
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Syst. Neurosci. , 11 December 2020
Volume 14 - 2020 | https://doi.org/10.3389/fnsys.2020.606345
This article is part of the Research Topic The Jilted Brain: Neglected Structures and Functions View all 6 articles
 Tanuja Bordia1
Tanuja Bordia1 Natalie M. Zahr1,2*
Natalie M. Zahr1,2*Post-mortem neuropathological and in vivo neuroimaging methods have demonstrated the vulnerability of the inferior colliculus to the sequelae of thiamine deficiency as occurs in Wernicke-Korsakoff Syndrome (WKS). A rich literature in animal models ranging from mice to monkeys—including our neuroimaging studies in rats—has shown involvement of the inferior colliculi in the neural response to thiamine depletion, frequently accomplished with pyrithiamine, an inhibitor of thiamine metabolism. In uncomplicated alcoholism (i.e., absent diagnosable neurological concomitants), the literature citing involvement of the inferior colliculus is scarce, has nearly all been accomplished in preclinical models, and is predominately discussed in the context of ethanol withdrawal. Our recent work using novel, voxel-based analysis of structural Magnetic Resonance Imaging (MRI) has demonstrated significant, persistent shrinkage of the inferior colliculus using acute and chronic ethanol exposure paradigms in two strains of rats. We speculate that these consistent findings should be considered from the perspective of the inferior colliculi having a relatively high CNS metabolic rate. As such, they are especially vulnerable to hypoxic injury and may be provide a common anatomical link among a variety of disparate insults. An argument will be made that the inferior colliculi have functions, possibly related to auditory gating, necessary for awareness of the external environment. Multimodal imaging including diffusion methods to provide more accurate in vivo visualization and quantification of the inferior colliculi may clarify the roles of brain stem nuclei such as the inferior colliculi in alcoholism and other neuropathologies marked by altered metabolism.
Alcohol Use Disorder (AUD, 12-month US prevalence 13.9%; lifetime US prevalence 29.1%) is a common mental-health issue and a leading global cause of illness and mortality (Grant et al., 2015). Neuroscience research has aimed at elucidating the brain structures and functions that are impaired by chronic alcohol consumption as well as those that are amenable to repair with sustained abstinence (Salib et al., 2018; Centanni et al., 2019; Fritz et al., 2019; Koob and Colrain, 2020). While it is recognized that alcoholism involves disrupted thalamocortical and pontocerebellar circuitry (Sullivan and Pfefferbaum, 2005), the purpose of this review is to draw attention to a brainstem structure (i.e., inferior colliculus) that has long been recognized as involved in certain aspects of alcohol exposure and withdrawal, but which nevertheless warrants greater attention in the field. The midbrain tectum (i.e., “roof” of the midbrain, also known as tectal plate, and includes the corpora quadrigemina comprising the superior and inferior colliculi) is relatively homologous in all vertebrates but has demonstrated an evolutionary trend toward increased complexity (Maximino, 2008). In considering the role of the inferior colliculus in alcoholism, an overview of the its involvement in the neurological disorder Wernicke-Korsakoff Syndrome and relevant preclinical animal models is first presented. Other disorders are reviewed to highlight a potentially common mechanism of inferior colliculus vulnerability to disruption of normal energy utilization. The review concludes with a hypothesis that alcoholism is associated with reduced synchronization of thalamocortical and pontocerebellar pathways due to inferior colliculi pathology.
The phosphate derivatives of the essential, water soluble nutrient—thiamine (vitamin B1) —are required for a number of aerobic processes. The citric acid cycle, for instance, responsible for the regulation of carbohydrate, lipid, and amino acid metabolism, requires thiamine pyrophosphate (TPP, also known as thiamine diphosphate, TDP); TPP is furthermore involved in production of neurotransmitters such as glutamate and GABA (Thompson and McGeer, 1985; Dodd et al., 1996). Thiamine has a half-life of 18 days; 2–3 weeks reserves in the human body are thus readily exhausted, particularly when metabolic demands exceed intake. Although rare in Western countries (Center for Disease Control and Prevention, 2012; National Institutes of Health Office of Dietary Supplements, 2017), thiamine deficiency expressed as Wernicke’s Encephalopathy (WE) may occur in diabetes (Page et al., 2011; Pácal et al., 2014), cancer (Isenberg-Grzeda et al., 2016), hyperemesis gravidarum (Oudman et al., 2019), HIV infection (Kv and Nguyễn, 2013), and in the critically ill (Attaluri et al., 2018); it also occurs following bariatric surgery (Aasheim, 2008; Oudman et al., 2018b) and in individuals with AUD (Thomson et al., 2008; Zahr et al., 2011). Classically, diagnosis of WE required the presence of a clinical triad of oculomotor abnormalities (nystagmus or ophthalmoplegia), cerebellar dysfunction (loss of equilibrium, incoordination of gait, trunk ataxia, dysdiadokokinesia and, rarely, limb ataxia or dysarthria), and altered mental state (mental sluggishness, apathy, impaired awareness, inability to concentrate, confusion or agitation, hallucinations, behavioral disturbances, or coma) (Harper et al., 1986; Victor et al., 1989; Sechi and Serra, 2007).
Practically, WE symptoms are subtle and nonspecific and include loss of appetite, headaches, fatigue, concentration difficulties, irritability, and abdominal discomfort (Jung et al., 2012). Indeed, a retrospective analysis of clinical symptoms of patients diagnosed with WE at autopsy revealed that only 20% presented with the full triad of clinical features and 30% exhibited only cognitive impairment (Harper et al., 1986). Operational criteria to improve clinical diagnosis found that the presence of just two of four signs—dietary deficiency, ocular motor abnormality, cerebellar dysfunction, and either altered mental state or mild memory impairment—was sufficient to identify patients at risk of WE (Caine et al., 1997). The prognosis of WE critically depends on the time of onset (Harper et al., 1986) and dose (Oudman et al., 2019) of thiamine supplementation. If left untreated (as is too frequently the case, e.g., Isenberg-Grzeda et al., 2012), WE can lead to Korsakoff’s syndrome (KS), a severe, typically permanent, neurological disorder characterized by anterograde amnesia (Butters, 1981). The term “Wernicke-Korsakoff syndrome (WKS)” is used to refer to the presence of both WE and KS, due to the close relationship between the disorders (Feinberg, 1980; Butters, 1985).
Archetypal neuropathological reports of the thiamine deficient, WKS brain describe bilateral, symmetric lesions affecting periventricular areas, midbrain tectum, and hypothalamus (Victor et al., 1971). Although damage to the mammillary bodies is frequently present, it is not a necessary concomitant of WE (Victor et al., 1989). More contemporary post-mortem studies of WKS brains also report neuronal loss in thalamus (anterior principal and mediodorsal nuclei) (Halliday et al., 1994; Harding et al., 2000), basal forebrain (Halliday et al., 1994; Cullen and Halliday, 1995), and cerebellar vermis (Phillips et al., 1987). In acute WE, in vivo Magnetic Resonance Imaging (MRI) studies recapitulate post-mortem findings in reporting bilateral, symmetrical signal intensity changes (hyperintense on T2-weighted and hypointense on T1-weighted images) representing edemic foci in medial thalamus, mamillary bodies, tectal plate (superior and inferior colliculi), periaqueductal gray, and tissue surrounding the third ventricle (Lenz et al., 2002; Hegde et al., 2011; Ha et al., 2012). Regions sporadically noted by imaging studies as affected in acute WE include the caudate, red nucleus, olivary bodies, cranial nerve nuclei, corpus callosum, cerebellum, pons, and cerebral cortices (Murata et al., 2001; Zuccoli and Motti, 2008; Zuccoli and Pipitone, 2009; Liou et al., 2012). Following the resolution of edema and inflammation of acute WE, quantitative neuroimaging studies indicate volume deficits in affected brain regions (Sullivan and Pfefferbaum, 2009) including mammillary bodies, other hypothalamic nuclei, hippocampus, cholinergic forebrain, pons, and cerebellum (Sheedy et al., 1999; Sullivan et al., 1999; Sullivan and Pfefferbaum, 2009).
Damage to the inferior colliculus, as occurs in WKS, implicates effects on hearing and the vestibular system. Case reports demonstrate impaired vestibulo-ocular reflexes (Probst, 1983; Kattah et al., 2013, 2018) and occasionally hearing loss (Buscaglia and Faris, 2005; Flabeau et al., 2008; Jethava and Dasanu, 2012; Walker et al., 2014) in WE. A retrospective study of 26 WE patients (14 female) showed altered signal intensities in midbrain tectum (superior and inferior colliculi) in 38% of cases (Zuccoli and Pipitone, 2009). The inferior colliculi may be especially vulnerable to rapid thiamine depletion, as may occur in the sequalae of parenteral hyperalimentation (Vortmeyer et al., 1992; Kishimoto et al., 2012). MRI studies occasionally report overt pathology involving the quadrigeminal plate such as the presence of cancer or cysts (e.g., Mancuso et al., 1988; Weindling et al., 1988; Herrmann et al., 1992; Fischer et al., 1994; Ono et al., 1998), but quantitative volumetric measures of the quadrigeminal plate are scarce (cf., Aiba et al., 1997; Angeles Fernandez-Gil et al., 2010; Columbano et al., 2010). To our knowledge, MRI-based inferior colliculi volume in WKS has not been reported.
Animal models permit the study of underlying mechanisms, enabling researchers to better interpret findings from human studies. Two experimental approaches are used to model WE in animals. The slower approach uses a thiamine-deficient diet (i.e., feeding with a thiamine-deficient chow), which can take 3–4 weeks to produce symptoms in rodents (Nakagawasai et al., 2001; Nakagawasai, 2005). Behavioral symptoms can be achieved in just 2 weeks using a combination of a thiamine-deficient chow and intraperitoneal (i.p.) administration of a thiamine pyrophosphokinase inhibitor such as pyrithiamine (Hakim and Pappius, 1981; Zhang et al., 1995; Pfefferbaum et al., 2007; Hazell and Butterworth, 2009). Both models result in symptoms that mimic those observed in human WE, including weight loss, ataxia, seizures, and memory impairment (Pitkin and Savage, 2001; Savage et al., 2012).
In rats, histopathological findings indicate significant neuronal loss and gliosis in the thalamus, hypothalamus, midbrain (vestibular nuclei, inferior olives), and collicular plate (Troncoso et al., 1981; Vortmeyer and Colmant, 1988); the basal forebrain, white matter, and cortical regions are also sometimes affected (Langlais et al., 1996; Langlais and Zhang, 1997). Similar findings showing damage common to periaqueductal gray, mammillary bodies, and inferior colliculi were reported in cats; mediodorsal thalamic damage was reported in fewer than half of animals (Irle and Markowitsch, 1982). Bouts of thiamine deficiency (1, 2, or 4) conducted in groups of three rhesus monkeys showed the inferior colliculi to be among the first affected structures. By contrast, damage to the mammillary bodies and mediodorsal thalamus was not evident even following four bouts of thiamine deficiency (Witt and Goldman-Rakic, 1983). A histological study conducted 6-months following resolution of thiamine deficiency in the rhesus monkey showed persistently significant neuronal loss specific to the inferior colliculi and midbrain cranial nerve nuclei (Cogan et al., 1985). Longitudinal structural MRI findings in thiamine-deficient animals show similar patterns of brain changes including hyperintense signals observed on T2-weighted images in thalamus, hypothalamus, mammillary bodies, hippocampus, and colliculi (Jordan et al., 1998; Pfefferbaum et al., 2007; Dror et al., 2010; Zahr et al., 2014a, 2016b). In cats (Palus et al., 2010; Moon et al., 2013) and dogs (Garosi et al., 2003), hyperintense lesions as a result of thiamine deficiency are also noted in thalamus and colliculi, as well as in cerebellum.
A number of reports using thiamine deficiency models observed that the intensity of neurological symptoms and the extent and location of lesions is complex and can vary greatly among individual animals (Witt, 1985; Read and Harrington, 1986; Mair et al., 1988). Selective vulnerability of thiamine-sensitive regions has been ascribed to their high metabolic demand (Hakim and Pappius, 1981), associated with low energy (ATP and phosphocreatine) (Aikawa et al., 1984), acidosis (reduced pH) (Hakim, 1984; Navarro et al., 2008), reduced carbon dioxide (CO2) (Gibson et al., 1989) and elevated nitric oxide (Kruse et al., 2004) production, altered perfusion (Hakim, 1986), compromised blood brain barrier integrity (Phillips and Cragg, 1984; Calingasan et al., 1995; Harata and Iwasaki, 1995; Chen et al., 1997), and gliosis (Leong et al., 1994, 1996). The variously sensitive regions may be vulnerable due to unique underlying mechanisms (e.g., Vortmeyer and Colmant, 1988; Matsushima et al., 1997; Hazell et al., 1998; Meng and Okeda, 2003; Ke and Gibson, 2004; Vemuganti et al., 2006). The edematous nature of inferior colliculus pathology (Watanabe and Kanabe, 1978), for example, may explain why it is detected early in the course of in thiamine deficiency by in vivo MRI, which is sensitive to brain injury caused by tissue edema (Jung et al., 2012).
Alcohol Use Disorder (AUD) is a prevalent, complex, dynamic condition with profound CNS effects (Grant et al., 2015; Sullivan and Pfefferbaum, 2019). Chronic alcohol abuse is associated with decreased absorption of thiamine (Hoyumpa, 1980; Lieber, 2003; Martin et al., 2003; Saad et al., 2010; Heirene et al., 2018; Oudman et al., 2018a; Karakonstantis et al., 2020) and impaired hepatic function (Levy et al., 2002; Butterworth, 2009), which may together contribute to subclinical thiamine deficiency. Genetic mutations of the thiamine transporters (SLC19A2/SLC19A3) (Kono et al., 2009) or transketolase enzymes (TKTL1) (Coy et al., 1996, 2005) may further predispose individual alcoholics to thiamine deficiency (Jung et al., 2012). Traditional clinical pathological methods demonstrate only mild cerebral atrophy and lower mean brain weight in cases of uncomplicated (i.e., absent diagnosable neurological complications) alcoholism relative to healthy controls (Harper and Blumbergs, 1982; Halliday et al., 1993).
Quantitative studies, required to characterize the relatively subtle structural abnormalities caused by the direct effects of alcohol, have demonstrated greater mean peri-cerebral spaces in the AUD than in the healthy control brain (Harper et al., 1985). Stereometric studies have suggested that this reduction in brain volume is largely accounted for by the shrinkage of white matter (Harper et al., 1985; de la Monte, 1988; Kril et al., 1997). Cerebellar white matter volume, especially in the vermis, is significantly smaller than in control brains (Phillips et al., 1987), and corpus callosum area is significantly thinned in alcoholics (Harper and Kril, 1988; Tarnowska-Dziduszko et al., 1995). Microscopic studies also reveal ∼25% loss of pyramidal neurons in the superior frontal and frontal association cortices of AUD relative to healthy brains (Harper and Kril, 1994; Kril et al., 1997). Although neuronal loss in the supraoptic and paraventricular nuclei of the hypothalamus correlates with maximum daily alcohol consumption (Harding et al., 1996), pathological studies have not consistently shown a decrease in the number of neurons in cerebellum, basal ganglia (Harper et al., 2003), hippocampus (Kril et al., 1997; Baker et al., 1999), or serotonergic raphe nuclei (Baker et al., 1996).
In general, cross-sectional magnetic resonance imaging (MRI) studies of AUD report volume deficits in cortical gray and white matter and anterior cerebellum (Zahr, 2014). Selective regions of frontal cortex are among the most commonly described volume deficits in alcoholism (Zahr et al., 2017). Large-scale longitudinal MRI studies demonstrate AUD-related volume deficits in frontal, temporal, parietal, cingulate, and insular cortices with evidence for accelerated aging in volumes of precentral and superior frontal cortices (Sullivan et al., 2018). Although less severe, the AUD brain shows volume deficits in regions affected by thiamine-deficiency-associated WKS including mammillary bodies, hippocampus, thalamus, cerebellum, and pons (Sullivan and Pfefferbaum, 2009; Le Berre et al., 2014; Pfefferbaum et al., 2018). These graded effects suggest that AUD individuals carry a history, or “scar,” from subclinical bouts of thiamine deficiency. This hypothesis is supported with reference to neuropsychological performance in studies which categorize AUD individuals by the operationalized criteria for determining history of preclinical WE (Ambrose et al., 2001a,b). Uncomplicated alcoholics meeting no WE criteria performed at normal levels on a large neuropsychological test battery; those meeting one criterion performed at impaired levels on a few of the test composites; those meeting two or more criteria were impaired on all test composites (Pitel et al., 2011; Fama et al., 2019). Thus, although these AUD individuals had no history of clinically diagnosable WE, performance impairment level conforms to the “dose effect” of a WE burden. To our knowledge, only two studies provide evidence for effects of AUD on the tectal plate. A post-mortem neuropathological study comparing alcoholic to control brains (n = 9 in each group) demonstrated higher levels of gangliosides in alcoholics relative to controls in the quadrigeminal plate (Alling and Bostrom, 1980). In a computed tomography (CT) study comparing 327 chronic alcoholics to 419 age-matched controls, the cistern of the quadrigeminal plate was one of six parameters that distinguished alcoholics and controls (Kohlmeyer et al., 1986).
Rat models of alcohol dependence, typically using intragastric (e.g., French, 2001), intraperitoneal (e.g., Correa et al., 2009; Fernandez-Lizarbe et al., 2009), or vapor (e.g., Roberts et al., 2000; Vendruscolo and Roberts, 2014) ethanol exposure, have revealed a variety of susceptible brain regions. Markers of degeneration typically highlight effects of ethanol on corticolimbic circuitry (Geisler et al., 1978; Grupp and Perlanski, 1979; Carlen and Corrigall, 1980; Devenport et al., 1981; Roulet et al., 1985; Cadete-Leite et al., 1989; Moghaddam and Bolinao, 1994; Collins et al., 1996, 1998; Nakano et al., 1996; Zou et al., 1996; Corso et al., 1998; Nixon and Crews, 2002; Obernier et al., 2002; Rice et al., 2004; Kelso et al., 2011; McClain et al., 2011; Maynard and Leasure, 2013). Studies determining localized central nervous system (CNS) changes in glucose metabolism—a marker for neuronal activity—show a more widespread signature of ethanol exposure in auditory circuitry (i.e., inferior colliculus, medial geniculate), and structures including thalamus, cerebellum, and pons (Eckardt et al., 1988; Grunwald et al., 1993; Williams-Hemby and Porrino, 1994; Dudek et al., 2015). Animals withdrawing from ethanol show elevated glucose metabolism in the limbic system (i.e., piriform cortex, amygdala, hippocampus), frontal sensorimotor systems, diencephalon (i.e., thalamus, hypothalamus), midbrain (i.e., inferior colliculus, locus coeruleus, median raphe), cerebellum (flocculus, paraflocculus, vermis, white matter), and brainstem (i.e., pons) (Campbell et al., 1982; Eckardt et al., 1986, 1992; Marietta et al., 1986). Similarly, upregulation of immediate early gene (e.g., c-Fos) expression (another marker of recent neuronal activity) during ethanol withdrawal is observed in regions including the olfactory bulbs, cerebral cortex, inferior colliculi, cerebellum, and brain stem (Matsumoto et al., 1993; Wilce et al., 1994; see also, Putzke et al., 1998; Smith et al., 2019).
One of the most consistent, translational findings made with structural MRI in ethanol -exposed rodents is ventricular enlargement, which may be influenced by both timing and exposure method. Rats that achieve binge-like blood alcohol levels via gavage feeding show a larger effect (Zahr et al., 2010b, 2013, 2014b) than rats exposed to ethanol chronically via vapor (Pfefferbaum et al., 2008; Zahr et al., 2020a). The effect on ventricle size is transient: ventricular volume recovers rapidly within one week of abstinence (Zahr et al., 2016a, 2020a,b). A high field strength 7T animal scanner and voxel-based, rather than region-of-interest (ROI) based morphological evaluation demonstrated in animals exposed to binge ethanol exposure reversible ventricular enlargement and thalamic shrinkage but enduring shrinkage of pretectal nuclei, and superior and inferior colliculi (Zhao et al., 2018). In a follow up study, two experiments [binge (4-day) intragastric ethanol in Fisher 344 rats and chronic (1-month) vaporized ethanol in Wistar rats] showed similarly affected brain regions including retrosplenial and cingulate cortices, dorsal hippocampi, central and ventroposterior thalami, superior and inferior colliculi, periaqueductal gray, and corpus callosum: volumes of the colliculi and periaqueductal gray showed persistent deficits with abstinence (Zhao et al., 2020). Although the inferior colliculi, in particular, have been studied as a substrate for ethanol -withdrawal induced seizures (McCown and Breese, 1990, 1993; Chakravarty and Faingold, 1998; Evans et al., 2000; Faingold et al., 2000; N’Gouemo and Morad, 2003, 2014; N’Gouemo et al., 2006, 2015; Akinfiresoye et al., 2016; Newton et al., 2018), this recent study (i.e., Zhao et al., 2020) demonstrates inferior colliculi shrinkage in the response to both acute and chronic ethanol exposure absent seizures and absent detectable thiamine deficiency (cf., Zahr et al., 2010a).
Beyond ethanol, lead (Bini and Bollea, 1947), mercury (Oyanagi et al., 1989; Nagashima, 1997; Kern et al., 2012), and methyl bromide (Goulon et al., 1975) poisoning can result in a WKS-like pattern of brain damage particularly affecting inferior colliculi and visual and auditory functions (Otto and Fox, 1993; Counter et al., 2011).
Lesions in Leigh’s disease—a genetic neurometabolic disorder associated with reduced or absent thiamine triphosphate—are cardinally present in dorsal midbrain (periaqueductal gray, superior and inferior colliculi) among other thiamine-sensitive regions (e.g., thalamus) (Cavanagh and Harding, 1994; Huang et al., 1996; Scalais et al., 2012; Wei et al., 2018) and may be associated with disturbed hearing and vision. Indeed, a recent report describes a woman with a history of bariatric surgery with near total external ophthalmoplegia and hearing loss that resolved with intravenous thiamine treatment (Nyce et al., 2020).
Fetal Alcohol Spectrum Disorder (FASD), which refers to the sequalae of alcohol abuse engaged in during pregnancy and is expressed as attention deficit hyperactivity disorder (ADHD) (Aronson et al., 1997), autistic-like behaviors (Nanson, 1992; Harris et al., 1995), and often exhibiting problems with vision, hearing, speech, and postural stability (Church, 1987; Church and Abel, 1998; Wozniak et al., 2019). Microcephaly is a common feature of FASD, but autopsy studies also report abnormalities of the corpus callosum, brainstem, and cerebellum as well as effects on hippocampus and basal ganglia. Post-mortem findings have been confirmed by in vivo MRI studies showing FASD effects on corpus callosum, basal ganglia, diencephalon, and cerebellum (Roebuck et al., 1998). Ethanol-exposed infant rodents (Ikonomidou et al., 2000; Olney et al., 2002; Dikranian et al., 2005) and monkeys (Farber et al., 2010) show brain damage to subcortical structures and cerebellum, with evidence for effects on periaqueductal gray and superior and inferior colliculi (Zajac et al., 1988; Phillips et al., 2000; N’Gouemo and Lovinger, 2012) possibly via mechanisms of impaired mitochondrial function or oxidative stress (Chu et al., 2007).
The complex developmental disorder autism may present with symptoms of oculomotor and auditory dysfunction (Rapin, 1988; Miller et al., 1998). Some studies have shown involvement of the inferior colliculi (e.g., Baldwin et al., 2016) among other brainstem structures such as inferior olives, corpus callosum, and cerebellum (Ritvo et al., 1986; Courchesne, 1997; Piven et al., 1997; Bailey et al., 1998; Kemper and Bauman, 1998) and links to mitochondrial dysfunction and disturbed brain energy metabolism (Lombard, 1998; Chauhan and Chauhan, 2006; Palmieri and Persico, 2010; Shoffner et al., 2010; Chauhan et al., 2011; Anitha et al., 2013; Siddiqui et al., 2016). In animal models, the genetic disorder Fragile X Syndrome likewise shows evidence for inferior colliculus involvement (Gonzalez et al., 2019; Kokash et al., 2019; Nguyen et al., 2020). Asphyxia at birth in humans (Schneider et al., 1975; Leech and Alvord, 1977; Roland et al., 1988) and in monkeys (Windle, 1969; Myers, 1972) results in disproportionate injury to thalamus and brainstem nuclei, particularly the inferior colliculi. In adult animals, the inferior colliculi are among the most frequently and seriously damaged regions in response to transient ischemia (Araki et al., 1990; Siman et al., 2005; Pan et al., 2019).
A 1955 study using radioactive tracers to determine local cerebral glucose utilization in the cat brain revealed greatest consumption in brainstem auditory nuclei, nearly three times more than cerebral white matter and two times more than most cortical regions (Landau et al., 1955–1956). Glucose utilization—in species ranging from mice to monkeys—has since been confirmed to be highest (among evaluated brain regions) in the auditory system, particularly in the inferior colliculi (Sokoloff et al., 1977; Sokoloff, 1981; Kennedy et al., 1982; Bryan, 1986). Capillary density (Gross et al., 1986; Klein et al., 1986; Song et al., 2011), blood volume (Cremer and Seville, 1983), blood flow (Jay et al., 1988), and levels of glucose transport proteins (e.g., GLUT1) (Zeller et al., 1997) are also higher in inferior colliculus than in other regions measured. This high metabolic demand may be due to the involvement of the inferior colliculus in the integration of sensory inputs (Houser et al., 2010). High rates of blood flow and aerobic metabolism (Sokoloff et al., 1977; Zeller et al., 1997) likely increase its vulnerability to toxic and traumatic brain injuries (Kanner and Eisenberg, 1957; Denny-Brown, 1962). Because a variety of syndromes with various pathogenic causes can have WKS-like neuropathology, and because the inferior colliculi are among the most metabolically demanding brain regions, it has been proposed that a common etiological factor may be energy deprivation (Simon, 1999) as would occur in mitochondrial disorders (Lestienne and Bataillé, 1994; Althoff et al., 2010).
Located on the dorsal surface of the mesencephalon caudal to the superior colliculus, the inferior colliculus is the largest nucleus of the auditory system. The inferior colliculus varies in size by more than 130-fold among mammals: relative to total brain size, rats have among the largest and humans have the smallest (Glendenning and Masterton, 1998; Figure 1). The inferior colliculus comprises core [central nucleus of the inferior colliculus (CNIC)] and shell (dorsal and ventral nuclei) regions (Oliver and Morest, 1984). Both structures are composed of excitatory glutamatergic and inhibitory GABAergic (∼25%) neurons (Merchán et al., 2005; Ono et al., 2005, 2017; Ito et al., 2011; Schofield and Beebe, 2019). Vascular supply to inferior colliculus is principally via paramedian branches of the basilar artery (Ruchalski and Hathout, 2012; Komune et al., 2015).
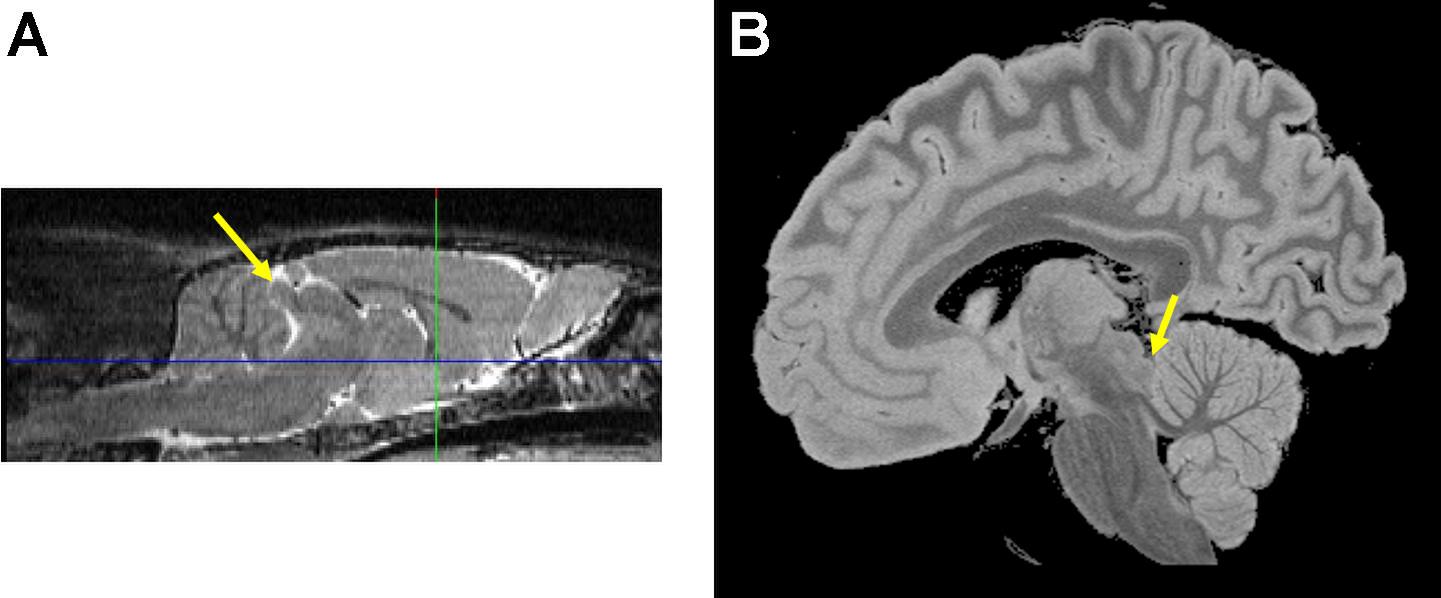
Figure 1. The inferior colliculus (yellow arrows) on sagittal images from a (A) wild type Wistar rat and a (B) postmortem formalin fixed human brain specimen. Images compliments of Adolf Pfefferbaum, M.D., SRI International.
The inferior colliculus is one of the earliest structures to become myelinated and functional in the developing human brain. Histological studies have demonstrated that the statoacoustic system begins myelination at the end of the fifth fetal month in the second trimester (Yakovlev and Lecours, 1967; Moore et al., 1995). In mature newborns, the inferior colliculus, superior olivary nucleus, and lateral lemniscus are nearly completely myelinated (Rorke et al., 1968). Early myelination of the inferior colliculi has also been demonstrated in vivo by MRI (Curnes et al., 1988; Counsell et al., 2002; Sano et al., 2007).
Afferents to the inferior colliculus are excitatory and inhibitory (González-Hernández et al., 1996; Patel et al., 2017; Figure 2). Brainstem ascending inputs from the cochlear nuclei and superior olives via the lateral lemniscus generally terminate bilaterally in the CNIC (Cant and Benson, 2006, 2007). Additional afferents, which tend to be non-auditory, principally target the shell and arise from other brainstem nuclei (e.g., substantia nigra pars compacta, ventral tegmental area, dorsolateral periaqueductal gray, olivary nuclei, locus coeruleus, dorsal raphe), spinal trigeminal nucleus (somatosensory input), deep layers of the superior colliculus, cerebellum, and target regions such as medial geniculate body, other thalamic structures (posterior limitans, suprapeduncular nucleus, and subparafascicular intralaminar nuclei of the thalamus), and auditory cortex (Adams, 1979; Glendenning and Masterton, 1983; Oliver, 1984a, 1987; Coleman and Clerici, 1987; Winer et al., 1998, 2005; Zhou and Shore, 2006; Loftus et al., 2008; Hurley and Sullivan, 2012; Chen et al., 2018). Indeed, a number of studies show that processing and integration in the inferior colliculi are significantly modulated by a massive descending corticofugal system (Jen et al., 1998; Jen and Zhou, 2003; Popelár et al., 2003; Yan et al., 2005; Zhou and Jen, 2007; Ma and Suga, 2008). Efferents from the inferior colliculus predominately targeting ipsilateral medial geniculate bodies travel through the inferior brachium (Calford and Aitkin, 1983); 40% of projections to the thalamus are GABAergic (Peruzzi et al., 1997). There is also evidence for efferent fibers to lateral lemniscus (Ito and Oliver, 2012; Ruchalski and Hathout, 2012), periaqueductal gray, and superior colliculus (Chen et al., 2018; Goyer et al., 2019).
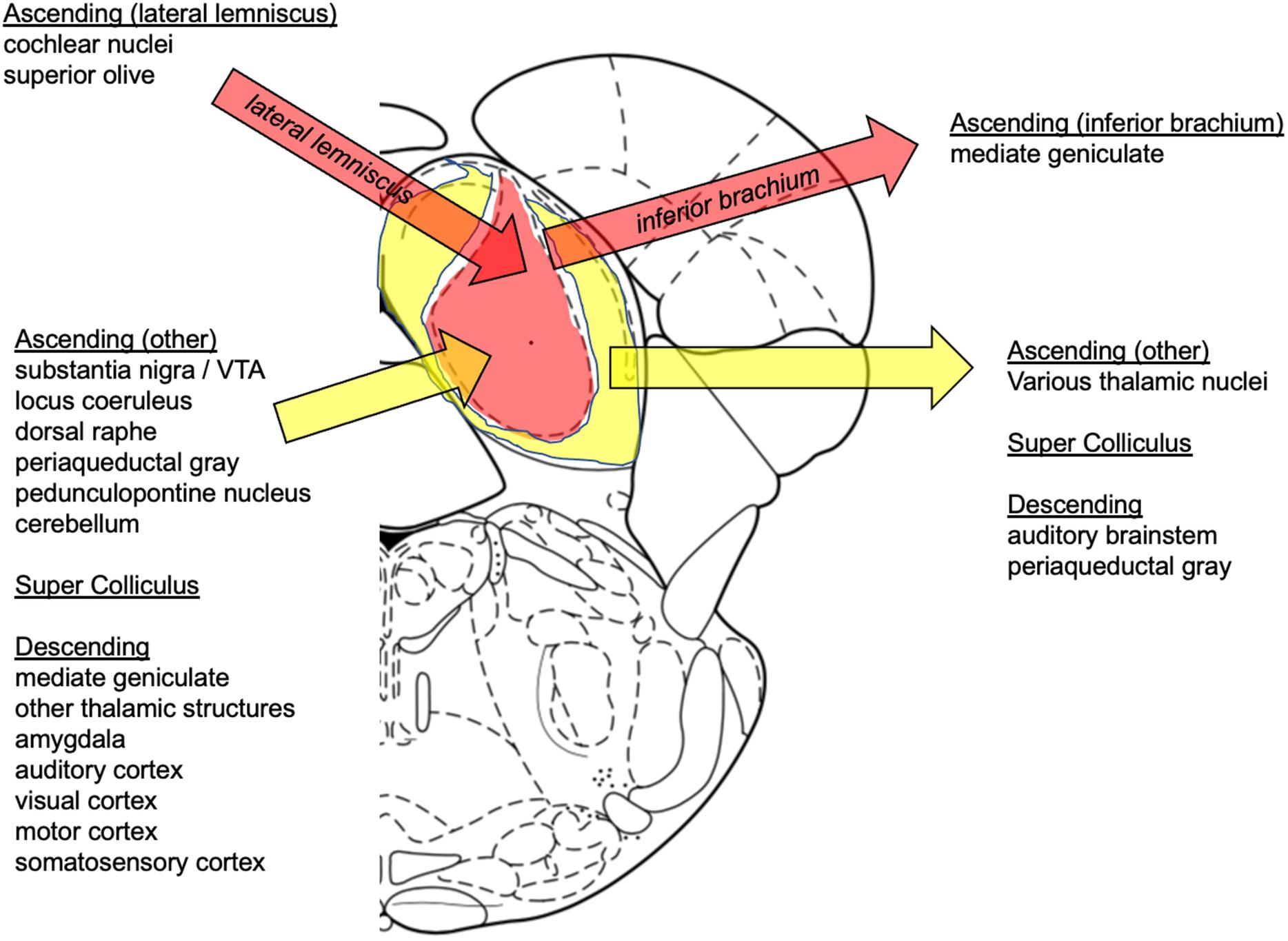
Figure 2. Summary of inferior colliculus afferent and efferent connections. Afferents: Ascending (later lemniscus): cochlear nuclei (Klepper and Herbert, 1991; Cant and Benson, 2006), superior olive (Cant and Benson, 2006), lateral lemniscus (Zhang et al., 1998; Saalmann et al., 2006; Kelly et al., 2009; Chen et al., 2018); Ascending (other): substantia nigra/VTA (Takada et al., 1987; Kemel et al., 1988; Yasui et al., 1991; Deniau and Chevalier, 1992; Moriizumi et al., 1992), locus coeruleus (Klepper and Herbert, 1991; Hormigo et al., 2012), dorsal raphe (Hurley and Pollak, 2001; Hurley, 2006; Hurley and Sullivan, 2012), pedunculopontine nucleus (Noftz et al., 2020), periaqueductal gray (Dujardin and Jürgens, 2005), cerebellum (Huffman and Henson, 1990); Super Colliculus (Adams, 1980; Clerici and Coleman, 1987; Stepniewska et al., 2000; Nodal et al., 2005); Descending: medial geniculate (Driscoll and Tadi, 2020), other thalamic structures (Kuwabara and Zook, 2000), amygdala (Hopkins and Holstege, 1978; Marsh et al., 2002), auditory cortex (Games and Winer, 1988); visual, motor, somatosensory cortices (Cooper and Young, 1976). Efferents: Ascending (inferior brachium): medial geniculate (Oliver, 1984b; Oliver et al., 1991; Peruzzi et al., 1997; Smith et al., 2006); Ascending (other): other thalamic nuclei (Kudo and Niimi, 1980; Senatorov and Hu, 2002; Smith et al., 2006), auditory cortex (Schofield et al., 2011; Xiong et al., 2015; Schofield and Beebe, 2019; Lesicko et al., 2020); Super Colliculus (Van Buskirk, 1983); Descending: auditory brainstem (Huffman and Henson, 1990), periaqueductal gray (Santos et al., 2003; Reimer et al., 2008).
Positioned to serve as a relay station to analyze, integrate, and route sound signals to higher level brain centers (Casseday et al., 1994; LeBeau et al., 2001; Pan et al., 2004), the inferior colliculus is also involved in multi-modal sensory perceptions such as the vestibulo-ocular reflex (Feng, 1992; Brandao et al., 1993), and startle response (Jordan and Leaton, 1982; Leitner and Cohen, 1985; Moore et al., 1995; Li et al., 1998; Li and Yeomans, 2000; Li and Yue, 2002; Heldt and Falls, 2003; Nobre et al., 2003; Satake et al., 2012). Specifically, converging anatomical and physiological evidence indicates that cells within the inferior colliculus are sensitive to visual, oculomotor, and somatosensory information as well as to signals relating to behavioral context and reward. Ethnologically, it is considered to drive acoustic-motor behaviors including predator escape (Xiong et al., 2015), prey localization (Knudsen and Konishi, 1978; King et al., 1998), and conspecific communication (Jürgens, 2002; Wilczynski and Ryan, 2010; Gruters and Groh, 2012). The inferior colliculus may also serve to enhance perception by decreasing attentional thresholds and increasing alertness (Hermans et al., 2011; LeDoux, 2012); indeed, arousal induced by sound can facilitate attention in a subsequent visual search (Lee et al., 2014; Asutay and Vastfjall, 2017), a behavior likely mediated by the inferior colliculus. The brainstem auditory evoked potential (BAEP) is easily recorded, has an invariant waveform and is stable and robust (Shaw, 1988). Both human (e.g., Kevanishvili, 1980; Zappia et al., 1996) and rodent (e.g., Funai and Funasaka, 1983; Shaw, 1987) studies have ascribed the inferior colliculus as the origin of portions of wave V of the BAEP (Kevanishvili, 1980). A comprehensive discussion of inferior colliculus organization and function is beyond the scope of this review. Instead, the interested reader is referred to Winer and Schreiner (2005) and Malmierca and Young (2015).
The remaining portions will discuss the inferior colliculus in the context of alcoholism-related circuitry. Behaviors associated with the use of ethanol include an initial stimulatory effect and a prominent depressant action; alcohol abuse can result in tolerance and physical dependence, which may express as withdrawal comprising symptoms of tremor, hallucinations, motor and autonomic hyperactivity, and seizures. Critically, the inferior colliculus has long been described as involved in ethanol-withdrawal seizures, typically induced by an auditory trigger in animal models (Riaz and Faingold, 1994) and supported by research in genetically epilepsy-prone rats (Faingold et al., 1992; Ribak and Morin, 1995). During ethanol withdrawal, inferior colliculus metabolism is elevated (Eckardt et al., 1986) allowing for enhanced responsivity to acoustic stimuli, thereby providing a basis for greater seizure susceptibility (Chakravarty and Faingold, 1997). This is due, in part, to the fact that GABA-mediated inhibition, which normally limits high intensity firing of inferior colliculus neurons, is less effective during ethanol withdrawal due to downregulation or desensitization of GABA-A receptors and over-activation of NMDA receptors (Faingold et al., 1993).
Independently, work has implicated the periaqueductal gray in responses to ethanol exposure in animal models (Yang et al., 2003; Ezequiel Leite and Nobre, 2012; Li et al., 2013). Both the periaqueductal gray (George et al., 2019) and the inferior colliculus (Nobre et al., 2010) may be involved in stress and anxiety. The quadrigeminal plate and dorsal periaqueductal gray (Bandler et al., 1991; Brandão et al., 1994) and likely amygdala and medial hypothalamus (Coimbra and Brandão, 1997) are the presumed neural substrates of aversion, integration of defensive behaviors, and analgesia (Lowe et al., 2007; Reimer et al., 2008). In particular, the inferior colliculus, which can respond to both rewarding (Nienhuis and Olds, 1978) and aversive (Ruth et al., 1974) stimuli, is thought to integrate information involved in modulating fear-related behaviors (Lamprea et al., 2002) via anatomical and functional connections with the amygdala (Maisonnette et al., 1996). Indeed, neuronal activity in a circuit between the medial prefrontal cortex and dorsal periaqueductal gray (Vander Weele et al., 2018) was recently shown to regulate ethanol drinking: inhibition of this cortico-brainstem pathway promoted compulsive (i.e., aversive-resistant) drinking (Siciliano et al., 2019). These findings, however, are derived from animal models.
Although there has been some support for inferior colliculus involvement in seizures associated with Leigh’s disease (e.g., Wei et al., 2018), direct evidence for their involvement in alcohol withdrawal in human studies is scarce. Instead, BAEP responses to alcohol indirectly suggest involvement of the inferior colliculi (e.g., Squires et al., 1978). BAEPs are particularly impaired in alcoholics with a history of seizures (Touchon et al., 1984; Neiman et al., 1991). Even in healthy human men, however, blood alcohol levels of 70 mg/dL were associated with depression of several components of the BAEP (Pfefferbaum et al., 1979). Ethanol may have greater effect on the BAEPs elicited under inattentive than under attentive conditions (Soveri and Fruhstorfer, 1969) suggesting that the generally observed depressant effect on BAEP is not due solely to the direct pharmacological ethanol but may be mediated or intensified by the general decline in attentiveness accompanying intoxication.
As described, our recent work using novel, voxel-based analysis of structural MRI data demonstrated in three independent studies [two ethanol intoxication models: “binge” (4-day) via intragastric gavage and “chronic” (1-month) via vaporized ethanol; two strains: wild-type Wistar and Fisher 344 rats] significant shrinkage of the inferior colliculi (Zhao et al., 2018, 2020). In Wistar rats exposed to the binge protocol, a single week of abstinence was insufficient for inferior colliculi volume recovery (Zhao et al., 2018). Notably, Wistar rats continued to show transient inferior colliculus volume loss even after three cycles of 1-month vaporized ethanol exposure (Zhao et al., 2020). In both intragastric binge and “chronic” vaporized ethanol models, earlier work failed to demonstrate quantifiable thiamine deficiency (cf., Zahr et al., 2009, 2010a).
Volume loss in response to ethanol in brain regions sensitive to thiamine deficiency may be interpreted in several ways. (1) Ethanol-exposed animals could be thiamine-deficient transiently (i.e., missed data collection time point) or below detection levels. This interpretation would also hold for human studies which are even more challenging than those in animals with respect to capturing a clinically relevant time point (i.e., individuals with AUD may experience transient thiamine deficiency that is not captured in a laboratory setting). (2) Alternatively, circulating thiamine levels may be adequate, but ethanol may disrupt tissue absorption of thiamine (Abdul-Muneer et al., 2018). (3) Finally, high doses of ethanol may disrupt energy metabolism without affecting thiamine utilization. Ethanol, per se, is disruptive to cellular respiration (Blachley et al., 1985; Cunningham and Ivester, 1999). AUD is associated with decreased brain glucose utilization (Volkow et al., 2006; Pawlosky et al., 2010) and increased acetate uptake (Sarkola et al., 2002; Volkow et al., 2013). Evidence suggests that even heavy drinking promotes the use of acetate rather than glucose as a substrate of mitochondrial energy oxidation (cf., Jiang et al., 2013). The ethanol-induced utilization of alternative energy substrates may initially target brain regions with high metabolic demand such as the inferior colliculi.
Beyond primary sensation (Sprague et al., 1961), the tectum is a “perceptual facilitating apparatus….a vital facilitator of thalamocortical function…” (so that) just as cortical activity is dependent on thalamic integrity, so both also require the colliculi (Denny-Brown, 1962). It is this more general role—that is, the “alerting” or “attention-focusing” function of the inferior colliculus (cf., Latash, 1990) that is proposed herein to be the initial insult in alcohol addiction. Disruption of thalamocortical circuitry in AUD has been associated with craving (Modell et al., 1990), reduced arousal (Jia et al., 2007; Eckle and Todorovic, 2010), and sleep impairments (Liu et al., 2019), whereas disordered pontocerebellar circuitry has been associated with impaired balance and visuospatial abilities (Sullivan, 2003; Sullivan et al., 2010). Thus, it is proposed that the primary impairment in alcohol exposure is due to a lack of thalamocortical and pontocerebellar synchronization by the inferior colliculi (Figure 3).
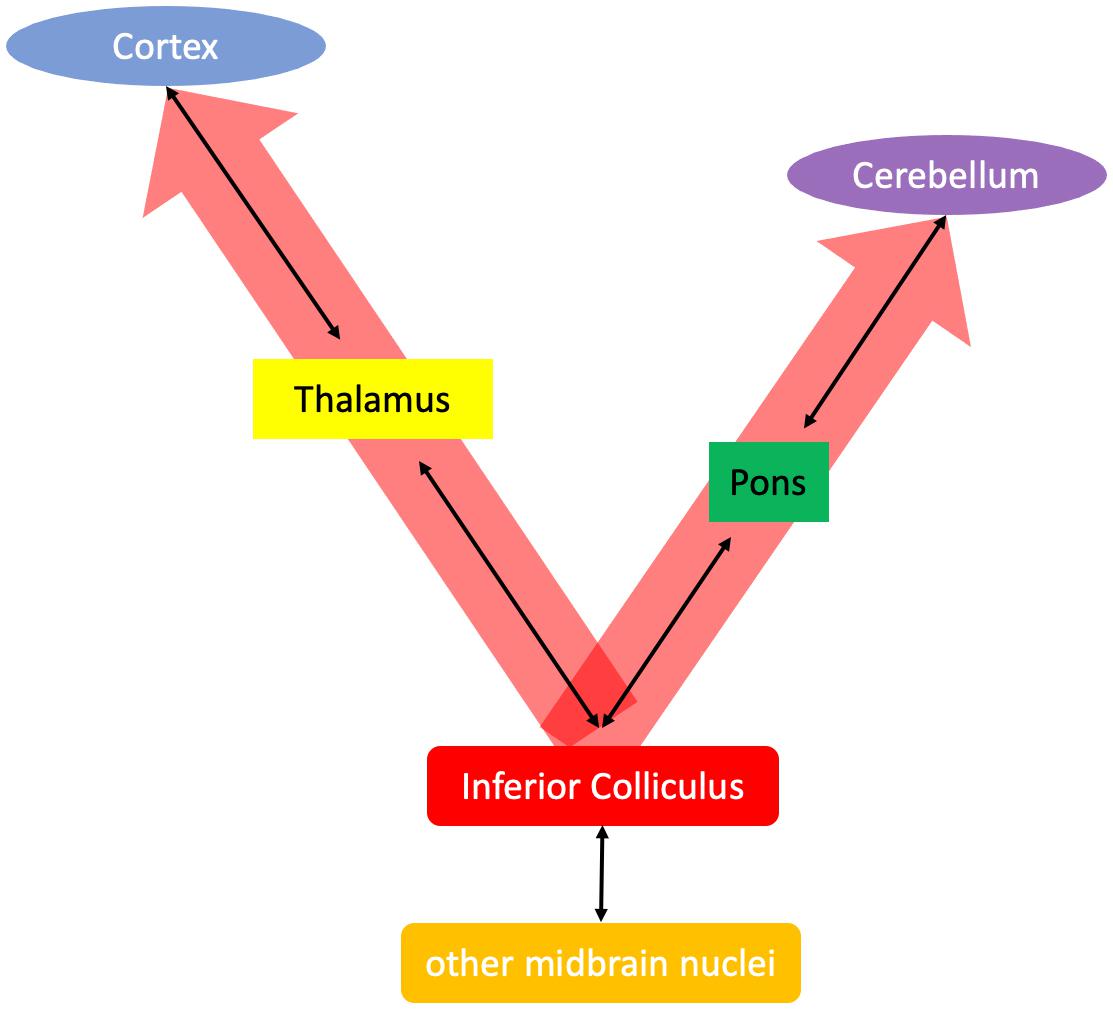
Figure 3. Proposed alerting function of the inferior colliculus accomplished by synchronizing thalamocortical and pontocerebellar systems.
A caveat to the proposed hypothesis is that most of the work highlighting ethanol effects on the inferior colliculi has been accomplished in animal models. A phylogenetically older structure such as the midbrain inferior colliculus may be more salient to rodent than human alcohol exposure. Further, the inferior colliculus is sensitive to anesthesia (Kuwada et al., 1989; Szalda and Burkard, 2005; Franken et al., 2008; Huang et al., 2019), adding complexity to interpretations of in vivo investigations requiring sedation. Alternatively, as already described, the inferior colliculi are a relatively large structure in rodents, but relatively small in humans making them rather difficult to study. To our knowledge, MRI-based volumetric analysis of the inferior colliculi in AUD has not been accomplished. In conclusion, although the contribution of brainstem nuclei (particularly periaqueductal gray, see George et al., 2019; Siciliano et al., 2019) to aspects of alcohol addiction are now under investigation, it is recommended that greater attention be given to the potential contribution of the inferior colliculus to AUD.
NMZ formulated the ideas and concepts expressed herein. TB helped write and endnote portions of the manuscript. Both authors contributed to the article and approved the submitted version.
This work was supported by the U.S. Department of Health & Human Services [NIH] National Institute on Alcohol Abuse and Alcoholism (NIAAA) grant numbers AA005965, AA013521, and AA017347.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Some of the concepts introduced in this review are based on the thoughts of Eileen Nicole Simon. Please see: http://www.conradsimon.org/index.html.
Aasheim, E. T. (2008). Wernicke encephalopathy after bariatric surgery: a systematic review. Ann. Surg. 248, 714–720.
Abdul-Muneer, P. M., Alikunju, S., Schuetz, H., Szlachetka, A. M., Ma, X., and Haorah, J. (2018). Impairment of thiamine transport at the GUT-BBB-AXIS contributes to Wernicke’s encephalopathy. Mol. Neurobiol. 55, 5937–5950. doi: 10.1007/s12035-017-0811-0
Adams, J. C. (1979). Ascending projections to the inferior colliculus. J. Comp. Neurol. 183, 519–538. doi: 10.1002/cne.901830305
Adams, J. C. (1980). Crossed and descending projections to the inferior colliculus. Neurosci. Lett. 19, 1–5. doi: 10.1016/0304-3940(80)90246-3
Aiba, I., Hashizume, Y., Yoshida, M., Okuda, S., Murakami, N., and Ujihira, N. (1997). Relationship between brainstem MRI and pathological findings in progressive supranuclear palsy–study in autopsy cases. J. Neurol. Sci. 152, 210–217. doi: 10.1016/s0022-510x(97)00166-4
Aikawa, H., Watanabe, I. S., Furuse, T., Iwasaki, Y., Satoyoshi, E., Sumi, T., et al. (1984). Low energy levels in thiamine-deficient encephalopathy. J. Neuropathol. Exp. Neurol. 43, 276–287. doi: 10.1097/00005072-198405000-00006
Akinfiresoye, L. R., Miranda, C., Lovinger, D. M., and N’gouemo, P. (2016). Alcohol withdrawal increases protein kinase A activity in the rat inferior colliculus. Alcohol. Clin. Exp. Res. 40, 2359–2367. doi: 10.1111/acer.13223
Alling, C., and Bostrom, K. (1980). Demyelination of the mamillary bodies in alcoholism. A combined morphological and biochemical study. Acta Neuropathol. 50, 77–80. doi: 10.1007/bf00688539
Althoff, K. N., Gebo, K. A., Gange, S. J., Klein, M. B., Brooks, J. T., Hogg, R. S., et al. (2010). CD4 count at presentation for HIV care in the United States and Canada: are those over 50 years more likely to have a delayed presentation? AIDS Res. Ther. 7:45. doi: 10.1186/1742-6405-7-45
Ambrose, M. L., Bowden, S. C., and Whelan, G. (2001a). Thiamin treatment and working memory function of alcohol-dependent people: preliminary findings. Alcohol. Clin. Exp. Res. 25, 112–116. doi: 10.1111/j.1530-0277.2001.tb02134.x
Ambrose, M. L., Bowden, S. C., and Whelan, G. (2001b). Working memory impairments in alcohol-dependent participants without clinical amnesia. Alcohol. Clin. Exp. Res. 25, 185–191. doi: 10.1111/j.1530-0277.2001.tb02197.x
Angeles Fernandez-Gil, M., Palacios-Bote, R., Leo-Barahona, M., and Mora-Encinas, J. P. (2010). Anatomy of the brainstem: a gaze into the stem of life. Semin. Ultrasound CT MR 31, 196–219. doi: 10.1053/j.sult.2010.03.006
Anitha, A., Nakamura, K., Thanseem, I., Matsuzaki, H., Miyachi, T., Tsujii, M., et al. (2013). Downregulation of the expression of mitochondrial electron transport complex genes in autism brains. Brain Pathol. 23, 294–302. doi: 10.1111/bpa.12002
Araki, T., Inoue, T., Kato, H., Kogure, K., and Murakami, M. (1990). Neuronal damage and calcium accumulation following transient cerebral ischemia in the rat. Mol. Chem. Neuropathol. 12, 203–213. doi: 10.1007/bf03159945
Aronson, M., Hagberg, B., and Gillberg, C. (1997). Attention deficits and autistic spectrum problems in children exposed to alcohol during gestation: a follow-up study. Dev. Med. Child. Neurol. 39, 583–587. doi: 10.1111/j.1469-8749.1997.tb07493.x
Asutay, E., and Vastfjall, D. (2017). Exposure to arousal-inducing sounds facilitates visual search. Sci. Rep. 7:10363.
Attaluri, P., Castillo, A., Edriss, H., and Nugent, K. (2018). Thiamine deficiency: an important consideration in critically Ill patients. Am. J. Med. Sci. 356, 382–390. doi: 10.1016/j.amjms.2018.06.015
Bailey, A., Luthert, P., Dean, A., Harding, B., Janota, I., Montgomery, M., et al. (1998). A clinicopathological study of autism. Brain 121(Pt 5), 889–905. doi: 10.1093/brain/121.5.889
Baker, K. G., Halliday, G. M., Kril, J. J., and Harper, C. G. (1996). Chronic alcoholics without Wernicke-Korsakoff syndrome or cirrhosis do not lose serotonergic neurons in the dorsal raphe nucleus. Alcohol. Clin. Exp. Res. 20, 61–66. doi: 10.1111/j.1530-0277.1996.tb01045.x
Baker, K. G., Harding, A. J., Halliday, G. M., Kril, J. J., and Harper, C. G. (1999). Neuronal loss in functional zones of the cerebellum of chronic alcoholics with and without Wernicke’s encephalopathy. Neuroscience 91, 429–438. doi: 10.1016/s0306-4522(98)90664-9
Baldwin, P. R., Curtis, K. N., Patriquin, M. A., Wolf, V., Viswanath, H., Shaw, C., et al. (2016). Identifying diagnostically-relevant resting state brain functional connectivity in the ventral posterior complex via genetic data mining in autism spectrum disorder. Autism Res. 9, 553–562. doi: 10.1002/aur.1559
Bandler, R., Carrive, P., and Zhang, S. P. (1991). Integration of somatic and autonomic reactions within the midbrain periaqueductal grey: viscerotopic, somatotopic and functional organization. Prog. Brain Res. 87, 269–305. doi: 10.1016/s0079-6123(08)63056-3
Bini, L., and Bollea, G. (1947). Fatal poisoning by lead-benzine (aclinicopathologic study). J. Neuropathol. Exp. Neurol. 6, 271–278. doi: 10.1097/00005072-194707000-00007
Blachley, J. D., Johnson, J. H., and Knochel, J. P. (1985). The harmful effects of ethanol on ion transport and cellular respiration. Am. J. Med. Sci. 289, 22–26. doi: 10.1097/00000441-198501000-00004
Brandão, M. L., Cardoso, S. H., Melo, L. L., Motta, V., and Coimbra, N. C. (1994). Neural substrate of defensive behavior in the midbrain tectum. Neurosci. Biobehav. Rev. 18, 339–346. doi: 10.1016/0149-7634(94)90047-7
Brandao, M. L., Melo, L. L., and Cardoso, S. H. (1993). Mechanisms of defense in the inferior colliculus. Behav. Brain Res. 58, 49–55. doi: 10.1016/0166-4328(93)90089-9
Bryan, R. M. Jr. (1986). A method for measuring regional cerebral blood flow in freely moving, unstressed rats. J. Neurosci. Methods 17, 311–322. doi: 10.1016/0165-0270(86)90132-9
Buscaglia, J., and Faris, J. (2005). Unsteady, unfocused, and unable to hear. Am. J. Med. 118, 1215–1217. doi: 10.1016/j.amjmed.2005.08.050
Butters, N. (1981). The Wernicke-Korsakoff syndrome: a review of psychological, neuropathological and etiological factors. Curr. Alcohol. 8, 205–232.
Butters, N. (1985). Alcoholic Korsakoff’s syndrome: some unresolved issues concerning etiology, neuropathology, and cognitive deficits. J. Clin. Exp. Neuropsychol. 7, 181–210. doi: 10.1080/01688638508401252
Butterworth, R. F. (2009). Thiamine deficiency-related brain dysfunction in chronic liver failure. Metab. Brain Dis. 24, 189–196. doi: 10.1007/s11011-008-9129-y
Cadete-Leite, A., Tavares, M. A., Pacheco, M. M., Volk, B., and Paula-Barbosa, M. M. (1989). Hippocampal mossy fiber-CA3 synapses after chronic alcohol consumption and withdrawal. Alcohol 6, 303–310. doi: 10.1016/0741-8329(89)90087-6
Caine, D., Halliday, G. M., Kril, J. J., and Harper, C. G. (1997). Operational criteria for the classification of chronic alcoholics: identification of Wernicke’s encephalopathy. J. Neurol. Neurosurg. Psychiatry 62, 51–60. doi: 10.1136/jnnp.62.1.51
Calford, M. B., and Aitkin, L. M. (1983). Ascending projections to the medial geniculate body of the cat: evidence for multiple, parallel auditory pathways through thalamus. J. Neurosci. 3, 2365–2380. doi: 10.1523/jneurosci.03-11-02365.1983
Calingasan, N. Y., Baker, H., Sheu, K. F., and Gibson, G. E. (1995). Blood-brain barrier abnormalities in vulnerable brain regions during thiamine deficiency. Exp. Neurol. 134, 64–72. doi: 10.1006/exnr.1995.1037
Campbell, G. A., Eckardt, M. J., Majchrowicz, E., Marietta, C. A., and Weight, F. F. (1982). Ethanol-withdrawal syndrome associated with both general and localized increases in glucose uptake in rat brain. Brain Res. 237, 517–522. doi: 10.1016/0006-8993(82)90465-6
Cant, N. B., and Benson, C. G. (2006). Organization of the inferior colliculus of the gerbil (Meriones unguiculatus): differences in distribution of projections from the cochlear nuclei and the superior olivary complex. J. Comp. Neurol. 495, 511–528. doi: 10.1002/cne.20888
Cant, N. B., and Benson, C. G. (2007). Multiple topographically organized projections connect the central nucleus of the inferior colliculus to the ventral division of the medial geniculate nucleus in the gerbil, Meriones unguiculatus. J. Comp. Neurol. 503, 432–453. doi: 10.1002/cne.21391
Carlen, P. L., and Corrigall, W. A. (1980). Ethanol tolerance measured electrophysiologically in hippocampal slices and not in neuromuscular junctions from chronically ethanol-fed rats. Neurosci. Lett. 17, 95–100. doi: 10.1016/0304-3940(80)90068-3
Casseday, J. H., Ehrlich, D., and Covey, E. (1994). Neural tuning for sound duration: role of inhibitory mechanisms in the inferior colliculus. Science 264, 847–850. doi: 10.1126/science.8171341
Cavanagh, J. B., and Harding, B. N. (1994). Pathogenic factors underlying the lesions in Leigh’s disease. Tissue responses to cellular energy deprivation and their clinico-pathological consequences. Brain 117(Pt 6), 1357–1376. doi: 10.1093/brain/117.6.1357
Centanni, S. W., Bedse, G., Patel, S., and Winder, D. G. (2019). Driving the downward spiral: alcohol-induced dysregulation of extended amygdala circuits and negative affect. Alcohol. Clin. Exp. Res. 43, 2000–2013. doi: 10.1111/acer.14178
Center for Disease Control and Prevention (2012). Nutrition and Growth Guidelines - Domestic Guidelines - Immigrant and Refugee Health. Atlanta, GA: Center for Disease Control, and Prevention.
Chakravarty, D. N., and Faingold, C. L. (1997). Aberrant neuronal responsiveness in the genetically epilepsy-prone rat: acoustic responses and influences of the central nucleus upon the external nucleus of inferior colliculus. Brain Res. 761, 263–270. doi: 10.1016/s0006-8993(97)00331-4
Chakravarty, D. N., and Faingold, C. L. (1998). Comparison of neuronal response patterns in the external and central nuclei of inferior colliculus during ethanol administration and ethanol withdrawal. Brain Res. 783, 102–108. doi: 10.1016/s0006-8993(97)01193-1
Chauhan, A., Gu, F., Essa, M. M., Wegiel, J., Kaur, K., Brown, W. T., et al. (2011). Brain region-specific deficit in mitochondrial electron transport chain complexes in children with autism. J. Neurochem. 117, 209–220. doi: 10.1111/j.1471-4159.2011.07189.x
Chen, C., Cheng, M., Ito, T., and Song, S. (2018). Neuronal organization in the inferior colliculus revisited with Cell-Type-dependent monosynaptic tracing. J. Neurosci. 38, 3318–3332. doi: 10.1523/jneurosci.2173-17.2018
Chen, Q., Okada, S., and Okeda, R. (1997). Causality of parenchymal and vascular changes in rats with experimental thiamine deficiency encephalopathy. Pathol. Int. 47, 748–756. doi: 10.1111/j.1440-1827.1997.tb04452.x
Chu, J., Tong, M., and De La Monte, S. M. (2007). Chronic ethanol exposure causes mitochondrial dysfunction and oxidative stress in immature central nervous system neurons. Acta Neuropathol. 113, 659–673. doi: 10.1007/s00401-007-0199-4
Church, M. W. (1987). Chronic in utero alcohol exposure affects auditory function in rats and in humans. Alcohol 4, 231–239. doi: 10.1016/0741-8329(87)90017-6
Church, M. W., and Abel, E. L. (1998). Fetal alcohol syndrome. Hearing, speech, language, and vestibular disorders. Obstet. Gynecol. Clin. N. Am. 25, 85–97.
Clerici, W. J., and Coleman, J. R. (1987). Resting and pure tone evoked metabolic responses in the inferior colliculus of young adult and senescent rats. Neurobiol. Aging 8, 171–178. doi: 10.1016/0197-4580(87)90028-5
Cogan, D. G., Witt, E. D., and Goldman-Rakic, P. S. (1985). Ocular signs in thiamine-deficient monkeys and in Wernicke’s disease in humans. Arch. Ophthalmol. 103, 1212–1220. doi: 10.1001/archopht.1985.01050080124032
Coimbra, N. C., and Brandão, M. L. (1997). Effects of 5-HT2 receptors blockade on fear-induced analgesia elicited by electrical stimulation of the deep layers of the superior colliculus and dorsal periaqueductal gray. Behav. Brain Res. 87, 97–103. doi: 10.1016/s0166-4328(96)02267-x
Coleman, J. R., and Clerici, W. J. (1987). Sources of projections to subdivisions of the inferior colliculus in the rat. J. Comp. Neurol. 262, 215–226. doi: 10.1002/cne.902620204
Collins, M. A., Corso, T. D., and Neafsey, E. J. (1996). Neuronal degeneration in rat cerebrocortical and olfactory regions during subchronic “binge” intoxication with ethanol: possible explanation for olfactory deficits in alcoholics. Alcohol. Clin. Exp. Res. 20, 284–292. doi: 10.1111/j.1530-0277.1996.tb01641.x
Collins, M. A., Zou, J. Y., and Neafsey, E. J. (1998). Brain damage due to episodic alcohol exposure in vivo and in vitro: furosemide neuroprotection implicates edema-based mechanism. FASEB J. 12, 221–230. doi: 10.1096/fasebj.12.2.221
Columbano, L., Stieglitz, L. H., Wrede, K. H., Samii, A., Samii, M., and Luedemann, W. O. (2010). Anatomic study of the quadrigeminal cistern in patients with 3-dimensional magnetic resonance cisternography. Neurosurgery 66, 991–998. doi: 10.1227/01.neu.0000368384.06288.4c
Cooper, M. H., and Young, P. A. (1976). Cortical projections to the inferior colliculus of the cat. Exp. Neurol. 51, 488–502. doi: 10.1016/0014-4886(76)90272-7
Correa, M., Viaggi, C., Escrig, M. A., Pascual, M., Guerri, C., Vaglini, F., et al. (2009). Ethanol intake and ethanol-induced locomotion and locomotor sensitization in Cyp2e1 knockout mice. Pharmacogenet. Genom. 19, 217–225. doi: 10.1097/fpc.0b013e328324e726
Corso, T. D., Mostafa, H. M., Collins, M. A., and Neafsey, E. J. (1998). Brain neuronal degeneration caused by episodic alcohol intoxication in rats: effects of nimodipine, 6,7-dinitro-quinoxaline-2,3-dione, and MK-801. Alcohol. Clin. Exp. Res. 22, 217–224. doi: 10.1097/00000374-199802000-00030
Counsell, S. J., Maalouf, E. F., Fletcher, A. M., Duggan, P., Battin, M., Lewis, H. J., et al. (2002). MR imaging assessment of myelination in the very preterm brain. AJNR Am. J. Neuroradiol. 23, 872–881.
Counter, S. A., Buchanan, L. H., Ortega, F., Van Der Velde, J., and Borg, E. (2011). Assessment of auditory brainstem function in lead-exposed children using stapedius muscle reflexes. J. Neurol. Sci. 306, 29–37. doi: 10.1016/j.jns.2011.04.003
Courchesne, E. (1997). Brainstem, cerebellar and limbic neuroanatomical abnormalities in autism. Curr. Opin. Neurobiol. 7, 269–278. doi: 10.1016/s0959-4388(97)80016-5
Coy, J. F., Dressler, D., Wilde, J., and Schubert, P. (2005). Mutations in the transketolase-like gene TKTL1: clinical implications for neurodegenerative diseases, diabetes and cancer. Clin. Lab. 51, 257–273.
Coy, J. F., Dubel, S., Kioschis, P., Thomas, K., Micklem, G., Delius, H., et al. (1996). Molecular cloning of tissue-specific transcripts of a transketolase-related gene: implications for the evolution of new vertebrate genes. Genomics 32, 309–316. doi: 10.1006/geno.1996.0124
Cremer, J. E., and Seville, M. P. (1983). Regional brain blood flow, blood volume, and haematocrit values in the adult rat. J. Cereb. Blood Flow Metab. 3, 254–256. doi: 10.1038/jcbfm.1983.35
Cullen, K. M., and Halliday, G. M. (1995). Mechanisms of cell death in cholinergic basal forebrain neurons in chronic alcoholics. Metab. Brain Dis. 10, 81–91. doi: 10.1007/bf01991785
Cunningham, C. C., and Ivester, P. (1999). Chronic ethanol, oxygen tension and hepatocyte energy metabolism. Front. Biosci. 4, D551–D556. doi: 10.2741/cunning
Curnes, J. T., Burger, P. C., Djang, W. T., and Boyko, O. B. (1988). MR imaging of compact white matter pathways. AJNR Am. J. Neuroradiol. 9, 1061–1068.
de la Monte, S. M. (1988). Disproportionate atrophy of cerebral white matter in chronic alcoholics. Arch. Neurol. 45, 990–992. doi: 10.1001/archneur.1988.00520330076013
Deniau, J. M., and Chevalier, G. (1992). The lamellar organization of the rat substantia nigra pars reticulata: distribution of projection neurons. Neuroscience 46, 361–377. doi: 10.1016/0306-4522(92)90058-a
Denny-Brown, D. (1962). The midbrain and motor integration. Proc. R. Soc. Med. 55, 527–538. doi: 10.1177/003591576205500701
Devenport, L. D., Devenport, J. A., and Holloway, F. A. (1981). Necessity of the hippocampus for alcohol’s indirect but not behavioral action. Behav. Neural Biol. 33, 476–487. doi: 10.1016/s0163-1047(81)91851-3
Dikranian, K., Qin, Y. Q., Labruyere, J., Nemmers, B., and Olney, J. W. (2005). Ethanol-induced neuroapoptosis in the developing rodent cerebellum and related brain stem structures. Brain Res. Dev. Brain Res. 155, 1–13. doi: 10.1016/j.devbrainres.2004.11.005
Dodd, P. R., Thomas, G. J., Mccloskey, A., Crane, D. I., and Smith, I. D. (1996). The neurochemical pathology of thiamine deficiency: GABAA and glutamateNMDA receptor binding sites in a goat model. Metab. Brain Dis. 11, 39–54. doi: 10.1007/bf02080930
Driscoll, M. E., and Tadi, P. (2020). “Neuroanatomy, inferior colliculus,” in StatPearls (Treasure Island, FL: StatPearls Publishing). Available online at: https://www.ncbi.nlm.nih.gov/books/NBK554468/
Dror, V., Eliash, S., Rehavi, M., Assaf, Y., Biton, I. E., and Fattal-Valevski, A. (2010). Neurodegeneration in thiamine deficient rats-A longitudinal MRI study. Brain Res. 1308, 176–184. doi: 10.1016/j.brainres.2009.10.032
Dudek, M., Abo-Ramadan, U., Hermann, D., Brown, M., Canals, S., Sommer, W. H., et al. (2015). Brain activation induced by voluntary alcohol and saccharin drinking in rats assessed with manganese-enhanced magnetic resonance imaging. Addict. Biol. 20, 1012–1021. doi: 10.1111/adb.12179
Dujardin, E., and Jürgens, U. (2005). Afferents of vocalization-controlling periaqueductal regions in the squirrel monkey. Brain Res. 1034, 114–131. doi: 10.1016/j.brainres.2004.11.048
Eckardt, M. J., Campbell, G. A., Marietta, C. A., Majchrowicz, E., Rawlings, R. R., and Weight, F. F. (1992). Ethanol dependence and withdrawal selectively alter localized cerebral glucose utilization. Brain Res. 584, 244–250. doi: 10.1016/0006-8993(92)90901-k
Eckardt, M. J., Campbell, G. A., Marietta, C. A., Majchrowicz, E., and Weight, F. F. (1988). Acute ethanol administration selectively alters localized cerebral glucose metabolism. Brain Res. 444, 53–58. doi: 10.1016/0006-8993(88)90912-2
Eckardt, M. J., Campbell, G. A., Marietta, C. A., Majchrowicz, E., Wixon, H. N., and Weight, F. F. (1986). Cerebral 2-deoxyglucose uptake in rats during ethanol withdrawal and postwithdrawal. Brain Res. 366, 1–9. doi: 10.1016/0006-8993(86)91276-x
Eckle, V. S., and Todorovic, S. M. (2010). Mechanisms of inhibition of CaV3.1 T-type calcium current by aliphatic alcohols. Neuropharmacology 59, 58–69. doi: 10.1016/j.neuropharm.2010.03.016
Evans, M. S., Li, Y., and Faingold, C. (2000). Inferior colliculus intracellular response abnormalities in vitro associated with susceptibility to ethanol withdrawal seizures. Alcohol. Clin. Exp. Res. 24, 1180–1186. doi: 10.1111/j.1530-0277.2000.tb02081.x
Ezequiel Leite, L., and Nobre, M. J. (2012). The negative effects of alcohol hangover on high-anxiety phenotype rats are influenced by the glutamate receptors of the dorsal midbrain. Neuroscience 213, 93–105. doi: 10.1016/j.neuroscience.2012.04.009
Faingold, C., Li, Y., and Evans, M. S. (2000). Decreased GABA and increased glutamate receptor-mediated activity on inferior colliculus neurons in vitro are associated with susceptibility to ethanol withdrawal seizures. Brain Res. 868, 287–295. doi: 10.1016/s0006-8993(00)02342-8
Faingold, C. L., Naritoku, D. K., Copley, C. A., Randall, M. E., Riaz, A., Anderson, C. A., et al. (1992). Glutamate in the inferior colliculus plays a critical role in audiogenic seizure initiation. Epilep. Res. 13, 95–105. doi: 10.1016/0920-1211(92)90064-z
Faingold, C. L., Randall, M. E., Naritoku, D. K., and Boersma Anderson, C. A. (1993). Noncompetitive and competitive NMDA antagonists exert anticonvulsant effects by actions on different sites within the neuronal network for audiogenic seizures. Exp. Neurol. 119, 198–204. doi: 10.1006/exnr.1993.1021
Fama, R., Le Berre, A. P., Hardcastle, C., Sassoon, S. A., Pfefferbaum, A., Sullivan, E. V., et al. (2019). Neurological, nutritional and alcohol consumption factors underlie cognitive and motor deficits in chronic alcoholism. Addict. Biol. 24, 290–302. doi: 10.1111/adb.12584
Farber, N. B., Creeley, C. E., and Olney, J. W. (2010). Alcohol-induced neuroapoptosis in the fetal macaque brain. Neurobiol. Dis. 40, 200–206. doi: 10.1016/j.nbd.2010.05.025
Feng, A. S. (1992). Information processing in the auditory brainstem. Curr. Opin. Neurobiol. 2, 511–515. doi: 10.1016/0959-4388(92)90189-r
Fernandez-Lizarbe, S., Pascual, M., and Guerri, C. (2009). Critical role of TLR4 response in the activation of microglia induced by ethanol. J. Immunol. 183, 4733–4744. doi: 10.4049/jimmunol.0803590
Fischer, C., Bognar, L., Turjman, F., Villanyi, E., and Lapras, C. (1994). Auditory early- and middle-latency evoked potentials in patients with quadrigeminal plate tumors. Neurosurgery 35, 45–51. doi: 10.1227/00006123-199407000-00007
Flabeau, O., Foubert-Samier, A., Meissner, W., and Tison, F. (2008). Hearing and seeing: Unusual early signs of Wernicke encephalopathy. Neurology 71:694. doi: 10.1212/01.wnl.0000324599.66359.b1
Franken, N. D., Van Oostrom, H., Stienen, P. J., Doornenbal, A., and Hellebrekers, L. J. (2008). Evaluation of analgesic and sedative effects of continuous infusion of dexmedetomidine by measuring somatosensory- and auditory-evoked potentials in the rat. Vet. Anaesth. Analg. 35, 424–431. doi: 10.1111/j.1467-2995.2008.00404.x
French, S. W. (2001). Intragastric ethanol infusion model for cellular and molecular studies of alcoholic liver disease. J. Biomed. Sci. 8, 20–27. doi: 10.1007/bf02255967
Fritz, M., Klawonn, A. M., and Zahr, N. M. (2019). Neuroimaging in alcohol use disorder: from mouse to man. J. Neurosci. Res. 2019:24423.
Malmierca, M. S., and Young, E. D. (2015). “Inferior colliculus microcircuits,” in Frontiers in Neural Circuits, eds M. S. Malmierca and E. D. Young (Lausanne: Frontiers Media).
Funai, H., and Funasaka, S. (1983). Experimental study on the effect of inferior colliculus lesions upon auditory brain stem response. Audiology 22, 9–19. doi: 10.3109/00206098309072766
Games, K. D., and Winer, J. A. (1988). Layer V in rat auditory cortex: projections to the inferior colliculus and contralateral cortex. Hear. Res. 34, 1–25. doi: 10.1016/0378-5955(88)90047-0
Garosi, L. S., Dennis, R., Platt, S. R., Corletto, F., De Lahunta, A., and Jakobs, C. (2003). Thiamine deficiency in a dog: clinical, clinicopathologic, and magnetic resonance imaging findings. J. Vet. Intern. Med. 17, 719–723. doi: 10.1111/j.1939-1676.2003.tb02507.x
Geisler, R. F., Hunter, B. E., and Walker, D. W. (1978). Ethanol dependence in the rat: temporal changes in neuroexcitability following withdrawal. Psychopharmacology 56, 287–292. doi: 10.1007/bf00432851
George, D. T., Ameli, R., and Koob, G. F. (2019). Periaqueductal gray sheds light on dark areas of psychopathology. Trends Neurosci. 42, 349–360. doi: 10.1016/j.tins.2019.03.004
Gibson, G., Nielsen, P., Mykytyn, V., Carlson, K., and Blass, J. (1989). Regionally selective alterations in enzymatic activities and metabolic fluxes during thiamin deficiency. Neurochem. Res. 14, 17–24. doi: 10.1007/bf00969752
Glendenning, K. K., and Masterton, R. B. (1983). Acoustic chiasm: efferent projections of the lateral superior olive. J. Neurosci. 3, 1521–1537. doi: 10.1523/jneurosci.03-08-01521.1983
Glendenning, K. K., and Masterton, R. B. (1998). Comparative morphometry of mammalian central auditory systems: variation in nuclei and form of the ascending system. Brain Behav. Evol. 51, 59–89. doi: 10.1159/000006530
Gonzalez, D., Tomasek, M., Hays, S., Sridhar, V., Ammanuel, S., Chang, C. W., et al. (2019). Audiogenic seizures in the Fmr1 knock-out mouse are induced by Fmr1 deletion in subcortical, VGlut2-expressing excitatory neurons and require deletion in the inferior colliculus. J. Neurosci. 39, 9852–9863. doi: 10.1523/jneurosci.0886-19.2019
González-Hernández, T., Mantolán-Sarmiento, B., González-González, B., and Pérez-González, H. (1996). Sources of GABAergic input to the inferior colliculus of the rat. J. Comp. Neurol. 372, 309–326. doi: 10.1002/(sici)1096-9861(19960819)372:2<309::aid-cne11>3.0.co;2-e
Goulon, M., Nouailhat, F., Escourolle, R., Zarranz-Imirizaldu, J. J., Grosbuis, S., and Lévy-Alcover, M. A. (1975). Methyl bromide poisoning. 3 cases, 1 fatal. Neruopathological study of one case of coma with myoclonus followed for 5 years. Rev. Neurol. 131, 445–468.
Goyer, D., Silveira, M. A., George, A. P., Beebe, N. L., Edelbrock, R. M., Malinski, P. T., et al. (2019). A novel class of inferior colliculus principal neurons labeled in vasoactive intestinal peptide-Cre mice. eLife 8:e43770.
Grant, B. F., Goldstein, R. B., Saha, T. D., Chou, S. P., Jung, J., Zhang, H., et al. (2015). Epidemiology of DSM-5 alcohol use disorder: results from the national epidemiologic survey on alcohol and related conditions III. JAMA Psychiatr. 72, 757–766.
Gross, P. M., Sposito, N. M., Pettersen, S. E., and Fenstermacher, J. D. (1986). Differences in function and structure of the capillary endothelium in gray matter, white matter and a circumventricular organ of rat brain. Blood Ves. 23, 261–270. doi: 10.1159/000158652
Grunwald, F., Schrock, H., Biersack, H. J., and Kuschinsky, W. (1993). Changes in local cerebral glucose utilization in the awake rat during acute and chronic administration of ethanol. J. Nucl. Med. 34, 793–798.
Grupp, L. A., and Perlanski, E. (1979). Ethanol-induced changes in the spontaneous activity of single units in the hippocampus of the awake rat: a dose-response study. Neuropharmacology 18, 63–70. doi: 10.1016/0028-3908(79)90010-8
Gruters, K. G., and Groh, J. M. (2012). Sounds and beyond: multisensory and other non-auditory signals in the inferior colliculus. Front. Neural Circ. 6:96. doi: 10.3389/fncir.2012.00096
Ha, N. D., Weon, Y. C., Jang, J. C., Kang, B. S., and Choi, S. H. (2012). Spectrum of MR imaging findings in Wernicke encephalopathy: are atypical areas of involvement only present in nonalcoholic patients? AJNR Am. J. Neuroradiol. 33, 1398–1402. doi: 10.3174/ajnr.a2979
Hakim, A. M. (1984). The induction and reversibility of cerebral acidosis in thiamine deficiency. Ann. Neurol. 16, 673–679. doi: 10.1002/ana.410160609
Hakim, A. M. (1986). Effect of thiamine deficiency and its reversal on cerebral blood flow in the rat. Observations on the phenomena of hyperperfusion, “no reflow,” and delayed hypoperfusion. J. Cereb. Blood Flow Metab. 6, 79–85. doi: 10.1038/jcbfm.1986.10
Hakim, A. M., and Pappius, H. M. (1981). The effect of thiamine deficiency on local cerebral glucose utilization. Ann. Neurol. 9, 334–339. doi: 10.1002/ana.410090404
Halliday, G., Cullen, K., and Harding, A. (1994). Neuropathological correlates of memory dysfunction in the Wernicke-Korsakoff syndrome. Alcohol. Alcohol. Suppl. 2, 245–251.
Halliday, G., Ellis, J., Heard, R., Caine, D., and Harper, C. (1993). Brainstem serotonergic neurons in chronic alcoholics with and without the memory impairment of Korsakoff’s psychosis. J. Neuropathol. Exp. Neurol. 52, 567–579. doi: 10.1097/00005072-199311000-00003
Harata, N., and Iwasaki, Y. (1995). Evidence for early blood-brain barrier breakdown in experimental thiamine deficiency in the mouse. Metab. Brain Dis. 10, 159–174. doi: 10.1007/bf01991863
Harding, A., Halliday, G., Caine, D., and Kril, J. (2000). Degeneration of anterior thalamic nuclei differentiates alcoholics with amnesia. Brain 123(Pt 1), 141–154. doi: 10.1093/brain/123.1.141
Harding, A. J., Halliday, G. M., Ng, J. L., Harper, C. G., and Kril, J. J. (1996). Loss of vasopressin-immunoreactive neurons in alcoholics is dose-related and time-dependent. Neuroscience 72, 699–708. doi: 10.1016/0306-4522(95)00577-3
Harper, C., Dixon, G., Sheedy, D., and Garrick, T. (2003). Neuropathological alterations in alcoholic brains. Studies arising from the New South Wales tissue resource centre. Prog. Neuropsychopharmacol. Biol. Psychiatry 27, 951–961. doi: 10.1016/s0278-5846(03)00155-6
Harper, C., and Kril, J. (1994). An introduction to alcohol-induced brain damage and its causes. Alcohol. Alcohol. Suppl. 2, 237–243.
Harper, C. G., and Blumbergs, P. C. (1982). Brain weights in alcoholics. J. Neurol. Neurosurg. Psychiatry 45, 838–840. doi: 10.1136/jnnp.45.9.838
Harper, C. G., Daly, J., and Kril, J. (1985). Brain water in chronic alcoholics: a necropsy study. Lancet 2:327. doi: 10.1016/s0140-6736(85)90368-x
Harper, C. G., Giles, M., and Finlay-Jones, R. (1986). Clinical signs in the Wernicke-Korsakoff complex: a retrospective analysis of 131 cases diagnosed at necropsy. J. Neurol. Neurosurg. Psychiatry 49, 341–345. doi: 10.1136/jnnp.49.4.341
Harper, C. G., and Kril, J. J. (1988). Corpus callosal thickness in alcoholics. Br. J. Addict. 83, 577–580. doi: 10.1111/j.1360-0443.1988.tb02577.x
Harris, S. R., Mackay, L. L., and Osborn, J. A. (1995). Autistic behaviors in offspring of mothers abusing alcohol and other drugs: a series of case reports. Alcohol. Clin. Exp. Res. 19, 660–665. doi: 10.1111/j.1530-0277.1995.tb01564.x
Hazell, A. S., and Butterworth, R. F. (2009). Update of cell damage mechanisms in thiamine deficiency: focus on oxidative stress, excitotoxicity and inflammation. Alcohol Alcohol. 44, 141–147. doi: 10.1093/alcalc/agn120
Hazell, A. S., Mcgahan, L., Tetzlaff, W., Bedard, A. M., Robertson, G. S., Nakabeppu, Y., et al. (1998). Immediate-early gene expression in the brain of the thiamine-deficient rat. J. Mol. Neurosci. 10, 1–15. doi: 10.1007/bf02737081
Hegde, A. N., Mohan, S., Lath, N., and Lim, C. C. (2011). Differential diagnosis for bilateral abnormalities of the basal ganglia and thalamus. Radiographics 31, 5–30. doi: 10.1148/rg.311105041
Heirene, R., John, B., and Roderique-Davies, G. (2018). Identification and evaluation of neuropsychological tools used in the assessment of alcohol-related cognitive impairment: a systematic review. Front. Psychol. 9:2618. doi: 10.3389/fpsyg.2018.02618
Heldt, S. A., and Falls, W. A. (2003). Destruction of the inferior colliculus disrupts the production and inhibition of fear conditioned to an acoustic stimulus. Behav. Brain Res. 144, 175–185. doi: 10.1016/s0166-4328(03)00092-5
Hermans, E. J., Van Marle, H. J., Ossewaarde, L., Henckens, M. J., Qin, S., Van Kesteren, M. T., et al. (2011). Stress-related noradrenergic activity prompts large-scale neural network reconfiguration. Science 334, 1151–1153. doi: 10.1126/science.1209603
Herrmann, H. D., Winkler, D., and Westphal, M. (1992). Treatment of tumours of the pineal region and posterior part of the third ventricle. Acta Neurochir. 116, 137–146. doi: 10.1007/bf01540866
Hopkins, D. A., and Holstege, G. (1978). Amygdaloid projections to the mesencephalon, pons and medulla oblongata in the cat. Exp. Brain Res. 32, 529–547.
Hormigo, S., E Horta Junior, J. D. A., Gómez-Nieto, R., and López García, D. (2012). The selective neurotoxin DSP-4 impairs the noradrenergic projections from the locus coeruleus to the inferior colliculus in rats. Front. Neural Circ. 6:41. doi: 10.3389/fncir.2012.00041
Houser, D. S., Moore, P. W., Johnson, S., Lutmerding, B., Branstetter, B., Ridgway, S. H., et al. (2010). Relationship of blood flow and metabolism to acoustic processing centers of the dolphin brain. J. Acoust. Soc. Am. 128, 1460–1466. doi: 10.1121/1.3442572
Hoyumpa, A. M. Jr. (1980). Mechanisms of thiamin deficiency in chronic alcoholism. Am. J. Clin. Nutr. 33, 2750–2761. doi: 10.1093/ajcn/33.12.2750
Huang, B., Yan, L., Zhang, Z., Yang, X., and Xiao, Z. (2019). General anesthetic induced differential changes in latency of auditory evoked potential in the central nucleus of inferior colliculus of mouse. Neurosci. Lett. 708:134325. doi: 10.1016/j.neulet.2019.134325
Huang, M. Y., Jong, Y. J., Tsai, J. L., Liu, G. C., Chiang, C. H., Pang, C. Y., et al. (1996). Mitochondrial NADH-coenzyme Q reductase deficiency in Leigh’s disease. J. Formos. Med. Assoc. 95, 325–328.
Huffman, R. F., and Henson, O. W. Jr. (1990). The descending auditory pathway and acousticomotor systems: connections with the inferior colliculus. Brain Res. Brain Res. Rev. 15, 295–323. doi: 10.1016/0165-0173(90)90005-9
Hurley, L. M. (2006). Different serotonin receptor agonists have distinct effects on sound-evoked responses in inferior colliculus. J. Neurophysiol. 96, 2177–2188. doi: 10.1152/jn.00046.2006
Hurley, L. M., and Pollak, G. D. (2001). Serotonin effects on frequency tuning of inferior colliculus neurons. J. Neurophysiol. 85, 828–842. doi: 10.1152/jn.2001.85.2.828
Hurley, L. M., and Sullivan, M. R. (2012). From behavioral context to receptors: serotonergic modulatory pathways in the IC. Front. Neural Circ. 6:58. doi: 10.3389/fncir.2012.00058
Ikonomidou, C., Bittigau, P., Ishimaru, M. J., Wozniak, D. F., Koch, C., Genz, K., et al. (2000). Ethanol-induced apoptotic neurodegeneration and fetal alcohol syndrome. Science 287, 1056–1060. doi: 10.1126/science.287.5455.1056
Irle, E., and Markowitsch, H. J. (1982). Thiamine deficiency in the cat leads to severe learning deficits and to widespread neuroanatomical damage. Exp. Brain Res. 48, 199–208.
Isenberg-Grzeda, E., Kutner, H. E., and Nicolson, S. E. (2012). Wernicke-Korsakoff-syndrome: under-recognized and under-treated. Psychosomatics 53, 507–516. doi: 10.1016/j.psym.2012.04.008
Isenberg-Grzeda, E., Rahane, S., Derosa, A. P., Ellis, J., and Nicolson, S. E. (2016). Wernicke-Korsakoff syndrome in patients with cancer: a systematic review. Lancet Oncol. 17, e142–e148.
Ito, T., Bishop, D. C., and Oliver, D. L. (2011). Expression of glutamate and inhibitory amino acid vesicular transporters in the rodent auditory brainstem. J. Comp. Neurol. 519, 316–340. doi: 10.1002/cne.22521
Ito, T., and Oliver, D. L. (2012). The basic circuit of the IC: tectothalamic neurons with different patterns of synaptic organization send different messages to the thalamus. Front. Neural Circ. 6:48. doi: 10.3389/fncir.2012.00048
Jay, T. M., Lucignani, G., Crane, A. M., Jehle, J., and Sokoloff, L. (1988). Measurement of local cerebral blood flow with [14C]iodoantipyrine in the mouse. J. Cereb. Blood Flow Metab. 8, 121–129. doi: 10.1038/jcbfm.1988.16
Jen, P. H., Chen, Q. C., and Sun, X. D. (1998). Corticofugal regulation of auditory sensitivity in the bat inferior colliculus. J. Comp. Physiol. A 183, 683–697. doi: 10.1007/s003590050291
Jen, P. H., and Zhou, X. (2003). Corticofugal modulation of amplitude domain processing in the midbrain of the big brown bat, Eptesicus fuscus. Hear. Res. 184, 91–106. doi: 10.1016/s0378-5955(03)00237-5
Jethava, A., and Dasanu, C. A. (2012). Acute Wernicke encephalopathy and sensorineural hearing loss complicating bariatric surgery. Conn. Med. 76, 603–605.
Jia, F., Pignataro, L., and Harrison, N. L. (2007). GABAA receptors in the thalamus: alpha4 subunit expression and alcohol sensitivity. Alcohol 41, 177–185. doi: 10.1016/j.alcohol.2007.03.010
Jiang, L., Gulanski, B. I., De Feyter, H. M., Weinzimer, S. A., Pittman, B., Guidone, E., et al. (2013). Increased brain uptake and oxidation of acetate in heavy drinkers. J. Clin. Invest. 123, 1605–1614. doi: 10.1172/jci65153
Jordan, L. R., Zelaya, F. O., Rose, S. E., Bower, A. J., Galloway, G., Wholohan, T., et al. (1998). Changes in the hippocampus induced by glucose in thiamin deficient rats detected by MRI. Brain Res. 791, 347–351. doi: 10.1016/s0006-8993(98)00203-0
Jordan, W. P., and Leaton, R. N. (1982). Startle habituation in rats after lesions in the brachium of the inferior colliculus. Physiol. Behav. 28, 253–258. doi: 10.1016/0031-9384(82)90071-3
Jung, Y. C., Chanraud, S., and Sullivan, E. V. (2012). Neuroimaging of Wernicke’s encephalopathy and Korsakoff’s syndrome. Neuropsychol. Rev. 22, 170–180.
Jürgens, U. (2002). Neural pathways underlying vocal control. Neurosci. Biobehav. Rev. 26, 235–258. doi: 10.1016/s0149-7634(01)00068-9
Kanner, L., and Eisenberg, L. (1957). Early infantile autism, 1943–1955. Psychiatr. Res. Rep. Am. Psychiatr. Assoc. 55–65. doi: 10.4159/harvard.9780674367012.c2
Karakonstantis, S., Galani, D., Korela, D., Maragou, S., Arna, D., and Basta, M. (2020). Missing the early signs of thiamine deficiency. A case associated with a liquid-only diet. Nutr. Neurosci. 23, 384–386. doi: 10.1080/1028415x.2018.1507964
Kattah, J. C., Dhanani, S. S., Pula, J. H., Mantokoudis, G., Tehrani, A. S. S., and Toker, D. E. N. (2013). Vestibular signs of thiamine deficiency during the early phase of suspected Wernicke encephalopathy. Neurol. Clin. Pract. 3, 460–468. doi: 10.1212/01.cpj.0000435749.32868.91
Kattah, J. C., Guede, C., and Hassanzadeh, B. (2018). The medial vestibular nuclei, a vulnerable target in thiamine deficiency. J. Neurol. 265, 213–215. doi: 10.1007/s00415-017-8670-1
Ke, Z. J., and Gibson, G. E. (2004). Selective response of various brain cell types during neurodegeneration induced by mild impairment of oxidative metabolism. Neurochem. Int. 45, 361–369. doi: 10.1016/j.neuint.2003.09.008
Kelly, J. B., Van Adel, B. A., and Ito, M. (2009). Anatomical projections of the nuclei of the lateral lemniscus in the albino rat (Rattus norvegicus). J. Comp. Neurol. 512, 573–593. doi: 10.1002/cne.21929
Kelso, M. L., Liput, D. J., Eaves, D. W., and Nixon, K. (2011). Upregulated vimentin suggests new areas of neurodegeneration in a model of an alcohol use disorder. Neuroscience 197, 381–393. doi: 10.1016/j.neuroscience.2011.09.019
Kemel, M. L., Desban, M., Gauchy, C., Glowinski, J., and Besson, M. J. (1988). Topographical organization of efferent projections from the cat substantia nigra pars reticulata. Brain Res. 455, 307–323. doi: 10.1016/0006-8993(88)90090-x
Kemper, T. L., and Bauman, M. (1998). Neuropathology of infantile autism. J. Neuropathol. Exp. Neurol. 57, 645–652. doi: 10.1097/00005072-199807000-00001
Kennedy, C., Sakurada, O., Shinohara, M., and Miyaoka, M. (1982). Local cerebral glucose utilization in the newborn macaque monkey. Ann. Neurol. 12, 333–340. doi: 10.1002/ana.410120404
Kern, J. K., Geier, D. A., Audhya, T., King, P. G., Sykes, L. K., and Geier, M. R. (2012). Evidence of parallels between mercury intoxication and the brain pathology in autism. Acta Neurobiol. Exp. 72, 113–153.
Kevanishvili, Z. (1980). Sources of the human brainstem auditory evoked potential. Scand. Audiol. 9, 75–82. doi: 10.3109/01050398009076339
King, A. J., Jiang, Z. D., and Moore, D. R. (1998). Auditory brainstem projections to the ferret superior colliculus: anatomical contribution to the neural coding of sound azimuth. J. Comp. Neurol. 390, 342–365. doi: 10.1002/(sici)1096-9861(19980119)390:3<342::aid-cne4>3.0.co;2-1
Kishimoto, Y., Ikeda, K., Murata, K., Kawabe, K., Hirayama, T., and Iwasaki, Y. (2012). Rapid development of central pontine myelinolysis after recovery from Wernicke encephalopathy: a non-alcoholic case without hyponatremia. Intern. Med. 51, 1599–1603. doi: 10.2169/internalmedicine.51.7498
Klein, B., Kuschinsky, W., Schröck, H., and Vetterlein, F. (1986). Interdependency of local capillary density, blood flow, and metabolism in rat brains. Am. J. Physiol. 251, H1333–H1340.
Klepper, A., and Herbert, H. (1991). Distribution and origin of noradrenergic and serotonergic fibers in the cochlear nucleus and inferior colliculus of the rat. Brain Res. 557, 190–201. doi: 10.1016/0006-8993(91)90134-h
Knudsen, E. I., and Konishi, M. (1978). A neural map of auditory space in the owl. Science 200, 795–797. doi: 10.1126/science.644324
Kohlmeyer, K., Stober, B., and Jennen, C. (1986). Computed tomography in chronic alcoholism. Acta Radiol. Suppl. 369, 393–395.
Kokash, J., Alderson, E. M., Reinhard, S. M., Crawford, C. A., Binder, D. K., Ethell, I. M., et al. (2019). Genetic reduction of MMP-9 in the Fmr1 KO mouse partially rescues prepulse inhibition of acoustic startle response. Brain Res. 1719, 24–29. doi: 10.1016/j.brainres.2019.05.029
Komune, N., Yagmurlu, K., Matsuo, S., Miki, K., Abe, H., and Rhoton, A. L. Jr. (2015). Auditory brainstem implantation: anatomy and approaches. Neurosurgery 11, (Suppl. 2), 306–320. doi: 10.1227/neu.0000000000000736
Kono, S., Miyajima, H., Yoshida, K., Togawa, A., Shirakawa, K., and Suzuki, H. (2009). Mutations in a thiamine-transporter gene and Wernicke’s-like encephalopathy. N. Engl. J. Med. 360, 1792–1794. doi: 10.1056/nejmc0809100
Koob, G. F., and Colrain, I. M. (2020). Alcohol use disorder and sleep disturbances: a feed-forward allostatic framework. Neuropsychopharmacology 45, 141–165. doi: 10.1038/s41386-019-0446-0
Kril, J. J., Halliday, G. M., Svoboda, M. D., and Cartwright, H. (1997). The cerebral cortex is damaged in chronic alcoholics. Neuroscience 79, 983–998. doi: 10.1016/s0306-4522(97)00083-3
Kruse, M., Navarro, D., Desjardins, P., and Butterworth, R. F. (2004). Increased brain endothelial nitric oxide synthase expression in thiamine deficiency: relationship to selective vulnerability. Neurochem. Int. 45, 49–56. doi: 10.1016/j.neuint.2003.12.007
Kudo, M., and Niimi, K. (1980). Ascending projections of the inferior colliculus in the cat: an autoradiographic study. J. Comp. Neurol. 191, 545–556. doi: 10.1002/cne.901910403
Kuwabara, N., and Zook, J. M. (2000). Geniculo-collicular descending projections in the gerbil. Brain Res. 878, 79–87. doi: 10.1016/s0006-8993(00)02695-0
Kuwada, S., Batra, R., and Stanford, T. R. (1989). Monaural and binaural response properties of neurons in the inferior colliculus of the rabbit: effects of sodium pentobarbital. J. Neurophysiol. 61, 269–282. doi: 10.1152/jn.1989.61.2.269
Kv, L. N., and Nguyễn, L. T. (2013). The role of thiamine in HIV infection. Int. J. Infect. Dis. 17, e221–e227.
Lamprea, M. R., Cardenas, F. P., Vianna, D. M., Castilho, V. M., Cruz-Morales, S. E., and Brandão, M. L. (2002). The distribution of fos immunoreactivity in rat brain following freezing and escape responses elicited by electrical stimulation of the inferior colliculus. Brain Res. 950, 186–194. doi: 10.1016/s0006-8993(02)03036-6
Landau, W. M., Freygang, W. H. Jr., Roland, L. P., Sokoloff, L., and Kety, S. S. (1955–1956). The local circulation of the living brain; values in the unanesthetized and anesthetized cat. Trans. Am. Neurol. Assoc. 125–129.
Langlais, P. J., and Zhang, S. X. (1997). Cortical and subcortical white matter damage without Wernicke’s encephalopathy after recovery from thiamine deficiency in the rat. Alcohol. Clin. Exp. Res. 21, 434–443. doi: 10.1097/00000374-199705000-00010
Langlais, P. J., Zhang, S. X., and Savage, L. M. (1996). Neuropathology of thiamine deficiency: an update on the comparative analysis of human disorders and experimental models. Metab. Brain Dis. 11, 19–37. doi: 10.1007/bf02080929
Latash, L. P. (1990). Orienting reaction, organizing for action, and emotional processes. Pavlov J. Biol. Sci. 25, 123–129.
Le Berre, A. P., Pitel, A. L., Chanraud, S., Beaunieux, H., Eustache, F., Martinot, J. L., et al. (2014). Chronic alcohol consumption and its effect on nodes of frontocerebellar and limbic circuitry: comparison of effects in France and the United States. Hum. Brain Mapp. 35, 4635–4653. doi: 10.1002/hbm.22500
LeBeau, F. E., Malmierca, M. S., and Rees, A. (2001). Iontophoresis in vivo demonstrates a key role for GABA(A) and glycinergic inhibition in shaping frequency response areas in the inferior colliculus of guinea pig. J. Neurosci. 21, 7303–7312. doi: 10.1523/jneurosci.21-18-07303.2001
LeDoux, J. (2012). Rethinking the emotional brain. Neuron 73, 653–676. doi: 10.1016/j.neuron.2012.02.004
Lee, T. H., Baek, J., Lu, Z. L., and Mather, M. (2014). How arousal modulates the visual contrast sensitivity function. Emotion 14, 978–984. doi: 10.1037/a0037047
Leech, R. W., and Alvord, E. C. Jr. (1977). Anoxic-ischemic encephalopathy in the human neonatal period. The significance of brain stem involvement. Arch. Neurol. 34, 109–113. doi: 10.1001/archneur.1977.00500140063013
Leitner, D. S., and Cohen, M. E. (1985). Role of the inferior colliculus in the inhibition of acoustic startle in the rat. Physiol. Behav. 34, 65–70. doi: 10.1016/0031-9384(85)90079-4
Lenz, V., Vargas, M. I., Bin, J. F., Bogorin, A., Grebici-Guessoum, M., Jacques, C., et al. (2002). Value of MRI findings in Gayet-Wernicke encephalopathy. J. Neuroradiol. 29, 153–160.
Leong, D. K., Le, O., Oliva, L., and Butterworth, R. F. (1994). Increased densities of binding sites for the “peripheral-type” benzodiazepine receptor ligand [3H]PK11195 in vulnerable regions of the rat brain in thiamine deficiency encephalopathy. J. Cereb. Blood Flow Metab. 14, 100–105. doi: 10.1038/jcbfm.1994.14
Leong, D. K., Oliva, L., and Butterworth, R. F. (1996). Quantitative autoradiography using selective radioligands for central and peripheral-type benzodiazepine receptors in experimental Wernicke’s encephalopathy: implications for positron emission tomography imaging. Alcohol. Clin. Exp. Res. 20, 601–605. doi: 10.1111/j.1530-0277.1996.tb01101.x
Lesicko, A. M. H., Sons, S. K., and Llano, D. A. (2020). Circuit mechanisms underlying the segregation and integration of parallel processing streams in the inferior colliculus. J. Neurosci. 40, 6328–6344. doi: 10.1523/jneurosci.0646-20.2020
Lestienne, P., and Bataillé, N. (1994). Mitochondrial DNA alterations and genetic diseases: a review. Biomed. Pharmacother. 48, 199–214. doi: 10.1016/0753-3322(94)90134-1
Levy, S., Herve, C., Delacoux, E., and Erlinger, S. (2002). Thiamine deficiency in hepatitis C virus and alcohol-related liver diseases. Dig. Dis. Sci. 47, 543–548.
Li, C., Mccall, N. M., Lopez, A. J., and Kash, T. L. (2013). Alcohol effects on synaptic transmission in periaqueductal gray dopamine neurons. Alcohol 47, 279–287. doi: 10.1016/j.alcohol.2013.02.002
Li, L., Priebe, R. P., and Yeomans, J. S. (1998). Prepulse inhibition of acoustic or trigeminal startle of rats by unilateral electrical stimulation of the inferior colliculus. Behav. Neurosci. 112, 1187–1198. doi: 10.1037/0735-7044.112.5.1187
Li, L., and Yeomans, J. S. (2000). Using intracranial electrical stimulation to study the timing of prepulse inhibition of the startle reflex. Brain Res. Brain Res. Protoc. 5, 67–74. doi: 10.1016/s1385-299x(99)00056-2
Li, L., and Yue, Q. (2002). Auditory gating processes and binaural inhibition in the inferior colliculus. Hear. Res. 168, 98–109. doi: 10.1016/s0378-5955(02)00356-8
Lieber, C. S. (2003). Relationships between nutrition, alcohol use, and liver disease. Alcohol. Res. Health 27, 220–231.
Liou, K. C., Kuo, S. F., and Chen, L. A. (2012). Wernicke encephalopathy with atypical magnetic resonance imaging. Am. J. Emerg. Med. 30, e2081–e2083.
Liu, J., Cai, W., Zhao, M., Cai, W., Sui, F., Hou, W., et al. (2019). Reduced resting-state functional connectivity and sleep impairment in abstinent male alcohol-dependent patients. Hum. Brain Mapp. 40, 4941–4951. doi: 10.1002/hbm.24749
Loftus, W. C., Malmierca, M. S., Bishop, D. C., and Oliver, D. L. (2008). The cytoarchitecture of the inferior colliculus revisited: a common organization of the lateral cortex in rat and cat. Neuroscience 154, 196–205. doi: 10.1016/j.neuroscience.2008.01.019
Lombard, J. (1998). Autism: a mitochondrial disorder? Med. Hypothes. 50, 497–500. doi: 10.1016/s0306-9877(98)90270-5
Lowe, A. S., Beech, J. S., and Williams, S. C. (2007). Small animal, whole brain fMRI: innocuous and nociceptive forepaw stimulation. Neuroimage 35, 719–728. doi: 10.1016/j.neuroimage.2006.12.014
Ma, X., and Suga, N. (2008). Corticofugal modulation of the paradoxical latency shifts of inferior collicular neurons. J. Neurophysiol. 100, 1127–1134. doi: 10.1152/jn.90508.2008
Mair, R. G., Anderson, C. D., Langlais, P. J., and Mcentee, W. J. (1988). Behavioral impairments, brain lesions and monoaminergic activity in the rat following recovery from a bout of thiamine deficiency. Behav. Brain Res. 27, 223–239. doi: 10.1016/0166-4328(88)90119-2
Maisonnette, S. S., Kawasaki, M. C., Coimbra, N. C., and Brandão, M. L. (1996). Effects of lesions of amygdaloid nuclei and substantia nigra on aversive responses induced by electrical stimulation of the inferior colliculus. Brain Res. Bull. 40, 93–98. doi: 10.1016/0361-9230(95)02136-1
Mancuso, P., Carpinteri, M., Guarnera, F., Augello, G., Chiaramonte, I., and Cristaudo, C. (1988). Diagnosis of quadrigeminal plate arachnoid cyst with CT and NMR. A case report. Acta Neurol. 10, 367–372. doi: 10.3171/jns.1970.32.3.0367
Marietta, C. A., Eckardt, M. J., Campbell, G. A., Majchrowicz, E., and Weight, F. F. (1986). Glucose uptake in brain during withdrawal from ethanol, phenobarbital, and diazepam. Alcohol. Clin. Exp. Res. 10, 233–236. doi: 10.1111/j.1530-0277.1986.tb05081.x
Marsh, R. A., Fuzessery, Z. M., Grose, C. D., and Wenstrup, J. J. (2002). Projection to the inferior colliculus from the basal nucleus of the amygdala. J. Neurosci. 22, 10449–10460. doi: 10.1523/jneurosci.22-23-10449.2002
Martin, P. R., Singleton, C. K., and Hiller-Sturmhofel, S. (2003). The role of thiamine deficiency in alcoholic brain disease. Alcohol. Res. Health 27, 134–142.
Matsumoto, I., Leah, J., Shanley, B., and Wilce, P. (1993). Immediate early gene expression in the rat brain during ethanol withdrawal. Mol. Cell. Neurosci. 4, 485–491. doi: 10.1006/mcne.1993.1060
Matsushima, K., Macmanus, J. P., and Hakim, A. M. (1997). Apoptosis is restricted to the thalamus in thiamine-deficient rats. Neuroreport 8, 867–870.
Maximino, C. (2008). Evolutionary changes in the complexity of the tectum of nontetrapods: a cladistic approach. PLoS One 3:e3582. doi: 10.1371/journal.pone.0003582
Maynard, M. E., and Leasure, J. L. (2013). Exercise enhances hippocampal recovery following binge ethanol exposure. PLoS One 8:e76644. doi: 10.1371/journal.pone.0076644
McClain, J. A., Hayes, D. M., Morris, S. A., and Nixon, K. (2011). Adolescent binge alcohol exposure alters hippocampal progenitor cell proliferation in rats: effects on cell cycle kinetics. J. Comp. Neurol. 519, 2697–2710. doi: 10.1002/cne.22647
McCown, T. J., and Breese, G. R. (1990). Multiple withdrawals from chronic ethanol “kindles” inferior collicular seizure activity: evidence for kindling of seizures associated with alcoholism. Alcohol. Clin. Exp. Res. 14, 394–399. doi: 10.1111/j.1530-0277.1990.tb00492.x
McCown, T. J., and Breese, G. R. (1993). A potential contribution to ethanol withdrawal kindling: reduced GABA function in the inferior collicular cortex. Alcohol. Clin. Exp. Res. 17, 1290–1294. doi: 10.1111/j.1530-0277.1993.tb05243.x
Meng, J. S., and Okeda, R. (2003). Neuropathological study of the role of mast cells and histamine-positive neurons in selective vulnerability of the thalamus and inferior colliculus in thiamine-deficient encephalopathy. Neuropathology 23, 25–35. doi: 10.1046/j.1440-1789.2003.00481.x
Merchán, M., Aguilar, L. A., Lopez-Poveda, E. A., and Malmierca, M. S. (2005). The inferior colliculus of the rat: quantitative immunocytochemical study of GABA and glycine. Neuroscience 136, 907–925. doi: 10.1016/j.neuroscience.2004.12.030
Miller, M. T., Strömland, K., Gillberg, C., Johansson, M., and Nilsson, E. W. (1998). The puzzle of autism: an ophthalmologic contribution. Trans. Am. Ophthalmol. Soc. 96, 369–385.
Modell, J. G., Mountz, J. M., and Beresford, T. P. (1990). Basal ganglia/limbic striatal and thalamocortical involvement in craving and loss of control in alcoholism. J. Neuropsychiatry Clin. Neurosci. 2, 123–144. doi: 10.1176/jnp.2.2.123
Moghaddam, B., and Bolinao, M. L. (1994). Biphasic effect of ethanol on extracellular accumulation of glutamate in the hippocampus and the nucleus accumbens. Neurosci. Lett. 178, 99–102. doi: 10.1016/0304-3940(94)90299-2
Moon, S. J., Kang, M. H., and Park, H. M. (2013). Clinical signs, MRI features, and outcomes of two cats with thiamine deficiency secondary to diet change. J. Vet. Sci. 14, 499–502. doi: 10.4142/jvs.2013.14.4.499
Moore, J. K., Perazzo, L. M., and Braun, A. (1995). Time course of axonal myelination in the human brainstem auditory pathway. Hear. Res. 87, 21–31. doi: 10.1016/0378-5955(95)00073-d
Moriizumi, T., Leduc-Cross, B., Wu, J. Y., and Hattori, T. (1992). Separate neuronal populations of the rat substantia nigra pars lateralis with distinct projection sites and transmitter phenotypes. Neuroscience 46, 711–720. doi: 10.1016/0306-4522(92)90157-w
Murata, T., Fujito, T., Kimura, H., Omori, M., Itoh, H., and Wada, Y. (2001). Serial MRI and (1)H-MRS of Wernicke’s encephalopathy: report of a case with remarkable cerebellar lesions on MRI. Psychiatry Res. 108, 49–55. doi: 10.1016/s0925-4927(01)00304-3
Myers, R. E. (1972). Two patterns of perinatal brain damage and their conditions of occurrence. Am. J. Obstet. Gynecol. 112, 246–276. doi: 10.1016/0002-9378(72)90124-x
Nagashima, K. (1997). A review of experimental methylmercury toxicity in rats: neuropathology and evidence for apoptosis. Toxicol. Pathol. 25, 624–631. doi: 10.1177/019262339702500613
Nakagawasai, O. (2005). Behavioral and neurochemical alterations following thiamine deficiency in rodents: relationship to functions of cholinergic neurons. Yakugaku Zasshi 125, 549–554. doi: 10.1248/yakushi.125.549
Nakagawasai, O., Tadano, T., Hozumi, S., Taniguchi, R., Tan-No, K., Esashi, A., et al. (2001). Characteristics of depressive behavior induced by feeding thiamine-deficient diet in mice. Life Sci. 69, 1181–1191. doi: 10.1016/s0024-3205(01)01206-1
Nakano, T., Fujimoto, T., Shimooki, S., Fukudome, T., Uchida, T., Tsuji, T., et al. (1996). Transient elevation of nerve growth factor content in the rat hippocampus and frontal cortex by chronic ethanol treatment. Psychiatry Clin. Neurosci. 50, 157–160. doi: 10.1111/j.1440-1819.1996.tb01681.x
Nanson, J. L. (1992). Autism in fetal alcohol syndrome: a report of six cases. Alcohol. Clin. Exp. Res. 16, 558–565. doi: 10.1111/j.1530-0277.1992.tb01417.x
Navarro, D., Zwingmann, C., Chatauret, N., and Butterworth, R. F. (2008). Glucose loading precipitates focal lactic acidosis in the vulnerable medial thalamus of thiamine-deficient rats. Metab. Brain Dis. 23, 115–122. doi: 10.1007/s11011-007-9076-z
Neiman, J., Noldy, N. E., El-Nesr, B., Mcdonough, M., and Carlen, P. L. (1991). Late auditory evoked potentials in alcoholics. Identifying those with a history of epileptic seizures during withdrawal. Ann. N.Y. Acad. Sci. 620, 73–81. doi: 10.1111/j.1749-6632.1991.tb51575.x
Newton, J., Suman, S., Akinfiresoye, L. R., Datta, K., Lovinger, D. M., and N’gouemo, P. (2018). Alcohol withdrawal upregulates mRNA encoding for Ca(V)2.1-α1 subunit in the rat inferior colliculus. Alcohol 66, 21–26. doi: 10.1016/j.alcohol.2017.07.007
N’Gouemo, P., Akinfiresoye, L. R., Allard, J. S., and Lovinger, D. M. (2015). Alcohol withdrawal-induced seizure susceptibility is associated with an upregulation of CaV1.3 channels in the rat inferior colliculus. Int. J. Neuropsychopharmacol. 18:yu123.
N’Gouemo, P., and Lovinger, D. M. (2012). Prenatal alcohol exposure enhances L- And R-type calcium channel currents in neonatal inferior colliculus neurons. Res. Soc. Alcohol. Alcohol. Clin. Exper. Res. 36:107A.
N’Gouemo, P., and Morad, M. (2003). Ethanol withdrawal seizure susceptibility is associated with upregulation of L- and P-type Ca2+ channel currents in rat inferior colliculus neurons. Neuropharmacology 45, 429–437. doi: 10.1016/s0028-3908(03)00191-6
N’Gouemo, P., and Morad, M. (2014). Alcohol withdrawal is associated with a downregulation of large-conductance Ca2 + -activated K+ channels in rat inferior colliculus neurons. Psychopharmacology 231, 2009–2018.
N’Gouemo, P., Yasuda, R. P., and Morad, M. (2006). Ethanol withdrawal is accompanied by downregulation of calcium channel alpha 1B subunit in rat inferior colliculus neurons. Brain Res. 1108, 216–220. doi: 10.1016/j.brainres.2006.06.028
Nguyen, A. O., Binder, D. K., Ethell, I. M., and Razak, K. A. (2020). Abnormal development of auditory responses in the inferior colliculus of a mouse model of Fragile X syndrome. J. Neurophysiol. 123, 2101–2121. doi: 10.1152/jn.00706.2019
Nienhuis, R., and Olds, J. (1978). Changes in unit responses to tones after food reinforcement in the auditory pathway of the rat: intertrial arousal. Exp. Neurol. 59, 229–242. doi: 10.1016/0014-4886(78)90152-8
Nixon, K., and Crews, F. T. (2002). Binge ethanol exposure decreases neurogenesis in adult rat hippocampus. J. Neurochem. 83, 1087–1093. doi: 10.1046/j.1471-4159.2002.01214.x
Nobre, M. J., Cabral, A., and Brandão, M. L. (2010). GABAergic regulation of auditory sensory gating in low- and high-anxiety rats submitted to a fear conditioning procedure. Neuroscience 171, 1152–1163. doi: 10.1016/j.neuroscience.2010.10.011
Nobre, M. J., Sandner, G., and Brandão, M. L. (2003). Enhancement of acoustic evoked potentials and impairment of startle reflex induced by reduction of GABAergic control of the neural substrates of aversion in the inferior colliculus. Hear. Res. 184, 82–90. doi: 10.1016/s0378-5955(03)00231-4
Nodal, F. R., Doubell, T. P., Jiang, Z. D., Thompson, I. D., and King, A. J. (2005). Development of the projection from the nucleus of the brachium of the inferior colliculus to the superior colliculus in the ferret. J. Comp. Neurol. 485, 202–217. doi: 10.1002/cne.20478
Noftz, W. A., Beebe, N. L., Mellott, J. G., and Schofield, B. R. (2020). Cholinergic projections from the pedunculopontine tegmental nucleus contact excitatory and inhibitory neurons in the inferior colliculus. Front. Neural Circ. 14:43. doi: 10.3389/fncir.2020.00043
Nyce, M. Q., Chisholm, J. S., Szmanda, J. A., Boyce, A. K., Boczar, C. M., and Kattah, J. C. (2020). Resolved external ophthalmoplegia and hearing loss in Wernicke’s encephalopathy with thiamine replacement. J. Neuroophthalmol. doi: 10.1097/WNO.0000000000001057
Obernier, J. A., Bouldin, T. W., and Crews, F. T. (2002). Binge ethanol exposure in adult rats causes necrotic cell death. Alcohol. Clin. Exp. Res. 26, 547–557. doi: 10.1111/j.1530-0277.2002.tb02573.x
National Institutes of Health Office of Dietary Supplements (2017). Thiamin Fact Sheet for Consumers. Bethesda, MD: National Institutes of Health Office of Dietary Supplements.
Oliver, D. L. (1984a). Dorsal cochlear nucleus projections to the inferior colliculus in the cat: a light and electron microscopic study. J. Comp. Neurol. 224, 155–172. doi: 10.1002/cne.902240202
Oliver, D. L. (1984b). Neuron types in the central nucleus of the inferior colliculus that project to the medial geniculate body. Neuroscience 11, 409–424. doi: 10.1016/0306-4522(84)90033-2
Oliver, D. L. (1987). Projections to the inferior colliculus from the anteroventral cochlear nucleus in the cat: possible substrates for binaural interaction. J. Comp. Neurol. 264, 24–46. doi: 10.1002/cne.902640104
Oliver, D. L., Kuwada, S., Yin, T. C., Haberly, L. B., and Henkel, C. K. (1991). Dendritic and axonal morphology of HRP-injected neurons in the inferior colliculus of the cat. J. Comp. Neurol. 303, 75–100. doi: 10.1002/cne.903030108
Oliver, D. L., and Morest, D. K. (1984). The central nucleus of the inferior colliculus in the cat. J. Comp. Neurol. 222, 237–264.
Olney, J. W., Tenkova, T., Dikranian, K., Muglia, L. J., Jermakowicz, W. J., D’sa, C., et al. (2002). Ethanol-induced caspase-3 activation in the in vivo developing mouse brain. Neurobiol. Dis. 9, 205–219. doi: 10.1006/nbdi.2001.0475
Ono, J., Ikeda, T., Imai, K., Mano, T., Matsuoka, T., Nagai, T., et al. (1998). Intracranial lipoma of the quadrigeminal region associated with complex partial seizures. Pediatr. Radiol. 28, 729–731. doi: 10.1007/s002470050453
Ono, M., Bishop, D. C., and Oliver, D. L. (2017). Identified GABAergic and glutamatergic neurons in the mouse inferior colliculus share similar response properties. J. Neurosci. 37, 8952–8964. doi: 10.1523/jneurosci.0745-17.2017
Ono, M., Yanagawa, Y., and Koyano, K. (2005). GABAergic neurons in inferior colliculus of the GAD67-GFP knock-in mouse: electrophysiological and morphological properties. Neurosci. Res. 51, 475–492. doi: 10.1016/j.neures.2004.12.019
Otto, D. A., and Fox, D. A. (1993). Auditory and visual dysfunction following lead exposure. Neurotoxicology 14, 191–207.
Oudman, E., Wijnia, J. W., Oey, M., Van Dam, M., Painter, R. C., and Postma, A. (2019). Wernicke’s encephalopathy in hyperemesis gravidarum: a systematic review. Eur. J. Obstet. Gynecol. Reprod. Biol. 236, 84–93.
Oudman, E., Wijnia, J. W., Oey, M. J., Van Dam, M. J., and Postma, A. (2018a). Preventing Wernicke’s encephalopathy in anorexia nervosa: a systematic review. Psychiatry Clin. Neurosci. 72, 774–779. doi: 10.1111/pcn.12735
Oudman, E., Wijnia, J. W., Van Dam, M., Biter, L. U., and Postma, A. (2018b). Preventing Wernicke encephalopathy after bariatric surgery. Obes. Surg. 28, 2060–2068. doi: 10.1007/s11695-018-3262-4
Oyanagi, K., Ohama, E., and Ikuta, F. (1989). The auditory system in methyl mercurial intoxication: a neuropathological investigation on 14 autopsy cases in Niigata, Japan. Acta Neuropathol. 77, 561–568. doi: 10.1007/bf00687882
Pácal, L., Kuricová, K., and Kaňková, K. (2014). Evidence for altered thiamine metabolism in diabetes: is there a potential to oppose gluco- and lipotoxicity by rational supplementation? World J. Diabetes 5, 288–295. doi: 10.4239/wjd.v5.i3.288
Page, G. L., Laight, D., and Cummings, M. H. (2011). Thiamine deficiency in diabetes mellitus and the impact of thiamine replacement on glucose metabolism and vascular disease. Int. J. Clin. Pract. 65, 684–690. doi: 10.1111/j.1742-1241.2011.02680.x
Palmieri, L., and Persico, A. M. (2010). Mitochondrial dysfunction in autism spectrum disorders: cause or effect? Biochim. Biophys. Acta 1797, 1130–1137. doi: 10.1016/j.bbabio.2010.04.018
Palus, V., Penderis, J., Jakovljevic, S., and Cherubini, G. B. (2010). Thiamine deficiency in a cat: resolution of MRI abnormalities following thiamine supplementation. J. Feline Med. Surg. 12, 807–810. doi: 10.1016/j.jfms.2010.04.005
Pan, C. L., Kuo, M. F., and Hsieh, S. T. (2004). Auditory agnosia caused by a tectal germinoma. Neurology 63, 2387–2389. doi: 10.1212/01.wnl.0000148592.92484.a9
Pan, X. C., Li, Z. X., Wu, D. Z., Li, S. Y., Xiang, H. B., and Song, Y. T. (2019). Mapping changes of whole brain blood flow in rats with myocardial ischemia/reperfusion injury assessed by positron emission tomography. Curr. Med. Sci. 39, 653–657. doi: 10.1007/s11596-019-2087-2
Patel, M. B., Sons, S., Yudintsev, G., Lesicko, A. M., Yang, L., Taha, G. A., et al. (2017). Anatomical characterization of subcortical descending projections to the inferior colliculus in mouse. J. Comp. Neurol. 525, 885–900. doi: 10.1002/cne.24106
Pawlosky, R. J., Kashiwaya, Y., Srivastava, S., King, M. T., Crutchfield, C., Volkow, N., et al. (2010). Alterations in brain glucose utilization accompanying elevations in blood ethanol and acetate concentrations in the rat. Alcohol. Clin. Exp. Res. 34, 375–381. doi: 10.1111/j.1530-0277.2009.01099.x
Peruzzi, D., Bartlett, E., Smith, P. H., and Oliver, D. L. (1997). A monosynaptic GABAergic input from the inferior colliculus to the medial geniculate body in rat. J. Neurosci. 17, 3766–3777. doi: 10.1523/jneurosci.17-10-03766.1997
Pfefferbaum, A., Adalsteinsson, E., Bell, R. L., and Sullivan, E. V. (2007). Development and resolution of brain lesions caused by pyrithiamine- and dietary-induced thiamine deficiency and alcohol exposure in the alcohol-preferring rat: a longitudinal magnetic resonance imaging and spectroscopy study. Neuropsychopharmacology 32, 1159–1177. doi: 10.1038/sj.npp.1301107
Pfefferbaum, A., Roth, W. T., Tinklenberg, J. R., Rosenbloom, M. J., and Kopell, B. S. (1979). The effects of ethanol and meperidine on auditory evoked potentials. Drug Alcohol. Depend. 4, 371–380. doi: 10.1016/0376-8716(79)90069-3
Pfefferbaum, A., Zahr, N. M., Mayer, D., Vinco, S., Orduna, J., Rohlfing, T., et al. (2008). Ventricular expansion in wild-type Wistar rats after alcohol exposure by vapor chamber. Alcohol. Clin. Exp. Res. 32, 1459–1467. doi: 10.1111/j.1530-0277.2008.00721.x
Pfefferbaum, A., Zahr, N. M., Sassoon, S. A., Kwon, D., Pohl, K. M., and Sullivan, E. V. (2018). Accelerated and premature aging characterizing regional cortical volume loss in human immunodeficiency virus infection: contributions from alcohol, substance use, and hepatitis C coinfection. Biol. Psychiatry Cogn. Neurosci. Neuroimag. 3, 844–859. doi: 10.1016/j.bpsc.2018.06.006
Phillips, D. E., Cummings, J. D., and Wall, K. A. (2000). Prenatal alcohol exposure decreases the number of nitric oxide synthase positive neurons in rat superior colliculus and periaqueductal gray. Alcohol 22, 75–84. doi: 10.1016/s0741-8329(00)00108-7
Phillips, S. C., and Cragg, B. G. (1984). Blood-brain barrier dysfunction in thiamine-deficient, alcohol-treated rats. Acta Neuropathol. 62, 235–241. doi: 10.1007/bf00691858
Phillips, S. C., Harper, C. G., and Kril, J. (1987). A quantitative histological study of the cerebellar vermis in alcoholic patients. Brain 110(Pt 2), 301–314. doi: 10.1093/brain/110.2.301
Pitel, A. L., Zahr, N. M., Jackson, K., Sassoon, S. A., Rosenbloom, M. J., Pfefferbaum, A., et al. (2011). Signs of preclinical Wernicke’s Encephalopathy and thiamine levels as predictors of neuropsychological deficits in alcoholism without Korsakoff’s syndrome. Neuropsychopharmacology 36, 580–588. doi: 10.1038/npp.2010.189
Pitkin, S. R., and Savage, L. M. (2001). Aging potentiates the acute and chronic neurological symptoms of pyrithiamine-induced thiamine deficiency in the rodent. Behav. Brain Res. 119, 167–177. doi: 10.1016/s0166-4328(00)00350-8
Piven, J., Bailey, J., Ranson, B. J., and Arndt, S. (1997). An MRI study of the corpus callosum in autism. Am. J. Psychiatry 154, 1051–1056. doi: 10.1176/ajp.154.8.1051
Popelár, J., Nwabueze-Ogbo, F. C., and Syka, J. (2003). Changes in neuronal activity of the inferior colliculus in rat after temporal inactivation of the auditory cortex. Physiol. Res. 52, 615–628.
Putzke, J., De Beun, R., Schreiber, R., De Vry, J., Tolle, T. R., Zieglgansberger, W., et al. (1998). Long-term alcohol self-administration and alcohol withdrawal differentially modulate microtubule-associated protein 2 (MAP2) gene expression in the rat brain. Brain Res. Mol. Brain Res. 62, 196–205. doi: 10.1016/s0169-328x(98)00253-8
Rapin, I. (1988). Verbal auditory agnosia in children. Dev. Med. Child Neurol. 30:685. doi: 10.1111/j.1469-8749.1988.tb04811.x
Read, D. H., and Harrington, D. D. (1986). Experimentally induced thiamine deficiency in beagle dogs: pathologic changes of the central nervous system. Am. J. Vet. Res. 47, 2281–2289.
Reimer, A. E., Oliveira, A. R., and Brandão, M. L. (2008). Selective involvement of GABAergic mechanisms of the dorsal periaqueductal gray and inferior colliculus on the memory of the contextual fear as assessed by the fear potentiated startle test. Brain Res. Bull. 76, 545–550. doi: 10.1016/j.brainresbull.2008.03.011
Riaz, A., and Faingold, C. L. (1994). Seizures during ethanol withdrawal are blocked by focal microinjection of excitant amino acid antagonists into the inferior colliculus and pontine reticular formation. Alcohol. Clin. Exp. Res. 18, 1456–1462. doi: 10.1111/j.1530-0277.1994.tb01450.x
Ribak, C. E., and Morin, C. L. (1995). The role of the inferior colliculus in a genetic model of audiogenic seizures. Anat. Embryol. 191, 279–295. doi: 10.1007/bf00534681
Rice, A. C., Bullock, M. R., and Shelton, K. L. (2004). Chronic ethanol consumption transiently reduces adult neural progenitor cell proliferation. Brain Res. 1011, 94–98. doi: 10.1016/j.brainres.2004.01.091
Ritvo, E. R., Freeman, B. J., Scheibel, A. B., Duong, T., Robinson, H., Guthrie, D., et al. (1986). Lower Purkinje cell counts in the cerebella of four autistic subjects: initial findings of the UCLA-NSAC autopsy research report. Am. J. Psychiatry 143, 862–866. doi: 10.1176/ajp.143.7.862
Roberts, A. J., Heyser, C. J., Cole, M., Griffin, P., and Koob, G. F. (2000). Excessive ethanol drinking following a history of dependence: animal model of allostasis. Neuropsychopharmacology 22, 581–594. doi: 10.1016/s0893-133x(99)00167-0
Roebuck, T. M., Mattson, S. N., and Riley, E. P. (1998). A review of the neuroanatomical findings in children with fetal alcohol syndrome or prenatal exposure to alcohol. Alcohol. Clin. Exp. Res. 22, 339–344. doi: 10.1111/j.1530-0277.1998.tb03658.x
Roland, E. H., Hill, A., Norman, M. G., Flodmark, O., and Macnab, A. J. (1988). Selective brainstem injury in an asphyxiated newborn. Ann. Neurol. 23, 89–92. doi: 10.1002/ana.410230115
Rorke, L. B., Riggs, H. E., and Fogelson, M. H. (1968). Cerebellar heterotopia in infancy. J. Neuropathol. Exp. Neurol. 27, 140–141.
Roulet, T., Van Den Bosch De Aguilar, P., and De Witte, P. (1985). Effects of ethanol on the rat brain: ultrastructural alterations in the temporal cortex and in the hippocampus. Alcohol 2, 227–230. doi: 10.1016/0741-8329(85)90050-3
Ruchalski, K., and Hathout, G. M. (2012). A medley of midbrain maladies: a brief review of midbrain anatomy and syndromology for radiologists. Radiol. Res. Pract. 2012:258524.
Ruth, R. E., Rosenfeld, J. P., Harris, D. M., and Birkel, P. (1974). Effects of aversive and rewarding electrical brain stimulation on auditory evoked responses in albino rat tectum. Physiol. Behav. 13, 729–735. doi: 10.1016/0031-9384(74)90254-6
Saad, L., Silva, L. F., Banzato, C. E., Dantas, C. R., and Garcia, C. Jr. (2010). Anorexia nervosa and Wernicke-Korsakoff syndrome: a case report. J. Med. Case Rep. 4:217.
Saalmann, Y. B., Morgan, I. G., and Calford, M. B. (2006). Neurosteroids involved in regulating inhibition in the inferior colliculus. J. Neurophysiol. 96, 3064–3073. doi: 10.1152/jn.00786.2006
Salib, A. N., Ho, A. L., Sussman, E. S., Pendharkar, A. V., and Halpern, C. H. (2018). Neuromodulatory treatments for alcohol use disorder: a review. Brain Sci. 8:95. doi: 10.3390/brainsci8060095
Sano, M., Kaga, K., Kuan, C. C., Ino, K., and Mima, K. (2007). Early myelination patterns in the brainstem auditory nuclei and pathway: MRI evaluation study. Int. J. Pediatr. Otorhinolaryngol. 71, 1105–1115. doi: 10.1016/j.ijporl.2007.04.002
Santos, N. R., Huston, J. P., and Brandão, M. L. (2003). Blockade of histamine H2 receptors of the periaqueductal gray and inferior colliculus induces fear-like behaviors. Pharmacol. Biochem. Behav. 75, 25–33. doi: 10.1016/s0091-3057(03)00033-9
Sarkola, T., Iles, M. R., Kohlenberg-Mueller, K., and Eriksson, C. J. (2002). Ethanol, acetaldehyde, acetate, and lactate levels after alcohol intake in white men and women: effect of 4-methylpyrazole. Alcohol. Clin. Exp. Res. 26, 239–245. doi: 10.1111/j.1530-0277.2002.tb02530.x
Satake, S., Yamada, K., Melo, L. L., and Barbosa Silva, R. (2012). Effects of microinjections of apomorphine and haloperidol into the inferior colliculus on prepulse inhibition of the acoustic startle reflex in rat. Neurosci. Lett. 509, 60–63. doi: 10.1016/j.neulet.2011.12.052
Savage, L. M., Hall, J. M., and Resende, L. S. (2012). Translational rodent models of Korsakoff syndrome reveal the critical neuroanatomical substrates of memory dysfunction and recovery. Neuropsychol. Rev. 22, 195–209. doi: 10.1007/s11065-012-9194-1
Scalais, E., Francois, B., Schlesser, P., Stevens, R., Nuttin, C., Martin, J. J., et al. (2012). Polymerase gamma deficiency (POLG): clinical course in a child with a two stage evolution from infantile myocerebrohepatopathy spectrum to an Alpers syndrome and neuropathological findings of Leigh’s encephalopathy. Eur. J. Paediatr. Neurol. 16, 542–548. doi: 10.1016/j.ejpn.2012.01.013
Schneider, H., Ballowitz, L., Schachinger, H., Hanefeld, F., and Dröszus, J. U. (1975). Anoxic encephalopathy with predominant involvement of basal ganglia, brain stem and spinal cord in the perinatal period. Report on seven newborns. Acta Neuropathol. 32, 287–298. doi: 10.1007/bf00696791
Schofield, B. R., and Beebe, N. L. (2019). Subtypes of GABAergic cells in the inferior colliculus. Hear. Res. 376, 1–10. doi: 10.1016/j.heares.2018.10.001
Schofield, B. R., Motts, S. D., and Mellott, J. G. (2011). Cholinergic cells of the pontomesencephalic tegmentum: connections with auditory structures from cochlear nucleus to cortex. Hear. Res. 279, 85–95. doi: 10.1016/j.heares.2010.12.019
Sechi, G., and Serra, A. (2007). Wernicke’s encephalopathy: new clinical settings and recent advances in diagnosis and management. Lancet Neurol. 6, 442–455. doi: 10.1016/s1474-4422(07)70104-7
Senatorov, V. V., and Hu, B. (2002). Extracortical descending projections to the rat inferior colliculus. Neuroscience 115, 243–250. doi: 10.1016/s0306-4522(02)00316-0
Shaw, N. A. (1987). Effects of low pass filtering on the brainstem auditory evoked potential in the rat. Exp. Brain Res. 65, 686–690.
Shaw, N. A. (1988). The auditory evoked potential in the rat–a review. Prog. Neurobiol. 31, 19–45. doi: 10.1016/0301-0082(88)90021-4
Sheedy, D., Lara, A., Garrick, T., and Harper, C. (1999). Size of mamillary bodies in health and disease: useful measurements in neuroradiological diagnosis of Wernicke’s encephalopathy. Alcohol. Clin. Exp. Res. 23, 1624–1628. doi: 10.1097/00000374-199910000-00009
Shoffner, J., Hyams, L., Langley, G. N., Cossette, S., Mylacraine, L., Dale, J., et al. (2010). Fever plus mitochondrial disease could be risk factors for autistic regression. J. Child Neurol. 25, 429–434. doi: 10.1177/0883073809342128
Siciliano, C. A., Noamany, H., Chang, C. J., Brown, A. R., Chen, X., Leible, D., et al. (2019). A cortical-brainstem circuit predicts and governs compulsive alcohol drinking. Science 366, 1008–1012. doi: 10.1126/science.aay1186
Siddiqui, M. F., Elwell, C., and Johnson, M. H. (2016). Mitochondrial dysfunction in autism spectrum disorders. Autism Open Access. 6:1000190.
Siman, R., Zhang, C., Roberts, V. L., Pitts-Kiefer, A., and Neumar, R. W. (2005). Novel surrogate markers for acute brain damage: cerebrospinal fluid levels corrrelate with severity of ischemic neurodegeneration in the rat. J. Cereb. Blood Flow Metab. 25, 1433–1444. doi: 10.1038/sj.jcbfm.9600138
Simon, E. N. (1999). The Brain and Disorders of Language and Social Awareness (Book 1). New York, NY: Barnes & Noble.
Smith, P. H., Bartlett, E. L., and Kowalkowski, A. (2006). Unique combination of anatomy and physiology in cells of the rat paralaminar thalamic nuclei adjacent to the medial geniculate body. J. Comp. Neurol. 496, 314–334. doi: 10.1002/cne.20913
Smith, R. J., Anderson, R. I., Haun, H. L., Mulholland, P. J., Griffin, W. C. III, Lopez, M. F., et al. (2019). Dynamic c-Fos changes in mouse brain during acute and protracted withdrawal from chronic intermittent ethanol exposure and relapse drinking. Addict. Biol. 25:e12804.
Sokoloff, L. (1981). Localization of functional activity in the central nervous system by measurement of glucose utilization with radioactive deoxyglucose. J. Cereb. Blood Flow Metab. 1, 7–36. doi: 10.1038/jcbfm.1981.4
Sokoloff, L., Reivich, M., Kennedy, C., Des Rosiers, M. H., Patlak, C. S., Pettigrew, K. D., et al. (1977). The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J. Neurochem. 28, 897–916. doi: 10.1111/j.1471-4159.1977.tb10649.x
Song, Y., Mellott, J. G., and Winer, J. A. (2011). Microvascular organization of the cat inferior colliculus. Hear. Res. 274, 5–12. doi: 10.1016/j.heares.2010.02.014
Soveri, P., and Fruhstorfer, H. (1969). The effect of alcohol on auditory evoked potentials in attentive and non-attentive states. Scand. J. Clin. Lab. Invest. 108:81.
Sprague, J. M., Chambers, W. W., and Stellar, E. (1961). Attentive, affective, and adaptive behavior in the cat: sensory deprivation of the forebrain by lesions in the brain stem results in striking behavioral abnormalities. Science 133, 165–173. doi: 10.1126/science.133.3447.165
Squires, K. C., Chu, N. S., and Starr, A. (1978). Acute effects of alcohol on auditory brainstem potentials in humans. Science 201, 174–176. doi: 10.1126/science.208148
Stepniewska, I., Ql, H. X., and Kaas, J. H. (2000). Projections of the superior colliculus to subdivisions of the inferior pulvinar in New World and Old World monkeys. Vis. Neurosci. 17, 529–549. doi: 10.1017/s0952523800174048
Sullivan, E. V. (2003). Compromised pontocerebellar and cerebellothalamocortical systems: speculations on their contributions to cognitive and motor impairment in nonamnesic alcoholism. Alcohol. Clin. Exp. Res. 27, 1409–1419. doi: 10.1097/01.alc.0000085586.91726.46
Sullivan, E. V., Lane, B., Deshmukh, A., Rosenbloom, M. J., Desmond, J. E., Lim, K. O., et al. (1999). In vivo mammillary body volume deficits in amnesic and nonamnesic alcoholics. Alcohol. Clin. Exp. Res. 23, 1629–1636. doi: 10.1111/j.1530-0277.1999.tb04054.x
Sullivan, E. V., and Pfefferbaum, A. (2005). Neurocircuitry in alcoholism: a substrate of disruption and repair. Psychopharmacology 180, 583–594. doi: 10.1007/s00213-005-2267-6
Sullivan, E. V., and Pfefferbaum, A. (2009). Neuroimaging of the Wernicke-Korsakoff syndrome. Alcohol Alcohol. 44, 155–165. doi: 10.1093/alcalc/agn103
Sullivan, E. V., and Pfefferbaum, A. (2019). Brain-behavior relations and effects of aging and common comorbidities in alcohol use disorder: a review. Neuropsychology 33, 760–780. doi: 10.1037/neu0000557
Sullivan, E. V., Rohlfing, T., and Pfefferbaum, A. (2010). Pontocerebellar volume deficits and ataxia in alcoholic men and women: no evidence for “telescoping”. Psychopharmacology 208, 279–290. doi: 10.1007/s00213-009-1729-7
Sullivan, E. V., Zahr, N. M., Sassoon, S. A., Thompson, W. K., Kwon, D., Pohl, K. M., et al. (2018). The role of aging, drug dependence, and Hepatitis C comorbidity in alcoholism cortical compromise. JAMA Psychiatry 75, 474–483. doi: 10.1001/jamapsychiatry.2018.0021
Szalda, K., and Burkard, R. (2005). The effects of nembutal anesthesia on the auditory steady-state response (ASSR) from the inferior colliculus and auditory cortex of the chinchilla. Hear. Res. 203, 32–44. doi: 10.1016/j.heares.2004.11.014
Takada, M., Li, Z. K., and Hattori, T. (1987). A note on the projections of pars compacta neurons within pars reticulata of the substantia nigra in the rat. Brain Res. Bull. 18, 285–290. doi: 10.1016/0361-9230(87)90004-9
Tarnowska-Dziduszko, E., Bertrand, E., and Szpak, G. M. (1995). Morphological changes in the corpus callosum in chronic alcoholism. Folia Neuropathol. 33, 25–29.
Thompson, S. G., and McGeer, E. G. (1985). GABA-transaminase and glutamic acid decarboxylase changes in the brain of rats treated with pyrithiamine. Neurochem. Res. 10, 1653–1660. doi: 10.1007/bf00988607
Thomson, A. D., Cook, C. C., Guerrini, I., Sheedy, D., Harper, C., and Marshall, E. J. (2008). Wernicke’s encephalopathy: ‘Plus ça change, plus c’est la même chose’. Alcohol Alcohol. 43, 180–186.
Touchon, J., Rondouin, G., De Lustrac, C., Billiard, M., Baldy-Moulinier, M., and Cadilhac, J. (1984). Brain stem auditory evoked potentials in alcoholic epilepsy. Rev. Electroencephalogr. Neurophysiol. Clin. 14, 133–137.
Troncoso, J. C., Johnston, M. V., Hess, K. M., Griffin, J. W., and Price, D. L. (1981). Model of Wernicke’s encephalopathy. Arch. Neurol. 38, 350–354.
Van Buskirk, R. L. (1983). Subcortical auditory and somatosensory afferents to hamster superior colliculus. Brain Res. Bull. 10, 583–587. doi: 10.1016/0361-9230(83)90025-4
Vander Weele, C. M., Siciliano, C. A., Matthews, G. A., Namburi, P., Izadmehr, E. M., Espinel, I. C., et al. (2018). Dopamine enhances signal-to-noise ratio in cortical-brainstem encoding of aversive stimuli. Nature 563, 397–401. doi: 10.1038/s41586-018-0682-1
Vemuganti, R., Kalluri, H., Yi, J. H., Bowen, K. K., and Hazell, A. S. (2006). Gene expression changes in thalamus and inferior colliculus associated with inflammation, cellular stress, metabolism and structural damage in thiamine deficiency. Eur. J. Neurosci. 23, 1172–1188. doi: 10.1111/j.1460-9568.2006.04651.x
Vendruscolo, L. F., and Roberts, A. J. (2014). Operant alcohol self-administration in dependent rats: focus on the vapor model. Alcohol 48, 277–286. doi: 10.1016/j.alcohol.2013.08.006
Victor, M., Adams, R. D., and Collins, G. H. (1971). The Wernicke-Korsakoff syndrome. A clinical and pathological study of 245 patients, 82 with post-mortem examinations. Contemp. Neurol. Ser. 7, 1–206.
Victor, M., Adams, R. D., and Collins, G. H. (1989). The Wernicke_Korsakoff Syndrome. Philadelphia, PA: F.A. Davis Company.
Volkow, N. D., Kim, S. W., Wang, G. J., Alexoff, D., Logan, J., Muench, L., et al. (2013). Acute alcohol intoxication decreases glucose metabolism but increases acetate uptake in the human brain. Neuroimage 64, 277–283. doi: 10.1016/j.neuroimage.2012.08.057
Volkow, N. D., Wang, G. J., Franceschi, D., Fowler, J. S., Thanos, P. P., Maynard, L., et al. (2006). Low doses of alcohol substantially decrease glucose metabolism in the human brain. Neuroimage 29, 295–301. doi: 10.1016/j.neuroimage.2005.07.004
Vortmeyer, A. O., and Colmant, H. J. (1988). Differentiation between brain lesions in experimental thiamine deficiency. Virchows Arch. A Pathol. Anat. Histopathol. 414, 61–67. doi: 10.1007/bf00749739
Vortmeyer, A. O., Hagel, C., and Laas, R. (1992). Haemorrhagic thiamine deficient encephalopathy following prolonged parenteral nutrition. J. Neurol. Neurosurg. Psychiatry 55, 826–829. doi: 10.1136/jnnp.55.9.826
Walker, M. A., Zepeda, R., Afari, H. A., and Cohen, A. B. (2014). Hearing loss in Wernicke encephalopathy. Neurol. Clin. Pract. 4, 511–515. doi: 10.1212/cpj.0000000000000072
Watanabe, I., and Kanabe, S. (1978). Early edematous lesion of pyrithiamine induced acute thiamine deficient encephalopathy in the mouse. J. Neuropathol. Exp. Neurol. 37, 401–413. doi: 10.1097/00005072-197807000-00004
Wei, Y., Cui, L., and Peng, B. (2018). Mitochondrial DNA mutations in late-onset Leigh syndrome. J. Neurol. 265, 2388–2395. doi: 10.1007/s00415-018-9014-5
Weindling, S. M., Press, G. A., and Hesselink, J. R. (1988). MR characteristics of a primary melanoma of the quadrigeminal plate. AJNR Am. J. Neuroradiol. 9, 214–215.
Wilce, P., Beckmann, A., Shanley, B., and Matsumoto, I. (1994). Gene expression during ethanol withdrawal. Alcohol Alcohol. Suppl. 2, 97–102.
Wilczynski, W., and Ryan, M. J. (2010). The behavioral neuroscience of anuran social signal processing. Curr. Opin. Neurobiol. 20, 754–763. doi: 10.1016/j.conb.2010.08.021
Williams-Hemby, L., and Porrino, L. J. (1994). Low and moderate doses of ethanol produce distinct patterns of cerebral metabolic changes in rats. Alcohol. Clin. Exp. Res. 18, 982–988. doi: 10.1111/j.1530-0277.1994.tb00070.x
Windle, W. F. (1969). Brain damage by asphyxia at birth. Sci. Am. 221, 76–84. doi: 10.1038/scientificamerican1069-76
Winer, J. A., Larue, D. T., Diehl, J. J., and Hefti, B. J. (1998). Auditory cortical projections to the cat inferior colliculus. J. Comp. Neurol. 400, 147–174. doi: 10.1002/(sici)1096-9861(19981019)400:2<147::aid-cne1>3.0.co;2-9
Winer, J. A., Miller, L. M., Lee, C. C., and Schreiner, C. E. (2005). Auditory thalamocortical transformation: structure and function. Trends Neurosci. 28, 255–263. doi: 10.1016/j.tins.2005.03.009
Witt, E. D. (1985). Neuroanatomical consequences of thiamine deficiency: a comparative analysis. Alcohol Alcohol. 20, 201–221.
Witt, E. D., and Goldman-Rakic, P. S. (1983). Intermittent thiamine deficiency in the rhesus monkey. I. Progression of neurological signs and neuroanatomical lesions. Ann. Neurol. 13, 376–395. doi: 10.1002/ana.410130404
Wozniak, J. R., Riley, E. P., and Charness, M. E. (2019). Clinical presentation, diagnosis, and management of fetal alcohol spectrum disorder. Lancet Neurol. 18, 760–770. doi: 10.1016/s1474-4422(19)30150-4
Xiong, X. R., Liang, F., Zingg, B., Ji, X. Y., Ibrahim, L. A., Tao, H. W., et al. (2015). Auditory cortex controls sound-driven innate defense behaviour through corticofugal projections to inferior colliculus. Nat. Commun. 6:7224.
Yakovlev, P. I., and Lecours, A. R. (1967). “The myelogenetic cycles of regional maturation of the brain,” in Regional Development of the Brain in Early Life, ed. A. Minkowski (Oxford: Blackwell Scientific Publications), 3–70.
Yan, J., Zhang, Y., and Ehret, G. (2005). Corticofugal shaping of frequency tuning curves in the central nucleus of the inferior colliculus of mice. J. Neurophysiol. 93, 71–83. doi: 10.1152/jn.00348.2004
Yang, L., Long, C., Randall, M. E., and Faingold, C. L. (2003). Neurons in the periaqueductal gray are critically involved in the neuronal network for audiogenic seizures during ethanol withdrawal. Neuropharmacology 44, 275–281. doi: 10.1016/s0028-3908(02)00367-2
Yasui, Y., Nakano, K., Kayahara, T., and Mizuno, N. (1991). Non-dopaminergic projections from the substantia nigra pars lateralis to the inferior colliculus in the rat. Brain Res. 559, 139–144. doi: 10.1016/0006-8993(91)90296-8
Zahr, N. M. (2014). Structural and microstructral imaging of the brain in alcohol use disorders. Handb. Clin. Neurol. 125, 275–290. doi: 10.1016/b978-0-444-62619-6.00017-3
Zahr, N. M., Alt, C., Mayer, D., Rohlfing, T., Manning-Bog, A., Luong, R., et al. (2014a). Associations between in vivo neuroimaging and postmortem brain cytokine markers in a rodent model of Wernicke’s encephalopathy. Exp. Neurol. 261, 109–119. doi: 10.1016/j.expneurol.2014.06.015
Zahr, N. M., Mayer, D., Rohlfing, T., Hsu, O., Vinco, S., Orduna, J., et al. (2014b). Rat strain differences in brain structure and neurochemistry in response to binge alcohol. Psychopharmacology 231, 429–445. doi: 10.1007/s00213-013-3253-z
Zahr, N. M., Kaufman, K. L., and Harper, C. G. (2011). Clinical and pathological features of alcohol-related brain damage. Nat. Rev. Neurol. 7, 284–294. doi: 10.1038/nrneurol.2011.42
Zahr, N. M., Lenart, A. M., Karpf, J. A., Casey, K. M., Pohl, K. M., Sullivan, E. V., et al. (2020a). Multi-modal imaging reveals differential brain volumetric, biochemical, and white matter fiber responsivity to repeated intermittent ethanol vapor exposure in male and female rats. Neuropharmacology 170:108066. doi: 10.1016/j.neuropharm.2020.108066
Zahr, N. M., Sullivan, E. V., Pohl, K. M., and Pfefferbaum, A. (2020b). Age differences in brain structural and metabolic responses to binge ethanol exposure in fisher 344 rats. Neuropsychopharmacology doi: 10.1038/s41386-020-0744-6
Zahr, N. M., Luong, R., Sullivan, E. V., and Pfefferbaum, A. (2010a). Measurement of serum, liver, and brain cytokine induction, thiamine levels, and hepatopathology in rats exposed to a 4-day alcohol binge protocol. Alcohol. Clin. Exp. Res. 34, 1858–1870. doi: 10.1111/j.1530-0277.2010.01274.x
Zahr, N. M., Mayer, D., Rohlfing, T., Hasak, M., Hsu, O., Vinco, S., et al. (2010b). Brain injury and recovery following binge ethanol: evidence from in vivo magnetic resonance spectroscopy. Biol. Psychiatry 67, 846–854. doi: 10.1016/j.biopsych.2009.10.028
Zahr, N. M., Mayer, D., Rohlfing, T., Orduna, J., Luong, R., Sullivan, E. V., et al. (2013). A mechanism of rapidly reversible cerebral ventricular enlargement independent of tissue atrophy. Neuropsychopharmacology 38, 1121–1129. doi: 10.1038/npp.2013.11
Zahr, N. M., Mayer, D., Vinco, S., Orduna, J., Luong, R., Sullivan, E. V., et al. (2009). In vivo evidence for alcohol-induced neurochemical changes in rat brain without protracted withdrawal, pronounced thiamine deficiency, or severe liver damage. Neuropsychopharmacology 34, 1427–1442. doi: 10.1038/npp.2008.119
Zahr, N. M., Pfefferbaum, A., and Sullivan, E. V. (2017). Perspectives on fronto-fugal circuitry from human imaging of alcohol use disorders. Neuropharmacology 122, 189–200. doi: 10.1016/j.neuropharm.2017.01.018
Zahr, N. M., Rohlfing, T., Mayer, D., Luong, R., Sullivan, E. V., and Pfefferbaum, A. (2016a). Transient CNS responses to repeated binge ethanol treatment. Addict. Biol. 21, 1199–1216. doi: 10.1111/adb.12290
Zahr, N. M., Sullivan, E. V., Rohlfing, T., Mayer, D., Collins, A. M., Luong, R., et al. (2016b). Concomitants of alcoholism: differential effects of thiamine deficiency, liver damage, and food deprivation on the rat brain in vivo. Psychopharmacology 233, 2675–2686. doi: 10.1007/s00213-016-4313-y
Zajac, C. S., Bunger, P. C., and Moore, J. C. (1988). Neuron development in the superior colliculus of the fetal mouse following maternal alcohol exposure. Teratology 38, 37–43. doi: 10.1002/tera.1420380106
Zappia, M., Cheek, J. C., and Lüders, H. (1996). Brain-stem auditory evoked potentials (BAEPs) from basal surface of temporal lobe recorded from chronic subdural electrodes. Electroencephalogr. Clin. Neurophysiol. 100, 141–151. doi: 10.1016/0013-4694(95)00180-8
Zeller, K., Rahner-Welsch, S., and Kuschinsky, W. (1997). Distribution of Glut1 glucose transporters in different brain structures compared to glucose utilization and capillary density of adult rat brains. J. Cereb. Blood Flow Metab. 17, 204–209. doi: 10.1097/00004647-199702000-00010
Zhang, D. X., Li, L., Kelly, J. B., and Wu, S. H. (1998). GABAergic projections from the lateral lemniscus to the inferior colliculus of the rat. Hear. Res. 117, 1–12. doi: 10.1016/s0378-5955(97)00202-5
Zhang, S. X., Weilersbacher, G. S., Henderson, S. W., Corso, T., Olney, J. W., and Langlais, P. J. (1995). Excitotoxic cytopathology, progression, and reversibility of thiamine deficiency-induced diencephalic lesions. J. Neuropathol. Exp. Neurol. 54, 255–267. doi: 10.1097/00005072-199503000-00012
Zhao, Q., Fritz, M., Pfefferbaum, A., Sullivan, E. V., Pohl, K. M., and Zahr, N. M. (2018). Jacobian maps reveal under-reported brain regions sensitive to extreme binge ethanol intoxication in the rat. Front. Neuroanat. 12:108. doi: 10.3389/fnana.2018.00108
Zhao, Q., Pohl, K. M., Sullivan, E. V., Pfefferbaum, A., and Zahr, N. M. (2020). Jacobian mapping reveals converging brain substrates of disruption and repair in response to ethanol exposure and abstinence in two strains of rats. Alcoholism Clin. Exp. Res. (in press).
Zhou, J., and Shore, S. (2006). Convergence of spinal trigeminal and cochlear nucleus projections in the inferior colliculus of the guinea pig. J. Comp. Neurol. 495, 100–112. doi: 10.1002/cne.20863
Zhou, X., and Jen, P. H. (2007). Corticofugal modulation of multi-parametric auditory selectivity in the midbrain of the big brown bat. J. Neurophysiol. 98, 2509–2516. doi: 10.1152/jn.00613.2007
Zou, J. Y., Martinez, D. B., Neafsey, E. J., and Collins, M. A. (1996). Binge ethanol-induced brain damage in rats: effect of inhibitors of nitric oxide synthase. Alcohol. Clin. Exp. Res. 20, 1406–1411. doi: 10.1111/j.1530-0277.1996.tb01141.x
Zuccoli, G., and Motti, L. (2008). Atypical Wernicke’s encephalopathy showing lesions in the cranial nerve nuclei and cerebellum. J. Neuroimag. 18, 194–197. doi: 10.1111/j.1552-6569.2007.00188.x
Keywords: Wernicke’s encephalopathy, Korsakoff’s syndrome, metabolism, energy, ethanol
Citation: Bordia T and Zahr NM (2020) The Inferior Colliculus in Alcoholism and Beyond. Front. Syst. Neurosci. 14:606345. doi: 10.3389/fnsys.2020.606345
Received: 14 September 2020; Accepted: 02 November 2020;
Published: 11 December 2020.
Edited by:
James W. Grau, Texas A&M University, United StatesReviewed by:
Erik Oudman, Slingedael Korsakoff Center, Rotterdam, NetherlandsCopyright © 2020 Bordia and Zahr. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Natalie M. Zahr, bnphaHJAc3RhbmZvcmQuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.