure, expression domains in adult and developing tissues, and comparison to the structures and expression domains of the other types V and XI procollagen chains. J Biol Chem (2000) 275(12):8749–59. doi:10.1074/jbc.275.12.8749
69. Ritzenthaler JD, Han S, Roman J. Stimulation of lung carcinoma cell growth by fibronectin-integrin signalling. Mol Biosyst (2008) 4(12):1160–9. doi:10.1039/b800533h
70. Williams CM, Engler AJ, Slone RD, Galante LL, Schwarzbauer JE. Fibronectin expression modulates mammary epithelial cell proliferation during acinar differentiation. Cancer Res (2008) 68(9):3185–92. doi:10.1158/0008-5472.CAN-07-2673
71. Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer (2010) 10(1):9–22. doi:10.1038/nrc2748
72. Sakai T, Johnson KJ, Murozono M, Sakai K, Magnuson MA, Wieloch T, et al. Plasma fibronectin supports neuronal survival and reduces brain injury following transient focal cerebral ischemia but is not essential for skin-wound healing and hemostasis. Nat Med (2001) 7(3):324–30. doi:10.1038/85471
73. Tucker RP, Chiquet-Ehrismann R. The regulation of tenascin expression by tissue microenvironments. Biochim Biophys Acta (2009) 1793(5):888–92. doi:10.1016/j.bbamcr.2008.12.012
74. Jones FS, Jones PL. The tenascin family of ECM glycoproteins: structure, function, and regulation during embryonic development and tissue remodeling. Dev Dyn (2000) 218(2):235–59. doi:10.1002/(SICI)1097-0177(200006)218:2<235::AID-DVDY2>3.0.CO;2-G
75. Didem T, Faruk T, Senem K, Derya D, Murat S, Murat G, et al. Clinical significance of serum tenascin-c levels in epithelial ovarian cancer. Tumour Biol (2014) 35(7):6777–82. doi:10.1007/s13277-014-1923-z
76. Orend G, Chiquet-Ehrismann R. Tenascin-C induced signaling in cancer. Cancer Lett (2006) 244(2):143–63. doi:10.1016/j.canlet.2006.02.017
77. Hsia HC, Schwarzbauer JE. Meet the tenascins: multifunctional and mysterious. J Biol Chem (2005) 280(29):26641–4. doi:10.1074/jbc.R500005200
78. Mithieux SM, Weiss AS. Elastin. Adv Protein Chem (2005) 70:437–61. doi:10.1016/S0065-3233(05)70013-9
79. Petersen E, Wågberg F, Ängquist KA. Serum concentrations of elastin-derived peptides in patients with specific manifestations of atherosclerotic disease. Eur J Vasc Endovasc Surg (2002) 24(5):440–4. doi:10.1053/ejvs.2002.1750
80. Kagan HM, Sullivan KA. Lysyl oxidase: preparation and role in elastin biosynthesis. Methods Enzymol. (1982) 82(Pt A):637–50. doi:10.1016/0076-6879(82)82092-2
81. Lapis K, Tímár J. Role of elastin-matrix interactions in tumor progression. Semin Cancer Biol (2002) 12(3):209–17. doi:10.1016/S1044-579X(02)00024-X
82. Toupance S, Brassart B, Rabenoelina F, Ghoneim C, Vallar L, Polette M, et al. Elastin-derived peptides increase invasive capacities of lung cancer cells by post-transcriptional regulation of MMP-2 and uPA. Clin Exp Metastasis (2012) 29(5):511–22. doi:10.1007/s10585-012-9467-3
83. Devy J, Duca L, Cantarelli B, Joseph-Pietras D, Scandolera A, Rusciani A, et al. Elastin-derived peptides enhance melanoma growth in vivo by upregulating the activation of Mcol-A (MMP-1) collagenase. Br J Cancer (2010) 103(10):1562–70. doi:10.1038/sj.bjc.6605926
84. Stewart CJ, Brennan BA, Crook ML, Russell P. Value of elastin staining in the assessment of peritoneal implants associated with ovarian serous borderline tumours. Histopathology (2007) 51(3):313–21. doi:10.1111/j.1365-2559.2007.02789.x
85. Colognato H, Yurchenco PD. Form and function: the laminin family of heterotrimers. Dev Dyn (2000) 218(2):213–34. doi:10.1002/(SICI)1097-0177(200006)218:2<213::AID-DVDY1>3.0.CO;2-R
86. Hamill KJ, Kligys K, Hopkinson SB, Jones JC. Laminin deposition in the extracellular matrix: a complex picture emerges. J Cell Sci (2009) 122(Pt 24):4409–17. doi:10.1242/jcs.041095
87. Aumailley M, Bruckner-Tuderman L, Carter WG, Deutzmann R, Edgar D, Ekblom P, et al. A simplified laminin nomenclature. Matrix Biol (2005) 24(5):326–32. doi:10.1016/j.matbio.2005.05.006
88. Tzu J, Marinkovich MP. Bridging structure with function: structural, regulatory, and developmental role of laminins. Int J Biochem Cell Biol (2008) 40(2):199–214. doi:10.1016/j.biocel.2007.07.015
89. Yurchenco PD, Quan Y, Colognato H, Mathus T, Harrison D, Yamada Y, et al. The α chain of laminin-1 is independently secreted and drives secretion of its β- and γ-chain partners. Proc Natl Acad Sci U S A (1997) 94(19):10189–94. doi:10.1073/pnas.94.19.10189
90. Lee VH, Britt JH, Dunbar BS. Localization of laminin proteins during early follicular development in pig and rabbit ovaries. J Reprod Fertil (1996) 108(1):115–22. doi:10.1530/jrf.0.1080115
91. Iozzo RV, Schaefer L. Proteoglycan form and function: a comprehensive nomenclature of proteoglycans. Matrix Biol (2015) 42(0):11–55. doi:10.1016/j.matbio.2015.02.003
92. Brezillon S, Zeltz C, Schneider L, Terryn C, Vuillermoz B, Ramont L, et al. Lumican inhibits B16F1 melanoma cell lung metastasis. J Physiol Pharmacol (2009) 60(Suppl 4):15–22.
93. Dellett M, Hu W, Papadaki V, Ohnuma S. Small leucine rich proteoglycan family regulates multiple signalling pathways in neural development and maintenance. Dev Growth Differ (2012) 54(3):327–40. doi:10.1111/j.1440-169X.2012.01339.x
94. Melchior-Becker A, Dai G, Ding Z, Schäfer L, Schrader J, Young MF, et al. Deficiency of biglycan causes cardiac fibroblasts to differentiate into a myofibroblast phenotype. J Biol Chem (2011) 286(19):17365–75. doi:10.1074/jbc.M110.192682
95. Nikitovic D, Berdiaki K, Chalkiadaki G, Karamanos N, Tzanakakis G. The role of SLRP-proteoglycans in osteosarcoma pathogenesis. Connect Tissue Res (2008) 49(3):235–8. doi:10.1080/03008200802147589
96. Shimizu-Hirota R, Sasamura H, Kuroda M, Kobayashi E, Hayashi M, Saruta T. Extracellular matrix glycoprotein biglycan enhances vascular smooth muscle cell proliferation and migration. Circ Res (2004) 94(8):1067–74. doi:10.1161/01.RES.0000126049.79800.CA
97. Iozzo RV. The biology of the small leucine-rich proteoglycans. Functional network of interactive proteins. J Biol Chem (1999) 274(27):18843–6.
98. Kalamajski S, Oldberg A. Homologous sequence in lumican and fibromodulin leucine-rich repeat 5-7 competes for collagen binding. J Biol Chem (2009) 284(1):534–9. doi:10.1074/jbc.M805721200
99. Kalamajski S, Oldberg A. The role of small leucine-rich proteoglycans in collagen fibrillogenesis. Matrix Biol (2010) 29(4):248–53. doi:10.1016/j.matbio.2010.01.001
100. Kresse H, Liszio C, Schönherr E, Fisher LW. Critical role of glutamate in a central leucine-rich repeat of decorin for interaction with type I collagen. J Biol Chem (1997) 272(29):18404–10. doi:10.1074/jbc.272.29.18404
101. Neame PJ, Kay CJ, McQuillan DJ, Beales MP, Hassell JR. Independent modulation of collagen fibrillogenesis by decorin and lumican. Cell Mol Life Sci (2000) 57(5):859–63. doi:10.1007/s000180050048
102. Reinboth B, Thomas J, Hanssen E, Gibson MA. Beta ig-h3 interacts directly with biglycan and decorin, promotes collagen VI aggregation, and participates in ternary complexing with these macromolecules. J Biol Chem (2006) 281(12):7816–24. doi:10.1074/jbc.M511316200
103. Keene DR, San Antonio JD, Mayne R, McQuillan DJ, Sarris G, Santoro SA, et al. Decorin binds near the C terminus of type I collagen. J Biol Chem (2000) 275(29):21801–4. doi:10.1074/jbc.C000278200
104. Iozzo RV. The family of the small leucine-rich proteoglycans: key regulators of matrix assembly and cellular growth. Crit Rev Biochem Mol Biol (1997) 32(2):141–74. doi:10.3109/10409239709108551
105. Iozzo RV, Cohen I. Altered proteoglycan gene expression and the tumor stroma. EXS (1994) 70:199–214.
106. McDoniels-Silvers AL, Nimri CF, Stoner GD, Lubet RA, You M. Differential gene expression in human lung adenocarcinomas and squamous cell carcinomas. Clin Cancer Res (2002) 8(4):1127–38.
107. Troup S, Njue C, Kliewer EV, Parisien M, Roskelley C, Chakravarti S, et al. Reduced expression of the small leucine-rich proteoglycans, lumican, and decorin is associated with poor outcome in node-negative invasive breast cancer. Clin Cancer Res (2003) 9(1):207–14.
108. Merle B, Durussel L, Delmas PD, Clézardin P. Decorin inhibits cell migration through a process requiring its glycosaminoglycan side chain. J Cell Biochem (1999) 75(3):538–46. doi:10.1002/(SICI)1097-4644(19991201)75:3<538::AID-JCB17>3.3.CO;2-P
109. Nash MA, Loercher AE, Freedman RS. In vitro growth inhibition of ovarian cancer cells by decorin: synergism of action between decorin and carboplatin. Cancer Res (1999) 59(24):6192–6.
110. Teicher BA, Maehara Y, Kakeji Y, Ara G, Keyes SR, Wong J, et al. Reversal of in vivo drug resistance by the transforming growth factor-beta inhibitor decorin. Int J Cancer (1997) 71(1):49–58. doi:10.1002/(SICI)1097-0215(19970328)71:1<49::AID-IJC10>3.0.CO;2-4
111. Hirsch CS, Ellner JJ, Blinkhorn R, Toossi Z. In vitro restoration of T cell responses in tuberculosis and augmentation of monocyte effector function against Mycobacterium tuberculosis by natural inhibitors of transforming growth factor beta. Proc Natl Acad Sci U S A (1997) 94(8):3926–31. doi:10.1073/pnas.94.8.3926
112. Iozzo RV, Moscatello DK, McQuillan DJ, Eichstetter I. Decorin is a biological ligand for the epidermal growth factor receptor. J Biol Chem (1999) 274(8):4489–92. doi:10.1074/jbc.274.8.4489
113. Moscatello DK, Santra M, Mann DM, McQuillan DJ, Wong AJ, Iozzo RV. Decorin suppresses tumor cell growth by activating the epidermal growth factor receptor. J Clin Invest (1998) 101(2):406–12. doi:10.1172/JCI846
114. Santra M, Reed CC, Iozzo RV. Decorin binds to a narrow region of the epidermal growth factor (EGF) receptor, partially overlapping but distinct from the EGF-binding epitope. J Biol Chem (2002) 277(38):35671–81. doi:10.1074/jbc.M205317200
115. Santra M, Eichstetter I, Iozzo RV. An anti-oncogenic role for decorin. Down-regulation of ErbB2 leads to growth suppression and cytodifferentiation of mammary carcinoma cells. J Biol Chem (2000) 275(45):35153–61. doi:10.1074/jbc.M006821200
116. Csordás G, Santra M, Reed CC, Eichstetter I, McQuillan DJ, Gross D, et al. Sustained down-regulation of the epidermal growth factor receptor by decorin. A mechanism for controlling tumor growth in vivo. J Biol Chem (2000) 275(42):32879–87. doi:10.1074/jbc.M005609200
117. Seidler DG, Goldoni S, Agnew C, Cardi C, Thakur ML, Owens RT, et al. Decorin protein core inhibits in vivo cancer growth and metabolism by hindering epidermal growth factor receptor function and triggering apoptosis via caspase-3 activation. J Biol Chem (2006) 281(36):26408–18. doi:10.1074/jbc.M602853200
118. Vij N, Roberts L, Joyce S, Chakravarti S. Lumican regulates corneal inflammatory responses by modulating Fas-Fas ligand signaling. Invest Ophthalmol Vis Sci (2005) 46(1):88–95. doi:10.1167/iovs.04-0833
119. Vuillermoz B, Khoruzhenko A, D’Onofrio MF, Ramont L, Venteo L, Perreau C, et al. The small leucine-rich proteoglycan lumican inhibits melanoma progression. Exp Cell Res (2004) 296(2):294–306. doi:10.1016/j.yexcr.2004.02.005
120. Albig AR, Roy TG, Becenti DJ, Schiemann WP. Transcriptome analysis of endothelial cell gene expression induced by growth on matrigel matrices: identification and characterization of MAGP-2 and lumican as novel regulators of angiogenesis. Angiogenesis (2007) 10(3):197–216. doi:10.1007/s10456-007-9075-z
121. Naito Z. Role of the small leucine-rich proteoglycan (SLRP) family in pathological lesions and cancer cell growth. J Nippon Med Sch (2005) 72(3):137–45. doi:10.1272/jnms.72.137
122. Leygue E, Snell L, Dotzlaw H, Troup S, Hiller-Hitchcock T, Murphy LC, et al. Lumican and decorin are differentially expressed in human breast carcinoma. J Pathol (2000) 192(3):313–20. doi:10.1002/1096-9896(200011)192:3<313::AID-PATH694>3.3.CO;2-2
123. Matsuda Y, Yamamoto T, Kudo M, Kawahara K, Kawamoto M, Nakajima Y, et al. Expression and roles of lumican in lung adenocarcinoma and squamous cell carcinoma. Int J Oncol (2008) 33(6):1177–85. doi:10.3892/ijo_00000107
124. Lu YP, Ishiwata T, Kawahara K, Watanabe M, Naito Z, Moriyama Y, et al. Expression of lumican in human colorectal cancer cells. Pathol Int (2002) 52(8):519–26. doi:10.1046/j.1440-1827.2002.01384.x
125. Seya T, Tanaka N, Shinji S, Yokoi K, Koizumi M, Teranishi N, et al. Lumican expression in advanced colorectal cancer with nodal metastasis correlates with poor prognosis. Oncol Rep (2006) 16(6):1225–30. doi:10.3892/or.16.6.1225
126. Köninger J, Giese T, di Mola FF, Wente MN, Esposito I, Bachem MG, et al. Pancreatic tumor cells influence the composition of the extracellular matrix. Biochem Biophys Res Commun (2004) 322(3):943–9. doi:10.1016/j.bbrc.2004.08.008
127. Leygue E, Snell L, Dotzlaw H, Hole K, Hiller-Hitchcock T, Roughley PJ, et al. Expression of lumican in human breast carcinoma. Cancer Res (1998) 58(7):1348–52.
128. Ishiwata T, Cho K, Kawahara K, Yamamoto T, Fujiwara Y, Uchida E, et al. Role of lumican in cancer cells and adjacent stromal tissues in human pancreatic cancer. Oncol Rep (2007) 18(3):537–43. doi:10.3892/or.18.3.537
129. Shinji S, Naito Z, Ishiwata T, Tanaka N, Furukawa K, Suzuki H, et al. Neuroendocrine cell differentiation of poorly differentiated colorectal adenocarcinoma correlates with liver metastasis. Int J Oncol (2006) 29(2):357–64. doi:10.3892/ijo.29.2.357
130. Russell DL, Ochsner SA, Hsieh M, Mulders S, Richards JS. Hormone-regulated expression and localization of versican in the rodent ovary. Endocrinology (2003) 144(3):1020–31. doi:10.1210/en.2002-220434
131. Cattaruzza S, Schiappacassi M, Ljungberg-Rose A, Spessotto P, Perissinotto D, Mörgelin M, et al. Distribution of PG-M/versican variants in human tissues and de novo expression of isoform V3 upon endothelial cell activation, migration, and neoangiogenesis in vitro. J Biol Chem (2002) 277(49):47626–35. doi:10.1074/jbc.M206521200
132. Häkkinen L, Westermarck J, Kähäri VM, Larjava H. Human granulation-tissue fibroblasts show enhanced proteoglycan gene expression and altered response to TGF-beta 1. J Dent Res (1996) 75(10):1767–78. doi:10.1177/00220345960750101001
133. Isogai Z, Shinomura T, Yamakawa N, Takeuchi J, Tsuji T, Heinegård D, et al. 2B1 antigen characteristically expressed on extracellular matrices of human malignant tumors is a large chondroitin sulfate proteoglycan, PG-M/versican. Cancer Res (1996) 56(17):3902–8.
134. Theocharis AD. Human colon adenocarcinoma is associated with specific post-translational modifications of versican and decorin. Biochim Biophys Acta (2002) 1588(2):165–72. doi:10.1016/S0925-4439(02)00161-8
135. Theocharis AD, Vynios DH, Papageorgakopoulou N, Skandalis SS, Theocharis DA. Altered content composition and structure of glycosaminoglycans and proteoglycans in gastric carcinoma. Int J Biochem Cell Biol (2003) 35(3):376–90. doi:10.1016/S1357-2725(02)00264-9
136. Tsara ME, Theocharis AD, Theocharis DA. Compositional and structural alterations of proteoglycans in human rectum carcinoma with special reference to versican and decorin. Anticancer Res (2002) 22(5):2893–8.
137. Touab M, Villena J, Barranco C, Arumí-Uría M, Bassols A. Versican is differentially expressed in human melanoma and may play a role in tumor development. Am J Pathol (2002) 160(2):549–57. doi:10.1016/S0002-9440(10)64874-2
138. Ito Y, Abiko Y, Tanaka Y, Rahemtulla F, Kaku T. Immunohistochemical localization of large chondroitin sulfate proteoglycan in odontogenic tumor. Med Electron Microsc (2002) 35(3):173–7. doi:10.1007/s007950200022
139. Nara Y, Kato Y, Torii Y, Tsuji Y, Nakagaki S, Goto S, et al. Immunohistochemical localization of extracellular matrix components in human breast tumours with special reference to PG-M/versican. Histochem J (1997) 29(1):21–30. doi:10.1023/A:1026460700592
140. Brown LF, Guidi AJ, Schnitt SJ, Van De Water L, Iruela-Arispe ML, Yeo TK, et al. Vascular stroma formation in carcinoma in situ, invasive carcinoma, and metastatic carcinoma of the breast. Clin Cancer Res (1999) 5(5):1041–56.
141. Dobra K, Andäng M, Syrokou A, Karamanos NK, Hjerpe A. Differentiation of mesothelioma cells is influenced by the expression of proteoglycans. Exp Cell Res (2000) 258(1):12–22. doi:10.1006/excr.2000.4915
142. Dours-Zimmermann MT, Zimmermann DR. A novel glycosaminoglycan attachment domain identified in two alternative splice variants of human versican. J Biol Chem (1994) 269(52):32992–8.
143. Bouterfa H, Darlapp AR, Klein E, Pietsch T, Roosen K, Tonn JC. Expression of different extracellular matrix components in human brain tumor and melanoma cells in respect to variant culture conditions. J Neurooncol (1999) 44(1):23–33. doi:10.1023/A:1006331416283
144. Sharma B, Handler M, Eichstetter I, Whitelock JM, Nugent MA, Iozzo RV. Antisense targeting of perlecan blocks tumor growth and angiogenesis in vivo. J Clin Invest (1998) 102(8):1599–608. doi:10.1172/JCI3793
145. Iozzo RV, San Antonio JD. Heparan sulfate proteoglycans: heavy hitters in the angiogenesis arena. J Clin Invest (2001) 108(3):349–55. doi:10.1172/JCI200113738
146. Cohen IR, Murdoch AD, Naso MF, Marchetti D, Berd D, Iozzo RV. Abnormal expression of perlecan proteoglycan in metastatic melanomas. Cancer Res (1994) 54(22):5771–4.
147. Datta MW, Hernandez AM, Schlicht MJ, Kahler AJ, DeGueme AM, Dhir R, et al. Perlecan, a candidate gene for the CAPB locus, regulates prostate cancer cell growth via the sonic hedgehog pathway. Mol Cancer (2006) 5:9. doi:10.1186/1476-4598-5-9
148. Iozzo RV, Cohen IR, Grässel S, Murdoch AD. The biology of perlecan: the multifaceted heparan sulphate proteoglycan of basement membranes and pericellular matrices. Biochem J (1994) 302(Pt 3):625–39. doi:10.1042/bj3020625
149. Blackhall FH, Merry CL, Davies EJ, Jayson GC. Heparan sulfate proteoglycans and cancer. Br J Cancer (2001) 85(8):1094–8. doi:10.1054/bjoc.2001.2054
150. Sanderson RD. Heparan sulfate proteoglycans in invasion and metastasis. Semin Cell Dev Biol (2001) 12(2):89–98. doi:10.1006/scdb.2000.0241
151. Aviezer D, Iozzo RV, Noonan DM, Yayon A. Suppression of autocrine and paracrine functions of basic fibroblast growth factor by stable expression of perlecan antisense cDNA. Mol Cell Biol (1997) 17(4):1938–46. doi:10.1128/MCB.17.4.1938
152. Evanko SP, Potter-Perigo S, Petty LJ, Workman GA, Wight TN. Hyaluronan controls the deposition of fibronectin and collagen and modulates TGF-β1 induction of lung myofibroblasts. Matrix Biol (2015) 42:74–92. doi:10.1016/j.matbio.2014.12.001
153. Laterra J, Culp LA. Differences in hyaluronate binding to plasma and cell surface fibronectins. Requirement for aggregation. J Biol Chem (1982) 257(2):719–26.
154. Yamada KM, Kennedy DW, Kimata K, Pratt RM. Characterization of fibronectin interactions with glycosaminoglycans and identification of active proteolytic fragments. J Biol Chem (1980) 255(13):6055–63.
155. Coleman PJ. Evidence for a role of hyaluronan in the spacing of fibrils within collagen bundles in rabbit synovium. Biochim Biophys Acta (2002) 1571(3):173–82. doi:10.1016/S0304-4165(02)00213-1
156. Toole BP. Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer (2004) 4(7):528–39. doi:10.1038/nrc1391
157. Evanko SP, Tammi MI, Tammi RH, Wight TN. Hyaluronan-dependent pericellular matrix. Adv Drug Deliv Rev (2007) 59(13):1351–65. doi:10.1016/j.addr.2007.08.008
158. Itano N. Simple primary structure, complex turnover regulation and multiple roles of hyaluronan. J Biochem (2008) 144(2):131–7. doi:10.1093/jb/mvn046
159. Hascall VC, Heinegard D. Aggregation of cartilage proteoglycans. I. The role of hyaluronic acid. J Biol Chem (1974) 249(13):4232–41.
160. Bonnet F, Dunham DG, Hardingham TE. Structure and interactions of cartilage proteoglycan binding region and link protein. Biochem J (1985) 228(1):77–85. doi:10.1042/bj2280077
161. Mörgelin M, Paulsson M, Hardingham TE, Heinegård D, Engel J. Cartilage proteoglycans. Assembly with hyaluronate and link protein as studied by electron microscopy. Biochem J (1988) 253(1):175–85. doi:10.1042/bj2530175
162. Toole BP, Wight TN, Tammi MI. Hyaluronan-cell interactions in cancer and vascular disease. J Biol Chem (2002) 277(7):4593–6. doi:10.1074/jbc.R100039200
163. Tammi RH, Kultti A, Kosma VM, Pirinen R, Auvinen P, Tammi MI. Hyaluronan in human tumors: pathobiological and prognostic messages from cell-associated and stromal hyaluronan. Semin Cancer Biol (2008) 18(4):288–95. doi:10.1016/j.semcancer.2008.03.005
164. Itano N, Zhuo L, Kimata K. Impact of the hyaluronan-rich tumor microenvironment on cancer initiation and progression. Cancer Sci (2008) 99(9):1720–5. doi:10.1111/j.1349-7006.2008.00885.x
165. Tolg C, Hamilton SR, Zalinska E, McCulloch L, Amin R, Akentieva N, et al. A RHAMM mimetic peptide blocks hyaluronan signaling and reduces inflammation and fibrogenesis in excisional skin wounds. Am J Pathol (2012) 181(4):1250–70. doi:10.1016/j.ajpath.2012.06.036
166. Tolg C, McCarthy JB, Yazdani A, Turley EA. Hyaluronan and RHAMM in wound repair and the “cancerization” of stromal tissues. Biomed Res Int (2014) 2014:103923. doi:10.1155/2014/103923
167. Dahl IM, Laurent TC. Concentration of hyaluronan in the serum of untreated cancer patients with special reference to patients with mesothelioma. Cancer (1988) 62(2):326–30. doi:10.1002/1097-0142(19880715)62:2<326::AID-CNCR2820620217>3.0.CO;2-Y
168. Delpech B, Bertrand P, Maingonnat C. Immunoenzymoassay of the hyaluronic acid-hyaluronectin interaction: application to the detection of hyaluronic acid in serum of normal subjects and cancer patients. Anal Biochem (1985) 149(2):555–65. doi:10.1016/0003-2697(85)90613-X
169. Yahya RS, El-Bindary AA, El-Mezayen HA, Abdelmasseh HM, Eissa MA. Biochemical evaluation of hyaluronic acid in breast cancer. Clin Lab (2014) 60(7):1115–21. doi:10.7754/Clin.Lab.2013.130413
170. Wu M, Cao M, He Y, Liu Y, Yang C, Du Y, et al. A novel role of low molecular weight hyaluronan in breast cancer metastasis. FASEB J (2015) 29(4):1290–8. doi:10.1096/fj.14-259978
171. Ween MP, Hummitzsch K, Rodgers RJ, Oehler MK, Ricciardelli C. Versican induces a pro-metastatic ovarian cancer cell behavior which can be inhibited by small hyaluronan oligosaccharides. Clin Exp Metastasis (2011) 28(2):113–25. doi:10.1007/s10585-010-9363-7
172. Vincent T, Molina L, Espert L, Mechti N. Hyaluronan, a major non-protein glycosaminoglycan component of the extracellular matrix in human bone marrow, mediates dexamethasone resistance in multiple myeloma. Br J Haematol (2003) 121(2):259–69. doi:10.1046/j.1365-2141.2003.04282.x
173. Wang SJ, Bourguignon LY. Hyaluronan and the interaction between CD44 and epidermal growth factor receptor in oncogenic signaling and chemotherapy resistance in head and neck cancer. Arch Otolaryngol Head Neck Surg (2006) 132(7):771–8. doi:10.1001/archotol.132.7.771
174. Bourguignon LY, Peyrollier K, Xia W, Gilad E. Hyaluronan-CD44 interaction activates stem cell marker Nanog, Stat-3-mediated MDR1 gene expression, and ankyrin-regulated multidrug efflux in breast and ovarian tumor cells. J Biol Chem (2008) 283(25):17635–51. doi:10.1074/jbc.M800109200
175. Torre C, Wang SJ, Xia W, Bourguignon LY. Reduction of hyaluronan-CD44-mediated growth, migration, and cisplatin resistance in head and neck cancer due to inhibition of Rho kinase and PI-3 kinase signaling. Arch Otolaryngol Head Neck Surg (2010) 136(5):493–501. doi:10.1001/archoto.2010.25
176. Lu P, Takai K, Weaver VM, Werb Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol (2011) 3(12):a005058. doi:10.1101/cshperspect.a005058
177. Stack MS, Ellerbroek SM, Fishman DA. The role of proteolytic enzymes in the pathology of epithelial ovarian carcinoma. Int J Oncol (1998) 12(3):569–76.
178. Kuhn W, Schmalfeldt B, Reuning U, Pache L, Berger U, Ulm K, et al. Prognostic significance of urokinase (uPA) and its inhibitor PAI-1 for survival in advanced ovarian carcinoma stage FIGO IIIc. Br J Cancer (1999) 79(11–12):1746–51. doi:10.1038/sj.bjc.6690278
179. Ozalp S, Tanir HM, Yalcin OT, Kabukcuoglu S, Oner U, Uray M. Prognostic value of matrix metalloproteinase-9 (gelatinase-B) expression in epithelial ovarian tumors. Eur J Gynaecol Oncol (2003) 24(5):417–20.
180. Schmalfeldt B, Prechtel D, Härting K, Späthe K, Rutke S, Konik E, et al. Increased expression of matrix metalloproteinases (MMP)-2, MMP-9, and the urokinase-type plasminogen activator is associated with progression from benign to advanced ovarian cancer. Clin Cancer Res (2001) 7(8):2396–404.
181. Brun JL, Cortez A, Commo F, Uzan S, Rouzier R, Daraï E. Serous and mucinous ovarian tumors express different profiles of MMP-2, -7, -9, MT1-MMP, and TIMP-1 and -2. Int J Oncol (2008) 33(6):1239–46. doi:10.3892/ijo_00000114
182. Moser TL, Young TN, Rodriguez GC, Pizzo SV, Bast RC Jr, Stack MS. Secretion of extracellular matrix-degrading proteinases is increased in epithelial ovarian carcinoma. Int J Cancer (1994) 56(4):552–9. doi:10.1002/ijc.2910560415
183. Young TN, Rodriguez GC, Rinehart AR, Bast RC Jr, Pizzo SV, Stack MS. Characterization of gelatinases linked to extracellular matrix invasion in ovarian adenocarcinoma: purification of matrix metalloproteinase 2. Gynecol Oncol (1996) 62(1):89–99. doi:10.1006/gyno.1996.0195
184. Fishman DA, Bafetti LM, Banionis S, Kearns AS, Chilukuri K, Stack MS. Production of extracellular matrix-degrading proteinases by primary cultures of human epithelial ovarian carcinoma cells. Cancer (1997) 80:1457–63. doi:10.1002/(SICI)1097-0142(19971015)80:8<1457::AID-CNCR13>3.0.CO;2-4
185. Afzal S, Lalani el-N, Foulkes WD, Boyce B, Tickle S, Cardillo MR, et al. Matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-2 expression and synthetic matrix metalloproteinase-2 inhibitor binding in ovarian carcinomas and tumor cell lines. Lab Invest (1996) 74(2):406–21.
186. Wang L, Jin X, Lin D, Liu Z, Zhang X, Lu Y, et al. Clinicopathologic significance of claudin-6, occludin, and matrix metalloproteinases-2 expression in ovarian carcinoma. Diagn Pathol (2013) 8:190. doi:10.1186/1746-1596-8-190
187. Che YL, Luo SJ, Li G, Cheng M, Gao YM, Li XM, et al. The C3G/Rap1 pathway promotes secretion of MMP-2 and MMP-9 and is involved in serous ovarian cancer metastasis. Cancer Lett (2015) 359(2):241–9. doi:10.1016/j.canlet.2015.01.019
188. Sillanpää S, Anttila M, Voutilainen K, Ropponen K, Turpeenniemi-Hujanen T, Puistola U, et al. Prognostic significance of matrix metalloproteinase-9 (MMP-9) in epithelial ovarian cancer. Gynecol Oncol (2007) 104(2):296–303. doi:10.1016/j.ygyno.2006.09.004
189. Kamat AA, Fletcher M, Gruman LM, Mueller P, Lopez A, Landen CN Jr, et al. The clinical relevance of stromal matrix metalloproteinase expression in ovarian cancer. Clin Cancer Res (2006) 12(6):1707–14. doi:10.1158/1078-0432.CCR-05-2338
190. Lengyel E, Schmalfeldt B, Konik E, Späthe K, Härting K, Fenn A, et al. Expression of latent matrix metalloproteinase 9 (MMP-9) predicts survival in advanced ovarian cancer. Gynecol Oncol (2001) 82(2):291–8. doi:10.1006/gyno.2001.6243
191. Sakata K, Shigemasa K, Nagai N, Ohama K. Expression of matrix metalloproteinases (MMP-2, MMP-9, MT1-MMP) and their inhibitors (TIMP-1, TIMP-2) in common epithelial tumors of the ovary. Int J Oncol (2000) 17(4):673–81. doi:10.3892/ijo.17.4.673
192. Hu X, Li D, Zhang W, Zhou J, Tang B, Li L. Matrix metalloproteinase-9 expression correlates with prognosis and involved in ovarian cancer cell invasion. Arch Gynecol Obstet (2012) 286(6):1537–43. doi:10.1007/s00404-012-2456-6
193. Wang FQ, So J, Reierstad S, Fishman DA. Matrilysin (MMP-7) promotes invasion of ovarian cancer cells by activation of progelatinase. Int J Cancer (2005) 114(1):19–31. doi:10.1002/ijc.20697
194. Zhao H, Yang Z, Wang X, Zhang X, Wang M, Wang Y, et al. Triptolide inhibits ovarian cancer cell invasion by repression of matrix metalloproteinase 7 and 19 and upregulation of E-cadherin. Exp Mol Med (2012) 44(11):633–41. doi:10.3858/emm.2012.44.11.072
195. Shigemasa K, Tanimoto H, Sakata K, Nagai N, Parmley TH, Ohama K, et al. Induction of matrix metalloprotease-7 is common in mucinous ovarian tumors including early stage disease. Med Oncol (2000) 17(1):52–8. doi:10.1007/BF02826217
196. Sillanpää SM, Anttila MA, Voutilainen KA, Ropponen KM, Sironen RK, Saarikoski SV, et al. Prognostic significance of matrix metalloproteinase-7 in epithelial ovarian cancer and its relation to beta-catenin expression. Int J Cancer (2006) 119(8):1792–9. doi:10.1002/ijc.22067
197. Wang X, Cong JL, Qu LY, Jiang L, Wang Y. Association between lysyl oxidase G473A polymorphism and ovarian cancer in the Han Chinese population. J Int Med Res (2012) 40(3):917–23. doi:10.1177/147323001204000310
198. Wu J, Cai C, Tong D, Hou H. Lysyl oxidase G473A polymorphism is associated with increased risk of ovarian cancer. Genet Test Mol Biomarkers (2012) 16(8):915–9. doi:10.1089/gtmb.2011.0374
199. Ji F, Wang Y, Qiu L, Li S, Zhu J, Liang Z, et al. Hypoxia inducible factor 1α-mediated LOX expression correlates with migration and invasion in epithelial ovarian cancer. Int J Oncol (2013) 42(5):1578–88. doi:10.3892/ijo.2013.1878
200. Wang Y, Ma J, Shen H, Wang C, Sun Y, Howell SB, et al. Reactive oxygen species promote ovarian cancer progression via the HIF-1alpha/LOX/E-cadherin pathway. Oncol Rep (2014) 32(5):2150–8. doi:10.3892/or.2014.3448
201. Barry-Hamilton V, Spangler R, Marshall D, McCauley S, Rodriguez HM, Oyasu M, et al. Allosteric inhibition of lysyl oxidase-like-2 impedes the development of a pathologic microenvironment. Nat Med (2010) 16(9):1009–17. doi:10.1038/nm.2208
202. Zaffryar-Eilot S, Marshall D, Voloshin T, Bar-Zion A, Spangler R, Kessler O, et al. Lysyl oxidase-like-2 promotes tumour angiogenesis and is a potential therapeutic target in angiogenic tumours. Carcinogenesis (2013) 34(10):2370–9. doi:10.1093/carcin/bgt241
203. Sebban S, Golan-Gerstl R, Karni R, Vaksman O, Davidson B, Reich R. Alternatively spliced lysyl oxidase-like 4 isoforms have a pro-metastatic role in cancer. Clin Exp Metastasis (2013) 30(1):103–17. doi:10.1007/s10585-012-9514-0
204. Yabushita H, Noguchi M, Kishida T, Fusano K, Noguchi Y, Itano N, et al. Hyaluronan synthase expression in ovarian cancer. Oncol Rep (2004) 12(4):739–43. doi:10.3892/or.12.4.739
205. Nykopp TK, Rilla K, Sironen R, Tammi MI, Tammi RH, Hämäläinen K, et al. Expression of hyaluronan synthases (HAS1-3) and hyaluronidases (HYAL1-2) in serous ovarian carcinomas: inverse correlation between HYAL1 and hyaluronan content. BMC Cancer (2009) 9:143. doi:10.1186/1471-2407-9-143
206. Weiss I, Trope CG, Reich R, Davidson B. Hyaluronan synthase and hyaluronidase expression in serous ovarian carcinoma is related to anatomic site and chemotherapy exposure. Int J Mol Sci (2012) 13(10):12925–38. doi:10.3390/ijms131012925
207. Tuhkanen H, Anttila M, Kosma VM, Ylä-Herttuala S, Heinonen S, Kuronen A, et al. Genetic alterations in the peritumoral stromal cells of malignant and borderline epithelial ovarian tumors as indicated by allelic imbalance on chromosome 3p. Int J Cancer (2004) 109(2):247–52. doi:10.1002/ijc.11733
208. Yoffou PH, Edjekouane L, Meunier L, Tremblay A, Provencher DM, Mes-Masson AM, et al. Subtype specific elevated expression of hyaluronidase-1 (HYAL-1) in epithelial ovarian cancer. PLoS One (2011) 6(6):e20705. doi:10.1371/journal.pone.0020705
209. Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res (2006) 69:562–73. doi:10.1016/j.cardiores.2005.12.002
210. Nagase H, Woessner JF Jr. Matrix metalloproteinases. J Biol Chem (1999) 274(31):21491–4. doi:10.1074/jbc.274.31.21491
211. Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature (2004) 432(7015):332–7. doi:10.1038/nature03096
212. Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer (2006) 6(5):392–401. doi:10.1038/nrc1877
213. Cathcart J, Pulkoski-Gross A, Cao J. Targeting matrix metalloproteinases in cancer: bringing new life to old ideas. Genes Dis (2015) 2(1):26–34. doi:10.1016/j.gendis.2014.12.002
214. Nelson AR, Fingleton B, Rothenberg ML, Matrisian LM. Matrix metalloproteinases: biologic activity and clinical implications. J Clin Oncol (2000) 18(5):1135.
215. Tamakoshi K, Kikkawa F, Nawa A, Maeda O, Kawai M, Sugamuma N, et al. Different pattern of zymography between human gynecologic normal and malignant tissues. Am J Obstet Gynecol (1994) 171(2):478–84. doi:10.1016/0002-9378(94)90286-0
216. Yu Y, Li H, Xue B, Jiang X, Huang K, Ge J, et al. SDF-1/CXCR7 axis enhances ovarian cancer cell invasion by MMP-9 expression through p38 MAPK pathway. DNA Cell Biol (2014) 33(8):543–9. doi:10.1089/dna.2013.2289
217. Ii M, Yamamoto H, Adachi Y, Maruyama Y, Shinomura Y. Role of matrix metalloproteinase-7 (matrilysin) in human cancer invasion, apoptosis, growth, and angiogenesis. Exp Biol Med (Maywood) (2006) 231(1):20–7.
218. Brew K, Nagase H. The tissue inhibitors of metalloproteinases (TIMPs): an ancient family with structural and functional diversity. Biochim Biophys Acta (2010) 1803(1):55–71. doi:10.1016/j.bbamcr.2010.01.003
219. Nagase H, Murphy G. Tailoring TIMPs for selective metalloproteinase inhibition. In: Edwards D, editors. The Cancer Degradome. New York, NY: Springer (2008). p. 787–810.
220. Goldberg GI, Strongin A, Collier IE, Genrich LT, Marmer BL. Interaction of 92-kDa type IV collagenase with the tissue inhibitor of metalloproteinases prevents dimerization, complex formation with interstitial collagenase, and activation of the proenzyme with stromelysin. J Biol Chem (1992) 267(7):4583–91.
221. Stetler-Stevenson WG, Krutzsch HC, Liotta LA. Tissue inhibitor of metalloproteinase (TIMP-2). A new member of the metalloproteinase inhibitor family. J Biol Chem (1989) 264(29):17374–8.
222. Okamoto T, Niu R, Yamada S. Increased expression of tissue inhibitor of metalloproteinase-2 in clear cell carcinoma of the ovary. Mol Hum Reprod (2003) 9(10):569–75. doi:10.1093/molehr/gag074
223. Huang LW, Garrett AP, Bell DA, Welch WR, Berkowitz RS, Mok SC. Differential expression of matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 protein and mRNA in epithelial ovarian tumors. Gynecol Oncol (2000) 77(3):369–76. doi:10.1006/gyno.2000.5806
224. Obayashi Y, Yabushita H, Kanyama K, Noguchi M, Zhuo L, Kimata K, et al. Role of serum-derived hyaluronan-associated protein-hyaluronan complex in ovarian cancer. Oncol Rep (2008) 19(5):1245–51. doi:10.3892/or.19.5.1245
225. Kikkawa F, Tamakoshi K, Nawa A, Shibata K, Yamagata S, Yamagata T, et al. Positive correlation between inhibitors of matrix metalloproteinase 1 and matrix metalloproteinases in malignant ovarian tumor tissues. Cancer Lett (1997) 120(1):109–15. doi:10.1016/S0304-3835(97)00295-4
226. Kagan HM, Li W. Lysyl oxidase: properties, specificity, and biological roles inside and outside of the cell. J Cell Biochem (2003) 88(4):660–72. doi:10.1002/jcb.10413
227. Mäki JM. Lysyl oxidases in mammalian development and certain pathological conditions. Histol Histopathol (2009) 24:651–60.
228. Li W, Nellaiappan K, Strassmaier T, Graham L, Thomas KM, Kagan HM. Localization and activity of lysyl oxidase within nuclei of fibrogeniccells. Proc Natl Acad Sci U S A (1997) 94(24):12817–22. doi:10.1073/pnas.94.24.12817
229. Erler JT, Bennewith KL, Nicolau M, Dornhöfer N, Kong C, Le QT, et al. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature (2006) 440(7088):1222–6. doi:10.1038/nature04695
230. Barker HE, Cox TR, Erler JT. The rationale for targeting the LOX family in cancer. Nat Rev Cancer (2012) 12(8):540–52. doi:10.1038/nrc3319
231. Himeno N. [Effect of prostaglandins on collagen synthesis in rabbit ovarian follicles during the ovulatory process]. Nihon Naibunpi Gakkai Zasshi (1986) 62(10):1181–93.
232. Slee RB, Hillier SG, Largue P, Harlow CR, Miele G, Clinton M. Differentiation-dependent expression of connective tissue growth factor and lysyl oxidase messenger ribonucleic acids in rat granulosa cells. Endocrinology (2001) 142(3):1082–9. doi:10.1210/en.142.3.1082
233. Kendall NR, Marsters P, Scaramuzzi RJ, Campbell BK. Expression of lysyl oxidase and effect of copper chloride and ammonium tetrathiomolybdate on bovine ovarian follicle granulosa cells cultured in serum-free media. Reproduction (2003) 125(5):657–65. doi:10.1530/rep.0.1250657
234. Harlow CR, Rae M, Davidson L, Trackman PC, Hillier SG. Lysyl oxidase gene expression and enzyme activity in the rat ovary: regulation by follicle-stimulating hormone, androgen, and transforming growth factor-beta superfamily members in vitro. Endocrinology (2003) 144(1):154–62. doi:10.1210/en.2002-220652
235. Akiri G, Sabo E, Dafni H, Vadasz Z, Kartvelishvily Y, Gan N, et al. Lysyl oxidase-related protein-1 promotes tumor fibrosis and tumor progression in vivo. Cancer Res (2003) 63(7):1657–66.
236. Peinado H, Moreno-Bueno G, Hardisson D, Pérez-Gómez E, Santos V, Mendiola M, et al. Lysyl oxidase-like 2 as a new poor prognosis marker of squamous cell carcinomas. Cancer Res (2008) 68(12):4541–50. doi:10.1158/0008-5472.CAN-07-6345
237. Fong SF, Dietzsch E, Fong KS, Hollosi P, Asuncion L, He Q, et al. Lysyl oxidase-like 2 expression is increased in colon and esophageal tumors and associated with less differentiated colon tumors. Genes Chromosomes Cancer (2007) 46(7):644–55. doi:10.1002/gcc.20444
238. Goel S, Duda DG, Xu L, Munn LL, Boucher Y, Fukumura D, et al. Normalization of the vasculature for treatment of cancer and other diseases. Physiol Rev (2011) 91(3):1071–121. doi:10.1152/physrev.00038.2010
239. Görögh T, Weise JB, Holtmeier C, Rudolph P, Hedderich J, Gottschlich S, et al. Selective upregulation and amplification of the lysyl oxidase like-4 (LOXL4) gene in head and neck squamous cell carcinoma. J Pathol (2007) 212(1):74–82. doi:10.1002/path.2137
240. Weise JB, Rudolph P, Heiser A, Kruse ML, Hedderich J, Cordes C, et al. LOXL4 is a selectively expressed candidate diagnostic antigen in head and neck cancer. Eur J Cancer (2008) 44(9):1323–31. doi:10.1016/j.ejca.2008.03.026
241. Li RK, Zhao WY, Fang F, Zhuang C, Zhang XX, Yang XM, et al. Lysyl oxidase-like 4 (LOXL4) promotes proliferation and metastasis of gastric cancer via FAK/Src pathway. J Cancer Res Clin Oncol (2015) 141(2):269–81. doi:10.1007/s00432-014-1823-z
242. DeAngelis PL. Hyaluronan synthases: fascinating glycosyltransferases from vertebrates, bacterial pathogens, and algal viruses. Cell Mol Life Sci (1999) 56(7–8):670–82. doi:10.1007/s000180050461
243. Weigel PH, Hascall VC, Tammi M. Hyaluronan synthases. J Biol Chem (1997) 272(22):13997–4000. doi:10.1074/jbc.272.22.13997
244. Itano N, Sawai T, Yoshida M, Lenas P, Yamada Y, Imagawa M, et al. Three isoforms of mammalian hyaluronan synthases have distinct enzymatic properties. J Biol Chem (1999) 274(35):25085–92. doi:10.1074/jbc.274.35.25085
245. Itano N, Yamada Y, Yoshida M, Kimata K. [Cancer metastasis and extracellular matrix]. Gan To Kagaku Ryoho (1999) 26(11):1663–8.
246. Kimata K, Honma Y, Okayama M, Oguri K, Hozumi M, Suzuki S. Increased synthesis of hyaluronic acid by mouse mammary carcinoma cell variants with high metastatic potential. Cancer Res (1983) 43(3):1347–54.
247. Itano N, Sawai T, Miyaishi O, Kimata K. Relationship between hyaluronan production and metastatic potential of mouse mammary carcinoma cells. Cancer Res (1999) 59(10):2499–504.
248. Simpson MA, Reiland J, Burger SR, Furcht LT, Spicer AP, Oegema TR Jr, et al. Hyaluronan synthase elevation in metastatic prostate carcinoma cells correlates with hyaluronan surface retention, a prerequisite for rapid adhesion to bone marrow endothelial cells. J Biol Chem (2001) 276(21):17949–57. doi:10.1074/jbc.M010064200
249. Simpson MA, Wilson CM, Furcht LT, Spicer AP, Oegema TR Jr, McCarthy JB. Manipulation of hyaluronan synthase expression in prostate adenocarcinoma cells alters pericellular matrix retention and adhesion to bone marrow endothelial cells. J Biol Chem (2002) 277(12):10050–7. doi:10.1074/jbc.M110069200
250. Csoka AB, Frost GI, Stern R. The six hyaluronidase-like genes in the human and mouse genomes. Matrix Biol (2001) 20(8):499–508. doi:10.1016/S0945-053X(01)00172-X
251. Stern R, Asari AA, Sugahara KN. Hyaluronan fragments: an information-rich system. Eur J Cell Biol (2006) 85(8):699–715. doi:10.1016/j.ejcb.2006.05.009
252. Montesano R, Kumar S, Orci L, Pepper MS. Synergistic effect of hyaluronan oligosaccharides and vascular endothelial growth factor on angiogenesis in vitro. Lab Invest (1996) 75(2):249–62.
253. Rozario T, DeSimone DW. The extracellular matrix in development and morphogenesis: a dynamic view. Dev Biol (2010) 341(1):126–40. doi:10.1016/j.ydbio.2009.10.026
Keywords: extracellular matrix, ovarian cancer, proteases, collagen, proteoglycan
Citation: Cho A, Howell VM and Colvin EK (2015) The extracellular matrix in epithelial ovarian cancer – a piece of a puzzle. Front. Oncol. 5:245. doi: 10.3389/fonc.2015.00245
Received: 28 August 2015; Accepted: 15 October 2015;
Published: 02 November 2015
Edited by:
Ben Davidson, Oslo University Hospital, NorwayReviewed by:
Reuven Reich, Hebrew University of Jerusalem, IsraelBjørn Åke Risberg, Oslo University Hospital, Norway
Copyright: © 2015 Cho, Howell and Colvin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Viive M. Howell, dmlpdmUuaG93ZWxsJiN4MDAwNDA7c3lkbmV5LmVkdS5hdQ==
 Angela Cho
Angela Cho Viive M. Howell
Viive M. Howell Emily K. Colvin
Emily K. Colvin