
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Synaptic Neurosci. , 29 August 2011
volume 3 - 2011 | https://doi.org/10.3389/fnsyn.2011.00004
This article is part of the Research Topic Spike-timing dependent plasticity View all 33 articles
How learning and memory is achieved in the brain is a central question in neuroscience. Key to today’s research into information storage in the brain is the concept of synaptic plasticity, a notion that has been heavily influenced by Hebb’s (1949) postulate. Hebb conjectured that repeatedly and persistently co-active cells should increase connective strength among populations of interconnected neurons as a means of storing a memory trace, also known as an engram. Hebb certainly was not the first to make such a conjecture, as we show in this history. Nevertheless, literally thousands of studies into the classical frequency-dependent paradigm of cellular learning rules were directly inspired by the Hebbian postulate. But in more recent years, a novel concept in cellular learning has emerged, where temporal order instead of frequency is emphasized. This new learning paradigm – known as spike-timing-dependent plasticity (STDP) – has rapidly gained tremendous interest, perhaps because of its combination of elegant simplicity, biological plausibility, and computational power. But what are the roots of today’s STDP concept? Here, we discuss several centuries of diverse thinking, beginning with philosophers such as Aristotle, Locke, and Ribot, traversing, e.g., Lugaro’s plasticità and Rosenblatt’s perceptron, and culminating with the discovery of STDP. We highlight interactions between theoretical and experimental fields, showing how discoveries sometimes occurred in parallel, seemingly without much knowledge of the other field, and sometimes via concrete back-and-forth communication. We point out where the future directions may lie, which includes interneuron STDP, the functional impact of STDP, its mechanisms and its neuromodulatory regulation, and the linking of STDP to the developmental formation and continuous plasticity of neuronal networks.
Already in antiquity, philosophers such as Aristotle observed the need for repeating sequences of activation in order to link mental representations (reviewed in Fregnac, (2002). In De Memoria Et Reminiscentia, Aristotle argued “Acts of recollection, as they occur in experience, are due to the fact that one movement has by nature another that succeeds it in regular order” (cited in Hartley, 1749; James, 1890). This is an intuitively appealing way of describing recollection, but it also implies causative chains of events. How can the mind establish causal relationships between events in the outside world? Indeed, it instinctively seems correct and very human to assume that the repeated and persistent temporal ordering of events A and B actually means that event A somehow causes event B. In fact, this mode of thinking is so human that concluding that B is caused by A in this scenario may make others accuse us of the logical fallacy of false cause, also known as post hoc ergo propter hoc.
Even so, this way of establishing causal and acausal relationships between events in the outside world seems to be key to how individual synaptic connections in the brain operate: typically, synapses are increased in strength if presynaptic spikes repeatedly occur before postsynaptic spikes within a few tens of milliseconds or less, whereas the opposite temporal order elicits synaptic weakening, a concept known as spike-timing-dependent plasticity (STDP; Figures 1A,B). It is as if synapses in the brain are rewarded via strengthening if its activity consistently predicts the postsynaptic activity, while repeated failure at predicting the postsynaptic cell’s activity – “postdiction” – results in punishment via synaptic weakening. As shall be discussed in more detail later, there are however many different types of STDP (Caporale and Dan, 2008; Sjöström et al., 2008). In this historical overview, we aim to briefly trace the historical background leading up to the STDP cellular learning paradigm in modern neuroscience research.
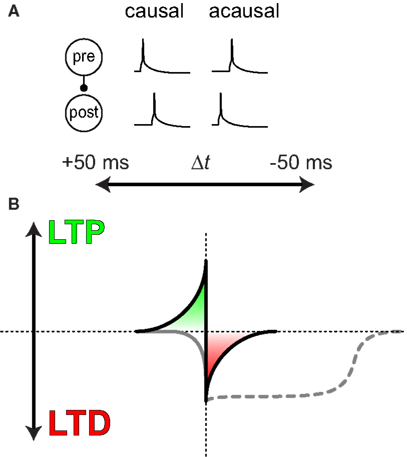
Figure 1. Defining Spike-Timing-Dependent Plasticity (A) A presynaptic cell connected to a postsynaptic cell repeatedly spiking just before the latter is in part causing it to spike, while the opposite order is acausal. (B) In typical STDP, causal activity results in long-term potentiation (LTP), while acausal activity elicits long-term depression (LTD; Markram et al., 1997b; Bi and Poo, 1998; Zhang et al., 1998). At some cortical synapses, the temporal window for LTD (dashed gray line) is extended (Feldman, 2000; Sjöström et al., 2001). These temporal windows are often also activity dependent, with LTP being absent at low-frequency (gray continuous line, Markram et al., 1997b; Sjöström et al., 2001), and postsynaptic bursting relaxing the LTD timing requirements to hundreds of milliseconds (Debanne et al., 1994; Sjöström et al., 2003).
Aristotle first introduced in his treatise De Anima the notion of the mind as a tabula rasa, or a blank slate, an idea that in the eleventh century was further developed by the Islamic philosopher Avicenna (also known as ibn-Sina), who argued that the mind was a blank slate at birth that was later developed through education. This idea was in stark contrast to that of Plato, Aristotle’s teacher, who argued in, e.g., Phaedo that the human mind was created in the heavens, pre-formed and ready, and was then sent to Earth to join the body. Philosophers have thus long argued as to whether we primarily are a product of nature or of nurture.
In modern times, the clean-slate view of the brain is normally accredited to the seventeenth century English philosopher, John Locke. Locke (1689) proposed that we are born without any preconceptions or innate ideas and that experience completely molds the brain, thus nurture determines who we are. This notion is central to Locke’s empiricism, which emphasizes the individual’s ability to author his or her own destiny. The tabula rasa view on learning in the brain had a powerful effect on subsequent philosophers and psychologists, and became generally accepted in psychology by the mid nineteenth century. It for example features in Sigmund Freud’s psychoanalysis, and is in fact still today a major paradigm in many respects.
The seventeenth and eighteenth century philosophers, such as Thomas Hobbes, David Hume, Étienne de Condillac, and David Hartley, drove the shift to empiricism by claiming a physical basis for behavior, learning, and memory. An important related question that these philosophers were trying to answer was how habits come about. These questions lead to a series of fundamental postulates of associative learning, contiguity, synchronization, and succession of events. Hartley, for example, wrote “Any sensations A, B, C etc., by being associated with one another a sufficient Number of Times, get such a power over the corresponding Ideas, a, b, c, etc., that any one of the sensations A, when impressed alone shall be able to excite in the Mind, b, c, etc., the ideas of the rest.” (Hartley, 1749).
By the mid nineteenth century, philosophers, psychologists, and early physiologists, neurosurgeons, and the first neuroscientists started seeking the mechanisms that form the physiological bases of learning and memory and locked on to the notion that associating information is the ultimate law governing brain function. Philosophers during this time even expressed their surprise at how the “ancient ones” could have thought otherwise. The influential French philosopher Théodule Ribot writes, “It is remarkable that this discovery was made so late. Nothing is simpler, apparently, than to notice that this law of association is the truly fundamental, irreducible phenomenon of our mental life; that it is at the bottom of all our acts; that it permits of no exception; that neither dream, revery, mystic ecstasy, nor the most abstract reasoning can exist without it; that its suppression would be equivalent to that of thought itself. Nevertheless no ancient author understood it, for one cannot seriously maintain that a few scattered lines in Aristotle and the Stoics constitute a theory and clear view of the subject. It is to Hobbes, Hume, and Hartley that we must attribute the origin of these studies on the connection of our ideas. The discovery of the ultimate law of our psychologic acts has this, then, in common with many other discoveries: it came late and seems so simple that it may justly astonish us.” (Ribot, 1870). The Scottish Philosopher, Alexander Bain writes, “Actions, sensations, and States of Feeling, occurring together or in succession, tend to grow together, or cohere, in such a way that, when any one of them is afterwards presented to the mind, the others are apt to be brought up in idea.” (Bain, 1855).
The idea that changes at junctions between neurons might account for learning and memory by changing the way information flows in the brain was already speculated in the later half of the nineteenth century. The earliest references that explicitly pins down the junctions between cells as the physical element that must change to enable learning and memory, even before the existence of synapses was known, is probably that of Bain; “For every act of memory, every exercise of bodily aptitude, every habit, recollection, train of ideas, there is a specific grouping or coordination of sensations and movements, by virtue of specific growth in cell junctions.” (Bain, 1873).
William James (Figure 2), a leading American psychologist, driven by the belief that truth was relative and shaped by the learned usefulness of events, lay down the foundations for many years of speculations on the specific causal conditions that would strengthen these junctions. “The psychological law of association of objects thought of through their previous contiguity in thought or experience would thus be an effect, within the mind, of the physical fact that nerve-currents propagate themselves easiest through those tracts of conduction which have been already most in use…the phenomenon of habit in living beings are due to the plasticity of the organic materials of which their bodies are composed…And it is too the infinitely attenuated currents that pour in through these latter channels (sensory nerve roots) that the hemispherical cortex shows itself to be so peculiarly susceptible. The currents, once in, must find a way out. In getting out they leave traces in the paths they take…So nothing is easier than to imagine how, when a current once traversed a path, it should traverse it more readily still the second time.” (James, 1890).

Figure 2. William James Source: Houghton Library, Harvard University, Call number pfMS Am 1092 (1185) #83, with permission.
James considered repetition, intensity, and competition key determinants of associations. “The amount of activity at any given point in the brain-cortex is the sum of tendencies of all other points to discharge into it, such tendencies being proportionate (1) to the number of times the excitement of each other point may have accompanied that of the point in question; (2) to the intensity of such excitement; and (3) to the absence of any rival point of functionality disconnected with the first point, into which the discharges might be diverted.” James also postulated a neural mechanism of associative learning, “After discrimination, association!…a stimulus which would be inadequate by itself to excite a nerve centre to effective discharge may, by acting with one or more other stimuli (equally ineffectual by themselves alone) bring the discharge about…Let us then assume as the basis of all our subsequent reasoning this law: When two elementary brain-processes have been active together or in immediate succession, one of them, on reoccurring, tends to propagate its excitement into the other.” (James, 1890). This associative learning rule is strikingly similar to that proposed by Donald Hebb about half a century later (see below).
One may be tempted to think that early philosophers and psychologists considered timing of events only vaguely, but in fact a remarkable number of psychophysical studies were conducted in the nineteenth century in an attempt to define the temporal unit of perception and the temporal unit of associations of perceptions. Measurements varied from 750 ms down to as little as 2 ms for the units of perception and as little as 50 ms for associations of events (see James, 1890). The sequential timing and succession of events was considered critical in these early theories of mind and in particular learning and memory. James writes, “Time-determinations apart,…objects once experienced together tend to become associated in the imagination, so that when any one of them is thought of, the others are likely to be thought of also, in the same order of sequence or coexistence as before. This statement was named the law of mental association by contiguity.” Shadworth Hodgson, an English philosopher and close colleague of James, writes, “Memory aims at filling the gap with an image which has at some particular time filled it before, reasoning with one which bears certain time-and space-relations to the images before and after.” (James, 1890).
The later half of the nineteenth century was also the period when the experimental foundations for classical conditioning where being laid down. Ivan Pavlov’s 12 years of experiments on conditioned salivation and digestion in his dog were published in 1897. The principle was laid down that there are pre-set physiological reactions (salivation) that can be triggered by an unconditioned stimulus (smell of food) and that any arbitrary neutral stimulus (e.g., the color of one’s shirt) can be converted into a conditioned stimulus if presented at the same time as the unconditioned stimulus. Temporal ordering on a timescale of seconds was essential (Pavlov, 1897).
The foundations for the electrical properties of the brain and the discovery of the action potential were laid down in the latter part of the nineteenth century. Building on the work of the Italians Luigi Galvani and Allesandro Volta in the 1790s, Matteucci (1838) showed that living organisms generate electricity, thus giving rise to the concept of bioelectricity – the electric fish was of course a great help in this scientific revolution (Sances et al., 1980). Following on from this work, the German physician Emil du Bois Reymond, with the theoretical help of Hermann von Helmholtz, went on to develop methods of extracellular electrical recording and stimulation, which he used to discover the action potential in 1848 (du Bois Reymond, 1848). His work essentially founded experimental neuroscience in general and electrophysiology in particular. By the late 1890s neurosurgeons, neurologists, and neurophysiologist were using these new electrophysiological methods to study changes in the flow of electrical potentials in the nervous system by stimulating and recording from nerve tracts. Julius Bernstein, a student of du Bois Reymond and Helmholtz succeeded in 1868 to record the time course of action potentials with sub millisecond resolution (Bernstein, 1868; reviewed in Schuetze, 1983) and later in his life developed the theory of the equilibrium membrane potential of neurons generated by separation of ionic charges by the cell membrane (Bernstein, 1902).
Perhaps the most important work during this time was by the early Oxford neuroscientists Sir Victor Horsley and Francis Gotch in the 1890s (Gotch and Horsley, 1891). Horsley and Gotch used in vivo extracellular field recording and stimulation to identify the locus of epileptic seizures in humans. They were among the early explorers of functional specialization and lateralization of the brain some 50 years before Penfield’s systematic study of the homunculus (Penfield and Boldrey, 1937). In relation to synaptic plasticity, they stimulated the cerebral cortex and recorded in the spinal cord and sciatic nerve of cats and monkeys while also monitoring changes in muscle contraction. “…the dura mater was exposed at the level of the motor area of the lower limb; the spinal cord was then exposed at the level of about the 7th dorsal vertebra; raised in air and connected to the non-polarized electrodes…These results indicate (1) that the rise in the (potential) difference is occasioned not merely by direct application of the stimulating agent to the cord, but as a consequence of the presence of a series of excitatory processes, whether these are produced by nerve impulses entering below by afferent channels, or from above by cortical efferent ones….(2) They also show that the rise is least in the case of the excitatory cord changes evoked by cortical stimulation, in which case the limit of rise is not only small, but soon attained, … when the columns of the cord itself are excited, the rise is greater, … It would thus appear that one of the main features in the rise is the extent to which the nerve structure of the cord are thrown into activity…” (Gotch and Horsley, 1891). Their records on woodcuts actually show initial facilitation followed by depression of the evoked local field potentials.
The German neuroanatomist von Waldeyer-Hartz (1891) among others lay down the neuron doctrine – the idea that the brain is a system composed of separate neurons. At the same time, the documentation of neuronal composition of the brain began with the work of the Spanish physician-turned-neuroanatomist Santiago Ramón y Cajal (Figure 3) and the Italian pathologist Camillo Golgi. Ramón y Cajal (1894) had also proposed that long-term memories do not need new neurons, but rather the growth of new connections between existing neurons. The junction between neurons only became known as a “synapse” at the turn of the century after Sir Charles Sherrington declared that the “tip of a twig of the arborescence is not continuous, but merely in contact with the substance of the dendrite or cell-body on which it impinges” and that “Such a special connection of one nerve cell with another might be called a ‘synapsis’” (Sherrington, 1897, 1909)).
Yet Sherrington did not speculate on the possible relation between synaptic plasticity and learning. Tanzi (1893), an Italian neuropsychiatrist put forward the very first hypothesis that associative memories and practice-dependent motor skills may depend on a localized facilitation of transmission of already existing connections some 4 years before Sherrington coined the term “synapsis.” Tanzi and his disciple Ernesto Lugaro clearly admired Ramón y Cajal and his ideas of the nervous system as an aggregate of neurons separated by small distances. Influenced by Ramón y Cajal’s ideas of neurotropism, they hypothesized that nervous excitation must encounter some difficulty in crossing this space between neurons and that repetitive activity of the neuronal path (such as during learning of a specific task) would lead to hypertrophy of the neurons and thus facilitate easier crossing of the space between them (Tanzi, 1893). Lugaro (1898, 1906, 1909) expanded on this view, combining it with his new insight on chemical neurotransmission which attempts to explain how nerves find their targets via gradients of diffusible messengers. He also argued that coincident activity drives modifications of connections between neurons and used familiar and modern-sounding terminology such as “The plasticity of the nervous elements” (“La plasticità degli elementi nervosi cerebrali”) and “plastic activity of neurons” (“attività plastica dei neuroni”). Lugaro was thus the first to coin the term plasticity to synaptic modification (Lugaro, 1898, 1906, 1909).
By the end of the nineteenth century, it was widely believed that information flow must change in the brain for learning and memory to occur, that synapses control the flow of information, that they are the neural substrate of learning and memory, and that learning requires repeated and persistent activation without competing inputs, and that it is the temporal organization of events that determines the strength of associations – the glue to build memories.
The first half of the twentieth century witnessed a number of landmark studies that had a great influence on our views of chemical synapses, neurotransmitters, neuronal processing, direction of information flow in neurons, learning, memory, and behavior. First, the notion of chemical synapses became well defined, building on the nineteenth century work of Claude Bernard, by contributions from many great scientists such as Langley, Elliot, Dale, Loewi, Feldberg, and Brown (for a review, see Bennett, 2000). Chemical synapses were more attractive for learning and memory processes than electrical synapses because they impose a sense of direction to the flow of information in the brain. The actual direction however was a topic of rather intense debate until the 1930s. Ramón y Cajal (1911) was preoccupied with the direction of flow of information between neurons, which he emphasized using artistic arrows in his many drawings, although Cajal’s arrows sometimes pointed in the wrong direction.
While these neural principles were laid down, Karl Lashley was literally trying to cut out memories from the brain. His failure to find “the engram” led to the important conclusion that memory – and brain function in general – depends on “mass functioning of many neurons.” (Lashley, 1929). In the 1930s, the Canadian neurosurgeon Wilder Penfield – who was greatly inspired by Sherrington – developed the Montréal procedure for treating patients with intractable epilepsy by destroying pathological tissue. By locally stimulating the brain of awake patients to ascertain the origin of the seizure, he could excise the epileptogenic area while at the same time preserving healthy brain tissue. This technique also permitted the creation of maps of the sensory and motor cortices of the brain, known as the cortical homunculus, a view that counter balanced Locke’s tabula rasa vision of the brain. Penfield thus contributed greatly to our understanding of localization and lateralization in the brain (Penfield and Boldrey, 1937). This was also around the time that John Watson, the founder of behaviorism, proposed that negative associations could just as easily replace positive ones, through his famous but ethically questionable experiments on Little Albert. With this young boy, he demonstrated that a previously rewarding conditioning stimulus (playing with a white rat) could easily become negatively associated (by a loud noise). Watson thus went to the extreme end of the nature-versus-nurture argument and claimed that the environment can create any personality (Watson and Rayner, 1920). Experiments such as the one on Little Albert reinforced the notion that the brain begins as a clean-slate – a tabula rasa – on which experience shapes the individual. The clean-slate hypothesis is central to synaptic plasticity as it implies that the connectivity and strength of synaptic connections are entirely shaped by experience. In other words, circuits have full freedom to reconfigure and existing synapses are unrestricted with respect to change following experience.
Later, Burrhus Skinner argued that classical conditioning was not sufficient to explain all habits, traits, and tendencies, and instead developed operant conditioning. This denotes the formation of an association with an event that is accidentally found to have a positive behavioral outcome, similar to what is today commonly known as trial-and-error learning (Skinner, 1938).
By the 1930s, it had become clear that information flowed from presynaptic axons to postsynaptic dendrites, that all inputs were integrated at the soma, and that – once the threshold for action potential generation was reached – the information propagated along the axon of the postsynaptic cell. Sir John Eccles, a student of Sherrington’s, was perhaps the first to speculate that once an action potential is generated and propagates down the axon, it would also be momentarily reflected back into the dendrites (Eccles and Sherrington, 1931).
The work of Rafael Lorente de Nó, a student of Cajal’s, however put forward the winning notion of the time that “The only possibility for… [a neuron]… using all the impulses seems to be, first, that each synapse sets only a subliminal (chemical or other) change able of summation and, second, that the conduction through the synapses is not followed by a refractory period. The subliminal changes are summated first in the dendrites then the surrounding of the axon. When the change reaches threshold value, an explosive discharge through the axon takes place…The axon… enters in a refractory state, but the cell body and dendrites do not do so, they continue receiving and adding subliminal changes until the threshold value is reached again and the axon has recovered….” (Lorente de Nó, 1934). Lorente de Nó also went on to develop the early concepts of neural network function with the concepts of recurrent chains of neurons in which activity would reverberate persistently without leaving. His work influenced his Chinese student Feng (1941) to produce some of the early twentieth century records of synaptic facilitation, which also sparked the early neural network theories by cybernetician Warren McCulloch and logician Walter Pitts (McCullogh and Pitts, 1943). It was these early recurrent network ideas that created the notion of “infinite loops within loops” – once information enters a neural system it may persistently reverberate and not easily leave.
The next leap in synaptic plasticity was made in the discoveries of synaptic changes that lasted for several minutes after the tetanic stimulus was over. Post-tetanic potentiation seemed to have been discovered in the early part of the twentieth century by the American neurophysiologist and behaviorist, Ralf Gerard (1930). Other important early works in the 1940s included those of Lloyd (1949) and Larrabee and Bronk (1947). “It is our purpose to describe certain observations which reveal long-lasting effects of nervous activity that increase the stimulating action of nerve impulses at a synapse. The transient effects of an electric stimulus and the brief duration of a nerve impulse have emphasized the role of rapidly occurring events in the nervous system. On the other hand, physiological and psychological observations reveal many phenomena, which must be due to long persistent effects of nerve impulses within the central nervous system. Among these are the after-effects which continue for many minutes following a visual stimulus…, the sensory effects of intense mechanical vibrations which may continue for days, and the process of learning. These are among the obscure and challenging problems of neurology. It is probable that such phenomena are due to long-lasting changes in the properties of neurones and of synapses caused by previous activity.” (Larrabee and Bronk, 1947).
Inspired by Pavlov’s work, Gerard also restated a long-held understanding from empiricist psychology that “in the course of establishing a conditioned reflex, a particular afferent system comes to exercise control over an efferent one upon which it normally has no action. In neurological terms, this means that two brain centers become able to interact physiologically as a consequence of having been repeatedly set into action together…On the other hand, it has long been known (Ralf Gerard, 1930), though often overlooked, that a few seconds tetanus may leave, even in nerve, considerable after-potentials which actually increase in magnitude during three or four minutes and endure for over fifteen.” (Gerard, 1949). Gerard (1949) realized the importance of these “after-effects” of an action potential for learning, memory and behavior. He noted, “What occurs at a given synapse can be highly variable…It is not over when an impulse flashes across a synapse and onto its destination. It leaves behind ripples in the state of the system. The fate of a later impulse can thus be at least a little influenced by the past history of the neurones involves, by what happened before – and when. So we begin to get some increased freedom in accounting for behavior.” (Gerard, 1949).
By the end of the first half of the twentieth century, the pieces were in place for an early unification of ideas and a comprehensive theory of learning and memory based on synaptic plasticity. Long-lasting changes in synaptic efficacy were widely speculated upon, speculations that were fuelled by these early discoveries of short-term plasticity and post-tetanic potentiation.
The Canadian neuropsychologist Donald Hebb (Figure 4) – who was a student of Wilder Penfield as well as of Karl Lashley – made considerable headway at developing the concept of the distributed location of memory. In his book “The Organization of Behavior,” Hebb brought together many of the earlier ideas and findings on plasticity and learning and memory in a tremendously influential formal postulate of the neural mechanisms of learning and memory (Hebb, 1949), although Hebb himself later claimed that he “was not proposing anything new” (Berlucchi and Buchtel, 2009). Memories could be stored if the connections that repeatedly drive activity in a cell become strengthened because this would couple specific groups of neurons together and explain how neurons could be molded together in an assembly as a function of past experience. “Let us assume that the persistence or repetition of a reverberatory activity (or “trace”) tends to induce lasting cellular changes that add to its stability.[…] When an axon of cell A is near enough to excite a cell B and repeatedly or persistently takes part in firing it, some growth process or metabolic change takes place in one or both cells such that A’s efficiency, as one of the cells firing B, is increased.” (Hebb, 1949). Even though Hebb explicitly stated that “The general idea is an old one, that any two cells or systems of cells that are repeatedly active at the same time will tend to become “associated,” so that activity in one facilitates activity in the other” (Hebb, 1949), strengthening of connections between co-active cells has become known as Hebbian plasticity and the resulting groups of cells joined together through this form of plasticity even today go under the moniker of Hebbian assemblies (Figure 5).
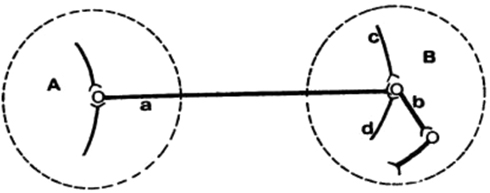
Figure 5. An illustration of the Hebbian postulate and a small assembly of cells. Here, presynaptic cell a, along with afferents c and d, repeatedly and persistently drive the postsynaptic cell b, thus leading to a long-term increase in the connective strength between cells a and b (reprinted with permission from Hebb, 1972).
Hebb considered these assemblies as representing percepts and the basis of thought. Key to this notion is the need for closed-loop circuits and re-entrant paths in the brain, thus leading to reverberating activity being held for some period of time by the circuit. In this view, this reverberating activity represents the environmental event that triggered it, and these re-entrant closed-loop circuits are wired up in the first place by the very processes of perceptual learning that Hebb proposed in his famed postulate. But it is key that this system can also be intrinsically excited in the brain in the absence of the sensory stimulus that originally helped organize it. As Hebb put it, “You need not have an elephant present to think about elephants” (Hebb, 1972). Hebb also went further to propose that assemblies are linked in chains to create a phase sequence, which he considered the neural basis of the thought process, via chains of percepts. The notion of phase sequences is perhaps not entirely clear, but one key element seems to be the idea that the same cells and assemblies can partake in several different percepts depending on which cells and assemblies are co-active as well as on which fired before and which fire after. Different phase sequences may thus represent different thought processes, and the same cells may be part of different thought processes via different phase sequences. What is clear is that a temporal ordering of activity in cells is central to the phase sequence in Hebbian assemblies (Hebb, 1949, 1972).
The idea that memories were held in cell assemblies was actually proposed before Hebb. For example, Joseph Edgar DeCamp stated that “From the neurological standpoint, in the learning of a series of syllables, we may assume that a certain group of synapses, nerve-cells, nerve paths, centres, etc., are involved. Immediately after the learning process that after-discharge continues for a short time, tending to set the associations between the just learned syllables.” (DeCamp, 1915). Hebb’s comprehensive unification of the many previous ideas was particularly important, because it laid the foundation for subsequent generations to build upon.
One year before Hebb published his 1949 book, the Polish neurophysiologist Konorski (1948) had already published remarkably similar ideas on synaptic plasticity and its relation to learning. In his book, Konorski aimed to show that morphological changes in neuronal synaptic connections are the substrate of learning (Zielinski, 2006). In other words, he argued against the view that the formation of new connections was important, and instead emphasized the role of changes in already existing pathways that were for some reason not already in use. Coincident activation of neuronal centers should lead to the formation of actual excitatory pathways between them, based on pre-existing potential connections, argued Konorski. But Konorski also conceived of a key role for inhibition in such processes: When the receiving neuronal center became less active after activation of the transmitting center, inhibitory connections are enabled. Either way, Konorski explicitly pointed out the role of repetition and repetition intervals in these processes. Interestingly, Konorski also proposed the existence of what we now jokingly refer to as grandmother cells, although he termed them “gnostic units,” thus predicting the existence of e.g., neurons that respond to particular faces (Quiroga et al., 2005).
Although Jerzy Konorski’s ideas sprung from those of Ivan Pavlov, they were not entirely in agreement. This posed a problem in the Communist East – Pavlov was religiously held in high esteem both in the Soviet Union and in Poland. Konorski thus found himself as well as his work being suppressed for political reasons. For more than a decade after and around the publication of his book, he became relatively isolated from the West, and the impact of his work was probably not as great in the West as it should have been. Researchers such as Hebb, Adrian, and Eccles, however, in all likelihood fully appreciated the importance of his proposals at a very early stage (Zielinski, 2006). Today, some researchers prefer to speak of Hebb–Konorski plasticity (e.g., Lamprecht and LeDoux, 2004), although the concept of Hebbian plasticity is clearly in wider use.
In the early 1990s, Carla Shatz (1992) summarized the Hebbian postulate as “cells that fire together wire together” to inputs in the visual system that strengthen together if they are active at the same time as the postsynaptic cell, thus leading to ocular dominance column formation in early development due to retinal waves. This Hebbian slogan caught on and is now in wide colloquial use in the field. It is important to note, however, that if interpreted superficially, this slogan does not reflect all of what Hebb meant, because, strictly speaking, Hebb’s rule is directional: cell A helps fire cell B. In addition, provided that they are persistently co-active, Hebb suggested the possible formation of assemblies of any neurons, even previously unconnected ones: “When one cell repeatedly assists in firing another, the axon of the first cell develops synaptic knobs (or enlarges them if they already exist)…” (Hebb, 1949). How a neuron assists the firing of target neuron that it is not connected was supposedly via the activation of other neurons that were connected to that neuron.
Nevertheless, in synaptically coupled assemblies of neurons, future stimulation of even a few of the members of the group would tend to reactivate the entire assembly of neurons, thus recreating the activity state that represented past experience and recalling a memory of the past event. The Hebbian principle was not only catchy because of its clear-cut and experimentally testable formulation; it also rendered synaptic plasticity immediately and intuitively meaningful by positioning it in the context of neuronal assemblies. Hebb’s postulate was also particularly powerful because it gave a possible neural explanation to two notions held by early philosophers and psychologists: that information enters the brain and reverberates, thus leaving persistent traces; and that information flow in the brain must change for learning and memory to occur (Hebb, 1949).
The Hebbian principle is fundamentally a causal selection principle based on rewarding synapses for successfully driving a postsynaptic neuron. It was therefore also a natural neural mechanism for association of simultaneous and sequential perceptual events, speculated for over a century (see above). Between 1950 and 1967, Hebb’s ideas spurred a plethora of studies by Shimbel, Brindley, Eccels, Ito, and Szentagothai, to mention but a few, who attempted to explain how synaptic plasticity could account for Pavlov and Watson’s classical conditioning as well as for Skinner’s operant conditioning.
In 1964, Eric Kandel and Ladislav Tauc showed that pairing an EPSP with a conditioning stimulus in the giant marine snail Aplysia caused a long-lasting facilitation of the EPSP (Kandel and Tauc, 1964). More importantly, Kandel’s work strongly linked synaptic plasticity with behavioral associative learning of the gill withdrawal reflex in Aplysia. Because the presumed link between synaptic plasticity and information storage in the mammalian brain has not yet been established (Stevens, 1998; Sjöström et al., 2008), the importance of Kandel’s (2001) research on learning in Aplysia is difficult to overstate. Presently, the molecular, biophysical and cellular mechanisms that underlie behavioral learning in Aplysia are known in great detail. Although this form of plasticity is not Hebbian, the firm evidence for a role of synaptic plasticity in learning in the marine snail – literally ranging all the way from molecules to memory – thus forms a solid foundation for on-going plasticity and memory research in mammals, where the role of synaptic plasticity in memory storage remains to be formally proven (Stevens, 1998). It should be pointed out, however that in mammals tremendous progress has been made in linking fear conditioning to synaptic plasticity in the amygdala (Maren and Fanselow, 1996; Rodrigues et al., 2004; Maren, 2005).
Approximately two decades after Hebb published his postulate, Terje Lømo (Figure 6) presented his work from Per Andersen’s laboratory at a conference of the Scandinavian Physiological Society, showing that high-frequency electrical stimulation in the dentate gyrus of the rabbit hippocampus elicited responses that kept growing (Lømo, 1964; Bliss and Lømo, 1970, 1973). Tim Bliss joined the Andersen group in 1968 and showed together with Lømo that the condition for persistent growth of response amplitude was the high-frequency stimulation itself (Lømo, 1964; Bliss and Lømo, 1970, 1973). While tetanic stimulation was already used for about 100 years, Bliss and Lømo’s study was the first to demonstrate that the effects could last much longer than short-term facilitation or post-tetanic potentiation. These findings lent experimental support to Hebb’s hypothesis that synapses are strengthened if they are involved in successfully driving a cell, since sufficiently strong high-frequency stimulation of afferent fibers could reasonably be assumed to drive activity in postsynaptic cells.
Hebb’s learning rule did not provide for an active mechanism to weaken synapses – he proposed that synapses would weaken if they were unused and that “less strongly established memories would gradually disappear unless reinforced” through a slow “synaptic decay.” (Hebb, 1949). His book spurred intense debate in the theoretical community whether memory can be stored in cell assemblies. In 1956, a group in IBM research labs including Rochester, Holland, Haibt, and Duda tested the formation of Hebbian cell assemblies in a simulation on one of the biggest computers at the time. They realized that a standard Hebb rule does not work and proposed a variant of Hebbian learning that essentially amounts to a co-variance learning rule, combined with an additional feature of weight normalization so that during learning the total sum of all synaptic weights onto the same postsynaptic neuron remains constant, a feature used later in many studies of cortical map formation and unsupervised learning. In their paper they review the ideas of Hebbian learning and stated: “It is evident that the mechanism that Hebb postulated would tend to cause recollections. The question of whether or not the postulate is sufficient is, in a sense, the main topic of this paper. If no additional rule were made, the Hebb postulate would cause synapse values to rise without bound. Therefore, an additional rule was established: The sum of the synapse values should remain constant. This meant that, if a synapse was used by one neuron to help cause another to fire, the synapse would grow. On the other hand, if a synapse was not used effectively, it would degenerate and become even less effective, because active synapses would grow and then, to obey the rule about a constant sum of magnitudes, all synapses would be reduced slightly, so the inactive synapses would decrease.” (Rochester et al., 1956). This study thus postulated the existence of heterosynaptic weakening via a competitive mechanism, based on two important insights: the co-variance learning rule in combination with overall weight normalization. In order to measure whether a synapse was effective in driving the postsynaptic neuron, the authors introduced local variables 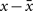 where x is the presynaptic activity and
where x is the presynaptic activity and 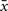 its average, and analogously
its average, and analogously 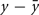 for postsynaptic activity. The co-variance rule was implemented by calculating
for postsynaptic activity. The co-variance rule was implemented by calculating 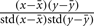 where std is the standard deviation (Rochester et al., 1956).
where std is the standard deviation (Rochester et al., 1956).
An early lasting mathematical formulation inspired by Hebb and his followers, was made by Frank Rosenblatt at Cornell University in his famous notion of the brain as a perceptron learning machine. Rosenblatt, influenced by many aspects of the brain’s plasticity and the early reports on the trillions of synapses in the human brain, was the first to introduce the concept of the “bivalent system” to “reward and punish” synaptic connections by making them stronger or weaker. He proposed a multi-layer perceptron where neurons in the middle layer, called A-units, received fixed random connections from the input layer. The projections from the A-units to the output were plastic. The output layer had a winner-takes-all connectivity, so that only one output was active at a time. He proposed a learning rule that would apply to all synapses from a given A-unit that had a connection to the active output. Hence, this rule was not Hebbian, as it would also apply to another connection from the same A-unit to an inactive output. His first rule distinguishes between two cases: active A-units with a projection to the active output and inactive A-units with a projection to the active output. In the main part of the paper, he studies unsupervised learning, but toward the end of the paper he continues: “In all of the systems analyzed up to this point, the increments of value gained by an active A-unit, as a result of reinforcement or experience, have always been positive, in the sense that an active unit has always gained in its power to activate the responses to which it is connected. In the gamma-system, it is true that some units lose value, but these are always the inactive units, the active ones gaining in proportion to their rate of activity. In a bivalent system, two types of reinforcement are possible (positive and negative), and an active unit may either gain or lose in value, depending on the momentary state of affairs in the system. If the positive and negative reinforcement can be controlled by the application of external stimuli, they become essentially equivalent to “reward” and “punishment,” and can be used in this sense by the experimenter. Under these conditions, a perceptron appears to be capable of trial-and-error learning.” (Rosenblatt, 1958).
Strengthening and weakening synaptic connections by the degree of their causality became a topic of debate in the mid 1960s. Some predicted that cerebellar parallel fiber inputs should strengthen when activated simultaneously with climbing fibers, whereas others argued that they should weaken: Brindley (1964), Marr (1969), and Grossberg (1969) voted in favor of potentiation, while Albus (1971) argued for depression. Although Marr (1971) erroneously favored potentiation, he was one of the first mathematicians to nevertheless claim that he could use Hebb’s rules to explain how the neocortex, cerebellum, and hippocampus operate.
A few years later, Gunter Stent tried to explain the loss of connections suggested by Hubel and Wiesel’s monocular deprivation experiments (Hubel and Wiesel, 1965; Wiesel and Hubel, 1965), by postulating the inverse to Hebbian learning (Stent, 1973). Stent proposed that “When the presynaptic axon of cell A repeatedly and persistently fails to excite the postsynaptic cell B while cell B is firing under the influence of other presynaptic axons, metabolic change takes place in one or both cells such that A’s efficiency, as one of the cells firing B, is decreased.” Stent also proposed a learning rule for inhibitory connections, whereby the failure of an inhibitory input to silence the postsynaptic cell would elicit weakening of that input, thus working in synergy with Hebbian excitatory inputs. This formulation is in fact precisely what Konorski conjectured regarding inhibitory plasticity more than two decades earlier (see above), except that Stent formulated his inhibitory learning rule the other way around. Von der Malsburg (1973) also implemented bidirectional plasticity, but indirectly by normalizing the changes induced by long-term potentiation (LTP). The concepts of Stent and von der Malsburg revived the nineteenth century views that intensity and competition was an important consideration in the decision to change a synapse.
In an attempt to explain ocular dominance column development and eye suture experiments carried out in the 1970s, Elie Bienenstock, Leon Cooper, and Paul Munro, unified the earlier key discoveries and developed a mathematical model whereby low-frequency activity of the postsynaptic neuron during presynaptic stimulation would lead to long-term depression (LTD) while high-frequency activity would lead to LTP with a variable frequency threshold marking the transition between the two. The model became known as the BCM learning rule (Bienenstock et al., 1982; also see Cooper, 2010). This was a landmark in the history of the theory of plasticity not only because of the computational power of the model, but also because it gave convincing theoretical arguments for the existence of a new form of plasticity: homosynaptic LTD. In this form of plasticity, synapses are depressed not because they are inactive during a competing input, nor because they are co-active with the wrong input, as in the cerebellum. Rather, in classical homosynaptic LTD, it is a specific frequency requirement that determines plasticity. Temporal order however plays little or no role. In addition, the BCM rule introduces key concepts in cellular learning rules, such as competition among inputs and metaplasticity. Metaplasticity – which denotes “the plasticity of plasticity” (Abraham and Bear, 1996) – ensures both a degree of stability in neurons and competition.
The period shortly after the publication of Hebb’s book was also an important time for synaptic and dendritic integration and neuronal computation. Sir John Eccles, another luminary student of Sherrington’s, carried out extensive studies on short-term plasticity until the 1960s (see Eccles et al., 1941; Eccles, 1946, 1964). Eccles (1964) felt that “[u]nder natural conditions synapses are activated by trains of impulses that may be of relatively high frequency…It is therefore imperative to study the operation of synapses during repetitive activation.” Sir Bernard Katz (Figure 7), a student of Eccles, took the study of short-term plasticity in a statistical direction to better understand its mechanisms (Del Castillo and Katz, 1954), which gave rise to the quantal hypothesis of neurotransmitter release. The quantal hypothesis became important for later synaptic plasticity studies because it provided a means to dissect the pre versus postsynaptic mechanism underlying synaptic plasticity.
Though a visionary of the dynamics of synaptic transmission, Eccles discarded the notion that dendrites are relevant for the integration of synaptic input (Eccles, 1960). A student of Eccles, Wilfred Rall disagreed and developed – in spite of many years of disagreement with Eccles – a comprehensive mathematical theory of how synaptic potentials are summated in the dendrites of a neuron, thereby giving rise to its axonal spiking output (Rall, 1955, 1957, 1959, 1960, 1962). The field of synaptic integration and dendritic computation had thus finally begun. Surprisingly, this field was to develop quite separately from the field of synaptic plasticity for many years, even though Rall and Rinzel (1971) did propose early on that changing spine neck resistance could alter synaptic weight. Similarly, Bliss and Lømo (1973) argued that alterations in spine structure could underlie LTP through the reduction of spine resistance. The idea that the back-propagating action potential has a role as an arbiter of causality in synaptic plasticity, however, required many more years to emerge (see below).
The excitement arising from the discovery of hippocampal plasticity triggered a veritable avalanche of studies. Douglas and Goddard (1975) showed that repeated high-frequency bursts were more effective in inducing LTP than a single long tetanic train. This was an important landmark in the history of synaptic plasticity, not only because repeated brief bursts became a popular protocol to induce LTP, but also because it demonstrated the importance of repeated and persistent periods of stimulation to induce LTP, which was predicted in the nineteenth century and elaborated by Hebb. Douglas and Goddard also named the phenomenon LTP at the suggestion of Per Andersen (Douglas and Goddard, 1975). A flood of experimental and theoretical studies followed in a race to test different aspects of Hebb’s postulate and to tease apart the underlying cellular, synaptic, and network mechanisms (Malenka, 2003). Much of this race was dominated by disputes over the pre or postsynaptic locus of the change, only to be settled by the fact that synapses can change in many ways, either pre or postsynaptically, or both (for a review, see Malenka and Nicoll, 1999).
Bruce McNaughton made the next landmark discovery that supported Hebb’s associative principle, when he experimentally tested James’ “law of association” and Hebb’s associative learning postulate. He showed that two weakly activated pathways, which would not succeed on their own to induce LTP after tetanic stimulation, could indeed cooperate to induce LTP in both their connections (McNaughton et al., 1978; McNaughton, 2003). This was central to Hebb’s hypothesis for associative memories where components of a memory can reinforce other components and even other related memories. This was a landmark study because it revealed a neural substrate for classical conditioning that had already become the bedrock of psychology. The same year, Baranyi and Feher (1978) found that pairing EPSPs recorded intracellularly with antidromic action potentials could trigger conditioned facilitation. They concluded that discharge of the postsynaptic action potential alone, without necessarily being triggered by synaptic input was important in the induction of the potentiation (Baranyi and Feher, 1978).
Gary Lynch and colleagues discovered LTD in the hippocampus around this time. They found that, while tetanic stimulation induced LTP of the activated pathway, the inactive pathway underwent LTD (Lynch et al., 1977). Moreover homosynaptic LTD was found to occur at the activated pathway provided that the activation frequency was low (Dunwiddie and Lynch, 1978). In psychological terms, this phenomenon may be seen as a neural correlate for passive extinction of memories, but is also reminiscent of James’ view that there should be no competition among pathways that carry different information.
William Levy and Oswald Steward soon after explored the effect on a weak pathway (contralateral entorhinal to dentate pathway) in the hippocampus that was not capable of LTP on its own, but only when combined with a strong pathway (ipsilateral). They also found LTD in the inactive pathway following potentiation of another pathway (as found by Lynch), but additionally found that the potentiated weak pathway could be depotentiated if tetanized on its own afterward (Levy and Steward, 1979) – a phenomenon that has since become known as “depotentiation.” Thus any future activity of the weak pathway without the conditioned stimulus would lead to depotentiation. The subsequent year, it was discovered that low-frequency stimulation of a potentiated pathway also induced depotentiation (Barrionuevo et al., 1980), thus emphasizing the extinction of a newly associated pathway that is weakly active or weakly synchronous with the conditioning pathway.
In contrast to what Brindley (1964), Marr (1969), and Grossberg (1969) postulated in the late 1960s (see above), Ito et al. (1982) found heterosynaptic LTD of the parallel fibers in the cerebellum caused when the climbing fibers where simultaneously activated. In this form of LTD, the synapses were active at the time that a conditioning stimulus was being applied, the inverse of Hebbian associative LTP as shown by McNaughton. This inverse of LTP was elegantly consistent with the growing notion that the parallel fibers carry an error, which must decrease during learning and was thus also consistent with notions of classical conditioning. It should be noted, however, that this form of plasticity is neither Hebbian nor classical STDP.
In 1988, Yves Frégnac et al reported a cellular analog of visual cortex plasticity in vivo (Frégnac et al., 1988). They found that by repeated pairing of visual stimulation with direct positive or negative iontophoretic stimulation of a cortical neuron, they could often restructure the functional preference of the cell in question in a manner consistent with Hebb’s postulate. The experimenter could thus alter a cell’s receptive field in a form of supervised learning paradigm, interestingly even in the mature brain. This study also provided some of the first results consistent with the existence of homosynaptic LTD in neocortex.
The discovery of homosynaptic LTD has been reported in many studies (e.g., Dunwiddie and Lynch, 1978; Bramham and Srebro, 1987; Frégnac et al., 1988), but is typically attributed to two studies, one by Serena Dudek and Mark Bear and the other by Rosel Mulkey and Robert Malenka, both conducted in the hippocampus (Dudek and Bear, 1992; Mulkey and Malenka, 1992). These teams used long periods of precisely timed low-frequency stimulation to achieve depression, an approach that is perhaps biologically implausible (see e.g., Perrett et al., 2001). Nevertheless, this particular induction protocol became a major LTD paradigm for years to come and is still in use, probably because it is quite reliable. In general, the problem of extracellular stimulation would haunt the search for true homosynaptic plasticity for some time, since extracellular stimulation potentially activates heterogeneous inputs and possibly even neuromodulatory fibers (see Bear, 1999).
The early 1980s was also the time when the molecular substrate for associative plasticity was discovered in the unique properties of the NMDA receptor. This remarkable receptor only opens to allow a calcium influx after the presynaptic terminal has released glutamate and the postsynaptic membrane has been depolarized (Collingridge et al., 1983; Harris et al., 1984; Wigström and Gustafsson, 1984; Slater et al., 1985) but not with either condition alone – an elegant molecular coincidence detector.
The German engineer Karl Steinbuch showed in (Steinbuch et al., 1965) that a Hebbian learning rule is useful for forming associations between inputs and outputs, a scenario that was later termed a hetero-associative memory. In his model system, learning happens at the “synaptic” connection points between a set of parallel input wires (transporting a binary coded pattern of input features representing the stimulus) and output wires (the pattern index of “meaning”) running orthogonally to the inputs. The learning rule he uses is motivated by conditioned reflexes between stimulus and response and is essentially Hebbian in nature. At the crossing point between an input line j carrying a binary signal xj and an output line i with binary signal yi, the synapse measures the correlation, cij = yi(2xj − 1), between pre and postsynaptic signals. The correlation cij takes a value of +1 if both input and outputs are active; it is −1 if the input is inactive, but the output active; and zero if the output is inactive. The correlation is summed over T time steps, and the connection is increased if the result passes a threshold. The up and down regulation of the correlation signal during the summation time in combination with the threshold process assures that spurious correlations do not lead to a change of the synapse, but only consistent associations between inputs and outputs. As an electrical engineer, Karl Steinbuch even proposed a possible implementation of such a Hebbian rule by a physical system built from contact points between silver and silver bromide – and thereby constructed the first associative learning memory system, essentially a correlation memory system. In 1965, Steinbuch was granted a patent for his concept learning machine (Steinbuch et al., 1965), which states:
“An electrical circuit arrangement is provided in which combinations of input information signals … are assigned to corresponding output meanings. Input and output leads are arranged in a matrix of column wires and row wires. A device at each crossing point or intersection of a column wire and a row wire is arranged to be altered to change its condition by means of currents flowing simultaneously in both these wires. The marking of a row wire by a current flowing therein however can only effect the change of condition of such a device upon repeated current signals being applied to its associated column wire while current is still flowing in the row wire. This repeated action with respect to intersections of the matrix is referred to hereinafter as the learning phase.”
The work of Steinbuch inspired Teuvo Kohonen, who cites Steinbuch in his article on correlation matrix memories (Kohonen, 1972). In his paper, which appeared at the same time as a similar study by Anderson (1972), Kohonen gives an elegant mathematical analysis of the properties of such a matrix memory system. Despite the abstract mathematical formulation, the biological inspiration of these studies is clear in both papers.
Other early models of associative memories around this time, such as that by Willshaw et al. (1969) formulated how a network of neurons could learn to associate a particular activity pattern involving a subset of neurons with one out of many other types of patterns. This would require a learning rule where synapses change during coincident activity in connected pairs of neurons, much like what Hebb suggested.
Also, in 1973, Leon Cooper proposed that, “for such modifications to occur, there must be a means of communication between the cell body and the dendrite ends in order that the information be available at the appropriate connections; this information must move in a direction opposite to the flow of electrical signals.” (Cooper, 1973). While Cooper did not emphasize that the back-propagating action potential could carry this information back into the dendrites to all the synapses, he did realize that all the synapses had to somehow be informed about the cell’s spiking output (cf. Cooper, 2010).
In all models of hetero-associative memories, the stimulus A is associated with a later response or output Y, but no temporal order is explicitly defined, and temporal asymmetry is thus absent from. Similarly, the Hopfield model for auto-associative memory and pattern completion, where memory items were regarded as static objects (Hopfield, 1982) and the BCM model (Bienenstock et al., 1982) also simplified spike-timing out of the equations. In the Hopfield model, time plays a key role during the retrieval of a stored pattern, since it takes several time steps until the memory pattern is completed and fully retrieved, but time is of no importance during learning. In the BCM formulation, the average firing rate of any synaptic pathway and that of the postsynaptic neuron was important. In neither of these models, however, did the learning rule need precise relative timing of spiking of pre and postsynaptic neurons to trigger LTP or LTD.
As discussed earlier, timing in the sequence and association of events have been considered vital for over a century. How neurons could orchestrate their timing was also extensively considered. For example, Gerard (1949) wrote: “Another form of interaction is manifested in the synchronized electrical beating of large numbers of neurones. This is widely manifest in neural masses - from the synchronized discharges of the uniformly illuminated retina (Adrian and Matthews, 1928), or the like impulse trains set up from the two respiratory centers and recorded in the phrenic nerves (Gasser and Newcomer, 1921), to the regular alpha rhythm of the human occipital cortex, and the equivalent regular beat of the isolated frog olfactory bulb (Libet and Gerard, 1939). How is this interaction achieved?” If observations such as these – which hint at neuronal synchrony – are taken at face value, at least two important questions arise. The first one concerns the timescale of neuronal events such as synchrony, coincidence, and causality. In fact, the precision of timing for effective synaptic plasticity perplexed David Marr in the early 1970s. He proposed that the coincidence between the parallel and climbing fiber inputs must be “about the same time.” He further clarified this approximate phrasing by saying: “At about the same time” is an intentionally inexact phrase: the period of sensitivity needs to be something like 50-100 msec” (Marr, 1969), which was around the same interval that the early psychologists proposed.
In 1977, Terry Sejnowski developed the first mathematical model for bidirectional associative synaptic modification driven by the proportion of coincident and anti-coincident spiking activity as part of a proposed competition between the timing of inputs. He called it the “time-dependent non-linear model” and proposed that “…the change in synaptic strength is proportional to the covariance between discharges of the parallel and climbing fiber: then the synapses increases in strength when the discharges are positively correlated, decreases in strength when the discharges are negatively correlated, and maintains a constant average strength when the discharges are uncorrelated.” (Sejnowski, 1977b). Sejnowski went beyond the typically loose phrasing of synchronous activity to precise coincidences of single spikes by proposing that the “coincidence window for strengthening is 2ms (comparable to the time course of an action potential)…” and about 20 ms for “single anti-coincidences” (Sejnowski, 1977a,b). However, Sejnowski simplified and reduced the precision of this statement by embedding these temporally precise events as discharge rates in the average membrane potential of his co-variance model. Nevertheless, this model marked the beginning of a movement of theory away from behavioral time scales to those of spiking neurons as a mechanism to judge whether pathways should potentiate or depress.
The second question concerns the organization of sequences of neuronal events in time. Despite the fact that all classical and operant conditioning experiments have an important temporal component, since the response happens after the stimulus, theories like the Rescorla–Wagner theory of conditioning (Rescorla and Wagner, 1972) do not include timing in their mathematical formulae. The reason for this is somewhat unclear. One possible explanation is that timing issues were considered so obvious that it was not necessary to overload the mathematical formalism and, if necessary, the reader would be able to add timing in his or her mind. Similarly, the hetero-associative memories of Steinbuch, Willshaw, Anderson, Kohonen, and others, did not focus on the relative timing of input and output. Regardless, one important distinction between the classical condition and these associative memory models should be pointed out: the timescale on which the former operates is in seconds rather than milliseconds.
In 1976, the German researcher Gerd Willwacher published an article where he considered an extension from instantaneous – or time-less – associations to those with a temporal dimension. He expands on his ideas of Hebbian learning: “If two neurons are activated at the same time, mutual symmetric links are formed as synaptic connections between them. The intensity of the connection is proportional to the duration and intensity of the synchronous activity. The symmetric connection implies the function of parallel association. In the case of temporally shifted activity of the two units, asymmetric connections will be formed. The asymmetric connections result in a sequential association. ” (Willwacher, 1976).
In 1984, Valentino Braitenberg popularized the concept of asymmetric learning rules in his book “Vehicles” (Braitenberg, 1984), as he introduced the rectifying “Ergotrix” wire to enable his animal-like vehicles to distinguish causal from non-causal relationships. The Hopfield model of the early 80s inspired a large number of physicists to enter the field of theoretical neuroscience. One of the intriguing questions at that time was whether the Hopfield model could be generalized so that it could replay sequences of patterns rather than only static patterns. Similar to the insights of Willwacher, researchers realized that the key was to have asymmetric connections: if in a spatio-temporal sequence neuron j has to fire before i, the connection should be directed from j to i. Andreas Herz and Leo van Hemmen showed that such asymmetric connections could arise naturally, if timing issues and transmission delays are taken correctly into account during Hebbian learning. They also considered generalizations of Hebbian learning, where synchrony was defined not necessarily between the momentary activity of pre- and postsynaptic neurons, but between the postsynaptic spike and a low-pass filtered form of the presynaptic activity (Herz et al., 1988). Neurons in these Hopfield-like networks were binary and did not have any refractory period or intrinsic neuronal dynamics, so that in this approach toward sequence learning in associative memories (Sompolinsky and Kanter, 1986; Herz et al., 1988; Kleinfeld and Sompolinsky, 1988), the time scale was not well defined. Whilst the learning rule lead to asymmetric connections that reflected temporal order, it was formulated in discrete steps of time that could represent anything, from 1 ms to 1 s. Activity of a formal artificial model neuron could thus be interpreted as an episode of high firing rate as well as a single spike – the unit of time was the duration of one memory item.
Until the end of the 1980s, it was common to consider average rates and membrane potentials as measures of activity. In the early 1990s, Misha Tsodyks, Wulfram Gerstner, and others translated associative memory models from firing rates to spiking neurons, both for stationary patterns (Amit and Tsodyks, 1991; Gerstner and van Hemmen, 1992) and for sequences of patterns (Gerstner et al., 1993). In 1993, Gerstner and colleagues proposed that potentiation of synaptic strength can only be triggered if a postsynaptic spike coincides with the EPSP caused by incoming synaptic input and theorized that crucial information for plasticity, necessary for the learning of spatio-temporal spike patterns, would be missed if the usual averaging of firing rates or postsynaptic membrane potentials were considered (Gerstner et al., 1993). This coincidence window was assumed to be in the range of 1 ms. In these models, the time scale of co-activation of pre- and postsynaptic neurons was rationalized by the need for a hypothetical back-propagating spike that had to provide an unknown signal, which had to coincide with neurotransmitter release to elicit potentiation. The model showed only the importance of the causal order of timing of presynaptic activity before the postsynaptic spike in driving potentiation and did not deal with temporally precise conditions for depression.
Although the learning of associations clearly requires the introduction of the concept of time, since associations should take place only for events that are coincident in time, surprisingly few early experimental studies directly examined the role of timing in plasticity. In many reports, it was thus not clear what “coincidence” referred to. Was it a matter of minutes, second, milliseconds?
McNaughton et al. (1978) were probably the first to experimentally explore the importance of timing of the postsynaptic spike relative to the input timing in plasticity as part of the “logic” conditions for the association of events. They pointed out that “the discharge of the postsynaptic cell plays a pivotal role in Hebb’s initial postulate…” and attempted various methods to block the discharge of postsynaptic neurons during the tetanic stimulation by activating recurrent inhibition 20–50 ms before the tetanic stimulation. At that time, the only way to confirm that the postsynaptic neurons were not spiking was to examine the population spike and they found that the associative LTP was unaffected when there was no detectable population spike during the conditioning tetanus. They reported that, “the timing of the postsynaptic discharge with respect to the high-frequency input is not important over, at least, a 25 msec interval.” (McNaughton et al., 1978). In 1981, Baranyi and Feher, published a follow-up study to their 1978 paper showing thatto induce LTP, the timing requires for EPSPs and a burst of spikes was 100 ms (Baranyi and Feher, 1981). However, the order of EPSPs and spikes in the pairing was not important, so no temporal asymmetry akin to that of classical STDP was found.
In 1983, Levy and Steward examined the timing constraints for associative plasticity by triggering a train of stimuli in one pathway before or after a train of stimuli in another pathway (Levy and Steward, 1983) They found a clear temporal asymmetry such that weak-before-strong activation evoked LTP in the weak, whereas strong-before-weak stimulation resulted in LTD in the weak input. They did not however explore the specific relative timing of single spikes. They concluded, “that perfect temporal contiguity is not a requirement of this prototypical elemental memory unit.” Like Cooper, Levy, and Steward also concluded that the associative signal is “in the postsynaptic cell or some portion thereof. Regardless of whether the critical signal is cell discharge, as Hebb reasoned, or simply a massive local dendritic depolarization… these processes eventually ‘feed back’ to regulate individual synapses…” (Levy and Steward, 1983).
A few years later, Gustafsson and Wigström (1986) too investigated the timing requirements of hippocampal plasticity using either two inputs or one input paired with postsynaptic current injection (Gustafsson et al., 1987). Interestingly, they studied the role of pairing with individual volleys (Wigström et al., 1985), in a manner very similar to some of the early STDP studies (e.g., Bi and Poo, 1998; Zhang et al., 1998; Feldman, 2000). Gustafsson and Wigström, however, did not report the temporal asymmetry of hippocampal plasticity that Levy and Steward reported and that is so characteristic of classical STDP (Caporale and Dan, 2008; Sjöström et al., 2008). But others have reproduced this variability of the timing requirements in hippocampal plasticity (e.g., Kelso et al., 1986; Wittenberg and Wang, 2006; Buchanan and Mellor, 2007), although its precise reasons remain unknown (for a review, see Buchanan and Mellor, 2010). Perhaps some important experimental parameter is yet unaccounted for.
After Masao Ito discovered parallel fiber LTD, Ekerot and Kano (1985) tested Marr’s timing predictions more explicitly, but found similar levels of LTD when the parallel fiber input arrived anywhere between 20 ms before and 150 ms after the climbing fiber input. They concluded that the precise relative timing was not critical for associative plasticity, as Marr had proposed (see Ito, 1989). In 1989, Stanton and Sejnowski reported a similar experiment to that of Levy and Steward, but in a different part of the hippocampus (Stanton and Sejnowski, 1989). They too found bidirectional LTP and LTD depending on the timing of weak and strong trains of stimulation, and they also observed LTD due to weak-after-strong input activation. This study suggested that LTD could be induced by simultaneous hyperpolarization of the postsynaptic neuron, suggesting that membrane potential can gate plasticity and that this may in fact underlie the timing rule. Although the findings of Stanton and Sejnowski have been called into question, with some studies reporting contradictory results (Kerr and Abraham, 1993; Paulsen et al., 1993), their study drove the research on timing in plasticity forward.
The year following Stanton and Sejnowski’s paper, Wolf Singer and colleagues reported that the level of hyperpolarization and depolarization determines whether LTP or LTD will result in the same pathway after the same tetanic conditioning (Artola et al., 1990). This study brought the focus of plasticity research further onto the postsynaptic neuron, because the key signal was dependent on the level of depolarization of the neuron, and not necessarily produced by any synaptic input in particular.
In 1994, Dominique Debanne and colleagues took the Singer study a step further and showed that the timing of a 250-ms-long depolarization relative to incoming inputs could determine whether LTD or LTP would result (Debanne et al., 1994). This added to the Singer study because now depolarization could act in the same way as hyperpolarization if it occurred before the input. In other words, it was the level of depolarization and hyperpolarization evoked in any way – even artificially – that determined the direction of synaptic plasticity.
These aforementioned studies thus introduced and parameterized the role of time in synaptic plasticity. Time is thus key not only to STDP, but also to classical rate and depolarization-dependent forms of plasticity. But timing is also key to for example ocular dominance column formation in the developing brain, as summarized colloquially in the early 1990s by Carla Shatz with “fire out of sync, lose your link” to depict how asynchronous activity in early development retinal waves results in visual system inputs weakening if they are consistently not able to drive the postsynaptic cell (personal communication, Carla Shatz, 1992). The notion of a critical role of timing in brain plasticity was thus bubbling for years and decades in the field before being directly discovered.
Lorente de Nó’s notion of the direction of information flow influenced interpretations of neuronal and synaptic processing until the 1990s. Eccles (1961) hypothesized that the spike can propagate in both directions, while Cooper (1973) and Levy and Steward (1983) hypothesized that some signal must propagate back to the synapses to prepare synapses for plasticity. Gerstner et al. (1993) also hypothesized that individual pre–post spike times contain more information for plasticity than average rates, so that the precise timing of a postsynaptic action potential needs to be communicated to the synapse.
It was the landmark discovery by Greg Stuart, in Bert Sakmann’s laboratory – using dual patch-clamp recordings from the soma and dendrites of the same neuron – that changed this field. This experiment unequivocally demonstrated that the action potential actively propagates back into the dendrites (Stuart and Sakmann, 1994). Henry Markram, also in Sakmann’s laboratory at the time, showed that single subthreshold synaptic potentials could trigger a low level of calcium influx (Markram and Sakmann, 1994) and that a single action potential left behind a much larger, 100 ms-long wake of calcium as it propagated back into the dendrite (Markram et al., 1995). Markram was also developed the technique of paired patch-clamp recordings of isolating monosynaptic connections between pyramidal neurons in the neocortex and he questioned how this wake of calcium triggered by the back-propagating action potential would impact synaptic input (see below).
Up until this stage, LTP and LTD had almost been exclusively studied using extracellular electrical shocks of input fibers to neurons. With this experimental paradigm, it is difficult to avoid heterosynaptic and polysynaptic effects even by attempting to stimulate a single afferent pathway. It is also difficult to avoid stimulating neuromodulatory afferents, which are known to exert profound effects on neurons, synaptic transmission, and synaptic plasticity. With this technique precise timing of activity in pre and postsynaptic neurons is not known. Up until this time, 60 years after Hebb, there was also still no direct demonstration that the synaptic connections between two neurons could change.
In 1991, Roberto Malinow reported the first such evidence. In a heroic study, he isolated four monosynaptically connected CA3–CA1 pyramidal pairs in the acute hippocampal slice. He then evoked LTP in these connections by simultaneously eliciting bursts of spikes in the pre and the postsynaptic neuron (Malinow, 1991). This was the first study that can be said to be truly homosynaptic and that most closely tested Hebb’s (1949) prediction that strengthening would occur “[w]hen an axon of cell A is near enough to excite a cell B and repeatedly or persistently takes part in firing it.”
In 1995, at the Annual Society for Neuroscience Meeting, Henry Markram reported the first experimental study on the importance of precise relative timing of spikes emitted by the pre and postsynaptic neurons at monosynaptic connections between pairs of neurons in the neocortex (Markram and Sakmann, (1995). A watershed marked by relative timing of single spikes on a timescale of a few tens of milliseconds – as opposed to relative timing of competing inputs, general depolarization or trains of stimuli – determined the direction and amplitude of the synaptic change (Markram et al., 1997b). The back-propagating spike could be seen as representing the integrated sum of all synaptic inputs and is therefore an ideal associative signal between all individual synaptic inputs coming in along the dendrite. The postsynaptic spike was generated by direct current injection and therefore these changes are also not heterosynaptic. The postsynaptic spike alone could act as an associative signal consistent with previous findings that merely polarizing the membrane during synaptic input can trigger synaptic plasticity. This study revealed LTP for causal pre-before-postsynaptics pike timings with 10 ms temporal displacement, while LTD was elicited by acausal pre-after-postsynaptic spike timings, even though both conditions were elicited at the same frequency. In other words, cells that fire together do not always wire together, because timing matters too. Larger timing differences of 100 ms, however, did not evoke any plasticity. This phenomenon was later named STDP (Song et al., 2000). These experiments also showed that blockade of postsynaptic spiking abolished the LTP, as did NMDA receptor antagonism. There was furthermore a tendency for synaptic depression if the presynaptic spike failed to evoke a postsynaptic spike, reminiscent of what Stent proposed for excitatory inputs (see above and Stent, 1973).
In 1996, two theoretical studies on STDP were published. Gerstner et al. (1996) extended their earlier idea of spike-based Hebbian potentiation to spike-based causal potentiation and non-causal depression, although still with a 1-ms time window to explain how the receptive fields in the barn owl auditory system could develop with such exquisite temporal precision. This paper was formulated at the level of spikes and contained a drawing of a theoretical STDP function without knowledge of the results of Markram et al. (1997b). Larry Abbott and Ken Blum also published a timing-dependent model of plasticity that year and applied it to a hippocampal model to explain rodent navigation experiments (Abbott and Blum, 1996; Blum and Abbott, 1996). The model was formulated as a rate model, with an asymmetric Hebbian rule for causal potentiation under the pre-before-post condition on a time scale of a few hundred milliseconds. In the Blum and Abbott (1996) study, depression for pre-after-post timings was furthermore mentioned as a possibility. Although formulated in terms of rates, it is straightforward to reinterpret the Blum and Abbott study in an STDP framework.
Henry Markram and Misha Tsodyks developed a now widely used test stimulus for synapses going beyond the single shock to test synaptic transmission to a train of presynaptic action potentials that could reveal the short-term plasticity of the connection and reported that Hebbian pairing does not necessarily change the synaptic efficacy of synapses, but also their short-term dynamics. This revived the earlier Eccles work on the importance of high-frequency stimulation in testing transmission in synaptic pathways and added a new facet to long-term plasticity – that short-term plasticity can change in the long-term, a notion they called redistribution of synaptic efficacy, or RSE (Markram and Tsodyks, 1996b). Tsodyks and Markram also developed a model of dynamic synaptic transmission that demonstrates how simply changing various synaptic parameters alters synaptic transmission and introduced the notion that the probability of release, synaptic depression and facilitation determine the coding of the transmitted signal (Tsodyks and Markram, 1997; Tsodyks et al., 1998). At the same time, Larry Abbott and Sacha Nelson reported similar findings, using a different phenomenological model that did not directly link short-term plasticity parameters to synaptic properties such as vesicle depletion or probability of release (Abbott et al., 1997; Varela et al., 1997).
The STDP study by Markram and colleagues was published in 1997 (Markram et al., 1997b), back-to-back with a report by Magee and Johnston (1997), in which dendritic recordings were used to show that LTP is more readily induced when the action potential propagates back into the dendrites than when it is not, thus acting as an associative signal.
In 1997, Curtis Bell and colleagues reported the timing requirements of synaptic plasticity in the cerebellar-like electric lobe of the mormyrid electric fish. This study followed up on findings going back more than a decade earlier (Bell, 1981). Bell et al. (1997) used a stimulus protocol similar to that which Stanton and Sejnowski used in the hippocampus, with extracellular stimulation of two independent parallel fiber inputs paired with depolarization of a single inhibitory Purkinje-like neuron. They revealed causally induced LTD and non-causally induced LTP by displacing the relative timing of the stimulated inputs from −600 ms across to +600 ms with respect to the depolarization of the postsynaptic neuron. In these experiments, the coincidence window was inverted, falling anti-symmetrically around 60 ms on either side of exact coincidence, with an additional non-associative potentiation component. This was a landmark finding, showing that dramatically different forms of STDP exist (Caporale and Dan, 2008; Sjöström et al., 2008). It should furthermore be noted that this form of STDP is nothing like the classical form of parallel fiber plasticity reported by Ito in the cerebellum (Ito et al., 1982). Not only does Bell’s STDP have different induction requirements and is partially non-associative (Bell et al., 1997), it also has a use-dependent form of depression (Han et al., 2000).
In 1998, Debanne et al. (1998) found that individual spike-pairings evoked STDP at connections between synaptically coupled neurons in hippocampal slice cultures. This was an extension to their 1994 study of temporal asymmetry with respect to postsynaptic depolarization (Debanne et al., 1994), inspired by Stent’s conjecture (Stent, 1973) and by prior work with Yves Frégnac (Debanne et al., 1995). As for the neocortex, they found potentiation for causal, pre-before-post spike-pairings, while the opposite temporal order resulted in LTD. In addition however, they also discovered a striking asymmetry in the width of the causal and acausal temporal windows such that the LTD window was considerably larger than that of LTP (Debanne et al., 1998). This type of imbalance in timing-dependent LTP and LTD was later reproduced in neocortical layer-2/3 by Feldman (2000) and layer-5 by Sjöström et al. (2001). In theoretical models, Kempter et al. (1999a) as well as Sen Song and Larry Abbott (Song et al., 2000) showed that this type of imbalance may help preserve stability, while the total width of the STDP function determines the correlation time scale in synaptic plasticity (Kempter et al., 1999a; Song and Abbott, 2001).
In 1998, Guo-qiang Bi, Li Zhang, and Mu-ming Poo (Bi and Poo, 1998; Zhang et al., 1998) examined the causal STDP window in great detail by mapping out the synaptic changes for a large number of timings covering essentially the entire coincidence window. For example, using paired recordings in dissociated neuronal cultures, Bi and Poo found a roughly 40-ms-long coincidence window, with an astoundingly rapid 1-ms transition between LTP and LTD for near-perfect coincidence between pre and postsynaptic cell activity. This sudden transition between LTP and LTD is in biological terms essentially instantaneous and thereby quite surprising, but was later reproduced in neocortex (Celikel et al., 2004) and is now considered one of several hallmark features of STDP.
Several more recent studies of STDP have focused on parameterizing STDP with respect to factors such as rate, higher-order spiking motifs, or dendritic location (for a review, see Froemke et al., 2010a). For example, Robert Froemke and Yang Dan reported in 2002 that the first spike pairing in a train of triplet or quadruplet spike-pairings determines whether LTP or LTD ensues in layer-2/3 pyramidal cells (Froemke and Dan, 2002). Similar although not entirely identical findings were reported in hippocampal cell culture by Guo-qiang Bi’s team (Wang et al., 2005). On the other hand, Sjöström et al. (2001) found that STDP is quite non-linear with frequency, so that LTD is promoted at low-frequency, while LTP is evoked at high-frequency regardless of temporal order (also see Markram et al., 1997b; Froemke et al., 2006). These findings thus link the older classical rate-dependent LTP literature with the newer STDP studies, by showing that rate and timing-dependent forms of plasticity co-exist at the same synapse type (Nelson et al., 2002).
Froemke et al. (2005) later also found that STDP depends on synaptic location in the dendritic arbor of layer-2/3 pyramidal cells, with more LTD farther from the soma. In 2006, a similar but more extreme case was reported by Sjöström and Häusser (2006) in neocortical layer-5 pyramidal cells, in which plasticity induced by high-frequency pairing at distal inputs is either Hebbian or non-Hebbian depending on the depolarization state of the dendrite. The same year, Letzkus et al. (2006) reported a striking reversal of the timing requirements for STDP along the apical dendrite of layer-5 pyramidal cells. These location-dependent forms of STDP have been extensively reviewed more recently (Sjöström et al., 2008; Froemke et al., 2010b).
Importantly, parameterizations such as these have been key to the development of well-tuned computer models of cellular learning rules, whether these models are phenomenological or mechanistic in nature, and whether they are formulated within timing or rate-dependent learning rule paradigms (Shouval et al., 2002; Clopath et al., 2010; Rackham et al., 2010; Mihalas, 2011). Parameterizations of the non-linear voltage and frequency dependence of STDP (Markram et al., 1997b; Sjöström et al., 2001; Froemke et al., 2006), for example, has led to the Claudia Clopath model which accounts for a large number of experimental results from slice experiments (also see Clopath and Gerstner, 2010) while making the crucial prediction that network connectivity motifs may be a reflection of the neural code (Clopath et al., 2010).
Most STDP studies have been carried out in vitro, in the acute slice (e.g., Markram et al., 1997b; Sjöström et al., 2001; Froemke and Dan, 2002) or using cultured neurons (Debanne et al., 1994; Bi and Poo, 1998). Studying cellular learning rules in vitro has many advantages, by providing excellent experimental control. But in vitro preparations are obviously also fraught with complications and alternative interpretations due to the simplifications and artifacts introduced by the preparation itself. The acute brain slice, for example, is entirely devoid of natural neuromodulation, and many connections are severed during dissection. Showing evidence for STDP in vivo, in the intact brain, is thus of utmost importance. Already in 1998, Mu-ming Poo and colleagues showed that STDP exists in vivo, using the retinotectal preparation of the Xenopus tadpole (Zhang et al., 1998). Evidence in support of STDP in vivo was subsequently also demonstrated in rodents, cats, and even in humans, chiefly in a set of studies by the groups of Yang Dan (Yao and Dan, 2001; Yao et al., 2004; Meliza and Dan, 2006), Joseph Classen (Stefan et al., 2000; Müller-Dahlhaus et al., 2010), Tobias Bonhoeffer (Schuett et al., 2001), Dan Feldman (Feldman, 2000; Allen et al., 2003; Celikel et al., 2004; Jacob et al., 2007), and Dan Shulz (Jacob et al., 2007; Shulz and Jacob, 2010). It should be noted, however, that many of these studies find results consistent with STDP, but in principle some of the same effects could also result from circuit phenomena in combination with co-variance learning rules.
Studies from Martin Heisenberg’s and Gilles Laurent’s laboratories also provide intriguing evidence for the existence of STDP in vivo in insects, such as the fruit fly Drosophila melanogaster (Tanimoto et al., 2004) and the locust Schistocerca americana (Cassenaer and Laurent, 2007). Here, this temporally sensitive form of plasticity appears to be key to olfactory learning and information transfer. Although some controversy remains regarding the general relevance of STDP as a cellular learning paradigm (Lisman and Spruston, 2005), this preservation of timing sensitivity in cellular learning across millions of years of evolution would seem to suggest that STDP is not just relevant but actually rather important (Lisman and Spruston, 2010; Shulz and Jacob, 2010). Precisely how important and for what remains to be elucidated, which leaves us neuroscientists with some very exciting future directions.
In this article, we covered the development of some of the ideas on learning, memory, and plasticity that led up to the discovery of STDP and further studies that revealed more intricate features of STDP and that demonstrated the ubiquity of this phenomenon. We overviewed philosophical, psychological, theoretical, and experimental developments, and we have seen how these interact and also how they often develop in relative isolation of each other. This (partial) history of timing in synaptic plasticity research takes us all the way back to Aristotle, beginning with the tabula rasa concept, through William James’s notion of the temporal needs for associative memories, passing via Hebb’s neural postulate for synaptic modifications, to the present. Since we have taken big strides through the centuries, we have necessarily had to leave out many important concepts that surely contributed to the evolution of these ideas. The Frontiers in Synaptic Neuroscience Special Topic on STDP provides us with a snapshot of the present-day state of research as well as a glimpse into the future. When combined with this history, one can perhaps better speculate on future directions. A number of core issues are worth pointing out.
Clearly not all forms of plasticity depend on the back-propagating action potential (Sjöström et al., 2008), but STDP in its classical form does provide a unique neural mechanism for the determination of causality and non-causality on the millisecond timescale. This timing-centric view of plasticity is not meant to imply that spike rate is irrelevant. Roughly synchronized bursts of activity in connected neurons also lead to potentiation regardless of the precise millisecond timing (Sjöström et al., 2001; Froemke et al., 2006; Butts et al., 2007). At high frequencies, synaptic plasticity is thus determined by rate rather than by timing, which potentially explains earlier conclusions drawn by McNaughton, Ito, Levy, Gustafsson, and others about the lack of timing dependence on the millisecond timescale. Synaptic plasticity is also most sensitive to timing within a spiking frequency window (Sjöström et al., 2001), suggesting that the relative spike-timing in connected neurons only mediates bidirectional weight changes in this mid-range of spiking frequencies. Rate-dependent models may therefore accurately describe synaptic plasticity for when firing rates are in a larger dynamic range, while the STDP model may more precisely describe synaptic plasticity induced across mid-range frequencies when spiking activity is furthermore temporally relatively precise (Sjöström et al., 2001). Indeed, recent modeling studies highlight how the dual timing and rate dependence of plasticity adds tremendous flexibility and computational power to the brain during the development of cortical circuits (Clopath and Gerstner, 2010; Clopath et al., 2010; Gilson et al., 2010).
The determinants of synaptic plasticity are however more complex than that. As detailed in this review, subthreshold depolarization can also determine the amplitude and sign of synaptic plasticity (Artola et al., 1990). Clearly, strong depolarization elicits more spikes and potentially stronger potentiation, but abolishing spikes does not necessarily prevent such strong depolarization-induced plasticity (Golding et al., 2002; Remy and Spruston, 2007; Hardie and Spruston, 2009). This suggests that strong depolarization is sufficient to evoke a similar amount of plasticity as a few spikes. On the other hand, slight depolarization that is not enough to trigger spiking tends to initiate depression if combined with synaptic input (Markram et al., 1997b; Sjöström et al., 2001, 2004; Sjöström and Häusser, 2006). Furthermore, the temporal asymmetry as well as the mechanistic underpinnings of spike-timing-dependent LTD (Sjöström et al., 2003) are indistinguishable from LTD triggered by pairing presynaptic input with subthreshold depolarization (Sjöström et al., 2004). The depression triggered by subthreshold depolarization during synaptic input may be analogous to the effects of low-frequency stimulation and is therefore consistent with earlier findings of low-frequency stimulation as a protocol to induce LTD and is also consistent with the BCM rate model (Clopath and Gerstner, 2010; Clopath et al., 2010; Cooper, 2010). The depression of synapses that participate in attempts to drive a neuron to spiking, but reaching only subthreshold levels is also consistent with the Stentian notion of punishment for failure (Stent, 1973). In the causal, Hebbian model for increasing synaptic transmission (i.e., pre driving post), synapses can therefore be punished for a failed attempt at driving a neuron or for a late arrival of the input – both cases representing a wasted synaptic effort – but these synaptic failures are pardoned when activity rates are high.
The profile of the STDP window can take on different degrees of asymmetry and can even be inverted such that post-after-pre leads to depression rather than strengthening (Abbott and Nelson, 2000; Caporale and Dan, 2008). As outlined above, such an inverted STDP window was first found for inputs onto inhibitory Purkinje-cell-like neurons in the mormyrid electric fish (Bell et al., 1997), and has since also been found, for example, at excitatory synapses onto inhibitory cells of the neocortex (Holmgren and Zilberter, 2001). In the electric fish, this form of STDP is thought to stabilize the membrane potential of the postsynaptic neuron by effectively canceling predictable variations in the input (Roberts and Bell, 2000). It is worth noting, however, that not all inhibitory cell types possess identical plasticity learning rules (Kullmann and Lamsa, 2007; Lamsa et al., 2010). A key question that thus remains open is: Why is it that a number of STDP learning rules are specific to certain types of synaptic connections?
Another key area for future experimental studies is the relationship between STDP and short-term plasticity. STDP and rate-dependent models have largely assumed that only the strength of synapses changes. But as pointed out above, Markram and Tsodyks demonstrated in 1996 that if the change is in the release probability then it is not a straightforward strengthening of the synapse (Markram and Tsodyks, 1996b). An increased probability of release due to LTP will enhance low but not high-frequency transmission, simply because synapses also depress faster. The converse decrease in release probability after LTD induction also produces less short-term depression or even facilitation (Sjöström et al., 2003). On the other hand, an increased rate of recovery from depression enhances only high-frequency transmission, while synaptic facilitation enhances transmission in mid-range frequencies (Markram et al., 1998). The conditions that would lead to a uniform strengthening of synapses across all frequencies may in fact be quite limited: increased postsynaptic receptor numbers, or increased numbers of synaptic contacts per connection, and similar. LTP studies have traditionally tried to pin down a single plasticity expression mechanism, but it has become clear that there are a plethora of such mechanisms (Malenka and Bear, 2004). It has also been easier to develop computer algorithms that deal with only changes in synaptic weights rather than with more complicated alterations in synaptic dynamics, which means there have been only a few studies relating STDP to short-term plasticity (for examples, see Senn et al., 2001; Carvalho and Buonomano, 2011). In reality, however, it is most likely that synapses can change in many different ways and that many of these ways lead to changes in the dynamics of synaptic transmission and not in a uniform change of efficacy across a high-frequency train of pulses. Changing synaptic dynamics generally changes the temporal sensitivity of synapses: an open question is how this temporal sensitivity relates to timing requirements in synaptic plasticity. Altering synaptic weights is a direct change in gain, while altering synaptic dynamics modifies a neuron’s sensitivity to the temporal coherence of its inputs (Markram and Tsodyks, 1996a; Abbott et al., 1997). How this change in temporal sensitivity reorganizes activity patterns in a recurrent local circuit with STDP remains entirely unknown.
An analogous problem exists with the structural plasticity of circuits. Learning algorithms use synaptic plasticity rules derived from already existing synapses to reorganize the connectivity within a group of neurons. Such models assume that it is valid to apply synaptic plasticity rules to reconfigure connectivity as well – i.e., to microcircuit plasticity. For example, wiring and rewiring of a neural circuit appears to face strikingly different problems that STDP might not sufficiently address, such as how axons and dendrites communicate when no synapse is present in order to decide whether to form a synapse. It is also not at all clear how a multi-synaptic connection can be switched on and off. Le Be and Markram (2006) provided the first direct demonstration of induced rewiring of a functional circuit in the neocortex, that is, the appearance and disappearance of multi-synaptic connections, which requires hours of general stimulation. It is however clear that glutamate release is a key determinant in synapse formation (Engert and Bonhoeffer, 1999; Kwon and Sabatini, 2011). Regardless, additional studies are therefore required to further investigate what Le Be and Markram termed long-term microcircuit plasticity, or LTMP, and to examine its links to other forms of plasticity, such as STDP.
As shown by the teams of Jason Kerr, Alfredo Kirkwood, and Guo-qiang Bi, STDP is also under powerful neuromodulatory control (Seol et al., 2007; Pawlak and Kerr, 2008; Zhang et al., 2009; Pawlak et al., 2010). These findings are crucially important, since it is quite unclear how STDP can do anything useful at all if it is not possible to somehow gate or modulate it. Indeed, blocking neuromodulation severely impairs virtually all known forms of learning and memory (Bear and Singer, 1986; Hasselmo, 1995; Kilgard and Merzenich, 1998; Li et al., 2006; Gelinas and Nguyen, 2007; Hasselmo and Sarter, 2011), and abnormalities in neuromodulation are implicated in virtually every known psychiatric disease (Toda and Abi-Dargham, 2007; Sara, 2009). A loss of neuromodulation is also implicated in neurodegenerative disease (Aleman and Torres-Aleman, 2009). From a clinical perspective, the role of neuromodulation in synaptic plasticity is arguably one of the most crucial issues to be resolved. Yet, the vast majority of experiments are conducted under conditions where the degree of neuromodulation is unknown or non-existent. A link between synaptic plasticity and the memory functions of neuromodulators emerged from the finding that acetylcholine modulates NMDA receptor activity (Markram and Segal, 1990). It is also worth noting that the neuromodulation of STDP potentially extends beyond the “big five” – acetylcholine, noradrenaline, serotonin, dopamine, and histamine – to neuropeptides, hormones, and immunological agents. Much more work is therefore needed to understand the functional role and mechanisms of neuromodulatory control in unsupervised learning rules such as STDP, for which neuromodulation may provide global supervision or, at the very least, a degree of regulation.
Future studies will also need to clarify the relationship between synaptic learning rules and homeostatic plasticity (Turrigiano et al., 1998; Turrigiano and Nelson, 2000, 2004). It is important to note that Hebbian plasticity algorithms are intrinsically unstable: persistent correlated firing in connected neurons results in synaptic strengthening, which in turn brings about increased levels of correlated firing, thus resulting in a form of positive feedback that can cause synapses to grow uncontrollably (Watt and Desai, 2010). Although certain forms of STDP keep the firing rate of the postsynaptic neuron in a stable regime (Kempter et al., 1999a,b, 2001; Song et al., 2000; van Rossum et al., 2000), at least below a critical frequency (Tsodyks, 2002), this intrinsic stabilization feature may not be quick enough to keep track of rapidly increasing rates in a highly connected network. Here is where homeostatic plasticity – discovered by Gina Turrigiano and colleagues in the mid 1990s (Turrigiano et al., 1994, 1998) – may provide negative feedback to keep postsynaptic activity within reasonable bounds. This form of plasticity may thus play a vital role in ensuring that synaptic plasticity rules do not drive synaptic weights into a range where the neuron cannot be driven to fire at all, or becomes excessively sensitive to any input (Toyoizumi et al., 2005, 2007a; Clopath and Gerstner, 2010; Clopath et al., 2010). Building models without homeostatic plasticity typically requires artificial compensatory assumptions such as global weight normalization, but even with homeostatic rate normalization, individual synapses may need to be additionally bounded. Finding links between STDP and the BCM learning rules seems key to future progress in our understanding of the relationship between intrinsically unstable synaptic plasticity and stability-promoting mechanisms such as synaptic scaling (Turrigiano et al., 1998; Turrigiano and Nelson, 2000; Izhikevich and Desai, 2003; Watt and Desai, 2010). It also is key that the link between homeostatic plasticity and long-term microcircuit plasticity is elucidated (Le Be and Markram, 2006).
Additionally, understanding the relationship between synaptic and homeostatic plasticity will likely require taking neuroenergetics into account since energy supply from mitochondria ultimately is what determines the membrane potential, restricts firing rates, speed of repolarization, synaptic plasticity, etc. Energetics may therefore provide interesting links between the BCM rule, STDP, and homeostasis (Toyoizumi et al., 2007b; Clopath and Gerstner, 2010; Clopath et al., 2010; Cooper, 2010; Watt and Desai, 2010).
Future studies will need to cast light on the network topology of Hebbian assemblies, that is how neurons of an assembly interconnect, a problem that presumably will at least in part require a graph theoretical approach (Bullmore and Sporns, 2009). One important question is whether topologies are determined by experience only, or by pre-defined experience-independent mechanisms. This lies at the heart of the nature-versus-nurture debate. Subsequent to the publication of Hebb’s postulate, theorists pointed out that these assemblies would not be very useful for storing multiple memories if synapses saturate (e.g., Fusi, 2002), since – with neurons strongly coupled via saturated synapses – assemblies would homogenize such that all produce similar outputs, which is not an ideal memory storage system. Theorists such as Rochester, Rosenblatt, Sejnowski, and Stent therefore proposed that synaptic depression must also be exist, to allow for more optimal information storage (see above). Paradoxically, Hebb’s proposal alone does not produce useful Hebbian assemblies – as LTP alone is not sufficient, there was a need for an extension to bidirectional plasticity. Yet, there is plenty of suggestive evidence for functional assemblies in terms of the patterned activity of neurons (Dudai, 1989; Abeles, 1991; Kandel, 2006; Byrne, 2008). In fact, we also know from paired recordings that synaptic connectivity often clusters, showing higher reciprocal coupling than expected from random uniform distributions (Markram et al., 1997a; Song et al., 2005; Perin et al., 2011). It also seems quite plausible that high-order motifs in clusters of local cells – a more intricate topology of connectivity (Song et al., 2005; Perin et al., 2011) – can be inferred from synaptic plasticity learning rules in combination with the history of activity of the neurons in question (Clopath and Gerstner, 2010; Clopath et al., 2010). Indeed, a recent study shows that, in mouse visual cortex, pyramidal neurons with similar feature selectivity are significantly more frequently interconnected, yet functionally dissimilar neurons are still connected at considerable albeit lower rates (Ko et al., 2011). This functional bias of cell connectivity is in basic agreement with a Hebbian-like learning rule such as frequency-dependent STDP (Markram et al., 1997b; Sjöström et al., 2001; Froemke et al., 2006) acting during the development of visual cortex neuronal receptive fields, with firing of sufficiently high-frequency to enable overrepresentation of reciprocal connections (Song et al., 2005; Clopath et al., 2010; Perin et al., 2011).

Structural assemblies imposed by topographic connectivity can furthermore be shaped by the region in which the neurons reside (Yoshimura and Callaway, 2005; Yoshimura et al., 2005; Kampa et al., 2006), their afferents and efferents (Le Be et al., 2007; Brown and Hestrin, 2009a,b) – many of which display cell–cell specific connectivity patterns (Markram et al., 1997a; Stepanyants et al., 2004; Song et al., 2005) – as well as by ontogenetic relationship among cells (Yu et al., 2009). How STDP and other forms of plasticity operate on top of these potential constraints remains to be shown. To reconcile activity dependent plasticity and its role in molding assemblies with pre-specified connectivity will depend on the nature of the configuration of connectivity and synaptic weight distributions that results within synaptically coupled assemblies. The nature of this connectivity may finally resolve the nature-versus-nurture debate that set the path for synaptic plasticity research and that has persistently remained unresolved for more than 2000 years. We suspect future research may show that the nature-versus-nurture debate is largely due to a false dichotomy (Traynor and Singleton, 2010). These two forces are in all likelihood inextricably linked in shaping the brain during development and therefore also in forming the self. Precisely how remains unknown, but we place our bets on timing-dependent plasticity being involved one way or the other.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Yves Frégnac, Carla Shatz, Alanna Watt, Rui P. Costa, Kate Buchanan, and Alex Moreau for help and useful discussions and Melissa Cochrane editorial help on locating references and providing editorial comments. We thank John Lisman for the photos of Bliss, Andersen, and Lømo, and of Bernard Katz. This work was funded by the The Blue Brain Project (Henry Markram), and the EU FACETS and EU BrainScales Project (Wulfram Gerstner and Henry Markram), the UK Medical Research Council Career Development Award G0700188 (Per Jesper Sjöström), FP7 grant #243914 “Brain-i-Nets” (Per Jesper Sjöström and Wulfram Gerstner).
Abbott, L. F., and Blum, K. I. (1996). Functional significance of long-term potentiation for sequence learning and prediction. Cereb. Cortex 6, 406–416.
Abbott, L. F., and Nelson, S. B. (2000). Synaptic plasticity: taming the beast. Nat. Neurosci. 3(Suppl.), 1178–1183.
Abbott, L. F., Varela, J. A., Sen, K., and Nelson, S. B. (1997). Synaptic depression and cortical gain control. Science 275, 220–224.
Abeles, M. (1991). Corticonics: Neural Circuits of the Cerebral Cortex. Cambridge: Cambridge University Press.
Abraham, W. C., and Bear, M. F. (1996). Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci. 19, 126–130.
Adrian, E. D., and Matthews, R. (1928). The action of light on the eye: part III. The interaction of retinal neurones. J. Physiol. 65, 273–298.
Aleman, A., and Torres-Aleman, I. (2009). Circulating insulin-like growth factor I and cognitive function: neuromodulation throughout the lifespan. Prog. Neurobiol. 89, 256–265.
Allen, C. B., Celikel, T., and Feldman, D. E. (2003). Long-term depression induced by sensory deprivation during cortical map plasticity in vivo. Nat. Neurosci. 6, 291–299.
Amit, D. J., and Tsodyks, M. V. (1991). Quantitative study of attractor neural network retrieving at low spike rates: I. Substrate-spikes, rates and neuronal gain. Network 2, 259.
Anderson, J. A. (1972). A simple neural network generating an interactive memory. Math. Biosci. 14, 197–220.
Artola, A., Brocher, S., and Singer, W. (1990). Different voltage-dependent thresholds for inducing long-term depression and long-term potentiation in slices of rat visual cortex. Nature 347, 69–72.
Baranyi, A., and Feher, O. (1978). Conditioned changes of synaptic transmission in the motor cortex of the cat. Exp. Brain Res. 33, 283–298.
Baranyi, A., and Feher, O. (1981). Intracellular studies on cortical synaptic plasticity. Conditioning effect of antidromic activation on test-EPSPs. Exp. Brain Res. 41, 124–134.
Barrionuevo, G., Schottler, F., and Lynch, G. (1980). The effects of repetitive low frequency stimulation on control and “potentiated” synaptic responses in the hippocampus. Life Sci. 27, 2385–2391.
Bear, M. F. (1999). Homosynaptic long-term depression: a mechanism for memory? Proc. Natl. Acad. Sci. U.S.A. 96, 9457–9458.
Bear, M. F., and Singer, W. (1986). Modulation of visual cortical plasticity by acetylcholine and noradrenaline. Nature 320, 172–176.
Bell, C. C., Han, V. Z., Sugawara, Y., and Grant, K. (1997). Synaptic plasticity in a cerebellum-like structure depends on temporal order. Nature 387, 278–281.
Bennett, M. R. (2000). The concept of transmitter receptors: 100 years on. Neuropharmacology 39, 523–546.
Berlucchi, G., and Buchtel, H. A. (2009). Neuronal plasticity: historical roots and evolution of meaning. Exp. Brain Res. 192, 307–319.
Bernstein, J. (1868). Ueber den zeitlichen Verlauf der negative Schwankung des Nervenstroms. Pflugers Arch. 1, 173–207.
Bernstein, J. (1902). Untersuchungen zur Thermodynamik der bioelektrischen Strome. Pflugers Arch. ges. Physiol. 92, 521–562.
Bi, G. Q., and Poo, M. M. (1998). Synaptic modifications in cultured hippocampal neurons: dependence on spike timing, synaptic strength, and postsynaptic cell type. J. Neurosci. 18, 10464–10472.
Bienenstock, E. L., Cooper, L. N., and Munro, P. W. (1982). Theory for the development of neuron selectivity: orientation specificity and binocular interaction in visual cortex. J. Neurosci. 2, 32–48.
Bliss, T. V., and Lømo, T. (1970). Plasticity in a monosynaptic cortical pathway. J. Physiol. (Lond.) 207, 61P.
Bliss, T. V., and Lømo, T. (1973). Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J. Physiol. 232, 331–356.
Blum, K. I., and Abbott, L. F. (1996). A model of spatial map formation in the hippocampus of the rat. Neural. Comput. 8, 85–93.
Braitenberg, V. (1984). Rules and Regularities. In Vehicles, Experiments in Synthetic Psychology. Cambridge, MA: The MIT Press, 55–61.
Bramham, C. R., and Srebro, B. (1987). Induction of long-term depression and potentiation by low- and high-frequency stimulation in the dentate area of the anesthetized rat: magnitude, time course and EEG. Brain Res. 405, 100–107.
Brindley, G. S. (1964). The use made by the cerebellum of the information that it receives from sense organs. I.B.R.O. Bull. 3, 80.
Brown, S. P., and Hestrin, S. (2009a). Cell-type identity: a key to unlocking the function of neocortical circuits. Curr. Opin. Neurobiol. 19, 415–421.
Brown, S. P., and Hestrin, S. (2009b). Intracortical circuits of pyramidal neurons reflect their long-range axonal targets. Nature 457, 1133–1136.
Buchanan, K. A., and Mellor, J. R. (2007). The development of synaptic plasticity induction rules and the requirement for postsynaptic spikes in rat hippocampal CA1 pyramidal neurones. J. Physiol. (Lond.) 585, 429–445.
Buchanan, K. A., and Mellor, J. R. (2010). The activity requirements for spike timing-dependent plasticity in the hippocampus. Front. Synaptic Neurosci. 2:11. doi: 10.3389/fnsyn.2010.00011
Bullmore, E., and Sporns, O. (2009). Complex brain networks: graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosci. 10, 186–198.
Butts, D. A., Kanold, P. O., and Shatz, C. J. (2007). A burst-based “Hebbian” learning rule at retinogeniculate synapses links retinal waves to activity-dependent refinement. PLoS Biol. 5, e61. doi: 10.1371/journal.pbio.0050061
Caporale, N., and Dan, Y. (2008). Spike timing-dependent plasticity: a Hebbian learning rule. Annu. Rev. Neurosci. 31, 25–46.
Carvalho, T. P., and Buonomano, D. (2011). A novel learning rule for long-term plasticity of short-term synaptic plasticity enhances temporal processing. Front. Integr. Neurosci. 5:20. doi: 10.3389/fnint.2011.00020
Cassenaer, S., and Laurent, G. (2007). Hebbian STDP in mushroom bodies facilitates the synchronous flow of olfactory information in locusts. Nature 448, 709–713.
Celikel, T., Szostak, V. A., and Feldman, D. E. (2004). Modulation of spike timing by sensory deprivation during induction of cortical map plasticity. Nat. Neurosci. 7, 534–541.
Clopath, C., Busing, L., Vasilaki, E., and Gerstner, W. (2010). Connectivity reflects coding: a model of voltage-based STDP with homeostasis. Nat. Neurosci. 13, 344–352.
Clopath, C., and Gerstner, W. (2010). Voltage and spike timing interact in STDP – a unified model. Front. Synaptic Neurosci. 2:25. doi: 10.3389/fnsyn.2010.00025
Collingridge, G. L., Kehl, S. J., and McLennan, H. (1983). Excitatory amino acids in synaptic transmission in the Schaffer collateral-commissural pathway of the rat hippocampus. J. Physiol. (Lond.) 334, 33–46.
Cooper, L. N. (1973). “A possible organization of learning and memory,” in Proceedings of the Nobel Symposium on Collective Properties of Physical Systems, eds B. Lundqvist, S. Lundqvist, and V. Runnstrom-Reio (Aspen Garden: Nobel), 252–264.
Cooper, L. N. (2010). STDP: spiking, timing, rates and beyond. Front. Synaptic Neurosci. 2:11. doi: 10.3389/fnsyn.2010.00014
Debanne, D., Gahwiler, B. H., and Thompson, S. M. (1994). Asynchronous pre- and postsynaptic activity induces associative long-term depression in area CA1 of the rat hippocampus in vitro. Proc. Natl. Acad. Sci U.S.A. 91, 1148–1152.
Debanne, D., Gähwiler, B. H., and Thompson, S. M. (1998). Long-term synaptic plasticity between pairs of individual CA3 pyramidal cells in rat hippocampal slice cultures. J. Physiol. 507, 237–247.
Debanne, D., Shulz, D. E., and Fregnac, Y. (1995). Temporal constraints in associative synaptic plasticity in hippocampus and neocortex. Can. J. Physiol. Pharmacol. 73, 1295–1311.
Del Castillo, J., and Katz, B. (1954). Statistical factors involved in neuromuscular facilitation and depression. J. Physiol. (Lond.) 124, 574–585.
Douglas, R. M., and Goddard, G. V. (1975). Long-term potentiation of the perforant path-granule cell synapse in the rat hippocampus. Brain Res. 86, 205–215.
Dudek, S. M., and Bear, M. F. (1992). Homosynaptic long-term depression in area CA1 of hippocampus and effects of N-methyl-D-aspartate receptor blockade. Proc. Natl. Acad. Sci. U.S.A. 89, 4363–4367.
Dunwiddie, T., and Lynch, G. (1978). Long-term potentiation and depression of synaptic responses in the rat hippocampus: localization and frequency dependency. J. Physiol. (Lond.) 276, 353–367.
Eccles, J. C. (1960). The Properties of the Dendrites, Structure and Function of the Cerebral Cortex. New York: American Elsevier.
Eccles, J. C., Katz, B., and Kuffler, S. W. (1941). Nature of the “endplate potential” in curarized muscle. J. Neurophysiol. 4, 362–387.
Eccles, J. C., and Sherrington, C. (1931). Studies on the Flexor Reflex. IV. After-Discharge. Proc. R. Soc. Lond. B Biol. Sci. 107, 586–596.
Ekerot, C. F., and Kano, M. (1985). Long-term depression of parallel fibre synapses following stimulation of climbing fibres. Brain Res. 342, 357–360.
Engert, F., and Bonhoeffer, T. (1999). Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature 399, 66–70.
Feldman, D. E. (2000). Timing-based LTP and LTD at vertical inputs to layer II/III pyramidal cells in rat barrel cortex. Neuron 27, 45–56.
Feng, T. P. (1941). Studies on the neuromuscular junction. XXVI. The changes of the end-plate potential during and after prolonged stimulation. Chin. J. Physiol. 16, 301–372.
Fregnac, Y. (2002). “Hebbian synapses,” in The Handbook of Brain Theory and Neural Networks, ed. M.A. Arbib (Cambridge, MA: The MIT Press), 515–521.
Frégnac, Y., Shulz, D., Thorpe, S., and Bienenstock, E. (1988). A cellular analogue of visual cortical plasticity. Nature 333, 367–370.
Froemke, R. C., and Dan, Y. (2002). Spike-timing-dependent synaptic modification induced by natural spike trains. Nature 416, 433–438.
Froemke, R. C., Debanne, D., and Bi, G.-Q. (2010a). Temporal modulation of spike-timing-dependent plasticity. Front. Synaptic Neurosci. 2:19. doi: 10.3389/fnsyn.2010.00019
Froemke, R. C., Letzkus, J. J., Kampa, B. M., Hang, G. B., and Stuart, G. J. (2010b). Dendritic synapse location and neocortical spike-timing-dependent plasticity. Front. Synaptic Neurosci. 2:29. doi: 10.3389/fnsyn.2010.00029
Froemke, R. C., Poo, M., and Dan, Y. (2005). Spike-timing-dependent plasticity depends on dendritic location. Nature 434, 221–225.
Froemke, R. C., Tsay, I. A., Raad, M., Long, J. D., and Dan, Y. (2006). Contribution of individual spikes in burst-induced long-term synaptic modification. J. Neurophysiol. 95, 1620–1629.
Fusi, S. (2002). Hebbian spike-driven synaptic plasticity for learning patterns of mean firing rates. Biol. Cybern. 87, 459–470.
Gasser, H. S., and Newcomer, H. S. (1921). Physiological action currents in the phrenic nerve. An application of the thermionic vacuum tube to nerve physiology. Am. J. Physiol. 57, 1–26.
Gelinas, J. N., and Nguyen, P. V. (2007). Neuromodulation of hippocampal synaptic plasticity, learning and memory by noradrenaline. Cent. Nerv. Syst. Agents Med. Chem. 7, 17–33.
Gerstner, W., Kempter, R., van Hemmen, J. L., and Wagner, H. (1996). A neuronal learning rule for sub-millisecond temporal coding. Nature 383, 76–81.
Gerstner, W., Ritz, R., and van Hemmen, J. L. (1993). Why spikes? Hebbian learning and retrieval of time-resolved excitation patterns. Biol. Cybern. 69, 503–515.
Gerstner, W., and van Hemmen, J. L. (1992). Universality in neural networks: the importance of the “mean firing rate.” Biol. Cybern. 67, 195–205.
Gilson, M., Burkitt, A., and Van Hemmen, L. J. (2010). STDP in recurrent neuronal networks. Front. Comput. Neurosci. 4:23. doi: 10.3389/fncom.2010.00023
Golding, N. L., Staff, N. P., and Spruston, N. (2002). Dendritic spikes as a mechanism for cooperative long-term potentiation. Nature 418, 326–331.
Gotch, F., and Horsley, V. (1891). Croonian lecture: on the mammalian nervous system; its functions and their localisation determined by an electrical method. Philos. Trans. R. Soc. Lond., B, Biol. Sci. 182, 267–526.
Grossberg, S. (1969). On the production and release of chemical transmitters and related topics in cellular control. J. Theor. Biol. 22, 325–364.
Gustafsson, B., and Wigström, H. (1986). Hippocampal long-lasting potentiation produced by pairing single volleys and brief conditioning tetani evoked in separate afferents. J. Neurosci. 6, 1575–1582.
Gustafsson, B., Wigström, H., Abraham, W. C., and Huang, Y. Y. (1987). Long-term potentiation in the hippocampus using depolarizing current pulses as the conditioning stimulus to single volley synaptic potentials. J. Neurosci. 7, 774–780.
Han, V. Z., Grant, K., and Bell, C. C. (2000). Reversible associative depression and nonassociative potentiation at a parallel fiber synapse. Neuron 27, 611–622.
Hardie, J., and Spruston, N. (2009). Synaptic depolarization is more effective than back-propagating action potentials during induction of associative long-term potentiation in hippocampal pyramidal neurons. J. Neurosci. 29, 3233–3241.
Harris, E. W., Ganong, A. H., and Cotman, C. W. (1984). Long-term potentiation in the hippocampus involves activation of N-methyl-D-aspartate receptors. Brain Res. 323, 132–137.
Hartley, D. (1749). Observations on Man, His Frame, His Duty, and His Expectations. London: Cambridge University Press.
Hasselmo, M. E. (1995). Neuromodulation and cortical function: modeling the physiological basis of behavior. Behav. Brain Res. 67, 1–27.
Hasselmo, M. E., and Sarter, M. (2011). Modes and models of forebrain cholinergic neuromodulation of cognition. Neuropsychopharmacology 36, 52–73.
Herz, A. V. M., Sulzer, B., Kühnvan, R., and van Hemmen, J. L. (1988). The Hebb rule: representation of static and dynamic objects in neural nets. Europhys. Lett. 7, 663–669.
Holmgren, C. D., and Zilberter, Y. (2001). Coincident spiking activity induces long-term changes in inhibition of neocortical pyramidal cells. J. Neurosci. 21, 8270–8277.
Hopfield, J. J. (1982). Neural networks and physical systems with emergent collective computational abilities. Proc. Natl. Acad. Sci. U.S.A. 79, 2554–2558.
Hubel, D. H., and Wiesel, T. N. (1965). Binocular interaction in striate cortex of kittens reared with artificial squint. J. Neurophysiol. 28, 1041–1059.
Ito, M., Sakurai, M., and Tongroach, P. (1982). Climbing fibre induced depression of both mossy fibre responsiveness and glutamate sensitivity of cerebellar Purkinje cells. J. Physiol. (Lond.) 324, 113–134.
Jacob, V., Brasier, D. J., Erchova, I., Feldman, D., and Shulz, D. E. (2007). Spike timing-dependent synaptic depression in the in vivo barrel cortex of the rat. J. Neurosci. 27, 1271–1284.
Kampa, B. M., Letzkus, J. J., and Stuart, G. J. (2006). Cortical feed-forward networks for binding different streams of sensory information. Nat. Neurosci. 9, 1472–1473.
Kandel, E. R. (2001). The molecular biology of memory storage: a dialog between genes and synapses. Biosci. Rep. 21, 565–611.
Kandel, E. R. (2006). In Search of Memory: The Emergence of a New Science of Mind, 1st Edn. New York: W. W. Norton & Company.
Kandel, E. R., and Tauc, L. (1964). Mechanism of prolonged heterosynaptic facilitation. Nature 202, 145–147.
Kelso, S. R., Ganong, A. H., and Brown, T. H. (1986). Hebbian synapses in hippocampus. Proc. Natl. Acad. Sci. U.S.A. 83, 5326–5330.
Kempter, R., Gerstner, W., and van Hemmen, J. L. (1999a). Hebbian learning and spiking neurons. Phys. Rev. E 59, 4498–4514.
Kempter, R., Gerstner, W., and van Hemmen, J. L. (1999b). “Spike-based compared to rate-based Hebbian learning,” in Advances in neural information processing systems, eds M.S. Kearns, S.A. Solla, and D.A. Cohn (Cambridge, MA: MIT Press), 125–131.
Kempter, R., Gerstner, W., and van Hemmen, J. L. (2001). Intrinsic stabilization of output rates by spike-based Hebbian learning. Neural Comput. 13, 2709–2741.
Kerr, D. S., and Abraham, W. C. (1993). Comparison of associative and non-associative conditioning procedures in the induction of LTD in CA1 of the hippocampus. Synapse 14, 305–313.
Kilgard, M. P., and Merzenich, M. M. (1998). Cortical map reorganization enabled by nucleus basalis activity. Science 279, 1714–1718.
Kleinfeld, D., and Sompolinsky, H. (1988). Associative neural network model for the generation of temporal patterns. Theory and application to central pattern generators. Biophys. J. 54, 1039–1051.
Ko, H., Hofer, S. B., Pichler, B., Buchanan, K. A., Sjöström, P. J., and Mrsic-Flogel, T. D. (2011). Functional specificity of local synaptic connections in neocortical networks. Nature 473, 87–91.
Konorski, J. (1948). Conditioned Reflexes and Neuron Organization. Cambridge: Cambridge University Press.
Kullmann, D. M., and Lamsa, K. P. (2007). Long-term synaptic plasticity in hippocampal interneurons. Nat. Rev. Neurosci. 8, 687–699.
Kwon, H. B., and Sabatini, B. L. (2011). Glutamate induces de novo growth of functional spines in developing cortex. Nature 474, 100–104.
Lamprecht, R., and LeDoux, J. (2004). Structural plasticity and memory. Nat. Rev. Neurosci. 5, 45–54.
Lamsa, K. P., Kullmann, D. M., and Woodin, M. A. (2010). Spike-timing dependent plasticity in inhibitory circuits. Front. Synaptic Neurosci. 2:8. doi: 10.3389/fnsyn.2010.00008
Larrabee, M. G., and Bronk, D. W. (1947). Prolonged facilitation of synaptic excitation in sympathetic ganglia. J. Neurophysiol. 10, 139–154.
Lashley, K. S. (1929). Brain Mechanisms and Intelligence. A Quantitative Study of Injuries to the Brain. Chicago: University of Chicago Press.
Le Be, J. V., and Markram, H. (2006). Spontaneous and evoked synaptic rewiring in the neonatal neocortex. Proc. Natl. Acad. Sci. U.S.A. 103, 13214–13219.
Le Be, J. V., Silberberg, G., Wang, Y., and Markram, H. (2007). Morphological, electrophysiological, and synaptic properties of corticocallosal pyramidal cells in the neonatal rat neocortex. Cereb. Cortex 17, 2204–2213.
Letzkus, J. J., Kampa, B. M., and Stuart, G. J. (2006). Learning rules for spike timing-dependent plasticity depend on dendritic synapse location. J. Neurosci. 26, 10420–10429.
Levy, W. B., and Steward, O. (1979). Synapses as associative memory elements in the hippocampal formation. Brain Res. 175, 233–245.
Levy, W. B., and Steward, O. (1983). Temporal contiguity requirements for long-term associative potentiation/depression in the hippocampus. Neuroscience 8, 791–797.
Li, S.-C., Brehmer, Y., Shing, Y. L., Werkle-Bergner, M., and Lindenberger, U. (2006). Neuromodulation of associative and organizational plasticity across the life span: empirical evidence and neurocomputational modeling. Neurosci. Biobehav. Rev. 30, 775–790.
Libet, B., and Gerard, R. W. (1939). Control of the potential rhythm of the isolated frog brain. J. Neurophysiol. 2, 153–169.
Lisman, J., and Spruston, N. (2005). Postsynaptic depolarization requirements for LTP and LTD: a critique of spike timing-dependent plasticity. Nat. Neurosci. 8, 839–841.
Lisman, J., and Spruston, N. (2010). Questions about STDP as a general model of synaptic plasticity. Front. Synaptic Neurosci. 2:140. doi: 10.3389/fnsyn.2010.00140
Lloyd, D. P. (1949). Post-tetanic potentiation of response in monosynaptic reflex pathways of the spinal cord. J. Gen. Physiol. 33, 147–170.
Lømo, T. (1964). Frequency potentiation of excitatory synaptic activity in the dentate area of the hippocampal formation. Acta Physiol. Scand. 68, 128.
Lorente de Nó, R. (1934). Studies on the structure of the cerebral cortex. II. Continuation of the study of the ammonic system. J. Psychol. Neurol. 46, 113–177.
Lugaro, E. (1909). Modern Problems in Psychiatry. (Translated by D. Orr, and R. G. Rows). Manchester: The University Press.
Lynch, G. S., Dunwiddie, T., and Gribkoff, V. (1977). Heterosynaptic depression: a postsynaptic correlate of long-term potentiation. Nature 266, 737–739.
Magee, J. C., and Johnston, D. (1997). A synaptically controlled, associative signal for Hebbian plasticity in hippocampal neurons. Science 275, 209–213.
Malenka, R. C., and Nicoll, R. A. (1999). Long-term potentiation – a decade of progress? Science 285, 1870–1874.
Malinow, R. (1991). Transmission between pairs of hippocampal slice neurons: quantal levels, oscillations, and LTP. Science 252, 722–724.
Maren, S., and Fanselow, M. S. (1996). The amygdala and fear conditioning: has the nut been cracked? Neuron 16, 237–240.
Markram, H., Gupta, A., Uziel, A., Wang, Y., and Tsodyks, M. (1998). Information processing with frequency-dependent synaptic connections. Neurobiol. Learn. Mem. 70, 101–112.
Markram, H., Helm, P. J., and Sakmann, B. (1995). Dendritic calcium transients evoked by single back-propagating action potentials in rat neocortical pyramidal neurons. J. Physiol. (Lond.) 485, 1–20.
Markram, H., Lubke, J., Frotscher, M., Roth, A., and Sakmann, B. (1997a). Physiology and anatomy of synaptic connections between thick tufted pyramidal neurones in the developing rat neocortex. J. Physiol. (Lond.) 500, 409–440.
Markram, H., Lübke, J., Frotscher, M., and Sakmann, B. (1997b). Regulation of synaptic efficacy by coincidence of postsynaptic APs and EPSPs. Science 275, 213–215.
Markram, H., and Sakmann, B. (1994). Calcium transients in dendrites of neocortical neurons evoked by single subthreshold excitatory postsynaptic potentials via low-voltage-activated calcium channels. Proc. Natl. Acad. Sci. U.S.A. 91, 5207–5211.
Markram, H., and Sakmann, B. (1995). Action potentials propogating back into dendrites triggers changes in efficacy of single-axon synapses between layer V pyramidal cells. Soc. Neurosci. Abstr. 21, 2007.
Markram, H., and Segal, M. (1990). Acetylcholine potentiates responses to N-methyl-d-aspartate in the rat hippocampus. Neurosci. Lett. 113, 62–65.
Markram, H., and Tsodyks, M. (1996a). Redistribution of synaptic efficacy between neocortical pyramidal neurons. Nature 382, 807–810.
Markram, H., and Tsodyks, M. (1996b). Redistribution of synaptic efficacy: a mechanism to generate infinite synaptic input diversity from a homogeneous population of neurons without changing absolute synaptic efficacies. J. Physiol. Paris 90, 229–232.
Marr, D. (1971). Simple memory: a theory for archicortex. Philos. Trans. R. Soc. Lond., B, Biol. Sci. 262, 23–81.
Matteucci, C. (1838). Sur le courant électrique ou propre de la grénouille. Bibl. Univ. Genève 15, 157–168.
McCullogh, W. S., and Pitts, W. H. (1943). A logical calculus of the ideas immanent in nervous activity. Bull. Math. Biophys. 5, 115–113.
McNaughton, B. L. (2003). Long-term potentiation, cooperativity and Hebb’s cell assemblies: a personal history. Philos. Trans. R. Soc. Lond., B, Biol. Sci. 358, 629–634.
McNaughton, B. L., Douglas, R. M., and Goddard, G. V. (1978). Synaptic enhancement in fascia dentata: cooperativity among coactive afferents. Brain Res. 157, 277–293.
Meliza, C. D., and Dan, Y. (2006). Receptive-field modification in rat visual cortex induced by paired visual stimulation and single-cell spiking. Neuron 49, 183–189.
Mihalas, S. (2011). Calcium messenger heterogeneity: a possible signal for spike-timing-dependent plasticity. Front. Comput. Neurosci. 4:158. doi: 10.3389/fncom.2010.00158
Mulkey, R. M., and Malenka, R. C. (1992). Mechanisms underlying induction of homosynaptic long-term depression in area CA1 of the hippocampus. Neuron 9, 967–975.
Müller-Dahlhaus, F., Ziemann, U., and Classen, J. (2010). Plasticity resembling spike-timing dependent synaptic plasticity: the evidence in human cortex. Front. Synaptic Neurosci. 2:34. doi: 10.3389/fnsyn.2010.00034
Nelson, S. B., Sjöström, P. J., and Turrigiano, G. G. (2002). Rate and timing in cortical synaptic plasticity. Philos. Trans. R. Soc. Lond., B, Biol. Sci. 357, 1851–1857.
Paulsen, O., Li, Y. G., Hvalby, O., Anderson, P., and Bliss, T. V. (1993). Failure to induce long-term depression by an anti-correlation procedure in area CA1 of the rat hippocampal slice. Eur. J. Neurosci. 5, 1241–1246.
Pavlov, I. P. (1897). The Work of the Digestive Glands English Translation by W. H. Thompson, London, 1902. (London: Griffin).
Pawlak, V., and Kerr, J. N. (2008). Dopamine receptor activation is required for corticostriatal spike-timing-dependent plasticity. J. Neurosci. 28, 2435–2446.
Pawlak, V., Wickens, J. R., Kirkwood, A., and Kerr, J. N. D. (2010). Timing is not everything: neuromodulation opens the STDP gate. Front. Synaptic Neurosci. 2, 146. doi: 10.3389/fnsyn.2010.00146
Penfield, W., and Boldrey, E. (1937). Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain Res. 60, 389–443.
Perin, R., Berger, T. K., and Markram, H. (2011). A synaptic organizing principle for cortical neuronal groups. Proc. Natl. Acad. Sci. U.S.A. 108, 5419–5424.
Perrett, S. P., Dudek, S. M., Eagleman, D., Montague, P. R., and Friedlander, M. J. (2001). LTD induction in adult visual cortex: role of stimulus timing and inhibition. J. Neurosci. 21, 2308–2319.
Quiroga, R. Q., Reddy, L., Kreiman, G., Koch, C., and Fried, I. (2005). Invariant visual representation by single neurons in the human brain. Nature 435, 1102–1107.
Rackham, O., Tsaneva-Atanasova, K., Ganesh, A., and Mellor, J. (2010). A Ca2+-based computational model for NDMA receptor-dependent synaptic plasticity at individual post-synaptic spines in the hippocampus. Front. Synaptic Neurosci. 2, 31. doi: 10.3389/fnsyn.2010.00031
Rall, W. (1955). A statistical theory of monosynaptic input-output relations. J. Cell. Physiol. 46, 373–411.
Rall, W. (1959). Branching dendritic trees and motoneuron membrane resistivity. Exp. Neurol. 1, 491–527.
Rall, W. (1960). Membrane potential transients and membrane time constant of motoneurons. Exp. Neurol. 2, 503–532.
Rall, W., and Rinzel, J. (1971). Dendritic spine function and synaptic attenuation calculations. Prog. Abstr. Soc. Neurosci. First Ann. Mtg. 64.
Ramón y Cajal, S. (1894). The Croonian Lecture: La Fine Structure Des Centres Nerveux. Proc. R. Soc. Lond., B, Biol. Sci. 4, 444–468.
Ramón y Cajal, S. (1911). Histologie du Système Nerveux de l’Homme et des Vertebrés, Vol. 2. Paris: Malone.
Remy, S., and Spruston, N. (2007). Dendritic Spikes induce single-burst long-term potentiation. Proc. Natl. Acad. Sci. U.S.A. 104, 17192–17197.
Rescorla, R. A., and Wagner, A. R. (1972). “A theory of Pavlovian conditioning: variations in the effectiveness of reinforcement and nonreinforcement,” in Classical Conditioning II: Current Research and Theory, eds A. H. Black, and W. F. Prokasy (New York: Appleton Century Crofts), 64–99.
Ribot, T. (1870). La Psychologie Anglaise Contemporaine. Paris: Librairie philosophique de Ladrange.
Roberts, P. D., and Bell, C. C. (2000). Computational consequences of temporally asymmetric learning rules: II. Sensory image cancellation. J. Comput. Neurosci. 9, 67–83.
Rochester, N., Holland, J., Haibt, L., and Duda, W. (1956). Tests on a cell assembly theory of the action of the brain, using a large digital computer. IRE Trans. Inf. Theory 2, 80–93.
Rodrigues, S. M., Schafe, G. E., and LeDoux, J. E. (2004). Molecular mechanisms underlying emotional learning and memory in the lateral amygdala. Neuron 44, 75–91.
Rosenblatt, F. (1958). The perceptron: a probabilistic model for information storage and organization in the brain. Psychol. Rev. 65, 386–408.
Sances, A., Myklebust, J., Larson, S. J., and Cusick, J. F. (1980). The evoked potential and early studies of bioelectricity. J. Clin. Eng. 5.
Sara, S. J. (2009). The locus coeruleus and noradrenergic modulation of cognition. Nat. Rev. 10, 211–223.
Schuett, S., Bonhoeffer, T., and Hubener, M. (2001). Pairing-induced changes of orientation maps in cat visual cortex. Neuron 32, 325–337.
Sejnowski, T. J. (1977a). Statistical constraints on synaptic plasticity. J. Theor. Biol. 69, 385–389.
Sejnowski, T. J. (1977b). Storing covariance with nonlinearly interacting neurons. J. Math. Biol. 4, 303–321.
Senn, W., Markram, H., and Tsodyks, M. (2001). An algorithm for modifying neurotransmitter release probability based on pre- and postsynaptic spike timing. Neural. Comput. 13, 35–67.
Seol, G. H., Ziburkus, J., Huang, S., Song, L., Kim, I. T., Takamiya, K., Huganir, R. L., Lee, H. K., and Kirkwood, A. (2007). Neuromodulators control the polarity of spike-timing-dependent synaptic plasticity. Neuron 55, 919–929.
Sherrington, C. S. (1897). “The central nervous system,” in A Textbook of Physiology, 7th Edn, ed. M. Foster (London: Macmillan), 3, :929.
Sherrington, C. S. (1909). On plastic tonus and proprioceptive reflexes. Q. J. Exp. Physiol. 2, 109–156.
Shouval, H. Z., Bear, M. F., and Cooper, L. N. (2002). A unified model of NMDA receptor-dependent bidirectional synaptic plasticity. Proc. Natl. Acad. Sci. U.S.A. 99, 10831–10836.
Shulz, D. E., and Jacob, V. (2010). Spike timing dependent plasticity in the intact brain: counteracting spurious spike coincidences. Front. Synaptic Neurosci. 2, 137. doi: 10.3389/fnsyn.2010.00137
Sjöström, P. J., and Häusser, M. (2006). A cooperative switch determines the sign of synaptic plasticity in distal dendrites of neocortical pyramidal neurons. Neuron 51, 227–238.
Sjöström, P. J., Rancz, E. A., Roth, A., and Hausser, M. (2008). Dendritic excitability and synaptic plasticity. Physiol. Rev. 88, 769–840.
Sjöström, P. J., Turrigiano, G. G., and Nelson, S. B. (2001). Rate, timing, and cooperativity jointly determine cortical synaptic plasticity. Neuron 32, 1149–1164.
Sjöström, P. J., Turrigiano, G. G., and Nelson, S. B. (2003). Neocortical LTD via coincident activation of presynaptic NMDA and cannabinoid receptors. Neuron 39, 641–654.
Sjöström, P. J., Turrigiano, G. G., and Nelson, S. B. (2004). Endocannabinoid-dependent neocortical layer-5 LTD in the absence of postsynaptic spiking. J. Neurophysiol. 92, 3338–3343.
Skinner, B. F. (1938). The Behavior or Organisms: An Experimental Analysis. New York: Appleton-Century-Crofts.
Slater, N. T., Stelzer, A., and Galvan, M. (1985). Kindling-like stimulus patterns induce epileptiform discharges in the guinea pig in vitro hippocampus. Neurosci. Lett. 60, 25–31.
Sompolinsky, H., and Kanter, I. I. (1986). Temporal association in asymmetric neural networks. Phys. Rev. Lett. 57, 2861–2864.
Song, S., and Abbott, L. F. (2001). Cortical development and remapping through spike timing-dependent plasticity. Neuron 32, 339–350.
Song, S., Miller, K. D., and Abbott, L. F. (2000). Competitive Hebbian learning through spike-timing-dependent synaptic plasticity. Nat. Neurosci. 3, 919–926.
Song, S., Sjöström, P. J., Reigl, M., Nelson, S., and Chklovskii, D. B. (2005). Highly nonrandom features of synaptic connectivity in local cortical circuits. PLoS Biol. 3, e68. doi: 10.1371/journal.pbio.0030068
Stanton, P. K., and Sejnowski, T. J. (1989). Associative long-term depression in the hippocampus induced by hebbian covariance. Nature 339, 215–218.
Stefan, K., Kunesch, E., Cohen, L. G., Benecke, R., and Classen, J. (2000). Induction of plasticity in the human motor cortex by paired associative stimulation. Brain 123, 572–584.
Steinbuch, K., Jaenicke, W., and Reiner, H. (1965). Learning Matrix. C.I.P. Office. Available at: http://brevets-patents.ic.gc.ca/opic-cipo/cpd/eng/patent/717227/summary.html
Stent, G. S. (1973). A physiological mechanism for Hebb’s postulate of learning. Proc. Natl. Acad. Sci. U.S.A. 70, 997–1001.
Stepanyants, A., Tamas, G., and Chklovskii, D. B. (2004). Class-specific features of neuronal wiring. Neuron 43, 251–259.
Stuart, G. J., and Sakmann, B. (1994). Active propagation of somatic action potentials into neocortical pyramidal cell dendrites. Nature 367, 69–72.
Tanimoto, H., Heisenberg, M., and Gerber, B. (2004). Experimental psychology: event timing turns punishment to reward. Nature 430, 983.
Tanzi, E. (1893). I fatti e le induzioni dell’odierna istologia del sistema nervoso. Riv Sper Fren Med Leg 19, 419–472.
Toda, M., and Abi-Dargham, A. (2007). Dopamine hypothesis of schizophrenia: making sense of it all. Curr. Psychiatry Rep. 9, 329–336.
Toyoizumi, T., Pfister, J.-P., Aihara, K., and Gerstner, W. (2005). Generalized Bienenstock–Cooper–Munro rule for spiking neurons that maximizes information transmission. Proc. Natl. Acad. Sci. U.S.A. 102, 5239–5244.
Toyoizumi, T., Pfister, J.-P., Aihara, K., and Gerstner, W. (2007a). Optimality model of unsupervised spike-timing-dependent plasticity: synaptic memory and weight distribution. Neural. Comput. 19, 639–671.
Toyoizumi, T., Pfister, J. P., Aihara, K., and Gerstner, W. (2007b). Optimality model of unsupervised spike-timing-dependent plasticity: synaptic memory and weight distribution. Neural. Comput. 19, 639–671.
Traynor, B. J., and Singleton, A. B. (2010). Nature versus nurture: death of a dogma, and the road ahead. Neuron 68, 196–200.
Tsodyks, M. (2002). Spike-timing-dependent synaptic plasticity - the long road towards understanding neuronal mechanisms of learning and memory. Trends Neurosci. 25, 599–600.
Tsodyks, M., Pawelzik, K., and Markram, H. (1998). Neural networks with dynamic synapses. Neural. Comput. 10, 821–835.
Tsodyks, M. V., and Markram, H. (1997). The neural code between neocortical pyramidal neurons depends on neurotransmitter release probability. Proc. Natl. Acad. Sci. U.S.A. 94, 719–723.
Turrigiano, G., Abbott, L. F., and Marder, E. (1994). Activity-dependent changes in the intrinsic properties of cultured neurons. Science 264, 974–977.
Turrigiano, G. G., Leslie, K. R., Desai, N. S., Rutherford, L. C., and Nelson, S. B. (1998). Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature 391, 892–896.
Turrigiano, G. G., and Nelson, S. B. (2000). Hebb and homeostasis in neuronal plasticity. Curr. Opin. Neurobiol. 10, 358–364.
Turrigiano, G. G., and Nelson, S. B. (2004). Homeostatic plasticity in the developing nervous system. Nat. Rev. Neurosci. 5, 97–107.
van Rossum, M. C. W., Bi, G. Q., and Turrigiano, G. G. (2000). Stable Hebbian learning from spike timing-dependent plasticity. J. Neurosci. 20, 8812–8821.
Varela, J. A., Sen, K., Gibson, J., Fost, J., Abbott, L. F., and Nelson, S. B. (1997). A quantitative description of short-term plasticity at excitatory synapses in layer 2/3 of rat primary visual cortex. J. Neurosci. 17, 7926–7940.
von der Malsburg, C. (1973). Self-organization of orientation sensitive cells in the striate cortex. Kybernetik. 14, 85–100.
von Waldeyer-Hartz, H. (1891). Ueber einige neuere Forschungen im Gebiete der Anatomie des Centralnervensystems. Dtsch. Med. Wochenschr. 17, 1352–1356.
Wang, H. X., Gerkin, R. C., Nauen, D. W., and Bi, G. Q. (2005). Coactivation and timing-dependent integration of synaptic potentiation and depression. Nat. Neurosci. 8, 187–193.
Watt, A. J., and Desai, N. S. (2010). Homeostatic plasticity and STDP: keeping a neuron’s cool in a fluctuating world. Front. Synaptic Neurosci. 2:5. doi: 10.3389/fnsyn.2010.00005
Wiesel, T. N., and Hubel, D. H. (1965). Comparison of the effects of unilateral and bilateral eye closure on cortical unit responses in kittens. J. Neurophysiol. 28, 1029–1040.
Wigström, H., and Gustafsson, B. (1984). A possible correlate of the postsynaptic condition for long-lasting potentiation in the guinea pig hippocampus in vitro. Neurosci. Lett. 44, 327–332.
Wigström, H., Gustafsson, B., and Huang, Y. Y. (1985). A synaptic potential following single volleys in the hippocampal CA1 region possibly involved in the induction of long-lasting potentiation. Acta Physiol. Scand. 124, 475–478.
Willshaw, D. J., Buneman, O. P., and Longuet-Higgins, H. C. (1969). Non-holographic associative memory. Nature 222, 960–962.
Willwacher, G. (1976). Fiihigkeiten eines assoziativen Speichersystems im. Vergleich zu Gehirnfunktionen. Biol. Cybernetics 24, 181–198.
Wittenberg, G. M., and Wang, S. S. -H. (2006). Malleability of spike-timing-dependent plasticity at the CA3-CA1 synapse. J. Neurosci. 26, 6610–6617.
Yao, H., and Dan, Y. (2001). Stimulus timing-dependent plasticity in cortical processing of orientation. Neuron 32, 315–323.
Yao, H., Shen, Y., and Dan, Y. (2004). Intracortical mechanism of stimulus-timing-dependent plasticity in visual cortical orientation tuning. Proc. Natl. Acad. Sci. U.S.A. 101, 5081–5086.
Yoshimura, Y., and Callaway, E. M. (2005). Fine-scale specificity of cortical networks depends on inhibitory cell type and connectivity. Nat. Neurosci. 8, 1552–1559.
Yoshimura, Y., Dantzker, J. L., and Dan, Y. (2005). Excitatory cortical neurons form fine-scale functional networks. Nature 433, 868–873.
Yu, Y. C., Bultje, R. S., Wang, X., and Shi, S. H. (2009). Specific synapses develop preferentially among sister excitatory neurons in the neocortex. Nature 458, 501–504.
Zhang, J. C., Lau, P. M., and Bi, G. Q. (2009). Gain in sensitivity and loss in temporal contrast of STDP by dopaminergic modulation at hippocampal synapses. Proc. Natl. Acad. Sci. U.S.A. 106, 13028–13033.
Zhang, L. I., Tao, H. W., Holt, C. E., Harris, W. A., and Poo, M. (1998). A critical window for cooperation and competition among developing retinotectal synapses. Nature 395, 37–44.
Keywords: synaptic plasticity, spike-timing-dependent plasticity, bidirectional plasticity, long term depression, long term plasticity, history, learning, memory
Citation: Markram H, Gerstner W and Sjöström PJ (2011) A history of spike-timing-dependent plasticity. Front. Syn. Neurosci. 3:4. doi: 10.3389/fnsyn.2011.00004
Received: 20 February 2011; Paper pending published: 19 April 2011;
Accepted: 25 July 2011; Published online: 29 August 2011.
Edited by:
Mary B. Kennedy, Caltech, USAReviewed by:
Kei Cho, University of Bristol, UKCopyright: © 2011 Markram, Gerstner and Sjöström. This is an open-access article subject to a non-exclusive license between the authors and Frontiers Media SA, which permits use, distribution and reproduction in other forums, provided the original authors and source are credited and other Frontiers conditions are complied with.
*Correspondence: Henry Markram, Brain Mind Institute, Ecole Polytechnique Fédérale de Lausanne, CH-1015 Lausanne, Switzerland. e-mail:aGVucnkubWFya3JhbUBlcGZsLmNo
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.