- 1Research Group Pharmacognosy and Phytotherapy, UCL School of Pharmacy, University London, London, United Kingdom
- 2Division of Pharmacognosy, Department of Pharmaceutical Sciences, University of Vienna, Vienna, Austria
- 3Institute of Genetics and Animal Biotechnology of the Polish Academy of Sciences, Warsaw, Poland
- 4Ludwig Boltzmann Institute Digital Health and Patient Safety, Medical University of Vienna, Vienna, Austria
- 5Laboratory of Natural Products and Medicinal Chemistry (LNPMC), Center for Global Health Research, Saveetha Medical College and Hospital, Saveetha Institute of Medical and Technical Sciences (SIMATS), Chennai, India
- 6Department of Pharmacy and Institutes for Systems Genetics, Center for High Altitude Medicine, Frontiers Science Center for Disease-related Molecular Network, West China Hospital, Sichuan University, Chengdu, Sichuan, China
- 7School of Pharmaceutical Sciences, Lovely Professional University, Phagwara, Punjab, India
- 8College of Pharmacy, The Ohio State University, Columbus, OH, United States
- 9Chinese Medicine Research Center, Department of Pharmaceutical Sciences and Chinese Medicine Resources, College of Chinese Medicine, China Medical University, Taichung, Taiwan
Natural products have long been a cornerstone of drug discovery, providing diverse and biologically relevant chemical scaffolds. This work aims to guide newcomers to natural product research and, specifically, drug discovery by presenting a curated list of 30 key publications selected through an international survey of experts and critical evaluation by the authors. The selected works span textbooks, review articles, and original research papers, covering various aspects of natural product research, including chemistry, pharmacology, analytical sciences, emerging open science, and computational approaches. We discuss historical milestones in natural product drug discovery, highlighting the specific contributions of the U.S. National Cancer Institute in developing anticancer and anti-HIV agents. The present work also addresses current challenges and innovations in the field, emphasizing the importance of data quality, interdisciplinary collaboration, and the integration of artificial intelligence. By providing this carefully selected reading list and accompanying analysis, we aim to offer a comprehensive yet accessible entry point for researchers new to natural product-based drug discovery and highlight future directions and opportunities in this dynamic field.
1 Introduction
Natural products from various organisms have always been a mainstream source of medications for treating and managing various diseases and disorders, especially cancer and infectious diseases (Agarwal et al., 2020; Atanasov et al., 2021). Natural products originate from plants, animals and microbes, including fungi (Singla et al., 2023b). Here, we use the term natural products or metabolites for any compound derived from these organisms. However, one must be aware that the term “natural products” is also often used to refer to multicomponent mixtures, often those used in supplements (botanicals). Therefore, the terminology is sometimes confusing. Misleading terms often found in the literature include “components” and “ingredient(s)”, and others. Traditional healers have long used natural products in their practice. Some of those employed in medical systems are well-documented via the “written” record, such as in the Indian system of medicine (Ayurveda) (Mohanraj et al., 2018), traditional Chinese medicine (TCM) (Wang et al., 2018), Japanese Kampo and Korean traditional medicine, Unani medicine, and European phytotherapy (Kalim et al., 2010). However, many natural products are complex structurally, and isolating bioactive metabolites from natural sources may be tedious and challenging.
Further, standardisation is another requirement encountered in the use of natural products. If not performed correctly for efficacious and safe products, they may not reach the bedside, thereby not having clinical utility. While many natural products indeed have potential therapeutic effects, the general lack of their in-depth mechanism-based analysis, as well as limitations from their toxicity, are factors that tend to reduce the enthusiasm of clinicians in utilizing natural products in therapeutic regimens.
Research on natural products is broad and often interdisciplinary, involving of all isolation, structure elucidation, formulation, pharmacological evaluation, artificial intelligence and machine learning, downstream processing, tissue culture, and other optimisation, along with additional biotechnological and genomics aspects. Therefore, it can be unclear for early career researchers to decide which published documents to access in order to develop a thorough basic understanding of natural products and their specific potential in drug discovery. When one inserts any of the terms “Natural product,” “Traditional medicine,” or “Medicinal plant,” in the PubMed database (https://pubmed.ncbi.nlm.nih.gov/), this will generate thousands of relevant documents in each case, including books, review articles, and reports on clinical trials and their meta-analysis. It is often challenging to extract the required information from a set of diverse documents to start understanding the topics concerned. On the other hand, academic supervisors with long-standing expertise in natural product research are often focused on their specific research questions, and, accordingly, there is a risk that newcomers are not provided with a broad overview of this diverse, complex, and interconnected field.
Keeping this in mind, the authors of the present article sought nominations for informative core papers and books when using an online platform from the global scientific community of natural products, including science-based netizens. Based on the nominations and core team suggestions, we have curated a list of about 30 relevant published documents that are considered valuable for any early career researcher to read and comprehend before deciding to engage in intensive research in the field of natural products. Although the recommended reading list should not be considered an absolute gold standard, it reflects diverse views and opinions on the fundamental scientific literature on biologically active natural products, which we hope will be helpful.
2 Aims
In this overview, the aim is to present publications that are widely accessible, written in such a way that they are understandable to a wide range of researchers, and representative of the present and past in order to primarily address newcomers to the field of natural product research and drug discovery. Hopefully, this compilation of the suggested papers and books will provide a solid and broad fundament for researchers interested in natural product research and drug discovery. It is conducted by compiling 30 papers/books, which are most often named and labelled as most influential, vital, and essential by the survey’s respondents, to facilitate access to and provide an overview of this tremendously rich and exciting research field. In addition, through the regular discussions of the authors of this paper and the critical evaluation of the feedback from the online survey, the authors pinpoint potential methodological weaknesses, gaps, and challenges that need to be addressed, hence, beneficial for newcomers to the field.
3 Approach and methods
The online survey was conducted to invite global nominations of important papers or books and those with the most significant influence in natural products and drug discovery research. Participants were also asked explicitly to include “overlooked” papers or books that were considered essential but should receive more attention since they are fundamental contributions to the field. The online survey comprised two main sections adapted from our previous work on core publications in ethnopharmacology (Jalil et al., 2023), with the first section including three questions covering (1) full bibliographic details of the nominated paper or book, (2) a short justification of why this paper or book is so essential; and (3) a summary of the main findings of the nominated paper or book. The second section contains two questions covering some demographic questions, including (1) the country/continent where the participant is located and (2) years of experience in natural product research. The survey was designed to be short and take 3–5 min to complete. Between March-November 2023, the survey link was distributed via a range of scientific society websites, official social media platforms (e.g., LinkedIn, Instagram, Facebook, and Twitter/X), and through personal networks of academics (i.e., using the snowballing approach) as well as the project flyer was also circulated during International Conferences and Congresses, such as the International Society of Medicinal Plants and Natural Products Research (GA) Congress (https://ga-online.org/), the International Conference on Natural Products Utilisations, from Plants to Pharmacy Shelf (https://icnpu2023.com/), and the Colombian Congress on Natural Products (https://nozomiscience.org/index.php/rpn/issue/view/523) webpages. All nominations were then solicited between December-April 2023. Our call received considerable attention from researchers all over the world. During data analysis of responses received, duplicates were merged. Each of the assessors, the authors of the present work, then discussed the remaining nominations, focussing on those selected papers or books with three or more nominations. It was followed by rounds of adjustments to prioritise between partially overlapping nominations, resulting in a final selection of 30 publications in Natural Products and Drug Discovery research. Core inclusion criteria were: All publications had to (a) be in English, published as an article (both original studies and reviews) or as a book; (b) be of general interest to the field based on their approach, topic, or methodology; (c) there was no direct overlap with other studies. Papers and books listed seven times or more in the survey were all included. Papers with a lower frequency of mention (e.g., publications nominated five times or less) were included on a selective basis. In general, preference was given to more widely cited publications.
4 Results and discussion
4.1 Demographic characteristics of the respondents
In total, 282 respondents completed the demographic information, including the country/continent where the respondents are based. Overall, based on the feedback on natural product experience, 270 respondents were active researchers in the field, with 60% thereof having more than 10 years of experience in this field of research, followed by 37% for those with 1–9 years of experience and less than 1-year experience (3%). Respondents (n = 282) from almost all continents have contributed and added their nominations. However, the response from Europe (34.40% from Western Europe and 14.89% from Eastern Europe) was particularly strong, followed by Central Asia (12.06%) and North America (10.64%) (Figure 1).
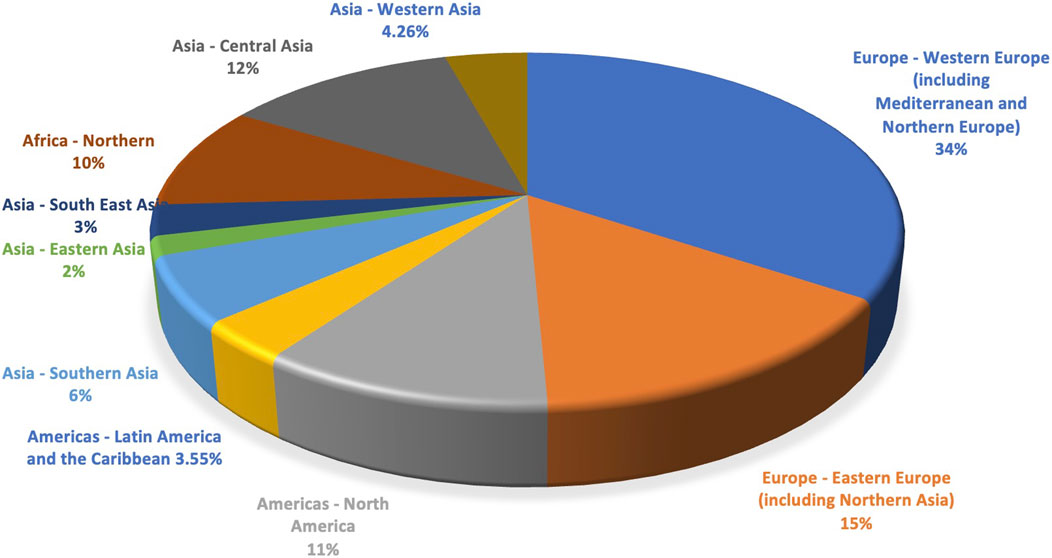
Figure 1. Continent* where the respondents are based (n = 282). (*) The division of the countries and territories of the world into regional and subregional groups is based on the United Nations geoscheme system. It was devised by the United Nations Statistics Division (UNSD) based on the M49 coding classification.
4.2 Survey nominations of most relevant publications - a comparison and analysis of the nominations received and included
In total, we received 320 nominations. After the merging of duplicates, 87 nominations remained. From these, ten books and papers suggested seven times or more and 16 books or papers suggested 5–6 times are all included in the 30 most relevant publications list. The authors then selected four additional publications to ascertain a comprehensive methodological and global coverage of the various approaches and the current advances in natural product and drug discovery research, most notably focusing on recent developments like artificial intelligence-/cheminformatics-driven approaches. These publications have had a high impact (e.g., highly cited) in the field (Table 1).
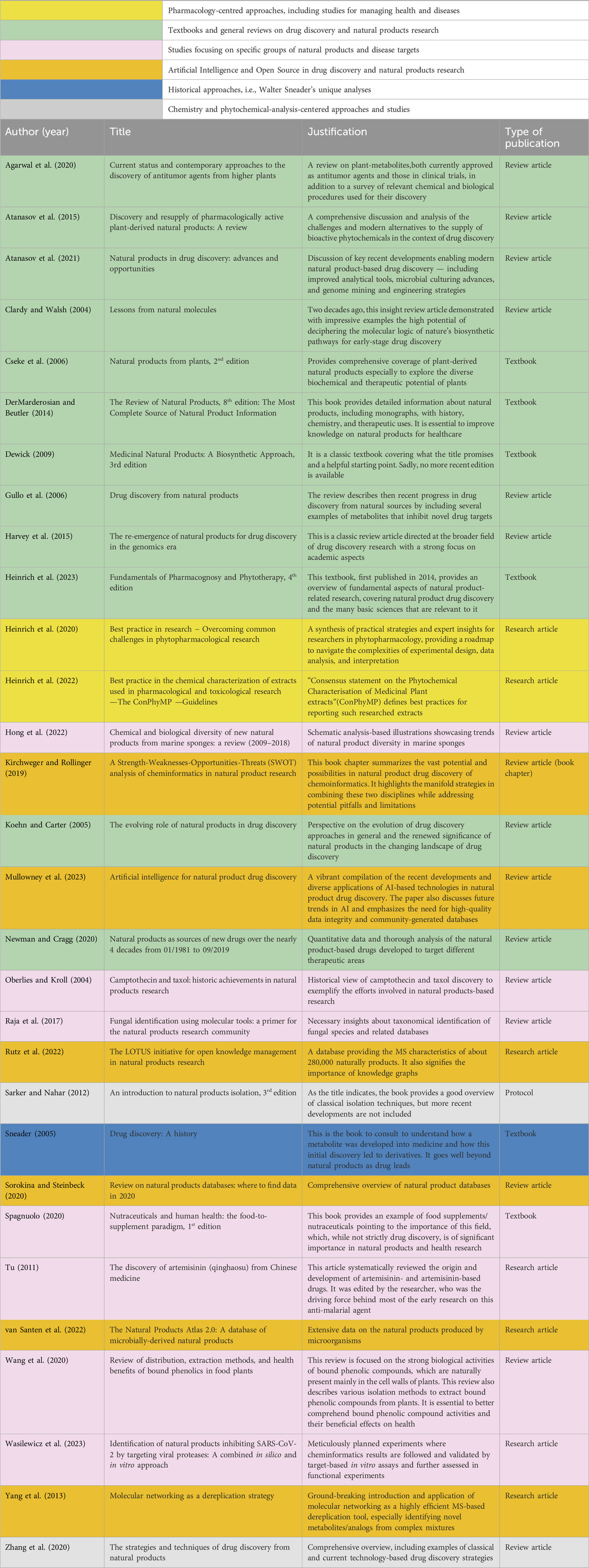
Table 1. Most relevant publications in Drug Discovery and Natural Product Research (n = 30) with a short summary of each paper or book included in the core list. Colour code for defining the main approaches of the studies included.
Out of the nominations for inclusion as the most valuable sources for researchers starting to work at the interface of natural products and drug discovery, one stands out: Walter Sneader (2005) Drug Discovery–a historical treatise on how specific structurally novel medicines and their derivatives were developed. He was a professor at the School of Pharmacy at the University of Strathclyde, Glasgow, Scotland. It was nominated 25 times, seven times more than the next set of sources, and is based on an earlier monumental study - Drug Prototypes and their Exploitation (Sneader, 1996) and an earlier history of drug development (Sneader, 1985). Uniquely, it covers the origins, development, and history of medicines derived from a wide range of sources, not only natural products but also essential medicines from 2005. Biologicals (e.g., hormones and their derivatives), medicines derived from plants and microorganisms, and synthetic small molecules are covered. The core focus is on prototypes and how these were developed. This makes it of particular interest to a newcomer, and it highlights the innovation needed to find a new medical substance and the subsequent steps in developing derivatives with (hopefully) improved medical properties. It is, however, not a text that gives an easy entry into the field, and one may well want to read some of the other publications suggested in this article before delving into this fascinating account of drug development’s successes (and failures).
4.2.1 Pharmacology-centred approaches, including studies for managing health and diseases ( )
)Based on the pharmacology-centered approaches and accurate study conduct and designs, Heinrich et al. (2020) is an invaluable resource for overcoming common obstacles encountered in phytopharmacological research. It is a practical approach, providing actionable strategies for addressing challenges in this field of research. The authors draw from their extensive experience in the field to offer evidence-based recommendations and best practices, empowering new researchers to navigate complex research processes confidently. It also highlights the importance of interdisciplinary collaboration, emphasising the need for collaboration between botanists, pharmacologists, chemists, and clinicians to ensure robust research outcomes. It is an indispensable resource for new researchers embarking on phytopharmacological studies, fostering excellence and innovation in the field.
Similarly, the ConPhyMP Guidelines (Heinrich et al., 2022) present a comprehensive framework for the chemical characterisation of extracts utilised in pharmacological and toxicological studies. It offers standardised protocols and methodologies for extract characterisation. These guidelines provide new researchers with clear and actionable steps to ensure chemical data’s reproducibility, reliability, and comparability across studies. The authors also emphasise the importance of transparency and rigour in reporting chemical analysis methods and results, promoting integrity and trustworthiness in research outcomes. It is an invaluable resource for new researchers in pharmacology and toxicology, facilitating high-quality research and advancing scientific knowledge in the field.
Regarding the insight into the journey of selected plant-derived metabolites from discovery to clinical application, Agarwal et al. (2020) focus on the development of an anti-cancer agent (taxol) and a major lead compound (camptothecin), made possible by the active support by the US National Cancer Institute. This review of taxol and camptothecin offers valuable lessons for researchers navigating similar paths in natural product drug discovery. The review is an inspirational resource for new researchers interested in harnessing the therapeutic potential of plant-derived metabolites for medical applications.
4.2.2 Textbooks and general reviews on drug discovery and natural products research ( )
)Natural products play a pivotal role in drug discovery due to their vast chemical diversity and evolutionary optimisation for biological activity. These metabolites, derived from plants, microbes, and marine organisms, offer a rich source of novel structures with unique pharmacological properties. For example, Dewick (2009) comprehensively covers medicinal natural products and their biosynthetic pathways. This classical textbook is a detailed exploration of the biosynthetic processes involved in producing natural products, providing readers with a deep understanding of how these metabolites are synthesised in living organisms. The book is praised for its clarity and accessibility, making complex biochemical concepts accessible to new researchers as a helpful resource for all in natural product chemistry and drug discovery. In an authoritative reference in natural product studies, DerMarderosian and Beutler (2014) include detailed information on botanicals, herbs, and dietary supplements. The book offers comprehensive monographs on individual natural products, including their botanical characteristics, chemical composition, pharmacological properties, and clinical uses. With its user-friendly format, detailed information, and critical reviews of scientific literature, the book can serve as a resource for new researchers seeking to explore the diverse world of natural products and their potential applications in healthcare.
The textbook by Heinrich et al. (2023) stands out as a comprehensive and up-to-date overview of pharmacognosy and phytotherapy research, emphasising herbal medicine’s fundamental principles and practical applications, including a focus on drug discovery from natural sources. The book integrates traditional knowledge with modern scientific approaches, providing a holistic understanding of medicinal plants and their therapeutic potential. It covers many topics, including plant identification, extraction techniques, phytochemical analysis, and evidence-based herbal medicine, making it a valuable resource for researchers exploring natural products. The authors also address issues such as quality control and regulatory considerations in the use of herbal medicines, offering insights into the challenges and opportunities in the field. Its comprehensive coverage and interdisciplinary approach equip new researchers with the knowledge and skills to navigate the complex landscape of herbal medicine research and practice.
By emphasising the practical aspects of natural product-based drug discovery, Gullo et al. (2006) offer comprehensive insights into the entire process, from sourcing natural materials to lead identification and optimisation. It also explores the diverse range of screening methods and bioassays used to evaluate the pharmacological potential of natural products. It equips new researchers with the necessary tools to characterise and identify lead compounds from complex natural extracts and provides insights for new researchers navigating the complexities of the field.
When it comes to chemical diversity, biosynthesis, and pharmacological properties of plant-derived natural products, Cseke et al. (2006) provide perspectives on various aspects of natural product research, including isolation techniques, structural elucidation, and bioassay-guided screening methods, as well as the ecological and evolutionary significance of plant natural products, highlighting their role in plant defense mechanisms and interactions with other organisms. It equips new researchers with the knowledge and tools necessary to explore the vast potential of plant-derived metabolites for drug discovery and other applications. Clardy and Walsh (2004) focus on the unique chemical diversity and structural complexity of natural products, emphasising their potential to serve as sources of inspiration for drug discovery efforts. It explores the various biosynthetic pathways and mechanisms responsible for producing natural products, elucidating the biochemical processes underlying their formation. The article can provide new researchers with valuable guidance for rational drug design strategies as well as understanding the significance of natural molecules in drug discovery and inspiring innovative approaches to drug design and development.
Regarding the historical evolution of natural products as a source of therapeutic agents, Koehn and Carter (2005) delve into tracing the trajectory of natural product research from traditional medicine to modern drug discovery. The article provides a contextual framework for understanding the enduring importance of natural products in the field. It highlights the unique chemical diversity and structural complexity of purified natural products, emphasising their potential as chemical scaffolds for developing novel drugs. Furthermore, the article addresses current trends and challenges in natural product research, including supply and intellectual property rights issues. It encourages new researchers to adopt innovative approaches and interdisciplinary collaborations to overcome obstacles and maximise the potential of natural products in drug discovery.
In the context of advancements in genomics and related technologies, Harvey et al. (2015) explore how genomics has revitalised interest in natural products as a source of novel drug leads. The authors discuss how genomic sequencing and bioinformatics tools have enabled researchers to understand the biosynthetic pathways of natural products better, facilitating the targeted discovery of bioactive metabolites with pharmaceutical potential. The article also highlights the concept of “genome mining,” wherein genomic data are mined systematically to identify gene clusters responsible for the biosynthesis of natural products. The article can serve as a valuable resource for new researchers by highlighting the transformative impact of genomics on natural product research, elucidating key concepts and methodologies, and inspiring innovative approaches to drug discovery in the twenty-first century. On the importance of natural products as sources of novel therapeutic agents, Atanasov et al. (2015) provide a comprehensive overview of the discovery and resupply processes of pharmacologically active plant-derived natural products. The authors discuss various strategies for discovering bioactive metabolites from plants, including bioassay-guided fractionation, high-throughput screening, and metabolomics approaches. It addresses challenges related to the resupply of natural products, such as sustainability, biodiversity conservation, and intellectual property issues. Its synthesis of research findings and critical analysis of current trends provides new researchers with valuable insights and guidance for navigating the complexities of natural product discovery and development. In addition, the extensive analysis of the contributions of natural products to drug discovery over nearly 4 decades is highlighted by Newman and Cragg (2020). This article is unique in its comprehensive scope, encompassing many natural sources and therapeutic areas. The authors systematically examine trends in the discovery and development of natural product-derived drugs, offering insights into their structural diversity, bioactivity profiles, and clinical applications. Through detailed case studies and statistical analyses, the article provides new researchers with valuable information on the success rates, challenges, and prospects of natural product-based drug discovery. By highlighting the enduring importance of natural products in pharmaceutical innovation, this publication serves as an invaluable resource for researchers interested in harnessing the potential of nature’s chemical diversity for the development of novel therapeutics.
Looking into the recent advances and emerging opportunities in the field, Atanasov et al. (2021) cover comprehensively and discuss the innovative technologies and methodologies, such as metabolomics, synthetic biology, and computational approaches, that are revolutionising natural product research. The review addresses integrating traditional knowledge systems and ethnopharmacology into modern drug discovery processes, highlighting the importance of cultural heritage in identifying potential therapeutic agents. Moreover, the authors examine the potential of natural product-inspired drug discovery strategies, including exploring biosynthetic gene clusters and developing hybrid compounds. Through its forward-looking perspective and multidisciplinary approach, it provides new researchers with valuable insights and inspiration for harnessing the full potential of natural products in the search for new medicines.
4.2.3 Studies focusing on specific groups of natural products and disease targets ( )
)The complex molecular structures of natural products inspired the development of new drugs, providing starting points for synthetic modification and optimisation. Delving into the chemistry and analysis-centered approaches and studies, Tu (2011) captivates the narrative of the discovery of artemisinin, a pivotal antimalarial metabolite derived from traditional Chinese medicine. The work explores the intersection between traditional medicines and modern pharmaceutical discovery processes. The author provides detailed insights into the research journey that led to the identification and isolation of artemisinin, highlighting the perseverance and ingenuity required to uncover its therapeutic properties. The article delves into Chinese medicine’s cultural and historical context, shedding light on the rich tradition of herbal medicine that inspires scientific inquiry today. The book is an educational resource for new researchers interested in the intersection of traditional knowledge and modern drug discovery.
A step-by-step primer focusing on molecular tools and applying molecular techniques, such as DNA barcoding and phylogenetic analysis, Raja et al. (2017) provides practical guidance specifically for accurate and reliable fungal identification. It discusses the advantages and limitations of molecular methods and considerations for choosing the most appropriate technique based on research objectives and sample characteristics. It supports new researchers in enhancing the precision and efficiency of fungal identification in their natural product research.
Several studies explore the role of nutraceuticals in promoting human health, emphasising the transition from traditional food consumption to supplementation. For example, Spagnuolo (2020) systematically examines the intersection of nutrition, pharmacology, and human physiology in nutraceuticals. The author provides in-depth insights into the bioactive metabolites in food and supplements, elucidating their mechanisms of action and potential health benefits. It addresses the regulatory framework surrounding nutraceuticals, offering new researchers valuable guidance on navigating the complex landscape of product development and marketing and the growing field of nutraceutical research via synthesising scientific evidence and practical considerations. In addition, Wang et al. (2020) provides an overview of the distribution of bound phenolics in various food plants, highlighting their potential health-promoting properties. This article also discusses extraction methods for releasing bound phenolics from plant matrices. It offers practical guidance for new researchers interested in studying these metabolites and exploring the bioactive metabolites in food plants and their potential health effects.
Regarding the chemical and biological diversity of natural products derived from marine sponges, Hong et al. (2022) offer a comprehensive review by focusing on the period from 2009 to 2018, providing a recent synthesis of research findings in the field. The authors explore the vast array of bioactive metabolites discovered from marine sponges during this timeframe, highlighting their potential pharmacological applications. The authors discuss the methodologies and technologies employed in discovering and characterising these natural products, offering insights into the innovative approaches driving advancements in the field. New researchers will benefit from this invaluable resource by exploring the rich potential of marine-derived natural products for drug discovery and other applications.
4.2.4 Artificial intelligence and open source in drug discovery and natural products research ( )
)Natural product databases compile diverse information helpful to researchers focusing on drug discovery, including chemical structures, biological activity data, and information on occurrences. The perceived value of such databases for the natural product research community is highlighted with the inclusion of the general review by Sorokina and Steinbeck (2020) on natural products databases and the focused article by van Santen et al. (2022) presenting specifically The Natural Products Atlas 2.0 (representing a database of microbial natural products). With the increased availability of large volumes of data related to natural products, the value of computational approaches that can analyse in different ways such large datasets is surging, and two of the included literature sources well reflect this trend, in particular the review by Kirchweger and Rollinger (2019) on cheminformatics in natural product research with the use of Strength-Weaknesses-Opportunities-Threats (SWOT) analysis and the review by Mullowney et al. (2023) on applications of artificial intelligence in the context of natural product drug discovery. Advancement of digital technologies promotes the vast datasets, which are increasing exponentially, and the diverse computational ways to analyze them. It also enables profound long-distance collaborations, including creating research networks and platforms that coordinate their activities online. One example of such an open innovation platform with high relevance to natural product-based drug discovery is The International Natural Product Sciences Taskforce (INPST) (Singla et al., 2023a).
Yang et al. (2013) and Rutz et al. (2022) significantly exemplify and underscore the importance of innovative computational tools and open data platforms in enhancing efficiency and collaboration in natural product research and drug discovery. In particular, Yang et al. (2013) introduce molecular networking as a dereplication strategy, which leverages mass spectrometry data to identify and organise chemical similarities within complex mixtures. This approach has proven especially useful in natural product discovery, enabling researchers to dereplicate known metabolites and identify novel analogues rapidly. The method’s ability to accommodate different ionisation platforms and its application to various microbial samples demonstrates its versatility and utility in streamlining the drug discovery process. Rutz et al. (2022) present The LOTUS initiative, which focuses on open knowledge management in natural products research. LOTUS represents a significant step toward harmonising and disseminating natural product data by providing a comprehensive database of mass spectrometry characteristics for approximately 280,000 metabolites. Integrating this data into the Wikidata framework facilitates interoperability and community curation, which is essential for advancing research and fostering collaboration across disciplines.
4.2.5 Chemistry and phytochemical-analysis-centered approaches and studies ( )
)The various strategies and techniques employed in the discovery process, such as bioactivity-guided fractionation, high-throughput screening, and computer-aided drug design, and Zhang et al. (2020) provide insights into their applications and limitations. The review discusses innovative approaches and emerging technologies that could enhance the efficiency and success rate of natural product-based drug discovery and the therapeutic potential of metabolites for drug development. Sarker and Nahar (2012) cover a wide range of isolation methods, including extraction, chromatography, and spectroscopy, providing readers with a solid foundation in the principles and applications of each technique. The authors also emphasise the importance of understanding natural products’ chemical and biological properties during isolation, facilitating their successful isolation and characterisation. Moreover, Wasilewicz et al. (2023) integrate in silico screening and in vitro assays to identify metabolites targeting viral proteases, a crucial step in the viral replication cycle. The authors provide a detailed methodology for the virtual screening of natural product databases followed by experimental validation of the identified candidates, offering a comprehensive framework for drug discovery. The study also highlights the potential of natural products as a source of novel antiviral agents, contributing to the global effort to combat COVID-19. By combining computational modelling with laboratory experimentation, this research provides new researchers with a roadmap for leveraging interdisciplinary approaches in drug discovery against emerging viral threats.
4.3 Overview of drug discovery and natural products with anticancer and anti-HIV activities sponsored by the U.S. National Cancer Institute
A cornerstone in natural product-driven drug discovery has emanated from the numerous studies focusing on developing novel anticancer and anti-HIV agents led by U.S. governmental agencies. The U.S. National Cancer Institute (NCI) was established in 1937. As part of its mission, it has fostered potential anticancer drug discovery and development by screening extracts obtained from various organisms. For example, 114,000 extracts prepared from taxonomically authenticated plants were screened for cancer cell line cytotoxicity from 1960 to 1982 (Suffness and Douros, 1982). Substantial progress in this early work occurred in isolation chemistry, biological testing, and preclinical and clinical development, with the significant involvement of the NCI. By the mid-1990s, four major groups of natural products derived from plants were available on the global markets as approved cancer chemotherapeutic agents, representing the bisindole and camptothecin alkaloid, epipodophyllotoxin lignan, and taxane diterpenoid classes (Cragg et al., 1997). In addition to their clinical utility, the cellular mechanisms of action of these four compound classes have proved very important. The following paragraph describes significant scientific progress for each of these groups of oncolytic agents.
The first two plant-derived anticancer agents were approved clinically in the early 1960s to treat various forms of cancer. They were the Vinca or bisindole alkaloids, vinblastine and vincristine, from the Madagascan periwinkle, Catharanthus roseus (L.) G. Don (syn.: Vinca rosea L.) (Johnson et al., 1963). Their purification and structure elucidation, then known as vincaleukoblastine and leurocristine, respectively, were conducted at Eli Lilly and Company, Indianapolis, Indiana (Neuss et al., 1962). In the 1940s, Dr. Jonathan Hartwell and his collaborators at the U.S. National Cancer Institute demonstrated that the lignan podophyllotoxin from the Mayapple (Podophyllum peltatum L.), showed antineoplastic effects in mice (Pettit, 1995). However, podophyllotoxin also showed some toxicity, so ultimately, two semi-synthetic epidopodophyllotoxin derivatives, etoposide and teniposide, were developed as clinically useful cancer chemotherapeutic agents (O'Dwyer et al., 1985). The team of Drs Monroe Wall and Mansukh Wani and their Research Triangle Institute, North Carolina associates made two significant discoveries that have led to useful anticancer drugs. Initially, the alkaloid camptothecin was purified and characterised structurally from the tree Camptotheca acuminata Decne., and shown to exhibit antileukemic activity in a murine in vivo model (Wall et al., 1966). While camptothecin itself is not used clinically to treat cancer, two semi-synthetic derivatives, topotecan and irinotecan, presently have wide use in treating various cancer types. In turn, the nitrogen-containing diterpenoid, taxol, was isolated and characterised as an antitumor metabolite of the stem bark of the western yew, Taxus brevifolia Nutt. (Wani et al., 1971). Taxol, now known as paclitaxel, is widely used worldwide as a cancer chemotherapeutic agent (Wall and Wani, 1996; Agarwal et al., 2020). The NCI has also sponsored research on marine organisms by the U.S. academic community, with two leaders in this area being Professors Paul Scheuer (University of Hawaii) (Scheuer, 1995) and George Pettit (Arizona State University) (Pettit, 1996). Over the last 20 years, about 15 antibody-drug conjugates (ADCs) have been approved by the U.S. FDA as cancer therapeutic agents, and several of these contain a component derived from marine blue-green algal sources (Newman, 2021).
Plant samples from the U.S. NCI Natural Products Repository were also tested for anti-HIV effects during the worldwide pandemic of acquired immune deficiency syndrome (AIDS) in the 1980s and 1990s. Two examples of plant-derived metabolites with anti-HIV activity discovered at NCI, Frederick, Maryland, and then subjected to further development are the coumarin (+)-calanolide A and the dimeric alkaloid michellamine B (Yang et al., 2001). The U.S. NCI has long been a leader in developing plant and other organism collection agreements with source countries in a manner consistent with the recommendations of international conventions (Baker et al., 1995). The above examples provide a rich set of details of the approaches used. While the methods have changed dramatically, the case studies demonstrate what is crucial for natural product-based drug development: Interdisciplinarity, perseverance, innovation in methods and approaches, and strong industry-based development opportunities.
4.4 Core innovations and challenges in the last decade
Innovation is coined by newness, improvement, and spreading ideas or technologies. According to Amabile and Pratt (2016), innovation (“the successful implementation of creative ideas within an organisation”) is distinguished from creativity (“the production of novel and useful ideas by an individual or small group of individuals working together”). The general focus is on health improvement in drug discovery and natural product research. In this field, thousands of researchers from academia and industry work intensively to explore new frontiers and ideas. This endeavor requires expertise, curiosity, and mental and (some) financial freedom to create a fertile ground for creative ideas. It may result in either practical new concepts or fanciful ideas and, as such, lay the ground for innovation as impressively formulated by Louis Pasteur in 1854 and recited by Hugo Kubinyi, “in the field of observation, chance only favors the prepared mind.” Discoveries are made by scientists practising good science, working closely together, sharing ideas, and testing hypotheses (Kubinyi, 1999).
It can be speculated that the most fruitful and, in some respects, more realistic ideas in terms of applicability are taking place in the border areas of natural product research, where an interface with other disciplines is created. Expert knowledge from manifold areas stimulates natural product research, such as microbiology, biodiversity, ecology, anthropology, systems biology, bioprospecting, imaging, chemistry in all its facets, analytics (-omics), genetics, synthetic biology, biophysics, engineering, bioinformatics, cheminformatics with all aspects of AI. For the translation into clinics, many further aspects have to be considered, such as IP rights, regulations, ethics, and economy. Some of the interfaces mentioned above are well-established and have already been covered in the respondents’ feedback. Nevertheless, we are far from imaging all the possible impacts of other disciplines in drug discovery and natural product research.
Looking 220 years back, in 1804, the German pharmacist Friedrich Sertürner discovered the first active (partially purified) metabolite, morphine, from opium. Meanwhile, the research community faces an unbelievable and continuously growing treasure of chemical and biological data from isolated metabolites. The opportunities from these billions of experimental data in the public domain cannot be embraced by a human brain in its complexity and richness. We also benefit from the increasing knowledge of macromolecular targets and their physiological role in humans. In sum, this infinite data source offers tremendous opportunities to use AI with continuous advances in machine learning algorithms, molecular dynamics, more accurate predictions, and overcoming the earlier computational power bottleneck (Mullowney et al., 2023). The more challenging questions, however, are: Can we rely on the data in the public domain? Are these data correct and solid enough to predict new events? How can we use the masses of data and the AI tools without getting lost? The reliability of data refers to the correct structure elucidation as well as to their bioactivity assessment. In a recent analysis by Landrum and Riniker (2024) performed on an extensive collection of literature data collected in and exported from the resource ChEMBL, the authors investigated the consistency of measured IC50 values from assays against the same target. Intriguingly, their analysis showed that in the case of minimal curation settings, the assay results have poor agreement with each other: almost 65% of data points differ by more than 0.3 log units. Even with the maximal curation settings, much noise remains in the combined data sets. One can imagine that feeding AI with this tremendous noise in data for training or validating machine learning models inevitably decreases the quality and accuracy of models, which will accordingly not result in a meaningful prediction. To minimizing the inaccuracy, the authors of that study recommend a maximal curation of data settings (and also offer an open-source license in their public GitHub repository: https://github.com/rinikerlab/overlapping_assays) as a prerequisite for high-quality public bioactivity data.
Similarly, success relies on chemical and pharmacological data, which are generated and assessed for correctness using a rigorous and state-of-the-art methodology. The scientific literature is now cluttered with data based on poor quality methods and approaches, and a much more rigorous approach to ascertaining high data quality is crucial (e.g., Heinrich et al., 2022). There are also numerous reasons for variabilities in assay results from different laboratories, e.g., differences in assay technologies, assay protocols, cell type, operator handling, substrate identity, and concentration. Further problems in assessing a “true” bioactive hit may arise from assay interferences. False assay readouts can result from colloidal aggregation, chelation, chemical reactivity, light signal attenuation, emission, membrane disruption, and other interferences. These “false” hits trigger expensive hit expansion and counteract every rationale toward hit optimization. A recent review article by Tan et al. (2024) offers critical insight into the challenges derived from biochemical and cell-based assays. It focuses on experimental and theoretical approaches for tackling assay interferences. Overcoming these very critical aspects of data reliability remains a challenge, and we more and more benefit from overwhelming initiatives of community efforts, which are strengthening and accumulating the publicly available information, thereby contributing to and sharing our mutual knowledge. Two examples are the Global Natural Products Social Molecular Networking GNPS - Analyze, Connect, and Network with your Mass Spectrometry Data (ucsd.edu) and the previously mentioned Lotus initiative (LOTUS: Natural Products Online). The drug discovery community faces increasing efforts towards FAIR data, open data, and open access, e.g., PubChem, PubChem bioassay, ChEMBL database. Although it might be tempting to uncritically publish results on metabolites wrongly assigned to be bioactive, we all must be aware that the correctness of data reaching the public will be our future foundation to build up discoveries, which lies in the hands of every researcher.
5 Conclusion
Current and upcoming societal needs require further sophisticated explorations by implementing tools and expert knowledge from different disciplines. There are enormous possibilities for natural product researchers to expand the exploration of natural product scaffolds as role models in different niches without limits to the imagination. Although forecasting novel aspects might be challenging with unknown and insecure spaces, it is one of the most significant opportunities researchers in this field face. It is upon us to share knowledge, collaborate with experts from neighbouring disciplines, and generate reliable data to build a solid foundation for current and upcoming research generations. The paper offers a perspective for newcomers in order to gain a broad overview of core areas. The selection was initially driven by feedback from the research-active community, but we acknowledge that it cannot be (nor should it be) comprehensive. The selection is based on what is most valuable, incorporating the scope of a publication, its relevance, and, importantly, how user-friendly the source is. It is a personal choice but a collective one of many leading experts. Accordingly, we refrain from the necessity and impossibility of full literature coverage of drug discovery and natural product research disciplines in this paper. However, with the list of 30 publications, from classics to very recent articles, a broad insight into different aspects of natural products and drug discovery is provided to newcomers in the field. It will help - so we hope–to assess a wide variety of natural product fields and, simultaneously, acquire the necessary know-how to extract the maximum usage for novel, innovative natural product strategies.
Author contributions
BJ: Conceptualization, Data curation, Investigation, Methodology, Writing–original draft, Writing–review and editing. JR: Investigation, Methodology, Writing–review and editing. AA: Investigation, Methodology, Writing–review and editing. RS: Investigation, Writing–review and editing. AK: Investigation, Methodology, Writing–review and editing. MH: Conceptualization, Investigation, Methodology, Writing–original draft, Writing–review and editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Agarwal, G., Blanco, C. P. J., Addo, E. M., and Kinghorn, A. D. (2020). Current status and contemporary approaches to the discovery of antitumor agents from higher plants. Biotechnol. Adv. 38, 107337. doi:10.1016/j.biotechadv.2019.01.004
Amabile, T. M., and Pratt, M. G. (2016). The dynamic componential model of creativity and innovation in organizations: making progress, making meaning. Res. Organ. Behav. 36, 157–183. doi:10.1016/j.riob.2016.10.001
Atanasov, A. G., Waltenberger, B., Pferschy-Wenzig, E.-M., Linder, T., Wawrosch, C., Uhrin, P., et al. (2015). Discovery and resupply of pharmacologically active plant-derived natural products: a review. Biotechnol. Adv. 33, 1582–1614. doi:10.1016/j.biotechadv.2015.08.001
Atanasov, A. G., Zotchev, S. B., Dirsch, V. M., and Supuran, C. T. (2021). Natural products in drug discovery: advances and opportunities. Nat. Rev. Drug Discov. 20, 200–216. doi:10.1038/s41573-020-00114-z
Baker, J. T., Borris, R. P., Carté, B., Cordell, G. A., Soejarto, D. D., Cragg, G. M., et al. (1995). Natural product drug discovery and development: new perspectives on international collaboration. J. Nat. Prod. 58, 1325–1357. doi:10.1021/np50123a003
Clardy, J., and Walsh, C. (2004). Lessons from natural molecules. Nature 432, 829–837. doi:10.1038/nature03194
Cragg, G. M., Newman, D. J., and Snader, K. M. (1997). Natural products in drug discovery and development. J. Nat. Prod. 60, 52–60. doi:10.1021/np9604893
Cseke, L. J., Kirakosyan, A., Kaufman, P. B., Warber, S., Duke, J. A., and Brielmann, H. L. (2006). Natural products from plants. Boca Raton, FL: CRC Press.
DerMarderosian, A., and Beutler, J. A. (Eds.), (2014). in The review of natural products: the most complete source of natural product information (St. Louis, MO: Wolters Kluwar Health).
Dewick, P. M. (2009). Medicinal natural products: a biosynthetic approach. Chichester, West Sussex, UK: John Wiley and Sons, Ltd.
Gullo, V. P., Mcalpine, J., Lam, K. S., Baker, D., and Petersen, F. (2006). Drug discovery from natural products. J. Industrial Microbiol. Biotechnol. 33, 523–531. doi:10.1007/s10295-006-0107-2
Harvey, A. L., Edrada-Ebel, R., and Quinn, R. J. (2015). The re-emergence of natural products for drug discovery in the genomics era. Nat. Rev. Drug Discov. 14, 111–129. doi:10.1038/nrd4510
Heinrich, M., Appendino, G., Efferth, T., Fürst, R., Izzo, A. A., Kayser, O., et al. (2020). Best practice in research–Overcoming common challenges in phytopharmacological research. J. Ethnopharmacol. 246, 112230. doi:10.1016/j.jep.2019.112230
Heinrich, M., Barnes, J., Prieto-Garcia, J., Gibbons, S., and Williamson, E. M. (2023). Fundamentals of pharmacognosy and phytotherapy. London, UK: Elsevier.
Heinrich, M., Jalil, B., Abdel-Tawab, M., Echeverria, J., Kulić, Ž., Mcgaw, L. J., et al. (2022). Best Practice in the chemical characterisation of extracts used in pharmacological and toxicological research—the ConPhyMP—guidelines. Front. Pharmacol. 13, 953205. doi:10.3389/fphar.2022.953205
Hong, L.-L., Ding, Y.-F., Zhang, W., and Lin, H.-W. (2022). Chemical and biological diversity of new natural products from marine sponges: a review (2009–2018). Mar. Life Sci. Technol. 4, 356–372. doi:10.1007/s42995-022-00132-3
Jalil, B., Schultz, F., and Heinrich, M. (2023). Where to begin? The best publications for newcomers to ethnopharmacology. Front. Pharmacol. 14, 278. doi:10.3389/fphar.2023.1141502
Johnson, I. S., Armstrong, J. G., Gorman, M., and Burnett Jr, J. P. (1963). The vinca alkaloids: a new class of oncolytic agents. Cancer Res. 23, 1390–1427.
Kalim, M. D., Bhattacharyya, D., Banerjee, A., and Chattopadhyay, S. (2010). Oxidative DNA damage preventive activity and antioxidant potential of plants used in Unani system of medicine. BMC Complementary Altern. Med. 10, 77. doi:10.1186/1472-6882-10-77
Kirchweger, B., and Rollinger, J. M. (2019). A Strength-Weaknesses-Opportunities-Threats (SWOT) analysis of cheminformatics in natural product research. Prog. Chem. Org. Nat. Prod. Cheminformatics Nat. Prod. Res. 110, 239–271. doi:10.1007/978-3-030-14632-0_7
Koehn, F. E., and Carter, G. T. (2005). The evolving role of natural products in drug discovery. Nat. Rev. Drug Discov. 4, 206–220. doi:10.1038/nrd1657
Kubinyi, H. (1999). Chance favors the prepared mind-from serendipity to rational drug design. J. Recept. Signal Transduct. 19, 15–39. doi:10.3109/10799899909036635
Landrum, G. A., and Riniker, S. (2024). Combining IC50 or Ki values from different sources is a source of significant noise. J. Chem. Inf. Model. 64, 1560–1567. doi:10.1021/acs.jcim.4c00049
Mohanraj, K., Karthikeyan, B., Vivek-Ananth, R., Chand, R., Aparna, S., Pattulingam Mangalapandi, P. M., et al. (2018). IMPPAT: a curated database of Indian Medicinal Plants, phytochemistry and therapeutics. Sci. Rep. 8, 4329. doi:10.1038/s41598-018-22631-z
Mullowney, M. W., Duncan, K. R., Elsayed, S. S., Garg, N., Van Der Hooft, J. J., Martin, N. I., et al. (2023). Artificial intelligence for natural product drug discovery. Nat. Rev. Drug Discov. 22, 895–916. doi:10.1038/s41573-023-00774-7
Neuss, N., Gorman, M., Boaz, H. E., and Cone, N. J. (1962). Vinca alkaloids. XI.1 structures of leurocristine (LCR) and Vincaleukoblastine (VLB)2. J. Am. Chem. Soc. 84, 1509–1510. doi:10.1021/ja00867a049
Newman, D. J. (2021). Natural product based antibody drug conjugates: clinical status as of November 9, 2020. J. Nat. Prod. 84, 917–931. doi:10.1021/acs.jnatprod.1c00065
Newman, D. J., and Cragg, G. M. (2020). Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 83, 770–803. doi:10.1021/acs.jnatprod.9b01285
Oberlies, N. H., and Kroll, D. J. (2004). Camptothecin and taxol: historic achievements in natural products research. J. Nat. Prod. 67, 129–135. doi:10.1021/np030498t
O'Dwyer, P. J., Leyland-Jones, B., Alonso, M. T., Marsoni, S., and Wittes, R. E. (1985). Etoposide (VP-16–213) current status of an active anticancer drug. N. Engl. J. Med. 312, 692–700. doi:10.1056/nejm198503143121106
Pettit, G. R. (1995). The scientific contributions of Jonathan L. Hartwell, PhD. J. Nat. Prod. 58, 359–364. doi:10.1021/np50117a003
Pettit, G. R. (1996). Progress in the discovery of biosynthetic anticancer drugs. J. Nat. Prod. 59, 812–821. doi:10.1021/np9604386
Raja, H. A., Miller, A. N., Pearce, C. J., and Oberlies, N. H. (2017). Fungal identification using molecular tools: a primer for the natural products research community. J. Nat. Prod. 80, 756–770. doi:10.1021/acs.jnatprod.6b01085
Rutz, A., Sorokina, M., Galgonek, J., Mietchen, D., Willighagen, E., Gaudry, A., et al. (2022). The LOTUS initiative for open knowledge management in natural products research. Elife 11, e70780. doi:10.7554/elife.70780
Sarker, S. D., and Nahar, L. (2012). An introduction to natural products isolation: methods and protocols. New York: Springer.
Scheuer, P. J. (1995). Marine natural products research: a look into the dive bag. J. Nat. Prod. 58, 335–343. doi:10.1021/np50117a001
Singla, R. K., De, R., Efferth, T., Mezzetti, B., Uddin, M. S., Ntie-Kang, F., et al. (2023a). The international natural product sciences Taskforce (INPST) and the power of twitter networking exemplified through# INPST hashtag analysis. Phytomedicine 108, 154520. doi:10.1016/j.phymed.2022.154520
Singla, R. K., Wang, X., Gundamaraju, R., Joon, S., Tsagkaris, C., Behzad, S., et al. (2023b). Natural products derived from medicinal plants and microbes might act as a game-changer in breast cancer: a comprehensive review of preclinical and clinical studies. Crit. Rev. Food Sci. Nutr. 63, 11880–11924. doi:10.1080/10408398.2022.2097196
Sneader, W. (1985). Drug discovery: the evolution of modern medicines. Chichester, UK: John Wiley and Sons Ltd.
Sneader, W. (1996). Drug prototypes and their exploitation. Chichester, UK: John Wiley and Sons Ltd.
Sorokina, M., and Steinbeck, C. (2020). Review on natural products databases: where to find data in 2020. J. Cheminformatics 12, 20. doi:10.1186/s13321-020-00424-9
Spagnuolo, P. A. (2020). Nutraceuticals and human health: the food-to-supplement paradigm. London, UK: Royal Society of Chemistry.
Suffness, M., and Douros, J. (1982). Current status of the NCI plant and animal product program. J. Nat. Prod. 45, 1–14. doi:10.1021/np50019a001
Tan, L., Hirte, S., Palmacci, V., Stork, C., and Kirchmair, J. (2024). Tackling assay interference associated with small molecules. Nat. Rev. Chem. 8, 319–339. doi:10.1038/s41570-024-00593-3
Tu, Y. (2011). The discovery of artemisinin (qinghaosu) and gifts from Chinese medicine. Nat. Med. 17, 1217–1220. doi:10.1038/nm.2471
Van Santen, J. A., Poynton, E. F., Iskakova, D., Mcmann, E., Alsup, T. A., Clark, T. N., et al. (2022). The Natural Products Atlas 2.0: a database of microbially-derived natural products. Nucleic Acids Res. 50, D1317–D1323. doi:10.1093/nar/gkab941
Wall, M. E., and Wani, M. C. (1996). Camptothecin and taxol: from discovery to clinic. J. Ethnopharmacol. 51, 239–254. doi:10.1016/0378-8741(95)01367-9
Wall, M. E., Wani, M. C., Cook, C. A., Palmer, K. H., Mcphail, A., and Sim, G. (1966). Plant antitumor agents. I. The isolation and structure of camptothecin, a novel alkaloidal leukemia and tumor inhibitor from Camptotheca acuminata. J. Am. Chem. Soc. 88, 3888–3890. doi:10.1021/ja00968a057
Wang, J., Wong, Y.-K., and Liao, F. (2018). What has traditional Chinese medicine delivered for modern medicine? Expert Rev. Mol. Med. 20, e4. doi:10.1017/erm.2018.3
Wang, Z., Li, S., Ge, S., and Lin, S. (2020). Review of distribution, extraction methods, and health benefits of bound phenolics in food plants. J. Agric. Food Chem. 68, 3330–3343. doi:10.1021/acs.jafc.9b06574
Wani, M. C., Taylor, H. L., Wall, M. E., Coggon, P., and Mcphail, A. T. (1971). Plant antitumor agents. VI. Isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J. Am. Chem. Soc. 93, 2325–2327. doi:10.1021/ja00738a045
Wasilewicz, A., Kirchweger, B., Bojkova, D., Abi Saad, M. J., Langeder, J., Butikofer, M., et al. (2023). Identification of natural products inhibiting SARS-CoV-2 by targeting viral proteases: a combined in silico and in vitro approach. J. Nat. Prod. 86, 264–275. doi:10.1021/acs.jnatprod.2c00843
Yang, J. Y., Sanchez, L. M., Rath, C. M., Liu, X., Boudreau, P. D., Bruns, N., et al. (2013). Molecular networking as a dereplication strategy. J. Nat. Prod. 76, 1686–1699. doi:10.1021/np400413s
Yang, S. S., Cragg, G. M., Newman, D. J., and Bader, J. P. (2001). Natural product-based anti-HIV drug discovery and development facilitated by the NCI developmental therapeutics program. J. Nat. Prod. 64, 554–277. doi:10.1021/np010136q
Keywords: natural products, drug discovery, medicinal plants, marine organisms, fungi, microorganisms
Citation: Jalil B, Rollinger JM, Atanasov AG, Singla RK, Kinghorn AD and Heinrich M (2024) Core publications in drug discovery and natural product research. Front. Nat. Produc. 3:1493720. doi: 10.3389/fntpr.2024.1493720
Received: 09 September 2024; Accepted: 15 October 2024;
Published: 11 November 2024.
Edited by:
Mohamed L. Ashour, Ain Shams University, EgyptReviewed by:
Lars Bohlin, Uppsala University, SwedenJuan J. Araya, University of Costa Rica, Costa Rica
Copyright © 2024 Jalil, Rollinger, Atanasov, Singla, Kinghorn and Heinrich. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Banaz Jalil, Yi5qYWxpbEB1Y2wuYWMudWs=; Michael Heinrich, bS5oZWlucmljaEB1Y2wuYWMudWs=
 Banaz Jalil
Banaz Jalil Judith M. Rollinger
Judith M. Rollinger Atanas G. Atanasov
Atanas G. Atanasov Rajeev K. Singla
Rajeev K. Singla A. Douglas Kinghorn
A. Douglas Kinghorn Michael Heinrich
Michael Heinrich