- 1Department of Gynecology and Obstetrics, Integrated Research Center for Fetal Medicine, Johns Hopkins University School of Medicine, Baltimore, MD, United States
- 2Department of Neurology, Johns Hopkins University School of Medicine, Baltimore, MD, United States
Synaptic signaling is integral for proper brain function. During fetal development, exposure to inflammation or mild hypoxic-ischemic insult may lead to synaptic changes and neurological damage that impairs future brain function. Preterm neonates are most susceptible to these deleterious outcomes. Evaluating clinically used and novel fetal neuroprotective measures is essential for expanding treatment options to mitigate the short and long-term consequences of fetal brain injury. Magnesium sulfate is a clinical fetal neuroprotective agent utilized in cases of imminent preterm birth. By blocking N-methyl-D-aspartate receptors, magnesium sulfate reduces glutamatergic signaling, which alters calcium influx, leading to a decrease in excitotoxicity. Emerging evidence suggests that melatonin and N-acetyl-L-cysteine (NAC) may also serve as novel putative fetal neuroprotective candidates. Melatonin has important anti-inflammatory and antioxidant properties and is a known mediator of synaptic plasticity and neuronal generation. While NAC acts as an antioxidant and a precursor to glutathione, it also modulates the glutamate system. Glutamate excitotoxicity and dysregulation can induce perinatal preterm brain injury through damage to maturing oligodendrocytes and neurons. The improved drug efficacy and delivery of the dendrimer-bound NAC conjugate provides an opportunity for enhanced pharmacological intervention. Here, we review recent literature on the synaptic pathways underlying these therapeutic strategies, discuss the current gaps in knowledge, and propose future directions for the field of fetal neuroprotective agents.
Introduction
Perinatal brain development is an intricate process that begins early in fetal development and can be subject to numerous insults and delays. Perinatal brain injury is a leading maternal-fetal health issue that is often associated with preterm birth, cerebral palsy (CP), neurodevelopmental delay, and a spectrum of negative health sequelae (Burd et al., 2012; Duncan and Matthews, 2018; Hagberg et al., 2018). The mechanisms driving perinatal brain injury are varied, but are frequently tied to inflammation, infection, or hypoxia-ischemia, and subsequent cerebral white and gray matter cell death and damage (Novak et al., 2018; Stolp et al., 2019; McClendon et al., 2019; Mülling et al., 2020). Hypoxic-ischemic contribution to brain injury is more clearly defined clinically in term vs. preterm infants (Laptook, 2016; Gilles et al., 2018). Additionally, sex-specific differences in perinatal brain injury and response to treatment are often observed, with males at higher risk for perinatal brain injury compared to females (Bronson and Bale, 2014; Alexander et al., 2016; Thagard et al., 2017; Bolton et al., 2017; Motta-Teixeira et al., 2018; Al-Haddad et al., 2019a). Although the exact effect of these insults on synaptogenesis are unknown, increasing evidence links such insults to aberrant synaptic development and perinatal neurological deficits.
There is a concerning link between maternal infection and an increased risk of neuropsychiatric disorders in exposed in utero children (Lee et al., 2015; Ursini et al., 2018; Al-Haddad et al., 2019a,b; Kepinska et al., 2020). A Swedish birth cohort found that exposure to maternal infection, like influenza, increased the risk for autism and depression during the child’s lifetime (Al-Haddad et al., 2019a). Exposure to mild and ubiquitous infections, including a maternal urinary tract infection, can negatively impact fetal neurological development (Al-Haddad et al., 2019b). The gestational age of the fetus upon exposure to maternal infection may further dictate the severity of brain injury. There is a stronger correlation between maternal infection during the first or second trimesters compared to the third trimester or at delivery and the subsequent development of schizophrenia and other psychotic disorders in offspring (Khandaker et al., 2013). Some level of intrauterine inflammation is observed in about 20% of all pregnancies and in about 85% of very premature births (Elovitz et al., 2011). Other factors, such as genetics, may also contribute independently or synergistically to synaptic dysfunction. To date, several genome-wide association studies have identified links between neuropsychiatric disorders and synaptic structural and functional genes (Lee et al., 2012; Zoghbi and Bear, 2012; Schizophrenia Working Group of the Psychiatric Genomics Consortium et al., 2014; Schmidt-Kastner et al., 2020).
The prevention of perinatal brain injury has far reaching applications, such as decreasing societal neurological disease burden and improving the quality of life for affected mothers and neonates. This review summarizes the emerging evidence for the synaptic origins of fetal neurological disorders and details the use of several clinical and experimental neuroprotective agents.
Fetal Synaptic Development and Excitotoxicity-Induced Synaptopathies
Within the dynamic context of fetal brain development, genetic, epigenetic, and environmental factors may contribute to the structure and function of fetal synapses as well as the development of synaptopathies (Fagiolini et al., 2009; Kundakovic and Ivana, 2017; Pozzi et al., 2018). In the human fetal brain, synaptogenesis normally begins around 5 weeks of gestation with development in an early cortical layer. Later in gestation, interaction with scaffolding cells such as astrocytes, microglia, radial glia, and oligodendrocytes leads to the initiation of neuronal migration. Functional synaptic transmission soon follows, and peak synaptogenesis is reached around 34 weeks of gestation with over 40,000 new synapses formed per second (Tau and Peterson, 2010). In addition, myelination appears to surge at 36 weeks of gestation (Hüppi et al., 1998). This critical period in fetal development ensures proper sensory, motor, and cognitive skills (Liberman et al., 2018). Neonates born or suffering injury before 34–36 weeks of gestation may thus be at higher risk for adverse neurodevelopmental outcomes compared to full-term neonates, due to deficits in synaptogenesis and myelination (Liberman et al., 2018; You et al., 2019). Postmortem tissue from preterm neonates confirms that the perinatal brain exhibits delayed synaptogenesis across several brain regions (Sarnat and Flores-Sarnat, 2016). Furthermore, there is a critical balance of apoptosis, or programmed cell death, in the developing brain that may be disrupted by preterm brain insult (Truttmann et al., 2020).
Glutamate acts as the main excitatory neurotransmitter in the central nervous system (CNS); however, glutamate is also implicated in fetal brain injury. Glutamate signals through both metabotropic and ionotropic post-synaptic receptors, initiating important cellular and molecular signaling cascades. Ionotropic glutamate receptors are membrane ion channel coupled and include N-methyl-D-aspartate (NMDA), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), and kainic acid (KA) receptors. Overexposure to glutamate and subsequent excess intracellular calcium influx, termed excitotoxicity, destroys neurons both in vivo and in vitro (Choi and Rothman, 1990; Lipton and Rosenberg, 1994; Mark et al., 2001; Park et al., 2010).
Following an initial mild hypoxic-ischemic injury or inflammatory trigger, the excitotoxic pathway culminates in neonatal brain injury (Silverstein et al., 1986, 1991; Hagberg et al., 1993; Burd et al., 2016). Impairment of oxidative metabolism results in ischemia, reducing oxygen delivery to cellular structures. These events, in turn, reduce neuronal depolarization. In a murine model of chorioamnionitis, this leads to a dependence on anaerobic metabolism and increases extracellular ATP release, likely as a direct response to inflammatory mediators (Lei et al., 2018).
Exposure to intrauterine inflammation may precipitate fetal neuroinflammation and the development of fetal-specific immune synaptopathies (Riazi et al., 2015; Pozzi et al., 2018; Figure 1). For instance, it is well established that intrauterine inflammation leads to activation of pro-inflammatory cytokines, which compromises the fetal blood brain barrier (BBB), leading to microglia activation and astrocyte signaling (Hagberg et al., 2015; Burd et al., 2016; Kelley et al., 2017). Microglial activation perpetuates pro-inflammatory cytokine expression and can lead to the build-up of reactive oxygen species (ROS), calcium, and glutamate-induced excitotoxicity (Vexler and Ferriero, 2001; Kaindl et al., 2012). Excessive intracellular calcium accumulation can also damage fetal oligodendrocyte precursor cells (Salter and Fern, 2005). Moreover, evidence indicates that maternal inflammation adversely impacts fetal neuronal morphology (Burd et al., 2010). The identification and mechanistic understanding of fetal-specific synaptopathies may provide the opportunity to develop fetal neuroprotective agents targeting and mitigating dysfunctional synaptic signaling.
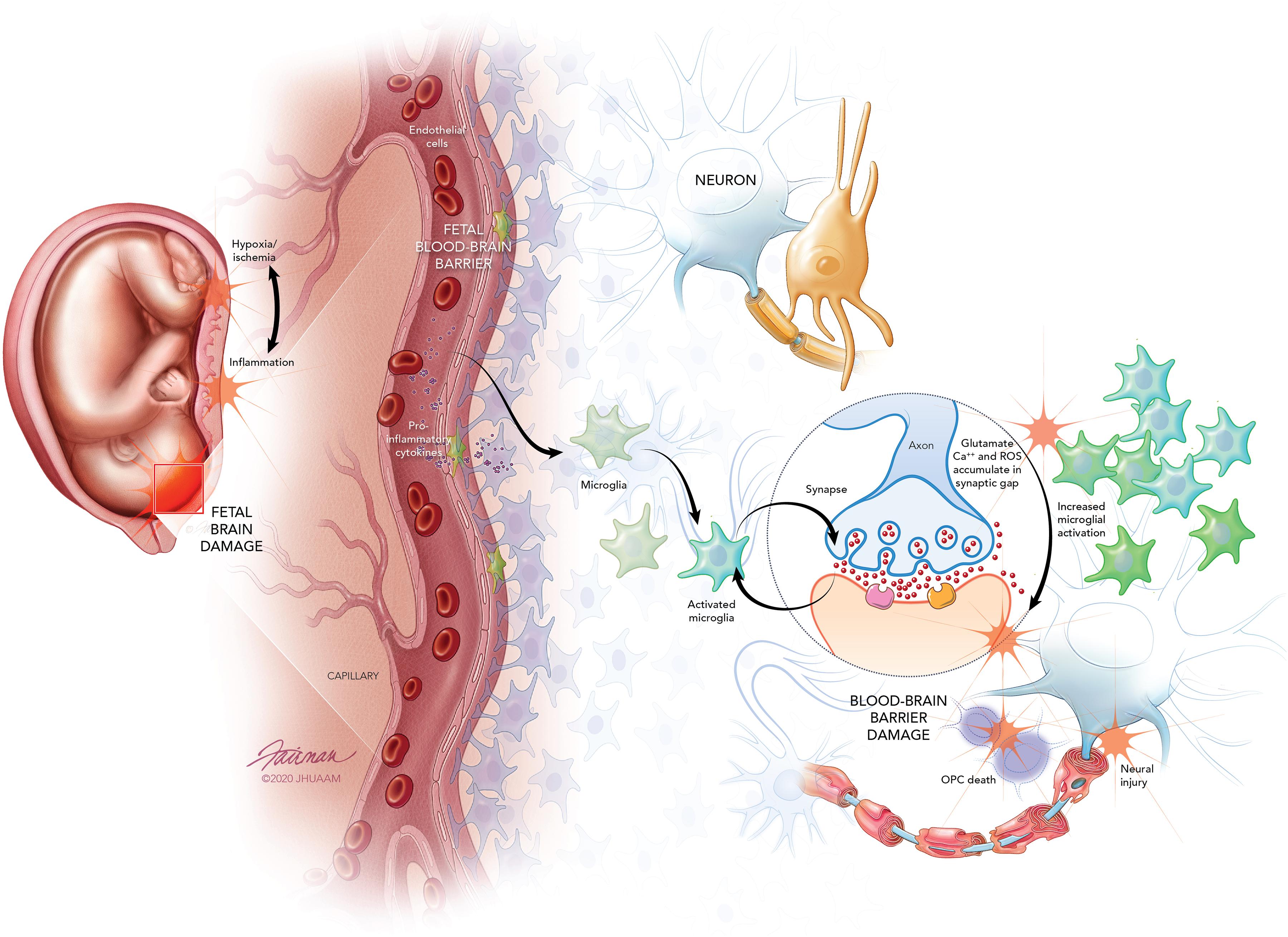
Figure 1. Fetal brain injury originates from inflammatory insult or mild hypoxic ischemic injury that triggers inflammation. Toll-like receptors on inflammatory cells of the placental membranes and decidua act as the inflammatory mediator leading to the induction and release of pro-inflammatory cytokines. The pro-inflammatory cytokines increase permeability of the developing fetal blood-brain-barrier (BBB), allowing recruitment of immune cells and increasing pro-inflammatory cytokines in the fetal brain. Fetal microglia are activated by the pro-inflammatory cytokines, and these activated microglia lead to the excessive release of glutamate. Overactivation of N-methyl-D-aspartate (NMDA), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), and kainate receptors expressed on neurons and oligodendrocyte precursor cells (OPCs) leads to the accumulation of glutamate in the synaptic gap. Calcium (Ca2+) and reactive oxygen species (ROS) also accumulate within the synaptic gap. Furthermore, NMDA receptors on microglia increase and exacerbate microglial activation. The overaccumulation of glutamate, Ca2+, and ROS leads to excitotoxic injury of neurons and OPCs culminating in fetal BBB damage, neural injury, and fetal brain damage.
Microglia, the resident immune cells of the CNS, are integral to maintaining CNS homeostasis, promoting neurogenesis, and mediating synaptic pruning (Paolicelli and Ferretti, 2017; Tay et al., 2018). In the early mouse postnatal brain, microglia make contact with synapses and engulf synaptic material (Paolicelli et al., 2011). Complement activation is key to this process of synaptic pruning, with complement molecules tagging synapses destined for removal by microglia (Tay et al., 2018; Druart and Le Magueresse, 2019). There is evidence that synaptic pruning begins prenatally, as microglia have been shown to accumulate in areas where synapses are nascent at the cortical plate-subplate junction (Verney et al., 2010). NMDA receptors are expressed on activated microglia and can perpetuate inflammation and cell death (Verney et al., 2010; Kaindl et al., 2012). Animal studies confirm that exposure to intrauterine inflammation leads to increased microglia activation and excitatory synaptic strength in the hippocampus (Kelley et al., 2017), with release of cytotoxic pro-inflammatory cytokines (Romero and Mazor, 1988; Yoon et al., 1997; Riazi et al., 2008; Welser-Alves and Milner, 2013). Aberrant microglia activation has been shown to continue into adulthood, particularly in the hippocampus (Kelley et al., 2017), which may result in long-term deficits in learning and memory if left untreated (Schaafsma et al., 2017; Bilbo and Stevens, 2017). This may be due to early priming of microglia in response to maternal inflammation, leading to loss of homeostatic function, increased sensitivity to future insults, and greater risk for neurodevelopmental and neuropsychiatric disorders (Tay et al., 2018). Inappropriate activation of the complement has also been implicated in neuropsychiatric disorders (Druart and Le Magueresse, 2019).
Magnesium Sulfate
Magnesium sulfate (MgSO4) is a fetal neuroprotective agent that is administered in cases of imminent preterm birth. Originally used as a seizure prophylactic for women suffering from pre-eclampsia and later as a tocolytic agent to prevent preterm birth, MgSO4 has proven to have numerous beneficial effects for mother and fetus (Pritchard and Pritchard, 1975; Crowther et al., 2014; Ozen et al., 2019). Researchers first noted the neuroprotective properties of MgSO4 in the 1990s when infants of women who received MgSO4 during pregnancy had a lower incidence rate of CP (Nelson and Grether, 1995; Schendel et al., 1996; Paneth et al., 1997). Since then, multiple meta-analyses of randomized controlled trials of MgSO4 reveal that it is effective in decreasing the incidence of CP without increasing the risk of death in infants (Conde-Agudelo and Romero, 2009; Costantine and Weiner, 2009; Doyle et al., 2009; Shepherd et al., 2017).
The exact mechanism by which MgSO4 mediates its neuroprotective effects is not fully understood, although growing evidence indicates it is at least partially due to modulation of glutamatergic signaling. MgSO4 permeates freely across the BBB in neonatal swine (Rivera et al., 1991) and non-competitively blocks NMDA receptors, reducing calcium ion intake and preventing excitotoxic cell injury and death (Nowak et al., 1984; Marret et al., 1995). MgSO4 reduces both NMDA-induced and hypoxia-ischemia-induced rat brain injury and accelerates the differentiation of oligodendrocytes (McDonald et al., 1990; Thordstein et al., 1993; Kang et al., 2011; Itoh et al., 2016). The timing of MgSO4 administration is critical; across multiple animal models, MgSO4 leads to deleterious brain effects when administered after hypoxic insult as opposed to before the insult (Penrice et al., 1997; Galvin and Oorschot, 1998; Sameshima et al., 1999; Greenwood et al., 2000). This may be because administration of MgSO4 prior to a perinatal brain insult leads to preconditioning via mitochondrial resistance and reduced inflammation (Koning et al., 2018, 2019). In human neonates, administration of MgSO4 after birth for hypoxic-ischemic encephalopathy shows inconsistent long-term benefits and a trend toward increased neonatal mortality, underscoring the need to better understand the ideal timing of MgSO4 administration (Tagin et al., 2013). Dosing is another important consideration, as higher doses of MgSO4 may not confer greater neuroprotection and instead negatively affect fetal hemodynamics in animals and humans (Lecuyer et al., 2017).
MgSO4 is not without risks; in human neonates, MgSO4 can cause mild hypothermia, respiratory depression, feeding difficulties and longer neonatal intensive care unit (NICU) admissions (Lyell et al., 2007; Greenberg et al., 2013). Data from both preclinical and clinical studies show inconsistencies in long-term benefits of MgSO4 (Tagin et al., 2013; Galinsky et al., 2014). Despite these limitations, the evidence paints an overwhelmingly positive picture of this therapeutic agent and it continues to be used for fetal neuroprotection.
Melatonin
Melatonin is an endogenous indolamine hormone synthesized and secreted by the pineal gland. It acts an antioxidant, neutralizing a number of ROS and reducing cellular damage (Tan et al., 1993, 2002; Reiter et al., 2009). Moreover, melatonin has anti-inflammatory properties, linked primarily to its reduction of nitric oxide levels and regulation of cytokine expression (Esposito and Cuzzocrea, 2010; Reiter et al., 2014).
In humans, the pineal gland does not fully develop until after birth, rendering the fetus dependent on its mother for melatonin. Maternal melatonin freely crosses the placenta to interact with ubiquitously expressed melatonin receptor MT1 in the fetus (Okatani et al., 1998; Thomas et al., 2002). Additionally, evidence indicates that the placenta itself contributes to melatonin synthesis (Lanoix et al., 2008). This provides a steady influence on the internal rhythm of the fetus along with protection from oxidative damage (Reiter et al., 2014; Motta-Teixeira et al., 2018). Preterm brain injury may interfere with this critical melatonin regulation, as data from a prospective multicenter study reveal that infants born before 34 weeks gestation have significantly lower levels of melatonin at birth and at 3 days of life compared to term infants (Biran et al., 2019).
Melatonin has low toxicity across a wide range of doses (Guardiola-Lemaitre, 1997; Jahnke et al., 1999) and has neuroprotective effects, regulating neuroexcitability and synaptic transmission in the hippocampus (Zeise and Semm, 1985; El-Sherif et al., 2002; Musshoff et al., 2002). Melatonin reduces impairments in long term potentiation and neuronal death in mouse models of Alzheimer’s disease and epilepsy (Liu et al., 2013; Ma et al., 2017), possibly by modulating AMPA subunit expression and preventing excitotoxic cell death (Soundarapandian et al., 2005; Ma et al., 2017).
Across multiple animal models of perinatal brain injury, melatonin has consistently been proven to be neuroprotective by increasing oligodendrocyte maturation and myelination repair, promoting axonal growth, and decreasing microglial activation after hypoxic, ischemic, inflammatory or drug-induced brain damage (Husson et al., 2002; Miller et al., 2005; Welin et al., 2007; Hutton et al., 2009; Olivier et al., 2009; Hamada et al., 2010; Kaur et al., 2010; Villapol et al., 2011). Melatonin is known to regulate neuronal generation and synaptic plasticity in the dentate gyrus, which is particularly susceptible to inflammatory and oxidative damage (Ramírez-Rodríguez et al., 2019). Furthermore, melatonin protects the dentate gyrus from neuroinflammation in neonatal rat models (Shah et al., 2017). Melatonin may offer this neuroprotection via activation of the SIRT1/Nrf2 signaling pathway, which is important for stimulating cell antioxidant responses (Shah et al., 2017; Lee et al., 2019a,b; Ren et al., 2019). Melatonin also reduces inflammatory damage and fetal death through reduction of hypoxic and oxidative stress (Chen et al., 2006; Wang et al., 2011; Lee et al., 2019a,b). Maternal melatonin deprivation during gestation and lactation in a rat model leads to neurobehavioral and neurodevelopmental deficits, primarily seen in male fetuses and rescued by administration of melatonin (Motta-Teixeira et al., 2018). An important consideration in future clinical use of melatonin is excipient influence, as observed neuroprotection in neonatal piglet asphyxia models differ based on excipient use (Robertson et al., 2020).
In human neonates, administration of melatonin for sepsis leads to decreased lipid peroxidation products and improved clinical outcome (Gitto et al., 2001). Multiple clinical trials of melatonin are currently underway to assess its ability to provide neuroprotection for growth restricted fetuses and in cases of neonatal asphyxia (Miller et al., 2014; Aly et al., 2015; Wilkinson et al., 2016). Preliminary results indicate that melatonin reduces placental oxidative stress in pregnancies complicated by fetal growth restriction (Miller et al., 2014) and improves neurodevelopmental outcomes in neonates with perinatal asphyxia (Aly et al., 2015). Melatonin shows a wealth of potential as a fetal neuroprotective agent, and the next decade will likely usher in more data supporting the use of this molecule in pregnancy.
N-Acetyl-L-Cysteine
N-acetyl-L-cysteine (NAC) is an acetylated amino acid approved by the United States Food and Drug Administration for the treatment of acetaminophen toxicity (Scalley and Conner, 1978; Smilkstein et al., 1988). Recently, NAC was found to have psychiatric and neurological applications and is experimentally used for the treatment of addiction, schizophrenia, bipolar disorder, and obsessive-compulsive disorder (Lafleur et al., 2006; Berk et al., 2008, 2012; Gray et al., 2010). Neuroinflammation is shown to disrupt synaptogenesis (Riazi et al., 2015; Pozzi et al., 2018), and NAC may exert therapeutic benefit through the decrease of synaptic glutamate release mitigating subsequent excitotoxic neurological damage (Dean et al., 2011).
NAC’s therapeutic properties stem from its action on the cystine-glutamate antiporter system and as an antioxidant to regulate the neuroinflammatory response (Dean et al., 2011; Durieux et al., 2015). The cystine-glutamate antiporter, system xc–, is a highly conserved heterodimeric amino acid transporter that exchanges extracellular cystine for intracellular glutamate across the plasma membrane. In animal models, regional distribution studies demonstrate the presence of the antiporter protein in the meninges, cortex, hippocampus, striatum, and cerebellum of embryonic and adult rat brains (Shih et al., 2006). Developmental age impacts the expression of the transporter, and immature neurons exhibit greater system xc– expression compared to mature neurons (Murphy et al., 1990; Shih et al., 2006). Within the CNS, system xc– is found in neurons and glial cells, such as astrocytes (Shih et al., 2006). It provides an essential non-vesicular pathway for extracellular glutamate release (Bridges et al., 2012; Lewerenz et al., 2013) and may inhibit the clustering of post-synaptic glutamate receptors (Augustin et al., 2007). Cysteine, the oxidized form of cystine, also acts as the rate-limiting substrate in the production of the tripeptide endogenous antioxidant glutathione (GSH) (Gunduz-Bruce et al., 2012). Astrocytes play a critical role in cystine uptake to supply the surrounding neurons with cysteine for GSH production (Wang and Cynader, 2002). System xc– mediates the link between the release of excitatory neurotransmitters, in the form of glutamate, and the modulation of oxidative stress through the generation of GSH.
NAC increases the availability of cysteine within the extracellular space. This increase leads to the production of GSH and regulates glutamate release through system xc–. Stimulation of the antiporter system increases glutamate within the extrasynaptic space and activates presynaptic inhibitory metabotropic glutamate receptors, mGluR2/3. Ultimately, this reduces the synaptic release of glutamate (Baker et al., 2002; Dean et al., 2011). Analysis of plasma GSH levels show oral NAC administration yields a greater GSH fraction compared to the administration of GSH or L-cysteine (Borges-Santos et al., 2012; Schmitt et al., 2015).
The clinical utility of NAC shows promise in clinical trials (Amin et al., 2008; Jenkins et al., 2016). In a double-blind Phase 1/2 trial of NAC as a neuroprotective agent in cases of maternal chorioamnionitis, IV NAC was maternally administered every 6 h until delivery. After delivery, IV NAC was administered to neonates every 12 h for five doses (Jenkins et al., 2016). During the study, no maternal or neonatal adverse events associated with NAC were observed. While neonatal cerebrovascular measures did not differ between the control and treatment groups, inflammatory cytokines quantified in maternal serum, cord serum, and neonatal cerebral spinal fluid samples improved following NAC administration (Jenkins et al., 2016). Both serum fibroblast growth factor 2 and IL-17 were reduced in the NAC treatment group compared to the control group. NAC also shows improved neuroprotective benefits compared to melatonin, another free radical scavenger (Wang et al., 2007). These findings demonstrate the translational ability of NAC for use as a fetal neuroprotective agent; however, larger clinical studies are needed to fully evaluate its safety and efficacy.
The impact of the physiological changes of pregnancy, including hemodynamic changes, on NAC pharmacokinetics remains largely unknown (Pavek et al., 2009). In healthy non-pregnant individuals, the half-life of oral NAC is 6.25 h, while the half-life of IV NAC is 5.58 h (Olsson et al., 1988; Prescott et al., 1989). However, a study of IV maternal NAC administration reports the half-life of NAC as 1.2 h at a dosage of 100 mg/kg within the pregnant population (Wiest et al., 2014). Additionally, differences in the bioavailability of oral NAC administration compared to maternal IV NAC administration should be further characterized.
The optimal timing of NAC administration to prevent fetal neurological damage remains an area of ongoing research. In murine models, NAC shows therapeutic benefit when administered prenatally or within 1 day of birth (Lanté et al., 2008). No neurological benefits of NAC are observed if treatment is delayed 2 days after delivery. Since NAC crosses the placental barrier and BBB in both humans and mice, it likely exerts its benefits through restoring the fetomaternal oxidative balance and decreasing the excitotoxic effects of excess glutamate (Horowitz et al., 1997; Lanté et al., 2008; Wiest et al., 2014). Additional studies are needed to provide guidance about the optimal timing of maternal NAC administration to achieve maximal therapeutic benefits for the fetus in humans.
Dendrimer-Bound N-Acetyl-L-Cysteine
While NAC has been found to be therapeutically usefully, NAC has presented with many adverse effects at the recommended dosage. Conjugating NAC with a vehicle, such as a dendrimer, may improve the bioavailability and tolerability of the drug (Kannan et al., 2012). The dendrimer-bound NAC conjugate (DNAC) shows promise in optimizing drug administration and mitigating deleterious medication side-effects. This demonstrates the improved possibilities of targeted fetal neuroprotective therapies. DNAC is a polyamidoamino (PAMAM) dendrimer conjugated with NAC (Lei et al., 2017). Disulfide linkers, cleavable by exposure to GSH, enable controlled intracellular release of NAC (Kurtoglu et al., 2009). In a randomized trial of the addition of NAC to 17-hydroxyprogesterone treatment in women with bacterial vaginosis and at high-risk of preterm birth, 11.4% of participants discontinued NAC due to nausea, vomiting, and/or gastric disruptions (Shahin et al., 2009). Therefore, there is a clear need to develop more tolerable alternatives to improve translational feasibility and treatment compliance.
A dendrimer-based complex therapy also presents the opportunity to improve drug delivery and to optimize drug functionality in vivo (Madaan et al., 2014). Dendrimers enhance solubility, stability, and the bioavailability of drugs through a three-dimensional branched polymer scaffolding surrounding the drug molecule (Chauhan, 2018). Varying surface groups and the charge of terminal moieties can modulate drug toxicity and tolerability (Gillies and Frechet, 2005; Janaszewska et al., 2019). Nanostructured molecules improve the permeability of drugs across physiological barriers, such as epithelial and mucosal barriers, and they enable targeted drug delivery to the specific sites of disease and injury (Kannan et al., 2014).
The surface groups of dendrimers are modified to improve targeted drug delivery to areas of interest (Wang et al., 2009; Zhang et al., 2016). The modification of dendrimers is especially relevant when considering their ability to cross the placental barrier and undergo selective fetal absorption (Menjoge et al., 2011; Burd et al., 2014). In an intra-amniotic delivery system of the neutrally charged hydroxyl-terminated (D-OH) dendrimer, the dendrimer was transported across the placental barrier and absorbed by the rabbit fetus. This showed superiority to the negatively charged carboxyl-functionalized dendrimer (D-COOH) when crossing the BBB of the fetus. The dendrimer was systemically administered to the mother following an inflammatory insult, and the D-OH dendrimer selectively localized in activated fetal microglia (Zhang et al., 2016). This localization continued into the postnatal period providing a continued level of protection.
Postnatal administration of DNAC shows promise in attenuating neonatal neurological injury following maternal inflammation (Kannan et al., 2012). This appears in contrast with the lack of efficacy of postnatal free NAC administration (Lanté et al., 2008). In a rabbit model of CP, DNAC administrated intravenously on postnatal day one improved neurological function of kits and decreased markers of neuroinflammation. CP kits in the DNAC treatment group also showed increased levels of myelin basic protein compared to CP kits that received phosphate buffered saline. Markers of oxidative stress to lipids, proteins, and RNA also decreased within the DNAC treatment group (Kannan et al., 2012).
Conclusion
There remains a paucity of data about fetal neuroprotective therapeutic agents and their underlying synaptic pathways. Given the growing prevalence of perinatal neurological injury, expanding the armamentarium of fetal neuroprotective agents is a pressing issue for researchers. Newer agents with potential to modulate neuronal plasticity and treat neonatal hypoxia-ischemia, such as erythropoietin, should be further explored (Oorschot et al., 2020). Future studies should also address sex-appropriate therapeutic agents to better characterize which neonates derive benefit from specific treatments.
Author Contributions
TB and NE contributed equally to the writing of this manuscript. IB reviewed and edited the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Johns Hopkins Integrated Research Center for Fetal Medicine Fund.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Alexander, N., Rosenlöcher, F., Dettenborn, L., Stalder, T., Linke, J., Distler, W., et al. (2016). Impact of antenatal glucocorticoid therapy and risk of preterm delivery on intelligence in term-born children. J. Clin. Endocrinol. Metab. 101, 581–589. doi: 10.1210/jc.2015-2453
Al-Haddad, B. J. S., Jacobsson, B., Chabra, S., Modzelewska, D., Olson, E. M., Bernier, R., et al. (2019a). Long-term risk of neuropsychiatric disease after exposure to infection in utero. JAMA Psychiatry 76, 594–602. doi: 10.1001/jamapsychiatry.2019.0029
Al-Haddad, B. J. S., Oler, E., Armistead, B., Elsayed, N. A., Weinberger, D. R., Bernier, R., et al. (2019b). The fetal origins of mental illness. Am. J. Obstet. Gynecol. 221, 549–562. doi: 10.1016/j.ajog.2019.06.013
Aly, H., Elmahdy, H., El-Dib, M., Rowisha, M., Awny, M., El-Gohary, T., et al. (2015). Melatonin use for neuroprotection in perinatal asphyxia: a randomized controlled pilot study. J. Perinatol. 35, 186–191. doi: 10.1038/jp.2014.186
Amin, A. F., Shaaban, O. M., and Bediawy, M. A. (2008). N-acetyl cysteine for treatment of recurrent unexplained pregnancy loss. Reprod. BioMed. Online 17, 722–726. doi: 10.1016/S1472-6483(10)60322-7
Augustin, H., Grosjean, Y., Chem, K., Sheng, Q., and Featherstone, D. E. (2007). Nonvesicular release of glutamate by glial xCT transporters suppresses glutamate receptor cluster in vivo. J. Neurosci. 27, 111–123. doi: 10.1523/JNEUROSCI.4770-06.2007
Baker, D. A., Xi, Z.-X., Shen, H., Swanson, C. J., and Kalivas, P. W. (2002). The origin and neuronal function of in vivo nonsynaptic glutamate. J. Neurosci. 22, 9134–9141. doi: 10.1523/JNEUROSCI.22-20-09134.2002
Berk, M., Copolov, D., Dean, O., Lu, K., Jeavons, S., Schapkaitz, I., et al. (2008). N-Acetyl cysteine as a glutathione precursor for schizophrenia – a double-blind, randomized, placebo-controlled trial. Biol. Psychiatry 64, 361–368. doi: 10.1016/j.biopsych.2008.03.004
Berk, M., Dean, O. M., Cotton, S. M., Gama, C. S., Kapczinski, F., Fernandes, B., et al. (2012). Maintenance N-acetyl cysteine treatment for bipolar disorder: a double-blind randomized placebo controlled trial. BMC Med. 10:91. doi: 10.1186/1741-7015-10-91
Bilbo, S., and Stevens, B. (2017). Microglia: the brain’s first responders. Cerebrum 2017, cer–14–17.
Biran, V., Decobert, F., Bednarek, N., Boizeau, P., Benoist, J. F., Claustrat, B., et al. (2019). Melatonin levels in preterm and term infants and their mothers. Int. J. Mol. Sci. 20:2077. doi: 10.3390/ijms20092077
Bolton, J. L., Marinero, S., Hassanzadeh, T., Natesan, D., Le, D., Belliveau, C., et al. (2017). Gestational exposure to air pollution alters cortical volume, microglial morphology, and microglia-neuron interactions in a sex-specific manner. Front. Synaptic. Neurosci. 9:10. doi: 10.3389/fnsyn.2017.00010
Borges-Santos, M. D., Moreto, F., Pereira, P. C. M., Ming-Yu, Y., and Burini, R. C. (2012). Plasma glutathione of HIV+ patients responded positively and differently to dietary supplementation with cysteine or glutamine. Nutrition 28, 753–756. doi: 10.1016/j.nut.2011.10.014
Bridges, R. J., Natale, N. R., and Patel, S. A. (2012). System xc- cystine/glutamate antiporter: an update on molecular pharmacology and roles within the CNS. Br. J. Pharmacol. 165, 20–34. doi: 10.1111/j.1476-5381.2011.01480.x
Bronson, S. L., and Bale, T. L. (2014). Prenatal stress-induced increases in placental inflammation and offspring hyperactivity are male-specific and ameliorated by maternal antiinflammatory treatment. Endocrinology 155, 2635–2646. doi: 10.1210/en.2014-1040
Burd, I., Balakrishnan, B., and Kannan, S. (2012). Models of fetal brain injury, intrauterine inflammation, and preterm birth. Am. J. Reprod. Immunol. 67, 287–294. doi: 10.1111/j.1600-0897.2012.01110.x
Burd, I., Bentz, A. I., Chai, J., Gonzalez, J., Monnerie, H., Roux, P. D. L., et al. (2010). Inflammation-induced preterm birth alters neuronal morphology in the mouse fetal brain. J. Neurosci. Res. 88, 1872–1881. doi: 10.1002/jnr.22368
Burd, I., Welling, J., Kannan, G., and Johnston, M. V. (2016). Excitotoxicity as a common mechanism for fetal neuronal injury with hypoxia and intrauterine inflammation. Adv. Pharmacol. 76, 85–101. doi: 10.1016/bs.apha.2016.02.003
Burd, I., Zhang, F., Dada, T., Mishra, M. K., Borbiev, T., Lesniak, W. G., et al. (2014). Fetal uptake of intra-amniotically delivered dendrimers in a mouse model of intrauterine inflammation and preterm birth. Nanomedicine 10, 1343–1351. doi: 10.1016/j.nano.2014.03.008
Chauhan, A. S. (2018). Dendrimers for drug delivery. Molecules 23:938. doi: 10.3390/molecules23040938
Chen, Y. H., Xu, D. X., Wang, J. P., Wang, H., Wei, L. Z., Sun, M. F., et al. (2006). Melatonin protects against lipopolysaccharide-induced intra-uterine fetal death and growth retardation in mice. J. Pineal Res. 40, 40–47. doi: 10.1111/j.1600-079X.2005.00274.x
Choi, D. W., and Rothman, S. M. (1990). The role of glutamate neurotoxicity in hypoxic-ischemic neuronal death. Annu. Rev. Neurosci. 13, 171–182. doi: 10.1146/annurev.ne.13.030190.001131
Conde-Agudelo, A., and Romero, R. (2009). Antenatal magnesium sulfate for the prevention of cerebral palsy in preterm infants less than 34 weeks’ gestation: a systematic review and metaanalysis. Am. J. Obstet. Gynecol. 200, 595–609. doi: 10.1016/j.ajog.2009.04.005
Costantine, M. M., and Weiner, S. J. (2009). Effects of antenatal exposure to magnesium sulfate on neuroprotection and mortality in preterm infants: a meta-analysis. Obstet. Gynecol. 114(2 Pt 1);354. doi: 10.1097/AOG.0b013e3181ae98c2
Crowther, C. A., Brown, J., McKinlay, C. J. D., and Middleton, P. (2014). Magnesium sulphate for preventing preterm birth in threatened preterm labour. Cochrane Database Syst. Rev. 8:CD001060. doi: 10.1002/14651858.CD001060.pub2
Dean, O., Giorlando, F., and Berk, M. (2011). N-acetylcysteine in psychiatry: current therapeutic evidence and potential mechanisms of action. J. Psychiatry Neurosci. 36, 78–86. doi: 10.1503/jpn.100057
Doyle, L. W., Crowther, C. A., Middleton, P., Marret, S., and Rouse, D. (2009). Magnesium sulphate for women at risk of preterm birth for neuroprotection of the fetus. Cochrane Database Syst. Rev. 1:CD004661. doi: 10.1002/14651858.CD004661.pub3
Druart, M., and Le Magueresse, C. (2019). Emerging roles of complement in psychiatric disorders. Front. Psychiatry 10:573. doi: 10.3389/fpsyt.2019.00573
Duncan, A. F., and Matthews, M. A. (2018). Neurodevelopmental outcomes in early childhood. Clin. Perinatol. 45, 377–392. doi: 10.1016/j.clp.2018.05.001
Durieux, A. M. S., Fernandes, C., Murphy, D., Labouesse, M. A., Giovanoli, S., Meyer, U., et al. (2015). Targeting glia with N-acetylcysteine modulates brain glutamate and behaviors relevant to neurodevelopmental disorders in C57BL/6J mice. Front. Behav. Neurosci. 9:343. doi: 10.3389/fnbeh.2015.00343
Elovitz, M. A., Brown, A. G., Breen, K., Anton, L., Maubert, M., and Burd, I. (2011). Intrauterine inflammation, insufficient to induce parturition, still evokes fetal and neonatal brain injury. Int. J. Dev. Neurosci. 29, 663–671. doi: 10.1016/j.ijdevneu.2011.02.011
El-Sherif, Y., Hogan, M. V., Tesoriero, J., and Wieraszko, A. (2002). Factors regulating the influence of melatonin on hippocampal evoked potentials: comparative studies on different strains of mice. Brain Res. 945, 191–201. doi: 10.1016/s0006-8993(02)02752-x
Esposito, E., and Cuzzocrea, S. (2010). Antiinflammatory activity of melatonin in central nervous system. Curr. Neuropharmacol. 8, 228–242. doi: 10.2174/157015910792246155
Fagiolini, M., Jensen, C. L., and Champagne, F. A. (2009). Epigenetic influences on brian development and plasticity. Curr. Opin. Neurobiol. 19, 207–212. doi: 10.1016/j.conb.2009.05.009
Galinsky, R., Bennet, L., Groenendaal, F., Lear, C. A., Tan, S., Van Bel, F., et al. (2014). Magnesium is not consistently neuroprotective for perinatal hypoxia-ischemia in term-equivalent models in preclinical studies: a systematic review. Dev. Neurosci. 36, 73–82. doi: 10.1159/000362206
Galvin, K. A., and Oorschot, D. E. (1998). Postinjury magnesium sulfate treatment is not markedly neuroprotective for striatal medium spiny neurons after perinatal hypoxia/ischemia in the rat. Pediatr. Res. 44, 740–745. doi: 10.1203/00006450-199811000-00017
Gilles, F., Gressens, P., Dammann, O., and Leviton, A. (2018). Hypoxia-ischemia is not an antecedent of most preterm brain damage: the illusion of validity. Dev. Med. Child. Neurol. 60, 120–125. doi: 10.1111/dmcn.13483
Gillies, E. R., and Frechet, J. M. (2005). Dendrimers and dendritic polymers in drug delivery. Drug Discov. Today 10, 35–43. doi: 10.1016/S1359-6446(04)03276-3
Gitto, E., Karbownik, M., Reiter, R. J., Tan, D. X., Cuzzocrea, S., Chiurazzi, P., et al. (2001). Effects of melatonin treatment in septic newborns. Pediatr. Res. 50, 756–760. doi: 10.1203/00006450-200112000-00021
Gray, K. M., Watson, N. L., Carpenter, M. J., and LaRowe, S. D. (2010). N-Acetylcysteine (NAC) in young marijuana users: an open-label pilot study. Am. J. Addict. 19, 187–189. doi: 10.1111/j.1521-0391.2009.00027.x
Greenberg, M. B., Penn, A. A., Whitaker, K. R., Kogut, E. A., El-Sayed, Y. Y., Caughey, A. B., et al. (2013). Effect of magnesium sulfate exposure on term neonates. J. Perinatol. 33, 188–193. doi: 10.1038/jp.2012.95
Greenwood, K., Cox, P., Mehmet, H., Penrice, J., Amess, P. N., Cady, E. B., et al. (2000). Magnesium sulfate treatment after transient hypoxia-ischemia in the newborn piglet does not protect against cerebral damage. Pediatr. Res. 48, 346–350. doi: 10.1203/00006450-199703000-00024
Guardiola-Lemaitre, B. (1997). Toxicology of melatonin. J. Biol. Rhythms 12, 697–706. doi: 10.1177/074873049701200627
Gunduz-Bruce, H., Reinhart, R. M. G., Roach, B. J., Gueorguieva, R., Oliver, S., D’Souza, D. C., et al. (2012). Glutamatergic modulation of auditory information processing in the human brain. Biol. Psychiatry 71, 969–977. doi: 10.1016/j.biopsych.2011.09.031
Hagberg, H., Mallard, C., Ferriero, D. M., Vannucci, S. J., Levison, S. W., Vexler, Z. S., et al. (2015). The role of inflammation in perinatal brain injury. Nat. Rev. Neurol. 11, 192–208. doi: 10.1038/nrneurol.2015.13
Hagberg, H., Thornberg, E., Blennow, M., Kjellmer, I., Lagercrantz, H., Thiringer, K., et al. (1993). Excitatory amino acids in the cerebral spinal fluid of asphyxiated infants: relationship to hypoxic-ischemic encephalopathy. Acta Paediatr. 82, 925–929. doi: 10.1111/j.1651-2227.1993.tb12601.x
Hagberg, H. A., Edwards, D., and Groenendaal, F. (2018). Perinatal brain damage: the term infant. Neurobiol. Dis. 92(Pt A), 102–112. doi: 10.1016/j.nbd.2015.09.011
Hamada, F., Watanabe, K., Wakatsuki, A., Nagai, R., Shinohara, K., Hayashi, Y., et al. (2010). Therapeutic effects of maternal melatonin administration on ischemia/reperfusion-induced oxidative cerebral damage in neonatal rats. Neonatology 98, 33–40. doi: 10.1159/000264205
Horowitz, R. S., Dart, R. C., Jarvie, D. R., Bearer, C. F., and Gupta, U. (1997). Placental transfer of N-acetylcysteine following human maternal acetaminophen toxicity. J. Toxicol. Clin. Toxicol. 35, 447–451. doi: 10.3109/15563659709001226
Hüppi, P. S., Warfield, S., Kikinis, R., Barnes, P. D., Zientara, G. P., Jolesz, F. A., et al. (1998). Quantitative magnetic resonance imaging of brain development in premature and mature newborns. Ann. Neurol. 43, 224–235. doi: 10.1002/ana.410430213
Husson, I., Mesples, B., Bac, P., Vamecq, J., Evrard, P., and Gressens, P. (2002). Melatoninergic neuroprotection of the murine periventricular white matter against neonatal excitotoxic challenge. Ann. Neurol. 51, 82–92. doi: 10.1002/ana.10072
Hutton, L. C., Abbass, M., Dickinson, H., Ireland, Z., and Walker, D. W. (2009). Neuroprotective properties of melatonin in a model of birth asphyxia in the spiny mouse (Acomys cahirinus). Dev. Neurosci. 31, 437–451. doi: 10.1159/000232562
Itoh, K., Maki, T., Shindo, A., Egawa, N., Liang, A. C., Itoh, N., et al. (2016). Magnesium sulfate protects oligodendrocyte lineage cells in a rat cell-culture model of hypoxic–ischemic injury. Neurosci. Res. 106, 66–69. doi: 10.1016/j.neures.2015.12.004
Jahnke, G., Marr, M., Myers, C., Wilson, R., Travlos, G., and Price, C. (1999). Maternal and developmental toxicity evaluation of melatonin administered orally to pregnant Sprague-Dawley rats. Toxicol. Sci. 50, 271–279. doi: 10.1093/toxsci/50.2.271
Janaszewska, A., Lazniewska, J., Trzepinski, P., Marcinkowska, M., and Klajnert-Maculewics, B. (2019). Cytotoxicity of dendrimers. Biomolecules 9:330. doi: 10.3390/biom9080330
Jenkins, D. D., Wiest, D. B., Mulvihill, D. M., Hlavacek, A. M., Majstoravich, S. J., Brown, T. R., et al. (2016). Fetal and neonatal effects of n-acetylcysteine when used for neuroprotection in maternal chorioamnionitis. J. Pediatr. 168, 67–76.e6. doi: 10.1016/j.jpeds.2015.09.076
Kaindl, A. M., Degos, V., Peineau, S., Gouadon, E., Chhor, V., Loron, G., et al. (2012). Activation of microglial N-methyl-D-aspartate receptors triggers inflammation and neuronal cell death in the developing and mature brain. Ann. Neurol. 72, 536–549. doi: 10.1002/ana.23626
Kang, S. W., Choi, S. K., Park, E., Chae, S. J., Choi, S., and Joo, H. J. (2011). Neuroprotective effects of magnesium-sulfate on ischemic injury mediated by modulating the release of glutamate and reduced of hyperreperfusion. Brain Res. 1371, 121–128. doi: 10.1016/j.brainres.2010.11.057
Kannan, R. M., Nance, E., Kannan, S., and Tomalia, D. A. (2014). Emerging concepts in dendrimer-based nanomedicine: from design principles to clinical applications. J. Intern. Med. 276, 579–617. doi: 10.1111/joim.12280
Kannan, S., Dai, H., Navath, R. S., Balakrishnan, B., Jyoti, A., Janisse, J., et al. (2012). Dendrimer-based postnatal therapy for neuroinflammation and cerebral palsy in a rabbit model. Sci. Transl. Med. 4:130ra46. doi: 10.1126/scitranslmed.3003162
Kaur, C., Sivakumar, V., and Ling, E. A. (2010). Melatonin protects periventricular white matter from damage due to hypoxia. J. Pineal Res. 48, 185–193. doi: 10.1111/j.1600-079X.2009.00740.x
Kelley, M. H., Wu, W. W., Lei, J., McLane, M., Xie, H., Hart, K. D., et al. (2017). Functional changes in hippocampal synaptic signaling in offspring survivors of a mouse model of intrauterine inflammation. J. Neuroinflammation 14:180. doi: 10.1186/s12974-017-0951-1
Kepinska, A. P., Iyegbe, C. O., Vernon, A. C., Yolken, R., Murray, R. M., and Pollak, T. A. (2020). Schizophrenia and influenza at the centenary of the 1918-1919 spanish influenza pandemic: mechanisms of psychosis risk. Front. Psychiatry 11:72. doi: 10.3389/fpsyt.2020.00072
Khandaker, G. M., Zimbron, J., Lewis, G., and Jones, P. B. (2013). Prenatal maternal infection, neurodevelopment and adult schizophrenia: a systemic review of population-based studies. Psychol. Med. 43, 239–257. doi: 10.1017/S0033291712000736
Koning, G., Leverin, A. L., Nair, S., Schwendimann, L., Ek, J., Carlsson, Y., et al. (2019). Magnesium induces preconditioning of the neonatal brain via profound mitochondrial protection. J. Cereb. Blood Flow Metab. 39, 1038–1055. doi: 10.1177/0271678X17746132
Koning, G., Lyngfelt, E., Svedin, P., Leverin, A. L., Jinnai, M., Gressens, P., et al. (2018). Magnesium sulphate induces preconditioning in preterm rodent models of cerebral hypoxia-ischemia. Int. J. Dev. Neurosci. 70, 56–66. doi: 10.1016/j.ijdevneu.2018.01.002
Kundakovic, M., and Ivana, J. (2017). The epigenetic link between prenatal adverse environments and neurodevelopmental disorders. Genes 8:104. doi: 10.3390/genes8030104
Kurtoglu, Y. E., Navath, R. S., Wang, B., Kannan, S., Romero, R., and Kannan, R. M. (2009). Poly(amidoamine) dendrimer-drug conjugates with disulfide linkages for intracellular drug delivery. Biomaterials 30, 2112–2121. doi: 10.1016/j.biomaterials.2008.12.054
Lafleur, D. L., Pittenger, C., Kelmendi, B., Gardner, T., Wasylink, S., and Mailson, R. T. (2006). N-acetylcysteine augmentation in serotonin reuptake inhibitor refectory obsessive-compulsive disorder. Psychopharmacology 184, 254–256. doi: 10.1007/s00213-005-0246-6
Lanoix, D., Beghdadi, H., Lafond, J., and Vaillancourt, C. (2008). Human placental trophoblasts synthesize melatonin and express its receptors. J. Pineal Res. 45, 50–60. doi: 10.1111/j.1600-079X.2008.00555.x
Lanté, F., Meunier, J., Guiramand, J., De Jesus Ferreira, M.-C., Cambonie, G., Aimar, R., et al. (2008). Late N-acetylcysteine treatment prevents the deficits induced in the offspring of dams exposed to an immune stress during gestation. Hippocampus 18, 602–609. doi: 10.1002/hipo.20421
Laptook, A. R. (2016). Birth asphyxia and hypoxic-ischemic brain injury in the preterm infant. Clin. Perinatol. 43, 529–545. doi: 10.1016/j.clp.2016.04.010
Lecuyer, M., Rubio, M., Chollat, C., Lecointre, M., Jégou, S., Leroux, P., et al. (2017). Experimental and clinical evidence of differential effects of magnesium sulfate on neuroprotection and angiogenesis in the fetal brain. Pharmacol. Res. Perspect. 5:e00315. doi: 10.1002/prp2.315
Lee, B. K., Magnusson, C., Gardner, R. M., Blomstrom, A., Newschaffer, C. J., Burstyn, I., et al. (2015). Maternal hospitalization with infection during pregnancy and risk of autism spectrum disorders. Brain Behav. Immun. 44, 100–105. doi: 10.1016/j.bbi.2014.09.001
Lee, J. Y., Li, S., Shin, N. E., Na, Q., Dong, J., Jia, B., et al. (2019b). Melatonin for prevention of placental malperfusion and fetal compromise associated with intrauterine inflammation-induced oxidative stress in a mouse model. J. Pineal Res. 67:e12591. doi: 10.1111/jpi.12591
Lee, J. Y., Song, H., Dash, O., Park, M., Shin, N. E., McLane, M. W., et al. (2019a). Administration of melatonin for prevention of preterm birth and fetal brain injury associated with premature birth in a mouse model. Am. J. Reprod. Immunol. 82:e13151. doi: 10.1111/aji.13151
Lee, P., Perlis, R., Jung, J. Y., Byrne, E. M., Rueckert, E., Siburian, R., et al. (2012). Multi-locus genome-wide association analysis supports the role of glutamatergic synaptic transmission in the etiology of major depressive disorder. Transl. Psychiatry 2:e184. doi: 10.1038/tp.2012.95
Lei, J., Rosenzweig, J. M., Mishra, M. K., Alshehri, W., Brancusi, F., McLane, M., et al. (2017). Maternal dendrimer-based therapy for inflammation-induced preterm birth and perinatal brain injury. Sci. Rep. 7:6106. doi: 10.1038/s41598-017-06113-2
Lei, J., Zhong, W., Almalki, A., Zhao, H., Arif, H., and Rozzah, R. (2018). Maternal glucose supplementation in a murine model of chorioamnionitis alleviates dysregulation of autophagy in fetal brain. Reprod. Sci. 25, 1175–1185. doi: 10.1177/1933719117734321
Lewerenz, J., Hewett, S. J., Huang, Y., Lambros, M., Gout, P. W., Kalivas, P. W., et al. (2013). The cystine/glutamate antiporter system xc- in health and disease: from molecular mechanisms to novel therapeutic opportunities. Antioxid. Redox. Signal. 18, 522–555. doi: 10.1089/ars.2011.4391
Liberman, A. C., Trias, E., da Silva Chagas, L., Trindade, P., dos Santos, Pereira, M., et al. (2018). Neuroimmune and inflammatory signals in complex disorders of the central nervous system. NIM 25, 246–270. doi: 10.1159/000494761
Lipton, S. A., and Rosenberg, P. A. (1994). Excitatory amino acids as a final common pathway for neurologic disorders. NEJM 330, 613–622. doi: 10.1056/NEJM199403033300907
Liu, X. J., Yuan, L., Yang, D., Han, W. N., Li, Q. S., Yang, W., et al. (2013). Melatonin protects against amyloid-β-induced impairments of hippocampal LTP and spatial learning in rats. Synapse 67, 626–636. doi: 10.1002/syn.21677
Lyell, D. J., Pullen, K., Campbell, L., Ching, S., Druzin, M. L., Chitkara, U., et al. (2007). Magnesium sulfate compared with nifedipine for acute tocolysis of preterm labor: a randomized controlled trial. Obstet. Gynecol. 110, 61–67. doi: 10.1097/01.AOG.0000269048.06634.35
Ma, Y., Sun, X., Li, J., Jia, R., Yuan, F., Wei, D., et al. (2017). Melatonin alleviates the epilepsy-associated impairments in hippocampal LTP and spatial learning through rescue of surface GluR2 expression at hippocampal CA1 synapses. Neurochem. Res. 42, 1438–1448. doi: 10.1007/s11064-017-2200-5
Madaan, K., Kumar, S., Poonia, N., Lather, V., and Pandita, D. (2014). Dendrimers in drug delivery and targeting: drug-dendrimer interactions and toxicity issues. J. Pharm. Bioallied. Sci. 6, 139–150. doi: 10.4103/0975-7406.130965
Mark, L. P., Prost, R. W., Ulmer, J. L., Smith, M. M., Daniels, D. L., Strottmann, J. M., et al. (2001). Pictorial review of glutamate excitotoxicity: fundamental concepts for neuroimaging. Am. J. Neuroradiol. 22, 1813–1824.
Marret, S., Gressens, P., Gadisseux, J. F., and Evrard, P. (1995). Prevention by magnesium of excitotoxic neuronal death in the developing brain: an animal model for clinical intervention studies. Dev. Med. Child Neurol. 37, 473–484. doi: 10.1111/j.1469-8749.1995.tb12035.x
McClendon, E., Wang, K., Degener-O’Brien, K., Hagen, M. W., Gong, X., Nguyen, T., et al. (2019). Transient hypoxemia disrupts anatomical and functional maturation of preterm fetal ovine CA1 pyramidal neurons. J. Neurosci. 39, 7853–7871. doi: 10.1523/JNEUROSCI.1364-19.2019
McDonald, J. W., Silverstein, F. S., and Johnston, M. V. (1990). Magnesium reduces N-methyl-D-aspartate (n.d.)-mediated brain injury in perinatal rats. Neurosci. Lett. 109, 234–238. doi: 10.1016/0304-3940(90)90569-u
Menjoge, A. R., Rinderknecht, A. L., Navath, R. S., Faridnia, M., Kim, C. J., Romero, R., et al. (2011). Transfer of PAMAM dendrimers across human placenta: prospects of its use as drug carrier during pregnancy. J. Control. Release 150, 326–338. doi: 10.1016/j.jconrel.2010.11.023
Miller, S. L., Yan, E. B., Castillo-Meléndez, M., Jenkin, G., and Walker, D. W. (2005). Melatonin provides neuroprotection in the late-gestation fetal sheep brain in response to umbilical cord occlusion. Dev. Neurosci. 27, 200–210. doi: 10.1159/000085993
Miller, S. L., Yawno, T., Alers, N. O., Castillo-Melendez, M., Supramaniam, V. G., VanZyl, N., et al. (2014). Antenatal antioxidant treatment with melatonin to decrease newborn neurodevelopmental deficits and brain injury caused by fetal growth restriction. J. Pineal Res. 56, 283–294. doi: 10.1111/jpi.12121
Motta-Teixeira, L. C., Machado-Nils, A. V., Battagello, D. S., Diniz, G. B., Andrade-Silva, J., and Silva, S. Jr., et al. (2018). The absence of maternal pineal melatonin rhythm during pregnancy and lactation impairs offspring physical growth, neurodevelopment, and behavior. Horm. Behav. 105, 146–156. doi: 10.1016/j.yhbeh.2018.08.006
Mülling, K., Fischer, A. J., Siakaeva, E., Richter, M., Bordbari, S., Spyra, I., et al. (2020). Neutrophil dynamics, plasticity and function in acute neurodegeneration following neonatal hypoxia-ischemia. Brain Behav. Immun. 92, 232–242. doi: 10.1016/j.bbi.2020.12.012
Murphy, T. H., Schnaar, R. L., and Coyle, J. T. (1990). Immature cortical neurons are uniquely sensitive to glutamate toxicity by inhibition of cystine uptake. FASEB 4, 1624–1633. doi: 10.1096/fasebj.4.6.2180770
Musshoff, U., Riewenherm, D., Berger, E., Fauteck, J. D., and Speckmann, E. J. (2002). Melatonin receptors in rat hippocampus: molecular and functional investigations. Hippocampus 12, 165–173. doi: 10.1002/hipo.1105
Nelson, K. B., and Grether, J. K. (1995). Can magnesium sulfate reduce the risk of cerebral palsy in very low birthweight infants? Obstet. Gynecol. Survey 50, 573–575.
Novak, C. M., Ozen, M., and Burd, I. (2018). Perinatal brain injury: mechanisms, prevention, and outcomes. Clin. Perinatol. 45, 357–375. doi: 10.1016/j.clp.2018.01.015
Nowak, L., Bregestovski, P., Ascher, P., Herbet, A., and Prochiantz, A. (1984). Magnesium gates glutamate-activated channels in mouse central neurones. Nature 307, 462–465. doi: 10.1038/307462a0
Okatani, Y., Okamoto, K., Hayashi, K., Wakatsuki, A., Tamura, S., and Sagara, Y. (1998). Maternal-fetal transfer of melatonin in pregnant women near term. J. Pineal Res. 25, 129–134. doi: 10.1111/j.1600-079x.1998.tb00550.x
Olivier, P., Fontaine, R. H., Loron, G., Van Steenwinckel, J., Biran, V., Massonneau, V., et al. (2009). Melatonin promotes oligodendroglial maturation of injured white matter in neonatal rats. PLoS One 4:e7128. doi: 10.1371/journal.pone.0007128
Olsson, B., Johansson, M., Gabrielsson, J., and Bolme, P. (1988). Pharmacokinetics and bioavailability of reduced and oxidized N-acetylcysteine. Eur. J. Clin. Pharmacol. 34, 77–82. doi: 10.1007/bf01061422
Oorschot, D. E., Sizemore, R. J., and Amer, A. R. (2020). Treatment of neonatal hypoxic-ischemic encephalopathy with erythropoietin alone, and erythropoietin combined with hypothermia: history, current status, and future research. Int. J. Mol. Sci. 21:1487. doi: 10.3390/ijms21041487
Ozen, M., Xie, H., Shin, N., Al Yousif, G., Clemens, J., McLane, M. W., et al. (2019). Magnesium sulfate inhibits inflammation through P2X7 receptors in human umbilical vein endothelial cells. Pediatr. Res. 87, 463–471. doi: 10.1038/s41390-019-0557-7
Paneth, N., Jetton, J., Pinto-Martin, J., and Susser, M. (1997). Magnesium sulfate in labor and risk of neonatal brain lesions and cerebral palsy in low birth weight infants. Pediatrics 99:e1. doi: 10.1542/peds.99.5.e1
Paolicelli, R. C., Bolasco, G., Pagani, F., Maggi, L., Scianni, M., Panzanelli, P., et al. (2011). Synaptic pruning by microglia is necessary for normal brain development. Science 333, 1456–1458. doi: 10.1126/science.1202529
Paolicelli, R. C., and Ferretti, M. T. (2017). Function and dysfunction of microglia during brain development: consequences for synapses and neural circuits. Front. Synaptic. Neurosci. 9:9. doi: 10.3389/fnsyn.2017.00009
Park, E., Lee, G. J., Choi, S., Choi, S. K., Chae, S. J., Kang, S. W., et al. (2010). Correlation between extracellular glutamate release and neuronal cell death in an eleven vessel occlusion model in rat. Brain Res. 1342, 160–166. doi: 10.1016/j.brainres.2010.04.054
Pavek, P., Ceckova, M., and Staud, F. (2009). Variation of drug kinetics in pregnancy. Curr. Drug Metab. 10, 520–529. doi: 10.2174/138920009788897993
Penrice, J., Amess, P. N., Punwani, S., Wylezinska, M., Tyszczuk, L., D’Souza, P., et al. (1997). Magnesium sulfate after transient hypoxia-ischemia fails to prevent delayed cerebral energy failure in the newborn piglet. Pediatr. Res. 41, 443–447.
Pozzi, D., Menna, E., Canzi, A., Desiato, G., Mantovani, C., and Matteoli, M. (2018). The communication between the immune and nervous systems: the role of IL-1β in synaptopathies. Front. Mol. Neurosci. 11:111. doi: 10.3389/fnmol.2018.00111
Prescott, L. F., Donovan, J. W., Jarvie, D. R., and Proudfoot, A. T. (1989). The disposition and kinetics of intravenous N-acetylcysteine in patients with paracetamol overdosage. Eur. J. Clinc. Pharmacol. 37, 501–506. doi: 10.1007/bf00558131
Pritchard, J. A., and Pritchard, S. A. (1975). Standardized treatment of 154 consecutive cases of eclampsia. Am. J. Obstet. Gynecol. 123, 543–552. doi: 10.1016/0002-9378(75)90042-3
Ramírez-Rodríguez, G. B., Olvera-Hernández, S., Vega-Rivera, N. M., and Ortiz-López, L. (2019). Melatonin influences structural plasticity in the axons of granule cells in the dentate gyrus of Balb/C mice. Int. J. Mol. Sci. 20:73. doi: 10.3390/ijms20010073
Reiter, R. J., Paredes, S. D., Manchester, L. C., and Tan, D. X. (2009). Reducing oxidative/nitrosative stress: a newly-discovered genre for melatonin. Crit. Rev. Biochem. Mol. Biol. 44, 175–200. doi: 10.1080/10409230903044914
Reiter, R. J., Tan, D. X., Korkmaz, A., and Rosales-Corral, S. A. (2014). Melatonin and stable circadian rhythms optimize maternal, placental and fetal physiology. Hum. Reprod. Update 20, 293–307. doi: 10.1093/humupd/dmt054
Ren, Z., He, H., Zuo, Z., Xu, Z., Wei, Z., and Deng, J. (2019). The role of different SIRT1-mediated signaling pathways in toxic injury. Cell. Mol. Biol. Lett. 24:36. doi: 10.1186/s11658-019-0158-9
Riazi, K., Galic, M. A., Kentner, A. C., Reid, A. Y., Sharkey, K. A., and Pittman, Q. J. (2015). Microglia-dependent alteration of glutamatergic synaptic transmission and plasticity in the hippocampus during peripheral inflammation. J. Neurosci. 35, 4942–4952. doi: 10.1523/JNEUROSCI.4485-14.2015
Riazi, K., Galic, M. A., Kuzmiski, J. B., Ho, W., Sharkey, K. A., and Pittman, Q. J. (2008). Microglial activation and TNFα production mediate altered CNS excitability following peripheral inflammation. Proc. Natl. Acad. Sci.U.S.A. 105, 17151–17156. doi: 10.1073/pnas.0806682105
Rivera, L. I., Gootman, P. M., Lin, R. H., and Gootman, N. (1991). Effects of elevated plasma magnesium concentration on cerebrospinal fluid levels of magnesium in neonatal swine. Proc. Soc. Exp. Biol. Med. 197, 98–101. doi: 10.3181/00379727-197-43231
Robertson, N. J., Lingam, I., Meehan, C., Martinello, K. A., Avdic-Belltheus, A., Stein, L., et al. (2020). High-dose melatonin and ethanol excipient combined with therapeutic hypothermia in a newborn piglet asphyxia model. Sci. Rep. 10:3898. doi: 10.1038/s41598-020-60858-x
Romero, R., and Mazor, M. (1988). Infection and preterm labor. Clin. Obstet. Gynecol. 31, 553–584. doi: 10.1097/00003081-198809000-00006
Salter, M. G., and Fern, R. (2005). NMDA receptors are expressing in developing oligodendrocyte processes and mediate injury. Nature 438, 1167–1171. doi: 10.1038/nature04301
Sameshima, H., Ota, A., and Ikenoue, T. (1999). Pretreatment with magnesium sulfate protects against hypoxic-ischemic brain injury but postasphyxial treatment worsens brain damage in seven-day-old rats. Am. J. Obstetr. Gynecol. 180, 725–730. doi: 10.1016/s0002-9378(99)70279-6
Sarnat, H. B., and Flores-Sarnat, L. (2016). Synaptogenesis and myelination in the nucleus/tractus solitarius: potential role in apnea of prematurity, congenital central hypoventilation, and sudden infant death syndrome. J. Child Neurol. 31, 722–732. doi: 10.1177/0883073815615227
Scalley, R. D., and Conner, C. S. (1978). Acetaminophen poisoning: a case report of the use of acetylcysteine. Am. J. Hosp. Pharm. 35, 964–967.
Schaafsma, W., Basterra, L. B., Jacobs, S., Brouwer, N., Meerlo, P., Schaafsma, A., et al. (2017). Maternal inflammation induces immune activation of fetal microglia and leads to disrupted microglia immune responses, behavior, and learning performance in adulthood. Neurobiol. Dis. 106, 291–300. doi: 10.1016/j.nbd.2017.07.017
Schendel, D. E., Berg, C. J., Yeargin-Allsopp, M., Boyle, C. A., and Decoufle, P. (1996). Prenatal magnesium sulfate exposure and the risk for cerebral palsy or mental retardation among very low-birth-weight children aged 3 to 5 years. JAMA 276, 1805–1810. doi: 10.1001/jama.1996.03540220029026
Schizophrenia Working Group of the Psychiatric Genomics Consortium, Ripke, S., Neale, B., Corvin, A., Walters, J. T., Farh, K. H., et al. (2014). Biological insights from 108 schizophrenia-associated genetic loci. Nature 511, 421–427. doi: 10.1038/nature13595
Schmidt-Kastner, R., Guloksuz, S., Kietzmann, T., Van Os, J., and Rutten, B. P. (2020). Analysis of GWAS-derived schizophrenia genes for links to ischemia-hypoxia response of the brain. Front. Psychiatry 11:393. doi: 10.3389/fpsyt.2020.00393
Schmitt, B., Vicenzi, M., Garrel, C., and Denis, F. M. (2015). Effects of N-acetylcysteine, oral glutathione (GSH) and a novel sublingual form of GSH on oxidative stress markers: a comparative crossover study. Redox Biol. 6, 198–205. doi: 10.1016/j.redox.2015.07.012
Shah, S. A., Khan, M., Jo, M. H., Jo, M. G., Amin, F. U., and Kim, M. O. (2017). Melatonin stimulates the SIRT 1/Nrf2 signaling pathway counteracting lipopolysaccharide (LPS)-induced oxidative stress to rescue postnatal rat brain. CNS Neurosci. Ther. 23, 33–44. doi: 10.1111/cns.12588
Shahin, A. Y., Hassanin, I. M. A., Ismail, A. M., Kruessel, J. S., and Hirchenhain, J. (2009). Effect of oral N-acetyl cysteine on recurrent preterm labor following treatment for bacterial vaginosis. Int. J. Gynecol. Obstet. 104, 44–48. doi: 10.1016/j.ijgo.2008.08.026
Shepherd, E., Salam, R. A., Middleton, P., Makrides, M., McIntyre, S., Badawi, N., et al. (2017). Antenatal and intrapartum interventions for preventing cerebral palsy: an overview of Cochrane systematic reviews. Cochrane Database Syst. Rev. 8:CD012077. doi: 10.1002/14651858.CD012077.pub2
Shih, A. Y., Erb, H., Sun, X., Toda, S., Kalivas, P. W., and Murphy, T. H. (2006). Cystine/glutamate exchange modulates glutathione supply for neuroprotection from oxidative stress and cell proliferation. J. Neurosci. 26, 10514–10523. doi: 10.1523/JNEUROSCI.3178-06.2006
Silverstein, F. S., Buchanan, K., and Johnston, M. V. (1986). Perinatal hypoxia-ischemia disrupts stiatal high affinity 3H-glutamate uptake into synaptosomes. J. Neurochem. 47, 1614–1619. doi: 10.1111/j.1471-4159.1986.tb00803.x
Silverstein, F. S., Naik, B., and Simpson, J. (1991). Hypoxia- ischemia stimulates hippocampal glutamate efflux in perinatal at brain: an in vivo microdialysis study. Pediatr. Res. 30, 587–590. doi: 10.1203/00006450-199112000-00021
Smilkstein, M. J., Knapp, G. L., Kulig, K. W., and Rumack, B. H. (1988). Efficacy of Oral N-acetylcysteine in the treatment of acetaminophen overdose. NEJM 319, 1557–1562. doi: 10.1056/NEJM198812153192401
Soundarapandian, M. M., Tu, W. H., Peng, P. L., Zervos, A. S., and Lu, Y. (2005). AMPA receptor subunit GluR2 gates injurious signals in ischemic stroke. Mol. Neurobiol. 32, 145–155. doi: 10.1385/MN:32:2:145
Stolp, H. B., Fleiss, B., Arai, Y., Supramaniam, V. G., Vontell, R., Birtles, S., et al. (2019). Interneuron development is disrupted in preterm brains with diffuse white matter injury: observations in mouse and human. Front. Physiol. 10:955. doi: 10.3389/fphys.2019.00955
Tagin, M., Shah, P. S., and Lee, K. S. (2013). Magnesium for newborns with hypoxic-ischemic encephalopathy: a systematic review and meta-analysis. J. Perinatol. 33, 663–669. doi: 10.1038/jp.2013.65
Tan, D. X., Chen, L. D., Poeggeler, B., Manchester, L. C., Reiter, R. J., and Poeggler, B. (1993). Melatonin a potent endogenous hydroxyl radical scavenger. Ann. N. Y. Acad. Sci. 738, 419–420. doi: 10.1111/j.1749-6632.1994.tb21831.x
Tan, D. X., Reiter, R. J., Manchester, L. C., Yan, M. T., El-Sawi, M., Sainz, R. M., et al. (2002). Chemical and physical properties and potential mechanisms: melatonin as a broad spectrum antioxidant and free radical scavenger. Curr. Top. Med. Chem. 2, 181–197. doi: 10.2174/1568026023394443
Tau, G. Z., and Peterson, B. S. (2010). Normal development of brain circuits. Neuropsychopharmacology 35, 147–168. doi: 10.1038/npp.2009.115
Tay, T. L., Béchade, C., D’Andrea, I., St-Pierre, M. K., Henry, M. S., Roumier, A., et al. (2018). Microglia gone rogue: impacts on psychiatric disorders across the lifespan. Front. Mol. Neurosci. 10:421. doi: 10.3389/fnmol.2017.00421
Thagard, A. S., Slack, J. L., Estrada, S. M., Kazanjian, A. A., Chan, S., Burd, I., et al. (2017). Long-term impact of intrauterine neuroinflammation and treatment with magnesium sulphate and betamethasone: sex-specific differences in a preterm labor murine model. Sci. Rep. 7, 1–13. doi: 10.1038/s41598-017-18197-x
Thomas, L., Purvis, C. C., Drew, J. E., Abramovich, D. R., and Williams, L. M. (2002). Melatonin receptors in human fetal brain: 2-[125I] iodomelatonin binding and MT1 gene expression. J. Pineal Res. 33, 218–224. doi: 10.1034/j.1600-079x.2002.02921.x
Thordstein, M., Bågenholm, R., Thiringer, K., and Kjellmer, I. (1993). Scavengers of free oxygen radicals in combination with magnesium ameliorate perinatal hypoxic-ischemic brain damage in the rat. Pediatr. Res. 34, 23–25. doi: 10.1203/00006450-199307000-00006
Truttmann, A. C., Ginet, V., and Puyal, J. (2020). Current evidence on cell death in preterm brain injury in human and preclinical models. Front. Cell Dev. Biol. 8:27. doi: 10.3389/fcell.2020.00027
Ursini, G., Punzi, G., Chen, Q., Marenco, S., Robinson, J. F., Porcelli, A., et al. (2018). Convergence of placenta biology and genetic risk for schizophrenia. Nat. Med. 24, 792–801. doi: 10.1038/s41591-018-0021-y
Verney, C., Monier, A., Fallet-Bianco, C., and Gressens, P. (2010). Early microglial colonization of the human forebrain and possible involvement in periventricular white-matter injury of preterm infants. J. Anat. 217, 436–448. doi: 10.1111/j.1469-7580.2010.01245.x
Vexler, Z. S., and Ferriero, D. M. (2001). Molecular and biochemical mechanisms of perinatal brain injury. Semin. Neonatol. 6, 99–108. doi: 10.1053/siny.2001.0041
Villapol, S., Fau, S., Renolleau, S., Biran, V., Charriaut-Marlangue, C., and Baud, O. (2011). Melatonin promotes myelination by decreasing white matter inflammation after neonatal stroke. Pediatr. Res. 69, 51–55. doi: 10.1203/PDR.0b013e3181fcb40b
Wang, B., Navath, R., Romero, R., Kannan, S., and Kannan, R. (2009). Anti-inflammatory and anti-oxidant activity of anionic dendrimer-N-acetyl cysteine conjugates in activated microglial cells. Int. J. Pharm. 377, 159–168. doi: 10.1016/j.ijpharm.2009.04.050
Wang, H., Li, L., Zhao, M., Chen, Y. H., Zhang, Z. H., Zhang, C., et al. (2011). Melatonin alleviates lipopolysaccharide-induced placental cellular stress response in mice. J. Pineal Res. 50, 418–426. doi: 10.1111/j.1600-079X.2011.00860.x
Wang, X., Svedin, P., Nie, C., Lapatto, R., Zhu, C., Gustavsson, M., et al. (2007). N-acetylcysteine reduces lipopolysaccharide-sensitized hypoxic-ischemic brain injury. Ann. Neurol. 61, 263–271. doi: 10.1002/ana.21066
Wang, X. F., and Cynader, M. S. (2002). Astrocytes provide cysteine to neurons by releasing glutathione. J. Neurochem. 74:4. doi: 10.1046/j.1471-4159.2000.0741434.x
Welin, A. K., Svedin, P., Lapatto, R., Sultan, B. O., Hagberg, H., Gressens, P., et al. (2007). Melatonin reduces inflammation and cell death in white matter in the mid-gestation fetal sheep following umbilical cord occlusion. Pediatr. Res. 61, 153–158. doi: 10.1203/01.pdr.0000252546.20451.1a
Welser-Alves, J. V., and Milner, R. (2013). Microglia are the major source of TNF-α and TGF-β1 in postnatal glial cultures; regulation by cytokines, lipopolysaccharide, and vitronectin. Neurochem. Int. 63, 47–53. doi: 10.1016/j.neuint.2013.04.007
Wiest, D. B., Chang, E., Fanning, D., Garner, S., Cox, T., and Jenkins, D. D. (2014). Antenatal pharmacokinetics and placental transfer of n-acetylcysteine in chorioamnionitis for fetal neuroprotection. J. Pediatr. 165, 672–677.e2. doi: 10.1016/j.jpeds.2014.06.044
Wilkinson, D., Shepherd, E., and Wallace, E. M. (2016). Melatonin for women in pregnancy for neuroprotection of the fetus. Cochrane Database Syst. Rev. 3:CD010527. doi: 10.1002/14651858.CD010527.pub2
Yoon, B. H., Romero, R., Kim, C. J., Koo, J. N., Choe, G., Syn, H. C., et al. (1997). High expression of tumor necrosis factor-α and interleukin-6 in periventricular leukomalacia. Am. J. Obstetr. Gynecol. 177, 406–411. doi: 10.1016/s0002-9378(97)70206-0
You, J., Shamsi, B. H., Hao, M., Cao, C., and Yang, W. (2019). A study on the neurodevelopment outcomes of late preterm infants. BMC Neurol. 19:108. doi: 10.1186/s12883-019-1336-0
Zeise, M. L., and Semm, P. (1985). Melatonin lowers excitability of guinea pig hippocampal neurons in vitro. J. Comp. Physiol. 57, 23–29. doi: 10.1007/BF00611091
Zhang, F., Nance, E., Zhang, Z., Jasty, V., Kambhampati, S. P., Mishra, M. K., et al. (2016). Surface functionality affects the biodistribution and microglia-targeting of intra-amniotically delivered dendrimers. J. Control. Release 237, 61–70. doi: 10.1016/j.jconrel.2016.06.046
Keywords: fetal neurodevelopment, fetal synaptopathy, magnesium sulfate, melatonin, N-acetyl-L-cysteine, DNAC
Citation: Elsayed NA, Boyer TM and Burd I (2021) Fetal Neuroprotective Strategies: Therapeutic Agents and Their Underlying Synaptic Pathways. Front. Synaptic Neurosci. 13:680899. doi: 10.3389/fnsyn.2021.680899
Received: 15 March 2021; Accepted: 28 May 2021;
Published: 23 June 2021.
Edited by:
Matilde Otero-Losada, Centro de Altos Estudios en Ciencias Humanas y de la Salud, Universidad Abierta Interamericana, Consejo Nacional de Investigaciones Científicas y Técnicas, CAECIHS.UAI-CONICET, ArgentinaReviewed by:
Pedro M. Pimentel-Coelho, Federal University of Rio de Janeiro, BrazilXiaodi Chen, Women & Infants Hospital of Rhode Island, United States
Copyright © 2021 Elsayed, Boyer and Burd. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Irina Burd, aWJ1cmRAamhtaS5lZHU=
†These authors share first authorship
 Nada A. Elsayed
Nada A. Elsayed Theresa M. Boyer
Theresa M. Boyer Irina Burd
Irina Burd