
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol. , 08 June 2015
Sec. Cancer Immunity and Immunotherapy
Volume 5 - 2015 | https://doi.org/10.3389/fonc.2015.00117
This article is part of the Research Topic Advances in Combination Tumor Immunotherapy View all 8 articles
Immunotherapy is a rapidly expanding field of oncology aimed at targeting, not the tumor itself, but the immune system combating the cancerous lesion. Of the many approaches currently under study to boost anti-tumor immune responses; modulation of immune co-receptors on lymphocytes in the tumor microenvironment has thus far proven to be the most effective. Antibody blockade of the T cell co-inhibitory receptor cytotoxic T lymphocyte antigen-4 (CTLA-4) has become the first FDA approved immune checkpoint blockade; however, tumor infiltrating lymphocytes express a diverse array of additional stimulatory and inhibitory co-receptors, which can be targeted to boost tumor immunity. Among these, the co-stimulatory receptor 4-1BB (CD137/TNFSF9) possesses an unequaled capacity for both activation and pro-inflammatory polarization of anti-tumor lymphocytes. While functional studies of 4-1BB have focused on its prominent role in augmenting cytotoxic CD8 T cells, 4-1BB can also modulate the activity of CD4 T cells, B cells, natural killer cells, monocytes, macrophages, and dendritic cells. 4-1BB’s expression on both T cells and antigen presenting cells, coupled with its capacity to promote survival, expansion, and enhanced effector function of activated T cells, has made it an alluring target for tumor immunotherapy. In contrast to immune checkpoint blocking antibodies, 4-1BB agonists can both potentiate anti-tumor and anti-viral immunity, while at the same time ameliorating autoimmune disease. Despite this, 4-1BB agonists can trigger high grade liver inflammation which has slowed their clinical development. In this review, we discuss how the underlying immunobiology of 4-1BB activation suggests the potential for therapeutically synergistic combination strategies in which immune adverse events can be minimized.
Current front-line therapies in the treatment of cancer seek to destroy large tumor lesions by either inducing irreparable DNA damage in the case of radiation therapy and certain chemotherapeutic agents (e.g., alkylating agents, anthracyclines), or by inhibiting protein synthesis, transport, or cell cycle progression (e.g., antimetabolites, topoisomerase inhibitors, mitotic inhibitors, proteasome inhibitors). Radiotherapy has proven effective against a variety of hematological and epithelial cancers including leukemia and lymphoma, head and neck, breast, cervical and prostate cancer. Similarly, DNA alkylating agents and antimetabolites are being used to treat lymphomas, leukemias, brain cancers, and some carcinomas. In most cases, however, tumors become refractory to treatment, leading to the development of therapy-resistant metastases. Furthermore, the delicate radio-sensitivity of many organs, inaccessibility of lesions to surgical resection, and toxicity of chemo- and radio-therapeutic agents further complicate the use of such therapies.
The ground-breaking revelation that the body’s own immune system can recognize tumor antigens as foreign and initiate anti-tumor responses against growing lesions has changed the field of tumor therapy (1, 2); boosting the immune response against tumors has become an exciting new avenue in the fight against cancer. Immunotherapy of cancer, as opposed to drug therapy, provides the added benefit of sustained protection often with less severe and less persistent side effects compared to those associated with chemotherapeutics or radiation therapy. Early immunotherapies sought to boost systemic immune responses by administration of prosurvival or proinflammatory cytokines with varied, and often suboptimal clinical outcomes (3–6). These early therapies failed, in part, due to our inadequate understanding of the immune relevance of conserved versus mutanome antigens, tumor immune escape mechanisms, and, foremost, the complex and highly immunosuppressive tumor microenvironment. With a better understanding of how the immune system targets tumors and tumor antigens, an entire arsenal of immunotherapeutics has been developed to augment anti-tumor immune responses from vaccine strategies to adoptive transfer of tumor-reactive T cells. The most successful approaches that have been translated from bench to bedside, however, aim at targeting co-receptors expressed on various immune cells in the tumor microenvironment. Therapeutic antibodies which block the co-inhibitory checkpoint receptors on T cells were among the first, and most effective, of the current generation of therapeutics targeting the immune system. Ipilimumab (αCTLA-4) became the first FDA approved T cell checkpoint antibody for use against melanoma, with patients showing a 13% objective response rate against Stage III/IV melanoma, with largely manageable immune-related adverse events (7–9). The approval of Ipilimumab paved the way for the transition of other checkpoint blockade antibodies into clinical trials. Nivolumab and Pembrolizumab [both antagonist antibodies targeting the programmed death receptor-1 (PD-1)] are currently approved for melanoma, and, more recently, non-small cell lung cancer (NSCLC) in the case of Nivolumab. Other trials of PD-1 blockade are ongoing for renal cell carcinoma (RCC), NSCLC (NCT01844505, NCT02041533, NCT01866319, NCT02212730), and glioblastoma (NCT02311920, NCT02311582) among others. Tumor-infiltrating lymphocytes (TIL) express a wide array of additional co-stimulatory and co-inhibitory receptors, though, that may serve as potential targets for novel immunotherapeutic interventions (10). One such immuno-stimulatory receptor with promising clinical applications is the tumor necrosis factor superfamily member 4-1BB (CD137/TNFRSF9).
Targeting 4-1BB with agonist antibodies elicits potent anti-tumor responses; however, clinical progress has been slowed by dose-limiting liver inflammation. This review will explore the current knowledge of the function of 4-1BB and its role in the immune response, potentiating both antiviral and antitumor responses, while alleviating certain autoimmune conditions. The means by which the 4-1BB co-receptor can be targeted to induce anti-tumor immunity will be highlighted, with a particular focus on the unique potential for synergism between 4-1BB co-stimulation and various other immune and non-immune therapies.
4-1BB belongs to the TNF receptor family, which includes multiple T cell co-stimulatory receptors which have been targeted with agonist antibodies including GITR, CD40, CD27, HVEM, LIGHT, APRIL, and TWEAK (11, 12). 4-1BB plays a critical role in sustaining effective T cell immune responses and in generating immunological memory. The expression profile of 4-1BB, as well as its unique ability to potentiate robust effector responses in multiple subsets of lymphocytes relevant for tumor immunity, makes 4-1BB a uniquely appealing target for immunotherapy (Figure 1).
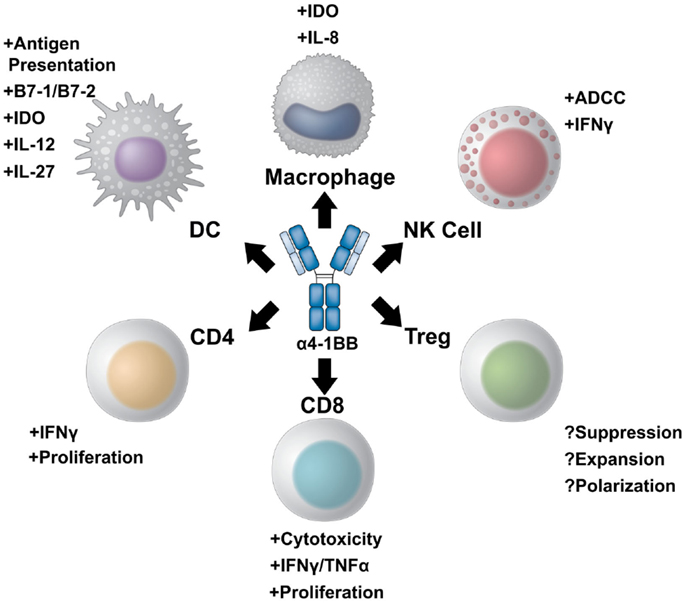
Figure 1. A multi-potent role for 4-1BB targeted immunotherapy. 4-1BB agonist therapies elicit diverse immune effector responses on both the innate and adaptive immune arms. The most potent of responses stimulate CD8+ cytotoxic T cells to proliferate and increase their effector potential through increased interferon gamma production and expression of multiple granzymes. CD4+ effector T cells can also be stimulated to expand and produce pro-inflammatory cytokines. The role of 4-1BB stimulation on regulatory T cells, however, is controversial. 4-1BB agonist therapy may either inhibit differentiation of conventional effector cells into Tregs while also inhibiting Treg suppression, or, conversely, maintain Treg expansion and suppressive capacity. NK cells also benefit from 4-1BB agonist therapy. Not only can α4-1BB antibodies stimulate antibody-dependent cell-mediated cytotoxicity through Fc/FcR interactions, but activated NK cells express 4-1BB to become targets of therapy. Additionally, cells of the myeloid lineage upregulate 4-1BB upon activation. 4-1BB agonists targeting dendritic cells induce DC maturation and antigen presentation. In addition, α4-1BB stimulated DCs begin to express IL-12 and IL-27 as well as the enzyme IDO to modulate T cell function. 4-1BB+ macrophages can also be stimulated to increase antigen presentation and produce IL-8 as well as IDO.
4-1BB is expressed on a multitude of cells of the hematopoietic lineage (13). While 4-1BB is widely known to be transiently upregulated on CD8 T cells following activation (14–18), 4-1BB can also be expressed on activated CD4 helper T cells (17, 19, 20), B cells (21, 22), regulatory T cells (23), natural killer (NK) cells (24–26), natural killer T (NKT) cells (27), gamma-delta T cells (28), dendritic cells (29, 30), mast cells (31), osteoclasts (32, 33), thymocytes (34), early myeloid progenitor cells (35, 36), and activated endothelium (37–40). Human neural cells also express 4-1BB, including neurons, astroglia, and microglia in the brain (41). Multiple subtypes of lymphomas and leukemias are also known to express 4-1BB, although its precise function remains unclear in the setting of malignancy (42–45). The broad range of 4-1BB expression on multiple cell types makes this receptor a dual-edged sword in the fight against cancer; as stimulation with 4-1BB agonists elicits strong anti-tumor responses from a myriad of cell types, however, sometimes at the cost of off-target immune pathology. Significant progress has been made in recent years in describing the complex regulation of 4-1BB expression; yet, further studies are needed to clarify the impact of each 4-1BB expressing cell population toward the anti-tumor versus auto-inflammatory effects of 4-1BB agonist antibodies.
Co-stimulation through the 4-1BB receptor activates multiple signaling cascades within the T cell, powerfully augmenting T cell activation. Upon receptor ligation, 4-1BB enhances signaling through the T cell receptor (18). 4-1BB forms a heterotrimer complex consisting of two TNF-receptor associated factor (TRAF)-2 complexes (46) in conjunction with TRAF-1 (47). This interaction, through leukocyte specific protein-1 (LSP-1) (48), potentiates signaling through the c-Jun N-terminal kinase (JNK) pathway (49), the extracellular signal-regulated kinase (ERK) pathways (50, 51), as well as through β-catenin and AKT (51). These signaling pathways converge on the master transcription factor NF-κB to regulate 4-1BB signaling, as well as effector immune responses (47, 52). As most of these signals are shared between TNF co-stimulatory receptors, yet none of the others can replicate the phenotypic changes associated with 4-1BB activation, it is likely that additional molecular pathways are triggered by 4-1BB which have yet to be described.
4-1BB signaling inhibits activation-induced cell death (AICD) in T cells (53), promotes survival (20), is critical for the formation of immunological memory through upregulation of the anti-apoptotic genes Bcl-2, Bcl-xl, and Bfl-1 (16, 17), and induces T cell proliferation and enhanced effector function (14). Interestingly, T cells from 4-1BB deficient mice demonstrate enhanced proliferative potential, while exhibiting reduced effector responses (54, 55). Moreover, 4-1BB−/− mice demonstrate defects in myelopoiesis as well as B cell deficiencies in the production of IgG2a and IgG3 (55).
In addition to enhancing IFNγ and TNFα production (56, 57), activation of 4-1BB has been shown to induce IL-13 production from both CD8 and CD4 T cells to limit inflammation (58). Moreover, enhanced IFNγ production leads to the generation of indoleamine 2,3-dioxygenase (IDO) by dendritic cells which can attenuate 4-1BB-mediated effector responses (59). In addition, 4-1BB signaling induces maturation of dendritic cells leading to the upregulation of B7 co-stimulatory ligands, increases DC survival, and boosts the production of inflammatory cytokines such as IL-6, IL-12, and IL-27 (60, 61). Further, DCs present in the mesenteric lymph nodes upregulate retinal dehydrogenase through 4-1BB signaling, which promotes Treg development in order to maintain homeostasis in the gut (62).
The ability of 4-1BB receptor signaling to evoke robust effector responses has been extensively demonstrated in infectious disease models. Several studies have shown a role for 4-1BB in mediating antiviral immune responses toward influenza (63–65), hepatitis C (66), cytomegalovirus (67), HIV (68), lymphocytic choriomeningitis virus (69), as well as poxviruses (70, 71). Additionally, 4-1BB mediates anti-bacterial immune responses particularly toward Streptococcus pneumoniae (72) and Listeria monocytogenes infection (73, 74).
Although 4-1BB potentiates strong immune responses, it also has the potential to alleviate autoimmune disease. Stimulation through 4-1BB ameliorates murine models of experimental autoimmune encephalomyelitis (EAE) (75, 76), systemic lupus erythematosus (SLE) (77–79), murine Sjögren’s disease (80), inflammatory bowel disease (81, 82), uveoretinitis (83), and rheumatoid arthritis (84). Conversely, 4-1BB may worsen type I diabetes (85–87), although one study demonstrated a role for 4-1BB in protecting mice from pathology by increasing CD4+CD25+ regulatory T cells (88). Further, 4-1BB may also play a role in alleviating allergic reactions (89, 90). The capacity of 4-1BB to mediate both potent immune responses and ameliorate autoimmunity likely stems from the unique ability this receptor possesses to promote Th1 type responses, while inhibiting Th2- and Th17-related pathologies (61, 76).
The dual ability of 4-1BB to stimulate strong effector T cell responses toward pathogens while restricting autoimmune disease has made this receptor an attractive target for cancer immunotherapy. While 4-1BB monotherapy is capable of mediating significant tumor regressions and even cures of numerous types of established murine tumors (Table 1), targeting 4-1BB with agonist antibodies as a monotherapy in the clinic has only yielded modest frequencies of RECIST partial responses and stabilization of disease. Although agonist antibodies have been the best studied modality for activating 4-1BB, the immune pathologies associated with their use have prompted the development of alternate therapeutics seeking to separate 4-1BB’s anti-tumor effects from its associated liver inflammation (91). Each of these potential drugs for activation of 4-1BB has unique advantages and disadvantages for use in combination with other therapies.
By far the most straightforward and most extensively studied approach to targeting 4-1BB relies on the exquisite specificity of targeted antibodies. Melero et al. were the first group to describe the potent anti-tumor properties of agonist 4-1BB antibodies in eliminating murine P815 mastocytoma and Ag104A sarcoma (122). This landmark work opened the field of 4-1BB-targeted immunotherapy. Kim et al., however, demonstrated that α4-1BB antibodies were ineffective as a monotherapy against subcutaneously implanted B16/D5 melanoma, MCA205 sarcoma, or GL261 glioblastoma when administered systemically over a range of doses (123). Interestingly though, systemic monotherapy was effective against intracranially implanted MCA205 and GL261 tumors, suggesting that efficacy of agonist therapy relies heavily on microenvironmental factors as well as intrinsic tumor-resistance mechanisms. In a model of plasmacytoma, May demonstrated that a critical effect of α4-1BB-mediated tumor regression lies in the ability of CD8 T cells from treated mice to survive and avoid AICD (124). Moreover, α4-1BB antibody therapy is dependent on IFNγ, as CD8 T cells were incapable of trafficking to the tumor site in IFNγ-deficient mice (125). The use of 4-1BB antibodies further provides unique advantages over other 4-1BB targeted modalities. For instance, binding of the Fc region of the 4-1BB antibody to Fc receptors may activate NK cells. Moreover, these NK cells then express 4-1BB and in so doing, become targets for immunotherapy (126). Additionally, Martinez-Forero et al. demonstrated the mechanism of α4-1BB antibody binding and internalization into endosomal compartments and subsequent K63 polyubiquitination necessary to recruit TRAF2 and initiate the 4-1BB signaling cascade (127). Importantly, Galectin-9 contributes to the stabilization of 4-1BB for multimerization and ligand binding and stimulation, demonstrating a role for cell surface glycoproteins in mediating receptor signaling (95). While the strong anti-tumor immunity promoted by 4-1BB agonist antibodies can engender serious immune-mediated pathology not evident through some other therapeutic modalities (91, 94, 128), the use of agonist antibodies in combination with other cancer therapeutics may help alleviate these detrimental side effects.
A surrogate approach to 4-1BB-targeted antibodies lies in the use of the natural 4-1BB ligand to stimulate anti-tumor T cell responses. Mouse forestomach cancer cells transfected with DNA encoding 4-1BBL were capable of doubling the cytotoxicity of tumor infiltrating T cells over untransfected cells, demonstrating the potential of 4-1BBL expression as an alternate means to target 4-1BB therapeutically (129). Transduction of 4-1BBL into other tumor cell lines has also shown therapeutic potential (130–132), particularly the transduction of 4-1BBL into K562 leukemic cells to expand both T cells and NK cells (133, 134). Furthermore, work from the Shirwan lab has elegantly demonstrated the therapeutic effect of a streptavidinated 4-1BBL (SA-4-1BBL) complex to induce effective anti-tumor immune responses. Firstly, subcutaneous administration of SA-4-1BBL potently stimulated both CD8 and CD4 T cell proliferation compared to equal doses of a 4-1BB agonist antibody (94) without a dramatic increase in inflammatory cytokines exhibited by antibody administration. Further, SA-4-1BBL induced less lymphadenopathy and splenomegaly than antibody therapy, suggesting that SA-4-1BBL has a higher therapeutic index. In this study, however, the therapeutic index of systemic administration of SA-4-1BBL was never tested. Moreover, SA-4-1BBL evoked strong anti-tumor effects when given as a vaccine adjuvant in the murine TC-1 HPV-driven tumor model (135–137), or in a Survivin+ lung carcinoma model (136, 138). This potential role of 4-1BB agonists as vaccine adjuvants suggests potential future combinations with other TNF (e.g., CD40) or innate (e.g., TLR9) agonists in this setting. The pre-clinical data with SA-4-1BBL suggest that if a clinically suitable multimeric form of 4-1BBL can be developed; it may offer a compelling alternative to 4-1BB agonist antibody mediated stimulation for tumor immunotherapy.
An alternate means of targeting 4-1BB entails the use of oligonucleotide aptamer technology, pioneered in the Gilboa lab (139). Aptamers are single-stranded oligonucleotides designed through an enrichment process to develop a unique structure capable of binding a given target protein, usually for therapeutic purposes. As 4-1BB most efficiently signals through a multimer complex, so too do 4-1BB bivalent aptamer conjugates more efficiently co-stimulate CD8 T cells. Bivalent aptamers co-stimulated CD8 T cell proliferation with similar potency to agonist antibodies when injected systemically, and elicited approximately two-fold more IFNγ production. These 4-1BB aptamers were thus able to protect mice from P815 mastocytoma tumors with comparable efficacy to antibody monotherapy (119). Much like antibodies, aptamers can be conjugated to various cargos in order to enhance therapeutic benefit (120). Berezhnoy et al. demonstrated that the addition of a siRNA targeting the mTOR pathway conjugated to a 4-1BB aptamer was able to efficiently inhibit Raptor to suppress mTORC1 activity and enhance the persistence of T cells exhibiting a memory phenotype. Moreover, T cells more effectively controlled growth of murine B16 melanomas when mice were administered 4-1BB/Raptor compared to 4-1BB aptamer or rapamycin monotherapies (121). Aptamers can also be conjugated to targeting motifs allowing for the trafficking and close juxtaposition of effector T cells with tumor tissue. Conjugation of a 4-1BB aptamer to a second aptamer targeting the prostate-specific membrane antigen (PSMA) inhibited growth of PSMA expressing tumors and prolonged survival in half of mice receiving the 4-1BB/PSMA aptamer conjugate (140). In a similar vein, 4-1BB/VEGF aptamer conjugates were able to enhance T cell proliferation when administered systemically to mice bearing subcutaneous 4T1 mammary carcinomas or MCA induced fibrosarcoma in a VEGF-dependent manner (128). Notably, the 4-1BB aptamer, 4-1BB/PSMA, and 4-1BB/VEGF conjugates did not induce significant pathology associated with systemic administration of 4-1BB antibodies, as CD8 T cell infiltratration into the spleens and livers was markedly reduced in aptamer-treated mice. Neither the pharmacodynamics of α4-1BB aptamers, nor how the 4-1BB aptamer/receptor interaction mediates signaling into the T cell has been extensively studied. Whether this interaction disrupts 4-1BB/4-1BBL interactions, and by what mechanism the aptamer lessens liver inflammation relative to 4-1BB antibody therapy remains to be determined. Further, 4-1BB targeted antibodies have the added benefit of inducing antibody-dependent cell-mediated cytotoxicity (ADCC) through Fc/Fc receptor interactions on NK cells as a dual arm of therapeutic efficacy (126, 141–143), which would be lacking in aptamer conjugates. Oligonucleotide aptamers, on the other hand, may act as stimuli for nucleotide sensing pathways in innate immune cells and thereby promote activation of antigen-presenting cells. Regardless, the enhanced therapeutic index of 4-1BB targeted aptamers supports their potential translation into the clinic.
Regardless of modality, 4-1BB-targeted therapies potently modulate anti-tumor immune responses to effectively treat a variety of cancers. Tumor cells expressing 4-1BB scFv potently stimulate anti-tumor effector responses (144, 145). In addition, 4-1BB targeted immunotherapy has demonstrated great potential in treating floor of mouth squamous cell cancer (146), lymphoma (96), hepatocellular carcinoma (147, 148), and colon cancer to name a few (149, 150).
The anti-tumor potential of α4-1BB therapy stems from the ability to modulate the tumor microenvironment, largely by promoting a type 1 cytokine response (151). Palazon et al. demonstrated that 4-1BB is up-regulated in limited oxygen environments (152), particularly in hypoxic tumor environments potentially enhancing the selectivity of 4-1BB agonists for cells in the tumor. Ye also established that 4-1BB can act as a marker for tumor-reactive T cells (153). Work of Ju et al. showed that 4-1BB agonist antibodies enhance anti-tumor responses by inducing a CD8+CD11c+ T cell population with enhanced IFNγ activity (56, 154). Perhaps most strikingly, α4-1BB therapy is capable of inducing a potently cytotoxic T cell phenotype mediated by the T-box transcription factor Eomesodermin (61, 155), which is required for 4-1BB-mediated tumor therapy (156). Further, perforin and granzyme act together during α4-1BB therapy to eradicate established murine lymphomas (157), adding to the body of work demonstrating the role of 4-1BB in enhancing cytotoxic responses. While 4-1BB predominantly acts on T cells, depletion of dendritic cells impairs the anti-tumor effects of α4-1BB, suggesting a role for DCs as well in anti-tumor 4-1BB agonist immunotherapy (158). Moreover, α4-1BB therapy can act on 4-1BB+ endothelial cells to increase T cell recruitment into tumor sites and sites of inflammation (159).
Expression of 4-1BB correlates well with effective anti-tumor immune responses (153); however, 4-1BB agonist antibodies can induce a variety of pathologies that may limit their utility in patients.
In the setting of natural immunity, 4-1BB signaling has been implicated in mediating the pathogenesis of herpetic stromal keratitis (an HSV-1 associated eye infection that can lead to glaucoma and/or corneal scarring) (160), atherosclerosis (161, 162), obesity-induced inflammation (163–165), allograft rejection (166–169), lung inflammation, and airway hyper-responsiveness (27), as well as infection-induced fetal rejection during pregnancy (170). Though, mild and manageable, the potential for 4-1BB to precipitate auto-reactive pathologies should be considered in planning the management of 4-1BB agonist treated patients.
The most clinically relevant adverse events associated with the use of 4-1BB agonist antibodies involve defects in immune homeostasis (e.g., neutropenia, thrombocytopenia, and reduced B cell numbers) (171, 172), as well as moderate to severe liver inflammation characterized by immune infiltration and concomitant elevation in serum levels of liver transaminases (e.g., AST, ALT). Dubrot et al. first demonstrated a polyclonal influx of CD8 T cells into the livers of mice bearing MC38 colon carcinomas which correlated with an increase in transaminase levels (173). Wang et al. further demonstrated in a mouse model of chronic Hepatitis C infection that, 4-1BB stimulation promotes hepatic fibrosis and progression to hepatocellular carcinoma (174). This progression was mediated by production of IFNγ from CD8 infiltrates which drove CD11b+ macrophages to increase production of inflammatory cytokines. Interestingly, Lin et al. established a role for the glucocorticoid-induced TNF-related receptor (GITR) in mediating 4-1BB-induced liver pathology (175). GITR−/− mice treated with 4-1BB antibodies demonstrated reduced splenomegaly as well as decreases in both ALT and AST levels associated with liver damage. GITR knockout mice also had fewer T cell infiltrates into the liver as well as fewer 4-1BB+ regulatory T cells and dendritic cells in the spleens and lymph nodes, suggesting that GITR may play a role in systemic 4-1BB expression.
In translating 4-1BB agonists into the setting of clinical oncology, responses to α4-1BB have been impressive, including partial remission and some stable disease; however, adverse events have complicated progression of 4-1BB agonists into late stage clinical trials. As reviewed by Ascierto et al. (91), in a Phase I/II trial conducted by Bristol–Myers Squibb (BMS) using α4-1BB monoclonal antibodies for advanced or metastatic solid tumors (NCT00309023), cases of low grade fatigue were witnessed as well as grade 2+ neutropenia, leukopenia, thrombocytopenia, and increases in AST and ALT. This mild and manageable toxicity profile led to a Phase II study of 4-1BB antibodies for previously treated stage IV melanoma patients (NCT00612664). Unfortunately, though, this study was terminated due to high incidence of severe (Grade IV), and potentially fatal, hepatitis. These severe adverse events led to withdrawal or termination of several other ongoing and approved Phase I trials designed to discover the breadth and potency of the anti-tumor effects of 4-1BB agonist immunotherapy (NCT00803374, NCT00309023, NCT00461110, NCT00351325). There is room for improvement in clinical management of 4-1BB induced hepatitis as steroids alone may not be sufficient to ameliorate severe hepatitis without the addition of myelosuppressive agents. Despite these setbacks, the therapeutic potential of 4-1BB agonist antibodies, particularly in combination with other immune and traditional cancer therapies, has led to a revival of clinical 4-1BB antibody development.
Though liver toxicity is a major concern in the treatment of advanced cancers, addition of 4-1BB agonists to other therapeutic modalities could potentiate stronger anti-tumor responses while necessitating reduced dosing, thus limiting severity of 4-1BB associated adverse events. Preclinical studies have shown cooperative and even synergistic therapeutic benefit by combining 4-1BB agonists with multiple anti-tumor therapies including IL-12 (176), IFNα (177), vaccination (102, 149, 178–180), as well as various other combinations (99, 101, 141–143). The most alluring combinations, however, are those that combine 4-1BB agonists with therapies that are already approved or in clinical trials, particularly T cell immune checkpoint blockade.
The use of oncolytic viruses to treat cancer or gene therapy to introduce novel genes into the tumor microenvironment has begun to gain new life in recent years (181). Early gene therapy approaches to tumor vaccination sought to activate 4-1BB as an adjuvant. Incorporating 4-1BB ligand co-stimulation into viral therapies has also proven especially fruitful (92, 93, 97, 98, 100, 103). Work from Melero first demonstrated that Ag104A sarcomas transfected with both 4-1BBL and B7-1, but neither alone, induced potent anti-tumor responses and cured 60% of treated mice (130). Work from Chen et al. further demonstrated the potent therapeutic benefit of combining 4-1BB targeted therapy with interleukin 12, a cytokine that robustly activates NK cells as well as induces Th1 responses. Intratumoral injection of an adenoviral vector containing the p35 and p40 IL-12 subunits in combination with systemic administration of a 4-1BB antibody led to a dose-dependent increase in survival and complete rejection of MCA26 colon adenocarcinomas and non-immunogenic B16-F10 melanomas (104, 106). Intratumoral and systemic administration of 4-1BBL and IL-12 showed similar effects (105, 131, 182), and, in fact, intratumoral 4-1BBL may induce a more robust secondary tumor response than systemic 4-1BB antibodies (131). In a similar vein, while intratumoral implantation of IL-12 transfected DCs showed some anti-tumor effects, addition of systemic 4-1BB agonist antibodies led to complete cures in some cases of both directly injected and untreated contralateral MC38 colon adenocarcinomas (150). These early experiments using viral vectors clearly demonstrated the adjuvant properties of 4-1BB co-stimulation and opened the doors to additional dual therapies involving 4-1BB. Clearly, 4-1BB activation may prove a potent combination strategy as second generation oncolytics emerge which co-express co-stimulatory ligands and/or cytokines.
The FDA approval of anti-CTLA-4 (Ipilimumab) checkpoint blockade for the treatment of advanced stage melanoma has made this an attractive therapeutic for combination with 4-1BB agonists. While a Phase I trial was approved to determine the therapeutic benefit of combining 4-1BB agonists with Ipilimumab (NCT00803374), this trial was withdrawn prior to opening enrollment due to the liver toxicity observed in the melanoma monotherapy trial. The synergistic therapeutic potential of the α4-1BB/αCTLA-4 dual therapy in preclinical models, however, cannot be overlooked. Three doses of α4-1BB/αCTLA-4 administered to mice bearing B16 melanomas demonstrated a potently synergistic curative effect (183). Combination therapy led to changes in the tumor microenvironment including increases in CD8 T cell infiltration as well as significantly increased CD8/Treg, CD4 Teff/Treg, and CD8/MDSC ratios, which favor tumor clearance and successful treatment. Moreover, dual therapy increased the effector capabilities of T cells in the tumor microenvironment. It should be noted though, that this synergism was only demonstrated in combination with an irradiated tumor vaccine expressing Flt-3 ligand (FVAX). Further evidence of the therapeutic benefit of α4-1BB/αCTLA-4 was demonstrated by the injection of prostate tumors transfected with a plasmid containing 4-1BBL. Enhanced survival benefit and complete tumor rejection were evidenced when combined with systemic αCTLA-4 antibody therapy, leading to long-term immunological protection (109).
The most convincing argument for combining α4-1BB with αCTLA-4 stems from the ability of each therapy to not only potentiate stronger anti-tumor responses, but to also mutually ameliorate the side effects of each monotherapy. Kocak et al. demonstrated that systemic administration of α4-1BB/αCTLA-4 antibody therapy, while ineffective in treating B16 melanoma, was effective in clearing poorly immunogenic MC38 colon adenocarcinoma (110). Most strikingly, whereas αCTLA-4 induced the production of anti-dsDNA antibodies, leading to autoimmune-like syndromes, addition of α4-1BB appeared to alleviate antibody deposition and development of lupus-like pathology in the kidney. Moreover, α4-1BB induced an influx of T cell infiltrates into the liver, leading to hepatitis; however, addition of αCTLA-4 reduced cellular infiltration and liver pathology. The clear therapeutic benefit and alleviation of pathology demonstrated by Kocak suggest that this α4-1BB/αCTLA-4 combination should be prioritized for clinical translation.
Another checkpoint receptor with potential therapeutic synergy in combination with α4-1BB is the programmed death-1 (PD-1) pathway. Targeting the PD-1 pathway by blocking PD-1 (108), or by blocking Programed death-ligand 1 (PD-L1) (107), has evoked impressive clinical responses to melanoma, non-small-cell lung cancer, and renal cancer. The recent approval of the PD-1 blocking antibodies Nivolumab and Pembrolizumab for the treatment of melanoma validates the effectiveness of αPD-1 as a monotherapy, confirms the attractiveness of a lower toxicity profile than Ipilimumab, and suggests that αPD-1 may offer a more appealing partner for α4-1BB co-therapy.
In one of the first preclinical models of dual therapies targeting the 4-1BB and PD-1 pathways, Xiao et al. demonstrated that systemic administration of soluble PD-1 to inhibit PD-L1 synergized well with implantation of H22 hepatocarinomas transfected with 4-1BBL by increasing anti-tumor cytotoxicity and decreasing tumor burden compared to either monotherapy (184). In an ovarian cancer model, co-administration of a PD-L1 antagonist with α4-1BB and a cellular vaccine expressing GM-CSF (GVAX) let to increases in both CD4 effector and CD8 T cell infiltrates into the tumor with a concomitant decrease in regulatory T cells (185). Much like with α4-1BB/αCTLA-4 therapy, this combination increased IFNγ and TNFα production in the tumor microenvironment. PD-1 blocking antibodies in combination with 4-1BB agonists have also shown increased therapeutic potential toward subcutaneously implanted CT26 colon carcinoma (186) or B16/F10 melanoma (187). Interestingly, Chen et al. showed that PD-1 blockade increased 4-1BB expression on CD8 T cells and α4-1BB conversely induced PD-1 expression, thus pointing toward a mechanism of potential synergy in the combination setting. Furthermore, in this study, dual therapy increased the CD8/Treg ratio in the tumor as well as the potent effector capacity of T cells. Unfortunately, though, co-administration of α4-1BB and αPD-1 appeared to exacerbate α4-1BB associated toxicities at moderate to high doses of 4-1BB agonist treatment in mice. At both 1 and 5 mg/kg doses of α4-1BB, ALT and AST levels were increased almost two-fold over α4-1BB alone. Moreover, α4-1BB/αPD-1 therapy failed to ameliorate, and even worsened, α4-1BB mediated thrombocytopenia, lymphopenia, and neutropenia at the higher doses. At the lowest dose of α4-1BB, however, no increased toxicity was observed for the α4-1BB/αPD-1 combination relative to the same dose of α4-1BB alone. These results strongly suggest caution in choosing combination therapies, and that trial design should include conservative dosing in initial cohorts with the potential for escalation after demonstration of safety.
Another avenue for combination therapy that may have therapeutic potential engages combination of α4-1BB with agonists of other co-stimulatory TNFR family members. Clinical trials targeting the OX-40 pathway for tumor therapy are currently underway (NCT01862900, NCT02274155, NCT01303705). Moreover, the co-stimulatory nature of both 4-1BB and OX-40 receptors as well as diversity in their expression patterns may offer potential synergism between these two therapies in the clinic (188, 189). Work from Lee et al. demonstrated that activating both OX-40 and 4-1BB enhanced CD8 T cell proliferation, survival, and effector function over either monotherapy in response to staphylococcal enterotoxin A (SEA) stimulation (190). In the context of a prime/boost vaccine using a recombinant vaccinia virus (VV) vector encoding the OVA-peptide, α4-1BB alone enhanced CD8 T cell expansion and memory formation, whereas the α4-1BB/αOX-40 combination was able to expand both CD4 and CD8 responses (191). Further, OX-40 and 4-1BB co-stimulation may advantageously regulate Treg function as well (192). For instance, work from St. Rose et al. suggests that enhanced IFNγ production during dual co-stimulation regulates expression of the IL-2 receptor (CD25), limiting the expansion of regulatory T cells (193). Contrarily though, one report demonstrated enhanced expansion of Tregs during 4-1BB stimulation (194), while another showed that α4-1BB inhibits the suppressive capacity of CD4+CD25+ regulatory T cells (195), and yet a third study reported suppressed conversion of CD4+ T effector cells into Tregs during 4-1BB stimulation (111). Thus, the functional consequences of 4-1BB activation upon Tregs remains contentious, and further studies are necessary, particularly to clarify the impact of α4-1BB on human Treg expansion and suppressive capacity.
Significant to the field of tumor immunology, Bandyopadhyay et al. showed that dual co-stimulation through α4-1BB/αOX-40 fueled T cell expansion but not effector function in a murine model of responses toward tolerized self-antigens (113). This study, while suggestive of implications on tumor immunotherapy, did not take into account the vast array of neoantigens or polyclonal nature of the adaptive immune response in a natural setting. Further, the therapeutic potential of combining dual co-stimulation with whole tumor vaccines compared to peptide vaccination was clearly demonstrated by Cuadros et al. This strategy produced stronger T cell responses compared to peptide vaccination and fostered complete tumor rejection when combined with α4-1BB/αOX-40 therapy, further demonstrating the need to generate an oligoclonal anti-tumor response to efficiently eliminate certain tumors (112). The ability to boost both CD8 and CD4 T cell responses while, at the same time, suppressing Treg expansion will likely make 4-1BB and OX-40 agonists attractive combination partners in the clinic.
Front-line radiation therapy is the current standard of care for many malignancies, often eliciting objective responses. More recently, the capacity of radiotherapy to awaken and augment dormant tumor immune responses has been demonstrated both in pre-clinical studies and clinical trials. Shi et al. demonstrated that high doses of radiation can induce expression of 4-1BBL on some murine tumors (196). In addition, a single dose or multiple, fractionated doses of radiation given prior to systemic administration of 4-1BB agonists induced partial tumor regression in murine models of lung and breast cancer. In particular, cancers of the brain and nervous system may benefit from the potential of α4-1BB/radiotherapy combinations. As neurological cancers are highly radiosensitive and high doses or repeated exposure lead to cognitive impairment, therapies that permit lower dosages with increased anti-tumor effects are sorely needed. In one of the first studies seeking to combine 4-1BB antibodies with radiation, mice implanted with intracranial GL261 gliomas were treated with whole brain irradiation in combination with systemic α4-1BB. This combination therapy dramatically increased survival, reduced total tumor volume, and increased lymphocyte infiltration with the acquisition of durable systemic immunological memory (116). Belcaid et al. further demonstrated that while αCTLA-4 therapy in combination with focal radiation increased the overall survival of mice intracranially implanted with GL261, radiotherapy in combination with αCTLA-4/α4-1BB further increased survival and led to long-term immunological protection (114). Interestingly, investigators found that CD4 T cells played a critical role in mediating this effect, as CD4 but not CD8 T cell depletion abrogated the therapeutic response. The capacity of 4-1BB agonist antibody to expand and empower tumor-specific T cell responses unleashed following radiotherapy clearly will make this a desirable and accessible therapy in the clinic for both corporeal and, potentially, CNS malignancies.
While various chemotherapeutic drugs have demonstrated anti-tumor responses and become the standard of care for both hematological and solid tissue malignancies, most tumors become refractive to therapy, demonstrating a need for combination therapies to overcome resistance. As chemotherapy regimens can induce T cell co-stimulatory receptors, in particular 4-1BB (115), and elicit tumor antigen release, immunotherapy has emerged as a key candidate for combination with chemotherapy.
Agonist 4-1BB therapy with chemotherapy has proven effective pre-clinically in multiple murine tumor models. Ju et al. demonstrated that either α4-1BB or 5-fluorouracil (5-FU) alone did little to treat RCC; however, the combination of α4-1BB with 5-FU led to profound tumor regressions and increased overall survival rates in dual treated mice (117). Further, adding α4-1BB with the DNA-alkylating platinum-containing derivatives, particularly cisplatin, produced cooperative anti-tumor responses and increased survival (118). Not only did α4-1BB/cisplatin induce complete rejection of CT26 colon adenocarcinoma, but addition of 4-1BB agonists also afforded protection from cisplatin-induced nephrotoxicity. In addition, cisplatin in combination with PD-1 blockade and α4-1BB antibody therapy improved responses in a murine model of ovarian cancer, increasing the overall survival rate in a CD8 T cell-dependent fashion (197). Moreover, α4-1BB acts cooperatively with cyclophosphamide (CTX) therapy. While CTX, given early, showed moderate increases in median survival, CTX in combination with α4-1BB increased overall survival by eliciting polyclonal expansion of anti-tumor T cells with significantly enhanced effector function (198). In a similar fashion, CTX treatment followed by intratumoral injection of an adenoviral vector encoding an agonistic scFv targeting 4-1BB synergized to treat murine TC-1 lung adenocarcinomas, boosting T cell proliferation while suppressing Treg expansion (199). Clinical application of 4-1BB agonist and chemotherapy combinations will require careful design to avoid bystander killing of the 4-1BB amplified T cells by the cytotoxic agent; however, the preclincal data suggest translational promise for 4-1BB to augment the effect of selected chemotherapies.
Ex vivo expansion and re-infusion of a patient’s own tumor-specific T cells, known as adoptive cell therapy (ACT), has become a potent new class of immunotherapy, particularly for melanoma. ACT seeks to either expand a patient’s own endogenous anti-tumor T cells, or alternatively, to genetically engineer endogenous T cells with chimeric antigen receptors (CARs) in order to redirect them to the tumor. While CARs offer exceptional anti-tumor specificity and effector function, adoptive transfer of a patient’s own tumor reactive TIL or PMBC initiates immunity against a broader range of tumor-associated antigens, thereby reducing the chance of tumor immune escape through antigen loss. Only recently has the role of 4-1BB in demarking tumor reactive T cells, and in rapidly and robustly expanding T cells for ACT, been appreciated and instituted into TIL expansion protocols (153).
Separate work from Strome et al. and Li et al. demonstrated the synergy of 4-1BB agonists used in combination with adoptively transferred T cells to treat murine lung metastases (200, 201). Moreover, in a hallmark paper, Maus et al. showed that the capacity of K562 cells used as artificial antigen presenting cells (aAPC) to expand patient TIL was dramatically enhanced by co-expression of 4-1BBL (133). This model has now become the standard protocol for ex vivo expansion of T cells for adoptive transfer. Work from Chacon et al. further uncovered the potential of adding 4-1BB agonist antibody stimulation after expansion of TIL in human melanoma, particularly in preventing AICD of TIL ex vivo (202). In order to gain enough T cells from patient TIL samples for ACT, TIL samples undergo a rapid expansion protocol (REP). By adding α4-1BB post-REP, Chacon demonstrated increased polyclonal expansion of CD8+ TIL. These cells were highly functional and capable of responding to antigenic restimulation. Choi et al. showed in similar fashion that tumor-antigen-specific T cells can be harvested and expanded from a patient’s peripheral blood much more rapidly than traditional TIL expansion protocols permit via the addition of 4-1BB agonists (203). Care should be taken, however, in using α4-1BB to expand patient lymphocytes prior to reinfusion, as, despite preferential expansion of CD8+ T cells, 4-1BB agonists may also augment other 4-1BB-expressing cell types including DCs, NK cells, and Tregs. Goldstein et al. showed, for example, that 4-1BB also is present on a population of Treg cells capable of suppressing anti-tumor effector function (204).
Engineering CAR T cells serves as an alternate means of adoptive T cell transfer in which patient T cells are transgenically altered ex vivo to express a tumor-targeted antibody Fab linked to the TCR signaling machinery. Early generation CARs bearing tumor-targeted scFV with CD3ζ demonstrated some anti-tumor potential, but failed to persist long term. Adding co-stimulatory endodomains (via CD28 and/or 4-1BB), however, greatly increased tumor-killing potential and in vivo persistence of adoptively transferred CAR T cells (205). These αCD19-BB-ζ CARs prove to be highly cytotoxic cells capable of potentiating strong anti-leukemia activity (206). Further, in a small pilot study, αCD20 CARs engineered with 4-1BB endodomains produced therapeutic benefit against relapsed indolent B-cell and mantle cell lymphomas (207). From this and many other CAR T cell trials, it appears clear that the presence of the 4-1BB signaling domain affords advantages in both persistence and effector function to adoptively transferred CAR T cells whether alone or in combination with the CD28 endodomain.
Manipulating 4-1BB in the adoptive transfer setting to treat cancer is an expanding area of interest within the field of immunotherapy. A multitude of upcoming CAR T cell trials in both hematologic and solid tumors will test the value of the 4-1BB endodomain in enhancing their anti-tumor activity and in vivo persistence. Also, the potency of 4-1BB agonist antibodies in selecting, expanding, and conditioning the most effective tumor-specific CD8+ T cells for ACT will also be thoroughly tested in upcoming studies.
Many other therapeutic modalities have incorporated 4-1BB agonists to enhance weak anti-tumor responses. Work from Kohrt et al. elegantly demonstrated the therapeutic potential of combining α4-1BB antibodies with approved tumor-targeted antibody therapies. In one study, combining α4-1BB with αCD20 antibodies profoundly enhanced NK cell-mediated anti-lymphoma activity. By combining sequential injections of αCD20 before administration of α4-1BB, Kohrt demonstrated that αCD20 administration enhanced NK cell tumor-killing capacity through antibody-dependent cell-mediated cytotoxicity (ADCC). This led to increases in anti-tumor 4-1BB+ NK cells that then served as targets for α4-1BB therapy (142). Kohrt went further, demonstrating that NK cells from patients with various B cell lymphomas and leukemias upregulated 4-1BB during culture with Rituximab-coated tumor cells, offering a proof of principle that Rituximab and α4-1BB work well as a dual therapy to treat B cell malignancies. In a similar fashion, treatment with trastuzumab or cetuximab prior to α4-1BB agonist therapy boosted ADCC and NK cell responses to HER2+ breast cancer or EGFR+ head and neck and colorectal carcinomas, respectively (143). These pre-clinical findings have fostered clinical trials of α4-1BB antibodies in combination with these tumor-specific antibodies.
Impressive preclinical anti-tumor potential (Table 1) has progressed 4-1BB targeted therapies into clinical trials. Pfizer is currently recruiting patients for a Phase I trial to determine the safety profile and potential of the combination of α4-1BB (PF-05082566) with Pembrolizumab for the treatment of advanced solid tumors (NCT02179918). Additionally, patients are being recruited for a Phase I trial of α4-1BB (PF-05082566) in conjunction with Rituximab (αCD20) for the treatment of Non-Hodgkin’s lymphoma (NCT01307267).
Further, BMS is currently recruiting for multiple trials including a Phase I safety trial of Urelumab (BMS-663513) for advanced stage metastatic solid tumors and relapsed/refractory B cell Non-Hodgkin’s lymphoma (NCT01471210). Recruitment is also ongoing for a trial combining Elotuzumab (targeting SLAMF7) in combination with Lirilumab (targeting KIR receptors on NK cells) or Urelumab (α4-1BB) for the treatment of multiple myeloma (NCT02252263). BMS has also opened Phase I trials combining Urelumab with Cetuximab for metastatic colorectal cancer and head and neck cancer (NCT02110082), combining Urelumab with Rituximab for Non-Hodgkin’s lymphoma (NCT01775631), and Urelumab with Nivolumab (αPD-1) for the treatment of solid tumors and Non-Hodkin’s lymphoma (NCT02253992). All of these trials are still Phase I, and results are eagerly awaited.
The majority of these trials focus on modulating NK cell responses in order to not only boost ADCC, but to also activate and expand anti-tumor NK cells. While both the BMS and Pfizer/Merck trials of α4-1BB/αPD-1 combination therapy seek to translate the impressive pre-clinical efficacy of this combination, it remains to be seen, especially for Urelumab, whether patients will be able to tolerate sufficient doses of these two antibodies to realize this potential. We believe the greatest clinical impact of 4-1BB agonist antibodies will come in future combination trials with CTLA-4 blockade in which both therapeutic synergy and reduction in one another’s immune related adverse events appears likely. This would be a unique case in which both agents, which are currently being under-dosed in the clinic to minimize side effects, could be administered in higher doses than as monotherapies and with a better safety profile. Tumor selective 4-1BB antibodies are also in development using, for example, the Cytomix probody technology, which would allow maximum efficacious activation of 4-1BB alone and in combinations without fear of high grade liver inflammation (208). These developments which separate the anti-tumor from the liver inflammatory effects of 4-1BB antibodies will dramatically accelerate the progress of this extraordinarily promising immunotherapy toward FDA approval.
Activation of the 4-1BB receptor has proven exquisitely effective in treating a wide range of murine tumors. Hepatic toxicities elicited by α4-1BB therapy, however, have limited its progression into Phase II/III clinical trials (91). Fortunately, new therapeutic modalities using 4-1BB targeted aptamers (119, 128, 140), as well as therapeutic combinations with other immuno-modulatory and traditional anti-cancer treatments, have renewed excitement for the use of 4-1BB agonists in the clinic. The most significant dual therapies may lie in combinations of α4-1BB with T cell immune checkpoint blockade, in particular αCTLA-4. This combination has demonstrated therapeutic synergy in multiple tumor models (109, 114, 183), and, remarkably, diminishes immune related toxicities compared to each antibody given alone (110). When selecting combination partners for 4-1BB agonist antibodies, both combinatorial efficacy and toxicity should be considered. For example, both αCTLA-4 and αPD-1 synergize with α4-1BB therapeutically; however, αCTLA-4 reduces α4-1BB mediated liver inflammation while αPD-1 seems to exacerbate it (110, 187). Additionally, unlike checkpoint blocking antibodies which restore T cell proliferation and functionality but do little to alter their phenotype, 4-1BB agonists strongly suppress Th17 T cell responses and favor a highly cytotoxic, tumoricidal ThEO T cell phenotype (61, 76). For tumors such as colon cancer in which Th17 polarized T cells play important roles in tumor formation and support, combinations involving α4-1BB which can alter the balance of T cell phenotypes away from a Th17 and toward a Th1 or ThEO polarity could offer uniquely effective therapy. Ongoing efforts to design tumor-selective 4-1BB agonists coupled with pre-clinical studies focused on revealing the detailed cellular and molecular mechanisms by which 4-1BB agonists enhance tumor immunity, alone and in combination with other therapies, predict a significant role for these agents in the future of clinical tumor immunotherapy.
4-1BB is a co-stimulatory receptor expressed on a variety of cells of the immune system, particularly on CD8+ T cells. This broad range of expression, coupled with the ability of 4-1BB to potentiate strong and durable immune effector responses, has made 4-1BB a clinically viable target for cancer immunotherapy. Although 4-1BB can be targeted through a variety of mechanisms, its capacity to treat advanced tumors as a monotherapy is limited. The unique and often synergistic advantages of 4-1BB activation in combination with other therapies, however, suggest a prominent role for these agents in the treatment of multiple types of cancer. Despite early setbacks, 4-1BB agonists may provide a critical piece in assembling combination therapies capable of achieving durable complete responses against advanced cancers.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
2. Burnet FM. Implications of immunological surveillance for cancer therapy. Isr J Med Sci (1971) 7(1):9–16.
3. Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol (1999) 17(7):2105–16.
4. Lotze MT, Matory YL, Rayner AA, Ettinghausen SE, Vetto JT, Seipp CA, et al. Clinical effects and toxicity of interleukin-2 in patients with cancer. Cancer (1986) 58(12):2764–72. doi: 10.1002/1097-0142(19861215)58:12<2764::AID-CNCR2820581235>3.0.CO;2-Z
5. Lotze MT, Chang AE, Seipp CA, Simpson C, Vetto JT, Rosenberg SA. High-dose recombinant interleukin 2 in the treatment of patients with disseminated cancer. Responses, treatment-related morbidity, and histologic findings. JAMA (1986) 256(22):3117–24. doi:10.1001/jama.256.22.3117
6. Lotze MT, Rosenberg SA. Results of clinical trials with the administration of interleukin 2 and adoptive immunotherapy with activated cells in patients with cancer. Immunobiology (1986) 172(3–5):420–37. doi:10.1016/S0171-2985(86)80122-X
7. Weber J. Review: anti-CTLA-4 antibody ipilimumab: case studies of clinical response and immune-related adverse events. Oncologist (2007) 12(7):864–72. doi:10.1634/theoncologist.12-7-864
8. Weber J. Overcoming immunologic tolerance to melanoma: targeting CTLA-4 with ipilimumab (MDX-010). Oncologist (2008) 13(Suppl 4):16–25. doi:10.1634/theoncologist.13-S4-16
9. Weber JS, O’Day S, Urba W, Powderly J, Nichol G, Yellin M, et al. Phase I/II study of ipilimumab for patients with metastatic melanoma. J Clin Oncol (2008) 26(36):5950–6. doi:10.1200/JCO.2008.16.1927
10. Ai M, Curran MA. Immune checkpoint combinations from mouse to man. Cancer Immunol Immunother (2015). doi:10.1007/s00262-014-1650-8
11. Moran AE, Kovacsovics-Bankowski M, Weinberg AD. The TNFRs OX40, 4-1BB, and CD40 as targets for cancer immunotherapy. Curr Opin Immunol (2013) 25(2):230–7. doi:10.1016/j.coi.2013.01.004
12. Croft M, Benedict CA, Ware CF. Clinical targeting of the TNF and TNFR superfamilies. Nat Rev Drug Discov (2013) 12(2):147–68. doi:10.1038/nrd3930
13. Vinay DS, Kwon BS. 4-1BB signaling beyond T cells. Cell Mol Immunol (2011) 8(4):281–4. doi:10.1038/cmi.2010.82
14. Shuford WW, Klussman K, Tritchler DD, Loo DT, Chalupny J, Siadak AW, et al. 4-1BB costimulatory signals preferentially induce CD8+ T cell proliferation and lead to the amplification in vivo of cytotoxic T cell responses. J Exp Med (1997) 186(1):47–55. doi:10.1084/jem.186.1.47
15. Cooper D, Bansal-Pakala P, Croft M. 4-1BB (CD137) controls the clonal expansion and survival of CD8 T cells in vivo but does not contribute to the development of cytotoxicity. Eur J Immunol (2002) 32(2):521–9. doi:10.1002/1521-4141(200202)32:2<521::AID-IMMU521>3.0.CO;2-X
16. Lee HW, Park SJ, Choi BK, Kim HH, Nam KO, Kwon BS. 4-1BB promotes the survival of CD8+ T lymphocytes by increasing expression of Bcl-xL and Bfl-1. J Immunol (2002) 169(9):4882–8. doi:10.4049/jimmunol.169.9.4882
17. Dawicki W, Watts TH. Expression and function of 4-1BB during CD4 versus CD8 T cell responses in vivo. Eur J Immunol (2004) 34(3):743–51. doi:10.1002/eji.200324278
18. Nam KO, Kang H, Shin SM, Cho KH, Kwon B, Kwon BS, et al. Cross-linking of 4-1BB activates TCR-signaling pathways in CD8+ T lymphocytes. J Immunol (2005) 174(4):1898–905. doi:10.4049/jimmunol.174.4.1898
19. Kim J, Choi SP, La S, Seo JS, Kim KK, Nam SH, et al. Constitutive expression of 4-1BB on T cells enhances CD4+ T cell responses. Exp Mol Med (2003) 35(6):509–17. doi:10.1038/emm.2003.66
20. Lee HW, Nam KO, Seo SK, Kim YH, Kang H, Kwon BS. 4-1BB cross-linking enhances the survival and cell cycle progression of CD4 T lymphocytes. Cell Immunol (2003) 223(2):143–50. doi:10.1016/S0008-8749(03)00169-2
21. Vinay DS, Lee SJ, Kim CH, Oh HS, Kwon BS. Exposure of a distinct PDCA-1+ (CD317) B cell population to agonistic anti-4-1BB (CD137) inhibits T and B cell responses both in vitro and in vivo. PLoS One (2012) 7(11):e50272. doi:10.1371/journal.pone.0050272
22. Zhang X, Voskens CJ, Sallin M, Maniar A, Montes CL, Zhang Y, et al. CD137 promotes proliferation and survival of human B cells. J Immunol (2010) 184(2):787–95. doi:10.4049/jimmunol.0901619
23. Schoenbrunn A, Frentsch M, Kohler S, Keye J, Dooms H, Moewes B, et al. A converse 4-1BB and CD40 ligand expression pattern delineates activated regulatory T cells (Treg) and conventional T cells enabling direct isolation of alloantigen-reactive natural Foxp3+ Treg. J Immunol (2012) 189(12):5985–94. doi:10.4049/jimmunol.1201090
24. Melero I, Johnston JV, Shufford WW, Mittler RS, Chen L. NK1.1 cells express 4-1BB (CDw137) costimulatory molecule and are required for tumor immunity elicited by anti-4-1BB monoclonal antibodies. Cell Immunol (1998) 190(2):167–72. doi:10.1006/cimm.1998.1396
25. Barao I. The TNF receptor-ligands 4-1BB-4-1BBL and GITR-GITRL in NK cell responses. Front Immunol (2012) 3:402. doi:10.3389/fimmu.2012.00402
26. Maniar A, Zhang X, Lin W, Gastman BR, Pauza CD, Strome SE, et al. Human gammadelta T lymphocytes induce robust NK cell-mediated antitumor cytotoxicity through CD137 engagement. Blood (2010) 116(10):1726–33. doi:10.1182/blood-2009-07-234211
27. Kim DH, Chang WS, Lee YS, Lee KA, Kim YK, Kwon BS, et al. 4-1BB engagement costimulates NKT cell activation and exacerbates NKT cell ligand-induced airway hyperresponsiveness and inflammation. J Immunol (2008) 180(4):2062–8. doi:10.4049/jimmunol.180.4.2062
28. Lee SJ, Kim YH, Hwang SH, Kim YI, Han IS, Vinay DS, et al. 4-1BB signal stimulates the activation, expansion, and effector functions of gammadelta T cells in mice and humans. Eur J Immunol (2013) 43(7):1839–48. doi:10.1002/eji.201242842
29. Pauly S, Broll K, Wittmann M, Giegerich G, Schwarz H. CD137 is expressed by follicular dendritic cells and costimulates B lymphocyte activation in germinal centers. J Leukoc Biol (2002) 72(1):35–42.
30. Choi BK, Kim YH, Kwon PM, Lee SC, Kang SW, Kim MS, et al. 4-1BB functions as a survival factor in dendritic cells. J Immunol (2009) 182(7):4107–15. doi:10.4049/jimmunol.0800459
31. Nishimoto H, Lee SW, Hong H, Potter KG, Maeda-Yamamoto M, Kinoshita T, et al. Costimulation of mast cells by 4-1BB, a member of the tumor necrosis factor receptor superfamily, with the high-affinity IgE receptor. Blood (2005) 106(13):4241–8. doi:10.1182/blood-2005-04-1358
32. Yang J, Park OJ, Lee YJ, Jung HM, Woo KM, Choi Y. The 4-1BB ligand and 4-1BB expressed on osteoclast precursors enhance RANKL-induced osteoclastogenesis via bi-directional signaling. Eur J Immunol (2008) 38(6):1598–609. doi:10.1002/eji.200737650
33. Shin HH, Lee JE, Lee EA, Kwon BS, Choi HS. Enhanced osteoclastogenesis in 4-1BB-deficient mice caused by reduced interleukin-10. J Bone Miner Res (2006) 21(12):1907–12. doi:10.1359/jbmr.060813
34. Kim YM, Kim HK, Kim HJ, Lee HW, Ju SA, Choi BK, et al. Expression of 4-1BB and 4-1BBL in thymocytes during thymus regeneration. Exp Mol Med (2009) 41(12):896–911. doi:10.3858/emm.2009.41.12.095
35. Jiang D, Schwarz H. Regulation of granulocyte and macrophage populations of murine bone marrow cells by G-CSF and CD137 protein. PLoS One (2010) 5(12):e15565. doi:10.1371/journal.pone.0015565
36. Lee SW, Park Y, So T, Kwon BS, Cheroutre H, Mittler RS, et al. Identification of regulatory functions for 4-1BB and 4-1BBL in myelopoiesis and the development of dendritic cells. Nat Immunol (2008) 9(8):917–26. doi:10.1038/ni.1632
37. Drenkard D, Becke FM, Langstein J, Spruss T, Kunz-Schughart LA, Tan TE, et al. CD137 is expressed on blood vessel walls at sites of inflammation and enhances monocyte migratory activity. FASEB J (2007) 21(2):456–63. doi:10.1096/fj.05-4739com
38. Broll K, Richter G, Pauly S, Hofstaedter F, Schwarz H. CD137 expression in tumor vessel walls. High correlation with malignant tumors. Am J Clin Pathol (2001) 115(4):543–9. doi:10.1309/6U88-357U-UKJ5-YPT3
39. Teijeira A, Palazon A, Garasa S, Marre D, Auba C, Rogel A, et al. CD137 on inflamed lymphatic endothelial cells enhances CCL21-guided migration of dendritic cells. FASEB J (2012) 26(8):3380–92. doi:10.1096/fj.11-201061
40. Yan J, Wang C, Wang Z, Yuan W. The effect of CD137-CD137 ligand interaction on phospholipase C signaling pathway in human endothelial cells. Chem Biol Interact (2013) 206(2):256–61. doi:10.1016/j.cbi.2013.09.014
41. Reali C, Curto M, Sogos V, Scintu F, Pauly S, Schwarz H, et al. Expression of CD137 and its ligand in human neurons, astrocytes, and microglia: modulation by FGF-2. J Neurosci Res (2003) 74(1):67–73. doi:10.1002/jnr.10727
42. Anderson MW, Zhao S, Freud AG, Czerwinski DK, Kohrt H, Alizadeh AA, et al. CD137 is expressed in follicular dendritic cell tumors and in classical Hodgkin and T-cell lymphomas: diagnostic and therapeutic implications. Am J Pathol (2012) 181(3):795–803. doi:10.1016/j.ajpath.2012.05.015
43. Wang Q, Zhang P, Zhang Q, Wang X, Li J, Ma C, et al. Analysis of CD137 and CD137L expression in human primary tumor tissues. Croat Med J (2008) 49(2):192–200. doi:10.3325/cmj.2008.2.192
44. Ho WT, Pang WL, Chong SM, Castella A, Al-Salam S, Tan TE, et al. Expression of CD137 on Hodgkin and Reed-Sternberg cells inhibits T-cell activation by eliminating CD137 ligand expression. Cancer Res (2013) 73(2):652–61. doi:10.1158/0008-5472.CAN-12-3849
45. Pang WL, Ho WT, Schwarz H. Ectopic CD137 expression facilitates the escape of Hodgkin and Reed-Sternberg cells from immunosurveillance. Oncoimmunology (2013) 2(4):e23441. doi:10.4161/onci.23441
46. Jang IK, Lee ZH, Kim YJ, Kim SH, Kwon BS. Human 4-1BB (CD137) signals are mediated by TRAF2 and activate nuclear factor-kappa B. Biochem Biophys Res Commun (1998) 242(3):613–20. doi:10.1006/bbrc.1997.8016
47. Oussa NA, Soumounou Y, Sabbagh L. TRAF1 phosphorylation on Serine 139 modulates NF-kappaB activity downstream of 4-1BB in T cells. Biochem Biophys Res Commun (2013) 432(1):129–34. doi:10.1016/j.bbrc.2013.01.073
48. Sabbagh L, Andreeva D, Laramee GD, Oussa NA, Lew D, Bisson N, et al. Leukocyte-specific protein 1 links TNF receptor-associated factor 1 to survival signaling downstream of 4-1BB in T cells. J Leukoc Biol (2013) 93(5):713–21. doi:10.1189/jlb.1112579
49. Kim HH, Kwack K, Lee ZH. Activation of c-Jun N-terminal kinase by 4-1BB (CD137), a T cell co-stimulatory molecule. Mol Cells (2000) 10(3):247–52.
50. Sabbagh L, Pulle G, Liu Y, Tsitsikov EN, Watts TH. ERK-dependent Bim modulation downstream of the 4-1BB-TRAF1 signaling axis is a critical mediator of CD8 T cell survival in vivo. J Immunol (2008) 180(12):8093–101. doi:10.4049/jimmunol.180.12.8093
51. Lee do Y, Choi BK, Lee DG, Kim YH, Kim CH, Lee SJ, et al. 4-1BB signaling activates the t cell factor 1 effector/beta-catenin pathway with delayed kinetics via ERK signaling and delayed PI3K/AKT activation to promote the proliferation of CD8+ T Cells. PLoS One (2013) 8(7):e69677. doi:10.1371/journal.pone.0069677
52. Kim JO, Kim HW, Baek KM, Kang CY. NF-kappaB and AP-1 regulate activation-dependent CD137 (4-1BB) expression in T cells. FEBS Lett (2003) 541(1–3):163–70. doi:10.1016/S0014-5793(03)00326-0
53. Hurtado JC, Kim YJ, Kwon BS. Signals through 4-1BB are costimulatory to previously activated splenic T cells and inhibit activation-induced cell death. J Immunol (1997) 158(6):2600–9.
54. Lee SW, Vella AT, Kwon BS, Croft M. Enhanced CD4 T cell responsiveness in the absence of 4-1BB. J Immunol (2005) 174(11):6803–8. doi:10.4049/jimmunol.174.11.6803
55. Kwon BS, Hurtado JC, Lee ZH, Kwack KB, Seo SK, Choi BK, et al. Immune responses in 4-1BB (CD137)-deficient mice. J Immunol (2002) 168(11):5483–90. doi:10.4049/jimmunol.168.11.5483
56. Ju SA, Park SM, Lee SC, Kwon BS, Kim BS. Marked expansion of CD11c+CD8+ T-cells in melanoma-bearing mice induced by anti-4-1BB monoclonal antibody. Mol Cells (2007) 24(1):132–8.
57. Choi BK, Kim YH, Choi JH, Kim CH, Kim KS, Sung YC, et al. Unified immune modulation by 4-1BB triggering leads to diverse effects on disease progression in vivo. Cytokine (2011) 55(3):420–8. doi:10.1016/j.cyto.2011.05.015
58. Shin SM, Kim YH, Choi BK, Kwon PM, Lee HW, Kwon BS. 4-1BB triggers IL-13 production from T cells to limit the polarized, Th1-mediated inflammation. J Leukoc Biol (2007) 81(6):1455–65. doi:10.1189/jlb.1006619
59. Kim YH, Choi BK, Kang WJ, Kim KH, Kang SW, Mellor AL, et al. IFN-gamma-indoleamine-2,3 dioxygenase acts as a major suppressive factor in 4-1BB-mediated immune suppression in vivo. J Leukoc Biol (2009) 85(5):817–25. doi:10.1189/jlb.0408246
60. Kuang Y, Weng X, Liu X, Zhu H, Chen Z, Chen H. Effects of 4-1BB signaling on the biological function of murine dendritic cells. Oncol Lett (2012) 3(2):477–81. doi:10.3892/ol.2011.506
61. Curran MA, Geiger TL, Montalvo W, Kim M, Reiner SL, Al-Shamkhani A, et al. Systemic 4-1BB activation induces a novel T cell phenotype driven by high expression of eomesodermin. J Exp Med (2013) 210(4):743–55. doi:10.1084/jem.20121190
62. Lee SW, Park Y, Eun SY, Madireddi S, Cheroutre H, Croft M. Cutting edge: 4-1BB controls regulatory activity in dendritic cells through promoting optimal expression of retinal dehydrogenase. J Immunol (2012) 189(6):2697–701. doi:10.4049/jimmunol.1201248
63. Bertram EM, Lau P, Watts TH. Temporal segregation of 4-1BB versus CD28-mediated costimulation: 4-1BB ligand influences T cell numbers late in the primary response and regulates the size of the T cell memory response following influenza infection. J Immunol (2002) 168(8):3777–85. doi:10.4049/jimmunol.168.8.3777
64. Vidric M, Suh WK, Dianzani U, Mak TW, Watts TH. Cooperation between 4-1BB and ICOS in the immune response to influenza virus revealed by studies of CD28/ICOS-deficient mice. J Immunol (2005) 175(11):7288–96. doi:10.4049/jimmunol.175.11.7288
65. Lin GH, Sedgmen BJ, Moraes TJ, Snell LM, Topham DJ, Watts TH. Endogenous 4-1BB ligand plays a critical role in protection from influenza-induced disease. J Immunol (2009) 182(2):934–47. doi:10.4049/jimmunol.182.2.934
66. Arribillaga L, Sarobe P, Arina A, Gorraiz M, Borras-Cuesta F, Ruiz J, et al. Enhancement of CD4 and CD8 immunity by anti-CD137 (4-1BB) monoclonal antibodies during hepatitis C vaccination with recombinant adenovirus. Vaccine (2005) 23(27):3493–9. doi:10.1016/j.vaccine.2005.02.003
67. Humphreys IR, Lee SW, Jones M, Loewendorf A, Gostick E, Price DA, et al. Biphasic role of 4-1BB in the regulation of mouse cytomegalovirus-specific CD8(+) T cells. Eur J Immunol (2010) 40(10):2762–8. doi:10.1002/eji.200940256
68. De Keersmaecker B, Heirman C, Corthals J, Empsen C, van Grunsven LA, Allard SD, et al. The combination of 4-1BBL and CD40L strongly enhances the capacity of dendritic cells to stimulate HIV-specific T cell responses. J Leukoc Biol (2011) 89(6):989–99. doi:10.1189/jlb.0810466
69. Zhang B, Maris CH, Foell J, Whitmire J, Niu L, Song J, et al. Immune suppression or enhancement by CD137 T cell costimulation during acute viral infection is time dependent. J Clin Invest (2007) 117(10):3029–41. doi:10.1172/JCI32426
70. Zhao Y, Croft M. Dispensable role for 4-1BB and 4-1BBL in development of vaccinia virus-specific CD8 T cells. Immunol Lett (2012) 141(2):220–6. doi:10.1016/j.imlet.2011.10.008
71. Zhao Y, Tahiliani V, Salek-Ardakani S, Croft M. Targeting 4-1BB (CD137) to enhance CD8 T cell responses with poxviruses and viral antigens. Front Immunol (2012) 3:332. doi:10.3389/fimmu.2012.00332
72. Wu ZQ, Khan AQ, Shen Y, Wolcott KM, Dawicki W, Watts TH, et al. 4-1BB (CD137) differentially regulates murine in vivo protein- and polysaccharide-specific immunoglobulin isotype responses to Streptococcus pneumoniae. Infect Immun (2003) 71(1):196–204. doi:10.1128/IAI.71.1.196-204.2003
73. Lee SC, Ju SA, Pack HN, Heo SK, Suh JH, Park SM, et al. 4-1BB (CD137) is required for rapid clearance of Listeria monocytogenes infection. Infect Immun (2005) 73(8):5144–51. doi:10.1128/IAI.73.8.5144-5151.2005
74. Lee SC, Ju SA, Sung BH, Heo SK, Cho HR, Lee EA, et al. Stimulation of the molecule 4-1BB enhances host defense against Listeria monocytogenes infection in mice by inducing rapid infiltration and activation of neutrophils and monocytes. Infect Immun (2009) 77(5):2168–76. doi:10.1128/IAI.01350-08
75. Sun Y, Lin X, Chen HM, Wu Q, Subudhi SK, Chen L, et al. Administration of agonistic anti-4-1BB monoclonal antibody leads to the amelioration of experimental autoimmune encephalomyelitis. J Immunol (2002) 168(3):1457–65. doi:10.4049/jimmunol.168.3.1457
76. Kim YH, Choi BK, Shin SM, Kim CH, Oh HS, Park SH, et al. 4-1BB triggering ameliorates experimental autoimmune encephalomyelitis by modulating the balance between Th17 and regulatory T cells. J Immunol (2011) 187(3):1120–8. doi:10.4049/jimmunol.1002681
77. Foell J, McCausland M, Burch J, Corriazzi N, Yan XJ, Suwyn C, et al. CD137-mediated T cell co-stimulation terminates existing autoimmune disease in SLE-prone NZB/NZW F1 mice. Ann N Y Acad Sci (2003) 987:230–5. doi:10.1111/j.1749-6632.2003.tb06052.x
78. Foell J, Strahotin S, O’Neil SP, McCausland MM, Suwyn C, Haber M, et al. CD137 costimulatory T cell receptor engagement reverses acute disease in lupus-prone NZB x NZW F1 mice. J Clin Invest (2003) 111(10):1505–18. doi:10.1172/JCI17662
79. Vinay DS, Choi JH, Kim JD, Choi BK, Kwon BS. Role of endogenous 4-1BB in the development of systemic lupus erythematosus. Immunology (2007) 122(3):394–400. doi:10.1111/j.1365-2567.2007.02653.x
80. Vinay DS, Kim JD, Asai T, Choi BK, Kwon BS. Absence of 4 1BB gene function exacerbates lacrimal gland inflammation in autoimmune-prone MRL-Faslpr mice. Invest Ophthalmol Vis Sci (2007) 48(10):4608–15. doi:10.1167/iovs.07-0153
81. Lee J, Lee EN, Kim EY, Park HJ, Chang CY, Jung DY, et al. Administration of agonistic anti-4-1BB monoclonal antibody leads to the amelioration of inflammatory bowel disease. Immunol Lett (2005) 101(2):210–6. doi:10.1016/j.imlet.2005.06.001
82. Martinez Gomez JM, Chen L, Schwarz H, Karrasch T. CD137 facilitates the resolution of acute DSS-induced colonic inflammation in mice. PLoS One (2013) 8(9):e73277. doi:10.1371/journal.pone.0073277
83. Choi BK, Asai T, Vinay DS, Kim YH, Kwon BS. 4-1BB-mediated amelioration of experimental autoimmune uveoretinitis is caused by indoleamine 2,3-dioxygenase-dependent mechanisms. Cytokine (2006) 34(5–6):233–42. doi:10.1016/j.cyto.2006.04.008
84. Seo SK, Choi JH, Kim YH, Kang WJ, Park HY, Suh JH, et al. 4-1BB-mediated immunotherapy of rheumatoid arthritis. Nat Med (2004) 10(10):1088–94. doi:10.1038/nm1104-1261
85. Sytwu HK, Lin WD, Roffler SR, Hung JT, Sung HS, Wang CH, et al. Anti-4-1BB-based immunotherapy for autoimmune diabetes: lessons from a transgenic non-obese diabetic (NOD) model. J Autoimmun (2003) 21(3):247–54. doi:10.1016/S0896-8411(03)00112-4
86. Cannons JL, Chamberlain G, Howson J, Smink LJ, Todd JA, Peterson LB, et al. Genetic and functional association of the immune signaling molecule 4-1BB (CD137/TNFRSF9) with type 1 diabetes. J Autoimmun (2005) 25(1):13–20. doi:10.1016/j.jaut.2005.04.007
87. Szypowska A, Stelmaszczyk-Emmel A, Demkow U, Luczynski W. High expression of OX40 (CD134) and 4-1BB (CD137) molecules on CD4(+)CD25(high) cells in children with type 1 diabetes. Adv Med Sci (2014) 59(1):39–43. doi:10.1016/j.advms.2013.07.003
88. Irie J, Wu Y, Kachapati K, Mittler RS, Ridgway WM. Modulating protective and pathogenic CD4+ subsets via CD137 in type 1 diabetes. Diabetes (2007) 56(1):186–96. doi:10.2337/db06-0793
89. Sun Y, Blink SE, Liu W, Lee Y, Chen B, Solway J, et al. Inhibition of Th2-mediated allergic airway inflammatory disease by CD137 costimulation. J Immunol (2006) 177(2):814–21. doi:10.4049/jimmunol.177.2.814
90. Kim BJ, Kwon JW, Seo JH, Choi WA, Kim YJ, Kang MJ, et al. Hu.4-1BB-Fc fusion protein inhibits allergic inflammation and airway hyperresponsiveness in a murine model of asthma. Korean J Pediatr (2011) 54(9):373–9. doi:10.3345/kjp.2011.54.9.373
91. Ascierto PA, Simeone E, Sznol M, Fu YX, Melero I. Clinical experiences with anti-CD137 and anti-PD1 therapeutic antibodies. Semin Oncol (2010) 37(5):508–16. doi:10.1053/j.seminoncol.2010.09.008
92. Huang JH, Zhang SN, Choi KJ, Choi IK, Kim JH, Lee MG, et al. Therapeutic and tumor-specific immunity induced by combination of dendritic cells and oncolytic adenovirus expressing IL-12 and 4-1BBL. Mol Ther (2010) 18(2):264–74. doi:10.1038/mt.2009.205
93. John LB, Howland LJ, Flynn JK, West AC, Devaud C, Duong CP, et al. Oncolytic virus and anti-4-1BB combination therapy elicits strong antitumor immunity against established cancer. Cancer Res (2012) 72(7):1651–60. doi:10.1158/0008-5472.CAN-11-2788
94. Schabowsky RH, Elpek KG, Madireddi S, Sharma RK, Yolcu ES, Bandura-Morgan L, et al. A novel form of 4-1BBL has better immunomodulatory activity than an agonistic anti-4-1BB Ab without Ab-associated severe toxicity. Vaccine (2009) 28(2):512–22. doi:10.1016/j.vaccine.2009.09.127
95. Madireddi S, Eun SY, Lee SW, Nemcovicova I, Mehta AK, Zajonc DM, et al. Galectin-9 controls the therapeutic activity of 4-1BB-targeting antibodies. J Exp Med (2014) 211(7):1433–48. doi:10.1084/jem.20132687
96. Houot R, Goldstein MJ, Kohrt HE, Myklebust JH, Alizadeh AA, Lin JT, et al. Therapeutic effect of CD137 immunomodulation in lymphoma and its enhancement by Treg depletion. Blood (2009) 114(16):3431–8. doi:10.1182/blood-2009-05-223958
97. Moraes TJ, Lin GH, Wen T, Watts TH. Incorporation of 4-1BB ligand into an adenovirus vaccine vector increases the number of functional antigen-specific CD8 T cells and enhances the duration of protection against influenza-induced respiratory disease. Vaccine (2011) 29(37):6301–12. doi:10.1016/j.vaccine.2011.06.022
98. Quetglas JI, Dubrot J, Bezunartea J, Sanmamed MF, Hervas-Stubbs S, Smerdou C, et al. Immunotherapeutic synergy between anti-CD137 mAb and intratumoral administration of a cytopathic Semliki forest virus encoding IL-12. Mol Ther (2012) 20(9):1664–75. doi:10.1038/mt.2012.56
99. Choi BK, Kim YH, Kang WJ, Lee SK, Kim KH, Shin SM, et al. Mechanisms involved in synergistic anticancer immunity of anti-4-1BB and anti-CD4 therapy. Cancer Res (2007) 67(18):8891–9. doi:10.1158/0008-5472.CAN-07-1056
100. Kudo-Saito C, Hodge JW, Kwak H, Kim-Schulze S, Schlom J, Kaufman HL. 4-1BB ligand enhances tumor-specific immunity of poxvirus vaccines. Vaccine (2006) 24(23):4975–86. doi:10.1016/j.vaccine.2006.03.042
101. Guo Z, Cheng D, Xia Z, Luan M, Wu L, Wang G, et al. Combined TIM-3 blockade and CD137 activation affords the long-term protection in a murine model of ovarian cancer. J Transl Med (2013) 11:215. doi:10.1186/1479-5876-11-215
102. Sin JI, Kim H, Ahn E, Jeon YH, Park WS, Lee SY, et al. Combined stimulation of TLR9 and 4.1BB augments Trp2 peptide vaccine-mediated melanoma rejection by increasing Ag-specific CTL activity and infiltration into tumor sites. Cancer Lett (2013) 330(2):190–9. doi:10.1016/j.canlet.2012.11.045
103. Kim HS, Kim-Schulze S, Kim DW, Kaufman HL. Host lymphodepletion enhances the therapeutic activity of an oncolytic vaccinia virus expressing 4-1BB ligand. Cancer Res (2009) 69(21):8516–25. doi:10.1158/0008-5472.CAN-09-2522
104. Chen SH, Pham-Nguyen KB, Martinet O, Huang Y, Yang W, Thung SN, et al. Rejection of disseminated metastases of colon carcinoma by synergism of IL-12 gene therapy and 4-1BB costimulation. Mol Ther (2000) 2(1):39–46. doi:10.1006/mthe.2000.0086
105. Martinet O, Ermekova V, Qiao JQ, Sauter B, Mandeli J, Chen L, et al. Immunomodulatory gene therapy with interleukin 12 and 4-1BB ligand: long- term remission of liver metastases in a mouse model. J Natl Cancer Inst (2000) 92(11):931–6. doi:10.1093/jnci/92.11.931
106. Xu D, Gu P, Pan PY, Li Q, Sato AI, Chen SH. NK and CD8+ T cell-mediated eradication of poorly immunogenic B16-F10 melanoma by the combined action of IL-12 gene therapy and 4-1BB costimulation. Int J Cancer (2004) 109(4):499–506. doi:10.1002/ijc.11696
107. Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med (2012) 366(26):2455–65. doi:10.1056/NEJMoa1200694
108. Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med (2012) 366(26):2443–54. doi:10.1056/NEJMoa1200690
109. Youlin K, Li Z, Xiaodong W, Xiuheng L, Hengchen Z. Combination immunotherapy with 4-1BBL and CTLA-4 blockade for the treatment of prostate cancer. Clin Dev Immunol (2012) 2012:439235. doi:10.1155/2012/439235
110. Kocak E, Lute K, Chang X, May KF Jr, Exten KR, Zhang H, et al. Combination therapy with anti-CTL antigen-4 and anti-4-1BB antibodies enhances cancer immunity and reduces autoimmunity. Cancer Res (2006) 66(14):7276–84. doi:10.1158/0008-5472.CAN-05-2128
111. Madireddi S, Schabowsky RH, Srivastava AK, Sharma RK, Yolcu ES, Shirwan H. SA-4-1BBL costimulation inhibits conversion of conventional CD4+ T cells into CD4+ FoxP3+ T regulatory cells by production of IFN-gamma. PLoS One (2012) 7(8):e42459. doi:10.1371/journal.pone.0042459
112. Cuadros C, Dominguez AL, Lollini PL, Croft M, Mittler RS, Borgstrom P, et al. Vaccination with dendritic cells pulsed with apoptotic tumors in combination with anti-OX40 and anti-4-1BB monoclonal antibodies induces T cell-mediated protective immunity in Her-2/neu transgenic mice. Int J Cancer (2005) 116(6):934–43. doi:10.1002/ijc.21098
113. Bandyopadhyay S, Long M, Qui HZ, Hagymasi AT, Slaiby AM, Mihalyo MA, et al. Self-antigen prevents CD8 T cell effector differentiation by CD134 and CD137 dual costimulation. J Immunol (2008) 181(11):7728–37. doi:10.4049/jimmunol.181.11.7728
114. Belcaid Z, Phallen JA, Zeng J, See AP, Mathios D, Gottschalk C, et al. Focal radiation therapy combined with 4-1BB activation and CTLA-4 blockade yields long-term survival and a protective antigen-specific memory response in a murine glioma model. PLoS One (2014) 9(7):e101764. doi:10.1371/journal.pone.0101764
115. Kim KM, Kim HW, Kim JO, Baek KM, Kim JG, Kang CY. Induction of 4-1BB (CD137) expression by DNA damaging agents in human T lymphocytes. Immunology (2002) 107(4):472–9. doi:10.1046/j.1365-2567.2002.01538.x
116. Newcomb EW, Lukyanov Y, Kawashima N, Alonso-Basanta M, Wang SC, Liu M, et al. Radiotherapy enhances antitumor effect of anti-CD137 therapy in a mouse Glioma model. Radiat Res (2010) 173(4):426–32. doi:10.1667/RR1904.1
117. Ju SA, Cheon SH, Park SM, Tam NQ, Kim YM, An WG, et al. Eradication of established renal cell carcinoma by a combination of 5-fluorouracil and anti-4-1BB monoclonal antibody in mice. Int J Cancer (2008) 122(12):2784–90. doi:10.1002/ijc.23457
118. Kim YH, Choi BK, Kim KH, Kang SW, Kwon BS. Combination therapy with cisplatin and anti-4-1BB: synergistic anticancer effects and amelioration of cisplatin-induced nephrotoxicity. Cancer Res (2008) 68(18):7264–9. doi:10.1158/0008-5472.CAN-08-1365
119. McNamara JO, Kolonias D, Pastor F, Mittler RS, Chen L, Giangrande PH, et al. Multivalent 4-1BB binding aptamers costimulate CD8+ T cells and inhibit tumor growth in mice. J Clin Invest (2008) 118(1):376–86. doi:10.1172/JCI33365
120. Sakib Hossain DM, Duttagupta P, Kortylewski M. The aptamer-siRNA conjugates: reprogramming T cells for cancer therapy. Ther Deliv (2015) 6(1):1–4. doi:10.4155/tde.14.92
121. Berezhnoy A, Castro I, Levay A, Malek TR, Gilboa E. Aptamer-targeted inhibition of mTOR in T cells enhances antitumor immunity. J Clin Invest (2014) 124(1):188–97. doi:10.1172/JCI69856
122. Melero I, Shuford WW, Newby SA, Aruffo A, Ledbetter JA, Hellstrom KE, et al. Monoclonal antibodies against the 4-1BB T-cell activation molecule eradicate established tumors. Nat Med (1997) 3(6):682–5. doi:10.1038/nm0697-682
123. Kim JA, Averbook BJ, Chambers K, Rothchild K, Kjaergaard J, Papay R, et al. Divergent effects of 4-1BB antibodies on antitumor immunity and on tumor-reactive T-cell generation. Cancer Res (2001) 61(5):2031–7.
124. May KF Jr, Chen L, Zheng P, Liu Y. Anti-4-1BB monoclonal antibody enhances rejection of large tumor burden by promoting survival but not clonal expansion of tumor-specific CD8+ T cells. Cancer Res (2002) 62(12):3459–65.
125. Wilcox RA, Flies DB, Zhu G, Johnson AJ, Tamada K, Chapoval AI, et al. Provision of antigen and CD137 signaling breaks immunological ignorance, promoting regression of poorly immunogenic tumors. J Clin Invest (2002) 109(5):651–9. doi:10.1172/JCI0214184
126. Lin W, Voskens CJ, Zhang X, Schindler DG, Wood A, Burch E, et al. Fc-dependent expression of CD137 on human NK cells: insights into “agonistic” effects of anti-CD137 monoclonal antibodies. Blood (2008) 112(3):699–707. doi:10.1182/blood-2007-11-122465
127. Martinez-Forero I, Azpilikueta A, Bolanos-Mateo E, Nistal-Villan E, Palazon A, Teijeira A, et al. T cell costimulation with anti-CD137 monoclonal antibodies is mediated by K63-polyubiquitin-dependent signals from endosomes. J Immunol (2013) 190(12):6694–706. doi:10.4049/jimmunol.1203010
128. Schrand B, Berezhnoy A, Brenneman R, Williams A, Levay A, Kong LY, et al. Targeting 4-1BB costimulation to the tumor stroma with bispecific aptamer conjugates enhances the therapeutic index of tumor immunotherapy. Cancer Immunol Res (2014) 2(9):867–77. doi:10.1158/2326-6066.CIR-14-0007
129. Li Q, Ai J, Song Z, Liu J, Shan B. 4-1BB (CD137) ligand enhanced anti-tumor immune response against mouse forestomach carcinoma in vivo. Cell Mol Immunol (2008) 5(5):379–84. doi:10.1038/cmi.2008.47
130. Melero I, Bach N, Hellstrom KE, Aruffo A, Mittler RS, Chen L. Amplification of tumor immunity by gene transfer of the co-stimulatory 4-1BB ligand: synergy with the CD28 co-stimulatory pathway. Eur J Immunol (1998) 28(3):1116–21. doi:10.1002/(SICI)1521-4141(199803)28:03<1116::AID-IMMU1116>3.0.CO;2-A
131. Martinet O, Divino CM, Zang Y, Gan Y, Mandeli J, Thung S, et al. T cell activation with systemic agonistic antibody versus local 4-1BB ligand gene delivery combined with interleukin-12 eradicate liver metastases of breast cancer. Gene Ther (2002) 9(12):786–92. doi:10.1038/sj.gt.3301687
132. Zhang H, Merchant MS, Chua KS, Khanna C, Helman LJ, Telford B, et al. Tumor expression of 4-1BB ligand sustains tumor lytic T cells. Cancer Biol Ther (2003) 2(5):579–86. doi:10.4161/cbt.2.5.545
133. Maus MV, Thomas AK, Leonard DG, Allman D, Addya K, Schlienger K, et al. Ex vivo expansion of polyclonal and antigen-specific cytotoxic T lymphocytes by artificial APCs expressing ligands for the T-cell receptor, CD28 and 4-1BB. Nat Biotechnol (2002) 20(2):143–8. doi:10.1038/nbt0202-143
134. Gong W, Xiao W, Hu M, Weng X, Qian L, Pan X, et al. Ex vivo expansion of natural killer cells with high cytotoxicity by K562 cells modified to co-express major histocompatibility complex class I chain-related protein A, 4-1BB ligand, and interleukin-15. Tissue Antigens (2010) 76(6):467–75. doi:10.1111/j.1399-0039.2010.01535.x
135. Sharma RK, Elpek KG, Yolcu ES, Schabowsky RH, Zhao H, Bandura-Morgan L, et al. Costimulation as a platform for the development of vaccines: a peptide-based vaccine containing a novel form of 4-1BB ligand eradicates established tumors. Cancer Res (2009) 69(10):4319–26. doi:10.1158/0008-5472.CAN-08-3141
136. Sharma RK, Schabowsky RH, Srivastava AK, Elpek KG, Madireddi S, Zhao H, et al. 4-1BB ligand as an effective multifunctional immunomodulator and antigen delivery vehicle for the development of therapeutic cancer vaccines. Cancer Res (2010) 70(10):3945–54. doi:10.1158/0008-5472.CAN-09-4480
137. Sharma RK, Srivastava AK, Yolcu ES, MacLeod KJ, Schabowsky RH, Madireddi S, et al. SA-4-1BBL as the immunomodulatory component of a HPV-16 E7 protein based vaccine shows robust therapeutic efficacy in a mouse cervical cancer model. Vaccine (2010) 28(36):5794–802. doi:10.1016/j.vaccine.2010.06.073
138. Srivastava AK, Sharma RK, Yolcu ES, Ulker V, MacLeod K, Dinc G, et al. Prime-boost vaccination with SA-4-1BBL costimulatory molecule and survivin eradicates lung carcinoma in CD8+ T and NK cell dependent manner. PLoS One (2012) 7(11):e48463. doi:10.1371/journal.pone.0048463
139. Gilboa E, McNamara J II, Pastor F. Use of oligonucleotide aptamer ligands to modulate the function of immune receptors. Clin Cancer Res (2013) 19(5):1054–62. doi:10.1158/1078-0432.CCR-12-2067
140. Pastor F, Kolonias D, McNamara JO II, Gilboa E. Targeting 4-1BB costimulation to disseminated tumor lesions with bi-specific oligonucleotide aptamers. Mol Ther (2011) 19(10):1878–86. doi:10.1038/mt.2011.145
141. Kohrt HE, Colevas AD, Houot R, Weiskopf K, Goldstein MJ, Lund P, et al. Targeting CD137 enhances the efficacy of cetuximab. J Clin Invest (2014) 124(6):2668–82. doi:10.1172/JCI73014
142. Kohrt HE, Houot R, Goldstein MJ, Weiskopf K, Alizadeh AA, Brody J, et al. CD137 stimulation enhances the antilymphoma activity of anti-CD20 antibodies. Blood (2011) 117(8):2423–32. doi:10.1182/blood-2010-08-301945
143. Kohrt HE, Houot R, Weiskopf K, Goldstein MJ, Scheeren F, Czerwinski D, et al. Stimulation of natural killer cells with a CD137-specific antibody enhances trastuzumab efficacy in xenotransplant models of breast cancer. J Clin Invest (2012) 122(3):1066–75. doi:10.1172/JCI61226
144. Zhang H, Knutson KL, Hellstrom KE, Disis ML, Hellstrom I. Antitumor efficacy of CD137 ligation is maximized by the use of a CD137 single-chain Fv-expressing whole-cell tumor vaccine compared with CD137-specific monoclonal antibody infusion. Mol Cancer Ther (2006) 5(1):149–55. doi:10.1158/1535-7163.MCT-05-0206
145. Yang Y, Yang S, Ye Z, Jaffar J, Zhou Y, Cutter E, et al. Tumor cells expressing anti-CD137 scFv induce a tumor-destructive environment. Cancer Res (2007) 67(5):2339–44. doi:10.1158/0008-5472.CAN-06-3593
146. Adappa ND, Sung CK, Choi B, Huang TG, Genden EM, Shin EJ. The administration of IL-12/GM-CSF and Ig-4-1BB ligand markedly decreases murine floor of mouth squamous cell cancer. Otolaryngol Head Neck Surg (2008) 139(3):442–8. doi:10.1016/j.otohns.2008.05.001
147. Li G, Wu X, Zhang F, Li X, Sun B, Yu Y, et al. Triple expression of B7-1, B7-2 and 4-1BBL enhanced antitumor immune response against mouse H22 hepatocellular carcinoma. J Cancer Res Clin Oncol (2011) 137(4):695–703. doi:10.1007/s00432-010-0905-9
148. Gauttier V, Judor JP, Le Guen V, Cany J, Ferry N, Conchon S. Agonistic anti-CD137 antibody treatment leads to antitumor response in mice with liver cancer. Int J Cancer (2014) 135(12):2857–67. doi:10.1002/ijc.28943
149. Li B, Lin J, Vanroey M, Jure-Kunkel M, Jooss K. Established B16 tumors are rejected following treatment with GM-CSF-secreting tumor cell immunotherapy in combination with anti-4-1BB mAb. Clin Immunol (2007) 125(1):76–87. doi:10.1016/j.clim.2007.07.005
150. Tirapu I, Arina A, Mazzolini G, Duarte M, Alfaro C, Feijoo E, et al. Improving efficacy of interleukin-12-transfected dendritic cells injected into murine colon cancer with anti-CD137 monoclonal antibodies and alloantigens. Int J Cancer (2004) 110(1):51–60. doi:10.1002/ijc.20093
151. Li Q, Carr A, Ito F, Teitz-Tennenbaum S, Chang AE. Polarization effects of 4-1BB during CD28 costimulation in generating tumor-reactive T cells for cancer immunotherapy. Cancer Res (2003) 63(10):2546–52.
152. Palazon A, Martinez-Forero I, Teijeira A, Morales-Kastresana A, Alfaro C, Sanmamed MF, et al. The HIF-1alpha hypoxia response in tumor-infiltrating T lymphocytes induces functional CD137 (4-1BB) for immunotherapy. Cancer Discov (2012) 2(7):608–23. doi:10.1158/2159-8290.CD-11-0314
153. Ye Q, Song DG, Poussin M, Yamamoto T, Best A, Li C, et al. CD137 accurately identifies and enriches for naturally occurring tumor-reactive T cells in tumor. Clin Cancer Res (2014) 20(1):44–55. doi:10.1158/1078-0432.CCR-13-0945
154. Ju SA, Lee SC, Kwon TH, Heo SK, Park SM, Paek HN, et al. Immunity to melanoma mediated by 4-1BB is associated with enhanced activity of tumour-infiltrating lymphocytes. Immunol Cell Biol (2005) 83(4):344–51. doi:10.1111/j.1440-1711.2005.01330.x
155. Qui HZ, Hagymasi AT, Bandyopadhyay S, St Rose MC, Ramanarasimhaiah R, Menoret A, et al. CD134 plus CD137 dual costimulation induces eomesodermin in CD4 T cells to program cytotoxic Th1 differentiation. J Immunol (2011) 187(7):3555–64. doi:10.4049/jimmunol.1101244
156. Song C, Sadashivaiah K, Furusawa A, Davila E, Tamada K, Banerjee A. Eomesodermin is required for antitumor immunity mediated by 4-1BB-agonist immunotherapy. Oncoimmunology (2014) 3(1):e27680. doi:10.4161/onci.27680
157. Morales-Kastresana A, Catalan E, Hervas-Stubbs S, Palazon A, Azpilikueta A, Bolanos E, et al. Essential complicity of perforin-granzyme and FAS-L mechanisms to achieve tumor rejection following treatment with anti-CD137 mAb. J Immunother Cancer (2013) 1:3. doi:10.1186/2051-1426-1-3
158. Murillo O, Dubrot J, Palazon A, Arina A, Azpilikueta A, Alfaro C, et al. In vivo depletion of DC impairs the anti-tumor effect of agonistic anti-CD137 mAb. Eur J Immunol (2009) 39(9):2424–36. doi:10.1002/eji.200838958
159. Palazon A, Teijeira A, Martinez-Forero I, Hervas-Stubbs S, Roncal C, Penuelas I, et al. Agonist anti-CD137 mAb act on tumor endothelial cells to enhance recruitment of activated T lymphocytes. Cancer Res (2011) 71(3):801–11. doi:10.1158/0008-5472.CAN-10-1733
160. Seo SK, Park HY, Choi JH, Kim WY, Kim YH, Jung HW, et al. Blocking 4-1BB/4-1BB ligand interactions prevents herpetic stromal keratitis. J Immunol (2003) 171(2):576–83. doi:10.4049/jimmunol.171.2.576
161. Jeon HJ, Choi JH, Jung IH, Park JG, Lee MR, Lee MN, et al. CD137 (4-1BB) deficiency reduces atherosclerosis in hyperlipidemic mice. Circulation (2010) 121(9):1124–33. doi:10.1161/CIRCULATIONAHA.109.882704
162. Li Y, Yan J, Wu C, Wang Z, Yuan W, Wang D. CD137-CD137L interaction regulates atherosclerosis via cyclophilin A in apolipoprotein E-deficient mice. PLoS One (2014) 9(2):e88563. doi:10.1371/journal.pone.0088563
163. Kim CS, Kim JG, Lee BJ, Choi MS, Choi HS, Kawada T, et al. Deficiency for costimulatory receptor 4-1BB protects against obesity-induced inflammation and metabolic disorders. Diabetes (2011) 60(12):3159–68. doi:10.2337/db10-1805
164. Tu TH, Kim CS, Goto T, Kawada T, Kim BS, Yu R. 4-1BB/4-1BBL interaction promotes obesity-induced adipose inflammation by triggering bidirectional inflammatory signaling in adipocytes/macrophages. Mediators Inflamm (2012) 2012:972629. doi:10.1155/2012/972629
165. Le NH, Kim CS, Tu TH, Choi HS, Kim BS, Kawada T, et al. Blockade of 4-1BB and 4-1BBL interaction reduces obesity-induced skeletal muscle inflammation. Mediators Inflamm (2013) 2013:865159. doi:10.1155/2013/865159
166. Wang J, Guo Z, Dong Y, Kim O, Hart J, Adams A, et al. Role of 4-1BB in allograft rejection mediated by CD8+ T cells. Am J Transplant (2003) 3(5):543–51. doi:10.1034/j.1600-6143.2003.00088.x
167. Cho HR, Kwon B, Yagita H, La S, Lee EA, Kim JE, et al. Blockade of 4-1BB (CD137)/4-1BB ligand interactions increases allograft survival. Transpl Int (2004) 17(7):351–61. doi:10.1007/s00147-004-0726-3
168. Asai T, Choi BK, Kwon PM, Kim WY, Kim JD, Vinay DS, et al. Blockade of the 4-1BB (CD137)/4-1BBL and/or CD28/CD80/CD86 costimulatory pathways promotes corneal allograft survival in mice. Immunology (2007) 121(3):349–58. doi:10.1111/j.1365-2567.2007.02581.x
169. Shi Y, Hu S, Song Q, Yu S, Zhou X, Yin J, et al. Gene silencing of 4-1BB by RNA interference inhibits acute rejection in rats with liver transplantation. Biomed Res Int (2013) 2013:192738. doi:10.1155/2013/192738
170. Kim KH, Choi BK, Kim JD, Kim YH, Lee SK, Suh JH, et al. 4-1BB signaling breaks the tolerance of maternal CD8+ T cells that are reactive with alloantigens. PLoS One (2012) 7(9):e45481. doi:10.1371/journal.pone.0045481
171. Lee SW, Salek-Ardakani S, Mittler RS, Croft M. Hypercostimulation through 4-1BB distorts homeostasis of immune cells. J Immunol (2009) 182(11):6753–62. doi:10.4049/jimmunol.0803241
172. Niu L, Strahotin S, Hewes B, Zhang B, Zhang Y, Archer D, et al. Cytokine-mediated disruption of lymphocyte trafficking, hemopoiesis, and induction of lymphopenia, anemia, and thrombocytopenia in anti-CD137-treated mice. J Immunol (2007) 178(7):4194–213. doi:10.4049/jimmunol.178.7.4194
173. Dubrot J, Milheiro F, Alfaro C, Palazon A, Martinez-Forero I, Perez-Gracia JL, et al. Treatment with anti-CD137 mAbs causes intense accumulations of liver T cells without selective antitumor immunotherapeutic effects in this organ. Cancer Immunol Immunother (2010) 59(8):1223–33. doi:10.1007/s00262-010-0846-9
174. Wang J, Zhao W, Cheng L, Guo M, Li D, Li X, et al. CD137-mediated pathogenesis from chronic hepatitis to hepatocellular carcinoma in hepatitis B virus-transgenic mice. J Immunol (2010) 185(12):7654–62. doi:10.4049/jimmunol.1000927
175. Lin GH, Snell LM, Wortzman ME, Clouthier DL, Watts TH. GITR-dependent regulation of 4-1BB expression: implications for T cell memory and anti-4-1BB-induced pathology. J Immunol (2013) 190(9):4627–39. doi:10.4049/jimmunol.1201854
176. Pan PY, Gu P, Li Q, Xu D, Weber K, Chen SH. Regulation of dendritic cell function by NK cells: mechanisms underlying the synergism in the combination therapy of IL-12 and 4-1BB activation. J Immunol (2004) 172(8):4779–89. doi:10.4049/jimmunol.172.8.4779
177. Dubrot J, Palazon A, Alfaro C, Azpilikueta A, Ochoa MC, Rouzaut A, et al. Intratumoral injection of interferon-alpha and systemic delivery of agonist anti-CD137 monoclonal antibodies synergize for immunotherapy. Int J Cancer (2011) 128(1):105–18. doi:10.1002/ijc.25333
178. Lin X, Zhou C, Wang S, Wang D, Ma W, Liang X, et al. Enhanced antitumor effect against human telomerase reverse transcriptase (hTERT) by vaccination with chemotactic-hTERT gene-modified tumor cell and the combination with anti-4-1BB monoclonal antibodies. Int J Cancer (2006) 119(8):1886–96. doi:10.1002/ijc.22048
179. Lee H, Park HJ, Sohn HJ, Kim JM, Kim SJ. Combinatorial therapy for liver metastatic colon cancer: dendritic cell vaccine and low-dose agonistic anti-4-1BB antibody co-stimulatory signal. J Surg Res (2011) 169(1):e43–50. doi:10.1016/j.jss.2011.03.067
180. Williams EL, Dunn SN, James S, Johnson PW, Cragg MS, Glennie MJ, et al. Immunomodulatory monoclonal antibodies combined with peptide vaccination provide potent immunotherapy in an aggressive murine neuroblastoma model. Clin Cancer Res (2013) 19(13):3545–55. doi:10.1158/1078-0432.CCR-12-3226
181. Quetglas JI, John LB, Kershaw MH, Alvarez-Vallina L, Melero I, Darcy PK, et al. Virotherapy, gene transfer and immunostimulatory monoclonal antibodies. Oncoimmunology (2012) 1(8):1344–54. doi:10.4161/onci.21679
182. Xu DP, Sauter BV, Huang TG, Meseck M, Woo SL, Chen SH. The systemic administration of Ig-4-1BB ligand in combination with IL-12 gene transfer eradicates hepatic colon carcinoma. Gene Ther (2005) 12(20):1526–33. doi:10.1038/sj.gt.3302556
183. Curran MA, Kim M, Montalvo W, Al-Shamkhani A, Allison JP. Combination CTLA-4 blockade and 4-1BB activation enhances tumor rejection by increasing T-cell infiltration, proliferation, and cytokine production. PLoS One (2011) 6(4):e19499. doi:10.1371/journal.pone.0019499
184. Xiao H, Huang B, Yuan Y, Li D, Han LF, Liu Y, et al. Soluble PD-1 facilitates 4-1BBL-triggered antitumor immunity against murine H22 hepatocarcinoma in vivo. Clin Cancer Res (2007) 13(6):1823–30. doi:10.1158/1078-0432.CCR-06-2154
185. Duraiswamy J, Freeman GJ, Coukos G. Therapeutic PD-1 pathway blockade augments with other modalities of immunotherapy T-cell function to prevent immune decline in ovarian cancer. Cancer Res (2013) 73(23):6900–12. doi:10.1158/0008-5472.CAN-13-1550
186. Shindo Y, Yoshimura K, Kuramasu A, Watanabe Y, Ito H, Kondo T, et al. Combination immunotherapy with 4-1BB activation and PD-1 blockade enhances antitumor efficacy in a mouse model of subcutaneous tumor. Anticancer Res (2015) 35(1):129–36.
187. Chen S, Lee LF, Fisher TS, Jessen B, Elliott M, Evering W, et al. Combination of 4-1BB agonist and PD-1 antagonist promotes antitumor effector/memory CD8 T cells in a poorly immunogenic tumor model. Cancer Immunol Res (2015) 3(2):149–60. doi:10.1158/2326-6066.CIR-14-0118
188. Dawicki W, Bertram EM, Sharpe AH, Watts TH. 4-1BB and OX40 act independently to facilitate robust CD8 and CD4 recall responses. J Immunol (2004) 173(10):5944–51. doi:10.4049/jimmunol.173.10.5944
189. Lee SW, Park Y, Song A, Cheroutre H, Kwon BS, Croft M. Functional dichotomy between OX40 and 4-1BB in modulating effector CD8 T cell responses. J Immunol (2006) 177(7):4464–72. doi:10.4049/jimmunol.177.7.4464
190. Lee SJ, Myers L, Muralimohan G, Dai J, Qiao Y, Li Z, et al. 4-1BB and OX40 dual costimulation synergistically stimulate primary specific CD8 T cells for robust effector function. J Immunol (2004) 173(5):3002–12. doi:10.4049/jimmunol.173.5.3002
191. Munks MW, Mourich DV, Mittler RS, Weinberg AD, Hill AB. 4-1BB and OX40 stimulation enhance CD8 and CD4 T-cell responses to a DNA prime, poxvirus boost vaccine. Immunology (2004) 112(4):559–66. doi:10.1111/j.1365-2567.2004.01917.x
192. So T, Lee SW, Croft M. Immune regulation and control of regulatory T cells by OX40 and 4-1BB. Cytokine Growth Factor Rev (2008) 19(3–4):253–62. doi:10.1016/j.cytogfr.2008.04.003
193. St Rose MC, Taylor RA, Bandyopadhyay S, Qui HZ, Hagymasi AT, Vella AT, et al. CD134/CD137 dual costimulation-elicited IFN-gamma maximizes effector T-cell function but limits Treg expansion. Immunol Cell Biol (2013) 91(2):173–83. doi:10.1038/icb.2012.74
194. Elpek KG, Yolcu ES, Franke DD, Lacelle C, Schabowsky RH, Shirwan H. Ex vivo expansion of CD4+CD25+FoxP3+ T regulatory cells based on synergy between IL-2 and 4-1BB signaling. J Immunol (2007) 179(11):7295–304. doi:10.4049/jimmunol.179.11.7295
195. Choi BK, Bae JS, Choi EM, Kang WJ, Sakaguchi S, Vinay DS, et al. 4-1BB-dependent inhibition of immunosuppression by activated CD4+CD25+ T cells. J Leukoc Biol (2004) 75(5):785–91. doi:10.1189/jlb.1003491
196. Shi W, Siemann DW. Augmented antitumor effects of radiation therapy by 4-1BB antibody (BMS-469492) treatment. Anticancer Res (2006) 26(5A):3445–53.
197. Wei H, Zhao L, Li W, Fan K, Qian W, Hou S, et al. Combinatorial PD-1 blockade and CD137 activation has therapeutic efficacy in murine cancer models and synergizes with cisplatin. PLoS One (2013) 8(12):e84927. doi:10.1371/journal.pone.0084927
198. Kim YH, Choi BK, Oh HS, Kang WJ, Mittler RS, Kwon BS. Mechanisms involved in synergistic anticancer effects of anti-4-1BB and cyclophosphamide therapy. Mol Cancer Ther (2009) 8(2):469–78. doi:10.1158/1535-7163.MCT-08-0993
199. Liu F, Fang C, Ye X, Lu Q, Yu DH, Zhang WW, et al. Anti-4-1BB scFv immunogene therapy and low dose cyclophosphamide exhibit a synergistic antitumor effect in established murine lung tumors. Cancer Biol Ther (2009) 8(8):707–13. doi:10.4161/cbt.8.8.7916
200. Strome SE, Martin B, Flies D, Tamada K, Chapoval AI, Sargent DJ, et al. Enhanced therapeutic potential of adoptive immunotherapy by in vitro CD28/4-1BB costimulation of tumor-reactive T cells against a poorly immunogenic, major histocompatibility complex class I-negative A9P melanoma. J Immunother (2000) 23(4):430–7. doi:10.1097/00002371-200007000-00006
201. Li Q, Iuchi T, Jure-Kunkel MN, Chang AE. Adjuvant effect of anti-4-1BB mAb administration in adoptive T cell therapy of cancer. Int J Biol Sci (2007) 3(7):455–62. doi:10.7150/ijbs.3.455
202. Hernandez-Chacon JA, Li Y, Wu RC, Bernatchez C, Wang Y, Weber JS, et al. Costimulation through the CD137/4-1BB pathway protects human melanoma tumor-infiltrating lymphocytes from activation-induced cell death and enhances antitumor effector function. J Immunother (2011) 34(3):236–50. doi:10.1097/CJI.0b013e318209e7ec
203. Choi BK, Lee SC, Lee MJ, Kim YH, Kim YW, Ryu KW, et al. 4-1BB-based isolation and expansion of CD8+ T cells specific for self-tumor and non-self-tumor antigens for adoptive T-cell therapy. J Immunother (2014) 37(4):225–36. doi:10.1097/CJI.0000000000000027
204. Goldstein MJ, Kohrt HE, Houot R, Varghese B, Lin JT, Swanson E, et al. Adoptive cell therapy for lymphoma with CD4 T cells depleted of CD137-expressing regulatory T cells. Cancer Res (2012) 72(5):1239–47. doi:10.1158/0008-5472.CAN-11-3375
205. Campana D, Schwarz H, Imai C. 4-1BB chimeric antigen receptors. Cancer J (2014) 20(2):134–40. doi:10.1097/PPO.0000000000000028
206. Imai C, Mihara K, Andreansky M, Nicholson IC, Pui CH, Geiger TL, et al. Chimeric receptors with 4-1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia (2004) 18(4):676–84. doi:10.1038/sj.leu.2403302
207. Till BG, Jensen MC, Wang J, Qian X, Gopal AK, Maloney DG, et al. CD20-specific adoptive immunotherapy for lymphoma using a chimeric antigen receptor with both CD28 and 4-1BB domains: pilot clinical trial results. Blood (2012) 119(17):3940–50. doi:10.1182/blood-2011-10-387969
Keywords: 4-1BB, immunotherapy, CD137, co-stimulation, combination therapy
Citation: Bartkowiak T and Curran MA (2015) 4-1BB agonists: multi-potent potentiators of tumor immunity. Front. Oncol. 5:117. doi: 10.3389/fonc.2015.00117
Received: 13 March 2015; Accepted: 11 May 2015;
Published: 08 June 2015
Edited by:
Emanuela Romano, Centre hospitalier universitaire vaudois, SwitzerlandReviewed by:
Dennis O. Adeegbe, Harvard Medical School, USACopyright: © 2015 Bartkowiak and Curran. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael A. Curran, Department of Immunology, MD Anderson Cancer Center, 1515 Holcombe Blvd., Unit 901, Houston, TX 77030, USA,bWN1cnJhbkBtZGFuZGVyc29uLm9yZw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.