
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
OPINION article
Front. Oncol. , 28 April 2015
Sec. Neuro-Oncology and Neurosurgical Oncology
Volume 5 - 2015 | https://doi.org/10.3389/fonc.2015.00099
This article is part of the Research Topic Immunotherapy for tumor in the brain: Insights from – and for – other tumor sites View all 9 articles
While historically viewed as an immune-privileged area fully isolated from the immune system, the central nervous system (CNS) is now appreciated to maintain dynamic bi-directional communication with the immune system across the blood–brain barrier (BBB) (1, 2). In no setting can this communication be more urgent that acute CNS infection – a dampened or delayed host response could allow an invading virus or bacterium to gain a foothold within the brain, while over-exuberant or protracted inflammation might cause substantial collateral damage to sensitive and non-renewable cells such as neurons. In this opinion piece, we compare host immunity against one prototype virus and one prototype bacterium known to cause disease either outside or within the CNS. Allowing for some variability in disease pathogenesis, and leaving aside complex issues related to chronic intrauterine or neonatal infections, we argue that antimicrobial host responses in both CNS and non-CNS tissue compartments of adult hosts who acquire these infections exhibit many more similarities than differences. In this setting, the concept of CNS immune privilege seems antiquated.
It is now accepted that there is a need for constant immune surveillance of the adult CNS as part of normal host defense, acknowledging that simultaneous mechanisms must keep local CNS inflammation strongly in check (1). Indeed, blockade of normal lymphocyte homing through the CNS can occasionally trigger local virus recrudescence (3), and low numbers of lymphocytes and antigen-presenting dendritic cells (DC) are found in perivascular spaces of the normal brain (4). For infectious particles that unexpectedly gain access to the CNS, some pathogen clearance occurs via bulk flow along paravenous routes by means of a process that depends on astrocytic water channels (4). This so-called “glymphatic system” of the brain ultimately carries antigenic material toward a specific group of large-caliber veins that drain to deep cervical lymph nodes (CLN) (5, 6). The CLN are increasingly appreciated as an important site where antigen-specific immune responses bound for the CNS are generated (7). Blood-borne pathogens, on the other hand, are mostly carried to the spleen where adaptive immunity occurs. Immune cells then migrate back to the CNS under the influence of chemokine gradients induced by infection, and bind and traverse the BBB via the actions of specific adhesion receptors and degradative enzymes.
Streptococcus pneumoniae is an important pathogen because it is the main cause of both community-acquired pneumonia and meningitis induced by a bacterial pathogen in otherwise healthy older children and adults. Much has also been learned about host immune responses elicited by pneumococcal pneumonia or meningitis using mouse models (8, 9). These infections develop in both mice and humans following pathogen inhalation and subsequent local tissue invasion (Table 1). Tissue-resident innate immune pathways are activated and host immunity is mobilized within hours. Outcome is determined over days to a few weeks. Morbidity and mortality, even in previously healthy hosts, is substantial, as many of the same mediators that have antibacterial activities also cause direct cellular damage (proteins, membrane lipids, DNA). Current polyvalent vaccines are effective in preventing both forms of invasive disease.
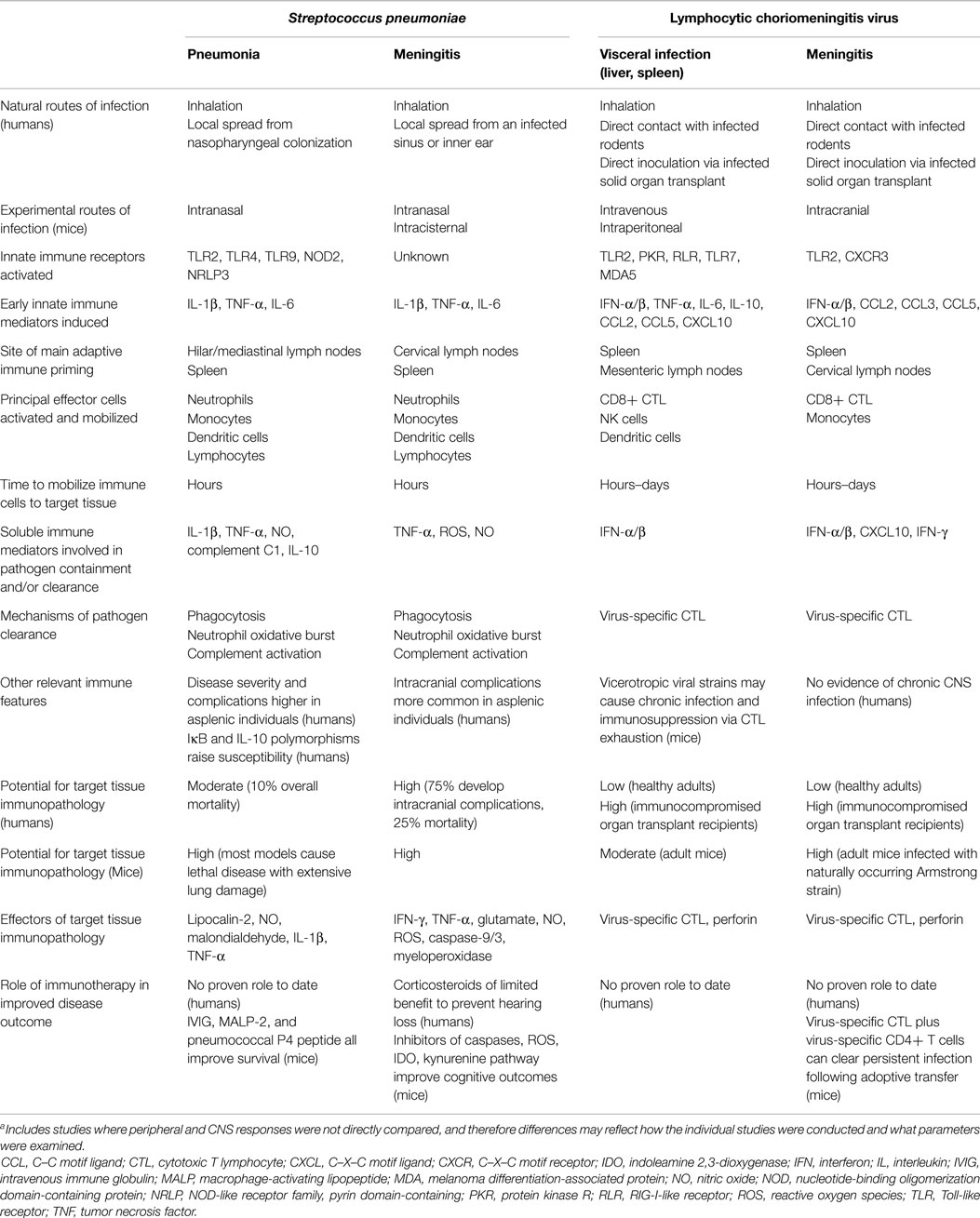
Table 1. Pathogenesis and host responses elicited during a prototype bacterial (Streptococcus pneumoniae) or viral (lymphocytic choriomeningitis virus) infection of adult hosts when localized in either the periphery or the CNS [data adapted from Ref. (10–15)]a.
Lymphocytic choriomeningitis virus (LCMV) is an arenavirus to which both mice and humans are susceptible and that causes varying combinations of visceral (hepatitis, pancreatitis, myocarditis) and/or CNS (meningitis, encephalitis) involvement in adult hosts. Murine LCMV infection has served as a prototype experimental system to study immunity to viruses for many years (10). This pathogen gets inhaled or directly inoculated into susceptible murine and human recipients (Table 1). Both innate and adaptive immune pathways are mobilized and disease can last several weeks. Healthy adults generally recover reasonably well, although the disease is more fulminant in immunocompromised hosts. In mice, the same virus-specific cytotoxic T cell (CTL) response that clears virus from infected tissues also causes tissue immunopathology. For acute CNS infection (choriomeningitis) in the setting of impaired CTL activity, survival is the trade-off for poor viral clearance.
The host responses provoked by these two naturally occurring pathogens are used here to compare how the immune system recognizes and responds to the same challenge in the periphery and the CNS (Table 1). The availability of mouse models for both pathogens that can be manipulated to cause infection either outside or within the CNS allows for the identification of immune determinants that directly influence disease outcome. It seems notable that many more similarities than differences in host immunity are seen, and that there is little evidence suggesting immune recognition is delayed or immune responses dampened for those infections localized primarily to the CNS compartment. Outcome differences for infections occurring at the two sites are likely driven by mechanisms independent of the host response.
Both the afferent and efferent arms of the immune system are efficiently activated in the context of either pneumococcal or LCMV infection, and the kinetics, magnitude, and composition of immune responses elicited in adult hosts appear remarkably similar when either a visceral organ or the CNS is the main target of disease (Table 1). Acute CNS infections generally result in less favorable outcomes than those localized to the periphery, but this is more likely explained by the exquisite sensitivity of the brain to cellular damage rather than any delayed immune recognition behind the BBB. This comparison leads us to suggest that the concept of CNS immune privilege in the adult host seems somewhat obsolete in the setting of acute CNS infection.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Huber AK, Duncker PC, Irani DN. Immune responses to non-tumor antigens in the central nervous system. Front Oncol (2014) 4:328. doi: 10.3389/fonc.2014.00328
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
2. Banks WA. The blood-brain barrier in neuroimmunology: tales of separation and assimilation. Brain Behav Immun (2014) 44:1–8. doi:10.1016/j.bbi.2014.08.007
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
3. Jelcic I, Jelcic I, Faigle W, Sospedra W, Martin R. Immunology of progressive multifocal leukoencephalopathy. J Neurovirol (2015). doi:10.1007/s13365-014-0294-y
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
4. Ransohoff RA, Engelhardt B. The anatomical and cellular basis of immune surveillance in the central nervous system. Nat Rev Immunol (2012) 12(9):623–35. doi:10.1038/nri3265
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
5. Illiff JJ, Nedergaard M. Is there a cerebral lymphatic system? Stroke (2013) 44(Suppl 1):S93–5. doi:10.1161/STROKEAHA.112.678698
6. Cserr HF, Harling-Berg CJ, Knopf PM. Drainage of brain extracellular fluid into blood and deep cervical lymph and its immunological significance. Brain Pathol (1992) 2:269–76. doi:10.1111/j.1750-3639.1992.tb00703.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
7. Mohammad MG, Tsai VW, Ruitenberg MJ, Hassanpour M, Li H, Hart PH, et al. Immune cell trafficking from the brain maintains CNS immune tolerance. J Clin Invest (2014) 124(3):1228–41. doi:10.1172/JCI71544
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
8. Warszawska JM, Gawish R, Sharif O, Sigel S, Doninger B, Lakovits K, et al. Lipocalin 2 deactivates macrophages and worsens pneumococcal pneumonia outcomes. J Clin Invest (2013) 123(3):3363–72. doi:10.1172/JCI67911
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
9. Too LK, Ball HJ, McGregor IS, Hunt NH. The pro-inflammatory cytokine interferon-gamma is an important driver of neuropathology and behavioral sequelae in experimental pneumococcal meningitis. Brain Behav Immun (2014) 40:252–68. doi:10.1016/j.bbi.2014.02.020
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
10. Zhou X, Ramachandran S, Mann M, Popkin DL. Role of lymphocytic choriomeningitis virus (LCMV) in understanding viral immunology: past, present and future. Viruses (2012) 4(11):2650–69. doi:10.3390/v4112650
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
11. Kastenbauer S, Pfister HW. Pneumococcal meningitis in adults: spectrum of complications and prognostic factors in a series of 87 cases. Brain (2003) 126(Pt 5):1015–25. doi:10.1093/brain/awg113
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
12. Calbo E, Garau J. Of mice and men: innate immunity in pneumococcal pneumonia. Int J Antimicrob Agents (2010) 35:107–13. doi:10.1016/j.ijantimicag.2009.10.002
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
13. Barichello T, Collodel A, Generoso JS, Simoes LR, Moreira AP, Ceretta RA, et al. Targets for adjunctive therapy in pneumococcal meningitis. J Neuroimmunol (2015) 278:262–70. doi:10.1016/j.jneuroim.2014.11.015
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
14. Kang SS, McGavern DB. Lymphocytic choriomeningitis infection of the central nervous system. Front Biosci (2008) 13:4529–43. doi:10.2741/3021
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Keywords: central nervous system, infection, host immunity, viruses, bacteria
Citation: Huber AK and Irani DN (2015) Is the concept of central nervous system immune privilege irrelevant in the setting of acute infection? Front. Oncol. 5:99. doi: 10.3389/fonc.2015.00099
Received: 20 March 2015; Accepted: 13 April 2015;
Published: 28 April 2015
Edited by:
Lois A. Lampson, Harvard Medical School, USAReviewed by:
Thomas E. Lane, University of Utah, USACopyright: © 2015 Huber and Irani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: David N. Irani,ZGF2aWRpcmFAbWVkLnVtaWNoLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.