- Nuclear Medicine Research Center, Mashhad University of Medical Sciences (MUMS), Mashhad, Iran
A 61-year-old woman with a history of metastatic follicular thyroid carcinoma became radioiodine-refractory following two doses of radioiodine (RAI) therapy (cumulative = 230 mCi). While no RAI-avid lesion was noticed in the last post-ablation whole-body radioiodine scan (WBIS), she reported sternal pain, which was accompanied by rapidly rising thyroglobulin levels. 18F-FDG and 68Ga-DOTA-TATE PET/CT was performed, showing metastatic pulmonary nodules and a lytic sternal lesion with acceptable avidity (i.e. uptake ≥ liver). Following four cycles of peptide receptor radionuclide therapy (PRRT) with 177Lu-DOTA-TATE, the thyroglobulin levels dropped significantly, and the sternal pain was partially alleviated. Despite only experiencing grade I thrombocytopenia, the treating physician decided to discontinue PRRT and repeat the diagnostic WBIS. Surprisingly, the scan revealed significantly increased tracer uptake in the sternum. The patient received 200 mCi 131I, and WBIS showed increased RAI uptake in all pulmonary nodules as well as bone metastases. We report a case of RAI-refractory thyroid carcinoma with a somatostatin-receptor expression that re-differentiated and gained significant RAI uptake capacity after PRRT.
Introduction
The most common endocrine malignancy, differentiated thyroid cancer (DTC), is usually associated with a good prognosis. However, about 10% of DTCs are metastatic, of which two-thirds lose their radioiodine (RAI) uptake and finally become RAI-refractory (RAIR-DTC), heralding a worse prognosis (1, 2). Multiple re-differentiation strategies have been proposed, including tyrosine kinase inhibitors (TKI) and retinoic acid administration (3).
A proportion of RAIR-DTCs also fall into the thyroglobulin (Tg)-elevated negative iodine scan (TENIS) category. These patients are candidates for a variety of imaging modalities (4). While FDG PET/CT is considered the primary imaging modality option in TENIS syndrome patients, imaging with somatostatin analogues (SSTR) is also noteworthy since it provides a chance for peptide receptor radionuclide therapy (PRRT) (4). Although the experience of PRRT in differentiated thyroid cancer is still in its infancy, studies have shown disease control rates similar to TKIs (5, 6). We report a case of TENIS syndrome regaining its RAI uptake following PRRT.
Case report
A 61-year-old woman with a history of follicular thyroid carcinoma (initial TNM: T3aN0bM0; restaging TNM: T3aN1aM1) was referred to our nuclear medicine department following a total thyroidectomy. She was under active follow-up for 11 years. The first stimulated serum Tg and anti-Tg antibody levels were 0.01 and 33 ng/ml, respectively. She received 30 mCi of I-131, and the post-ablation WBIS showed a post-surgical thyroid remnant (Figure 1A). During follow-up, she developed TENIS syndrome, and the stimulated Tg levels reached 351 ng/ml (Tg doubling time = 26 months) with negative diagnostic WBIS. After five years of follow-up, she complained of sternal pain, swelling, and elevated serum suppressed-Tg levels reaching >500 ng/ml. The lung CT correlation was suggestive of a lytic sternal lesion and bilateral pulmonary metastases (Figures 1B,C). Therefore, another dose of I-131 (200 mCi) was applied 13 months following a negative diagnostic WBIS, and the metastatic foci revealed no iodine avidity in the post-ablation scan (Figure 1D). Thus, the patient underwent external beam radiation therapy (EBRT) for the sternum. Subsequently, 18F-FDG PET/CT was done because of significantly rising serum-suppressed Tg (i.e., >30,000 ng/ml). Also, for evaluation of SSTR expression, 68Ga-DOTA-TATE PET/CT was performed. These two scans revealed multiple pulmonary nodules and a sternal lytic lesion with acceptable uptake of both radiotracers (Figure 2). After being discussed in the tumor board meeting, the patient was deemed eligible for PRRT, and four cycles of 177Lu-DOTA-TATE were administered (cumulative = 810 mCi). Post-treatment PRRT SPECT/CT depicted increased radiotracer uptake in the aforementioned areas (Figure 3). Following PRRT, clinical symptoms improved, and serum suppressed-Tg level was reduced to 1884 ng/ml. During PRRT, the patient experienced grade I thrombocytopenia according to the 5th version of the common terminology criteria for adverse events. On this account, the treating physician decided to withhold additional cycles requesting a diagnostic WBIS. Surprisingly, the WBIS showed significant iodine avidity in the sternal lesion (Figures 4A–D). The patient received a trial of 200 mCi 131I, and the post-treatment whole-body scan confirmed the regaining RAI uptake in all of the metastatic foci found in the SSTR PET/CT study (Figures 4E–H). The interval between this WBIS and the previous negative study was 70 months.
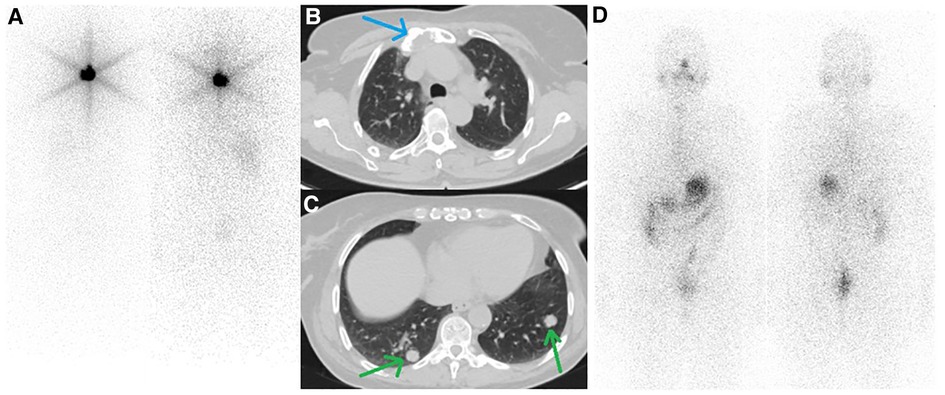
Figure 1. (A) First whole-body iodine scan after administration of 30 mCi of 131I showed post-surgical thyroid remnant. (B, C) follow-up CT scan from the thoracic region showed a lytic lesion in the sternum (blue arrow) along with multiple bilateral pulmonary nodules (green arrows). (D) The second post-treatment whole-body iodine scan after administration of 200 mCi 131I revealed no radioiodine-avidity in the tumoral lesion.
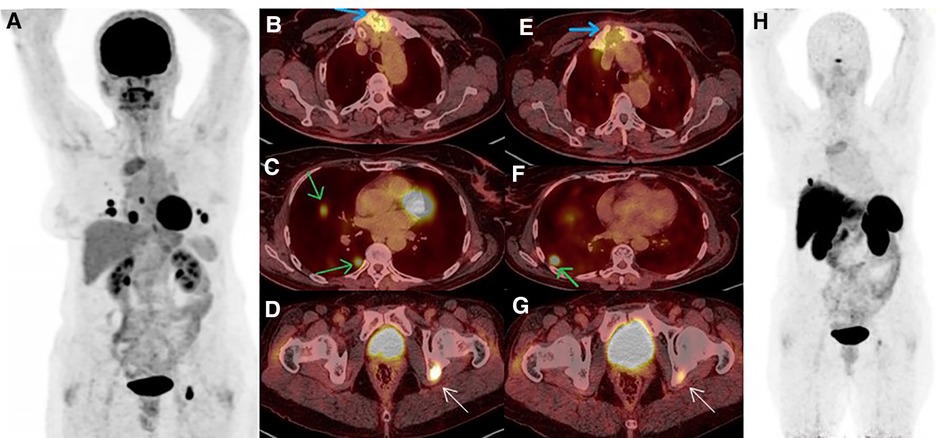
Figure 2. (A–D) 18F-FDG PET/CT MIP revealed increased tracer uptake in pulmonary nodules, sternal, and acetabular lesions (green, blue and white arrows, respectively). (E–H) A few days later, 68Ga-DOTA-TATE PET/CT confirmed somatostatin-receptor (SSTR) uptake in the above-mentioned areas, albeit most of the pulmonary nodules were non-SSTR-avid.
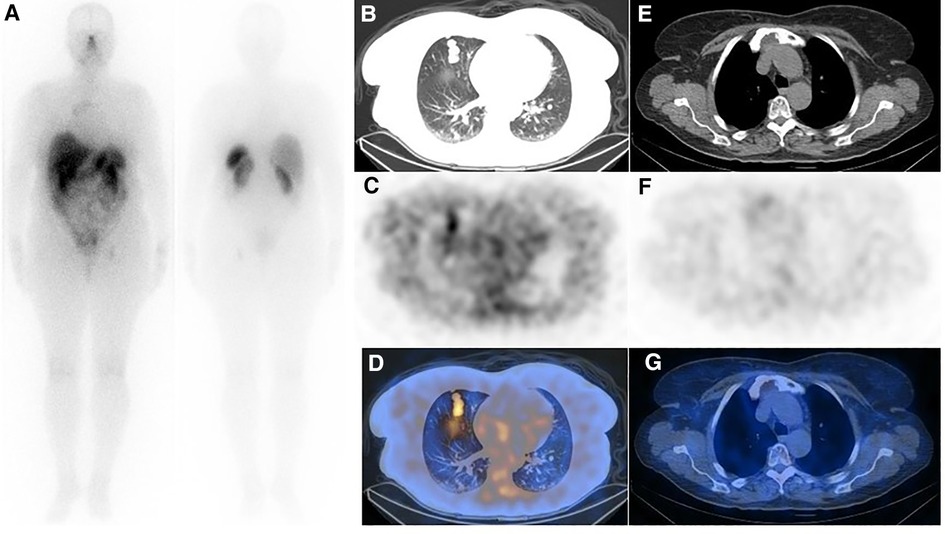
Figure 3. (A) Post-treatment whole-body imaging following administration of 200 mCi of 177Lu-DOTA-TATE (the second cycle is shown as an example). (B–D) Post-treatment SPECT/CT correlation showed moderate SSTR avidity in the lung metastases (E–G), while the uptake in the sternal lesion was unremarkable. Although interesting, the discordance of uptake in the pulmonary nodules and sternum between SSTR-targeted imaging (refer to figure 2) and therapy is confusing, and the underlying mechanism is unknown to us.
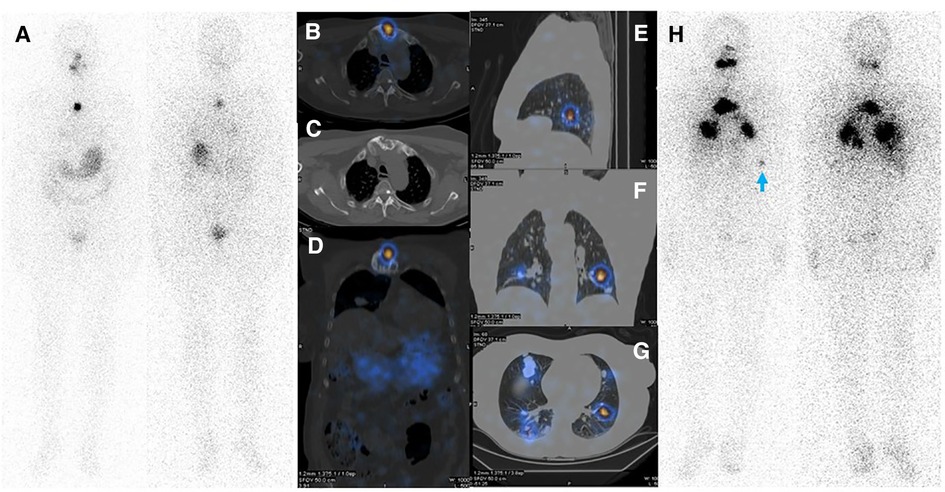
Figure 4. (A–D) Diagnostic WBIS following administration of 3 mCi of 131I showing regained sternal uptake. (E–H) Whole body WBIS after administration of 200 mCi of 131I depicted increased uptake in pulmonary metastases and a sternal lesion. Considering the appearance of new lesions as well as the increasing size of the main pulmonary nodules in the right middle lobe, disease progression is evident. Faint activity is also apparent in the left acetabulum (red arrowhead).
Discussion
RAI therapy is the treatment of choice for differentiated thyroid carcinoma after total thyroidectomy, with about two-thirds of patients demonstrating appropriate radioiodine accumulation in metastatic sites (1). Nearly 30% of patients with progressive metastatic differentiated thyroid carcinoma develop a radioiodine-refractory disease (7). Treatment options are limited in these patients (8). In asymptomatic patients as well as in stable, or minimally progressive disease, monitoring during TSH suppression is recommended (9).
Interventions should be done only in symptomatic patients in order to decrease morbidity. Surgery, radiation therapy, PRRT or TKIs are alternative treatment options. However, because of the severe adverse effects of TKIs, they should be used cautiously (4). In patients with radioiodine-refractory thyroid carcinoma, PRRT is a therapeutic option with minimal toxicity and probably survival benefits (10, 11).
A comprehensive systematic review investigated the safety and efficacy of PRRT in treating progressive RAIR-DTC and metastatic medullary thyroid cancer. The results showed that PRRT could stabilize the disease with few side effects (5, 6). It needs over-expression of somatostatin receptor subtype II (10), which can be assessed by SSTR scintigraphy like 68Ga-DOTA-TATE PET/CT to determine the somatostatin receptor density in the metastases and recurrent tumor lesions as well as evaluating treatment response (12).
One may suggest that non-visualization of the metastatic foci following the second dose of RAI can be due stunning effect secondary to prior diagnostic WBIS. However, to the best of our knowledge, no study has pointed out the persistence of this phenomenon for more than several weeks to months (12–16). In the current case, the interval between diagnostic and therapeutic RAI was 13 months. Therefore, the stunning effect phenomenon is unlikely to be the plausible explanation for what we have observed here.
Despite doing a thorough literature search, we could not find any hypothetical mechanism for re-differentiation following PRRT. Indeed, data in this research domain is still limited, as fewer than 200 cases of RAIR-DTC have been treated with PRRT (5, 6). Whether regaining radioiodine uptake in the current case was serendipitously found during our observations or really happened because of PRRT-induced irradiation to the tumoral cells needs to be elucidated in future studies.
Conclusion
We report a case of RAIR-DTC that showed re-differentiation and gained RAI uptake capacity after four cycles of PRRT. This hypothesis may be a ray of hope. It may propose PRRT as a possible re-differentiation strategy in patients with RAIR-DTC who have lost their RAI avidity if repeated and confirmed by future studies.
Data availability statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
AA treated the patient and supervised the treatment process and revised the article. HB wrote the initial draft of the manuscript. AE critically revised the text and illustrations. AK and SRZ supervised the treatment process. SP and SE helped BH in data gathering and writing the manuscript. NN edited the English language. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Durante C, Haddy N, Baudin E, Leboulleux S, Hartl D, Travagli JP, et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab. (2006) 91(8):2892–9. doi: 10.1210/jc.2005-2838
2. Liu J, Liu Y, Lin Y, Liang J. Radioactive iodine-refractory differentiated thyroid cancer and redifferentiation therapy. Endocrinol Metab (Seoul). (2019) 34:215–25. doi: 10.3803/EnM.2019.34.3.215
3. Buffet C, Wassermann J, Hecht F, Leenhardt L, Dupuy C, Groussin L, et al. Redifferentiation of radioiodine-refractory thyroid cancers. Endocr Relat Cancer. (2020) 27(5):R113–32. doi: 10.1530/ERC-19-0491
4. Avram AM, Giovanella L, Greenspan B, Lawson SA, Luster M, Van Nostrand D, et al. SNMMI procedure standard/EANM practice guideline for nuclear medicine evaluation and therapy of differentiated thyroid cancer: abbreviated version. J Nucl Med. (2022) 63(6):15N–35N. PMID: 35649660
5. Lee DY, Kim YI. Peptide receptor radionuclide therapy in patients with differentiated thyroid cancer: a meta-analysis. Clin Nucl Med. (2020) 45:604–10. doi: 10.1097/RLU.0000000000003110
6. Maghsoomi Z, Emami Z, Malboosbaf R, Malek M, Khamseh ME. Efficacy and safety of peptide receptor radionuclide therapy in advanced radioiodine-refractory differentiated thyroid cancer and metastatic medullary thyroid cancer: a systematic review. BMC Cancer. (2021) 21(1):579. doi: 10.1186/s12885-021-08257-x
7. Spitzweg C, Bible KC, Hofbauer LC, Morris JC. Advanced radioiodine-refractory differentiated thyroid cancer: the sodium iodide symporter and other emerging therapeutic targets. Lancet Diabetes Endocrinol. (2014) 2(10):830–42. doi: 10.1016/S2213-8587(14)70051-8
8. Oliván-Sasot P, Falgás-Lacueva M, García-Sánchez J, Vera-Pinto V, Olivas-Arroyo C, Bello-Arques P. Use of 177Lu-dotatate in the treatment of iodine refractory thyroid carcinomas. Rev Esp Med Nucl Imagen Mol. (2017) 36(2):116–9.
9. de Vries LH, Lodewijk L, Braat AJAT, Krijger GC, Valk GD, Lam MGEH, et al. 68Ga-PSMA PET/CT in radioactive iodine-refractory differentiated thyroid cancer and first treatment results with 177Lu-PSMA-617. EJNMMI Res. (2020) 10(1):18. doi: 10.1186/s13550-020-0610-x
10. Budiawan H, Salavati A, Kulkarni HR, Baum RP. Peptide receptor radionuclide therapy of treatment-refractory metastatic thyroid cancer using (90) Yttrium and (177) Lutetium labeled somatostatin analogs: toxicity, response and survival analysis. Am J Nucl Med Mol Imaging. (2013) 4(1):39–52. PMID: 24380044; PMCID: 3867728
11. Ataei-Nakhaei S, Aryana K, Mostafavi SM, Kosari HM, Esmatinia M, Aghaee A. Peptide receptor radionuclide therapy (PRRT) in radioiodine-refractory thyroid cancer: a case report of significant response to lu177 DOTA-TATE treatment. Arch Endocrinol Metab. (2022) 66(2):269–71. doi: 10.20945/2359-3997000000451
12. Lapa C, Werner RA, Schmid JS, Papp L, Zsótér N, Biko J, et al. Prognostic value of positron emission tomography-assessed tumor heterogeneity in patients with thyroid cancer undergoing treatment with radiopeptide therapy. Nucl Med Biol. (2015) 42(4):349–54. doi: 10.1016/j.nucmedbio.2014.12.006
13. Waxman A, Ramanna L, Chapman N, Chapman D, Brachman M, Tanasescu D, et al. The significance of 1-131 scan dose in patients with thyroid cancer: determination of ablation: concise communication. J Nucl Med. (1981) 22(10):861–5. PMID: 7288483
14. Leger FA, Izembart M, Dagousset F, Barritault L, Baillet G, Chevalier A, et al. Decreased uptake of therapeutic doses of iodine-131 after 185-MBq iodine-131 diagnostic imaging for thyroid remnants in differentiated thyroid carcinoma. Eur J Nucl Med. (1998) 25(3):242–6. doi: 10.1007/s002590050223
15. Hu YH, Wang PW, Wang ST, Lee CH, Chen HY, Chou FF, et al. Influence of 131I diagnostic dose on subsequent ablation in patients with differentiated thyroid carcinoma: discrepancy between the presence of visually apparent stunning and the impairment of successful ablation. Nucl Med Commun. (2004) 25(8):793–7. doi: 10.1097/01.mnm.0000126626.17166.ed
Keywords: RAI-refractory, thyroid cancer, PRRT, somatostatin receptor, lu177-dotatate
Citation: Hadad B, Askari E, Zakavi SR, Aryana K, Erfani S, Sahafi P, Nabavi N and Aghaee A (2023) Case Report: Regaining radioiodine uptake following PRRT in radioiodine-refractory thyroid cancer: A new re-differentiation strategy?. Front. Nucl. Med. 2:1071022. doi: 10.3389/fnume.2022.1071022
Received: 15 October 2022; Accepted: 29 December 2022;
Published: 17 January 2023.
Edited by:
Egesta Lopci, University of Milan, ItalyReviewed by:
Jules Zhang-Yin, Clinique Sud Luxembourg, BelgiumFlávia Dornelas Kurkowski, Pontifical Catholic University of Rio Grande do Sul, Brazil
© 2023 Hadad, Askari, Zakavi, Aryana, Erfani, Sahafi, Nabavi and Aghaee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Atena Aghaee YWdoYWVlYXRAZ21haWwuY29t
Specialty Section: This article was submitted to Radionuclide Therapy, a section of the journal Frontiers in Nuclear Medicine
 Bentolhoda Hadad
Bentolhoda Hadad Atena Aghaee
Atena Aghaee