Prom1 Function in Development, Intestinal Inflammation, and Intestinal Tumorigenesis
A commentary on
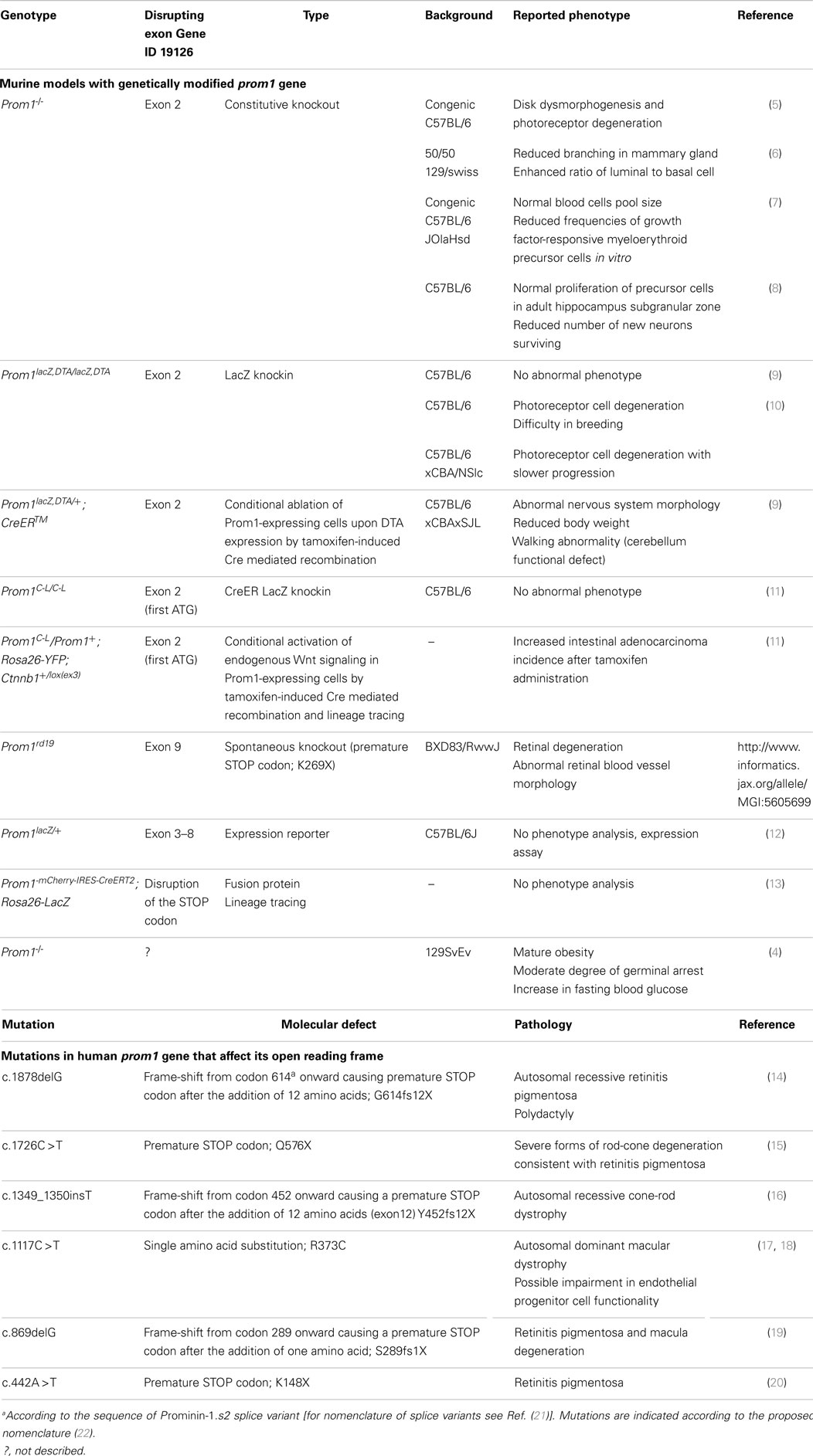
A large body of publications mentioning prominin-1 (Prom1, CD133) is related to its use as a stem and cancer stem cell marker (1). Besides progenitor cells, its expression is widespread throughout terminally differentiated cells as those found in glandular organs (e.g., pancreas) and retina (2). Yet, the amino acid sequence of Prom1 didn’t reveal any motif or potential enzymatic activity that could explain its molecular function (3). Nonetheless, its implication in cellular physiology has been addressed in several cellular systems and animal models. In particular, the effect of Prom1 gene ablation has been investigated in different tissues and organs (Table 1, top). In the above-mentioned article (4), erroneously qualified by its authors as “the first study to provide an in-depth evaluation of the role and function of Prom1”, several features of a new Prom1-/- mouse were highlighted that were not described in previous Prom1-deficient murine models, raising questions about the influence of the genetic backgrounds on the penetrance and variable expressivity. To understand these issues, it is important to have an accurate view on the implication of Prom1 in molecular processes leading to tissue homeostasis and diseases. Unfortunately, valuable information that could potentially enlighten some of the data reported was omitted (4). Moreover, the genetic construct and the characterization of their model (protein expression) were missing, thus preventing direct comparison with previous works. We propose to provide the readers of Frontiers in Oncology with a larger context for the observations of Karim et al. and the potential functions of Prom1.
In humans, several mutations in PROM1 gene affecting the open reading frame were found to be associated with retinitis pigmentosa, macular degeneration, and cone-rod dystrophy (Table 1, bottom). Despite the extended expression of PROM1, little phenotypical effects were observed beyond the visual system. Extensive clinical analysis of patients carrying PROM1 R373C mutation suggested that endothelial function could be affected despite apparently normal levels of endothelial progenitor cells (23). Neuro-imaging revealed many small lesions in the cerebral white matter in three patients. The expression of Prom1 in myelinating oligodendrocytes might be relevant in this context (24). Some carriers of the R373C mutation showed memory disturbance and impairment in measured executive functions, suggesting that the lack of functional PROM1 may not solely affect photoreceptor cells. Yet, the penetrance was variable, calling for larger studies to confirm these findings (23).
In the first description of Prom-1-/- mice, they were reported as viable and fertile, with a normal lifespan and no obvious abnormalities upon macroscopic inspection and histological analysis of various organs other than a progressive photoreceptor degeneration leading to complete loss of vision (5), thus constituting a mouse model of the human diseases. These observations have been recently confirmed in an independent Prom1-deficient murine model that also revealed that this degeneration was light dependent, consistent with what is observed in Stargardt’s disease patients (10). Interestingly, variations in the genetic background influenced the progression of photoreceptor cell degeneration (10). In addition to engineered animal models, a spontaneous knockout mouse (Prom1rd19) carrying single point mutation in Prom1 gene was reported with retinal degeneration and abnormal retinal blood vessel morphology (Table 1). Moreover, using a transgenic knock-in mouse carrying the human dominant R373C mutation, Yang et al. could demonstrate the structural involvement of Prom1 in photoreceptor morphogenesis (17). As a cholesterol-binding protein associated with membrane microdomains, Prom1 could provide a proper membrane lipid composition and synchronize various steps in the outer segment biogenesis. Whether the new mouse line of Karim et al. also suffers visual impairment is not known but it appears to present several features not yet described (4).
Notably, they noted compromised spermatogenesis in “some Prom1-/- males” (4). It would be interesting to know what proportion is affected, since Prom1 is expressed in the male reproductive tract of mouse and human (25, 26), and is detected in mouse spermatozoa found in the testes and the epididymis (25, 27, 28). Similarly, the mature obesity in Prom1-/- mice reported by Karim et al. based on ≈15% increase in body weight over a 13-week period compared to wild type is different from earlier studies (Table 1), and may reflect the influence of genetic background on the permeability to metabolic disorders. In fact, in a conditional ablation model, tamoxifen-induced ablation of Prom1-expressing cells caused body weight loss (9). Studies have pointed to the potential involvement of Prom1 in cellular metabolism in rat myotubes and mouse pancreatic islets with contrasting findings (29, 30), and an increase in Prom1 expression in young mice with induced obesity has been reported (31). In other respects, PROM1 was shown to promote glucose uptake in a human hepatocellular carcinoma cell line (32). Karim et al. also indicated an increase in blood glucose levels and therefore suggested a link between Prom1 and pancreatic function (4). Although the metabolic features of Prom1-/- mice need to be more thoroughly investigated (e.g., insulinemia, gain in adipose tissue, glucose tolerance), it is worth mentioning that Prom1 labels fetal mouse and human islet progenitor cells (33, 34) and was used for isolation of pancreatic ductal progenitor cells (35). PROM1 is expressed in ductal cells of the exocrine component of adult pancreas (36–38).
The novel Prom1-/- mouse was maintained in 129SvEv, a less phenotypically characterized background than the frequent C57BL/6 and might be more permissive to the expression of these characters. Therefore, whether the differences in various Prom1-deficient mice are related to strain, the different background (39), environmental factors, or the construct would require further studies. Yet, the various cellular activities with which Prom1 has been associated are in line with our early suggestion that Prom1 can act as regulator in the organization and functionality of plasmalemma protrusions (40). Hence, its absence may cause alterations in cellular adhesion and/or signaling pathways. Although the phenotypic consequences of Prom1 defect seem to be limited, despite a broad tissue expression, to a restricted number of organs especially those devoid of prominin-2 (e.g., retina and testes) (3, 27), a careful examination over longer periods of time may uncover the expression of subtle changes in functionality in specific tissues.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Grant support: DFG (SFB655-B3); SMWK (4-7531.60/29/31).
References
1. Grosse-Gehling P, Fargeas CA, Dittfeld C, Garbe Y, Alison MR, Corbeil D, et al. CD133 as a biomarker for putative cancer stem cells in solid tumours: limitations, problems and challenges. J Pathol (2013) 229(3):355–78. doi:10.1002/path.4086
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
2. Karbanová J, Missol-Kolka E, Fonseca AV, Lorra C, Janich P, Hollerova H, et al. The stem cell marker CD133 (prominin-1) is expressed in various human glandular epithelia. J Histochem Cytochem (2008) 56(11):977–93. doi:10.1369/jhc.2008.951897
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
3. Fargeas CA, Florek M, Huttner WB, Corbeil D. Characterization of prominin-2, a new member of the prominin family of pentaspan membrane glycoproteins. J Biol Chem (2003) 278(10):8586–96. doi:10.1074/jbc.M210640200
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
4. Karim BO, Rhee KJ, Liu G, Yun K, Brant SR. Prom1 function in development, intestinal inflammation, and intestinal tumorigenesis. Front Oncol (2014) 4:323. doi:10.3389/fonc.2014.00323
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
5. Zacchigna S, Oh H, Wilsch-Bräuninger M, Missol-Kolka E, Jászai J, Jansen S, et al. Loss of the cholesterol-binding protein prominin-1/CD133 causes disk dysmorphogenesis and photoreceptor degeneration. J Neurosci (2009) 29(7):2297–308. doi:10.1523/JNEUROSCI.2034-08.2009
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
6. Anderson LH, Boulanger CA, Smith GH, Carmeliet P, Watson CJ. Stem cell marker prominin-1 regulates branching morphogenesis, but not regenerative capacity, in the mammary gland. Dev Dyn (2011) 240(3):674–81. doi:10.1002/dvdy.22539
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
7. Arndt K, Grinenko T, Mende N, Reichert D, Portz M, Ripich T, et al. CD133 is a modifier of hematopoietic progenitor frequencies but is dispensable for the maintenance of mouse hematopoietic stem cells. Proc Natl Acad Sci U S A (2013) 110(14):5582–7. doi:10.1073/pnas.1215438110
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
8. Walker TL, Wierick A, Sykes AM, Waldau B, Corbeil D, Carmeliet P, et al. Prominin-1 allows prospective isolation of neural stem cells from the adult murine hippocampus. J Neurosci (2013) 33(7):3010–24. doi:10.1523/JNEUROSCI.3363-12.2013
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
9. Nishide K, Nakatani Y, Kiyonari H, Kondo T. Glioblastoma formation from cell population depleted of prominin1-expressing cells. PLoS One (2009) 4(8):e6869. doi:10.1371/journal.pone.0006869
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
10. Dellett M, Sasai N, Nishide K, Becker S, Papadaki V, Limb GA, et al. Genetic background and light-dependent progression of photoreceptor cell degeneration in prominin-1 knockout mice. Invest Ophthalmol Vis Sci (2015) 56(1):164–76. doi:10.1167/iovs.14-15479
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
11. Zhu L, Gibson P, Currle DS, Tong Y, Richardson RJ, Bayazitov IT, et al. Prominin 1 marks intestinal stem cells that are susceptible to neoplastic transformation. Nature (2009) 457(7229):603–7. doi:10.1038/nature07589
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
12. Shmelkov SV, Butler JM, Hooper AT, Hormigo A, Kushner J, Milde T, et al. CD133 expression is not restricted to stem cells, and both CD133+ and CD133- metastatic colon cancer cells initiate tumors. J Clin Invest (2008) 118(6):2111–20. doi:10.1172/JCI34401
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
13. Snippert HJ, van Es JH, van den Born M, Begthel H, Stange DE, Barker N, et al. Prominin-1/CD133 marks stem cells and early progenitors in mouse small intestine. Gastroenterology (2009) 136(7):2187–94 e1. doi:10.1053/j.gastro.2009.03.002
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
14. Maw MA, Corbeil D, Koch J, Hellwig A, Wilson-Wheeler JC, Bridges RJ, et al. A frameshift mutation in prominin (mouse)-like 1 causes human retinal degeneration. Hum Mol Genet (2000) 9(1):27–34. doi:10.1093/hmg/9.1.27
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
15. Zhang Q, Zulfiqar F, Xiao X, Riazuddin SA, Ahmad Z, Caruso R, et al. Severe retinitis pigmentosa mapped to 4p15 and associated with a novel mutation in the PROM1 gene. Hum Genet (2007) 122(3–4):293–9. doi:10.1007/s00439-007-0395-2
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
16. Pras E, Abu A, Rotenstreich Y, Avni I, Reish O, Morad Y, et al. Cone-rod dystrophy and a frameshift mutation in the PROM1 gene. Mol Vis (2009) 15:1709–16.
17. Yang Z, Chen Y, Lillo C, Chien J, Yu Z, Michaelides M, et al. Mutant prominin 1 found in patients with macular degeneration disrupts photoreceptor disk morphogenesis in mice. J Clin Invest (2008) 118(8):2908–16. doi:10.1172/JCI35891
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
18. Michaelides M, Gaillard MC, Escher P, Tiab L, Bedell M, Borruat FX, et al. The PROM1 mutation p.R373C causes an autosomal dominant bull’s eye maculopathy associated with rod, rod-cone, and macular dystrophy. Invest Ophthalmol Vis Sci (2010) 51(9):4771–80. doi:10.1167/iovs.09-4561
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
19. Permanyer J, Navarro R, Friedman J, Pomares E, Castro-Navarro J, Marfany G, et al. Autosomal recessive retinitis pigmentosa with early macular affectation caused by premature truncation in PROM1. Invest Ophthalmol Vis Sci (2010) 51(5):2656–63. doi:10.1167/iovs.09-4857
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
20. Jinda W, Taylor TD, Suzuki Y, Thongnoppakhun W, Limwongse C, Lertrit P, et al. Whole exome sequencing in Thai patients with retinitis pigmentosa reveals novel mutations in six genes. Invest Ophthalmol Vis Sci (2014) 55(4):2259–68. doi:10.1167/iovs.13-13567
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
21. Fargeas CA, Huttner WB, Corbeil D. Nomenclature of prominin-1 (CD133) splice variants – an update. Tissue Antigens (2007) 69(6):602–6. doi:10.1111/j.1399-0039.2007.00825.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
22. Antonarakis SE. Recommendations for a nomenclature system for human gene mutations. Nomenclature working group. Hum Mutat (1998) 11(1):1–3. doi:10.1002/(SICI)1098-1004(1998)11:1<1::AID-HUMU1>3.0.CO;2-O
23. Arrigoni FI, Matarin M, Thompson PJ, Michaelides M, McClements ME, Redmond E, et al. Extended extraocular phenotype of PROM1 mutation in kindreds with known autosomal dominant macular dystrophy. Eur J Hum Genet (2011) 19(2):131–7. doi:10.1038/ejhg.2010.147
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
24. Corbeil D, Joester A, Fargeas CA, Jászai J, Garwood J, Hellwig A, et al. Expression of distinct splice variants of the stem cell marker prominin-1 (CD133) in glial cells. Glia (2009) 57(8):860–74. doi:10.1002/glia.20812
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
25. Fargeas CA, Joester A, Missol-Kolka E, Hellwig A, Huttner WB, Corbeil D. Identification of novel prominin-1/CD133 splice variants with alternative C-termini and their expression in epididymis and testis. J Cell Sci (2004) 117(Pt 18):4301–11. doi:10.1242/jcs.01315
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
26. Gashaw I, Dushaj O, Behr R, Biermann K, Brehm R, Rubben H, et al. Novel germ cell markers characterize testicular seminoma and fetal testis. Mol Hum Reprod (2007) 13(10):721–7. doi:10.1093/molehr/gam059
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
27. Jászai J, Fargeas CA, Haase M, Farkas LM, Huttner WB, Corbeil D. Robust expression of prominin-2 all along the adult male reproductive system and urinary bladder. Histochem Cell Biol (2008) 130(4):749–59. doi:10.1007/s00418-008-0445-4
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
28. Asano A, Nelson JL, Zhang S, Travis AJ. Characterization of the proteomes associating with three distinct membrane raft sub-types in murine sperm. Proteomics (2010) 10(19):3494–505. doi:10.1002/pmic.201000002
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
29. Yang C, Yang Y, Gupta N, Liu X, He A, Liu L, et al. Pentaspan membrane glycoprotein, prominin-1, is involved in glucose metabolism and cytoskeleton alteration. Biochemistry (Mosc) (2007) 72(8):854–62. doi:10.1134/S000629790708007X
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
30. Singh H, Farouk M, Bose BB, Singh P. Novel genes underlying beta cell survival in metabolic stress. Bioinformation (2013) 9(1):37–41. doi:10.6026/97320630009037
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
31. Thole AA, Rodrigues-Cunha AC, Carvalho SN, Garcia-Souza EP, Cortez E, Stumbo AC, et al. Progenitor cells and TNF-alpha involvement during morphological changes in pancreatic islets of obese mice. Tissue Cell (2012) 44(4):238–48. doi:10.1016/j.tice.2012.04.001
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
32. Chen H, Luo Z, Dong L, Tan Y, Yang J, Feng G, et al. CD133/prominin-1-mediated autophagy and glucose uptake beneficial for hepatoma cell survival. PLoS One (2013) 8(2):e56878. doi:10.1371/journal.pone.0056878
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
33. Sugiyama T, Rodriguez RT, McLean GW, Kim SK. Conserved markers of fetal pancreatic epithelium permit prospective isolation of islet progenitor cells by FACS. Proc Natl Acad Sci U S A (2007) 104(1):175–80. doi:10.1073/pnas.0609490104
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
34. Koblas T, Pektorova L, Zacharovova K, Berkova Z, Girman P, Dovolilova E, et al. Differentiation of CD133-positive pancreatic cells into insulin-producing islet-like cell clusters. Transplant Proc (2008) 40(2):415–8. doi:10.1016/j.transproceed.2008.02.017
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
35. Oshima Y, Suzuki A, Kawashimo K, Ishikawa M, Ohkohchi N, Taniguchi H. Isolation of mouse pancreatic ductal progenitor cells expressing CD133 and c-Met by flow cytometric cell sorting. Gastroenterology (2007) 132(2):720–32. doi:10.1053/j.gastro.2006.11.027
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
36. Lardon J, Corbeil D, Huttner WB, Ling Z, Bouwens L. Stem cell marker prominin-1/AC133 is expressed in duct cells of the adult human pancreas. Pancreas (2008) 36(1):e1–6. doi:10.1097/mpa.0b013e318149f2dc
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
37. Immervoll H, Hoem D, Sakariassen PO, Steffensen OJ, Molven A. Expression of the “stem cell marker” CD133 in pancreas and pancreatic ductal adenocarcinomas. BMC Cancer (2008) 8:48. doi:10.1186/1471-2407-8-48
38. Shimizu K, Itoh T, Shimizu M, Ku Y, Hori Y. CD133 expression pattern distinguishes intraductal papillary mucinous neoplasms from ductal adenocarcinomas of the pancreas. Pancreas (2009) 38(8):e207–14. doi:10.1097/MPA.0b013e3181bb5037
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
39. Montagutelli X. Effect of the genetic background on the phenotype of mouse mutations. J Am Soc Nephrol (2000) 11(Suppl 16):S101–5.
40. Corbeil D, Röper K, Fargeas CA, Joester A, Huttner WB. Prominin: a story of cholesterol, plasma membrane protrusions and human pathology. Traffic (2001) 2(2):82–91. doi:10.1034/j.1600-0854.2001.020202.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Keywords: cancer, CD133, prominin-1, murine model, retinal degeneration, testis
Citation: Fargeas CA, Büttner E and Corbeil D (2015) Commentary: “Prom1 function in development, intestinal inflammation, and intestinal tumorigenesis”. Front. Oncol. 5:91. doi: 10.3389/fonc.2015.00091
Received: 01 February 2015; Accepted: 30 March 2015;
Published online: 21 April 2015.
Edited by:
Rupert Langer, University of Bern, SwitzerlandReviewed by:
Yi Zhong, Kyoto University, JapanCopyright: © 2015 Fargeas, Büttner and Corbeil. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: corbeil@biotec.tu-dresden.de
 Christine A. Fargeas
Christine A. Fargeas Edgar Büttner
Edgar Büttner Denis Corbeil
Denis Corbeil