- 1HEARing Co-operative Research Center, Carlton, VIC, Australia
- 2Department of Linguistics, Macquarie University, Sydney, NSW, Australia
- 3School of Psychology, University of Auckland, Auckland, New Zealand
- 4Eisdell Moore Centre for Hearing and Balance Research, University of Auckland, Auckland, New Zealand
A novel experimental paradigm, “deconvolution of ears' activity” (DEA), is presented which allows to disentangle overlapping neural activity from both auditory cortices when two auditory stimuli are presented closely together in time in each ear. Pairs of multi-tone complexes were presented either binaurally, or sequentially by alternating presentation order in each ear (i.e., first tone complex of the pair presented to one ear and second tone complex to the other ear), using stimulus onset asynchronies (SOAs) shorter than the neural response length. This timing strategy creates overlapping responses, which can be mathematically separated using least-squares deconvolution. The DEA paradigm allowed the evaluation of the neural representation in the auditory cortex of responses to stimuli presented at syllabic rates (i.e., SOAs between 120 and 260 ms). Analysis of the neuromagnetic responses in each cortex offered a sensitive technique to study hemispheric lateralization, ear representation (right vs. left), pathway advantage (contra- vs. ipsi-lateral) and cortical binaural interaction. To provide a proof-of-concept of the DEA paradigm, data was recorded from three normal-hearing adults. Results showed good test-retest reliability, and indicated that the difference score between hemispheres can potentially be used to assess central auditory processing. This suggests that the method could be a potentially valuable tool for generating an objective “auditory profile” by assessing individual fine-grained auditory processing using a non-invasive recording method.
Introduction
The auditory system is a binaural system. Auditory cortices in right and left hemispheres receive ascending projections originating from each ear. The resulting activity in one cortex is a mixture of signals from both ears. The effects of monaural and binaural stimulation on cortical responses have been studied considerably in humans, using techniques such as magnetoencephalography (MEG) (Pantev et al., 1986). MEG is well suited to study hemispheric processing differences given the low dispersion of the magnetic field and the location of the cerebral auditory cortical centers in the temporal lobe of each hemisphere. For monaural sound presentation, there is evidence of a predominant contra-lateral pathway in the human auditory system (Pantev et al., 1986, 1998; Mäkel et al., 1993). The contra-lateral advantage is characterized by shorter latencies and larger amplitudes of the N100m. These measures reflect anatomical differences, especially the larger number of neurons projecting on the contra-lateral compared to the ipsi-lateral side of the ascending auditory pathways. For binaural presentation at the cortical level, MEG frequency-tagging of cortical steady-state responses can be employed (Fujiki et al., 2002). Here, stimuli receive a marker, or tag, using a specific modulation frequency. This makes it possible to identify which stimulus evoked the observed cortical response.
The auditory system is a temporally fast system. It can process acoustic stimuli presented with short temporal disparities between the ears. Processing rapidly changing sounds encompasses several levels of transformation from one cochlea to the auditory cortex of both hemispheres. Unfortunately, a non-invasive objective measure of binaural interaction in the auditory cortex during rapid stimulation with temporally restricted sounds is not yet available. However, if such a method were to be available, research on the interaction and/or integration of signals in the auditory cortex for stimuli presented at syllabic rates (i.e., between 4 and 10 Hz) could provide new insights into normally developed and disordered central auditory processing systems.
This report describes a novel experimental paradigm, named “deconvolution of ears' activity” (DEA), which makes use of the least-squares (LS) deconvolution technique to allow separation of left and right ear activity in each hemisphere to rapidly presented stimuli (Bardy et al., 2014a,b). The LS deconvolution technique is a mathematical algorithm designed to disentangle temporally overlapping brain responses. The technique, described in Bardy et al. (2014a), relies on the timing characteristics of the stimulus sequence to be unequally spaced. This specific property is called “jitter”. The LS deconvolution has been validated in a pair paradigm using EEG data (Bardy et al., 2014b). In the DEA paradigm, LS deconvolution is applied to a sequence of stimuli presented in pairs either binaurally or sequentially, using stimulus onset asynchronies (SOAs) shorter than the duration of the cortical. Right and left ear activity is extracted from the mixture of signals in both auditory cortices such that, using this method, the signal propagation from each ear to each auditory cortex can be tracked. The DEA paradigm is introduced in this paper, and is evaluated on three normal hearing adults as a proof-of-concept.
Two hypotheses were investigated: (1) the LS deconvolution technique can disentangle temporally overlapping brain responses in each auditory cortex originating from both ears with a high test-retest reliability; and (2) an auditory profile can be generated based on measures of the auditory pathway lateralization, hemispheric advantage, ear advantage and binaural cortical interaction.
Methods
Subjects
Test and retest MEG data were obtained from 3 right-handed adult subjects (3 males, age: 37, 32, 29) on two separate occasions. Subjects had no history of neurological or audiological problems and had pure tone audiometric thresholds ≤20 dB HL in all octave frequencies between 250 to 8,000 Hz. This study was approved by and conducted under oversight of the Macquarie University Human Research Ethics Committee. All subjects gave written informed consent to participate in this study.
Stimulation
Two multi-tone (MT) stimuli, selected to optimize the amplitude of the cortical response (Bardy et al., 2015), were obtained by amplitude-modulated tone-bursts composed of carrier frequencies of 2 and 1 kHz with modulation frequencies 800 and 400 Hz respectively. Changing the frequency of the stimuli was used to minimize the habituation of the cortical neural response. The stimuli were presented through custom insert earphones, using pneumatic tubes to deliver sound to the subject, with a frequency response relatively flat between 500 and 8 kHz and an approximate 10 dB/octave roll-off for frequencies below 500 Hz (Raicevich et al., 2010). The two MTs were presented in pairs, using jittered SOAs with means of 120, 190, or 260 ms. The jitter distribution, permitting the deconvolution, was rectangular with a width of 70 ms and a step size of 13.3 ms. The inter-pair interval (IPI), representing the time interval between the onset of two successive pairs of stimuli, was jittered with 400 ms around an average of 1,400 ms. The MTs had a rise and decay time of 10 ms, a duration of 50 ms and an rms intensity of 70 dB SPL. They were presented through shielded transducers (Oldfield, 1971). The stimuli were presented in three presentation conditions. The first presentation condition was binaural (both stimuli of the pair presented simultaneously to the right and left ears). In the two other presentation conditions, stimuli were alternated sequentially in each ear (i.e., when the left ear received the first tone, the right ear received the second tone of the pair, and vice versa). All 9 conditions (3 SOAs x 3 presentation conditions) were randomly presented in a 25-min-long stimulus sequence.
In conditions where the cortical response was longer than the SOA, brain responses overlapped in time, and LS deconvolution described by Bardy et al. (2014a) was employed to disentangle the occurring overlapping responses. Thus, for example, in the alternating sequential condition, it was possible within each auditory cortex to separate the activity elicited by the stimulus to the right and left ears respectively from the overlapping cortical response (Figure 1).
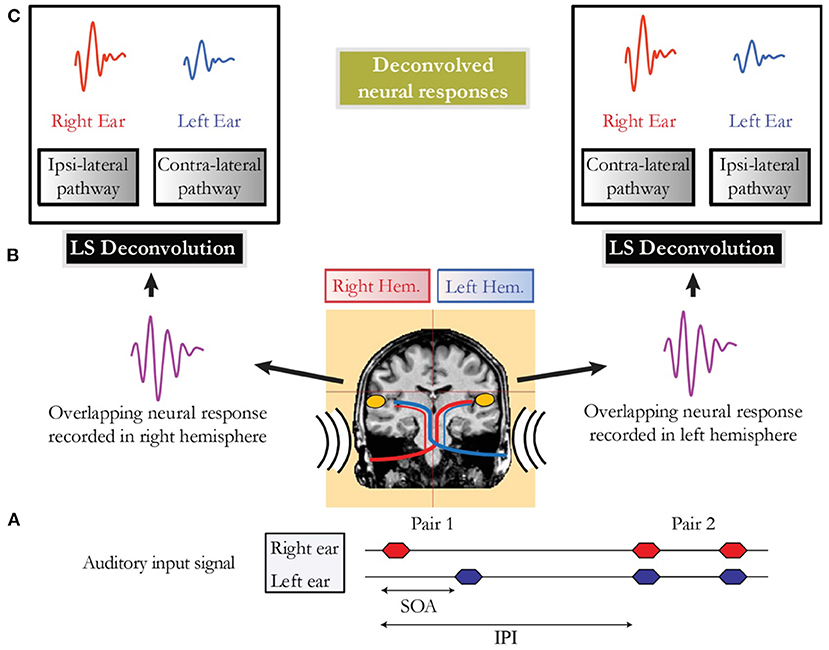
Figure 1. (A) Schematic representation of the auditory input signal for a sequential presentation condition (e.g., Pair 1) followed by a binaural presentation condition (e.g., Pair 2). The stimulus onset asynchrony (SOA) that represents the timing between the onset of the two auditory stimuli of a pair is jittered to allow deconvolution. The time interval between the onset of two pairs is referred as the inter-pair interval (IPI). (B) Representation of the overlapping neural activity of Pair 1. The MEG signal is recorded in both hemispheres in response to the first auditory stimulus presented to the right ear, followed closely by a stimulus presented in the left ear. (C) Deconvolved neural responses in each auditory cortex obtained using the least-squares (LS) deconvolution technique.
Procedure
MEG data were continuously recorded using a whole-head MEG system (Model PQ1160R-N2, KIT, Kanazawa, Japan) consisting of 160 coaxial first-order gradiometers with a 50 mm baseline (Kado et al., 1999; Uehara et al., 2003). MEG data were acquired in a magnetically shielded room using a sampling rate of 1,000 Hz with a bandpass filter of 0.1–200 Hz and a 50 Hz notch filter. For co-registration, the location of five indicator coils placed on the participant's head were digitized. A pen digitizer (Polhemus Fastrack, Colchester, VT) was used to measure the shape of each participant's head which was then carefully centered in the MEG dewar (position error <10 mm for each subject). Artifact removal from MEG data included signals exceeding amplitude (>2,700 fT/cm) and magnetic gradient (>800 fT/cm/sample) criteria (Yetkin et al., 2004). Averaging and band-pass filtering between 3 Hz (6 dB/octave, forward) and 30 Hz (48 dB/octave, zero-phase) was performed for each trigger condition using the non-contaminated epochs. The accepted epochs after artifact rejection were exported from BESA 5.3 into MATLAB (MathWorks, Natick, MA) and downsampled to 100 Hz. Deconvolution was performed for each of the 160 channels to disentangle overlapping responses. For each condition, recovered responses were defined by epochs of 100 ms pre-stimulus to 380 ms post-stimulus.
Statistical Analysis
Amplitudes and latencies were defined by peak measures of magnetic global field power (mGFP) calculated on 40 sensors located over the temporal lobe in each hemisphere. For each subject and each condition, the N100m was defined as the most positive peak in the 80–150 ms following the sound onset. The selected time window for the P200m was 120–200 ms. A repeated measures ANOVAs was performed. Greenhouse-Geisser corrections for sphericity were applied, as indicated by the cited ε value (Greenhouse and Geisser, 1959). Bonferroni corrections were applied for post hoc analysis.
Individual laterality indices (LIs) for hemisphere, pathway, ear and cortical binaural interaction were calculated. For each subject, LIs were calculated based on the relevant mGFP response amplitudes, time-averaged over a 200-ms window post-onset. Figure 2 displays an example of auditory cortical responses elicited by pairs of auditory stimuli presented binaurally or alternated sequentially for an individual subject with SOAs jittered around 190 ms. For hemispheric lateralization, the LI was calculated as the difference between left and right mGFP response amplitudes (bottom vs. top 6 panels in Figure 2B) normalized by the sum of left and right mGFP responses (i.e. ). The LI was +1 for a response geared completely asymmetrical toward the left hemisphere, zero for a symmetrical response, and −1 for a response geared completely asymmetrical toward the right hemisphere. For pathway advantage, the LI was calculated employing the same method using the responses associated with the contra- (panels labeled 3R, 4L, 5L and 6R in Figure 2B) and the ipsi-lateral pathways (panels labeled 3L, 4R, 5R, 6L in Figure 2B). The ear LI was calculated by comparing mGFP responses from the left ear (3rd and 6th columns in Figure 2B) to the responses from the right ear (4th and 5th columns in Figure 2B). Finally, the binaural interaction LI was computed by comparing binaural stimulation (first 2 columns in Figure 2B) and monaural stimulation responses (last 4 columns in Figure 2B). The binaural interaction LI was computed for both hemispheres and for each pathway (i.e., ipsi- and contra-lateral). For each subject, the difference between the means for each LI was checked by the Student's t-test. The threshold for significance after Bonferroni correction was p < 0.0041. Test-retest reliability indices were obtained using the mean squared error for each measure of LI as well as the intra-class correlation coefficients (ICCs) on mGFP waveforms.
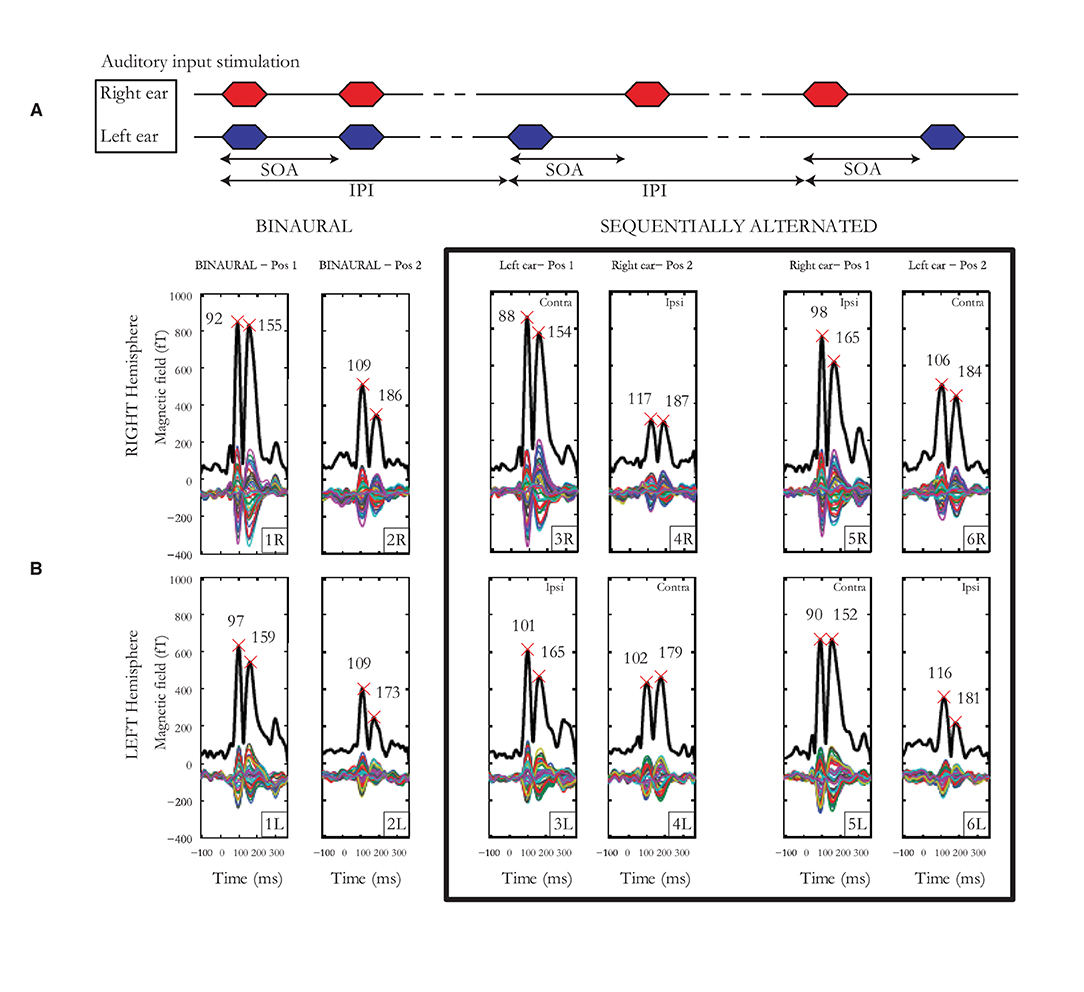
Figure 2. (A) Auditory input stimulation representing the binaural condition to the left, the sequentially alternated conditions “left ear” followed by “right ear” in the middle and then the sequentially alternated “right ear” followed by “left ear” on the right. The stimulus onset asynchrony (SOA) represents the time between the start of the two stimuli of a pair, while the inter-pair interval (IPI) represents the time interval between the onset of two successive pairs of stimuli. (B) Cortical responses from subject 1 for SOAs jittered around 190 ms. Multiple thin waveforms represent activity recorded by each of the 40 sensors located over the temporal lobe, in each hemisphere, after LS deconvolution, from −100 to 380 ms after stimulus onset. mGFP waveforms are represented with a thick black line, provided for both right and left hemispheres, the 3 presentation conditions (1 x binaural, 2 x sequentially alternated) and both first and second tone-bursts. Latencies of the N100m and P200m are indicated by crosses.
Results
Cortical Responses to Rapidly Presented Stimuli
Figure 3 presents means and standard deviations of N100m and P200m amplitudes and latencies for ear, stimulus, pathway, and hemisphere. Data analysis was conducted on the amplitude and latency of N100m and P200m in response to the second stimulus of the pair. A repeated measure ANOVA was computed with these factors: hemisphere (right, left), presentation condition (binaural, sequentially alternated left ear first, sequentially alternated right ear first), and SOA (~120, ~190, ~260 ms). The effect of SOA was found to be significant for both amplitudes and latencies of N100m (Amp. F(2,10)=46.48, p = 0.000009, ε = 0.58; Lat. F(2,10) = 7.30, p = 0.03, ε = 0.54) and for P200m amplitude (Amp. F(2,10) = 53.95, p = 0.000004, ε = 0.78). Post hoc analysis for N100m and P200m Amp showed a significant increase in amplitude from SOA ~120 to SOA ~190 ms. The amplitude increased between SOA ~190 to SOA ~260 ms was only significant for N100m. A significant interaction was present between SOA and presentation condition for both N100m (F(4,20) = 10.07; p = 0.001, ε = 0.60) and P200m [F(4,20) = 8.29; p = 0.004, ε = 0.60] latencies. Post hoc analysis revealed a decrease in N100m response latency when SOA increased from ~120 to ~190ms (p < 0.003) and from ~120 to ~ 260 ms (p < 0.02) for both sequentially alternated presentation conditions, while this trend was absent in the binaural presentation conditions. For P200m, the only significant difference was between binaural presentation and right-left sequential for SOA ~260 ms. A significant interaction was observed between hemisphere and presentation condition for N100m [Lat. F(2,10) = 41.78, p = 0.00001, ε = 0.75] and for P200m [Amp. F(2,10)=16.18, p = 0.0007, ε = 0.87; Lat. F(2,10)=14.60, p=0.001, ε = 0.82]. For N100m latencies, post hoc analysis revealed shorter latencies in the right hemisphere compared to the left hemisphere when stimuli were presented binaurally (p<0.04). Moreover, pairwise comparisons revealed longer latency for the ipsilateral pathway compared to the contralateral pathway in the sequential stimulation mode for both N100m and P200m when the second stimulus of the pair was presented to the left ear (p < 0.009) while this difference was significant only for P200m (p < 0.037) when the second stimulus was presented to the right ear. The amplitude of P200m was also significantly larger in the left hemisphere when the second stimulus of the pair was presented to the right ear. Lastly, an interaction between hemisphere, SOA and presentation condition was significant for N100m [F(4,20) = 3.34, p = 0.02, ε = 0.68]. Post hoc analysis revealed a significant difference between hemispheres for all SOAs in sequential presentation condition when the second stimulus of the pair was presented to the right ear (p < 0.008). The SOA ~ 260 ms for the binaural presentation was the only other condition that showed a significant hemispheric difference.
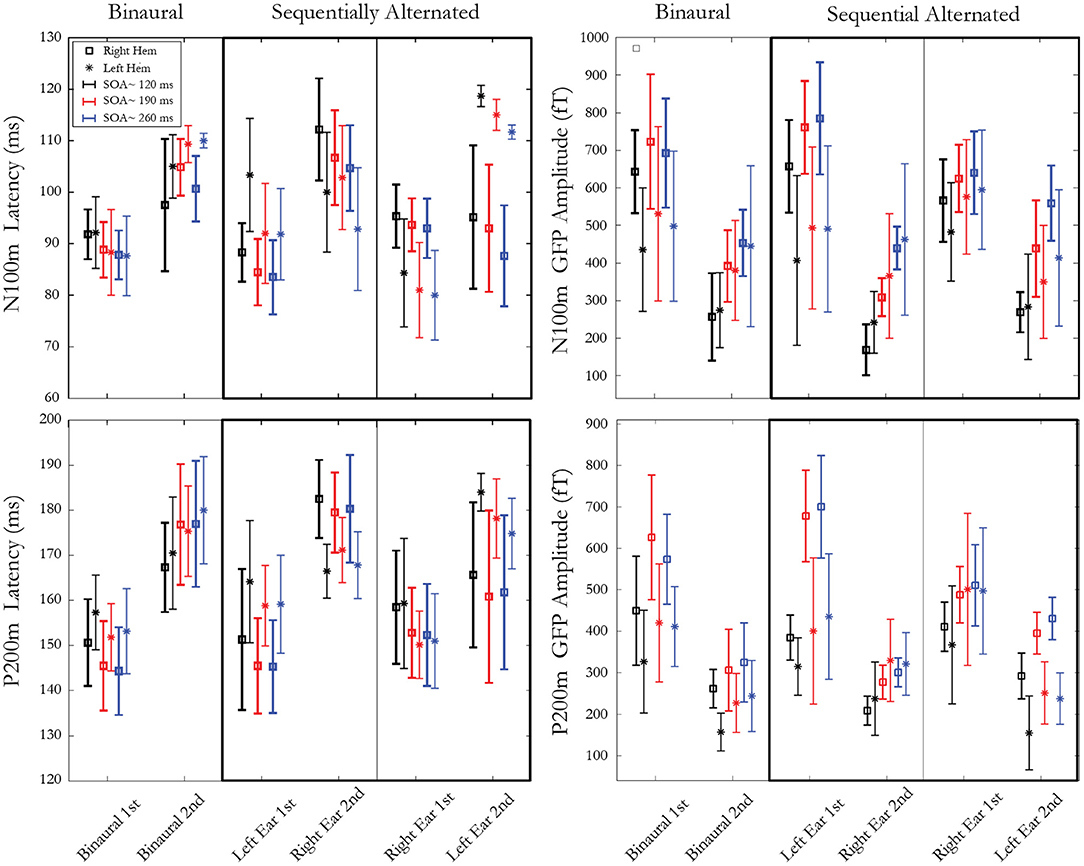
Figure 3. Latencies (left) and amplitudes (right) of mGFP N100m (top) and P200m (bottom) components. Each panel represents 3 presentation conditions: 1 binaural condition and 2 sequentially alternated conditions (stimulus presented first at either left or right ear). Within each presentation condition, three SOAs (~120 ms, ~190 ms and ~ 260 ms) are used, resulting in two responses to both stimuli of the pair, recorded from both right and left hemispheres. Error bars denote standard deviations between participants.
Hemispheric Lateralization
The hemispheric lateralization index (LI) for response amplitude presented in Figure 4A shows intra-subject differences on the vertical abscissa, and inter-subject differences on the horizontal abscissa. Subject 1 presented a rightward, subject 2 a large rightward, and subject 3 a slightly leftward lateralization. The t-test, which allows comparing the hemispheric LI to 0, was significant for each subject (p<0.001) after Bonferroni correction. No differences in symmetrical activation were found for the latencies either for subject 1 (p = 0.86) or subject 2 (p = 0.51). However, significantly earlier latencies were found in the right hemisphere for subject 3 (p = 0.0003).
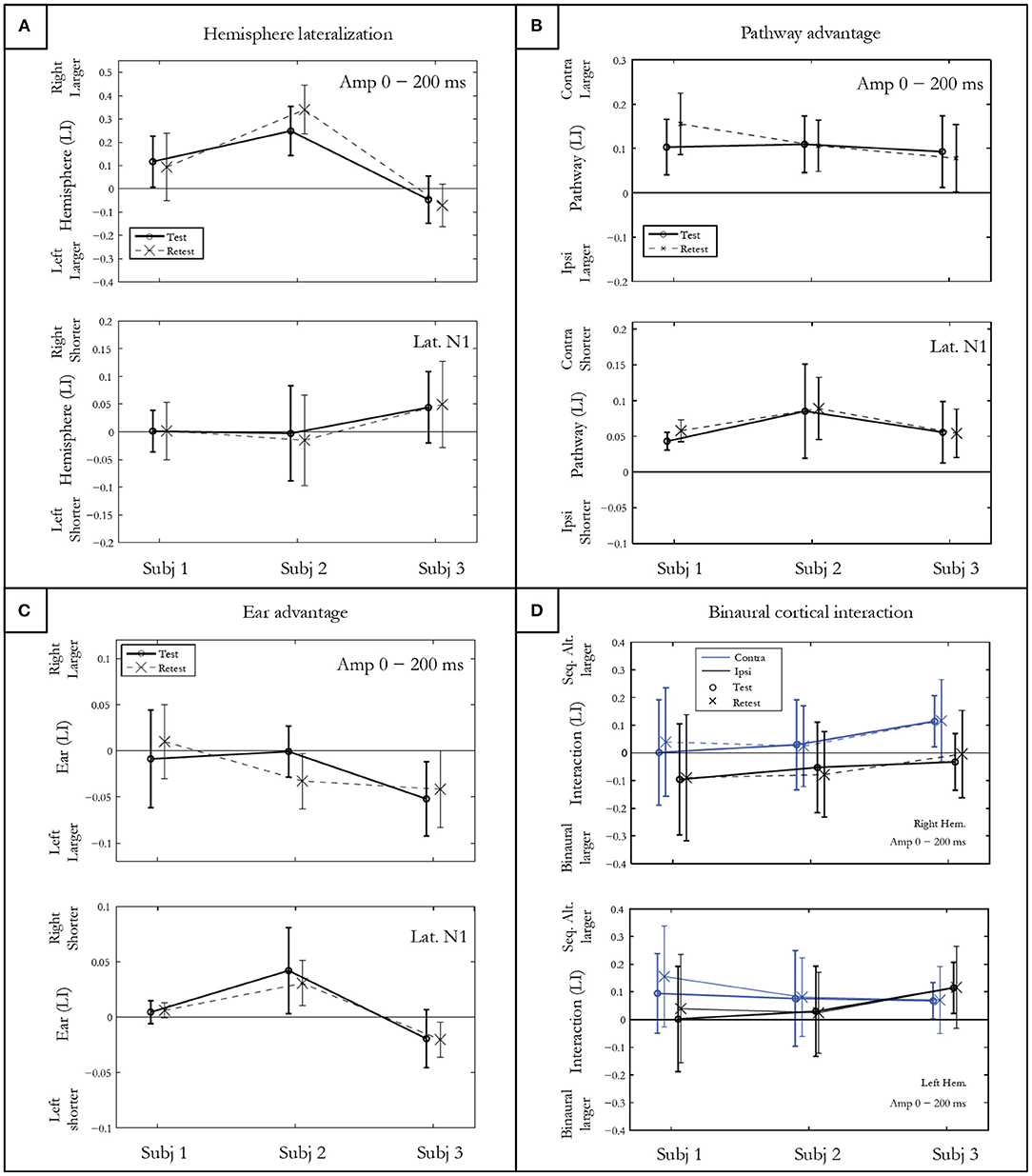
Figure 4. Indices of hemispheric lateralization (A), pathway advantage (B), and ear advantage (C) for mGFP amplitudes in a 200 ms post onset window, and for N100m latency. Both test and retest conditions are shown. The binaural cortical interaction (D) is represented for the mean mGFP amplitude in the right and left hemisphere for the contra- and ipsi-lateral pathways. Error bars denote standard deviations between conditions for each participant.
Pathway Advantage
The pathway LI calculated by contrasting contra- vs. ipsi-lateral pathway responses in the sequential conditions is represented in Figure 4B. After Bonferroni correction, significantly larger amplitudes and shorter latencies for the N100m and P200m were measured in the contra-lateral pathway for all subjects (p < 0.0001).
Ear Advantage
The statistical results of ear LI presented in Figure 4C indicated no significant amplitude difference between the activity elicited by the right and the left ear for subject 1 (p = 0.96) and for subject 2 (p = 0.01). A left ear advantage was observed for subject 3 for both amplitude (p = 0.002) and latency (p = 0.002).
Cortical Binaural Interaction (CBI)
Figure 4D shows the CBI for the three subjects in both hemispheres for contra- and ipsi-lateral pathways. The finding of a positive CBI LI indicates that the response recorded in the sequentially alternated condition is larger compared to the response in the ipsi-lateral pathway. CBI of different natures are observed for each subject. When collapsed across hemispheres, the t-test showed that CBI was close to significance only for subject 3 (subject 1: p = 0.02; subject 2: p =0.10; subject 3: p = 0.006).
Test-Retest Reliability
Two different test-retest reliability measures were computed. First, the mGFP waveforms were compared for test and retest conditions by computing the intra-class correlation coefficients (ICCs) for the three subjects in a 250 ms window post onset. A mean ICC value larger than 0.75 for each subject (i.e., subject 1 = 0.78, subject 2 = 0.79; subject 3 = 0.84) demonstrated a good test-retest reliability.
Second, a test-retest index was calculated using the mean squared error (mean = 0.057; SD = 0.026) of all four indices presented in Figure 4 (i.e., hemispheric lateralization, pathway advantage, ear advantage and CBI).
Discussion
The central aim of this paper was to introduce the deconvolution of ears' activity (DEA) paradigm which disentangles the activity in both auditory cortices elicited by stimuli presented to both ears simultaneously or separately. In this paradigm, the LS deconvolution technique was applied to MEG data recorded using pairs of stimuli presented either binaurally or alternating sequentially (i.e., right-left and left-right). The DEA paradigm allowed the investigation of auditory information transfer from one specific ear to both auditory cortices. It could also be used to explore response lateralization, the strength of crossed auditory pathways and the response adaptation properties to auditory stimuli closely separated in time. Furthermore, it allowed for the investigation of non-linear processing in the brain and CBI, mainly caused by inhibition mechanisms (Imig and Brugge, 1978; Imig and Reale, 1981; Reite et al., 1981; Papanicolaou et al., 1990).
We demonstrated the feasibility and test–retest reproducibility of this non-invasive measure on 3 right-handed normal-hearing subjects. The case studies provided examples of different auditory processing characteristics at the cortical level, identifiable at the individual level. The inter-individual differences were detectable by assessment of the difference in response between experimental conditions. For example, hemispheric lateralization was assessed by computation of the LI calculated from the responses in each hemisphere. The CBI was investigated by contrasting binaural and monaural stimulation both in contra- and ipsi-lateral pathways. The results collected using the DEA paradigm allows an objective auditory processing characterization and the generation of an individual “auditory profile” in a relatively quick time (i.e., 25 min).
Experimental Results
The data recorded from three normal-hearing subjects confirmed that both ears were represented in each cortical hemisphere. However, differences in latency and amplitude were observed for each response to various conditions.
Beyond the idea proposed by Poeppel (2003) that sound processing in the brain is a bilateral phenomenon, the present study revealed inter-individual differences in the hemispheric lateralization of the cortical response. While two subjects showed a rightward hemisphere lateralization for response amplitude, the third subject had a leftward lateralization. These hemispheric asymmetries and specializations for processing auditory stimuli were also reported previously by Mäkel et al. (1993) and Jamison et al. (2006). The cerebral lateralization of the auditory cortical area however is still highly debated (Bishop, 2013; Scott and McGettigan, 2013).
For all subjects tested, the N100m was larger and approximately 10 ms shorter for the contra-lateral compared to the ipsi-lateral auditory pathway in the sequentially alternated conditions. These results are in agreement with several studies showing a contra-lateral dominance based on lateralization of the N100m component (Pantev et al., 1986, 1998; Tiihonen et al., 1989; Woldorff et al., 1999).
Individual differences were also observed when comparing ear activity. Further research will need to investigate whether this objective measure of ear advantage is correlated with behavioral performance on a dichotic listening task such as the Dichotic Digits Test (Musiek, 1983).
The DEA paradigm allowed to investigate the suppression-type interaction and neural mechanisms underlying the processing of rapidly presented signals. As shown in Figure 4D, different binaural interactions were observed. Amplitudes of responses elicited in the sequentially alternated presentation condition were found to be either slightly larger, slightly smaller or of similar amplitude compared to the binaural presentation condition. Inter-subject differences were observed with different interactions depending on hemisphere and pathway involved. A MEG study using complex tones showed that responses to ipsi-lateral stimuli over the right auditory cortex are inhibited by the stimuli presented in the contra-lateral (left) ear (Brancucci et al., 2004).
Lastly, cortical responses to stimulus pairs separated by short SOAs allowed the study of the representation in the auditory cortex of stimuli presented closely together. The significant interactions between hemisphere, presentation condition, and SOA revealed by ANOVA indicate the complex binaural interactions occurring in the brain when processing rapidly presented stimuli.
We conclude that the DEA paradigm could represent a technique to study interesting properties of the central auditory system. Individual differences are of special interest as they provide an alternative characterization of the hearing profile of a person which could potentially be useful to for example objectively identify auditory processing disorder (APD) subjects. Using the LS deconvolution technique to separate overlapping ear activity in both auditory cortices, recorded MEG data can provide a measure for rapid temporal processing, response lateralization, auditory pathway and ear advantage, and CBI for rapidly presented sound stimuli. Such a test would allow studying the temporal acuity of the human auditory system when processing rapid changes in the acoustic signal. Moreover, it could provide insights concerning the flow of neural signals from the cochlea to the cerebral cortex. From a clinical perspective, tests are needed to better evaluate and understand the neurological characteristics of binaural processing occurring in the auditory system. Such tests could contribute to the diagnosis of neurodevelopment disorders, such as specific language impairment (SLI) or dyslexia where abnormal crossing pathways or the disability to process rapid auditory stimuli has been identified (Lamminmäki et al., 2012). However, further studies are needed to record normative data on normal hearing subjects, that can then be used as a benchmark to characterize other populations. Moreover, other complex sounds, such as speech syllables (using carefully selected jitter parameters), could be used in the future to investigate the influence of stimuli on binaural interaction mechanisms and lateralization of the response.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Macquarie University Ethics Committee. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
FB confirms being the sole contributor of this work and has approved it for publication.
Funding
This work was supported in part by: the HEARing CRC, established and supported under the Australian Cooperative Research Centers Program, an Australian Government Initiative, by the Australian Government Department of Health and by the Oticon Foundation.
Conflict of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The author gratefully thank Bram Van Dun, Harvey Dillon, Catherine McMahon, Robert Cowan, Mark Seeto and Ramesh Rajan for their suggestions during the preparation of this manuscript.
References
Bardy, F., Dillon, H., and Van Dun, B. (2014a). Least-squares deconvolution of evoked potentials and sequence optimization for multiple stimuli under low-jitter conditions. Clin. Neurophysiol. 125, 727–737. doi: 10.1016/j.clinph.2013.09.030
Bardy, F., Van Dun, B., and Dillon, H. (2014b). Mc,Mahon CM: Deconvolution of overlapping cortical auditory evoked potentials (caeps) recorded using short stimulus onset-asynchrony (soa) ranges. Clin. Neurophysiol. 125, 814–826. doi: 10.1016/j.clinph.2013.09.031
Bardy, F., Van Dun, B., and Dillon, H. (2015). “Bigger is better: Increasing cortical response amplitude via stimulus spectral complexity”. Ear Hear. 36, 677–687. doi: 10.1097/AUD.0000000000000183
Bishop, D. V. (2013). Cerebral asymmetry and language development: cause, correlate, or consequence? Science. 2013, 340. doi: 10.1126/science.1230531
Brancucci, A., Babiloni, C., Babiloni, F., Galderisi, S., Mucci, A., Tecchio, F., et al. (2004). Inhibition of auditory cortical responses to ipsilateral stimuli during dichotic listening: Evidence from magnetoencephalography. Eur. J. Neurosci. 19, 2329–2336. doi: 10.1111/j.0953-816X.2004.03302.x
Fujiki, N., Jousmaki, V., and Hari, R. (2002). Neuromagnetic responses to frequency-tagged sounds: A new method to follow inputs from each ear to the human auditory cortex during binaural hearing. J. Neurosc.i 22, 201–204. doi: 10.1523/JNEUROSCI.22-03-j0003.2002
Greenhouse, S. W., and Geisser, S. (1959). On methods in the analysis of profile data. Psychometrika. 24, 95–112. doi: 10.1007/BF02289823
Imig, T. J., and Brugge, J. F. (1978). Sources and terminations of callosal axons related to binaural and frequency maps in primary auditory cortex of the cat. J. Comp. Neurol. 182, 637–660. doi: 10.1002/cne.901820406
Imig, T. J., and Reale, R. A. (1981). Ipsilateral corticocortical projections related to binaural columns in cat primary auditory cortex. J. Comp. Neurol. 203, 1–14. doi: 10.1002/cne.902030102
Jamison, H. L., Watkins, K. E., Bishop, D. V., and Matthews, P. M. (2006). Hemispheric specialization for processing auditory nonspeech stimuli. Cereb. Cortex. 16, 1266–1275. doi: 10.1093/cercor/bhj068
Kado, H., Higuchi, M., Shimogawara, M., Haruta, Y., Adachi, Y., Kawai, J., et al. (1999). Magnetoencephalogram systems developed at kit. IEEE Trans. Appl. Supercond. 9, 4057–4062. doi: 10.1109/77.783918
Lamminmäki, S., Massinen, S., Nopola-Hemmi, J., Kere, J., and Hari, R. (2012). Human robo1 regulates interaural interaction in auditory pathways. J. Neurosci. 32, 966–971. doi: 10.1523/JNEUROSCI.4007-11.2012
Mäkel,ä, J. P., Ahonen, A., Hämäläinen, M., Hari, R., Llmoniemi, R., Kajola, M., et al. (1993). McEvoy L, Salmelin R: Functional differences between auditory cortices of the two hemispheres revealed by whole-head neuromagnetic recordings. Hum. Brain Mapp. 1, 48–56. doi: 10.1002/hbm.460010106
Musiek, F. E. (1983). Assessment of central auditory dysfunction: the dichotic digit test revisited. Ear Hear. 4, 79–83. doi: 10.1097/00003446-198303000-00002
Oldfield, R. C. (1971). The assessment and analysis of handedness: the edinburgh inventory. Neuropsychologia. 9, 97–113. doi: 10.1016/0028-3932(71)90067-4
Pantev, C., Lütkenhöner, B., Hoke, M., and Lehnertz, K. (1986). Comparison between simultaneously recorded auditory-evoked magnetic fields and potentials elicited by ipsilateral, contralateral and binaural tone burst stimulation. Int. J. Audiol. 25, 54–61. doi: 10.3109/00206098609078369
Pantev, C., Ross, B., Berg, P., Elbert, T., and Rockstroh, B. (1998). Study of the human auditory cortices using a whole-head magnetometer: left vs. right hemisphere and ipsilateral vs. Contralateral stimulation. Audiol. Neurootol. 3, 183–190. doi: 10.1159/000013789
Papanicolaou, A. C., Baumann, S., Rogers, R. L., Saydjari, C., Amparo, E. G., and Eisenberg, H. M. (1990). Localization of auditory response sources using magnetoencephalography and magnetic resonance imaging. Arch. Neurol. 47, 33–37. doi: 10.1001/archneur.1990.00530010041016
Poeppel, D. (2003). The analysis of speech in different temporal integration windows: Cerebral lateralization as ‘asymmetric sampling in time'. Speech Commun. 41, 245–255. doi: 10.1016/S0167-6393(02)00107-3
Raicevich, G., Burwood, E., Dillon, H., Johnson, B. W., and Crain, S. (2010). Wide band pneumatic sound system for MEG. 20th International Congress on Acoustics. Sydney: ICA 2010, 1–5. Available online at: http://www.acoustics.asn.au/conference_proceedings/ICA2010/cdrom-ICA2010/papers/p570.pdf
Reite, M., Zimmerman, J. T., and Zimmerman, J. E. (1981). Magnetic auditory evoked fields: Interhemispheric asymmetry. Electroencephalogr Clin. Neurophysiol. 51, 388–392. doi: 10.1016/0013-4694(81)90102-4
Scott, S. K., and McGettigan, C. (2013). Do temporal processes underlie left hemisphere dominance in speech perception? Brain Lang. 127, 36–45. doi: 10.1016/j.bandl.2013.07.006
Tiihonen, J., Hari, R., Kaukoranta, E., and Kajola, M. (1989). Interaural interaction in the human auditory cortex. Int. J. Audiol. 28, 37–48. doi: 10.3109/00206098909081609
Uehara, G., Adachi, Y., Kawai, J., Shimogawara, M., Higuchi, M., Haruta, Y., et al. (2003). Multi-channel squid systems for biomagnetic measurement. IEICE Trans. Electron. 86, 43–54. Available online at: https://search.ieice.org/bin/summary.php?id=e86-c_1_43
Woldorff, M. G., Tempelmann, C., Fell, J., Tegeler, C., Gaschler-Markefski, B., Hinrichs, H., et al. (1999). Lateralized auditory spatial perception and the contralaterality of cortical processing as studied with functional magnetic resonance imaging and magnetoencephalography. Hum. Brain Mapp. 7, 49–66. doi: 10.1002/(SICI)1097-0193(1999)7:1<49::AID-HBM5>3.0.CO;2-J
Keywords: auditory cortical responses, overlapping neural responses, auditory stimulation, least-squares deconvolution, rapid acoustic stimulation
Citation: Bardy F (2022) Deconvolution of Ears' Activity (DEA): A New Experimental Paradigm to Investigate Central Auditory Processing. Front. Syst. Neurosci. 16:892198. doi: 10.3389/fnsys.2022.892198
Received: 08 March 2022; Accepted: 16 June 2022;
Published: 14 July 2022.
Edited by:
Norbert Dillier, University of Zurich, SwitzerlandReviewed by:
Lina Reiss, Oregon Health and Science University, United StatesPavel Zahorik, University of Louisville, United States
Copyright © 2022 Bardy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fabrice Bardy, ZmFicmljZS5iYXJkeUBhdWNrbGFuZC5hYy5ueg==
 Fabrice Bardy
Fabrice Bardy